Abstract
Objective
To follow toddlers referred for risk for autism using standardized observational measures administered frequently from ages 18 to 36 months.
Method
65 consecutive referrals and 13 children from other research projects were seen approximately every 2 months from 18 to 36 months for standardized assessments and clinical judgments by the same examiner, and every 6 months by an examiner “blind” to previous scores.
Results
30 children never received an ASD diagnosis; 48 children (all referrals) received at least one diagnosis of ASD. Best trajectory typology using ADOS scores revealed 4 trajectory classes with high probabilities for fit to most likely class: severe persistent (21%), worsening (21%), improving (19%) and nonspectrum (40%). Classes differed by trajectories in verbal and nonverbal mental ages; never/ever ASD groups differed on ADI-R domain scores and clinician judgments, but improving/worsening trajectory groups did not.
Conclusions
Results replicated findings from studies of infant siblings of children with autism in siblings and non-siblings, suggesting variability in early trajectories and supporting the need for early identification, regular monitoring and standardized assessments of young children suspected of ASD.
keywords or short phrases: Autism, ADOS, Longitudinal, Toddlers, Trajectory
Developmental changes are an important focus within the growing body of autism spectrum disorder (ASD) research and research in other neurodevelopmental disorders. ASD includes several related disorders, including autism, Asperger’s disorder and pervasive developmental disorder-not otherwise specified (PDD-NOS) (Chawarska, Klin, Paul & Volkmar, 2007). The unifying symptoms across these conditions include early impairments in reciprocal social interaction and communication and the presence of restricted and repetitive behaviors (American Psychiatric Association, 1994).
Longitudinal research addressing the progression of ASD over time has both clinical and theoretical implications. In the clinical realm, these investigations illustrate the course of symptoms over development (Soke et al., 2011). In addition, they permit a better understanding of prognosis, including the ways in which we might intervene in development to achieve more positive outcomes (Baker, Smith, Greenberg, Selzer & Taylor, 2011). Longitudinal studies also inform theoretical discussions of ASD. An important example of this which has been increasingly drawing attention is that clusters of trajectories (that is, groups of children who change in similar ways across time) might be used as phenotypic markers for different neurobiological subgroups (Lord, Leventhal & Cook, 2001). Historically, most longitudinal research in ASD enrolled children when they were preschool age, or later, and followed them to late childhood and beyond (e.g., Pellicano, 2010).
Age of enrollment has been tied closely to the limits of our diagnostic expertise. Until recently, the earliest consistently reliable diagnosis of ASD was typically possible between 2 and 3 years of age (Charman, Taylor, Drew, Cockerill, Brown & Baird, 2005; Chawarska, Klin, Paul, Macari & Volkmar, 2009; Lord, 1995; Turner, Stone, Pozdol, & Coonrod, 2006). A consistent finding from longitudinal studies was that the vast majority of individuals with ASD showed enduring and pervasive impairments into adulthood (Billstedt, Gillberg, & Gillberg, 2005; Seltzer et al., 2003; Sigman & McGovern, 2005). In these samples, early individual differences were clearly associated with meaningful differences in later outcome. Children who had better early language skills (Billstedt, Gillberg, & Gillberg, 2007; Sigman & McGovern, 2005) and those with higher early cognitive skills (Eaves & Ho, 2008; Howlin, Magiati, & Charman, 2009; Stevens et al., 2000) had more positive outcomes in school-age years and beyond. Many children showed gradual improvements as they developed, with a small minority exhibiting very marked developmental gains with time (Anderson et al., 2007; Howlin, Goode, Hutton, & Rutter, 2004; Seltzer, Shattuck, Abbeduto, & Greenberg, 2004).
In recent years, much attention has been paid to early symptoms of ASD. Behavioral differences between children who later received diagnoses of ASD and typically developing children have been identified as early as the first year of life (Ozonoff et al., 2008; Wetherby, Woods, Allen, Cleary, Dickinson & Lord, 2004; Zwaigenbaum et al., 2005). Yet within children later diagnosed with ASD, trajectories from infant development, often not identifiable as obviously atypical to clear autism seem to be variable (Bryson et al., 2007; Landa et al., 2007; Ozonoff et al., 2010; (Werner & Dawson, 2005). Many of these findings have arisen from studies of younger siblings of children with ASD because the siblings can be identified as at high risk from birth (Bryson et al., 2007; Landa, Holmes & Garrett-Mayer, 2007; Ozonoff et al., 2009). For example, siblings of children with ASD who developed ASD have shown several different global patterns of change over time. Bryson and colleagues (Bryson et al., 2007) reported that their prospective sample of younger siblings who went on to receive ASD diagnoses at age 3 showed two distinct patterns of early development. A first pattern was more common and was distinguished by dramatic decreases in standardized developmental or IQ scores (usually between 12 and 24 months of age), along with marked diminishment of social behaviors and an increase in temperamental difficulties (generally by 12 or 18 months of age). A second pattern of development was less common and characterized by persistent average or near average developmental/IQ scores and more variable trends of social development. Because the symptoms of ASD were earlier and more pronounced in the former group than in the latter, the authors referred to this larger group of children as the “early onset” cases (Bryson et al., 2007).
Similar results were reported by Landa et al.,(2007) in their prospective study of younger siblings of children on the autism spectrum. Their “early diagnosis” group, identified with ASD by 14 months of age, was compared to a “later diagnosis” group, who could not be identified by 14 months but was diagnosed with ASD by 30 or 36 months of age. Younger siblings who did not have ASD and siblings of non-ASD controls were also studied. The early diagnosis group showed fewer prosocial and typical communicative behaviors than the later diagnosis group at 14 months; the groups looked more similar at a 24 month assessment. The degree and direction of developmental change in social communication between 14 and 24 months differed most strongly between ASD and non-ASD groups rather than between the different ASD-onset groups or between the different non-ASD high-risk siblings and others. Whereas the non-ASD groups showed robust and pervasive increases in social communication skills from 14 to 24 months, both ASD groups showed decreases, plateaus or slowed gains (Landa et al., 2007). Using a partially overlapping sample, parallel findings were reported by Landa & Garrett-Mayer (2006), such that younger siblings diagnosed with ASD showed a slowing in cognitive gains between 14 and 24 months of age.
Although not yet thoroughly documented in the prospective infant literature, the phenomenon of “regression” is one that is central to the study of early development in ASD. Regression refers to the loss of previously established skills, usually sometime in the second year of life, and has been estimated, based on parent retrospective reports, to occur in 15–40 percent of children with ASD (Baird et al., 2008; Hansen et al., 2008; Pickles et al., 2009; Rozga et al., 2010). Retrospective reports, including some using home videotapes, have documented regression as affecting children’s early language and social communication (Goldberg, Thorsen, Osann, & Spence, 2008; Lord, Shulman, & DiLavore, 2004; Luyster et al., 2005; Werner & Dawson, 2005). The prospective investigations described earlier reported some preliminary evidence of regression. Bryson et al. (2007) observed loss of language in a subset of cases, as well as decreases in reciprocal social behaviors in a number of participants, and Landa and colleagues (Landa et al., 2007) noted a decrease in the ASD group in shared positive affect and gestures between 14 and 24 months of age.
Investigations of early development in ASD have revealed that there is much to learn about the different trajectories taken by these children, but the richness of the data has been limited because observations generally took place approximately every 6 to 10 months. An exception to this is a recent study by Ozonoff and colleagues. Ozonoff et al., (2010) used observational methods to show trajectories from 6 to 36 months of declining social-communicative behaviors of siblings of children with autism who later developed ASD compared to typical children who were not siblings of ASD children. In the first few years of life, however, children are changing rapidly. Therefore, a fine-grained approach to measuring individual trajectories has the potential to draw a much more complete picture of developmental change. In addition, because of the time lapses between observations, trajectories have often been generated by comparing group means and variation at each time point. This permits the detection of global paths of development (i.e., showing that, overall, children went from point A to point B) but may not clarify what the variation was within that group or the paths individual children took from one point in time to the next.
These projects followed large numbers of younger siblings. However, because not all siblings develop ASD, on the whole, the numbers of children with ASD have often been small, and we do not know yet how representative affected siblings are of singletons with ASD in early years.
Gaining a better understanding of the trajectories that characterize early development in ASD will aid in determining how similar these paths in the first few years of life are to those observed across childhood and adolescence. One prediction is that the patterns observed in children with ASD in the first years of life will look similar to those seen later in life; most individuals with earlier ASD diagnoses have enduring and pervasive impairments, while many show gradual but slight improvement in autistic symptoms, and only very few make marked gains (Anderson, Oti, Lord & Welch, 2009; Gotham, Pickles & Lord, in press). On the other hand, it could also be hypothesized that, due to neuronal plasticity early in development (Bjorklund, Periss, & Causey, 2009; Pennington, 2009) and the availability of effective intensive intervention (Howlin et al., 2009), early trajectories could be different than later in life. Indeed, in recent years, there are reports of higher proportions of children showing marked reductions in symptoms or even “moving off” the spectrum (Helt et al., 2008; Kleinman et al., 2008; Sutera et al., 2007).
The current study attempts to address these questions through a more “microgenetic” (Siegler & Crowley, 1991) technique; that is, we sought to collect a high density of observations throughout a shorter period of development in order to monitor change as it occurred. Moreover, rather than comparing group means at specific time points to identify general paths of development, the focus was individual trajectories across time. In the present investigation, children with ASD and a small comparison group of children with languge delays or typical development were identified in the first year and a half of life and enrolled into the study, with the goal of carrying out frequent assessments up to age three. Standardized direct observations of behavior and ratings of diagnostic certainty were conducted at each visit. Children without any concern about possible ASD were included so that cognitive and diagnostic assessments by a clinician “blind” to referral status and to previous results could be conducted every six months. These frequently collected data-points were then used to plot individual differences from the point of enrollment to three years of age in an attempt to illustrate distinct patterns of development over time.
Method
Participants
Recruitment targeted children between the ages of 12 and 18 months of age from two sources: (1) 65 consecutive referrals of children from the clinic at the University of Michigan Autism and Communication Disorders Center because of concerns about possible ASD, and (2) 13 children who were part of typically-developing and language-delayed comparison groups specifically recruited for University of Michigan projects studying early development of children with communication delays. All participants could walk independently, and none had sensory (visual or hearing) impairments or severe motor impairments.
As part of the ongoing longitudinal studies, every participant was seen more than once and by at least two different examiners. Altogether, data were used for 78 individuals (31 children referred for possible ASD who were not siblings of children with ASD, 34 younger siblings of children with ASD whose parents referred them because of some concern, and 13 developmentally-delayed or typical children), who were seen 490 times in total. Initial appointments were between age 12 and 19 months; children were seen an average of six times over an average of 20 months. Fifteen children were seen for more than 10 visits (10 ever ASD, 5 never), 18 were seen 5–10 times (11 ever ASD, 7 never), 27 were seen 3–4 times (17 ever ASD, 11 never), and 16 were seen twice (8 ever ASD, 8 never). Children were seen by the same clinician for most of their monthly visits because of the potential stress on parents coming in frequently of seeing a different clinician every visit. Every six months each child was evaluated by an independent clinician who had not met the child before and who had no knowledge of his or her previous performance and earlier diagnoses.
At each appointment, a “best-estimate” diagnosis was assigned based on the impressions of the examiner. Examiners were clinical psychologists or advanced graduate students in psychology, all of whom had extensive experience working with young children with developmental concerns and ASD. A clinical “probability rating” (discussed below) accompanied each visit’s diagnosis in order to capture the degree of clinician certainty about the classification. Consequently, each child had several diagnostic judgments made throughout the study based on current clinical estimates in the monthly visits; judgments could vary from month-to-month. These diagnostic judgments were used to create two groups based on whether a child had ever been classified during their monthly visits as being on the autism spectrum, including 48 children who had been judged by a clinician to be on the autism spectrum at least once (the “ever ASD” group) and 30 children who were never classified as ASD (the “never ASD” group), which included all 13 children in the comparison group (see Table 1).
Table 1.
Demographics according to Ever ASD vs Never ASD diagnoses
| Ever ASD n=48 |
Never ASD n=30 |
|
|---|---|---|
| Number male (%) | 38 (79.16) | 22 (73.33) |
| Chronological age (Baseline) | 17.25 (5.58) | 17.00 (5.82) |
| Chronological age at (Final) | 35.04 (7.01) | 31.70 (7.09) |
| Number of observations | 6.79 (4.72) | 5.43 (4.25) |
| Mullen receptive language age equivalent* (Baseline) | 12.69 (7.39) | 15.10 (7.50) |
| Mullen expressive language age equivalent* (Baseline) | 12.02 (6.20) | 14.20 (6.01) |
| Mullen verbal IQ* (Baseline) | 66.07 (24.64) | 79.00 (28.66) |
| Mullen nonverbal IQ* (Baseline) | 92.96 (20.44) | 94.97 (27.56) |
| ADOS Social Affect subtotal (Baseline) | 11.42 (4.89) | 5.60 (4.01) |
| ADOS RRB subtotal (Baseline) | 3.54 (1.72) | 1.77 (1.41) |
| ADOS Algorithm total (Baseline) | 14.96 (5.88) | 7.37 (4.22) |
| Clinician diagnostic certainty rating (Baseline) | 10.56 (4.63) | 4.12 (1.05) |
| ADI-R subtotals (at approximately 30 months) | n=47 | n=25 |
| Reciprocal Social Interaction | 12.23 (6.48) | 6.16 (4.70) |
| Verbal Communication | 8.94 (6.11) | 4.60 (3.44) |
| Nonverbal Communication | 8.17 (4.52) | 5.48 (4.24) |
| Restricted and Repetitive Interests | 4.06 (2.64) | 1.68 (1.63) |
Note: Values above are averages, with standard deviations in parentheses, unless otherwise indicated.
T1 = Time 1; ADOS=Autism Diagnostic Observation Schedule; ADI-R=Autism Diagnostic Interview-Revised (for 47 “ever” ASD; 25 “never ASD”).
At each child’s final research appointment (mean age 34 months, standard deviation = 7 months), an overall consensus diagnosis was made by two clinicians, at least one of whom had been previously unfamiliar with the child until the last appointment so that there was less bias, even though, at this point, they had access to historical information. The final diagnostic decision was based on clinician observation, earlier summary reports and recent monthly diagnoses, certainty ratings, psychometric and diagnostic algorithm scores, and videotaped observations of the child. Using these final diagnoses, the sample included 39 children with ASD, 20 with typical development (TD) and 19 with non-spectrum diagnoses (NS). All individuals with NS and TD did not meet standard ADI-R criteria for autism or ASD (Risi et al., 2006) and received best-estimate diagnoses outside the autism spectrum. Nonspectrum participants had a range of diagnoses, including six children with expressive language disorders, five children with general developmental delays, three children with non-specific intellectual disability, three children with Down syndrome, one child with mild cerebral palsy and one child with fetal alcohol syndrome. Ethnicity was 9.5% African-American, 2% Hispanic, 2% Asian-American, 73% Caucasian, and 13.5% multi-racial. Maternal education was equally distributed between completed university or some college (37% each) with 25% high school graduates and only one mother with less than high school. There were no differences in ethnicity or maternal education for the diagnostic groups (“ever ASD,” “ never ASD”) or the trajectory groups.
Measures
Diagnostic Instruments
The Autism Diagnostic Interview – Revised (ADI-R: LeCouteur, Lord, & Rutter, 2003) is a standardized 90-minute caregiver interview that generates separate scores for socialization, communication and restricted and repetitive behaviors in children referred for possible autism. A toddler version of the ADI-R, which includes a number of additional items specific to the first two years of life (Lord, Shulman, & DiLavore, 2004), was used.
The Autism Diagnostic Observation Schedule (Lord et al., 2000) is a semi-structured, standardized assessment of communication, social interaction and play for children who have been referred for possible autism. A module is selected based on the child’s language level. Children in this study were administered a modified version intended for children between 12 and 30 months of age called the “Toddler Module” (Lord, Luyster, Gotham, & Guthrie, in press; Luyster at al., 2009), Module 1 (preverbal or single words for children over 30 months of age), or Module 2 (phrase speech at any age). The ADOS involves a variety of social “presses” designed to elicit behaviors relevant to a diagnosis of autism. A standardized diagnostic algorithm can be calculated, consistent with autism criteria in DSM-IV/ICD-10, (American Psychiatric Association, 1994; World Health Organization, 1993) and yields subscores for social affect (SA) and restricted and repetitive behaviors (RRB) (Gotham et al., 2007; Luyster et al., 2009). Established cut-off scores based on algorithm totals are used to differentiate autism, autism spectrum, and non-autism spectrum participants. As such, higher algorithm totals indicate more abnormality. The algorithm which was most appropriate for the child’s age and language level was used.
Each member of the clinical research team established inter-rater reliability exceeding 90% exact agreement for all items on the ADI-R and algorithm, and 80% exact agreement on codes for the ADOS and algorithm, for three consecutive administrations prior to active data collection. Reliability was maintained through regular (at least one in five administrations) consensus coding with a second rater (with percent exact item agreement ranging from 75–96, mean = 92 for the ADI-R, all ICC’s >.95 and from 70–100, mean = 85 for the ADOS, all ICC’s >.91). Inter-rater reliability for unblinded versus blinded ADOS examiners (all ADI-R examiners were blinded) was lower, but not significantly lower (mean = 83) for blinded – unblinded pairs than blinded-blinded pairs (mean = 87).
A probability-of-diagnosis rating scale was used by clinicians at each monthly visit to characterize their certainty of their own judgments of diagnoses of ASD or not ASD. The scale required the clinicians to rate whether or not they judged the child as having autism or non-autism ASD and how certain they were this diagnosis was correct. These judgments were translated onto a 15-point scale, with lower scores indicating that the clinician was certain the child was not on the autism spectrum and higher scores indicating greater certainty for autism. The same scale has been used in several previous projects and shown to have a good predictive validity of ASD diagnosis several years later (Lord, 1995), and to have good concurrent validity with DSM-IV best estimate diagnoses across sites in a multi-site study (Lord et al., in press).
At each visit, parents were asked to report the number of hours of treatment their child had received and the type of treatment. Very few children under 24 months of age had received any treatment and none exceeded more than an hour a week. After 24 months, amount of treatment increased, most often consisting of non-categorical preschool/toddler classes, home-based weekly non-categorical early childhood sessions, individual speech therapy, or a few hours of usually home-based applied behavior analysis (ABA). In order to reduce the data, treatment data were analyzed in three ways: receipt of any treatment by age 30 months, average weekly hours of treatment beginning at 24 months, and whether ABA (mean = 5 hours a week) was delivered.
Psychometric Instruments
The Mullen Scales of Early Learning (MSEL) (Mullen, 1995) evaluates cognitive functioning for children from birth to 68 months of age. The MSEL provides an overall cognitive score and yields subtest scores for motor skills (gross and fine), visual reception, and receptive and expressive language. In three cases where scores were too low to use standard scores, subtest scores were used to derive ratio nonverbal, verbal, and full-scale IQs by averaging age equivalents for the two nonverbal, two verbal and all four subtests, respectively, dividing by chronological age and multiplying by 100. For all other children, T-scores were used to estimate standard nonverbal and verbal scores from the full-scale distributions.
Procedures
Testing was generally administered in a clinic room with tables and chairs appropriate for young children. A familiar caregiver was always present in the room. Consent, which was approved by the University of Michigan Medical School Institutional Review Board for Human Subject Research, was given by parents.
Each child’s initial evaluation included the Toddler ADI-R, the ADOS and the MSEL. Monthly follow-up assessments included the ADOS. The MSEL was re-administered every six months during the “blind” assessment. Each child’s final evaluation again included both diagnostic measures (ADI-R and ADOS) and the MSEL. After each assessment, families received oral feedback and a brief report.
Statistical Analyses
All analysis was undertaken in Stata 10 (StataCorp, 2007). ROC curves of algorithm scores for predicting a child’s final diagnosis were estimated using the rocgold procedure.
ADOS algorithm scores for participants with longitudinal data [yit] were analyzed for patterns of stability or change using a discrete class age-related growth model. The model assumed that participants belonged to one of k=1,…, K latent classes each with their own expected trajectory. The trajectories were allowed to vary in their intercept (level at the mean age of the sample), and linear and quadratic slopes with respect to time. Models were estimated using the gllamm procedure (Generalized Linear Latent and Mixed Model, www.gllamm.org; Rabe-Hesketh, Skrondal, & Pickles, 2004). Models with between two to five trajectory classes varying in intercept, linear and quadratic age trends were compared for goodness of fit with Bayesian Information Criterion (BIC) scores of 1916.98 (intercept), 1915.68 (intercept and linear slope) and 1924.39 (intercept, linear and quadratic slope) with consistently higher scores for other numbers of classes. The model with the lowest Bayesian Information Criterion (BIC = −2log-likelihood + number of parameters × log (number of observations)) was chosen as being the simplest empirically-plausible model.
The models were estimated using full information maximum likelihood, which not only allows use of all the available assessments, but takes account of variation in data availability (ie., missing data) associated with the observed scores and their trajectory, and any included covariates such as age of child (Rubin, 1976).
In the fixed part of the model, linear and quadratic age effects were included as well as dummy variables to account for scores from the five different algorithms (Toddler NV; Toddler SW; Mod 1 NV, Mod 1 SW, Mod 2) and for observations that were made blind or unblind to previous diagnoses and scores. This allows for systematic mean shifts in scores arising from moving between algorithms. Participants were assigned to their most likely class using empirical Bayes’ posterior probabilities. The association of class membership with family and child characteristics was assessed using likelihood ratio chi-square tests from ordinary and logistic regression, while association with profiles of measures used marginal regression models from which p-values and confidence intervals were calculated from the robust covariance matrix-based Wald test criterion (Froot, 1989).
Results
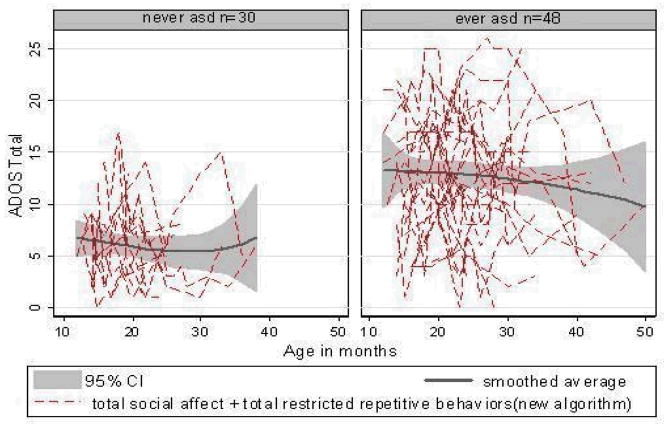
Table 1 describes the sample of 78 children at their first assessment. As stated earlier, 48 were judged clinically to be ASD on one or more occasions, and 30 were not. “Never ASD” children were seen on average 5.4 times over 20.5 months and the “ever ASD” group 6.8 times over 20.5 months. As shown in Table 1, “ever ASD” and “never ASD” classes did not differ in proportion of males, number of observations or age at baseline, but “ever ASD” children were older at final assessments, t (76) = 2.83, p <0.01. “Ever ASD” participants had higher ADOS Social Affect scores, t (76) = 5.76, p <0.01; ADOS RRB scores, t (76) = 3.66, p <0.05; total ADOS algorithm scores, t (76) = 6.66, p <0.001; clinician diagnostic probability ratings, t (76) = 11.33, p <0.001; and all ADI-R domain scores, t (76) ≥ 4.86, p <0 .01, as well as lower Verbal IQs, t (76) = 2.05, p<.05. There were no significant class differences for nonverbal IQs or MSEL language age equivalents. Figure 1 displays ADOS total algorithm score histories.
Figure 1.
ADOS Total Algorithm Score Trajectories for “Ever ASD” and “Never ASD” Children
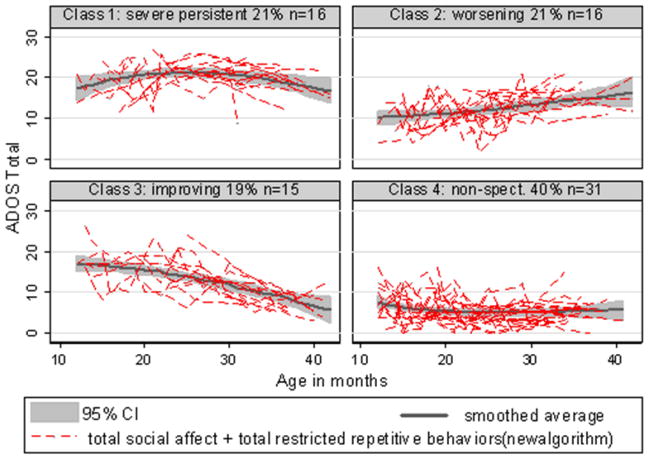
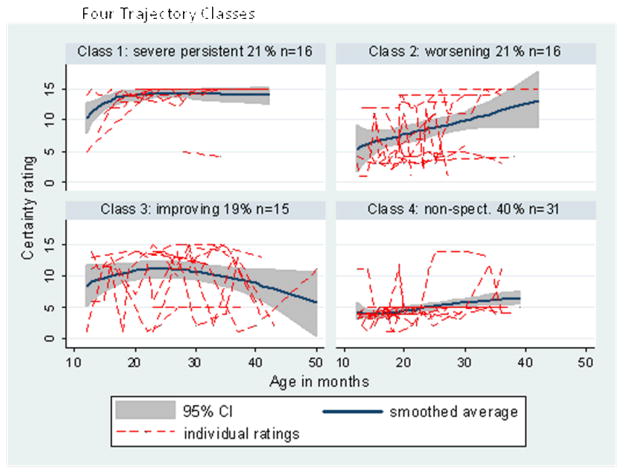
Using the minimum BIC as the selection criterion, the best trajectory typology was a model with four classes that varied in intercepts and linear slopes, as shown in Table 2. Figure 2 displays the ADOS histories and fitted mean trends for these four classes. The average probabilities for being assigned to the most likely class were high, being 0.94 for class 1 (severe persistent ~ 21% of sample), 0.87 for class 2 (worsening ~ 21%), 0.84 for class 3 (improving ~ 19%), and 0.99 for class 4 (nonspectrum ~ 40%). Confirming the validity of this categorization, the last class included all children who had never received an ASD diagnosis at any point and one child who had received an ASD diagnosis only once.
Table 2.
Trajectory Model BIC scores for parsimonious fit
| Classes Dimensions |
2 | 3 | 4 |
|---|---|---|---|
| Intercept | 1936.45 | 1921.69 | 1916.98 |
| Intercept Linear slope |
20003.29 | 1934.56 | 1915.68 |
| Intercept Linear slope Quad slope |
2009.02 | 1930.84 | 1924.39 |
Figure 2.
ADOS Total Algorithm Score Trajectories for Four Empirically Derived Classes
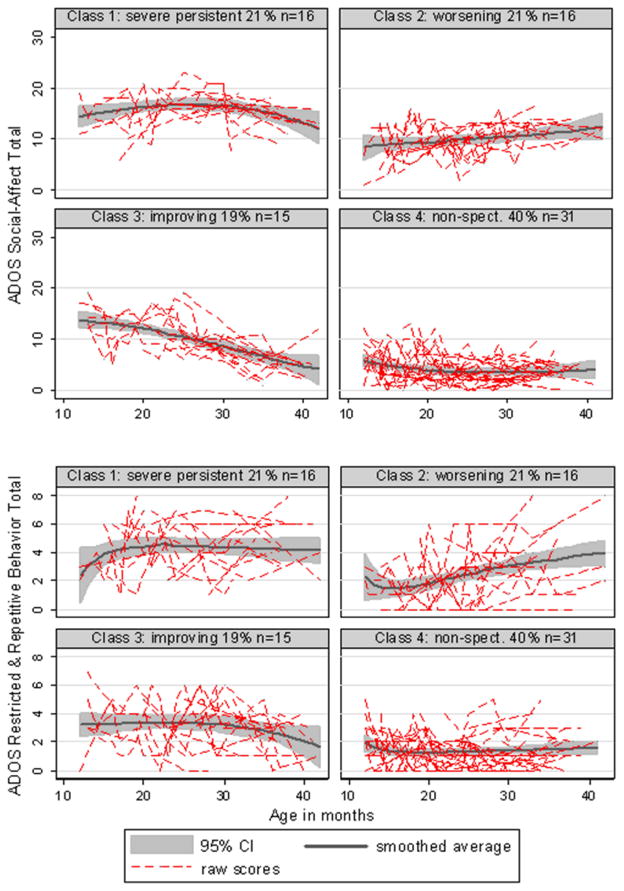
Figure 3 displays the ADOS SA and RRB histories and mean trends for the four classes. Marginal regression models confirmed our labeling of the severe persistent class, with there being no significant evidence of change over time in either SA or RRB. Among the worsening class, both SA (p=0.012) and RRB (p=0.001) increased (became more abnormal) over time. Among the improving class, while SA decreased (p<0.001), the RRB score showed little evidence of improvement (p=0.176). Within the nonspectrum class, there was a marginal decrease in SA (p=0.058) but not RRB (p=0.904).
Figure 3.
ADOS Scores for Four Trajectory Classes: Social-Affect and Restricted, Repetitive Behaviors
Baseline ADOS domain scores discriminated among all four classes (SA χ2(3)=56.48, p<0.001; RRB χ2(3)=28.12, p<0.001) and among the three ASD classes alone (SA χ2(2)=15.15, p<0.001; RRB χ2(2)=12.25, p=0.002). Compared to the persistent class, those in the worsening class started significantly lower (less abnormal) on SA (p=0.002) and on RRB (p=0.003) and the improving class started marginally lower on SA (p=0.054), though similar on RRB (p=0.228).
In contrast, taking the final observation for each child, compared to those in the persistent class, those in the improving class scored 8.10 points lower (less abnormal) on SA (p<0.001) and 2.74 points lower on RRB (p=0.002). Those in the worsening class had scores that were still not yet as severe as the persistent class for either SA (3.18 points lower, p=0.006) or RRB (1.5 points lower, p=0.05).
We examined the association of class membership with a variety of factors and covariates. Tables 3 and 4 show class demographics and means for a variety of measures. The classes did not differ by gender, χ2(3)=2.02, p=0.567, nor maternal education, χ2(3)=3.56, p=0.313, referral status, χ2(6)=5.24, p=0.513, (clinic non-sibling/sibling/control) nor with ADI-R report of any regression, χ2(3)=6.91, p=0.075. All three baseline ADI-R domain scores were strongly associated with ASD vs non-ASD class membership (social χ2(3)=33.26, p<0.001; nonverbal communication χ2(3)=13.40, p=0.004; repetitive χ2(3)=18.23, p<0.001), but did not discriminate among the three ASD trajectory classes when the nonspectrum class was excluded (social χ2(2)=3.24, p=0.198; nonverbal communication χ2(2)=2.44, p=0.295; repetitive χ2(3)=1.37, p=0.503).
Table 3.
Demographics by ADOS trajectories
| Severe Persistent n=16 |
Worsening n=16 |
Improving n=15 |
Nonspectrum n=31 |
|
|---|---|---|---|---|
| Male | 14 (87.50) | 13 (81.25) | 11 (73.33) | 22 (70.96) |
| Younger sibling of child with ASD | 8 (50.00) | 6 (37.50) | 6 (40.00) | 14 (45.16) |
| Race/Ethnicity* | ||||
| African-American | 2 (12.50) | 1 (3.2) | ||
| Asian/Pacific Islander | 1 (6.25) | |||
| Bi-racial | 2 (12.50) | 2 (13.33) | 3 (9.67) | |
| Caucasian | 14 (87.50) | 13 (81.25) | 13 (86.67) | 25 (80.64) |
| Hispanic | 1 (3.22) | |||
| Maternal education† | ||||
| Graduate/professional degree | 4 (25.00) | 7 (43.75) | 3 (20.00) | 13 (41.93) |
| College degree | 8 (50.00) | 3 (18.75) | 7 (46.67) | 9 (29.03) |
| Some college | 4 (25.00) | 4 (25.00) | 1 (6.67) | 4 (12.90) |
| High school diploma/GED | 2 (13.33) | |||
| Recruitment source | ||||
| Concerns - sibling | 8 (50.00) | 6 (37.50) | 6 (40.00) | 14 (45.16) |
| Concerns non-sibling | 6 (37.50) | 8 (50.00) | 8 (53.33) | 9 (29.04) |
| Other research | 2 (12.50) | 2 (12.50) | 1 (6.67) | 8 (25.80) |
| Final diagnosis | ||||
| Autism | 15 (93.75) | 2 (12.50) | 2 (13.33) | |
| PDD-NOS | 8 (50.00) | 9 (60.00) | 2 (6.45) | |
| Nonspectrum disorder | 1 (6.25) | 3 (18.75) | 4 (26.67) | 10 (32.25) |
| Typically developing | 1 (6.25) | 19 (61.29) | ||
Note: Values reported are counts per class, with percentage per class indicated in parentheses.
Data missing for one child
Data missing for nine children
Table 4.
Means and Standard Deviations for Trajectory Diagnoses
| Severe Persistent n=16 |
Worsening n=16 |
Improving n=15 |
Nonspectrum n=31 |
|
|---|---|---|---|---|
| Chronological age in months (Initial) | 19.62 (5.69) | 17.50 (4.59) | 19.87 (6.45) | 16.90 (5.56) |
| Chronological age in months (final) | 33.75 (7.95) | 33.47 (6.82) | 37.00 (7.16) | 32.06 (6.67) |
| Baseline cognitive evaluation (MSEL)* | ||||
| Verbal IQ | 49.03 (23.75) | 73.31 (19.03) | 66.73 (23.69) | 82.16 (26.03) |
| Nonverbal IQ | 86.06 (22.07) | 91.44 (20.30) | 93.80 (20.36) | 97.87 (26.09) |
| Final cognitive evaluation (MSEL)* | ||||
| Verbal IQ | 56.84 (18.31) | 88.40 (30.61) | 93.46 (23.27) | 97.11 (24.95) |
| Nonverbal IQ | 71.62 (20.18) | 95.00 (29.46) | 102.13 (25.96) | 103.50 (24.61) |
| Baseline ADOS evaluation | ||||
| SA subtotal | 14.88 (4.08) | 9.13 (3.52) | 11.93 (4.18) | 4.94 (3.39) |
| RRB subtotal | 4.56 (1.97) | 2.31 (1.62) | 3.67 (1.63) | 1.87 (1.43) |
| Final ADOS evaluation | ||||
| SA subtotal | 14.56 (3.58) | 11.37 (2.85) | 6.47 (3.90) | 3.61 (2.01) |
| RRB subtotal | 4.81 (1.97) | 3.31 (2.27) | 2.07 (1.28) | 1.45 (1.29) |
| Baseline diagnostic probability rating | 12.00 (4.69) | 9.00 (5.38) | 10.60 (4.26) | 2.81 (2.61) |
| Final diagnostic probability rating | 14.00 (3.46) | 9.50 (5.52) | 9.36 (5.03) | 2.15 (2.91) |
| Number (%) ADI-R reported regression | ||||
| No loss reported | 12 (75.00) | 14 (93.33) | 12 (80.00) | 25 (96.15) |
| Word loss reported | 3 (18.75) | 1 (6.67) | 2 (13.33) | 1 (3.85) |
| Other loss reported | 1 (6.25) | 1 (6.67) | ||
| Final ADI-R subtotals | ||||
| Reciprocal Social Interaction | 16.37 (5.3) | 10.67 (5.39) | 11.13 (7.39) | 5.23 (2.79) |
| Verbal Communication | 11 (NA) | 13.17 (8.06) | 7.25 (3.20) | 5.00 (4.04) |
| Nonverbal Communication | 11.06 (2.65) | 7.73 (4.92) | 7.33 (4.39) | 4.54 (3.74) |
| Restricted and Repetitive Interests | 5.12 (2.25) | 4.07 (3.13) | 3.47 (1.89) | 1.31 (1.54) |
| Mean weekly treatment hours (24–36 months) | 4.23 (4.71) | 3.79 (4.62) | 4.5 (3.59) | 1.1 (1.28) |
Note: Values above are means, with standard deviations in parentheses, unless otherwise indicated.
MSEL = Mullen Scales of Early Learning; ADOS=Autism Diagnostic Observation Schedule; ADI-R=Autism Diagnostic Interview-Revised.
Date missing for one child in the Nonspectrum class.
Final cognitive data missing for 3 children in the Severe Persistent class and 4 in the Nonspectrum class. Differential Ability Scales used for 11 children in the Nonspectrum class, and 1 child per each of the other three classes.
Final certainty data missing for 2 participants in Severe Persistent, 2 in Worsening, 1 in Improving and 4 in Nonspectrum.
ADI-R missing for 1 participant in the Worsening class and 5 participants in the Nonspectrum class.
Similarly, ASD vs non-ASD children, irrespective of trajectory, differed in whether they had ever received any treatment after 24 months (ASD trajectory groups ranged from 77 –100% of participants; non-ASD 50%; χ2(3) = 7.25, p = .046), but not whether they had received ABA (ASD trajectory groups ranged from 14 – 27% vs no non-ASD; χ2(3) = 2.35, p = 0.67). All ASD trajectory groups, when combined, received more treatment hours than the non-ASD group, t (44) = −1.87, p = 0.07, but there were no differences among ASD trajectory groups.
Relatively large mean differences were accompanied by substantial variation. Half of the nonspectrum group did not receive any treatment; the three ASD groups, 77% (worsening), 80% (persistent) and 100% (improving), received at least some treatment, χ2(3)=7.25, p=0.046. There was no difference among the three ASD groups, χ2(2) = 3.55, p =.17. There were no significant differences in the proportion of children who received at least five hours a week of ABA beginning sometime before 30 months (27% persistent, 15% worsening, 14% improving, and 0% nonspectrum), exact p’s = 0.67). The large variability in the hours of treatment received after 24 months meant that the differences between groups remained non-significant, F (3.44) = 1.12 p = 0.35. Thus overall, there was no association between treatment and trajectory.
Though not significantly different, more of the children in the severe-pervasive group received ABA (27%) vs the other two ASD groups (15% and 14%), with little difference in absolute number of hours of treatment across the three ASD groups.
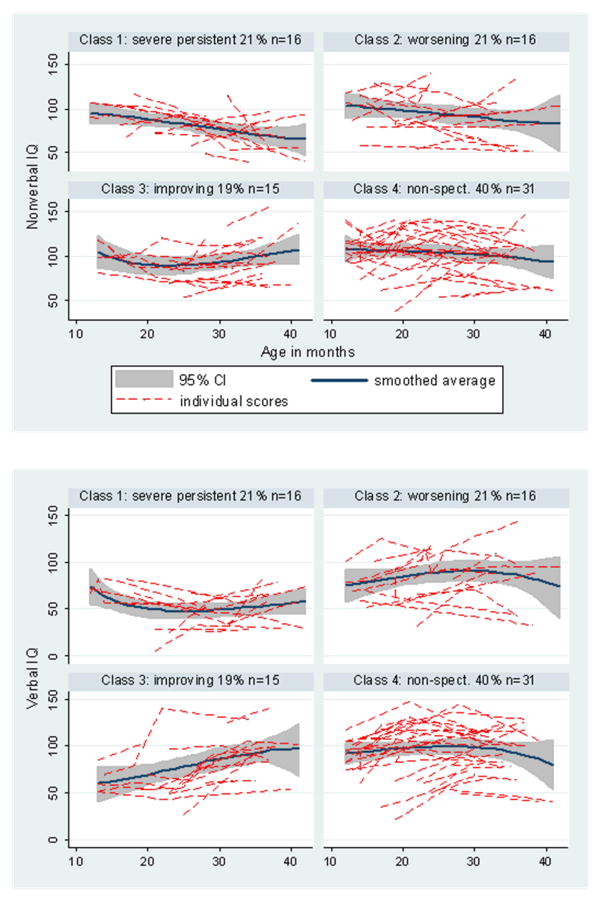
We examined the trajectories of the cognitive scores for which we had repeated measures over the same period. Figure 4 shows the histories for estimated verbal and nonverbal IQs. Differences by class in linear trend were found for verbal IQ, F(3.77)=5.26, p=0.002, but not nonverbal IQ, F(3.77)=2.03, p=0.116.
Figure 4.
IQ Changes for Four Trajectory Classes: Nonverbal and Verbal
For nonverbal mental age, F(3.77)=3.36, p=0.023, the improving group showed increases in scores more quickly than the persistent group, while for verbal mental age, F(3.77)=8.28, p<0.001, the improving and the NS classes increased more quickly than the persistent class. More specifically, there were class differences in time trends in the MSEL expressive and receptive age-equivalents, F(3.77)=5.13, p=0.003; F(3.77)=7.21, p<0.001, with the improving class increasing more rapidly relative to the persistent class in both measures.
Clinicians’ probability-of-diagnosis estimates made after each assessment are depicted according to each trajectory group in Figure 5. These estimates shared a common trend (trend × class interaction, F (3, 74) = 2.05, p = 0.11) but differed in mean level, F (3, 74) = 80.70, p < 0.001. The severe persistent and nonspectrum trajectory groups differed from each other and differed from the improving and worsening groups, which did not differ from each other.
Figure 5.
Changes in Clinician Ratings of Probability of ASD Diagnosis for the Four Trajectory Classes.
For the prediction of final diagnosis we compared the performance of an ADOS algorithm total score from a single assessment with the sum from the two assessments for 57 children where a previous assessment had occurred within three months before the final diagnosis. The second assessment significantly improved prediction (Area-Under-Curve 0.85 vs 0.77, p=0.015).
Discussion
Trajectories of growth for core features of autism, including social-affective development and repetitive behaviors and interests, measured during standardized semi-structured observations, were quantifiable and predictable in children as young as 15 – 18 months of age. Trajectories of development of both positive and negative ASD symptoms and language-related cognitive skills up to the third year of life were predicted from ADOS scores at baseline. Trajectories of children who were never judged to have had ASD during numerous visits were very clearly differentiated from the children who, in at least one visit, received a “possible ASD” diagnosis. Early ADI-R interviews also differentiated children who were never judged as having ASD from those who had received an ASD diagnosis during at least one visit.
However, as noted in the prospective studies of infant siblings of children with ASD (Bryson et al, 2007; Chawarska et al, 2009; Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010), there was significant variability in two year trajectories among children who showed ASD symptoms between 12 – 18 months. About half of the participants in this study were younger siblings of children with ASD and about half were not, allowing us to address whether the trajectories for “ever ASD” differed by sibling status. They did not. Approximately one-third of the children for whom ASD was suspected during at least one visit consistently showed clear severe and persistent deficits in social-affect and repetitive behaviors during standardized observations from 12 – 36 months. These same children showed slower gains in both receptive and language skills than the improving group, which also constituted about one-third of the “ever ASD” sample. A second group of children with “ever ASD” improved in social-affect and also improved more in verbal (both receptive and expressive language) skills and nonverbal skills compared to the severe persistent group, but did not differ in trajectory in repetitive behaviors and interests. Early scores on the ADI-R did not differentiate between these trajectory classes.
On the other hand, a third group of children showed a clear pattern of worsening in total ADOS scores, accounted for by absolute increases in repetitive behaviors and social-affect deficits compared to baseline, as well as in relative discrepancies compared to other groups, particularly the nonspectrum group. The worsening group was not differentiable from the severe persistent group or the improving group by changes in nonverbal cognitive or language scores. There was little evidence of actual “losses” of skills, but there were several children in the worsening and severe persistent group, and a few in the nonspectrum group, who made little or no progress in language and nonverbal problem-solving over the nearly two years of the study.
The children who showed worsening symptoms were marginally different (more normal) in social-affect than the children who improved when compared at baseline at 18 months and significantly different (more normal) than children with severe, persistent ASD. Even though their scores grew markedly more severe at 30 months, they were not any more likely to have been described by their parents as having had a regression than any of the other children, similar to findings by Ozonoff et al. (2010).
Another surprising finding was that the scores for clinician diagnostic certainty for the worsening group also did not differ over time from the improving group. That is, the clinicians were more certain about diagnoses for the children who did not change compared to children who changed in either direction. Clinicians’ diagnostic judgments were not as sensitive to trajectories of improvement or worsening as were the standardized instruments.
Nevertheless, the children who worsened differed from the “never ASD” group from the very start of our data collection. As in the sibling studies, by 30 – 36 months the worsening group had only slightly less severe ASD symptoms than the severe, persistent group, though they continued to have higher nonverbal and verbal cognitive scores. It is also interesting, though not surprising, that, in this study, where siblings were all part of the “concern” group in which a family expressed concerns about possible autism (in contrast to infant sibling studies where the purpose is to follow all infant siblings), we had a much higher rate of diagnosis of ASD in the siblings than typically found in the Autism Speaks Baby Siblings Research Consortium (Rozga et al, 2010). Not all siblings did have ASD as a final diagnosis, but parents’ explicit concern was associated with a greater likelihood of diagnosis. Follow-up into older ages, in studies of infant siblings and in this population, will be extremely interesting.
These results support the call for careful early screening for ASD of children by 18 months. Given that, for many of these children, including 30 children who were never judged to have ASD, someone (most often a parent) was concerned enough to refer the child for an assessment, the study supports the importance of systematic observation as a follow-up to parent-report screens in differentiating children when ASD is a likely possibility. The finding that nearly one-third of the children who had at least one diagnosis of ASD showed steady improvements in social-affect and increases in the slope of acquisition of verbal skills between 12 and 36 months is extremely encouraging. This pattern highlights the need for continued assessment and monitoring, not only for children who do not progress, but also for children who show marked improvements. These improvements were not the result of one poor assessment but were predictable trajectories over time that reflected gradual growth in verbal skills and gains in sociability.
That one-third of the children with an “ever ASD” diagnosis showed worsening between 12 and 36 months further supports the need for careful follow-up that continues beyond 12 and 18 month screens. It seems time for a broadening focus beyond concerns about the most dramatic regressions (which do occur, but perhaps less frequently than more gradual changes) (Rozga et al, 2010), to include concerns about slowing of development and gradual social withdrawal in toddlers (Landa et al., 2007; Ozonoff et al., 2010).
The finding that two assessments within three months improved prediction of later trajectory supports a recommendation for repeated assessments, sooner rather than later, in unclear cases. The finding that standardized observational diagnostic measures (e.g., the ADOS) and cognitive measures (e.g., the MSEL) differentiated trajectories more than clinician probability-of-diagnosis ratings or parent reports supports the need for standardized assessments that go beyond judgments even by experienced clinicians.
In Michigan, where this study was conducted, early intervention for children under age three is limited and nonspecific. Only one of the children in the study received intensive behavioral treatment. With very active advocacy, many families were eventually able to get placements in noncategorical preschool programs, often intended for older children. Several children had families that arranged various kinds of home-based structured and unstructured treatments to be carried out on a regular basis, and many had some individual speech therapy once or twice a week. There was no evidence of an association between treatment and trajectory within the “ever ASD” groups. This is not evidence that early treatment cannot have an effect (see Dawson et al., 2010), but is evidence that minimal, nonspecific early treatment-as-usual of toddlers with ASD had relatively little measurable effect on core symptoms or cognitive measures. Nevertheless, something internal or external to the children produced significant variability in trajectories within the 48 toddlers where ASD concerns brought parents to the project and for whom a clinical diagnosis was made during at least one visit. The challenge is to begin with such individual differences to seek links to neurobiology to better understand how children with ASD learn and develop and to use this information to develop more effective treatments for all of the children and families who need them.
Acknowledgments
Portions of this research were presented at the 2007 International Meeting for Autism Research (IMFAR), Seattle, Washington.
Dr. Lord receives royalties for the ADOS and ADI-R; all royalties from clinics and projects in which she is involved are donated to charity. This research was supported by funding from the Department of Education (H324 C030112), National Institute of Mental Health (R01 MH066496), and a gift from the Simons Foundation. We gratefully acknowledge the help of Mia Coffing, Rachel Petrak and Susan Risi, as well as the parents and children who participated in the study, and the UMACC staff, present and past.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ccp
Contributor Information
Catherine Lord, Weill Cornell Medical College.
Rhiannon Luyster, Harvard University.
Whitney Guthrie, Florida State University.
Andrew Pickles, Institute of Psychiatry, University of London.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Anderson DK, Lord C, Risi S, Shulman C, Welch K, DiLavore PS, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37(7):1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, Simonoff E. Regression, developmental trajectory and associated problems in disorders in the autism spectrum: The SNAP study. Journal of Autism and Developmental Disorders. 2008;38(10):1827–1836. doi: 10.1007/s10803-008-0571-9. [DOI] [PubMed] [Google Scholar]
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. Journal of Abnormal Psychology. 2011 May;120(2):465–475. doi: 10.1037/a0021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt E, Gillberg C, Gillberg C. Autism after adolescence: Population-based 13-to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. Journal of Autism and Developmental Disorders. 2005;35(3):351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism in adults: Symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology and Psychiatry. 2007;48(11):1102–1110. doi: 10.1111/j.1469-7610.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Bjorklund D, Periss V, Causey K. The benefits of youth. European Journal of Developmental Psychology. 2009;6(1):120–137. [Google Scholar]
- Bryson S, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown J, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: Predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers withASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50(10):1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e-23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L, Ho H. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(4):739–747. doi: 10.1007/s10803-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. Journal of Financial and Quantitative Analysis. 1989;24:333–355. [Google Scholar]
- Goldberg W, Thorsen P, Osann K, Spence M. Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders. 2008;38(6):1136–1146. doi: 10.1007/s10803-007-0498-6. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Modeling trajectories of autism severity in children using standardized ADOS scores. doi: 10.1542/peds.2011-3668. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule (ADOS): Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Depry LJ, De DN, Croen LA, Hertz-Picciotto I. Regression in autism: Prevalence and associated factors in the CHARGE study. Ambulatory Pediatrics. 2008;8(1):25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert MR, Fein D. Can children with autism recover? If so, how? Neuropsychology Review. 2008;18(4):339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. American Journal on Intellectual and Developmental Disabilities. 2009;114(1):23–41. doi: 10.1352/2009.114:23;nd41. [DOI] [PubMed] [Google Scholar]
- Kleinman JM, Ventola PE, Pandey J, Verbalis AD, Barton M, Hodgson S, Fein D. Diagnostic stability in very young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(4):606–615. doi: 10.1007/s10803-007-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa R, Holman K, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The Autism Diagnostic Interview - Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Lord C. Follow-up of two-year-olds referred for possible autism. Journal of Child Psychology and Psychiatry. 1995;36:1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH. Quantifying the phenotype in autism spectrum disorders. American Journal of Medical Genetics: Neuropsychiatric Genetics. 2001;105(1):36–38. [PubMed] [Google Scholar]
- Lord C, Luyster R, Gotham K, Guthrie W. The Autism Diagnostic Observation Schedule, Toddler Module. Los Angeles, CA: Western Psychological Services; (in press) [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore P, … &, Rutter M. The Autism Diagnostic Observation Schedule -- Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Shulman C, DiLavore P. Regression and word loss in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(5):936–955. doi: 10.1111/j.1469-7610.2004.t01-1-00287.x. [DOI] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, DiLavore P, Lord C. The Autism Diagnostic Observation Schedule -- Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;399:1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu W, Dawson G, Bernier R, Lord C. Early regression in social communication in autistic spectrum disorders: A CPEA study. Developmental Neuropsychology. 2005;27(3):311–336. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12:457. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld M, Hill MM, Cook I, Hutman T, … &, Sigman M. How early do parent concerns predict later autism diagnosis? Journal of Developmental & Behavioral Pediatrics. 2009;20I(5):367–375. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E. The development of core cognirtive skills in autism: A 3-year prospective study. Child Development. 2010;81(5):1400–1416. doi: 10.1111/j.1467-8624.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- Pennington B. How neuropsychology informs our understanding of developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(1–2):72–78. doi: 10.1111/j.1469-7610.2008.01977.x. [DOI] [PubMed] [Google Scholar]
- Pickles A, Simonoff E, Conti-Ramsden G, et al. Loss of language in early development of autism and specific language impairment. Journal of Child Psychology and Psychiatry. 2009;50(7):843–852. doi: 10.1111/j.1469-7610.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, Pickles A. Generqalized multilevel structural equation modeling. Psychometrika. 2004;69:167–190. [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, … &, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1094. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2010 doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. Journal of Autism and Developmental Disorders. 2003;33(6):565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Siegler RS, Crowley K. The microgenetic method: A direct means for studying cognitive development. American Psychologist. 1991;46:606–620. doi: 10.1037//0003-066x.46.6.606. [DOI] [PubMed] [Google Scholar]
- Sigman M, McGovern C. Improvement in cognitive and language skills from preschool to adolescence in autism. Journal of Autism and Developmental Disorders. 2005;35(1):15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Soke GN, Philofsky A, Diguiseppi C, Lezotte D, Rogers S, Hepburn S. Longitudinal changes in scores on the Autism Diagnostic Interview-Revised (ADI-R) in pre-school children with autism: Implications for diagnostic classification and symptom stability. Autism. 2011 May 17; doi: 10.1177/1362361309358332. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Stevens M, Fein D, Dunn M, Allen D, Waterhouse L, Feinstein C, Rapin I. Subgroups of children with autism by cluster analysis: A longitudinal investigation. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(3):346–352. doi: 10.1097/00004583-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser E, Rosenthal M, Wilson L, Barton M, … &, Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Turner L, Stone WL, Pozdol S, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10(3):243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Archives of General Psychiatry. 2005;62(8):889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- Wetherby A, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the Communication and Symbolic Behavior Scales Developmental Profile with very young children. Journal of Speech, Language and Hearing Research. 2002;45(6):1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Wetherby A, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2004;34(5):473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International classification of diseases and related health problems. 10. Geneva, Switzerland: 1993. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]