Abstract
We report the development of burr cell anemia in an infant with short bowel syndrome who received parenteral fish oil (Omegaven, Fresenius-Kabi, Graz, Austria) after development of total parenteral nutrition–associated liver disease. Parenteral fish oil was discontinued, and the burr cell anemia disappeared, suggesting that parenteral fish oil might be associated with hemolytic anemia.
Total parenteral nutrition (TPN)–associated liver disease develops in 40% to 60% of infants who require long-term TPN for intestinal failure.1 The pathogenesis is multifactorial, and standard omega-6 soy-based intravenous lipid is one of the suspected culprits. Parenteral fish oil (Omegaven, Fresenius-Kabi, Graz, Austria) is a recently introduced intravenous omega-3 fatty acid (fish oil) for TPN administration. Parenteral fish oil has been shown to reverse cholestasis resulting from conventional TPN with intravenous omega-6 fat emulsion administration.2–5 The explanation for this reversal remains under investigation. One possibility is that, in distinction to soy-based formulation, parenteral fish oil contains docosahexaenoic acid and eicosapentaenoic acid, which both have antiinflammatory properties.
Burr cells (echinocytes), first described in 1949, are red blood cells with short, uniform spikes emanating from the edges of the cell membrane. They are induced by either extrinsic or intrinsic factors.6 Extrinsic factors include lysolecithin, high levels of fatty acid or physiological levels of fatty acid in the presence of lysolecithin. The intrinsic factors can include aging of red blood cells, which is probably related to decreased adenosine triphosphate. The extrinsically induced echinocyte transformation is generally reversible by washing the cells in fresh plasma; the intrinsically induced transformation is not. Burr cells have been described in association with a variety of disorders, including the following: hemolytic anemia of various causes, kidney disease, liver disease, vitamin E deficiency, increased intracellular calcium, alkalosis, and drug-induced (mesna, 5-fluorouracil, and benzodiazepines). Alternatively, burr cells may represent an artifact while preparing the smear.7
Recently, Fischler et al8 demonstrated burr cell formation in red blood cells incubated in the presence of parenteral fish oil. We report a patient who is unique in that echinocytes developed while she was receiving parenteral fish oil.
Patient Presentation
The patient is a female born at 34 weeks’ gestation (birth weight 2210 g) with gastroschisis and jejunal atresia. At surgery on the first day of life, only 40 cm of viable small bowel was present. TPN with intravenous omega-6–based lipid (Intralipid, Fresenius-Kabi, Uppsala, Sweden) was started on the first day of life at a dose of 0.5 g/kg/d. The dose was increased gradually to 3 g/kg/d. During TPN administration, TPN-associated cholestasis developed. At 6 weeks of age, total bilirubin was 2.7 mg/dL (normal: 0.3–1.5), conjugated bilirubin 2.2 mg/dL (0.0–0.5), aspartate aminotransferase 75 U/L (0–34), and alanine aminotransferase 57 U/L (10–44). In an attempt to halt the progression of the liver disease, parenteral fish oil was started at age 2 months. Before its administration, an investigational new drug application was submitted and approved by the Food and Drug Administration. The patient’s mother signed a consent form to allow the use and administration of parenteral fish oil. The initial dose was 0.5 g/kg/d and was increased to the currently accepted dose of 1 g/kg/d. The hepatic biochemical profile normalized by age 3 months with total bilirubin of 0.4 mg/dL, conjugated bilirubin 0.3 mg/dL, aspartate aminotransferase 28 U/L, and alanine aminotransferase 26 U/L.
While receiving parenteral fish oil, it was noted that recurrent anemia developed, and the patient required a total of 8 red blood cell transfusions throughout the 5 months of administration. Upper and lower endoscopies were performed and did not show any sources of bleeding.
Evaluation to determine the cause of persistent anemia included the following: mean corpuscular volume of 82 fL (71–84), ferritin 155 ng/mL (10–291), total iron binding capacity 247 μg/dL (250–450), transferrin saturation 20% (15%–50%), reticulocytes 2% (0.5%–1.5%), haptoglobin 62 mg/dL (40–240), PT/PTT 13.4/25.7, folate >24 ng/mL (>5.4), and vitamin B12 830 pg/mL (211–911). Parvovirus immunoglobulin M and G results were negative. Hemoglobin electrophoresis showed hemoglobin A1 97.4% and A2 2.6% (normal electrophoresis). Serum vitamin E was 11.2 mg/L (5.0–9.0), and the vitamin E/total blood lipid (total cholesterol + triglycerides) ratio was 6.32 μmol/mmol (lower limit of normal is 1.6 μmol/mmol).9 Serum essential fatty acids were monitored and measured serially while parenteral fish oil was administered and demonstrated elevated levels of do-cosahexaenoic acid 890 μmol/L (10–220) and eicosapentaenoic acid 629 μmol/L (2–60). Family history was negative for hemolytic anemia.
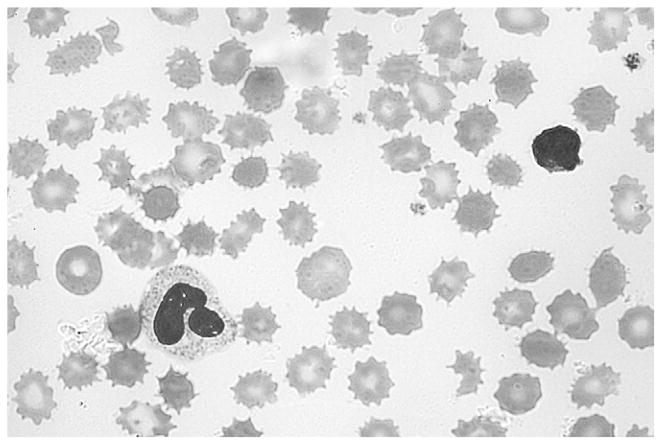
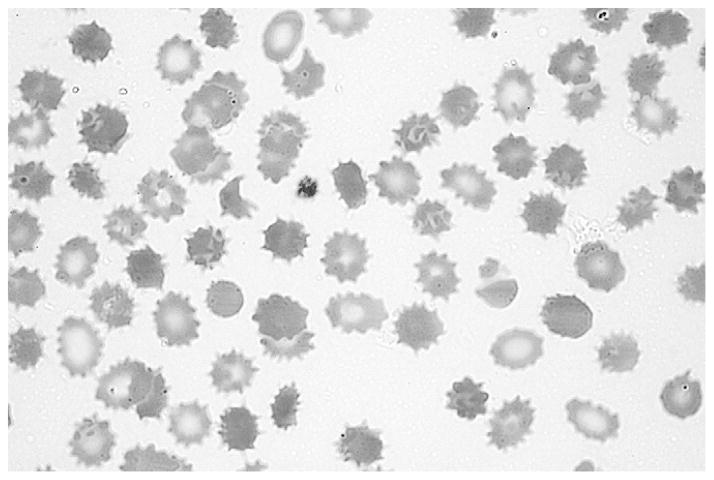
The patient’s peripheral blood smear showed extensive echinocytes (burr cells), red blood cell fragments, spherocytes, and a mild reticulocytosis (Figure 1 and Figure 2; available at www.jpeds.com). The mother’s blood smear was evaluated and was normal. Parenteral fish oil use was suspected as the cause of the anemia and discontinued because alterations in blood lipids can cause morphologic abnormalities in the red blood cell membrane and eventually hemolysis.10
Figure 1.
Patient’s peripheral blood smear showing extensive echinocytes (burr cells), red blood cell fragments, spherocytes, and a mild reticulocytosis.
Figure 2.
Patient’s peripheral blood smear showing extensive echinocytes (burr cells), red blood cell fragments, spherocytes, and a mild reticulocytosis.
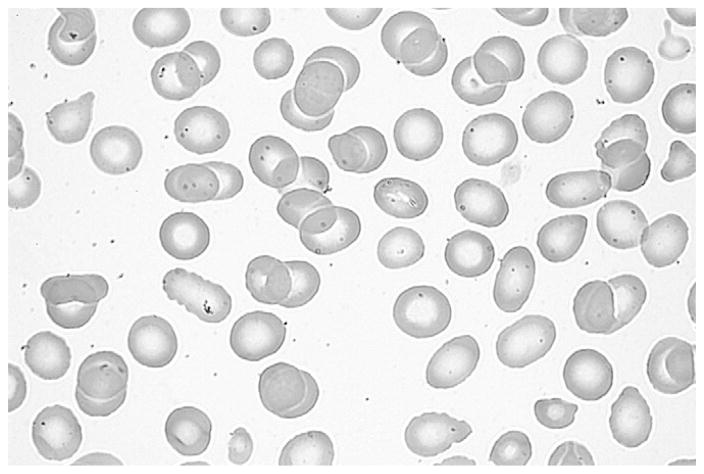
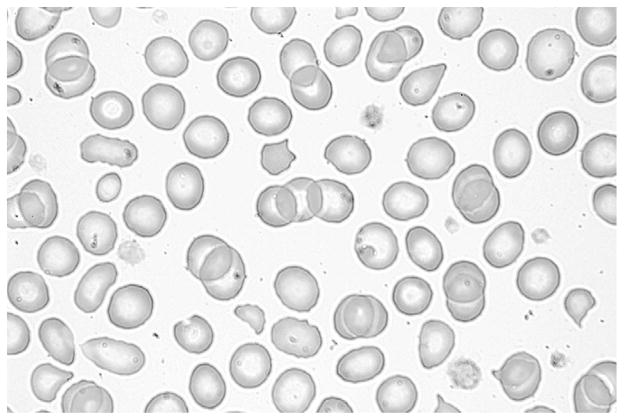
The burr cells disappeared from the blood smear after parenteral fish oil was discontinued. At that time, the result of the smear was mildly abnormal with microcytes, red blood cell fragments, and poikilocytes (Figure 3 and Figure 4; available at www.jpeds.com).
Figure 3.
Mildy abnormal blood smear with microcytes, red blood cell fragments, and poikilocytes after parenteral fish oil was discontinued.
Figure 4.
Mildy abnormal blood smear with microcytes, red blood cell fragments, and poikilocytes after parenteral fish oil was discontinued.
The patient continued to have anemia, but blood transfusions were no longer needed. Six months after parenteral fish oil was discontinued, the anemia had resolved, and the result of the repeat blood smear appeared normal.
The patient had an appropriate growth rate while on parenteral fish oil, gaining an average of 18 g/kg/d. Her growth continued at 22 g/d once Intralipid was substituted for Omegaven at 7 months of age. Enteral feeds were started early in life with an amino acid–based formula, and close to 40% of her daily caloric requirement was provided by enteral feeds at the time parenteral fish oil was discontinued. The patient continued to advance enteral feedings and by 2 months after stopping parenteral fish oil, she was receiving 60% of calories enterally. Micronutrients, including copper, iron, and zinc, remained within normal limits.
Discussion
The appearance and subsequent resolution of burr cells coinciding with the use and discontinuation of parenteral fish oil, respectively, incriminate this preparation as the cause of these abnormal cells. In vitro and in vivo observations support this hypothesis. Fischler et al8 incubated red blood cells with increasing concentrations of parenteral fish oil and observed red blood cell membrane changes resulting in burr cell formation. In their study, these effects were fully reversible on wash-out of parenteral fish oil. Escudero et al10 reported higher echinocyte formation in fish oil–fed rats compared with others fed with either olive or sun-flower oil.
Burr cell anemia is not listed by the manufacturer of parenteral fish oil. Is duration of parenteral fish oil usage a factor in the development of burr cells? In case series that cumulatively included 33 patients with an infusion duration range of 7 to 37 weeks, no deleterious adverse effects were noted. Our patient received 23 weeks of parenteral fish oil, which is within the reported range.4,5
The mechanisms responsible for formation of burr cells are unknown but have been attributed to changes in composition of the lipids of the red blood cell membrane. These changes affect erythrocyte fragility and ultimately cause shape alterations, which make the red blood cells more susceptible to trapping and destruction by the spleen.7,11 For example, phosphatidylcholine, which is another constituent of parenteral fish oil, incorporates into the outer hemileaflet of cell membranes and may alter the shape of erythrocytes, leukocytes, and other endothelial cells and thus may contribute to echinocytosis.
Acknowledgments
Supported by a grant from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research (KL2 RR 024136) (R.C.B). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Glossary
- TPN
Total parenteral nutrition
Footnotes
Information on NCRR is available at http://www.ncrr.nih.gov. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
The authors declare no conflicts of interest.
References
- 1.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70–7. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 2.Gura K, Duggan C, Collier S, Jennings R, Folkman J, Bistrian B, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 3.Ekema G, Falchetti D, Boroni G, Tanca A, Altana C, Righetti L, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (Omega-3 fatty acids) J Pediatr Surg. 2008;43:1191–5. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Gura K, Lee S, Valim C, Zhou J, Kim S, Modi B. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 5.Diamond I, Sterescu A, Pencharz P, Kim J, Wales P. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 6.Brecher G, Bessis M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: a critical review. Blood. 1972;40:3. [PubMed] [Google Scholar]
- 7.Altomare I, Desman G, Aledort L. Echinocytosis-an unusual manifestation of hemangioma. Am J Hematol. 2006;81:532–4. doi: 10.1002/ajh.20558. [DOI] [PubMed] [Google Scholar]
- 8.Fischler L, Meredith D, Reinhart W. Influence of a parenteral fish-oil preparation (Omegaven) on erythrocyte morphology and blood viscosity in vitro. Clin Hemorheol Microcirc. 2003;28:79–88. [PubMed] [Google Scholar]
- 9.Traber M. Vitamin E: metabolism and requirements. In: Caballero B, Allen L, Prentice A, editors. Encyclopedia of human nutrition. 2. Oxford (UK): Elsevier; 2006. pp. 383–8. [Google Scholar]
- 10.Liu Y, Yang H, Su Q, Sakanishi A. Influence of parenteral fat emulsion Intralipos and citric acid on blood viscosity and erythrocyte morphology in vitro. Colloids Surf B Biointerfaces. 2006;53:51–4. doi: 10.1016/j.colsurfb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Escudero A, Montilla J, García J, Sánchez-Quevedo M, Periago J, Hortelano P, et al. Effect of dietary n-9, n-6 and n-3 fatty acids on membrane lipid composition and morphology of rat erythrocytes. Biochim Biophysica Acta. 998;1394:65–73. doi: 10.1016/s0005-2760(98)00095-2. [DOI] [PubMed] [Google Scholar]