Abstract

Mix in water, then stir. That is all that is required in this new approach to stereoselective sp3-sp2 cross-couplings between an alkyl and alkenyl halide. Prior formation of organozinc reagents is not required.
Palladium-catalyzed cross-couplings involving E- or Z-alkenyl halides lie at the very origin of several of the most commonly used ‘name’ reactions.1 These include Negishi couplings between organozinc halides and alkenyl or aryl halides, usually in THF. Recently, we have shown that by simply combining an aryl bromide with an alkyl iodide in the presence of zinc powder and a palladium catalyst, C-C bond formation smoothly occurs within nanomicelles at room temperature in water.2 In this report we disclose newly investigated cross-couplings of alkenyl halides. Our approach provides high levels of maintenance of both E- and Z-olefin geometry in the products of zinc-mediated reactions, which in some cases compare very favorably to traditional two-step Negishi cross-couplings in ethereal media.
Crucial to the success of this technology are: (a) the use of TMEDA as additive,3 presumably functioning both as a surface-cleaning/activating agent for the metal and ligand for the in situ-formed organozinc reagent; (b) the palladium(II) precursor PdCl2(Amphos)2;4 other sources of Pd(0) or Pd(II) catalysts led to inferior results; (c) the amphiphile PTS (the diester made from PEG-600, α-Tocopherol, Sebacic acid),5 which presumably supplies the hydrophobic pocket in which the in situ-generated water-sensitive organozinc halide reacts;6 and (d) Zn dust, rather than powder. Only two equivalents of the corresponding alkyl halide and 1 mol % of PdCl2(Amphos)2 are required for high levels of conversion and good isolated yields under mild, ambient temperatures.
In the case of cross-couplings of stereoisomerically pure E-alkenyl halides (Table 1, entries 1 and 2), complete retention of E-stereochemistry was observed. Mixtures of E/Z-alkenyl halides led to slight increases of the resulting E/Z-isomer ratio in the final products (entries 3, 4). This observation is expected based on relative rates of cross-couplings between E- and Z-disubstituted isomers. We could clearly observe much faster consumption of the E-isomer by monitoring reactions by GC. A second explanation relies on either isomerization of the starting material or final product during the course of reaction to the more thermodynamically stable E-isomer. Lastly, isomerization could happen after generation of a Pd(II) intermediate following a likely stereospecific7 Pd-insertion/transmetallation.
Table 1.
Representative Couplings of E-Alkenyl Halides in PTS/H2Oa
 | |||
|---|---|---|---|
| entry | coupling partners | productb | Z/Ec |
 |
|||
| 1 | R = CH2CH2CH3 | (89%) | >99/1 |
| 2 | R = OCH2Ph | (85%) | >99/1 |
 |
|||
| 3 | (83%)d | 96/4 | |
 |
 |
||
| 4 | (92%)d | 91/9 | |
Conditions: alkyl halide (2 mmol), alkenyl halide (1 mmol), PdCl2(Amphos)2 (0.01 mmol), zinc dust (3 mmol), TMEDA (2 mmol), 2% PTS/H2O (2 mL), rt, 24 h.
Isolated yields.
Determined by proton NMR and GC of the crude.
12 h.
To gain further insight, cross-couplings of Z-alkenyl halides were studied (Table 2). In all representative cases examined, ≥95% retention of Z-stereochemistry was observed. Various functionalized Z-olefins could be prepared in good to excellent yields (entries 1–14, Table 2). When both substrates are unactivated, the “homohalide” pairs: alkenyl iodide + alkyl iodide; alkenyl bromide + alkyl bromide, led to efficient cross-couplings with anticipated Z-stereochemistry (entries 1, 4). Curiously, the “mixed halide” pairs, under otherwise identical conditions, gave low levels of conversion and/or significant amounts of the product of alkenyl halide reduction. In one case (entry 2), competitive zinc insertion occurs with the alkenyl iodide, while in the other case (entry 3), consumption of the alkyl iodide is too rapid. Nonetheless, stereoselectivity is unaffected. As illustrated in entries 7–9 and 11–14, functional groups such as benzyl ether, ketone, chloride, ester, and trimethylsilyl are tolerated within the starting halides.8
Table 2.
Representative Couplings of Z-Alkenyl Halides in PTS/H2Oa
 | |||
|---|---|---|---|
| entry | coupling partners | product b | Z/Ec |
 |
|||
| 1 | X = I, Y = I | (93%)d | 99/1 |
| 2 | X = I, Y = Br | (67%)e | 96/4 |
| 3 | X = Br, Y = I | (51%)f | 95/5 |
| 4 | X = Br, Y = Br | (87%)g | 96/4 |
 |
 |
||
| 5 | X, Y = Br | (86%) | 97/3 |
| 6 | X, Y = I | (79%) | 99/1 |
 |
 |
||
| 7 | R = H | (52%)h | 98/2 |
| 8 | R = n-C5H11 | (93%) | 97/3 |
 |
|||
| 9 | R = Cl | (85%)i | 97/3 |
| 10 | R = C2H5 | (90%)i | 98/2 |
 |
|||
| 11 | X = Br | (94%)d | 99/1 |
| 12 | X = I | (95%)d | 99/1 |
 |
 |
||
| 13 | n = 2 | (74%) | 96/4 |
| 14 | n = 3 | (85%) | 99/1 |
Conditions: alkyl halide (2 mmol), alkenyl halide (1 mmol), PdCl2(Amphos)2 (0.01 mmol), zinc dust (3 mmol), TMEDA (2 mmol), 2% PTS/H2O (2 mL), rt, 48 h.
Isolated yields.
Determined by proton NMR and GC of the crude.
12 h.
~20% reduction of alkenyl iodide.
60% conversion.
81% yield with 1.5 equiv of alkyl bromide.
GC yield 94%.
24 h.
β–Bromostyrene showed anomalous behavior in PTS/H2O, losing 21–30% of its originally all Z constitution (Scheme 1). After stirring the reaction for one additional day upon completion, only 5% Z→E isomerization occurred. Moreover, a control experiment run in the absence of alkyl halide also showed insignificant isomerization of starting material under these reaction conditions. By way of comparison, in THF, Pd(PPh3)4 as catalyst leads mainly to the Z-isomer, albeit in low isolated yield, while PdCl2(Amphos)2 gave virtually all E-olefinic product (Scheme 1).9 Clearly, further experimental and theoretical studies on the role of ligands and reaction media in Negishi cross-couplings seem warranted.
Scheme 1.
Negishi-like Couplings in Water and in THF
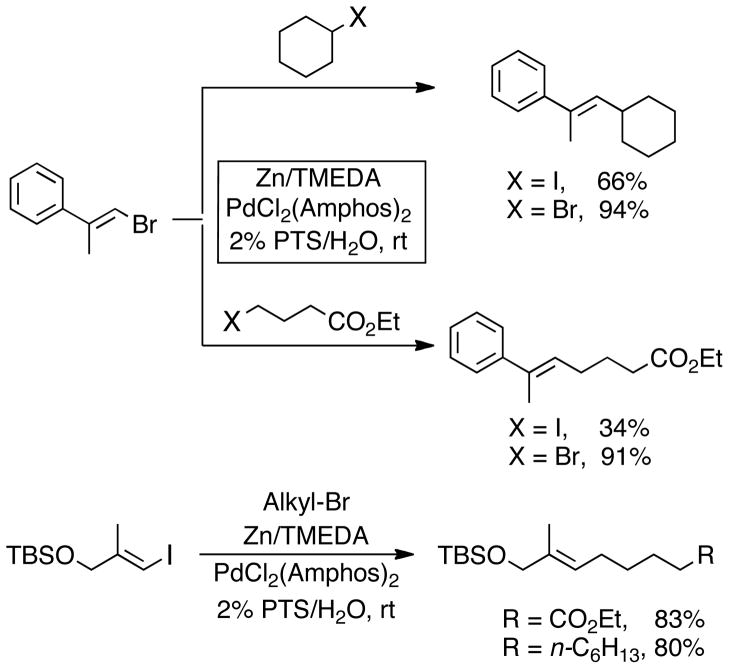
Also noteworthy are the couplings with unsymmetrically 2,2-disubstituted alkenyl halides, found to readily undergo cross-coupling reactions under our conditions with complete retention of configuration (Scheme 2). Both primary and secondary alkyl halides react smoothly, although use of alkyl bromides is preferred for more sterically hindered disubstituted alkenyl halides. In these latter cases, alkyl iodides were readily consumed; however, a considerable amount of their homocoupled Wurtz-type side-products were formed. Under our reaction conditions, dimerization products from starting alkenyl halides were not observed. These overall trends seem to be general for both alkenyl bromides and vinyl iodides. On the other hand, we were unable to obtain reasonable yields with more sterically hindered alpha-substituted cyclic or acyclic halides.
Scheme 2.
Stereoelective Coupling of 2,2-Disubstituted Alkenyl Halides
In summary, a new micellar technology is described for effecting net Negishi-like cross-couplings of stereo-defined alkenyl halides with alkyl halides. This method is not only an alternative to the generally accepted two-step procedures that rely on initial generation of stoichiometric organozinc reagents, but also allows replacement of organic solvent with water as the only medium.
Supplementary Material
Acknowledgments
Financial support provided by the NIH is warmly acknowledged. We are grateful to Johnson Matthey for generously supplying catalyst PdCl2(Amphos)2, and to Boulder Scientific for the Cp2ZrCl2 used to make the alkenyl halides.
Footnotes
Supporting Information Available Experimental procedures and product spectral data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Negishi E-I, Gagneur S. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. Vol. 1. Wiley; New York: 2002. p. 597. [Google Scholar]; (b) Negishi EI. Acc Chem Res. 1982;15:340. [Google Scholar]; (c) Negishi EI, Hu Q, Huang Z, Qian M, Wang G. Aldrichimica Acta. 2005;38:71. [Google Scholar]; (d) Negishi EI, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Acc Chem Res. 2008;41:1474. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]; (e) Knochel P, Singer RD. Chem Rev. 1993;93:2117. [Google Scholar]
- 2.Krasovskiy A, Duplais C, Lipshutz BH. J Am Chem Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Without TMEDA, no product is formed.
- 4.PdCl2(Amphos)2 (Dichlorobis(p-dimethylaminophenyl-πditbutylphosphine)palladium(II) is commercially available from Johnson Matthey.
- 5.Lipshutz BH, Ghorai S. Aldrichimica Acta. 2008;41:59. [PMC free article] [PubMed] [Google Scholar]
- 6.In the absence of PTS, low levels of conversion (<20%) were observed after 48 h.
- 7.Jutand A, Negry S. Organometallics. 2003;22:4229. [Google Scholar]
- 8.General Procedure: In a 5 mL round-bottom flask under argon containing zinc dust (197 mg, 3 mmol) and PdCl2(Amphos)2 (7 mg, 0.01 mmol) was added 2% PTS solution in water (2 mL). N,N,N′,N′-Tetramethylethylenediamine (TMEDA, 232 mg, 2 mmol) was added at rt followed by the addition of the alkyl halide (2 mmol) and the vinyl halide (1 mmol). The flask was stirred vigorously at rt for the indicated time. The product was extracted with EtOAc. Silica gel (1 g) was added to the combined organic phase and solvents were removed under vacuum. The resulting dry, crude silica was introduced on top of a silica gel chromatography column to purify the product.
- 9.Rare examples of cross-couplings between alkylzinc reagents and Z-vinyl halides: Negishi EI, Luo FT, Rand CL. Tetrahedron Lett. 1982;23:27.Koumaglo K, Chan TH. Tetrahedron Lett. 1984;25:717.Chan TH, Koumaglo K. J Organomet Chem. 1985;285:109.Nakamura E, Kuwajima I. Tetrahedron Lett. 1986;27:83.Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Tetrahedron Lett. 1986;27:955.Millar JG. Tetrahedron Lett. 1989;30:4913.Negishi EI, Ay M, Gulevich YV, Noda Y. Tetrahedron Lett. 1993;34:1437.Meyer C, Marek I, Courtemanche G, Normant JF. J Org Chem. 1995;60:863.Nakamura E, Aoki S, Sekiya K, Oshino H, Kuwajima I. J Am Chem Soc. 1987;109:8056.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.