Abstract
PURPOSE
Imatinib is an inhibitor of the Bcr-Abl tyrosine kinase; however, resistance is common. Flavopiridol, a cyclin-dependent kinase (CDK) inhibitor, down-regulates short-lived anti-apoptotic proteins via inhibition of transcription. In preclinical studies, flavopiridol synergizes with imatinib to induce apoptosis. We investigated this novel combination regimen in patients with Bcr-Abl+ malignancies.
METHODS
In a phase I dose-escalation study, imatinib was administered orally daily, and flavopiridol by 1-hour intravenous infusion weekly for three weeks every four weeks. Adults with chronic myelogenous leukemia (CML) or Philadelphia chromosome-positive (Ph+) acute leukemias were eligible. Patients were divided into two strata based on peripheral blood and bone marrow blast counts. The primary objective was to identify the recommended phase II doses (RPTD) for the combination. Correlative pharmacokinetic and pharmacodynamic studies were also performed.
RESULTS
A total of 21 patients received study treatment. Four dose levels were evaluated before the study was closed following the approval of the second generation Bcr-Abl tyrosine kinase inhibitors (TKIs). Five patients responded, including four sustained responses. Four patients had stable disease. All but one responder, and all patients with stable disease had previously been treated with imatinib. One patient had a complete response sustained for 30 months. Changes in expression of phospho-Bcr/Abl, -Stat5, and Mcl-1 were monitored. No major pharmacokinetic interaction was observed.
CONCLUSIONS
This is the first study to evaluate the combination of a CDK inhibitor and a TKI in humans. The combination of flavopiridol and imatinib is tolerable and produces encouraging responses, including in some patients with imatinib-resistant disease.
Keywords: Imatinib, flavopiridol, cyclin dependent kinase inhibitor, CDK inhibitor, Bcr-Abl, tyrosine kinase inhibitor
Introduction
The Bcr-Abl tyrosine kinase (TK) is responsible for the malignant phenotype [1] in essentially all patients with chronic myelogenous leukemia (CML), 20% to 30% of patients with adult acute lymphoblastic leukemia (ALL) and rare patients with acute myelogenous leukemia (AML). Imatinib, a competitive inhibitor of the Bcr-Abl and other TKs, is standard therapy [2] for all phases of CML, as well as (along with conventional chemotherapy) for Philadelphia chromosome-positive (Ph+) ALL [3]. However, imatinib is unable to eradicate leukemic stem cells [4]. Indefinite therapy is usually needed, and the drug is associated with side effects [5]. Importantly, resistance to imatinib is a significant problem [6]. Primary cytogenetic resistance is seen in 15–25% of patients, and secondary resistance at 18 months to 2 years develops in 80%, 40–50%, and 10–15% of patients initially in blastic (BP), accelerated (AP) or chronic phases (CP), respectively [7]. Improved therapies for Bcr-Abl+ malignancies are therefore clearly needed.
The cyclin-dependent kinases (CDKs) have become attractive new targets for novel anticancer drug development [8]. Flavopiridol was the first CDK inhibitor to enter clinical trials in humans. Besides inhibiting CDKs, leading to cell cycle arrest at both the G1/S and G2/M checkpoints, flavopiridol globally affects cellular transcription, at least in preclinical studies, by inhibiting cyclin T-CDK 9 [8–10]. This latter action is noncompetitive (11) with adenosine triphosphate (ATP), and may be the principal mechanism by which the drug exerts its anti-tumor activity [9, 12]. The most profound effect of transcriptional repression by flavopiridol is on messenger RNAs (mRNAs) and proteins of short half-life, such as cyclins B and D, Bcl-2, Mcl-1, XIAP and other inhibitors of apoptosis, Mdm-2, p21, p27, survivin and vascular endothelial growth factor [9]. Flavopiridol also induces apoptosis by promoting inappropriate persistence of the transcription factor E2F1 during S phase, an effect mediated through CDK inhibition [10]. Other mechanisms of action include inhibition of angiogenesis and induction of differentiation [13].
Synergistic interactions between signal transduction and CDK inhibitors in human leukemia cells have previously been demonstrated [14]. For example, in Bcr-Abl+ human leukemia (K562) cells, co-administration of marginally toxic concentrations of imatinib (200 nM) and flavopiridol (150 nM) for 48 hours resulted in a marked increase in mitochondrial damage (cytochrome c release), caspase activation and apoptosis [15], including in imatinib-resistant K562 cells displaying Bcr-Abl amplification.
In light of these considerations, a phase I trial of the combination of imatinib and flavopiridol was conducted in patients with Bcr-Abl+ malignancies (CML, ALL and AML). The primary objective of the study was to identify recommended phase II doses (RPTDs) for the combination in this setting. Secondary objectives included determining the toxicities and MTD of the combination, observing the disease-related effects of the combination, and characterizing the pharmacokinetics of each agent. Additional secondary objectives were to observe pharmacological effects of the combination in peripheral blood (PB) and/or bone marrow (BM) Bcr-Abl+ cells on Bcr-Abl expression and activity of Bcr-Abl downstream signaling pathways, and to observe responses in patients previously treated with imatinib.
Methods
Drug sources and formulation
Flavopiridol was provided by Aventis Pharmaceuticals, Inc. and distributed by the Clinical Trials Evaluation Program (CTEP) of the National Cancer Institute (NCI). It was supplied as a sterile, 10 mg/ml solution in glass vials. The contents of the vial were diluted prior to administration with 0.9% sodium chloride or 5% dextrose. The diluted solutions were iso-osmotic, with a pH of about 3.5. A final concentration of 0.5 mg/ml was recommended to decrease the risk of thrombotic complications. Imatinib was purchased from commercially available sources.
Eligibility criteria
Inclusion criteria were: (a) age ≥ 18 years and able to give informed consent; (b) Bcr-Abl+ CML in either CP or AP with (1) hematologic progression during imatinib treatment, (2) less than a complete hematologic response (CHR) following at least three months of imatinib treatment, or (3) less than a partial cytogenetic response (PCyR) following at least six months of imatinib treatment, or Bcr-Abl+ CML in BP, regardless of prior therapy, or Bcr-Abl+ ALL or Bcr-Abl+ AML, regardless of prior therapy; (c) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; (d) adequate organ function as evidenced by serum creatinine ≤ 2 × upper limit of normal (ULN), total bilirubin ≤ 2.5 × ULN and aspartate and alanine aminotransferases (AST/ALT) ≤ 2.5 × ULN, unless, for patients enrolled onto stratum 2, liver involvement was suspected, in which case AST/ALT could be ≤ 5 × ULN; (e) not pregnant or nursing and willing to use adequate contraception for the duration of study treatment and for three months after the end of treatment. For diagnostic purposes, Bcr-Abl+ could be identified by karyotype, fluorescence in situ hybridization (FISH), or polymerase chain reaction (PCR) in BM or PB. Baseline status and cytogenetic response were documented by karyotype or FISH (not PCR) on BM (not PB). PCyR was defined as ≤35% Bcr-Abl+ cells in the BM. CP was defined as <15% blasts in PB and BM. BP was defined as >30% blasts in PB or BM or the presence of extramedullary blasts other than hepatosplenomegaly. Patients in AP were those not in CP or BP. Patients were enrolled onto one of two strata based on BM status and PB counts at the time of enrollment, in order to allow treatment of patients with more aggressive disease for whom standard hematologic toxicity criteria were felt to be inappropriate. Those with <15% blasts in PB and BM, and no recent myelosuppressive therapy were assigned to stratum 1. Patients with ≥15% blasts in the BM or PB were assigned to stratum 2, as were patients with <15% blasts who had grade 1 or 2 neutropenia or thrombocytopenia from previous myelosuppressive therapy. Exclusion criteria were: (a) persistent symptoms, signs or anticipated effects from prior treatment, other than imatinib, hydroxyurea, leukapheresis or anagrelide; (b) history of allergy to study drugs or compounds of similar composition; (c) known central nervous system (CNS) malignancy or progressive disease-related neurologic dysfunction; (d) uncontrolled intercurrent illness; (e) human immunodeficiency virus (HIV)-positive patients receiving combination anti-retroviral therapy. Characteristics of the enrolled patients are shown in Table 1.
Table 1.
Patient enrollment and characteristics
| Stratum I* | Stratum II* | |
|---|---|---|
| Gender (no. of patients) | ||
| Men | 5 | 2 |
| Women | 10 | 4 |
| Total | 15 | 6 |
| Age (years) | ||
| Median | 52 | 58.5 |
| Range | 24–71 | 30–71 |
| Performance Status (no. of patients) | ||
| 0 | 10 | 2 |
| 1 | 5 | 4 |
| Total | 15 | 6 |
| Diagnosis (no. of patients) | ||
| CML | 13 | 4 |
| ALL | 2 | 2 |
| Total | 15 | 6 |
| Prior Treatment (no. of regimens) | ||
| Median | 2 | 2 |
| Range | 1–4 | 0–3 |
| Study Treatment Received (no. of courses) | ||
| Mean | 5.69 | 7 |
| Median | 3 | 1.5 |
| Range | 1–28 | 1–32 |
| Total | 91 | 42 |
Patients with <15% blasts in PB and BM, and no recent myelosuppressive therapy were assigned to stratum 1. Patients with ≥15% blasts in the BM or PB were assigned to stratum 2, as were patients with <15% blasts who had grade 1 or 2 neutropenia or thrombocytopenia from previous myelosuppressive therapy.
Treatment plan
Flavopiridol was administered as a 1 hour infusion once a week for three weeks every four weeks. Imatinib was started at least one day prior to flavopiridol and given by continuous oral dosing. Cycles were defined by initiation of flavopiridol. The starting doses of flavopiridol and imatinib were 30 mg/m2 and 400 mg, respectively. The plan was to escalate the doses of both agents, with the doses of flavopiridol (mg/m2)/imatinib (mg) being 30/400, 30/600, 45/600, 60/600, 60/800 and 60/1000 for dose levels one through six, respectively. The starting doses were selected keeping in mind the overlapping toxicities of the two drugs, i.e., diarrhea and myelosuppression, as well as the established success of imatinib in Bcr-Abl+ disease at daily doses of 400 mg and higher. In patients receiving imatinib prior to registration, imatinib was continued at the registration dose. Hydroxyurea and/or leukaphereis were allowed as clinically indicated for control of leukocytosis, as was anagrelide for thrombocytosis, but were discontinued as soon as possible. Prophylactic antiemetic treatment, preferably with a 5HT3 antagonist, was required for flavopiridol doses ≥30 mg/m2. Diarrhea ≥ grade 1 was treated with loperamide or diphenoxylate/atropine. Octreotide or cholestyramine could be added if diarrhea was persistent. Prophylactic antidiarrheal treatment was recommended for patients who experienced ≥ grade 3 diarrhea with any previous course, but could be used at any time at the investigator’s discretion. Allopurinol, hydration and urinary alkalinization were recommended for patients at risk for hyperuricemia and tumor lysis syndrome, as was transfusion to maintain hemoglobin >8 g/dl and platelets >10,000/µl. Erythropoietin was not initiated during the first course of treatment but could be continued in patients who were receiving it prior to enrollment. White blood cell (WBC) growth factors and interleukin-11 were not administered during the first course of treatment unless clinically indicated for the management of febrile neutropenia or thrombocytopenia with complications. Patients continued on treatment as clinically indicated considering the risks and prospects of benefit. Flavopiridol could be discontinued after six months at the discretion of the patient and investigator. Imatinib was continued until disease progression or unacceptable toxicity. Flavopiridol, with or without imatinib, could be restarted if clinically indicated at a previous dose tolerated by the patient as long as the study remained open and the drug was available. Patients were followed for one year from the end of treatment.
Toxicity evaluation
Adverse events were characterized in terms of nature, attribution and severity according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Adverse events considered to be possibly, probably, or definitely related to treatment were scored as toxicities.
Dose levels, definition of DLT, and identification of MTDs
Dose limiting toxicity (DLT) was defined during the first course of treatment. Non-hematologic DLT was defined as ≥ grade 3 with these exceptions: nausea and vomiting in the absence of prophylactic and/or symptomatic treatment, and grade 3 AST/ALT elevation in stratum 2 patients. Hematologic DLT was defined for stratum 1 patients as grade 4 neutropenia or thrombocytopenia for at least one week. Hematologic DLT was defined for stratum 2 patients as simultaneous presence of BM cellularity <10% and <10% bone marrow blasts. Patients were enrolled in cohorts of three according to a modified “three plus three” dose escalation scheme [16]. Patient enrollment and dose escalation were conducted independently for stratum 1 and stratum 2, with at least 3 patients enrolled at each dose level, except that if a dose level was determined to be safe for stratum 1 and that dose level had not yet completed enrollment for stratum 2, both strata escalated to the next dose level. Dose level escalation was based upon observation of DLTs. The maximum tolerated dose (MTD) was the highest dose level at which fewer than two of six patients experienced DLT. If 2 patients in a cohort (of 3 or 6) experienced DLT, this exceeded the MTD and the previous dose level was expanded to 6 if necessary. The MTD was to be the RPTD, unless the nature of toxicities or the frequency of dose adjustments suggested that a lower dose level would be more appropriate. Doses of both imatinib and flavopiridol were required to be omitted if grade ≥3 non-hematologic toxicity occurred; both drugs were to be restarted at the next lower dose level upon resolution of toxicity to grade ≤2 for imatinib and ≤1 for flavopiridol. This was optional for grade 2 non-hematologic toxicity. If grade ≥3 hematologic toxicity occurred in stratum 1, doses of both drugs were omitted and resumed at the next lower dose level upon resolution of the toxicity to grade ≤1. For hematologic DLT in stratum 2, doses of both drugs were omitted and resumed at the next lower dose level upon resolution. Doses of flavopiridol and/or imatinib for an individual patient could also be increased following two cycles at a dose level with acceptable toxicity. Intrapatient dose escalation, however, was limited to the highest dose level known to not exceed the MTD for a patient’s stratum.
Study treatment evaluation
A complete history and physical examination, medication review, performance status assessment, routine laboratory tests and toxicity assessment were performed prior to treatment and then weekly during treatment. A BM aspirate and biopsy, including Bcr-Abl status by karyotype and FISH was done prior to treatment and then every 3 months while on treatment. Additional testing for Bcr-Abl was done if clinically indicated. Response was assessed based on PB counts, BM cellularity and blast percentage, and cytogenetic status of the BM. A response observed on two occasions at least 4 weeks apart was considered a sustained response. CHR was defined as the absence of PB blasts, WBC count between 2000/µl and the ULN, absolute neutrophil count (ANC) between 1000/µl and the ULN, and no extramedullary disease other than persistent hepatosplenomegaly. BM cellularity had to be ≥10%, with <5% blasts. A return to CP was defined as <15% blasts in the blood and marrow, with ≥10% BM cellularity. Cytogenetic responses were determined by the percentage of Ph+ cells in the BM as assessed by conventional karyotyping or FISH, with complete, partial, minor, minimal and no cytogenetic response defined by 0%, 1–35%, 36–65%, 66–95% and >95% Ph+ cells, respectively. For patients with grade 4 neutropenia or thrombocytopenia of greater than 2 weeks’ duration, a BM evaluation every 2 weeks was recommended. For patients in stratum 2 only, a BM aspirate and biopsy with cytogenetics was recommended to document complete response (CR) if PB counts suggested this.
Correlative laboratory studies
Please see the Supplementary Appendix for details. Blood samples for imatinib pharmacokinetics were collected before treatment, and at 1, 2, 4, 8, 12 and 24 hours after the day 1 dose; and at the same times around the day 2 imatinib dose, which was taken 25 hours after the first dose, at the end of the 1 h infusion of flavopiridol. Those for flavopiridol pharmacokinetics were collected before drug administration, at the end of the 1-hr infusion, and at 1, 2, 4, 8, 12, and 24 hours after the end of infusion on day 2 of cycle 1. Pharmacodynamic samples were obtained prior to initiation of imatinib (if feasible) and immediately prior to and 48 hours following the course 1 week 1 flavopiridol dose. Pharmacodynamic sampling could be repeated as frequently as weekly if appropriate, given the clinical situation. Peripheral blood mononuclear cells were obtained by centrifugation over Ficoll-Hypaque, lysed and proteins isolated. Western Blot analysis (Western Lightning Plus - ECL, PerkinElmer, Waltham, MA) was employed to monitor expression, pre- and post-flavopiridol, of phospho-Bcr-Abl, cyclin D1, p21CIP1, Mcl-1, phospho-Akt and phospho-Rb, as previously described [17]. In addition, circulating CD34+ blast cells were analyzed for phospho-Stat5 (pStat5) activity and deoxyribonucleic acid (DNA) content by multi-color flow cytometry (Supplementary Appendix, Figure S1) at a reference laboratory (Case Western Reserve University, Cleveland, OH). Stat5 is constitutively phosphorylated in CML [18–20] and anti-phospho-Stat5 (pStat5) immunofluorescence can be used to monitor Bcr-Abl activity in CML cell lines by flow cytometry [21]. Patients were evaluated before treatment, 24 hours after initiation of imatinib and 24 hours after flavopiridol treatment. Ancillary goals were to monitor changes in pStat5 expression and correlate changes with responses to imatinib and flavopiridol. For patients with prior exposure to imatinib in whom imatinib resistance was suspected, peripheral blood and/or bone marrow samples were sent to a reference laboratory (Oregon Health Sciences University, Portland, OR) for characterization of mechanisms of resistance.
Results
Patients
A total of twenty-two patients were enrolled onto the study between July 28, 2003 and July 29, 2005 at any of four participating centers (Table 1). The majority (59%) were heavily pretreated (≥3 prior therapies), and 19 (86%) had received imatinib in the past. One imatinib-pretreated patient with CML in stratum 1 was found ineligible after enrollment and did not receive study treatment; thus, twenty one patients were evaluable for toxicity. Data for this individual do not appear in Table I.
DLTs and MTDs
Dose levels and DLT events are depicted in Table 2. The only frequent toxicities were hematological. Four dose levels of flavopiridol (mg/m2) /imatinib (mg) were evaluated: 30/400, 30/600, 45/600 and 60/600. In stratum 1, one DLT occurred at dose level 4 in the form of grade III cholecystitis requiring cholecystectomy that was felt to be possibly related to treatment. This dose level was expanded to 6 patients with no further occurrence of DLT. In stratum 2, one grade 5 adverse event occurred at dose level 3 (death resulting from sepsis/multi-organ failure that was probably not related to treatment, and therefore not considered a DLT). At this point in the study, however, accrual dramatically declined, coinciding with the introduction of the 2nd generation Bcr-Abl TKIs nilotinib and dasatinib, and the study was closed. As noted above, there was one death on the study. The patient had CML-BP and WBC count at presentation was 67,000/µl, with a predominance of blasts. He was placed on hydroxyurea, 1000 mg twice daily, and imatinib, 400 mg once daily. ANC was >1000/µl. Because of an increasing WBC count, the dose of imatinib was increased to 600 mg once daily and that of hydroxyurea doubled. He was then enrolled on the study (stratum 2, dose level 3) and received his first two doses of flavopiridol without incident; however, his ANC fell to zero. Hydroxyurea was continued because of persistent fevers thought to be related to his disease and leukocytosis (22,000–27,000/µl) despite an ANC of zero (all blasts). The third (day 15) dose of flavopiridol was held, and he was discharged with a WBC count of 14,000/µl (ANC zero, 98% blasts). He presented a week later with neutropenic sepsis and died of multi-organ failure. This event was attributed to severe neutropenia resulting both from the patient’s underlying disease and the concomitant use of high doses of the cytoreductive agent, hydroxyurea, and therefore felt most likely not to be related to study treatment.
Table 2.
Strata, Dose levels & Dose-Limiting Toxicity (DLT) events
| Stratum 1* | ||||
|---|---|---|---|---|
| Dose Level |
Flavopiridol | Imatinib | No. of patients treated | DLT |
| 1 | 30 | 400 | 3 | |
| 2 | 30 | 600 | 3 | |
| 3 | 45 | 600 | 3 | |
| 4 | 60 | 600 | 6 | Grade 3 cholecystitis |
| Stratum 2* | ||||
| Dose Level |
Flavopiridol | Imatinib | No. of patients treated | DLT |
| 1 | 30 | 400 | 3 | |
| 2 | 30 | 600 | 0 | |
| 3 | 45 | 600 | 3 | |
Patients with <15% blasts in PB and BM, and no recent myelosuppressive therapy were assigned to stratum 1. Patients with ≥15% blasts in the BM or PB were assigned to stratum 2, as were patients with <15% blasts who had grade 1 or 2 neutropenia or thrombocytopenia from previous myelosuppressive therapy.
Other toxicities and tolerability
The only frequent toxicities were hematologic. Table 3 lists the grade 3 and 4 hematologic and non-hematologic toxicities that were felt to be possibly, probably or definitely related to study treatment for the duration of the study.
Table 3.
Grade 3 & 4 hematologic and non-hematologic toxicities possibly, probably, or definitely related to study treatment occurring during any treatment course
| Hematologic Toxicities | Stratum 1* no. of patients (%) |
Stratum 2* no. of patients (%) |
|---|---|---|
| Anemia | 0 (%) | 3 (50%) |
| Leukopenia | 0 (0%) | 2 (33%) |
| Lymphopenia | 0 (0%) | 1 (17%) |
| Neutropenia | 4 (27%) | 2 (33%) |
| Thrombocytopenia | 1 (7%) | 3 (50%) |
| Non-Hematologic Toxicities | Stratum 1* no. of patients (% patients) |
Stratum 2* no. of patients (% patients) |
| Cholecystitis | 1 (7%) | 0 (0%) |
| Diarrhea | 1 (7%) | 0 (0%) |
| Febrile Neutropenia | 0 (0%) | 1 (17%) |
| Hypophosphatemia | 1 (7%) | 0 (0%) |
| Infection-NOS | 1 (7%) | 0 (0%) |
| Infection with normal ANC: upper respiratory-NOS | 0 (0%) | 1 (17%) |
| Multi-organ failure (grade 5) | 0 (0%) | 1 (17%) |
Patients with <15% blasts in PB and BM, and no recent myelosuppressive therapy were assigned to stratum 1. Patients with ≥15% blasts in the BM or PB were assigned to stratum 2, as were patients with <15% blasts who had grade 1 or 2 neutropenia or thrombocytopenia from previous myelosuppressive therapy.
Disease response
Twenty patients were evaluable for response. Five patients (4 with CML, 1 with ALL) met criteria for a response based on PB counts, BM evaluation, or cytogenetic status, as described above. All five patients had a response based on BM cytogenetics, and two of the five patients met criteria for a response based on PB and BM evaluation. Four of these five patients had a sustained response, for 2, 4, 15 and 30 months, respectively.
Two of the five responses were complete. Both occurred in stratum 1 patients with CML. One patient had a complete response based solely on BM cytogenetics, but the response was not sustained. The second patient had a complete response based on all three criteria, and the response was sustained for 30 months. Both patients had previously been treated with imatinib. Two other patients with CML in stratum 1 who had received imatinib in the past experienced sustained partial responses, for 4 and 15 months, respectively. The fifth response occurred in a patient with ALL in stratum 2, and was sustained for 2 months. This patient had not previously received imatinib. Response rates to the combination were 23.5% in imatinib-pretreated patients (4 responders out of 17 evaluable) and 33% in imatinib-naïve patients (1 responder out of 3 evaluable). The small numbers of patients involved preclude any conclusions from being drawn.
Four patients had stable disease for at least three cycles. All four had CML, were enrolled in stratum 1, and had received imatinib in the past. One of these decided to discontinue treatment after seven cycles, even though their disease remained stable. The other three patients had disease progression after 3, 5 and 24 months, respectively.
Eleven patients had disease progression and stopped treatment without a documented response. All eleven patients stopped treatment by the end of cycle 3. One other patient decided to stop treatment during cycle 3, before the first BM biopsy, although there was no evidence of disease progression.
Correlative laboratory studies
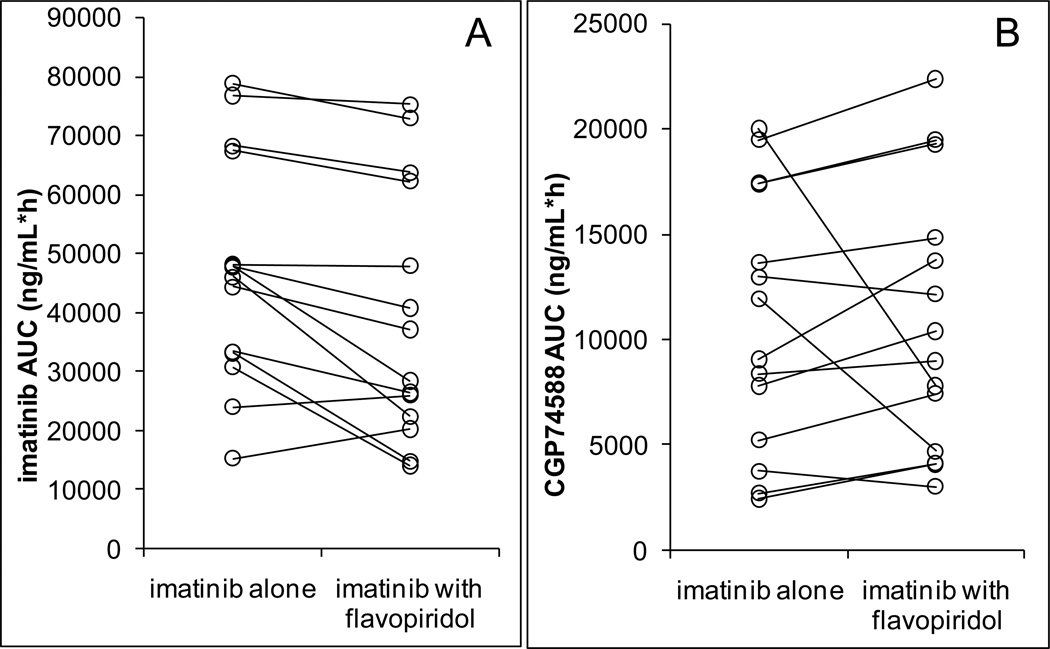
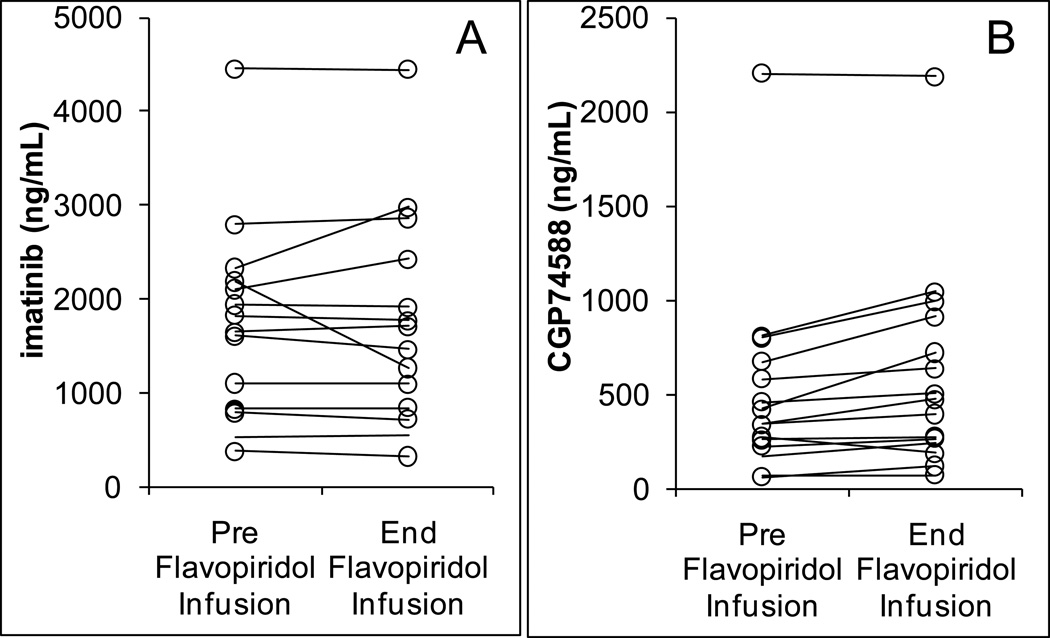
17 patients had pharmacokinetic data for imatinib. AUC values of imatinib were on average 21% lower in the presence of flavopiridol (p=0.022) (Figure 1A). The AUC of the metabolite CGP74588 was not significantly affected (Figure 1B). In comparing concentrations immediately prior to and at the end of the one hour administration of flavopiridol on day 2, flavopiridol did increase the concentrations of metabolite CGP74588, but did not affect imatinib plasma concentrations (Figure 2). Although statistically significant, the magnitude of these effects was small.
Figure 1.
AUC values of imatinib (A), P=0.022, and its metabolite CGP74588 (B), P=0.84, in the absence and presence of flavopiridol (N=14).
Figure 2.
Concentration values of imatinib (A), P=0.44, and its metabolite CGP74588 (B), P=0.007, in the absence of flavopiridol, immediately prior to infusion (at 24 h) and in the presence of flavopiridol, immediately after the 1-hr infusion (at 25 h) (N=15).
Flavopiridol pharmacokinetic analyses were performed in 11 patients treated with 1-hr infusions of 30, 45 or 60 mg/m2 flavopiridol with either 400 or 600 mg/day imatinib in cycle 1. After the end of the infusion, flavopiridol concentrations rapidly declined in a multi-exponential manner and fell below the lower limit of quantification within 12 hours (Figure S3, Supplementary Appendix). Few patients had measurable flavopiridol concentrations 24 hours post-infusion. Table S1 (Supplementary Appendix) summarizes estimated pharmacokinetic parameters of flavopiridol at various dose levels. The mean flavopiridol clearance was estimated to be 25.63 ± 9.14 L/h (13.2 L/hr/m2), very similar to previously reported values (13.8 L/hr/m2) for 1-hr infusions of flavopiridol as a single agent [22]. The mean volume of distribution at steady state (66.9 ± 33.8 L) and mean terminal half-life (2.33 ± 0.97 hr) were lower in this study compared to earlier studies [22, 23], probably because of no detectable drug concentrations 24 hours post-infusion in most patients.
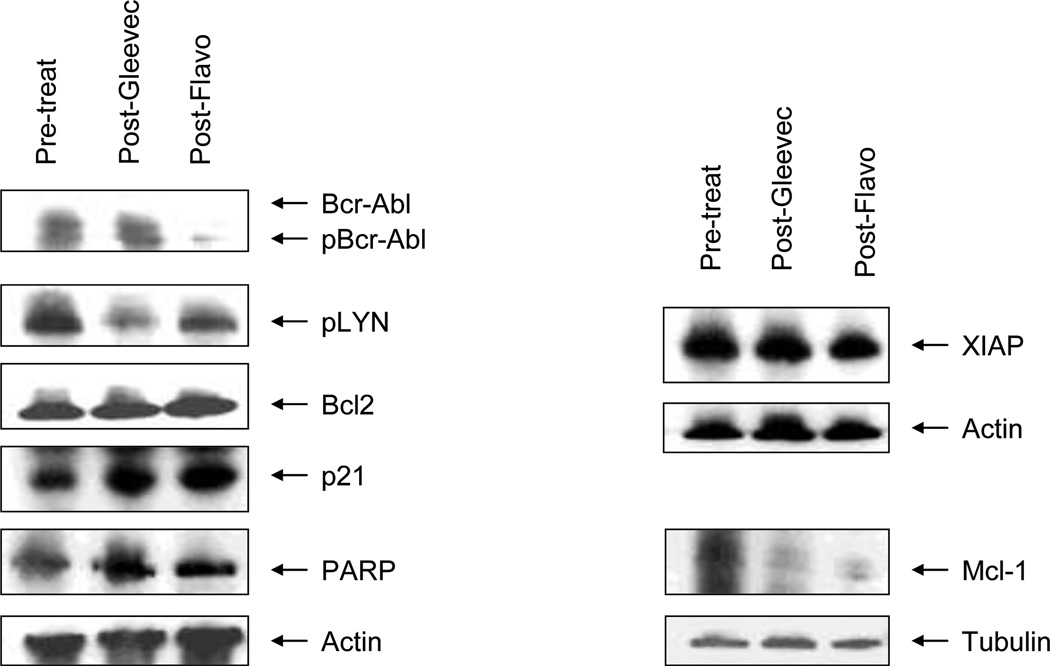
Due to technical and other issues, pharmacodynamic studies involving Western blot analyses could only be performed on a limited number of samples, an example of which is shown in Figure 3. Cells obtained from this patient revealed decreased expression of total and phospho-Bcr-Abl as well as Mcl-1 following administration of the combination of flavopiridol and imatinib. Interestingly, this patient had a sustained response. The small number of samples assayed by this method precluded reliable interpretation of results, and additional studies will be necessary to establish the feasibility of this approach.
Figure 3.
Western Blot analysis of protein expression pre-treatment, post-imatinib, and post-flavopiridol, in a patient with chronic myelogenous leukemia in stratum 1. This patient had a sustained response, and had received prior imatinib. Marked diminution of the expression of Bcr-Abl and Mcl-1 are seen after the administration of flavopiridol.
Studies involving flow cytometric analysis of phospho Stat5 were also performed. Of 9 patients with baseline data, 5 demonstrated abnormal constitutive expression of pStat5 in blasts compared to samples from healthy volunteers. Expression of pStat5 was correlated with higher numbers of blasts with DNA content >2C (Figure S2, Supplementary Appendix). The majority of patients exhibited a decline in the fraction of pStat5+ blasts as well as G1 arrest after imatinib, and in some, these effects were amplified or maintained after flavopiridol administration. However, no correlation between these findings and clinical outcomes was observed.
Nineteen samples were processed for characterization of mechanisms of resistance to imatinib. Eight of the 19 patients had >50% phosphorylation of CRKL, suggesting reactivation of the Bcr-Abl kinase as a contributing mechanism (data not shown). Interestingly, of these patients, the four that were tested did not have detectable Bcr-Abl kinase domain mutations (data not shown), suggesting that mutations were either present at very low levels, outside of the domain that was sequenced, or that resistance occurred through other mechanisms.
Discussion
Early phase I trials of flavopiridol aimed at safely achieving the nanomolar concentrations needed for CDK inhibition in preclinical models employed 72-hour infusions every 2 weeks [24]. Aside from inconvenience, such schedules were both ineffective in the phase II setting [25–27], and associated with substantial toxicity. Subsequent phase I studies of daily 1-hour infusions of flavopiridol every 3 weeks found 37.5 mg/m2/d for 5 days, 50 mg/m2/d for 3 days, and 62.5 mg/m2/d for 1 day to be safe and effective in achieving micromolar concentrations [28]. Encouraging results have been reported with a pharmacologically derived “hybrid” schedule of administration (30-minute loading dose followed by 4-hour infusion) in patients with refractory, genetically high-risk chronic lymphocytic leukemia (CLL) [29]. However, the role of flavopiridol may ultimately lie in combination regimens rather than as a single agent. With standard cytotoxic chemotherapy, interactions have been found in vitro to be highly sequence-dependent, with optimal synergism seen when flavopiridol follows chemotherapeutic agents. Several early-phase clinical trials have evaluated the drug in combination with taxanes and with irinotecan in solid tumors [30–32]. Recently, a phase 1 study of flavopiridol administered as a 30-minute bolus infusion followed by a 4-hour continuous infusion in conjunction with bortezomib showed promising results in patients with refractory multiple myeloma or indolent non-Hodgkin’s lymphoma (NHL) [17]. In a phase I trial in combination with fludarabine and rituximab in patients with mantle cell lymphoma (MCL) and indolent B-cell NHL including CLL, both 1-day and 2-day bolus schedules and two different hybrid schedules of flavopiridol administration were evaluated [33]. Interestingly, the hybrid schedule did not improve outcomes compared to 1-hr bolus dosing in this study [33]. Recently, the sequential administration of flavopiridol, cytarabine and mitoxantrone (“FLAM”) has been investigated in patients with acute leukemia with encouraging response rates in both untreated and relapsed/refractory patients [34, 35]. A phase II trial in newly-diagnosed adults with poor-risk AML employed a daily 1-hour infusion of flavopiridol for 3 consecutive days [34]. In a phase I trial of “hybrid FLAM” in relapsed/refractory acute leukemias, the RPTD of flavopiridol was a 30 mg/m2 bolus followed by a 60 mg/m2 infusion daily for 3 days [35]. While our study predated the development of the hybrid schedules of flavopiridol administration, evidence to suggest that hybrid schedules are superior to 1-hr infusions in the combination setting are lacking, particularly for patients with acute leukemias. Indeed, a recent randomized phase II comparison of two schedules of flavopiridol (as part of “FLAM”) in patients with newly diagnosed poor-risk AML found no major difference in toxicity or responses between the bolus and "hybrid" schedules [36].
During the course of the study, it became apparent that for patients requiring cytoreductive agents such as hydroxyurea to control counts, such measures could confound the interpretation of marrow toxicity. A protocol amendment was then executed, expanding the eligibility criteria for enrollment to stratum 2 to allow entry of patients with pre-existing hematologic toxicity secondary either to their underlying disease or to medically indicated concurrent treatment (e.g., hydroxyurea). The combination of imatinib and flavopiridol was well-tolerated by patients, most of whom were previously treated. Several sustained responses, one complete lasting 30 months, were noted in patients with prior exposure to imatinib, and others had prolonged disease stabilization. The death that occurred at dose level 3 in a stratum 2 patient was an unfortunate event not directly related to study treatment.
No clinically significant pharmacokinetic interactions between imatinib and flavopiridol were observed in this study, and imatinib exposures were comparable to those reported previously [37]. The slight decrease of imatinib AUC in the presence of flavopiridol could be due to an effect of flavopiridol on one of the clearance mechanisms of imatinib, which is not uncommon for flavonoids [38]. Another possible explanation might be the displacement of imatinib from plasma proteins by flavopiridol, which is known to bind tightly to human serum proteins [39], resulting in lower total plasma concentrations. The estimated flavopiridol clearance in this study, when co-administered with daily oral imatinib, was similar to that reported for flavopiridol alone [22, 23], suggesting no significant effect of imatinib on the total systemic exposure of flavopiridol. The primary differences in parameter estimates between studies included volume of distribution and terminal half-life. Concomitant imatinib may contribute to differences in these parameters as imatinib is an inhibitor of ABCG2 [40] of which flavopiridol is a substrate [41]. Interpretation of our studies of protein expression pre-treatment, post-imatinib and post-flavopiridol were hampered by technical issues related to Western Blot assays. Nevertheless, in at least one specimen, pronounced down-regulation of Bcr-Abl, phospho-Bcr/Abl, and Mcl-1 occurred after treatment with the combination, analogous to results observed in the preclinical setting. Whether this phenomenon occurs more generally, and if so, whether such findings correlate with disease responsiveness, will require a much larger sample size. Similarly, the flow cytometry data does not permit definitive conclusions to be drawn regarding changes in pStat5 in relation to patient outcome. However, it appears to be feasible to monitor pStat5 expression in CML by flow cytometry within the context of a multi-institutional clinical trial. Multi-parameter flow cytometry assays hold the promise of highly correlated, quantitative, cell type specific information on drug activity and drug-drug interactions at both target and functional levels. This is important in view of recent work suggesting that Stat5 may be an important additional target in Bcr-Abl dependent malignancies [42, 43]. The functional significance of changes in pStat5 expression will best be determined in more highly powered successor studies (e.g., phase II) employing uniform drug doses. Surprisingly, in this study, we noted a high frequency of imatinib resistance not attributable to Bcr-Abl kinase domain mutations. Potential explanations for this phenomenon include mutations distant from the active site (P-loop), diminished expression (and dependence upon) Bcr-Abl, possibly associated with activation of Lyn, and increased expression of Bcl-2, which was seen in some of our samples and has been described in other phase I trials of flavopiridol [35]. As no Bcr-Abl kinase domain mutations were identified, it was not possible to correlate responses to the drug combination with mutation status.
In summary, this study is the first to evaluate the combination of a CDK inhibitor and a Bcr-Abl TKI in humans. The combination was well-tolerated, and showed promising clinical activity, including in some patients with documented resistance to imatinib. Due to the introduction of the 2nd generation Bcr-Abl TKIs dasatinib and nilotinib for imatinib-intolerant and –resistant patients, the MTD and RPTD of the combination were not able to be determined. However, despite the success of these newer agents, “gatekeeper” mutations in the Bcr-Abl kinase domain such as T315I remain a formidable challenge. Similarly, although the addition of the Bcr-Abl TKIs to conventional chemotherapy in Ph+ ALL represents a significant advance, long term outcomes remain unknown in this subgroup that historically has had a poor prognosis. Newer CDK inhibitors, such as roscovtine and SCH 727965, are currently in clinical development [44]. In addition, novel TKIs currently in various stages of development include several molecules that inhibit both Bcr-Abl and Aurora kinases, leading to enhanced activity against refractory Bcr-Abl kinase domain mutations such as T315I [45]. Trials of CDK inhibitors in combination with 2nd (dasatinib, nilotinib) and 3rd (bosutinib) generation Bcr-Abl TKIs, as well as those in the pipeline, represent a potentially promising avenue of investigation for patients with TKI-refractory disease. However, given uncertainties concerning the future clinical development of flavopiridol, such trials may have to involve the newer CDK inhibitors mentioned above, provided preclinical evidence of synergy can be demonstrated. Studies designed to address this issue are currently underway.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Mary Beth Tombes, R.N., M.N. for assistance in preparing the manuscript tables, Lora Kramer for the Western Blot figure, Sookyung Woo and Shawn Spencer for the manuscript and supplemental sections on flavopiridol pharmacokinetics and Brian J Druker, Director, Knight Cancer Institute, Oregon Health and Science University, for performing correlative studies on characterization of mechanisms of resistance to imatinib in patients previously treated with imatinib.
This work was supported by the following NIH grants: R01 CA93738-05, CA 100866, and R21 CA106139, NCI Cooperative Agreement U01 CA70095, Massey Cancer Center Support Grant P30 CA016059, General Clinical Research Center Grant M01 RR00065, Leukemia and Lymphoma Society of America award 6181-10, Multiple Myeloma Research Foundation, Myeloma SPORE award 1P50CA142509, and Lymphoma SPORE award 1P50CA130805.
EK was supported by a scholarship from the Hellenic Society of Medical Oncology. JHB and MJE were supported by grant P30-CA47904 from the National Cancer Institute. MJE was the recipient of an American Society of Clinical Oncology Cancer Foundation Translational Research Professorship.
Footnotes
Conflict of interest statement: JHB discloses having received research support from Novartis. None of the other authors have any conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Deininger MWN, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib Compared with Interferon and Low-Dose Cytarabine for Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2010;116:3409–3417. doi: 10.1182/blood-2010-01-242750. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MF. Chronic Myelogenous Leukemia - Identifying the Hydra’s Heads. N Engl J Med. 2004;351:634–636. doi: 10.1056/NEJMp048120. [DOI] [PubMed] [Google Scholar]
- 5.Schiffer CA. BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myelogenous Leukemia. N Engl J Med. 2007;357:258–265. doi: 10.1056/NEJMct071828. [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, Van Etten RA. Tyrosine Kinases as Targets for Cancer Therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood. 2007;110:2828–2837. doi: 10.1182/blood-2007-04-038943. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Grant S. Cyclin-dependent kinase inhibitors. Curr Opin Pharmacol. 2003;3:362–370. doi: 10.1016/s1471-4892(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 9.Blagosklonny MV. Flavopiridol, an Inhibitor of Transcription. Cell Cycle. 2004;3:1537–1542. doi: 10.4161/cc.3.12.1278. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro GI. Preclinical and Clinical Development of the Cyclin-Deprendent Kinase Inhibitor Flavopiridol. Clin Cancer Res. 2004;10:4270s–4275s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- 11.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 12.Grant S, Dent P. Gene profiling and the cyclin-dependent kinase inhibitor flavopiridol: What’s in a name? Mol Cancer Ther. 2004;3:873–875. [PubMed] [Google Scholar]
- 13.Senderowicz AM. The Cell Cycle as a Target for Cancer Therapy: Basic and Clinical Findings with the Small Molecule Inhibitors Flavopiridol and UCN-01. Oncologist. 2002;7(suppl 3):12–19. doi: 10.1634/theoncologist.7-suppl_3-12. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Yu C, Singh V, et al. Pharmacological Inhibitors of the Mitogen-activated Protein Kinase (MAPK) Cascade Interact Synergistically with UCN-01 to Induce Mitochondrial Dysfunction and Apoptosis in Human Leukemia Cells. Cancer Res. 2001;61:5106–5115. [PubMed] [Google Scholar]
- 15.Yu C, Krystal G, Dent P, Grant S. Flavopiridol Potentiates STI571-induced Mitochondrial Damage and Apoptosis in BCR-ABL-positive Human Leukemia Cells. Clin Cancer Res. 2002;8:2976–2984. [PubMed] [Google Scholar]
- 16.Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 17.Holkova B, Perkins EB, Ramakrishnan V, et al. Phase I trial of bortezomib (PS341; NSC681239) and alvocidib (flavopiridol; NSC649890) in patients with relapsed or refractory B-cell neoplasms. Clin Cancer Res. 2011;17(10):3388–3397. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlesso N, Frank DA, Griffin JD. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183(3):811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilaria RL, Jr, Van Etten RA. P210 and P190 (BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271(49):31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 20.Shuai K, Halpern J, ten Hoeve J, et al. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13(2):247–254. [PubMed] [Google Scholar]
- 21.Jacobberger JW, Sramkoski RM, Frisa PS, et al. Immunoreactivity of Stat5 phosphorylated on tyrosine as a cell-based measure of Bcr/Abl kinase activity. Cytometry A. 2003;54(2):75–88. doi: 10.1002/cyto.a.10063. [DOI] [PubMed] [Google Scholar]
- 22.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res. 2005;11(23):8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 23.Tan AR, Yang X, Berman A, et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res. 2004;10(15):5038–5047. doi: 10.1158/1078-0432.CCR-04-0025. [DOI] [PubMed] [Google Scholar]
- 24.Senderowicz AM, Headlee D, Stinson SF, et al. Phase I Trial of Continuous Infusion Flavopiridol, a Novel Cyclin-Dependent Kinase Inhibitor, in Patients With Refractory Neoplasms. J Clin Oncol. 1998;16:2986–2999. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GK, Ilson D, Saltz L, et al. Phase II Study of the Cyclin-Dependent Kinase Inhibitor Flavopiridol Administered to Patients with Advanced Gastric Carcinoma. J Clin Oncol. 2001;19:1985–1992. doi: 10.1200/JCO.2001.19.7.1985. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro GI, Supko JG, Patterson A, et al. A Phase II Trial of the Cyclin-Dependent Kinase Inhibitor Flavopiridol in Patients With Previously Untreated Stage IV Non-Small Cell Lung Cancer. Clin Cancer Res. 2001;7:1590–1599. [PubMed] [Google Scholar]
- 27.Stadler WM, Vogelzang NJ, Amato R, et al. Flavopiridol, a Novel Cyclin-Dependent Kinase Inhibitor, in Metastatic Renal Cancer: a University of Chicago Phase II Consortium Study. J Clin Oncol. 2000;18:371–375. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 28.Tan AR, Headlee D, Messman R, et al. Phase I Clinical and Pharmacokinetic Study of Flavopiridol Administered as a Daily 1-Hour Infusion in Patients With Advanced Neoplasms. J Clin Oncol. 2002;20:4074–4082. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathkopf DE, Ilson DH, Yi S, et al. A phase II trial of sequential paclitaxel and flavopiridol in patients with metastatic paclitaxel-refractory esophageal cancer. Presented at the American Society of Clinical Oncology Gastrointestinal Cancers Synposium; January 22–24, 2004; San Francisco, CA. 2004. (abstract 67). [Google Scholar]
- 31.Rathkopf DE, Fornier M, Shah MA, et al. A phase I dose-finding sudy of weekly, sequential docetaxel (Doc) followed by flavopiridol (F) in patients with advanced, solid tumors. J Clin Oncol 2004. 2004;22:213s. doi: 10.1158/1078-0432.CCR-07-1218. (suppl; abstr 3072). [DOI] [PubMed] [Google Scholar]
- 32.Shah MA, Kortmansky J, Motwani M, et al. A Phase I Clinical Trial of the Sequential Combination of Irinotecan Followed by Flavopiridol. Clin Cancer Res. 2005;11:3836–3845. doi: 10.1158/1078-0432.CCR-04-2651. [DOI] [PubMed] [Google Scholar]
- 33.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28(3):418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karp JE, Blackford A, Smith BD, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34(7):877–882. doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp JE, Smith BD, Resar LS, et al. Phase I and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood. 2011;117:3302–3310. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karp JE, Pagel JM, Smith BD, et al. Randomized phase II study of two schedules of flavopiridol (alvocidib, F) given as timed sequential therapy (TST) with Ara-C and mitoxantrone (FLAM) for adults with newly diagnosed, poor-risk acute myelogenous leukemia (AML) Blood (ASH Annual Meeting Abstracts) 2010. 2010;116 Abstract 186. [Google Scholar]
- 37.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 38.Deferme S, Van Gelder J, Augustijns P. Inhibitory effect of fruit extracts on P-glycoprotein-related efflux carriers: an in-vitro screening. J Pharm Pharmacol. 2002;54(9):1213–1219. doi: 10.1211/002235702320402053. [DOI] [PubMed] [Google Scholar]
- 39.Blum W, Klisovic MA, Phelps RB, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed and refractory acute leukemias. Haematologica. 2010;95(7):1098–1105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houghton PJ, Germain GS, Harwood FC, et al. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64(7):2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 41.Robey RW, Medina-Pérez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7(1):145–152. [PubMed] [Google Scholar]
- 42.Nelson EA, Walker SR, Weisberg E, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warsch W, Kollmann K, Eckelhart E, et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood. 2011;117:3409–3420. doi: 10.1182/blood-2009-10-248211. [DOI] [PubMed] [Google Scholar]
- 44.Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16(2):36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore AS, Blagg J, Linardopoulos S, Pearson AD. Aurora kinase inhibitors: novel small molecules with promising activity in acute myeloid and Philadelphia-positive leukemias. Leukemia. 2010;24(4):671–678. doi: 10.1038/leu.2010.15. Epub 2010 Feb 11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.