Abstract
Objectives
To noninvasively image the electophysiologic substrate of human ventricles after myocardial infarction and define its characteristics.
Background
Ventricular infarct border zone is characterized by abnormal cellular electrophsyiology and altered structural architecture and is a key contributor to arrhythmogenesis. The ability to noninvasively image its electrical characteristics could contribute to understanding of mechanisms and to risk-stratification for ventricular arrhythmia.
Methods
Electrocardiographic Imaging (ECGI), a noninvasive functional electrophysiologic imaging modality, was performed during sinus rhythm in 24 subjects with infarct-related myocardial scar. The abnormal electrophysiologic substrate on the epicardial aspect of the scar was identified and its location, size, and morphology were compared to the anatomic scar imaged by other noninvasive modalities.
Results
ECGI constructs epicardial electrograms which have characteristics of reduced amplitude (low voltage) and fractionation. ECGI co-localizes the epicardial electrical scar to the anatomic scar with a high degree of accuracy (sensitivity 89%, specificity 85%). In nearly all subjects, sinus rhythm activation patterns were affected by the presence of myocardial scar. Late potentials could be identified and were almost always within ventricular scar.
Conclusions
ECGI accurately identifies areas of anatomic scar and complements standard anatomic imaging by providing scar - related electrophysiologic characteristics of low voltages, altered sinus rhythm activation, electrogram fragmentation and presence of late potentials.
Keywords: ECGI, Infarct, Noninvasive Imaging, Border Zone, Ventricular Substrate
BACKGROUND
Delayed enhanced magnetic resonance imaging (DE-MRI) has been used to identify noninvasively anatomic myocardial scars in patients with prior myocardial infarction (MI)(1). DE-MRI has been used to improve prediction of ventricular arrhythmia inducibility at electrophysiology study(2,3), and of spontaneous ventricular arrhythmia and mortality(4).
Typically, the abnormal electrophysiological substrate extends beyond the dense anatomical scar, into regions of heterogeneous tissue containing excitable “islands” of myocardia(5). This “border zone” (BZ) is characterized by abnormal cellular electrophsyiology and altered structural properties due to remodeling processes. The BZ has been recognized as a contributor to scar-related arrhythmias and a target for catheter ablation. Recognizing its important role in arrhythmogenesis, attempts have been made to identify the BZ as the “gray zone” in DE-MRI images. However, this is not a natural application of MRI, which images anatomy, not electrophysiology. This distinction is of utmost importance, as electrophysiological properties of the scar determine arrhythmogenesis.
Currently, scar electrophysiology after MI is studied using electrophysiologic (EP) catheter mapping. However, this process is invasive, time-consuming, and carries a modest risk. Additionally, bipolar mapping incompletely represents the transmurality of the scar architecture(6,7). Although invasive unipolar endocardial mapping was recently reported to address some of the challenges related to identification of scar beyond the endocardial surface(8,9). the time constraints and patient risks prohibit it from becoming a routine cardiac test.
Experimental, theoretical and clinical studies have shown that scar-related abnormal electrical properties are reflected in specific characteristics of cardiac potential distributions and electrograms. Canine studies in a 5-day old infarct showed low-level fractionated electrograms in the infarct, which correlated with slow, discontinuous conduction, a property that supports arrhythmogenicity(10). Low-amplitude potentials, broad fractionated electrograms, and delayed local activation are seen in invasive mapping during sinus rhythm in patients with history of MI(11–13). Our laboratory has demonstrated the ability of a new noninvasive EP imaging modality (Electrocardiographic Imaging, ECGI) to reconstruct, from body surface potentials, such characteristics of epicardial potentials and electrograms over the scar region in canine experiments and human intraoperative studies(14–16). The purpose of this study is to explore the limits of ECGI to noninvasively identify areas of human infarct-related ventricular scar and to characterize the local electrophysiological properties in relation to its anatomic substrate.
METHODS
All protocols were approved by the Institutional Review Board at Washington University, and informed consent was obtained from all patients. Subjects with history of MI were identified and recruited during inpatient and outpatient visits.
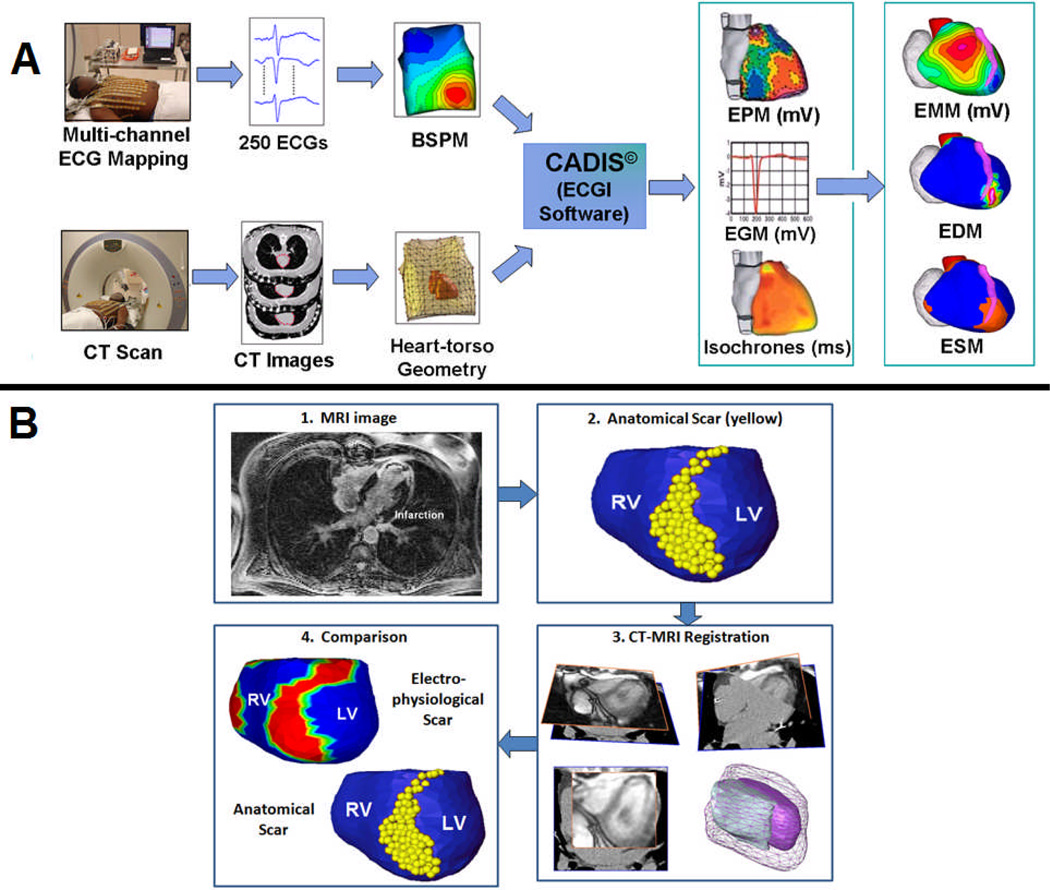
ECGI methodology was described previously (Figure 1)(17,18). 256 electrodes on strips were applied to the patient’s torso. CT markers were attached to each electrode. All strips were connected to a portable mapping system (BioSemi, Netherlands). After electrodes application, patients underwent thoracic non-contrast gated CT with axial resolution of 3mm. Scans were gated at 70% of the R-R interval (ventricular diastole). Patient-specific ventricular epicardial surface geometry and body surface electrode positions were labeled and digitized from CT images.
Figure 1.
A: The ECGI Procedure. Recorded body surface potentials and CT-imaged geometry are processed mathematically to obtain noninvasively potential maps (EPM), electrograms (EGM), and activation sequences (isochrones). From these data, electrogram magnitude maps (EMM), electrogram deflection maps (EDM) and electrical scar maps (ESM) are constructed (see text for details). ESM, defined by combining low magnitude potentials and EGMs with multiple deflections, is shown in red. B: Comparing electrical scar to anatomical scar. Anatomical scar is imaged with DE-MRI and, annotated (yellow dots) on the reconstructed cardiac geometry. The MRI image and CT from ECGI are co-registered to construct the anatomic scar map. A comparison of electrical (red) and anatomical (yellow) maps of scar on the anterior epicardial aspect of the interventricular septum is shown in B.4.
The 256 channels of body surface potentials (BSP) were sampled at 1-ms intervals from the start of the QRS complex through the ST segment (ventricular activation) during sinus rhythm (SR). The BSP and geometrical information (torso-heart geometrical relationship) were combined by ECGI algorithms to noninvasively construct epicardial potentials, unipolar epicardial electrograms, and epicardial activation sequences (isochrones). ECGI is constructed on a beat-by-beat basis and does not require accumulating data from many identical beats. Fewer than 1% of body surface electrocardiograms were rejected prior to the ECGI reconstruction. After ECGI reconstruction, none of the EGMs were rejected.
Electrogram Magnitude (Voltage)
For each subject, evenly-distributed reconstructed unipolar epicardial EGMs were used for data analysis. EGMs from valvular regions were excluded. EGM magnitude (voltage) was measured peak-to-peak. Because absolute EGM magnitude is patient-specific, EGMs were indexed to the maximum value for each patient. Low voltage regions and very low voltage regions were defined by EGMs with amplitudes less than 30% or 15% of the maximum, respectively. Epicardial Electrogram Magnitude Maps (EMM) were created using patient-specific geometry.
Electrogram Characterization and Localization
Electrogram morphology was characterized by degree of fractionation (number of deflections; low pass filtering at 120Hz), and displayed as epicardial Electrogram Deflection Maps (EDM). Combining both voltage and fractionation of EGMs, the “Electrical Scar” is defined as the area with electrograms that demonstrated both low voltage (< 30% of maximum value) and multiple deflections. Electrical Scar Maps (ESM) represent this combined criterion visually.
Comparison of ECGI-Determined Electrical Scar to Conventionally-Imaged Anatomic Scar
Clinical noninvasive cardiac imaging was available for comparison, including DE-MRI for patients without cardiac devices, and myocardial perfusion imaging with single-photon emission computed tomography (SPECT) for patients with cardiac devices. For DE-MRI, coregistration of ECGI and MRI images was performed to demonstrate relationship between electrical and anatomic scar (Figure 1B). The epicardial aspect of the DE-MRI scar was used for analysis. For SPECT comparison, determination of myocardial scar was performed in an automated fashion, using AutoQuant software (Phillips Medical Systems). ECGI maps were divided into a standard 17-segment classification and scored based on presence or absence of electrical scar. Because ECGI reconstructs epicardial electrograms, septal segments were not scored. The anatomic scar imaging and ECGI scar imaging were analyzed independently to eliminate bias.
Functional Imaging: Sinus Rhythm Activation, Late Potentials and Electrical Scar
During sinus rhythm, activation times were determined by the maximal negative slope of EGMs and activation isochrone (AI) maps were created(17,18). Lines/regions of block (thick black lines) were inferred if adjacent activation times differed by more than 50ms. Slow conduction is represented by crowded isochrones. Deflections in EGMs above the ambient electrical noise level that occurred after the surface QRS were labeled as late potentials (LP).
RESULTS
24 patients with history of MI were included in the study (mean age 62 years, mean LVEF 30%). The ECGI procedure was completed successfully in all subjects without adverse events. Patient demographics are in Table 1. Representative examples are presented in the paper; all data are in the Online Supplement.
Table 1.
Patient Characteristics.
| Patient | Age | Gender | Race | Infarct | LVEF | AAD | ACE/ ARB |
BB | Digoxin | VT |
|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic Cardiomyopathy | ||||||||||
| 1 | 80 | Male | White | Inferior | 33 | Yes | Yes | |||
| 2 | 66 | Male | White | Inferolateral | 17 | Amiodarone | Yes | Yes | Yes | |
| 3 | 48 | Male | White | Apical | 17 | Amiodarone | Yes | Yes | Yes | Yes |
| 4 | 62 | Male | White | Inferobasal | 30 | Amiodarone Mexiletine |
Yes | Yes | Yes | |
| 5 | 71 | Male | White | Anteroapical | 27 | Amiodarone Mexiletine |
Yes | Yes | Yes | Yes |
| 6 | 27 | Female | Black | Apical | 10 | Amiodarone | Yes | Yes | Yes | |
| 7 | 50 | Male | White | Inferobasal | 23 | Sotalol | Yes | Yes | Yes | Yes |
| 8 | 68 | Male | White | Anteroapical | 20 | Amiodarone | Yes | Yes | Yes | |
| 9 | 52 | Male | Other | Inferoapical | 32 | Yes | Yes | |||
| 10 | 85 | Male | White | Inferior | 40 | Yes | Yes | Yes | ||
| 11 | 76 | Male | White | Inferolateral | 35 | Yes | Yes | |||
| 12 | 77 | Male | White | Inferolateral | 40 | Yes | Yes | |||
| 13 | 66 | Male | White | Inferoseptal | 32 | Amiodarone | Yes | Yes | Yes | |
| 14 | 74 | Male | White | Inferoseptal | 24 | Amiodarone | Yes | Yes | Yes | |
| 15 | 58 | Male | White | Apical | 15 | Amiodarone | Yes | Yes | ||
| 16 | 52 | Male | White | Anterior | 30 | Sotalol | Yes | Yes | Yes | |
| 17 | 59 | Male | White | Inferior | 33 | Amiodarone | Yes | Yes | Yes | |
| 18 | 60 | Male | White | Apical | 55 | Yes | Yes | Yes | ||
| 19 | 50 | Male | White | Anteroapical | 35 | Yes | Yes | |||
| 20 | 55 | Male | White | Anteroapical | 34 | Yes | Yes | |||
| 21 | 50 | Female | White | Anteroapical | 35 | Yes | Yes | |||
| 22 | 64 | Male | Black | Septal | 45 | Yes | Yes | |||
| 23 | 62 | Male | White | Apical | 15 | Yes | Yes | Yes | ||
| 24 | 76 | Male | White | Septal | 35 | Yes | Yes | |||
AAD: antiarrhythmic drug; ACE: angiotensin converting enzyme-inhibitor; ARB: angiotensin receptor-blocker; BB: beta-blocker; LVEF: left ventricular ejection fraction; VT: ventricular tachycardia
For each subject, average of 769 EGMs were used for analysis (range 620 – 885). The average maximum voltage was 8.6mV ± 2.8mV (range 2.3mV to 14.9mV),and average minimum 0.17mV ± 0.14mV (range 0.02mV to 0.58mV). Average mean voltage was 2.0mV ± 0.83mV (range 0.51mV to 3.9mV).
Low voltage EGMs comprised 55% +/− 9% of all EGMs for each patient (range 36% to 73%), and very-low-voltage EGMs 19% +/− 9% (range 6% to 43%). Fractionated EGMs (2 or more deflections) were observed in every patient. On average, they comprised 18% +/− 11% of EGMs in each patient (range 5% to 48%), with 91% of fractionated EGMs inside low voltage regions and 51% inside very-low-voltage regions.
Electrogram Characterization and Localization
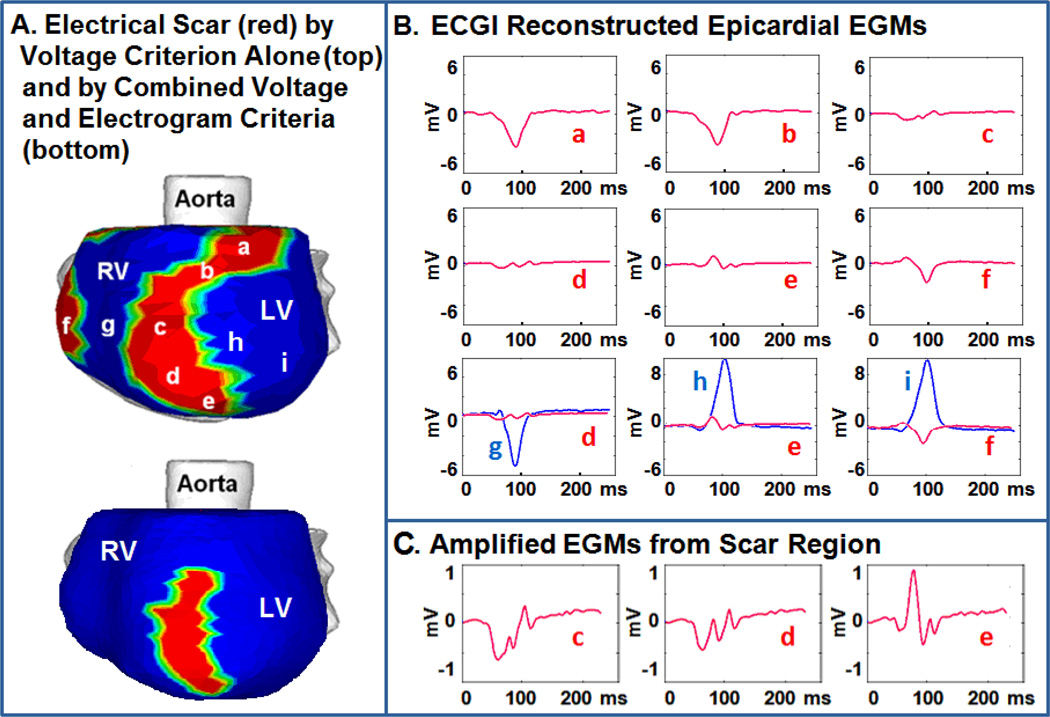
Examples of constructed epicardial EGMs from inside and around an electrical scar is shown in Figure 2. Panel A shows the electrical scar in red. Top image uses a conservative criteron of reduced amplitude (low voltage) alone. Bottom image uses a strict criterion combining low voltage with EGM fractionation to define electrical scar. Panel B shows representative EGMs from areas within the low voltage regions (a–f; red). For comparison, EGMs from neighboring myocardium outside the scar (g–i) are shown in blue. Panel C shows amplified EGM signals from the electrical scar, demonstrating high degree of fractionation.
Figure 2.
EGM Characteristics A: Electrical scar (red) is shown in the left anterior obligue view. Top image uses a reduced voltage criterion to identify scar. Bottom image uses reduced voltage and EGM fractionation to identify scar. B: EGMs a,b and f (red) from low voltage regions demonstrate low amplitude alone. EGMs c–e (red) demonstrate both fractionation and low amplitude. EGMs g–i (blue) from neighboring regions outside the scar demonstrate considerably larger amplitude and single deflection. C: EGMs c–e amplified to clearly demonstrate multiple deflections.
Comparison of ECGI-Determined Electrical Scar to Anatomic Scar from DE-MRI
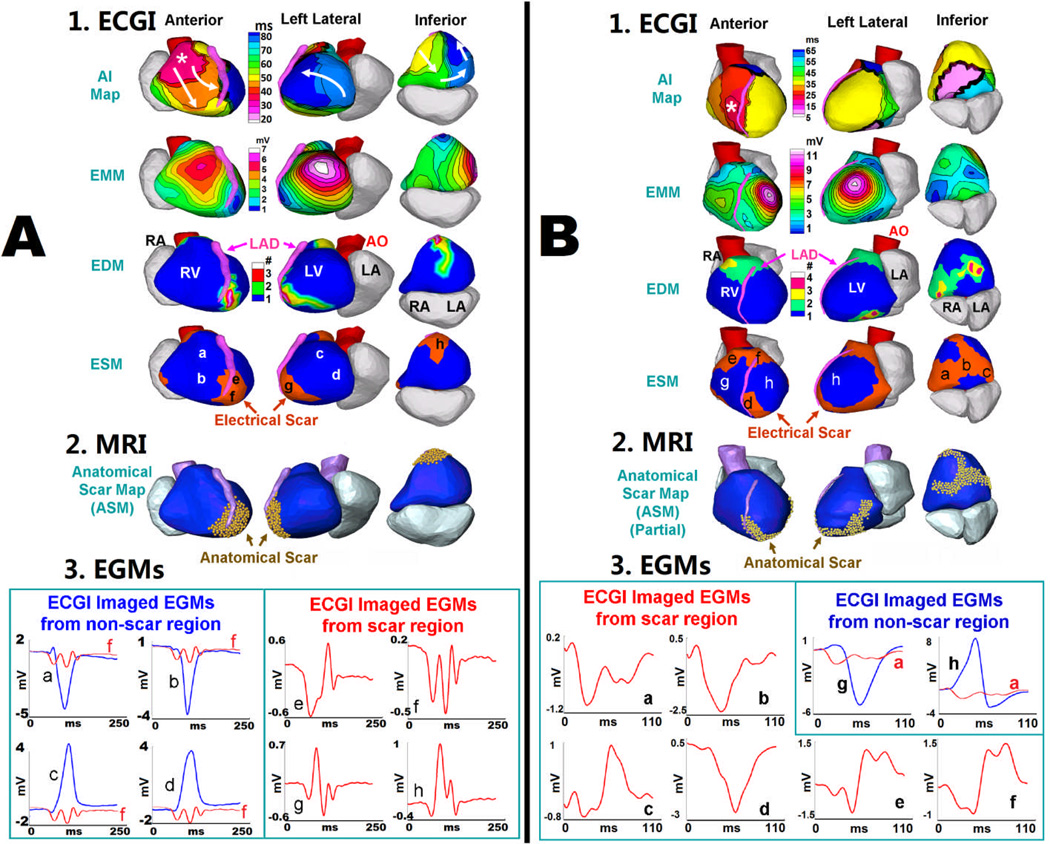
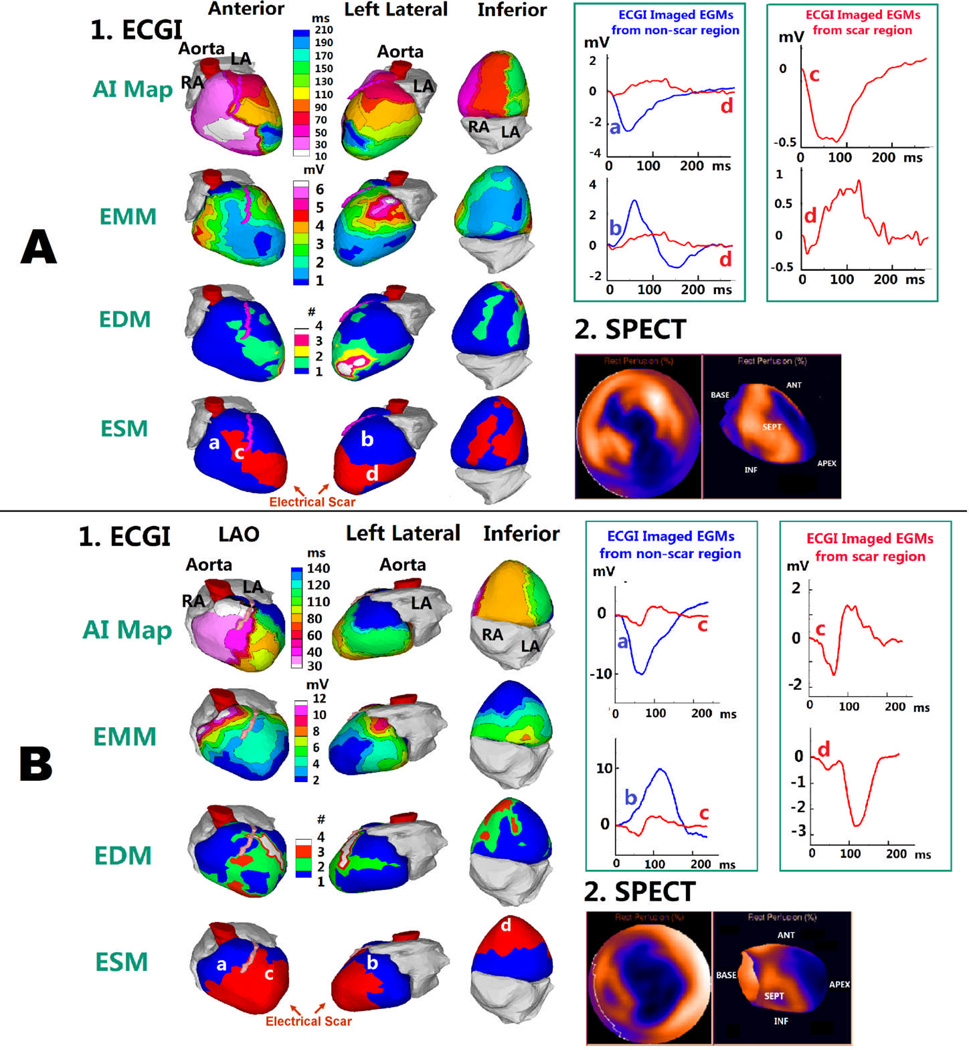
Anatomical scar from DE-MRI was compared to ECGI electrical scar for five subjects without a cardiac device in a blinded fashion. An example of anterior scar is shown in Figure 1B. Figure 3 shows two additional examples: one with a localized apical infarction and the other with a complex morphology inferoapical infarction. ECGI co-localizes the epicardial electrical scar to the anatomic scar with high accuracy. The two-dimensional shape of the anatomic scar is accurately reproduced with electrical scar imaging, as highlighted by the serpiginous scar shape in Figure 3B. Representative EGMs from non-scar regions (blue) and electrical scar regions (red) are shown below each example, highlighting the low amplitude and fractionated EGM qualities seen in electrical scar.
Figure 3.
Relationship between ECGI-derived Electrical Scar and DE-MRI Anatomic Scar. A: Apical MI. 1, top to bottom: SR activation map (AI map), EMM, EDM and ESM (three views). Asterisk in AI map marks the RV breakthrough site of earliest activation; arrows show wavefront propagation. Latest activation is in LV apex (dark blue) which is abnormal. ESM demonstrates an electrical scar at apex (red). 2: Anatomical scar map from DE-MRI (gold) shows similar apical distribution of scar. 3: Four selected EGMs from non-scar region (blue) and from scar region (red) (locations indicated on ESM). Scar EGMs are shown together with non-scar EGMs to demonstrate magnitude difference, and on amplified scale to show clearly multiple deflections (fractionation). B: Inferior MI. Similar format to panel A. SR activation of the inferior septum is abnormal (pink region in AI map). ESM demonstrates electrical scar that extends across the inferior wall and toward the apex, similar to the anatomical scar
Comparison of ECGI-Determined Electrical Scar to Anatomic Scar from SPECT
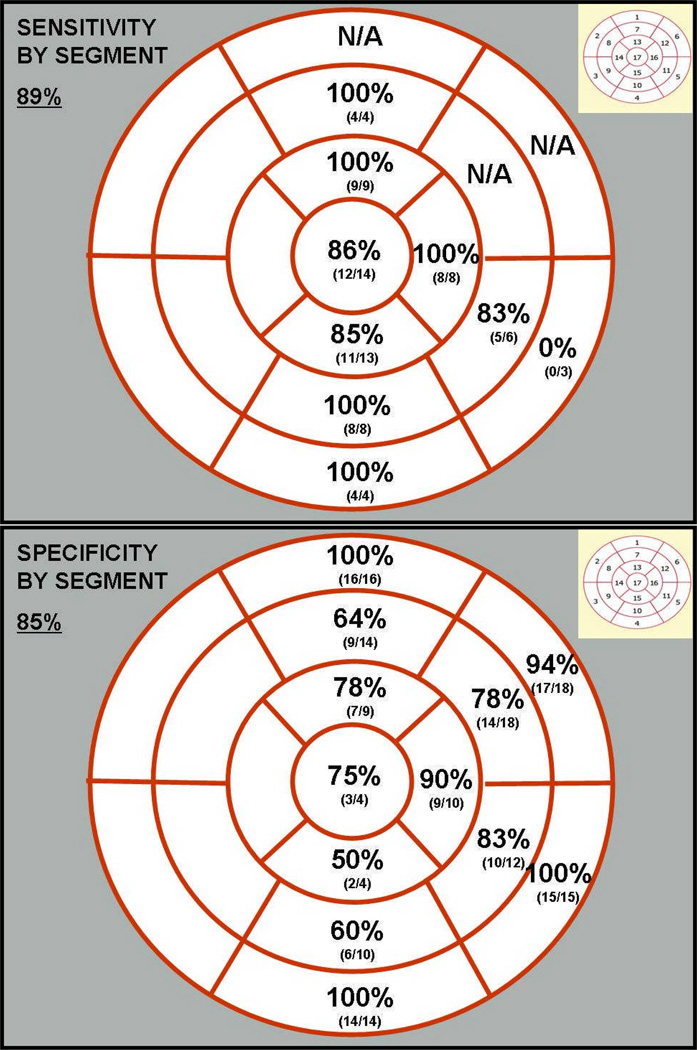
Anatomical scar from SPECT was compared to ECGI electrical scar in 18 subjects in a blinded fashion. Segmental sensitivity and specificity for ECGI is shown in Figure 4. Overall, the sensitivity and specificity of ECGI to detect scar in each segment of myocardium compared to SPECT imaging was 89% and 85%, respectively. Apical and mid-cavitary segments had the highest sensitivity, while basal segments had the lowest sensitivity. This is most likely due to the purposeful exclusion of basal valvular regions from ECGI analysis. Inferior segments had the lowest specificity.
Figure 4.
Segmental sensitivity and specificity of ECGI electrical scar imaging compared to rest myocardial perfusion imaging (SPECT). Standard 17-segment classification for sensitivity (top) and specificity (bottom) analyses. Septal segments were excluded.
Figure 5 shows two examples comparing ECGI electrical scar with SPECT imaging for a subject with an inferoapical infarct and a subject with a large apical infarct/aneurysm. ECGI co-localizes the epicardial electrical scar (red region, ESM) to the anatomic scar with high accuracy. EGMs from the scar region (red) demonstrate fractionated, low-amplitude qualities of electrical scar.
Figure 5.
Relationship between ECGI-derived Electrical Scar and SPECT Anatomic Scar. Similar format to Figure 3, except SPECT images replace MRI scar maps. A: Apical MI. Latest activation is in the anterior apex (dark blue in AI map), which is abnormal. ESM demonstrates an electrical scar in the apex, extending anteriorly and inferiorly (red). Resting myocardial perfusion images (SPECT), shown in a standard “bullseye” configuration (left) and long-axis view (right), demonstrate large area of infarction in the anterior, apical and inferior LV.
B: Apical Aneurysm. ESM demonstrates a large electrical scar across the apex. SPECT imaging shows similar extensive apical distribution of scar.
Functional Imaging: Alteration in Sinus Rhythm Activation and Late Potentials
Normal epicardial activation patterns have been reported previously(18). In general, earliest epicardial activation is in the anterior right ventricle (RV) and latest is along the basal lateral left ventricle (LV). AI maps in Figures 3 and 5 show how the presence and location of electrical scar alters the epicardial activation pattern during SR. Figure 3A shows normal location for earliest epicardial breakthrough (asterisk) but a line of block along the inferior and apical aspect of the electrical scar (thick black line). This forces LV activation to progress in a base-to-apex pattern, with the area of latest activation near the apical scar. Figure 5A shows a similar pattern, with earliest activation in the RV (white) and a line of block along the septal border of the electrical scar. Latest activation (dark blue) occurs at the LV side of the scar, nearly 200ms after the first breakthrough. The activation pattern of unidirectional block at one border of a scar, followed by marked conduction delay and latest activation near the other border of a scar is often associated with initiation of reentry and reentrant ventricular arrhythmia.
Of the 19 subjects imaged during SR, 16 (84%) demonstrated altered or delayed epicardial activation at the location of electrical scar. Ten (52%) had latest activation at the electrical scar location. Ten (52%) had a line of block at the scar border.
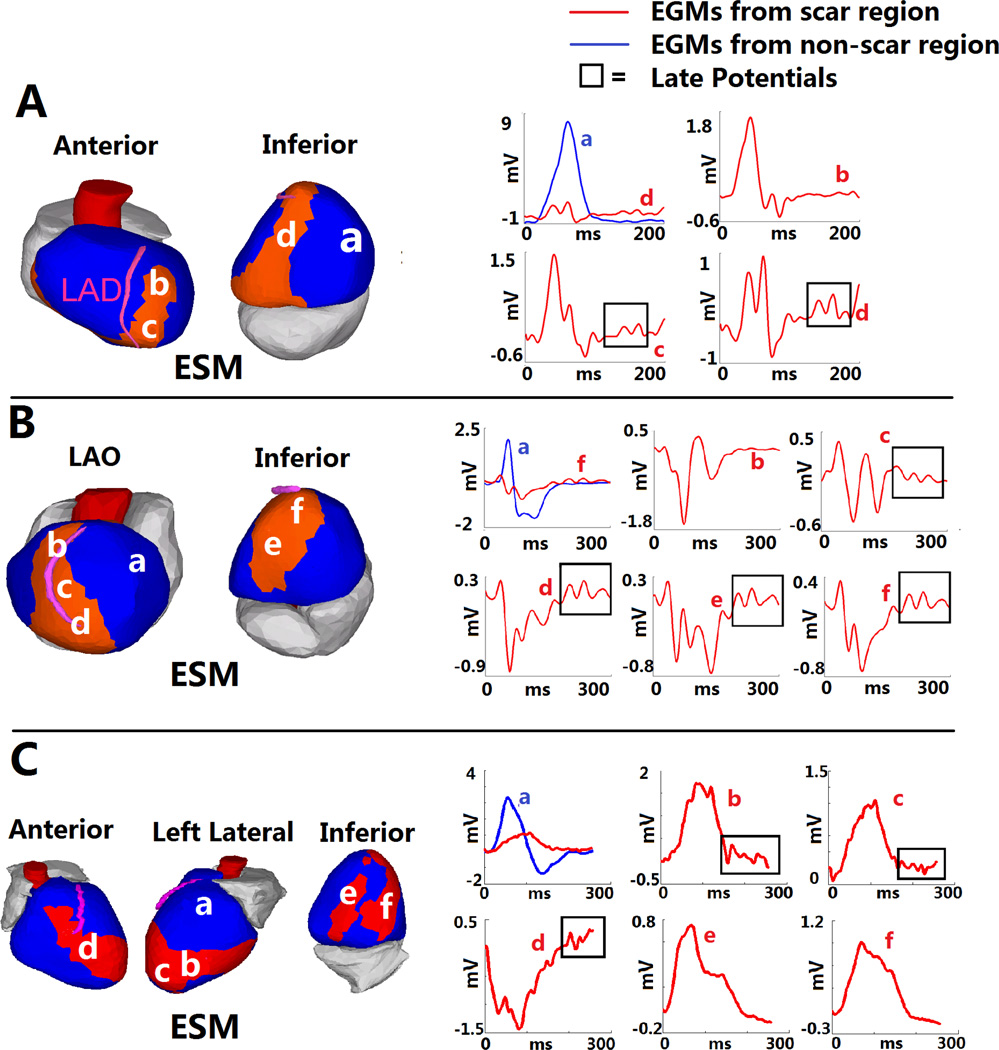
In addition to the functional relationship between electrical scar and SR activation patterns, EGMs with late potentials (LP) were observed. LP were present in an average of 8.4% of all EGMs for each patient (range 0% to 35%). 17 of 24 subjects had > 3% LP. Almost all LP were found within the electrical scar (94%); 62% were found within the very-low-voltage region. Figure 6 shows three examples of LP EGMs within the electrical scar.
Figure 6.
Late Potentials within Electrical Scar. A: ESM from a patient with inferoapical scar. Three EGMs from the scar are shown on the right (b,c and d; red). Scar EGM d is also shown together with non-scar EGM a (blue) in the upper left panel, to highlight differences. EGMs c and d demonstrate late deflections (“late potentials”) (box). LPs were observed almost exclusively within electrical scar. However (EGM b), LPs were not present in all scar regions. B: Anteroapical infarct. LPs are present in EGMs from inferior and apical regions (c–f), but not the anterior region (b). C: Complex anterior, apical and inferior infarction. LPs are present in several EGMs from the anterior and apical regions (b–d), but not the inferior region (e,f).
DISCUSSION
Key findings of this study include: 1) within anatomic myocardial scar in post-MI patients, ECGI reconstructs noninvasively EGMs characterized by reduced amplitude (low voltage) and fractionation; 2) using combination of low voltage and fractionated EGMs, ECGI images the “electrical scar,” which co-localizes with the anatomical scar as determined by DE-MRI or SPECT; 3) ECGI is a functional imaging modality that complements MRI and SPECT by imaging scar-related electrophysiologic characteristics of altered SR activation, EGM fragmentation and presence of late potentials. We define the periphery of the epicardial electrical scar, outside the epicardial anatomical scar, as the border zone. This study is a step toward noninvasively imaging the relationship between myocardial anatomic scar and its electrophysiologic properties.
Fractionated, low-magnitude electrograms are commonly found in post-infarct myocardium, and it is well-accepted that they reflect slow, non-uniform and discontinuous conduction through the heterogeneous scar substrate(10,11,19). Consistent with invasive studies, the ECGI noninvasive EGMs from scar regions are fragmented, of small magnitude and prolonged duration, reflecting similar substrate properties.
Low-amplitude, high-frequency signals have been recorded from the body surface in post-MI patients at the end and following the QRS complex(20). These late potentials are thought to originate from the infarct border zone and correspond to late deflections on electrograms recorded directly from the heart(21,22); they are thought to reflect late activation via slow discontinuous conduction along viable myocardial fibers. Endocardial catheter mapping of patients with coronary artery disease identified LPs in 12% of the sampled electrograms, similar to the results of this study(23). The ECGI reconstructed LPs were localized to electrical scar regions.
The presence of scar provides electrophysiological substrate that supports asymmetrical electrical loading on a propagating wavefront, a property that favors formation of unidirectional block. Using ECGI, lines of block and altered wavefront propagation were clearly seen and were associated with the borders of electrical scar. Similarly, scar related regions of slow conduction were detected. The combination of unidirectional block and slow conduction is highly arrhythmogenic, providing conditions for reentrant VT. “Patchiness” of the scar substrate (islands of viable myocardium within scar tissue) supports slow discontinuous conduction and conditions for block; it manifests electrophysiologically in fragmented EGMs and presence of late potentials. Typically, each feature of the electrical scar (location, size and shape, EGM fragmentation, late potentials, ventricular activation patterns) is assessed in isolation during a clinical EP study, and EGMs from different parts of the scar are recorded sequentially with a roving-catheter process that requires a long time to cover the entire scar. ECGI provides the ability to gather this information from the entire epicardial scar in a single noninvasive study, during a single beat.
A noninvasive method for identifying post- MI patients at risk of arrhythmia would aid in making decisions regarding preventative intervention, such as ICD implantation. Also, with increasing numbers of catheter-based VT ablation procedures, there is great interest in defining scar borderzone prior to a procedure. ECGI is still a novel research tool and its limited availability precludes large-scale multicenter clinical studies. However, our results suggest potential role for ECGI in arrhythmia risk stratification or identification of targets for ablation based on the electrophysiological properties of the scar.
Several limitations of the study should be recognized. At this stage of development, ECGI is limited to imaging the epicardium, and cannot image the ventricular septum. Intraoperative mapping suggest a purely epicardial location of the arrhythmia substrate in 33% of patients(24,25) although this number is increasing with epicardial mapping becoming more common. ECGI reconstructs epicardial potentials and unipolar EGMs, which are more affected by far field influences than bipolar EGMs. However, evolution in time of the epicardial potential pattern and unipolar EGM properties provide information on the intramural depth of the VT circuit(8,9,26) which partially addresses the limitation above. Because of small signal amplitude in regions of scar and because ECGI cannot image active depolarization during a repolarization period (T wave), it is likely that ECGI does not image all late potentials.
The extent of the ECGI-determined electrophysiological scar depends on the threshold chosen for scar EGM magnitude. With our choice of 30% of the maximum value for each patient, the electrophysiological scars and DE-MRI-imaged anatomical scars were highly correlated in our patient population, and ECGI achieved a reasonable level of specificity and sensitivity compared with SPECT. As shown in Figure 2, compared to using EGM magnitude alone, the use of multiple criteria (EGM magnitude and fractionation) increases the specificity of scar determination, and reduces the confounding influence of epicardial fat(27). It will require a larger-scale study to establish a universal criterion for ventricular electrical scar with high level of statistical significance.
Supplementary Material
Acknowledgments
Yoram Rudy is the Fred Saigh distinguished professor at Washington University in St Louis. This study was supported by NIH-NHLBI grants R01-HL-033343-26 and R01HL-049054-18 (to Yoram Rudy) and Grants 1 UL1 RR024992-01, 1 TL1 RR024995-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR) of the NIH. Dr. Rudy co-chairs the scientific advisory board of and holds equity in CardioInsight Technologies. CardioInsight Technologies does not support any research conducted by Dr Rudy, including that presented here.
ABBREVIATIONS
- AI Map
activation isochrone map—Image of the heart that displays chronological progression of the epicardial electrical activation.
- EMM
electrogram magnitude map—Image of the heart that displays the magnitude (voltage) of computed epicardial electrograms.
- EDM
electrogram deflection map—Image of the heart that displays the number of deflections (fractionation) in the computed epicardial electrograms.
- ESM
electrical scar map—Image of the heart that combines abnormal features of computed epicardial electrograms (low voltage and fractionation) to determine the presence of “electrical scar” (a region of abnormal electrical substrate associated with post-MI anatomical scar).
- EGM
electrogram
REFERENCES
- 1.Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. doi: 10.1161/01.cir.0000036368.63317.1c. [DOI] [PubMed] [Google Scholar]
- 2.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan AT, Shayne AJ, Brown KA, et al. Characterization of peri-infarct zone by contrast-enhanced magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 5.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 6.Codreanu A, Ollie F, Aliot E, et al. Electroanatomic Characterization of Post-Infarct Scars. Journal of Amer Coll Cardiol. 2008;52:839–842. doi: 10.1016/j.jacc.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins B, Crawford T, Good E, et al. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polin G, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson M, Gerstenfeld E, Desjardins B, et al. Endocardial Unipolar Voltage Mapping to Detect Epicardial VT Substrate in Patients with Nonischemic Left Ventricular Cardiomyopathy. Circulation Arrhythmia and Electrophysiol. 2011;4:49–55. doi: 10.1161/CIRCEP.110.959957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner PI, Ursell PC, Fenoglio JJ, Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 11.J. M. T. de Bakker JMT, Janse MJ, van Cappelle FJL, Durrer D. Endocardial mapping by simultaneous recording of endocardial electrograms during cardiac surgery for ventricular aneurysm. J Am Coll Cardiol. 1983;2:947–953. doi: 10.1016/s0735-1097(83)80244-7. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz LN, Harken AH, Kastor JA, Josephson ME. Ventricular resection guided by epicardial and endocardial mapping for treatment of recurrent ventricular tachycardia. N Engl J Med. 1980;302:589–593. doi: 10.1056/NEJM198003133021101. [DOI] [PubMed] [Google Scholar]
- 13.Klein H, Karp RB, Kouchoukos NT, Zorn GL, Jr, James TN, Waldo AL. Intraoperative electrophysiologic mapping of the ventricles during sinus rhythm in patients with a previous myocardial infarction. Identification of the electrophysiologic substrate of ventricular arrhythmias. Circulation. 1982;66:847–853. doi: 10.1161/01.cir.66.4.847. [DOI] [PubMed] [Google Scholar]
- 14.Burnes JE, Taccardi B, Ershler PR, Rudy Y. Noninvasive electrocardiographic imaging of substrate and intramural ventricular tachycardia in infarcted hearts. J Am Coll Cardiol. 2001;38:2071–2078. doi: 10.1016/s0735-1097(01)01653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnes JE, Taccardi B, MacLeod RS, Rudy Y. Noninvasive ECG Imaging of Electrophysiologically Abnormal Substrates in Infarcted Hearts. Circulation. 2000;101:533–540. doi: 10.1161/01.cir.101.5.533. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem RN, Jia P, Ramanathan C, Ryu K, Markowitz A, Rudy Y. Noninvasive electrocardiographic imaging (ECGI): comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2:339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat. Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephson ME, Wit AL. Fractionated electrical activity and continuous electrical activity: fact or artifact? Circulation. 1984;70:529–532. doi: 10.1161/01.cir.70.4.529. [DOI] [PubMed] [Google Scholar]
- 20.Denes P, Santarelli P, Hauser RG, Uretz EF. Quantitative analysis of the high-frequency components of the terminal portion of the body surface QRS in normal subjects and in patients with ventricular tachycardia. Circulation. 1983;67:1129–1138. doi: 10.1161/01.cir.67.5.1129. [DOI] [PubMed] [Google Scholar]
- 21.Berbari EJ, Scherlag BJ, Hope RR, Lazzara R. Recording from the body surface of arrhythmogenic ventricular activity during the S-T segment. Am J Cardiol. 1978;41:697–702. doi: 10.1016/0002-9149(78)90820-2. [DOI] [PubMed] [Google Scholar]
- 22.Simson MB, Untereker WJ, Spielman SR, et al. Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am J Cardiol. 1983;51:105–112. doi: 10.1016/s0002-9149(83)80020-4. [DOI] [PubMed] [Google Scholar]
- 23.Cassidy DM, Vassallo JA, Miller JM, et al. Endocardial catheter mapping in patients in sinus rhythm: relationship to underlying heart disease and ventricular arrhythmias. Circulation. 1986;73:645–652. doi: 10.1161/01.cir.73.4.645. [DOI] [PubMed] [Google Scholar]
- 24.Kaltenbrunner W, Cardinal R, Dubuc M, et al. Epicardial and endocardial mapping of ventricular tachycardia in patients with myocardial infarction. Is the origin of the tachycardia always subendocardially localized? Circulation. 1991;84:1058–1071. doi: 10.1161/01.cir.84.3.1058. [DOI] [PubMed] [Google Scholar]
- 25.Littmann L, Svenson RH, Gallagher JJ, et al. Functional role of the epicardium in postinfarction ventricular tachycardia. Observations derived from computerized epicardial activation mapping, entrainment, and epicardial laser photoablation. Circulation. 1991;83:1577–1591. doi: 10.1161/01.cir.83.5.1577. [DOI] [PubMed] [Google Scholar]
- 26.Oster HS, Taccardi B, Lux RL, Ersher PR, Rudy Y. Electrocardiographic imaging: noninvasive characterization of intramural myocardial activation from inverse-reconstructed epicardial potentials and electrograms. Circulation. 1998;97:1496–1507. doi: 10.1161/01.cir.97.15.1496. [DOI] [PubMed] [Google Scholar]
- 27.Tung R, Nakahara S, Ramirez R, et al. Distinguishing epicardial fat from scar: analysis of electrograms using high-density electroanatomic mapping in a novel infarct model. Heart Rhythm. 2010;7:389–395. doi: 10.1016/j.hrthm.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.