Abstract
Epithelia ensure many critical functions of the body, including protection against the external environment, nutrition, respiration, and reproduction. Stem cells (SCs) located in the various epithelia ensure the homeostasis and repair of these tissues throughout the lifetime of the animal. Genetic lineage tracing in mice has allowed the labeling of SCs and their progeny. This technique has been instrumental in characterizing the origin and heterogeneity of epithelial SCs, their tissue location, and their differentiation potential under physiological conditions and during tissue regeneration.
Introduction
Epithelia are sheets of cells that constitute the lining of most of the organs of the body—such as the skin, the digestive and respiratory tracts, and the urogenital system—and separate the inside of the body from the outside (Blanpain et al., 2007). An epithelium can be composed of one (simple epithelium) or multiple layers of cells (stratified epithelium), and forms the majority of the glands. A variety of specialized differentiated cells present in these epithelia ensure the important diversity of physiological functions they control. The skin epidermis acts as a barrier that keeps fluids in and protects the animal from the aggressions of the external environment. The lungs allow the gas exchange necessary for cellular respiration. The gut together with its associated glands allows the absorption of water and nutrients. The urologic system allows the evacuation of ions, water, and toxic byproducts of metabolism. The genital tract ensures reproductive function. Due to their vital functions, epithelia have mechanisms to ensure their proper maintenance and functionality. Some epithelia such as the skin or the intestine have a very high cellular turnover to replace the cells that are continuously lost (Barker et al., 2010a). Other epithelia, such as those of the airway tracts, renew much more slowly under physiological conditions but nevertheless can rapidly and extensively proliferate to repair tissue upon damage or injuries (Rock and Hogan, 2011). Stem cells (SCs) located in these different adult tissues are essential to sustain tissue turnover and repair these different epithelia upon injuries. SCs can renew throughout life and can differentiate into the different cell lineages of their tissue of origin. The balance between SC proliferation and differentiation must be precisely controlled, as deregulation of this process may lead to tissue atrophy and cancer formation. Carcinoma, which are tumors arising from epithelium, are by far the most common cancers in humans and lead to millions of death per year throughout the world.
Different assays have been developed to study the function of epithelial SCs. Inspired by the field of hematopoietic SCs, the most common assay to assess the renewal and differentiation of putative epithelial SCs is their transplantation into immunodeficient animals (Blanpain et al., 2007). Although transplantation assays are very informative about the differentiation potential of SCs, they do not necessarily reflect physiological conditions because SCs are dissociated from their normal environment and very often transplanted into a heterotopic site, such as into the renal capsule (the fibrous layer surrounding the kidney) or the dermis, with or without their normal underlying mesenchyme. These assays recapitulate embryonic development or severe regeneration conditions rather than normal tissue homeostasis. Lineage tracing has now become the method of choice to study the renewal and the differentiation potential of SCs in intact tissue, as this approach avoids the many drawbacks of transplantation experiments. In this technique, a particular cell lineage is labeled and the fate of the labeled cells and their progeny is analyzed over time. These experiments are usually performed in mice coexpressing two different transgenes: a CRE recombinase expressed under a lineage-specific promoter, and a reporter gene only expressed when the CRE removes a stop cassette that precedes the reporter gene. Upon CRE excision of the stop cassette, the reporter transgene is permanently expressed in these cells and all their future progeny. Two different CRE genes can be used to perform such experiments: a constitutive CRE, which is naturally active, and an inducible CRE, which is usually fused to a mutated nuclear hormone receptor such as the estrogen receptor (ER) called CREER or progesterone receptor (PR) called CREPR. In the absence of their synthetic ligands (such as tamoxifen for the CREER or RU486 for the CREPR), the CREER is maintained inactive in the cytoplasm. Administration of the synthetic ligand induces activation of the inducible CRE recombinase, which in turn induces the expression of the reporter gene in the cells expressing the CRE and all their subsequent progeny, allowing the fate of labeled cells to be followed over time. The limitation of the inducible lineage tracing experiments is the identification of a specific promoter that targets the SC of interest and the mosaic expression of the reporter gene within the SC population. The latter precludes one from drawing any firm conclusions about the fate of the nonlabeled cells. In these studies, it is generally assumed that the labeled cells are representative of the whole SC population. Because there is an important heterogeneity within epithelial SCs, one should always leave open the possibility of the existence of another population of epithelial SCs not labeled by this approach. The frequency of labeled cells over time is a good indication of whether these cells are in equilibrium with other SC populations; if nonlabeled SCs replace labeled cells, the frequency of labeled cells should decrease over time. Clonal analysis allows following the fate of single marked cells. To perform clonal analysis, it is necessary to mark individual cells sufficiently distant from each other to follow the fate of individual clones. This can be achieved by lowering the dose of the TAM until it induces CREER activity in isolated cells. Lineage-tracing experiments provide important information about the cellular hierarchy that governs epithelium development and homeostasis, and uncover the diversity of epithelial SCs, their origin, tissue location, renewal, migration, and differentiation potential, as well as their ability to contribute to tissue repair and tumor initiation. Different methods to perform lineage tracing in vertebrates and invertebrates have been recently thoroughly reviewed elsewhere (Buckingham and Meilhac, 2011; Kretzschmar and Watt, 2012). In this review, we discuss the novel insights into epithelial SCs and their lineages that have been gathered thanks to the use of this technique in mice.
The skin epidermis
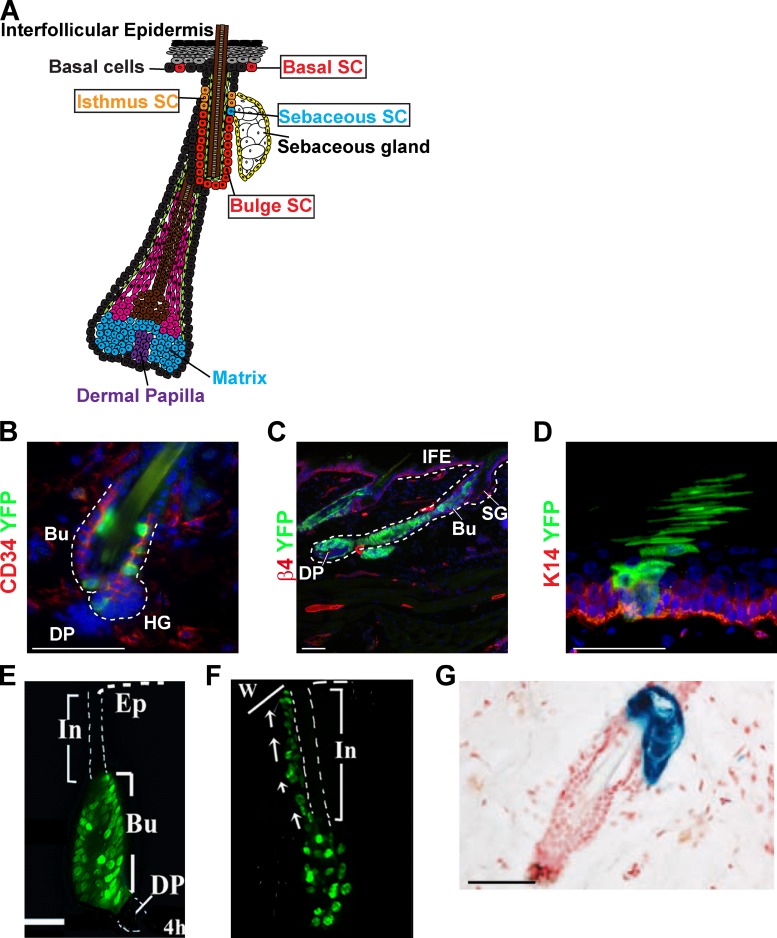
The skin epidermis is composed of the interfollicular epidermis, which forms the skin barrier, and its various appendages such as the hair follicle (HF), the sebaceous gland, and the sweat gland (Fig. 1 A; Blanpain and Fuchs, 2006). During mouse embryonic development, HFs are specified through an epithelial–mesenchymal interaction around embryonic day (E) 16. At this stage, a group of cells that present an elongated morphology called the placode progress to form a bud called the hair germ, which keeps growing and differentiates into the seven concentric cell lineages that form the mature HF (Blanpain and Fuchs, 2006). Lineage tracing during embryonic epidermal development using ShhCRE (Levy et al., 2005) or Sox9CRE (Nowak et al., 2008), which are specifically expressed in the hair germ, demonstrated that all HF lineages including adult HF SCs and the sebaceous gland arise from embryonic progenitors expressing these two markers during HF morphogenesis. Similar long-term labeling of the HF lineages has been obtained using Lgr6CREER lineage tracing at E17.5, which is also expressed in embryonic hair germ, although its expression is broader and included cells of the interfollicular epidermis (Snippert et al., 2010a). Embryonic lineage tracing using ShhCRE (Levy et al., 2005) and Sox9CRE (Nowak et al., 2008) also revealed that HFs were entirely labeled whereas the interfollicular epidermis was not labeled, demonstrating that the interfollicular epidermis and the HF lineages arise from two independent sources of cells that are maintained independently in adult animals.
Figure 1.
Lineage tracing of the skin epidermis. (A) Schematic representation of the skin epidermis and the different epidermal SCs: the interfollicular epidermis (IFE) SCs, the isthmus SCs, and the bulge SCs. (B and C) Lineage tracing of bulge stem cells (green) using K19CREER-RosaYFP mice induced with 10 mg tamoxifen between d 21 and 25, and analyzed 1 wk (B) or 5 wk (C) after induction. Immunostaining of CD34 (B) or integrin β4 (C) and YFP show the initial labeling of CD34+ bulge SC (B) and the presence of YFP cells in the newly formed hair follicle 5 wk after (C). (D) Lineage tracing of basal cells (green) from the interfollicular epidermis using K14CREER-RosaYFP mice induced with 1 mg tamoxifen and analyzed 5 wk later. Immunostaining of K14 (red) and YFP (green) shows the presence of a column of YFP-marked cells spanning from the basal layer (red) to the cornified layer, corresponding to a unit of interfollicular epidermis maintained by a single SC. (E and F) Migration of H2B-GFP label-retaining bulge SCs to the interfollicular epidermis after wounding, showing the early contribution of bulge SC to the wound repair. Adapted from Tumbar et al. (2004) with permission from AAAS. (G) Lineage tracing using Lgr6-GFP-IresCREER/Rosa-LacZ mice induced at d 20 and analyzed 1 yr later, showing the labeling of the sebaceous gland, and demonstrating the existence of long-lived SC of the sebaceous gland. Adapted from Snippert et al. (2010a) with permission from AAAS. Bu, bulge; DP, dermal papilla; HG, hair germ; IFE, interfollicular epidermis; SG, sebaceous gland; In, infudibulum; Ep, epidermis; w, wound. Bars, 50 µM.
In addition to ensuring the barrier function and thermoregulation, the skin epidermis is also a sensory organ that perceives all kinds of sensory stimuli. Merkel cells are neuroendocrine cells scattered in the epidermis that are responsible for the fine touch sensation (Maricich et al., 2009; Lumpkin et al., 2010). Merkel cells present a number of characteristics of presynaptic neurons. They are excitable cells that express pro-neural transcription factors, possess many components of the presynaptic machinery, and contact the end of sensory nerves (Haeberle et al., 2004). Due to their resemblance with neuronal cells, there has been a long-standing debate whether Merkel cells originate from the neural crest derivatives or from epidermal cells. Two independent groups have recently demonstrated using lineage-tracing experiments that Merkel cells do not originate from neural crest cells, but rather from epidermal progenitors by a mechanism depending on the expression of the proneural transcription factor Atoh1 in epidermal progenitors (Morrison et al., 2009; Van Keymeulen et al., 2009).
Transplantation experiments suggested that the permanent portion of the HF called the bulge contains multipotent SCs able to differentiate into all epidermal lineages including HF, sebaceous gland, and interfollicular epidermis upon transplantation with embryonic skin mesenchyme into immunodeficient mice (Rochat et al., 1994; Oshima et al., 2001; Blanpain et al., 2004; Morris et al., 2004; Claudinot et al., 2005; Jaks et al., 2008). Lineage tracing of adult bulge SCs using the K15CREPR (Morris et al., 2004), ShhCRE (Levy et al., 2005), Sox9CRE (Nowak et al., 2008), Lgr5CREER (Jaks et al., 2008), and K19CREER (Youssef et al., 2010) markers confirmed that bulge SCs are indeed multipotent SCs responsible for the maintenance of all HF lineages under physiological conditions (Fig. 1, B and C). Clonal analysis of bulge SCs indicated that some bulge SCs can differentiate into all HF lineages, whereas others are committed to either the outer or the inner cell layers (Legué et al., 2010; Zhang et al., 2010). These results suggest that the heterogeneity in the differentiation potential of bulge SCs is either related to an intrinsic heterogeneity of bulge cells containing multipotent and unipotent SCs or through a regulation of the lineage differentiation potential of multipotent SCs. Similarly to the embryonic HF lineage tracing, lineage tracing of adult bulge SCs also revealed that the HF lineages do not contribute to the homeostasis the interfollicular epidermis under physiological conditions (Morris et al., 2004; Jaks et al., 2008; Youssef et al., 2010), consistent with the presence of two separate pools of SCs that separately maintain HF and interfollicular epidermis turnover (Fig. 1 D). However, upon wounding, bulge SCs are rapidly activated and actively participate in the repair of the damaged interfollicular epidermis (Fig. 1, E and F; Tumbar et al., 2004; Ito et al., 2005; Levy et al., 2007). These experiments suggested that the multipotency of bulge SCs observed in transplantation assays represents the fate of bulge SCs in a wounding or regenerative environment. Lineage tracing of HF matrix cells demonstrated that these cells are transient amplifying cells that differentiate into one of the six lineages depending on their spatial position within the matrix (Legué and Nicolas, 2005; Youssef et al., 2010).
Sebaceous gland SCs arise from multipotent embryonic HF progenitors expressing Shh, Lgr5, Sox9, and Lgr6 (Levy et al., 2005; Nowak et al., 2008; Snippert et al., 2010a). Within the next few days, Blimp1-expressing progenitors are specified along the HF and give rise to the cells of the adult sebaceous gland including sebaceous gland SCs (Horsley et al., 2006). The long-term labeling of the sebaceous gland observed after Lgr6 lineage tracing in adult skin demonstrates the presence of resident Lgr6-expressing sebaceous gland SCs (Fig. 1 G). Similar to bulge SCs, Lgr6-expressing isthmus SCs are also recruited toward the interfollicular epidermis upon wounding and contribute to the repair of the damaged epidermis (Snippert et al., 2010a). A recent study using new K15CREER transgenic mice suggests that a fraction of bulge SCs migrate upward toward the isthmus region and replenish the sebaceous gland under homeostatic and physiopathological conditions (Petersson et al., 2011). Further studies will be required to better understand the respective contribution of bulge versus resident Lgr6+ SCs in maintaining the homeostasis of the sebaceous gland and defining the mechanisms leading to the recruitment and the migration of bulge SCs to this gland.
As previously mentioned, the interfollicular epidermis is maintained by a source of SCs that is distinct from the HF lineages during postnatal physiological conditions. Many small units of proliferation called epidermal proliferative units (EPUs) scattered all along the interfollicular epidermis ensure the constant turnover of the epidermis and its proper differentiation. Based on morphological and proliferation studies, it has been proposed that the interfollicular epidermis is maintained by hexagonal shaped EPUs containing one SC and ∼10 transit-amplifying cells (Potten, 1974, 1981). Retroviral fate mapping indeed demonstrated the presence of columns of labeled cells spanning from the basal layer to the top of the cornified layer a long time after the clonal marking (Ghazizadeh and Taichman, 2001), consistent with the presence of long-lived SCs ensuring the long-term homeostasis of an EPU. However, genetic lineage tracing showed that the EPU was not hexagonal in shape as suggested by histological analysis and was sometimes bigger than the 10 basal cells expected from the model based on morphological observations (Ro and Rannala, 2004, 2005). These data were interpreted as the consequence of SC migration from one EPU to another. Recently, clonal analysis studies labeling basal epidermal cells of the tail and the ear epidermis using a nontissue-specific drug inducible CREER (AhCREER) showed that the majority of labeled cells are not maintained long-term and are lost over time. However, the surviving clones seemed to expand continuously, in contrast to what would be expected if these cells were maintaining a discrete and predetermined unit of the epidermis. Mathematical modeling of these data suggests that the epidermis could be maintained by one type of progenitor, without the presence of transit-amplifying cells, and these progenitors undergo population asymmetric renewal by balancing asymmetric and symmetric cell division in a stochastic manner (Clayton et al., 2007; Doupé et al., 2010). Although this model is appealing in its simplicity, it relies on the assumption that all basal cells of the epidermis behave like the basal cells labeled by the AhCREER. The model also does not easily explain the ability of the tissue to respond rapidly to increased cell demand, such as during wound healing. Importantly, these data cannot rule out the presence of a small proportion of more quiescent SCs that would exist in equilibrium with more committed basal cells, as it has been previously suggested. New studies combining clonal analysis with different epidermal-specific CREER and proliferation kinetic data will be helpful to address these open questions.
In summary, lineage-tracing experiments in the skin epidermis indicate the existence of multiple populations of stem cells that ensure the maintenance of different compartments of the epidermis and play distinct roles during homeostasis and repair.
The mammary gland
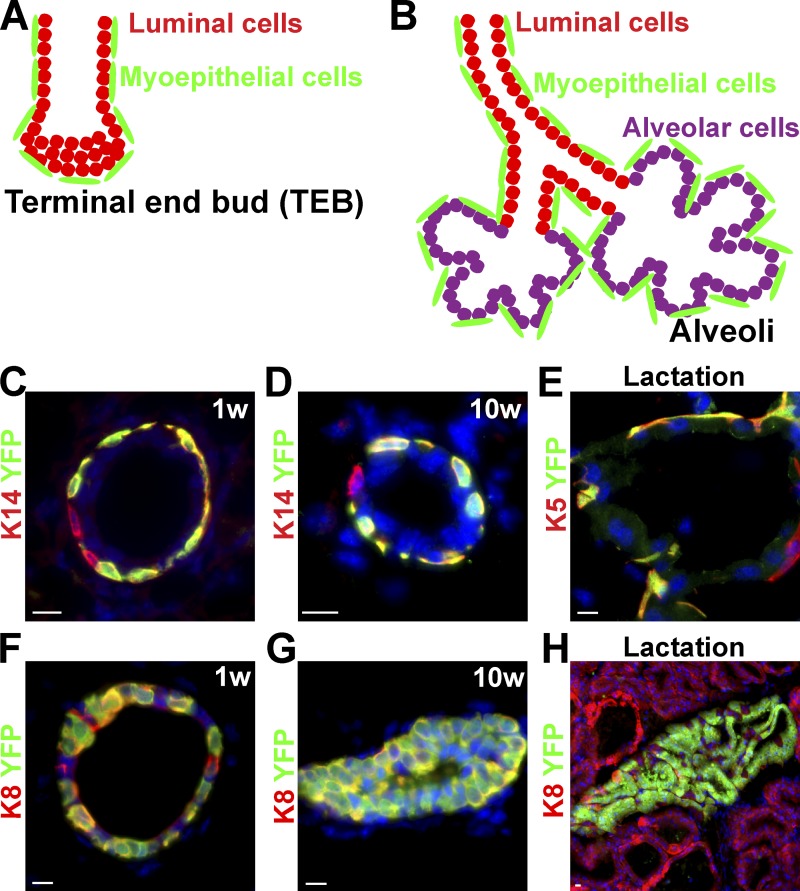
The mammary gland of the mother plays an essential role during the early postnatal life of young mammalian offspring by providing them nutrients, water, and electrolytes, and immune defense until they reach the size and maturity to survive independently. Mammary glands are epidermal appendages that are specified along the ventral epidermis around E12 during mouse embryonic development. These glands are initially visible as placode-like structures that progressively invade the underlying mesenchyme, called the mammary fat pad. At puberty, the mammary gland expands considerably to form a highly branched tubular structure that progressively fills the entire fat pad. The epithelial part of the mammary gland comprises two main cell types, the basal myoepithelial cells and the luminal cells, which can differentiate either into ductal cells or milk-producing alveolar cells (Fig. 2, A and B). During pregnancy, the mammary gland further expands and differentiates into milk-producing cells. After each cycle of pregnancy, the mammary gland involutes through a massive apoptosis of alveolar cells after which the gland returns to a similar morphology as before pregnancy (Watson and Khaled, 2008). Transplantation studies using different populations of mammary epithelial cells isolated by fluorescence-activated cell sorting have demonstrated that rare single cells are able to reconstitute an entire functional mammary gland (Shackleton et al., 2006; Stingl et al., 2006). These results suggest the presence of rare multipotent mammary SCs at the top of the cellular hierarchy of the breast epithelium (Visvader, 2009). The whey acidic protein (WAP) promoter is active in luminal cells during pregnancy and lactation. Lineage-tracing experiments using the WAP-CRE, which labeled luminal cells during pregnancy, identified luminal cells capable of resisting apoptosis during involution and which clonally expand upon the succeeding pregnancy to give rise to luminal and alveolar cells. These cells were therefore described as alveolar progenitors and called parity-induced cells (Wagner et al., 2002). More recently, lineage tracing of the mammary gland using inducible CRE expressed either in myoepithelial cells (K14- or K5-expressing cells) or in luminal cells (K8- or K18-expressing cells) demonstrated that the mammary gland initially develops from multipotent embryonic K14-expressing progenitors, which give rise to both myoepithelial cells and luminal cells. However, postnatal mammary gland development that occurred during puberty, as well as mammary gland expansion that accompanied pregnancies, are ensured by the presence of two types of long-lived SCs. K14/K5-expressing myoepithelial and K8/18-expressing luminal unipotent SCs are able to differentiate at the clonal level into myoepithelial or luminal lineages, respectively, rather than being maintained by rare multipotent SCs (Fig. 2, C–H). Decreasing the ratio of luminal to myoepithelial SCs stimulates the luminal differentiation of myoepithelial SCs in transplantation assay (Van Keymeulen et al., 2011). Further studies will be necessary to define the mechanisms that restrict the differentiation potential of myoepithelial SCs under physiological conditions, and whether pathophysiological conditions can expand the differentiation potential of SCs in the intact mammary gland. Also, it would be interesting to determine whether other glandular epithelia such as the prostate or sweat glands are also maintained by the presence of distinct classes of SCs during postnatal development and adult homeostasis.
Figure 2.
Lineage tracing of the mammary gland. (A and B) Schematic representation of the main epithelial cell types (myoepithelial, luminal, and alveolar cells) of the breast during post-natal development (A) and pregnancy (B). (C–E) Lineage tracing of myoepithelial cells (green) using K14rtTA/TetOCre/RosaYFP mice induced at the onset of puberty (4 wk old) and analyzed 1 wk (C) or 10 wk (D) later or during lactation (E). Immunostaining of K14 (C and D) or K5 (E) (red) and YFP (green) demonstrates the labeling of unipotent SCs that ensure myoepithelial lineage expansion during puberty and pregnancy. (F–H) Lineage tracing of luminal cells (green) using K8CREER/RosaYFP mice induced at the onset of puberty (4 wk old) and analyzed 1 wk (F) or 10 wk later (G), or during lactation (H) shows the labeling of unipotent SCs that ensure luminal lineage expansion during puberty and pregnancy. Adapted from Van Keymeulen et al. (2011) with permission from Nature Publishing Group. Bars, 10 µM.
The gut
The gut regulates the absorption of water, electrolytes, nutrients, and vitamins essential for the survival of the animal. The gut can be subdivided into the esophagus, stomach, intestine, and colon. The esophagus is a stratified epithelium resembling skin epidermis, whereas the stomach, intestine, and colon are simple epithelia composed of only one cell layer that contains the different cell lineages present in the different parts of the gut (Barker et al., 2010a).
The stomach can be subdivided into the corpus, the pylorus, the cardia, and the fundus. In the corpus, the parietal cells secrete the acid necessary to activate digestion, while the mucus cells secrete a protective barrier and the enteroendocrine cells regulate gut contractility and the secretion of the various digestive enzymes. Although the mucus cells renew quite rapidly, the chief cells and the parietal cells responsible for acid secretion display a slow turnover. Random marking of stomach SCs after chemical mutagen administration demonstrated the coexistence of long-lived multipotent and unipotent stomach SCs (Bjerknes and Cheng, 2002). In the pylorus, the vast majority of the proliferating cells are located in the middle zone called the isthmus. Lgr5 lineage tracing, which marked cells located at the bottom of the gland, demonstrated that the progeny of Lgr5-expressing cells migrate into the isthmus region, proliferate, and give rise to all lineages of the stomach (Barker et al., 2010b). Recently, Sox2 lineage tracing, which appears to mark cells distinct from Lgr5+ cells, showed that Sox2 also marked long-lived multipotent stomach SCs (Arnold et al., 2011). Further studies will be required to understand the relationship between Sox2+ and Lgr5+ stomach SCs.
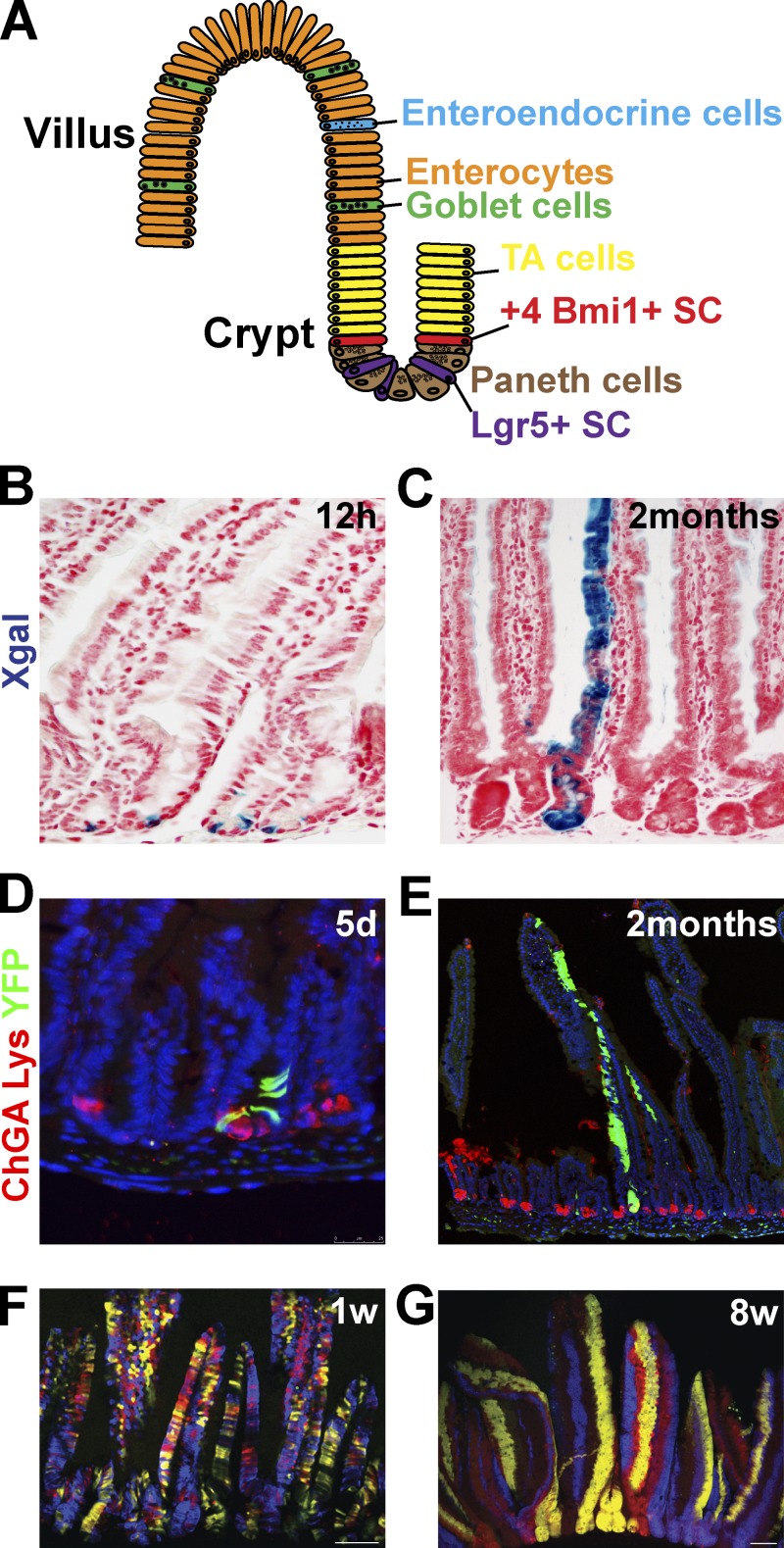
The small intestine ensures the absorption of nutrients. The intestine is composed of proliferating crypts that produce the cells giving rise to the differentiated villi. Villi are protrusions of the epithelium in the gut lumen that massively increase the surface area of the gut to allow for greater absorption. The intestine is one of the most rapidly proliferating tissues in the body, which completely self-renews in less than a week. Several cell lineages are present in the intestine: the absorptive enterocytes are the most abundant cells, the goblet cells produce the mucus, and the enteroendocrine cells regulate the motility of the gut and the secretion of the digestive enzymes while the paneth cells residing at the base of the crypt produce microbicide proteins (Fig. 3 A). Based on proliferation kinetics experiments, it has been suggested that intestinal SCs reside in the crypt at position +4 with respect to the base of the crypt (Potten et al., 1978).
Figure 3.
Lineage tracing of the intestine. (A) Schematic representation of the intestinal epithelium lineages (enterocytes, enteroendocrine, goblet, and paneth cells) and its SCs. (B and C) Lineage tracing of Lgr5+ SCs (blue) using Lgr5-GFP-IresCREER/RosaLacZ mice analyzed 12 h (B) or 60 d (C) after induction demonstrates the initial labeling of columnar basal cells (B) that give rise to the differentiated cells of a villus (C). Adapted from Barker et al. (2007) with permission from Nature Publishing Group. (D and E) Lineage tracing of Bmi1+ SCs (green) using Bmi1CREER/Rosa YFP mice analyzed 5 d (D) or 2 mo (E) after induction. Staining of YFP (green) and of chromogranin A (ChGA) labeling enteroendocrine cells (red); and lysozyme (Lys) antibody, labeling Paneth cells (red), showing the initial labeling of cells above the paneth cells (D) that give rise to the differentiated cells of a villus (E). Images courtesy of E. Sangiorgi and M. Capecchi. (F and G) Lineage tracing of crypts using Ah-CREER/RosaConfetti mice analyzed 1 wk (F) or 8 wk (G) after induction, showing the initial multicolor labeling of the crypt and villus unit that progressively become monoclonal (one color per crypt) over time. Adapted from Snippert et al. (2010b) with permission from Elsevier.
The existence of multipotent SCs in the intestine has been suggested by the presence of long-lived clones of marked cells that contain several types of differentiated intestinal cells (Bjerknes and Cheng, 1999). Barker et al. (2007) identified Lgr5 as a Wnt target gene in colonic cancer lines, and demonstrated that Lgr5 is expressed at the base of the crypt in intestinal crypt base columnar cells intercalated between paneth cells. In contrast to the vast majority of Wnt target genes, which are expressed throughout the crypt, including in the transit amplifying cells, Lgr5 expression is restricted to crypt base columnar cells. Lgr5CREER-IRES-GFP lineage tracing demonstrated that indeed Lgr5-expressing crypt base columnar cells are rapidly cycling multipotent SCs of the intestine giving rise to all cell lineages of the intestine including enterocytes, goblet cells, and neuroendocrine cells, as well as paneth cells (Fig. 3, B and C; Barker et al., 2007).
Bmi1, a polycomb repressor, is expressed in the proximal intestine and preferentially marks the cells located in position +4. Cells labeled in lineage-tracing experiments using Bmi1CREER, similarly to Lgr5+ cells, give rise to all cell lineages of the intestine (Fig. 3, D and E; Sangiorgi and Capecchi, 2008), suggesting the existence of two distinct classes of SCs in the small intestine. Two independent studies using CREER show that preferentially but not exclusively labeled +4 cells (mTertCREER and HopxCREER) give rise to all intestinal lineages (Montgomery et al., 2011; Takeda et al., 2011), confirming the presence of multipotent intestinal SCs in the +4 position. The faster expansion of Lgr5 progeny in comparison to Bmi1- or mTert-derived cells suggests that +4 intestinal SCs could be more quiescent than Lgr5+ SCs, but yet long-lived. Interestingly, although the ablation of Bmi1+ cells leads to the degeneration of the crypts (Sangiorgi and Capecchi, 2008), the ablation of Lgr5 cells has no effect on intestinal homeostasis, as Bmi1+ SCs can compensate for the loss of Lgr5 cells (Tian et al., 2011). Under physiological conditions or after the destruction of Lgr5+ cells, Bmi1 cells can replenish the Lgr5 pool. Similarly, mTert and Hopx labeled cells also give rise to Lgr5+ cells and conversely Lgr5 cells can give rise to Hopx cells (Takeda et al., 2011), suggesting that the Lgr5 SCs are in equilibrium with the +4 cells SCs.
Although there are around 16 Lgr5+ cells per crypt, Lgr5 lineage tracing using a multicolor reporter mouse demonstrated that the crypt rapidly evolves from polyclonal labeling (multicolor) to monoclonal labeling (monocolor) (Fig. 3, F and G). Analysis of Lgr5 cell division revealed that they mostly divide symmetrically giving rise to 2 Lgr5+ cells, incompatible with a model in which homeostasis is maintained by pure asymmetric cell division (one SC gives one transit-amplifying cell). Instead, this supports a model in which SC divisions are symmetric at the cellular level, but, at the level of the SC pool, the divisions seem asymmetric because for one SC that divides, one SC is lost, leading to neutral drift dynamics in which SCs expand or are lost at random (Lopez-Garcia et al., 2010; Snippert et al., 2010b).
How to reconcile the data supporting the existence of two populations of intestinal SCs in equilibrium with each other, with the presence of neutral drift toward monoclonality of the intestinal crypt? One possibility is that, despite their relatively distinct tissue localization, Lgr5 and Bmi1 are functionally equipotent SCs competing with each other in the neutral drift proliferation dynamics. Another possibility is that the long-term lineage tracing in both models results from the labeling of the same multipotent intestinal SCs, as there is a small overlap between the cells traced with Lgr5CREER and the cells traced with Bmi1, Hopx, mTER CREER. Further studies analyzing the rate of the drift toward monoclonality using Lgr5+CREER and the other +4 CREER will help to address this open question.
Although Lgr5 can also mark colonic SCs (Barker et al., 2007), the low frequency of Lgr5-marked crypts due to the mosaic expression Lgr5CREER in the colon renders it difficult to ascertain whether there is one or more colonic SCs contributing to the homeostasis and the regenerative potential of the colonic epithelium.
The airway system
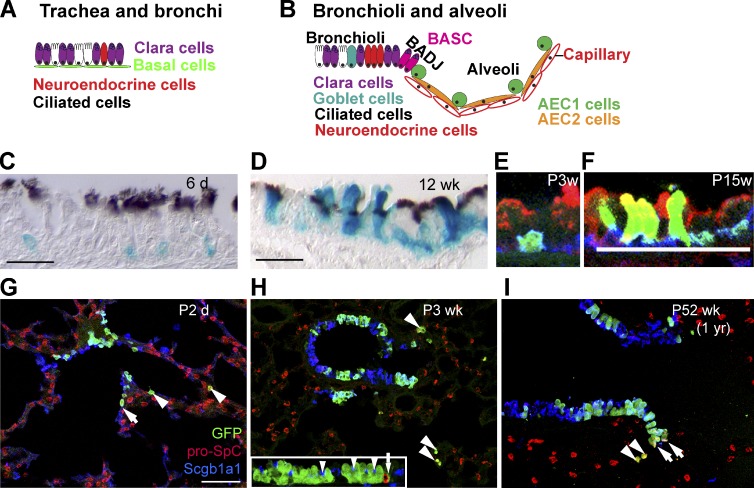
The airway system is compartmentalized along the proximal–distal axis into three anatomically distinct regions: the trachea and bronchi, the bronchioles, and the alveoli. The cellular composition of the lung epithelium varies in the different regions, as well as the cellular hierarchy that regulates the maintenance and repair of these epithelia (Rock and Hogan, 2011). The trachea and bronchi are pseudostratified epithelia containing ciliated cells, secretory cells (Clara cells and goblet cells), and basal cells (∼30% of tracheal epithelial cells; Fig. 4 A). The function of this first part of the airway tract is to allow the circulation of the air but also to protect the lung epithelium by absorbing dust particles and microbes that are constantly inhaled. Through movement of the cilia, these cells chase the dirty mucus out of the respiratory tract. Rare neuroendocrine cells are also dispersed in the luminal layer of the trachea, which regulate the contraction of the respiratory tracts as well as the secretion of the mucus (Rock and Hogan, 2011).
Figure 4.
Lineage tracing of the airway epithelium. (A and B) Schematic representation of the airway epithelium that can be divided into trachea and bronchi, bronchioli, and alveoli. (C–F) Lineage tracing of tracheal basal cells using K5CREER/Rosa-LacZ and analyzed 6 d (C and E), 3 wk (F), or 12 wk (D) after TAM administration. Paraffin sections of X-gal–stained (blue) (K5-labeled cells and their progeny) and anti-acetylated tubulin (brown, cilia), showing the initial labeling of basal cells (C) that give rise to luminal cells during postnatal development (D). (E and F) Lineage tracing of tracheal basal cells using K5CREER/Rosa-YFP and analyzed 3 and 15 wk after TAM administration showing the initial labeling of basal cells (green) that give rise to Clara cells (red). Adapted from Rock et al. (2009) with permission from Proc. Natl. Acad. Sci. USA. (G–I) Lineage tracing of the Clara cells in the bronchioli using Scgb1a1CREER/RosaYFP mice induced at E18.5 and analyzed 2 d (G), 3 wk (H), or 1 yr (I) after induction. Immunostaining of GFP (green), pro-SPC (AEC2) (red), and Scgb1a1 (Clara cells) (blue) shows the long-term renewal of Clara cells and the expansion of ciliated cells over time. Arrowheads represent lineage-labeled AEC2 cells. Arrows represent lineage-labeled putative BASCs. Inset in H shows labeled ciliated cells (arrowheads), but no neuroendocrine cells (red, arrow). The stable frequency of lineage-labeled AEC2 cells over time (1–3%) suggests that BASC cells do not contribute to alveoli expansion during postnatal growth. Adapted from Rawlins et al. (2009) with permission from Elsevier. AEC1, alveolar type I; AEC2, alveolar type II; BADJ, bronchioalveolar duct junction; BASC, bronchioalveolar stem cell. Bars: (C and D) 20 µM; (E and F) 25 µM; (G) 50 µM.
In contrast to the constant and rapid turnover occurring in the intestine or the skin epidermis, the airway system presents a very low renewal under steady-state conditions. For example, lineage tracing using Foxj1-CREER, which labeled ciliated cells, supports the view that ciliated cells are postmitotic with a half time of several months under homeostatic conditions (Rawlins et al., 2007). There is increasing evidence that basal cells function as multipotent SCs in the trachea and bronchi, able to self-renew and differentiate into both Clara cells and ciliated cells under steady-state conditions and after injury. The first evidence that basal cells contain multipotent SCs came from lineage-tracing experiments using the K14-CREER to label basal cells in the trachea and bronchi during naphthalene-induced epithelium regeneration, demonstrating the massive contribution of basal cells during trachea and bronchi regeneration (Hong et al., 2004a,b). However, under physiological conditions most mouse basal cells express K5, whereas only a subset of basal cells expresses K14, which is up-regulated in the basal cell population upon injury. These studies did not assess the contribution of the basal cells under steady-state conditions. Rock et al. (2009) used a K5-CREER to label basal cells of the upper airway tract postnatally under physiological conditions and demonstrated that basal cells contain self-renewing multipotent SCs, giving rise to Clara and ciliated cells during postnatal growth, adult homeostasis, and epithelial repair (Fig. 4, C–F). Lineage tracing using Scgb1a1 (also called Secretoglobin 1a1 or CC10) CREER mice, which specifically marked Clara cells in the trachea and the main bronchi, demonstrated that some of these cells can undergo several rounds of replication giving rise to Clara and ciliated cells. However, most of them are progressively lost and replaced over time by cells that are not marked by Scgb1a1CREER (Rawlins et al., 2009), suggesting that Clara cells in the trachea and the main bronchi do not contain SCs and behave as a transit-amplifying cell population.
Bronchioles lack basal cells but are surrounded instead by myofibroblast cells (Fig. 4 B). They are mainly composed of ciliated and secretory cells, and also contain clusters of neuroendocrine cells. Lineage tracing of Clara cells with Scgb1a1-CREER showed that Clara cells from bronchioles self-renew over a long period of time and give rise to ciliated cells, during postnatal growth, homeostasis, and repair of the bronchiolar epithelium. This is consistent with the presence of bipotent Scgb1a1-expressing SCs, which ensure the homeostasis of the bronchioles (Fig. 4, G–I; Rawlins et al., 2009).
Alveoli are the sites of exchange between the inhaled air and the gas of the blood. To maximize the surface of exchange between the blood and the air, the bronchioles end in multiple sacs, called alveoli, which are encased by a dense capillary network. The alveoli contain two major cell types: the alveolar type 1 cells, which are squamous cells that comprise the major surface of the lung and express different ion transporters critical for fluidity of the mucus produced, whereas alveolar type 2 cells are cuboidal cells that secrete the surfactant protein C (SPC) essential to maintain the alveoli open. Proliferation kinetic experiments suggested that the alveolar type 1 cells are terminally differentiated cells that do not divide, neither under physiological conditions nor during tissue regeneration. On the other hand, type 2 cells divide during homeostasis and repair, and were assumed to contain SCs of the alveoli (Adamson and Bowden, 1975), although no lineage-tracing experiment has formally demonstrated this hypothesis. Cells expressing both Scgb1a1 (the Clara marker) and pro-SPC (a marker of cuboidal alveolar type 2 cells) have been described at the bronchioalveolar junction. These cells do not die upon injury, become proliferatively active, and exhibit SC properties in culture, and therefore were called bronchioalveolar SCs (Kim et al., 2005). Labeling of bronchiolar cells, including the bronchioalveolar SCs, with the Scgb1a1-CREER demonstrated that these cells do not contribute to the alveoli during postnatal growth and after hyperoxia injury but rather contribute to the bronchiolar regeneration (Rawlins et al., 2009). However, a new study showed that cells labeled with the Scgb1a1-CREER contribute extensively to the alveoli regeneration after bleomycin-induced lung injury, suggesting that the contribution of Clara cells to lung regeneration can vary depending on the type of injury (Rock et al., 2011). Consistent with the role of Scgb1a1+ cells during lung repair after bleomycin-induced injury, lineage tracing of alveolar type 2 cells using SPC-CREER demonstrated that SPC-derived cells are replaced by SPC-negative progenitors during lung repair after bleomycin inhalation (Chapman et al., 2011). K14-CREER lineage tracing suggested that K14/K5-positive cells appear in the bronchioles a few days post-infection of H1N1 virus and give rise to migrating K5-positive clusters of cells at the sites of interbronchiolar lung damage (Kumar et al., 2011). However, the origin of these K5-positive cells remains unclear. Do they arise from Sgbd1a1 cells that begin to express K5/K14 upon injury or do they come from already K5-positive cells from the main bronchia? What is their long-term contribution to alveoli lineage? Further work will be needed to clarify these open questions.
Perspectives
Lineage-tracing experiments in different epithelia reveal the coexistence of several types of SCs in each epithelium. Epithelia such as the epidermis and the intestine contain rapidly cycling SCs, dividing asymmetrically at the level of the population but symmetrically at the SC level, which balanced renewal and differentiation stochastically. In addition, these tissues present a slower cycling population of cells that represent a reserve pool of SCs in case of sudden need. More studies will be required to precisely determine how the equilibrium between these two pools of SCs is achieved and what the molecular mechanisms are that allow the stochastic choice between renewal and differentiation.
Another theme that emerges from these lineage-tracing studies is the existence of both multipotent and unipotent SCs maintaining the diversity of cell lineages found in these epithelia during postnatal life. Further studies will be necessary to better understand when the switch from multipotency to unipotency occurs during morphogenesis, how the differentiation of these epithelial SCs is controlled, and what the relative importance of intrinsic versus extrinsic determinants is in regulating their fate.
New lineage-tracing studies will be required to identify new types of SCs in the different epithelial tissues. For example, how are the esophagus, pancreas, bladder, prostate, and ovary developed, maintained, and repaired? Do these tissues contain multipotent SCs or different classes of unipotent SCs? The identification of the different SCs, transit-amplifying cells, and differentiated cells in these different epithelia will be instrumental to uncover the cell lineages at the origin of the different epithelial cancers (Visvader, 2011). What is the respective importance of oncogenic mutations versus the cellular origin in dictating the tumor phenotypes? Also, the identification of cellular origin of the different epithelial cancers will allow one to define more precisely the transcriptional and genetic changes accompanying tumor initiation and to understand the molecular mechanisms that shape the fate of tumor-initiating cells. Cancer SCs have been identified in several human and mouse cancers based on their ability to reform primary tumors after transplantation into immunodeficient mice (Lobo et al., 2007). Further studies will be required to demonstrate the existence of cancer SCs during in vivo tumor growth in intact tissues using lineage-tracing and clonal analysis.
Acknowledgments
We thank Brigid Hogan and Ben Simons for insightful discussions. We apologize to those whose work could not be cited due to space constraints.
C. Blanpain and A. Van Keymeulen are Chercheur Qualifié of the FRS/FNRS. C. Blanpain is an investigator of Welbio. This work was supported by the FNRS, TELEVIE, and the program d’excellence CIBLES of the Wallonia Region; research grants from the Fondation Contre le Cancer, the ULB foundation, and the fond Gaston Ithier; a starting grant of the European Research Council (ERC); and the EMBO Young Investigator Program.
Footnotes
Abbreviations used in this paper:
- HF
- hair follicle
- SC
- stem cell
References
- Adamson I.Y., Bowden D.H. 1975. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab. Invest. 32:736–745 [PubMed] [Google Scholar]
- Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. 2011. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 9:317–329 10.1016/j.stem.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449:1003–1007 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barker N., Bartfeld S., Clevers H. 2010a. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 7:656–670 10.1016/j.stem.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., et al. 2010b. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. 1999. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 116:7–14 10.1016/S0016-5085(99)70222-2 [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. 2002. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G767–G777 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. 2006. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22:339–373 10.1146/annurev.cellbio.22.010305.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W.E., Geoghegan A., Polak L., Fuchs E. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118:635–648 10.1016/j.cell.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Horsley V., Fuchs E. 2007. Epithelial stem cells: turning over new leaves. Cell. 128:445–458 10.1016/j.cell.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M.E., Meilhac S.M. 2011. Tracing cells for tracking cell lineage and clonal behavior. Dev. Cell. 21:394–409 10.1016/j.devcel.2011.07.019 [DOI] [PubMed] [Google Scholar]
- Chapman H.A., Li X., Alexander J.P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T.H. 2011. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121:2855–2862 10.1172/JCI57673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S., Nicolas M., Oshima H., Rochat A., Barrandon Y. 2005. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA. 102:14677–14682 10.1073/pnas.0507250102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E., Doupé D.P., Klein A.M., Winton D.J., Simons B.D., Jones P.H. 2007. A single type of progenitor cell maintains normal epidermis. Nature. 446:185–189 10.1038/nature05574 [DOI] [PubMed] [Google Scholar]
- Doupé D.P., Klein A.M., Simons B.D., Jones P.H. 2010. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell. 18:317–323 10.1016/j.devcel.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S., Taichman L.B. 2001. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20:1215–1222 10.1093/emboj/20.6.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H., Fujiwara M., Chuang J., Medina M.M., Panditrao M.V., Bechstedt S., Howard J., Lumpkin E.A. 2004. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc. Natl. Acad. Sci. USA. 101:14503–14508 10.1073/pnas.0406308101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. 2004a. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164:577–588 10.1016/S0002-9440(10)63147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. 2004b. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L643–L649 10.1152/ajplung.00155.2003 [DOI] [PubMed] [Google Scholar]
- Horsley V., O’Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., Fuchs E. 2006. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 126:597–609 10.1016/j.cell.2006.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R.J., Cotsarelis G. 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11:1351–1354 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Jaks V., Barker N., Kasper M., van Es J.H., Snippert H.J., Clevers H., Toftgård R. 2008. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40:1291–1299 10.1038/ng.239 [DOI] [PubMed] [Google Scholar]
- Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 121:823–835 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K., Watt F.M. 2012. Lineage tracing. Cell. 148:33–45 10.1016/j.cell.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D., Sun Y., Joo L.S., Dagher R., Zielonka E.M., et al. 2011. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 147:525–538 10.1016/j.cell.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué E., Nicolas J.F. 2005. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 132:4143–4154 10.1242/dev.01975 [DOI] [PubMed] [Google Scholar]
- Legué E., Sequeira I., Nicolas J.F. 2010. Hair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineages. Development. 137:569–577 10.1242/dev.044123 [DOI] [PubMed] [Google Scholar]
- Levy V., Lindon C., Harfe B.D., Morgan B.A. 2005. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 9:855–861 10.1016/j.devcel.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Levy V., Lindon C., Zheng Y., Harfe B.D., Morgan B.A. 2007. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 21:1358–1366 10.1096/fj.06-6926com [DOI] [PubMed] [Google Scholar]
- Lobo N.A., Shimono Y., Qian D., Clarke M.F. 2007. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23:675–699 10.1146/annurev.cellbio.22.010305.104154 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C., Klein A.M., Simons B.D., Winton D.J. 2010. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 330:822–825 10.1126/science.1196236 [DOI] [PubMed] [Google Scholar]
- Lumpkin E.A., Marshall K.L., Nelson A.M. 2010. The cell biology of touch. J. Cell Biol. 191:237–248 10.1083/jcb.201006074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich S.M., Wellnitz S.A., Nelson A.M., Lesniak D.R., Gerling G.J., Lumpkin E.A., Zoghbi H.Y. 2009. Merkel cells are essential for light-touch responses. Science. 324:1580–1582 10.1126/science.1172890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R.K., Carlone D.L., Richmond C.A., Farilla L., Kranendonk M.E., Henderson D.E., Baffour-Awuah N.Y., Ambruzs D.M., Fogli L.K., Algra S., Breault D.T. 2011. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA. 108:179–184 10.1073/pnas.1013004108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.J., Liu Y., Marles L., Yang Z., Trempus C., Li S., Lin J.S., Sawicki J.A., Cotsarelis G. 2004. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22:411–417 10.1038/nbt950 [DOI] [PubMed] [Google Scholar]
- Morrison K.M., Miesegaes G.R., Lumpkin E.A., Maricich S.M. 2009. Mammalian Merkel cells are descended from the epidermal lineage. Dev. Biol. 336:76–83 10.1016/j.ydbio.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J.A., Polak L., Pasolli H.A., Fuchs E. 2008. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 3:33–43 10.1016/j.stem.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H., Rochat A., Kedzia C., Kobayashi K., Barrandon Y. 2001. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 104:233–245 10.1016/S0092-8674(01)00208-2 [DOI] [PubMed] [Google Scholar]
- Petersson M., Brylka H., Kraus A., John S., Rappl G., Schettina P., Niemann C. 2011. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 30:3004–3018 10.1038/emboj.2011.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C.S. 1974. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 7:77–88 [DOI] [PubMed] [Google Scholar]
- Potten C.S. 1981. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int. Rev. Cytol. 69:271–318 10.1016/S0074-7696(08)62326-8 [DOI] [PubMed] [Google Scholar]
- Potten C.S., Hume W.J., Reid P., Cairns J. 1978. The segregation of DNA in epithelial stem cells. Cell. 15:899–906 10.1016/0092-8674(78)90274-X [DOI] [PubMed] [Google Scholar]
- Rawlins E.L., Ostrowski L.E., Randell S.H., Hogan B.L. 2007. Lung development and repair: contribution of the ciliated lineage. Proc. Natl. Acad. Sci. USA. 104:410–417 10.1073/pnas.0610770104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H., Wang F., Hogan B.L. 2009. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 4:525–534 10.1016/j.stem.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S., Rannala B. 2004. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep. 5:914–920 10.1038/sj.embor.7400218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S., Rannala B. 2005. Evidence from the stop-EGFP mouse supports a niche-sharing model of epidermal proliferative units. Exp. Dermatol. 14:838–843 10.1111/j.1600-0625.2005.00366.x [DOI] [PubMed] [Google Scholar]
- Rochat A., Kobayashi K., Barrandon Y. 1994. Location of stem cells of human hair follicles by clonal analysis. Cell. 76:1063–1073 10.1016/0092-8674(94)90383-2 [DOI] [PubMed] [Google Scholar]
- Rock J.R., Hogan B.L. 2011. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell Dev. Biol. 27:493–512 10.1146/annurev-cellbio-100109-104040 [DOI] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. 2009. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 106:12771–12775 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. 2011. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA. 108:E1475–E1483 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E., Capecchi M.R. 2008. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40:915–920 10.1038/ng.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K.J., Stingl J., Smyth G.K., Asselin-Labat M.L., Wu L., Lindeman G.J., Visvader J.E. 2006. Generation of a functional mammary gland from a single stem cell. Nature. 439:84–88 10.1038/nature04372 [DOI] [PubMed] [Google Scholar]
- Snippert H.J., Haegebarth A., Kasper M., Jaks V., van Es J.H., Barker N., van de Wetering M., van den Born M., Begthel H., Vries R.G., et al. 2010a. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 327:1385–1389 10.1126/science.1184733 [DOI] [PubMed] [Google Scholar]
- Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., Clevers H. 2010b. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 143:134–144 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H.I., Eaves C.J. 2006. Purification and unique properties of mammary epithelial stem cells. Nature. 439:993–997 [DOI] [PubMed] [Google Scholar]
- Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. 2011. Interconversion between intestinal stem cell populations in distinct niches. Science. 334:1420–1424 10.1126/science.1213214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. 2011. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 478:255–259 10.1038/nature10408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W.E., Rendl M., Fuchs E. 2004. Defining the epithelial stem cell niche in skin. Science. 303:359–363 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Mascre G., Youseff K.K., Harel I., Michaux C., De Geest N., Szpalski C., Achouri Y., Bloch W., Hassan B.A., Blanpain C. 2009. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 187:91–100 10.1083/jcb.200907080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Rocha A.S., Ousset M., Beck B., Bouvencourt G., Rock J., Sharma N., Dekoninck S., Blanpain C. 2011. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 479:189–193 10.1038/nature10573 [DOI] [PubMed] [Google Scholar]
- Visvader J.E. 2009. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 23:2563–2577 10.1101/gad.1849509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J.E. 2011. Cells of origin in cancer. Nature. 469:314–322 10.1038/nature09781 [DOI] [PubMed] [Google Scholar]
- Wagner K.U., Boulanger C.A., Henry M.D., Sgagias M., Hennighausen L., Smith G.H. 2002. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 129:1377–1386 [DOI] [PubMed] [Google Scholar]
- Watson C.J., Khaled W.T. 2008. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 135:995–1003 10.1242/dev.005439 [DOI] [PubMed] [Google Scholar]
- Youssef K.K., Van Keymeulen A., Lapouge G., Beck B., Michaux C., Achouri Y., Sotiropoulou P.A., Blanpain C. 2010. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 12:299–305 [DOI] [PubMed] [Google Scholar]
- Zhang Y.V., White B.S., Shalloway D.I., Tumbar T. 2010. Stem cell dynamics in mouse hair follicles: a story from cell division counting and single cell lineage tracing. Cell Cycle. 9:1504–1510 10.4161/cc.9.8.11252 [DOI] [PMC free article] [PubMed] [Google Scholar]