Abstract
Signal honesty may be compromised when heightened competition provides incentive for signal exaggeration. Some degree of honesty might be maintained by intrinsic handicap costs on signalling or through imposition of extrinsic costs, such as social punishment of low quality cheaters. Thus, theory predicts a delicate balance between signal enhancement and signal reliability that varies with degree of social competition, handicap cost, and social cost. We investigated whether male sexual signals of the electric fish Brachyhypopomus gauderio would become less reliable predictors of body length when competition provides incentives for males to boost electric signal amplitude. As expected, social competition under natural field conditions and in controlled lab experiments drove males to enhance their signals. However, signal enhancement improved the reliability of the information conveyed by the signal, as revealed in the tightening of the relationship between signal amplitude and body length. Signal augmentation in male B. gauderio was independent of body length, and thus appeared not to be curtailed through punishment of low quality (small) individuals. Rather, all individuals boosted their signals under high competition, but those whose signals were farthest from the predicted value under low competition boosted signal amplitude the most. By elimination, intrinsic handicap cost of signal production, rather than extrinsic social cost, appears to be the basis for the unexpected reinforcement of electric signal honesty under social competition. Signal modulation may provide its greatest advantage to the signaller as a mechanism for handicap disposal under low competition rather than as a mechanism for exaggeration of quality under high competition.
Keywords: Honest signalling, competition, signal enhancement, signal reliability, electric fish, handicap hypothesis, handicap disposal
Introduction
Dynamic signals may be reliable indicators of current quality since they can respond rapidly to changes in the individual’s condition (Hill et al. 1999; Torres & Velando 2003). However, the lability of dynamic signals may allow signallers to transiently escape signalling constraints and temporarily exaggerate their quality and thereby decrease signal reliability. Signal exaggeration can benefit a signaller when there is high reward incentive for increased signalling (Andersson 1994; Searcy & Nowicki 2005). Although signalling systems can survive a small degree of unreliability without completely impairing the stability of the communication system (Johnstone & Grafen 1993; Kokko 1997), a high prevalence of cheating in the population would render signals meaningless for receivers and result in receivers ignoring the signals (Dawkins & Krebs 1978; Johnstone & Grafen 1993). Thus, for signals to remain evolutionarily stable, dishonesty has to be contained.
According to the handicap principle, signal honesty is maintained when the advertising signal meets the following three conditions: 1) the expression of the signal depends on a sexually selected phenotypic trait of the signaller, 2) signals are expensive to produce or maintain and, 3) the costs of signalling rises faster than its benefits (Grafen 1990; Zahavi 1975; Zahavi 1977). Asignal that satisfies the former condition is referred to as an “index” if it is physically associated with the sexually selected trait of the signaller (Maynard Smith & Harper 1995). Signalling cost may include the physiological cost of producing or maintaining the signal, the cost of predation risk that comes with increased conspicuousness due to signalling, and/or social cost from increased confrontation with conspecific males.
Signallers are thought to benefit by producing costly signals when signalling incentive is high, such as during intensified competition, high resource value, or when future reproductive opportunities are reduced. Empirical studies show that competition often induces males to increase signalling effort, producing more conspicuous and costly signals (Endler 1995; Franchina et al. 2001; Kodric-Brown & Brown 1984; Ryan 1985; Salazar & Stoddard 2009). Likewise, males typically signal more in the presence of females (Akre & Ryan 2011), and particularly in the presence of good quality females (Wong & Svensson 2009). Males also tend to signal more intensively when they are close to their last reproductive opportunity (Candolin 2000a; Hall et al. 2009; Proulx et al. 2002). Older males may benefit by reallocating energy from self-maintenance and survival to signalling if their survival probability has been reduced by age or time of season (Kokko 1997; Lindstrom et al. 2009; Proulx et al. 2002). Still, theory is unclear what effect signal enhancement should have on the reliability of the signal (Johnstone et al. 2009). Theoretical models predict that the marginal cost of signal augmentation should ultimately determine how signal enhancement affects honesty (Johnstone et al. 2009).1) Low augmentation costs favour signal enhancement that reduces signal reliability(Grafen 1990; Wagner 1992; Zahavi 1975). 2) If signal enhancement is prevented by social costs, such as punishment of low quality cheaters by social dominants, signal reliability should increase under social competition as low quality signallers contain their signal output more than high quality signallers (Candolin 2000b; Rohwer & Ewald 1981). 3) Likewise, where signal enhancement disproportionally increases signal production costs, signal enhancement could reinforce the handicap effect and result in increased signal honesty (Johnstone et al. 2009; Wagner 1992).
An imbalance between incentive and signalling costs can decrease signal reliability. When the benefits of signalling outweigh the costs, signal reliability is decreased by “cheating up” wherein low quality individuals signal at a level higher than expected for their quality (Bee et al. 2000; Candolin 2000c; Wagner 1989a). However, when the costs of signalling outweigh its benefits, signal reliability can also be reduced by “cheating down” wherein high quality individuals reduce their signalling output and reallocate that energy to self-maintenance as an investment in future reproduction (Lindstrom et al. 2009)or predation avoidance (Endler 1987). An expensive signal that can be attenuated to reduce costs under low competition is effectively a “disposable handicap”.
From this disposable handicap hypothesis we can derive three testable predictions: 1) Signalling output should track incentive, such as social competition. 2) Signal reliability should increase under social competition as individuals increase their handicap magnitude and approach their physical limits. 3) Individuals that have lowered their signals the farthest below the level predicted for their quality have disposed of most of their handicap but will have the most to catch-up to when competition resumes. Thus low signalling individuals, rather than low quality individuals, should enhance their signals the most when social competition increases.
We tested these predictions in Brachyhypopomus gauderio (Giora & Malabarba 2009), a South American electric fish that produces a biphasic electric organ discharge (EOD). As with all gymnotiforms, the signal functions for active electrolocation and communication during their nocturnal active phase (Moller 1995). EODs of male B. gauderio and its sister species B. pinnicaudatus are greater in amplitude and 2nd phase duration than those of females (Franchina & Stoddard 1998; Hopkins et al. 1990). Moreover, the EOD of B. gauderio has the potential to serve as an indicator of body length since the amplitude of the signal physically depends on the length of the electric organ, which runs the length of the fish’s body (Curtis & Stoddard 2003; Hopkins et al. 1990; Hopkins 1999). Body length information is relevant for mate-choice and male-male interactions, since longer males are more attractive to females (Curtis & Stoddard 2003) and more likely to win agonistic encounters (Salazar 2009; Silva et al. 2010). Additionally, electric signals of males are costly handicaps; they are energetically expensive, consuming an average of 22% of the daily energetic budget (compared to 3% for females), and their energetic costs rise with the amplitude of the EOD (Salazar & Stoddard 2008). However, the EOD of B. gauderio is highly plastic, varying in magnitude along multiple time scales (Stoddard et al. 2006). EOD amplitude and duration display short-term changes, both increasing during social interaction (Franchina et al. 2001; Silva et al. 2007)and in anticipation of night, when fish become active (Franchina & Stoddard 1998; Stoddard et al. 2007). These short-term signal increases are mediated by melanocortin peptide hormones such as ACTH and α-MSH (Markham et al. 2009a; Markham & Stoddard 2005; Stoddard & Markham 2008). Social interaction also induces long-term changes across several days (Franchina et al. 2001), and signals vary seasonally with the transition into reproductive condition (Silva et al. 2002). These long-term signal enhancements are mediated by androgens (Allee et al. 2009; Gavassa et al. 2011; Goldina et al. 2011; Silva et al. 2002).
Here we test whether signal enhancement affects signal reliability, and if so, in which direction. We studied the reliability of EOD amplitude as an indicator of body length, the main determinant of male-male competition and female choice. Given the cost of the electric signal of B. gauderio and the intrinsic relation between body length and EOD amplitude, signal modulation could be used to reduce signalling cost when signalling incentives are low(i.e. “cheating down”). Conversely, the cost of signal enhancement may not be high enough to prevent dishonest exaggeration, in which case some individuals should be able to enhance their signal beyond their quality (i.e. “cheating up’).
Materials and Methods
Subjects
We studied Brachyhypopomus gauderio (Giora & Malabarba 2009), sister species of B. pinnicaudatus(Hopkins et al. 1990), native to marshes and slow waters of Argentina, Uruguay, and southern Brazil. Males have longer and broader caudal filaments than females and produce EODs of greater amplitude and longer duration than females. B. gauderio is a short-lived species; adults disappear from natural populations after breeding during the austral summer (Silva et al. 2003), and males disappear faster than females (Miranda et al. 2008). Males maintain territories and females move freely between male territories, consistent with exploded lek polygyny (Miranda et al. 2008). We studied B. gauderio from a native population in Uruguay, and from our 18th generation captive-reared colony in Miami, FL. Collections and experimental procedures were performed under the guidelines and approval of the Comisión Honoraria de Experimentación Animal, Universidad de la República, Montevideo, Uruguay, and by the IACUC at Florida International University, Miami, FL (protocols 08-027 and 10-020).
Field study
Detailed field methods are described by Gavassa et al. (2011). In brief, we recorded the EODs of B. gauderio in situ from Laguna Lavalle (32° 01.259′ S, 055° 22.498′W), Department of Tacuarembó, Uruguay. We sampled during Oct. 15th–16th, 27th–28th, Nov. 16th–18th, Dec. 04th, 12th and 13th. All sampling took place during the breeding season reported for this species in this region of the Southern Hemisphere (Silva et al. 2003). All sampling occurred during the day (11:00–17:59 UYST) on sites with similar depth and vegetation located along the shore of the lagoon. We located fish in their hiding places using an amplifier to convert their electric signals into sound. We recorded EODs of captured fish in a floating digitizer rig within a minute of initial disturbance, before melanocortin-mediated EOD waveform modulation could take effect (Stoddard et al. 2006). We performed a census in each of the seasonal sampling sites to estimate population abundance. We estimated the abundance of B. gauderio by recording the time a skilled fishing team took to capture 30 fish, and defined population abundance in terms of fish captured per unit of effort (CPUE fish h−1). This method has been very successful for estimating population abundance in this species since these fish are nocturnal and spend the day motionless in their hiding place where they are easily captured (Miranda et al. 2008; Silva et al. 2003).
We confirmed the sex of the fish by gonadal inspection after sacrifice by immersion in an anaesthetic solution (eugenol 8 mg l−1). The body lengths of males collected ranged between 10.5 and 19.3 cm, corresponding to the size of adult males (Franchina 1997; Silva et al. 2003). Caudal filament damage affects the EOD waveform (Hopkins et al. 1990), thus only fish with intact caudal filaments were retained for EOD analysis. In each sampling event we captured 10 or more males, except for 12–13 Dec when we only captured eight males, two of which had damage to their caudal filaments and could not be used for EOD analysis.
Lab experiment
We tagged 18 females and 18 males with alphanumeric elastomer tags and randomly placed them in 450 l pools outdoors of 2, 6, 12, or 18 fish, keeping sex ratio at unity. The body length of males used in this experiment ranged between 15.5 and 24.0 cm. At a given time we had 6 pools with 2 fish each, 2 pools with 6 fish each and one pool with 12 fish, this set up was replicated three times and followed by two pools with 18 fish each. This design manipulated fish density while controlling for sex ratio. After a week of social treatment, we recorded their EODs. In between social treatments, fish remained with an opposite sex companion for a week. Fish went through all treatments except one male and one female that were replaced partway through after they became sick. Treatments were temporally interspersed, except for the 18-fish pool, which was performed at the end since we couldn’t run it simultaneously with the other treatments because its great fish density required the use of most experimental individuals at once.
In B. gauderio temperature affects EOD amplitude with a non-linear Q10(Silva et al. 2002) wherein EODs of sexually mature males partially resist effects of declining temperature (Silva et al. 1999). An early cold front arrived during the week of the 18-fish/pool treatment and lowered the temperature of the holding pools 6°C below the average for the other treatments. B. gauderio remains socially active over wide temperature swings, and all fish in the 18-fish treatment experienced the same temperature, so we expect the intragroup signal dynamics to continue reflecting the pool density. Thus, while we cannot compare directly the absolute values of EOD amplitude in the 18 fish per pool treatment with the other treatment groups that experienced narrower temperature ranges, we feel more comfortable using these data in within-group amplitude analyses.
EOD recordings and analysis
In gymnotiforms the repeatability of the recordings depends on the orientation of the fish and its relative distance to the recording electrodes (Hopkins 1986). Field EOD recordings took place inside the lagoon in a submerged plastic sheet cage (100 × 50 × 50 cm) with recording electrodes located at either end, 100 cm apart. A mesh tube held the fish lengthwise, equidistant from the recording electrodes and 25 cm below the water surface. A ground electrode was located perpendicular to the fish. This EOD recording geometry was designed to permit high repeatability of amplitude measurements (Franchina & Stoddard 1998). Water conductivity was 38–57 μS cm−1 and water temperature was 19–28°C throughout the field study. The variability in environmental conditions and the underlying seasonal variability associated with field observations makes lab tests crucial to confirm field observations. In the lab, we recorded the EODs inside a glass aquarium (120 × 40 × 40 cm), water conductivity adjusted to 100 ± 6 μS/cm and 25.8 ±1.3° C. Since we did not cross-calibrate the recording rigs, which differed in dimensions and water conductivity between field and lab, we cannot directly compare absolute amplitude measurements of these populations.
EODs were differentially amplified 100x (World Precision Instruments, Inc., Sarasota, FL.DAM-50, AC-coupled, lowpass filter corner 0.1 Hz, highpass filter corner 10 kHz). Signals were digitized by an RM1 mobile processor (Tucker Davis Technologies, Alachua, FL), and stored and analyzed on a portable computer using custom software developed in MATLAB. We analyzed the EODs using custom MATLAB software to calculate median values of peak-to-peak amplitude (mV) (Stoddard et al. 2003).
Model fitting and statistics
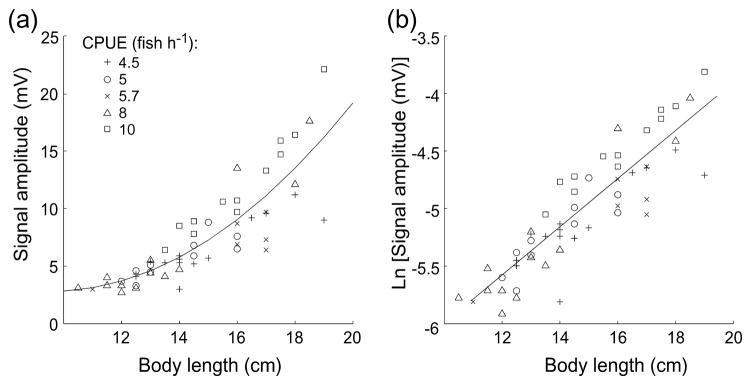
Following Hughes (2000), we analyzed the honesty of the signal as a remote indicator of body length by using signal residuals obtained from the regression of signal amplitude and body length. Based on the visual inspection of the scatter plots, we tested the fit of several regression model s: linear, exponential, non-integer exponential, and power function. We tested the validity of these regression models by checking whether their residuals were normally distributed, with a mean of zero, independent, uncorrelated, and homeoskedastic. When we regressed EOD amplitude against body length using a simple linear regression model, the residuals were still correlated with body length (r=0.68, p<0.0001), indicating higher order or non-linear relationship (Fig. 1). The residual plot also showed heteroskedasticity of the error. Linear regression of ln[EOD amplitude] against body length provided a strong fit while satisfying all the assumptions of linear regression; therefore we used ln[EOD amplitude] to determine reliability of EOD amplitude as an honest indicator of body length. Differences among group means were tested using ANOVA for field observations and repeated-measures ANOVA for lab manipulations. When significant differences were detected (p<0.05) by the ANOVA models, we used protected Tukey post hoc analysis to determine pairwise statistical differences at α=0.05 level. All statistical analyses were performed using the MATLAB Statistical Toolbox and SPSS V18.
Figure 1.
Amplitude varies with body length. In (a) body length is plotted against amplitude in a linear scale. Note that the relationship between body length and EOD amplitude is not linear, showing that EOD amplitude rises faster for longer males. In ( b) body length is plotted against the natural logarithm of EOD amplitude, after this correction we now have a linear correlation between body length and ln[EOD amplitude]. CPUE is “catch per unit effort”, our index of population density in the field.
Results
I. Signalling incentive and signalling output
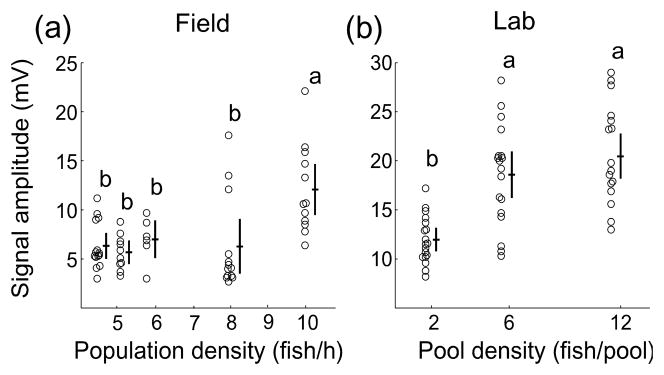
The first prediction of the disposable handicap hypothesis is that males should modulate their signal output in proportion to social incentive, such as intensity of competition. In the field, the local population density in the breeding marshes changed from one sampling event to the next by as much as a factor of two (CPUE: 5, 8, 4.5, 10 and 5.7 fish h−1). The highest mean signal amplitude in the field occurred at the highest population density (ANOVA, F4,50=6.39, p<0.001, n=14,10,6,13,12. Fig. 2a). Moreover, residual signal amplitude adjusted for body length correlated significantly with population density, while sex-ratio, season, and temperature showed no relation to signal amplitude(Spearman correlations between amplitude and: population density: rs=0.55, p<0.001, n=55; sex-ratio: rs=−0.08, p=0.54; season: rs=0.11, p=0.40; temperature: rs=0.23, p=0.08, n=55). Our experimental lab study confirmed the effect of population density on signal amplitude: males increased the signal amplitude in the high-density treatments (6, 12, and 18 fish per pool) with respect to the low-density treatment (Repeated measures ANOVA, F1,17=71.64, p<0.001, n=18, Fig. 2b).
Figure 2.
Signal amplitude increased with population density both under natural field conditions (a) and, under experimentally controlled lab conditions (b). Circles indicate raw data, while crosses indicate mean ± s.e. Significant differences among groups, based on Tukey post-hoc analysis(α=0.05), are noted by lower case letters. In the field (a) and in the lab (b), the highest signal amplitude recorded corresponded to the highest fish densities, while the lowest signal amplitude corresponded to low density.
II. Signalling incentive and signal reliability
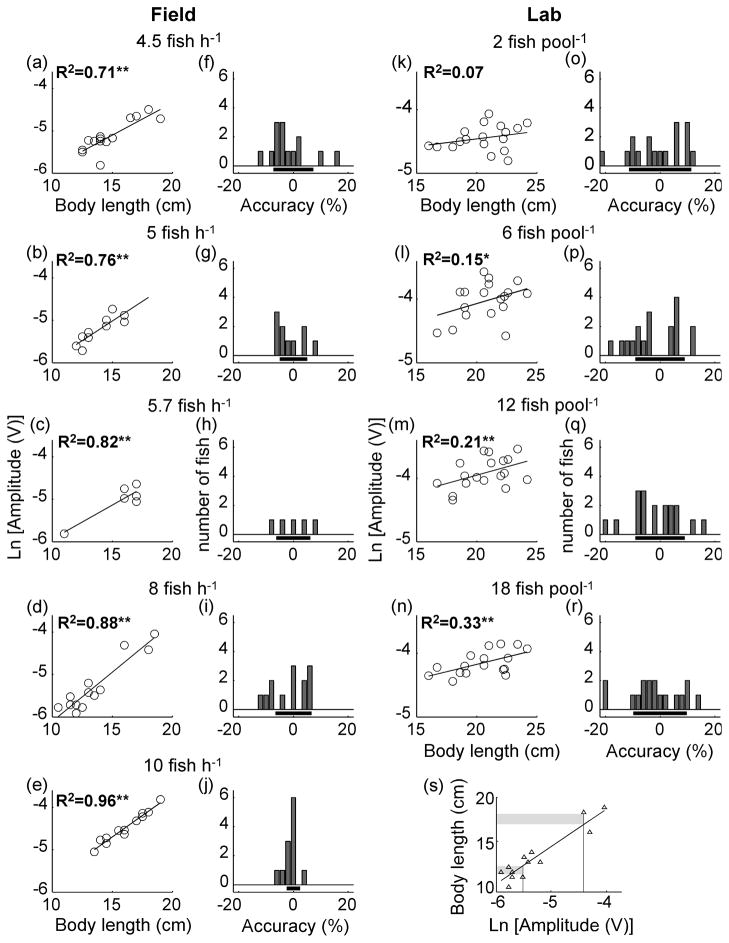
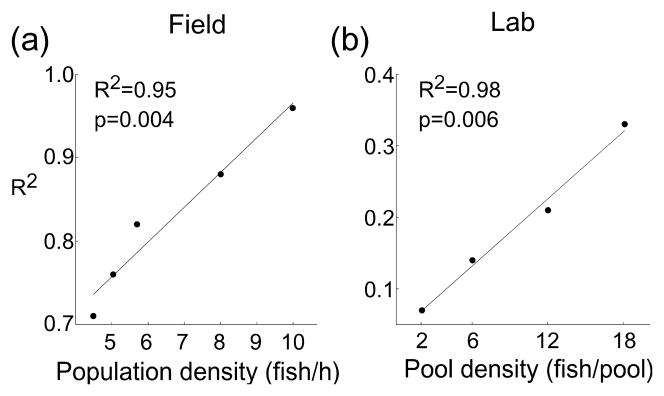
The 2nd prediction of the disposable handicap hypothesis is that signal reliability should increase with competition. In the field, we found a strong relationship between EOD amplitude and body length throughout the season, however the strength of the association varied with population density(Fig. 3a–e). When field population density was the highest, the residuals from the regression between ln[EOD amplitude] and body length had the lowest variance compared to any other sample (Bartlett’s statistics = 10.95, d.f. = 4, p = 0.027). In the lab, only the highest density treatments had significant relationships between EOD amplitude and body length (Fig. 3k–n). As predicted from the field data, experimental manipulation offish density in the lab determined the reliability of body length as a predictor of electric signal amplitude. Under both natural field conditions and experimental lab conditions, the relation between body length and signal amplitude became stronger with population density(field: R2=0.95, p=0.004, n=5, post-hoc statistical power=0.99; lab: R2=0.98, p=0.006, n=4, post-hoc statistical power=0.99; Fig. 4). Thus, the signal became more reliable with increased competition.
Figure 3.
Signal amplitude becomes a more accurate predictor of body length as population density increases, both in the field and the lab. Scatter plots and linear regressions show relationships between body length and the natural logarithm of signal amplitude, both in the field (a–e), and in the lab (k–n). All regressions in the field were statistically significant (a: p=0.0002, n=14; b: p=0.001, n=10; c: p=0.013, n=6; d: p<0.0001, n=13;e: p<0.0001, n=12). In the lab, only the highest two density treatments produced statistically significant relationships between signal amplitude and body length (k: p=0.3, n=18; l: p=0.10, n=18; m: p=0.05, n=18; n: p=0.01, n=17). Single asterisks denote marginally significant regressions (p≤0.1) and double asterisks denote significant regressions (p≤0.05). Bar plots show the accuracy of EOD amplitude as a predictor of body length in the field (f–j), and in the lab (o–r). The x axis values represent the accuracy of EOD amplitude predicting body length, calculated from the residuals of the regression between ln[EOD amplitude] and body length (s), as a percentage of body length. The height of the bars indicate the number of fish whose length can be predicted from the EOD at a given accuracy level. Horizontal bars indicate ± standard deviation of the accuracy of EOD amplitude predicting body length. Note that the accuracy of EOD amplitude as a predictor of body length was within 2.4 % of the fish’s actual body length (j) for the highest population density in the field (e).
Figure 4.
Signal reliability as a predictor of body length increases with population density, under (a) natural field conditions and (b) experimental lab manipulation. Linear regression of the R-squares obtained from the linear regressions between body length and ln[EOD amplitude] (Fig. 3a–e & k–n) plotted against fish density.
We defined the accuracy of EOD amplitude as a predictor of body length by calculating the standard deviation of the residuals from the regression of body length and ln[EOD amplitude]as a percentage of body length (100 * body length residuals/body length), encompassing 68% of the population (Fig. 3s). In the field, the accuracy with which EOD amplitude predicted the size of males varied from 7.3% at low density (Fig. 3f–i) to 2.4% at the highest density (Fig. 3j). That is, at the highest density in the field EOD amplitude could be used to predict the body length of 68% of males to within 2.4% of actual body length. The accuracy of EOD amplitude as a predictor of body length was lower in the lab, where EOD amplitude could predict body length with an accuracy of 9.1% at most(Fig. 3o–r).
III. Handicap disposal vs. social cost
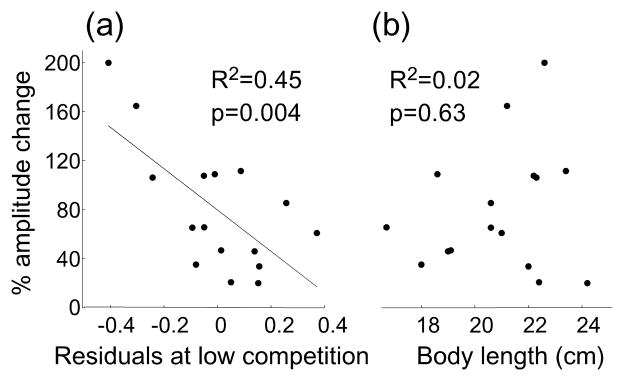
The 3rd prediction of the disposable handicap hypothesis is that males with signal amplitudes the farthest below those predicted for their body length sunder low competition should enhance their signals the most under high competition to reveal their full signalling capability. Comparison of males in the lab experiment that had experienced both low and high density (2 vs. 12 fish per pool, random order) revealed that every male generated signals of higher amplitude under high social competition than under low social competition (paired t=8.2, p<0.001 one-tailed, n=16). Likewise, males whose signal amplitudes were the farthest below the body length regression line under low competition (2 fish per pool) increased signal amplitude the most under high competition (12 fish per pool) (R2=0.45, p=0.004, n=16; Fig. 5a). Body length did not predict amount of amplitude change (R2=0.02, p=0.63, n=16; Fig. 5b), inconsistent with the hypothesis that social punishment might suppress small males from enhancing their signals.
Figure 5.
(a) In the lab experiment, the amount of change in signal amplitude between low competition (2 fish/pool) and high competition (12 fish/pool) depended on how far males had deviated under low competition from the signal amplitude predicted for their body length. The significant negative slope shows that the farther below their predicted signal amplitude at low competition (negative residuals of regression between body length and ln[EOD amplitude]) the greater males increased their signal amplitudes under high competition. (b) Body length is not correlated with the change in amplitude, contrary to expectation if signal reliability were maintained by social costs.
If social costs are constraining signal reliability, we would predict larger males to increase signal amplitude more than small males during high competition (Candolin 2000b). However, in neither the field nor in the lab did the slope or the intercept of the regressions between body length and ln[EOD amplitude] differ significantly among population densities (field: ANCOVA, Fstat=1.82, d.f.=45, p=0.141; lab: ANCOVA, Fstat=0.36, d.f.=63, p=0.78). Thus the data provide no evidence that social costs constrain enhancement of EOD amplitude.
Discussion
We sought to determine whether the ability of gymnotiforms to augment their electric signals could allow individuals to exaggerate their quality, in the process, reducing the reliability of their signals as indicators of body size. Alternatively, given the high energetic expense of the EOD, males might modulate EOD amplitude for the opposite function, to reduce signal magnitude and dispose of handicap costs when incentive for costly signalling is low. Under the disposable handicap model, high competitive incentive would pull signallers upward toward the physiological bound of their signalling capability where the signal serves as a reliable index of size or quality. In our studies, EOD amplitude was the highest when population abundance was the highest, showing that competition did provide incentive for enhanced signalling. Such enhanced signalling also increased reliability of the signal as an index of body length. Thus, signal enhancement no longer functions to exaggerate body size; on the contrary, it results in a more reliable indication of body size. These findings support the predictions of the “disposable handicap” hypothesis and contradict the hypothesis that signal enhancement would degrade signal reliability.
Information coded by the amplitude of the EOD
We expected electric signals of male B. gauderio to code information about body length since body length is the main determinant both of female preference and of male-male contest outcome. In the lab, females select longer males, which typically produce EODs of greater amplitude and duration (Curtis & Stoddard 2003). Moreover, in staged resident-intruder contests, the longest male always won the encounter despite residential status or body condition (Salazar 2009; Silva et al. 2010). From the tight relationship between body length and EOD amplitude, a receiver could use the amplitude of the signaller’s EOD as an indicator of his resource holding potential. Although signal reliability increased with competition in both the lab and the field, signal reliability was slightly greater in the field. Our lab fish are accustomed to high densities, possibly making it harder to simulate increased competition.
Although it has not been shown that gymnotiforms use electric signals to estimate body length of conspecifics, detailed lab studies indicate that electric fish have the sensory capability to disambiguate strong, distant signals, from weak, close signals. Electric fish can determine the distance of an active signal source from the curvature of the field lines. Gymnotus carapo and Brachyhypopomus diazi follow the curvature of field lines to locate electric sources (Davis & Hopkins 1988; Shieh et al. 1996), but Gymnotus will cut across the field lines to attack a familiar signaller, showing it has analyzed the field structure to separate signal intensity and source location (Scudamore & McGregor 1993). Mormyrids have been shown to distinguish between a large distant object, and a close small object (von der Emde et al. 1998). From these studies we infer that signal amplitude is not made ambiguous by source distance, so receivers can likely ascertain EOD amplitude independently of distance to obtain information about the signaller.
EOD modulation as a mechanism for handicap disposal
Both field and lab data satisfy the first prediction of the disposable handicap hypothesis, that males should modulate their signals in proportion to signalling incentive, such as competition intensity. Prior lab studies had also found that EOD amplitude of male B. gauderio varied with social context (Franchina et al. 2001), increasing with the number and proportion of male fish in the pool (Salazar & Stoddard 2009).
The 2nd prediction of the disposable handicap hypothesis is that signal reliability increases with competition. This prediction is key to the hypothesis since it distinguishes between signal enhancement for dishonest vs. honest signalling. If signal modulation is used to exaggerate body length (cheating up), the signal should be the least reliable under high competition, whereas if signal modulation is used to economize under low competition (cheating down), the signal should be least reliable under low competition. Consistent with the disposable handicap hypothesis, the reliability of the signal was the lowest when signal intensity and signalling incentives were low (cheating down), while signal reliability increased with competition intensity.
Johnstone et al. (2009) proposed that when the marginal cost of signal enhancement rises disproportionally with signal intensity, the quality of the information conveyed by the signal improves with signal enhancement. Accordingly in B. gauderio, the energetic cost of producing a male EOD rises in proportion to EOD amplitude (Salazar & Stoddard 2008). Given the exponential relationship between EOD amplitude and body length (Fig. 1a), for a male to effectively exaggerate his size, he would have to boost his EOD amplitude exponentially and bear the exponential cost of doing so. Furthermore, Salazar and Stoddard (2008) showed that males spend 10 times more of their total energy budget in signalling than females. Moreover, males (but not females)trade-off between energy allocated to signal sversus other metabolic compartments(Stoddard & Salazar 2011). Additionally, by extending the duration of the EOD’s 2nd phase of the EOD, males divert energy to the low frequency spectrum, detected by ampullary electroreceptors of predators (Stoddard & Markham 2008), making male electric signals more conspicuous than female signals, and increasing their predation risk (Hanika & Kramer 1999; Hanika & Kramer 2000; Stoddard 1999; Stoddard 2002). The increased predation risk and energetic metabolic trade-off of male signals should provide great incentive for handicap disposal during low competition.
The 3rd prediction of the disposable handicap hypothesis is that social competition drives signal catch-up. Under low competition, males with EOD amplitudes the farthest below those predicted for their body length, should enhance their signals the most under high competition. In other words, the farther a male’s amplitude has strayed from his individual limit under low signalling incentive, the greater his catch-up under high signalling incentive. This prediction allows us to distinguish between signal modulation for energy savings and signal modulation to avoid social costs; the latter would be concentrated among smaller individuals who face punishment for appearing larger than they are. We found the magnitude of male EOD enhancement under social competition depends on the male’s prior signal magnitude and capacity for increase rather than on his quality (size). Under a social costs constraint, we also would have expected larger males to increase signal amplitude more than small males during high competition, resulting in a steeper relationship between body length and ln[EOD amplitude]under high competition than low (Candolin 2000b). But we found no evidence that social costs prevent small, low quality males from increasing signal amplitude. T he slopes of the regressions between body length and ln[EOD amplitude] did not differ significantly between low and high competition, disfavouring the idea that signal enhancement is constrained by social costs.
Mechanisms ensuring honesty
In addition to constraints from energetic expense and predation risk, signal exaggeration by male in B. gauderio might be subject to allometric or biophysical constraints. For instance, electric signal amplitude might be limited by the number of electrocytes that can be packed into a given length of fish, or limits on sodium channel retention in electrocyte membranes. Animals in which degree of signal expression is physically linked to the sexually selected trait of the signaller presumably cannot exaggerate their signals (cheat up) and thus their signals should be intrinsically honest (Maynard Smith & Harper 1995). In frogs, the spectral frequency of the male’s call is allometrically related to body size. The fundamental frequency of the call is determined in part by the size of the laryngeal apparatus, which varies with body length, resulting in a negative correlation between body length and fundamental frequency (Gerhardt 1994; Ryan 1980; Ryan 1985). Similar to the effect of competition on signal reliability in our electric fish B. gauderio, in the toad, Bufo americanus, a decrease in fundamental frequency of the call results in a tighter relationship between frequency and body size (Howard & Young 1998). However, some allometric constrains can be circumvented through evolution of by-pass mechanisms wherein signal enhancement reduces signal honesty. For instance, in most of the anuran species studied, exaggeration of male body size through the dynamic reduction of call frequency degrades reliability of the signal as an indicator of body size (Bee & Perrill 1996; Bee et al. 2000; Wagner 1989b; Wagner 1992).
Selection on signallers to circumvent allometric relationships may hit additional and more restricting constraints. Although the fundamental frequency of acoustic signals is determined by the size of the larynx, resonance in the acoustic path from the larynx to the external environment reinforces particular spectral frequencies, producing formant peaks. The frequency of those formants depends on the characteristics of the vocal tract, primarily the distance from the larynx to the external environment (Fitch & Hauser 2010). Animals could potentially exaggerate their size by positioning the larynx deeper in the body, closer to the chest, therefore elongating their trachea to lower the pitch (Fitch 1999). Howbeit, a new constraint arises, as the larynx cannot be lowered beyond the top of the sternum (Reby & McComb 2003). As a result, the active lowering of the larynx reinforces the relationship between body size and formant frequency, enhancing the honesty of formant frequency as an indicator of body size (Reby & McComb2003).
In electric fish, the amplitude of the EOD physically depends on the length of the fish, longer fish can accommodate more electrogenic cells, electrocytes, along their bodies and therefore produce EODs of greater amplitude (Curtis & Stoddard 2003; Hopkins et al. 1990; Hopkins 1999). Further, the electric field of Brachyhypopomus resembles a dipole, the separation of the dipole is proportional to the fish’s length (Stoddard et al. 1999). Thus, longer fish have longer electric organs with greater dipole separation resulting in an exponential relationship between body length and EOD amplitude. At least two potential mechanisms could allow an increase in EOD amplitude outside the intrinsic relationship with body length. First, gymnotiform electric fish can increase the amplitude of their signals by augmenting the number of voltage-gated sodium channels in their electrocyte membranes (Markham et al. 2009b). Second, male B. gauderio can increase the temporal offset of the opposing action potentials in the biphasic EOD, which increases the EOD amplitude up to 25% (Markham et al. 2009a; Markham & Stoddard 2005). The latter mechanism merely unmasks the signal and thus should not require additional metabolic energy, whereas the former increases energy consumption in proportion to the opening of added ion channels. These mechanisms possibly evolved to exaggerate the signaller’s quality. Nonetheless, these mechanisms could have reached their own intrinsic limits,1) the maximum sodium channels that can fit in the electrocyte’s membrane, 2)the maximum temporal offset of the two phases of the EOD, 3) maximum energy that can be diverted to EOD production before other bodily functions begin to fail, and 4) increased predation risk. Therefore, further constraints on size-independent mechanisms for electric signal enhancement still maintain a strong one-to-one relationship between body length and EOD amplitude.
Adaptive significance of the “disposable handicap” hypothesis for receivers
Receivers will benefit from obtaining the most accurate information from the signal, however the benefits of receiving reliable signals may be greater under high competition than under low competition. Wiley’s (1994)“a daptive gullibility hypothesis” predicts that under low competition it costs more to incorrectly reject an honest signal than to believe a dishonest one, while under high competition it costs more to believe a dishonest signal than to reject an honest one. For example, under low competition the cost of finding a new territory might be lower than the cost of failing to believe a signal from a high quality individual who could inflict damage while defending a contested territory. Conversely, under high competition, when territories are scarce, the cost of an agonistic encounter may be lower than the cost of suckering for a dishonest signal and abandoning attempts to obtain a particular territory. So if signals become unreliable under high competition, they become virtually worthless to receivers, and thus not worth producing, whereas under low competition, receivers benefit by being more trusting, allowing signallers to cheat down a bit without losing their option for honesty under high competition when the reliability of signals matters more.
Conclusion
While signal modulation by B. gauderio may have arisen as a mechanism for signal exaggeration, in the present day when every male has this capacity, signal modulation appears to benefit the individual by allowing a reduction in signalling costs during low competition (cheating down) rather than by effective exaggeration of body length during high competition (cheating up). This conclusion is supported by 1) downward modulation of signal amplitude during low competition, 2) increased predictive value of the signal (greater honesty) with elevated signal amplitude during increased social competition, 3) ubiquitous increase in signal amplitude with social incentive, independent of signaller quality, and 4) the dependence of the magnitude of amplitude increase under high social incentive on deviance from the predicted value measured under low social incentive.
Signal modulation mechanisms that may have initially evolved under the selective advantage of exaggerating the signaller’s quality could be maintained in the signaller by the advantages of energy conservation under low competition, i.e., a disposable handicap. Under this scenario, competition should improve the reliability of disposable handicaps as indicators of quality. The enhancing effect of signal augmentation on signal reliability suggests an evolutionary stable strategy where signals still increase, but no receivers are fooled, and no signaller can afford not to boost his signal. Thus attention to signals by receivers should be reinforced, rather than degraded, by the signaller’s capacity to enhance the signal, provided the signal costs rise with the signal output faster than the increasing benefits of enhanced signalling. Further, handicap disposal (energetic savings) could have served as the originating driver of signal modulation just as easily as exaggeration of body size.
Highlights.
We studied the effect of signal enhancement on signal reliability
As expected, increased competition promotes signal enhancement
Unexpectedly, signal reliability improved with competition and signal enhancement
Downward signal modulation reduces signal handicap cost during low competition
Acknowledgments
We thank R. Perrone, L. Zubizarreta, G. Batista, T. de los Campos, D. Colacce, I. Becerra, P. Pouso, A. Cabana, and J. E. Campbell for assistance in the field. We thank Dr. O. Macadar, Min. de Cultura y Educación, Uruguay, and Fac. de Ciencias, Universidad de la República, Montevideo, Uruguay, for field logistics. We thank J. Molina, A. Goldina, C. Curtis, P. Perez and J. Roach for assistance in the lab. We thank two anonymous reviewers for their helpful comments on the manuscript. This work was supported by Tinker Travel Grant (LACC at FIU), FIU Graduate School’s Dissertation Evidence Acquisition Fellowship, Judith Evans Parker Scholarship, Animal Behavior Society Graduate Student Award to S. G., National Institutes of Health MBRS RISE R25 award GM061347 to C. H. Bigger, and National Science Foundation grant IOS 0956603 to P.K.S. This paper is contribution # *** to the FIU Tropical Biology Program.
Abbreviations
- EOD
electric organ discharge
- CPUE
capture per unit of effort
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akre KL, Ryan MJ. Female túngara frogs elicit more complex mating signals from males. Behavioral Ecology. 2011;22:846–853. [Google Scholar]
- Allee SJ, Markham MR, Stoddard PK. Androgens enhance plasticity of an electric communication signal in female knifefish, Brachyhypopomus pinnicaudatus. Hormones and Behavior. 2009;56:264–273. doi: 10.1016/j.yhbeh.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Sexual Selection. New Jersey: Princeton University Press; 1994. [Google Scholar]
- Bee MA, Perrill SA. Responses to conspecific advertisement calls in the green frog (Rana clamitans) and their role in male-male communication. Behaviour. 1996;133:283–301. [Google Scholar]
- Bee MA, Perrill SA, Owen PC. Male green frogs lower the pitch of acoustic signals in defense of territories: a possible dishonest signal of size? Behavioral Ecology. 2000;11:169–177. [Google Scholar]
- Candolin U. Changes in expression and honesty of sexual signalling over the reproductive lifetime of sticklebacks. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000a;267:2425–2430. doi: 10.1098/rspb.2000.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U. Male-male competition ensures honest signaling of male parental ability in the three-spined stickleback (Gasterosteus aculeatus) Behavioral Ecology and Sociobiology. 2000b;49:57–61. [Google Scholar]
- Candolin U. Increased signalling effort when survival prospects decrease: male-male competition ensures honesty. Animal Behaviour. 2000c;60:417–422. doi: 10.1006/anbe.2000.1481. [DOI] [PubMed] [Google Scholar]
- Curtis CC, Stoddard PK. Mate preference in female electric fish, Brachyhypopomus pinnicaudatus. Animal Behaviour. 2003;66:329–336. [Google Scholar]
- Davis EA, Hopkins CD. Behavioural analysis of electric signal localization in the electric fish, Gymnotus carapo (Gymnotiformes) Animal Behaviour. 1988;36:1658–1671. [Google Scholar]
- Dawkins R, Krebs JR. Animal signals: information or manipulation. In: Krebs JR, Davies NB, editors. Behavioural Ecology: An Evolutionary Approach. Oxford: Blackwell; 1978. pp. 282–309. [Google Scholar]
- Endler JA. Predation, light intensity and courtship behaviour in Poecilia reticulata(Pisces: Poeciliidae) Animal Behaviour. 1987;35:1376–1385. [Google Scholar]
- Endler JA. Multiple-trait coevolution and environmental gradients in guppies. Trends in Ecology & Evolution. 1995;10:22–29. doi: 10.1016/s0169-5347(00)88956-9. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Acoustic exaggeration of size in birds via tracheal elongation: comparative and theoretical analyses. Journal of Zoology. 1999;248:31–48. [Google Scholar]
- Fitch WT, Hauser MD. Unpacking “honesty”: vertebrate vocal production and the evolution of acoustic signals. In: Megela Simmons A, Popper AN, editors. Acoustic Communication. New York, NY, USA: Springer-Verlag; 2010. pp. 65–137. [Google Scholar]
- Franchina CR. Ontogeny of the electric organ discharge and the electric organ in the weakly electric pulse fish Brachyhypopomus pinnicaudatus(Hypopomidae, Gymnotiformes) Journal of Comparative Physiology A. 1997;181:111–119. doi: 10.1007/s003590050098. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus. I. Quantification of day-night changes. Journal of Comparative Physiology A. 1998;183:759–768. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus II. Social effects. Journal of Comparative Physiology A. 2001;187:45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- Gavassa S, Silva AC, Stoddard PK. Tight hormonal phenotypic integration ensures honesty of the electric signal of male and female Brachyhypopomus gauderio. Hormones and Behavior. 2011;60:420–426. doi: 10.1016/j.yhbeh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. The evolution of vocalization in frogs and toads. Annual Review of Ecology and Systematics. 1994;25:293–324. [Google Scholar]
- Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fish in the southern South America (Gymnotiformes: Hypopomidae) Zootaxa. 2009;2093:60–68. [Google Scholar]
- Goldina A, Gavassa S, Stoddard PK. Testosterone and 11-ketotestosteone have different regulatory effects on electric communication signals of male Brachyhypopomus gauderio. Hormones and Behavior. 2011;60:139–147. doi: 10.1016/j.yhbeh.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A. Biological signals as handicaps. Journal of Theoretical Biology. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Hall ML, Molles LE, Illes AE, Vehrencamp SL. Singing in the face of death: male banded wrens Thryophilus pleurostictus sing more to playback in their last breeding season. Journal of Avian Biology. 2009;40:217–224. doi: 10.1111/j.1600-048X.2009.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanika S, Kramer B. Electric organ discharges of mormyrid fish as a possible cue for predatory catfish. Naturwissenschaften. 1999;86:286–288. [Google Scholar]
- Hanika S, Kramer B. Electrosensory prey detection in the African sharptooth catfish, Clarias gariepinus (Clariidae), of a weakly electric mormyrid fish, the bulldog (Marcusenius macrolepidotus) Behavioral Ecology and Sociobiology. 2000;48:218–228. [Google Scholar]
- Hill JA, Enstrom DA, Ketterson ED, Nolan V, Ziegenfus C. Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behavioral Ecology. 1999;10:91–96. [Google Scholar]
- Hopkins CD. Temporal structure of non-propagated electric communication signals. Brain Behavior and Evolution. 1986;28:43–59. doi: 10.1159/000118691. [DOI] [PubMed] [Google Scholar]
- Hopkins CD, Comfort NC, Bastian J, Bass AH. Functional analysis of sexual dimorphism in an electric fish, Hypopomus pinnicaudatus, order Gymnotiformes. Brain Behavior and Evolution. 1990;35:350–67. doi: 10.1159/000115880. [DOI] [PubMed] [Google Scholar]
- Hopkins CD. Design features for electric communication. Journal of Experimental Biology. 1999;202:1217–1228. doi: 10.1242/jeb.202.10.1217. [DOI] [PubMed] [Google Scholar]
- Howard RD, Young JR. Individual variation in male vocal traits and female mating preferences in Bufo americanus. Animal Behaviour. 1998;55:1165–1179. doi: 10.1006/anbe.1997.0683. [DOI] [PubMed] [Google Scholar]
- Hughes M. Deception with honest signals: signal residuals and signal function in snapping shrimp. Behavioral Ecology. 2000;11:614–623. [Google Scholar]
- Johnstone RA, Grafen A. Dishonesty and the handicap principle. Animal Behaviour. 1993;46:759–764. [Google Scholar]
- Johnstone RA, Rands SA, Evans MR. Sexual selection and condition-dependence. Journal of Evolutionary Biology. 2009;22:2387–2394. doi: 10.1111/j.1420-9101.2009.01822.x. [DOI] [PubMed] [Google Scholar]
- Kodric-Brown A, Brown JH. Truth in advertising: the kinds of traits favored by sexual selection. American Naturalist. 1984;124:309–323. [Google Scholar]
- Kokko H. Evolutionarily stable strategies of age-dependent sexual advertisement. Behavioral Ecology and Sociobiology. 1997;41:99–107. [Google Scholar]
- Lindstrom J, Pike TW, Blount JD, Metcalfe NB. Optimization of resource allocation can explain the temporal dynamics and honesty of sexual signals. American Naturalist. 2009;174:515–525. doi: 10.1086/606008. [DOI] [PubMed] [Google Scholar]
- Markham M, Allee S, Goldina A, Stoddard P. Melanocortins regulate the electric waveforms of gymnotiform electric fish. Hormones and Behavior. 2009a;55:306–313. doi: 10.1016/j.yhbeh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham MR, Stoddard PK. Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. Journal of Neuroscience. 2005;25:8746–8754. doi: 10.1523/JNEUROSCI.2809-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham MR, McAnelly ML, Stoddard PK, Zakon HH. Circadian and social cues regulate ion channel trafficking. PLoS Biology. 2009b;7:1–14. doi: 10.1371/journal.pbio.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Harper DGC. Animal signals: models and terminology. Journal of Theoretical Biology. 1995;177:305–311. [Google Scholar]
- Miranda M, Silva A, Stoddard PK. Use of space is consistent with exploded lek polygyny in the gymnotiform electric fish Brachyhypopomus pinnicaudatus. Environmental Biology of Fishes. 2008;83:379–389. [Google Scholar]
- Moller P. Electric Fish: History and Behavior. London, NY, USA: Chapman & Hall; 1995. [Google Scholar]
- Proulx SR, Day T, Rowe L. Older males signal more reliably. Proceedings of the Royal Society B-Biological Sciences. 2002;269:2291–2299. doi: 10.1098/rspb.2002.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Animal Behaviour. 2003;65:519–530. [Google Scholar]
- Rohwer S, Ewald PW. The cost of dominance and advantage of subordination in a badge signaling system. Evolution. 1981;35:441–454. doi: 10.1111/j.1558-5646.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. The Túngara Frog. Chicago, IL, USA: The University of Chicago Press; 1985. [Google Scholar]
- Salazar VK. Ph D dissertation. Florida International University; 2009. The effect of male-male competition and its underlying regulatory mechanisms on the electric signal of the gymnotiform fish Brachyhypopomus gauderio. [Google Scholar]
- Salazar VL, Stoddard PK. Sex differences in energetic costs explain sexual dimorphism in the circadian rhythm modulation of the electrocommunication signal of the gymnotiform fish Brachyhypopomus pinnicaudatus. Journal of Experimental Biology. 2008;211:1012–1020. doi: 10.1242/jeb.014795. [DOI] [PubMed] [Google Scholar]
- Salazar VL, Stoddard PK. Social competition affect electric signal plasticity and steroid hormone levels in the gymnotiform fish Brachyhypopomus gauderio. Hormones and Behavior. 2009;56:399–409. doi: 10.1016/j.yhbeh.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore RE, McGregor PK. Approach paths of electric fish to an active electrode are affected by playback stimulus. Animal Behaviour. 1993;46:1240–1242. [Google Scholar]
- Searcy WA, Nowicki S. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Princeton, NJ, USA: Princeton University Press; 2005. [Google Scholar]
- Shieh KT, Wilson W, Winslow M, McBride DW, Hopkins CD. Short-range orientation in electric fish: an experimental study of passive electrolocation. Journal of Experimental Biology. 1996;199:2383–93. doi: 10.1242/jeb.199.11.2383. [DOI] [PubMed] [Google Scholar]
- Silva A, Quintana L, Galeano M, Errandonea P, Macadar O. Water temperature sensitivity of EOD waveform in Brachyhypopomus pinnicaudatus. Journal of Comparative Physiology A. 1999;185:187–197. [Google Scholar]
- Silva A, Quintana L, Ardanaz JL, Macadar O. Environmental and hormonal influences upon EOD waveform in gymnotiform pulse fish. Journal of Physiology-Paris. 2002;96:473–484. doi: 10.1016/S0928-4257(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Silva A, Quintana L, Galeano M, Errandonea P. Biogeography and breeding in gymnotiformes from Uruguay. Environmental Biology of Fishes. 2003;66:329–338. [Google Scholar]
- Silva A, Perrone R, Macadar O. Environmental, seasonal, and social modulations of basal activity in a weakly electric fish. Physiology & Behavior. 2007;90:525–536. doi: 10.1016/j.physbeh.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Silva A, Zubizarreta L, Costa G. Interspecific differences in agonistic behavior and its serotonergic modulation. International Congress of Neuroethology; Salamanca, Spain. 2010. [Google Scholar]
- Stoddard PK. Predation enhances complexity in the evolution of electric fish signals. Nature. 1999;400:254–256. doi: 10.1038/22301. [DOI] [PubMed] [Google Scholar]
- Stoddard PK, Rasnow B, Assad C. Electric organ discharges of the gymnotiform fishes: III. Brachyhypopomus. Journal of Comparative Physiology A. 1999;184:609–630. doi: 10.1007/s003590050359. [DOI] [PubMed] [Google Scholar]
- Stoddard PK. Electric signals: Predation, sex, and environmental constraints. Advances in the Study of Behavior. 2002;31:201–242. [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL. Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. Journal of Experimental Biology. 2003;206:1353–1362. doi: 10.1242/jeb.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Zakon HH, Markham MR, McAnelly L. Regulation and modulation of electric waveforms in gymnotiform electric fish. Journal of Comparative Physiology A. 2006;192:613–624. doi: 10.1007/s00359-006-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL, Allee S. Circadian rhythms in electric waveform structure and rate in the electric fish Brachyhypopomus pinnicaudatus. Physiology & Behavior. 2007;90:11–20. doi: 10.1016/j.physbeh.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Markham MR. Signal cloaking by electric fish. BioScience. 2008;58:415–425. doi: 10.1641/B580508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Salazar VL. Energetic cost of communication. Journal of Experimental Biology. 2011;214:200–205. doi: 10.1242/jeb.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Velando A. A dynamic trait affects continuous pair assessment in the blue-footed booby, Sula nebouxii. Behavioral Ecology and Sociobiology. 2003;55:65–72. [Google Scholar]
- von der Emde G, Schwarz S, Gomez L, Budelli R, Grant K. Electric fish measure distance in the dark. Nature. 1998;395:890–894. doi: 10.1038/27655. [DOI] [PubMed] [Google Scholar]
- Wagner WE. Fighting, assessment, and frequency alteration in Blanchard’s cricket frog. Behavioral Ecologyand Sociobiology. 1989a;25:429–436. [Google Scholar]
- Wagner WE. Social correlates of variation in male calling behavior in Blanchard’s cricket frog, Acris crepitans blanchardi. Ethology. 1989b;82:27–45. [Google Scholar]
- Wagner WE. Deceptive or honest signalling of fighting ability? A test of alternative hypotheses for the function of changes in call dominant frequency by male cricket frogs. Animal Behaviour. 1992;44:449–462. [Google Scholar]
- Wiley RH. Errors, exaggeration, and deception in animal communication. In: Real LA, editor. Behavioral mechanisms in evolutionary ecology. Chicago: University of Chicago Press; 1994. pp. 157–189. [Google Scholar]
- Wong B, Svensson P. Strategic male signalling effort in a desert-dwelling fish. Behavioral Ecologyand Sociobiology. 2009;63:543–549. [Google Scholar]
- Zahavi A. Mate selection: a selection for a handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zahavi A. The cost of honesty: further remarks on the handicap principle. Journal of Theoretical Biology. 1977;67:603–605. doi: 10.1016/0022-5193(77)90061-3. [DOI] [PubMed] [Google Scholar]