Abstract
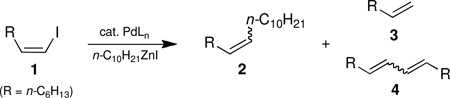
Negishi couplings at olefinic centers do not always occur with the anticipated maintenance of stereochemistry. The source of erosion has been traced to the ligand, and a modified method has been developed that solves the stereochemical issue and significantly improves yields of Negishi couplings in general.
Virtually every review article and textbook discussing Pd-catalyzed cross-coupling reactions of aryl or alkenyl halides illustrates the same catalytic cycle.1 Implied in this sequence is the expectation that while the nature of the ligands on Pd(0) may vary, there are no mechanistic consequences due to catalyst variations along these lines. The same assumption applies to the organometallic partner, usually represented in generic form as “R-M,” regardless of the metal (e.g., boron, tin, silicon, zirconium, zinc, etc.). Indeed, the hallmark of Pd-mediated C-C bond constructions is their normally strict maintenance of stereochemistry, where applicable, in going from educt to product.
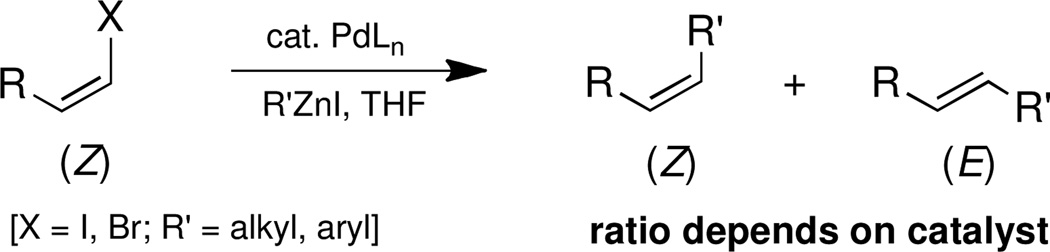
Several name reactions have their origins tied to couplings involving E- or Z-alkenyl halides.1 Included among these fundamental processes is the Negishi reaction, traditionally focused on an sp2 center undergoing coupling with an sp3-based organozinc halide (R-M = RZnX).
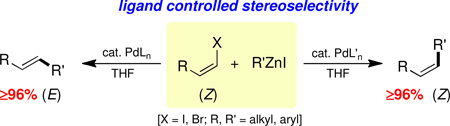
For most, where an aryl or E-vinyl halide is involved, there is either no associated stereochemistry or the starting E-olefinic geometry is pre-disposed toward remaining E- in the product. Only Z-alkenyl halides in unbiased substrates might offer insight regarding the potential role of ligands in affecting Negishi couplings.2 We now report that the stereochemical outcome in standard Negishi couplings, contrary to the commonly held view, is highly ligand dependent (Scheme 1),3 and that it can be easily compromised even at ambient temperatures.
Scheme 1.
Negishi Cross-couplings with Z-alkenyl halides.
Educt 1 (Table 1; >99% Z) was examined initially under traditional conditions using catalytic amounts of Pd(PPh3)4 or trans-PdCl2(PPh3)2 in THF (entries 1 and 2). Although each reaction led to a mixture of products, including significant amounts of protio-quench product 3 and/or homocoupling products 4,4,5 almost complete retention of geometry in the resulting Z-olefin 2 was observed. However, in efforts to improve the otherwise poor yields of desired product Z-2, screening commercially available phosphine- or carbene-based ligands of more recent vintage revealed a most unexpected finding: Z-olefin geometry can be easily lost even at room temperature (entries 5–8).
Table 1.
Effects of Catalyst and Additive on Z/E Ratios in 2a
 | |||
|---|---|---|---|
| entry | catalyst | 2 / 3 / 4b | 2, Z / Ec |
| 1 | Pd(PPh3)4 | 39/31/30 | 98/2 |
| 2 | PdCl2(PPh3)2 | 53/32/15 | 98/2 |
| 3 | PdCl2 + 3P(2-Fur)3 | 80/3/17 | 98/2 |
| 4 | PdCl2 + 3P(2-Tol)3 | 96/2/2 | 99/1 |
| 5 | PdCl2(Amphos)2d | 88/<1/12 | 68/32 |
| 6 | Pd[P(t-Bu)3]2 | 38/26/36 | 73/27 |
| 7 | PdCl2(PCy3)2 | 95/4/1 | 33/67 |
| 8 | PEPPSI | 55/33/12 | 45/55 |
| 9 | PdCl2(PCy3)2 + 3PPh3 | 73/9/18 | 98/2 |
| 10 | PdCl2(PPh3)2 + TMEDAe | 99/<1/<1 | >99/1 |
| 11 | PdCl2(PPh3)2 + Et3Nf | 79/5/16 | 98/2 |
Conditions: alkylzinc iodide (1.1 mmol, 1.0 M in THF), vinyl iodide (1 mmol), Pd catalyst (2 mol %). Reactions were run at 0.33 M at rt, 4 h (24 h for entries 10 and 11).
by GC.
Z/E-ratio determined by NMR or GC on crude material.
Defined as dichloro-bis(p-dimethylaminophenyl-di-tert-butylphosphine) palladium(II).
1.1 equiv.
2.2 equiv.
Maintenance of stereoselectivity and high levels of chemoselectivity6 are apparently favored by bulky aromatic phosphine ligands (entry 4). An attempt to combine the benefits in chemoselectivity imparted by tricyclohexylphosphine (entry 7) with the stereo-selectivity maintained in the presence of triphenylphosphine was unsuccessful at preventing significant amounts of side-products 3 and 4 (entry 9).
On the basis of the critical role played by N,N,N’,N’- tetramethylethylenediamine (TMEDA) in Zn-mediated cross-couplings between two halides performed in water at room temperature observed previously,7 the impact of TMEDA in these Negishi couplings was investigated. Remarkably, in the presence of TMEDA under otherwise identical (standard) Negishi conditions, virtually complete maintanence of olefin geometry was realized, as was the preference for adduct Z-2 (Table 1, entry 10). The amount of TMEDA (1.1 equiv) is critical for controlling selectivity. Presumably, the presence of an additive in stoichiometric amounts provides a coordinating ligand for both catalytic palladium and stoichiometric zinc. Addition of triethylamine also had a beneficial impact on product ratios, but not to the extent seen with TMEDA (compare entries 2 vs. 11).
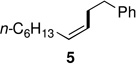
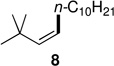
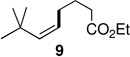
The powerful combination of PdCl2(PPh3)2 as catalyst and TMEDA as complexing agent could be applied to a variety of (functionalized) reaction partners (Table 2). Notably, sterically hindered (Z)-1-iodo-3,3-dimethylbut-1-ene afforded stereoisomerically pure cross-coupled products 8 and 9 in good yields, while PdCl2(PPh3)2 as catalyst alone led to low conversions even after 48 hours. It should also be noted that in the coupling of phenethylzinc iodide with (Z)-1-iodooct-1-ene (giving product 5), and coupling of n-decylzinc iodide with (Z)-1-iodo-3,3-dimethylbut-1-ene (giving product 8), in the absence of TMEDA, byproducts were observed in which the double bond had partly migrated.8 By contrast, these undesired products were not seen using cat. PdCl2(PPh3)2/TMEDA.
Table 2.
Effect of Ligand and TMEDA on Negishi Cross-couplings of Z-Alkenyl Halidesa
| entry | Ratios: Z/E | entry | Ratios: Z/E |
|---|---|---|---|
 |
 |
||
| 1 | A: 99/1 (49%)b | 3 | A: 99/1 (91%)b |
| 2 | B: 99/1 (92%)c | 4 | B: 99/1 (96%)c |
 |
 |
||
| 5 | A: 96/4 (73%)b | 7 | A: <20% conv |
| 6 | B: 98/2 (94%)c | 8 | B: 99/1 (63%)c |
 |
 |
||
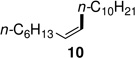
| 9 | A: <20% conv | 11 | A: 98/2 (64%)b |
| 10 | B: 99/1 (84%)c | 12 | B: 99/1 (96%)c |
 |
13 | B: 99/1 (91%)c,d | |
| 14 | C: 99/1 (98%)c,d | ||
| 15 | A: 90/10 (37%)b,d | 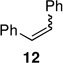 |
|
| 16 | B: 98/2 (94%)b,d | ||
| 17 | C: 99/1 (96%)c,d | 20 | A: 97/3 (78%)b,d |
| 18 | D: 2/98 (85%)c,d | 21 | B: 98/2 (93%)c,d |
| 19 | E: 96/4 (90%)b,d | 22 | D: 1/99 (85%)c,d |
Conditions: organozinc iodide (1.1 mmol, 1.0 M in THF), alkenyl iodide (1 mmol), Pd catalyst (A: PdCl2(PPh3)2 (2 mol %), rt, 4–24 h; B: PdCl2(PPh3)2 (2 mol %) + TMEDA (1.1 equiv), rt, 24–36 h; C: PdCl2(PPh3)2 (1 mol %) + TMEDA (1.1 equiv), 60 °C, 2–3 h; D: PdCl2(Amphos)2 (2 mol %), rt, 24–36 h; E: PdCl2(Amphos)2 (2 mol %) + TMEDA (1.1 equiv), rt, 24 h). Reactions were run at 0.33 M; Z/E-ratio determined by NMR and GC on crude material.
GC yield.
Isolated yield.
From alkenyl bromide.
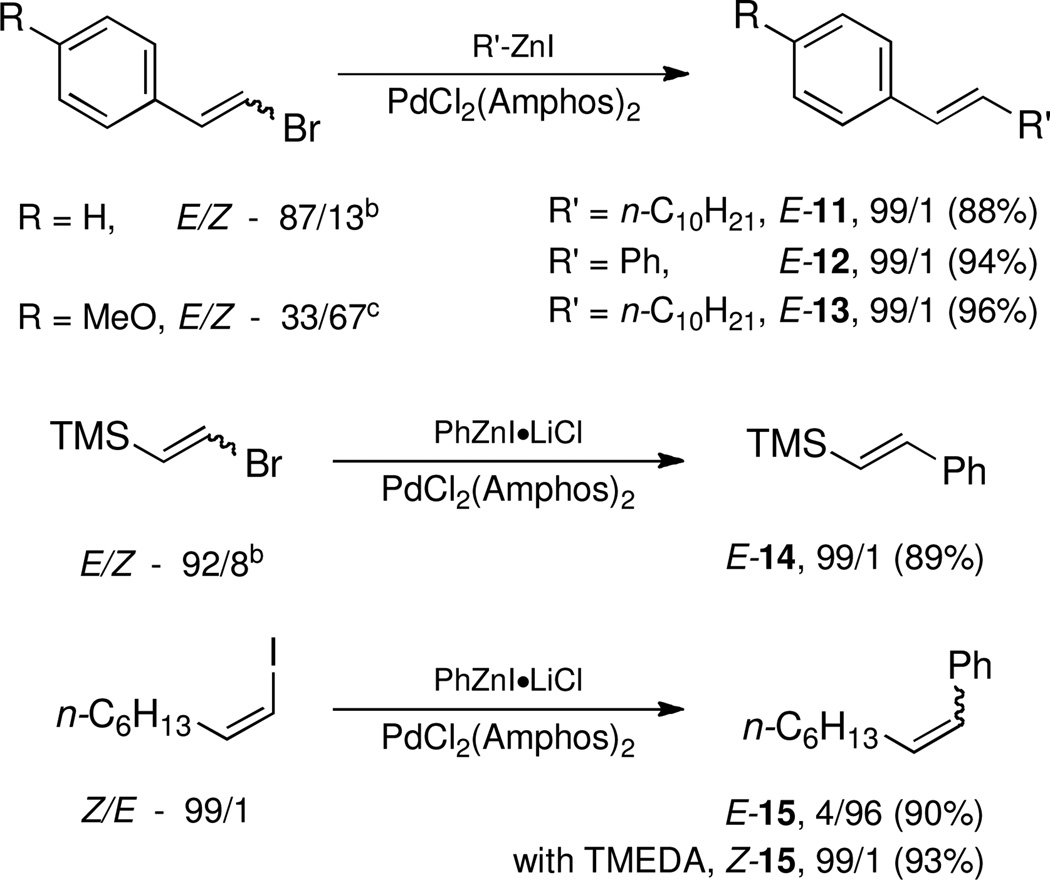
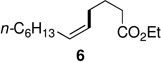
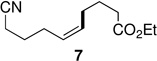
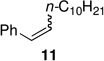
Alkenyl bromides could also be successfully coupled with alkylzinc reagents. Coupling of n-decylzinc iodide with Z-1-bromooct-1-ene led to the desired isomerically pure product 10 in high yield (entry 13). In general, use of TMEDA tends to slow rates of cross-couplings run at room temperature. When performed at 60 °C, however, they are complete in 2–3 hours, require less catalyst (only 1% of PdCl2(PPh3)2, and lead to stereoisomerically clean products 10 and 11 in close to quantitative yield (entries 14 and 17). Cross-couplings of both (Z)-1-iodo- and (Z)-1-bromooct-1-enes with secondary cyclohexylzinc iodide, however, resulted in less then 50% conversion in each case under a variety of conditions. Interestingly, although PdCl2(Amphos)2 catalyzed the alkylation and arylation of Z-β-bromostyrene in high yields, complete isomerization to the E-product took place (entries 18, 22). The presence of TMEDA, however, negates this pathway (compare entries 18 vs. 19). Best results are again obtained using cat. PdCl2(PPh3)2/TMEDA to afford products 11 and 12 (entries 17 and 21).
A controlled Z-to-E isomerization can be used to synthetic advantage, in particular when starting with a mixture of E/Z-isomers of β-bromostyrenes (Scheme 2). Using PdCl2(Amphos)2 as catalyst only the E-products are obtained. Likewise, a mixture of E/Z-isomers of (2-bromovinyl)trimethylsilane led to E-14 in good yield Scheme 2). Retention or inversion of configuration could also be achieved for the coupling of PhZnI·LiCl9 with an unbiased alkenyl iodide, depending upon the catalyst selected, leading to E- or Z-15. This new method obviates not only losses of the unwanted Z-isomer due to preferential reactivity of E-alkenyl halides,10 but also averts the need for separation of starting material and/or unwanted side products.
Scheme 2.
Negishi Cross-couplings of Z- and Mixed Z/E-Alkenyl Halides.a
a Conditions: organozinc iodide (1.1 mmol, 1.0 M in THF), alkenyl halide (1 mmol), PdCl2(Amphos)2 (2 mol %). Isolated yields. Reactions were run at 0.33 M at rt for 24 h. Z/E-ratio determined by NMR and GC on crude material. b As available commercially. c As obtained from 4-MeO-cinnamic acid via Grob fragmentation.
TMEDA, which is present in excess, likely serves several roles, such as (1) a ligand for Pd that may shift equilibria away from bridging dimeric species and toward a more highly (5- and/or 6-) coordinated intermediate. This would minimize ligand exchange that otherwise could lead to species from which homocoupling products arise;4 (2) inhibition of elimination of palladium hydride from more highly coordinated intermediates may also add to the enhanced chemoselectivity observed; (3) as a coordinating agent for RZnX11 and/or in situ-derived ZnX2,12 thereby negating its Lewis acidity (which could influence the mechanism of isomerization; vide infra); (4) facilitate conversion of any in situ-generated PdH (that could be potentially responsible for Z to E isomerization) to Pd(0).13
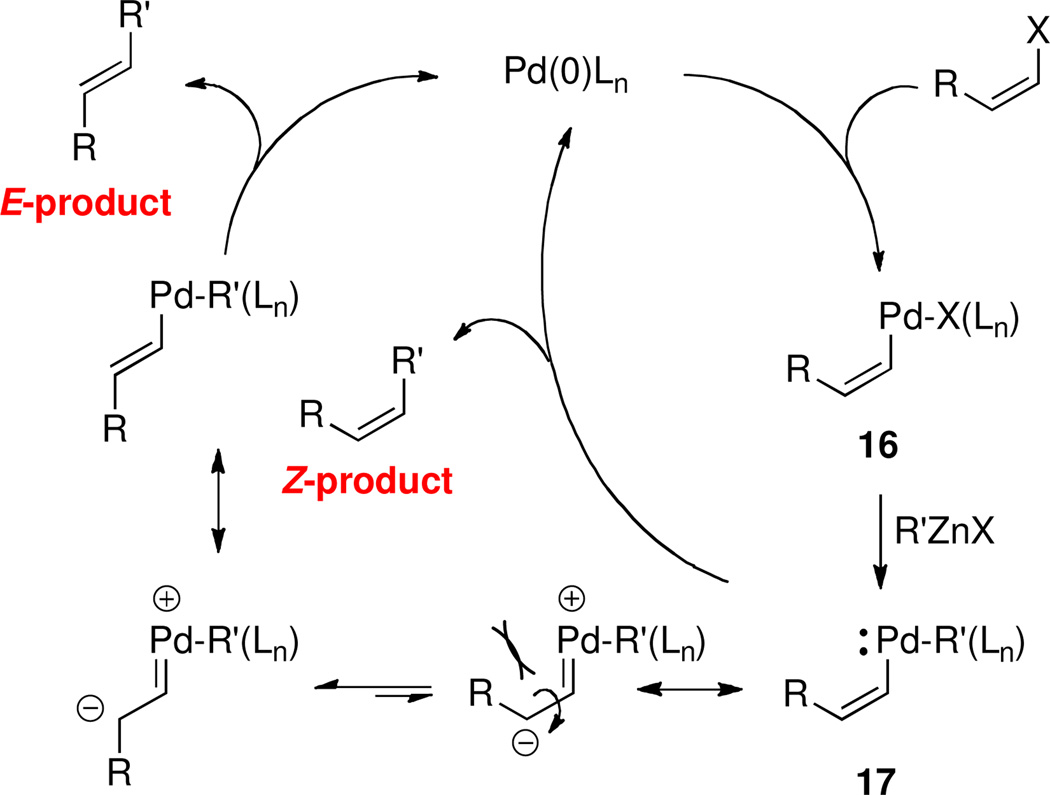
One explanation for the ligand-related loss of stereointegrity observed during Negishi cross-couplings of Z-vinylic halides calls for isomerization of a Pd(II) intermediate 17 following a likely initial stereospecific14 Pd-insertion to 16 (Scheme 3). A pathway involving either an anionic15 (as shown) or cationic1c,16 zwitterion-metal carbene may follow. Alternatively, isomerization can occur through an η2-vinylphosphonium complex,17 or possibly via reversible PdH elimination.18
Scheme 3.
Potential Pathways for Loss of Z-Olefinic Geometry.
These findings potentially offer immediate benefits to all types of Negishi couplings with respect to avoidance of homocoupling and protio-quenching, if not stereoselectivity; e.g., in cross-couplings of aryl halides. For the case of an electron-rich aryl bromide such as 1-bromo-4-methoxybenzene, coupling under standard Negishi conditions with n-decylzinc iodide resulted in very low conversion (Table 3, entry 1).
Table 3.
Effects of Catalyst and Additive on Cross-coupling of Aryl Bromides with n-C10H21ZnI.a
 | |||
|---|---|---|---|
| entry | Ar-Br | catalyst | 19 / 20 / 21b |
| 1 | PdCl2(PPh3)2 | < 5% conv | |
| 2 | 18a | PdCl2(dppf) | 7/91/2 |
| 3 | PdCl2(PPh3)2 + TMEDAc | <1/99/0 (95%)d | |
| 4 | PdCl2(PPh3)2 | 4/26/70 | |
| 5 | 18b | PdCl2(dppf) | 5/91/4 |
| 6 | PdCl2(PPh3)2 + TMEDAc | <1/98/1 (93%)d | |
Conditions: alkylzinc iodide (1.1 mmol, 1.0 M in THF), aryl bromide (1 mmol), Pd catalyst (2 mol %). Reactions were run at 0.33 M at 40 °C, 12 h for entries 1–3, and at rt, 20 h for entries 4–6.
by GC on crude material.
1.1 equiv.
Isolated yield.
While switching to catalyst PdCl2(dppf)19 the starting halide was fully consumed, and good selectivity was observed favoring product 20 (entry 2). In reactions of electron-deficient ethyl 4-bromobenzoate, which proceed smoothly even at room temperature, similar levels of chemoselectivity were observed with both catalysts (entries 4, 5). Thus, while use of this bidentate ferrocene-based ligand on Pd dramatically improves the reaction outcome, chemoselectivity issues remain unresolved.4 In the presence of TMEDA, however, use of the least expensive of palladium catalysts, PdCl2(PPh3)2,20 leads to full conversion together with virtually complete control of ratios favoring the desired cross-coupling products for both representative electron-rich and electron-poor aryl bromides (entries 3, 6).
In summary, the stereochemical outcome of Negishi couplings on Z-alkenyl halides has been found to vary, contrary to prevailing thinking, as a function of the ligand(s) on the Pd catalyst.21 These ligand effects on both stereoselectivity and reaction pathway can be fully negated using a method that relies on catalytic PdCl2(PPh3)2 in the presence of an equivalent of TMEDA.22 This new combination applies to Negishi couplings in general, where enhanced product yields are to be expected. These results suggest that additional new insights regarding mechanistic subtleties associated with Pd-catalyzed C-C bond-forming reactions, perhaps involving other organometallic coupling partners as well, lie ahead. Ongoing studies aimed at further elucidating the interplay between ligands and additives, and their effects on both Stille and Suzuki-Miyaura couplings will be reported in due course.
Supplementary Material
Acknowledgment
Financial support provided by the NIH is warmly acknowledged. We are grateful to Johnson Matthey for generously supplying PdCl2(Amphos)2.
Footnotes
Supporting Information Available. Experimental procedures and product spectral data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hegedus LS, Söderberg BCG. Transition Metals in the Synthesis of Complex Organic Molecules. 3rd ed. Sausalito, CA: University science Books; 2009. [Google Scholar]; (b) Tsuji J. Palladium in Organic Synthesis. Berlin, Germany: Springer; 2005. [Google Scholar]; (c) Negishi EI, Gagneur S. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E-I, editor. Vol. 1. New York: WILEY-VCH; 2002. p. 597. [Google Scholar]; (d) Negishi E-I. Acc. Chem. Res. 1982;15:340. [Google Scholar]; (e) Negishi E-I, Hu Q, Huang Z, Qian M, Wang G. Aldrichimica Acta. 2005;38:71. [Google Scholar]; (f) Negishi E-I, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Acc. Chem. Res. 2008;41:1474. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]; (g) Krasovskiy A, Duplais C, Lipshutz BH. J. Am. Chem. Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Negishi E-I, Luo F-T, Rand CL. Tetrahedron Lett. 1982;23:27. [Google Scholar]; (b) Koumaglo K, Chan TH. Tetrahedron Lett. 1984;25:717. [Google Scholar]; (c) Chan TH, Koumaglo K. J. Organomet. Chem. 1985;285:109. [Google Scholar]; (d) Nakamura E, Kuwajima I. Tetrahedron Lett. 1986;27:83. [Google Scholar]; (e) Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Tetrahedron Lett. 1986;27:955. [Google Scholar]; (f) Millar JG. Tetrahedron Lett. 1989;30:4913. [Google Scholar]; (g) Negishi E-I, Ay M, Gulevich YV, Noda Y. Tetrahedron Lett. 1993;34:1437. [Google Scholar]; (h) Meyer C, Marek I, Courtemanche G, Normant J-F. J. Org. Chem. 1995;60:863. [Google Scholar]
- 3.Loss of olefin geometry in Negishi couplings has been noted previously; however, these tend to be special cases: Zhao J, Yu Y, Ma S. Chem. Eur. J. 2010;16:74. doi: 10.1002/chem.200901287. Fauvarque JF, Jutand A. J. Organomet. Chem. 1981;209:109..
- 4.(a) Jin L, Zhang H, Sowa JR, Lei A. J. Am. Chem. Soc. 2009;131:9892. doi: 10.1021/ja903833u. [DOI] [PubMed] [Google Scholar]; (b) Liu Q, Lan Y, Liu J, Li G, Wu Y-D, Lei A. J. Am. Chem. Soc. 2009;131:10201. doi: 10.1021/ja903277d. [DOI] [PubMed] [Google Scholar]
- 5.Negishi E-I. Acc. Chem. Res. 1982;15:340. [Google Scholar]
- 6.Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Tetrahedron Lett. 1986;27:955. [Google Scholar]
- 7.(a) Krasovskiy A, Lipshutz BH. Org. Lett. 2010;12:4742. doi: 10.1021/ol101885t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Krasovskiy A, Duplais C, Lipshutz BH. J. Am. Chem. Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See Supporting Information.
- 9.Krasovskiy A, Malakhov V, Gavryushin A, Knochel P. Angew. Chem. Int. Ed. 2006;45:6040. doi: 10.1002/anie.200601450. [DOI] [PubMed] [Google Scholar]
- 10.(a) Andreini BP, Carpita A, Rossi R. Tetrahedron Lett. 1986;27:5533. [Google Scholar]; (b) Jutand A, Negri S. Organometallics. 2003;22:4229. [Google Scholar]
- 11.For recent work on the structure/basicity of organozinc halide complexes with TMEDA, see: Ross AJ, Dreiocker F, Schaefer M, Oomens J, Meijer AJHM, Pickup BT, Jackson RFW. J. Org. Chem. 2011;76:1727. doi: 10.1021/jo102334c..
- 12.(a) Sen Gupta PK, Houk LW, van der Helm D, Hossain MB. Acta Cryst. 1982;B38:1818. [Google Scholar]; (b) Citeau H, Conrad O, Gulando DM. Acta Cryst. 2001;E57:m5. [Google Scholar]; (c) Htoon C, Ladd MFC. J. Cryst. Mol. Struct. 1974;4:357. [Google Scholar]; (d) Yasuda H, Ohnuma Y, Nakamura A, Kai Y, Yasuoka N, Kasai N. Bull. Chem. Soc. Jpn. 1980;53:1101. [Google Scholar]; (e) Andrews PC, Raston CL, Skelton BW, White AH. Organometallics. 1998;17:779. [Google Scholar]
- 13.Hills D, Fu GC. J. Am. Chem. Soc. 2004;126:13178. doi: 10.1021/ja0471424. [DOI] [PubMed] [Google Scholar]
- 14.Recovered Z-vinyl iodide from a partially consumed reaction mixture retained its stereointegrity.
- 15.Amatore C, Bensalem S, Ghalem S, Jutand A. J. Organomet. Chem. 2004;689:4642. [Google Scholar]
- 16.(a) Blackmore T, Bruce MI, Stone FGA. J. Chem. Soc. Dalton Trans. 1974:106. [Google Scholar]; (b) Hart D, Schwartz J. J. Organomet. Chem. 1975;87:C11. [Google Scholar]; (c) Huggins JM, Bergman RG. J. Am. Chem. Soc. 1981;103:3002. [Google Scholar]; (d) Zargarian D, Alper H. Organometallics. 1993;12:712. [Google Scholar]
- 17.(a) Wakioka M, Ozawa F. Organometallics. 2010;29:5570. [Google Scholar]; (b) Wakioka M, Nakajima Y, Ozawa F. Organometallics. 2009;28:2527. [Google Scholar]
- 18.(a) Gauthier D, Lindhardt AT, Olsen EPK, Overgaard J, Skrydstrup T. J. Am. Chem. Soc. 2010;132:7998. doi: 10.1021/ja9108424. [DOI] [PubMed] [Google Scholar]; (b) Limmert ME, Roy AH, Hartwig JF. J. Org. Chem. 2005;70:9364. doi: 10.1021/jo051394l. [DOI] [PubMed] [Google Scholar]; (c) Ebran J-P, Hansen AL, Gøgsig TM, Skrydstrup T. J. Am. Chem. Soc. 2007;129:6931. doi: 10.1021/ja070321b. [DOI] [PubMed] [Google Scholar]; (d) Lindhardt AT, Gøgsig TM, Skrydstrup T. J. Org. Chem. 2009;74:135. doi: 10.1021/jo801824e. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Konishi M, Kobori Y, Kumada M, Higuchi T, Hirotsu K. J. Am. Chem. Soc. 1984;106:158. [Google Scholar]
- 20.For aryl-aryl couplings, only 1% catalyst is required under these conditions.
- 21.The outcome of the reaction depends on the source of Zn used for the preparation of the organozinc halide. Best results were reproducibly achieved using Zn dust, rather than Zn powder, independent of supplier.
- 22.Although, Z to E isomerization happens during the coupling event, normally it is incomplete and thus, additional stirring is needed to ensure full isomerization. Checking the reaction of β-bromostyrene with n-decylzinc iodide every 5 min during the first 30 min by GC aliquots showed a constant ~70/30 Z/E ratio of product dodec-1-en-1-ylbenzene (Z-11) independent of the extent of conversion. After an additional 24 h of stirring at rt, isomerization was complete leading to the final product in 85% yield in a 2/98 Z/E ratio (Table 2, entry 18). No isomerization was observed when purified product Z-11 was stirred for 1 day in THF at rt in the presence of PdCl2(Amphos)2(2 mol %) and ZnCl2(1 equiv).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.