Abstract
We describe a case of multiorgan dysfunction secondary to Trypanosoma brucei rhodesiense infection acquired on safari in Zambia. This case was one of several recently reported to ProMED-mail in persons who had traveled to this region. Trypanosomiasis remains rare in travelers but should be considered in febrile patients who have returned from trypanosomiasis-endemic areas of Africa.
Keywords: Trypanosomiasis, African, African sleeping sickness, trypanosomes, parasites, trypanocidal agents, suramin, adverse drug reaction, drug toxicity, tsetse fly, Trypanosoma brucei rhodesiense, multi-organ dysfunction, travel, Zambia
We describe a British safari tourist with multi-organ dysfunction and shock secondary to African trypanosomiasis. This case illustrates the complications associated with treatment of Trypanosoma brucei rhodesiense infection and highlights a recent increase in cases reported to ProMED (www.promedmail.org) of trypanosomiasis in travelers to Zambia.
The Case-Patient
A 49-year-old woman with a 5-day history of fever, malaise, headache, dizziness, abdominal discomfort, diarrhea, and vomiting sought treatment 1 day after returning to the United Kingdom from a 2-week safari in Zambia. During the safari, she spent 3 days in the South Luangwa National Park, 3 days in the Lower Zambezi National Park, and 6 days in Kafue National Park. Initial blood films examined at Furness General Hospital were negative for malaria parasites but positive for trypanosomes. Urgent transfer of the patient to the Tropical and Infectious Disease Unit in Liverpool, UK, was arranged.
Upon arrival, the patient was dehydrated and had jaundice and tachycardia, but she initially was normotensive. Examination revealed reduced breath sounds at the lung bases and a distended, nontender abdomen. There was a mild erythematous rash on the patient’s abdomen, but no chancres. Results of a neurologic examination were unremarkable.
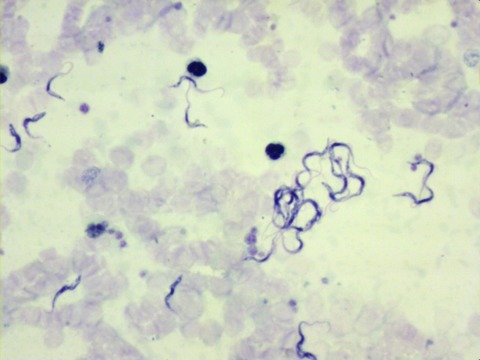
Repeat blood films confirmed numerous trypanosomes (Figure), which, given the patient’s travel history, were considered likely to be T. b. rhodesiense. PCR results confirmed the trypanosomes positive for the T. b. rhodesiense–specific serum resistance–associated gene (1). Blood test results were as follows: urea 9.2 mmol/L, creatinine 146 μmol/L, leukocytes 3.7 × 109 cells/L, platelets 13 × 109/L, C-reactive protein 234 mg/L, alanine aminotransferase 179 U/L, bilirubin 38 μmol/L, and prothrombin time 13.5 s.
Figure.
Thick film using Field’s stain showing trypanosomes under ×400 magnification. Motile trypanosomes are shown in the Video.
Video.
Motile trypanosomes.
Direct Video Link: http://streaming.cdc.gov/vod.php?id=7e517b3f1a94f72aad563c8c5d8ceec720120120160142637
Despite fluid resuscitation, the patient became increasingly hypotensive over the next 12 hours, prompting transfer to the intensive care unit. A 100-mg test dose of suramin was well tolerated by the patient; however, the first full treatment dose was complicated by circulatory collapse and bronchoconstriction, which required administration of hydrocortisone and chlorphenamine and immediate discontinuation of the suramin infusion. Subsequent investigation showed that the suramin dose had been infused more rapidly than prescribed. Further doses administered as slow infusions were uncomplicated.
Hypotension persisted for 4 days but did not necessitate vasopressors. Results of a short synacthen test, electrocardiogram, and echocardiogram were normal.
After the patient received 2 treatment doses of suramin and analysis of repeat blood films confirmed that parasitemia had cleared, a lumbar puncture was performed. The cerebrospinal fluid (CSF) had 4 leukocytes/μL and normal levels of protein and glucose, and no trypanosomes were detected after double centrifugation.
The patient received a full course of suramin for early-stage disease, (regimen in Table 1), which resulted in full recovery. As follow-up care, the patient will receive repeat lumbar punctures every 3 months for 2 years to exclude occult invasion of the central nervous system (CNS). Thus far, 3 repeat lumbar punctures have shown no evidence of CNS invasion.
Table 1. Treatment regimen for Trypanosoma brucei rhodesiense infection in adults*.
| Disease stage | Drug, route of administration | Regimen | Adverse effects |
|---|---|---|---|
| First | Suramin, intravenous | Test dose of 100 mg in 100 mL 0.9% saline over 30 min on day 0; and 5 doses of 20 mg/kg (maximum 1 g/dose) in 250 mL 0.9% saline over 3 h on days 1, 3, 7, 14, 21 | Hypersensitivity reactions (early and late); nephrotoxicity, hepatotoxicity, hemolytic anemia, peripheral neuropathy, agranulocytosis, thrombocytopenia, and cutaneous reactions |
| Second | Melarsoprol, intravenous | 2.0–3.6 mg/kg/d (maximum 180 mg/d) for 3 d; after 7 d, 3.6 mg/kg/d for 3 d; after 7 more d, 3.6 mg/kg/d for 3 d† | Encephalopathy, cutaneous reactions, peripheral neuropathy, cardiac arrhythmias, thrombophlebitis, fever, and gastric upset |
African trypanosomiasis is caused by the protozoan parasite T. brucei, which is transmitted by tsetse flies. Two subspecies are pathogenic in humans: T. b. gambiense in central and western Africa, and T. b. rhodesiense in eastern and southern Africa.
Disease progresses in 2 stages. In the first stage, parasites spread in the blood to the lymph nodes, liver, spleen, heart, endocrine system, and eyes (4). Untreated, they invade the CNS, which leads to second-stage or meningoencephalitic disease with characteristic sleep disturbances. Progression to the second stage may take months in T. b. gambiense infection but only weeks in T. b. rhodesiense infection.
Although trypanosomiasis is uncommon in travelers, it should be considered in the differential diagnosis of patients with fever who have returned from trypanosomiasis-endemic areas of Africa (5). Recent reports suggest an increase in cases emerging from Zambia, particularly from the South Luangwa Valley (Table 2) (6). Whether these cases reflect an increased risk for infection in that region or increasing tourism in a trypanosomiasis-endemic area is unclear. Infection in travelers is usually characterized by an acute febrile illness, sometimes associated with a macular evanescent rash or chancre (2,4). Laboratory tests often indicate anemia, thrombocytopenia, leukopenia, impaired renal function, electrolyte disturbances, coagulation abnormalities, and elevation in hepatic transaminase and C-reactive protein levels (2,7).
Table 2. Reports to ProMED-mail of Trypanosoma brucei rhodesiense infections associated with travel to or bordering Zambia, 2010*.
| Month of report | ProMED-mail archive no. | Nationality of patient | Travel activity | Area visited |
|---|---|---|---|---|
| September | 20100915.3338 | Zambian | Visiting game ranch | South Luangwa Valley, Zambia |
| 20100915.3338 | American | Hunting safari | South Luangwa Valley, Zambia | |
| October† | 20101022.3833 | British | Camping safari | South Luangwa National Park, Lower Zambez National Park, Kafu National Park, Zambia |
| 20101022.3833 | British | Visiting national park | Mana Pool National Park, Zimbabwe (bordering Zambia) | |
| November | 20101111.4093 | South African national of Scandinavian origin | Hiking | Luangwa River area, Zambia |
*Reports in (6). Since the start of 2005, 11 other cases of T. b. rhodesiense infection in travelers have been reported in ProMED-mail; those cases were acquired in Uganda (1), Tanzania (3), and Malawi (7). †Case presented in this report.
Conditions that should be considered in patients with persistent hypotension are adrenal insufficiency and cardiac dysfunction. The prevalence of adrenal insufficiency was 27% in a study of Ugandan patients with trypanosomiasis (8). Myocarditis, pericarditis, and congestive cardiac failure have been described and should be excluded by electrocardiogram and echocardiography (4).
The treatment for first-stage T. b. rhodesiense infection is intravenous suramin, given as 5 injections of 20 mg/kg each over 3–4 weeks (2,4). Early hypersensitivity reactions to suramin (i.e., nausea, circulatory collapse, and urticaria) are described in 0.1%–0.3% of patients; thus, an initial test dose is advocated (9).
Second-stage T. b. rhodesiense infection is treated with melarsoprol, a highly toxic arsenical which causes a severe reactive encephalopathy in ≈10% of patients, half of whom die as a result (10). This toxicity among patients emphasizes the importance of accurate staging, which is determined by CSF examination. According to World Health Organization guidelines, the presence of >5 leukocytes/μL and/or the presence of trypanosomes in the CSF indicates second-stage disease (11). Lumbar puncture should be deferred until clearance of blood parasitemia has been confirmed.
In view of our patient’s rapid onset of a high level of parasitemia, we investigated the possibility of a genetic susceptibility to trypanosomal infection. Human plasma contains a trypanosome lytic factor called apolipoprotein L-1 (APOL1) (12). This protein causes lysis of T. brucei subspecies other than rhodesiense and gambiense, both of which have acquired resistance to it (13). In 2006, Vanhollebeke et al. (14) described a patient infected with T. evansi, which is usually sensitive to APOL1. The patient’s serum lacked APOL1 due to mutations in the APOL1 gene, rendering him susceptible to a species regarded as nonpathogenic in humans. We sequenced the APOL1 gene of our patient, but no substantial variations suggesting enhanced susceptibility were detected.
Conclusions
In summary, trypanosomiasis remains rare in travelers, but possible infection should be considered in patients with fever who have returned from trypanosomiasis-endemic areas of Africa. Early reporting of trypanosomiasis cases to ProMED-mail allows timely recognition of emerging safari destinations that present an increased risk for infection to travelers. In patients with T. b. rhodesiense infection, multi-organ dysfunction may develop in early-stage disease. Treatment of such cases should be managed with critical care support, and it should be remembered that rapid infusion of suramin may precipitate circulatory collapse.
Acknowledgments
We acknowledge Anthony Macheta from Furness General Hospital.
Biography
Dr Cottle is a specialist registrar in infectious diseases at the Tropical and Infectious Disease Unit in Liverpool, UK.
Footnotes
Suggested citation for this article: Cottle LE, Peters JR, Hall A, Bailey JW, Noyes HA, Rimington JE. Multiorgan dysfunction caused by travel-associated African trypanosomiasis. Emerg Infect Dis [serial on the Internet]. 2012 Feb [date cited]. Epub 2012 Jan 4. http://dx.doi.org/10.3201/eid1802.111479
Current affiliation: Worthing Hospital, Worthing, UK.
References
- 1.Welburn SC, Picozzi K, Fèvre EM, Coleman PG, Odiit M, Carrington M, et al. Identification of human-infective trypanosomes in animal reservoir of sleeping sickness in Uganda by means of serum-resistance-associated (SRA) gene. Lancet. 2001;358:2017–9. 10.1016/S0140-6736(01)07096-9 [DOI] [PubMed] [Google Scholar]
- 2.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010;375:148–59. 10.1016/S0140-6736(09)60829-1 [DOI] [PubMed] [Google Scholar]
- 3.Abramowicz M. Drugs for parasitic infections. In: Abramowicz M, editor. The medical letter on drugs and therapeutics. New Rochelle (NY): The Medical Letter, Inc; 2000. p. 1–12. [Google Scholar]
- 4.Kennedy PG. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurol. 2008;64:116–26. 10.1002/ana.21429 [DOI] [PubMed] [Google Scholar]
- 5.Gautret P, Clerinx J, Caumes E, Simon F, Jensenius M, Loutan L, et al. Imported human African trypanosomiasis in Europe, 2005–2009. Euro Surveill. 2009;14:pii:19327. [PubMed] [Google Scholar]
- 6.ProMED-mail. Archive nos. 20100915.3338, 20101022.3833, and 20101111.4093 [cited 2011 Sep 22]. http://www.promedmail.org
- 7.Sinha A, Grace C, Alston WK, Westenfeld F, Maguire JH. African trypanosomiasis in two travelers from the United States. Clin Infect Dis. 1999;29:840–4. 10.1086/520446 [DOI] [PubMed] [Google Scholar]
- 8.Reincke M, Arlt W, Heppner C, Petzke F, Chrousos GP, Allolio B. Neuroendocrine dysfunction in African trypanosomiasis. Ann N Y Acad Sci. 1998;840:809–21. 10.1111/j.1749-6632.1998.tb09619.x [DOI] [PubMed] [Google Scholar]
- 9.Burri C. Chemotherapy against human African trypanosomiasis: is there a road to success? Parasitology. 2010;137:1987–94. 10.1017/S0031182010001137 [DOI] [PubMed] [Google Scholar]
- 10.Rodgers J. Human African trypanosomiasis, chemotherapy and CNS disease. J Neuroimmunol. 2009;211:16–22. 10.1016/j.jneuroim.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Control and surveillance of African trypanosomiasis. Report of a WHO expert committee. WHO Technical Report Series. 1998;881:I–VI, 1–114. [PubMed]
- 12.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, et al. Apolipoprotein L–I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–7. 10.1038/nature01461 [DOI] [PubMed] [Google Scholar]
- 13.Pays E, Vanhollebeke B. Human innate immunity against African trypanosomes. Curr Opin Immunol. 2009;21:493–8. 10.1016/j.coi.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 14.Vanhollebeke B, Truc P, Poelvoorde P, Pays A, Joshi PP, Katti R, et al. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L–I. N Engl J Med. 2006;355:2752–6. 10.1056/NEJMoa063265 [DOI] [PubMed] [Google Scholar]