Abstract
Unrelated donor (URD) BMT is an effective treatment for leukemia in children, but success is limited by graft versus host disease (GVHD) and relapse. In this study we describe the incidence and risk factors for GVHD over time in children receiving URD BMT. We analyzed outcomes of 638 myeloablative URD BMT performed between 1990 and 2003 for treatment of AML, ALL, CML or MDS, using the Center for International Blood & Marrow Transplant Research (CIBMTR) database. Recipients were <18 years of age, and had available high resolution HLA typing for HLA A, B, C and DRB1. Overall, 27% of recipients developed aGVHD grades III-IV and risk was significantly higher in recipients of T-replete compared with T-depleted grafts (OR 3.12, 95%CI 2.02-4.83; p< 0.0001). Acute GVHD significantly reduced risk of relapse in children with ALL (OR 0.34 95% CI 0.13-0.86, p=0.0052) but not AML (OR 0.58, 95% CI 0.22-2.98; p=0.26). Risk of aGVHD was higher in children transplanted in earlier years (1990-1998, n=365) compared to 1999-2003 (OR 1.93 95%CI 1.27-2.91; p=0.002). We conclude that outcomes have changed significantly over time, with reduced risk of aGVHD in more recent transplants.
Introduction
Bone marrow transplantation (BMT) has been used for over 30 years as a curative therapy to treat malignant and non-malignant diseases in children (1-3). Early success of the procedure in pediatric populations allowed extension of the treatment to adult populations and now the majority of transplants are performed in adults. The distributions of diagnoses, co-morbid conditions and the impact of prior therapies differ significantly between pediatric and adult populations. The majority of studies show superior outcomes in younger patient populations (4-8). However patient numbers are usually too small to analyze in detail the impact of younger age on specific outcomes. The occurrence of graft versus host disease (GVHD) significantly limits the success of unrelated donor (URD) BMT and is a frequent cause of morbidity and mortality. In this study we have used a large CIBMTR database of myeloablative URD BMT to analyze in detail risk factors and clinical impact of acute and chronic GVHD in a pediatric population. The data indicate some significant temporal changes in outcomes of URD BMT for children with leukemia. In particular, risk of aGVHD was higher in children transplanted in earlier years (1990-1998) compared to 1999-2003; in contrast, risk of relapse was lower in children transplanted 1990-1998 compared with 1999-2003.
Patients and Methods
The CIBMTR
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic stem cell transplants to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee or the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. The CIBMTR collects data at two levels: Registration and Research. Registration data include disease type, age, sex, pretransplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. Requests for data on progression or death for registered patients are at six-month intervals. All CIBMTR teams contribute Registration data. Research data are collected on subset of registered patients selected using a weighted randomization scheme and include detailed disease, and pre- and post-transplant clinical information.
Patients and Transplant Procedure
The analysis was restricted to recipients less than 18 years of age who had available high resolution typing reported by the transplant center or performed retrospectively by the National Marrow Donor Program (NMDP) (9,10). All patients received unrelated donor bone marrow as the primary stem cell source. Patients receiving peripheral blood stem cell or cord blood grafts were excluded as the natural history of GVHD may differ in these cases.
Study Population
The NMDP retrospectively obtained consent for data submission and study participation from surviving patients or their parent/legal guardian for transplants it facilitated in the U.S. prior to 2002. Thereafter, informed consent was obtained prospectively. The Institutional Review Board of the NMDP waived consent for patients who had died prior to soliciting consent (transplantations facilitated prior to 2002) and non-consenting surviving patients were excluded. To overcome the bias associated by the inclusion of a proportion of surviving patients (those consenting) but all deceased recipients, and hence their over-representation, a sample of deceased patients was selected using a weighted randomized scheme that adjusts for over-representation of deceased patients in the consented cohort (11). This weighted randomized scheme was developed based on all survivors in the NMDP database. A logistic regression model was fit to identify the factors that predicted whether a patient had consented or not consented to use of data collected by the NMDP. This analysis found the following factors were associated with the likelihood of a patient consenting: age, disease type, race, sex, cytomegalovirus serostatus and country of transplantation (US vs. non US). Using estimated consenting probabilities from this model based on the characteristics of dead patients, the biased coin method of randomization was performed to determine which of the dead patients likely would have consented to participate had they been alive. Thus this procedure includes the dead patients at the same probability as surviving patients who consented to participate.
The NMDP facilitated 2013 pediatric transplants in patients with acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML) or myelodysplasia disorders (MDS), as their first transplant between 1990 and 2003 using bone marrow as the stem cell source. Of these, 1043 had high resolution HLA typing available. Approximately 12% of dead patients (n=123) were deleted by the weighted randomized method. Patients with more than one antigen mismatch (n=208) or non-myeloablative preparative regimens (n=28) were excluded. An additional 46 patients were subsequently excluded because of presence of > 1 allele mismatch (n= 30) or missing acute GVHD or chronic GVHD information (n=16). The final study population consisted of 638 children.
Patient characteristics are summarized in Table 1. The most frequent diagnosis was acute lymphoblastic leukemia (ALL) and median patient age was 10 years (range <1-17.9 years). Median donor age was 36 years (range 19-60 years).
Table 1.
Characteristics of pediatric patients (age < 18 years) who underwent an unrelated bone marrow donor transplant receiving a myeloblative conditioning regimen, reported to CIBMTR between 1990 and 2003.
| Characteristics | N (%) |
|---|---|
| Number of patients | 638 |
| Age at transplant, median (range), years | 10 (<1-17.9) |
| Age at transplant, years | |
| <5 | 151 (24) |
| 6-10 | 183 (29) |
| 11-15 | 167 (26) |
| >15 | 137 (21) |
| Male sex | 365 (57) |
| Performance score | |
| <90 | 111 (17) |
| 90-100 | 513 (80) |
| Unknown | 14 ( 2) |
| Disease | |
| AML | 168 (26) |
| ALL | 329 (52) |
| CML | 75 (12) |
| MDS | 66 (10) |
| Disease status at transplanta | |
| Early | 175 (27) |
| Intermediate | 310 (49) |
| Advanced | 128 (20) |
| Otherb | 25 (4) |
| Donor age, median (range), years | 36 (19-60) |
| Donor age, years | |
| 18-30 | 169 (26) |
| 31-40 | 240 (38) |
| 41-60 | 229 (36) |
| Donor-recipient sex match | |
| Male -> Male | 230 (36) |
| Male -> Female | 132 (21) |
| Female -> Male | 135 (21) |
| Female -> Female | 141 (22) |
| Donor-recipient CMV match | |
| D(−)/R(−) | 277 (43) |
| D(−)/R(+) | 136 (21) |
| D(+)/R(−) | 125 (20) |
| D(+)/R(+) | 88 (14) |
| Unknown | 12 ( 2) |
| Donor pregnancy | |
| Male donor | 362 (57) |
| Female, no pregnancy | 117 (18) |
| 1 or more pregnancies | 153 (24) |
| Unknown | 6 ( 1) |
| Conditioning regimen | |
| Cy+TBI+/−other | 543 (85) |
| TBI+other | 29 ( 5) |
| Bu+Cy+/−other | 57 ( 9) |
| Bu+/other | 9 ( 1) |
| GVHD prophylaxis | |
| T-cell depletion | 234 (37) |
| CSA+MTX+/−other | 338 (53) |
| CSA+/−other | 18 ( 3) |
| FK506+/−other | 46 ( 7) |
| MTX+/−other | 1 (<1) |
| Missing | 1 (<1) |
| HLA match | |
| Matched | 296 (46) |
| Single allele mismatch | 84 (13) |
| Single antigen mismatch | 258 (40) |
| Median follow-up of survivors, months | 87 (3-195) |
Risk groups are defined as: Early - includes AML or ALL in CR1; CML in CP1; or MDS with RA or RARS; Intermediate - includes AML or ALL in ≥ CR2; CML accelerated phase or ≥CP2 and advanced - includes AML or ALL in relapse or PIF; CML in blast phase or MDS with RAEB-T or IPSS-4.
Other includes (N=25): 1) ALL, disease status prior to transplant is missing (n=1) 2) MDS, NOS (n=3) 3) CMML Chronic myelomonocytic leukemia (n=3) 4) PNH Paroxysmal nocturnal hemoglobinuria (n=1)
Patients received a variety of preparative regimens determined by the individual transplant centers. The majority of recipients (90%) received total body irradiation (550cGy-1500cGy); 10% received chemotherapy alone. Chemotherapy drugs administered as part of the preparative regimen included cyclophosphamide, busulfan, cytosine arabinoside, etoposide and thiotepa. Methods for GVHD prophylaxis varied according to the transplant center. The graft was depleted of T-cells ex vivo (TCD) in 37% of cases, who may or may not have received treatment with a calcineurin inhibitor, based on the degree of T-cell depletion; the majority of children (67%) received a non-T depleted graft together with GVHD prophylaxis using the calcineurin inhibitors cyclosporine A or tacrolimus. Risk groups were defined as: Early - includes AML or ALL in CR1; CML in CP1; or MDS with RA or RARS; Intermediate - includes AML or ALL in ≥ CR2; CML accelerated phase or ≥CP2 and advanced - includes AML or ALL in relapse or PIF; CML in blast phase or MDS with RAEB-T or IPSS-4.
Donor Selection and Processing of Bone Marrow
The characteristics of the donors are shown in Table 1. High resolution typing at HLA-A, B, C and DRB1 was performed for all donor recipient pairs (12). Forty six percent of recipients were matched with their recipient at all 8 loci; 13% had a single allele mismatch and forty percent had a single antigen mismatch.
Statistical Analysis
Univariate analysis of survival was calculated using the method of Kaplan and Meier and was compared using the log rank statistic (13,14). Rates of acute and chronic GVHD, and relapse were calculated using cumulative incidence, with death acting as a competing risk (15). Cumulative incidences were compared for acute and chronic GVHD and relapse by using a Taylor series linear approximation to estimate the variance (16).
The proportional hazard model was used for the multivariate analysis of survival. (17). Cumulative incidence estimates of acute and chronic GVHD, relapse and treatment-related mortality (TRM) were estimated using the Pseudo-value approach of Klein (18). Additional models were constructed for relapse and TRM treating acute GVHD as a time dependent variable. Variables considered in the analysis included recipient age, gender, performance score, diagnosis and stage of disease at transplantation. Transplant-related variables included donor age, donor-recipient gender match, donor-recipient CMV serology, donor parity and radiation vs non-radiation preparative therapy, GVHD prophylaxis (T-cell depletion vs no T-cell depletion), HLA matching (8 allele match vs 7/8 match, which includes single allele mismatch but antigen match, and single antigen mismatch) and year of transplant (1990-1998 vs 1999-2003).
Results
Acute GVHD
The cumulative incidence (CI) of grades II-IV GVHD was 45% (95% CI 42-49%) and of grades III-IV GVHD was 27%, indicating that most cases of acute GVHD were severe. Multivariate analysis showed similar risk factors for grades II-IV and III-IV acute GVHD . As expected, T-replete grafts were associated with a significantly increased risk of acute GVHD compared with T-depleted grafts (OR 3.12; 95% CI 2.02-4.83; p< 0.0001). In addition, transplant in the earlier era (1990-1998) was associated with significantly increased risk of acute GVHD compared to transplant in recent years (1999-2003) (OR 1.93; 95% CI 1.27-2.91; p=0.002). Notably, HLA mismatch at a single locus did not increase risk of GVHD (OR 1.00; 95% CI 0.68-1.46; p=0.98), and age and diagnosis did not significantly modify risk.
Chronic GVHD
The cumulative incidence of chronic GVHD was 35% (95% CI 31-38%) 3 years after BMT. Multivariate analysis of risk factors for chronic GVHD revealed only a modest non-significant increase in risk with parous female donors (OR 1.45; 95% CI 0.92-2.29; p=0.11), and no effect of HLA-mismatch (OR 1.19; 95% CI 0.78-1.79; p=0.41) or T-cell depletion on risk of chronic GVHD.
Relapse
The cumulative incidence of relapse was 26% (95% CI 22-29%) at 3 years. Multivariate analysis of risk factors for relapse was conducted for the entire group and separately for children with AML and with ALL, as we hypothesized that risk factors might vary in the two categories. Risk of relapse was significantly lower in children with MDS compared with those with acute leukemia, and risk was very markedly increased in children with the most advanced disease (OR 15.37, 95% CI 6.1-38.7; p <0.0001). Risk of relapse declined markedly with increasing recipient age (OR 0.23 for recipients 16-18 years compared with reference group of children less than 5 years old (95% CI 0.10-0.54, p=0.0004)). Risk of relapse was reduced in recipients of marrow from parous female donors (OR 0.43; 95% CI 0.23-0.83; p=0.01), perhaps reflecting the modest increase in chronic GVHD in these children, and was not increased in recipients of T-cell depleted marrow. A perhaps surprising observation was a decreased risk of relapse in children transplanted in the earlier era compared with more recent transplants (OR 0.47, 95% CI 0.28-0.78; p=0.004).
A multivariate analysis of risk factors for relapse restricted to children with AML (n=156) showed a non significant reduction in incidence of relapse (OR 0.58 comparing relapse incidence in children with GVHD grades III-IV with those without, 95% CI 0.22-1.58; p=0.26) (Table 2). However, relapse risk was significantly higher in the recent era (1999-2003), in children younger than 5 years and in those with advanced disease, as seen in the group overall. In contrast, the occurrence of acute GVHD significantly reduced risk of relapse in children with ALL (OR 0.34 comparing relapse incidence in children with GVHD grades II-IV with those without, 95% CI 0.13-0.86; p=0.0052) (Table 3). Similar to AML, children with ALL older than 5 years and with less advanced disease had a reduced risk of relapse. While there was an increased risk of relapse in the recent era, this was of marginal significance in the children with ALL.
Table 2.
Multivariate analysis for relapse in children who were diagnosed with AML (N=156) and received unrelated donor BMT.
| Variables | N | Odds Ratio (95% Confidence Interval ) |
P-value |
|---|---|---|---|
| HLA matching | |||
| (1) Matched | 69 | 1.00 a | |
| (2) 7/8 matched | 87 | 0.63 (0.26, 1.55) | 0.36 |
|
Other significant covariates: |
|||
| Grade III-IV aGVHD | |||
| (1) No | 89 | 1.00 a | |
| (2) Yes | 67 | 0.58 (0.22, 1.58) | 0.26 |
| Disease stage | < 0.0001 | ||
| (1) Early | 33 | 1.00 a | |
| (2) Intermediate | 69 | 0.90 (0.23, 3.54) | 0.88 |
| (3) Advanced | 54 | 12.59 (3.09, 51.32) | 0.0004 |
| Recipient age categories | 0.0059 | ||
| (1) < 5 years | 46 | 1.00 a | |
| (2) 6-10 years | 32 | 0.40 (0.13, 1.28) | 0.12 |
| (3) 11-15 years | 47 | 0.12 (0.04, 0.39) | 0.0005 |
| (4) 16-17.9 years | 31 | 0.12 (0.02, 0.65) | 0.014 |
| Transplant year group | |||
| 1999 - 2003 | 64 | 1.00 a | |
| 1990 - 1998 | 92 | 0.14 (0.13, 0.73) | 0.0075 |
Reference group.
Acute GVHD III-IV is treated as a time-dependent variable.
Table 3.
Multivariate analysis for relapse in children who were diagnosed with ALL (N=311) and received unrelated donor BMT.
| Variables | N | Odds Ratio (95% Confidence Interval ) |
P-value |
|---|---|---|---|
| HLA matching | |||
| Matched | 147 | 1.00 | |
| 7/8 matched | 164 | 0.72 (0.37, 1.43) | 0.45 |
| Grade III-IV aGVHD | |||
| No | 168 | 1.00 | |
| Yes | 143 | 0.34 (0.13, 0.86) | 0.0052 |
| Disease stage | 0.0012 | ||
| Early | 62 | 1.00 | |
| Intermediate | 214 | 1.96 (0.74, 5.17) | 0.17 |
| Advanced | 35 | 12.77 (3.83, 42.55) | < 0.0001 |
| Recipient age categories | 0.05 | ||
| < 5 years | 67 | 1.00 | |
| 6-10 years | 114 | 0.38 (0.17, 0.89) | 0.025 |
| 11-15 years | 75 | 0.22 (0.07, 0.66) | 0.0069 |
| 16-17.9 years | 55 | 0.31 (0.11, 0.87) | 0.025 |
| Transplant year group | |||
| 1999 - 2003 | 113 | 1.00 a | |
| 1990 - 1998 | 198 | 0.51 (0.26, 1.01) | 0.054 |
Survival and Treatment-Related Mortality
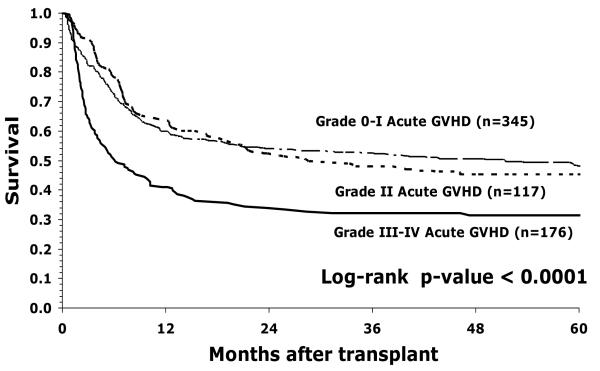
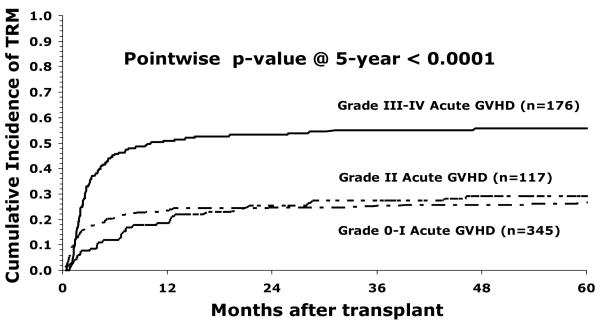
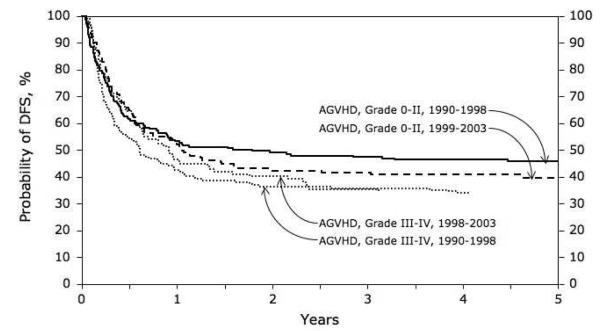
Fifty six percent (95% CI 52-59%) of children were surviving at 1 year and 43% (95% CI 39-47%) at 5 years. The occurrence of acute GVHD had an important effect on survival (Figure1). Survival was notably reduced in children with grades III-IV GVHD, but not in children with grade II GVHD. In a multivariate model of risk factors for mortality, the occurrence of acute GVHD was associated with an almost doubled risk of death (RR 1.96, 95% CI 1.46-2.29, p <0.0001). However, survival was not improved by T-cell depletion, despite significant reduction in GVHD. Mortality was increased in older children, as a consequence of increased treatment related mortality (OR 1.72 in children 16-18 years compared with < 5 years old, 95% CI 1.11-2.67; p = 0.016). Children receiving marrow mismatched at a single locus had significantly reduced survival, despite the absence of a significant effect on GVHD (OR 1.42, 95% CI 1.05-1.92; p=0.02). Treatment related mortality was significantly increased in children with grades III-IV acute GVHD, but not in children with grade II GVHD (Figure 2). Treatment related mortality was higher in the earlier era (1990-1998), OR 1.61 (95% CI 1.03-2.51; p=0.027) compared with more recent transplants (1999-2003). The overall effect of era of transplant and GVHD is shown in Figure 3. Survival is reduced in children with GVHD transplanted in early or later years. The increased risk of relapse in children transplanted in recent years leads to slightly lower DFS in those children, whether or not they have GVHD but is not statistically significantly different (p=0.81).
Figure 1.
Probability of overall survival by acute GVHD grade in children with leukemia receiving unrelated donor bone marrow transplants
Figure 2.
Treatment-related mortality according to grade of acute GVHD in children with leukemia receiving unrelated donor bone marrow transplant
Figure 3.
DFS in recent and earlier years, adjusted for the occurrence of GVHD (p=0.81).
Discussion
Children constitute an important subset of patients undergoing BMT. Despite differences in diagnoses, organ function and immune reconstitution capability, children are often considered together with adults in studies of GVHD, and reports of the effect of age on incidence of GVHD have been inconsistent (4,5,7,8,19-25). Some reports have described a reduced risk of GVHD in younger patients, while in other reports this has not been seen. The small number of children receiving BMT relative to adults has limited studies focused specifically on children. We examined a large database to examine the incidence, risk factors and outcome of GVHD in children to determine whether children with GVHD have similar risk factors and outcomes as adults, and to examine temporal trends in outcomes of unrelated donor transplants in children.
Our analysis showed that T-cell depletion for GVHD prophylaxis significantly reduced risk of GVHD, as might be expected. In addition, year of transplant was a significant risk factor, with lower risk of GVHD in more recent transplants. Perhaps unexpectedly, risk of GVHD was not increased by an allele or antigen mismatch at a single HLA locus, in contrast to findings in adult studies (20,21,23,24). This may indicate greater tolerance of modest degrees of HLA disparity in pediatric transplant recipients compared with adults, or greater success of GVHD prophylaxis in controlling alloreactivity. It should be noted, however, that survival was reduced in recipients of marrow with an allele or antigen mismatched bone marrow, perhaps indicating inferior immune reconstitution in this circumstance, despite lack of a measurable excess of GVHD.
Our analysis of risk factors for chronic GVHD failed to identify any important risk factors, aside form a marginal increase in risk with multiparous female donors, as described by others (26-33). Of note, HLA mismatch did not increase risk of chronic GVHD in agreement with our findings in acute GVHD, and risk was not decreased by T-cell depletion of the graft. It should be noted that the overall incidence of chronic GVHD was significant, with approximately one third of children affected, including even the youngest children, and efforts should be made to provide effective treatments for all chronic GVHD for all ages.
Our study showed a marked reduction in relapse in children with ALL who developed grades III/IV acute GVHD. These data are perhaps surprising as clinical experience of quite limited responses to donor lymphocyte infusions in ALL suggest that any graft-versus-leukemia effect in ALL might be modest (reviewed in 34). However, data from previous non-pediatric registry studies of sibling and URD BMT support the observation of a reduced relapse rate in cases with ALL who develop GVHD (35,36). Horowitz et al analyzed 2,254 sibling donor transplants performed in children and adults with early stage leukemia (ALL or AML in CR1 and CML in first chronic phase) reported to the International Bone Marrow Transplant Registry (IBMTR) (35). This study included 439 ALL cases and showed an almost 3-fold reduction in risk of relapse associated with acute GVHD, but no effect of chronic GVHD. Similarly, a low relapse rate, attributed to graft-versus-leukemia, has been described in a report of 127 adults receiving URD BMT for high risk ALL, facilitated by the NMDP (36). It is unclear, and perhaps equally surprising that a reduction of relapse was not seen in children with AML. It is possible that competing mortality from severe GVHD has obscured any benefit from GVHD, or that small sample size has rendered a biologically meaningful effect statistically insignificant. Future studies will address this question again as additional transplant data are accrued.
The data in our study show a significant effect of grades III/IV acute GVHD on mortality, with an almost doubled risk of death in children with acute GVHD, demonstrating that current treatments for acute GVHD are inadequate. In contrast, grade II GVHD had only a minor effect on survival. Performance of randomized controlled trials to optimize treatment of acute GVHD is an essential step in improving survival for children receiving URD BMT.
Our analysis has identified some striking temporal changes in outcome of URD BMT for children with leukemia. The risk of acute GVHD is reduced in more recent transplants (1999-2003) compared with those performed prior to 1999, even when T-cell depletion and HLA-match are included in the multivariate model. These data may indicate greater success in optimizing use of calcineurin inhibitors, and or methotrexate as GVHD prophylaxis in recent years. In contrast to this improvement in outcome, risk of relapse is increased in children transplanted in the more recent era and the increased risk is most notable in children with AML. This may reflect the use of increasingly intense chemotherapy in the primary treatment of children with AML. Of note, 35% of children with AML were transplanted with advanced disease compared with 11% of children with ALL. In addition, criteria for selection of children with leukemia for transplantation change frequently, based on improvements in identification of genetic characteristics that alter risk of relapse, for example and improvements in the effectiveness of chemotherapy. Notably, the era of transplant was not a significant risk factor for overall survival, perhaps indicating that recent reductions in GVHD are counterbalanced by the increase in relapse, illustrating the dynamic nature of leukemia patient populations as chemotherapy outcomes continue to evolve.
This study is a registry analysis and so is limited by the heterogeneity of treatment regimes and clinical practices at different institutions. The strength of the study is the size of the population, allowing analyses of subsets that could not be examined in single institution studies.
In summary, this study is the largest single analysis of GVHD in URD BMT in children to date. We have identified important temporal changes in clinical outcomes that will merit continued observation as chemotherapy and transplant strategies both continue to improve. In addition we have shown a significant effect of GVHD in reducing relapse in children with ALL, but not AML. However, overall, acute GVHD has a significant negative effect on survival, demonstrating the need for further improvements in both GVHD prophylaxis and the treatment of acute GVHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED. Bone marrow transplantation: a review. Semin Hematol. 1999;36:95–103. [PubMed] [Google Scholar]
- 2.Perry AR, Linch DC. The history of bone-marrow transplantation. Blood Rev. 1996;10:215–219. doi: 10.1016/s0268-960x(96)90004-1. [DOI] [PubMed] [Google Scholar]
- 3.Storek J, Joseph A, Espino G, et al. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood. 2001;98:3505–3512. doi: 10.1182/blood.v98.13.3505. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 5.Woolfrey AE, Anasetti C, Storer B, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99:2002–2008. doi: 10.1182/blood.v99.6.2002. [DOI] [PubMed] [Google Scholar]
- 6.Green A, Clarke E, Hunt L, et al. Children with acute lymphoblastic leukemia who receive T-cell-depleted HLA mismatched marrow allografts from unrelated donors have an increased incidence of primary graft failure but a similar overall transplant outcome. Blood. 1999;94:2236–2246. [PubMed] [Google Scholar]
- 7.Davies SM, Shu XO, Blazar BR, et al. Unrelated donor bone marrow transplantation: influence of HLA A and B incompatibility on outcome. Blood. 1996;86:1636–1642. [PubMed] [Google Scholar]
- 8.Anderson JE, Anasetti C, Appelbaum FR, et al. Unrelated donor marrow transplantation for myelodysplasia (MDS) and MDS-related acute myeloid leukaemia. Br J Haematol. 1996;93:59–67. doi: 10.1046/j.1365-2141.1996.4811022.x. [DOI] [PubMed] [Google Scholar]
- 9.Dodson KL, Coppo PA, Confer DL. The National Marrow Donor Program: improving access to hematopoietic stem cell transplantation. Clin Transpl. 1999;121:127. [PubMed] [Google Scholar]
- 10.Confer DL. The National Marrow Donor Program: meeting the needs of the medically underserved. Cancer. 2001;91:274–278. doi: 10.1002/1097-0142(20010101)91:1+<274::aid-cncr18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Farag S, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–84. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Mantel M. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 15.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 17.Cox DR. Regression models and line tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Klein JP. Modeling Competing Risks in Cancer Studies. Statistics in Medicine. 2006;25:1015–1034. doi: 10.1002/sim.2246. [DOI] [PubMed] [Google Scholar]
- 19.Dini G, Cancedda R, Locatelli F, et al. Unrelated donor marrow transplantation: an update of the experience of the Italian Bone Marrow Transplant Group (GITMO) Haematologica. 2001;86:451–456. [PubMed] [Google Scholar]
- 20.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 21.Beatty PG, Anasetti C, Hansen JA, et al. Marrow transplantation from unrelated donors for treatment of hematologic malignancies: effect of mismatching for one HLA locus. Blood. 1993;81:249–253. [PubMed] [Google Scholar]
- 22.Ash RC, Casper JT, Chitambar CR, et al. Successful allogeneic transplantation of T-cell-depleted bone marrow from closely HLA-matched unrelated donors. N Engl J Med. 1990;322:485–494. doi: 10.1056/NEJM199002223220801. [DOI] [PubMed] [Google Scholar]
- 23.Nagler A, Brautbar C, Slavin S, Bishara A. Bone marrow transplantation using unrelated and family related donors: the impact of HLA-C disparity. Bone Marrow Transplant. 1996;18:891–897. [PubMed] [Google Scholar]
- 24.El Kassar N, Legouvello S, Joseph CM, et al. High resolution HLA class I and II typing and CTLp frequency in unrelated donor transplantation: a single-institution retrospective study of 69 BMTs. Bone Marrow Transplant. 2001;27:35–43. doi: 10.1038/sj.bmt.1702733. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer A, Allebes WA, Paaardekooper J, Ruiter J, Joosten I. HLA-C mismatches induce strong cytotoxic T-cell reactivity in the presence of an additional DRB/DQB mismatch and affect NK cell-mediated alloreactivity. Transplantation. 2001;72:923–929. doi: 10.1097/00007890-200109150-00030. [DOI] [PubMed] [Google Scholar]
- 26.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 27.Arai S, Vogelsang GB. Management of graft-versus-host disease. Blood Rev. 2000;14:190–204. doi: 10.1054/blre.2000.0137. [DOI] [PubMed] [Google Scholar]
- 28.Przepiorka D, Anderlini P, Saliba R, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–1700. doi: 10.1182/blood.v98.6.1695. [DOI] [PubMed] [Google Scholar]
- 29.Carlens S, Ringden O, Remberger M, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998;22:755–61. doi: 10.1038/sj.bmt.1701423. [DOI] [PubMed] [Google Scholar]
- 30.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15:663–668. [PubMed] [Google Scholar]
- 31.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 32.Wagner JL, Seidel K, Boeckh M, Storb R. De novo chronic graft-versus-host disease in marrow graft recipients given methotrexate and cyclosporine: risk factors and survival. Biol Blood Marrow Transplant. 2000;6:633–639. doi: 10.1016/s1083-8791(00)70029-2. [DOI] [PubMed] [Google Scholar]
- 33.Kondo M, Kojima S, Horibe K, Kato K, Matsuyama T. Risk factors for chronic graft-versus-host disease after allogeneic stem cell transplantation in children. Bone Marrow Transplant. 2001;27:727–730. doi: 10.1038/sj.bmt.1702868. [DOI] [PubMed] [Google Scholar]
- 34.Peggs KS, Mackinnon S. Cellular therapy: donor lymphocyte infusion. Curr Opin Hematol. 2001;8:349–354. doi: 10.1097/00062752-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 36.Cornelissen JJ, Carston M, Kollman C, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97:1572–1577. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]