Abstract
The risk of death for hemodialysis patients is thought to be highest on the days following the longest interval without dialysis (usually Mondays and Tuesdays); however, existing results are inconclusive. To clarify this we analyzed Dialysis Outcomes and Practice Patterns Study (DOPPS) data of 22,163 hemodialysis patients from the United States, Europe and Japan. Our study focused on the association between dialysis schedule and day-of-week of all-cause, cardiovascular and non-cardiovascular mortality with day-of-week coding as a time-dependent covariate. The models were adjusted for dialysis schedule, age, country, DOPPS Phase I or II, and other demographic and clinical covariates comparing mortality on each day to the 7-day average. Patients on a Monday-Wednesday-Friday (MFW) schedule had elevated all-cause mortality on Monday, and those on a Tuesday-Thursday-Saturday (TTS) schedule increased risk of mortality on Tuesday in all 3 regions. The association between day-of-week mortality and schedule was generally stronger for cardiovascular than non-cardiovascular mortality, and most pronounced in the United States. Unexpectedly, Japanese patients on a MWF schedule had a higher risk of non-cardiovascular mortality on Fridays, and European patients on a TTS schedule experienced an elevated cardiovascular mortality on Saturdays. Thus, future studies are needed to evaluate the influence of practice patterns on schedule-specific mortality and factors that could modulate this effect.
Keywords: hemodialysis, schedule, mortality, day of week, DOPPS
Introduction
Hemodialysis (HD) patients usually experience relatively high mortality rates; for example, approximately 23% per annum in the U.S., 15% in Europe and 9% in Japan. As the most common treatment of advanced kidney failure, HD typically requires patients to follow a strict treatment schedule, which typically entails receiving dialysis on a thrice weekly schedule; either Monday-Wednesday-Friday (MWF) or Tuesday-Thursday-Saturday (TTS). During the intervals between dialysis sessions, electrolytes, fluid and various uremic toxins accumulate and, as a result, contribute to an increased risk of mortality. Therefore, the intermittent dialysis schedule, MWF or TTS, may put patients at higher risk of death on certain days. In particular, patients on a MWF schedule may have higher risk of death on Monday, whereas those on a TTS schedule may experience an elevated risk on Tuesday; since these days follow the longest intervals without the benefit of dialysis.
Various studies have assessed the association between day-of-week-specific mortality risk and dialysis schedule. For example, Bleyer, Russell, and Satko (1) found a significantly higher risk of sudden death and cardiac-related death on Monday for MWF schedule patients, and on Tuesday for TTS schedule patients. Karnik et al. (2) identified modifiable risk factors for cardiac arrest in dialysis units, including age, diabetes, using a catheter for vascular access, and being hospitalized within the past 30 days. In addition, results from Karnik et al. (2) revealed that there is a higher risk of cardiac arrest on Monday for MWF schedule patients. Bleyer et al. (3) applied a strict definition of sudden death and investigated the association between the timing of HD and occurrences of sudden death among HD patients. The authors found an increased risk of sudden death in the 12-hour period after starting dialysis, and also in the 12-hour period at the end of the weekend interval (i.e., before starting HD, namely Monday and Tuesday).
For several reasons, previously published studies should be interpreted with caution. Most prior studies were based on relatively small sample sizes; most were confined to U.S. patients, limiting their generalizability. Moreover, statistical analyses were lacking in several respects. For example, results were sometimes based on crude death rates without covariate adjustment. Additionally, for the most part, these analyses did not examine interactions between weekday and patient characteristics. For example, it is possible that mortality elevations on given days may be accentuated for various patient subgroups; e.g., older patients, diabetics, and patients with various comorbid conditions.
Our study uses data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), an international prospective observational study of hemodialysis treatment and patient outcomes. The DOPPS-I (1996-2001) comprised over 17,000 patients in seven countries (France, Germany, Italy, Japan, Spain, United Kingdom, and the U.S.) from more than 300 dialysis facilities. The DOPPS-II (2002-2004) population included over 12,000 patients in 12 countries (DOPPS-I countries, plus Australia/New Zealand, Belgium, Canada, and Sweden) from more than 300 dialysis facilities. The sampled facilities have at least 20-25 HD patients and represent all geographic regions and all types of facilities in each country. The DOPPS selects a random sample of HD patients within each participating facility. In this study, we carry out a comprehensive investigation of the association between day-of-week-specific death rates and dialysis schedule, in the U.S., Japan, and several European countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom). In addition to having a very large sample size and long follow-up period, the DOPPS database contains information on many demographic variables and comorbid conditions. Since the endpoint is time-to-death, we used survival analysis (Cox regression) with day-of-the-week serving as a time-dependent covariate. Cox regression is well-suited for this purpose since it is designed to handle a data structure where time until death is potentially censored and accurately tracks which patients are at risk on a particular weekday. Thus, Cox regression appropriately accounts for deaths and right censoring as they occur.
Results
Baseline characteristics of the prevalent cross-section of patients are presented in Table 1 by region. Compared with U.S. patients, European and Japanese patients were, on average, more likely to be male, to have lower BMI, less likely to have diabetes and less likely to have cardiovascular disease (CVD). European patients tended to be older than U.S. patients, while Japanese patients were on average younger than U.S. patients. Each of these differences was statistically significant (p<0.05). Subsequent models were constructed from both prevalent (cross-sectional) and incident patients.
Table 1. Demographics by Region.
| US (N=4,666) | Europe (N=5,623) | Japan (N=3,531) | |
|---|---|---|---|
| Black (%) | 35.8 | 1.5 | 0 |
| Male (%) | 54.2 | 57.8 | 61.8 |
| Age (years) | 61.1 (15.2) | 62.1 (15.1) | 59.7 (12.6) |
| BMI (kg/m2) | 25.1 (6.1) | 23.9 (4.7) | 20.3 (3.1) |
| Diabetes | 48.5 | 23.3 | 26.9 |
| CVD | 74.9 | 65.8 | 46.3 |
- Values in the parentheses are the standard deviations.
- This table is based on prevalent cross sections of HD patients participating either in DOPPS I or II.
- CVD corresponds to any of comorbid conditions cerebrovascular disease, congest heart failure, coronary heart disease or other cardiovascular disease.
In total, 4,395 deaths were reported during the study period. Of the deaths, 2,663 were among U.S patients, 1,391 among European patients, and 341 among Japanese patients. In addition, 1,744 out of 4,395 (40%) death were caused by CVD. A total of 2,489 out of 4,395 (57%) deaths were among patients on a MWF schedule, while 1,906 (43%) were on a TTS schedule.
The distribution of deaths by day-of-week for each dialysis schedule group is shown in Figure 1. For MWF schedule patients, Monday had a much higher percentage of deaths for U.S., European and Japanese patients (19.7%, 19.6% and 17.9%, respectively). For TTS schedule patients, Tuesday had the highest percentage of deaths for U.S., European and Japanese patients (19.2%, 16.9% and 19.1%, respectively). The distributions varied a little by region. For MWF schedule patients, Friday appears to have the highest risk of death in Japan, with approximately 1/5 of deaths occurring on that day. For TTS schedule patients, Monday appears to have a higher risk of death in the U.S. (15.7%), while Saturday appears to have a higher risk of death in Europe and Japan (18% for both).
Figure 1.
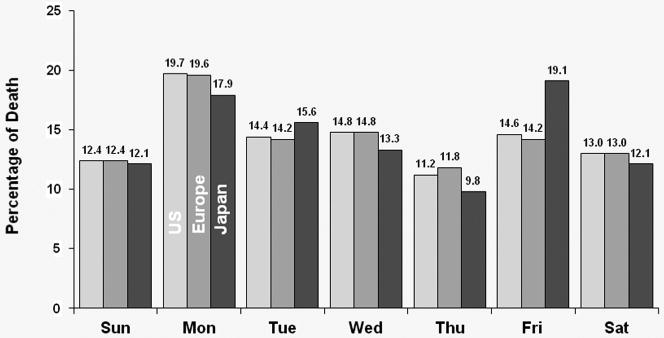
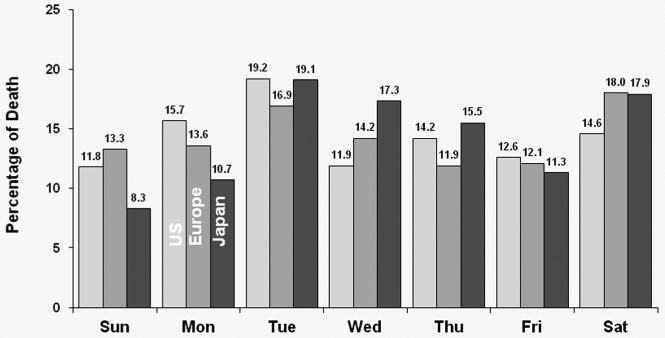
Figure 1a: Distribution of deaths by day of the week for patients receiving dialysis on MWF (all patients).
For MWF schedule patients, Monday had a much higher percentage of deaths for the U.S., European and Japanese patients.
Figure 1b: Distribution of deaths by day of the week for patients receiving dialysis on TTS (all patients).
For TTS schedule patients, Tuesday had the highest percentage of deaths for the U.S., European and Japanese patients
For each region, Cox regression models were used to estimate the covariate-adjusted day-of-week effect on hazard of death, with day-of-week coded as a time-dependent covariate. The model was set up so that mortality hazard on each day was compared to the seven-day average. Figure 2 contains three sets of covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with MWF schedule for U.S. patients [Figure 2(a)], for European patients [Figure 2(b)], and for Japanese patients [Figure 2(c)]. Patients on a MWF dialysis schedule from the U.S. experienced a significant (p<0.0001) 41% higher risk of all-cause death on Mondays relative to the seven-day average. European patients experienced a significant 34% higher risk of all-cause death on Mondays versus the seven-day average (p=0.001), while patients from Japan experienced a 27% higher risk of all-cause death on Monday (p=0.154) as well as a 44% higher mortality risk on Friday (p= 0.04).
Figure 2.
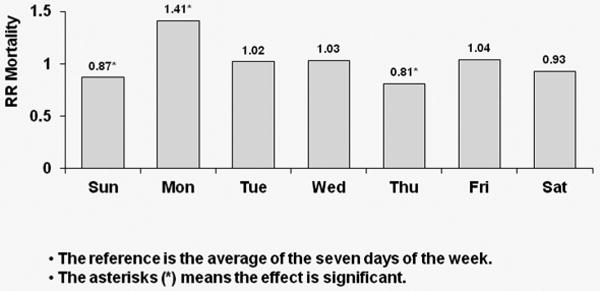
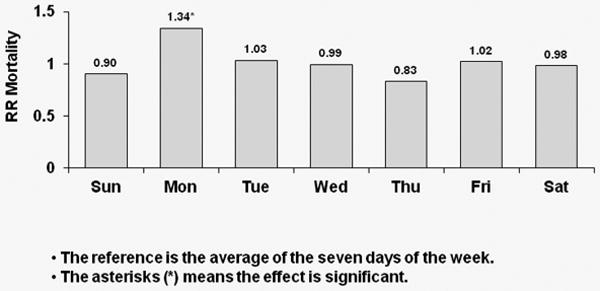
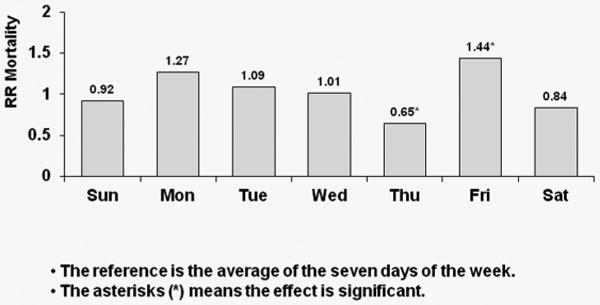
Figure 2a: Relative Risk of Mortality (all-cause) by Day: U.S. patients receiving dialysis on MWF
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with MWF schedule for U.S. patients
Figure 2b: Relative Risk of Mortality (all-cause) by Day: European patients receiving dialysis on MWF
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with MWF schedule for European patients
Figure 2c: Relative Risk of Mortality (all-cause) by Day: Japanese patients receiving dialysis on MWF
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with MWF schedule for Japanese patients
Corresponding results for TTS schedule patients are shown in Figure 3; again, with each week day compared to the overall average. A significant 39% higher risk of all-cause mortality was observed on Tuesdays for U.S. patients (p<0.0001). European patients experienced a significant 22% higher risk of all-cause death on Tuesdays (p=0.043) and a significant 31% higher risk of all-cause death on Saturdays (p=0.013). Japanese patients experienced a significant 43% higher risk of all-cause death on Tuesday (p=0.044) as well as a 43% higher risk on Saturdays (p=0.07).
Figure 3.
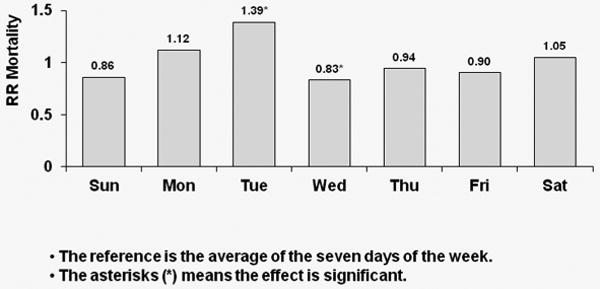
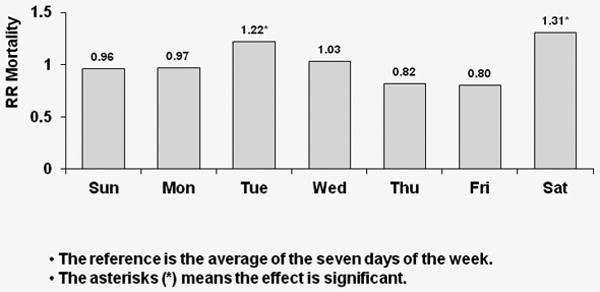
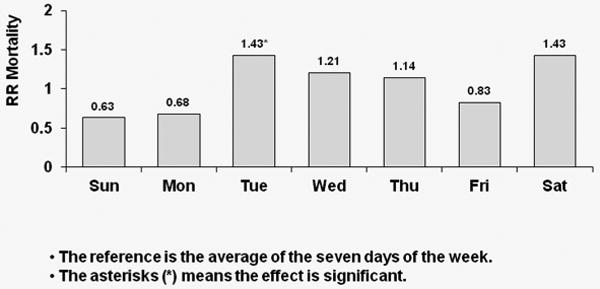
Figure 3a: Relative Risk of Mortality (all-cause) by Day: U.S. patients receiving dialysis on TTS
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with TTS schedule for U.S. patients
Figure 3b: Relative Risk of Mortality (all-cause) by Day: European patients receiving dialysis on TTS
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with TTS schedule for European patients
Figure 3c: Relative Risk of Mortality (all-cause) by Day: Japanese patients receiving dialysis on TTS
Covariate-adjusted hazard ratios (HRs) of all-cause mortality by day-of-week with TTS schedule for Japanese patients
Figure 4 shows covariate-adjusted HRs of CVD and non-CVD mortality by day-of-week for MWF schedule patients. In the U.S., CVD mortality risk was higher by 45% on Mondays relative to the seven-day average (p<0.0001); the corresponding value for non-CVD mortality was 38% (p<0.0001). In Europe, patients experienced a significant 55% higher risk of CVD mortality on Mondays (p=0.006), while also experiencing a significant 27% higher risk of non-CVD mortality on Mondays (p=0.023). In Japan, CVD mortality risk was higher by 62% on Mondays (p=0.098); the corresponding value for non-CVD mortality was 10% (p=0.674). Japanese patients, however, experienced a significant 100% higher non-CVD mortality risk on Fridays versus the seven-day average (p=0.001).
Figure 4.
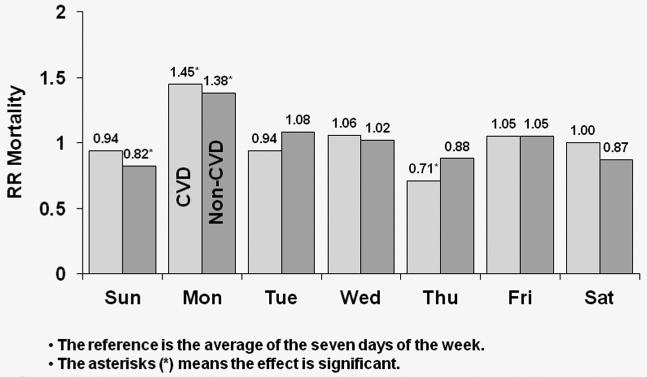
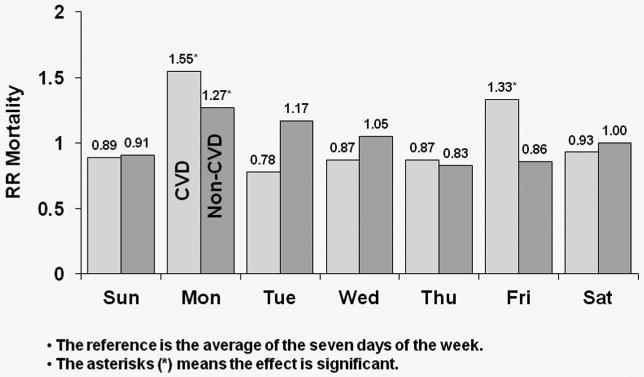
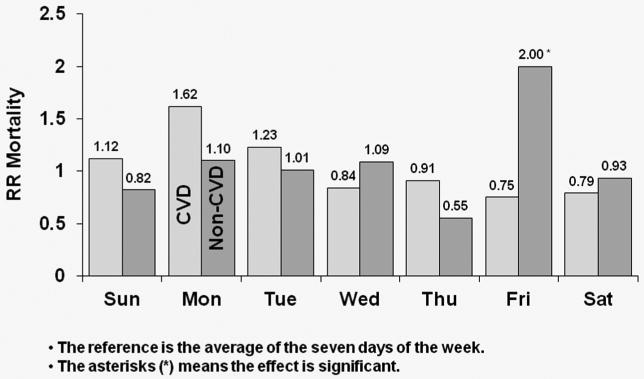
Figure 4a: Relative Risk of Mortality (CVD and non-CVD) by Day: U.S. patients receiving dialysis on MWF
Covariate-adjusted HRs of cardiovascular disease (CVD) and non-CVD mortality by day-of-week for MWF schedule for U.S. patients
Figure 4b: Relative Risk of Mortality (CVD and non-CVD) by Day: European patients receiving dialysis on MWF
Covariate-adjusted HRs of cardiovascular disease (CVD) and non-CVD mortality by day-of-week for MWF schedule for European patients
Figure 4c: Relative Risk of Mortality (CVD and non-CVD) by Day: Japanese patients receiving dialysis on MWF
Covariate-adjusted HRs of cardiovascular disease (CVD) and non-CVD mortality by day-of-week for MWF schedule for Japanese patients
In Figure 5, HRs of CVD and non-CVD mortality by day-of-week for TTS schedule patients is displayed. In the U.S., CVD mortality risk was higher by 56% on Tuesdays relative to the seven-day average (p<0.0001); patients also experienced a significant 26% higher non-CVD mortality risk on Tuesdays (p=0.016). In Europe, patients experienced 43% higher risk of CVD mortality on Tuesdays (p=0.058), with the corresponding value for non-CVD mortality being 15% (p=0.236). However, European patients had an 88% higher risk of CVD death on Saturdays (p=0.001). In Japan, patients experienced a significant 75% higher risk of CVD mortality on Tuesdays (p=0.037) and also experienced a 93% higher CVD mortality risk on Saturdays (p=0.034). However, in Japan there was no evidence of higher non-CVD mortality on Tuesdays compared to the seven-day average (HR=1.26; p=0.300).
Figure 5.

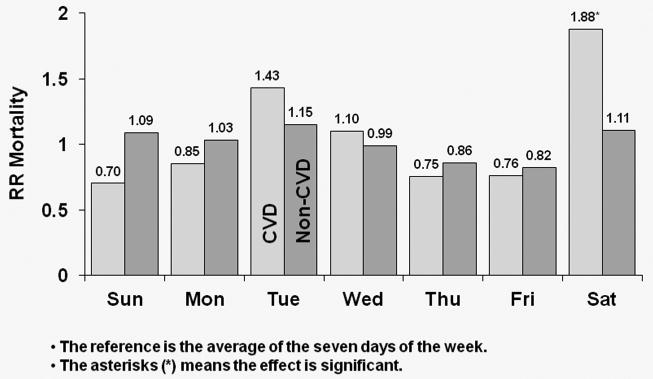
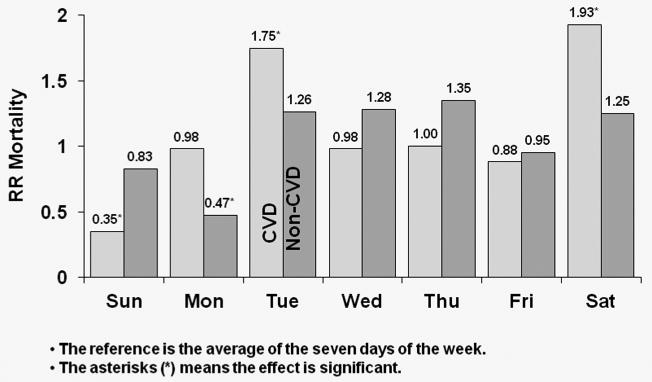
Figure 5a: Relative Risk of Mortality (CVD and non-CVD) by Day: U.S. patients receiving dialysis on TTS
HRs of CVD and non-CVD mortality by day-of-week for TTS schedule patients in U.S. patients
Figure 5b: Relative Risk of Mortality (CVD and non-CVD) by Day: European patients receiving dialysis on TTS
HRs of CVD and non-CVD mortality by day-of-week for TTS schedule patients in European patients
Figure 5c: Relative Risk of Mortality (CVD and non-CVD) by Day: Japanese patients receiving dialysis on TTS
HRs of CVD and non-CVD mortality by day-of-week for TTS schedule patients in Japanese patients
Cox models were fitted to all patients (i.e., combining the U.S., Europe and Japan) to study which patient characteristics were associated with the day-of-week effect on all-cause mortality. For this part of the analysis, we focused specifically on the impact of sex, 14 comorbid conditions and vascular access type on each of two effects: the mortality elevation on Mondays (for MWF schedule patients), and that on Tuesdays (for TTS patients). Results are listed in Tables 2, and 3. None of the above listed factors seemed to modulate or to account for the effects, with the possible exceptions of cancer (other than skin) being associated with a lower risk of all-cause mortality on Mondays (for MWF schedule patients) and on Tuesdays (for TTS patients) (HR = 1.18 for patients with cancer [other than skin], HR = 1.40 for patients without cancer [other than skin], p-value = 0.033); neurological disease being associated with a higher risk of all-cause mortality on Mondays (MWF schedule), and on Tuesdays (TTS schedule) (HR = 1.59 for patients with neurologic disease, HR = 1.33 for patients without neurologic disease, p-value = 0.041); and sex, where the effect of Tuesday was higher for males on a TTS schedule.
Table 2. RR of Death (all-cause) by Sex (US, Europe and Japan).
| Comparison | Schedule | RR Male | RR Female | p-value (difference) |
|---|---|---|---|---|
| Monday vs Avg | MWF | 1.32 | 1.47 | 0.152 |
| Tuesday vs Avg | TTS | 1.45 | 1.20 | 0.028 |
Table 3. Modification of Monday & Tuesday Effect by Comorbid Conditions and by Using Catheter as Vascular Access (US, Europe and Japan).
| Comorbid Conditions | Interaction with Monday and Tuesday | Comorbid Conditions | Interaction with Monday and Tuesday |
|---|---|---|---|
| Cerebrovascular Disease | 1.03 (0.699) | HIV | 0.79 (0.383) |
| Congest Heart Failure | 0.99 (0.891) | Hypertension | 1.09 (0.210) |
| Coronary Heart Disease | 1.05 (0.380) | Lung Disease | 1.00 (0.956) |
| Other Cardiovascular Disease | 1.02 (0.696) | Neurologic Disease | 1.20 (0.039) |
| Cancer (other than skin) | 0.84 (0.034) | Periph Vasc Disease | 1.05 (0.400) |
| Diabetes | 1.01 (0.924) | Psychiatric Disorder | 0.92 (0.166) |
| GI Bleeding | 1.01 (0.955) | Recur Cellulitis, Gangrene | 1.01 (0.899) |
| Catheter Use | Interaction with Monday and Tuesday | ||
|
| |||
| Catheter | 0.98 (0.760) | ||
- Numbers in the parentheses are the corresponding p-values.
A value < 1 (> 1) means the factor was associated with a decreased (increased) risk of all-cause mortality on Mondays with MWF schedule and on Tuesdays with TTS schedules.
A final Cox model was fitted which combined DOPPS-I patients from the U.S., Europe and Japan to determine whether time-dependent measures of blood pressure, potassium, sodium, ultrafiltration rate, intradialytic weight loss, residual renal function (RRF) and the use of diuretics modify the Monday and Tuesday effects. Table 4 shows that low ultrafiltration rate was associated with higher risk of all-cause mortality on Mondays with MWF schedule and on Tuesdays with TTS schedule. Diuretic use was associated with lower risk of all-cause mortality on Mondays with MWF schedule and on Tuesdays with TTS schedules.
Table 4. Modification of Monday & Tuesday Effect by Time Dependent Factors (DOPPS-I: US, Europe and Japan).
| Time dependent covariate | Interaction with Monday and Tuesday | Time dependent covariate | Interaction with Monday and Tuesday |
|---|---|---|---|
| Blood pressure low | 0.84 (0.054) | Blood pressure high | 1.19 (0.140) |
| Potassium low | 0.85 (0.142) | Potassium high | 1.15 (0.275) |
| Sodium low | 1.08 (0.496) | Sodium high | 1.06 (0.662) |
| Ultrafiltration rate low | 1.28 (0.019) | Ultrafiltration rate high | 0.94 (0.570) |
| Weight loss low | 1.11 (0.292) | Weight loss high | 1.07 (0.609) |
| RRF | 0.80 (0.096) | Diuretic use | 0.62 (0.003) |
- Numbers in the parentheses are the corresponding p-values.
- A value < 1 (> 1) means the factor was associated with a decreased (increased) risk of all-cause mortality on Mondays with MWF schedule and on Tuesdays with TTS schedules.
Discussion
Despite its proven value as a life-saving therapy, HD remains an intermittent intervention, most typically administered thrice weekly. Harmful waste products and fluid accumulated over the extended weekend interval may therefore put patients at a higher risk of death on certain days. Our results from the DOPPS in U.S., European, and Japanese patients indicate that in all three regions, HD patients have a higher risk of all-cause death on Mondays if they are on MWF schedule, or Tuesdays if they are on TTS schedule. Thus findings from prior reports (1, 3) are corroborated and expanded upon here. This day-of-week effect tends to be stronger for CVD than non-CVD death overall.
The European and Japanese data tend to confirm the effect, quite evident in the US data, of increased mortality on the day of the first dialysis session in the week. On the other hand, there are unique features to the results in Europe and Japan, such as high mortality on Saturday in the TTS schedule which may be due to practice patterns unique to those countries/regions. Such practice patterns could, for example, include continued aggressive/usual ultrafiltration and dialysis on Fridays or Saturdays, (the last dialysis session of the week) in order to allow for the anticipated longer weekend gap in dialysis, despite the patient being closer to their target post dialysis weight at the time of the last dialysis session of the week. This could predispose to a greater degree of intradialytic hypotension and/or hypokalemia which in turn would increase the tendency for CVD events, including sudden death. However, this mechanism while plausible, remains speculative.
Non-dialysis days had lower RRs for mortality compared to dialysis days perhaps because dialysis itself increases the risk of mortality. For HD patients, very large amounts of fluids and toxins are removed in a relatively short time period, particularly if the time elapsed since the last dialysis is long. This increases the potential for occurrence of intradialytic hypotension, which has previously been found to be a risk factor for mortality among HD patients (4, 5). Rapid reduction in post dialysis potassium may also be a contributory factor, as hypokalemia can enhance the risk for cardiac arrhythmia and sudden death, and this is more likely to occur on a dialysis day as opposed to the day preceding dialysis.
This study presents some evidence that the day-of-week effect is modulated by cancer (other than skin), neurological disease, sex, low ultrafiltration rate and use of diuretics; but apparently not by other suspected factors including other comorbid conditions, blood pressure, serum potassium, serum sodium, intradialytic weight loss and RRF (the hazard ratio for the interaction with day of the week in this instance was protective but statistically non significant). Potential explanations toward the interpretation of some of these interactions are again speculative. For those with cancer, it could be speculated that the interaction with first day of the week was associated with lower mortality because the mechanism of death may predominantly be through non-cardiovascular mechanisms. Those with neurologic disease may have an accentuated early in the week effect because of vascular diseases being the underlying cause for many types of neurologic disease (e.g., cerebrovascular disease). Similarly males have a greater association with cardiovascular disease and hence may have an interaction with early in the week mortality. Low ultrafiltration rate may at least in part reflect patients especially prone to dialysis-related hypotension which is itself a predictor of worse patient outcomes. In particular, the finding that modification of the early-in-the-week mortality risk in association with diuretic use has therapeutic implications, especially in patients with significant RRF who are likely to be responsive to diuretic therapy. This is consistent with previously published DOPPS findings showing a lower mortality among those receiving diuretics (6). Low ultrafiltration may represent patients with low blood pressure to begin with or those that are malnourished, thus being associated with higher early in the week mortality. Clearly, associations found in these exploratory analyses require confirmation in future studies.
Some limitations of our study are as follows. Dialysis schedule was inferred from the reported date of dose of dialysis reporting, which was collected once every four months. It was not possible to obtain the schedule information directly. In addition, the DOPPS data do not include the exact time of death, nor the timing of dialysis sessions; both of which would be useful additional data in further understanding the day-of-week effect. The secondary endpoint of CVD death is certainly of interest, but CVD death may not be ascertained completely or determined consistently across centers. Such limitations are typical of studies using large observational databases. Due to both the smaller sample size and lower event rate, inferences pertaining to Japanese HD patients are subject to substantially more uncertainty than that for U.S. patients. Countries within Europe also have smaller sample sizes. However, these countries share similar genetic and environmental factors as well as practice patterns, at least to some extent. In the analyses reported here, Europeans were considered as a single group and conclusions were made about average effects over the 5 to 7 countries from this entire region. Larger samples from each country could examine differences within Europe. DOPPS II also includes data from Canada, Australia and New Zealand, but the sample sizes in these countries were too small to support reliable conclusions in this study. Noncompliance has been observed to be most common in the U.S. and least common in Japan. Non adherence with dialysis sessions (‘shortening’ or ‘skipping’ sessions) has been associated with higher mortality as detailed in a previous DOPPS paper (7), and would be expected to raise overall mortality rates. Unfortunately, there is insufficient information to assess the rate or effect of noncompliance in the DOPPS data. Finally, a substantial proportion, 66% of patients died in the hospital. Often, patients who are hospitalized would no longer follow their regular dialysis schedule. In this case, one would expect the observed day of week effect in this study to be somewhat attenuated. Further study that considered the potential confounding effect of hospitalization would be valuable.
Our results imply that there may be an advantage to a more frequent dialysis schedule in Europe, the U.S and potentially Japan. This is supported by the relatively low rates of mortality for patients receiving “daily dialysis” based on various reports (8, 9, 10, 11). One could hypothesize that more frequent dialysis would reduce or eliminate the day-of-week effect and, therefore, lower mortality on HD. Many previous reports have found that daily dialysis is beneficial. For example, Woods et al. (8) found that for certain patients, daily HD might have advantages over thrice weekly HD. Blagg et al. (9) observed that patients treated by short-daily HD had a better survival rate than those treated by conventional HD. Kjellstrand et al. (10) reported that the survival of United States Renal Data System patients on short daily HD was 2-3 times lower than that of matched thrice weekly HD patients. Johansen et al. (11) examined survival and hospitalization between frequent HD patients and patients undergoing thrice-weekly conventional HD. Their study found that there was a non-significant reduction in death risk for patients using short-daily HD, while hospitalization patterns did not differ significantly between daily HD patients and their matched counterparts. There are many reasons why daily HD may improve patient survival. Daily HD can minimize fluid volumes (10), improve many abnormal physiological parameters and metabolic markers associated with a high mortality (12, 13, 14, 15, 16). Quality of life has been reported to improve for patients on daily HD (17, 18). However, the term “daily” has been defined differently in these studies. It can mean 5 or 6, 5-7, or 4-7 times of dialysis per week. Hence there is the possibility that death rates are still elevated on the first and/or last day of dialysis. Therefore, our results do not imply directly that a more frequent dialysis could reduce mortality. We would need to analyze the data using a cohort that received daily dialysis in order to determine whether day-of-week effect really disappeared and, hence, whether mortality was reduced.
Bliwise et al. (19) investigated the association of HD treatment shift with continued survival among elderly patients. Their study found that the morning-shift HD patients survived significantly longer than the afternoon-shift patients. In the present study, we observed a higher risk of all-cause mortality among European patients on Saturdays on a TTS schedule and a higher risk of CVD mortality among European and Japanese patients on Saturdays on a TTS schedule.
In summary, we have carried out a comprehensive analysis of the interaction between dialysis schedule and day-of-week specific mortality among HD patients in the U.S., Europe and Japan. Limitations associated with the DOPPS database suggest future directions for research in this area. In particular, future studies aimed at evaluating the benefit of additional weekly dialysis sessions or alternate-day dialysis should also assess the impact of schedule on day-of-week-specific mortality. Utilization of diuretics among those with significant RRF is another potential area fertile for further study, in addition to examination of other practice patterns that can reduce early-in-the-week mortality for those on conventional thrice weekly HD schedule, as this is likely to remain the norm for the vast majority of HD patients, at least for the foreseeable future. Further study of data with the exact time of death and the timing of dialysis sessions would be valuable in further understanding the day-of-week effect.
Methods
Data were obtained on U.S., Japanese, and European (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom) patients from the DOPPS Phase I and Phase II. The overall design of the DOPPS has been described in some detail previously (20, 21). In total, the study population consisted of n=22,163 patients; 9,227 were from the U.S., 8,517 from Europe, and 4,419 from Japan. For each patient, follow up began at entry into the DOPPS. Patients were then observed until the earliest of death, transplant, loss to follow-up, or the end of the observation period: December 31, 2001 for Phase I patients, and December 31, 2004 for Phase II patients. The mean follow-up time is 569 days for Phase I patients and 564 days for Phase II patients. About 5% of patients received transplantations, and 4% of patients withdrew from the study. The primary outcome of interest is death [all-cause, CVD and non-CVD]. The date and cause of death were recorded. CVD mortality was defined as the death with primary cause being any of acute myocardial infarction, hyperkalemia, pericarditis, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest, valvular heart disease, pulmonary edema due to exogenous fluid or congestive heart failure.
Baseline data on demographics, laboratory values, medical history and medications were collected at the start of participation in the DOPPS. Thereafter, longitudinal data were collected at four-month intervals, including updated hospitalization events, vascular access, laboratory values, medications, dialysis prescription, dialysis dose and RRF. Detailed information is also reported for the final dialysis session in the four-month period, including the date dialysis was received. For purposes of analysis, if the reported date was a Monday, Wednesday or Friday, the dialysis schedule was considered to be MWF in the corresponding four-month interval. The TTS dialysis schedule was defined in a similar manner. If this date was missing or a Sunday, the dialysis schedule was carried over from the preceding four-month reporting interval. About 79% of patients did not switch the dialysis schedule in this study, 13% patients switched once, either from MWF to TTS, or from TTS to MWF, and 8% patients switched more than once.
Baseline characteristics for prevalent patients (at the start of DOPPS I and II, n=13,820) were summarized, with simple means for continuous variables and proportions for categorical variables.
The covariate of interest is the day-of-week (Sunday, Monday,…, Saturday), which was coded as a time-dependent covariate in a Cox model with time origin as the first ever HD day. Since patients were not under observation until they entered the DOPPS study, using time scale of days since study entry can be subject to left-truncation bias, therefore, we used days since onset of dialysis (dialysis vintage) as the time scale. Adjustment covariates included sex, race, 14 comorbid conditions (coronary heart disease, cancer other than skin, other cardiovascular disease, cerebrovascular disease, congestive heart failure, diabetes, gastrointestinal bleeding, HIV/AIDS, hypertension, lung disease, neurologic disease, psychiatric disorder, peripheral vascular disease, and recurrent cellulitis), body mass index (grouped as <20, [20-25), [25-30), ≥ 30 kg/m2), and vascular access (catheter use). Dialysis schedule, country, phase and age group (18-29, 30-39, 40-44, 45-49, 50-54, 55-59, 60-64, 65-69, ≥ 70 years) were adjusted through stratification. Since patients from the same facility may be correlated due to shared practice patterns, valid statistical procedures must account for intra-cluster dependence, which was done using a robust (“sandwich”) estimator of the covariance (22).
Cox models were fitted to each region (U.S., Europe and Japan) separately. In all models, day of the week was coded such that each day was compared to the average of the seven days of the week. Table 5 shows the coding of the time-dependent covariates related to day of the week. The average of the seven days of the week is constrained to be zero in the model; therefore, each day was compared to this geometric average (i.e., zero). The comparison of each level of a categorical predictor to the average is as defensible statistically as the comparison of each level to a reference level. The former is used much less frequently than the latter, but is the best choice for our analysis.
Table 5. Coding for Time-Dependent Covariates.
| Time dependent covariate | Sun | Mon | Tue | Wed | Thu | Fri | Sat |
|---|---|---|---|---|---|---|---|
| Z1 | -1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Z2 | -1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Z3 | -1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Z4 | -1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Z5 | -1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Z6 | -1 | 0 | 0 | 0 | 0 | 0 | 1 |
Further analyses used Cox models fitted to patients from all three regions combined, in order to study whether sex, 14 comorbid conditions and vascular access (catheter use) modified the day-of-week effect. Models including interactions between region and day-of-week have been compared to those without such interactions. Since only small differences in estimates are observed between these two models, only results from the latter models are reported in this article. Also, in assessing the potential modifiers of the day of week effect, we found that the interactions between the modifiers and Mondays (for MWF schedule patients) are similar to those between the modifiers and Tuesdays (for TTS schedule patients); thus, in the models reported, these interactions are assumed equal and combined. Further, another Cox model was fitted to all patients' laboratory covariates (blood pressure, potassium, sodium, ultrafiltration rate, weight loss, RRF, and use of diuretics). Each of these variables, except RRF and the diuretic use, was categorized into three groups: low (lowest quartile), normal (middle two quartiles) and high (highest quartile). These time-dependent covariates were measured monthly in the unit and weight loss was measured with each treatment. However, the DOPPS updated such measures only every four months. In this last time-dependent analysis, results were based on DOPPS-I data because only baseline potassium and sodium values were available in DOPPS-II.
In this study, the covariate of interest is day of the week (Sunday, Monday, …, Saturday). We defined time-dependent covariates based on the day of the week. Cox regression analyses employed the PHREG procedure, and handled time-dependent covariates and left-truncation through the counting process style of input. Facility clustering effects were accounted for through the robust sandwich covariance estimates. In SAS PHREG procedure, this is implemented using COVS(AGGREGATE) option, with ID specified as facility [SAS 9.2 documentation, Example 64.11 in the PHREG procedure (23)]. As implied, all statistical analyses were conducted using SAS V9.2 (SAS Institute; Cary, NC).
Acknowledgments
Editorial assistance was provided by Ms. Shauna Leighton, a Medical Editor at the Arbor Research Collaborative for Health, Ann Arbor, Michigan
Sources of Support: The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications. The statistical methodology development and analysis for this investigation were supported in part by a National Institutes of Health grant (R01DK070869) to Professors Schaubel and Kalbfleisch.
Footnotes
Disclosure: The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications. The statistical methodology development and analysis for this investigation was supported by National Institutes of Health grant R01 DK070869 to Drs. Schaubel and Kalbfleisch.
References
- 1.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 2.Karnik JA, Young BS, Lew NL, et al. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60:350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer AJ, Hartman J, Brannon PC, et al. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 4.Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 5.Shoji T, Tsubakihara Y, Fujii M, et al. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Bragg-Gresham JL, Fissell RB, Mason NA, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007;49:426–31. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney In. 2003;64:254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 8.Woods JD, Port FK, Orzol S, et al. Clinical and biochemical correlates of starting “daily” hemodialysis. Kidney Int. 1999;55:2467–2476. doi: 10.1046/j.1523-1755.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 9.Blagg CR, Kjellstrand CM, Ting GO, et al. Comparison of survival between short-daily hemodialysis and conventional hemodialysis using the standardized mortality ratio. Hemodialysis Int. 2006;10:371–374. doi: 10.1111/j.1542-4758.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 10.Kjellstrand CM, Buoncristiani U, Ting G, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transpant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- 11.Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:984–990. doi: 10.1038/ki.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AW, Chebrolu S, Ing TS, et al. Early clinical quality of life and biochemical changes of “daily hemodialysis”. Am J Kidney Dis. 2004;43:90–102. doi: 10.1053/j.ajkd.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Ting GO, Freitas T, Kjellstrand C, et al. Five-year comparison of conventional versus short daily hemodialysis in high-comorbidity end stage renal disease patients. Am J Kid Dis. 2003;42:1020–1035. doi: 10.1016/j.ajkd.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Silverburg JS, Barre PE, Prichard SS, et al. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 15.Galland R, Traeger J, Arkouche W, et al. Short daily haemodialysis rapidly improves nutritional status in haemodialysis patients. Kidney Int. 2001;60:1555–1560. doi: 10.1046/j.1523-1755.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinciaroli AR. Hormonal changes in daily hemodialysis. Semin Dial. 1999;12:455–461. [Google Scholar]
- 17.Mohr P, Neumann P, Franco S, et al. The case for daily dialysis: its impact on costs and quality of life. Am J Kid Dis. 2001;37:777–789. doi: 10.1016/s0272-6386(01)80127-x. [DOI] [PubMed] [Google Scholar]
- 18.Heidenheim PA, Muirhead N, Moist L, et al. Patient quality of life on quotidian hemodialysis. Am J Kid Dis. 2003;42(Suppl 1):S36–S41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL, Kutner NG, Zhang R, et al. Survival by time of day of hemodialysis in an elderly cohort. JAMA. 2001;286:2690–2694. doi: 10.1001/jama.286.21.2690. [DOI] [PubMed] [Google Scholar]
- 20.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(suppl74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 21.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(suppl2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL, editors. The Survival Analysis of Failure Time Data. 2nd. New York: Wiley; 2002. [Google Scholar]
- 23.SAS Institute Inc. SAS 9.2 Software. Cary, NC: SAS Institute Inc.; 2008. The PHREG Procedure. [Google Scholar]


