Abstract
The NADPH oxidase (Nox) enzymes are critical mediators of cardiovascular physiology and pathophysiology. These proteins are expressed in virtually all cardiovascular cells, and regulate such diverse functions as differentiation, proliferation, apoptosis, senescence, inflammatory responses and oxygen sensing. They target a number of important signaling molecules, including kinases, phosphatases, transcription factors, ion channels and proteins that regulate the cytoskeleton. Nox enzymes have been implicated in many different cardiovascular pathologies: atherosclerosis, hypertension, cardiac hypertrophy and remodeling, angiogenesis and collateral formation, stroke and heart failure. In this review, we discuss in detail the biochemistry of Nox enzymes expressed in the cardiovascular system (Nox1, 2, 4 and 5), their roles in cardiovascular cell biology, and their contributions to disease development.
Keywords: NADPH oxidases, vascular smooth muscle, endothelial cells, cardiomyocytes, atherosclerosis, hypertension, cardiac hypertrophy
INTRODUCTION
In the dozen years since the discovery that the neutrophil respiratory burst NADPH oxidase was only one member of a family of homologous enzymes, tremendous progress has been made in understanding the role of these proteins in the cardiovascular system. The NADPH oxidase (Nox) family is comprised of 7 catalytic subunits termed Nox1-5 and Duox1 and Duox2 (for Dual Oxidase), regulatory subunits p22phox, p47phox or Noxo1, p67phox or Noxa1, p40phox and the major binding partner Rac. Of these, the Nox1, 2, 4 and 5 enzymes are expressed in cardiovascular tissues and not only participate in normal vascular and cardiac function, but also contribute to the development of cardiovascular disease.
Nox enzymes are heteroprotein complexes (except Nox5) with very specific regulatory mechanisms, tissue and subcellular patterns of expression, downstream targets and functions. They are found in endothelial cells (ECs), vascular smooth muscle cells (VSMCs), macrophages, adventitial fibroblasts, cardiac myocytes and fibroblasts, as well as adipocytes and stem cells. These proteins work independently or in concert to control not only cell survival, growth and death, but also cell-specific functions such as differentiation, angiogenesis and contraction. When acutely or chronically upregulated, they have been implicated in hypertension, atherosclerosis, heart failure, ischemia reperfusion injury and cardiac remodeling, but upregulation can be physiologically advantageous, as in angiogenesis and collateral formation. The molecular mechanisms that differentially control the expression, activation and cellular pathways linked to Nox enzymes, as well as the physiological and pathophysiological contexts in which they exert their effects, are the focus of this review.
BIOCHEMISTRY
Nox2
Historically the first discovered member of the NADPH oxidase family,1 also known as gp91phox, Nox2 is the catalytic subunit of an enzymatic complex responsible for the phagocyte respiratory burst. Upon activation, Nox2 uses NADPH to reduce molecular oxygen to superoxide anion, which, in concert with its metabolites, is used by phagocytes to destroy invading microorganisms. Nox2 is also found throughout the cardiovascular system (Table 1), where its expression and activity are much lower than in phagocytes,2-4 consistent with signaling functions described in a following section. However, in pathological circumstances, excess Nox2 can lead to oxidative stress and disease development (see Physiology and Pathophysiology in vivo). Here, we will first summarize salient features of Nox2, mostly derived from studies conducted in phagocytes, that apply to the cardiovascular enzyme. This enzyme will later serve as a reference against which to compare other NADPH oxidases.
Table 1.
Selected examples of characterization and interventions affecting Nox subunits
| Cell/tissue distribution | Upregulators | Transcription factors | Subcellular localization | Genetic models | Inhibitors | |
|---|---|---|---|---|---|---|
| Nox1 | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiomyocytes361 |
Ang II PDGF PGF2α, LDL4 TNF-α196 Oscillatory shear stress BMP4362 Aldosterone+salt363 IFN-γ364 ET-1365 T3177 Urokinase178 Oxidized LDL180 Vascular injury4 |
AP-1366 ATF-1367 MEF2B368 NFκB369 STAT1/3364 |
Plasma membrane Caveolae105 Endosomes106 Perinuclear74 |
Overexpressor289 Knockout170, 287 |
Apocynin DPI GK-136901 ML171 Nox2dstat370 |
| Nox2 | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiomyocytes371 |
Ang II ET-1 TGF-β IFN-γ Oxidized LDL4 Oscillatory shear stress232,362 Aldosterone +salt372 Ischemia373 Vascular injury4 |
Plasma membrane374 Perinuclear2, 74, 375 Nuclear pore75 |
Knockout376 |
Apocynin DPI Nox2dstat VAS2870370 |
|
| Nox4 | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiac fibroblasts236 Cardiomyocytes357 Heart374 |
TGF-β235, 236, 255 Thromboxane A2 TNF-α145, 377 IFN-γ364 Urotensin116 Urokinase178 Oscillatory shear stress232 Hypoxia238 Hyperoxia217 Vascular injury4 |
E2F378 NFκB369 STAT1/3364 HIF-1α379 Nrf2380, 381 Oct-1381 |
ER259, 374 Perinuclear74 Nucleus105, 129, 217, 374 Mitochondria139, 357, 374 Focaladhesions105, 229 Stress fibers229 Cytoplasm382 |
Overexpressor139, 337 Knockout337, 347, 357, 383 |
DPI GK-136901 Plumbag in VAS2870370 |
| Nox5 | EC153, 377 VSMC146, 154 Cardiac FB236 |
Ang II ET-1153 Thromboxane A2 TNF-α377 Atherosclerosis329 |
Plasma membrane Intracellular155, 162 |
DPI370 | ||
| p22phox | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiomyocytes371 |
Ang II4, 384 Thrombin385 PDGF TGF-β4 Urotensin116 TNF-α4, 145, 384 IFN-γ386 LPS387 Pulsatile shear stress4 Stretch + Ang II388 Aldosterone + salt372 Vascular injury4 |
AP-1384 STAT1/3364 NFκB389 |
Plasma membrane Caveolae Focal adhesions105 Perinuclear2, 375 Nucleus105,217 Nuclear pore75 |
Overexpressor299 Point mutation121,167 |
|
| p47phox | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiomyocytes371 |
Ang II4, 384 Thrombin4 TNF-α145, 384 Aldosterone + salt372 |
HBP167 Ets-1390 STAT1/3364 |
Perinuclear375 Nuclear pore75 Actin fibers32 |
Spontaneous null35 Knockout36, 37 |
Apocynin Nox2dstat370 |
| p67phox | EC VSMC Adventitial fibroblasts Whole vessels4 Cardiomyocytes371 |
Ang II4, 384 TNF-α145, 384 |
STAT1/3364 | Perinuclear375 Actin fibers32 |
Spontaneous mutant (requires confirmation)57 | |
| p40phox | EC VSMC Whole vessels4, 67 |
Ang II25 | HBP167 | Point mutation Knockout65 |
||
| Noxo1 | EC86 Whole vessels85 |
Spontaneousnull91 | ||||
| Noxal | EC86, 145, 391 VSMC83, 84 Whole vessels85 |
Atherosclerosis83 | Knockout97 | |||
The structure of Nox2 is not known in complete detail, but current models are based on homology to proteins of known architecture, mapped epitopes of monoclonal antibodies and mutations from X-linked chronic granulomatous disease (CGD) patients or site-directed mutagenesis.5 Briefly, Nox2 is composed of two main domains of equal sizes with very different properties. The amino-terminal moiety includes six transmembrane α-helices (numbered I-VI) connected by five loops (numbered A-E, Figure 1). Because both amino-and carboxy-termini are cytosolic, three loops are extracellular and include consensus asparagine glycosylation sites, while the other two are intracellular and accessible to cytosolic regulators. The degree of Nox2 glycosylation and apparent molecular mass differ between species. In western blots of human neutrophil proteins, Nox2 is detected as a smear from about 65 to 91 kDa. Transmembrane helices III and V each include two conserved histidine residues that coordinate iron atoms at the center of two heme molecules.6 The cytosolic carboxy-terminal moiety of Nox2 constitutes a dehydrogenase domain that includes consensus binding sites for its NADPH substrate and FAD cofactor. Upon activation, two electrons are transferred from cytosolic NADPH to FAD and in succession across the membrane, via redox changes in heme irons. Finally, each electron reduces a molecule of oxygen to a superoxide radical, which is subsequently released outside the cell or in a topologically equivalent compartment, such as a vesicle lumen. Superoxide is the first reactive oxygen species (ROS) in a cascade of metabolites including hydrogen peroxide and peroxynitrite.7 A charge compensation mechanism, required to balance electron transport by Nox2 and sustain its activity, is provided by a voltage-gated proton channel8 and the chloride/proton antiporter ClC-3.9
Figure 1. Structure and activation of Nox enzymes.
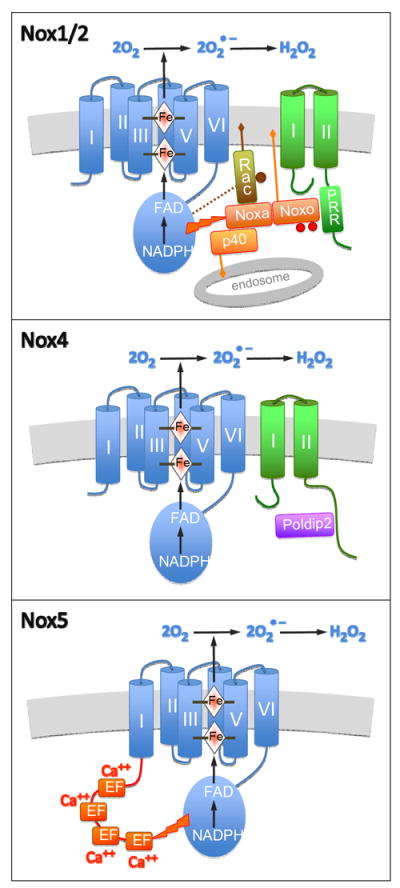
Catalytic Nox subunits, represented in blue, include an N-terminal domain composed of six transmembrane helices, numbered I-VI. Four histidine residues in helices III and V coordinate two heme iron atoms. A cytosolic C-terminal dehydrogenase domain includes an FAD cofactor and an NADPH substrate binding site. Upon activation, electrons are transferred from NADPH to FAD and across the membrane, via heme irons, to molecular oxygen, thus producing superoxide anion, which can be dismutated into hydrogen peroxide.
Upper panel. Both Nox1 and Nox2 (blue) form a complex with p22phox (green), with its two transmembrane domains and C-terminal proline-rich region (PRR). A cytosolic complex (orange), is composed of an organizer (Noxo1 or p47phox), an activator (Noxa1 or p67phox) and p40phox (only with p67phox). The organizer, stimulated by phosphorylation (red dots) in the case of p47phox, binds the proline-rich region of p22phox and membrane lipids. Likewise, p40phox binds lipids in endosomal membranes. Rac, activated by GTP (brown dot), binds membrane, Nox and Noxa. The latter subunit triggers FAD reduction.
Middle panel. Nox4 (blue) also forms a complex with p22phox (green). Its activity, constitutive in the absence of cytosolic subunits, can be increased by binding of Poldip2 to the cytosolic C-terminal of p22phox.
Lower panel. While Nox5S is composed of a catalytic subunit similar to the other oxidases (blue), Nox5L includes an additional N-terminal segment (red) with four EF-hands. Binding of cytosolic calcium to the EF hands triggers Nox5L activation.
Tight regulation, critical to avoid excessive production of deleterious superoxide, is evident from the large number of proteins involved in oxidase assembly (Figure 1). These include Nox2 itself, p22phox, p47phox, p67phox and p40phox; all essential subunits whose mutations can cause CGD.10, 11 Also crucial is Rac GTPase, which binds to the dehydrogenase domain of Nox2.12 In the resting state, Nox2 and p22phox form an inactive membrane complex known as cytochrome b558,1, 5 while the other dormant subunits form a trimer in the cytosol with p67phox linking p47phox and p40phox.13 Signaling cascades activate Rac, p47phox and p40phox, allowing translocation of cytosolic subunits to the membrane and association with the cytochrome to form an active enzyme.1, 5 Phosphorylation of Nox2 by protein kinase C (PKC) also promotes activity and recruitment of cytosolic subunits.14 We will now summarize essential features of these regulatory subunits.
Numerous structural and functional studies revealed that p47phox is composed of an N-terminal PX domain, two central SH3 domains, an autoinhibitory region and a C-terminal proline-rich region that binds p67phox.5 At rest, intramolecular folding masks the PX domain and the SH3 tandem, which binds to the autoinhibitory region.15 In leukocytes and transfected cells, unfolding of p47phox is triggered by PKC phosphorylation of serine residues, including 303, 304 and 328 in the autoinhibitory region.16-20 Other kinases that can phosphorylate p47phox on serine and tyrosine may contribute to oxidase activation.21-23 Similar phosphorylation events occur in vascular cells24-26 as well as tyrosine phosphorylation by Src.27, 28 Once exposed, the PX domain allows translocation of p47phox to the membrane by binding to phosphoinositide 3-kinase (PI3K) lipid products20, 29 and actin fibers,30-32 while the SH3 domains interact with the cytosolic C-terminal proline-rich region of p22phox.33, 34 Thus, PKC, PI3K, and Src can cause oxidase assembly by recruiting p47phox bound to p67phox and p40phox. Because p47phox is an essential component of the oxidase in vivo, many pathophysiological studies have taken advantage of a spontaneous null mutant,35 as well as two independent knockout mouse lines.36,37 These models are useful, but it should be remembered that p47phox can also be part of the Nox1-based oxidase (see below).
Functionally, p67phox is indispensible for Nox2 activation, whereas p47phox can be omitted from cell-free assays.38, 39 Results from many studies indicate that p67phox is composed of an N-terminal region including four tetratricopeptide repeat (TPR) motifs, followed by an activation domain, an SH3 domain, a PB1 domain and finally a second SH3 domain at the C-terminus. The TPR motifs, important for Rac-GTP binding, are defective in many CGD mutants.10, 40-42 Interestingly, in the cell-free system, a fragment of p67phox, including the N-terminus and the activation domain, fused with Rac1, is highly active presumably because it is targeted to the membrane.43 Most importantly, the activation domain of p67phox triggers FAD reduction by Nox2.39 The V204A mutant in this region is a competitive inhibitor of wild-type p67phox.44 The first SH3 domain increases oxidase activity, but its target is not known.45 The PB1 domain allows binding to p40phox, which is abolished by a K355A mutation.46, 47 Finally, the C-terminal SH3 domain of p67phox is responsible for binding the proline-rich region of p47phox and therefore allows p67phox translocation to the membrane after activation.48-50 An additional degree of regulation is provided by phosphorylation, which is both constitutive and stimulated by agonists.51-53 Phosphorylation of p67phox, mediated by PKCδ, ERK2 and p38MAPK, increases superoxide production by Nox2.54-56 Physiological studies still await the creation of a p67phox knockout model. Although a line of mice with a mutation in the PB1 domain of p67phox and increased susceptibility to infections has been reported, additional work is required to verify that this mutation is the only cause of the phenotype.57
Structural and functional studies indicate that p40phox is composed of an N-terminal PX domain,40 a central SH3 domain and a C-terminal PB1 domain. While masked by interaction with the PB1 domain at rest,58 the PX domain sustains oxidase activation after stimulation by interaction with lipid products of PI3K.59-61 Because p40phox binds inositol headgroups with lower phosphate content than p47phox,59 it preferentially interacts with endosomes. Thus, p40phox appears to increase oxidase activity in cooperation with p47phox47, 62, 63 not by inducing translocation to the membrane, but by retaining the oxidase at the phagosome.15, 61, 64 Depletion or mutation of p40phox impairs ROS production in neutrophils65, 66 and ECs.67 While the PX domain is clearly essential to p40phox function, the target of the SH3 domain is unknown and its function is controversial.68, 69 The C-terminal PB1 domain of p40phox interacts with the PB1 domain of p67phox, as shown using multiple approaches.13, 47 In addition, phosphorylation of p40phox on serine and threonine by PKC is increased during stimulation,67, 70, 71 particularly at threonine 154, which is usually,69 but not always,72 required for full oxidase activation. The significance of p40phox is emphasized by a single case of CGD known to date. In this patient a point mutation in the PX domain does not prevent extracellular superoxide production, but impairs oxidase activation following phagocytosis.73
Because Nox2 produces superoxide, a highly reactive and short-lived ROS, its subcellular localization is important for function. Superoxide can be released outside the cell when Nox2 is located at the plasma membrane, thus allowing it, for example, to intercept endothelial-derived nitric oxide (NO) before it reaches the adjacent smooth muscle cell layer in vessels.7 However, Nox2 is also frequently found in intracellular compartments, around the nucleus and colocalized with the endoplasmic reticulum (ER) in vascular cells (Table 1).2, 74 In ischemic cardiomyocytes, Nox2 is upregulated in the cytosol and targeted to the nuclear pore complex.75 Production inside cells is consistent with a direct signaling role of superoxide or its metabolites, as will be seen later.
Nox1
Nox1 is most highly expressed in colon epithelium, where it is thought to play a role in host defense.76 It is also found in many other tissues and in all layers of the vascular wall, particularly after induction by agonists and in pathological conditions (Table 1). Nox1, like Nox2, associates with p22phox to form a membrane-bound cytochrome.77-79 However, in colon the cytosolic subunits p47phox and p67phox are not expressed and are replaced by Noxo1 and Noxa1.80-82 We now understand that Nox organizers include both p47phox and its homologue, Noxo1, while Nox activators comprise p67phox and the structurally similar Noxa1. Because Nox2 and Nox1 are closely related, both enzymes can be activated in transfected cells by various organizer and activator pairs.80 Examples are also known in vivo, as in VSMC from mouse conduit arteries, where p47phox and Noxa1 activate Nox1.83, 84 To understand the significance of these substitutions, a brief description of Noxo1 and Noxa1 is required.
Similar to p47phox, Noxo1 facilitates oxidase assembly by binding both an activator subunit and p22phox. The proline-rich region of Noxo1 binds to an SH3 domain of the activator, while the tandem SH3 domains of Noxo1 bind to the proline-rich region of p22phox.80, 87, 88 Noxo1 also binds to the dehydrogenase domain of Nox1.89 Furthermore, the PX domain of Noxo1 provides an essential affinity for membrane phosphoinositides.90 However, unlike p47phox, because Noxo1 lacks an autoinhibitory domain, it is thought to constitutively bind the cytochrome.80, 81, 90 Surprisingly, in spite of coexpression with Nox1 in colon and some vascular cells (Table 1), mice lacking Noxo1 apparently only present a deficiency in Nox3 activity during development of the inner ear,91 perhaps because other organizers may compensate for its loss in other tissues. Besides p47phox, other possible organizers include Tks4 and Tks5, two Src substrates with a PX domain and multiple SH3 domains capable of binding p22phox and Noxa1, but not p67phox.92
Several important features of p67phox are conserved in Noxa1, allowing it to serve as an activator subunit. These include four Rac-binding TPR motifs, a Nox activation domain and an SH3 domain that interacts with the proline-rich region of an organizer subunit. However, the p40phox-binding PB1 domain is not well conserved and the SH3 domain in the middle of the molecule is missing.80, 81 In contrast to its Noxo1 partner, Noxa1 activity appears to be tightly regulated. Phosphorylation of serine 461 by protein kinase A (PKA), serine 282 by ERK1/2 or p38 MAPK, or serine 172 by PKA or PKC, decrease ROS production by Nox1.93-95 Phosphorylation of Noxa1 by PKA favors binding to 14-3-3 and dissociation from Nox1,93 while other kinases appear to decrease Noxa1 affinity for Rac1 and Nox1.95 In contrast, phosphorylation of Noxa1 by Src on tyrosine 110 increases Nox1 activity.96 These regulatory mechanisms may be important in vessels that express Noxa1 (Table 1). The recent creation of a knockout model will likely allow future detailed in vivo studies of Noxa1 function.97
In addition to cytosolic organizers and activators, Nox1 also requires Rac1 for activity, as might be expected from its resemblance to Nox2. Nox1 is stimulated by constitutively active Rac1 and inhibited by Rac1 knockdown.87, 98 In addition to binding an activator subunit, as mentioned above, Rac1 also has affinity for the plasma membrane due to prenylation99 and interacts directly with the carboxy terminus of Nox1, even in the absence of Noxa1.12, 98, 100 Another related small monomeric GTPase, Cdc42 cannot activate Nox1,98 but could be a competitive inhibitor, as in the case of Nox2.101 Interestingly, βPix, a Rac1 guanine nucleotide exchange factor, appears to be constitutively bound to Nox1 and essential for its activity.89, 100 These results suggest that Rac1 provides a crucial mechanism for activation by agonists, particularly in cells that exclusively express Nox1/Noxo1/Noxa1, as colon epithelial HT29 cells.102
Although the structure of Nox1 is not known, it is expected to be very close to other Nox enzymes (Figure 1),1, 5 with a dehydrogenase domain binding FAD and NADPH, as well as four conserved histidine residues in transmembrane helices III and V, thought to coordinate two heme molecules. Similar to Nox2, mutation of these histidines abolishes binding to p22phox.78 Family resemblance is further substantiated by the creation of a functional chimera comprised of the transmembrane domain of Nox1 and the cytosolic moiety of Nox4 (abbreviated as Nox1-Nox4 below).103, 104 However, the signal peptide at the N-terminus of Nox1 is important for localization at the plasma membrane, as it is prevented by replacement with the N-terminus of Nox4.103 In VSMCs, Nox1 can be found at the plasma membrane, in caveolae105 and in endosomes (Table 1).106 Interestingly, agonists appear to stimulate Nox1 in specific locations, thus determining where superoxide is produced: extracellularly by muscarinic agonists and thrombin; in endosomes by IL-1β and TNFα; both inside and outside cells by Ang II.106-108 Of importance, Nox1 stimulation in endosomes is dependent on ClC-3, where this ion exchanger is required to balance the electrogenic activity of Nox1.106, 109 Interestingly, Nox1 activity is also dependent on chaperones Hsp90104 and PDI,110, 111 which appear to be necessary not only for protein folding after synthesis, but also to maintain enzyme stability.
The primary biochemical function of vascular Nox1 is superoxide production, which is then rapidly converted to hydrogen peroxide. The moderate physiological activity of Nox1, compared to the phagocytic Nox2, can be attributed to its low expression as well as specific regulatory subunits and signaling cascades. However, in pathological conditions, upregulation of Nox1 can lead to oxidative stress in the cardiovascular system.
Nox4
Abundant in kidney cortex where it was originally discovered,112, 113 Nox4 appears to be expressed almost ubiquitously, including throughout the cardiovascular system and in macrophages important in atherosclerosis.114 Nox4 is usually coexpressed with other homologues such as Nox1 and Nox2, but at significantly higher levels. Upregulation by prolonged exposure to agonists such as TGF-β appears to be a major route of Nox4 regulation (Table 1).
Similar to Nox1 and Nox2, coprecipitation and colocalization studies showed that Nox4 binds to p22phox (Figure 1).74, 78, 115 Furthermore, overexpression and siRNA experiments indicate that association with p22phox is required for Nox4 activity and that the two proteins stabilize each other.78, 79, 115-117 As in Nox1 and Nox2, mutation of heme-binding histidine 115 also abolishes Nox4 interaction with p22phox.78 In contrast, transfection of organizer and activator subunits does not increase ROS production, suggesting that Nox4 activity is constitutive.78, 79, 115 This interpretation is supported by other observations in transfected cells: addition of cytosol to membrane fractions does not increase Nox4 activity;118 mutation of the proline-rich domain of p22phox required for docking organizers does not affect Nox4 activity;79 Nox1-Nox4 and Nox2-Nox4 chimeras are active without transfection of cytosolic subunits,103, 104, 119 while the opposite Nox4-Nox2 chimera requires activation.117, 119 However, ROS production is enhanced by the multifunctional Poldip2, which also interacts with p22phox,120 presumably at the beginning of the cytosolic C-terminus, upstream of the region dispensable for Nox4 activity.121 Unlike Nox1 and Nox2, Rac1 does not activate Nox4 in transfected cells,115, 122 in spite of evidence compatible with an indirect effect.123-126 A number of reports indicate that agonists can acutely increase Nox4 activity or signaling,122, 123, 127-134 but only a few instances of possible coupling mechanisms are known: very rapid protein upregulation;134, 135 control of NADPH availability;136, 137 or direct interaction of the TLR4 receptor with the oxidase.127, 131, 138 These results suggest that Nox4 can respond to agonist stimulation in addition to its widely accepted role as a constitutively active enzyme mostly regulated by transcription.
In the absence of a complete model, structural information about Nox4 can be inferred from comparison of its primary sequence to other oxidases. Similar to Nox1 and Nox2, the dehydrogenase domain of Nox4 requires FAD and NADPH.119 A P437H mutation in the canonical NADPH binding motif, analogous to the Nox2 mutation of a CGD patient, abolishes activity,117, 139 while deletion of the NADPH binding domain produces a dominant-negative Nox4.128, 140 Similar to Nox2, Nox4 is inhibited by an R96E mutation in the cytosolic B loop, a region of the amino-terminal domain that interacts with the NADPH binding site.117, 141 In spite of their sequence homology, Nox4 can produce a higher hydrogen peroxide to superoxide ratio than Nox1 and Nox2.104, 115, 117, 118, 142 However, the structural determinants allowing hydrogen peroxide production by Nox4 are not well understood. The Nox4 carboxy-terminal dehydrogenase domain would seem responsible, considering that Nox1-Nox4 or Nox2-Nox4 chimeras produce only peroxide in some studies.104, 119 But in other reports the Nox1-Nox4 chimera generates superoxide,103 and Nox4-Nox2 peroxide.117, 119 In addition, replacing the first transmembrane domain of Nox4 by that of Nox1,103 or altering the last extracellular loop of Nox4, makes it produce superoxide, rather than peroxide.143 Additional studies will be required to resolve these discrepancies and determine if Nox4 can catalyze superoxide dismutation (presumably via the extracellular region), or even directly produce hydrogen peroxide (possibly at the C-terminus).
Besides the type of ROS produced by Nox4 and equally important for signaling is the subcellular location in which they are released. The distribution of Nox4 is surprisingly wide, including endoplasmic reticulum, plasma membrane, nucleus and mitochondria (Table 1), perhaps because its location varies according to cell type. It is also possible that each antibody preferentially labels the enzyme in a specific compartment.144 Alternatively, different Nox4 isoforms may be present in specific subcellular locations. Five splice variants, named Nox4A through E, are found in lung epithelial cells. The most intriguing isoform, also reported in ECs,145 is Nox4D because it appears to be fully active in a DCFH-DA assay, although it lacks most of the transmembrane domain.140 One could speculate, by analogy with dehydrogenase domain assays,119 that Nox4D retains activity by coupling to electron acceptors, such as cytochrome c in mitochondria, which might also be an alternative route of hydrogen peroxide formation by full-length Nox4. Regardless of its activity, because Nox4D is expected to be hydrophilic, it could be present in compartments devoid of membranes. However, the prevalence and functional significance of Nox4 isoforms remain to be explored.
Nox5
Compared to that of its homologues described above, Nox5 expression is restricted to fewer tissues. Although found in human VSMCs, ECs and whole vessels (Table 1), Nox5 is unexpectedly absent in rodents.5 The most striking structural difference with other Nox enzymes is the presence of an additional cytosolic N-terminal segment, containing four calcium binding EF-hands (Figure 1).146, 147 However, this segment is missing in Nox5S, a short calcium-insensitive variant, which is the dominant isoform in carcinoma cells,148 and expressed together with the long Nox5L in ECs.149, 150 Nox5S may be constitutively active150 or could be a competitive inhibitor of calcium-dependent activation when present in the same tetrameric complex as Nox5L.151 Except for the N-terminus of the long isoform, Nox5 is similar to other Nox enzymes, with six transmembrane helices expected to bind two hemes and a cytosolic dehydrogenase domain including FAD and NADPH binding sites.146 However, neither Nox5 isoform appears to require cytosolic subunits or p22phox. Thus, Nox5 is active in a cell-free system without addition of cytosol147 and is unaffected by expression or knockdown p22phox in transfected cells.79, 150 Furthermore, Rac1 may participate in Nox5 activation in some systems,152 but not others.153 Upon stimulation with agonists, such as PDGF, Ang II or endothelin-1 in VSMCs or ECs,153, 154 or after addition of a calcium ionophore to transfected cells, an increase in cytosolic calcium concentration triggers high superoxide production by Nox5.146, 155 Calcium induces binding of the N-terminal domain of Nox5 to the dehydrogenease domain, thus relieving autoinhibition.147, 156 Moreover, two mechanisms may increase Nox5 sensitivity, allowing activation by resting resting calcium concentrations: 1) calcium-dependent binding of calmodulin to another site in the dehydrogenase domain,157 and 2) phosphorylation of serine and threonine residues by PKC and calcium/calmodulin-dependent kinase II.155, 158, 159 In addition, as Nox5 can be upregulated and activated by minute concentrations of hydrogen peroxide, ROS production may be sustained by a positive feed-back loop involving the tyrosine kinase c-Abl.160, 161 Finally, binding of heat shock protein 90 to the C-terminus of Nox5 appears to stabilize the protein and enhance expression and activity.104 Because Nox5 is found in intracellular compartments and at the plasma membrane, superoxide is expected to be produced both inside118, 146 and outside155 cells. Furthermore, a polybasic region in Nox5 with affinity for phosphoinositides may favor translocation to the plasma membrane and extracellular superoxide release.162
p22phox
The foregoing sections highlight the catalytic subunits of NADPH oxidases and their cytosolic regulators. Because p22phox is an integral part of the Nox1, 2 and 4 complexes (as well as Nox3, which is not a vascular oxidase), it is worth a separate discussion. Among all NADPH oxidase subunits, p22phox is the most ubiquitous. It is highly expressed throughout the cardiovascular system, including heart and vessels and further upregulated by many stimuli (Table 1). Although the complete crystal structure of p22phox is not known, it is clearly an integral membrane protein and colocalizes with Nox proteins in numerous cell types and subcellular compartments. Current molecular models of p22phox indicate that two transmembrane helices are located in the middle of the molecule, leaving both N and C termini in the cytosol (Figure 1).163, 164 The suggestion that histidine 94 in the first transmembrane region might coordinate a heme molecule in conjunction with a Nox subunit appears unlikely because this residue can be replaced without affecting the cytochrome.165 How p22phox interacts with Nox catalytic subunits is still elusive. Maturation of Nox requires the presence of p22phox,121, 164, 166 and conversely, p22phox stability is increased by the presence of a Nox subunit.78 Interestingly, a point mutation in p22phox at tyrosine 121, abolishes interaction with Nox2, but does not affect Nox4.117, 121, 167 Many studies have demonstrated that the proline-rich region in the C-terminal tail of p22phox is a docking site for organizer subunits80, 87, 88 and therefore required for activity of Nox179 and Nox2,164 but not Nox4.121 Point mutations at residues 152, 156 or 158 in this region of p22phox abolish cytosolic subunit translocation and activity. Interaction of p22phox with p47phox also appears to require phosphorylation on threonine 147 in the cytosolic tail.168 Thus, p22phox is not only required for stability of the Nox complex, but also serves to regulate interaction with cytosolic regulators.
CELL PHYSIOLOGY AND PATHOPHYSIOLOGY
At present, evidence suggests that under physiological conditions Nox proteins and their products superoxide and hydrogen peroxide participate in cardiovascular homeostasis by contributing to cell differentiation, repair of damaged tissue and vascular tone. They achieve all these functions through their roles as structural and signaling molecules. On the other hand, it is also well established that their dysregulation promotes and maintains pathological conditions, as outlined in the next section. Here, we will consider how NADPH oxidases signal distinct functional outcomes.
Nox1
Much effort has been directed towards understanding the specific roles of Nox1 in specific functions of cardiovascular cells. Animal studies show that neointimal formation following femoral artery injury as well as hypertensive remodeling in conduit arteries is significantly reduced in Nox1 knockout mice 169, 170, suggesting a role of this oxidase in the proliferative and migratory functions of phenotypically modulated VSMCs. Other studies implicate Nox1 in vascular inflammation, as oxidase activity and Nox1 expression are increased in VSMCs exposed to advanced glycation end-products (AGEs),171 as well as in aortas of rats and mice with experimental diabetes 172, 173. It should be noted that the involvement of Nox1 in these processes is not necessarily disease promoting: outward remodeling induced by high shear stress in a model of arteriovenous fistula, a positive adaptation, is likely mediated by Nox1,.174 and Nox1 has been implicated in angiogenesis, as necessary step in would healing. 175
A role for Nox1 in proliferation has been established on many fronts. First, Nox1 is activated by agonists that stimulate growth in cell culture, such as Ang II176, thyroid hormone,177, PDGF,176 urokinase plasminogen activator,178 prostaglandin F2α,179 thrombin83 or oxLDL.180 Serum- and PDGF-induced proliferation are increased in VSMCs from Nox1 transgenic mice;169 conversely, targeting Nox1 with antisense or siRNA or genetic deletion of Nox1 in VSMCs inhibits proliferation induced by serum,181 thyroid hormone 177 and PDGF.169 In an interesting study, Stanic et al.182 showed that a more oxidized extracellular environment increases Nox1 expression and induces Nox1-dependent proliferation of VSMCs by activation of EGF-receptor signaling. Conversely, in human abdominal aortic ECs, endothelin-1, via the ETB1 receptor, attenuates NADPH oxidase activity by inhibiting the Pyk2-Rac1-Nox1 pathway and thus inhibits Ang II-induced proliferation.183
The mechanisms by which Nox1 mediates proliferation are varied. In VSMCs stimulated with Ang II, p38MAPK and Akt are downstream of Nox1 and are required for the hypertrophic response.184, 185 Similarly, shRNA against the Nox1 activator Noxa1 inhibits the activation of Janus kinase 2, Akt, and p38MAPK by thrombin.83 In actively cycling mouse lung epithelial cells, Nox1 stimulates cell proliferation by reducing the requirement for growth factors to maintain expression of cyclin D1, and stimulates transcriptional activation of Fos family genes required for induction of cell cycle re-entry.186
However, Nox1 may have a dual role in the regulation of the balance between cell proliferation and death. Although in cancer cells downregulation of Nox1 increases caspase-3 activity and cell death,187 necrosis has been positively linked to Nox1 in mouse fibroblasts exposed to TNF-α.188 Moreover, Nox1, together with Nox4, appears to mediate arrest in S phase and senescence, a hallmark of atherosclerosis and cause of vascular dysfunction, in ECs exposed to resveratrol.189
Nox1 has also been implicated in migration in different cell types. In VSMCs stimulated with thrombin, Nox1-derived ROS mediate migration in part through regulation of calcium influx via l-type Ca2+ channels.190 Similarly, Nox1 mediates PDGF and bFGF-induced migration.169, 191 In the case of PDGF-induced migration, one role of Nox1 is to mediate the activation of the cofilin phosphatase, Slingshot,192 an activity shown to be necessary for migration after PDGF stimulation in VSMC. 193 When bFGF is the stimulus, Nox1 is required for activation of JNK and subsequent phosphorylation of the focal adhesion protein paxillin.191 Another mechanism by which Nox1 may affect migration is via regulation of matrix metalloproteinases. In fibroblasts, it has been demonstrated that Nox1-derived ROS induce the promoter activity of matrix metalloprotease-9 by a NFκB-mediated mechanism.194 Finally, in ECs, Nox1 mediates migration through PPAR-α-dependent pathways.175
A causal role for Nox1 in vascular inflammation has also been investigated. Exposure of VSMCs to thrombin increases IL-6 secretion, Nox1 expression and NFκB nuclear translocation.195 These effects may be mediated by Nox1, as they were inhibited by atorvastatin, which is known to downregulate this oxidase.195 Furthermore, stimulation of VSMCs with TNFα, IL-1β or AGEs activates NFκB via Nox1 stimulation, which, at least in the case of AGEs, leads to upregulation of inducible NO synthase (iNOS).171, 196 Nox1 is also expressed in macrophages and is required for foam cell formation,;197 thus, Nox1 mediates the vascular inflammatory response via several independent mechanisms.
Nox 2
Because of its expression pattern in the cardiovascular system, Nox2 functions mainly to regulate endothelial and cardiac function. It is upregulated and/or activated by a number of vasoactive stimuli, including Ang II, atrial natriuretic peptide, shear stress, activation of adenosine A(2A) receptors, deletion of α1-AMP kinase, and nonselective nonsteroidal anti-inflammatory drugs.122, 198-202 Nox2 has been implicated in the control of vessel tone, inflammation, EC proliferation and migration (and therefore angiogenesis), as well as cardiac hypertrophy and remodeling.
Because superoxide produced in the endothelium can rapidly neutralize NO, Nox2 is expected to impair NO-mediated relaxation and lead to vessel contraction. Indeed, a negative correlation is observed between Nox2 expression and endothelium-dependent relaxation in isolated aorta.203, 204 In CGD patients who have a specific mutation in Nox2, flow-mediated vasodilation (which is largely dependent on NO) is significantly higher than in normal controls, corresponding with higher levels of serum nitrite and nitrate and suggesting that these patients have more bioactive NO.205 However, Nox2 also appears to affect vasodilatation via other mechanisms as well. For example, activation of Nox2 by high glucose leads to eNOS downregulation in human ECs.206 Conversely, activation of NO production can downregulate Nox2 expression,198 suggesting tight regulation between these two important molecules.
Nox2 has also been closely linked to inflammation. Hwang et al.207 showed that in ECs exposed to oscillatory shear stress (a stimulus known to promote inflammation and lesion formation), nox2 mRNA expression correlates with superoxide production and monocyte binding. This suggests that Nox2 activation may be proinflammatory, a conclusion supported by its role in macrophage activation,208 and by the observation that in VSMCs treated with IL-17, p38MAPK-mediated Nox2 activation is required for release of the proinflammatory cytokines IL-6, G-CSF, GM-CSF and MCP-1.209 Moreover, shear stress-dependent activation of p47phox-dependent NADPH oxidases, presumably Nox2 in ECs, causes eNOS uncoupling and xanthine/xanthine oxidase activation, one of many examples of ROS-induced ROS release.210,211
In contrast to Nox1, the role of Nox2 in proliferation and cell cycle progression is more controversial. In human ECs, it has been demonstrated that alone or in coordination with Nox4, Nox2 contributes to cell proliferation by a mechanism that involves p38MAPK and Akt.122 Similarly, in adventitial fibroblasts, Nox2-ds-tat (formerly gp91phox-ds-tat), a peptide that prevents the interaction of Nox2 and p47phox, inhibits serum-induced proliferation.212 Along the same lines, it was suggested that Nox2 prevents apoptosis by inhibition of caspase 3/7 activity.213 On the other hand, in human microvascular ECs, Nox2 mediates serum starvation-induced cell cycle arrest by a mechanism that targets the expression of p21cip1 and p53.214 Similarly, Ang II/AT1R-induced senescence in endothelial progenitor cells (EPCs) was shown to require Nox2. 215 These disparate observations suggest that Nox2 regulation of the cell cycle is cell and context specific.
Like Nox1, Nox2 has been implicated in migration, but in this case almost exclusively in ECs and monocytes/macrophages.216-218 It also plays a role in EPC mobilization.219 In ECs, Nox2 accumulation at the leading edge of migrating cells is dependent upon an intact actin cytoskeleton, and associates with both actin and the scaffold protein IQGAP1.216 Depletion of IQGAP1 prevents Nox2 localization at the leading edge, reduces ROS production in migrating cells, and impairs migration. During EPC mobilization by erythropoietin, Nox2-derived ROS inactivate the protein-tyrosine phosphatase SHP-2 that activates STAT5.219 In macrophages, Nox2 is required for LPS–induced, ER1/2–mediated MMP-9, -10, and -12 expression and cell migration.218
In the heart, Nox2 has been shown to participate in hypertrophy and remodeling after injury or aging. 220, 221 Indeed, it has been demonstrated that after Ang II/AT1R activation in cardiomyocytes, Nox2-derived superoxide induces hypertrophy by a mechanism that requires Akt activation 222 and Wnt-dependent pathways. 223 The profibrotic effects of Ang II are also mediated by Nox2. In rats overexpressing renin, septal wall thickness and perivascular fibrosis are increased. These responses are attenuated when animals are treated with the mineralocorticoid receptor blocker spironolactone, in part due to reduction in Nox2-mediated ROS production. 224, 225 The increase in Nox2 that accompanies mineralcorticoid receptor activation depends upon apoptosis signal-regulating kinase 1 (ASK1) activation by a mechanism yet to be determined.226
Nox 4
Nox4 is the most highly expressed Nox family member in all cells of the cardiovascular system, but its role is perhaps the most controversial. Its expression is marginally higher in arteries than in veins,227 and significantly higher in the cerebral than systemic vasculature.228 Of relevance, in different cell types, Nox4 is thought to regulate basal ROS production,229, 230 suggesting that it may function to control the redox set-point and thus influence cellular metabolism.
Nox4 is upregulated by a wide variety of agonists and cellular stresses. In ECs, Nox4 is sensitive to mechanical forces: it has been reported to be downregulated by cyclic strain or pulsatile flow and upregulated by oscillatory shear stress.231, 232 The proinflammatory mediators TNF-α and oxidized 1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine (Ox-PAPC) increase the expression or activity of Nox4. 125, 145 In both endothelial and VSMCs, serum withdrawal also increases Nox4 expression.229, 233 While growth-promoting agonists and drugs such as Ang II, PDGF, interleukin-1β, thrombin and phorbol myristate acetate downregulate Nox4 in systemic VSMCs and adventitial fibroblasts, 176, 230, 234 the proproliferative hormone urotensin upregulates Nox4 in pulmonary artery SMCs.116 Only TGF-β has been consistently shown to upregulate Nox4 in all cell types tested, and to activate it acutely. 235-237 In fact, TGF-β is responsible for activation of Nox4 in response to other stimuli, such as hypoxia. 238 Nox4 expression is also increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGF-β1-induced fibroblast differentiation into myofibroblasts. 239 The ability of Nox4 to respond to such diverse agonists suggests that it mediates many different cellular responses. Indeed, the variety of reported functions of Nox4 in the vasculature is truly remarkable. Nox4 has been linked to differentiation, 229, 236, 240, 241 migration,234, 242 growth, 74, 178, 235 apoptosis, 243 senescence, 244, 245 proinflammatory responses,125, 131, 246 and oxygen sensing.243, 247, 248
Evidence linking Nox4 to differentiation is abundant. Correlative studies show that upregulation of Nox4 in the redifferentiating VSMCs in the neointima coincides with induction of SMC differentiation markers,249 while Nox4 in human atherosclerotic lesions correlates with smooth muscle α-actin expression.250 Moreover, electron microscopic analysis of SMCs in human atherosclerotic lesions indicate that only those VSMCs that maintain the contractile phenotype continue to express Nox4, while those that become de-differentiated lose Nox4.251 Treatment of vascular adventitial fibroblasts with TGF-β, which leads to differentiation to myofibroblasts, upregulates Nox4.252 Conversely, Nox4 expression is high in preadipocytes and goes down as these cells differentiate into adipocytes.253
A mechanistic link between Nox4 and differentiation was established in a number of studies using strategies to attenuate Nox4 expression or transfection to overexpress it. Nox4 depletion leads to a loss of differentiation marker gene expression in adult VSMCs and a reduction in the conversion of cardiac fibroblasts to myofibroblasts.229, 236 Similar results were found in mouse embryonic stem cells, in which Nox4 overexpression increased SMC differentiation markers and Nox4 depletion decreased the ability of the cells to differentiate to VSMCs.254 In both adult and embryonic cells, Nox4 appears to exert its effects by regulating SMC-specific transcription factors serum response factor (SRF) and/or myocardin, in some cases via a p38MAPK-dependent mechanism.229, 254, 255 Differentiation of mouse embryonic stem cells into cardiomyocytes has also been shown to require Nox4.241 During differentiation into embryoid bodies, Nox4 expression initially decreases (day 1), and then gradually increases by day 8. Depletion of Nox4 using ribozymes reduces cardiac commitment (as assessed by a decrease in Nkx2.5 and MEF2C transcripts and a consequent reduction in ventricular myosin regulatory light chain 2) and dramatically reduces the percentage of spontaneously beating cells.241
Although agonists such as TGF-β do not generally induce migration, clear evidence has accumulated implicating Nox4 in this biologically critical function. Inhibition of Nox4 using siRNA decreases PDGF-induced VSMC migration and wounding-activated EC migration.120, 256 Paradoxically, overexpression of Nox4 also decreases migration in VSMCs stimulated with PDGF and in adventitial fibroblasts exposed to Ang II.120, 234 One potential explanation for these apparently opposing observations lies in the fact that the Nox4/Poldip2 complex activates RhoA in SMCs, leading to focal adhesion turnover and stress fiber formation.120 Thus, too little Nox4 prevents focal adhesion formation, while too much Nox4 prevents focal adhesion dissolution, both of which are required for cell motility. However, Nox4 can also inhibit motility by oxidizing the sarco-/ER Ca2+ ATPase (SERCA) and blocking the antimigratory action of NO, as was shown in VSMCs from Zucker obese rats.257. The opposite is true in ECs, where overexpression of Nox4 upregulates eNOS, leading to an enhanced migratory response.258
Nox4 has also been implicated in progression through the cell cycle. It has been shown that both Nox2 and Nox4 are required for basal ROS production and EC proliferation.74 Moreover, knockdown of Nox4 impairs EGF-and HIV-tat induced EC proliferation, while overexpression of Nox4 enhances EC growth.256, 259 Mechanistically, overexpression of Nox4 in HEK293 cells has been shown to activate ERK1/2 and JNK and to enhance insulin-induced phosphorylation of p38MAPK, Akt, and GSK3β, any of which might contribute to proliferation.122 In pulmonary artery SMCs, urotensin-induced proliferation, TGFβ-mediated hypoxia-induced proliferation,238 and TGFβ induced proliferation 235 all require Nox4. Nox4 also mediates hypoxia-induced proliferation in pulmonary artery adventitial fibroblasts.260 Growth-related signaling pathways downstream of Nox4 are incompletely understood, but may involve ERK1/2, p38MAPK, JNK and Akt.116, 235, 256, 261 In addition, Nox4 inhibits protein tyrosine phosphatase 1B (PTP1B) in the ER, leading to a constant activation of Akt and ultimately increased EC proliferation.128, 258, 259
With regard to apoptosis, reports of the role of Nox4 are again mixed. TNF-α-induced apoptosis in microvascular ECs is dependent upon Nox4,262 but apoptosis in response to serum deprivation is inhibited by Nox4 overexpression.256 Knockdown of Nox4 inhibits BMP4-induced ROS production, phosphorylation of p38MAPK and JNK, and ultimately apoptosis in ECs.263 In the heart, transgenic overexpression of Nox4 leads to increased apoptosis by 13 -14 months of age.139 In adventitial fibroblasts, however, Nox4 inhibits hypoxia-induced apoptosis.260
Nox4 has also been implicated in senescence in a number of cell types and tissues,264, 265 and this function of Nox4 may be an important contributor to cardiovascular diseases as well. Correlative data show that Nox4 expression increases with aging, particularly in the aorta, renal cortex and medulla.266-268 Functionally, Nox4 has been linked to senescence in ECs and polyploidy in VSMCs.268 In human umbilical vein ECs, knockdown of Nox4 reduces lifespan-associated increases in DNA damage and extends replicative lifespan independent of telomere shortening.269 Ang II-induced senescence of EPCs is also dependent on Nox4,270 and resveratrol, an antioxidant compound found in red wine, causes eventual endothelial senescence in a Nox4-dependent manner.189 Of interest, overexpression of miR-146a, an miRNA whose expression increases with age, inhibits Nox4 protein expression and decreases senescence, although a causal link between these two endpoints was not established in this study.271 In VSMCs, overexpression of Nox4 increases polyploidy and decreases expression of the chromosome passenger protein, survivin, that has previously been implicated in polyploidization, suggesting that excess Nox4 impairs passage through the cell cycle.268 In the heart, NADPH oxidase inhibition normalizes age-dependent deceleration of shortening/re-lengthening of ventricular myocytes, although the responsible NADPH oxidase homologue has not been identified.272
Another potential role of Nox4 is in regulating proinflammatory/profibrotic gene expression in the vasculature. Urotensin-mediated induction of the pro-fibrotic gene PAI-1 in pulmonary artery SMCs is dependent upon Nox4, and downregulation of Nox4 blocks IL-8, MCP-1, LDLR and ROS production in ECs stimulated with Ox-PAPC.116, 125 Basal production of PAI-1 in human umbilical vein ECs is also dependent upon Nox4 via p38MAPK and NFκB.273 Another lipid agonist, palmitate, activates NFκB in ECs via a TLR-4, Nox4-dependent mechanism.274 A similar relationship between Nox4 and inflammatory gene expression was found in cardiomyocytes, where both Nox2 and Nox4 siRNA significantly inhibited TNF-α-induced upregulation of IL-1β and IL-6.275 Similarly, activation of the FcgammaRIIa by CRP leads to Nox4-dependent ROS production in VSMCs, and depletion of Nox4 attenuates AP-1 and NFκB activation, as well as MCP-1, IL-6, and ET-1 production.276 These types of responses would support a proatherosclerotic response in intact vessels.
Finally, Nox4 has been proposed to be an oxygen sensor. The most convincing data in this regard was obtained in skeletal muscle, where Nox4-dependent ROS production is proportional to pO2 and leads to oxidation of ryanodine receptor cysteine thiols to increase Ca2+ release in isolated sarcoplasmic reticulum.248 Another molecule proposed to be downstream of oxygen-dependent Nox4 activation is TASK-1, a pH-sensitive K+-channel. Hypoxia inhibits TASK-1 activity, a response abolished by Nox4 siRNA.247 Further studies are needed to determine if Nox4 has similar functions in the cardiovascular system.
The apparent ability of Nox4 to participate in such disparate cellular functions suggests that Nox4 may regulate fundamental cellular processes that contribute to each of these responses. Candidate pathways include, for example, the actin cytoskeleton, microtubules or cellular metabolism. Although some data exist linking Nox4 to the cytoskeleton, further investigation is needed to understand Nox4’s fundamental role in cell biology.
Nox5
The role of Nox5 in the cardiovascular system has not been well studied, in part due to the lack of suitable animal models. Our knowledge of the potential functions of Nox5 is thus derived mainly from cultured cells and isolated tissue, as well as Drosophila, which express an ortholog of Nox5. In the latter system, proctolin, a neuropeptide that causes smooth muscle contraction to move eggs out of the ovary, utilizes Nox5 to stimulate calcium flux and subsequent contraction.277 In cultured human VSMCs, Nox5 is required for PDGF-induced proliferation.154 Knockdown of Nox5 using siRNA impairs the ability of PDGF to activate the JAK/STAT pathway, thus providing a potential mechanism for its growth-modulating effects. In microvascular ECs, overexpression of Nox5S or Nox5L leads to a modest increase in proliferation and the formation of capillary-like structures, while depletion of Nox5 partially reduces thrombin-stimulated growth and tube formation.150 In these cells, Nox5 expression is increased by endothelin-1 and Ang II,153 and mediates activation of ERK1/2, but not p38MAPK or SAPK/JNK.153 In intact vessels, overexpression of Nox5 via adenovirus paradoxically increases eNOS activity, but as expected, reduces bioavailable NO via inactivation by superoxide.278 As a result, Nox5 overexpression in the endothelium impairs acetylcholine-induced endothelium-dependent relaxation and potentiates the contractile responses to phenylephrine.
PHYSIOLOGY AND PATHOPHYSIOLOGY IN VIVO
Hypertension
Twenty years ago, the concept that ROS may play a role in hypertension was established by studies in which administration of heparin-bound SOD to spontaneously hypertensive rats reduced blood pressure.279 Numerous reports since then have verified these initial observations and implicate NADPH oxidases as important sources of ROS in blood pressure regulation. Many investigators found upregulation of NADPH oxidase subunits in hypertensive animals and a reversal of blood pressure and vascular remodeling with agents such as the superoxide dismutase mimetic tempol or the putative NADPH oxidase inhibitor apocynin (for review see 280, 281). Human studies are limited mostly to showing increased activity of NADPH oxidases in hypertensive subjects and association studies linking p22phox polymorphisms to hypertension.282 However, it was not until the development of animals with specific genetic modifications of Nox subunits that the function of each homologue in hypertension could be rigorously interrogated (Table 2).
Table 2.
Phenotypes of mice in which Nox enzymes are genetically modified
| Genotype | Challenge | Phenotype | Reference |
|---|---|---|---|
| Nox1-/y | Ang II | Acute increase of blood pressure & chronic reduction of blood pressure | 170,287 |
| Nox1-/y | Ang II | No change in endothelium-dependent relaxation to acetylcholine | 287 |
| Nox1-/y | High dose Ang II | Reduction in hypertrophy | 170 |
| Nox1 overexpression in VSMC | Ang II | Increased blood pressure & Increased hypertrophy | 288 |
| Nox1 overexpression in VSMC | Basal | Impaired endothelium-dependent relaxation | 289 |
| Nox1-/y | 30 min Brain ischemia | Unchanged neurological score, cerebral infarct volume and edema; higher cortical infarct volume | 339 |
| Nox1-/y | 1 hour Brain ischemia | Improved neurological outcome, preservation of blood–brain barrier integrity and reduced cerebral edema | 340 |
| Nox2-/y | Ischemia/reperfusion | Smaller infarct volume | 341, 342 |
| Nox2-/y | Ischemia/reperfusion | Loss of protection with ischemic preconditioning in the heart and brain | 343 |
| Nox2-/y | Basal | Reduction in blood pressure | 295 |
| Nox2-/y | Ang II | No change in blood pressure | 295 |
| p47phox-/- | Ang II | Reduced hypertensive effect | 296 |
| p22phox overexpression in VSMC | Ang II | Exacerbated hypertensive response | 299 |
| Nox2 overexpression in EC | Ang II | Exacerbated hypertensive response | 300 |
| p47phox deletion in T cells | Ang II | Reduction in Ang II-dependent hypertension | 304 |
| Nox4 overexpression in EC | Acetylcholine & histamine | Enhanced agonist-mediated relaxation | 307 |
| Nox4-/- | Basal | No effect on blood pressure | 311 |
| Nox1-/y | Ang II | Protected from aortic dissection | 312 |
| Nox1-/y | Wire injury | Attenuated neointimal response | 169 |
| Nox1-/y | ApoE-/- | Reduced lesion area and macrophage infiltration in the ascending and thoracic aorta | 313 |
| Nox2-/y | ApoE-/- & high fat diet | No difference in lesion formation in the ascending aorta | 319 |
| Nox2-/y | ApoE-/- & high fat diet | Reduced atherosclerosis in the descending aorta | 320 |
| Nox2-/y | Femoral artery ligation | Reduced neovascularization | 333 |
| Nox2-/y plus wt bone marrow | Femoral artery ligation | Restoration of collateral formation | 334 |
| Nox2-/y | Arterial injury | Reduced lesion formation | 323 |
| Nox2-/y | Subpressor & pressor dose of Ang II | Attenuation of the hypertrophic response | 349, 351 |
| Nox2-/y | Left coronary ligation | Less post-MI remodeling & unchanged infarct size | 220, 353 |
| Nox2-/y | Aortic constriction | No difference in myocardial hypertrophy | 354, 355 |
| Nox2-/y | Aortic constriction | Less interstitial hypertrophy and contractile dysfunction | 356 |
| Nox2-/y | Hypercholesterolemia or diabetes | Protection against the loss of neovascularization | 335, 336 |
| p47phox-/- | ApoE-/- | Unchanged lesions in the ascending aorta | 321 |
| p47phox-/- | ApoE-/- | Reduced lesions in the descending aorta | 322 |
| p47phox-/- | ApoE-/- | No effect on lesion formation in the aortic sinus | 321, 322 |
| p47phox-/- | Myocardial infarction | Attenuation of cardiomyocyte hypertrophy, apoptosis, and interstitial fibrosis | 352 |
| Nox4-/- | bFGF | Unchanged neovascularization | 175 |
| Nox4 overexpression in cardiomyocytes | Pressure overload | Impaired angiogenesis | 337 |
| Nox4 overexpression in EC | Hindlimb ischemia | eNOS-dependent accelerated neovascularization | 258 |
| Nox4-/- | Transient occlusion of middle cerebral artery | Less oxidative stress, blood-brain barrier leakage and neuronal apoptosis | 347 |
| Nox4-/- | Aortic constriction | Greater cardiac hypertrophy, contractile dysfunction and ventricular dilatation | 337 |
| Nox4 overexpression in cardiomyocytes | Aortic constriction | Preservation of capillary density, protection against hypertrophy | 337 |
| Cardiac-specific Nox4 deletion | Aortic constriction | Reduced hypertrophy and contractile dysfunction | 357 |
The possibility that Nox1 might be implicated in hypertension is supported by descriptive data and correlative evidence. Indeed, in the kidney Nox1 is upregulated in various models of hypertension, including renin transgenic rats,283 2-kidney 2-clip rats,284 and Dahl salt sensitive rats on a high salt diet.285 In rats infused with Ang II for 7 days, increased aortic Nox1 expression and hypertension are both blunted by administration of the PKC antagonist chelerythrine, which may also block PKC-dependent phosphorylation of p47phox.286 While a causal role for Nox1 in the acute response to hypertension is supported by a number of studies, its importance in chronic hypertension is controversial. Mice in which Nox1 is genetically deleted initially respond to Ang II with an increase in blood pressure, but sustained blood pressure elevation is reduced in these mice compared to their wild-type controls.170, 287 Endothelium-dependent relaxation to acetylcholine is impaired in wild type mice exposed to Ang II, but is unaffected in Nox1 knockout mice, suggesting that it is Nox1-derived superoxide that inhibits endothelial function in Ang II-induced hypertension.287 The effect of Nox1 deletion on vascular hypertrophy is less clear: it has been reported to have no effect after a moderate dose of Ang II287 or to reduce hypertrophy by reducing matrix deposition after a high dose of Ang II.170 This discrepancy may result from the extent of hypertension induced in these animals, as overexpression of Nox1 specifically in smooth muscle cells exacerbates the blood pressure response and enhances vascular hypertrophy to the lower dose of Ang II.288 Mechanistically, Nox1 overexpressing animals exhibit impaired endothelium-dependent relaxation that is a consequence of NO inactivation, implying cross-talk between cell types.289 In contrast to these results, when Nox1 knockout animals are crossed with animals that express human renin and so have a chronically activated renin angiotensin system, no effect on blood pressure is observed even though ROS levels are reduced in plasma.290 These results are difficult to reconcile with the acute studies, although as in all work involving genetically modified animals, possible compensation must be considered. It should be noted, however, that deletion of Nox1 in this chronic model significantly reduced expression of the proinflammatory molecule VCAM-1.
Studies using recently designed Nox inhibitors or other therapeutic agents indirectly support the genetic studies implicating Nox1 in hypertension. Treatment of aged spontaneously hypertensive rats (which have increased Nox1 and Nox2 but not Nox4 compared to WKY animals) with VAS2870, a Nox1/Nox4 inhibitor, improved endothelium-dependent relaxation.291
Nox2 was also studied extensively with regard to its potential role in blood pressure control, and is implicated in hypertension by virtue of its ability to inactivate NO and affect vessel wall remodeling, as well as its ability to influence central and renal control of blood pressure. It is upregulated in the vessel wall, the kidney and brain in many forms of hypertension.280, 292-295 In Nox2 knockout mice, basal blood pressure is reduced, but the response to Ang II is intact.295 However, infusion of Nox2ds-tat, an inhibitor of Nox2 interaction with p47phox, or genetic deletion of p47phox reduces the hypertensive effect of angiotensin II.296, 297 In Dahl salt-sensitive rats treated with Nox2ds-tat, ROS and endothelial-dependent relaxation are normalized, but blood pressure is not affected,298 while in mice with smooth muscle-specific overexpression of p22phox, the blood pressure response to Ang II is exacerbated.299 It should be noted that interfering with p47phox or p22phox likely affects other Nox homologues in addition to Nox2, but the predominance of Nox2 in resistance arteries, the site of blood pressure control, supports the concept that Nox2 may play a role in hypertension. More recent work has shown that endothelial-specific overexpression of Nox2 does not affect basal blood pressure, but exacerbates the hypertensive response to Ang II.300 Because this mimics the upregulation of Nox2 that occurs in hypertension, these observations suggest that vascular Nox2 may not be involved in the initiation of hypertension, but that once upregulated, it can exacerbate the disease.
Another potential contribution of Nox2 to hypertension lies in its ability to regulate central and renal function. Work from the Davisson group showed that intracellular superoxide production in the subfornical organ of the brain mediates the blood pressure response to Ang II.301 Experiments involving administration of small interfering RNAs to Nox2 or Nox4 to this region showed that both Nox2 and Nox4 are required for the full vasopressor effects of Ang II, while only Nox2 regulates the dipsogenic response.302 Similarly, administration of p22phox antisense systemically abolishes the contribution of renal ROS production to the slow pressor response to Ang II.303 One mechanism that has been proposed to be the signal that integrates brain, vascular and renal control of blood pressure is the T-cell. Guzik et al.304 showed that the blood pressure response to Ang II is reduced in mice lacking T-and B-cells (Rag1-/- mice), and that adoptive transfer of T cells lacking p47phox only partially restores Ang II-dependent hypertension, while wild-type T cells completely reverse the response. Adoptive transfer of T cells derived from either gp91phox-/y or wild-type mice into Rag-1-/- mice fully restore the prothrombotic phenotype induced by Ang II, suggesting that T-cell derived ROS are not involved in the thrombotic response that accompanies hypertension. 305
A role for Nox4 in hypertension is quite controversial. Nox4 has been reported to be elevated in the vasculature in several models of hypertension,283, 306 but not in others.286 This increase in Nox4 may be compensatory, however, since Ray et al.307 found that endothelial-specific Nox4 overexpression enhances agonist-mediated vasodilation by inducing hyperpolarization and decreases blood pressure by ~10 mmHg. In agreement with a potential pro-vasodilatory effect of Nox4, Paravicini et al. 308 showed that Nox4 expression in basilar arteries (which is 10-fold higher than in systemic arteries228 and is even greater in SHR) is associated with enhanced vasodilation in response to NADPH due to H2O2-mediated activation of BK(Ca) channels. In the brain, however, Nox4 in the subfornical organ clearly plays a role in the pressor response to Ang II.302 Nox4 in the kidney may also mediate hypertension or the renal injury that accompanies hypertension, as it is upregulated in the macula densa and distal nephron in the SHR model, in the kidney of transgenic hypertensive rats overexpressing the Ren2 gene and in the cortex of rats treated with aldosterone.283, 309, 310 However, Nox4 is unlikely to be a major regulator of blood pressure, since knockout of Nox4 has no effect on basal blood pressure.311
Taken together, data from multiple genetically modified animal models confirms distinct roles for Nox homologues in hypertension. Depending on the cause of hypertension (genetic, diet-induced, hormone–regulated), its duration (acute vs. chronic), the contributing organ (brain, kidney, vasculature), and the endpoint (blood pressure per se vs. end organ damage), different homologues may play a predominant role. Translating these observations to human hypertension is obviously the next step, but may have to await the development of safe, homologue-specific Nox inhibitors.
Atherosclerosis
Even more so than with hypertension, a significant body of literature implicates ROS in the development of atherosclerosis, at least in animal models. While early work focused on oxidation of LDL, more recent studies tested the notion that NADPH oxidases in the vessel wall or in infiltrating macrophages and T-cells can affect atherogenesis on multiple levels. Conflicting reports in the literature leave the question of the relative importance of NADPH oxidases in this disease unsettled at this time.
Direct and indirect evidence for a role of Nox1 in atherogenesis is just now emerging. Gavazzi et al.312 showed that Nox1 deficient mice infused with the proatherogenic peptide Ang II were protected from aortic dissection, potentially because they express higher levels of tissue inhibitor of metalloproteinase 1 than their wild type controls, suggesting that Nox1 may regulate turnover of extracellular matrix. Mice in which Nox1 is deleted also exhibit an attenuated neointimal response to arterial injury, due in part to reduced VSMC proliferation and migration.169 As all of these cellular processes contribute to atherosclerosis, one would predict that atherosclerotic lesions formation would be inhibited in the absence of Nox1. In fact, in the only study to directly assess the role of Nox1 in atherosclerosis, in which Nox1 knockout mice were crossed into the ApoE-/- background and fed a high fat diet for 18 weeks, just such an outcome was observed.313 Compared with ApoE-/- mice, lesion area in the ascending and thoracic aorta was reduced by 20-30% in Nox1-/yApoE-/- mice, and macrophage content was reduced by about 50%. Following carotid injury in mice fed a chow diet, cell proliferation was decreased but collagen content was increased in the double knockout mice compared to ApoE-/- controls. Moreover, Niu et al.83 showed that adenovirus-mediated overexpression of the Nox1 activator Noxa1 enhanced neointimal hyperplasia in a model of mouse carotid injury. It is worth noting that Noxa1 is increased in atherosclerotic lesions in ApoE-/- mice and in humans.83 Additional evidence comes from a study showing that GKT136901, a newly-developed inhibitor of Nox1 and Nox4 oxidases, attenuates ROS generation and expression of the adhesion molecule CD44 and its principal ligand, hyaluronan, in atherosclerotic lesions.314 Finally, a study using the janus kinase-2 inhibitor tyrphostin AG490 found that in ApoE-/- mice fed a high fat diet, Nox1, 2 and 4 were elevated in the aorta, and that treatment with the inhibitor reduced expression and activity of all three homologues and reduced lesion area.315
The contribution of Nox2 to atherosclerosis is controversial. Because mononuclear cells express high levels of Nox2 and macrophages are critical to lesion development, it was originally assumed that Nox2-derived ROS in macrophages would contribute to atherogenesis. This notion was supported by the observation that p22phox and Nox2 expression is high in atherosclerotic lesions, especially in the shoulder region of the plaque,250, 316-318 and the findings that risk factors for atherosclerosis such as hypertension, oscillatory shear stress, and diabetes increase Nox2 expression in the vasculature.317 However, Kirk et al. 319 called this assumption into question when they observed no difference in lesion formation in the ascending aortas of WT and Nox2 knockout mice fed a high fat diet or crossed with ApoE-/- mice. In contrast, a more recent study found evidence for reduced atherosclerotic burden in the descending aortas of Nox2-/yApoE-/- mice compared with ApoE-/- mice fed a high fat diet.320 These observations are similar to those in p47phox-/-ApoE-/- mice, in which lesion area in the ascending aorta is identical between p47phox-/-ApoE-/- mice and ApoE-/-mice,321 but lesions in the descending aorta are reduced.322 Lesion formation is also reduced in Nox2-/y mice subjected to arterial injury due to reduced cellular proliferation and reduced leukocyte accumulation.323 Further experiments are necessary to define the contributions of cell-specific Nox2 to atherogenesis.
The mechanism for increased Nox expression and activity in atherosclerotic arteries is unclear. There is some evidence for ROS-dependent upregulation of NADPH oxidase subunits, suggesting a potential feed-forward mechanism that could exacerbate disease development.324 Moreover, proatherogenic cytokines that activate NFκB, such as TNF-α, upregulate Nox2 and its cytosolic activators.325 Ox-PAPC has been shown to increase Nox4 activity in ECs.125 Because the role of the various Nox isoforms in atherosclerosis is complex, their regulation is likely to be complicated as well, and measurements of different homologues in identical experimental conditions is needed.
Compared to Nox2 and Nox4, much less is known about the role of Nox4 in lesion formation because mice with genetically modified Nox4 alleles have only recently become available. As is the case for Nox2, risk factors such as diabetes173, 326, 327 and hypertension (see above) increase Nox4 expression in the vasculature. ApoE-/- mice treated with a high fat diet also exhibit enhanced expression of Nox4,315 and Nox4 expression is high in human atherosclerotic lesions.250 Interestingly, treatment with statins, which has been shown to be beneficial in atherosclerosis, decreases Nox4 expression.327, 328 Functionally, Nox4 has been implicated in neointimal formation after vascular injury. Adenoviral-mediated knockdown of Nox4 at the time of carotid injury in Zucker rats reduced the extent of oxidation of SERCA and inhibited the development of the neointima.257
Even less is known about the role of Nox5 in atherosclerosis, largely because it is not expressed in rodents, making genetic manipulation much more difficult. However, Guzik et al.329 showed that Nox5 expression is higher in human coronary arteries that exhibit coronary artery disease than in those that are disease free. This increased expression was accompanied by a 7-fold increase in calcium-dependent NADPH oxidase activity. Nox5 was observed in ECs in early lesions and in VSMCs in advanced lesions. This is one of the few human studies linking any Nox homologue to atherosclerosis.
Angiogenesis and collateral formation
Growth of new blood vessels and expansion of existing ones is important not only during development, but can also occur in response to ischemic injury or during tumor development. Thus, depending on the context, these processes can either be beneficial or detrimental to the health of the organism. ROS both promote and inhibit blood vessel formation, in part depending on the amount of ROS produced. Yun et al.330 proposed the “redox window” hypothesis, which states that while collateral formation requires ROS, either excess or insufficient ROS can inhibit vessel growth. A series of recent studies support a role for several of the Nox homologues in vessel formation and expansion.
Direct evidence for a role of Nox1 in angiogenesis comes from Garrido-Urbani et al.,175 who showed that Nox1 is upregulated by proangiogenic factors, that mice deficient in Nox1 exhibit impaired angiogenesis in the matrigel assay, and that tumor vascularization is inhibited either by genetic deletion of Nox1 in the host or by treatment with the Nox inhibitor GKT136901. They further demonstrated that Nox1 appears to promote tumor angiogenesis by inhibiting the anti-angiogenic factor PPARα. This supports earlier work by Arbiser et al.,331 who found that Nox1-expressing NIH 3T3 cells injected into athymic mice cause highly vascularized, aggressive tumors, due to upregulation of VEGF and its receptors as well as increased MMP-9 activity. Komatsu et al.332 found that Nox1 is necessary, but not sufficient, for induction of VEGF, indicating other factors are also involved.
Strong data exist both supporting and refuting a role for Nox2 in revascularization following ischemia. Ushio-Fukai’s laboratory showed that Nox2 expression and superoxide production are increased in wild-type mice up to 7 days following femoral artery ligation, and that the neovascularization response is reduced in Nox2 knockout mice.333 Subsequent work suggests that it is Nox2 in bone marrow-derived cells that mediates the neovascularization response. Transplantation of wild-type bone marrow into Nox2-deficient mice restores blood flow recovery.334 In contrast, Nox2 knockout mice are protected against the loss of neovascularization induced by hypercholesterolemia or diabetes, suggesting that the increase in Nox2 in response to these conditions is responsible for the observed impaired blood flow recovery in wild-type mice.335, 336 Moreover, in the tumor angiogenesis matrigel plug model, loss of Nox2 has no effect on vasculogenesis.175 Some of these apparent inconsistencies may relate to the level of ROS present basally in these models, per the redox window hypothesis, or to mechanistic differences between different types of vascular neogenesis.
The role of Nox4 in angiogenesis also seems to be context-specific. Subcutaneous implantation of a matrigel plug loaded with bFGF into Nox4-/- mice leads to vascularization indistinguishable from that observed in wild type mice.175 However, the angiogenesis (defined as an increase in capillary density) that accompanies pressure overload-induced cardiac hypertrophy is impaired in Nox4 null mice and enhanced in cardiomyocyte-specific Nox4 overexpressing mice compared to wild type controls.337 This appears to be a consequence of Nox4-mediated activation of hypoxia inducible factor-1 and the release of vascular endothelial growth factor from cardiomyocytes, and is supported by in vitro studies in which overexpression of Nox4 enhanced, whereas dominant negative Nox4 or knockdown of Nox4 by siRNA impaired, tube formation in ECs.256 Similarly, Craige et al.258 showed that endothelial-specific overexpression of Nox4 leads to eNOS-dependent accelerated recovery from hindlimb ischemia and enhanced aortic capillary sprouting. Finally, Bhandarkar et al.338 found that formation of hemangiomas (endothelial-derived neoplasias) induced by grafting polyoma middle T-transformed brain ECs from angiopoetin 2 heterozygotes into nude mice is greatly reduced by lentiviral expression of siNox4. Thus, the bulk of the evidence suggests that Nox4 is pro-angiogenic, although its role in tumor formation and angiogenesis remains unclear.
Ischemia/reperfusion in the brain, heart and lungs
Transient or sustained ischemia in the heart, brain or lung can lead to infarct, stroke, or pulmonary hypertension, respectively. NADPH oxidases have been implicated in the pathophysiology of all three diseases in animal models, with similar mechanisms.
In Nox1 knockout mice, the effects of ischemia have only been studied in the brain. Transient occlusion (30 minutes) of the middle cerebral artery results in similar neurological score, cerebral infarct volume and edema volume in wild-type and Nox1-/y mice, but cortical infarct volume is much higher in brains of Nox1-/y mice compared to wild-type mice after 24 hours.339 This suggests that Nox1 may actually limit infarct development following cerebral ischemia. However, in another study, a 1-hour, but not a 2-hour, occlusion of the middle cerebral artery resulted in attenuation of lesion size at 24 hours after induction of ischemia, accompanied by a significant improvement of neurological outcome, preservation of blood–brain barrier integrity and reduced cerebral edema. Clearly, more studies are needed to understand the role of Nox1 in stroke.340
More is known about the role of Nox2 in ischemic injury. Using a similar experimental procedure to that described for Nox1, transient middle cerebral artery occlusion followed by 22 hours of reperfusion, two groups showed that male Nox2-/y mice had a significantly smaller infarct volume compared with controls.341, 342 Bone marrow transplants of Nox2-/y cells into wild type mice fail to induce this protection, and if wild type cells are injected into Nox2-/y mice, infarct volumes are not reduced, suggesting that both circulating Nox2 and neural or endothelial Nox2 contribute to ischemic injury in this model. Similarly, in a Langendorff-perfused, isolated mouse heart model of ischemia/reperfusion injury, ischemic preconditioning confers protection against necrotic injury in wild type, but not Nox2-/y hearts, indicating that in the heart as well as the brain, Nox2 is activated during either ischemia or reperfusion.343 Finally, administration of intermittent hypoxia to mice induces a phenotype of pulmonary hypertension, including elevated right ventricular systolic pressure, right ventricular hypertrophy, and increased muscularization of the distal pulmonary vessels.344 These changes are accompanied by an increase in p22phox and Nox4 expression, but interestingly are attenuated in Nox2-/y mice, suggesting that Nox2 may in fact regulate expression of other NADPH oxidases, and raise the possibility of a feed-forward mechanism that exacerbates pulmonary hypertension.
Emerging evidence strongly suggests a link between Nox4 and ischemic injury. In pulmonary hypertension induced by chronic intermittent hypoxia, Nox4 is upregulated as a consequence of Nox2 activation.344 Cortical Nox4 is upregulated in neurons early (24 hours) after middle cerebral artery occlusion, as well as 7-14 days later in newly formed capillaries in the peri-infarct region.345 Transient upregulation of Nox4 in the cortex is also observed after endothelin-induced stroke.346 A functional role for Nox4 in ischemia-induced neurodegeneration was proposed based on the observation that compared to wild type mice, Nox4 knockout mice have less oxidative stress, less blood-brain barrier leakage and less neuronal apoptosis after either transient occlusion of the middle cerebral artery or permanent stroke induced by cortical photothrombosis.347 Importantly, post-stroke treatment with the putative Nox1/Nox4 inhibitor VAS2870 improves function, suggesting that Nox4 may be a viable therapeutic target for stroke.
Nox4 has also been implicated in cardiac performance following myocardial infarction (MI). Infanger et al.348 found that surgical ligation of the left anterior descending coronary artery induces a two-fold increase in Nox4 expression in the paraventricular nucleus (PVN) of the brain compared to sham animals, correlating with the increased sympathoexcitation and cardiac function that occurs following MI. Adenoviral-mediated silencing of Nox in the PVN improves both ejection fraction and fractional shortening two weeks post-MI, apparently by reducing sympathetic outflow. Surprisingly, these outcomes appeared to be a result of superoxide, rather than hydrogen peroxide, signaling in the PVN.
Cardiac hypertrophy, post-myocardial infarction remodeling and heart failure
As noted, Nox2 and Nox4 are the most highly expressed Nox homologues in the heart. While both are important for normal physiology, they seem to regulate distinct aspects of cardiac function and thus have discrete roles in heart disease.
Early work by Ajay Shah’s group implicated Nox2 in cardiac hypertrophy and fibrosis. Administration of a subpressor dose of Ang II to wild type mice increases heart/body weight ratio, myocyte area, and cardiac collagen content in wild-type mice, but these responses are attenuated in Nox2-/y mice.349 A similar reduction in cardiac hypertrophy is observed in mice with a cardiomyocyte-specific deletion of Rac1, which as expected, exhibit reduced ROS formation and reduced NADPH oxidase assembly after Ang II infusion compared with wild type mice.350 Johar et al.351 showed that in response to a pressor dose of Ang II, interstitial fibrosis, as well as upregulation of matrix proteins and matrix metalloproteinase 2 activity, is inhibited in Nox2-/y animals compared with controls. This Nox2-dependent profibrotic effect can be reversed by the aldosterone antagonist spironolactone. Cardiac remodeling also occurs after MI, and Doerries et al.352 found that cardiomyocyte hypertrophy and apoptosis, as well as interstitial fibrosis, are attenuated in mice lacking p47phox. Similarly, Nox2 knockout mice subjected to permanent left coronary ligation have less post-MI remodeling (as reflected by these same parameters) at 4 weeks despite no difference in infarct size.220, 353 Thus, in multiple murine models, there is a clear link between Nox2, cardiac remodeling and fibrosis.
This link is not incontrovertible, however, and in some situations, Nox4 appears to be involved in cardiac remodeling. In contrast to its role in Ang II-induced cardiac hypertrophy, Nox2 does not appear to contribute to hypertrophy induced by pressure overload. Two separate groups reported no difference in myocardial hypertrophy, cardiac mass or atrial natriuretic factor expression in Nox2-/y and wild type mice subjected to aortic constriction.354, 355 However, Nox2 does appear to contribute to interstitial hypertrophy and contractile dysfunction in this model.356 Several studies have reported an increase in Nox4 expression in the heart in response to pressure overload,337, 354, 357 but only two groups have directly addressed the role of Nox4 in cardiac hypertrophy in this model. Using Nox4 knockout mice and cardiomyocyte-specific Nox4 overexpressors, Zhang et al.337 showed that there were no basal differences in cardiac function, but after pressure overload induced by aortic constriction, mice in which Nox4 is deleted exhibited greater cardiac hypertrophy, contractile dysfunction and ventricular dilatation, while Nox4 transgenic mice were protected from these pathologies due to Nox4-dependent preservation of capillary density. However, Kuroda et al.357 found different results using mice with cardiac-specific Nox4 deletion or overexpression. In their hands, pressure overload-induced cardiac hypertrophy and contractile dysfunction were reduced in Nox4 knockout mice in parallel with improved mitochondrial function, but exacerbated in Nox4 overexpressing mice. With the exception of the Nox4 transgenic mice generated by Kuroda et al., mice used in both studies were on the C57/Bl6 background. However, the extent of pressure overload induced in the latter study was greater than that seen by Zhang et al., raising the possibility that the role of Nox4 may change with the severity of disease.
INTERACTIONS AMONG NOX PROTEINS
From the above summaries, it is evident that cells and tissues express multiple Nox homologues (Figure 2). Because their ultimate products (superoxide and hydrogen peroxide) are the same, the question arises as to the need for multiple oxidases in the same system. In some cases, their function appears to be redundant, while in others, each oxidase appears to regulate a distinct function. Moreover, there is cross-talk among oxidase systems, so that activation of Nox enzymes leads to subsequent activation of other sources of ROS, creating a positive feedback loop that can lead to vascular pathology.
Figure 2. Physiological and pathophysiological responses mediated by Nox homologues in cardiovascular cells.
This summary of the roles of Nox enzymes in different cell types of the cardiovascular system illustrates the paradox of their mediating both specialized and redundant functions. VSMC, vascular smooth muscle cells; EC, endothelial cells; FB, fibroblasts; CM, cardiomyocytes, MΦ, macrophages; and EPC, endothelial progenitor cells.
In ECs, which express primarily Nox2 and Nox4, agonists often activate both enzymes and each Nox contributes similarly to the resultant physiological response. As an example, Nox2 and Nox4 are both required for the angiogenic response, and both participate in serum-induced proliferation. 74, 256, 358 Both are upregulated by oscillatory shear stress and downregulated by pulsatile stimuli.232 Interestingly, in these cells, Nox2 and Nox4 are perinuclear, and colocalize with ER markers, perhaps explaining their redundant functions.74 Moreover, knockdown of either of these genes using siRNA leads to an increase in the other, further supporting the notion that their functions are overlapping.359 One report, however, implicates both Nox2 and Nox4 in the regulation of cell proliferation, but provides evidence that only Nox2 prevents apoptosis and modifies the cytoskeleton in ECs.213 In VSMCs from large arteries, where Nox1 and Nox4 are expressed in distinct subcellular compartments (caveolae and the nucleus and focal adhesions, respectively),105 they mediate completely different physiological functions. Nox1 is required for hypertrophy and proliferation,176, 181 while Nox4 mediates cell differentiation.229, 254 Both participate in cell migration, although via different mechanisms.120, 169 In this cell type, Nox1 and Nox4 are also differentially responsive to agonists176, 229, most likely a consequence of the differences in their subcellular localization. This hypothesis is supported by observations in HEK293 cells transfected with Nox2 or Nox4, in which each enzyme exhibits a specific subcellular localization (plasma membrane and ER, respectively) and each enzyme responds to specific agonists (Ang II and TNF-α vs. no stimulation and insulin) and activates distinct signaling pathways.122 Ultimately, the subcellular localization of different Nox enzymes and their activation by specific external stimuli may dictate the integrated response to Nox activation.
It is also likely that Nox enzymes contribute to cardiovascular pathophysiology in a coordinated fashion. This has been best established in hypertension, where, as noted, T-cells coordinate the rise in blood pressure In Ang II-induced hypertension, and brain, vascular and kidney Nox enzymes contribute to blood pressure regulation.304 A similar coordinated response may occur in the vessel wall in atherosclerosis, where macrophages release cytokines in a Nox2-dependent manner, which then activate Nox1 and Nox2 in VSMCs and ECs, respectively.
It should also be noted that ROS production by Nox enzymes often activates other oxidase systems, thus amplifying the response. For example, in ECs exposed to oscillatory shear stress, xanthine oxidase activation depends upon prior activation of NADPH oxidases. 210 In addition, Ang II stimulation of these cells increases mitochondrial ROS production in a Nox-dependent manner.360 In many forms of hypertension, ROS production by Nox enzymes leads to the formation of peroxynitrite, which uncouples eNOS, greatly increasing superoxide production.211 The converse is true as well. Hydrogen peroxide from these sources, or even from initial Nox activation, can feedback to further activate Nox enzymes and amplify ROS production. Thus, Nox enzymes appear to act as both initiators and integrators of redox signaling via cross-talk with other ROS producing systems. The notion of cross-talk leads to an important concept: inappropriate feed forward activation of NADPH oxidases can lead to self-reinforcing loops that maintain and worsen pathology.
CONCLUSION
Tremendous progress since the discovery of the Nox family of enzymes has firmly established their essential role in cardiovascular physiology and pathophysiology in animal models. The field has progressed to a point where the next major challenge is to assess their role in human disease, and then translate these findings into treatments, while continuing to dissect the molecular mechanisms and pathways specific to individual Nox homologues. Several research groups and companies have developed homologue-specific inhibitors that are only now beginning to undergo clinical testing. Because of the far-reaching effects of Nox enzyme activation, the therapeutic potential of these compounds is enormous compared with previously used, non-specific antioxidants. It is likely that these new agents will enable, for the first time, a true test of the redox hypothesis of human disease.
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL38206, HL095070, HL058863 and HL093115.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- AGEs
Advanced glycation end-products
- Ang II
Angiotensin II
- AP-1
Activator protein 1
- ASK1
Apoptosis signal-regulating kinase 1
- AT1R
Angiotensin II type 1 receptor
- bFGF
basic fibroblast growth factor
- BMP4
Bone morphogenetic protein 4
- CGD
Chronic granulomatous disease
- CRP
C-reactive protein
- Duox
Dual oxidase
- EGF
Epidermal growth factor
- ECs
Endothelial cells
- EPCs
Endothelial progenitor cells
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- ET-1
Endothelin 1
- G/GM-CSF
Granulocyte/granulocyte macrophage colony-stimulating factor
- GSK3
Glycogen synthase kinase 3
- Hsp
Heat shock protein
- IL
Interleukin
- iNOS
inducible NO synthase
- IQGAP1
IQ motif-containing GTPase-activating protein 1
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- LDLR
Low-density lipoprotein receptor
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemotactic protein-1
- MEF2C
Myocyte enhancer factor-2C
- MI
myocardial infarction
- miRNA
microRNA
- MMP
Matrix metalloproteinase
- NFκB
Nuclear factor kappa B
- NO
nitric oxide
- Nox
NADPH oxidase
- Nox5S/L
Nox5 short/long
- Noxa
Nox activator
- Noxo
Nox organizer
- PDI
Protein disulfide isomerase
- oxLDL
oxidized low-density lipoprotein
- Ox-PAPC
oxidized 1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine
- PAI-1
Plasminogen activator inhibitor-1
- PVN
paraventricular nucleus
- PDGF
Platelet-derived growth factor
- PI3K
phosphoinositide 3-kinase
- Poldip2
Polymerase delta-interacting protein 2
- PKA/C
Protein kinase A/C
- PPAR
Peroxisome proliferator-activated receptor
- PTP1B
Protein tyrosine phosphatase 1B
- ROS
Reactive oxygen species
- SAPK
Stress-activated protein kinase
- STAT
Signal-transducer and activator of transcription
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- SRF
serum response factor
- SH2/3
Src homology 2/3
- SHP-2
SH2 domain tyrosine phosphatase 2
- SHR
Spontaneously hypertensive rat
- si/shRNA
small interfering/short hairpin RNA
- TASK-1
TWIK-related acid-sensitive potassium channel
- TGF
Transforming growth factor
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- TPR
Tetratricopeptide repeat
- VSMCs
Vascular smooth muscle cells
- VCAM
vascular cell adhesion molecule
- WKY
Wistar-Kyoto rats
Footnotes
DISCLOSURES
Emory University is the owner of a patent on Nox1 with BL and KKG cited in the list of inventors.
References
- 1.Bedard K, Krause KH. The NOX family of ros-generating NADPH oxidases: Physiology and pathophysiology. Physiological reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 3.Hohler B, Holzapfel B, Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochemistry and cell biology. 2000;114:29–37. doi: 10.1007/s004180000160. [DOI] [PubMed] [Google Scholar]
- 4.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 5.Sumimoto H. Structure, regulation and evolution of nox-family NADPH oxidases that produce reactive oxygen species. The FEBS journal. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 6.Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b(558): Identification of specific histidines in gp91(phox) The Journal of biological chemistry. 2001;276:31105–31112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- 7.Lassegue B, Griendling KK. Reactive oxygen species in hypertension; an update. American journal of hypertension. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.DeCoursey TE. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 2010;25:27–40. doi: 10.1152/physiol.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreland JG, Davis AP, Bailey G, Nauseef WM, Lamb FS. Anion channels, including clc-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. The Journal of biological chemistry. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 10.Roos D, Kuhns DB, Maddalena A, Bustamante J, Kannengiesser C, de Boer M, van Leeuwen K, Koker MY, Wolach B, Roesler J, Malech HL, Holland SM, Gallin JI, Stasia MJ. Hematologically important mutations: The autosomal recessive forms of chronic granulomatous disease (second update) Blood cells, molecules & diseases. 2010;44:291–299. doi: 10.1016/j.bcmd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, Avcin T, de Boer M, Bustamante J, Condino-Neto A, Di Matteo G, He J, Hill HR, Holland SM, Kannengiesser C, Koker MY, Kondratenko I, van Leeuwen K, Malech HL, Marodi L, Nunoi H, Stasia MJ, Ventura AM, Witwer CT, Wolach B, Gallin JI. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood cells, molecules & diseases. 2010;45:246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao YY, Gianni D, Bohl B, Taylor RM, Bokoch GM. Identification of a conserved rac-binding site on NADPH oxidases supports a direct gtpase regulatory mechanism. The Journal of biological chemistry. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapouge K, Smith SJ, Groemping Y, Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. The Journal of biological chemistry. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 14.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: Phosphorylation of gp91phox/nox2 by protein kinase c enhances its diaphorase activity and binding to rac2, p67phox, and p47phox. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueyama T, Kusakabe T, Karasawa S, Kawasaki T, Shimizu A, Son J, Leto TL, Miyawaki A, Saito N. Sequential binding of cytosolic phox complex to phagosomes through regulated adaptor proteins: Evaluation using the novel monomeric kusabira-green system and live imaging of phagocytosis. Journal of immunology. 2008;181:629–640. doi: 10.4049/jimmunol.181.1.629. [DOI] [PubMed] [Google Scholar]
- 16.Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the sh3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. The Journal of biological chemistry. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 17.Reeves EP, Dekker LV, Forbes LV, Wientjes FB, Grogan A, Pappin DJ, Segal AW. Direct interaction between p47phox and protein kinase c: Evidence for targeting of protein kinase c by p47phox in neutrophils. The Biochemical journal. 1999;344(Pt 3):859–866. [PMC free article] [PubMed] [Google Scholar]
- 18.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase c zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. Journal of immunology. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 19.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by pkc alpha, beta ii, delta, and zeta: Effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 20.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from sh3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyal CR, Gutierrez A, Young BM, Catz SD, Lin JH, Tsichlis PN, Babior BM. Modulation of p47phox activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, McLeish KR. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. Journal of immunology. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 23.Martyn KD, Kim MJ, Quinn MT, Dinauer MC, Knaus UG. P21-activated kinase (pak) regulates NADPH oxidase activation in human neutrophils. Blood. 2005;106:3962–3969. doi: 10.1182/blood-2005-03-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. Pkczeta regulates tnf-alpha-induced activation of NADPH oxidase in endothelial cells. Circulation research. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 25.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin ii. Circulation research. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin ii. Role of the p47phox subunit. The Journal of biological chemistry. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 27.Touyz RM, Yao G, Schiffrin EL. C-src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin ii in human vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. The Journal of biological chemistry. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 29.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The px domains of p47phox and p40phox bind to lipid products of pi(3)k. Nature cell biology. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 30.Zhan Y, He D, Newburger PE, Zhou GW. P47(phox) px domain of NADPH oxidase targets cell membrane via moesin-mediated association with the actin cytoskeleton. Journal of cellular biochemistry. 2004;92:795–809. doi: 10.1002/jcb.20084. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. P47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: Role in NAD(P)H oxidase regulation by angiotensin ii. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 32.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of nox2 and nox4 in primary human endothelial cells. Antioxidants & redox signaling. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 33.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 34.Ogura K, Nobuhisa I, Yuzawa S, Takeya R, Torikai S, Saikawa K, Sumimoto H, Inagaki F. Nmr solution structure of the tandem src homology 3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. The Journal of biological chemistry. 2006;281:3660–3668. doi: 10.1074/jbc.M505193200. [DOI] [PubMed] [Google Scholar]
- 35.Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, c57bl/6j-m db/db and db/+ Journal of leukocyte biology. 2000;67:210–215. doi: 10.1002/jlb.67.2.210. [DOI] [PubMed] [Google Scholar]
- 36.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. The Journal of experimental medicine. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harbord M, Novelli M, Canas B, Power D, Davis C, Godovac-Zimmermann J, Roes J, Segal AW. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. The Journal of biological chemistry. 2002;277:5468–5475. doi: 10.1074/jbc.M110635200. [DOI] [PubMed] [Google Scholar]
- 38.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. The Journal of biological chemistry. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 39.Nisimoto Y, Motalebi S, Han CH, Lambeth JD. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558) The Journal of biological chemistry. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 40.Ponting CP. Novel domains in NADPH oxidase subunits, sorting nexins, and ptdins 3-kinases: Binding partners of sh3 domains? Protein science : a publication of the Protein Society. 1996;5:2353–2357. doi: 10.1002/pro.5560051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leusen JH, de Klein A, Hilarius PM, Ahlin A, Palmblad J, Smith CI, Diekmann D, Hall A, Verhoeven AJ, Roos D. Disturbed interaction of p21-rac with mutated p67-phox causes chronic granulomatous disease. The Journal of experimental medicine. 1996;184:1243–1249. doi: 10.1084/jem.184.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (tpr) motifs of p67(phox) participate in interaction with the small gtpase rac and activation of the phagocyte NADPH oxidase. The Journal of biological chemistry. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 43.Alloul N, Gorzalczany Y, Itan M, Sigal N, Pick E. Activation of the superoxide-generating NADPH oxidase by chimeric proteins consisting of segments of the cytosolic component p67(phox) and the small gtpase rac1. Biochemistry. 2001;40:14557–14566. doi: 10.1021/bi0117347. [DOI] [PubMed] [Google Scholar]
- 44.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) The Journal of biological chemistry. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 45.Yuzawa S, Miyano K, Honbou K, Inagaki F, Sumimoto H. The domain organization of p67 phox, a protein required for activation of the superoxide-producing NADPH oxidase in phagocytes. Journal of innate immunity. 2009;1:543–555. doi: 10.1159/000235656. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura R, Sumimoto H, Mizuki K, Hata K, Ago T, Kitajima S, Takeshige K, Sakaki Y, Ito T. The pc motif: A novel and evolutionarily conserved sequence involved in interaction between p40phox and p67phox, sh3 domain-containing cytosolic factors of the phagocyte NADPH oxidase. European journal of biochemistry / FEBS. 1998;251:583–589. doi: 10.1046/j.1432-1327.1998.2510583.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuribayashi F, Nunoi H, Wakamatsu K, Tsunawaki S, Sato K, Ito T, Sumimoto H. The adaptor protein p40(phox) as a positive regulator of the superoxide-producing phagocyte oxidase. The EMBO journal. 2002;21:6312–6320. doi: 10.1093/emboj/cdf642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: Binding of src homology 3 domains to proline-rich targets. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finan P, Shimizu Y, Gout I, Hsuan J, Truong O, Butcher C, Bennett P, Waterfield MD, Kellie S. An sh3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. The Journal of biological chemistry. 1994;269:13752–13755. [PubMed] [Google Scholar]
- 50.Mizuki K, Takeya R, Kuribayashi F, Nobuhisa I, Kohda D, Nunoi H, Takeshige K, Sumimoto H. A region c-terminal to the proline-rich core of p47phox regulates activation of the phagocyte NADPH oxidase by interacting with the c-terminal sh3 domain of p67phox. Archives of biochemistry and biophysics. 2005;444:185–194. doi: 10.1016/j.abb.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Dusi S, Rossi F. Activation of NADPH oxidase of human neutrophils involves the phosphorylation and the translocation of cytosolic p67phox. The Biochemical journal. 1993;296(Pt 2):367–371. doi: 10.1042/bj2960367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benna JE, Dang PM, Gaudry M, Fay M, Morel F, Hakim J, Gougerot-Pocidalo MA. Phosphorylation of the respiratory burst oxidase subunit p67(phox) during human neutrophil activation. Regulation by protein kinase c-dependent and independent pathways. The Journal of biological chemistry. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 53.Forbes LV, Truong O, Wientjes FB, Moss SJ, Segal AW. The major phosphorylation site of the NADPH oxidase component p67phox is thr233. The Biochemical journal. 1999;338(Pt 1):99–105. [PMC free article] [PubMed] [Google Scholar]
- 54.Dang PM, Morel F, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of the NADPH oxidase component p67(phox) by erk2 and p38mapk: Selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003;42:4520–4526. doi: 10.1021/bi0205754. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK. Protein kinase cdelta regulates p67phox phosphorylation in human monocytes. Journal of leukocyte biology. 2005;77:414–420. doi: 10.1189/jlb.0504284. [DOI] [PubMed] [Google Scholar]
- 56.Dang PM, Raad H, Derkawi RA, Boussetta T, Paclet MH, Belambri SA, Makni-Maalej K, Kroviarski Y, Morel F, Gougerot-Pocidalo MA, El-Benna J. The NADPH oxidase cytosolic component p67phox is constitutively phosphorylated in human neutrophils: Regulation by a protein tyrosine kinase, mek1/2 and phosphatases 1/2a. Biochemical pharmacology. 2011;82:1145–1152. doi: 10.1016/j.bcp.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 57.Sancho-Shimizu V, Malo D. Sequencing, expression, and functional analyses support the candidacy of ncf2 in susceptibility to salmonella typhimurium infection in wild-derived mice. Journal of immunology. 2006;176:6954–6961. doi: 10.4049/jimmunol.176.11.6954. [DOI] [PubMed] [Google Scholar]
- 58.Ueyama T, Tatsuno T, Kawasaki T, Tsujibe S, Shirai Y, Sumimoto H, Leto TL, Saito N. A regulated adaptor function of p40phox: Distinct p67phox membrane targeting by p40phox and by p47phox. Molecular biology of the cell. 2007;18:441–454. doi: 10.1091/mbc.E06-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ago T, Takeya R, Hiroaki H, Kuribayashi F, Ito T, Kohda D, Sumimoto H. The px domain as a novel phosphoinositide- binding module. Biochemical and biophysical research communications. 2001;287:733–738. doi: 10.1006/bbrc.2001.5629. [DOI] [PubMed] [Google Scholar]
- 60.Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. The p40phox and p47phox px domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. The Journal of biological chemistry. 2002;277:4512–4518. doi: 10.1074/jbc.M109520200. [DOI] [PubMed] [Google Scholar]
- 61.Tian W, Li XJ, Stull ND, Ming W, Suh CI, Bissonnette SA, Yaffe MB, Grinstein S, Atkinson SJ, Dinauer MC. Fc gamma r-stimulated activation of the NADPH oxidase: Phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cross AR. P40(phox) participates in the activation of NADPH oxidase by increasing the affinity of p47(phox) for flavocytochrome b(558) The Biochemical journal. 2000;349:113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ueyama T, Nakakita J, Nakamura T, Kobayashi T, Kobayashi T, Son J, Sakuma M, Sakaguchi H, Leto TL, Saito N. Cooperation of p40phox with p47phox for nox2 activation during fc{gamma}r-mediated phagocytosis --- mechanism for acquisition of p40phox pi(3)p binding. The Journal of biological chemistry. 2011;286:40693–40705. doi: 10.1074/jbc.M111.237289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dusi S, Donini M, Rossi F. Mechanisms of NADPH oxidase activation: Translocation of p40phox, rac1 and rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. The Biochemical journal. 1996;314(Pt 2):409–412. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellson C, Davidson K, Anderson K, Stephens LR, Hawkins PT. Ptdins3p binding to the px domain of p40phox is a physiological signal in NADPH oxidase activation. The EMBO journal. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bissonnette SA, Glazier CM, Stewart MQ, Brown GE, Ellson CD, Yaffe MB. Phosphatidylinositol 3-phosphate-dependent and -independent functions of p40phox in activation of the neutrophil NADPH oxidase. The Journal of biological chemistry. 2008;283:2108–2119. doi: 10.1074/jbc.M706639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan LM, Teng L, Li JM. Knockout of p47 phox uncovers a critical role of p40 phox in reactive oxygen species production in microvascular endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1651–1656. doi: 10.1161/ATVBAHA.109.191502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh CI, Stull ND, Li XJ, Tian W, Price MO, Grinstein S, Yaffe MB, Atkinson S, Dinauer MC. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during fcgammaiia receptor-induced phagocytosis. The Journal of experimental medicine. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chessa TA, Anderson KE, Hu Y, Xu Q, Rausch O, Stephens LR, Hawkins PT. Phosphorylation of threonine 154 in p40phox is an important physiological signal for activation of the neutrophil NADPH oxidase. Blood. 2010;116:6027–6036. doi: 10.1182/blood-2010-08-300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. P40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. The Journal of biological chemistry. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 71.Someya A, Nunoi H, Hasebe T, Nagaoka I. Phosphorylation of p40-phox during activation of neutrophil NADPH oxidase. Journal of leukocyte biology. 1999;66:851–857. doi: 10.1002/jlb.66.5.851. [DOI] [PubMed] [Google Scholar]
- 72.Lopes LR, Dagher MC, Gutierrez A, Young B, Bouin AP, Fuchs A, Babior BM. Phosphorylated p40phox as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- 73.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, Marchal CC, Stull ND, Lewis DB, Steele M, Kellner JD, Yu W, Meroueh SO, Nauseef WM, Dinauer MC. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–3315. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. Nox2 and nox4 mediate proliferative response in endothelial cells. Antioxidants & redox signaling. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 75.Hahn NE, Meischl C, Wijnker PJ, Musters RJ, Fornerod M, Janssen HW, Paulus WJ, van Rossum AC, Niessen HW, Krijnen PA. Nox2, p22phox and p47phox are targeted to the nuclear pore complex in ischemic cardiomyocytes colocalizing with local reactive oxygen species. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;27:471–478. doi: 10.1159/000329968. [DOI] [PubMed] [Google Scholar]
- 76.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH. Expression of nox1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. The Journal of pathology. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 77.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free radical biology & medicine. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. The Journal of biological chemistry. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 79.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of nox1- and nox2-dependent reactive oxygen generation. The Journal of biological chemistry. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 80.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. The Journal of biological chemistry. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 81.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase nox1. The Journal of biological chemistry. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 82.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. The Journal of biological chemistry. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 83.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: A potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free radical biology & medicine. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 85.Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circulation research. 2009;105:1013–1022. doi: 10.1161/CIRCRESAHA.109.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, Wakisaka M, Kawahara T, Rokutan K, Ibayashi S, Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke; a journal of cerebral circulation. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 87.Ueyama T, Geiszt M, Leto TL. Involvement of rac1 in activation of multicomponent nox1- and nox3-based NADPH oxidases. Molecular and cellular biology. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dutta S, Rittinger K. Regulation of noxo1 activity through reversible interactions with p22 and noxa1. PloS one. 2010;5:e10478. doi: 10.1371/journal.pone.0010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park HS, Park D, Bae YS. Molecular interaction of NADPH oxidase 1 with betapix and nox organizer 1. Biochemical and biophysical research communications. 2006;339:985–990. doi: 10.1016/j.bbrc.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 90.Cheng G, Lambeth JD. Noxo1, regulation of lipid binding, localization, and activation of nox1 by the phox homology (px) domain. The Journal of biological chemistry. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 91.Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, Thalmann R, Smith RJ, Verpy E, Banfi B. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Current biology : CB. 2006;16:208–213. doi: 10.1016/j.cub.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 92.Gianni D, DerMardirossian C, Bokoch GM. Direct interaction between tks proteins and the n-terminal proline-rich region (prr) of noxa1 mediates nox1-dependent ros generation. European journal of cell biology. 2011;90:164–171. doi: 10.1016/j.ejcb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of nox1 activity via protein kinase a-mediated phosphorylation of noxa1 and 14-3-3 binding. The Journal of biological chemistry. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 94.Oh H, Jung HY, Kim J, Bae YS. Phosphorylation of serine282 in NADPH oxidase activator 1 by erk desensitizes egf-induced ros generation. Biochemical and biophysical research communications. 2010;394:691–696. doi: 10.1016/j.bbrc.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 95.Kroviarski Y, Debbabi M, Bachoual R, Perianin A, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Phosphorylation of NADPH oxidase activator 1 (noxa1) on serine 282 by map kinases and on serine 172 by protein kinase c and protein kinase a prevents nox1 hyperactivation. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:2077–2092. doi: 10.1096/fj.09-147629. [DOI] [PubMed] [Google Scholar]
- 96.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. C-src-mediated phosphorylation of noxa1 and tks4 induces the reactive oxygen species (ros)-dependent formation of functional invadopodia in human colon cancer cells. Molecular biology of the cell. 2010;21:4287–4298. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flaherty JP, Spruce CA, Fairfield HE, Bergstrom DE. Generation of a conditional null allele of NADPH oxidase activator 1 (noxa1) Genesis. 2010;48:568–575. doi: 10.1002/dvg.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by rac1. The Journal of biological chemistry. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 99.Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by rac gtpase. Antioxidants & redox signaling. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 100.Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta pix, rac1, and nox1 in growth factor-induced production of h2o2. Molecular and cellular biology. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diebold BA, Fowler B, Lu J, Dinauer MC, Bokoch GM. Antagonistic cross-talk between rac and cdc42 gtpases regulates generation of reactive oxygen species. The Journal of biological chemistry. 2004;279:28136–28142. doi: 10.1074/jbc.M313891200. [DOI] [PubMed] [Google Scholar]
- 102.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Molecular biology of the cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in nox1 and nox4 controlling localization and activity. Antioxidants & redox signaling. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 104.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxidants & redox signaling. 2011;14:2107–2119. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of nox1 and nox4 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 106.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa b in vascular smooth muscle cells requires signaling endosomes containing nox1 and clc-3. Circulation research. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 107.Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. American journal of physiology. Heart and circulatory physiology. 2007;292:H483–495. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- 108.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of nox1. Antioxidants & redox signaling. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chu X, Filali M, Stanic B, Takapoo M, Sheehan A, Bhalla R, Lamb FS, Miller FJ., Jr A critical role for chloride channel-3 (cic-3) in smooth muscle cell activation and neointima formation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:345–351. doi: 10.1161/ATVBAHA.110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. The Journal of biological chemistry. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 111.Fernandes DC, Manoel AH, Wosniak J, Jr, Laurindo FR. Protein disulfide isomerase overexpression in vascular smooth muscle cells induces spontaneous preemptive NADPH oxidase activation and nox1 mrna expression: Effects of nitrosothiol exposure. Archives of biochemistry and biophysics. 2009;484:197–204. doi: 10.1016/j.abb.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 112.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. The Journal of biological chemistry. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 114.Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circulation research. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 116.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Gorlach A. Human urotensin ii is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- 117.von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into nox4 and nox2: Motifs involved in function and cellular localization. Molecular and cellular biology. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. Nox4 activity is determined by mrna levels and reveals a unique pattern of ros generation. The Biochemical journal. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the nox4-p22 phox complex. The Journal of biological chemistry. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 123.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin ii-induced activation of akt/protein kinase b in mesangial cells. American journal of physiology Renal physiology. 2003;285:F219–229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 124.Wu RF, Ma Z, Myers DP, Terada LS. Hiv-1 tat activates dual nox pathways leading to independent activation of erk and jnk map kinases. The Journal of biological chemistry. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 125.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free radical biology & medicine. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhuang J, Jiang T, Lu D, Luo Y, Zheng C, Feng J, Yang D, Chen C, Yan X. NADPH oxidase 4 mediates reactive oxygen species induction of cd146 dimerization in vegf signal transduction. Free radical biology & medicine. 2010;49:227–236. doi: 10.1016/j.freeradbiomed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 127.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: Direct interaction of tlr4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of nf-kappa b. Journal of immunology. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 128.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog nox4 modulates insulin-stimulated generation of h2o2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase nox4 in the nucleus of human vascular endothelial cells. Genes to cells : devoted to molecular & cellular mechanisms. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 130.Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates tgf-beta-induced cytoskeletal alterations in endothelial cells. American journal of physiology Renal physiology. 2005;289:F816–825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovascular research. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Grange L, Nguyen MV, Lardy B, Derouazi M, Campion Y, Trocme C, Paclet MH, Gaudin P, Morel F. NAD(P)H oxidase activity of nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxidants & redox signaling. 2006;8:1485–1496. doi: 10.1089/ars.2006.8.1485. [DOI] [PubMed] [Google Scholar]
- 133.Basuroy S, Tcheranova D, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during tnf-alpha-induced apoptosis. American journal of physiology Cell physiology. 2011;300:C256–265. doi: 10.1152/ajpcell.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.New D, Block K, Bhandari B, Gorin YC, Abboud HE. Igf-1 increases the expression of fibronectin by nox4-dependent akt phosphorylation in renal tubular epithelial cells. American journal of physiology Cell physiology. 2011 doi: 10.1152/ajpcell.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates tgf-beta1-induced activation of kidney myofibroblasts. Journal of the American Society of Nephrology : JASN. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. American journal of physiology Heart and circulatory physiology. 2005;288:H13–21. doi: 10.1152/ajpheart.00629.2004. [DOI] [PubMed] [Google Scholar]
- 137.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. The Journal of biological chemistry. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ben Mkaddem S, Pedruzzi E, Werts C, Coant N, Bens M, Cluzeaud F, Goujon JM, Ogier-Denis E, Vandewalle A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell death and differentiation. 2010;17:1474–1485. doi: 10.1038/cdd.2010.26. [DOI] [PubMed] [Google Scholar]
- 139.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation research. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goyal P, Weissmann N, Rose F, Grimminger F, Schafers HJ, Seeger W, Hanze J. Identification of novel nox4 splice variants with impact on ros levels in a549 cells. Biochemical and biophysical research communications. 2005;329:32–39. doi: 10.1016/j.bbrc.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 141.Jackson HM, Kawahara T, Nisimoto Y, Smith SM, Lambeth JD. Nox4 b-loop creates an interface between the transmembrane and dehydrogenase domains. The Journal of biological chemistry. 2010;285:10281–10290. doi: 10.1074/jbc.M109.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of nox1 and nox4 in basal and angiotensin ii-stimulated superoxide and hydrogen peroxide production. Free radical biology & medicine. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The e-loop is involved in hydrogen peroxide formation by the NADPH oxidase nox4. The Journal of biological chemistry. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang L, Nguyen MV, Lardy B, Jesaitis AJ, Grichine A, Rousset F, Talbot M, Paclet MH, Qian G, Morel F. New insight into the nox4 subcellular localization in hek293 cells: First monoclonal antibodies against nox4. Biochimie. 2011;93:457–468. doi: 10.1016/j.biochi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Yoshida LS, Tsunawaki S. Expression of NADPH oxidases and enhanced h(2)o(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. International immunopharmacology. 2008;8:1377–1385. doi: 10.1016/j.intimp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 146.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. The Journal of biological chemistry. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 147.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of ca2+ activation of the NADPH oxidase 5 (nox5) The Journal of biological chemistry. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 148.Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. Camp-response element-binding protein mediates acid-induced NADPH oxidase nox5-s expression in barrett esophageal adenocarcinoma cells. The Journal of biological chemistry. 2006;281:20368–20382. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- 149.Si J, Fu X, Behar J, Wands J, Beer DG, Souza RF, Spechler SJ, Lambeth D, Cao W. NADPH oxidase nox5-s mediates acid-induced cyclooxygenase-2 expression via activation of nf-kappab in barrett’s esophageal adenocarcinoma cells. The Journal of biological chemistry. 2007;282:16244–16255. doi: 10.1074/jbc.M700297200. [DOI] [PubMed] [Google Scholar]
- 150.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. Nox5 variants are functionally active in endothelial cells. Free radical biology & medicine. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 151.Kawahara T, Jackson HM, Smith SM, Simpson PD, Lambeth JD. Nox5 forms a functional oligomer mediated by self-association of its dehydrogenase domain. Biochemistry. 2011;50:2013–2025. doi: 10.1021/bi1020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hong J, Resnick M, Behar J, Wands J, DeLellis RA, Cao W. Role of rac1 in regulation of nox5-s function in barrett’s esophageal adenocarcinoma cells. American journal of physiology Cell physiology. 2011;301:C413–420. doi: 10.1152/ajpcell.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (nox5) regulation by angiotensin ii and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circulation research. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates pdgf-induced proliferation in human aortic smooth muscle cells. Free radical biology & medicine. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. Nox5 is expressed at the plasma membrane and generates superoxide in response to protein kinase c activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 156.Tirone F, Radu L, Craescu CT, Cox JA. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of nox5. Biochemistry. 2010;49:761–771. doi: 10.1021/bi901846y. [DOI] [PubMed] [Google Scholar]
- 157.Tirone F, Cox JA. NADPH oxidase 5 (nox5) interacts with and is regulated by calmodulin. FEBS letters. 2007;581:1202–1208. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 158.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. Calcium sensitization via phosphorylation. The Journal of biological chemistry. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 159.Pandey D, Gratton JP, Rafikov R, Black SM, Fulton DJ. Calcium/calmodulin-dependent kinase ii mediates the phosphorylation and activation of NADPH oxidase 5. Molecular pharmacology. 2011;80:407–415. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA. Novel redox-dependent regulation of nox5 by the tyrosine kinase c-abl. Free radical biology & medicine. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou X, Li D, Resnick MB, Behar J, Wands J, Cao W. Signaling in h2o2-induced increase in cell proliferation in barrett’s esophageal adenocarcinoma cells. The Journal of pharmacology and experimental therapeutics. 2011;339:218–227. doi: 10.1124/jpet.111.182352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates nox5 localization via an n-terminal polybasic region. Molecular biology of the cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor RM, Burritt JB, Baniulis D, Foubert TR, Lord CI, Dinauer MC, Parkos CA, Jesaitis AJ. Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: Characterization of six monoclonal antibodies to the p22phox subunit. Journal of immunology. 2004;173:7349–7357. doi: 10.4049/jimmunol.173.12.7349. [DOI] [PubMed] [Google Scholar]
- 164.Zhu Y, Marchal CC, Casbon AJ, Stull N, von Lohneysen K, Knaus UG, Jesaitis AJ, McCormick S, Nauseef WM, Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: Identification of regions critical for gp91phox maturation and NADPH oxidase activity. The Journal of biological chemistry. 2006;281:30336–30346. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- 165.Biberstine-Kinkade KJ, Yu L, Stull N, LeRoy B, Bennett S, Cross A, Dinauer MC. Mutagenesis of p22(phox) histidine 94. A histidine in this position is not required for flavocytochrome b558 function. The Journal of biological chemistry. 2002;277:30368–30374. doi: 10.1074/jbc.M203993200. [DOI] [PubMed] [Google Scholar]
- 166.Porter CD, Parkar MH, Verhoeven AJ, Levinsky RJ, Collins MK, Kinnon C. P22-phox-deficient chronic granulomatous disease: Reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood. 1994;84:2767–2775. [PubMed] [Google Scholar]
- 167.Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Banfi B. Mutation of the cyba gene encoding p22phox causes vestibular and immune defects in mice. The Journal of clinical investigation. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lewis EM, Sergeant S, Ledford B, Stull N, Dinauer MC, McPhail LC. Phosphorylation of p22phox on threonine 147 enhances NADPH oxidase activity by promoting p47phox binding. The Journal of biological chemistry. 2010;285:2959–2967. doi: 10.1074/jbc.M109.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in nox1-deficient mice. FEBS letters. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 171.San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for vsmc activation by advanced glycation end-products. Free radical biology & medicine. 2007;42:1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 172.Wendt MC, Daiber A, Kleschyov AL, Mulsch A, Sydow K, Schulz E, Chen K, Keaney JF, Jr, Lassegue B, Walter U, Griendling KK, Munzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med. 2005;39:381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 173.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. American journal of physiology Heart and circulatory physiology. 2007;292:H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 174.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. P47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 175.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a pparalpha mediated mechanism. PLoS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : Nox1 mediates angiotensin ii-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 177.Wang X, Sun Z. Thyroid hormone induces artery smooth muscle cell proliferation: Discovery of a new tralpha1-nox1 pathway. Journal of cellular and molecular medicine. 2010;14:368–380. doi: 10.1111/j.1582-4934.2008.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bashtrikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- 179.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin f2alpha-induced hypertrophy of vascular smooth muscle cells: Induction of nox1 by pgf2alpha. The Journal of biological chemistry. 2002;277:13438–13442. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 180.Yin CC, Huang KT. H2o2 but not o2- elevated by oxidized ldl enhances human aortic smooth muscle cell proliferation. J Biomed Sci. 2007;14:245–254. doi: 10.1007/s11373-006-9132-4. [DOI] [PubMed] [Google Scholar]
- 181.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 182.Stanic B, Katsuyama M, Miller FJ., Jr An oxidized extracellular oxidation-reduction state increases nox1 expression and proliferation in vascular smooth muscle cells via epidermal growth factor receptor activation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2234–2241. doi: 10.1161/ATVBAHA.110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dammanahalli JK, Sun Z. Endothelin (et)-1 inhibits nicotinamide adenine dinucleotide phosphate oxidase activity in human abdominal aortic endothelial cells: A novel function of etb1 receptors. Endocrinology. 2008;149:4979–4987. doi: 10.1210/en.2008-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. P38map kinase is a critical component of the redox-sensitive signaling pathways by angiotensin ii: Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 185.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of akt/protein kinase b by angiotensin ii in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 186.Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH. Redox-dependent expression of cyclin d1 and cell proliferation by nox1 in mouse lung epithelial cells. Antioxidants & redox signaling. 2006;8:1447–1459. doi: 10.1089/ars.2006.8.1447. [DOI] [PubMed] [Google Scholar]
- 187.Sancho P, Bertran E, Caja L, Carmona-Cuenca I, Murillo MM, Fabregat I. The inhibition of the epidermal growth factor (egf) pathway enhances tgf-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim Biophys Acta. 2009;1793:253–263. doi: 10.1016/j.bbamcr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 188.Kim YS, Morgan MJ, Choksi S, Liu ZG. Tnf-induced activation of the nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 189.Schilder YD, Heiss EH, Schachner D, Ziegler J, Reznicek G, Sorescu D, Dirsch VM. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic Biol Med. 2009;46:1598–1606. doi: 10.1016/j.freeradbiomed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 190.Zimmerman MC, Takapoo M, Jagadeesha DK, Stanic B, Banfi B, Bhalla RC, Miller FJ., Jr Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension. 2011;58:446–453. doi: 10.1161/HYPERTENSIONAHA.111.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 192.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, San Martin A. Platelet-derived growth factor (pdgf) regulates slingshot phosphatase activity via nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem. 2011;286:35430–35437. doi: 10.1074/jbc.M111.268284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by lim kinase and slingshot-1l phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res. 2008;102:432–438. doi: 10.1161/CIRCRESAHA.107.158923. [DOI] [PubMed] [Google Scholar]
- 194.Shinohara M, Adachi Y, Mitsushita J, Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K, Miyazaki H, Kamata T. Reactive oxygen generated by NADPH oxidase 1 (nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem. 2010;285:4481–4488. doi: 10.1074/jbc.M109.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haloui M, Meilhac O, Jandrot-Perrus M, Michel JB. Atorvastatin limits the proinflammatory response of rat aortic smooth muscle cells to thrombin. Eur J Pharmacol. 2003;474:175–184. doi: 10.1016/s0014-2999(03)02043-0. [DOI] [PubMed] [Google Scholar]
- 196.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: Role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 197.Lee JG, Lim EJ, Park DW, Lee SH, Kim JR, Baek SH. A combination of lox-1 and nox1 regulates tlr9-mediated foam cell formation. Cell Signal. 2008;20:2266–2275. doi: 10.1016/j.cellsig.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 198.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. No-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol. 2006;576:557–567. doi: 10.1113/jphysiol.2006.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via rac1 and NAD(P)H oxidase/nox2-activation. Circulation research. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 200.Li H, Hortmann M, Daiber A, Oelze M, Ostad MA, Schwarz PM, Xu H, Xia N, Kleschyov AL, Mang C, Warnholtz A, Munzel T, Forstermann U. Cyclooxygenase 2-selective and nonselective nonsteroidal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. The Journal of pharmacology and experimental therapeutics. 2008;326:745–753. doi: 10.1124/jpet.108.139030. [DOI] [PubMed] [Google Scholar]
- 201.Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF, Jr, Wegener G, Lackner K, Munzel T, Viollet B, Schulz E. Alpha1amp-activated protein kinase preserves endothelial function during chronic angiotensin ii treatment by limiting nox2 upregulation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:560–566. doi: 10.1161/ATVBAHA.110.219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thakur S, Du J, Hourani S, Ledent C, Li JM. Inactivation of adenosine a2a receptor attenuates basal and angiotensin ii-induced ros production by nox2 in endothelial cells. The Journal of biological chemistry. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takenouchi Y, Kobayashi T, Matsumoto T, Kamata K. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis. 2009;206:397–404. doi: 10.1016/j.atherosclerosis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 204.Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ang ii in murine aortic rings. Am J Physiol Heart Circ Physiol. 2007;292:H3103–3108. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
- 205.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: Results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 206.Taye A, Saad AH, Kumar AH, Morawietz H. Effect of apocynin on NADPH oxidase-mediated oxidative stress-lox-1-enos pathway in human endothelial cells exposed to high glucose. European journal of pharmacology. 2010;627:42–48. doi: 10.1016/j.ejphar.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 207.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of o2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 208.Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: Contributions to atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 209.Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CR. Proinflammatory effects of interleukin-17a on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J Vasc Res. 2011;48:52–58. doi: 10.1159/000317400. [DOI] [PubMed] [Google Scholar]
- 210.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. American journal of physiology. Heart and circulatory physiology. 2003;285:H2290–2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 211.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weaver M, Liu J, Pimentel D, Reddy DJ, Harding P, Peterson EL, Pagano PJ. Adventitial delivery of dominant-negative p67phox attenuates neointimal hyperplasia of the rat carotid artery. American journal of physiology. Heart and circulatory physiology. 2006;290:H1933–1941. doi: 10.1152/ajpheart.00690.2005. [DOI] [PubMed] [Google Scholar]
- 213.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 214.Li JM, Fan LM, George VT, Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Imanishi T, Kobayashi K, Kuroi A, Ikejima H, Akasaka T. Pioglitazone inhibits angiotensin ii-induced senescence of endothelial progenitor cell. Hypertens Res. 2008;31:757–765. doi: 10.1291/hypres.31.757. [DOI] [PubMed] [Google Scholar]
- 216.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. Iqgap1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 217.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of nox4 and nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxidants & redox signaling. 2009;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kim SY, Lee JG, Cho WS, Cho KH, Sakong J, Kim JR, Chin BR, Baek SH. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol Cell Biol. 2010;88:197–204. doi: 10.1038/icb.2009.87. [DOI] [PubMed] [Google Scholar]
- 219.Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, Brandes RP. NADPH oxidase nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- 220.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 221.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and akt activation play a key role in angiotensin ii-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 223.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, Chandrasekar B. Angiotensin-ii type 1 receptor and nox2 mediate tcf/lef and creb dependent wisp1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 225.Wilson P, Morgan J, Funder JW, Fuller PJ, Young MJ. Mediators of mineralocorticoid receptor-induced profibrotic inflammatory responses in the heart. Clinical science. 2009;116:731–739. doi: 10.1042/CS20080247. [DOI] [PubMed] [Google Scholar]
- 226.Nakamura T, Kataoka K, Fukuda M, Nako H, Tokutomi Y, Dong YF, Ichijo H, Ogawa H, Kim-Mitsuyama S. Critical role of apoptosis signal-regulating kinase 1 in aldosterone/salt-induced cardiac inflammation and fibrosis. Hypertension. 2009;54:544–551. doi: 10.1161/HYPERTENSIONAHA.109.135392. [DOI] [PubMed] [Google Scholar]
- 227.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. 2004;24:1614–1620. doi: 10.1161/01.ATV.0000139011.94634.9d. [DOI] [PubMed] [Google Scholar]
- 228.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circulation research. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 229.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 231.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-term cyclic strain downregulates endothelial nox4. Antioxid Redox Signal. 2009;11:2385–2397. doi: 10.1089/ars.2009.2561. [DOI] [PubMed] [Google Scholar]
- 232.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: Implication for native ldl oxidation. Circulation research. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Peshavariya H, Jiang F, Taylor CJ, Selemidis S, Chang CW, Dusting G. Translation-linked mrna destabilization accompanying serum-induced nox4 expression in human endothelial cells. Antioxidants & redox signaling. 2009 doi: 10.1089/ars.2009.2579. [DOI] [PubMed] [Google Scholar]
- 234.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous nox4 mrna and inhibits angiotensin ii-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 236.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 237.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK. NADPH oxidase 4 mediates tgf-beta-induced smooth muscle alpha-actin via p38mapk and serum response factor. Free radical biology & medicine. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. Nox4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: The role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. Nox4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates tgfbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang S, Zhang Y, Ries W, Key L. Expression of nox4 in osteoclasts. J Cell Biochem. 2004;92:238–248. doi: 10.1002/jcb.20048. [DOI] [PubMed] [Google Scholar]
- 241.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase nox4 drives cardiac differentiation: Role in regulating cardiac transcription factors and map kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation research. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Molecular and cellular biology. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. The Journal of biological chemistry. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 246.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. The Journal of biological chemistry. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 247.Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. Nox4 as an oxygen sensor to regulate task-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 248.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-ca2+ release channel by NADPH oxidase 4. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of nox-based NAD(P)H oxidases in restenosis after carotid injury. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 250.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 251.Perrotta I, Sciangula A, Perrotta E, Donato G, Cassese M. Ultrastructural analysis and electron microscopic localization of nox4 in healthy and atherosclerotic human aorta. Ultrastruct Pathol. 2011;35:1–6. doi: 10.3109/01913123.2010.510261. [DOI] [PubMed] [Google Scholar]
- 252.Guo SJ, Wu LY, Shen WL, Chen WD, Wei J, Gao PJ, Zhu DL. Gene profile for differentiation of vascular adventitial myofibroblasts. Sheng li xue bao : [Acta physiologica Sinica] 2006;58:337–344. [PubMed] [Google Scholar]
- 253.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, Steger K, Foti M, Meier CA, Muzzin P, Kahn CR, Ogier-Denis E, Szanto I. Reduced expression of the NADPH oxidase nox4 is a hallmark of adipocyte differentiation. Biochimica et biophysica acta. 2007;1773:1015–1027. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 254.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by nox4-produced h2o2. American journal of physiology Cell physiology. 2009;296:C711–723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- 255.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK. NADPH oxidase 4 mediates tgf-beta-induced smooth muscle alpha-actin via p38mapk and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 257.Tong X, Hou X, Jourd’heuil D, Weisbrod RM, Cohen RA. Upregulation of nox4 by tgf{beta}1 oxidizes serca and inhibits no in arterial smooth muscle of the prediabetic zucker rat. Circulation research. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ros signal transduction by NADPH oxidase 4 localization. The Journal of cell biology. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. Nox4 regulates ros levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxidants & redox signaling. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 261.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 262.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by tnf-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:C422–432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tian XY, Yung LH, Wong WT, Liu J, Leung FP, Liu L, Chen Y, Kong SK, Kwan KM, Ng SM, Lai PB, Yung LM, Yao X, Huang Y. Bone morphogenic protein-4 induces endothelial cell apoptosis through oxidative stress-dependent p38mapk and jnk pathway. Journal of molecular and cellular cardiology. 2011 doi: 10.1016/j.yjmcc.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 264.Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 265.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al Ghuzlan A, Bidart JM, Schlumberger M, Dupuy C. Ros-generating NADPH oxidase nox4 is a critical mediator in oncogenic h-ras-induced DNA damage and subsequent senescence. Oncogene. 2011 doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, Serrao MP, Pinho MJ, Soares-da-Silva P. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev. 2009;2:138–145. doi: 10.4161/oxim.2.3.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li L, Smith A, Hagen TM, Frei B. Vascular oxidative stress and inflammation increase with age: Ameliorating effects of alpha-lipoic acid supplementation. Annals of the New York Academy of Sciences. 2010;1203:151–159. doi: 10.1111/j.1749-6632.2010.05555.x. [DOI] [PubMed] [Google Scholar]
- 268.McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulation of nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009;8:902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lener B, Koziel R, Pircher H, Hutter E, Greussing R, Herndler-Brandstetter D, Hermann M, Unterluggauer H, Jansen-Durr P. The NADPH oxidase nox4 restricts the replicative lifespan of human endothelial cells. The Biochemical journal. 2009;423:363–374. doi: 10.1042/BJ20090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li H, Liu Q, Wang N, Xu J. Correlation of different NADPH oxidase homologues with late endothelial progenitor cell senescence induced by angiotensin ii: Effect of telmisartan. Intern Med. 2011;50:1631–1642. doi: 10.2169/internalmedicine.50.5250. [DOI] [PubMed] [Google Scholar]
- 271.Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, Mahe C, Agostini M, Knight RA, Melino G, Federici M. Mir-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 272.Rueckschloss U, Villmow M, Klockner U. NADPH oxidase-derived superoxide impairs calcium transients and contraction in aged murine ventricular myocytes. Exp Gerontol. 2010;45:788–796. doi: 10.1016/j.exger.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 273.Jaulmes A, Sansilvestri-Morel P, Rolland-Valognes G, Bernhardt F, Gaertner R, Lockhart BP, Cordi A, Wierzbicki M, Rupin A, Verbeuren TJ. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 mapk pathway in cultured human endothelial cells. Thromb Res. 2009;124:439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 274.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of nf-kappab by palmitate in endothelial cells: A key role for NADPH oxidase-derived superoxide in response to tlr4 activation. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Moe KT, Yin NO, Naylynn TM, Khairunnisa K, Wutyi MA, Gu Y, Atan MS, Wong MC, Koh TH, Wong P. Nox2 and nox4 mediate tumour necrosis factor-alpha-induced ventricular remodelling in mice. Journal of cellular and molecular medicine. 2011;15:2601–2613. doi: 10.1111/j.1582-4934.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ryu J, Lee CW, Shin JA, Park CS, Kim JJ, Park SJ, Han KH. Fcgammariia mediates c-reactive protein-induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovascular research. 2007;75:555–565. doi: 10.1016/j.cardiores.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 277.Ritsick DR, Edens WA, Finnerty V, Lambeth JD. Nox regulation of smooth muscle contraction. Free radical biology & medicine. 2007;43:31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lassegue B, Griendling KK. NADPH oxidases: Functions and pathologies in the vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic research in cardiology. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zalba G, San Jose G, Moreno MU, Fortuno A, Diez J. NADPH oxidase-mediated oxidative stress: Genetic studies of the p22(phox) gene in hypertension. Antioxidants & redox signaling. 2005;7:1327–1336. doi: 10.1089/ars.2005.7.1327. [DOI] [PubMed] [Google Scholar]
- 283.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms nox1 and nox4 by the renin-angiotensin system in vitro and in vivo. Free radical biology & medicine. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 284.Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, Huang H. Contribution of different nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: Effect of valsartan. Pharmacol Res. 2007;55:408–417. doi: 10.1016/j.phrs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 285.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of at1 receptor blockade on renal injury and mitogen-activated protein activity in dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin ii infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cgmp signaling. Circ Res. 2002;90:E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 287.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin ii-mediated hypertension: A study in nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 288.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin ii-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 289.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via enos uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by nox1 knockout in angiotensin ii-dependent chronic hypertension. Hypertension. 2008;51:500–506. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- 291.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by nox1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 292.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Cook-Mills JM. Reactive oxygen species regulation of immune function. Mol Immunol. 2002;39:497–498. doi: 10.1016/s0161-5890(02)00205-5. [DOI] [PubMed] [Google Scholar]
- 294.Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin ii-infused mice. Am J Physiol Heart Circ Physiol. 2000;279:H2234–2240. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 295.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin ii in mice. Circulation research. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 296.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin ii. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular o(2)(-) and systolic blood pressure in mice. Circulation research. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 298.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: Link among angiotensin ii, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 299.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin ii-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. American journal of physiology Heart and circulatory physiology. 2005;288:H37–42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 300.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial nox2 NADPH oxidase in angiotensin ii-induced hypertension and vasomotor dysfunction. Basic research in cardiology. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin ii infusion involves increased superoxide production in the central nervous system. Circulation research. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 302.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of nox2 and nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin ii. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. Rna silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 304.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Senchenkova EY, Russell J, Kurmaeva E, Ostanin D, Granger DN. Role of t lymphocytes in angiotensin ii-mediated microvascular thrombosis. Hypertension. 2011;58:959–965. doi: 10.1161/HYPERTENSIONAHA.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: Involvement of the renin-angiotensin system. Hypertension research : official journal of the Japanese Society of Hypertension. 2006;29:813–820. doi: 10.1291/hypres.29.813. [DOI] [PubMed] [Google Scholar]
- 307.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 308.Paravicini TM, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke; a journal of cerebral circulation. 2004;35:584–589. doi: 10.1161/01.STR.0000112974.37028.58. [DOI] [PubMed] [Google Scholar]
- 309.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 310.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 311.Schmidt H, Hermans J, Schiffers P, Kleikers P, Radermacher K, Altenhofer S, Wingler K. NADPH oxidase as a pharmacological target in disease-relevant oxidative stress. Acta Physiologica. 2011;203(Supplement 687):O–22. [Google Scholar]
- 312.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. Nox1 deficiency protects from aortic dissection in response to angiotensin ii. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 313.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr Role for nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS. NADPH oxidases regulate cd44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. The Journal of biological chemistry. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Fenyo IM, Florea IC, Raicu M, Manea A. Tyrphostin ag490 reduces napdh oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein e-deficient mice. Vascular pharmacology. 2011;54:100–106. doi: 10.1016/j.vph.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 316.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 317.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22(phox) in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 318.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2002;22:2037–2043. doi: 10.1161/01.atv.0000040222.02255.0f. [DOI] [PubMed] [Google Scholar]
- 319.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 320.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in apoe-/- mice. American journal of physiology Heart and circulatory physiology. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 321.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47(phox): An essential component of NADPH oxidase. Circulation. 2000;101:1234–1236. doi: 10.1161/01.cir.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 322.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. P47phox is required for atherosclerotic lesion progression in apoe(-/-) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, IV, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. P22phox mrna expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 325.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, Quinn MT. Role of nf-kappab in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. Journal of leukocyte biology. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 326.Ding H, Hashem M, Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoe-deficient mouse: Changes in expression of NADPH oxidase subunits and enos. European journal of pharmacology. 2007;561:121–128. doi: 10.1016/j.ejphar.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 327.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Tian XY, Wong WT, Xu A, Chen ZY, Lu Y, Liu LM, Lee VW, Lau CW, Yao X, Huang Y. Rosuvastatin improves endothelial function in db/db mice: Role of angiotensin ii type 1 receptors and oxidative stress. British journal of pharmacology. 2011;164:598–606. doi: 10.1111/j.1476-5381.2011.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent nox5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. Journal of the American College of Cardiology. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: The “redox window” hypothesis. Antioxidants & redox signaling. 2009;11:1961–1974. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by nox1 triggers the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Komatsu D, Kato M, Nakayama J, Miyagawa S, Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic ras-induced vascular endothelial growth factor expression. Oncogene. 2008;27:4724–4732. doi: 10.1038/onc.2008.102. [DOI] [PubMed] [Google Scholar]
- 333.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 334.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circulation research. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Haddad P, Dussault S, Groleau J, Turgeon J, Maingrette F, Rivard A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: Effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis. 2011;217:340–349. doi: 10.1016/j.atherosclerosis.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 336.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. The American journal of pathology. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jackman KA, Miller AA, Drummond GR, Sobey CG. Importance of nox1 for angiotensin ii-induced cerebrovascular superoxide production and cortical infarct volume following ischemic stroke. Brain research. 2009;1286:215–220. doi: 10.1016/j.brainres.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 340.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiology of disease. 2010;40:185–192. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 341.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke; a journal of cerebral circulation. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 342.Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: Gender, reperfusion, t lymphocytes, and nox2-derived superoxide. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 344.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. American journal of respiratory cell and molecular biology. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase nox4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 346.McCann SK, Dusting GJ, Roulston CL. Early increase of nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. Journal of neuroscience research. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
- 347.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circulation research. 2010;106:1763–1774. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin ii-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 350.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of rac1 in the development of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin ii-induced interstitial cardiac fibrosis via a nox2-containing NADPH oxidase. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 352.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circulation research. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 353.Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circulation research. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 354.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin ii-induced cardiac hypertrophy. Circulation research. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 355.Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R, Colucci WS. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 356.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform nox2 in cardiac contractile dysfunction occurring in response to pressure overload. Journal of the American College of Cardiology. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 357.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 359.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxidants & redox signaling. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circulation research. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 361.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in h9c2 cells: The role of map kinases and NAD(P)H oxidase. Cardiovascular research. 2006;69:736–745. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 362.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circulation research. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 363.Fan C, Kawai Y, Inaba S, Arakawa K, Katsuyama M, Kajinami K, Yasuda T, Yabe-Nishimura C, Konoshita T, Miyamori I. Synergy of aldosterone and high salt induces vascular smooth muscle hypertrophy through up-regulation of nox1. The Journal of steroid biochemistry and molecular biology. 2008;111:29–36. doi: 10.1016/j.jsbmb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 364.Manea A, Tanase LI, Raicu M, Simionescu M. Jak/stat signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:105–112. doi: 10.1161/ATVBAHA.109.193896. [DOI] [PubMed] [Google Scholar]
- 365.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of nox1, nox2 and nox4 in vascular smooth muscle cells from wky and shr. Journal of the American Society of Hypertension : JASH. 2011;5:137–153. doi: 10.1016/j.jash.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 366.Cevik MO, Katsuyama M, Kanda S, Kaneko T, Iwata K, Ibi M, Matsuno K, Kakehi T, Cui W, Sasaki M, Yabe-Nishimura C. The ap-1 site is essential for the promoter activity of nox1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the erk1/2-junb pathway. Biochemical and biophysical research communications. 2008;374:351–355. doi: 10.1016/j.bbrc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 367.Katsuyama M, Fan C, Arakawa N, Nishinaka T, Miyagishi M, Taira K, Yabe-Nishimura C. Essential role of atf-1 in induction of nox1, a catalytic subunit of NADPH oxidase: Involvement of mitochondrial respiratory chain. The Biochemical journal. 2005;386:255–261. doi: 10.1042/BJ20041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Katsuyama M, Ozgur Cevik M, Arakawa N, Kakehi T, Nishinaka T, Iwata K, Ibi M, Matsuno K, Yabe-Nishimura C. Myocyte enhancer factor 2b is involved in the inducible expression of nox1/NADPH oxidase, a vascular superoxide-producing enzyme. The FEBS journal. 2007;274:5128–5136. doi: 10.1111/j.1742-4658.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 369.Manea A, Tanase LI, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, nox1 and nox4, by nuclear factor-kappab in human aortic smooth muscle cells. Biochemical and biophysical research communications. 2010;396:901–907. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 370.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature reviews. Drug discovery. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. Journal of the American College of Cardiology. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 372.Park YM, Lim BH, Touyz RM, Park JB. Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. Journal of Korean medical science. 2008;23:1039–1045. doi: 10.3346/jkms.2008.23.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Meischl C, Krijnen PA, Sipkens JA, Cillessen SA, Munoz IG, Okroj M, Ramska M, Muller A, Visser CA, Musters RJ, Simonides WS, Hack CE, Roos D, Niessen HW. Ischemia induces nuclear nox2 expression in cardiomyocytes and subsequently activates apoptosis. Apoptosis : an international journal on programmed cell death. 2006;11:913–921. doi: 10.1007/s10495-006-6304-7. [DOI] [PubMed] [Google Scholar]
- 374.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. Journal of molecular and cellular cardiology. 2011;50:408–416. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. The Journal of biological chemistry. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 376.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of x-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature genetics. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 377.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. NADPH oxidase 4 mediates upregulation of type 4 phosphodiesterases in human endothelial cells. Journal of cellular physiology. 2011 doi: 10.1002/jcp.22922. [DOI] [PubMed] [Google Scholar]
- 378.Zhang L, Sheppard OR, Shah AM, Brewer AC. Positive regulation of the NADPH oxidase nox4 promoter in vascular smooth muscle cells by e2f. Free radical biology & medicine. 2008;45:679–685. doi: 10.1016/j.freeradbiomed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 379.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit nox4 is a new target gene of the hypoxia-inducible factor-1. Molecular biology of the cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, Natarajan V. Nrf2 regulates hyperoxia-induced nox4 expression in human lung endothelium: Identification of functional antioxidant response elements on the nox4 promoter. Free radical biology & medicine. 2011;50:1749–1759. doi: 10.1016/j.freeradbiomed.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, Bornstein SR, Duerrschmidt N, Wagner AH, Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic research in cardiology. 2011;106:551–561. doi: 10.1007/s00395-011-0170-3. [DOI] [PubMed] [Google Scholar]
- 382.Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, Morawietz H. Nox4 overexpression activates reactive oxygen species and p38 mapk in human endothelial cells. Biochemical and biophysical research communications. 2009;380:355–360. doi: 10.1016/j.bbrc.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 383.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH. A key role for nox4 in epithelial cell death during development of lung fibrosis. Antioxidants & redox signaling. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M. Ap-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: Role of p22phox subunit. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 385.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Gorlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free radical biology & medicine. 2005;38:616–630. doi: 10.1016/j.freeradbiomed.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 386.Moriwaki K, Kiyomoto H, Hitomi H, Ihara G, Kaifu K, Matsubara K, Hara T, Kondo N, Ohmori K, Nishiyama A, Fukui T, Kohno M. Interferon-gamma enhances superoxide production in human mesangial cells via the jak-stat pathway. Kidney international. 2006;70:788–793. doi: 10.1038/sj.ki.5001639. [DOI] [PubMed] [Google Scholar]
- 387.Li L, Frei B. Prolonged exposure to lps increases iron, heme, and p22phox levels and NADPH oxidase activity in human aortic endothelial cells: Inhibition by desferrioxamine. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:732–738. doi: 10.1161/ATVBAHA.108.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hitomi H, Fukui T, Moriwaki K, Matsubara K, Sun GP, Rahman M, Nishiyama A, Kiyomoto H, Kimura S, Ohmori K, Abe Y, Kohno M. Synergistic effect of mechanical stretch and angiotensin ii on superoxide production via NADPH oxidase in vascular smooth muscle cells. Journal of hypertension. 2006;24:1089–1095. doi: 10.1097/01.hjh.0000226199.51805.88. [DOI] [PubMed] [Google Scholar]
- 389.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by nf-kb in human aortic smooth muscle cells. Archives of physiology and biochemistry. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 390.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin ii. Circulation research. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 391.Honjo T, Otsui K, Shiraki R, Kawashima S, Sawamura T, Yokoyama M, Inoue N. Essential role of noxa1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium : journal of endothelial cell research. 2008;15:137–141. doi: 10.1080/10623320802125433. [DOI] [PubMed] [Google Scholar]


