Abstract
Treatment of refractory gout remains a challenge on drug development. While pegloticase, a recombinant mammalian uricase modified with monomethoxyl-poly(ethylene glycol) (mPEG) is effective in treating refractory gout, after continued treatment for three months biweekly at a therapeutic dose of 0.14 mg/kg body weight, it elicits an immune response against mPEG in nearly 20% of patients. For continued treatment of refractory gout PEGylated uricases at monthly therapeutic doses below 4 μg/kg body weight have promise. To formulate uricases to achieve monthly therapeutic regimens requires pharmacodynamics simulation and experimentation including: (a) molecular engineering of uricases based on rational design and evolution biotechnology in combination to improve their inherent catalytic efficiency, thermostability and selectivity for urate over xanthine and; (b) optimization of the number and distribution of accessible reactive amino acid residues in native uricases for site-specific PEGylation with PEG derivatives with lower of immunogenicity than mPEG to retain activity, minimize immunogenicity and enhance the pharmacokinetics of the PEGylated uricase. These issues are briefly reviewed as a means to stimulate the development of safer uricase formulations for continued treatment of refractory gout.
Keywords: Hyperuricemia, uricase, refractory gout, molecular engineering
Introduction
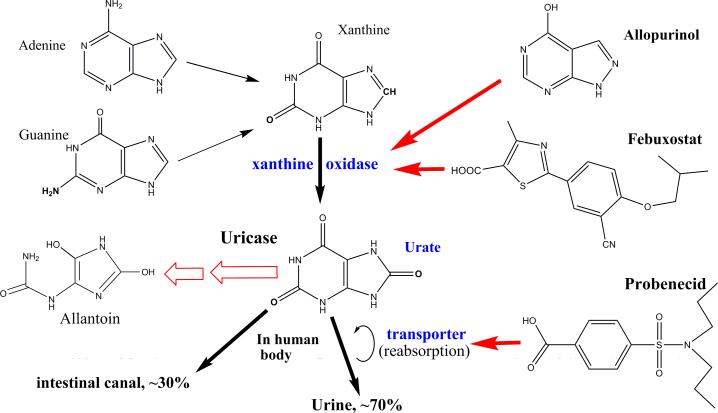
In humans, xanthine is oxidized by xanthine oxidase (XO) into urate as the end-product of purine catabolism [Ramazzina et al, 2006; Richette and Bardin, 2006]. Urate is mainly excreted through the kidney and partially through the gastrointestinal tract, but is re-absorbed in kidney (Fig. 1). Pathophysiological processes that accelerate purine catabolism and/or reduce kidney excretion of urate will elevate plasma urate levels. Hyperuricemia occurs when plasma urate levels are over the solubility of urate. The latter is usually associated with hyperxanthinemia, i.e., plasma xanthine levels over the average in healthy individuals that can lead to the precipitation of xanthine resulting in kidney dysfunction [Band et al, 1970; Mughal et al, 2010]. Hyperuricemia is associated with urate precipitation with the formation of crystals that are involved in gout, tumor lysis syndrome (TLS), chronic kidney disease (CKD) and various cardiovascular diseases [Davidson et al, 2004; Edwards, 2009; Feig et al, 2008; Feig, 2009; Kang and Nakagawa, 2005; Richette and Bardin, 2010].
Fig. 1.
Catabolism of purine bases and targets of anti-hyperuricemia agents. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Therapy for hyperuricemia-associated diseases are treated by agents that keep plasma urate concentrations from precipitating with the additional benefit of removing existing urate crystals. TLS is characterized by the acute onset of hyperuricemia during cancer chemotherapy [Davidson et al, 2004; Edwards, 2009; Mughal et al, 2010]. Gout, with a prevalence of about 1%, is characterized by the sustained elevation of plasma urate levels [Chohan and Becker, 2009; Davidson et al, 2004; Kang and Nakagawa, 2005; Richette and Bardin, 2010]. CKD is usually associated with hyperuricemia and has a prevalence of about 5% [Feig, 2009; Kang and Nakagawa, 2005; Sestigiani et al, 2008].
Currently, available anti-hyperuricemia drugs act on XO, the urate transporter or urate itself. Allopurinol and febuxostat are anti-hyperuricemia drugs that competitively inhibit XO (Fig. 1). Allopurinol is the first-line drug for treating gout and TLS, but refractory gout occurs when patients suffer from hypersensitivity or non-responsiveness to allopurinol, intolerance to allopurinol toxicity, or drug-drug interactions with allopurinol [Chohan and Becker, 2009; Edwards, 2009; Fels and Sundy, 2008; Keenan and Pillinger, 2009; Mughal et al, 2010; Richette and Bardin, 2006; Richette and Bardin, 2010; Terkeltaub, 2010; Vogt, 2005]. Febuxostat is tolerated by most patients [Chohan and Becker, 2009; Keenan and Pillinger, 2009; Terkeltaub, 2010], but its efficacy in treating refractory gout has not been proven. Probenecid is anti-hyperuricemia drug acting on the renal urate transporter (Fig. 1) [Chohan and Becker, 2009; Richette and Bardin, 2010] but it is not widely used due to associated kidney and liver toxicity. Uricase is a unique anti-hyperuricemia drug acting on urate [Bessmertny et al, 2005; Chohan and Becker, 2009; Fels and Sundy, 2008; Mughal et al, 2010; Richette and Bardin, 2006; Richette and Bardin, 2010; Schlesinger et al, 2011; Sherman et al, 2008; Sestigiani et al, 2008; Terkeltaub, 2010; Vogt, 2005] catalyzing the oxidation of urate into hydrogen peroxide and 5-hydroxyisourate (HIU) [Ramazzina et al, 2006]. HIU spontaneously decomposes to allantoin and carbon dioxide. Allantoin is highly soluble in plasma with few pathological actions and is easily excreted through kidney.
Uricases are effective in diverse forms of hyperuricemia including refractory gout removing pre-existing urate crystals in joints [Chohan and Becker, 2009; Edwards, 2008; Fels and Sundy, 2008; Richette and Bardin, 2006; Schlesinger et al, 2011; Vogt, 2005] and have negligible drug-drug interactions. Only uricases are effective in refractory gout, but available uricase formulations are unsuitable for continuous treatment.
Problem of uricases to treat refractory gout and potential solutions
Any protein drug should have poteny activity, negligible immunogenicity and a long circulation half-life in vivo. The modification of exogenous proteins with monomethoxyl poly(ethylene glycol) (mPEG), i.e., PEGylation, is a routine formulation approach for exogenous proteins to reduce their immunogenicity and improve their pharmacokinetics [Gaberc-Porekar et al, 2008; Haag and Kratz, 2006]. PEGylation routineely decreases protein activity while mPEG is weakly immunogenic. Eukaryote and prokaryote uricases have low catalytic capacity with lower apparent activities at physiological pH and are sensitive to xanthine inhibition. Eukaryote uricases usually have lower thermostability, lower solubility and a higher sensitivity to xanthine [Fridovich, 1965; Liao et al, 2005; Liu et al, 2009]. Hence, natural uricases themselves are unsatisfactory as therapeutic forumations for treating refractory gout.
Raburicase and pegloticase are approved uricase formulations for treating hyperuricemia.. Raburicase is a recombinant fungal uricase without structural modification and is used for short-term treatment of TLS [Bessmertny, et al, 2005; Richette and Bardin, 2006; Sestigiani et al, 2008; Vogt, 2005]. Pegloticase is a chimera of porcine and baboon liver uricases and is designed for the continuous treatment of refractory gout [Chohan and Becker, 2009; Fels and Sundy, 2008; Schlesinger et al, 2011; Sherman et al, 2008; Sundy et al, 2007; Terkeltaub, 2010; Yue et al, 2008]. Following the initial i.v. infusion, pegloticase has a circulation half-life of 3-7 days. However, after 6 administrations at the required biweekly therapeutic dose of approximately 8 mg, an immune response develops against the mPEG moiety of pegloticase in nearly 20% patients [Sundy et al, 2007; Yue et al, 2008]. Repeated administration of other PEGylated drugs also result in detectable antibodies specifically against mPEG moiety with few antibodies being developed against the drug itself causing an accelerated clearance [Cheng et al, 2000; Ganson et al, 2006; Ishida et al, 2006; Ishida and Kiwada, 2008; Sundy et al, 2007; Tsuji et al, 1985; Yue et al, 2008]. Despite the weak immunogenicity of mPEG, some PEGylated proteins can be used to treat chronic diseases for years [Foster, 2010; Gaspar et al, 2009; Pasut et al, 2008; Rodriguez-Torres et al, 2009]. For example, adenosine deaminase (ADA) from bovine intestine modified with mPEG can be safely used for nearly 10 years in patients severe compliant immunodeficiency at weekly doses of about 20μg/kg body weight [Gaspar et al, 2009]. PEGylated human interferon-α is successfully used to treat chronic hepatitis C at weekly therapeutic dose of about 4μg/kg body weight for up to a year [Foster, 2010; Rodriguez-Torres et al, 2009] while PEGylated asparginase from E. coli can be used to treat leukemia patients at weekly therapeutic dose of about 30μg/kg body weight for up to 2 years [Pasut et al, 2008]. Finally, monthly iv infusion of 10 mg raburicase, which can elicit an immune response in healthy individuals, was safely used for 3 years to treat a patient co-treated with immunosuppressors after kidney transplantation [Vogt, 2005].
In light of these data, a PEGylated uricase has promise for safe, continuous treatment of refractory gout, and even CKD when: patients have low immunoresponse capacity; the uricase is PEGylated with new PEG derivatives of lower immunogenicity and its therapeutic dose is too low to elicit an immune response. In practice, it is rare to find a patient with refractory gout with a low immunoresponse capacity a problem that is exacerbated by co-administration of immunosuppressors.
There have been few advances in the design of new PEG derivatives with lower immunogenicity than mPEG with PEG backbones having low immunogenicity [Su et al, 2010]. Without novel hydrophilic polymers of lower immunogenicity to formulate proteins, any PEGylated uricase for continued use in the treatment of refractory gout must have a therapeutic dose low enough to avoid eliciting an immunoresponse after repeated administrations.
In light of the safe continued administrations of a single-site PEGylated human interferon-α-a for several years, a PEGylated uricase at a monthly therapeutic dose below 4μg/kg body weight may have promise for continued treatment of refractory gout. In clinical practice, the recommended biweekly iv dose of Pegloticase is approximately 0.14 mg/kg body weight [Schlesinger, 2011; Sundy et al, 2007; Yue et al, 2008]. This dose is nearly 70 times of the threshold of the safe monthly dose with more PEG chains in PEGylated uricase than in a PEGylated interferon-α-a molecule. Continued administration of pegloticase will inevitably elicit a host immunoresponse via the mPEG moiety.
PEGylated proteins typically exhibit improved thermostability with higher solubility but lower activity than the native protein. A PEGylated uricase for chronic treatment of refractory gout under physiological conditions should have high catalytic capacity, a long thermo-inactivation half-life and high residual activity after PEGylation and should have a small quantity of PEG chains to mask its immunogenic sites. Therefore, molecular engineering should begin with a uricase of high activity and/or superior thermostability as to produce uricase mutants with the number and distribution of accessible amino acid residues reacting with common activated PEG derivatives optimized via site-specific PEGylation to mask immunogenic sites; new PEG chains or other suitable polymers of low immunogenicity should be designed for site-specific modification of uricases. Therapeutic doses of PEGylated uricases can be preliminarily evaluated by pharmacodynamics (PD) simulation followed by experimentation. These strategies should be integrated for site-specific PEGylation of uricases.
Molecular engineering and site-specific PEGylation of uricases
Molecular engineering to enhance inherent catalytic efficiency of enzymes
Molecular engineering can be initiated via rational design, evolutionary biotechnology or a combination of the two. Due to the low thermostability of most eukaryotic uricases [Liu et al, 2009], bacterial uricases with better thermostability can be used as a starting point [Zhao et al, 2006, 2009].
The first technique for molecular engineering of an enzyme is rational design based on the detailed understanding of three-dimensional structure, the catalytic mechanism, and structure-activity and/or thermostability correlation of the enzyme [Chen et al, 2009; Gao et al, 2006, 2009; Kumarasiri et al, 2009; Lonsdale et al, 2010; Pan et al, 2005, 2007, 2008; Yang et al, 2009; Zheng et al, 2008]. Recently, computational design approaches have been successfully employed with some enzymes to improve their catalytic activities and thermostability [Gao et al, 2009]. Knowledge of the putative reaction pathway and free energy profile of an enzyme reaction determined by computation can be used to rationally design essential amino acid residue(s) mutants of uricase to decrease the free energy barrier of the reaction and increase uricase catalytic efficiency [Chen et al, 2009; Gao et al, 2006; Kumarasiri et al, 2009; Lonsdale et al, 2010; Pan et al, 2005, 2007, 2008; Yang et al, 2009]. Comparative computational studies on the interactions of uricase with urate and xanthine can facilitate rational design of uricase mutants to improve selectivity for urate. To improve the thermostability of uricase, determination of its thermo-inactivation pathway can be used to design thermostable mutants based [Gao et al, 2009].
Another technique for molecular engineering of an enzyme is evolutionary biotechnology in vitro mimicking the natural evolution of the enzyme in vivo. This does not require a three-dimensional structure of the enzyme but usually suffers from higher cost and longer time in generating and screening mutant libraries [Chica et al, 2005; Labrou, 2010; Petrounia and Arnold, 2000; Voigt et al, 2000]. Uisng this technique, a library of uricase mutants from a suitable starting uricase can be constructed for screening. The mutant library is ideally focused on amino acid residues in the vicinity of the active site [Chica et al, 2005]. During the mutant screen, xanthine saturated in physiological buffer with thermoinactivation at 37°C can accelerate the discovery of desirable mutants. Efficient, validated screening methods for uricase mutants are currently unavailable.
Site-specific PEGylation of uricase using PEG chains of lower immunogenicity
Once engineered uricases with desirable inherent properties are identified, their modification with mPEG or similar hydrophilic polymers of lower immunogenicity can be used to improve their pharmacokinetics [Gaberc-Porekar et al, 2008; Haag and Kratz, 2006]. In the present review, only site-specific PEGylation and the design of new PEG derivatives will be briefly discussed.
Site-specific PEGylation is always preferred for the formulation of proteins based on the following considerations [Marsac et al, 2006; Sato, 2002];a) After optimization of the number and distribution of accessible reactive amino acid residues of uricases for PEGylation, small or even negligible decreases in uricase activity after PEGylation can be sought with the immunogenic sites effectively masked; b) using lower degrees of PEGylation there will be fewer PEG chains in PEGylated uricases and hence lower immunoresponsivity’ c) fewer modified isomers simplify the purification of PEGylated uricases and evaluation of their effectiveness. Immunogenic sites on uricases can be predicted using bioinformatics [Bryson et al, 2010]. Knowledge of uricase structure-activity relationships together with predicted immunogenic sites can facilitate the engineering of limited numbers of accessible reactive amino acid residues while the PEG chain structure can be optimized for lower immunogenicity with lower degrees of PEGylation masking all predicted immunogenic sites. Due to the weak immunogenicity of mPEG, design of new PEG chains or suitable new hydrophilic polymers of lower immunogenicity while mandatory is challenging. PEG is a linear polymer; after PEGylation, one end of a PEG chain is linked to accessible reactive amino acid residues and the other end remains free. Antibodies detected in animals after repeat administration of diverse PEGylated drugs are typically specific for the methoxyl group of mPEG [Cheng et al, 2000; Ganson et al, 2006; Ishida et al, 2006; Ishida and Kiwada, 2008; Sundy et al, 2007; Tsuji et al, 1985; Yue et al, 2008]. Thus PEG chains with groups other than a methoxyl at the free end are preferred; however, few of such PEG derivatives are known.
Therapeutic doses of formulated uricases
Properties affecting therapeutic doses of formulated uricases and their evaluations
Maximal catalytic efficiency of a formulated uricase in vivo is crucial in determining the therapeutic dose. PEGylated uricases usually have lower activity than the native enzyme. Under optimum reaction conditions, it is feasible to continuously monitor uricase reaction curve for kinetic analysis of reaction process to estimate the maximal catalytic efficiency [Liao et al, 2001, 2005]. Due to the interference from HIU with the UV absorbance of urate, the quantification of hydrogen peroxide is an alternative [Kahn and Tipton, 1998], but current conventional methods are able to quantify hydrogen peroxide for measuring uricase activities.
The circulation half-life in vivo of a formulated uricase is also critical in determining the therapeutic dose [Feng et al, 2010; Sarkissian et al, 2008; Zhang et al, 2010]. PEGylation usually enhances the thermostability of formulated proteins, but the circulation half-life of any formulated enzyme in vivo is inevitably shorter than its thermo-inactivation half-life in vitro under the same temperature and pH due to complex interactions with biomolecules in vivo. Usually, a fully-modified enzyme has the highest thermostability and the longest in vivo half-life. If there are large enough differences in the thermo-inactivation and/or circulation half-life of modified uricase isoforms, kinetic analysis of the decrease in the total activities of a mixture of modification isoforms of the uricasecan be used to estimate the thermo-inactivation or circulation half-life of the fully modified uricase [Feng et al, 2010].
The sensitivity of common uricases to xanthine and the association of hyperxanthinemia with hyperuricemia necessitates consideration of xanthine inhibition on the actions of uricases in vivo [Band et al, 1970; Fridovich, 1965; Liao et al, 2005]. In the plasma of TLS patients, the peak concentration of xanthine can be over 0.8 mM [Hande et al,1979] and the treatment of TLS patients with allopurinol results in kidney dysfunction due to hyperxanthinemia [Band et al, 1970]. Thus, uricases with affinities for urate higher than those for xanthine are desirable but none are known [Fridovich, 1965; Liao et al, 2005]. At a saturated level of xanthine (~ 0.22 mM at 37 °C), eukaryotic uricases have negligible advantages over prokaryotic uricases in the elimination of plasma urate [Feng et al, 2010]. To screen uricase mutants of higher affinities for urate than those for xanthine, kinetic analysis of reaction curves to estimate the competitive inhibition constants of xanthine is desirable [Liao et al, 2005].
Estimation of therapeutic doses of formulated uricases
PD simulation for drugs can be used to estimate therapeutic doses and optimized administration intervals [Danhof et al, 2007, 2008]. When formulated uricases with different inherent properties are prepared, PD simulation of actions of each can be carried out to estimate the potential monthly therapeutic dose for practical efficiency and cost. For PD simulation, the properties of a formulated uricase that affect its therapeutic dose and the factors affecting urate levels in vivo including the production of urate by XO, the transportation of urate from tissue pools into plasma, the excretion of urate via kidney, etc., should be taken into account. Such PD simulation techniques are still under development for enzymes.
After comparison of simulated monthly therapeutic doses of uricase formulations, the detailed experimental evaluation of their pharmacokinetics and pharmacodynamics are still required. However, the development of hyperuricemic animal models is also challenging. Hyperuricemic animals treated with uricase inhibitors are unsuitable as uricase inhibitors have no selectivity for mammalian uricases over a formulated uricase as a candidate therapeutic agent. Furthermore, uricase-deficient miceare expensive, have a short lifespan suffer from severe urate nephropathy [Kelly et al, 2001; Wu et al, 1994]. Alternatively, chicken and duck can be used as models to estimate the therapeutic dose of a modified uricase mutant in vivo [Ramazzina et al, 2006]. In addition, PD simulation techniques can be verified with experimental data of pharmacokinetics and pharmacodynamics of formulated uricases.
Conclusion
Currently available formulated uricases are unsuitable for the chronic treatment of refractory gout although a formulated uricase at a monthly therapeutic dose of below 4μg/kg body weight shows some promise to refractory gout. Molecular engineering to enhance the efficacy of mutant uricases together with site-specific PEGylation can lead to improved forms of formulated uricase. These will require efficient screens and and PD simulation to estimate monthly therapeutic dosing for chronic treatment of refractory gout.
Acknowledgments
Funding/Support information: This work was supported in part by the Program for New Century Excellent Talents in University (NCET-09), High-technology-program “863” of China (2011AA02A108), National Natural Sciences Foundation of China (Nos. 30672009, 81071427), Chinese Scholarship Council (2006[3085]), and NIH (R01 DA025100 and R01 DA013930).
References
- Band PR, Silverberg DS, Henderson JF, Ulan RA, Wensel RH, Banerjee TK, Little AS. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. N Engl J Med. 1970;283(7):354–7. doi: 10.1056/NEJM197008132830708. [DOI] [PubMed] [Google Scholar]
- Bessmertny O, Robitaille LM, Cairo MS. Rasburicase: a new approach for preventing and/or treating tumor lysis syndrome. Curr Pharm Des. 2005;11(32):4177–85. doi: 10.2174/138161205774913291. [DOI] [PubMed] [Google Scholar]
- Bryson CJ, Jones TD, Baker MP. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs. 2010;24(1):1–8. doi: 10.2165/11318560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Chen CY, Georgiev I, Anderson AC, Donald BR. Computational structure-based redesign of enzyme activity. Proc Natl Acad Sci U S A. 2009;106(10):3764–9. doi: 10.1073/pnas.0900266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TL, Chen BM, Chern JW, Wu MF, Roffler SR. Efficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapy. Bioconjug Chem. 2000;11(2):258–66. doi: 10.1021/bc990147j. [DOI] [PubMed] [Google Scholar]
- Chica RA, Doucet N, Pelletier JN. Semi-rational approaches to engineering enzyme activity: combining the benefits of directed evolution and rational design. Curr Opin Biotechnol. 2005;16(4):378–84. doi: 10.1016/j.copbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chohan S, Becker MA. Update on emerging urate-lowering therapies. Curr Opin Rheumatol. 2009;21(2):143–9. doi: 10.1097/BOR.0b013e328325bd94. [DOI] [PubMed] [Google Scholar]
- Danhof M, de Jongh J, De Lange EC, Della Pasqua O, Ploeger BA, Voskuyl RA. Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu Rev Pharmacol Toxicol. 2007;47:357–400. doi: 10.1146/annurev.pharmtox.47.120505.105154. [DOI] [PubMed] [Google Scholar]
- Danhof M, de Lange EC, Della Pasqua OE, Ploeger BA, Voskuyl RA. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol Sci. 2008;29(4):186–91. doi: 10.1016/j.tips.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A, Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116(8):546–54. doi: 10.1016/j.amjmed.2003.09.045. [DOI] [PubMed] [Google Scholar]
- Edwards NL. Treatment-failure gout: a moving target. Arthritis Rheum. 2008;58:2587–2590. doi: 10.1002/art.23803. [DOI] [PubMed] [Google Scholar]
- Feig DI. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18(6):526–30. doi: 10.1097/MNH.0b013e328330d9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels E, Sundy JS. Refractory gout: what is it and what to do about it? Curr Opin Rheumatol. 2008;20(2):198–202. doi: 10.1097/BOR.0b013e3282f4eff5. [DOI] [PubMed] [Google Scholar]
- Feng J, Li X, Yang XL, Zhang C, Yuan YH, Pu J, Zhao YS, Xie YL, Yuan HD, Bu YQ. A new practical system for evaluating pharmacological properties of uricase as potential drug to handle hyperuricemia. Arch Pharm Res. 2010;33(11):1761–1769. doi: 10.1007/s12272-010-1108-2. others. [DOI] [PubMed] [Google Scholar]
- Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010;70(2):147–65. doi: 10.2165/11531990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The Competitive Inhibition of Uricase by Oxonate and by Related Derivatives of S-Triazines. J Biol Chem. 1965;240:2491–4. [PubMed] [Google Scholar]
- Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11(2):242–50. [PubMed] [Google Scholar]
- Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 2006;8(1):R12. doi: 10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Cho H, Yang W, Pan Y, Yang G, Tai HH, Zhan CG. Computational design of a human butyrylcholinesterase mutant for accelerating cocaine hydrolysis based on the transition-state simulation. Angew Chem Int Ed Engl. 2006;45(4):653–7. doi: 10.1002/anie.200503025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol. 2009;75(2):318–23. doi: 10.1124/mol.108.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009;114(17):3524–32. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag R, Kratz F. Polymer therapeutics: concepts and applications. Angew Chem Int Ed Engl. 2006;45(8):1198–215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- Hande KR, Perini F, Putterman G, Elin R. Hyperxanthinemia interferes with serum uric acid determinations by the uricase method. Clin Chem. 1979;25(8):1492–4. [PubMed] [Google Scholar]
- Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115(3):251–8. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354(1-2):56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kahn K, Tipton PA. Spectroscopic characterization of intermediates in the urate oxidase reaction. Biochemistry. 1998;37(33):11651–9. doi: 10.1021/bi980446g. [DOI] [PubMed] [Google Scholar]
- Kang DH, Nakagawa T. Uric acid and chronic renal disease: possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005;25(1):43–9. doi: 10.1016/j.semnephrol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Keenan RT, Pillinger MH. Febuxostat: a new agent for lowering serum urate. Drugs Today (Barc) 2009;45(4):247–60. doi: 10.1358/dot.2009.45.4.1354217. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Delnomdedieu M, Oliverio MI, Williams LD, Saifer MG, Sherman MR, Coffman TM, Johnson GA, Hershfield MS. Diabetes insipidus in uricase-deficient mice: a model for evaluating therapy with poly(ethylene glycol)-modified uricase. J Am Soc Nephrol. 2001;12(5):1001–9. doi: 10.1681/ASN.V1251001. [DOI] [PubMed] [Google Scholar]
- Kumarasiri M, Baker GA, Soudackov AV, Hammes-Schiffer S. Computational approach for ranking mutant enzymes according to catalytic reaction rates. J Phys Chem B. 2009;113(11):3579–83. doi: 10.1021/jp810363k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrou NE. Random mutagenesis methods for in vitro directed enzyme evolution. Curr Protein Pept Sci. 2010;11(1):91–100. doi: 10.2174/138920310790274617. [DOI] [PubMed] [Google Scholar]
- Liao F, Liu WL, Zhou QX, Zeng ZC, Zuo YP. Assay of serum arylesterase activity by fitting to the reaction curve with an integrated rate equation. Clin Chim Acta. 2001;314(1-2):67–76. doi: 10.1016/s0009-8981(01)00631-3. [DOI] [PubMed] [Google Scholar]
- Liao F, Zhu XY, Wang YM, Zuo YP. The comparison of the estimation of enzyme kinetic parameters by fitting reaction curve to the integrated Michaelis-Menten rate equations of different predictor variables. J Biochem Biophys Methods. 2005;62(1):13–24. doi: 10.1016/j.jbbm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Liu J, Hamza A, Zhan CG. Fundamental reaction mechanism and free energy profile for (-)-cocaine hydrolysis catalyzed by cocaine esterase. J Am Chem Soc. 2009;131(33):11964–75. doi: 10.1021/ja903990p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lu D, Li J, Chen W, Liu Z. Strengthening intersubunit hydrogen bonds for enhanced stability of recombinant urate oxidase from Aspergillus flavus: molecular simulations and experimental validation. Phys Chem Chem Phys. 2009;11(2):333–40. doi: 10.1039/b811496j. [DOI] [PubMed] [Google Scholar]
- Lonsdale R, Ranaghan KE, Mulholland AJ. Computational enzymology. Chem Commun (Camb) 2010;46(14):2354–72. doi: 10.1039/b925647d. [DOI] [PubMed] [Google Scholar]
- Marsac Y, Cramer J, Olschewski D, Alexandrov K, Becker CF. Site-specific attachment of polyethylene glycol-like oligomers to proteins and peptides. Bioconjug Chem. 2006;17(6):1492–8. doi: 10.1021/bc0601931. [DOI] [PubMed] [Google Scholar]
- Mughal TI, Ejaz AA, Foringer JR, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36(2):164–76. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci U S A. 2005;102(46):16656–61. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Zhan CG. Free energy perturbation (FEP) simulation on the transition states of cocaine hydrolysis catalyzed by human butyrylcholinesterase and its mutants. J Am Chem Soc. 2007;129(44):13537–43. doi: 10.1021/ja073724k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gao D, Zhan CG. Modeling the catalysis of anti-cocaine catalytic antibody: competing reaction pathways and free energy barriers. J Am Chem Soc. 2008;130(15):5140–9. doi: 10.1021/ja077972s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasut G, Sergi M, Veronese FM. Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv Drug Deliv Rev. 2008;60(1):69–78. doi: 10.1016/j.addr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Petrounia IP, Arnold FH. Designed evolution of enzymatic properties. Curr Opin Biotechnol. 2000;11(4):325–30. doi: 10.1016/s0958-1669(00)00107-5. [DOI] [PubMed] [Google Scholar]
- Ramazzina I, Folli C, Secchi A, Berni R, Percudani R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat Chem Biol. 2006;2(3):144–8. doi: 10.1038/nchembio768. [DOI] [PubMed] [Google Scholar]
- Richette P, Bardin T. Successful treatment with rasburicase of a tophaceous gout in a patient allergic to allopurinol. Nat Clin Pract Rheumatol. 2006;2(6):338–42. doi: 10.1038/ncprheum0214. [DOI] [PubMed] [Google Scholar]
- Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Torres M, Jeffers LJ, Sheikh MY, Rossaro L, Ankoma-Sey V, Hamzeh FM, Martin P. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360(3):257–67. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- Sarkissian CN, Gamez A, Wang L, Charbonneau M, Fitzpatrick P, Lemontt JF, Zhao B, Vellard M, Bell SM, Henschell C. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc Natl Acad Sci U S A. 2008;105(52):20894–9. doi: 10.1073/pnas.0808421105. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H. Enzymatic procedure for site-specific pegylation of proteins. Adv Drug Deliv Rev. 2002;54(4):487–504. doi: 10.1016/s0169-409x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase. Nat. Rev. Drug Discov. 2011;10:17–18. doi: 10.1038/nrd3349. [DOI] [PubMed] [Google Scholar]
- Sestigiani E, Mandreoli M, Guardigli M, Roda A, Ramazzotti E, Boni P, Santoro A. Efficacy and (pharmaco)kinetics of one single dose of rasburicase in patients with chronic kidney disease. Nephron Clin Pract. 2008;108(4):c265–71. doi: 10.1159/000126906. [DOI] [PubMed] [Google Scholar]
- Sherman MR, Saifer MG, Perez-Ruiz F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv Drug Deliv Rev. 2008;60(1):59–68. doi: 10.1016/j.addr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Su YC, Chen BM, Chuang KH, Cheng TL, Roffler SR. Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjug Chem. 2010;21:1264–1270. doi: 10.1021/bc100067t. [DOI] [PubMed] [Google Scholar]
- Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, Hershfield MS. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 2007;56(3):1021–8. doi: 10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6(1):30–8. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Hirose K, Kasahara E, Naitoh M, Yamamoto I. Studies on antigenicity of the polyethylene glycol (PEG)-modified uricase. Int J Immunopharmacol. 1985;7(5):725–30. doi: 10.1016/0192-0561(85)90158-4. [DOI] [PubMed] [Google Scholar]
- Vogt B. Urate oxidase (rasburicase) for treatment of severe tophaceous gout. Nephrol Dial Transplant. 2005;20(2):431–3. doi: 10.1093/ndt/gfh629. [DOI] [PubMed] [Google Scholar]
- Voigt CA, Kauffman S, Wang ZG. Rational evolutionary design: the theory of in vitro protein evolution. Adv Protein Chem. 2000;55:79–160. doi: 10.1016/s0065-3233(01)55003-2. [DOI] [PubMed] [Google Scholar]
- Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C, Jr., Jones P, Bradley A, Caskey CT. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91(2):742–6. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Pan Y, Zheng F, Cho H, Tai HH, Zhan CG. Free-energy perturbation simulation on transition states and redesign of butyrylcholinesterase. Biophys J. 2009;96(5):1931–8. doi: 10.1016/j.bpj.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue CS, Huang W, Alton M, Maroli AN, Waltrip RW, Wright D, Marco MD. Population pharmacokinetic and pharmacodynamic analysis of pegloticase in subjects with hyperuricemia and treatment-failure gout. J Clin Pharmacol. 2008;48(6):708–18. doi: 10.1177/0091270008317589. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang X, Feng J, Yuan Y, Li X, Bu Y, Xie Y, Yuan H, Liao F. Effects of modification of amino groups with poly(ethylene glycol) on a recombinant uricase from Bacillus fastidiosus. Biosci Biotechnol Biochem. 2010;74(6):1298–301. doi: 10.1271/bbb.100080. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang X, Li X, Bu Y, Deng P, Zhang C, Feng J, Xie Y, Zhu S, Yuan H. Reversible inactivation of an intracellular uricase from Bacillus fastidiosus via dissociation of homotetramer into homodimers in solutions of low ionic strength. Biosci Biotechnol Biochem. 2009;73(9):2141–4. doi: 10.1271/bbb.90347. others. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao L, Yang G, Tao J, Bu Y, Liao F. Characterization of a uricase from Bacillus fastidious A.T.C.C. 26904 and its application to serum uric acid assay by a patented kinetic uricase method. Biotechnol Appl Biochem. 2006;45(2):75–80. doi: 10.1042/BA20060028. [DOI] [PubMed] [Google Scholar]
- Zheng F, Yang W, Ko M-C, Liu J, Cho H, Gao D, Tong M, Tai H-H, Woods JH, Zhan CG. Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc. 2008;130:12148–155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]