Abstract
INTRODUCTION
Perineal wound breakdown with delayed wound healing represents a significant cause of morbidity following surgery and radiotherapy to the perineum. The rectus abdominis myocutaneous (RAM) flap has been used increasingly to reconstruct the perineum with good effect. We describe our six-year experience of reconstruction of the perineum with the RAM flap and share some surgical adjuncts we believe are useful.
METHODS
We conducted a retrospective case note review of all patients who underwent a reconstruction of the perineum using the RAM flap between August 2003 and October 2009. Indications for the flap, complication rates and outcomes were all observed.
RESULTS
We conducted 16 RAM flap procedures, 15 of which (94%) were primary repairs and 1 (6%) a secondary repair. Three (19%) developed donor site hernias, two (12.5%) developed minor perineal wound infections, eight (50%) developed minor perineal wound breakdown and in one (6%) flap failure was observed. No perineal hernias were observed. There were no surgical mortalities.
CONCLUSIONS
The RAM flap has a high success rate and an acceptable morbidity rate and is a useful tool in the reconstruction of complex perineal wounds. Modifications to the standard surgical technique may reduce complications and improve the versatility of this flap.
Keywords: Abdominoperineal resection, Perineal wound, Rectus abdominis flap
Since Miles first described the operation of abdominoperineal resection (APR) in 1908,1 delayed perineal wound healing has been recognised as a problem. Over the last few decades, based on documented reduced local recurrence rates,2 there has been an increased utilisation of pelvic radiotherapy in the treatment of anal and rectal carcinomas. This has had a detrimental effect on wound healing in those patients subsequently coming to surgery. More recently, the adoption of the cylindrical extralevator abdominoperineal excision with resultant larger perineal defects and pelvic dead space may also have compounded the problem. There can be considerable morbidity from these perineal wounds and their increased use has demanded newer strategies to deal with the problem. One such strategy is the use of myocutaneous or myofascial flaps to fill these defects.
First described by Mathes and Bostwick in 1977,3 the rectus abdominis myocutaneous (RAM) flap has been used in the reconstruction of surgical defects at various sites of the body.4–6 Its use in filling complex perineal defects, however, was first described by Shkula and Hughes in 19847 and later popularised by Tobin and Day.8 Due to its robustness, simplicity in execution and versatility, the RAM flap is now an established technique in the armamentarium of many colorectal and reconstructive surgeons across the UK.
We present our experience of this technique in a large teaching hospital over a six-year period and highlight various modifications to the technique in an attempt to minimise morbidity based on our growing experience, which others may find useful in their practice.
Methods
Patients who had undergone an APR and had reconstruction with a RAM flap over a six-year period between August 2003 and October 2009 were identified from an operative database. The case notes of these patients were reviewed. Patient demographics and the utilisation of neoadjuvant radiotherapy were recorded. The surgical diagnosis and whether the flap was employed with the primary surgery or as a secondary salvage procedure was also noted.
The initial APR technique was supine. However, the authors have since changed their technique to prone APR and have not found this to be an impedance to using a RAM flap. The perineal part is an extralevator cylindrical dissection in all patients. After mobilising the flap, it is sutured in the correct orientation to the specimen. The abdomen is closed and the patient is turned. On completion of the perineal part of the operation, the specimen is extracted and the flap is delivered with it. The suture will have held it in the correct orientation.
A standard technique for flap reconstruction was employed and any variations on this technique along with the reasons for this variation were recorded. The patients were followed up in the outpatient clinic according to the standard cancer protocol in our institution. They were seen at six weeks after surgery and then every three months for the first year and every six months for the second year. Following this, the patients were reviewed annually until discharge at five years after the resection.
Postoperative data on general or specific complications were noted. Specific data sought included perineal wound breakdown, perineal wound infection, flap necrosis and flap failure. Furthermore, donor site incisional hernias and functional defects left from removal of the rectus muscle were also specifically sought at follow up and the presence of perineal hernias was documented. Recurrence of disease in the case of carcinoma diagnosis was recorded.
Our standard technique
A RAM flap based on the deep inferior epigastric artery and vein was employed. A marked skin paddle was incised starting at the level of the umbilicus and extending cephalad to the costal margin. The width of the skin paddle was determined by a combination of the size of the perineal defect to be filled and the mobility of the abdominal wall so that the resultant defect could be closed. The subcutaneous fat was incised to the anterior rectus sheath. The rectus sheath was incised as an ellipse, extending the incision inferiorly to expose the whole of the rectus muscle. The muscle was divided by coagulation several centimetres superior to the skin and fascial paddle.
The flap was raised out of the rectus sheath from superior to inferior. At the level of the arcuate line, the inferior epigastric artery and vein was identified as it enters the muscle laterally. The attachment of the rectus muscle to the symphysis pubis is generally left intact to add support to the inferior epigastric vessel and prevent ‘bowstringing’ and twisting of this vessel. An incision was made in the peritoneum suprapubically to create a gutter for the flap to lie in and the flap was rotated through a 270° somersault to reach the pelvic floor. The flap was sutured in place with subcuticular sutures and reinforced with interrupted nylon sutures.
The abdominal wall was closed with nylon sutures, incorporating the anterior and posterior rectus sheath from where the rectus muscle had been removed. This was always possible and was made easier if not too large a fascial defect had been created in the anterior rectus sheath at the site of flap elevation as the flap can survive adequately on the umbilical perforators alone. Care was again taken when incorporating the posterior peritoneal layer below the arcuate line so as not to compromise the flap, bearing in mind that this layer would not add strength to the closure.
If the patient had had surgery such as an open appendicectomy or a previous stoma brought through the rectus abdominis muscle, preference was given to the use of the other rectus muscle to create the flap. Where this was impractical, we reviewed the preoperative contrast enhanced computed tomography with a radiologist and confirmed patency of the appropriate inferior epigastric vessel prior to using this side for the flap.
Results
Patient demographics
Sixteen patients were identified. The mean age was 63.6 years (range: 29–83 years). All patients underwent a RAM flap for reconstruction of their perineum. There were eight men and eight women. One patient (6%) underwent surgery for extensive perineal sepsis from Crohn's disease whereas the remainder had surgery for a malignant process. The most common diagnoses were low rectal carcinoma following radiotherapy or perineal squamous cell carcinoma where chemoradiotherapy had failed to control the disease (Table 1).
Table 1.
Initial diagnosis
| Initial diagnosis | Numbers involved |
|---|---|
| Low rectal carcinoma | 9 (56%) |
| Anal canal squamous carcinoma | 4 (25%) |
| Rectal/perianal Crohn's disease | 1 (6%) |
| Vulval carcinoma | 1 (6%) |
| Ischiorectal sarcoma | 1 (6%) |
Fifteen patients (94%) underwent a primary repair and one patient (6%) had a secondary repair two years after primary surgery. Six female patients had part of their posterior vaginal wall excised and reconstructed with the flap. Two of sixteen flaps were muscle-only flaps without the cutaneous paddle, one due to morbid obesity and the other due to technical ease. Both were used for vaginal reconstruction and both healed primarily. One RAM flap was an oblique flap whereas the other fifteen flaps were vertical RAM flaps. The oblique flap was fashioned as it allowed for a larger cutaneous paddle than was possible with the vertical flap to fill a particularly large cutaneous defect following excision of a large vulval carcinoma.
Eleven patients (69%) had risk factors that would predict poor wound healing, nine having received preoperative long course radiotherapy, one with diabetes and one on long-term steroids. In three of the sixteen flaps, the origin of the rectus abdominis muscle was detached from the pubic tubercle to gain extra length, thus leaving the flap suspended on the vascular pedicle alone. In three patients the donor site defect left after removal of the rectus muscle and sheath was reinforced with a Prolene® mesh.
Complications
Five patients (31%) had a partial superficial wound dehiscence of the perineal wound in the early postoperative period requiring resuturing under local anaesthesia. Four of these (25%) occurred in our first eight patients and prompted a change from subcuticular absorbable sutures to bolstering the wound closure with interrupted nylon sutures to deal with this problem. Four patients (25%) developed minor degrees of perineal cellulitis as judged clinically. These were treated successfully with antibiotics. We experienced one (6%) flap failure requiring excision of the flap. This occurred in one of three patients who had the origin of the rectus abdominis detached from the symphysis pubis, possibly resulting in tension or torsion to the vascular pedicle. One patient (6%) developed early postoperative bleeding from the flap in the perineum, requiring a three-unit blood transfusion, a return to the operating theatre and diathermy of the offending muscular branch.
Five of fifteen patients (33%) having surgery for a cancer diagnosis developed a recurrence of their disease: two patients developed distant metastases (adenocarcinomas), one a pelvic nodal recurrence (squamous carcinoma) and two a deep pelvic local recurrence (one adenocarcinoma, one squamous cell carcinoma). Three of these patients subsequently died of their disease.
Donor site hernias were seen in three of our first eight patients. There were no hernias evident in the latter eight patients. We employed Prolene® mesh support on three of the latter eight donor sites as well as making some technical modifications to the harvest technique in these patients. To date, we have had no donor site hernias in these patients. In addition, we have had no instances of perineal hernias for the duration of follow-up. No patients have reported functional deficits from the loss of the rectus abdominis muscle used for the reconstruction. A summary of complications is given in Table 2, together with the rate of complications in Table 3.
Table 2.
Demographics and complications
| Case | Initial diagnosis | CT | RT | Other medical/surgical treatment | Risk factors for healing | Surgical procedure | Complications | Recurrent disease | Age at operation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Low rectal carcinoma | No | Yes | Nil | RT | APR + 1° VRAM flap | Superficial wound breakdown | Nil | 76 |
| 2 | Sarcoma left buttock | No | No | Previous resection with positive margins | Nil | APR + 1° VRAM flap; sacrectomy | Superficial wound breakdown | Nil | 68 |
| 3 | Low rectal carcinoma | No | No | Previous APR with wound breakdown | Nil | Debridement + 2° VRAM flap | Nil | Nil | 83 |
| 4 | Advanced low rectal carcinoma | No | No | Defunctioning colostomy | Nil | APR + 1° VRAM flap | Low haemoglobin requiring 3-unit blood transfusion; superficial wound breakdown; flap failure | Developed pulmonary metastasis | 29 |
| 5 | Vulval carcinoma | Yes | Yes | Multiple vaginal dilatations | NIDDM | APR + 1° VRAM flap | Nil | Deep seated recurrence | 69 |
| 6 | Low rectal carcinoma | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap | Nil | Nil | 63 |
| 7 | Anal squamous cell carcinoma | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap | Superficial wound breakdown; abdominal wall hernia | Nil | 80 |
| 8 | Low rectal carcinoma | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap | Minor wound infection; abdominal wall hernia | Nil | 75 |
| 9 | Low rectal carcinoma involving vagina | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap; TAH | Nil | Nil | 57 |
| 10 | Anal squamous cell carcinoma | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap | Superficial wound breakdown | Nil | 84 |
| 11 | Anal squamous cell carcinoma, rectovaginal fistula | Yes | Yes | Nil | Nil | APR + 1° VRAM flap | Superficial wound breakdown | Pelvic recurrence; patient died | 70 |
| 12 | Low rectal carcinoma | No | Yes | Nil | Nil | Total proctocolectomy + 1° VRAM flap | Superficial wound breakdown | Nil | 67 |
| 13 | Crohn's disease | No | No | Laparotomy/ileostomy; seton – severe perineal disease | Long course steroids | Panproctocolectomy + 1° VRAM flap | Superficial wound breakdown | Nil | 30 |
| 14 | Anal squamous cell carcinoma | Yes | Yes | Nil | RT/CT | APR + 1° VRAM flap | Nil | Groin recurrence; patient died | 59 |
| 15 | Low rectal carcinoma | No | Yes | Defunctioning colostomy | RT | APR + 1° APR | Perineal abscess – drained | Nil | 50 |
| 16 | Low rectal carcinoma | Yes | No | Nil | CT | APR + 1° APR | Relaparotomy for small bowel hernia | Nil | 54 |
APR = abdominoperineal resection; CT = chemotherapy; NIDDM = non-insulin-dependent diabetes mellitus; RT = radiotherapy; TAH = total abdominal hysterectomy; VRAM = vertical rectus abdominis myocutaneous
Table 3.
Complication rates
| Complication | Numbers involved |
|---|---|
| Minor perineal dehiscence | 5 (31%) |
| Perineal cellulitis | 4 (25%) |
| Postoperative bleeding from flap | 1 (6%) |
| Flap failure | 1 (6%) |
| Delayed donor site hernia | 3 (19%) |
| Recurrence of carcinoma | 5 (31%) |
| Deaths within 30 days | 0 (0%) |
| after 30 days | 3 (19%) |
Modifications to the technique
We have developed several technical modifications to the above described technique, which we believe are useful surgical adjuncts:
The addition of interrupted nylon sutures to the monofilament subcuticular closure was employed due to the minor wound dehiscences that occurred. Since this modification to the technique, there have been no further dehiscences (Fig 1).
In order to reduce the incidence of incisional hernias, we have altered the way the flap is harvested. Instead of taking the anterior rectus sheath to correspond to the full width of the rectus muscle, we now take only the medial part of the sheath, leaving the lateral 50%. The flap therefore runs on fewer perforating vessels but the major perforators are preserved. In addition, we now occasionally reinforce the donor site with mesh in selected patients, which is sandwiched between the anterior and posterior rectus sheaths. Since this modification, we have had no incisional hernia occurrences (Fig 2).
If the flap will not comfortably reach the perineum and extra length is required, the muscle origin from the symphysis pubis can be detached. This creates extra length but possibly makes the flap more vulnerable. Figure 3 shows the rectus flap pedicle with the origin still attached to the symphisis pubis and Figure 4 shows the pedicle after it has been detached.
When a very large defect needed to be reconstructed, then a larger flap could be taken, using a transverse or oblique RAM flap. The shape and size of the flap could be matched directly to the shape and size of the defect (Fig 5).
There are occasions when the posterior vaginal wall has been excised and the perineal defect is not very large. In this instance, we took a muscle-only flap to reconstruct the posterior vaginal wall (Fig 6). This technique is also useful in the very obese, where skin paddle necrosis is a significant risk, as was the case in one of our patients.
Figure 1.
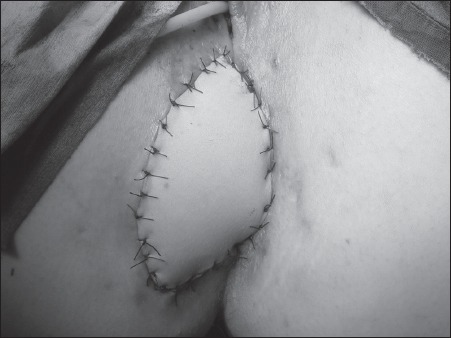
Finished rectus abdominis myocutaneous flap with the addition of interrupted nylon sutures to bolster the flap to withstand the shearing forces created by sitting
Figure 2.
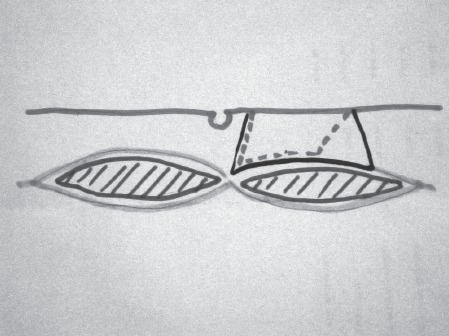
Schematic diagram to show the modification developed taking only the medial part of the rectus sheath. This is to reduce the incidence of incisional hernias. Dotted line indicates the modified incision.
Figure 3.
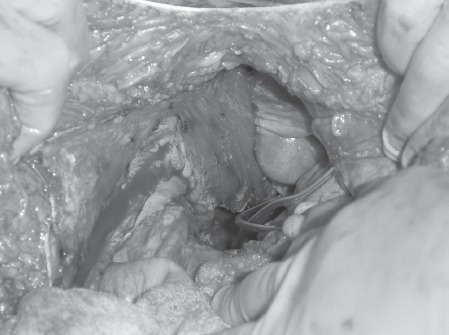
Rectus flap pedicle with the origin still attached to the symphisis pubis
Figure 4.
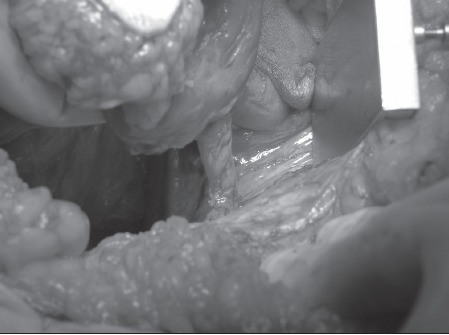
Pedicle only after it has been detached from the symphisis pubis. The flap is shown at the top left hand side of the picture in the surgeon's hand. The vertical structure in the centre of the picture is the pedicle.
Figure 5.
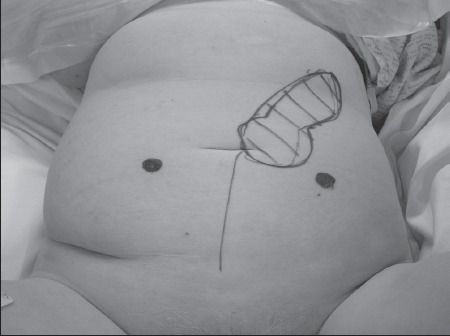
For reconstructing a very large defect a larger oblique rectus flap can be taken.
Figure 6.
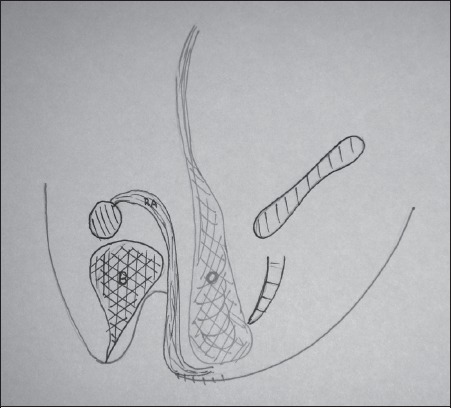
Muscle-only flap for reconstructing the posterior vaginal wall. The ‘O’ indicates omentum, which has been mobilised and placed into the pelvis, and the muscle-only flap is indicated with ‘RA’ (rectus abdominis).
Discussion
Colorectal surgeons are encountering patients who have had neoadjuvant radiotherapy with increasing frequency. This is due to the results of several trials, of which the most recent was CR07.2 The decrease in local recurrence achieved may be offset by the increase in wound morbidity seen in irradiated patients. While it is perfectly achievable to primarily close the perineum in non-irradiated patients or in those with a small defect, those patients post irradiation who have had an extralevator dissection pose a challenge in terms of wound closure and healing. It has been shown that a non-healing wound can complicate an APR in up to 51% of patients.9 Bullard et al reported a major wound complication rate of 35% out of 160 APR patients.10 These results have meant that advancements in surgical technique have been necessary to improve wound healing.
The transfer of vascularised non-irradiated tissue into the wound has been shown to improve healing and prevent wound dehiscence. This is due to the fact that blood flow to the area is increased, bringing with it oxygen and healthy, functioning leukocytes.11
The use of the RAM flap to close the perineal defect has been reported in 19 papers and in 5 of these outcomes were improved with the use of a RAM flap reconstruction. A series of 49 patients who had primary reconstruction with a RAM flap has been published,9,12 with major wound breakdown occurring in only one patient. In the most recent paper published, this figure was 17% for all types of myocutaneous flaps.13 Our results compare favourably with the above, with only one flap failure but otherwise no major wound breakdown and minor wound breakdown in 31%.
We had only one flap loss (6%) in our series and this occurred in a patient in whom we divided the muscular attachment to the symphysis pubis and so the pedicle was unsupported. This is similar to the reported rate of 2% by Sunesen12 and the <5% flap necrosis reported previously in the literature.14 We believe that if the origin is divided, then the flap does need a suture to support it to the bladder or pelvic side wall to minimise traction on the pedicle. We have modified our technique to include this suture since we had the flap failure.
The only other major complication we experienced was an episode of postoperative bleeding from the flap, which required a return to theatre and diathermy of a small muscular branch. There were three instances of similar haemorrhage reported in the large review paper.9
One of the concerns regarding the raising of a RAM flap is the potential increase in abdominal wall complications. In the paper by Abbott et al abdominal wound dehiscence was reported.15 However, this was a paper that looked exclusively at the oblique RAM flap. Nisar and Scott reported 10 fascial dehiscences in 385 patients.9 This gave a dehiscence rate of 2%. Others have reported rates of between 0% and 11%.12,16–18 In our series we had a 19% incisional hernia rate, which is comparable with the literature. As all of these (3) occurred in our first eight patients, we have adopted the practice of reinforcing the donor site with mesh if it appears to be at risk of an incisional hernia due to patient size, build or being under tension at closure. Mesh was used by Weiwei et al in all five of their reported cases18 and Petrie et al in 50% of their cases.11
We experienced minor wound dehiscence in a few cases. This was due to the fact that the flap was exposed to increased pressure and shearing forces from sitting. We therefore began closing the wound with subcuticular monofilament sutures followed by interrupted nylon, a technique not reported elsewhere. Since this modification, we have had no further problems with superficial dehiscence. The nylon interrupted sutures are removed after two weeks.
Abbott et al described the oblique RAM flap, taking a larger paddle of skin in an oblique direction in their case series of 16 patients.15 We have also used this modification when there is a very large defect to close. In our series this occurred after the excision of a large vulval carcinoma.
We have also harvested a muscle-only flap in the case of vaginal reconstruction, leaving the fascia and the skin from the donor site intact. The muscle reconstruction is left to epithelialise itself over time. The reconstructed vagina is packed initially and the patient is taught subsequently to use dilators in order to prevent stenosis. This modification has not been described elsewhere.
During the course of the series of patients, we changed our technique from supine to prone APR. This has been thought to be a hinderance to using a RAM flap. However, we have developed a method of ensuring the flap remains in the correct orientation while the patient is turned on the operating table. Just before the abdomen is closed, the flap is sutured in the correct orientation to the specimen. When the specimen is delivered through the perineum, the flap is drawn through in the correct orientation. This modification has also not been described elsewhere.
There are alternative techniques to the RAM flap to reconstruct the perineum. The inferior gluteal artery flap has been described,19 as has the use of biological mesh.20 We have used both these techniques. The inferior gluteal artery flap, however, has no bulk to fill the pelvis and instead just covers over the defect. In addition, we found the use of biological mesh to be associated with a significant incidence of seroma formation and infection.
Conclusions
Comparison of our case series with previously published results is favourable, with complication rates similar to previously published series. The RAM flap is a robust solution to the problem of reconstructing the perineum after extralevator APR. We have presented our case series together with our modifications, which we feel are useful in the treatment of this difficult problem.
References
- 1.Miles WE. A method of performing abdominoperineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon. Lancet. 1908;2:1812–1813. [Google Scholar]
- 2.Sebag-Montefiore D, Steele R, Quirke P, et al. Routine short course pre-op radiotherapy or selected post-op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. J Clin Oncol. 2006;24:18S. [Google Scholar]
- 3.Mathes S, Bostwick J., 3rd A rectus abdominis myocutaneous flap to reconstruct abdominal wall defects. Br J Plast Surg. 1977;30:282–283. doi: 10.1016/0007-1226(77)90118-7. [DOI] [PubMed] [Google Scholar]
- 4.Deo SV, Nootan KS, Niranjan B, Dinesh K. Vertical rectus abdominis myocutaneous flap cover for lower abdomen, chest wall, groin and thigh defects following resection of malignant tumours. Indian J Cancer. 2001;38:33–37. [PubMed] [Google Scholar]
- 5.Brierly RD, Pereira JA, Arnstein PM. Use of a vertical rectus abdominis myocutaneous flap in bilateral groin dissection for recurrent carcinoma of the penis. Urol Int. 1998;61:243–246. doi: 10.1159/000030339. [DOI] [PubMed] [Google Scholar]
- 6.Sakai S, Takahashi H, Tanabe H. The extended vertical rectus abdominis myocutaneous flap for breast reconstruction. Plast Reconstr Surg. 1989;83:1061–1067. [PubMed] [Google Scholar]
- 7.Shkula HS, Hughes LE. The rectus abdominis flap for perineal wounds. Ann R Coll Surg Engl. 1984;66:337–339. [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin GR, Day TG. Vaginal and pelvic reconstruction with distally based rectus abdominis myocutaneous flaps. Plast Reconstr Surg. 1988;81:62–73. doi: 10.1097/00006534-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Nisar PJ, Scott HJ. Myocutaneous flap reconstruction of the pelvis after abdominoperineal reconstruction. Colorectal Dis. 2009;11:806–816. doi: 10.1111/j.1463-1318.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 10.Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–443. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 11.Petrie N, Branagan G, McGuiness C, et al. Reconstruction of the perineum following anorectal cancer excision. Int J Colorectal Dis. 2009;24:97–104. doi: 10.1007/s00384-008-0557-2. [DOI] [PubMed] [Google Scholar]
- 12.Sunesen KG, Buntzen S, Tei T, et al. Perineal healing and survival after anal cancer salvage surgery: 10-year experience with primary perineal reconstruction using the vertical rectus abdominis myocutaneous (VRAM) flap. Ann Surg Oncol. 2009;16:68–77. doi: 10.1245/s10434-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 13.Chan S, Miller M, Ng R, et al. Use of myocutaneous flaps for perineal closure following abdominoperineal excision of the rectum for adenocarcinoma. Colorectal Dis. 2010;12:555–560. doi: 10.1111/j.1463-1318.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghouti L, Houvenaeghel G, Moutardier V, et al. Salvage abdominoperineal resection after failure of conservative treatment in anal epidermoid cancer. Dis Colon Rectum. 2005;48:16–22. doi: 10.1007/s10350-004-0746-1. [DOI] [PubMed] [Google Scholar]
- 15.Abott DE, Halversn AL, Wayne JD, et al. The oblique rectus abdominal myocutaneous flap for complex pelvic wound reconstruction. Dis Colon Rectum. 2008;51:1237–1241. doi: 10.1007/s10350-008-9359-4. [DOI] [PubMed] [Google Scholar]
- 16.Pocard M, Tiret E, Nugent K, et al. Results of salvage abdominoperineal resection for anal cancer after radiotherapy. Dis Colon Rectum. 1998;41:1488–1493. doi: 10.1007/BF02237294. [DOI] [PubMed] [Google Scholar]
- 17.Allal AS, Laurencent FM, Reymond MA, et al. Effectiveness of surgical salvage therapy for patients with locally uncontrolled anal carcinoma after sphincter-conserving treatment. Cancer. 1999;86:405–409. doi: 10.1002/(sici)1097-0142(19990801)86:3<405::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Weiwei L, Zhifei L, Ang Z, et al. Vaginal reconstruction with the muscle-sparing vertical rectus abdominus myocutaneous flap. J Plast Reconstr Aesthet Surg. 2009;62:335–340. doi: 10.1016/j.bjps.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Boccola MA, Rozen WM, Ek EW, et al. Inferior gluteal artery myocutaneous island transposition flap reconstruction of irradiated perineal defects. J Plast Reconstr Aesthet Surg. 2010;63:1169–1175. doi: 10.1016/j.bjps.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Wille-Jørgensen P, Pilsgaard B, Møller P. Reconstruction of the pelvic floor with a biological mesh after abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2009;24:323–325. doi: 10.1007/s00384-008-0607-9. [DOI] [PubMed] [Google Scholar]


