Abstract
Detection of asymmetries has been a mainstay of using vestibular reflexes to assess semicircular canal function. However, there has been relatively little work on how vestibular stimuli are perceived. Suprathreshold vestibular perception was measured in 13 normal healthy controls by having them compare the relative sizes of two yaw (vertical-axis rotation) or sway (right-left translation) stimuli. Both stimuli were 1.5 s in duration with a staircase used to adjust the relative size of the stimuli to find a pair of stimuli perceived as equal. Motion stimuli were delivered in darkness using a hexapod motion platform, and visual stimuli simulating motion were presented on a screen in the absence of platform motion. Both same direction (SD) and opposite direction (OD) stimuli were delivered in separate runs. After a two-interval stimulus, subjects reported which movement they perceived as larger. Cumulative distribution functions were fit to the responses so that the relative magnitudes of the two stimuli perceived as equal could be determined. For OD trial blocks, a directional asymmetry index (DAI) was calculated to compare the relative size of perceived rightward and leftward motion. For all trial blocks, a temporal asymmetry index (TAI) was used to compare the relative size of the first and second intervals. Motion OD stimuli were perceived as equal in all subjects in yaw and all but one in sway. For visual OD stimuli, two subjects had slightly asymmetric responses for both sway and yaw. The TAI demonstrated asymmetry in 54% in yaw, in which the second interval was perceived to be larger in all but one subject who had an asymmetry. For sway, only two subjects had a significant asymmetry. Visual stimuli produced a similar rate of asymmetry. The direction and magnitude of these asymmetries were not significantly correlated with those seen for motion stimuli. Asymmetries were found in a fraction with the TAI in SD stimuli for motion in yaw (42%) and sway (33%), as well as for vision in yaw (60%) and sway (43%). The precision at discriminating SD motion stimuli decreased significantly with age, but there was no difference with OD motion or visual stimuli.
Keywords: Vestibular, semi-circular canals, otoliths
Introduction
Detection of asymmetry has been a corner stone of measuring human vestibular function. The earliest and still most widely used test of vestibular symmetry is caloric irrigation (Bárány 1921) which makes use of the vestibulo-ocular reflex. Rapid head rotation has also been shown to localize vestibular asymmetries in humans (Halmagyi and Curthoys 1988; Crane and Demer 1998) as have vestibular evoked myogenic potentials (VEMP) (Welgampola and Colebatch 2005). Both of these are now commonly used clinical tests. An acute asymmetry in vestibular function is perceived as vertigo, for example, as commonly occurs with vestibular neuronitis (Strupp and Brandt 2009) or acute surgical lesions (Levine et al. 1990). However, in many patients, the vertigo will resolve even if the lost peripheral vestibular function does not recover. Also, there is evidence that vestibular reflexes and vestibular perception arise from different mechanisms (Merfeld et al. 2005b; Merfeld et al. 2005a). Thus, it is not surprising that patient’s subjective vertigo symptoms are often poorly correlated with vestibular function measured using reflex testing (Kanayama et al. 1995; Perez et al. 2003).
There have been studies to establish the motion perception thresholds in healthy humans (Benson and Brown 1989; Kingma 2005; Grabherr et al. 2008; MacNeilage et al. 2010; Mallery et al. 2010), but these studies have made the assumption that perceptual thresholds are symmetric with similar thresholds in opposite directions of movement. However, there is some evidence that normal subjects may have asymmetric motion perception thresholds for fore-aft motion (Benson et al. 1986), and directional bias may also be the source of some asymmetry in perceptual thresholds (Merfeld 2011). A recent study in the current laboratory has demonstrated that significant rotational and translational perceptual asymmetries occur in about a third of healthy individuals for a given test condition (Roditi and Crane 2012). The goal of the current study is to determine if such perceptual asymmetries extend into the realm of suprathreshold perception in healthy humans.
Methods
Subjects
Thirteen healthy human subjects (6 female, 7 male) aged 27 to 68 years (mean 41) participated in the study. All subjects gave informed consent. All subjects completed standard video nystagmography (VNG) testing including caloric testing and had results in the normal range (≤20% asymmetry on caloric testing). Subjects also completed visual acuity testing and audiometry to verify normal hearing and vision. The protocol was approved by the University of Rochester Research Science Review Board.
Platform Motion
Motion stimuli were delivered using a 6-degree-of-freedom motion platform (Moog, East Aurora, NY, model 6DOF2000E) similar to that used in other laboratories for human motion perception studies (Grabherr et al. 2008; Fetsch et al. 2009; MacNeilage et al. 2010) and in the current laboratory (Roditi and Crane 2012). Subjects were seated in a padded racing seat (Corbeau, Sandy UT, model FX-1) mounted on the platform. A four-point racing style harness held the body in place. The head was held in an open-face motorcycle helmet with a chinstrap. Helmets were available in 6 sizes to allow each subject to be fit appropriately. Once the subject was seated, the helmet was firmly attached to the motion platform using a custom built structure that allowed adjustment for the subject’s size and comfort. The back of the helmet was pushed against a headrest to ensure head motion was coupled to the platform.
Sounds from the platform were masked using a white noise stimulus reproduced from two platform-mounted speakers on either side of the subject. No masking noise was used between stimuli. The motion stimuli were delivered in complete darkness with no visual feedback available.
Visual Flow
During visual flow, the platform remained stationary and subjects viewed a visual stimulus consisting of a star field that simulated movement of the observer through a random-dot cloud. Visual coherence was fixed at 90%. Even though the stimulus delivery made no sound, a white noise masking sound similar to that used during motion stimuli was used. The stimuli were presented on a horizontal color LCD screen which measured 115.6 by 64.8 cm with a resolution of 1920 × 1080 pixels (Samsung model LN52B75OU1FXZA). The subject was seated 50 cm from the screen that filled a 98° field of view in azimuth.
Experimental procedure
Prior to stimulus delivery, the subject heard a 500 Hz, 0.125 s single tone to signal that the next stimulus was ready. The stimulus was delivered immediately after the subject pressed the start button. After the stimulus was delivered, two 0.125s tones were played in rapid succession to indicate that the stimulus had been delivered and suggest that one of two response buttons should be pressed. Thus, this was a two interval forced choice task. Tones were played from speakers mounted to the motion platform to eliminate any potential auditory localization cues. When a response button was pressed, a key click sound was played which did not depend on the accuracy of the response, but indicated that the subject’s selection had been recognized by the program. If no response was entered within 2 s, a “timeout sound” was played (a low frequency buzz).
Opposite Direction (OD) Stimuli
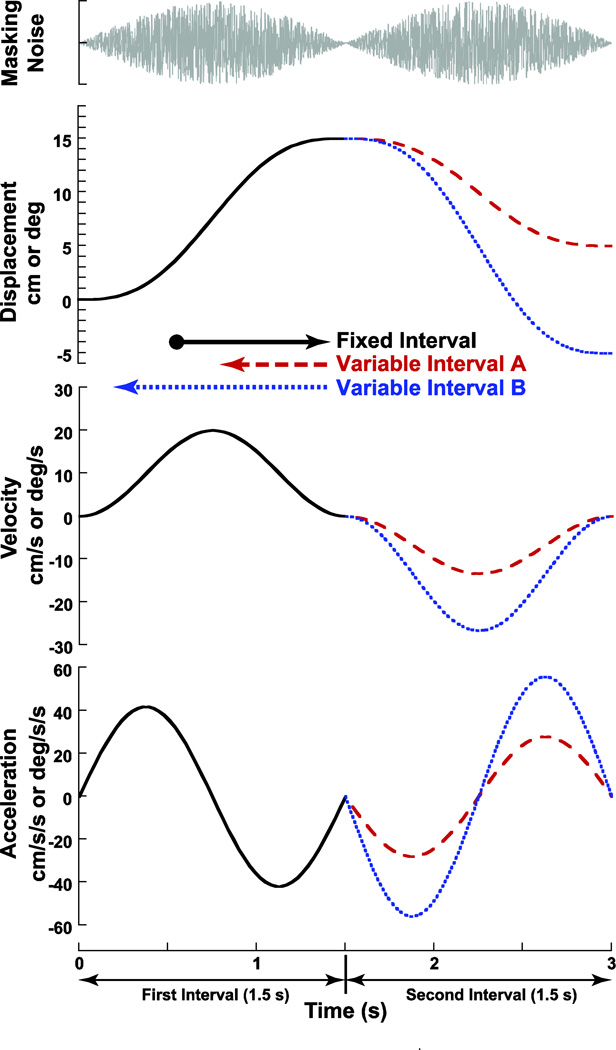
The subject was moved in one direction followed by a motion in the opposite direction. Each direction of motion was a sine wave in acceleration lasting 1.5 s (0.66 Hz) with the second stimulus starting immediately after the first stimulus ended (Fig. 1). It was important that the subject make their decision by comparing the two intervals. To achieve this, one of the two intervals always had a fixed displacement of 15° or 15 cm, thus the peak velocity was 20° or cm/s, and the peak acceleration was 42° cm/s/s.
Figure 1.
Motion profile. Each presentation of the stimulus consisted of 2-intervals of motion: one fixed and one variable. Both intervals are 1.5 s in duration. In this example the first interval (solid black line) was fixed at a displacement of 15 units (either degrees or centimeters) and peak velocity of 20 units/s. The second interval had a variable amplitude. Variable interval A has a smaller amplitude than the fixed interval by 5 units. Variable interval B is larger amplitude than the fixed interval by 5 units.
Each run of trials included 4 sets of interleaved conditions which were independently randomized for each subject: 1) a set in which the first interval was of variable displacement and to the right, 2) a set in which the first interval was variable and to the left, 3) a set with the first interval fixed and to the right, and 4) a set with the first interval fixed and to the left (Table 1). Each set included two staircases: One, in which the variable interval started at the minimum displacement (1 cm or deg), and one in which the variable interval started at the maximum displacement (29 cm or deg). The minimum displacement was sub-threshold in some subjects yielding a peak velocity of 1.3 cm or deg/s. The mean threshold previously found in the current laboratory was 1.3 deg/s in yaw and 1.7 cm/s in sway (Roditi and Crane 2012). Thus, each block of trials included 8 independent, interleaved staircases. The stimuli were adjusted such that if the subject indicated the variable interval was larger, the next stimulus was reduced by 4 cm. Conversely, if it was perceived to be smaller, the next stimulus was 4 cm larger. A reversal in the perceived size of the stimulus within a staircase reduced the step size by half, until a minimum step size of 1 cm was reached. The staircase then continued at the minimum step size, unless there were 3 responses in the same direction. In this scenario, the step size doubled up to the maximum of 4 cm. The variable interval was adjusted based on prior responses to converge on the point of subjective equality (PSE) when the subject was equally likely to identify either interval as larger. This method tended to focus the majority of stimuli near the PSE. Each staircase contained 21 pairs of stimuli, thus a block included 168 pairs of stimuli and took about 45 minutes to complete.
Table 1.
Summary of both two interval forced choice techniques used. The columns to the right show the correct responses for the perceived magnitudes shown on the left. The upper arrow in each series represents the first interval, with the lower arrow representing the second stimulus. Using the magnitude comparison (MC) task subjects were asked to identify if the first or second interval was larger. Using the vector addition (VA) task subjects were asked to determine if the end point was right or left of the starting point.
| Stimulus Combination | Magnitude Comparison |
Vector Addition |
|---|---|---|
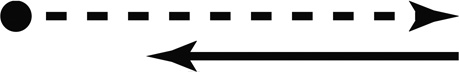 |
First | Right |
 |
First | Left |
 |
Second | Left |
 |
Second | Right |
Same Direction (SD) Stimuli
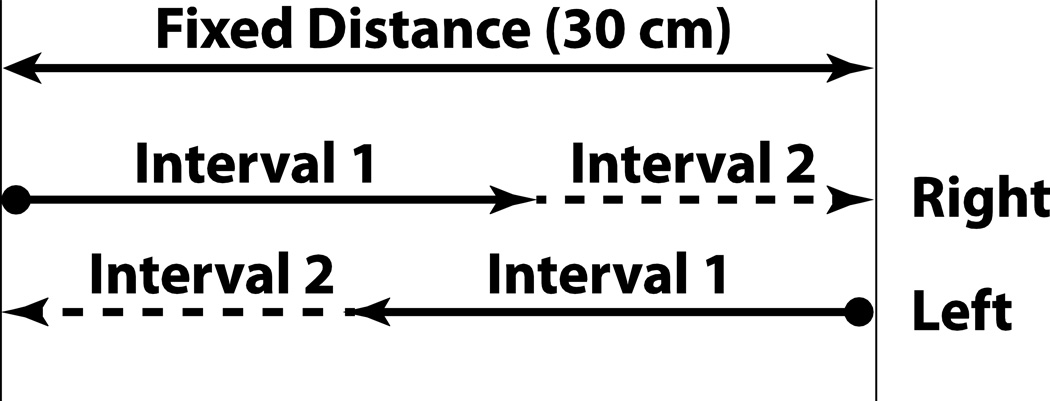
Similar to OD stimuli runs, each interval consisted of a sine wave in acceleration. Because the displacement of the platform was limited to about 35 cm, the method used to deliver the stimuli was changed so that the sum of the intervals was a constant 30 cm. However, when the two intervals were equal, each would be 15 cm, the same as the fixed interval in the OD task. Sets of stimuli in the right and left direction were interleaved which allowed the full range of platform motion to be used (Fig. 2). Each movement direction still had two randomly interleaved staircases, which started at the minimum and maximum displacement respectively. Thus, each SD block included a total of four independent staircases, and a total of 84 stimulus pair presentations. The stimuli were adjusted based on subjects’ responses, as in the OD stimuli runs.
Figure 2.
Diagram demonstrating same direction (SD) stimulus times.
Responses
Responses were collected using a three-button control box that the subject held. The center button was pressed by the subject to initiate each stimulus. The two buttons at either end were used to identify the relative magnitudes of the two motion intervals in this forced choice task. Two strategies for reporting the relative magnitudes of motion or tasks were investigated. The first method was magnitude comparison (MC). Using this method, the subject was encouraged to press one button to indicate that the first stimulus was larger and another button to indicate that the second stimulus was larger. A second method using vector addition (VA) was also investigated. Using this method subjects were asked to indicate the end point relative to the starting point (Table 1). Both methods required that the answer be entered within 2 s, and if no response was entered the trial was ignored.
Data Analysis
A Gaussian cumulative distribution function was fit over the fraction of rightward (VA task) or second interval larger (MC task) 0 to 1.0. The mean of the function occurs at the point were the responses are divided equally between the two possible responses; also known as the point of subjective equality (PSE). The function was fit to the data using an established Monte Carlo maximum-likelihood criteria allowing for a small lapse rate as previously described (Wichmann and Hill 2001a; Wichmann and Hill 2001b), used by others (Fetsch et al. 2009; MacNeilage et al. 2010), and previously described in the current laboratory (Roditi and Crane 2012). The bootstrapping method of this technique permitted fits to be repeated several times to determine the range of uncertainty in the curve fit parameters.
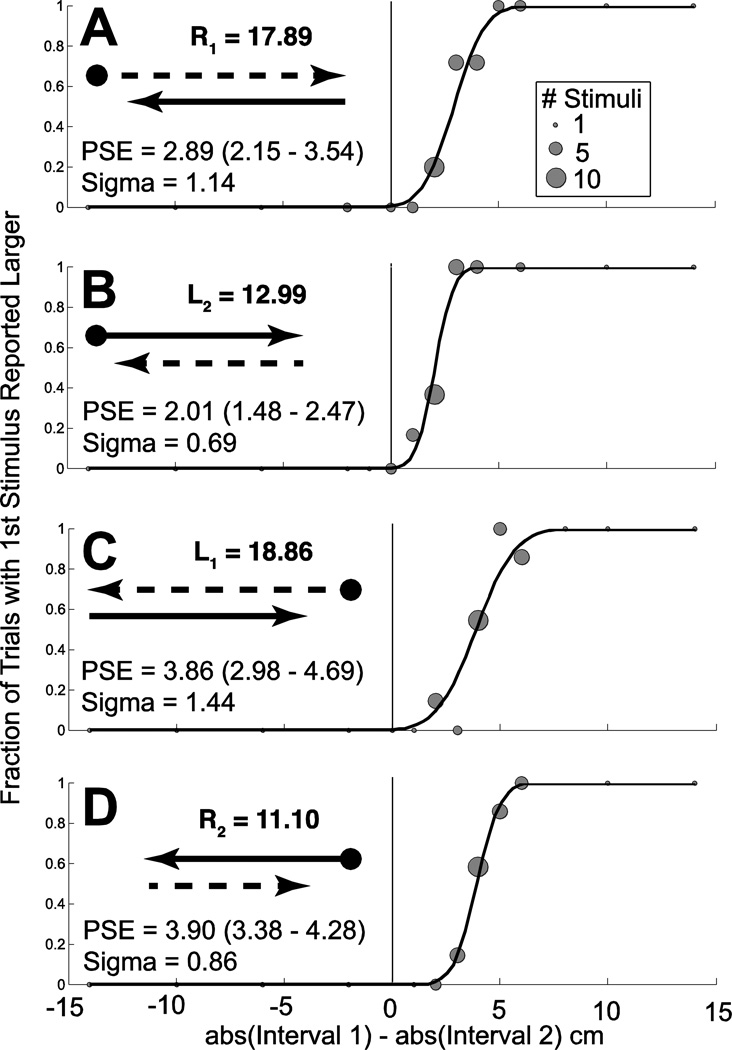
Directional asymmetry could be determined for OD trials by determining the magnitude of the variable intervals of leftward (L1 and L2) and rightward (R1 and R2) displacement at the PSE (Fig. 3). A directional asymmetry index (DAI) was calculated using the following formula:
| (1) |
Thus, the DAI is the base 2 log of the sum of all rightward displacements over the sum of all leftward displacements at the point for each test condition where these were perceived as equal. Because each of the four sets of staircases contains a constant interval of 15 cm or deg, 30 is added to both the rightward and leftward displacements. R1 is the magnitude of rightward motion when it was varied during the first interval, and R2 is the magnitude of rightward motion when it was varied during the second interval. Similarly, L1 and L2 represent the staircases were the leftward motion was variable. Thus, this method has the effect of providing an average across all the staircases within a trial block. It would not be appropriate to report a DAI for only a subset of test conditions because it would not be able to differentiate between a dynamic and temporal asymmetry. With this calculation the DAI would be zero when the right and left motion were perceived equally, 1.0 when the rightward motion had to be twice the left motion to be perceived as equal and −1.0 when the leftward motion had to be twice the right motion.
Figure 3.
Sample data from a trial in an individual subject (#1) for a rare trial block demonstrating both a directional and temporal asymmetry. The subject was shown visual motion at 90% coherence consistent with rotation. The task was to identify which interval was larger (MC). Results from 8 independent randomly interleaved staircases are shown. Each panel represents two staircases, one in which the variable interval started at a maximum value (29°) and the other at which it started at a minimum value (1°) which tended to converge to a similar PSE during the trial block. Data points are sized proportional to the number of stimulus presentations at each level. The value of the PSE in deg is given with the 95% confidence interval in parenthesis. In every panel, the PSE is positive indicating the first interval was larger than the second interval when they were perceived as equal. When similar stimuli in opposite directions are compared (A & D, B & D), the PSE was significantly larger when leftward motion was presented first (panels C & D). This indicates a directional asymmetry where the leftward stimuli were presented larger to be perceived as equal. The magnitudes of the variable stimuli at the PSE are shown. Panel A: The first interval is variable and to the right. Panel B: The first interval is fixed and to the right. Panel C: The first interval is variable and to the left. Panel D: The first interval is fixed and to the left.
A temporal asymmetry index (TAI) was determined for OD trials by comparing the relative magnitude of the first interval with the second interval at the point they were perceived as equal, using an equation analogous to the one used to calculate the DAI:
| (2) |
For SD trials, the TAI was also calculated similarly. However, because there was no constant interval and the sum of the two intervals was always 30 cm or deg the equation is slightly different:
| (3) |
Using this scale, a TAI of zero indicates the two intervals are perceived equally. A positive TAI indicates that the first interval had to be larger to be perceived equal to the second interval, thus, a positive TAI implies that if the two intervals were the same size the second would be perceived as larger.
Because the duration of the two intervals was constant, and DAI and TAI are calculated based on the ratio of two values, it does not matter if the magnitude is measured in distance, peak velocity, or peak acceleration. However, the constant used here (30 deg or cm) would have to be adjusted appropriately if the calculation were to be done in the velocity or acceleration domains.
The Spearman rank order coefficient was used to measure the significance of correlation between continuous variables. Two different axes of motion were examined (yaw and sway), each with both self-motion and visual motion. Pairs of stimuli were both SD and OD, with the method of comparison being vector addition (VA) or magnitude comparison (MC). The student’s t-test was used to compare results between the VA and MC tasks with the level of statistical significance defined as p < 0.05.
Results
A total of 13 human subjects participated in these experiments although due to time constraints and subject availability not every subject completed every test condition (Table 2). The vector addition (VA) task was not well suited to visual motion because many subjects found it ambiguous if the stimulus represented self-motion through a fixed environment or an environmental motion, thus magnitude comparison (MC) was predominately used for visual motion tasks. For each run of trials, curve fits were performed on the responses to determine the PSE between the two stimuli (Fig. 3).
Table 2.
Subjects with conditions completed. The vector addition (VA) task was only meaningful with opposite direction (OD) stimuli. The magnitude comparison (MC) task could be used for both OD and same direction (SD) stimuli. The columns contain MC or VA to indicate which conditions were completed in each subject. All but one of the subjects (#11) participated in a prior study of symmetry near the threshold (Roditi and Crane 2012), the subject numbers used in the prior study are listed under ‘Prior’ for comparison.
| Motion | Vision | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Opposite Direction | Same Direction | Opposite Direction | Same Direction | |||||||
| Subject | Prior | Age | Yaw | Sway | Yaw | Sway | Yaw | Sway | Yaw | Sway |
| 1 | 1 | 38 | MC,VA | MC,VA | MC | MC | MC | MC | MC | MC |
| 2 | 21 | 30 | MC,VA | MC,VA | MC | MC | ||||
| 3 | 20 | 30 | VA | VA | MC | MC | MC | MC | MC | MC |
| 4 | 8 | 29 | VA | MC,VA | MC | MC | MC | |||
| 5 | 18 | 29 | MC,VA | MC,VA | MC | MC | MC | MC | ||
| 6 | 19 | 29 | MC,VA | MC,VA | MC | MC | MC | MC | MC | MC |
| 7 | 10 | 27 | MC,VA | MC,VA | MC | MC | MC | MC | MC | MC |
| 8 | 14 | 68 | VA | VA | ||||||
| 9 | 13 | 45 | MC,VA | MC | MC | MC | MC | MC | MC | MC |
| 10 | 16 | 30 | MC | MC | MC | MC | MC | MC | ||
| 11 | 50 | MC | MC | MC | MC | MC | MC | MC | MC | |
| 12 | 4 | 65 | MC | MC | MC | MC | MC | |||
| 13 | 2 | 68 | MC | MC | MC | MC | MC | MC | MC | MC |
In a group of subjects, both the VA and MC tasks were applied in different testing sessions (Table 2). In these sets of runs, the two tasks yielded similar results (95% confidence intervals over lapped) in all but one set (subject #4, sway motion). When these conditions were compared using paired t-tests, the results were not significantly different (p > 0.1 for all). Results from the VA and MC tasks are shown using separate symbols (Fig. 4 and 5) but are otherwise averaged and discussed together since both tasks produced similar results.
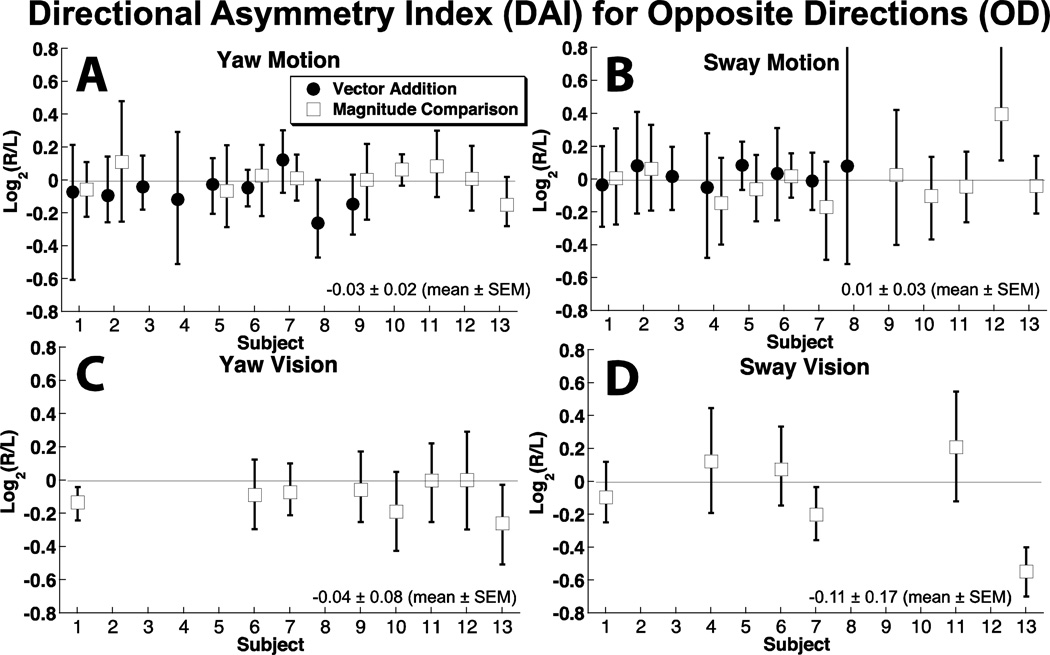
Figure 4.
Asymmetry in motion perception based on direction of stimulus.
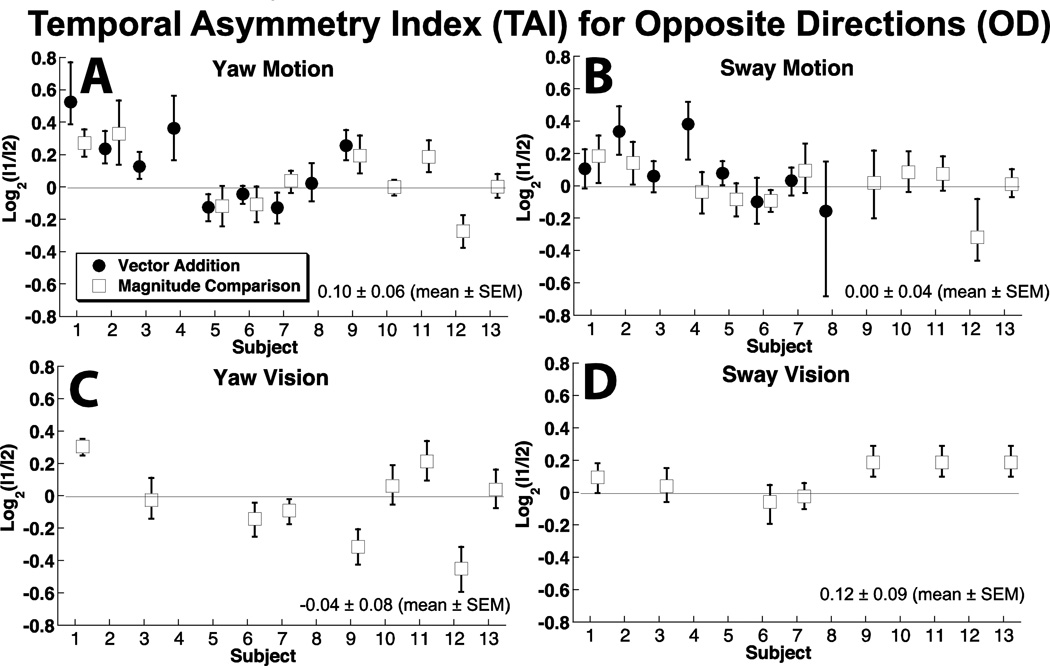
Figure 5.
Asymmetry in motion perception based on order of stimulus presentation.
Directional symmetry
The degree of directional asymmetry was measured using the DAI. With the motion stimulus, the 95% confidence interval of the DAI included zero in all 13 subjects for yaw motion (Fig. 4A), and in 12 of 13 subjects for sway (Fig. 4B). The mean DAI was 0.04 ± 0.10 (mean ± SD) for yaw and 0.02 ± 0.13 for sway.
A visual stimulus simulating yaw rotation was performed in a subset of 8 subjects, 2 of which had a DAI slightly but significantly below zero (Fig. 4C). The mean value was −0.09 ± 0.09. For visual sway, a group of 7 subjects were tested and 2 also had a DAI significantly below zero (Fig. 4D) with a mean of −0.09 ± 0.27.
Thus, directional asymmetries were rare and when present, were small when comparing OD yaw or sway motion. Asymmetries were slightly more common with visual motion.
Order symmetry
The perceived magnitude of the stimulus often depended on the order in which OD stimuli were presented (Fig. 5). For yaw motion, 7 subjects had a TAI significantly different from zero. In 6 subjects (46%), the first interval stimulus had to be significantly larger for the two stimuli to be perceived as equal, in 1 subject (8%) the second stimulus was significantly larger at the PSE, and for the remaining 6 subjects there was no significant difference between the magnitude of the first and second intervals at the PSE (Fig. 5A). For sway, in only two subjects was the PSE reached when the intervals were of significantly different magnitudes: in subject #2 the first interval was larger, and in #12 the second interval was larger. Average TAI for yaw motion was 0.09 ± 0.20 (mean ± SD), for sway motion was −0.01 ± 0.21. For yaw vision, the TAI averaged −0.04 ± 0.25, and for sway vision it was 0.09 ± 0.11.
For visual OD stimuli simulating yaw (Fig. 5C), 2 of 9 required significantly larger first intervals to reach the PSE and 3 of 9 required smaller first intervals. For visual sway stimuli, 3 of 7 required significantly larger first stimuli to reach the PSE (Fig. 5D).
There was a significant correlation between the TAI for OD sway and yaw motion (R = 0.74, p = 0.004, two-tailed Spearman rank order coefficient) but the correlation was not significant between sway and yaw vision (R = 0.39, p = 0.4). There was not a significant correlation between TAI for yaw motion and visual (R = 0.58, p = 0.09) or for sway motion and visual (R = 0.27, p > 0.5)
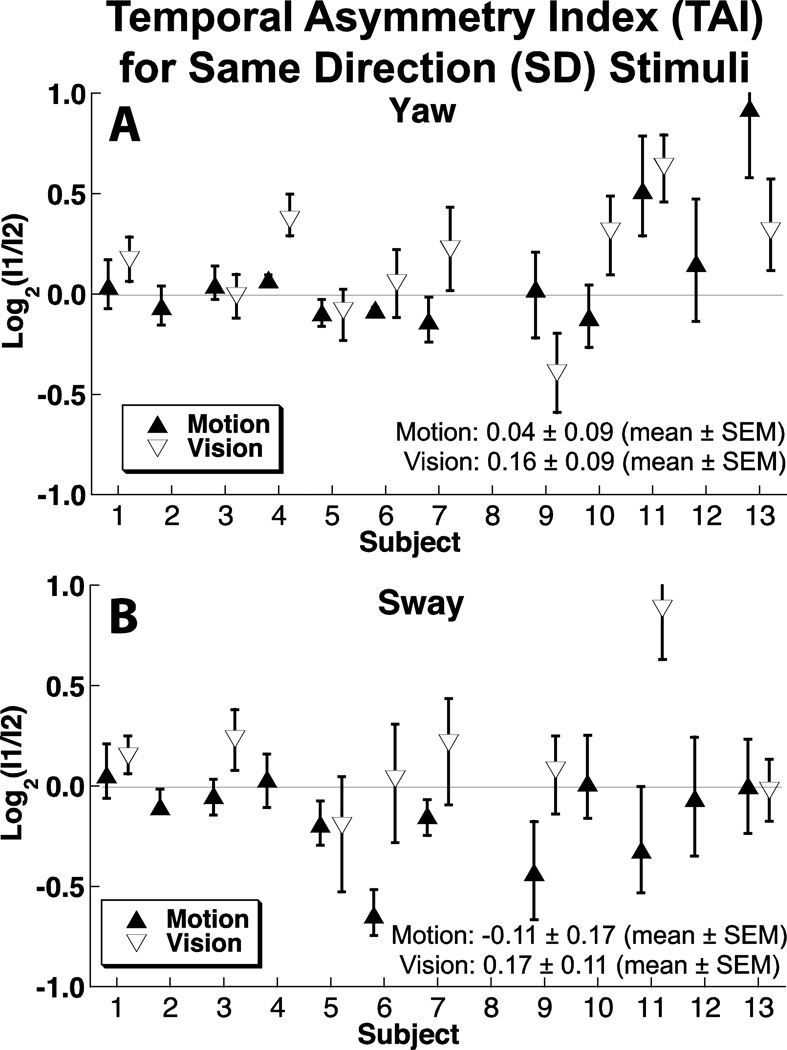
Order effects were significantly different from zero in 5 of 12 subjects when evaluating SD yaw motion stimuli (Fig. 6A). In two of these subjects (12 & 13), the effect was large enough that the first interval had to be at least 50% larger than the second for them to be perceived as equal. With a visual yaw stimulus, 6 out of 10 had a significant order effect.
Figure 6.
Order asymmetry for SD stimuli. Solid symbols represent motion in darkness, open symbols represent visual stimuli.
Similarly, for SD sway motion, 4 out of 12 subjects (Fig. 6B) demonstrated a significant difference in how the magnitude of stimuli were perceived based on their order. With visual sway stimuli, 3 out of 7 demonstrated a significant order asymmetry.
There was no correlation between the TAI rotation and sway motion (R = 0.23, p = 0.4) or rotation and sway vision (R = 0.38, p = 0.35) for SD motion. When visual and motion stimuli were correlated, there was no significant effect for rotation (R = 0.45, p = 0.19) or sway (R = 0.07, p > 0.5).
Age effects
There was no significant correlation between subject age and either TAI or DAI (both signed values, and the absolute value, p > 0.1 for all).
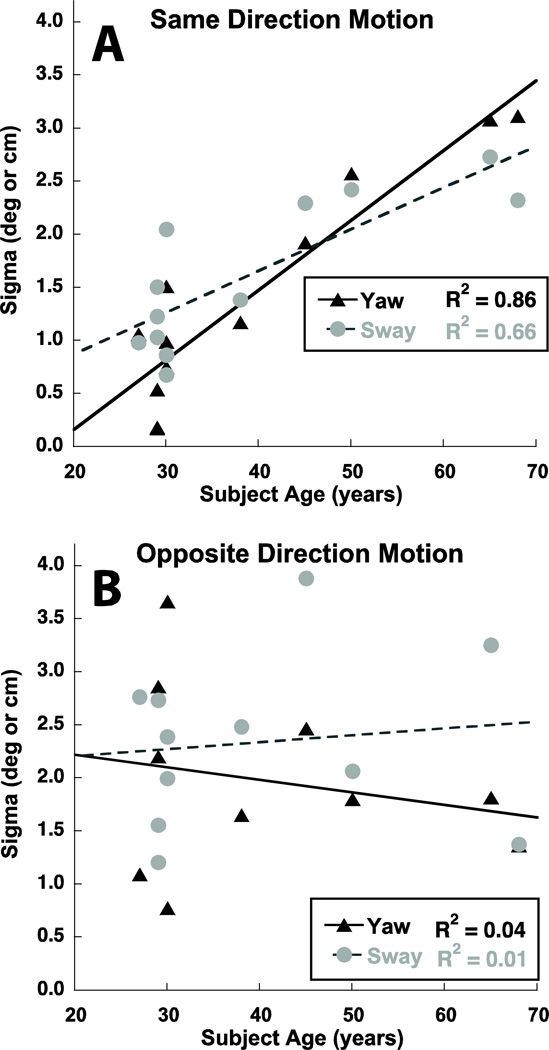
The precision of the response can be measured as the sigma or width of the psychometric function. The sigma was similar in both vision and motion trials (p > 0.1). The sigma in sway and yaw trials could not be directly compared due to differences in units (e.g. degrees vs. cm) even though the values were similar. There was a surprisingly strong correlation between age and sigma in SD motion stimuli for both sway and yaw (Fig. 7A, p < 0.001, two-tailed Spearman rank order coefficient). However, there was no correlation between sigma and age for visual stimuli (p > 0.1) or age and either sway or yaw OD motion stimuli (p > 0.1, Fig. 7B).
Figure 7.
Sigma was significantly correlated with subject age for both yaw and sway (p < 0.001).
Discussion
Studies of vestibular perception have historically focused on measuring perception at or near the threshold (Walsh 1961; Clark 1967; Gundry 1978; Melvill Jones and Young 1978; Benson and Brown 1989; Gianna et al. 1996; Grabherr et al. 2008; Mallery et al. 2010; Soyka et al. 2011) with studies of suprathreshold perception focusing on heading perception (MacNeilage et al. 2010) and cycles of sinusoids (Mallery et al. 2010). Previous studies have not investigated the possibility of directional asymmetry in suprathreshold vestibular perception. It has been demonstrated that vestibular perception near the threshold can be asymmetric in normal controls (Benson et al. 1986) and this has been recently demonstrated in the current laboratory (Roditi and Crane 2011). All but one of the subjects used in this study also participated in the prior study (Table 2). A recent review suggested that that these asymmetries may be caused by a bias (Merfeld 2011) although there is also evidence that they may be due to direction specific differences in sensitivity near the threshold (Roditi and Crane 2011). The current study attempts to extend knowledge of vestibular thresholds to stimuli that are well above the threshold of human perception. It was found that the magnitude of yaw and sway motion in opposite directions was perceived as equal, and when the differences were significant they were small.
There were no significant directional asymmetries in this study despite these same subjects often showing directional asymmetries near the threshold of perception as described in a prior study in this laboratory (Roditi and Crane 2012). Subject numbers are provided in Table 2. In the prior study, subject #1 had a significant and consistent asymmetry in sway thresholds, which was not evident here. Significant threshold asymmetries were also previously reported in subjects #4, 12, and 13. Yaw threshold asymmetry was less commonly present in subject #1. Thus, it seems asymmetries at the perceptual threshold do not translate into asymmetry in suprathreshold perception.
An unexpected result was that the order in which the stimuli were presented has a significant effect on their perceived magnitude in a large subset of subjects. In yaw motion, it was most common for the second interval to be perceived as larger than the first. Thus, the first interval was actually larger at the PSE. In sway, fewer subjects demonstrated the bias with a nearly equal number of subjects describing the first and second interval as appearing larger.
Using both MC and VA in otherwise similar conditions tested the possibility of the existence of the order effect being due to chance or the task itself. In 11 of the 12 conditions that were repeated using both methods, the results were similar (Fig. 5), indicating that the task did not lead to a difference in how these stimuli were perceived. Also, if asymmetries occurred by chance, we would also expect to see a similar rate of directional specific asymmetries and this was not observed.
The level at which this order asymmetry occurred was investigated using a visual only stimulus that was designed to be analogous to the motion stimulus. The results were similar to a motion only stimulus in that there was usually no directional asymmetry (the exception being subject #13 for a visual sway stimulus). The order asymmetry continued, but was not well correlated with the order asymmetry seen with motion. For example, in subject #1 there was a similar trend, but in subject #9 the effect reversed direction for a visual stimulus (Fig. 5). Thus, the order effects with motion stimuli were not directly duplicated with visual stimuli.
Order effects were not limited to comparisons of OD stimuli. With SD stimuli, about half the subjects demonstrated a significant order effect with both visual and motion stimuli, however, the effect was not well correlated between the visual and motion tests or between sway and yaw stimuli (Fig. 6).
One difference between OD and SD stimulus pairs was that the OD stimuli compared a variable interval to a constant interval while the SD stimuli compared two intervals of variable magnitude. This was necessary because the range of the motion platform was limited so that OD stimuli could potentially be of larger magnitude than SD stimuli. The largest differences (Fig. 6) were such that one interval was just less than twice as large as the other, which would correspond to PSEs at 10 and 20 cm. Most stimulus pairs were considerably smaller such that both intervals would be much closer to 15 cm, the constant interval used in the OD blocks. Due to the similar range of motion between the two, it is thought that this difference did not substantially influence the results.
One possible explanation for the order effects seen would be a perceptual after effect. The most extensively described of these is the visual motion after effect (MAE) or “waterfall illusion” initially described more than 175 years ago (Addams 1834). More recently, this has been found to influence other areas of visual perception (Kohn 2007; Thompson and Burr 2009). Similar perceptual after effects have been described with sound intensity (Reinhardt-Rutland 1998), voice (Bestelmeyer et al. 2010), proprioception (Seizova-Cajic et al. 2007) and even the vestibular system (Crane 2011). The visual component of this study was not designed to maximize visual MAE because the stimuli had an intentionally degraded coherence and the second stimulus was the same duration and coherence as the initial stimulus, which would tend to overpower any MAE. If a MAE were to occur in two similar visual stimuli in opposite directions, the second would appear larger, and this would cause the TAI to be positive. Although a slight positive was seen in sway (Fig. 5D) and for the visual SD stimuli (Fig. 6), the opposite effect was seen in yaw (Fig. 5C), implying that this was not a major effect. After effects in the vestibular system with translation and are more subtle than visual MAE (Crane 2011), and these results demonstrate only small and inconsistent effects (Figs. 5 & 6). The most consistent vestibular effect was seen with the TAI with OD yaw stimuli (Fig. 5A). A false perception of continued rotation after yaw rotation has been described as is thought to be related to velocity storage (Bertolini et al. 2011), although the initial stimulus here was likely too short for velocity storage to have been in issue. The effect may have been due to an after effect in yaw, but such a phenomenon has not yet been described with the relatively short stimuli used here. Such effects would be expected to show up as a non-zero TAI, but the mean TAI was near zero (Figs. 5 & 6) suggesting after effects likely did not have a significant influence. After effects may explain the positive TAI seen with yaw rotation in some subjects and in the average data (Fig. 5A).
It is possible that these temporal asymmetries may have been reduced by including a gap or inter-stimulus interval between the stimulus pairs. This could occur because the magnitude of the vestibular after effect (Crane 2011), as well as velocity storage effects (Bertolini et al. 2011) decay with time although some effect can still be present several seconds after the initial movement. Including a long inter-stimulus interval may have caused some recall bias as subjects may not be able to accurately remember the initial stimulus. Due to the large number of stimulus presentations in each block, adding a long inter-stimulus interval would add significant time to the experiment which would contribute to subject fatigue. In this study, potential vestibular aftereffects were instead controlled for by including stimulus presentations in which the initial stimulus could be in either direction so that any effect would cancel out when determining direction specific asymmetries. However the observed temporal asymmetries are likely to be a result of an aftereffect.
The standard deviation of the psychometric function was a measure of the precision of differentiating the two stimuli. It is known that function of the vestibular reflexes declines with age (Stefansson and Imoto 1986; Tian et al. 2001), and this has been also shown for fore-aft perception (Kingma 2005), as well as perception of motion in other directions (Roditi and Crane 2012). Based on these data it would be expected that the precision of responses might decline with age. However, this did not occur with visual stimuli or with motion stimuli in opposite directions (Fig. 7B). There was, conversely, a strong correlation between advancing age and decreased precision in differentiating same direction stimuli (Fig 7A). Although some of this may represent degeneration of the peripheral otolith organs, it cannot explain why the decline in precision with age is limited to comparing two stimuli in the same direction. It may be because comparison of opposite direction stimuli involves evaluation of vestibular signals that are less consistent than those from same direction. Thus, precision in comparison of opposite direction stimuli may be less dependent on end organ performance.
Conclusions
Perception of suprathreshold yaw and sway is directionally symmetric but, the perceived magnitude is often dependent on the order the stimuli are presented. Stimulus order effects are present in both visual and vestibular stimuli suggesting a higher order cognitive effect. With age, the accuracy in comparing two movements in the same direction declines while comparison of movements in opposite directions does not.
Acknowledgements
This study was funded by 1K23DC011298 from the NIDCD and a clinician-scientist award from the American Otological Society. We thank Justin Chan and Dan Stratz for providing technical support for this project.
Bibliography
- Addams R. An account of a peculiar optical phaenomenon seen after having looked at a moving body etc. Magaz. J Sci. 3rd series. 1834;5:373–374. [Google Scholar]
- Bárány R. Diagnose von Krankheitserscheinungen im Bereiche des Otolithenapparates. Acta Otolaryngol. 1921:434–437. [Google Scholar]
- Benson AJ, Brown SF. Visual display lowers detection threshold of angular, but not linear, whole-body motion stimuli. Aviat Space Environ Med. 1989;60:629–633. [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57:1088–1096. [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105:209–223. doi: 10.1152/jn.00154.2010. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PE, Rouger J, DeBruine LM, Belin P. Auditory adaptation in vocal affect perception. Cognition. 2010;117:217–223. doi: 10.1016/j.cognition.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Clark B. Thresholds for the perception of angular acceleration in man. Aerosp Med. 1967;38:443–450. [PubMed] [Google Scholar]
- Crane BT. Society for Neuroscience. Washington, DC: 2011. Vestibular motion after effects in humans; p. 580.516. [Google Scholar]
- Crane BT, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of acceleration, target distance, and unilateral deafferentation. J Neurophysiol. 1998;80:1151–1166. doi: 10.1152/jn.1998.80.3.1151. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, Deangelis GC, Angelaki DE. Dynamic re-weighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29:15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianna C, Heimbrand S, Gresty M. Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull. 1996;40:443–447. doi: 10.1016/0361-9230(96)00140-2. discussion 448–449. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Gundry AJ. Thresholds of perception for periodic linear motion. Aviat Space Environ Med. 1978;49:679–686. [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Kanayama R, Bronstein AM, Gresty MA, Brookes GB, Faldon ME, Nakamura T. Perceptual studies in patients with vestibular neurectomy. Acta Otolaryngol Suppl. 1995;520(Pt 2):408–411. doi: 10.3109/00016489509125284. [DOI] [PubMed] [Google Scholar]
- Kingma H. Thresholds for perception of direction of linear acceleration as a possible evaluation of the otolith function. BMC Ear Nose Throat Disord. 2005;5:5. doi: 10.1186/1472-6815-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Levine SC, Glasscock M, McKennan KX. Long-term results of labyrinthectomy. The Laryngoscope. 1990;100:125–127. doi: 10.1288/00005537-199002000-00003. [DOI] [PubMed] [Google Scholar]
- MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30:9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. 10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery RM, Olomu OU, Uchanski RM, Militchin VA, Hullar TE. Human discrimination of rotational velocities. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2010;204:11–20. doi: 10.1007/s00221-010-2288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvill Jones GM, Young LR. Subjective detection of vertical acceleration: a velocity-dependent response? Acta Otolaryngol. 1978;85:45–53. doi: 10.3109/00016487809121422. [DOI] [PubMed] [Google Scholar]
- Merfeld DM. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011 doi: 10.1007/s00221-011-2557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol. 2005a;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt&Translation. J Neurophysiol. 2005b;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Perez N, Martin E, Garcia-Tapia R. Dizziness: relating the severity of vertigo to the degree of handicap by measuring vestibular impairment. Otolaryngol Head Neck Surg. 2003;128:372–381. doi: 10.1067/mhn.2003.102. [DOI] [PubMed] [Google Scholar]
- Reinhardt-Rutland AH. Increasing-loudness aftereffect following decreasingintensity adaptation: spectral dependence in interotic and monotic testing. Perception. 1998;27:473–482. doi: 10.1068/p270473. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Asymmetries in human vestibular perception thresholds. Association for Research in Otolarngology; 34th Annual Meeting; Baltimore, MD. 2011. p. 1006. [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol In press. 2012 doi: 10.1007/s10162-012-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PloS one. 2007;2:e1037. doi: 10.1371/journal.pone.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011;209:95–107. doi: 10.1007/s00221-010-2523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson S, Imoto T. Age-related changes in optokinetic and rotational tests. Am J Otol. 1986;7:193–196. [PubMed] [Google Scholar]
- Strupp M, Brandt T. Vestibular neuritis. Seminars in neurology. 2009;29:509–519. doi: 10.1055/s-0029-1241040. [DOI] [PubMed] [Google Scholar]
- Thompson P, Burr D. Visual aftereffects. Current biology : CB. 2009;19:R11–R14. doi: 10.1016/j.cub.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Tian JR, Shubayev I, Baloh RW, Demer JL. Impairments in the initial horizontal vestibulo-ocular reflex of older humans. Exp Brain Res. 2001;137:309–322. doi: 10.1007/s002210000671. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol. 1961;155:506–513. doi: 10.1113/jphysiol.1961.sp006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–1688. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001a;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]