Abstract
Objectives
To determine whether an “optimal” DPOAE protocol including (1) optimal stimulus levels and primary-frequency ratios for each f2, (2) simultaneously measuring 2f2-f1 and 2f1-f2 distortion products, (3) controlling source contribution, (4) implementing improved calibration techniques, (5) accounting for the influence of middle-ear reflectance, and (6) applying multivariate analyses to DPOAE data results in improved accuracy in differentiating between normal-hearing and hearing-impaired ears, compared to a standard clinical protocol.
Design
Data were collected for f2 frequencies ranging from 0.75 to 8 kHz in 28 normal-hearing and 78 hearing-impaired subjects. The protocol included a control condition incorporating standard stimulus levels and primary-frequency ratios calibrated with a standard sound pressure level (SPL) method and three experimental conditions using optimized stimuli calibrated with an alternative forward pressure level (FPL) method. The experimental conditions differed with respect to the level of the reflection-source suppressor tone, and included conditions referred to as the null-suppressor (i.e., no suppressor tone presented), low-level suppressor (i.e., suppressor tone presented at 58 dB SPL), and high-level suppressor (i.e., suppressor tone presented at 68 dB SPL) conditions. The area under receiver operating characteristic (AROC) curves and sensitivities for fixed specificities (and vice versa) were estimated to evaluate test performance in each condition.
Results
AROC analyses indicated (1) improved test performance in all conditions using multivariate analyses, (2) improved performance in the null-suppressor and low-suppressor experimental conditions compared to the control condition, and (3) poorer performance below 4 kHz with the high-level suppressor. As expected from AROC, sensitivities for fixed specificities and specificities for fixed sensitivities were highest for the null-suppressor and low-suppressor conditions and lowest for standard clinical procedures. The influence of 2f2-f1 and reflectance on test performance was negligible.
Conclusions
Predictions of auditory status based on DPOAE measurements in clinical protocols may be improved by the inclusion of (1) optimized stimuli, (2) alternative calibration techniques, (3) low-level suppressors, and (4) multivariate analyses.
INTRODUCTION
The utility of distortion product otoacoustic emissions (DPOAEs) in the objective assessment of cochlear function in humans has been described in many studies (e.g., Nelson and Kimberly, 1992; Gorga et al. 1993, 1997; Lonsbury-Martin et al., 1993; Kim et al., 1996). Peripheral hearing loss caused by damage to the outer hair cells of the cochlea is known to result in a reduction in otoacoustic emissions (Brownell, 1990; Martin et al., 1990). Standard DPOAE protocols accurately differentiate between normal-hearing and hearing-impaired ears at audiometric frequencies from 2 to 6 kHz, and are often used in universal newborn hearing screening (UNHS) programs for this purpose. Unfortunately, DPOAE performance is not perfect in that some ears with normal hearing are misdiagnosed as hearing-impaired while some ears with hearing loss are incorrectly diagnosed as normal-hearing (e.g., Gorga et al., 1993, 1997; Kim et al., 1996). Performance is even worse for frequencies between 0.75 and 1.5 kHz and at 8 kHz.
Several strategies have been used in attempts to improve the sensitivity and specificity of DPOAE measures at all test frequencies. For example, the earliest descriptions of DPOAE test performance used equal-level primaries (L1 = L2) to elicit the response (Martin et al., 1990). Recognizing that the representation of the two primaries (f1 and f2) differ at the place(s) of generation of distortion products (presumably close to the f2 place), later efforts used unequal primary levels, with L1 higher than L2 (Gorga et al., 1993, 1997). Several studies showed that moderate stimulus levels result in better differentiation of normal-hearing and hearing-impaired ears. This occurs because low-level primaries do not elicit responses from all normal-hearing ears (resulting in an increase in false-positive errors) and high-level primaries may increase the likelihood of producing emissions from ears that are hearing-impaired (increasing the false-negative rate) (Whitehead et al. 1995; Stover et al., 1996; Johnson et al., 2010). Moderate-level stimuli appear to reduce both the false-positive and false-negative errors.
Kummer et al. (1998) developed an approach for setting primary levels that recognized the compressive growth of the response to f2 close to the f2 place, which differed from the response to f1 at the same place. They advocated the use of a primary-level relationship that took this differential growth into account, and proposed an alternative approach in which L1 = 0.4 L2 + 39 dB independent of f2 (Kummer et al., 1998; 2000). In many respects, this recommendation is similar to previous descriptions of “optimal” primary levels based on data from animal studies (Whitehead et al. 1992). Recently, Neely et al. (2005) found that the primary-level difference that produced the largest response in normal-hearing ears was frequency dependent, in that larger differences in L1 and L2 resulted in larger DPOAEs at higher frequencies. Johnson et al., (2006a) described effects for both primary levels and primary-frequency ratios, deriving formulas for ideal primary level [L1 = 80 + 0.137 · log2(18/f2) × (L2 − 80)] and frequency ratio [f2/f1 = 1.22 + log2 (9.6/f2) × (L2/415)2] which took frequency into account. The stimuli described by these equations resulted in larger DPOAE levels in normal-hearing subjects than those observed using the primary levels described by Kummer et al. Somewhat surprisingly, Johnson et al. (2010) found no improvement in test performance when these “optimal” primary-frequency ratios and primary-level differences were used in a group of normal-hearing and hearing-impaired subjects. Although the reason for the lack of improvement was not obvious, it was speculated that the optimal conditions caused an increase in DPOAE level for both normal and impaired ears (at least at some frequencies), resulting in no net increase in the separation of the distribution of responses from the two groups of subjects.
Another factor potentially affecting DPOAE test performance relates to source contribution. DPOAEs, as measured at the plane of the probe tip in the ear canal, arise primarily from the interaction of two sources: the distortion source, located near the f2 place and thought to be the main generator, and the coherent-reflection source resulting from a linear reflection from the characteristic place for the DPOAE frequency along the basilar membrane (e.g., Heitmann et al., 1998; Shera and Guinan, 1999). The phase relationship of the two emission sources varies as a function of frequency, resulting in a pattern of constructive and destructive interference known as “fine-structure” which can be observed in plots of DPOAE level as a function of frequency, especially when frequency is incremented in small steps (Talmadge et al., 1999). This interaction between distortion and reflection sources may cause changes in measured DPOAE level that affect test performance. Specifically, destructive interaction might result in a reduction in DPOAE level in a normal ear, causing it to be misdiagnosed as hearing impaired, while constructive interaction might increase the magnitude of the measured response in an ear with (mild or moderate) hearing loss, resulting in a diagnosis of normal hearing. Fine structure is reduced when a suppressor tone, f3, with a frequency slightly lower than that of the 2f1-f2 frequency, is presented simultaneously with the primaries (Heitmann et al, 1998; Talmadge et al. 1999). However, Johnson et al. (2007), using stimulus conditions that reduce fine structure based on work in subjects with normal hearing (Johnson et al. 2006b), failed to demonstrate an improvement in test performance when a suppressor tone was presented, compared to the performance achieved when there was no suppressor tone.
Poorer DPOAE test performance, especially for lower frequencies, might relate to the distortion product that typically is measured during clinical assessments. The 2f1-f2 DPOAE emission is measured to predict auditory status, as it is typically the highest level distortion product in humans. However, the 2f1-f2 emission occurs at a frequency approximately ½ octave below f2, where the noise floor surrounding the emission may be higher (e.g., Gorga et al. 1993). The poor signal-to-noise ratio (SNR) might make it more difficult to measure responses in ears with normal hearing, thus driving up the false-positive rate. There are other distortion products produced as a result of the presentation of two primaries, one of which occurs at a frequency that is about ½ octave above f1 (namely 2f2-f1) where noise levels typically are lower. There has been some interest in using both the 2f2-f1 and 2f1-f2 emissions to determine auditory status (Gorga et al., 2000; Fitzgerald and Prieve, 2005); however, the results from these two studies differ. In one case, multivariate analyses incorporating both emissions resulted in slight improvements in DPOAE test performance (Gorga et al. 2000), although in the other case, no significant improvement in test performance was observed when the levels of the two emissions were combined or with the application of logistic regression analysis (Fitzgerald and Prieve, 2005).
Stimulus calibration prior to DPOAE measurements also might influence DPOAE test performance. Typically, stimulus sound pressure level (SPL) is measured at the plane of the probe, and is assumed to represent the level of the stimulus at the eardrum. However, standing waves introduce errors in SPL calibrations at and above 3 kHz (Siegel, 1994; Siegel and Hirohata, 1994; Neely and Gorga, 1998, Dreisbach and Siegel, 2001; Scheperle et al., 2008). Thus, the level at the eardrum may be higher than the level at the probe or (less frequently) the level at the eardrum might be lower than the level estimated at the plane of the probe. Given the influences of stimulus level on test performance (Whitehead et al., 1992; Stover et al., 1996; Johnson et al. 2010), calibration errors might affect the accuracy with which auditory status is determined from DPOAE measurements. Several alternative calibration methods have been described that are unaffected by standing waves (Neely and Gorga, 1998; Scheperle et al., 2009), and thus might result in improvements in DPOAE test performance. Burke et al. (2010) showed that using alternative calibration methods that are not susceptible to standing-wave errors (e.g. forward pressure level, FPL) can improve DPOAE test performance, but the effect was restricted to 8 kHz, where test performance was poorest when stimuli were calibrated in SPL. At lower frequencies, differences were observed related to calibration procedure, but the effects were small and not always as predicted.
Middle-ear transfer of energy may have an influence on the accuracy with which auditory status is predicted from DPOAE measurements. The true input level of the primaries at the plane of the tympanic membrane and the level of the emission as recorded at the plane of the probe microphone depend on the forward and reverse transmission characteristics of the middle ear (Keefe et al., 2000). Keefe et al. (2003) described the relationship between high-frequency (2–8 kHz) reflectance properties of the ear and the level of recorded emissions in neonates. They found that a significant percentage of the variance in OAE levels (up to 28%) could be accounted for by middle-ear transmission characteristics, with OAE levels decreasing with increasing high-frequency energy reflectance. Evidence suggests that the typically greater DPOAE levels observed in infants arise from differences in ear-canal area which affect reverse middle-ear transmittance properties (Keefe and Abdala, 2007). Sanford et al. (2009) observed greater reflectance in neonatal ears that failed a DPOAE-based UNHS test, compared to infants who passed the same test. Given that reflectance can be measured at atmospheric pressure using the same probe assembly that is used to measure OAEs (Sanford et al., 2009), it might be advantageous to use reflectance measures to assist in the classification of cochlear status based on DPOAE measurements. To date, however, there are no studies on test performance in children and adults that incorporate measures of middle-ear energy reflectance with DPOAE measurements.
Finally, test performance may be affected by the approach that is taken to analyze DPOAE data. Typically, predictions of auditory status are based on simple univariate procedures, in which either DPOAE level (Ld) or SNR at 2f1-f2 is used to determine cochlear status. Multivariate analyses, which incorporate several factors as decision variables in classifying ears as either normal hearing or hearing impaired, have been shown to result in improvements in test performance, especially for frequencies at which univariate analyses result in poor performance (Dorn et al., 1999; Gorga et al., 1999, 2005). Although relatively simple to implement, it remains the case that multivariate analyses have not been incorporated into clinical assessments. One possible factor contributing to this may relate to the idiosyncratic nature of multivariate solutions. Further studies to validate the approach, therefore, may be needed prior to their acceptance in the clinic.
The purpose of the present study is to combine several techniques that have been used in efforts to improve DPOAE test performance. Specifically, DPOAE data were collected with “optimal” primary-level and primary-frequency ratios, using calibration procedures unaffected by standing waves, measuring two DPOAEs simultaneously, controlling for source contribution, and incorporating measurements of middle-ear reflectance, all of which were combined in a multivariate analytical framework. The test performance achieved under these conditions was compared to the results achieved when traditional stimulus conditions were used, only one DPOAE was measured, and data were analyzed with a univariate or a multivariate approach. The hypothesis is that measurements and analyses using several factors will result in improved test performance compared to what is achieved with simpler conditions in common clinical use.
METHODS
Instrumentation
An ER-10C probe microphone (Etymōtic Research, Elk Grove Village, IL) was used for presentation of stimuli, calibration, and recording of responses. Stimuli were generated with a 24-bit sound card (CardDeluxe, Digital Audio Labs, Chanhassen, MN) at a 32-kHz sampling rate. A custom-software system (EMAV Version 3.07; Neely and Liu, 1994) was used to (1) calibrate the probe source, (2) present stimuli (including primary and suppressor stimuli), (3) measure DPOAEs, and (4) measure reflectance of the ear.
Calibration
For the control condition, calibration was completed in the ear canal, using a standard sound pressure level (SPL) method and wideband chirp stimulus. The experimental conditions implemented an FPL technique to estimate the Thévenin source acoustic properties of the probe microphone and, in turn, isolate the incident from reflected components of the calibration signal pressure wave (Neely and Gorga, 1998; Scheperle et al., 2008). FPL calibration was performed daily using cavities (acoustic loads) warmed to approximate body temperature (95–105°F) and with resonant peaks occurring at approximately 2, 3, 4, 6 and 8 kHz (Burke et al., 2010).
Subjects
Data were collected from 28 normal-hearing and 78 hearing-impaired subjects ranging in age from 11 to 75 years. Whenever possible, data were collected from both ears of each subject, resulting in data from 147 ears with hearing loss and 51 ears with normal hearing. Audiometric thresholds were obtained by conventional audiometry, in 5-dB increments, using either insert (ER-3A) or supra-aural (TDH-50P) headphones. Normal hearing was defined as audiometric thresholds ≤ 20 dB HL (ANSI, 2004) for frequencies of 0.75, 1, 1.5, 2, 3, 4, 6, and 8 kHz, while hearing loss was defined as thresholds exceeding this level at one or more of those frequencies. Only hearing-impaired subjects with confirmed sensorineural hearing loss, defined as having air-bone gaps of < 15 dB for frequencies 0.5, 1.0, 2.0, and 4.0 kHz, were eligible for inclusion. During data analyses, the assignment to hearing category (normal or impaired) was made on a frequency-by-frequency basis. The distribution of ears across hearing-loss categories is provided in Table 1.
Table 1.
Number of ears in each hearing loss category by audiometric frequency. Normal hearing was defined as ≤20 dB HL, mild hearing loss as thresholds between 21 and 40 dB HL, moderate between 41 and 60 dB HL, severe between 61 and 80 dB HL, and profound as thresholds greater than 80 dB HL.
| Normal | Mild | Moderate | Severe | Profound | |
|---|---|---|---|---|---|
| 750 Hz | 112 | 38 | 29 | 10 | 9 |
| 1000 Hz | 112 | 35 | 31 | 10 | 10 |
| 1500 Hz | 95 | 46 | 34 | 10 | 13 |
| 2000 Hz | 83 | 43 | 46 | 12 | 14 |
| 3000 Hz | 76 | 40 | 55 | 13 | 14 |
| 4000 Hz | 70 | 33 | 56 | 22 | 17 |
| 6000 Hz | 67 | 33 | 52 | 27 | 19 |
| 8000 Hz | 68 | 29 | 51 | 32 | 18 |
Prior to DPOAE data collection, middle-ear status was assessed using tympanometry with a 226-Hz probe tone. In order to qualify for inclusion, the following tympanometric criteria had to be met: peak-compensated, static acoustic admittance of 0.3–2.5 mmho and tympanometric peak pressure between −100 to +50 daPa. Otoscopic examination also was completed as a way to further assess ear-canal and middle-ear status. Subjects were seated in a comfortable reclining chair in a sound-attenuating booth, and were allowed to watch closed-captioned videos with the sound turned off. The work described in this paper was conducted under an approved Institutional Review Board protocol. Following the acquisition of informed consent, audiometric, tympanometric and otoscopic assessments, data collection commenced, which required approximately 40 minutes per ear.
Procedures
The protocol consisted of a control condition and three experimental conditions. In the control condition, DPOAEs were measured at octave and interoctave frequencies from 0.75 to 8 kHz with L2 = 50 dB SPL, L1 = 59 dB SPL, and the f2/f1 fixed at 1.22. The primary-frequency ratio was chosen based on previous work (e.g., Gaskill and Brown, 1990) and was the same for all test frequencies, while L1 was set according to the formula described by Kummer et al. (1998). In the experimental conditions, L2 was also set to 50 dB SPL and L1 was determined for the same frequencies that were tested under the standard protocol using a modified version of the formula derived by Johnson et al., 2006a:
The f2/f1 was determined for those same frequencies also using a formula derived by Johnson et al., 2006a:
These stimulus conditions were chosen because they have been found to result in the largest DPOAEs for subjects with normal hearing (Neely et al., 2005; Johnson et al., 2006a). Two of the three experimental conditions included simultaneous presentation of a suppressor tone 16 Hz lower than the 2f1-f2 distortion-product frequency. Suppressor levels of 58 and 68 dB SPL were selected to bracket the range shown to reduce fine structure and, therefore, presumably reduce contributions from the reflection source (Johnson et al., 2006b). Though Johnson et al. (2007) demonstrated no improvements in DPOAE test performance with simultaneous suppression, experimental suppressor conditions were included in the present study to determine possible interaction effects with other factors, such as calibration method. Simultaneous measurement of the 2f1-f2 and 2f2-f1 distortion products and ear-canal reflectance at f1 and f2 were obtained in all conditions, although the contribution of these variables was only considered in the analysis of the experimental conditions.
During data collection for each frequency and condition, DPOAE data were alternately stored in two separate buffers. The level of the DPOAE, Ld, was estimated from the level in the frequency bin containing the emissions of interest, namely 2f1-f2 or 2f2-f1, after summing the contents of the two buffers. Frequency resolution for each bin was approximately 4 Hz. Estimates of the noise floor were derived by subtracting the contents of the two buffers and then using the bin containing the DPOAE and the two closest frequency bins above and below it, for a total of five bins. Measurement-based stopping criteria were used during data collection such that measurements terminated for any condition when (1) the noise floor ≤ −25 dB SPL, (2) the SNR exceeded 60 dB, or (3) 32 seconds of artifact-free averaging passed. These rules were chosen so that the test never stopped on SNR, stopped mainly on the noise-floor criterion (for higher f2 frequencies), but also stopped on averaging time (for lower f2 frequencies). Test time could have been extended so that all measurement stopped when the noise-floor criterion was met. However, this would have resulted in excessive test time for some subjects and some conditions, and would not be representative of test conditions that could be used clinically.
Analysis
Multivariate logistic-regression analyses were used to describe the relative contribution of the experimental variables to the distribution of responses from normal-hearing and hearing-impaired ears. These analyses produced a logit function (LF) for each audiometric frequency and were completed independently for each experimental condition. LF coefficients for those variables which contributed positively to DPOAE test performance in the null experimental condition are listed in Table 2. In the control condition, LF coefficients derived from previous work (Dorn et al. 1999; Gorga et al. 2005) were applied to the DPOAE data.
Table 2.
Logit function coefficients derived from multivariate analysis of experimental variables. Coefficients are listed for noise and DP level at 2f1-f2 in the null-suppressor condition. Coefficients for 2f2-f1 and R1/R2 are omitted.
| 750 | 1000 | 1500 | 2000 | 3000 | 4000 | 6000 | 8000 | |
|---|---|---|---|---|---|---|---|---|
| Predictor | ||||||||
| Ldp750 | 0.112 | 0.086 | −0.004 | 0.008 | 0.026 | 0.009 | −0.003 | 0.042 |
| Ldp1000 | 0.136 | 0.108 | 0.022 | 0.002 | −0.053 | −0.037 | −0.055 | 0.031 |
| Ldp1500 | 0.072 | 0.078 | 0.148 | 0.087 | 0.045 | 0.019 | 0.015 | 0.019 |
| Ldp2000 | 0.044 | 0.066 | 0.161 | 0.164 | 0.063 | 0.012 | 0.015 | −0.005 |
| Ldp3000 | −0.022 | 0.007 | 0.021 | 0.100 | 0.137 | 0.079 | 0.058 | 0.022 |
| Ldp4000 | −0.034 | −0.029 | −0.072 | −0.067 | 0.016 | 0.077 | 0.017 | 0.039 |
| Ldp6000 | 0.030 | 0.015 | 0.008 | 0.064 | 0.099 | 0.079 | 0.132 | 0.082 |
| Ldp8000 | 0.089 | 0.057 | 0.118 | 0.051 | 0.069 | 0.062 | 0.167 | 0.117 |
| Ndp750 | −0.046 | −0.035 | −0.031 | −0.010 | 0.008 | 0.005 | 0.017 | 0.003 |
| Ndp1000 | −0.040 | −0.031 | −0.027 | −0.014 | −0.031 | −0.019 | 0.007 | 0.008 |
| Ndp1500 | −0.080 | −0.052 | −0.014 | −0.006 | −0.008 | −0.004 | 0.018 | 0.005 |
| Ndp2000 | −0.057 | −0.036 | −0.021 | −0.004 | −0.014 | −0.009 | 0.013 | 0.009 |
| Ndp3000 | −0.017 | −0.010 | −0.014 | 0.001 | −0.006 | −0.006 | −0.003 | −0.001 |
| Ndp4000 | −0.008 | −0.006 | −0.009 | −0.002 | 0.008 | 0.005 | 0.004 | 0.001 |
| Ndp6000 | −0.002 | −0.002 | −0.003 | −0.006 | 0.000 | 0.002 | 0.001 | 0.001 |
| Ndp8000 | 0.000 | 0.000 | −0.002 | 0.000 | 0.000 | −0.001 | −0.002 | −0.002 |
Receiver operating characteristic (ROC) curves were then constructed for the control and experimental conditions and area under these curves (AROC) was estimated to evaluate test performance. In addition, test performance was evaluated using standard univariate analyses (SNR and DPOAE level at 2f1-f2). Finally, test performance was assessed by determining the sensitivity for fixed specificities and vice versa.
RESULTS
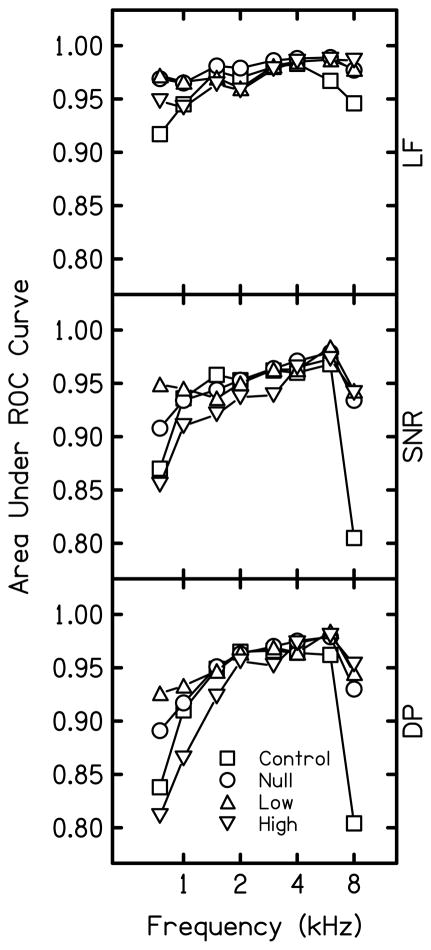
Results of the AROC analyses, shown in Fig. 1, revealed improved test performance in all conditions using the multivariate approach (compare AROC in the top panel to AROC in the two lower panels). These results are consistent with previous findings that demonstrated improved performance when multivariate analyses are applied (Dorn et al. 1999; Gorga et al., 2005). Greater AROC values were observed below 2 and at 8 kHz in the null-suppressor (circles) and low-suppressor (upright triangles) conditions compared to the control condition (squares). This was true using DPOAE level (Ld) and SNR criteria (compare circles and upright triangles to squares in the middle and bottom panels), and the case in which multivariate analyses (LF) were applied to both data sets (compare circles and upright triangles to squares in the top panel). The fact that test performance improved in the null-suppressor and low-suppressor conditions for univariate analyses (SNR and Ld) indicates that there was an influence of calibration method (SPL vs. FPL), optimized stimuli, and low-level suppression on test performance. Little effect of low-level suppression (to reduce the reflection source) on AROC was found in comparison to the null-suppressor condition when multivariate analyses were applied. Small improvements in test performance were observed for the low-level suppressor condition, compared to the null-suppressor condition, when univariate analyses were applied, but only at low frequencies (compare circles and upright triangles in middle and bottom panels). A decline in AROC relative to the null- and low-level suppressor conditions was observed in the low frequencies when a high-level suppressor was presented (inverted triangles). Application of multivariate analyses improved the situation, but not to the same extent as it was improved for null-suppressor and low-suppressor conditions.
Figure 1.
AROC for control and experimental conditions. The upper panel shows AROC using multivariate logistic regression analysis (LF), the middle panel shows AROC using univariate analysis of SNR at 2f1-f2 (SNR), and the bottom panel shows AROC using univariate analysis of distortion product level at 2f1-f2 (DP). Squares represent results for the control condition, circles represent the null-suppressor condition, upright triangles represent the low-suppressor condition, and inverted triangles represent the high-suppressor condition.
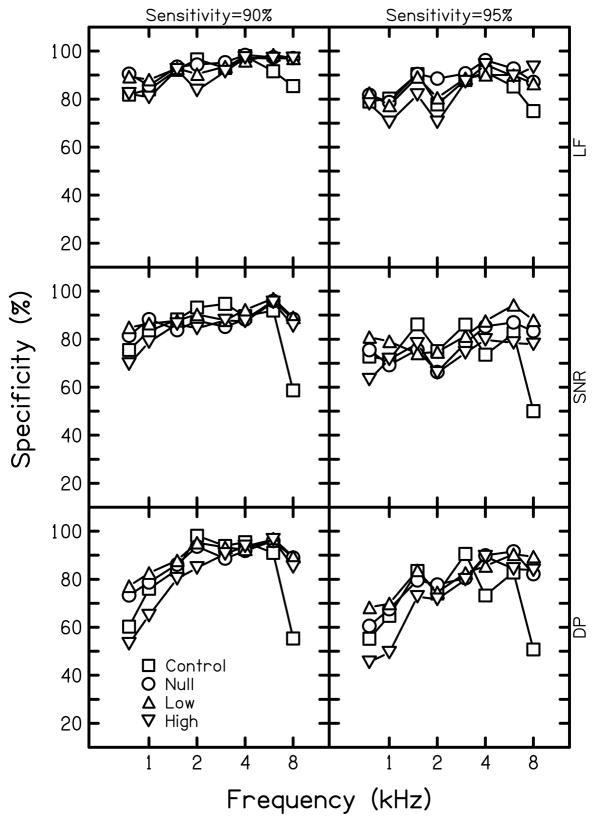
Figure 2 shows specificity at each audiometric frequency for fixed sensitivities of 90% (left column) and 95% (right column) for each analysis method, the results for which are shown in separate rows. As expected from previously reported data and the results shown in Fig. 1, the greatest specificity was found when using multivariate analyses, regardless of condition. Specificity was greater at 2 and 8 kHz with a fixed sensitivity of 95% in the null-suppressor and low-level suppressor conditions compared to the control condition. The differences in specificity between the null-suppressor and the low-level suppressor conditions were negligible for multivariate analyses, but the low-level suppressor condition resulted in higher specificities when univariate analyses (SNR or Ld) were used. The reasons for the improvements for these two conditions, which mostly had lower specificities compared to when multivariate analyses were used, are not obvious. Consistent with the AROC shown in Fig. 1, decreased specificity below 2 kHz was found with high-level suppression, most notably when sensitivity is fixed at 95%.
Figure 2.
Specificity at fixed sensitivities of 90% and 95% for each condition. Results using multivariate logistic regression analysis (LF) are shown in the top row, followed by univariate analysis of SNR at 2f1-f2 (SNR) and univariate analysis of distortion product level at 2f1-f2 (DP) methods, in descending order. Squares represent specificity for the control condition, circles represent the null-suppressor condition, upright triangles represent the low-suppressor condition, and inverted triangles represent the high-suppressor condition.
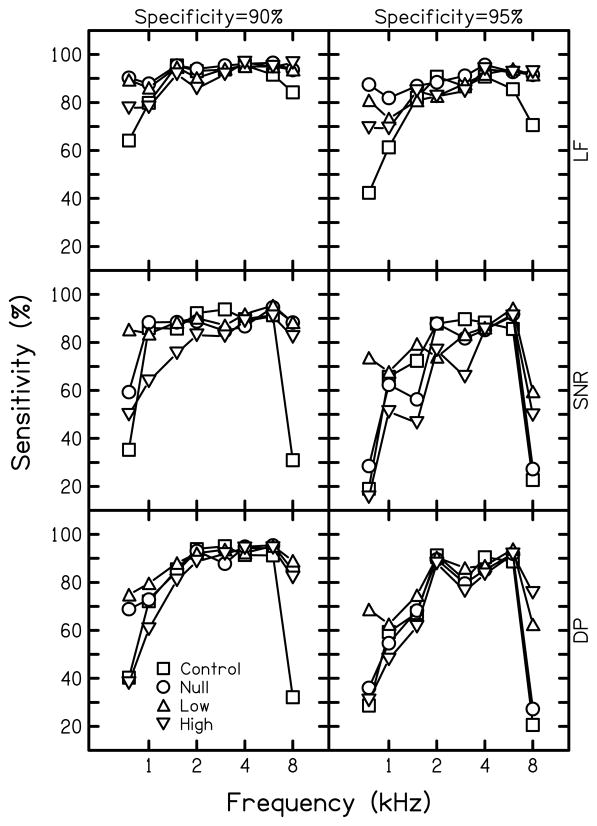
Figure 3 shows sensitivity at fixed specificities of 90% (left column) and 95% (right column) for all four test conditions following the convention used in Fig. 2. As in previous examples, the best performance (greatest sensitivity) in each condition was found using the multivariate approach. Sensitivity was greater below 2 kHz and at 8 kHz in the null-suppressor and low-level suppressor conditions compared to the standard control condition. As expected from previous results, the null-suppressor and both suppressor conditions achieved higher sensitivities for univariate conditions, compared to the control condition. These effects were not uniform across frequency, with some variability in which experimental conditions produced the highest sengsitivity, especially at 0.75 and 8 kHz. However, the highest sensitivity was achieved in the null-suppressor condition when multivariate analyses were applied to the data. Decreased sensitivity below 2 kHz was found with high-level suppression when univariate analyses were applied to the data, with the effect most notable when specificity was fixed at 95%. In contrast, both suppressor conditions resulted in higher sensitivities, compared to the null condition, when univariate analyses were applied at 8 kHz.
Figure 3.
Sensitivity at fixed specificities of 90% and 95% for each condition. Results using multivariate logistic regression analysis (LF) are shown in the top row, followed by univariate analysis of SNR at 2f1-f2 (SNR) and univariate analysis of distortion product level at 2f1-f2 (DP) methods, in descending order. Squares represent sensitivity for the control condition, circles represent the null-suppressor condition, upright triangles represent the low-suppressor condition, and inverted triangles represent the high-suppressor condition.
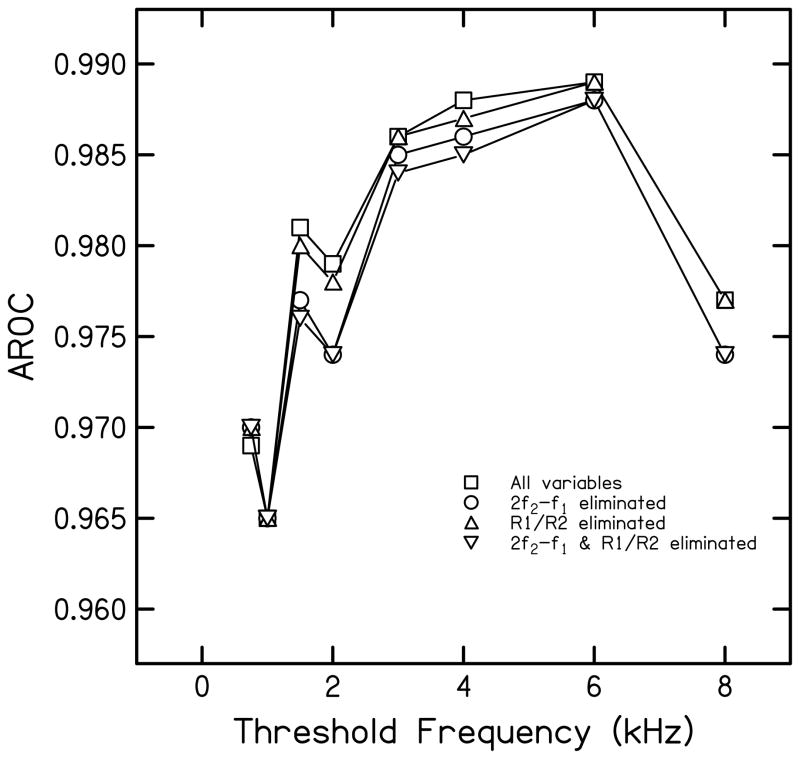
Figure 4 shows the influence of 2f2-f1 and reflectance (R1/R2) on AROC. Note the expanded Y-axis scale, ranging from 0.96 to 0.99. This scale was necessary in order to assess the size of the effect of removing these variables from the analyses. Removing reflectance (R1/R2) from the multivariate analysis had a negligible effect. Removing 2f2-f1 resulted in at most a 0.005 decrease in AROC at audiometric frequencies 1.5 and 2 kHz. Removing both reflectance estimates and data for the 2f2-f1 distortion product had the largest and most consistent effect across frequency, but even then, the maximum decrease in AROC was 0.005 at 2 kHz.
Figure 4.
Influence of 2f2-f1 and reflectance (R1/R2) on AROC. Squares represent AROC with all variables included in the LF analysis, circles represent AROC with 2f2-f1 eliminated from the LF analysis, upright triangles represent AROC with R1/R2 eliminated, and inverted triangles represent AROC with both 2f2-f1 and R1/R2 eliminated from the LF analysis.
DISCUSSION
The purpose of this study was to determine whether an “optimal” protocol which (1) used optimal stimulus levels and primary-frequency ratios for each f2, (2) simultaneously measured 2f2-f1 and 2f1-f2 emissions, (3) controlled source contribution, (4) implemented improved calibration techniques, (5) accounted for the influence of middle-ear reflectance, and (6) applied multivariate analyses to DPOAE data resulted in improved accuracy in differentiating between normal-hearing and hearing-impaired ears, compared to a standard clinical protocol.
The results of this study confirm and extend the findings of other studies (Dorn et al. 1999, Gorga et al. 2005) regarding the superiority of multivariate analyses of DPOAE data. AROC was greatest when previously derived coefficients from a multivariate analysis were applied to the control condition or when newly derived coefficients from a logistic regression analysis were applied to the experimental conditions (Fig. 1), especially at the frequencies where standard univariate analyses of Ld or SNR at 2f1-f2 show the poorest performance, specifically at 8 kHz and at 1.5 kHz and below. Although not studied in the present experiment, another factor in the poorer performance at 8 kHz for each analysis method may be the output limitations of the ER-10C transducer at this frequency.
FPL calibration and group-optimized stimulus levels, implemented in the experimental conditions, were found to increase AROC over the control condition (Fig. 1) independent of the analysis method. Our results confirmed that accurate estimates of the level of the stimulus at the plane of the probe with FPL calibration, when combined with group-optimized stimulus levels, can help to improve DPOAE test performance, although others have only found improvements at 8 kHz (Burke et al., 2010). The relative contribution of either stimulus optimization or calibration method to test performance could not be determined because test conditions were not included in which these two variables were separated.
The impacts of playing a suppressor tone just below the distortion-product frequency were mixed. Mostly, the inclusion of a low-level suppressor resulted in outcomes that were close to those observed in the null-suppressor condition, but the pattern was variable. Test performance with low-level suppression was slightly better, slightly worse or the same as it was for the null-suppressor condition. These small differences were even less obvious when multivariate analyses were used. Suppressor tones have been found to reduce fine structure in recorded DPOAEs (e.g., Heitmann et al. 1998, Johnson et al. 2006b). However, in agreement with the findings of Johnson et al. (2007), no consistent improvement in test accuracy was observed with the inclusion of a suppressor tone in the stimulus complex. It is possible that the suppressor levels used in the present study did not reduce or suppress the reflection source in some subjects. The levels that were used were chosen on the basis of previous work in an entirely different group of subjects (Johnson et al., 2007). However, determining suppressor levels in individual subjects would be prohibitive and perhaps impossible during clinical applications. Given that the inclusion of a suppressor creates a more complex stimulus, perhaps it is unnecessary to include a low-level suppressor when attempting to identify ears with hearing loss. In contrast, inclusion of a high-level suppressor almost invariably reduced test accuracy, just as it did previously (Johnson et al., 2007). This finding indicates that the inclusion of a high-level suppressor would be detrimental for clinical purposes.
Little or no advantage was gained by (1) measurement of middle-ear reflectance or (2) inclusion of the 2f2-f1 distortion product in the multivariate analysis. A lack of significant contribution of middle-ear reflectance measurements at the two primary frequencies (Fig. 4) suggests no benefit to their inclusion in clinical DPOAE protocols. This finding is not inconsistent with the results from other studies showing the influence of middle-ear status on DPOAE test results (Keefe et al., 2000, 2003; Sanford et al., 2009). Those previous studies were conducted in neonates, whose middle-ear status likely differed from the status in the present group of older subjects, who were included only if there was no evidence of middle-ear dysfunction. That the addition of the 2f2-f1 DPOAE in the multivariate analysis contributed little to improving test performance (Fig. 4) contradicts some previous studies (Gorga et al., 2000) which showed a small effect of including both DPOAEs in the analyses. The results, however, are consistent with the findings of others (Fitzgerald and Prieve, 2005) who found no such advantage.
In summary, our findings provide evidence in favor of adopting optimized stimulus parameters, multivariate analyses, and FPL calibration in clinical DPOAE test protocols. These variables resulted in the largest improvements in test performance as assessed by AROC, specificities for fixed sensitivities, and sensitivities for fixed specificities. Support for low-level suppression in DPOAE protocols was equivocal. The inclusion of other variables had little or no impact on test performance and in some cases (high-suppressor condition) actually caused a decrease in test performance. The use of optimized stimulus parameters and multivariate analyses would result in no additional test time in the clinic and no more complicated stimulus paradigm than the one in current clinical use. However, FPL calibrations require additional efforts over current calibration procedures, due to the need to determine the Thévenin-equivalent acoustic-source properties. Even so, the additional time required for these calibrations may be warranted if they result in fewer calibration errors and better test performance when combined with optimized stimuli and multivariate analyses.
Acknowledgments
Work supported by the NIH T35 DC8757, R01 DC2251, R01 DC6350, and P30 DC4662. Special thanks to Sandy Estee for her help in subject recruitment, to Megan J. Thorson and Rachel A. Tomasek for their assistance in data collection, and to Yi-Wen Liu for helping with data analysis.
References
- ANSI. Specifications for audiometers, ANSI Report No S36-2004. American National Standards Institute; New York: 2004. [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear and Hearing. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SR, Rogers AR, Neely ST, Kopun JG, Tan H, Gorga MP. Influence of calibration method on DPOAE measurements: I. Test performance. Ear and Hearing. 2010 doi: 10.1097/AUD.0b013e3181d86b3d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn PA, Piskorski P, Gorga MP, Neely ST, Keefe DH. Predicting Audiometric Status from Distortion Product Otoacoustic Emissions Using Multivariate Analyses. Ear and Hearing. 1999;20:149–163. doi: 10.1097/00003446-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Dreisbach LE, Siegel JH. Distortion-product otoacoustic emissions measured at high frequencies in humans. Journal of the Acoustical Society of America. 2001;110:2456–2469. doi: 10.1121/1.1406497. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TS, Prieve BA. Detection of hearing loss using 2f2-f1 and 2f1-f2 distortion-product otoacoustic emissions. Journal of Speech, Language, and Hearing Research. 2005;48:1165–1186. doi: 10.1044/1092-4388(2005/082). [DOI] [PubMed] [Google Scholar]
- Gaskill SA, Brown AM. The behavior of the acoustic distortion product, 2f1-f2, from the human ear and its relation to auditory sensitivity. Journal of the Acoustical Society of America. 1990;88:821–839. doi: 10.1121/1.399732. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinical application of multivariate analyses of distortion-product otoacoustic emission data. Ear and Hearing. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, Beauchaine KL, Kaminski JR, Peters J, Jesteadt W. Otoacoustic emissions from normal-hearing and hearing-impaired subjects. Journal of the Acoustical Society of America. 1993;93:2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dorn PA. Distortion product otoacoustic emission test performance for a priori criteria and for multifrequency audiometric standards. Ear and Hearing. 1999;20:345–362. doi: 10.1097/00003446-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Orlich B, Hoover B, Redner J, Peters J. From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear and Hearing. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Nelson K, Davis T, Dorn P. Distortion product otoacoustic emission test performance when both 2f1-f2 and 2f2-f1 are used to predict auditory status. Journal of the Acoustical Society of America. 2000;107:2128–2135. doi: 10.1121/1.428494. [DOI] [PubMed] [Google Scholar]
- Heitmann J, Waldmann B, Schnitzler H, Plinkert PK, Zenner H. Suppression of distortion product otoacoustic emissions (DPOAE) near 2f1-f2 removes DP-gram fine structure – Evidence for a secondary generator. Journal of the Acoustical Society of America. 1998;103:1527–1531. [Google Scholar]
- Johnson TA, Neely ST, Garner CA, Gorga MP. Influence of primary-level and primary-frequency ratios on human distortion product otoacoustic emissions. Journal of the Acoustical Society of America. 2006a;119:418–428. doi: 10.1121/1.2133714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Neely ST, Kopun JG, Dierking DM, Tan H, Converse C, Kennedy E, Gorga MP. Distortion product otoacoustic emissions: Cochlear-source contributions and clinical test performance. Journal of the Acoustical Society of America. 2007;122:3539–3553. doi: 10.1121/1.2799474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Neely ST, Kopun JD, Gorga MP. Reducing reflected contributions to ear-canal distortion product otoacoustic emissions in humans. Journal of the Acoustical Society of America. 2006b;119:3896–3907. doi: 10.1121/1.2200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Neely ST, Kopun JG, Dierking DM, Tan H, Gorga MP. Clinical test performance of distortion product otoacoustic emissions using new stimulus conditions. Ear and Hearing. 2010;31:74–83. doi: 10.1097/AUD.0b013e3181b71924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. Journal of the Acoustical Society of America. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Folsom RC, Gorga MP, Vohr BR, Bulen JC, Norton SJ. Identification of neonatal hearing impairment: Ear-canal measurements of acoustic admittance and reflectance in neonates. Ear and Hearing. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Zhao F, Neely ST, Gorga MP, Vohr B. Ear canal acoustic admittance and reflectance effects in human neonates. I. Predictions of otoacoustic emission and auditory brainstem responses. Journal of the Acoustical Society of America. 2003;113:389–406. doi: 10.1121/1.1523387. [DOI] [PubMed] [Google Scholar]
- Kim DO, Paparello J, Jung MD, Smurzynski J, Sun X. Distortion product otoacoustic emission test of sensorineural hearing loss: Performance regarding sensitivity, specificity, and receiver operating characteristics. Acta Otolaryngology (Stockholm) 1996;116:3–11. doi: 10.3109/00016489609137705. [DOI] [PubMed] [Google Scholar]
- Kummer P, Janssen T, Arnold W. The level and growth behavior of the 2f1-f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss. Journal of the Acoustical Society of America. 1998;103:3431–3444. doi: 10.1121/1.423054. [DOI] [PubMed] [Google Scholar]
- Kummer P, Janssen T, Hulin P, Arnold W. Optimal L1-L2 primary tone level separation remains independent of test frequency in humans. Hearing Research. 2000;146:47–56. doi: 10.1016/s0378-5955(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, McCoy MJ, Whitehead ML, Martin KM. Clinical testing of distortion product otoacoustic emissions. Ear and Hearing. 1993;14:11–22. doi: 10.1097/00003446-199302000-00003. [DOI] [PubMed] [Google Scholar]
- Martin GK, Ohlms LA, Franklin DJ, Harris FP, Lonsbury-Martin BL. Distortion product emissions in humans: III. Influence of sensorineural hearing loss. Annals of Otology, Rhinology, and Laryngology Supplement. 1990;147:30–42. [PubMed] [Google Scholar]
- Neely ST, Gorga MP. Comparison between intensity and pressure as measures of sound level in the ear canal. Journal of the Acoustical Society of America. 1998;104:2925–2934. doi: 10.1121/1.423876. [DOI] [PubMed] [Google Scholar]
- Neely ST, Johnson TA, Gorga MP. Distortion product otoacoustic emission measured with continuously varying stimulus level. Journal of the Acoustical Society of America. 2005;117:1248–1259. doi: 10.1121/1.1853253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Liu Z. Technical Memo No 17. Boys Town Research Hospital; Omaha, NE: 1994. EMAV: Otoacoustic emission averager. [Google Scholar]
- Nelson DA, Kimberley BP. Distortion-product emissions and auditory sensitivity in human ears with normal hearing and cochlear hearing loss. Journal of Speech and Hearing Research. 1992;35:1142–1159. doi: 10.1044/jshr.3505.1142. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Keefe DH, Liu Y, Fitzpatrick D, McCreery RW, Lewis DE, Gorga MP. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: Test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear and Hearing. 2009;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle RA, Neely ST, Kopun JG, Gorga MP. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. Journal of the Acoustical Society of America. 2008;124:288–300. doi: 10.1121/1.2931953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. Journal of the Acoustical Society of America. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Siegel JH. Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes. Journal of the Acoustical Society of America. 1994;95:2589–2597. [Google Scholar]
- Siegel JH, Hirohata ET. Sound calibration and distortion product otoacoustic emissions at high frequencies. Hearing Research. 1994;80:146–152. doi: 10.1016/0378-5955(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Stover L, Gorga MP, Neely ST, Montoya D. Toward optimizing the clinical utility of distortion product otoacoustic emissions. Journal of the Acoustical Society of America. 1996;100:956–967. doi: 10.1121/1.416207. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Tubis A, Dhar S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. Journal of the Acoustical Society of America. 1999;105:275–292. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK. Evidence for two discrete sources of 2f1-f2 distortion product otoacoustic emission in rabbit: I. Differential dependence on stimulus parameters. Journal of the Acoustical Society of America. 1992;91:1587–1607. doi: 10.1121/1.402440. [DOI] [PubMed] [Google Scholar]
- Whitehead ML, McCoy MJ, Lonsbury-Martin BL, Martin GK. Dependence of distortion product otoacoustic emissions on primary levels in normal and impaired ears: I. Effects of decreasing L2 below L1. Journal of the Acoustical Society of America. 1995;97:2346–2358. doi: 10.1121/1.411959. [DOI] [PubMed] [Google Scholar]