Abstract
Despite overlapping structural aspects with other phospholipids, lysophosphatidylserine (lysoPS), the monoacyl derivative of phosphatidylserine (diacylPS), appears to exert unique signaling characteristics important in both the early stages of initiating acute inflammation and in the orchestration of its resolution. LysoPS has long been known as a signaling phospholipid in mast cell biology, markedly enhancing stimulated histamine release and eicosanoid production. More recently, there has been a resurgence of interest in lysoPS as new roles in the promotion of phagocytosis of apoptotic cells, so-called efferocytosis, and resolution of inflammation have been identified. With regard to the latter, lysoPS generated in/on activated or aged apoptotic neutrophils enhances their clearance by macrophages via signaling through the macrophage G-protein coupled receptor G2A. In macrophages, this early acting pathway results in PKA-dependent augmentation of Rac1 activity via increased production of PGE2 and cAMP. As such, macrophages stimulated with lysoPS demonstrate significantly increased efferocytic capacity necessary to clear large numbers of recruited neutrophils typical of acute inflammation. Given that clearance of these cells is critical for restoration of tissue function, lysoPS, as a pro-resolving lipid mediator, is hypothesized to play a key role in promoting timely resolution of inflammation. This article will review our current knowledge of lysoPS biology including receptor signaling and mechanisms of generation as well as summarize the more recent evidence of its expanding roles in inflammation.
1. Introduction
LysoPS is among a growing list of bioactive lysophospholipids (lysoPLs) gaining attention as important lipid mediators (Table 1), and despite their structural similarities, each signals in a distinct manner allowing for divergent signaling. LysoPLs, originally named for their ability to lyse erythrocytes due to their detergent-like properties, are the monoacyl derivatives of the diacyl glycerophospholipid parent molecules. Once thought of simply as metabolic byproducts or intermediates in phospholipid synthesis, these molecules are now recognized as signaling lipids and their generation or metabolism may have profound impacts on cellular functions. In addition to altering the physical or mechanical properties of the lipid bilayer, they show receptor mediated signaling either via direct binding with their receptors and/or by indirect effects on the membrane in which the receptor resides. Much is known about the biological activities of lysoPC, the most abundant lysoPL present in serum at concentrations of several hundred μM, as well as lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), and these have been recently reviewed [1–3]. Relatively less, however, is known about the biological activity of lysoPS where, unlike lysoPC or S1P, its presence in serum is virtually undetected. New data demonstrate intriguing pro-resolution properties in addition to its older roles in acute inflammation. These properties are discussed below.
Table 1.
Bioactive lysophospholipids
| LysoPLs | Cellular effect | Responding cell | Receptor | Ref |
|---|---|---|---|---|
| S1P | Survival | T-cells; B-cells; Macrophage | S1P1-2,4 | [3] |
| Migration | T-cells; B-cells, Mast cells; Macrophages; Dendritic cells | S1P1 | “ | |
| Angiogenesis | Endothelial cells | S1P1 | “ | |
| LPA | Migration | T-cells; Dendritic cells; Fibroblasts | LPA1-3 | [1] |
| Proliferation | Neuronal cells; Astrocytes | LPA1-2 | “ | |
| Survival | Neuronal cells; Endothelial cells; Leukocytes | LPA1-2 | “ | |
| LysoPC | Calcium mobilization | Neutrophils | G2A | [35] |
| T-cells | G2A ? | [77] | ||
| Enhance ROS | Neutrophil | ? | [19, 78] | |
| Inhibit ROS | Neutrophil | G2A | [79] | |
| Migration | T-cells; Monocytes | G2A | [54, 80, 81] | |
| LysoPS | Degranulation | Mast cells; Neutrophils | GPR34; G2A | [35, 42] |
| Enhance ROS | Neutrophil | ? | [19, 78] | |
| Calcium mobilization | Neutrophils; T-cells | G2A; ? | [35, 82] | |
| Leukemic cells | ? | [83] | ||
| Enhanced efferocytosis | Macrophages | G2A | [22, 23] | |
| Migration | Fibroblasts | ? | [17] | |
| Glioma cells | ? | [16] | ||
| Differentiation | PC12 | ? | [84] | |
| LysoPE | Calcium mobilization | Neutrophils | G2A | [35] |
| Migration | Ovarian cancer cells | ? | [85] |
2. LysoPS old and new roles in inflammation
2.1. Mast cell priming
DiacylPS was originally found in brain extracts by Folch in 1941 [4, 5], and in 1971 was shown as an endogenous enhancer of mast cell histamine release in rats [6]. DiacylPS, when added simultaneously with a histamine releasing agent (e.g. dextran, antigen or nerve growth factor), potentiated histamine release by 5–10 fold which was increased even more if added 20–30 minutes ahead of time, suggesting perhaps either incorporation and/or conversion of diacylPS was important for this response. In 1979, Smith et al. demonstrated that lysoPS, was 1000 times more potent than diacylPS [7]. This effect was specific to lysoPS as other lysoPLs were unable to substitute for lysoPS in augmenting the response [8]. Additionally, this lysoPS enhancing effect was found to be stereospecific in that the lysophosphatidyl-D-serine stereoisomer was largely ineffective [9]. Interestingly, both 1-acyl-2-lyso-PS and 1-lyso-2-acyl-PS were equally effective with a minimum fatty acid chain length of C14 being required [7]. To rule out a simple membrane permeabilization effect, the concentration required for increased histamine release (~10−7M) was shown to be far below the critical micellar concentration (~10−5M) and there was no evidence of lactate dehydrogenase release, indicating that the mast cell membranes were intact [7, 10]. Furthermore, pretreatment of the mast cells with pertussis toxin inhibited the lysoPS response implicating the involvement of a Gαi G-protein coupled receptor (GPCR) [11], though the identity of the receptor is yet to be established (see section 3). The signal transduction pathway downstream of the GPCR led to calcium mobilization, PLCγ activation and generation of polyphosphoinositides [12, 13]. In addition to histamine release, lysoPS also enhanced immediate nerve growth factor-dependent PGD2 production via cyclooxygenase (COX) 1, as well as delayed PGD2 production through stimulation of COX2 expression via an as yet unknown signal transduction pathway [14]. Whether the signaling for histamine release and lipid mediator production require the same or divergent pathways, has yet to be elucidated. Notably, lysoPS was unable to induce histamine release or PGD2 production on its own in rat mast cells and required a co-signal, such as antigen or nerve growth factor. This may suggest that the lysoPS response is geared to avoid inappropriate or excessive mast cell activation as was seen following direct infusion of lysoPS in rodents: intravenous infusion resulted in a rapid increase in cAMP in the hypothalamus followed by hyperglycemia in the brain and anaphylaxis [9, 15]. These effects were attributed to systemic histamine release by mast cells into the vasculature and was abrogated in mast cell deficient mice [9].
2.2. Beyond the mast cell: roles for lysoPS in the resolution of inflammation
In addition to effects on mast cells, lysoPS has also been shown to exert biological effects on other cells including stimulating calcium flux and influencing cell survival, proliferation, differentiation and activation (Table 1). It also induced migration of glioma cells [16] and mouse fibroblasts [17] but not migration of macrophages [18] or neutrophils (our unpublished observations). In almost all cases, studies using exogenous lysoPS to test these effects used 1-oleoyl-2-lyso-PS because of its commercial availability. Therefore, varying species of lysoPS have not been fully investigated; variable effects related to chain length and the degree of saturation of the esterified fatty acid in lysoPC species have been observed [19]. The effect of structural features on lysoPS signaling such as the position of acyl chain esterification (e.g. in either the sn-1 or sn-2 position), or the D or L stereoisomer of the phosphoserine headgroup, have not been fully investigated as they have been in mast cell activation. Furthermore, the mode of presentation, either bound to albumin or embedded in a membrane, appears to alter lysoPS signaling (see section 4).
LysoPS (both 1-stearoyl-2-lyso-PS and 1-oleoyl-2-lyso-PS; see section 5.2) is made by neutrophils and recently, pro-resolving roles in the resolution phase of neutrophilic inflammation have been identified. Following tissue injury and/or microbial invasion, large numbers of neutrophils are recruited from the blood and activated as “first responders” to unleash a potent arsenal of microbicidal constituents. Once the site has been sterilized, the activated neutrophils must be cleared for restoration of tissue function. While a few neutrophils may exit tissues either outside the body e.g. gingival crevice, or migrate to lymph nodes [20], most undergo apoptosis and are removed by macrophages by a process referred to as efferocytosis (“to carry to the grave” [21]). Signals generated in/on activated and apoptosing neutrophils mark them for removal by macrophages and lysoPS is one such signal. LysoPS displayed by activated and aged apoptotic neutrophils signals via the macrophage orphan receptor, G2A, collaborating with other recognition ligands to enhance neutrophil clearance (below) [22, 23]. LysoPS presented on cells, or in liposomes to simulate this, increased both the number of macrophages ingesting and the number of apoptotic cells ingested per macrophage (Fig. 1A–B), whereas lysoPC and lysoPE were ineffective. In vivo, lysoPS significantly amplified the capacity of macrophages to efferocytose and clear neutrophils which vastly outnumber them during acute inflammation [20]. Since large numbers of apoptotic neutrophils are rarely seen unless a clearance defect exists, the recognition and removal process appears to be quite efficient. Indeed, lysoPS generated in/on activated neutrophils may promote their clearance long before there is obvious evidence of their having undergone apoptosis [20, 22].
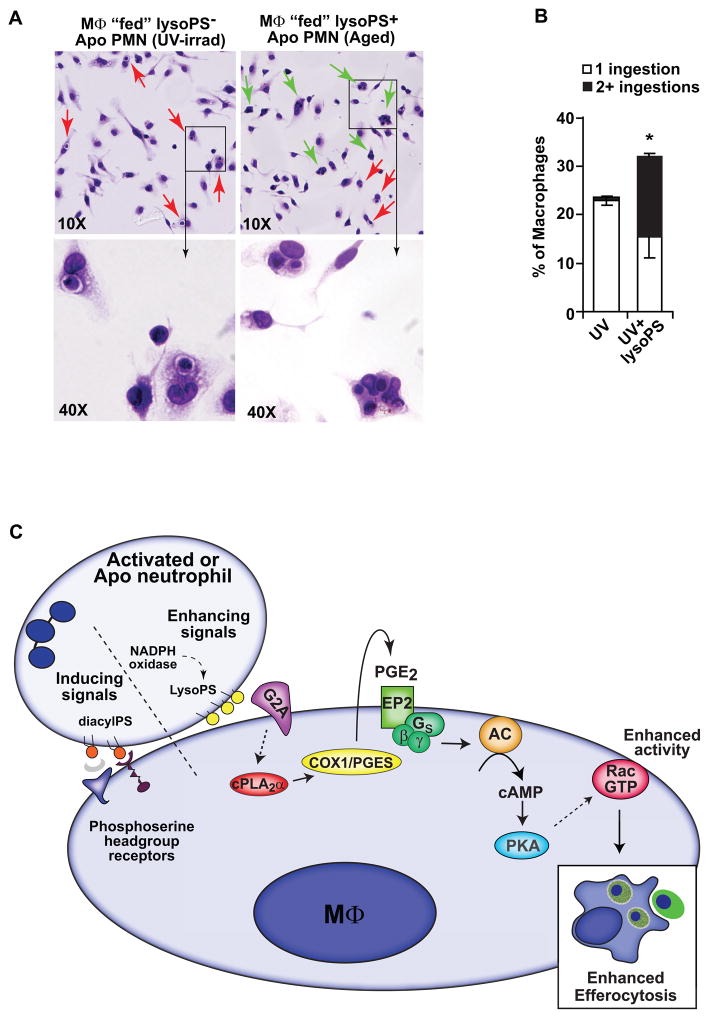
Figure 1.
LysoPS enhances efferocytosis. A. Multiple ingestions are observed in resident peritoneal macrophages (MΦ) co-cultured for 1 hour with lysoPS positive (aged apoptotic neutrophils (PMN)) but not lysoPS negative (UV-irradiated apoptotic PMN) targets, though both showed equivalent phosphoserine headgroup exposure as measured by annexin V binding. Red arrows indicate single ingestions and green arrows indicate multiple ingestions. B. Resident peritoneal MΦ were co-cultured for 1 hour with lysoPS negative PMN in the absence or presence of liposomes containing 30 mole % lysoPS and multiple ingestions were quantified. C. Early acting pathway for lysoPS enhancement of efferocytosis downstream of G2A. LysoPS signals coordinately with inducing signals such as the phosphoserine headgroup (see Table 2), to enhance efferocytosis. This figure has been adapted from the original published figure in Journal of Biological Chemistry. Frasch SC, Fernandez-Boyanapalli RF, Berry KZ, Leslie CC, Bonventre JV, Murphy RC, Henson PM, Bratton DL. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J. Biol. Chem. 2011; 286:12108-22. © the American Society for Biochemistry and Molecular Biology.
Of note, lysoPS by itself, e.g. when added to viable cells, did not induce efferocytosis, and hence, lysoPS is defined as an efferocytosis enhancer, working cooperatively with other signals, for apoptotic cell clearance (Table 2) (Fig. 1C). Conversely, exofacial exposure of the phosphoserine headgroup, the best known of ligands presented by apoptosing cells, or diacylPS when added to viable cells, induced efferocytosis via its headgroup. The phosphoserine headgroup is recognized steriospecifically by a growing number of macrophage receptors and interacting bridge molecules in plasma (Table 2) [20]. Why the phosphoserine headgroup of lysoPS is not recognized in this manner is unclear, but may relate to its altered conformation in the membrane or differences in packing [24, 25]. Interestingly, oxidation of diacylPS, yielding a truncated, oxidized sn-2 fatty acid chain that protrudes into the aqueous phase (oxPS) induces efferocytosis by signaling, in this case, through CD36, a pattern recognition receptor in the scavenger receptor family [26]. Thus, distinct signaling by phosphatidylserine species in efferocytosis has been documented, however, the relationship between the production, display and signaling by these phosphatidylserine species during cellular apoptosis have yet to be defined. DiacylPS, the precursor of both lysoPS and oxPS (Fig. 3), represents only 2–10% of the total plasma membrane phospholipid depending on the cell [27], and is asymmetrically distributed, exclusively found in the inner leaflet of viable, quiescent cells. Energy requiring mechanisms exist to maintain this asymmetry. During apoptosis, and even transiently during activation [28, 29], phospholipid asymmetry collapses with the most notable change being surface exposure of the phosphoserine headgroup, which can be detected by annexin V or Factor Va binding [29]. This exposure is the result of increased bidirectional movement, or “flip-flop” of all phospholipids, and in the case of apoptosis, a decreased aminophospholipid translocase (APLT) activity which specifically transports aminophospholipids, diacylPS and to a lesser extent diacylPE, from the outer leaflet back to the inner leaflet [30, 31]. Of note, lysoPS, as a cone shaped lipid, may itself contribute to enhanced phospholipid flip-flop across the membrane, perhaps leading to enhanced display of not only itself but other phosphatidylserine species as well [32]. Additionally, lysoPS that has reached the plasma membrane outer leaflet is not recognized by the APLT and is consequently not transported back to the inner leaflet, further enhancing its surface accumulation and availability for signaling [33, 34]. How much lysoPS is actually on the surface and the critical concentration required for downstream signaling is unknown. In neutrophils activating their NADPH oxidase, we estimate up to 20% of the plasma membrane diacylPS pool was converted to lysoPS while oxPS species were not detected despite robust oxidant production (see section 5.2). The relationship between the distinct signaling and potential interconversion that may exist between lysoPS, the phosphoserine headgroup and oxPS is just now being sorted out (see section 5 and Fig. 3).
Table 2.
Phosphatidylserine species in efferocytosis: inducers and enhancers
| Phosphatidylserine species | Bridge molecule | Receptor | Rolea | Ref. |
|---|---|---|---|---|
| Phosphoserine | - | RAGE | Inducer | [86] |
| Headgroup | - | TIM4 | [20, 87] | |
| - | BAI1 | “ | ||
| - | Stabilin1 | “ | ||
| - | RAGE | “ | ||
| MFG-E8 | αvβ5 integrins | “ | ||
| TSP | CD36; αvβ3/β5 | “ | ||
| GAS6 | TAM Rs (Tryo, Axl, Mer) | “ | ||
| C1q | LRP (CD91); C1qR | “ | ||
| Collectins | LPR (CD91) | “ | ||
| OxPS | - | CD36 Scavenger receptors |
Inducer | [26] |
| LysoPS | - | G2A | Enhancer | [22, 23] |
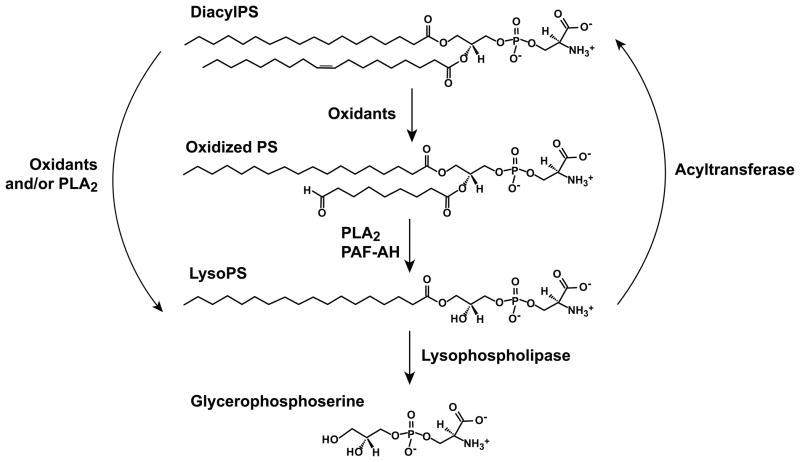
Figure 3.
Schematic for the mechanisms of lysoPS degradation as well as potential interconversion between diacylPS, oxidized PS and lysoPS species.
3. Receptors mediating lysoPS signaling
3.1. G2A Receptor
The newly described roles of lysoPS have been attributed to signaling through the G-protein coupled receptor G2A [22, 35]. G2A is expressed on all hematopoetic cells as well as on keratinocytes and endothelial cells [2, 36]. LysoPC was originally described as a specific ligand for G2A, but this was later retracted as data suggested that its signaling through G2A occurred in a ligand-independent manner [37, 38]. These studies have been hampered due, in part, to the difficulty in demonstrating direct receptor binding by amphipathic lipids that have the ability to adsorb onto the membrane. Further, G2A has also been shown to display significant promiscuity with regard to activating lipids [2]. This feature is highlighted by the fact that quite disparate lipid molecules including lysoPC, lysoPS, lysoPE and oxidized fatty acids (e.g. 9-HODE) are able to elicit signaling responses via G2A, however, differences in response to oxidized fatty acids between human and murine G2A also exist [35, 39]. The G2A signaling mechanism has been proposed to occur at the level of altering the receptor oligomerization and/or surface expression [35, 40]. Monitoring calcium mobilization in human neutrophils, it was hypothesized that lysoPL-dependent non-permeabilizing membrane perturbation resulted in G2A receptor dimerization or oligomerization and mobilization from secretory vesicles. Further, G-protein activation by lysoPS presented on albumin was self- and cross-desensitized by lysoPC and lysoPE for subsequent stimulation, a typical mechanism for limiting prolonged GPCR signaling [35].
Investigation of signaling downstream from G2A during efferocytosis, increased production of PGE2 was demonstrated within minutes. Autocrine signaling by PGE2 via the EP2 receptor enhanced cAMP production resulting in PKA-dependent augmentation of Rac1 activity (Fig. 1C). G2A-dependent enhanced efferocytosis was confirmed using blocking antibodies to G2A as well as using macrophages from G2A−/− mice [23]. In both instances, macrophages were insensitive to lysoPS-mediated enhancement of apoptotic cell engulfment. Importantly, blockade of G2A signaling during zymosan-induced peritonitis using a blocking antibody in wild type mice delayed the resolution of neutrophilia and apoptotic neutrophils accumulated. Similarly, G2A−/− mice also displayed delayed resolution of recruited neutrophils and accumulation of apoptotic neutrophils [23]. The observation that the G2A−/− mouse develops late onset autoimmunity similar to systemic lupus erythymetosus (SLE) supports the hypothesis that dysregulation of the timely removal of recruited inflammatory cells contributes to autoimmunity [41].
3.2. Other possible receptors mediating lysoPS signaling
To understand the mechanism by which lysoPS enhanced mast cell histamine release, Sugo et al., seeking the identity of a lysoPS receptor, identified the orphan receptor GPR34, highly expressed on mast cells [42]. Subsequently, however, GPR34−/− mice showed no defect in mast cell histamine release in response to lysoPS [43] suggesting that while GPR34 may be able to mediate lysoPS signaling in some circumstances, it does not appear to be required or other receptor(s) exist to perform this function as well. One likely receptor to consider would be G2A, but as yet, G2A deficient mast cells have not been examined to determine whether they respond to lysoPS for enhanced histamine release or PGD2 production.
The TLR2 receptor has also been shown to mediate lysoPS-dependent dendritic cell polarization during schistosome infection [44]. During infection, schistosomes generate a unique lysoPS species containing a 20:1 fatty acid esterified to the sn-2 position resulting in a 1-lyso-2-acyl-PS. This molecule, but not 1-acyl-2-lyso-PS or diacylPS, appeared to specifically signal through TLR2 on human monocyte-derived dendritic cells resulting in differential polarization. Dendritic cells matured in the presence of the schistosome lysoPS stimulated T cells toward IL-10 production in contrast to dendritic cells matured in the presence of schistosome diacylPS (in the absence of lysoPS) which stimulated T cells toward TGFβ production. The mechanism for this specificity remains unknown but may contribute to schistosome evasion of the host immune response. In our investigation, TLR2 was not required for lysoPS-mediated enhanced efferocytosis by macrophages (unpublished observations).
4. Altered signaling by mode of presentation
Importantly, downstream signaling of lysoPS and other lysoPLs also appears to depend on the mode of presentation. Lacking one fatty acid chain, lysoPLs can exist in plasma (or serum) bound to proteins such as albumin. Accordingly, it has been shown that lysoPS can signal to cells, e.g. for calcium mobilization, when presented on albumin or solubilized in small concentrations of methanol [35]. However, when presented in this manner, lysoPS was unable to enhance efferocytosis [23]. To enhance efferocytosis, lysoPS required presentation in a membrane on the neutrophil or on a liposome (Fig. 2). Differences in signaling based on soluble or surface presentation are not fully understood. Presentation on a membrane may alter the way the molecule is recognized, but may also allow engagement or clustering of other ligands and corresponding macrophage receptors resulting in varying downstream effects (Fig. 1C). LysoPS detected in the murine peritonitis model was always cell associated and it has rarely been found as a soluble lipid in vivo [22]. The phosphoserine headgroup, being less hydrated and therefore less bulky than the phosphocholine headgroup, may facilitate membrane association of lysoPS. In contrast, the hydrated and bulky headgroup of lysoPC may promote its dissociation from the membrane to albumin or other lipoproteins in serum and inflammatory exudates [25]. LysoPC was unable to enhance efferocytosis regardless of whether it was in a liposome or on albumin [23], perhaps because its bulky headgroup prevents it from remaining in the liposome (or membrane surface) or alternatively, it is unable to engage or cluster other ligand/receptors necessary for enhanced efferocytosis. These differences may account for some of the complex signaling, overlapping in some systems and divergent in others, observed by these structurally similar lipids.
Figure 2.
Equivalent amounts of lysoPS enhanced efferocytosis when presented in liposomes but not when presented on albumin. Resident peritoneal MΦ were co-cultured with lysoPS negative targets (UV-irradiated apoptotic PMN) in the absence or presence of liposomes containing 30 mole % lysoPS, or 30 nmoles lysoPS presented on 0.05% BSA and efferocytosis was determined after 1 hour. LysoPC does not enhance efferocytosis regardless of presentation in a liposome (30 mole%) or on albumin (30 nmoles on 0.05% BSA). This figure has been adapted from the original published figure in Journal of Biological Chemistry. Frasch SC, Fernandez-Boyanapalli RF, Berry KZ, Leslie CC, Bonventre JV, Murphy RC, Henson PM, Bratton DL. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J. Biol. Chem. 2011; 286:12108-22. © the American Society for Biochemistry and Molecular Biology.
5. Generation, accumulation and recycling of lysoPS
Production of lysoPS has not been well studied in cells other than neutrophils and platelets where it appears to be generated by oxidant and phospholipase mediated mechanisms, respectively [22, 45]. However, given the ubiquitous presence of oxidases and phospholipases, it is likely that lysoPS may be generated in other cells and have signaling roles in other contexts of cell activation or apoptosis. Furthermore, there may be rapid interconversion between diacylPS, oxPS and lysoPS (Fig. 3). Both known and potential pathways for its production and catabolism are discussed in sections 5.1–5.3 below. A further caveat requires mention: given the highly anionic and amphipathic nature of lysoPS, its isolation and detection have proven to be difficult. LysoPS has been measured by thin layer chromatography [46], LC/MS/MS and multiple reaction monitoring in negative ion mode [22, 47] and several limitations for its detection exist. First, as stated in section 2.2 the diacylPS precursor pool represents a small proportion of the total membrane phospholipid (2–10%; [27]) and the amount of lysoPS generated and accumulated at any given time is estimated to be 20% of total plasma membrane diacylPS (approximated 0.34 nmol/mg protein) in optimally stimulated neutrophils [22]. Therefore, large quantities of starting material are required for detection. Also, subsequent quantitative analysis requires further separation from the more abundant phospholipid species (e.g. diacyl- and lysoPC and diacyl- and lysoPE) to avoid ion suppression during LC/MS/MS. Furthermore, the anionic phosphoserine headgroup also creates challenges with separation by liquid chromatography, and we and others have attempted to optimize this [22, 48]. Given the unique behavior of the phosphoserine headgroup coupled with the amphipathic nature of the monoacyl form, recovery following extraction has been estimated to be at best 50%. This equates to detectable lysoPS being less than 0.5% of the total plasma membrane lipid content thereby ultimately testing the limits of detection by any method. Therefore, given the difficulty in extracting and detecting phosphatidylserine species (diacylPS, oxPS and lysoPS), progress in identifying and quantifying these products at any given time during cell activation or apoptosis has been limited.
5.1. Phospholipases
A Phosphatidylserine-specific PLA1 (PS-PLA1), hydrolyzing the fatty acid from the sn-1 position of diacylPS (E.C.3.1.1.32; reviewed in Richmond [49]) and secreted from platelets, has been identified for the production of lysoPS [50, 51]. The PS-PLA1 was shown to preferentially hydrolyze diacylPS while having very little activity on diacylPC, diacylPE, diacylPA or diacylPI. While this enzyme can act directly on the platelet membrane generating 0.33 nmol/mg protein, it has also been shown to be functional on other membranes where diacylPS is exposed on the outer leaflet, such as activated mast cells or other activated or apoptotic cells [45].
The PLA2 enzymes (reviewed in [52, 53]), of which there are 22 distinct proteins, are grouped together according to their enzymatic activity, similarity in protein structure, catalytic mechanism, requirement for calcium and whether they are cytosolic or secreted. As potential sources of lysoPS, candidates from each of the major groups are considered. Bone marrow derived macrophages activated with zymosan generated lysoPS (0.25 nmol/mg protein) over the course of 120 minutes of stimulation, with peak amounts observed at 30 minutes, of which half appeared to depend on the activity of cPLA2 (based on comparisons from wild type vs. cPLA2 knockout macrophages; Frasch SC, Leslie CC, Bratton DL, unpublished observations). Given the substrate specificity of this enzyme for arachidonic acid esterified in the sn-2 position, the availability of arachidonate containing diacylPS would be expected to determine the degree to which cPLA2 activity contributes to the generation of lysoPS in a given cell type. For instance, in neutrophils, the fatty acid repertoire is fairly limited to saturated and monounsaturated species containing very little arachidonic acid, unlike that found in macrophages where there is a higher proportion of polyunsaturated fatty acids including arachidonate [22]. Therefore, cPLA2 is an unlikely mechanism for lysoPS generation in neutrophils, but may play a role in lysoPS generation in other cells.
Other PLA2s deserve mention as potential candidate enzymes involved in lysoPS generation. The calcium independent PLA2 (iPLA2) [53], often activated in apoptosis, cleaves all classes of diacylPL including diacylPS and has a less restricted substrate requirement for fatty acid chain length/saturation than cPLA2. It has been shown to be responsible for lysoPC generation in apoptosing MCF7 cells [54] but to date, has not been investigated for lysoPS production. Whether lysoPS production is the result of a two-step process with an oxPS intermediate (see section 5.2) followed by the activity of a ‘clean-up’ PLA2 such as PAF-AH II, which requires an oxidized sn-2 group but has no headgroup specificity [55], is unknown. Several secreted PLA2 enzymes demonstrate a requirement for anionic phospholipids for membrane binding, such as sPLA2-IIA, IB and V and would all be candidate enzymes for lysoPS production. sPLA2-IIA has been shown to use diacylPS as a substrate, however it is apparently much less efficient than the PS-PLA1 [45, 56].
5.2. Oxidant-mediated production of lysoPS
We have recently shown that lysoPS is generated in a manner requiring the NADPH oxidase in human and murine neutrophils [22]. LysoPS species, specifically 1-oleoyl-2-lyso-PS and 1-stearoyl-2-lyso-PS, were readily detected following in vitro stimulation of the NADPH oxidase with phorbol myristate acetate (PMA), opsonized zymosan or induction of apoptosis by aging. LysoPS production was inhibited in the presence of the NADPH oxidase inhibitor diphenylene iodonium (DPI). Stimuli that did not activate the NADPH oxidase (fMLP stimulation or apoptosis induced by UV-irradiation) did not increase lysoPS production [22]. Similarly, during the course of zymosan-induced murine peritonitis, increasing amounts of lysoPS were detected associated with recruited neutrophils. Requirement for the NADPH oxidase was further demonstrated in mice lacking a functional NADPH oxidase (gp91phox−/−) where no increase in lysoPS was evident during the course of inflammation.
Precisely how NADPH oxidase derived oxidants contribute to generation of lysoPS remains unknown but two possibilities exist. First, neutrophils produce many reactive oxygen species (ROS) such as superoxide anion from the action of the NADPH oxidase in a reaction that converts O2 → O2−• which can be dismutated to H2O2 via superoxide dismutase. H2O2 can then be converted to hypochlorous acid (HOCl) by myeloperoxidase (MPO). We have detected lysoPS accumulation in both H2O2 treated neutrophils and stimulated MPO−/− neutrophils, suggesting that H2O2 may be all that is required (unpublished observations). As mentioned, oxPS intermediates, including hydroperoxides or chain shortened fatty acids, can be formed in the presence of these ROS and are substrates for PLA2 such as PAF-AH. In this case, lysoPS would result following the removal of the modified fatty acid from the sn-2 position. Alternatively, and more speculative, it has been shown that lysoPC can be generated from diacylPC directly from HOCl in an enzyme-independent manner by a mechanism involving spontaneous deacylation of an oxPC intermediate [57, 58]. While spontaneous deacylation has not yet been demonstrated for diacylPS species, given the robust production of ROS including HOCl in recruited and activated neutrophils, this remains a viable possibility. Notably, oxPS species have not been detected in activated or aged neutrophils in our investigations suggesting that these species, if generated, are rapidly converted or recycled.
5.3. Acyltransferases and lysophospholipases
On the flip side of production, there are acyltransferases for lysoPL recycling and degradative enzymes limiting the availability of the bioactive lipid. Several acyltransferases have been identified [59], and two have been shown to utilize lysoPS as a substrate, membrane bound O-acyltransferase (MBOAT) 1 and MBOAT 5 [34, 60, 61]. We hypothesized that decreased acyltransferase activity in activated or apoptotic neutrophils may contribute to the accumulation of lysoPS. To this end, microsomes from opsonized zymosan stimulated neutrophils were analyzed for lysoPS reacylation by either of these acyltransferases. Increased, rather than decreased acyltransferase activity by MBOAT 1 and MBOAT 5 was demonstrated (Frasch SC, Gijon M, Murphy RC, Bratton DL, unpublished observations) suggesting that diminished acyltransferase activity is an unlikely mechanism for lysoPS accumulation in activated neutrophils. Since diacylPS represents a quantitatively small proportion of the total membrane phospholipid, but clearly subserves multiple signaling functions depending on species (Table 1), reacylation of lysoPS may also function to maintain diacylPS in the membrane necessary for other signaling events. LysoPS accumulation may also be terminated by further degradation of the lysoPL by the action of a lysophospholipase (Fig. 3); for instance, PS-PLA1 reportedly is also capable of lysophospholipase activity specifically cleaving lysoPS [51]. Mechanisms likely exist to limit accumulation of lysoPS and lysoPLs in general; the amount of lysoPL present in quiescent cells (1–2% of phospholipids) is tightly controlled and likely required to maintain membrane integrity [62]. Given the lytic nature of lysoPLs, we speculate that lysoPS or other lysoPLs may result in the dissolution of membranes such as that seen during neutrophil extracellular trap (NET) formation [63, 64].
6. Initiating and resolving roles of lysoPS in inflammation
Based on what is known, it is hypothesized that lysoPS plays roles both in the initiation of acute inflammatory responses and in resolution. At sites of vascular injury, platelets are activated, aggregate and initiate the clotting response. Activated platelets degranulate and secrete a multitude of mediators including PS-PLA1 which acts on surface exposed diacylPS generating lysoPS [51]. Both diacylPS and lysoPS present on the surface of platelets support the assembly of the prothrombinase complex necessary for clotting [65]. LysoPS on the platelet surface can also interact with mast cells associated with the vasculature, enhancing localized histamine release [66]. Histamine, in turn, acts as an early activator causing vasodilation, extravasation of plasma, and upregulation of adhesion molecules on endothelium (e.g. P-selectin) that assist in the initial recruitment of inflammatory cells, such as neutrophils, for sterilization of the site [67, 68]. In our investigations, lysoPS has always been found associated with cells and not as a free lysoPL in the supernatant [22, 66, 69]. This strict cell association is hypothesized to limit the response to cells in direct contact. Alternatively, release of platelet-derived microparticles or exosomes from activated cells may provide additional and dispersible sources of membrane-bound lysoPS. Finally, in a few instances, lysoPS has been shown to reside in serum or exudate fluid (though generally less than that remaining cell-associated) [45, 69]. Such lysoPS may also act in a diffusible manner exerting other effects, e.g. chemotactic for fibroblasts [17] which are necessary for providing the structural framework for wound repair.
Following tissue injury, neutrophils are robustly recruited and activated and ultimately die by apoptosis. LysoPS in/on their surface signaling via G2A on macrophages enhances their clearance. The signaling pathway results in the generation of PGE2 and enhanced cAMP production which in combination with PKA activation, are anti-inflammatory [70] (Figure 1C) and likely result in downstream macrophage programming changes. Macrophages have been shown to exhibit substantial plasticity in their programming during the course of inflammation: early in inflammation, they are “classically” or so-called M1 programmed with increased microbicidal activity and are poorly efferocytic, and as inflammation resolves, they demonstrate M2 programming, are consequently less microbicidal, but more efferocytic and restorative [71]. Signals that drive this programming switch are not clearly defined, but local production of PGE2/cAMP has been shown pivotal for the transition of macrophages from being inflammatory to restorative in peritonitis [72]. Further, other pro-resolving lipid mediators such as lipoxins, resolvins and protectins, which are metabolites of arachidonic acid and omega-3-fatty acids, appear to be generated in response to PGE2/cAMP signaling [73] and together may also participate in orchestrating the resolution of inflammation.
The biological relevance of lysoPS signaling during resolution of inflammation is suggested in mice lacking a functional NADPH oxidase (gp91phox−/−), the murine model of chronic granulomatous disease (CGD). CGD neutrophils recruited in sterile peritonitis fail to generate lysoPS, neutrophilia is prolonged, apoptotic neutrophils accumulate and production of cAMP in macrophages is diminished [22, 74, 75]. Accordingly, CGD macrophages demonstrate a persistent inflammatory phenotype and are poorly efferocytic even for normal activated or apoptotic neutrophils [74, 76]. Though all NADPH oxidase-dependent functions are deficient in this model system, it is reasonable to attribute at least some of these defects to the lack of lysoPS in CGD neutrophils. In its absence, efferocytosis is diminished, apoptotic cells accumulate and exaggerated inflammation ensues.
7. Concluding remarks
LysoPS has emerged as a bioactive lysoPL playing unique and important roles during both acute inflammation and its resolution. Signaling through the G protein-coupled receptor G2A, lysoPS may act as a pro-resolving lipid mediator involved in the transition from an inflammatory milieu to a restorative environment. Part of its uniqueness in signaling may be determined by its mode of presentation: cell associated versus solubilized in plasma or serum. Whether other receptors, aside from G2A are involved in its various roles is not clear, and its role in cooperative signaling with other ligands and receptors appears to be a recurring theme. Aside from the many questions remaining regarding its signaling, lysoPS production has not been well studied to date. However, given the multitude of potential pathways for its production involving both oxidants and phospholipases, its generation by many different cell types and in many settings is likely and warrants address. Future investigation will undoubtedly clarify the biology of this unique and only recently “rediscovered” bioactive lysoPL.
Acknowledgments
This work was supported by A1058288, HL034303 and a grant from the Chronic Granulomatous Disorder Society (U.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Courtney Frasch, Email: fraschc@njhealth.org.
Donna L. Bratton, Email: brattond@njhealth.org.
References
- 1.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–86. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 2.Kabarowski JH. G2A and LPC: regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009;89:73–81. doi: 10.1016/j.prostaglandins.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–70. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 4.Folch J. The isolation of phosphatidyl serine from brain cephalin, and identification of the serine component. Journal of Biological Chemistry. 1941;139:973–4. [Google Scholar]
- 5.Folch J, Schneider HA. An amino acid constituent of ox brain cephalin. Journal of Biological Chemistry. 1941;137:51–62. [Google Scholar]
- 6.Goth A, Adams HR, Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971;173:1034–5. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- 7.Smith GA, Hesketh TR, Plumb RW, Metcalfe JC. The exogenous lipid requirement for histamine release from rat peritoneal mast cells stimulated by concanavalin A. FEBS Lett. 1979;105:58–62. doi: 10.1016/0014-5793(79)80887-x. [DOI] [PubMed] [Google Scholar]
- 8.Bruni A, Bigon E, Battistella A, Boarato E, Mietto L, Toffano G. Lysophosphatidylserine as histamine releaser in mice and rats. Agents Actions. 1984;14:619–25. doi: 10.1007/BF01978896. [DOI] [PubMed] [Google Scholar]
- 9.Chang HW, Inoue K, Bruni A, Boarato E, Toffano G. Stereoselective effects of lysophosphatidylserine in rodents. Br J Pharmacol. 1988;93:647–53. doi: 10.1111/j.1476-5381.1988.tb10322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruni A, Toffano G. Lysophosphatidylserine, a short-lived intermediate with plasma membrane regulatory properties. Pharmacol Res Commun. 1982;14:469–84. doi: 10.1016/s0031-6989(82)80038-6. [DOI] [PubMed] [Google Scholar]
- 11.Lloret S, Moreno JJ. Ca2+ influx, phosphoinositide hydrolysis, and histamine release induced by lysophosphatidylserine in mast cells. J Cell Physiol. 1995;165:89–95. doi: 10.1002/jcp.1041650112. [DOI] [PubMed] [Google Scholar]
- 12.Bellini F, Viola G, Menegus AM, Toffano G, Bruni A. Signalling mechanism in the lysophosphatidylserine-induced activation of mouse mast cells. Biochim Biophys Acta. 1990;1052:216–20. doi: 10.1016/0167-4889(90)90079-s. [DOI] [PubMed] [Google Scholar]
- 13.Bruni A, Monastra G, Bellini F, Toffano G. Autacoid properties of lysophosphatidylserine. Prog Clin Biol Res. 1988;282:165–79. [PubMed] [Google Scholar]
- 14.Murakami M, Tada K, Nakajima K, Kudo I. Cyclooxygenase-2-dependent delayed prostaglandin D2 generation is initiated by nerve growth factor in rat peritoneal mast cells: its augmentation by extracellular type II secretory phospholipase A2. J Immunol. 1997;159:439–46. [PubMed] [Google Scholar]
- 15.Calderini G, Teolato S, Bonetti AC, Battistella A, Toffano G. Effect of lyso-phosphatidylserine on rat hypothalamic cAMP, in vivo. Life Sci. 1981;28:2367–75. doi: 10.1016/0024-3205(81)90502-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Lee HY, Kim SD, Jo SH, Shim JW, Lee HJ, et al. Lysophosphatidylserine stimulates chemotactic migration in U87 human glioma cells. Biochem Biophys Res Commun. 2008;374:147–51. doi: 10.1016/j.bbrc.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 17.Park KS, Lee HY, Kim MK, Shin EH, Jo SH, Kim SD, et al. Lysophosphatidylserine stimulates L2071 mouse fibroblast chemotactic migration via a process involving pertussis toxin-sensitive trimeric G-proteins. Mol Pharmacol. 2006;69:1066–73. doi: 10.1124/mol.105.018960. [DOI] [PubMed] [Google Scholar]
- 18.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, et al. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 19.Ojala PJ, Hirvonen TE, Hermansson M, Somerharju P, Parkkinen J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J Leukoc Biol. 2007;82:1501–9. doi: 10.1189/jlb.0507292. [DOI] [PubMed] [Google Scholar]
- 20.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, cleanup begins. Trends Immunol. 2011 doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–17. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 22.Frasch SC, Berry KZ, Fernandez-Boyanapalli R, Jin HS, Leslie C, Henson PM, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283:33736–49. doi: 10.1074/jbc.M807047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frasch SC, Fernandez-Boyanapalli RF, Berry KZ, Leslie CC, Bonventre JV, Murphy RC, et al. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J Biol Chem. 2011;286:12108–22. doi: 10.1074/jbc.M110.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott JI, Sardini A, Cooper JC, Alexander DR, Davanture S, Chimini G, et al. Phosphatidylserine exposure in B lymphocytes: a role for lipid packing. Blood. 2006;108:1611–7. doi: 10.1182/blood-2005-11-012328. [DOI] [PubMed] [Google Scholar]
- 25.Lundbaek JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994;104:645–73. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–25. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–87. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–62. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 29.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625–33. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 30.Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584:2724–30. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–42. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–32. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 33.Paterson JK, Renkema K, Burden L, Halleck MS, Schlegel RA, Williamson P, et al. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–76. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- 34.Riekhof WR, Wu J, Gijon MA, Zarini S, Murphy RC, Voelker DR. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J Biol Chem. 2007;282:36853–61. doi: 10.1074/jbc.M706718200. [DOI] [PubMed] [Google Scholar]
- 35.Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 2007;178:6540–8. doi: 10.4049/jimmunol.178.10.6540. [DOI] [PubMed] [Google Scholar]
- 36.Bolick DT, Whetzel AM, Skaflen M, Deem TL, Lee J, Hedrick CC. Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ Res. 2007;100:572–80. doi: 10.1161/01.RES.0000258877.57836.d2. [DOI] [PubMed] [Google Scholar]
- 37.Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–5. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 38.Witte ON, Kabarowski JH, Xu Y, Le LQ, Zhu K. Retraction. Science. 2005;307:206. doi: 10.1126/science.307.5707.206b. [DOI] [PubMed] [Google Scholar]
- 39.Obinata H, Izumi T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. 2009;89:66–72. doi: 10.1016/j.prostaglandins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell. 2005;16:2234–47. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le LQ, Kabarowski JH, Weng Z, Satterthwaite AB, Harvill ET, Jensen ER, et al. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity. 2001;14:561–71. doi: 10.1016/s1074-7613(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 42.Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341:1078–87. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 43.Liebscher I, Muller U, Teupser D, Engemaier E, Engel KM, Ritscher L, et al. Altered immune response in mice deficient for the G protein-coupled receptor GPR34. J Biol Chem. 2011;286:2101–10. doi: 10.1074/jbc.M110.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 45.Hosono H, Aoki J, Nagai Y, Bandoh K, Ishida M, Taguchi R, et al. Phosphatidylserine-specific phospholipase A1 stimulates histamine release from rat peritoneal mast cells through production of 2-acyl-1-lysophosphatidylserine. J Biol Chem. 2001;276:29664–70. doi: 10.1074/jbc.M104597200. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama K, Shimizu F, Setaka M. Simultaneous separation of lysophospholipids from the total lipid fraction of crude biological samples using two-dimensional thin-layer chromatography. J Lipid Res. 2000;41:142–7. [PubMed] [Google Scholar]
- 47.Lee JY, Min HK, Moon MH. Simultaneous profiling of lysophospholipids and phospholipids from human plasma by nanoflow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;400:2953–61. doi: 10.1007/s00216-011-4958-7. [DOI] [PubMed] [Google Scholar]
- 48.Ogiso H, Suzuki T, Taguchi R. Development of a reverse-phase liquid chromatography electrospray ionization mass spectrometry method for lipidomics, improving detection of phosphatidic acid and phosphatidylserine. Anal Biochem. 2008;375:124–31. doi: 10.1016/j.ab.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Richmond GS, Smith TK. Phospholipases a(1) Int J Mol Sci. 2011;12:588–612. doi: 10.3390/ijms12010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 2007;89:197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, et al. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem. 1997;272:2192–8. doi: 10.1074/jbc.272.4.2192. [DOI] [PubMed] [Google Scholar]
- 52.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50 (Suppl):S237–42. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–92. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 55.McIntyre TM, Prescott SM, Stafforini DM. The emerging roles of PAF acetylhydrolase. J Lipid Res. 2009;50 (Suppl):S255–9. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami M, Hara N, Kudo I, Inoue K. Triggering of degranulation in mast cells by exogenous type II phospholipase A2. J Immunol. 1993;151:5675–84. [PubMed] [Google Scholar]
- 57.Choi J, Zhang W, Gu X, Chen X, Hong L, Laird JM, et al. Lysophosphatidylcholine is generated by spontaneous deacylation of oxidized phospholipids. Chem Res Toxicol. 2011;24:111–8. doi: 10.1021/tx100305b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panasenko OM, Spalteholz H, Schiller J, Arnhold J. Leukocytic myeloperoxidase-mediated formation of bromohydrins and lysophospholipids from unsaturated phosphatidylcholines. Biochemistry (Mosc) 2006;71:571–80. doi: 10.1134/s0006297906050178. [DOI] [PubMed] [Google Scholar]
- 59.Jackson SK, Abate W, Tonks AJ. Lysophospholipid acyltransferases: novel potential regulators of the inflammatory response and target for new drug discovery. Pharmacol Ther. 2008;119:104–14. doi: 10.1016/j.pharmthera.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Gijon MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem. 2008;283:30235–45. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc Natl Acad Sci U S A. 2008;105:2830–5. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson HA, Waldrip JB, Nielson KH, Judd AM, Han SK, Cho W, et al. Mechanisms by which elevated intracellular calcium induces S49 cell membranes to become susceptible to the action of secretory phospholipase A2. J Biol Chem. 1999;274:11494–504. doi: 10.1074/jbc.274.17.11494. [DOI] [PubMed] [Google Scholar]
- 63.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone MD, Nelsestuen GL. Efficacy of soluble phospholipids in the prothrombinase reaction. Biochemistry. 2005;44:4037–41. doi: 10.1021/bi047655n. [DOI] [PubMed] [Google Scholar]
- 66.Kawamoto K, Aoki J, Tanaka A, Itakura A, Hosono H, Arai H, et al. Nerve growth factor activates mast cells through the collaborative interaction with lysophosphatidylserine expressed on the membrane surface of activated platelets. J Immunol. 2002;168:6412–9. doi: 10.4049/jimmunol.168.12.6412. [DOI] [PubMed] [Google Scholar]
- 67.Bachert C. Histamine--a major role in allergy? Clin Exp Allergy. 1998;28 (Suppl 6):15–9. doi: 10.1046/j.1365-2222.1998.0280s6015.x. [DOI] [PubMed] [Google Scholar]
- 68.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Mietto L, Boarato E, Toffano G, Bruni A. Lysophosphatidylserine-dependent interaction between rat leukocytes and mast cells. Biochim Biophys Acta. 1987;930:145–53. doi: 10.1016/0167-4889(87)90026-7. [DOI] [PubMed] [Google Scholar]
- 70.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–32. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 72.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–27. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–55. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajakariar R, Newson J, Jackson EK, Sawmynaden P, Smith A, Rahman F, et al. Nonresolving inflammation in gp91phox−/− mice, a model of human chronic granulomatous disease, has lower adenosine and cyclic adenosine 5′-monophosphate. J Immunol. 2009;182:3262–9. doi: 10.4049/jimmunol.0801739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez-Boyanapalli R, Frasch SC, Riches DW, Vandivier RW, Henson PM, Bratton DL. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116:4512–22. doi: 10.1182/blood-2010-02-272005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Legradi A, Chitu V, Szukacsov V, Fajka-Boja R, Szekely Szucs K, Monostori E. Lysophosphatidylcholine is a regulator of tyrosine kinase activity and intracellular Ca(2+) level in Jurkat T cell line. Immunol Lett. 2004;91:17–21. doi: 10.1016/j.imlet.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Ginsburg I, Ward PA, Varani J. Lysophosphatides enhance superoxide responses of stimulated human neutrophils. Inflammation. 1989;13:163–74. doi: 10.1007/BF00924787. [DOI] [PubMed] [Google Scholar]
- 79.Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174:2981–9. doi: 10.4049/jimmunol.174.5.2981. [DOI] [PubMed] [Google Scholar]
- 80.Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci U S A. 2004;101:245–50. doi: 10.1073/pnas.2536801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2005;105:1127–34. doi: 10.1182/blood-2004-05-1916. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y, Casey G, Mills GB. Effect of lysophospholipids on signaling in the human Jurkat T cell line. J Cell Physiol. 1995;163:441–50. doi: 10.1002/jcp.1041630303. [DOI] [PubMed] [Google Scholar]
- 83.Park KS, Lee HY, Kim MK, Shin EH, Bae YS. Lysophosphatidylserine stimulates leukemic cells but not normal leukocytes. Biochem Biophys Res Commun. 2005;333:353–8. doi: 10.1016/j.bbrc.2005.05.109. [DOI] [PubMed] [Google Scholar]
- 84.Lourenssen S, Blennerhassett MG. Lysophosphatidylserine potentiates nerve growth factor-induced differentiation of PC12 cells. Neurosci Lett. 1998;248:77–80. doi: 10.1016/s0304-3940(98)00275-4. [DOI] [PubMed] [Google Scholar]
- 85.Park KS, Lee HY, Lee SY, Kim MK, Kim SD, Kim JM, et al. Lysophosphatidylethanolamine stimulates chemotactic migration and cellular invasion in SK-OV3 human ovarian cancer cells: involvement of pertussis toxin-sensitive G-protein coupled receptor. FEBS Lett. 2007;581:4411–6. doi: 10.1016/j.febslet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 86.He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–64. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henson P, Bratton D. Recognition and removal of apoptotic cells. In: Russell D, Gordon S, editors. Phagocyte-pathogen interactions. Washington, D.C: ASM Press; 2009. pp. 341–65. [Google Scholar]