Abstract
INTRODUCTION
The extent of calcified ductal carcinoma in situ (DCIS) detected by screening mammography is a determinant for treatment with breast conserving surgery (BCS). However, DCIS may be uncalcified and almost a quarter of patients with DCIS treated initially by BCS either require a second operation or are found to have unexpected invasive disease following surgery. Identification of these cases might guide selective implementation of additional diagnostic procedures.
METHODS
A retrospective review of patients with a preoperative diagnosis of pure high-grade DCIS at the Southampton and Salisbury Breast Screening Unit over a ten-year period was carried out. Mammograms were reviewed independently by a consultant radiologist and additional factors including the Breast Imaging Reporting and Data System (BI-RADS®) breast density score, DCIS extent and disease location within the breast recorded.
RESULTS
Unexpected invasive disease was found in 35 of 144 patients (24%). Within our unit the re-excision rate for all screen-detected DCIS is currently 23% but for patients included in this study with high-grade DCIS the re-excision rate was 39% (34/87). The extent of DCIS (p=0.008) and lack of expression of the oestrogen receptor (ER) predicted the requirement for re-excision in both univariate (p=0.004) and multivariate analysis (p=0.005).
CONCLUSIONS
High-grade DCIS may be focally uncalcified, leading to underestimation of disease extent, which might be related to ER status. Invasive foci associated with high-grade DCIS are often mammographically occult. Exploration of additional biomarkers and targeted use of further diagnostic techniques may improve the preoperative staging of DCIS.
Keywords: Breast, Carcinoma in situ, Screening, Estrogen receptor
Since the introduction of mammographic breast screening there has been a significant increase in the rate of detection of ductal carcinoma in situ (DCIS) without an invasive component (pure DCIS). This now comprises approximately 20% of all screen-detected breast malignancies in the UK.1 DCIS may progress to invasive breast cancer. Apparently, pure DCIS frequently coexists with concurrent invasive breast cancer, which in cases of widespread high-grade DCIS is found unexpectedly on final histology following surgery in up to 10–25% of cases and necessitates additional staging of the axilla if axillary surgery is not performed.2
Screening mammography is very sensitive at detecting calcified high-grade DCIS. Approximately 80–90% of DCIS lesions are calcified,3,4 which enables accurate prediction of disease extent. This is a major determinant of the suitability to treatment by wide local excision (WLE) and therefore breast conservation. It is recognised, however, that the distribution of calcification seen mammographically as well as on specimen radiographs is not always representative of the full extent of disease, leading to an underestimation of extent, both preoperatively and in intraoperative specimen radiographs.5 For example, DCIS is often found at or within a few millimetres of the initial excision margins. Re-excision procedures are often required as DCIS present at the surgical margin leads to a greater incidence of local recurrence.6–8
Annual returns to the NHS Breast Screening Programme (NHSBSP) indicate that our re-excision rates for all pure screen-detected DCIS were 23% between 2008 and 2009. This figure is within normal limits for a large breast screening unit and is given as an example. As an NHSBSP centre, our unit is audited on a yearly basis against national standards. These audits confirmed that the overall rates of re-excision for DCIS historically over the period of this study were also within acceptable levels. However, we suspected from anecdotal evidence that the re-excision rates for pure high-grade DCIS might be higher. This theory is biologically plausible since it has been reported that high-grade DCIS contains distinct genomic changes when compared with low and intermediate grade DCIS9 and has a higher risk of developing invasive foci, axillary metastases and local recurrence.10–13 We therefore elected specifically to investigate high-grade DCIS within this study.
The aim of this study was to determine the re-excision rate for screen-detected pure high-grade DCIS. We also wanted to document the effectiveness of conventional breast imaging for predicting the extent of DCIS and the ability to detect unexpected invasive disease, and to assess whether this might be influenced by conventional clinicopathological features.
Methods
The Southampton and Salisbury Breast Screening Unit national breast screening service database was used to identify patients over a ten-year period (1999–2009) presenting through the screening programme with a preoperative diagnosis of pure high-grade DCIS. There were 144 consecutive patients over this period who met the inclusion criteria. Case records and mammograms were systematically reviewed. Factors including the Breast Imaging Reporting and Data System (BI-RADS®) breast density score, DCIS extent and location of the mammographic abnormality within the breast were recorded. Patients with a preoperative diagnosis that included an invasive component or non-high grade DCIS were excluded from the study. All patients were managed in a large teaching hospital participating in the NHSBSP with a high-case volume.
Radiological assessment
Mammograms (mediolateral oblique and craniocaudal views) from all 144 cases were individually reviewed by a consultant radiologist. The extent of DCIS was measured in millimetres by recording the maximal length of microcalcifications. Cases consisting of two or more areas of calcification separated by apparently normal breast tissue (multi-focal DCIS) were measured by recording the largest diameter of the lesion, regarding multiple clusters as a single entity. The location of microcalcifications within the breast was classified into five categories: lower inner quadrant, lower outer quadrant, subareolar region, upper inner quadrant and upper outer quadrant. Breast density was graded using the BI-RADS® classification system.14,15 The distribution of calcifications (segmental/diffuse/cluster) and overall radiological opinion (R score)16 were also documented.
Pathological assessment
Pathology reports were reviewed both pre and postoperatively. Pathological size of DCIS was measured by the reporting pathologist using direct measurement from slides. Data collected included preoperative pathological opinion (biopsy/B score), grade of DCIS (DCIS of mixed cytological grade was recorded according to the highest grade), postoperative size of DCIS, histological type, presence of invasive disease (including grade, size and type) and distance from the nearest surgical margin. Microinvasion was classified as invasive disease.
Surgical assessment
The surgical treatment was recorded: either WLE or mastectomy with or without axillary sampling, clearance or sentinel lymph node biopsy (SLNB). All re-excision procedures were recorded along with the subsequent pathological diagnosis. Oestrogen receptor (ER) status and adjuvant therapy were also documented.
Statistical analysis
Descriptive analysis was used to report the primary outcomes, including the percentage of patients requiring a second surgical procedure and those who had invasive disease. Confidence intervals for these percentages were calculated using the Confidence Interval Analysis (CIA) software package (available on a disk with: Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence. 2nd edn. Wiley-Blackwell; 2000). Statistical analysis was performed using SPSS® v17.0 (SPSS Inc, Chicago, IL, US). A comparison of DCIS extent before and after surgery was analysed using the Bland–Altman method of agreement.17 A paired samples t-test was used to compare the extent of DCIS before and after surgery in those who required a second operation to assess whether there was a significant difference between the values. Logistic regression was used to identify univariate and multivariate predictors for invasive disease, involved surgical margins and the need for a second operation in WLE cases. The variables analysed included age, mammographic size of DCIS, BI-RADS® score, R score, asymmetrical density, stromal deformity, microcalcification, associated mass, B score, surgical margins and ER status. P-values of <0.05 were considered significant.
Results
There were 144 patients with a preoperative diagnosis of pure high-grade DCIS who met the inclusion criteria within the time period studied. The median age was 60 years (range: 49–78 years). The mean radiographic extent of microcalcifications was 33mm (median: 25mm, range: 5–120mm).
Breast conservation (WLE) was the primary surgical treatment modality for 87 patients (60%), while 57 patients (40%) had a mastectomy with or without reconstruction. Fifty-seven patients underwent axillary staging at the first operation (sampling (n=48), SLNB (n=4), clearance (n=5)).
Surgical margins, second operations and invasive disease
For all cases treated initially by WLE, 47% (95% confidence interval [CI]: 37–58) had involved surgical margins and a second surgical procedure was required in 39% (95% CI: 30–50). Reasons for DCIS at the surgical margin not leading to a second operation included an isolated involved deep (pectoral) margin. Unexpected invasive disease was found postoperatively in 24% (95% CI: 18–32).
Extent of DCIS and requirement for second operation
The preoperative mammographic estimation of DCIS extent was accurately predicted in 46% of cases where the discrepancy between pre and postoperative size of DCIS was ≤10mm. In cases selected for treatment by WLE, mammography more frequently underestimated the pathological size by ≥10mm (29%) compared with overestimation by ≥10mm (13%). The accuracy of mammographic assessment of DCIS extent reduced with increased size of DCIS (Fig 1). The median mammographic extent of calcification in cases treated by mastectomy was 50mm (mean: 53.7mm).
Figure 1.
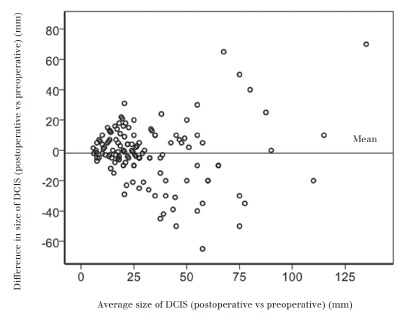
The difference between the mammographically predicted preoperative and histological postoperative size of DCIS. There is increased scatter as the size of DCIS increases, indicating that mammography is less accurate at predicting the extent of DCIS in larger lesions.
The surgical treatment of the breast in all 144 cases of high grade DCIS is illustrated in Table 1. Of those treated by WLE, 34 required a second operation. In these 34 patients, the extent of DCIS was underestimated preoperatively by a mean 6.75mm (p=0.049, 95% CI: 0.02–13.48) (Table 2). In contrast, for WLE cases requiring no further surgery, no significant difference was found between pre and postoperative size of DCIS (Table 2). Of the 34 cases requiring re-excision, 25 underwent a mastectomy. Residual disease was present on re-excision in 25 cases (74%) with a mean extent of further disease of 8mm (SD: 13mm).
Table 1.
Surgical treatment of the breast in 144 cases of high-grade ductal carcinoma in situ
| Primary operation | n | Second operation | n | Third operation | n |
|---|---|---|---|---|---|
| WLE | 87 (60%) | WLE | 9 (26%) | Mastectomy | 4 (100%) |
| Mastectomy | 57 (40%) | Mastectomy | 25 (74%) | ||
| Total | 144 (100%) | 34 (100%) | 4 (100%) |
WLE = wide local excision
Table 2.
Mean size of ductal carcinoma in situ (DCIS) before and after surgery in wide local excision cases requiring no further surgery/further surgery
| No further surgery* (n=53) | Further surgery** (n=34) | |
|---|---|---|
| DCIS size before surgery (mm) | 15.7 (SD: 8) | 24.0 (SD: 16) |
| DCIS size after surgery (mm) | 15.5 (SD: 7) | 30.6 (SD: 16) |
Mean difference between pre and postoperative DCIS size 0.16mm (p=0.907)
Mean difference between pre and postoperative DCIS size 6.75mm (p=0.049)
ER status and size of DCIS predicting requirement for re-excision
Logistic regression was used to identify significant predictors for second operation after primary WLE (Table 3). The extent of DCIS (p=0.008, odds ratio [OR]: 1.07, 95% CI: 1.02–1.12) and lack of expression of the ER predicted the requirement for a second operation in both univariate (ER negative) (p=0.004, OR: 4.10, 95% CI: 1.56–10.75) and multivariate analysis (ER negative) (p=0.005, OR: 8.61, 95% CI: 1.91–38.85). ER negative status was also a significant predictor for involved surgical margins (p=0.048, OR: 2.59, 95% CI: 1.01–6.63) (Fig 2).
Table 3.
Significant predictors of involved margins and second operation/no second operation calculated by logistic regression for cases undergoing breast conservation (wide local excision) as their first surgical treatment (n=87)
| Characteristic | Clear margins (n=46) | Involved margins (n=41) | Odds ratio (95% confidence interval) | p-value* | No further surgery (n=53) | Further surgery (n=34) | Odds ratio (95% confidence interval) | p-value* | |
|---|---|---|---|---|---|---|---|---|---|
| Mammographic size of DCIS (mm) | 0.9–9.9 | 9 (20%) | 9 (22%) | 12 (23%) | 6 (18%) | ||||
| 10.0–19.9 | 17 (37%) | 9 (22%) | 20 (38%) | 6 (18%) | |||||
| 20.0–29.9 | 12 (26%) | 14 (34%) | 14 (26%) | 12 (35%) | 1.07 (1.01–1.21) | p=0.008 | |||
| 30.0–39.9 | 5 (11%) | 5 (12%) | 5 (9%) | 5 (15%) | |||||
| 40+ | 3 (7%) | 3 (7%) | 2 (4%) | 4 (12%) | |||||
| BI-RADS®a | 1 | 5 (11%) | 3 (7%) | 6 (11%) | 2 (6%) | ||||
| 2 | 21 (46%) | 15 (37%) | 23 (43%) | 13 (38%) | |||||
| 3 | 17 (37%) | 19 (46%) | 21 (40%) | 15 (44%) | |||||
| 4 | 3 (7%) | 4 (10%) | 3 (6%) | 4 (12%) | |||||
| R scoreb | 1 | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | ||||
| 2 | 2 (4%) | 2 (5%) | 3 (6%) | 1 (3%) | |||||
| 3 | 12 (26%) | 11 (27%) | 11 (21%) | 12 (35%) | |||||
| 4 | 7 (15%) | 12 (30%) | 3.29 (1.03–10.53) | p=0.045 | 12 (23%) | 7 (21%) | |||
| 5 | 23 (50%) | 12 (30%) | 24 (45%) | 11 (32%) | |||||
| Location of radiological abnormality | LIQ | 10 (22%) | 4 (10%) | 8 (15%) | 6 (18%) | 0.15 (0.30–0.76) | p=0.022 | ||
| LOQ | 10 (22%) | 4 (10%) | 12 (23%) | 2 (6%) | |||||
| SAR | 1 (2%) | 2 (5%) | 2 (4%) | 1 (3%) | |||||
| UIQ | 7 (15%) | 7 (17%) | 11 (21%) | 3 (9%) | |||||
| UOQ | 18 (39%) | 24 (59%) | 20 (38%) | 22 (65%) | |||||
| Asymmetric density | No | 42 (91%) | 37 (90%) | 48 (91%) | 31 (91%) | ||||
| Yes | 2 (4%) | 4 (10%) | 3 (6%) | 3 (9%) | |||||
| Stromal deformity | No | 43 (94%) | 41 (100%) | 50 (94%) | 34 (100%) | ||||
| Yes | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | |||||
| Microcalcification | No | 2 (4%) | 1 (2%) | 3 (6%) | 0 (0%) | ||||
| Yes | 42 (91%) | 40 (98% | 48 (91%) | 34 (100%) | |||||
| Distribution of microcalcification | Cluster | 20 (44%) | 11 (27%) | 24 (45%) | 7 (21%) | ||||
| Diffuse | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | 0.26 (0.09–0.77) | p=0.015 | |||
| Segmental | 14 (30%) | 18 (44%) | 15 (28%) | 17 (50%) | |||||
| Missing data | 12 (26%) | 11 (27%) | 14 (26%) | 9 (27%) | |||||
| Associated mass | No | 40 (87%) | 36 (89%) | 44 (83%) | 32 (94%) | ||||
| Yes | 6 (13%) | 5 (12%) | 9 (17%) | 2 (6%) | |||||
| B scorec | 4 | 2 (4%) | 0 (0%) | 2 (4%) | 0 (0%) | ||||
| 5a | 43 (94%) | 41 (100%) | 50 (94%) | 34 (100%) | |||||
| Surgical margins | Clear of margins | 46 (100%) | 0 (0%) | 43 (81%) | 3 (9%) | 69.29 (16.03–299.43) | p<0.001 | ||
| At margin/uncertain | 0 (0%) | 41 (100%) | 10 (19%) | 31 91%) | |||||
| Oestrogen receptor status | Positive | 35 (76%) | 23 (56%) | 41 (77%) | 17 (50%) | ||||
| Negative | 10 (22%) | 17 (41%) | 2.59 (1.01–6.63) | p=0.048 | 10 (19%) | 17 (50%) | 4.10 (1.56–10.75) | p=0.004 | |
| Missing data | 1 (2%) | 1 (2%) | 2 (4%) | 0 (0%) | |||||
BI-RADS® = Breast Imaging Reporting and Data System; LIQ = lower inner quadrant; LOQ = lower outer quadrant; SAR = subareolar region; UIQ = upper inner quadrant; UOQ = upper outer quadrant
1 = almost entirely fat; 2 = scattered fibroglandular densities; 3 = heterogeneously dense; 4 = extremely dense
1 = normal; 2 = benign; 3 = probably benign; 4 = probably malignant; 5 = malignant
4 = indeterminate probably malignant; 5a = malignant (non invasive)
Variables where p-value is not stated were not significant.
Figure 2.
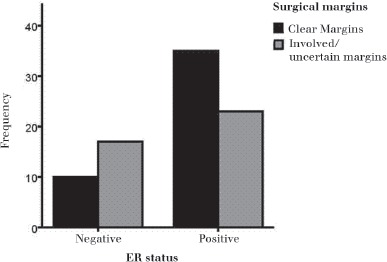
The distribution of involved margins in oestrogen receptor (ER) positive and ER negative cases. A greater proportion of ER negative patients (74% involved margins vs 26% clear margins) had involved margins compared with ER positive patients (36% involved margins vs 64% clear margins).
Unexpected invasive disease
Invasive disease was diagnosed postoperatively in 24% of patients. When all axillary surgical procedures were included (n=83 including second and subsequent operations) only three patients had positive nodes. No significant predictors of invasive disease were identified.
Discussion
This study demonstrates decreasing accuracy of mammographic prediction of disease extent with increasing dimension of microcalcification. In particular, DCIS extent was underestimated by an average of 6.75mm (p=0.049) in the 39.6% of patients selected for treatment with WLE who required a second operation. Involved margins and the requirement for a second operation were significantly more frequent in patients with ER negative disease.
Current literature has focused mainly on the effect of ER status on disease recurrence in DCIS18,19 and indications for adjuvant hormonal therapy20 but few studies have commented specifically on its effect on accurately predicting the extent of high-grade DCIS preoperatively. Results from the Sloane Project demonstrated that lesion size has a strong effect on the radiological features of calcified DCIS. However, this study did not comment on the effect of ER status.21 Further research into the importance of ER status is required to ascertain its effect on accurate diagnosis of disease extent and subsequent achievement of adequate surgical margins. ER status could potentially help in the identification of cases likely to harbour mammographically occult, uncalcified disease. This is biologically plausible since the chemical composition of microcalcification differs between DCIS and differing grades of breast cancer.22
These results show that mammography appears to underestimate the size of DCIS by ≥10mm (29%) more frequently than overestimating it in cases selected for treatment with WLE. Similarly, studies by Coombs et al23 and Chakrabarti et al5 have reported a higher incidence of underestimation (15% and 17% respectively) in patients undergoing WLE. The Association of Breast Surgery audit indicated that 23% of B5a (non-invasive) cancers required a second operation after WLE1 and, consistent with this, the overall rate of re-excision of pure DCIS of all grades within our unit was 23% between 2008 and 2009. This is to be compared with a much higher re-excision rate of 39% in this study in the subset of patients with pure-high grade DCIS. Since we specifically selected patients with high-grade DCIS, this higher re-excision rate is interesting and implies increased difficulty in successful management of high grade lesions at first operation. Uncalcified DCIS could be a possible explanation for cases where disease extent was underestimated. Holland and Hendriks outlined the prevalence of uncalcified DCIS, stating that after pathological specimen analysis of 119 cases, DCIS does not generally form a multicentric distribution, ie if two separate areas of microcalcification are seen, the probability of uncalcified disease between these areas is high.24
The number of patients requiring re-excision (n=34) was lower than the number of cases identified as having involved/uncertain margins (n=46). Involvement of DCIS at deep or superficial surgical margins may be less likely to lead to re-excision than at circumferential margins as no further breast tissue can be resected from these margins and it may explain why some patients with positive margins had no further surgery.
It has been reported that greater breast density is related to an increased incidence of associated invasive disease in DCIS25 as dense rather than fatty mammographic parenchymal density surrounding a lesion is more likely to obscure a potential soft tissue abnormality associated with invasive disease. However, the BI-RADS® breast density score was not a significant predictor for invasive disease. Greater extent of microcalcifications on mammography was also not a significant predictor of invasive disease. This corresponds with findings by Stomper et al,26 where lesions greater than 10mm showed no correlation with greater incidence of invasion. However, other studies have shown an increased risk of invasive disease with more extensive mammographic microcalcifications,27,28 leading to recommendations that staging of the axilla should be performed in these cases. No other significant predictors for invasive disease were identified.
It has emerged that the use of breast magnetic resonance imaging (MRI) could improve the diagnosis of DCIS. An observational study by Kuhl et al found that 48% of high-grade DCIS lesions diagnosed by MRI were missed by mammography.29 MRI may offer improved diagnosis for patients with areas of uncalcified high-grade DCIS that is otherwise mammographically occult. The current literature suggests MRI is effective for detection of high-grade DCIS but more research is needed to determine whether it provides greater accuracy in predicting disease extent since we demonstrate here that mammography is less accurate at predicting extent of DCIS in larger (>25mm), ER negative lesions, which may be due to uncalcified disease.
Conclusions
Current modern technological advances such as contrast-enhanced MRI and vacuum core biopsy could potentially assist in providing a more accurate diagnosis of both extent of disease and presence of invasion in a significant proportion of cases,29,30 particularly in cases of ER negative DCIS since our study suggests that underestimation of disease extent might be related to ER status. High-grade DCIS may be focally uncalcified, leading to underestimation of disease extent and an increased probability of the requirement for more than one therapeutic operation.
Acknowledgments
Ramsey Cutress is supported by Cancer Research UK.
References
- 1.NHS Breast Screening Programme, Association of Breast Surgery. An Audit of Screen Detected Breast Cancers for the Year of Screening April 2007 to March 2008. London: ABS; 2009. [Google Scholar]
- 2.O’Flynn EA, Morel JC, Gonzalez J, et al. Prediction of the presence of invasive disease from the measurement of extent of malignant microcalcification on mammography and ductal carcinoma in situ grade at core biopsy. Clin Radiol. 2009;64:178–183. doi: 10.1016/j.crad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989;170:411–415. doi: 10.1148/radiology.170.2.2536185. [DOI] [PubMed] [Google Scholar]
- 4.Muttarak M, Kongmebhol P, Sukhamwang N. Breast calcifications: which are malignant? Singapore Med J. 2009;50:907–913. [PubMed] [Google Scholar]
- 5.Chakrabarti J, Evans AJ, James J, et al. Accuracy of mammography in predicting histological extent of ductal carcinoma in situ (DCIS) Eur J Surg Oncol. 2006;32:1,089–1,092. doi: 10.1016/j.ejso.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald HR, Silverstein MJ, Mabry H, et al. Local control in ductal carcinoma in situ treated by excision alone: incremental benefit of larger margins. Am J Surg. 2005;190:521–525. doi: 10.1016/j.amjsurg.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein MJ Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1,455–1,461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 8.Douglas-Jones AG, Logan J, Morgan JM, et al. Effect of margins of excision on recurrence after local excision of ductal carcinoma in situ of the breast. J Clin Pathol. 2002;55:581–586. doi: 10.1136/jcp.55.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang ES, DeVries S, Chew KL, et al. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res. 2004;10:5,160–5,167. doi: 10.1158/1078-0432.CCR-04-0165. [DOI] [PubMed] [Google Scholar]
- 10.Lagios MD. Heterogeneity of duct carcinoma in situ (DCIS): relationship of grade and subtype analysis to local recurrence and risk of invasive transformation. Cancer Lett. 1995;90:97–102. doi: 10.1016/0304-3835(94)03683-a. [DOI] [PubMed] [Google Scholar]
- 11.Allred DC, O’Connell P, Fuqua SA, Osborne CK. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat. 1994;32:13–18. doi: 10.1007/BF00666202. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345:1,154–1,157. doi: 10.1016/s0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 13.Allegra CJ, Aberle DR, Ganschow P, et al. NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS) NIH Consens State Sci Statements. 2009;26:1–27. [PubMed] [Google Scholar]
- 14.Balleyguier C, Ayadi S, Van Nguyen K, et al. BIRADS classification in mammography. Eur J Radiol. 2007;61:192–194. doi: 10.1016/j.ejrad.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Eberl M, Fox CH, Edge SB, et al. BI-RADS classification for management of abnormal mammograms. J Am Board Fam Med. 2006;19:161–164. doi: 10.3122/jabfm.19.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell AJ, Ridley NT, Rubin G, et al. The Royal College of Radiologists Breast Group breast imaging classification. Clin Radiol. 2009;64:624–627. doi: 10.1016/j.crad.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Roka S, Rudas M, Taucher S, et al. High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur J Surg Oncol. 2004;30:243–247. doi: 10.1016/j.ejso.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Provenzano E, Hopper JL, Giles GG, et al. Biological markers that predict clinical recurrence in ductal carcinoma in situ of the breast. Eur J Cancer. 2003;39:622–630. doi: 10.1016/s0959-8049(02)00666-4. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1,993–2,000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 21.Evans A, Clements K, Maxwell A, et al. Lesion size is a major determinant of the mammographic features of ductal carcinoma in situ: findings from the Sloane project. Clin Radiol. 2010;65:181–184. doi: 10.1016/j.crad.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Baker R, Rogers KD, Shepherd N, Stone N. New relationships between breast microcalcifications and cancer. Br J Cancer. 2010;103:1,034–1,039. doi: 10.1038/sj.bjc.6605873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombs JH, Hubbard E, Hudson K, et al. Ductal carcinoma in situ of the breast: correlation of pathologic and mammographic features with extent of disease. Am Surg. 1997;63:1,079–1,083. [PubMed] [Google Scholar]
- 24.Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol. 1994;11:181–192. [PubMed] [Google Scholar]
- 25.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1,159–1,169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 26.Stomper PC, Geradts J, Edge SB, Levine EG. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. Am J Roentgenol. 2003;181:1,679–1,684. doi: 10.2214/ajr.181.6.1811679. [DOI] [PubMed] [Google Scholar]
- 27.Bagnall MJ, Evans AJ, Wilson AR, et al. Predicting invasion in mammographically detected microcalcification. Clin Radiol. 2001;56:828–832. doi: 10.1053/crad.2001.0779. [DOI] [PubMed] [Google Scholar]
- 28.Lagios MD, Westdahl PR, Margolin FR, Rose MR. Duct carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50:1,309–1,314. doi: 10.1002/1097-0142(19821001)50:7<1309::aid-cncr2820500716>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 30.Malhaire C, El Khoury C, Thibault F, et al. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol. 2010;20:1,554–1,562. doi: 10.1007/s00330-009-1707-9. [DOI] [PubMed] [Google Scholar]


