Abstract
TCR specific antibodies may modulate the TCR engagement with antigen-MHC complexes, and in turn regulate in vivo T-cell responses to allo-antigens. Herein, we found that in vivo administration of mAbs specific for mouse TCRβ (H57-597), TCRα, or CD3 promptly reduced the number of CD4+ and CD8+ T-cells in normal mice, but H57-597 mAb most potently increased the frequency of CD4+Foxp3+ Treg cells. When mice were injected with staphylococcal enterotoxin B (SEB) superantigen and H57-597 mAb, the expansion of SEB-reactive Vβ8+ T-cells was completely abrogated while SEB-non-reactive Vβ2+ T-cells remained unaffected. More importantly, transient H57-597 mAb treatment exerted long-lasting effect in preventing T-cell responses to allo-antigens, and produced long-term cardiac allograft survival (>100 days) in 10 out of 11 recipients. While Treg cells were involved in maintaining donor-specific long-term graft survival, T-cell homeostasis recovered over time and immunity was retained against third party allografts. Moreover, transient H57-597 mAb treatment significantly prolonged survival of skin allografts in naïve recipients as well as heart allografts in skin-sensitized recipients. Thus, transient modulation of the TCRβ chain by H57-597 mAb exhibits potent, long-lasting therapeutic effects to control allo-immune responses.
Keywords: tolerance, T-cell receptor, T regulatory cells, allograft survival
INTRODUCTION
The TCR that is made up of TCRα and TCRβ chains has an exquisite specificity for its peptide antigen presented by MHC expressed on antigen presenting cells (APCs). Engagement of the TCR by peptide/MHC ligand controls T-cell generation and function. In transplantation, an acute rejection is mainly mediated by direct recognition of donor allo-MHC on “passenger APCs,” while chronic rejection is mediated by the processed allopeptides presented by the recipient’s self-MHC. Great efforts have been devoted to study the peptide-MHC/TCR interaction [1] and associated fates of T-cells during clonal expansion followed by clonal contraction [2–4]. Moreover, manipulation of TCR engagement by modification of peptide/antigen or TCR engineering has been explored for potential therapeutic applications [5–7].
Binding of surface receptors by mAbs may result in the depletion of cells or in the agonistic/antagonistic effects mimicking/blocking the action of the receptor’s natural ligands [8]. The first mAb approved for clinical application was mouse anti-human CD3 agonist (OKT3) mAb, which targets the CD3ε chain of the TCR/CD3 complex [9;10]. Initially, OKT3 mAb was used in organ transplantation as an effective agent to prevent or reverse acute rejection. Nevertheless, OKT3 mAb was shown to be a potent mitogenic agent for T-cells; almost immediately after administration, OKT3 mAb elicited cytokine release syndrome with symptoms ranging in severity from mild to life threatening [11]. To reduce such side effects, the FcR-non-binding humanized anti-CD3 mAb (e.g. teplizumab or otelixizumab) was developed [12]. Because the Protégé Encore phase III clinical study with teplizumab was suspended for the lack of sufficient efficacy for type 1 diabetes and other clinical trials with anti-CD3 mAb are still underway [13;14], its long-term therapeutic effects remain obscure. Thus, safer and more effective methods to modulate the TCR signal are still needed for induction of tolerance.
In 1994, we applied a mouse anti-human TCR mAb (BMA 031) as an induction therapy for kidney transplant patients. Transient administration of BMA 031 mAb improved kidney allograft survival, and none of the treated patients showed even moderately adverse effects as seen in OKT3 mAb-treated patients [15]. Other groups also showed that a different anti-human TCR mAb (T10B9) provided prevention and treatment for allograft rejection as effective as that of OKT3 mAb with fewer untoward effects, namely less cytokine release and fewer serious infections [16–18]. We and other groups confirmed the effectiveness of anti-TCR mAbs in preventing skin allograft rejection [19], graft-versus-host disease [20], and in the synergistic interaction with cyclosporine to prolong rat heart allograft survivals [21]. Recent clinical reports re-emphasized the importance of using antibodies for induction therapies not only to prevent initial acute rejection but also to promote long-term allograft survival [22]. The aim of this study was to determine the therapeutic effects and underlying mechanisms of TCR-specific mAb in models of organ allograft transplantation.
MATERIALS AND METHODS
Mice
BALB/c, C57BL/6 (B6), C3H, C57BL/6-Tg(BCL2)25Wehi/J (Bcl-2 Tg), B6.Cg-FoxP3tm2Tch/J (FoxP3/GFP), and B6.129S7-Rag1tm1Mom/J (Rag1−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.SJL mice were obtained from Taconic Farms, Inc. (Hudson, NY). Animals were maintained at the University of Toledo specific pathogen-free animal facility according to institutional guidelines.
Reagents and software
All fluorescence-conjugated mAbs were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA). Purified anti-CD3 (145-2C11) and anti-TCR (H57-597) mAbs were obtained from eBioscience or Bio-X-Cell (West Lebanon, NH). Purified anti-TCRα (H28-710) and anti-mouse Thymoglobulin (ATG) were obtained from Santa-Cruz Biotechnology (SC, CA) and Fitzgerald Industries (North Acton, MA), respectively. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Invitrogen (Carlsbad, CA). Murine rIL-2 was purchased from Peprotech (Rocky Hill, NJ). Staphylococcal enterotoxin B (SEB) was obtained from Sigma-Aldrich (St. Louis, MO). For data generated from flow cytometry, the analysis was performed using FlowJo and WinMdi software.
Cell preparation and cultures
Single cell suspensions from spleens of 8–10 week old B6, FoxP3/GFP, or B6-SJL mice (2.0 × 105 cells/well) were cultured in 96-well round-bottom plates with or without appropriate stimulations as described in the text. For measuring cell proliferation, cell cultures were labeled with 1 μCi/well [H3]-thymidine during the final 18 hrs and incorporated radioactivity was determined by a microplate scintillation counter (Packard, Ramsey, MN).
In vivo response to SEB
6–8 wks old B6 mice were injected i.v. with 150 μg SEB alone or co-injected i.p. once with 1 mg/kg of H57-597 mAb. Single cell suspensions from spleens and lymph nodes of controls and treated groups were examined at days 0, 3, 6 and 10, and analyzed for frequencies and total numbers of Vβ8+ and Vβ2+ cells within CD4 and CD8 T-cell populations by flow cytometric analysis.
Heart transplantation and Histology
Heart transplantation was performed by a previously described method [23]. In brief, hearts from either C3H (H-2k) or Balb/c donors were transplanted to B6 or Rag1−/− recipients. Heart graft survival was monitored daily by palpation, and the day of complete cessation of heartbeat was considered as the day of rejection. Allografts were sectioned and stained with Hematoxylin/Eosin for microscopic evaluation.
Detection of anti-donor IgG
Sera were harvested from recipients (3–4 per group) of heart transplants and skin transplants in some cases at different days post-transplant as indicated in the text, and were analyzed for the presence of anti-donor (Balb/c) antibody levels using a previously described method [24]. Donor-type Balb/c splenocytes were incubated with Fc blocking anti-mouse CD16/CD32 antibody (clone 2.4G2; BD Biosciences) for 20–25 minutes on ice. Next, cells were washed twice with 2% FBS in PBS followed by incubation with 1:10 dilutions of serum harvested from recipients for 45 minutes at 4° C. The cells were then washed twice and stained with FITC-conjugated anti-mouse IgG (5 μg/ml) for 20 minutes on ice. Finally, the cells were washed twice and analyzed by flow cytometry for IgG levels.
Immunohistochemistry
Heart allografts were collected from recipients for immunohistologic evaluation at different days post-transplant, as indicated in the text. Samples (two to four per group) were snap frozen, and cryostat sections were stained using mAbs to murine CD3, CD4, CD8, CD11b, CD22 or isotype-matched control mAbs (BD Pharmingen), as well as mAb to murine Foxp3 (eBioscience). C4d deposition was assessed using a rat anti-mouse C4 mAb (Novus Biologicals), which recognizes C4, C4b, and C4d complement components. Primary Abs were localized using an Envision kit (DakoCytomation).
Skin Transplantation
Skin transplantation was performed as described previously [25]. Briefly, ear skin (1.0 cm2) from the donor mice (8–12 weeks old) was grafted onto the flank of anesthetized recipient mice. The graft was covered with a sterile bandage, which was removed on day 6–7. Grafts were scored daily until rejection (defined as 80% of grafted tissue becoming necrotic and reduced in size).
Mixed lymphocyte reaction
Splenocytes from heart graft recipient mice as well as B6 naïve mice were used as responders, while irradiated splenocytes from donor Balb/c, B6 and C3H mice were used as stimulators. 3 × 105 responder cells were cultured with 3 × 105 irradiated (15 Gy) stimulator cells supplemented with 10 IU/mL IL2 in a 96-well culture plate for 72 hours. Cellular proliferation was measured using [H3] thymidine incorporation as described before.
Statistical Analysis
The results of the graft survival data were analyzed by the Mann Whitney test. All other statistics were evaluated using the unpaired Student’s t-test method to document statistical significance. The p values of < 0.05 were considered as statistically significant.
RESULTS
H57-597 mAb enriches FoxP3-expressing Treg cells and diminishes antigen-reactive T-cells in vivo
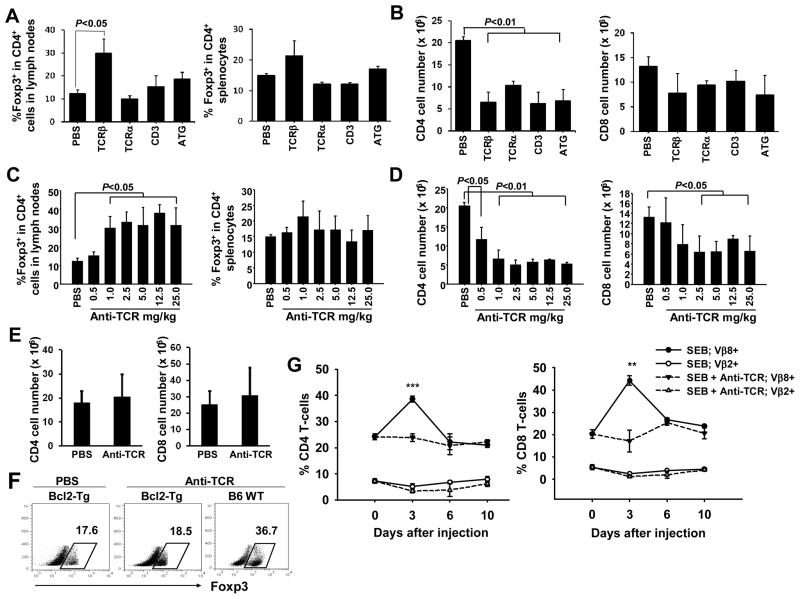
To investigate the in vivo immune regulatory effects of targeting TCR/CD3 complex by various mAbs, we assessed B6 mice 5 days after injection with PBS, anti-TCRα mAb (H28-710), anti-TCRβ mAb (H57-597), anti-CD3 mAb (145-2C11), or ATG. While H57-597 mAb most potently increased the frequencies of Treg cells in the secondary lymphoid organs, all tested antibodies reduced the T-cell numbers (Fig. 1A and B). We further treated B6 mice with various doses of H57-597 mAb, and found that 1 mg/kg and higher doses of H57-597 mAb elevated the frequency of CD4+FoxP3+ Treg cells among CD4+ cells in lymph nodes by ~3-fold to 30–40% and in spleens by ~2-fold to 20% (Fig. 1C). Because H57-597 mAb reduced the numbers of CD4+ cells by ~60% and CD8+ cells by ~40% (Fig. 1D), it is possible that the Treg enrichment resulted from the anti-TCRβ mAb-induced death of conventional T-cells but not Treg cells. To test this possibility, we used Bcl-2 Tg mice in which conventional T-cells are resistant to apoptosis [26]. In contrast to the WT B6 mice, H57-597 mAb failed to reduce T-cell numbers (Fig. 1E) and consequently did not increase the frequency of Treg cells in Bcl-2 Tg mice (Fig. 1F left two panels). Thus, Treg cells in B6 mice were relatively resistant to H57-597 mAb-induced death compared to conventional T-cells. A gradual recovery of the T-cell homeostasis in H57-597 mAb-treated B6 mice was observed within 40–100 days (Fig. S1). Consistent with the in vivo finding of Treg cell enrichment, H57-597 mAb (but not its isotype control or 145-2C11 mAb) significantly increased the frequency of Treg cells in an in vitro assay, which was due to the actual enrichment of existing Treg cells but not the conversion of naïve CD4+ T-cells into inducible Treg cells (Fig. S2).
Figure 1. H57-597 mAb enriches Treg cells and arrests T-cell response to SEB.
(A–D) In A & B, B6 mice were injected once with either PBS, indicated mAbs at 1mg/kg, or ATG at 5mg/kg. In C & D, B6 mice were injected with increasing doses of H57-597 mAb as indicated. Five days later, cells were isolated from the secondary lymphoid organs for ex vivo analysis. In A & C, bar graphs show the frequencies of FoxP3+ cells among the CD4 cell population in lymph nodes (left panel) and spleens (right panel). In B & D, bar graphs show the number of CD4 (left panel) or CD8 (right panel) cells in spleens. (E) Bcl-2 Tg or wild type B6 mice received a single injection of 1 mg/kg H57-597 mAb or PBS. Bar graphs show the number of CD4 (left panel) or CD8 (right panel) cells in spleens of Bcl-2 Tg mice treated with PBS or anti-TCRβ mAb. (F) Dot plots show the frequencies of FoxP3+ cells among CD4 cell population in spleens of Bcl-2 Tg mice treated with PBS (left panel), Bcl-2 Tg mice treated with H57-597 mAb (middle panel), and B6 treated with H57-597 mAb (right panel) mice at day 5 after injection. (G) B6 mice were injected with either 150 μg SEB alone (solid lines) or together with 1 mg/kg H57-597 mAb (dashed lines). Splenocytes were isolated at the indicated days after injection, and the frequencies of Vβ8+ and Vβ2+ among CD4 (left panel) or CD8 (right panel) cell populations are shown (** p < 0.005 and *** p < 0.0005).
We next determined the effects of TCRβ-specific mAb during an ongoing T-cell response to antigen. B6 mice injected with 150 μg SEB were treated once with 1 mg/kg H57-597 mAb or PBS. The frequencies of SEB-reactive Vβ8+ and SEB-nonreactive Vβ2+ T-cells in the lymphoid organs were tracked on days 0, 3, 6 and 10 after SEB injection. In the PBS-treated group, the percentage of Vβ8+ (but not Vβ2+) CD4+ and CD8+ T-cells was dramatically expanded on day 3 and quickly contracted on day 6. In contrast, H57-597 mAb abrogated this dramatic surge of Vβ8+ T-cells (both frequency and total numbers) with little effect on the percentage of Vβ2+ T-cells (Fig. 1G & S3). Therefore, H57-597 mAb not only reduces the total T-cell numbers and enriches Treg frequencies, but also selectively arrests the expansion of antigen-reactive T-cells.
H57-597 mAb induces long-term heart allograft survival
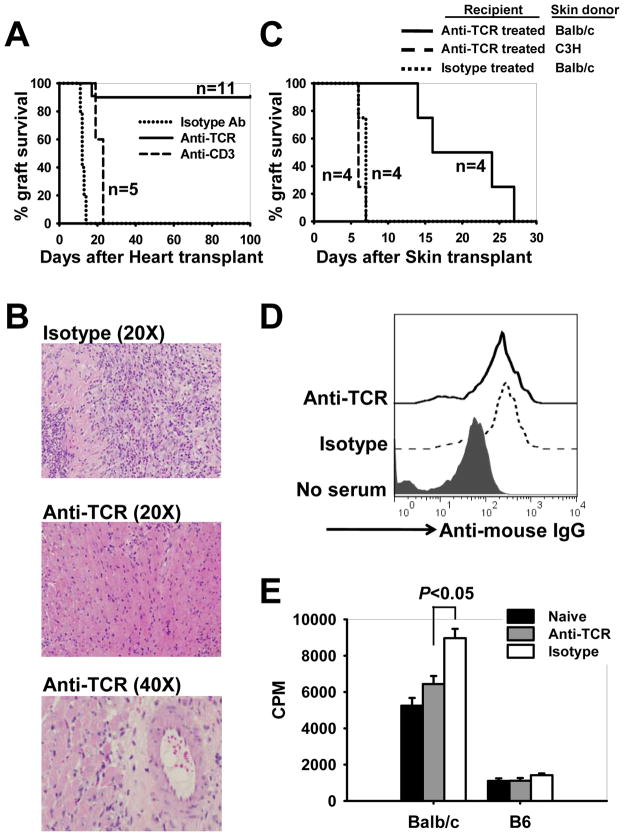
The in vivo effects of H57-597 mAb on T-cells encouraged us to investigate its potency in preventing allograft rejection by using an MHC/non-MHC-mismatched heart transplantation model. When Balb/c (H-2d) heart allografts were transplanted into isotype Ab or anti-CD3 mAb-treated B6 (H-2b) recipients, they all were acutely rejected by days 14 and 22, respectively (Fig. 2A). Following H57-597 mAb therapy (1 mg/kg on days 0, 1, 3, 7 and 11 post-grafting), 10 out of 11 heart allografts remained beating at a mean survival time (MST) >100 days (Fig. 2A) with little graft infiltration and minimal signs of coronary artery intimal thickening (Fig. 2B).
Figure 2. Transient H57-597 mAb treatment induces long-term survival of cardiac allografts.
(A) B6 mice were transplanted with Balb/c heart grafts, and were injected with a short course of either 1 mg/kg H57-597 mAb, 145-2C11 mAb, or isotype Ab on days 0, 1, 3, 7 and 11 post-grafting. The graft survival was monitored by daily palpation. The graph shows the percentage graft survival after transplantation. (B) Representative H & E stained sections of the heart allografts from isotype Ab- (top panel: 12 days post-grafting) or H57-597 mAb-treated (middle panel: 300 days post-grafting; and bottom panel: 100 days post-grafting) recipients at the indicated magnification. (C–E) B6 mice were transplanted with Balb/c hearts and treated with either H57-597 or isotype Ab as described in A. 60–80 days later these recipients were transplanted with skin allografts from Balb/c or C3H donors. In C, the survival of skin grafts is shown in the graph, respectively. In D and E, sera and splenocytes were obtained at day 11 after Balb/c skin-grafting onto H57-597 treated long-term heart graft surviving mice (Anti-TCR group) and isotype treated heart graft rejected mice (Isotype group). In D, histograms show serum levels of anti-mouse IgG in indicated samples. In E, isolated splenocytes were stimulated with irradiated Balb/c donor splenocytes or B6 syngenic splenocytes. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation.
We further determined whether transient H57-597 mAb treatment induced an antigen-specific diminution of anti-donor responses. H57-597 mAb-treated and long-term heart graft-accepting B6 mice were transplanted with either a donor-specific Balb/c or a third party C3H skin allograft. To serve as a control group, isotype Ab-treated B6 mice, which rejected their Balb/c heart allografts, also received Balb/c skin grafts. As shown in Figure 2C, Balb/c skin allografts survived significantly longer in Balb/c heart graft-accepting mice than in mice which rejected their Balb/c heart allografts. Importantly, heart allograft-accepting mice maintained potent immunity to third party C3H skins (Fig. 2C). Taken together, these findings show for the first time in a murine model that a “remodeling” of the immune response by anti-TCRβ mAb protected the long-term survival of heart allografts in an antigen-specific fashion.
To determine the mechanism underlying delayed rejection of Balb/c skin allografts by Balb/c heart allograft-accepting mice, we assessed the levels of donor-reactive T-cell and donor-specific antibody reactivities. B6 recipients were transplanted with Balb/c heart allografts, and treated with H57-597 mAb (anti-TCR group) or isotype Ab (Isotype group). Sixty to Eighty days later, Balb/c skin allografts were transplanted onto these B6 recipients. Eleven days after skin transplantation, sera and splenocytes were obtained from these recipients for analysis. Sera from both groups contained similar levels of anti-donor IgG (Fig. 2D). However, upon stimulation with irradiated Balb/c stimulators, splenocytes isolated from H57-597 mAb-treated group exhibited a lower level of cell proliferation when compare to the splenocytes from isotype Ab-treated group (Fig. 2E). Therefore, anti-donor T cells remained in the periphery of H57-597 mAb-treated recipients but displayed a reduced proliferation response, which may explain the prolonged (but not long-term) Balb/c skin graft survival in heart-graft-accepting mice.
Treg cells are involved in H57-597 mAb-induced long-term heart graft survival
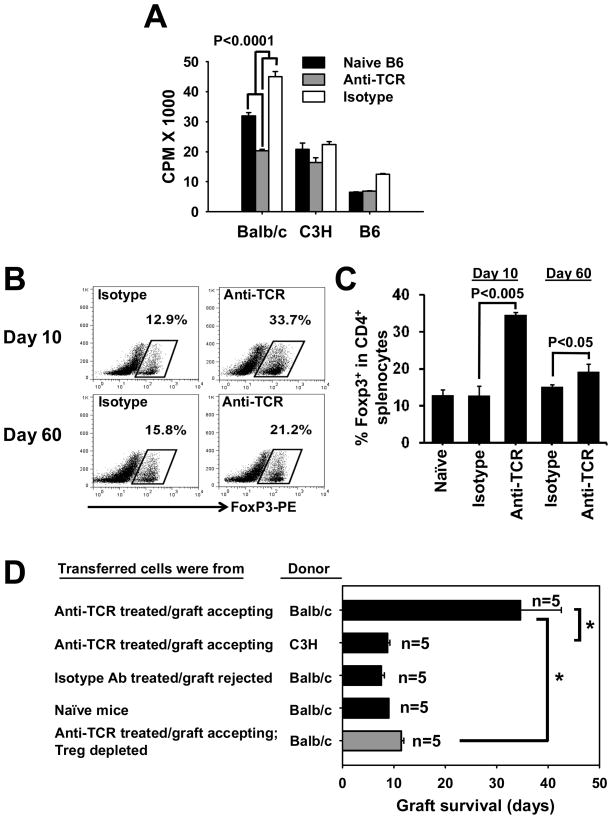
To further determine the cellular mechanisms by which H57-597 mAb prevents heart allograft rejection, we performed additional analyses of cells obtained from recipients treated with H57-597 mAb or isotype Ab. In these experiments naive mice were transplanted with Balb/c heart allografts and did not receive second grafts. Splenocytes isolated from H57-597 mAb-treated B6 recipients (~60 days post heart grafting) exhibited a reduced anti-donor cell proliferative response (to irradiated Balb/c stimulators) compared to those from the isotype Ab-treated B6 recipients or non-treated naïve B6 mice (Fig. 3A; p<0.0001). Moreover, H57-597 mAb-treatment induced a significant increase of Treg cell frequency in spleens (Fig. 3B & 3C) and lymph nodes (Fig. S4A & S4B) at days 10 and 60 in heart allograft accepting recipients.
Figure 3. Long-term heart graft survival induced by H57-597 mAb involves Treg cells.
(A) Splenocytes from H57-597 treated long-term heart graft surviving mice, isotype treated mice, or naïve B6 mice were stimulated with irradiated donor splenocytes from Balb/c, C3H or B6 mice. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. (B–C) Splenocytes were isolated from heart transplanted mice treated with H57-597 mAb or isotype Ab at Day 10 or Day 60 after the transplant. The figure shows the dot plots (B) and bar graphs (C) representative of the frequencies of FoxP3+ cells among the CD4 T-cell populations. (D) Rag1−/− mice were adoptively transferred with 3 × 107 splenocytes from naïve B6 mice, anti-TCR-treated and long-term Balb/c graft-accepting (> 100 days) B6 mice, or isotype-Ab-treated and Balb/c graft-rejected (< 15 days) B6 mice. Rag1−/− mice were then transplanted with either donor-specific Balb/c or third-party C3H hearts, the mean ± SD survival is shown in the bar graph. The grey bar shows Balb/c heart-graft survival in Rag1−/− mice injected with the CD25− (Treg depleted) fraction of splenocytes from anti-TCR treated heart graft accepting mice (* p<0.01).
To determine whether these Treg cells are involved in maintaining the hypo-responsiveness of anti-donor T-cells, Rag1−/− mice were adoptively transferred with 3 × 107 splenocytes from the same H57-597 mAb-treated graft-accepting mice, isotype Ab-treated mice which rejected skin allografts, or non-treated naïve mice, followed by transplantation with donor-specific Balb/c or third-party C3H (H-2k) hearts. Splenocytes from Balb/c allograft-accepting recipients robustly rejected third-party C3H hearts at a MST of 8.8 ± 0.4 days, but exhibited significantly delayed rejection of donor-specific Balb/c heart allografts at 34.6 ± 8 days (Fig. 3D top two bars; p<0.01). Rag1−/− mice transferred with splenocytes from naïve B6 mice or B6 mice which rejected Balb/c heart allografts all mounted rapid rejection of Balb/c heart allografts at 9.0 ± 0.0 days and 7.6 ± 0.5 days, respectively (Fig. 3D). Furthermore, when Rag1−/− mice were transferred with 3 × 107 CD25− splenocytes (Treg depleted; Fig. 3D gray bar) from Balb/c allograft-accepting recipients, they displayed a minimal prolongation of heart allograft survival to 11.4 ± 0.5 days (in contrast to the significant prolongation upon transferring whole splenocytes; Fig. 3D; gray bar vs. top black bar; p<0.01). These results demonstrate that remodeling of the immune response by transient H57-597 mAb treatment exhibited long-term therapeutic effects with donor-specific regulation, which involves Treg cells.
H57-597 mAb prolongs allograft survival in stringent transplantation models
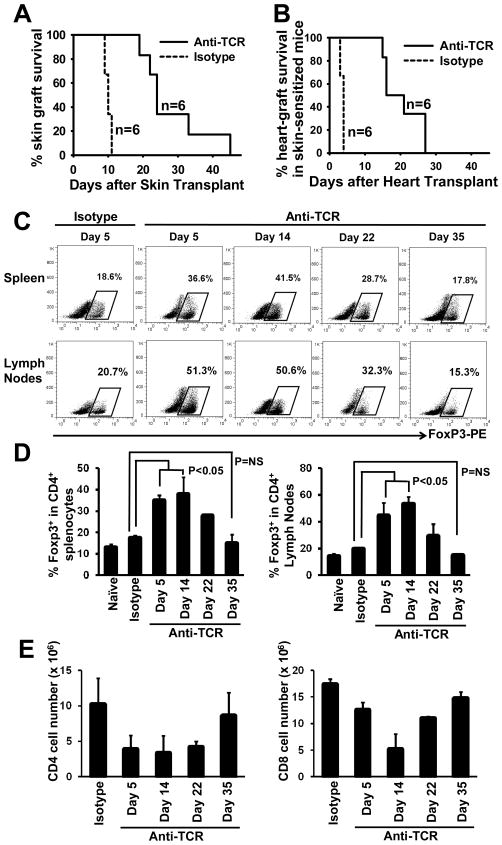
To further determine the potency of H57-597 mAb in controlling anti-allograft responses, naïve B6 mice were transplanted with Balb/c skin grafts and treated with low dose H57-597 mAb or isotype mAb (1 mg/kg on days 0, 1, 3, 7 and 11 post-grafting). H57-597 mAb prolonged the skin graft survival to 27.7 ± 9.8 days while skin allografts in recipients treated with isotype Ab were acutely rejected within 9.5 ± 0.6 days (Fig. 4A; p< 0.005).
Figure 4. H57-597 therapy for stringent transplantation models.
(A) B6 mice were transplanted with Balb/c skin grafts, and were injected with a short course of either 1 mg/kg H57-597 mAb or its isotype Ab on days 0, 1, 3, 7 and 11 post-grafting. The graph shows the percentage graft survival after transplantation. (B) B6 mice were pre-sensitized against Balb/c antigens by transplantation with Balb/c-donor skin grafts. 2-3 weeks after the skin transplantation, these recipient mice were then transplanted with Balb/c heart allografts, and injected with a short course of either 1 mg/kg H57-597 mAb or its isotype Ab on days 0, 1, 3, 7 and 11 post-heart grafting. The graph shows percentage survival of heart grafts in the sensitized mice. (C–E) Splenocytes and lymph nodes were isolated at indicated days after secondary heart transplantion from skin-sensitized, recipient mice treated with H57-597 mAb or isotype Ab. The figure shows the dot plots (C) and bar graphs (D) representative of the frequencies of FoxP3+ cells among the CD4 T-cell populations. (E) The figure shows the bar graphs representative of the number of CD4 (left panel) or CD8 (right panel) cells in spleens of the above mice.
We also assessed the protective effects of the same H57-597 mAb treatment of Balb/c heart allograft survivals in Balb/c skin transplant-sensitized recipients. This is one of the most stringent transplantation models, since donor-type skin transplantation induces donor-antigen experienced T-cells and memory T-cells that are resistant to various immune interventions (e.g. blockade of T-cell co-stimulatory signals) and induces the production of donor-specific Abs [27]. Indeed, preventing memory T-cell and antibody mediated allograft rejection are the major goals for transplant immunologists [28]. Herein, B6 mice that had previously rejected Balb/c skin allografts (2–3 weeks before) rapidly rejected all Balb/c heart allografts within 3.7 ± 0.5 days. However, transient H57-597 mAb treatment post heart grafting prolonged the heart allograft survival to 20.3 ± 5.6 days in skin-sensitized recipients (Fig. 4B; p<0.005). Thus, although H57-597 mAb significantly prolonged the heart graft survival in skin-sensitized mice, the long-term graft survival (>100 days) observed in naïve mice was not achieved.
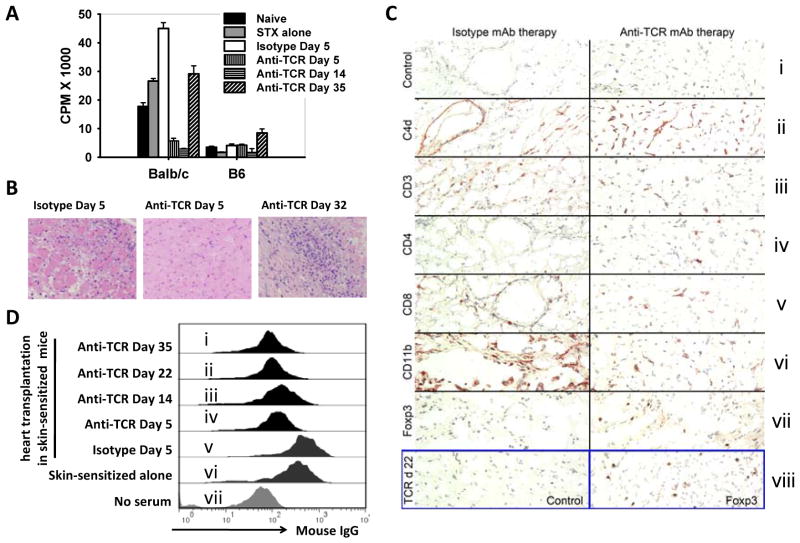
To assess the difference in mechanisms underlying heart allograft rejection in skin-sensitized recipients, we performed additional ex vivo analyses. At early time points after H57-597 mAb-treatment and heart transplantation (anti-TCR group), skin-sensitized recipients displayed an increased frequency of CD4+Foxp3+ Treg cells among the CD4+ population, but these levels promptly decreased over time to a level similar to isotype-treated recipients (Fig. 4C & 4D). In addition, the total numbers of both CD4+ and CD8+ splenocytes in the anti-TCR group also quickly recovered after heart transplantation reaching normal levels at day 35 (Fig. 4E). Moreover, splenocytes isolated from the anti-TCR group at days 5 and 14 post heart-grafting exhibited minimal proliferation against irradiated Balb/c donor splenocytes, but splenocytes from recipients at day 35 had shown significant anti-donor T-cell proliferation (Fig. 5A). These results correlated with histological analysis, as at day 5 post-grafting heart allografts in skin sensitized mice treated with H57-597 mAb showed intact myocytes with only minimal cell infiltration whereas the allografts in the isotype Ab-treated group were rejected. However, skin-sensitized recipients treated with H57-597 mAb displayed massive cell infiltration at day 32 in rejected heart allografts (Fig. 5B). Thus, the recovered anti-donor T-cell response is responsible for the rejection of heart allografts in donor antigen-experienced and anti-TCR mAb-treated recipients.
Figure 5. Recovery of anti-donor responses in skin-sensitized recipients.
Splenocytes and sera were isolated from naïve B6 mice (Naïve group), skin-sensitized B6 recipients (Skin-sensitized alone group; at 3 weeks after transplantation with Balb/c skins), or skin-sensitized B6 recipients that were further transplanted with Balb/c hearts and treated with isotype Ab (Isotype group; at 5 days after heart transplantation) or H57-597 mAb (anti-TCR group; at the indicated days after heart transplantation). (A) isolated splenocytes were stimulated with irradiated Balb/c donor splenocytes or B6 syngenic splenocytes. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. (B) Representative H & E stained sections of the Balb/c heart allografts from the isotype group or anti-TCR group at indicated days after heart transplantation into Balb/c skin-sensitized B6 recipients. (C) Immunoperoxidase staining of cryostat sections of cardiac allografts harvested at days 5 (panels i–vii) or 22 (panel viii) from sensitized mice; panels are representative of 4 allografts/group. (D) Splenocytes were isolated from Balb/c mice and incubated with 1:10 dilutions of serum harvested from the above groups. The histograms show the anti-donor antibody levels measured by flow cytometric analysis using anti-mouse IgG staining.
Immunohistologic comparison of cardiac allografts harvested from sensitized recipients at day 5 post-transplant showed comparable C4d deposition along graft endothelial cells (Fig. 5C; ii), whereas TCR mAb therapy reduced overall infiltration by CD3+ T-cells (Fig. 5C; iii) and CD11b+ macrophages and neutrophils (Fig. 5C; vi) compared to isotype control mAb therapy. The T cells in control allografts consisted almost entirely of CD8+ cells (Fig. 5C; v), whereas TCR mAb-treated recipients had allografts containing both CD4+ and CD8+ infiltrating T cells (Fig. 5C; iv & v). The latter group was also different in staining for Foxp3, a transcription factor characteristic of Treg cells; these cells were diffusely distributed in allografts from TCR-treated but not isotype control-treated mice (Fig. 5C; vii). In addition, Foxp3+ Treg cells were still detected within allografts from TCR mAb-treated mice at day 22 post-transplant (Fig. 5C; viii), despite increasing T cell and macrophage infiltration and development of end-stage cardiac allograft rejection. B cells were not a significant component of the graft infiltrate at any time (data not shown).
A significant serum level of anti-donor IgG was detected in skin-sensitized mice (Fig. 5D; vi). After heart allograft transplantation to these skin-sensitized mice, H57-597 mAb (but not isotype Ab) reduced the serum level of anti-donor IgG on days 5, 14, 22 and 35 (Fig. 5D; panels i–iv). In fact, direct parallel comparison on day 5 between the isotype Ab-treated group and the H57-597 mAb-treated group (Fig. 5D; iv & v) showed dramatic reduction of serum anti-donor IgG by anti-TCR mAb treatment. Although C4d deposition was detectable in the cardiac allografts of H57-597 mAb treated recipients at day 5 post-transplant (Fig. 5C; ii), anti-TCR mAb may have impaired Ab-mediated allograft rejection and remodeled the cellular composition of infiltrates at the allograft site.
Taken together, anti-TCR mAb treatment did not induce long-term heart allograft survival in skin sensitized hosts, again showing resistance of memory T-cell models to induction of long-term survivals even with initial re-programming of the immune response and reducing/altering cellular infiltration in allografts. We are further investigating the effects of high dose H57-597 mAb alone or in combination with other bio-reagents on graft survival in sensitized models, aiming to abrogate memory T-cell-mediated allograft rejection.
DISCUSSION
Overall, we found that transient TCR engagement with H57-597 mAb dramatically “resets” the T-cell composition, resulting in an initial reduction of conventional CD4+ and CD8+ T-cells with enrichment of CD4+FoxP3+ Treg cells. This reset period correlated with robust arrest of antigen-specific T-cell responses, as shown in SEB-treated mice. Following this reset period, long-term survival of heart allografts in B6 mice was achieved. Moreover, in heart graft-accepting recipient mice, T-cell function was intact against third-party alloantigens but remained hypo-responsive to donor alloantigens. This demonstrates that transient therapy with H57-597 mAb during initial exposure to donor alloantigens induced immune regulatory effects preferentially toward those alloantigens.
Mature T-cells in peripheral compartments are activated upon recognizing foreign- but also auto-antigen and their fate is controlled by signaling through TCR/CD3 complex as well as multiple other cell surface molecules [29-31]. Anti-CD3 mAb therapy targeting the CD3ε chain in the TCR/CD3 complex exerts agonistic effects on TCR signaling but elicits tolerogenic mechanisms such as induction of anergy, perturbation of Th1/Th2 balance, increased TGF-β production, and expansion of Treg cells [12;32]. Our work investigated the immune regulatory effects of targeting the TCRβ chain with H57-597 mAb, which has been previously shown to be effective in preventing experimental autoimmune encephalomyelitis and collagen induced arthritis in animal models [33;34]. Herein, we show that transient H57-597 mAb treatment potently arrested ongoing T-cell mediated immune responses to SEB superantigen exposure and to alloantigen exposure in heart graft recipients. Because Treg cell frequency was dramatically increased upon H57-597 mAb treatment, the Treg-mediated mechanism might be involved in the immune regulatory effects. Indeed, when Rag1−/− mice were transferred with Treg cell-depleted splenocytes from Balb/c allograft-accepting recipients, they displayed a minimal prolongation of heart graft survival, in contrast to the significant prolongation upon transferring whole splenocytes from graft-accepting recipients. Therefore, Treg cells are involved in the long-lasting immune regulatory effects of anti-TCRβ mAb.
Skin-sensitized mice contain alloantigen-experienced effector and memory T-cells, and promptly reject the subsequently transplanted heart allografts within 3 to 4 days. Transient H57-597 mAb-treatment still robustly abrogated the anti-donor T-cell response (Fig. 5A) and dramatically enriched Treg cells to delay heart graft rejection in these mice. However, the anti-donor T-cell response and Treg cell frequency quickly recovered after withdrawing the treatment, which was associated with massive cell infiltration in the heart grafts. We are continuously investigating the therapeutic effects of anti-TCR mAb in this model, by modifying the therapeutic approach as well as characterizing the cellular and molecular mechanisms underlying the alloreactive memory T-cell responses. Interestingly, H57-597 mAb-treatment reduced the serum anti-donor IgG levels in sensitized recipients. The effects of anti-TCR mAb on Ab-mediated rejection should also be investigated in future studies.
Previous clinical trials with T10B9 and BMA031 show the effectiveness of targeting the TCR using anti-TCR mAbs for treating renal and heart transplantations [15;17;18]. Our results further indicate the potency of anti-TCR mAb in preventing allograft rejection, and show that a transient treatment of anti-TCR mAb induces long-term cardiac allograft survival. Most importantly, we provide insights into the mechanisms underlying the effects of anti-TCRβ mAb therapy, including prompt and robust abrogation of the expanding antigen-reactive T-cells during an ongoing T-cell response with simultaneous initial enrichment of Treg cells, and the later involvement of the Treg cell-mediated regulation exerting long-term allograft protection. The results obtained from this study will further facilitate the design of TCR-based therapies for re-programming of the immune response to alloantigens for future clinical use.
Supplementary Material
Figure S1. Recovery of T-cell homeostasis in B6 mice after treatment with H57-597 mAb.
Figure S2. H57-597 mAb enriches suppressive FoxP3+ Treg cells in vitro.
Figure S3. H57-597 mAb treatment reduced total numbers of SEB-reactive T-cells in vivo.
Figure S4. H57-597 treatment enriched Tregs in heart allograft recipient mice.
Acknowledgments
The authors of this article would like to thank the NIH (grant number HL 69723) and AHA (grant number 11SDG7690000) for research funding.
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information: Supplemental Figures S1-S4 hosted by Wiley-Blackwell
Additional Supporting Information may be found in the online version of this article:
Reference List
- 1.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 2.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323(5913):502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 3.Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463(7283):963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielekova B, Martin R. Antigen-specific immunomodulation via altered peptide ligands. J Mol Med. 2001;79(10):552–565. doi: 10.1007/s001090100259. [DOI] [PubMed] [Google Scholar]
- 7.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 8.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10(5):301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 9.Kjer-Nielsen L, Dunstone MA, Kostenko L, Ely LK, Beddoe T, Mifsud NA, et al. Crystal structure of the human T cell receptor CD3 epsilon gamma heterodimer complexed to the therapeutic mAb OKT3. Proc Natl Acad Sci U S A. 2004;101(20):7675–7680. doi: 10.1073/pnas.0402295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmeron A, Sanchez-Madrid F, Ursa MA, Fresno M, Alarcon B. A conformational epitope expressed upon association of CD3-epsilon with either CD3-delta or CD3-gamma is the main target for recognition by anti-CD3 monoclonal antibodies. J Immunol. 1991;147(9):3047–3052. [PubMed] [Google Scholar]
- 11.Gaston RS, Deierhoi MH, Patterson T, Prasthofer E, Julian BA, Barber WH, et al. OKT3 first-dose reaction: association with T cell subsets and cytokine release. Kidney Int. 1991;39(1):141–148. doi: 10.1038/ki.1991.18. [DOI] [PubMed] [Google Scholar]
- 12.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 13.You S, Chatenoud L. New generation CD3 monoclonal antibodies: are we ready to have them back in clinical transplantation? Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e3283402bd8. [DOI] [PubMed] [Google Scholar]
- 14.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight RJ, Kurrle R, McClain J, Racenberg J, Baghdahsarian V, Kerman R, et al. Clinical evaluation of induction immunosuppression with a murine IgG2b monoclonal antibody (BMA 031) directed toward the human alpha/beta-T cell receptor. Transplantation. 1994;57(11):1581–1588. [PubMed] [Google Scholar]
- 16.Waid TH, Thompson JS, Siemionow M, Brown SA. T10B9 monoclonal antibody: A short-acting nonstimulating monoclonal antibody that spares gammadelta T-cells and treats and prevents cellular rejection. Drug Des Devel Ther. 2009;3:205–212. doi: 10.2147/dddt.s2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waid TH, Thompson JS, McKeown JW, Brown SA, Sekela ME. Induction immunotherapy in heart transplantation with T10B9. 1A-31: a phase I study. J Heart Lung Transplant. 1997;16(9):913–916. [PubMed] [Google Scholar]
- 18.Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown S, Kryscio R, et al. Treatment of renal allograft rejection with T10B9. 1A31 or OKT3: final analysis of a phase II clinical trial. Transplantation. 1997;64(2):274–281. doi: 10.1097/00007890-199707270-00017. [DOI] [PubMed] [Google Scholar]
- 19.Henrickson M, Reid J, Bellet JS, Sawchuk SS, Hirsch R. Comparison of in vivo efficacy and mechanism of action of antimurine monoclonal antibodies directed against TCR alpha beta (H57-597) and CD3 (145-2C11) Transplantation. 1995;60(8):828–835. [PubMed] [Google Scholar]
- 20.Drobyski WR, Majewski D. Treatment of donor mice with an alpha beta T-cell receptor monoclonal antibody induces prolonged T-cell nonresponsiveness and effectively prevents lethal graft-versus-host disease in murine recipients of major histocompatibility complex (MHC)-matched and MHC-mismatched donor marrow grafts. Blood. 1996;87(12):5355–5369. [PubMed] [Google Scholar]
- 21.Knight RJ, Kurrle R, Stepkowski S, Serino F, Chou TC, Kahan BD. Synergistic immunosuppressive actions of cyclosporine with a mouse anti-rat alpha/beta-T cell receptor monoclonal antibody. Transplantation. 1994;57(11):1544–1548. [PubMed] [Google Scholar]
- 22.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90(12):1511–1515. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 23.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Ferraresso M, Tian L, Ghobrial R, Stepkowski SM, Kahan BD. Rapamycin inhibits production of cytotoxic but not noncytotoxic antibodies and preferentially activates T helper 2 cells that mediate long-term survival of heart allografts in rats. J Immunol. 1994;153(7):3307–3318. [PubMed] [Google Scholar]
- 25.Garrod KR, Cahalan MD. Murine skin transplantation. J Vis Exp. 2008;(11):634. doi: 10.3791/634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM, et al. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr Top Microbiol Immunol. 1990;166:175–181. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185(1):71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Khattar M, Guo Z, Miyahara Y, Linkes SP, Sun Z, et al. IL-2-deprivation and TGF-beta are two non-redundant suppressor mechanisms of CD4+CD25+ regulatory T cell which jointly restrain CD4+CD25− cell activation. Immunol Lett. 2010;132(1–2):61–68. doi: 10.1016/j.imlet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, Wang G, Miyahara Y, Khattar M, Linkes SP, Wang C, et al. IL-7, but not thymic stromal lymphopoietin (TSLP), during priming enhances the generation of memory CD4+ T cells. Immunol Lett. 2010;128(2):116–123. doi: 10.1016/j.imlet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9(9):1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 33.Lavasani S, Dzhambazov B, Andersson M. Monoclonal antibody against T-cell receptor alphabeta induces self-tolerance in chronic experimental autoimmune encephalomyelitis. Scand J Immunol. 2007;65(1):39–47. doi: 10.1111/j.1365-3083.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 34.Moder KG, Luthra HS, Kubo R, Griffiths M, David CS. Prevention of collagen induced arthritis in mice by treatment with an antibody directed against the T cell receptor alpha beta framework. Autoimmunity. 1992;11(4):219–224. doi: 10.3109/08916939209035158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recovery of T-cell homeostasis in B6 mice after treatment with H57-597 mAb.
Figure S2. H57-597 mAb enriches suppressive FoxP3+ Treg cells in vitro.
Figure S3. H57-597 mAb treatment reduced total numbers of SEB-reactive T-cells in vivo.
Figure S4. H57-597 treatment enriched Tregs in heart allograft recipient mice.