Abstract
Aphids are important pests of plants that use their stylets to tap into the sieve elements to consume phloem sap. Besides the removal of photosynthates, aphid infestation also alters source-sink patterns. Most aphids also vector viral diseases. In this chapter, we will summarize on recent significant findings in plant-aphid interaction, and how studies involving Arabidopsis thaliana and Myzus persicae (Sülzer), more commonly known as the green peach aphid (GPA), are beginning to provide important insights into the molecular basis of plant defense and susceptibility to aphids. The recent demonstration that expression of dsRNA in Arabidopsis can be used to silence expression of genes in GPA has further expanded the utility of Arabidopsis for evaluating the contribution of the aphid genome-encoded proteins to this interaction.
INTRODUCTION
Over the past twenty five years, Arabidopsis thaliana has been utilized as a model plant to study plant growth, development and adaptation to the environment (Koornneef and Meinke, 2010). Arabidopsis has also provided valuable information on plant-insect interactions, including those involving insects in the orders Coleoptera, Diptera, Hemiptera, Lepidoptera and Thysanoptera. One of the early studies with Arabidopsis and the fungus gnat, Bradysia impatiens, shed light into the critical role of jasmonic acid signaling as a positive regulator of plant defense against dipteran insects (McConn et al., 1997). Since then several groups have successfully utilized Arabidopsis to gain important insights about genes and mechanisms that contribute to plant resistance and susceptibility to insects (Table 1).
Table 1.
Arabidopsis thaliana as a model system to study different plant-insect interactions
Insects are broadly classified into two groups based on their feeding behavior — (i) the chewing insects, and (ii) the piercing-sucking group of insects. Chewing insects, for example, caterpillars and beetles, have very strong mandibles which allow them to chew on plant tissue and thus cause extensive physical damage to the plant (Karban and Baldwin, 1997; Kandoth et al., 2007). By contrast, insects with piercing-sucking mouthparts (for example aphids, whiteflies, leafhoppers, and thrips) feed on plant sap or cell contents by piercing plant tissue and extracting plant fluids (Walling, 2000). In comparison to chewing insects, feeding by piercing-sucking insects causes minimal physical damage to plant tissues (Walling, 2000). Many piercing-sucking insects have specialized to feed from the sieve elements, which are conduit for transport of phloem sap (Tjallingii and Hogen Esch, 1993; Prado and Tjallingii, 1994; Kehr, 2006). These phloem-feeding insects are adapted to consuming a diet that is rich in sugars and have evolved a variety of mechanisms to cope with the unbalanced and the high osmolarity diet, including oligomerization of sugars in the gut, expulsion of sugars in the form of honeydew, and diluting the gut content with water consumed from xylem (Spiller et al., 1990; Pompon et al., 2010).
APHIDS
Aphids (Hemiptera: Aphididae) constitute the major group of phloem-feeding insects that utilize their slender stylets, which are modified mouthparts (Figure 1a and 1b), to tap into the sieve elements (Pollard, 1973; Blackman and Eastop, 2000). On their way to the vascular tissue, the aphid stylet follows a predominantly intercellular route (Tjallingii, 1990; Walling, 2000), thus minimizing physical damage to the plant tissue. As discussed later, salivary components of aphids also help in minimizing physical damage. Occasionally, the aphid stylets also penetrate host cells, presumably sampling cell contents. Aphid infestation results in the creation of a strong sink in the aphid-infested organ which results in increased flow of nutrients to aphid-infested tissues thereby reducing the mass flow of nutrients to the primary growth zone of plants (Mittler and Sylvester, 1961; Dixon, 1998; Girousse et al., 2005). In addition, aphids have the capacity to achieve high population growth and importantly, many aphid species vector economically important viral diseases of plants (Kennedy et al., 1962; Matthews, 1991).
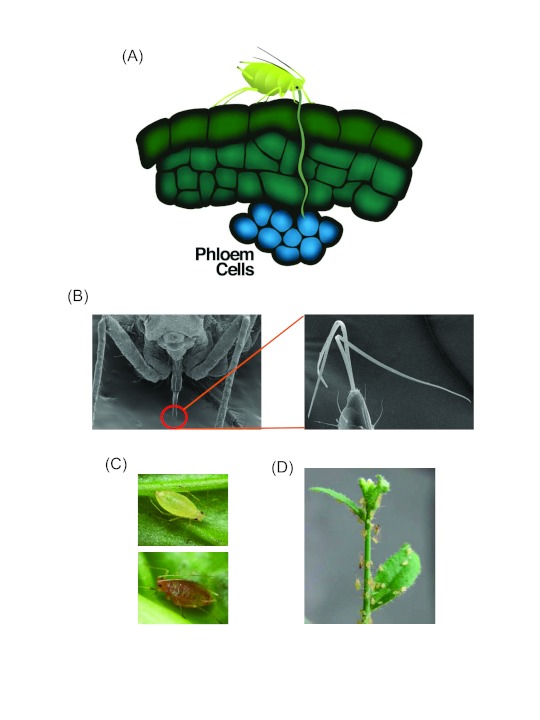
Figure 1.
(A) Aphid feeding track. Aphids use their slender stylets to penetrate between cells on their way to the phloem tissue to consume copious amount of phloem sap (Illustration by Nick Sloff).
(B) Scanning electron microscopy (SEM) images showing aphid mouthpart. Right panel shows the close-up image of aphid stylet. Images provided by John Diaz-Montano.
(C) Green and red morph of the green peach aphid (Images by Nick Sloff).
(D) Colonization by green peach aphid on the model plant Arabidopsis thaliana.
Specialist v/s Generalist
Amongst the 4,000 aphid species that have been described, approximately 250 are considered a pest (Dixon, 1998; Blackman and Eastop, 2000). Based on their host range, aphids are classified as specialist or generalists. Specialist aphids feed only on a restricted set of related plant species (Lankau, 2007). For instance, mustard aphid (Lipaphis erysimi) and cabbage aphid (Brevicoryne brassicae) feed only on cruciferous plants (Blackman and Eastop, 2000). On the other hand, a generalist aphid, such as the green peach aphid (GPA; Myzus persicae) (Figure 1c and 1d) feeds on a wide array of plant species and is considered polyphagous (Lankau, 2007; Blackman and Eastop, 2000). It has been suggested that specialist aphids have the ability to locate their host plant based on the presence of unique secondary metabolite(s) that act as cues for host recognition, feeding and oviposition (Raybould and Moyes, 2001; Macel and Vrieling, 2003). By comparison, it has been suggested that generalist aphids cue in on a combination of plant primary and secondary metabolites to make their host selection (Powell et al., 2006).
Life Cycle
Aphids are capable of sexual and asexual reproduction. They have a heteroecious holocyclic life cycle, involving alternate hosts. For instance, peach (Prunus persicae) is the primary host for GPA. Other plants that can serve as primary hosts for GPA include black cherry, (P. serotina), Canadian plum (P. nigra), dwarf Russian almond (P. tenella), and peach-almond hybrids (Blackman and Eastop, 2000). Secondary hosts for GPA include more than 50 families of plants that comprise, amongst others, vegetable crops like squash, cabbage, radish, mustard, celery, lettuce, tomato, potato and eggplant (Blackman and Eastop, 2000). Sexual reproduction, which occurs only during a portion of their life cycle, results in the production of female and male sexual morphs. The females lay their eggs on the primary host plant, where they can overwinter. In places where a primary host plant is absent or not available, and if climate permits the asexual stages to survive winter, GPA exhibits an anholocyclic life cycle and reproduces parthenogenically (offsprings produced without fertilization). In the lab, GPA exhibits an anholocylic life cycle under typical Arabidopsis growth conditions. However, in many GPA lineages, sexual reproduction can be induced in the laboratory by maintaining colonies at cool temperatures under short-day conditions (Blackman, 1974).
Feeding Patterns
Aphids display a variety of feeding patterns on plants. The electrical penetration graph (EPG) has provided a powerful technique to characterize the feeding patterns of phloem-feeding insects (Tjallingii, 1990; Tjallingii and Esch, 1993; Reese et al., 2000; Walker, 2000). This technique has been extensively utilized to investigate the details of plant resistance to aphids, whiteflies and leafhoppers (Diaz-Montano et al., 2007; Pegadaraju et al., 2007; Jiang et al., 2000; Stafford and Walker, 2009). In an EPG set up (Figure 2a), the insect on the plant is part of a low-voltage circuit. The patterns of electrical waveforms generated provide information on the feeding activity of the insect (van Helden and Tjallingii, 2000). In case of aphids, the three different phases that constitute the EPG waveforms include pathway phase, sieve element or phloem phase, and xylem phase (Reese et al., 2000; Tjallingii, 2006). During the pathway phase, the stylet is inserted in the tissue but outside the phloem and xylem. The pathway phase includes both the intercellular and/or intracellular stylet insertion or withdrawal during the brief sampling of cells (Tjallingii and Esch, 1993). The sieve element or phloem phase occurs when the stylet tips are in a phloem sieve element. EPG studies have indicated that during the sieve element phase, when the insect is ingesting phloem sap, it periodically resorts to intermittent salivation into the sieve elements. Xylem phase occurs when the insect is ingesting the xylem sap and is often thought to be related to water uptake (Spiller et al., 1990). Water uptake has been suggested as a means for the insect to dilute out the gut contents, and thus minimize dehydration (Pompon et al., 2010). A representative EPG waveform of a GPA feeding on wild type Arabidopsis accession Columbia plant is shown in Figure 2b. EPG has been used to study the impact of mutations in Arabidopsis genes on aphid feeding behavior and thus the contribution of individual genes and mechanisms to different aspects of Arabidopsis defense and susceptibility to aphids (Pegadaraju et al., 2007; Louis et al., 2010a, 2010b, 2012; Singh et al., 2011; Nalam et al., 2012).
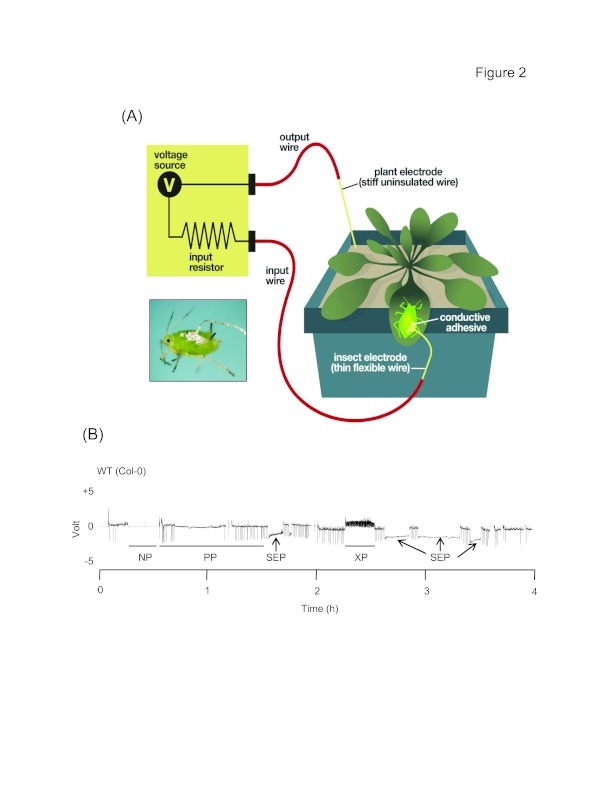
Figure 2.
Electrical penetration graph (EPG) setup used for characterizing the feeding behavior of aphids on its host plant.
(A) Diagram depicting the components of EPG system (Illustration by Nick Sloff). The EPG set-up has two components -the insect and plant electrodes. A very thin, gold wire is glued to the dorsum of the aphid using a conductive silver paint. This thin wire (insect electrode), which helps in the free movement of the aphid on the plant surface is connected to the EPG probe. A stiff copper wire is inserted into the soil of the pot, in which the plant is rooted (plant electrode). It electrifies the plant with a low-voltage, low amperage current. As the stylet of the aphid comes into contact with the electrified plant, the circuit is closed and current flows through the insect and the different signals (waveforms) are thus produced. Inset shows an aphid wired with the thin gold wire using silver conductive paint (Picture provided by Gregory Zolnerowich, Kansas State University).
(B) A representative EPG waveform of GPA feeding on Arabidopsis wild type accession Columbia plant. The different waveform patterns shown here represent different phases of aphid probing of the plant. In the pathway phase (PP) the aphid stylet has penetrated the tissue but is outside the sieve elements and xylem. The pathway phase includes both the intercellular and/or intracellular stylet insertion or withdrawal during the brief sampling of cells. The non-probing (NP) phase represents a state when there is relatively no stylet movement (NP). The sieve element phase (SEP) and the xylem phase (XP) represent the stages when the stylet is in a sieve element or a xylem tissue, respectively.
Saliva
Salivary secretions of aphids have a vital role in plant-aphid interactions (Mutti et al., 2008; De Vos and Jander, 2009). The aphid stylet (Figure 1b) punctures the plant tissue and injects aphid salivary secretions into the host (Tjallingii, 1990). Two types of saliva are injected by aphids into the host plant - a gelling saliva and a watery saliva (Miles, 1999). The proteinaceous gelling saliva, which is secreted as the stylet penetrates the plant tissue, forms a supportive sheath around the stylet and a tight seal around the sites on the cell surface that may have been punctured by the stylet, thus further limiting wounding damage to the plant. Intracellular puncture by the stylet is followed by a rapid switch to secretion of non-gelling (watery) saliva and only this watery saliva is delivered into the penetrated cell or sieve elements (Cherqui and Tjallingii, 2000; Powell, 2005). The watery saliva is suggested to contain various enzymes, including pectinases, cellulases, polyphenol oxidases, peroxidases, and lipases (Campbell and Dreyer 1990; Miles, 1990, 1999). These enzymes may perform vital roles in insect feeding, including lubrication of the stylets, maintaining favorable oxidative-reduction (redox) conditions and detoxification of phenolics and other chemical factors (Miles and Oertli, 1993). Furthermore, once the aphid reaches the sieve elements of the plant, salivary secretions help to limit phloem sealing and callose deposition, which enables the aphid to feed continuously for many hours or even days from a single sieve element (Will and van Bel, 2006; Will et al., 2007, 2009). As discussed below, salivary components could potentially also function as effectors that modulate plant physiology, including defense responses.
APHID DIET
The aphid diet (phloem sap) is very rich in sugars but relatively poor in amino acids, which are essential nutrients for aphids and could thus limit aphid growth. In general, 10 amino acids are considered as essential nutrients for insects: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine (Dadd, 1985). Insects cannot synthesize these amino acids and thus depend on their diet or endosymbiont bacteria to obtain adequate essential amino acids. Additionally, since tyrosine and cysteine, which are non-essential amino acids, are synthesized in the insect from phenylalanine and methionine, respectively, the availability of phenylalanine and methionine likely impact tyrosine and cysteine content as well (Dadd, 1985). Hence, aphids need to ingest large amounts of phloem sap in order to acquire sufficient amount of nitrogen. Mutation in Arabidopsis AMINO ACID PERMEASE6 (AAP6; At5g49630) gene resulted in significant reduction of the essential and non-essential amino acid content in the sieve element and phloem sap of aap6 mutant as compared to the wild type plant (Hunt et al., 2010). However, GPA proliferation and feeding behavior on the aap6 mutant was comparable to the wild type plant (Hunt et al., 2010), suggesting that something other than amino acid availability is limiting aphid growth.
All phloem-feeding insects rely on endosymbiont bacteria to synthesize essential amino acids to complement the unbalanced nitrogen content of the phloem sap. In the case of aphids, an obligate bacterial endosymbiont, Buchnera aphidicola, which lives inside aphids in specialized cells called bactehocytes, synthesize essential amino acids required by the aphids, but that are limiting in the phloem sap (Baumann, 2005; Douglas, 2006). Selective disruption of aphid symbionts with Chlortetracycline severely affected aphid growth and development, suggesting that the bacterial symbionts are essential for supplementing nutrients required by aphids (Prosser and Douglas, 1991). In contrast to the aphid host, Buchnera spp. lack the non-essential amino acid biosynthetic pathways and thus depend on the host for these amino acids (Hansen and Moran, 2011).
REPROGRAMMING OF PLANT METABOLISM
Carbohydrate Metabolism
Aphid infestation alters source-sink patterns in the infested plant, leading to the generation of a strong sink in the aphid-infested organ and thus increasing flow of nutrients to the infested organ at the cost of nutrient flow to the natural sink tissues (Mittler and Sylvester, 1961; Dixon, 1998; Girousse et al., 2005). Indeed, expression of genes involved in sugar transport and metabolism were altered in GPA infested Arabidopsis (Moran and Thompson, 2001; Moran et al., 2002; Pegadaraju, 2005). The concentration of sucrose and starch increased in GPA-infested leaves of Arabidopsis (Singh et al., 2011). Since sucrose has a large osmolyte effect, at first thought it would seem that this increase in sucrose and the corresponding increase in osmolahty of the phloem sap would be detrimental to the aphid, which expends lot of energy to counter the high osmolarity of the diet it consumes. However, Singh et al. (2011) demonstrated that GPA numbers were higher on the Arabidopsis tps11 (At2g18700) mutant plant, which accumulates higher levels of sucrose and lower amounts of starch in response to GPA infestation, than in the wild type plant, suggesting that increased sucrose levels are unlikely to be a mechanism utilized by plant to control GPA infestation. Instead, Singh et al. (2011) proposed that the accumulation of starch, at the expense of sucrose, is a mechanism utilized by plants to counter the insect. They demonstrated that in comparison to the wild type plant, insect numbers were higher on the Arabidopsis pgm1 (At5g51820) mutant plant, which is unable to synthesize starch. Similarly, in comparison to the wild type plant, the increment in starch content was lower in the tps11 mutant on which the insect population was larger. It has been suggested that starch accumulation at the expense of sucrose results in a secondary intracellular sink that counters the ability of the insect to alter host physiology to make the plant more suitable for the insect (Singh et al., 2011).
Aphid infestation also impacts nitrogen metabolism in the plants. Pea aphid (Acyrthosiphon pisum) infestation on alfalfa (Medicago sativa) resulted in an evident shift from nitrogen sinks to nitrogen sources (Girousse et al., 2005). Furthermore, aphid settlement on the actively growing zones of stem resulted in a systemic reduction of C and N fluxes, especially at the apical zones of the stem (Girousse et al., 2005). Molecular studies have also indicated changes in expression and/or activity of genes/proteins involved in N metabolism. For example, GPA infestation resulted in increased nitrate reductase activity in cabbage (Wilson et al., 2011). Voelckel et al. (2004) showed that tobacco aphid (Myzus nicotianae) infestation resulted in the induction of glutamate synthase expression. Similarly expression of glutamine synthase was upregulated in GPA infested celery (Divol et al., 2005).
Premature Senescence
GPA infestation resulted in the up-regulation of a subset of SENESCENCE ASSOCIATED GENES (SAG) (Pegadaraju et al., 2005; De Vos et al., 2005), and the activation of cell death and senescence in Arabidopsis (Pegadaraju et al., 2005). Some of these SAG genes were also induced in leaves infiltrated with synthetic diet containing aphid saliva (De Vos and Jander, 2009), suggesting that aphid salivary components elicit cell death in Arabidopsis. Indeed, the putative salivary protein Mp10 from GPA when transiently overexpressed in Nicotiana benthamiana promoted chlorosis (Bos et al., 2010). When compared to wild type Arabidopsis, the hyper-senescent ssi2 (At2g43710) and cpr5 (At5g64930) mutants exhibited enhanced resistance to GPA, and insect population size was larger on the pad4 (At3g52430) mutant, which exhibited delayed onset of senescence and expression of SAG genes in response to GPA infestation. Hence, it was suggested that premature senescence is a defense mechanism employed by the host to control GPA infestation (Pegadaraju et al., 2005). Senescence likely counters the efforts of the insect to increase sink nature of the infested tissues.
Plant Hormones
Plants produce a variety of hormones including auxins, gibberellins, abscisic acid, cytokinins, salicylic acid, jasmonic acid, ethylene, brassinosteroids and peptide hormones. Of these, salicylic acid (SA) and jasmonic acid (JA) are more widely studied for their signaling role in plant defense against various biotic stresses (Shah, 2003; Lorenzo and Solano, 2005; Broekaert et al., 2006; Bari and Jones, 2009; Pieterse et al., 2009). Several studies have shown that aphid feeding on host plant activates SA and JA-mediated defense pathways (Moran and Thompson, 2001; Mewis et al., 2005). GPA feeding on Arabidopsis activates genes that are involved in SA biosynthesis and signaling (Moran and Thompson, 2001; Pegadaraju, 2005). However, GPA population size on the SA biosynthesis mutant sid2 (salicylic acid-induction deficient2; At1g74710), the SA insensitive mutant npr1 (non-expressor of PR-1; At1g64280) and a SA deficient NahG transgenic plant that expresses a SA-degrading salicylate hydroxylase, were comparable to that on wild type plants, suggesting that SA is not a key player in Arabidopsis defense against GPA (Moran and Thompson, 2001; Pegadaraju et al., 2005). Quite to the contrary, Mewis et al., (2005) showed that both the GPA and cabbage aphid populations on the npr1 mutant and the NahG plant were smaller as compared to the wild type plant, suggesting that lack of NPR1 function and SA accumulation resulted in increased resistance. However, the role of SA may differ depending on the plant and insect involved. In contrast to the studies in Arabidopsis, in tomato, it was shown that hyperaccumulation of SA due to knock-down of FAD7 activity resulted in enhanced resistance to potato aphid (Macrosiphum euphorbiae) (Avila et al., 2012).
Since SA signaling is known to attenuate the activation of JA signaling, Mewis et al., (2005) suggested that the higher level of resistance against GPA observed in the npr1 mutant and NahG plants could possibly result from the stronger activation of the JA signaling pathway in these plants. Similarly, in the case of another sap-sucking insect, silverleaf whitefly (Bemisia tabaci), larval growth was reduced on npr1 and NahG plants, whereas the growth was higher on SA-overexpressing cim10 plants as compared to the wild type plants (Zarate et al., 2007). Zarate et al., (2007) suggested a ‘decoy’ hypothesis in which the insect evades host defenses by tricking the host plant into inappropriately activating SA signaling and thus depressing the activation of JA signaling. Indeed, other studies also have shown that resistance against aphids is mediated through the JA pathway (Ellis et al., 2002; Zhu-Salzman et al., 2004; Gao et al., 2007). For example, the GPA population was smaller on the Arabidopsis cev1 (At5g05170) mutant, which has high JA content (Ellis et al., 2002). In contrast, GPA numbers were higher on JA-insensitive mutant coi1 (At2g39940) mutant compared to the wild type plant (Ellis et al., 2002). JA has also been suggested to have an important role in defense against aphids in other plant species. For example, methyl jasmonate (MeJA) treatment of sorghum (Sorghum bicolor) seedlings resulted in fewer numbers of greenbug aphids (Schizaphis graminum) as compared to the untreated control plants (Zhu-Salzman et al., 2004). However, JA-responsive genes were only weakly induced in greenbug-infested sorghum leaves. By comparison, SA responsive genes were strongly induced in greenbug-infested sorghum (Zhu-Salzman et al., 2004). It is likely that, as in the case of whitefly infestation of Arabidopsis, greenbug tricks sorghum plants to induce SA signaling to prevent the timely activation of JA signaling. Similarly, the JA pathway is also required for AKR (Acyrthosiphon kondoi Resistance) mediated resistance against the blue green aphid (Acyrthosiphon kondoi) in Medicago truncatula (Gao et al., 2007).
JA also contributes to basal resistance against cabbage aphid in Arabidopsis. Cabbage aphid populations were smaller on the Arabidopsis cev1 and fou2 (Arabidopsis fatty acid oxygenation up-regulated2; At4g03560) mutants, respectively, both of which have high JA content (Kuśnierczyk et al., 2011). However, EPG studies revealed that the fou2 allele does not limit cabbage aphid feeding from sieve elements, thus suggesting that mechanisms other than feeding deterrence contribute to fou2 and JA-determined resistance against cabbage aphid. In addition to elevated levels of JA, Arabidopsis fou2 mutants also had higher levels of the JA precursor, oxo-phytodienoic acid (OPDA) and the related dinor oxo-phytodienoic acid (dnOPDA) (Bonaventure et al., 2007; Kuśnierczyk et al., 2011). Hence, further experiments are required to tease apart the contributions of JA and/or OPDA to fou2-determined heightened resistance against cabbage aphid. Besides JA, OPDA is also known to function as a signal molecule in Arabidopsis (Taki et al., 2005).
PERCEPTION OF APHIDS
During the last 20 years immense progress has been made in understanding the molecular mechanism underlying plant recognition of microbes. Several membrane spanning as well as intracellular Resistance (R) genes facilitate plant recognition of specific pathogen-derived molecules and plant-derived factors that are produced in response to infection (Chisholm et al., 2006; Jones and Dangl, 2006). For instance, a bacterial flagellin protein-derived Pathogen Associated Molecular Pattern (PAMP) - is recognized by Pattern Reception Receptors (PPR) in the plant resulting in the activation of PAMP-triggered immunity (PTI) (Chisholm et al., 2006; Schwessinger and Zipfel, 2008). However, over the years, pathogens have evolved effector molecules that can suppress the PTI. In comparison, plants have evolved additional R genes that recognize these effector molecules leading to the activation of effector-triggered immunity (ETI) (Chisholm et al., 2006). How aphids are perceived by plants has not been elucidated. As described below, in recent years a few R genes involved in recognizing aphids have been identified and some progress has been made in identifying effector molecules that either stimulate plant defense or promote insect infestation by manipulating plant metabolism/physiology.
Effectors
Oral secretions from caterpillars contain elicitors of plant defenses (e.g. volicitin, caeliferins and inceptins) (Alborn et al., 1997, 2007; Schmelz et al., 2006). Aphids also intermittently inject into sieve elements a watery saliva that contains proteins and other metabolites. One or more of these salivary components could elicit and/or modulate plant responses. For example, aphid saliva contains oxidases (e.g. glucose oxidase) (Harmel et al., 2008) that could potentially produce hydrogen peroxide (H2O2), a key modulator of plant defense (Bede et al., 2006; Maffei et al., 2006), similar to that observed with glucose oxidase from corn earworm (Helicoverpa zea) (Musser et al., 2002). Although the exact function of glucose oxidase in aphid saliva is not known, recently it was shown that the Arabidopsis RBOHD (RESPIRATORY BURST OXIDASE HOMOLOG D; At5g47910) gene, which is responsible for accumulation of reactive oxygen species (ROS), including local accumulation of H2O2 at the wound/injury site, modulates defense against GPA (Miller et al., 2009). GPA proliferation was higher on rbohD mutant plants than the wild type plant. These results suggest that any changes in the ROS status in the sieve elements could influence plant resistance/susceptibility to aphids. Further, evidence indicating a potential role for aphid salivary components in modulating plant response against aphids was provided by experiments with Arabidopsis leaves infiltrated with an artificial diet on which GPA had fed and thus contained aphid salivary secretions. Infiltration of diet containing aphid saliva resulted in differential expression of Arabidopsis genes related to signal transduction, response to stress and secondary metabolite biosynthesis (e.g. glucosinolate), and genes encoding various stress associated transcription factors (De Vos and Jander, 2009).
RNAi experiments with pea aphid provided the first evidence of importance of salivary components in facilitating aphid infestation on plants. Silencing expression of the pea aphid gene encoding the C002 protein, which is normally expressed in the salivary glands, adversely impacted the ability of the C002-silenced aphids to feed from the host plant (Mutti et al., 2008), thus suggesting that C002 is critical for aphids to continuously feed from the host plant (Mutti et al., 2008). Similarly, silencing expression of the C002 homolog of GPA by expression of dsRNA in transgenic Arabidopsis resulted in the production of less progeny by the C002-silenced GPA (Pitino et al., 2011). By contrast, agro-infiltration mediated overexpression of the GPA C002 protein in Nicotiana benthamiana enhanced GPA fecundity as compared to the vector control (Bos et al., 2010). These results suggest that C002 facilitates aphid infestation, most likely due to its action in the host plant. By contrast to C002, the Mp10 and MP42 proteins from GPA, which are encoded by genes expressed in the salivary glands of the aphid, when overexpressed in N. benthamiana resulted in reduced fecundity of GPA. Mp10 overexpression in N. benthamiana also resulted in chlorosis and attenuated ROS production in response to treatment with flg22, a bacterial effector peptide (Bos et al., 2010), suggesting that Mp10 impacts plant responses. Although, whether plants perceive the presence of aphids by recognizing these effectors is not known, the above studies suggest that aphid salivary components can elicit plant responses that likely impact the interaction between the host plant and aphid.
Resistance genes
The Arabidopsis-GPA and Arabidopsis-cabbage aphid systems have been successfully utilized by several groups to identify a number of plant genes (Table 2 and 3) and mechanisms that contribute to plant defense against aphids. However, to date, R gene type resistance against aphids in Arabidopsis has not been reported. Only very few examples of gene-for-gene resistance in plant-aphid interactions are known. For example, the Mi-1.2 gene in tomato (Solanum lycopersicum) and the Vat gene in melon are involved in providing protection against certain isolates of potato aphid and melon aphid (Aphis gossypii), respectively (Rossi et al., 1998; Dogimont et al., 2007). Mi-1.2 was the first aphid resistance gene to be cloned. Both Mi-1.2 and Vat genes are members of plant R genes consisting of nucleotide binding site (NBS) and leucine-hch-repeat (LRR) motifs (Milligan et al., 1998, Dogimont et al., 2007). In addition to potato aphids, Mi-1.2 also confers resistance against certain isolates of root-knot nematodes and biotypes of whitefly (B. tabaci), and the tomato psyllid (Bactericera cockerelli) (Nombela et al., 2003; Casteel et al., 2006). Mi-1.2 mediated resistance against root-knot nematodes functions through the activation of hypersensitive response (HR), which is associated with cell death at the feeding site, which results in the blockage of nematode feeding (Dropkin et al., 1969; Roberts and Thomason, 1986). In contrast, the Mi-1.2-mediated resistance to potato aphid is independent of HR (de Illarduya et al., 2003). Presence of Mi-1.2 confers resistance to potato aphid by limiting the insect's ability to feed continuously from the phloem sap (Kaloshian et al., 2000; Palliparambil et al., 2010). The bluegreen aphid resistance gene, AKR, identified in M. truncatula, also maps to a region containing NBS-LRR class of genes (Klingler et al., 2005). In addition, Nr in lettuce, Sd1 in apple, RAG1 and RAG2 in soybean, and RAP1 in M. truncatula have also been reported in conferring resistance against lettuce aphid, rosy-leaf curling aphid, soybean aphid and pea aphid, respectively (Helden et al., 1993; Roche et al., 1997; Hill et al., 2006; Stewart et al., 2009, Kim et al., 2010). Several loci conferring biotype-specific resistance, including Aph in maize, and Gby and Dn in wheat, have also been reported in providing resistance against corn leaf aphid, greenbug and Russian wheat aphid, respectively (Marais and Du Toit, 1993; Boyko et al., 2004; Castro et al., 2005; So et al., 2010).
Table 2.
Arabidopsis mutants and transgenic plants exhibiting altered resistance to green peach aphid
CATEGORIES OF PLANT RESISTANCE
In the early 1950's, Dr. R.H. Painter, a well renowned entomologist at Kansas State University, introduced, three categories of plant resistance mechanisms to aphids: non-preference, antibiosis and tolerance (Painter, 1951). The term non-preference was then replaced to ‘antixenosis’ by Kogan and Ortman (1978). Antixenosis is a resistance mechanism, in which a plant either fails to serve as a host for the insect pest, or the insects prefer an alternate host plant (Smith, 2005). Strong antixenosis may result in starvation even when no alternative host is available. In other cases, antixenosis adversely impacts insect behavior, for example its ability to find sieve elements, thus deterring infestation. Antibiosis is the category in which aphid physiology is affected, resulting in adverse impact on the growth, development and/or, reproduction or survival of the insect (Smith, 2005). Antibiosis may result from chemical and physical defenses of the plant. It could also result from the absence of sufficient nutrients in the plant (Pedigo, 1999; Smith, 2005). The third resistance category, tolerance, is the ability of the plant to withstand and/or recover from damage caused by the insect at a scale that is comparable to that on a plant with-out any resistant characteristics (Pegido, 1999). In many cases, tolerance is considered as the most durable kind of resistance against insects, because of the reduced selection pressure for new insect biotypes and also less deleterious effects on natural enemies (Flinn et al., 2001). Both constitutive (preformed physical and chemical factors) and inducible defenses involving small metabolites and macromolecules contribute to overall plant resistance to aphids by impacting insect behavior and physiology (Chen, 2008).
Table 3.
Arabidopsis mutants and transgenic plants exhibiting altered resistance to the cabbage aphid
Besides the above categories of resistance, plants also attract predatory insects to control aphid infestation. Plant volatiles produced in response to aphid infestation attract several natural enemies of aphids including lacewings, hoverflies, parasitoid wasps and coccinellid beetles (Francis et al., 2004; Zhu et al., 2005; Hatano et al., 2008). For instance, methyl salicylate (MeSA), a soybean aphid-induced plant volatile, attracts its predatory beetle, Coccinella septempunctata, to the aphid-infested plant and this predatory insect helps to curtail the aphid population (Zhu and Park, 2005). Volatiles produced by aphids also contribute to tritrophic interactions. For example, (E)-β-Farnesene (EBF), which is a key component of the aphid alarm pheromone (Pickett et al., 1992), was shown to attract predatory insects. Plants overexpressing EBF provided enhanced resistance by deterring aphids and also by attracting the aphid parasitoid, Diaeretiella rapae, to the infested plant which resulted in the reduction of aphid settlement on host plants (Gibson and Pickett, 1983; Beale et al., 2006; De Vos et al., 2010). Whether plant volatiles that mimic alarm pheromones could be targeted for durable resistance, remains to be determined. In Arabidopsis, it has been shown that EBF overexpression leads to habituation in three successive GPA generations (De Vos et al., 2010). Although Arabidopsis constitutive EBF-emitting transgenic plants supported higher proliferation of EBF-habituated GPA as compared to EBF-non-habituated GPA, these transgenic plants also recruited increased predators of attacking aphids (De Vos et al., 2010), suggesting that aphid alarm pheromone production could be engineered to promote indirect defense mechanism in plants.
CONTROLLING INFESTATION AT PLANT SURFACE
Insects utilize surface cues present on the plant to make decisions on feeding and/or oviposition (Walling, 2008). Thus, the leaf surface, in particular the waxy cuticle, could influence the ability of the aphid to colonize a plant and act as one of the first layers of defense against aphids. Indeed, cabbage aphid population size was adversely impacted on the Arabidopsis wax mutant, cer3 (At5g02310), which accumulates elevated levels of the C30 alcohol, triacontanol (Rashotte, 1999). However, the GPA population size on cer3 mutant was comparable to that of wild type plants (Rashotte, 1999), thus suggesting that the detrimental impact of C30 alcohol is aphid species specific.
Aphids also encounter trichomes on plant surface, which provide a physical barrier to insect movement on the plant. Trichomes in some plant species have glands at their base, which contain secondary metabolites (Wagner et al., 2004). Damage to these trichomes, as a result of insect activity, results in the exudation of glandular contents. The sugar esters present in these exudates from tobacco are known to adversely impact GPA population size (Wang et al., 2004; Jin et al., 2011), thus indicating that trichomes also contribute to chemical defenses in plants. Trichomes present in Arabidopsis are non-glandular, and so far there are no reports in Arabidopsis of trichomes providing defense against aphids.
Both cabbage aphid and GPA infestation on Arabidopsis induce changes in expression of genes involved in cell wall metabolism and remodeling (Divol et al., 2007; Kuśnierczyk et al., 2008). For example, aphid feeding on Arabidopsis altered expression of XTH33 (At5g02310), which encodes a xyloglucan endotransglycosylase/ hydrolase that is involved in cell wall remodeling (Divol et al., 2005, 2007; Kusnierczyk et al., 2008). GPA preferred to settle on the xth33 mutant as compared to the wild type plant, suggesting that XTH33 is involved in providing cell wall mediated defense against GPA (Divol et al., 2007). However, tissue specific expression of XTH33 in the companion cells did not provide enhanced resistance against GPA than the wild type plant (Divol et al., 2007), suggesting that XTH33-mediated resistance against GPA is exerted at a step outside of vascular tissues. Alternatively, XTH33 might work in conjunction with another gene(s) in Arabidopsis to control aphid infestation.
DEFENSE IN THE PHLOEM
When the aphids are feeding on a non-host or a resistant plant, they ingest phloem sap initially at normal rates, but will subsequently stop feeding, withdraw their stylet and leave the plant (Kloft, 1977), suggesting that aphids tend to move away from the plant possibly because the phloem sap is nutritionally unfavorable. Studies with Arabidopsis indicate that the sap contains a factor(s) that is detrimental to the insect. Petiole exudates, which are enriched in phloem sap, collected from leaves of wild type Arabidopsis, when added to a synthetic diet had a detrimental effect on GPA population (Louis et al., 2010a, 2010b). As mentioned later, the pad4 and the mpl1 mutant, both of which are deficient in this antibiotic activity, exhibited lowered resistance to GPA (Louis et al., 2010a, 2010b). By contrast, insect populations were smaller on mutant plants that constitutively accumulate high levels of this activity, for example the ssi2 (suppressor of SA-insensitivity2) mutant, than on the wild type plant (Louis et al., 2010b). However, despite the presence of this detrimental activity in petiole exudates of wild type plants, GPA manages to successfully colonize Arabidopsis, suggesting that over time it can overcome this detrimental factor(s), presumably by either detoxifying it in planta, and/or suppressing its production by the host plant, or activating mechanisms that inactivate this factor in the insect body. Previous studies have indicated that aphid infestation results in alterations in the composition of phloem sap (Sandström et al., 2000). Indeed, petiole exudates collected from GPA-infested leaves of wild type Arabidopsis lacked this inhibitory activity (Nalam et al., 2012). Quite to the contrary, these exudates now contained an activity that promoted GPA proliferation on an artificial diet, thus suggesting that aphids infestation results in the destruction or suppression of this inhibitory activity.
Several stress and defense associated proteins are present in the sap of plants (Walz et al., 2004; Gaupels et al., 2008). The PP2-A1 (Phloem Protein2-A1; At4g19840) in Arabidopsis, which is associated with the sieve elements (Beneteau et al., 2010), possesses lectin activity (Dinant et al., 2003; Beneteau et al., 2010). Lectins are proteins that have an affinity for carbohydrates and have insecticidal activities, presumably due to interference with processes in the aphid gut (Carlini and Grosside-Sa, 2002; Vasconcelos and Oliveira, 2004). This insecticidal effect of lectins has been successfully used to engineer plants with enhanced resistance against several aphids. For example, potato plants engineered to express high levels of snowdrop lectin were more resistant to GPA (Gatehouse et al., 1996). Recombinant PP2-A1 protein when added to a synthetic diet, also exhibited inhibitory activity against GPA (Beneteau et al., 2010). Furthermore, GPA had difficulty feeding from sieve elements of transgenic Arabidopsis plants that overexpressed PP2-A1 (Zhang et al., 2011), thus indicating that in planta produced PP2-A1 is also detrimental to GPA.
Phloem proteins are also involved in occlusion of sieve elements upon wounding. Plants also deposit callose in sieve elements penetrated by stylets, thereby preventing the prolonged feeding of phloem-feeding insects. In plants, callose synthesis is Ca2+-dependent (King and Zeevaart, 1974). It has been shown that sieve tube occlusion upon wounding in legumes involves the dispersion of spindle like proteins (forisomes) with the aid of Ca2+ influx to the injury site (Knoblauch et al., 2001; Thorpe et al., 2010). Occlusion of sieve elements by forisome plugging resulted in a change in feeding behavior of aphids on Vicia faba (Will et al., 2007). As a counter-mechanism, the Ca2+-binding proteins present in the aphid saliva have been suggested to participate in reverse phloem occlusion, which allows the aphids to feed continuously from the phloem sap (Will et al., 2007). In Arabidopsis, cabbage aphid infestation upregulated the expression of CALLOSE SYNTHASE (CALS1; At1g05570) gene (Kuśnierczyk et al., 2008), thus suggesting that aphid infestation induces the synthesis of callose by stimulating expression of plant genes involved in callose biosynthesis. Similarly, infestation by sliverleaf whitefly (B. tabaci) and brown leafhopper (Nilaparvata lugens), on Arabidopsis and rice, respectively, induced the accumulation of callose near the vascular tissues (Kempema et al., 2007; Hao et al., 2008).
Phloem sap also contains defensive compounds, such as gluconiolates and non-protein amino acids that impact Arabidopsis interaction with aphid. These are discussed in more detail below.
Glucosinolates—a Brassicaceae-specific Chemical Defense
Plants in the Brassicaceae family, which includes Arabidopsis thaliana, accumulate glucosinolates, a family of secondary metabolites that are sources of thioacyanates and other breakdown products that are toxic to some aphids (Rask et al., 2000; Halkier and Gershenzon, 2006). Levy et al., (2005) showed that the expression level of the IQD1 (At3g09710) gene, which encodes a transcription factor that is responsible for glucosinolate accumulation, impacts host plant choice by GPA. Another study showed that the fecundity of both generalist (GPA) and specialist aphid (cabbage aphid) were higher on Arabidopsis coi1 mutant that contained lower amount of glucosinolates than the wild type plant (Mewis et al., 2005). Differences have been observed in the profile of glucosinolates in aphid-infested compared to uninfested Arabidopsis. For example, although the total glucosinolate content does not change in GPA-infested (Kim and Jander, 2007, Louis et al., 2010a), compared to uninfested plants, the GPA-infested plants contain higher levels of indole glucosinolates. Kim et al., (2008) reported that GPA population size was smaller on the Arabidopsis atr1D (At5g60890) mutant plant, which accumulates elevated levels of indole glusocinolates, thus suggesting that indole glucosinolates are detrimental to GPA (Kim et al., 2008).
Glucosinolates themselves are not insecticidal. However, when acted upon by myrosinases, glucosinolates produce toxic thiocyanates and other breakdown products that act as defensive compounds against insects (Chew, 1988; Louda and Mole 1991; Rask et al., 2000). In Arabidopsis, β-thioglucoside glucohydrolases encoded by TGG1 and TGG2 (At5g25980 and At5g26000) contribute to the majority of the myrosinase activity (Barth and Jander, 2006). However, both GPA and cabbage aphid populations were unaffected on Arabidopsis tgg1 and tgg2 single and the ttg1 ttg2 double mutant plants, compared to the wild type plant, suggesting that aphids evade the production of toxic thiocyanates or modulate the activity of enzymes that synthesize these thiocyanates (Barth and Jander, 2006). Instead, Kim et al., (2008) showed that the adverse effect of glucosinolates on aphid performance correlated with the accumulation of indole glucosinolate breakdown products in the insect body, indicating that aphids consume glucosinolates produced by the host plant. In particular, diindolylmethylcysteines and other amino acid conjugates, which form after indole glucosinolate breakdown during aphid feeding from Arabidopsis, reduce aphid reproduction on artificial diets (Kim et al., 2008). Further studies identified the indol-3-ylmethyl glucosinolate -derived 4-methoxyindol-3-ylmethyl glucosinolate and 1-methoxyindol-3-yl-methyl glucosinolate as strong deterrents of GPA proliferation (Kim and Jander, 2007; Pfalz et al., 2009). GPA reproduction was improved on cyp81F2 (At5g57220) mutants, which are defective in the production of 4-methoxyindol-3-ylmethyl glucosinolate (Pfalz et al., 2009; De Vos and Jander, 2009). Unlike in the generalist GPA, the effect of glucosinolate breakdown products against the specialist cabbage aphids have not been reported. It is possible that the specialist aphids are able to evade, suppress and/or adapt to these glucosinolate breakdown products as opposed to the generalist aphids. Readers are directed to some excellent reviews that have summarized glucosinolate biosynthesis and metabolism, and its role in plant defense (Bednarek et al., 2009; Wittstock and Burow, 2010).
Non-protein Amino Acids
Plants produce several non-protein amino acids that serve as intermediates in the synthesis of primary metabolites. In addition, these compounds are also reported to have defense related functions against insects (Rosenthal, 1991). For instance, L-canavanine, an L-arginine analog, is a major storage compound in legumes and also has insecticidal allelochemical properties (Rosenthal, 2001).  -acetylornithine has been identified as a new class of defense related compound in Arabidopsis (Adio et al., 2011). This non-protein amino acid has been identified in phloem sap collected from methyl jasmonate-treated Arabidopsis. The Arabidopsis NATA1 gene (At2g39030), which encodes a protein with N-acetyltransferase activity, is involved in the biosynthesis of
-acetylornithine has been identified as a new class of defense related compound in Arabidopsis (Adio et al., 2011). This non-protein amino acid has been identified in phloem sap collected from methyl jasmonate-treated Arabidopsis. The Arabidopsis NATA1 gene (At2g39030), which encodes a protein with N-acetyltransferase activity, is involved in the biosynthesis of  -acetylornithine and is expressed in the phloemassociated tissues. Expression of the NATA1 gene was induced and
-acetylornithine and is expressed in the phloemassociated tissues. Expression of the NATA1 gene was induced and 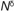 -acetylornithine content was higher in GPA infested Arabidopsis compared to uninfested plants. Furthermore, GPA population on aphid diet containing
-acetylornithine content was higher in GPA infested Arabidopsis compared to uninfested plants. Furthermore, GPA population on aphid diet containing 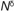 -acetylornithine was significantly reduced, suggesting that this compound has a direct toxic and/or deterrent effect on GPA (Adio et al., 2011). Transient expression of NATA1 in tobacco significantly reduced GPA population size as compared to the vector control plants (Adio et al., 2011). Resistance in these experiments correlated with the level of
-acetylornithine was significantly reduced, suggesting that this compound has a direct toxic and/or deterrent effect on GPA (Adio et al., 2011). Transient expression of NATA1 in tobacco significantly reduced GPA population size as compared to the vector control plants (Adio et al., 2011). Resistance in these experiments correlated with the level of 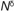 -acetylornithine. Whether
-acetylornithine. Whether 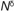 -acetylornithine has any effect on specialist cabbage aphids, is not known. However, by contrast to aphids, although NATA1 expression and
-acetylornithine has any effect on specialist cabbage aphids, is not known. However, by contrast to aphids, although NATA1 expression and 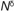 -acetylornithine accumulation were also induced in response to infestation by chewing herbivores, the nata1-1 mutation did not affect Pieris rapae (white cabbage butterfly) and Plutella xylostella (diamondback moth) caterpillar growth, suggesting that
-acetylornithine accumulation were also induced in response to infestation by chewing herbivores, the nata1-1 mutation did not affect Pieris rapae (white cabbage butterfly) and Plutella xylostella (diamondback moth) caterpillar growth, suggesting that  -acetylornithine accumulation is either not important or not sufficient to deter chewing insects. A recent review by Huang et al. (2011) summarizes the role of non-protein amino acids in plant defense against various insect pests.
-acetylornithine accumulation is either not important or not sufficient to deter chewing insects. A recent review by Huang et al. (2011) summarizes the role of non-protein amino acids in plant defense against various insect pests.
CONTRIBUTION OF HOST LIPIDS TO ARABIDOPSIS-APHID INTERACTION
Lipids are considered as vital structural components of biological membranes (Somerville et al., 2000). In addition, lipids also function as signaling molecules in plant growth, development and stress response (Wang 2004; Shah, 2005; Upchurch, 2008; Scherer, 2010). Fatty acids serve as substrates for enzymes that produce lipid-based signaling molecules, for instance, several oxylipins. Oxylipins are oxidized fatty acids and their synthesis in plants is initiated by the action of lipoxygenases (LOXs). LOXs are classified as 9- or 13-LOXs based on their ability to add oxygen at the 9- or 13-C position of the fatty acids to yield the corresponding fatty acid hydroperoxides. JA is derived from the 13-LOX pathway. As mentioned above, JA promotes host defense against a variety of aphids in several plants (Ellis ef al., 2002; Zhu-Salzman et al., 2004; Gao et al., 2007).
9-LOX-derived oxylipins
Recently, it was shown that 9-LOX-derived oxylipins contribute to host susceptibility to aphids (Nalam et al., 2012). In Arabidopsis, GPA infestation resulted in an increase in the level of 9-LOX-derived oxylipins in the petiole exudates (Nalam et al., 2012). Genetic and physiological evidence indicates that GPA cues in on 9-LOX pathway derived oxylipins in Arabidopsis to facilitate infestation (Nalam et al., 2012). GPA population was significantly reduced on Arabidopsis lox5 (At3g22400) mutants, in which the accumulation of 9-LOX-derived oxylipins is attenuated, as compared to the wild type plants. EPG studies indicated that insects on the lox5 mutant had difficulty feeding from sieve elements and xylem tissues. This reduction in feeding activity of GPA on the lox5 mutant also resulted in a reduction in water content in the aphids. Application of the 9-LOX-derived 9-hydroxyoctadecadienoic acid (9-HOD) restored water content and insect population size on the lox5 mutant, thus confirming that 9-LOX products have an important contribution in facilitating insect feeding. 9-HOD and 9-hydroperoxyoctadecadienoic acid (9-HPOD) when added to an artificial diet enhanced GPA population size, suggesting that 9-LOX-derived oxylipins are susceptibility factors that facilitate colonization of GPA on Arabidopsis. Micro-grafting experiments (wild type LOX scions and lox5 mutant rootstock, and vice-versa) demonstrated that the oxylipins that promote GPA susceptibility in Arabidopsis leaves are root-derived (Nalam et al., 2012). GPA infestation of Arabidopsis foliage was found to induce expression of LOX5 and promote accumulation of 9-LOX products in roots from where they are likely transported to shoots via the vasculature. Experiments in potato plants (Solanum tuberosum) have also indicated that accumulation of 9-LOX products is increased in GPA infested plants (Gosset et al., 2009).
MPL1
In Arabidopsis a lipase encoded by the MPL1 (MYZUS PERSICAE INDUCED LIPASE1; At5g14180) gene, is required for the accumulation of an activity in petiole exudates that is detrimental to GPA (Louis et al., 2010a). Loss of this antibiosis activity in the petiole exudates of the mpl1 mutant was accompanied by larger population size of GPA on the mpl1 mutant compared to wild type Arabidopsis plants. Loss of MPL1 function in the mpl1 mutant did not impact insect feeding behavior. MPL1 expression is induced in GPA-infested plants and is constitutively elevated in the ssi2 mutant, which exhibits enhanced resistance to GPA (Louis et al., 2010a, 2010b). Indeed, MPL1 function was required for the ssi2-determined resistance against GPA and accumulation of antibiosis activity in petiole exudates. Furthermore, constitutive overexpression of MPL1 from the Cauliflower mosaic virus 35S promoter resulted in enhanced resistance against GPA, suggesting that the induction of MPL1 expression is important for controlling GPA infestation (Louis et al., 2010a). Constitutive overexpression of PAD4 (described later) and MPL1 in mpl1 and pad4 plants, respectively, rescued the antibiosis deficiency of the mpl1 and pad4 mutants, suggesting that MPL1 and PAD4 contribute to two parallel antibiosis mechanisms and the elevated levels of one component/mechanism, can overcome the deficiency of the other.
Fatty Acid Desaturases
The stearoyl-ACP desaturase activity encoded by the Arabidopsis SSI2 gene catalyzes the desaturation of stearic acid to oleic acid (Shah et al., 2001; Kachroo et al., 2001) and thus contributes to Arabidopsis membrane lipid composition (Nandi et al., 2003). The ssi2 mutant plants constitutively accumulate high levels of SA, which is responsible for the heightened resistance of the ssi2 mutant to some pathogens (Shah et al., 2001; Kachroo et al., 2001). In comparison to the wild type plant, GPA reproduction was lower on the Arabidopsis ssi2 mutant plant as compared to its wild type plant (Pegadaraju et al., 2005). However, GPA growth was comparable on the ssi2 single mutant and the ssi2 nahG (SA-deficient) plant, suggesting that the high levels of SA present in the ssi2 plants do not contribute to the ssi2-conferred resistance to GPA. In addition, GPA counts on the suppressor of npr1-1, constitutive 1 (snc1; At4g16890) mutant, which accumulates high levels of SA (Zhang et al., 2003), was comparable to those on the wild type plant (Pegadaraju et al., 2005). These results suggested that some factor(s) other than SA contributes to the ssi2-conferred hyper-resistance against GPA. Petiole exudates collected from uninfested ssi2 mutant contain elevated levels of antibiosis activity against GPA, than similar exudates collected from the wild type plant. Whether this antibiosis factor is a lipid is not known. However, the ssi2-determined enhanced antibiosis and resistance to GPA was attenuated in the absence of MPL1 activity in the ssi2 mpl1 double mutant (Louis et al., 2010b). Phloem sap contains lipids (Madey et al., 2002; Nalam et al., 2012), and the salivary glands of phloem-feeding insects contain putative lipases (Shukle et al., 2009), thus suggesting that these insects likely encounter lipids in their diet, one or more of which could be detrimental to GPA.
More recently, loss of FAD7 and FAD8 (At3g11170 and At5g05580)-encoded fatty acid desaturases in Arabidopsis and the LeFAD7 activity in tomato (Solanum lycopersicum) were also shown to result in enhanced resistance against GPA and potato aphid (Macrosiphum euphorbiae), respectively (Avila et al., 2012). In Arabidopsis, FAD7 and FAD8 catalyze the desaturation of dienoic acyl chains in plastid galactolipids to yield lipids with thenoic fatty acyl chains. FAD7/FAD8 synthesized thenoic acyl chains are substrates for the synthesis of signaling oxylipids like OPDA and JA. The tomato fad7 plants are deficient in JA, yet they are more resistant to potato aphid. Similarly, the Arabidopsis fad7 fad8 mutant was more resistant to GPA. These results suggest that the impact of fad7 on aphid colonization is independent of fad7's effect on JA. Indeed, potato aphid numbers were comparable on wild type plants and in tomato mutants impaired in JA synthesis (acx1) or perception (jai1-1) (Avila et al., 2012), thus further supporting the suggestion that the impact of fad7 mutation on aphids is unrelated to FAD7's role in JA accumulation. In tomato, loss of FAD7 function resulted in increased antibiotic and antixenotic resistance to potato aphids, which was dependent on the accumulation of elevated SA levels in the fad7 tomato plants as evident from experiments with fad7 NahG plants in which attenuation of SA accumulation by expression of the NahG-encoded salicylate hydroxylase, resulted in suppression of fad7-conferred enhanced resistance to potato aphid. Whether, fad7 fad8 determined enhanced resistance against GPA in Arabidopsis is similarly dependent on SA remains to be determined, although as mentioned above, in Arabidopsis SA is not required for basal resistance against GPA (Moran and Thompson, 2001; Pegadaraju et al., 2005).
REGULATION OF PLANT DEFENSES
PAD4
In Arabidopsis, the PAD4 (PHYTOALEXIN DEFICIENT4) gene modulates antibiotic and antixenotic defense against GPA (Pegadaraju et al., 2005, 2007). In addition, PAD4 promotes premature leaf senescence and SAG13, SAG21 and SAG27 (At2g29350, At4g02380, and At4g44300) expression in GPA-infested plants (Pegadaraju et al., 2005). PAD4 was required for deterring insect settling on plants (Pegadaraju et al., 2007) and the accumulation of a factor in petiole exudates of uninfested plants that was detrimental to GPA (Louis et al., 2010b, 2012). In addition, EPG analysis indicated that PAD4 controls insect feeding from sieve elements (Pegadaraju et al., 2007). PAD4 is also involved in plant defense against a subset of pathogens, where it modulates the synthesis of SA and the phytoalexin, camalexin (Glazebrook et al., 1997; Zhou et al., 1998; Jirage et al., 1999). However, genetic studies have suggested that the involvement of PAD4 in plant defense against GPA is not due to its involvement in SA and camalexin metabolism (Pegadaraju et al., 2005). The N-terminal half of PAD4 shares homology with α/β-fold acyl hydrolases that include lipases and esterases (Zhou et al., 1998; Jirage et al., 1999). Although, the biochemical function of PAD4 is not known, at the molecular level PAD4 protein interacts with its signaling partner EDS1 (ENHANCED DISEASE SUSCEPTIBILITY1; At3g48090) thereby promoting defense against pathogens (Falk et al., 1999; Feys et al., 2005). EDS1, like PAD4, has homology to lipases/acylhydrolases, and like PAD4, EDS1 expression is also induced in GPA-infested leaves (Feys et al., 2005; Pegadaraju et al., 2007). However, PAD4-mediated defense against GPA does not require EDS1 (Pegadaraju et al., 2007; Louis et al., 2012), suggesting that PAD4s involvement in Arabidopsis defense against aphids is distinct from its involvement in defense against pathogens.
Mutational analysis of PAD4 indicated that Ser118, which is a catalytic residue in α/β fold acyl hydrolases, although not required for PAD4's involvement in defense against pathogens, is required for a subset of PAD4 activities in defense against GPA (Louis et al., 2012). It was essential for limiting insect feeding from sieve elements and for accumulation of the antibiosis factor in petiole exudates of uninfested plants (Louis et al., 2012). However, Ser118 is not required for controlling insect settling and premature senescence in GPA-infested plants. These results suggest that distinct molecular activities of PAD4 modulate different functions in Arabidopsis defense against GPA.
In Arabidopsis, PAD4 also modulates accumulation of camalexin (Tsuji et al., 1992; Rogers et al., 1996). Camalexin levels increase in GPA-infested Arabidopsis leaves. However, there was no significant difference in camalexin content in GPA infested leaves of pad4 mutant and wild type plants (J Louis, J Keerantweep and J Shah, unpublished data), suggesting that PAD4 is not required for camalexin accumulation in GPA-infested Arabidopsis. Camalexin synthesis also requires the PAD3 (At3g26830) gene, which encodes an enzyme that catalyzes the terminal step in the synthesis of camalexin (Schuhegger et al., 2006). PAD3 expression is induced in GPA-infested plants (Pegadaraju et al., 2005). However, GPA population size on the pad3 mutant was comparable to that on the wild type plant (Pegadaraju et al., 2005), thus indicating that PAD3 and camalexin are not essential for controlling GPA infestation on Arabidopsis. Cabbage aphid infestation also results in an increase in camalexin accumulation (Kuśnierczyk et al., 2008). PAD3 expression was induced in response to cabbage aphid infestation (Kuśnierczyk et al., 2008). Furthermore, fecundity of cabbage aphid was significantly higher on the pad3 mutant than on the wild type plant (Kuśnierczyk et al., 2008), thus indicating that unlike GPA infestation, camalexin accumulation has a role in controlling cabbage aphid infestation on Arabidopsis. Comparative analysis of EST sequences from the GPA with the whole genome sequence of the specialist pea aphid revealed that a generalist aphid like GPA has more detoxification enzymes, including cytochrome P450 monooxygenases, glutathione S-transferases, and carboxy/cholinesterases, likely because GPA encounters more diverse host plant species (Ramsey et al., 2010). Presence of these detoxification mechanisms could explain the lack of any obvious effect of camalexin on GPA.
TPS11 and Trehalose
Trehalose is a non-reducing disacchahde composed of two molecules of glucose. It is present in a wide spectrum of living organisms, as varying as bacteria, fungi, insects and angiosperms. Singh and co-workers (2011) recently demonstrated that transient accumulation of trehalose, dependent on the TPS11-encoded trehalose-6-phosphate synthase, modulates Arabidopsis defense against GPA. TPS11 was required for the timely induction of PAD4 in GPA-infested leaves. In addition, TPS11 was required for promoting starch accumulation at the expense of sucrose in GPA-infested leaves. As mentioned earlier, genetic studies have indicated that starch accumulation in Arabidopsis contributes to controlling GPA infestation. Petiole exudates of the tps11 mutant lacked the antibiosis activity that was present in petiole exudates of wild type plants. In addition, EPG analysis indicated that GPA spent more time in sieve element phase on the tps11 mutant, and given a choice the insects preferred to settle on the tps11 mutant than the wild type plant. Trehalose application restored wild type level of resistance against GPA in the tps11 mutant, indicating that the tps11 mutant phenotypes are due to its inability to accumulate elevated levels of trehalose in response to GPA infestation. The role of trehalose in defense against GPA is further evident from experiments with the trehalose hyper-accumulating tre1 (At4g24040) mutant, which contains a T-DNA insertion in the trehalase encoding TRE1 gene. GPA population was smaller on the tre1 mutant, compared to the wild type plant. Similarly, GPA population was also smaller on transgenic plants expressing the bacterial otsB gene, which encodes a trehalose-6-phosphate phosphatase involved in trehalose synthesis. Singh et al. (2011) suggested that TPS11 provides a threshold level of ‘signaling’ trehalose that is essential for regulating Arabidopsis defense against GPA, including the up-regulation of PAD4 expression and full extent of starch accumulation. Trehalose application promotes starch accumulation in Arabidopsis leaves (Wingler et al., 2000; Kolbe et al., 2005; Singh et al., 2011). As shown in other studies, the ability of trehalose to promote starch accumulation in GPA-infested plants could be due to the inhibition of starch turnover (Ramon et al., 2007) and/or the redox activation of AGPase, a key enzyme in starch synthesis, by trehalose-6-phosphate (Paul et al., 2008).
FINAL REMARKS
In the last decade, the use of Arabidopsis as a model plant system has helped us to better understand the genetic, biochemical and molecular aspects of plant interaction with aphids. Undoubtedly, applying the basic information gained from Arabidopsis to economically important crop plants will be a big step forward in improving our understanding of defense signaling in economically important crop species. In the future, the availability of new molecular tools and progress of genome sequences of several phloem-feeding insects will enable exploring Arabidopsis—phloem-feeding insect interactions from the perspective of both the plant and the insect. These tools will allow determining how alterations in activity of Arabidopsis genes and mechanisms involved in defense and susceptibility impact gene expression in the insect, and thus provide clues on how insect physiology is impacted on these Arabidopsis mutant and transgenic plants. The ability to silence expression of aphid genes by expressing dsRNA in Arabidopsis will permit in identifying and characterizing aphid-derived effectors and elicitors of plant defenses, and in assessing the function of aphid genes and the impact of these genes to the interaction with varied Arabidopsis genotypes.
Aphids also vector many economically important plant pathogenic viruses. Aphid settlement, growth and development are impacted on virus-infected plants (Colvin et al., 2006; Mauck et al., 2010). However, the molecular and biochemical mechanisms that contribute to this effect of viral infection on aphid performance are poorly understood. Recently, it was shown that microRNA (miRNA) profiles of resistant and susceptible melons are altered upon aphid herbivory (Sattar et al., 2012). However, the role of phloem-specific miRNAs in plant-aphid interaction is not characterized. Likewise, the role of phloem proteins and phloem-translocated small molecules in plant-aphid interaction is also poorly understood. Arabidopsis provides an excellent model system to characterize the role of these small and macromolecules, and the molecular and physiological impact of viral infection on plant-aphid interaction.
ACKNOWLEDGEMENTS
We thank Nick Sloff for artwork and photographs. In addition, we would like to acknowledge John Diaz-Montano and Gregory Zolnerowich, Kansas State University, for contributing photographs. This work was made possible by grants from the National Science Foundation (MCB-0920600 and IOS-0919192) and a Research Opportunities Program award from the University of North Texas to JS.
Footnotes
Citation: Joe Louis, Vijay Singh and Jyoti Shah (2012) Arabidopsis thaliana—Aphid Interaction The Arabidopsis Book 10:e0159. doi:10.1199/tab.0159
elocation-id: e0159
First published on May 22, 2012: e0159. doi: 10.1199/tab.0159
REFERENCES
-
Adio A.M., Casteel C.L., De Vos M., Kim J.H., Joshi V., Li B., Juery C., Daron J., Kliebenstein D.J., Jander G. Biosynthesis and defensive function of
 -acetylomithine, a jasmonate-induced Arabidopsis metabolite. Plant Cell. 2011;23:3303–3318. doi: 10.1105/tpc.111.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
-acetylomithine, a jasmonate-induced Arabidopsis metabolite. Plant Cell. 2011;23:3303–3318. doi: 10.1105/tpc.111.088989. [DOI] [PMC free article] [PubMed] [Google Scholar] - Alborn H., Turlings T., Jones T., Stenhage G., Loughrin J.H., Tumlinson J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Alborn H.T., Hansen T.V., Jones T.H., Bennett D.C., Tumlinson J.H., Schmelz E.A., Teal P.E.A. Disulfoxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila A.V., Arevalo-Soliz L.M., Jia L., Navarre D. A., Chen Z., Howe G.A., Meng Q-W., Smith J.E., Goggin F.L. Loss of function of FATTY ACID DESATURASE 7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012;158:2028–2041. doi: 10.1104/pp.111.191262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R., Jones J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Barth C., Jander G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 2006;46:549–562. doi: 10.1111/j.1365-313X.2006.02716.x. [DOI] [PubMed] [Google Scholar]
- Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- Beale M.H., Birkett M.A., Bruce T.J., Chamberlain K., Field L.M., Huttly A.K., Martin J.L., Parker R., Phillips A.L., Pickett J.A., Prosser I.M., Shewry P.R., Smart L.E., Wadhams L.J., Woodcock C.M., Zhang Y. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. USA. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede J., Musser R., Felton G., Korth K.L. Caterpillar herbivory and salivary enzymes decrease transcript Levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol. Biol. 2006;60:519–531. doi: 10.1007/s11103-005-4923-y. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Piślewska-Bednarek M., Svatoš A., Schneider B., Doubsky J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., Molina A., Schulze-Lefert P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- Beneteau J., Renard D., Marché L., Douville E., Lavenant L., Rahbé Y., Dupont D., F. Vilaine, Dinant S. Binding properties of the N-acetylglucosamine and high-mannose N-glycan PP2-A1 phloem lectin in Arabidopsis. Plant Physiol. 2010;153:1345–1361. doi: 10.1104/pp.110.153882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R.L. Life-cycle variation of Myzus persicae (Sulz.) (Hom., Aphididae) in different parts of the world, in relation to genotype and environment. Bull. Ent. Res. 1974;63:595–607. [Google Scholar]
- Blackman R.L., Eastop V.F. Aphids on the World's Crops. John Wiley & Sons; Chichester: 2000. [Google Scholar]
- Bonaventure G., Gfeller A., Proebsting W.M., Hortensteiner S., Chetelat A., Martinoia E., Farmer E.E. Again-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007;49:889–898. doi: 10.1111/j.1365-313X.2006.03002.x. [DOI] [PubMed] [Google Scholar]
- Bos J.I.B., Prince D., Pitino M., Maffei M.E., Win J., Hogenhout S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (Green Peach Aphid). PLoS Genet. 2010;6:e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko E., Starkey S., Smith C.M. Molecular genetic mapping of Gby, a new greenbug resistance gene in bread wheat. Theor. Appl. Genet. 2004;109:1230–1236. doi: 10.1007/s00122-004-1729-2. [DOI] [PubMed] [Google Scholar]
- Bressan A., Purcell A.H. Effect of benzothiadiazole on transmission of X-disease phytoplasma by the vector Colladonus montanus to Arabidopsis thaliana, a new experimental host plant. Plant Dis. 2005;89:1121–1124. doi: 10.1094/PD-89-1121. [DOI] [PubMed] [Google Scholar]
- Broekaert W.F., Delaure S.L., De Bolle M.F., Cammue B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Bruce T.J.A., Matthes M.C., Chamberlain K., Woodcock C.M., Mohib A., Webster B., Smart L.E., Birkett M.A., Pickett J.A., Napier J.A. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA. 2008;105:4553–4558. doi: 10.1073/pnas.0710305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Rutitzky M., Ballaré C.L. Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia. 2006;149:81–90. doi: 10.1007/s00442-006-0422-3. [DOI] [PubMed] [Google Scholar]
- Cardoza Y.M. Arabidopsis thaliana resistance to insects, mediated by an earthworm-produced organic soil amendment. Pest Mang. Sci. 2011;67:233–238. doi: 10.1002/ps.2059. [DOI] [PubMed] [Google Scholar]
- Carlini C.R., Grossi-de-Sa M.F. Plant toxic proteins with insecticidal properties: a review on their potentialities as bioinsecticides. Toxicon. 2002;40:1515–1539. doi: 10.1016/s0041-0101(02)00240-4. [DOI] [PubMed] [Google Scholar]
- Casteel C.L., Walling L.L., Paine T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006;121:67–72. [Google Scholar]
- Castro A.M., Vasicek A., Manifiesto M., Gimenez D.O., Tacaliti M.S., Dobrovolskaya O., Roder M.S., Snape J.W., Borner A. Mapping antixenosis genes on chromosome 6A of wheat to greenbug and to a new biotype of Russian wheat aphid. Plant Breed. 2005;124:229–233. [Google Scholar]
- Campbell B.C., Dreyer D.L. The role of plant matrix polysaccharides in aphid-plant interactions. In: Campbell RK, Eikenbary RD, editors. Aphid-plant genotype interactions. Amsterdam: Elsevier; 1990. pp. 149–169. [Google Scholar]
- Chen M. Inducible direct plant defense against insect herbivores a review. Insect Sci. 2008;15:101–114. doi: 10.1111/1744-7917.12436. [DOI] [PubMed] [Google Scholar]
- Cherqui A., Tjallingii W.F. Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J. Insect Physiol. 2000;46:1177–1186. doi: 10.1016/s0022-1910(00)00037-8. [DOI] [PubMed] [Google Scholar]
- Chew F.S. Biological effects of glucosionlates. In biologically active natural products (Cutler. H.G., ed). 1988.
- Chisholm S.T., Coaker G., Day B., Staskawicz B.J. Host—microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Cipollini D., Enright S., Traw M.B., Bergelson J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004;13:1643–1653. doi: 10.1111/j.1365-294X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- Colvin J., Omongo C.A., Govindappa M.R., Stevenson P.C., Maruthi M.N., Gibson G., Seal S. E., Muniyappa V. Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: management and epidemiological implications. Adv. Virus Res. 2006;67:419–452. doi: 10.1016/S0065-3527(06)67011-5. [DOI] [PubMed] [Google Scholar]
- Cui J., Bahrami A.K., Pringle E.G., Hernandez-Guzman G., Bender C.L., Pierce N.E., Ausubel F.M. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA. 2005;102:1791–1796. doi: 10.1073/pnas.0409450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadd R.H. Nutrition: Organisms. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology, vol. 4. Pergamon Press; Oxford: 1985. pp. 313–390. [Google Scholar]
- de Ilarduya O.M., Xie Q.G., Kaloshian I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant-Microbe Inter. 2003;16:699–708. doi: 10.1094/MPMI.2003.16.8.699. [DOI] [PubMed] [Google Scholar]
- De Vos M., Van Oosten V.R., Van Poecke R.M., Van Pelt J.A., Pozo M.J., Mueller M.J., Buchala A.J., Métraux J-P., Van Loon L.C., Dicke M., Pieterse C.M.J. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Inter. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- De Vos M., Kriksunov K.L., Jander G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008;146:916–926. doi: 10.1104/pp.107.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M., Jander G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009;32:1548–1560. doi: 10.1111/j.1365-3040.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- De Vos M., Cheng W.Y., Summers H.E., Raguso R.A., Jander G. Alarm pheromone habituation in Myzus persicae has fitness consequences and causes extensive gene expression changes. Proc. Natl. Acad. Sci. USA. 2010;17:14673–14678. doi: 10.1073/pnas.1001539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montano J., Reese J.C., Louis J., Campbell L.R., Schapaugh W.T. Feeding behavior by the soybean aphid (Hemiptera: Aphididae) on resistance and susceptible soybean genotypes. J. Econ. Entomol. 2007;100:984–989. doi: 10.1603/0022-0493(2007)100[984:fbbtsa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dinant S., Clark A.M., Zhu Y., Vilaine F., Palauqui J.C., Kusiak C., Thompson G.A. Diversity of the superfamily of phloem lectins (phloem protein 2) in angiosperms. Plant Physiol. 2003;131:114–128. doi: 10.1104/pp.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divol F., Vilaine F., Thibivilliers S., Amselem J., Palauqui J.C., Kusiak C., Dinant S. Systemic response to aphid infestation by Myzus persicae in the phloem of Apium graveolens. Plant Mol. Biol. 2005;57:517–540. doi: 10.1007/s11103-005-0338-z. [DOI] [PubMed] [Google Scholar]
- Divol F., Vilaine F., Thibivilliers S., Kusiak C., Sauge M.H., Dinant S. Involvement of the xyloglucan endotransglycosylase/hydrolases encoded by celery XTH1 and Arabidopsis XTH33 in the phloem response to aphids. Plant Cell Environ. 2007;30:187–201. doi: 10.1111/j.1365-3040.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Dixon A.F.G. Aphid Ecology: An Optimization Approach, Ed 2. Chapman and Hall; New York: 1998. [Google Scholar]
- Dogimont C., Bendahmane A., Pitrat M., Burget-Bigeard E., Hagen L., Le Menn A., Pauquet J., Rouselle P., Caboche M., Chovelon V. Gene resistant to Aphis gossypii. US Patent Application US 2007/0016977 A1. 2007.
- Douglas A.E. Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 2006;57:747–754. doi: 10.1093/jxb/erj067. [DOI] [PubMed] [Google Scholar]
- Dropkin V.H., Helgeson J.P., Upper C.D. The hypersensitivity reaction of tomato resistant to Meloidogyne incognita: Reversal by cytokinins. J. Nematol. 1969;1:55–61. [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Turner J.G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant-Microbe Interact. 2002;15:1025–1030. doi: 10.1094/MPMI.2002.15.10.1025. [DOI] [PubMed] [Google Scholar]
- Falk A., Feys B.J., Frost L.N., Jones J.D.G., Daniels M.J., Parker J.E. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA. 1999;96:3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B.J., Wiermer M., Bhat R.A., Moisan L.J., Medina-Escobar N., Neu C., de Cruz-Cabral A., Parker J.E. Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell. 2005;17:2601–2613. doi: 10.1105/tpc.105.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn M., Smith C.M., Reese J.C., Gill B. Categories of resistance to greenbug (Homoptera: Aphididae) Biotype I in Aegilops tauschii germplasm. J. Econ. Entom. 2001;94:558–563. doi: 10.1603/0022-0493-94.2.558. [DOI] [PubMed] [Google Scholar]
- Francis F., Lognay G., Haubruge E. Olfactory responses to aphid and host plant volatile releases: (E)-beta-farnesene an effective kairomone for the predator Adalia bipunctata. J. Chem. Ecol. 2004;30:741–755. doi: 10.1023/b:joec.0000028429.13413.a2. [DOI] [PubMed] [Google Scholar]
- Gao L-L., Anderson J.P., Klingler J.P., Nair R.M., Edwards O.R., Singh K.B. Involvement of the octadecanoid pathway in blue green aphid resistance in Medicago truncatula. Mol. Plant-Microbe Interact. 2007;20:82–93. doi: 10.1094/MPMI-20-0082. [DOI] [PubMed] [Google Scholar]
- Gatehouse A.M.R., Down R.E., Powell K.S., Sauvion N., Rabbe Y., Newell C.A., Merryweather A., Hamilton W.D.O., Gatehouse J.A. Transgenic potato plants with enhanced resistance to the peach-potato aphid Myzus persicae. Entomol. Exp. Appl. 1996;79:295–307. [Google Scholar]
- Gaupels F., Knauer T., van Bel A.J.E. A combinatory approach for analysis of protein sets in barley sieve-tube samples using EDTA facilitated exudation and aphid stylectomy. J. Plant Physiol. 2008;165:95–103. doi: 10.1016/j.jplph.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Gibson R.W., Pickett J.A. Wild potato repels aphids by release of aphid alarm pheromone. Nature. 1983;302:608–609. [Google Scholar]
- Girousse C., Moulia B., Silk W., Bonnemain J.L. Aphid infestation causes different changes in carbon and nitrogen allocation in alfalfa stems as well as different inhibitions of longitudinal and radial expansion. Plant Physiol. 2005;137:1474–1484. doi: 10.1104/pp.104.057430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J., Zook M., Mert F., Kagan I., Rogers E.E., Crute I.R., Houlb E.B., Hammerschmidt R., Ausubel F.M. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosset V., Harmel N., Göbel C., Francis F., Haubruge E., Wathelet J-P., du Jardin P., Feussner I., Fauconnier M-L. Attacks by a piercing-sucking insect (Myzus persicae Sulzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009;60:1231–1240. doi: 10.1093/jxb/erp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B.A., Gershenzon J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Hansen A.K., Moran N.A. Aphid genome expression reveals host—symbiont cooperation in the production of amino acids. Proc. Natl. Acad. Sci. USA. 2011;108:2849–2854. doi: 10.1073/pnas.1013465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P., Liu C., Wang Y., Chen R., Tang M., Du B., Zhu L., He G. Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol. 2008;146:1810–1820. doi: 10.1104/pp.107.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmel N., Letocart E., Cherqui A., Giordanengo P., Mazzucchelli G., Guillonneau F., De Pauw E., Haubruge E., Francis F. Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol. Biol. 2008;17:165–174. doi: 10.1111/j.1365-2583.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- Hatano E., Kunert G., Michaud J.P., Weisser W.W. Chemical cues mediating aphid location by natural enemies. Eur. J. Ent. 2008;105:797–806. [Google Scholar]
- Helden M.C., Tjallingii W.F., Dieleman F.L. The resistance of lettuce (Lactuca sativa L.) to Nasonovia ribisnigri: Bionomics of Nasonavia ribisnigri on near isogenic lettuce lines. Entomol. Exp. Appl. 1993;66:653–658. [Google Scholar]
- Hill C.B., Li Y., Hartman G.L. A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Sci. 2006;46:1601–1605. [Google Scholar]
- Huang T., Jander G., DeVos M. Non-protein amino acids in plant defense against insect herbivores: representative cases and opportunities for further functional analysis. Phytochemistry. 2011;72:1531–1537. doi: 10.1016/j.phytochem.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Hunt E., Gattolin S., Newbury H.J., Bale J.S., Tseng H.M., Barrett D.A., Pritchard J. A mutation in amino acid permease AAP6 reduces the amino acid content of the Arabidopsis sieve elements but leaves aphid herbivores unaffected. J. Exp. Bot. 2010;61:55–64. doi: 10.1093/jxb/erp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.X., de Blas C., Barrios L., Fereres A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000;93:573–579. [Google Scholar]
- Jin S., Kanagaraj A., Verma D., Lange T., Daniell H. Release of hormones from conjugates: Chloroplast expression of β-Glucosidase results in elevated phytohormone levels associated with significant increase in biomass and protection from aphids or whiteflies conferred by sucrose esters. Plant Physiol. 2011;155:222–235. doi: 10.1104/pp.110.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D., Tootle T.L., Reuber T.L., Frost L.N., Feys B.J., Parker J.E., Ausubel F.M., Glazebrook J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA. 1999;96:13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;44:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kachroo P., Shanklin J., Shah J., Whittle E.J., Klessig D.F. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloshian I., Kinsey M.G., Williamson V.M., Ullman D.E. Mi-mediated resistance against the potato aphid Macrosiphum euphorbiae (Hemiptera: Aphididae) limits sieve element ingestion. Environ. Entomol. 2000;29:690–695. [Google Scholar]
- Kandoth P.K., Ranf S., Pancholi S.S., Jayanty S., Walla M.D., Miller W., Howe G.A., Lincoln D.E., Stratmann J.W. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. USA. 2007;104:12205–12210. doi: 10.1073/pnas.0700344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R., Baldwin I.T. Induced responses to herbivory. Chicago: University of Chicago Press; 1997. pp. 1–319. [Google Scholar]
- Kehr J. Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J. Exp. Bot. 2006;57:767–774. doi: 10.1093/jxb/erj087. [DOI] [PubMed] [Google Scholar]
- Kempema L.A., Cui X., Holzer F.M., Walling L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs: similarities and distinctions in responses to aphids. Plant Physiol. 2007;143:849–865. doi: 10.1104/pp.106.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J.S., Day M.F., Eastop V.F. A Conspectus of Aphids as Vectors of Plant Viruses. London: Commonwealth Institute of Entomology; 1962. p. 114. [Google Scholar]
- Kim J.K., Jander G. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 2007;49:1008–1019. doi: 10.1111/j.1365-313X.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Lee B. W., Schroeder F. C., Jander G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 2008;54:1015–1026. doi: 10.1111/j.1365-313X.2008.03476.x. [DOI] [PubMed] [Google Scholar]
- Kim K., Hill C.B., Hartman G.L., Hyten D.L., Hudson M.E., Diers B.W. Fine mapping of the soybean aphid-resistance gene Rag2 in soybean PI 200538. Theor. Appl. Genet. 2010;121:599–610. doi: 10.1007/s00122-010-1333-6. [DOI] [PubMed] [Google Scholar]
- King R.W., Zeevaart J.A.D. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974;53:96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler J.P., Creasy R., Gao L., Nair R.M., Calix A.S., Spafford J.H., Edwards O.R., Singh K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005;137:1445–1455. doi: 10.1104/pp.104.051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloft W.J. Radioisotopes in aphid research. In: Harris KF, Maramorosch K, editors. Aphids as virus vectors. Academic Press; New York: 1977. pp. 291–310. [Google Scholar]
- Knoblauch M., Peters W.S., Ehlers K., Van Bel A.J.E. Reversible calcium-regulated stopcocks in legume sieve tubes. Plant Cell. 2001;13:1221–1230. doi: 10.1105/tpc.13.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan M., Ortman E.F. Antixenosis-a new term proposed to define Painter's “nonpreference” modality of resistance. Entomol. Soc. America Bull. 1978;24:175–176. [Google Scholar]
- Kolbe A., Tiessen A., Schluepmann H., Paul M., Ulrich S., Geigenberger P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. 2005;102:11118–11123. doi: 10.1073/pnas.0503410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Meinke D. The development of Arabidopsis as a model plant. Plant J. 2010;61:909–921. doi: 10.1111/j.1365-313X.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- Kusnierczyk A., Winge P., Jørstad T.S., Troczyńska J., Rossiter J.T., Bones A.M. Towards global understanding of plant defence against aphids — timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 2008;31:1097–1115. doi: 10.1111/j.1365-3040.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- Kuśnierczyk A., Tran D.H.T., Winge P., Jørstad T.S., Reese J.C., Troczyńska J., Bones A.M. Testing the importance of jasmonate signalling in induction of plant defences upon cabbage aphid (Brevicoryne brassicae) attack. BMC Geomics. 2011;12:423. doi: 10.1186/1471-2164-12-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau R.A. Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytol. 2007;175:176–184. doi: 10.1111/j.1469-8137.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- Levy M., Wang Q., Kaspi R., Parrella M.P., Abel S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J. 2005;43:79–96. doi: 10.1111/j.1365-313X.2005.02435.x. [DOI] [PubMed] [Google Scholar]
- Little D., Gouhier-Darimont C., Bruessow F., Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Solano R. Molecular players regulating the jasmonate signaling network. Curr. Opin. Plant Biol. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Louda S., Mole S. Glucosinolates: chemistry and ecology. In: Rosenthal G.A., Berenbaum M.R., editors. Herbivores. Their interactions with secondary plant metabolites. San Diego, CA.: Academic Press; 1991. pp. 123–164. [Google Scholar]
- Louis J., Gobbato E., Mondal H.A., Feys B.J., Parker J.E., Shah J. Discrimination of Arabidopsis PAD4 activities in defense against green peach aphid and pathogens. Plant Physiol. 2012;158:1860–1872. doi: 10.1104/pp.112.193417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J., Lorenc-Kukula K., Singh V., Reese J., Jander G., Shah J. Antibiosis against the green peach aphid requires the Arabidopsis thaliana MYZUS PERSICAE-INDUCED LIPASE1 gene. Plant J. 2010a;64:800–811. doi: 10.1111/j.1365-313X.2010.04378.x. [DOI] [PubMed] [Google Scholar]
- Louis J., Leung Q., Pegadaraju V., Reese J., Shah J. PAD4-dependent antibiosis contributes to the ssi2-conferred hyper-resistance to the green peach aphid. Mol. Plant-Microbe Interact. 2010b;23:618–627. doi: 10.1094/MPMI-23-5-0618. [DOI] [PubMed] [Google Scholar]
- Macel M., Vrieling K. Pyrrolizidine alkaloids as oviposition stimulants for the cinnabar moth, Tyria jacobaeae. J. Chem. Ecol. 2003;29:1435–1446. doi: 10.1023/a:1024269621284. [DOI] [PubMed] [Google Scholar]
- Madey E., Nowack L.M., Thompson J.E. Isolation and characterization of lipid in phloem sap of canola. Planta. 2002;214:625–634. doi: 10.1007/s004250100649. [DOI] [PubMed] [Google Scholar]
- Maffei M.E., Mithofer A., Arimura G.I., Uchtenhagen H., Bossi S., Bertea C.M., Cucuzza L.S., Novero M., Volpe V., Quadro S., Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–1035. doi: 10.1104/pp.105.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais G.F., Du Toit F. A monosomic analysis of Russian wheat aphid resistance in the common wheat PI 294994. Plant Breed. 1993;111:246–248. [Google Scholar]
- Matthews R.E.F. Relationships between plant viruses and invertebrates. In: Matthews R.E.F., editor. Plant Virology. 3rd edn. NY: Academic Press; 1991. pp. 520–561. [Google Scholar]
- Mauck K.E., De Moraes C.M., Mescher M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA. 2010;107:3600–3605. doi: 10.1073/pnas.0907191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M., Creelman R.A., Bell E., Mullet J.E., Browse J. Jasmonate is essential for insect defense Arabidopsis. Proc. Natl. Acad. Sci. USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meur G., Budatha M., Srinivasan T., Kumar K.R.R., Gupta A.D., Kirti P.B. Constitutive expression of Arabidopsis NPR1 confers enhanced resistance to the early instars of Spodoptera litura in transgenic tobacco. Physiol. Plant. 2008;133:765–775. doi: 10.1111/j.1399-3054.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- Mewis I., Appel H.M., Hom A., Raina R., Schultz J.C. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005;138:1149–1162. doi: 10.1104/pp.104.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I., Tokuhisa J.G., Schultz J.C., Appel H.M., Ulrichs C., Gershenzon J. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry. 2006;67:2450–2462. doi: 10.1016/j.phytochem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Miles P.W. Aphid salivary secretions and their involvement in plant toxicoses. In: Campbell RK, Eikenbary RD, editors. Aphid-plant geno-type interactions. Amsterdam: Elsevier; 1990. pp. 131–147. [Google Scholar]
- Miles P.W. Aphid saliva. Biol. Rev. 1999;74:41–85. [Google Scholar]
- Miles P.W., Oertli J.J. The significance of antioxidants in the aphid-plant interaction: the redox hypothesis. Entomol. Exp. Appl. 1993;67:273–285. [Google Scholar]
- Miller G., Schlauch K., Tarn R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. The plant NADPH oxidase RbohD mediates rapid, systemic signaling in response to diverse stimuli. Sci. Signal. 2009;2:84. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- Milligan S.B., Bodeau J., Yaghoobi J., Kaloshian I., Zabel P., Williamson V.M. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998;10:1307–1319. doi: 10.1105/tpc.10.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler T.E., Sylvester E.S. A comparison of the injury of alfalfa by the aphids Therioaphis maculata and Macrosiphum pisi. J. Econ. Entomol. 1961;54:615–622. [Google Scholar]
- Moran P., Thompson G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P.J., Cheng Y., Cassell J.L., Thompson G.A. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch. Insect Biochem. Physiol. 2002;51:182–203. doi: 10.1002/arch.10064. [DOI] [PubMed] [Google Scholar]
- Moreno J.E., Tao Y., Chory J., Ballare C.L. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA. 2009;106:4935–4940. doi: 10.1073/pnas.0900701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser R.O., Hum-Musser S.M., Eichenseer H., Peiffer M., Ervin G., Murphy B., Felton G. W. Herbivory: Caterpillar saliva beats plant defences - A new weapon emerges in the evolutionary arms race between plants and herbivores. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- Mutti N.S., Louis J., Pappan L.K., Pappan K., Begum K., Chen M.S., Park Y., Dittmer N., Marshall J., Reese J.C., Reeck G.R. A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. USA. 2008;105:9965–9969. doi: 10.1073/pnas.0708958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam V. J., Keeretaweep J., Sarowar S., Shah J. Root-derived oxylipins promote green peach aphid performance on Arabidopsis thaliana foliage. 2012. Plant Cell, doi:10.1105/tpc.111.094110. [DOI] [PMC free article] [PubMed]
- Nandi A., Krothapalli K., Buseman C., Li M., Welti R., Enyedi A., Shah J. The Arabidopsis thaliana sfd mutants affect plastidic lipid composition and suppress dwarfing, cell death and the enhanced disease resistance phenotypes resulting from the deficiency of a fatty acid desaturase. Plant Cell. 2003;15:2383–2398. doi: 10.1105/tpc.015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.K., Hansen M.D., Agerbirk N., Petersen B.L., Halkier B.A. Responses of the flea beetles Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile. Chemoecology. 2001;11:75–83. [Google Scholar]
- Nombela G., Williamson V.M., Muniz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant-Microbe Interact. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- Painter R.H. Insect resistance in crop plants. The Macmillan Co.; New York: 1951. p. 520. [Google Scholar]
- Pallipparambil G.R., Reese J.C., Avila C.A., Louis J., Goggin F.L. Mi-mediated aphid resistance in tomato: tissue localization and impact on the feeding behavior of two potato aphid isolates with differing levels of virulence. Entomol. Exp. Appl. 2010;135:295–307. [Google Scholar]
- Paul M.J., Primavesi L.F., Jhurreea D., Zhang Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008;59:417–441. doi: 10.1146/annurev.arplant.59.032607.092945. [DOI] [PubMed] [Google Scholar]
- Pedigo L.P. In Entomology and Pest Management. 3rd edition. Prentice-Hall, Englewood Cliffs; NJ: 1999. [Google Scholar]
- Pegadaraju V. Molecular insights into Arabidopsis response to Myzus persicae Sülzer (green peach aphid). Ph.D. thesis, Kansas State University; Manhattan, USA: 2005. [Google Scholar]
- Pegadaraju V., Knepper C., Reese J.C., Shah J. Premature leaf senescence modulated by the Arabidopsis PAD4 gene is associated with defense against the phloem-feeding green peach aphid. Plant Physiol. 2005;139:1927–1934. doi: 10.1104/pp.105.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegadaraju V., Louis J., Singh V., Reese J., Bautor J., Feys B., Cook G., Parker J., Shah J. Phloem-based resistance to green peach aphid is controlled by Arabidopsis PHYTOALEXIN DEFICIENT4 without its signaling partner ENHANCED DISEASE SUSCEPTIBILITY1. Plant J. 2007;52:332–341. doi: 10.1111/j.1365-313X.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- Pfalz M., Vogel H., Kroymann J. The gene controlling the Indole Glucosinolate Modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell. 2009;21:985–999. doi: 10.1105/tpc.108.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett J.A., Wadhams L.J., Woodcock C.M., Hardie J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992;37:67–90. [Google Scholar]
- Pieterse C. M. J., Leon-Reyes A., Van der Ent S., Van Wees S. C. M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Pitino M., Coleman A.D., Maffei M.E., Ridout C.J., Hogenhout S.A. Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE. 2011;(10):6. e25709. doi: 10.1371/journal.pone.0025709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D.G. Plant penetration by feeding aphids (Hemiptera: Aphidoidea): a review. Bull. Entomol. Res. 1973;62:631–714. [Google Scholar]
- Pompon J., Quiring D., Giordanengo P., Pelletier Y. Role of xylem consumption on osmoregulation in Macrosiphum euphorbiae (Thomas). J. Insect Physiol. 2010;56:610–615. doi: 10.1016/j.jinsphys.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Powell G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005;86:469–472. doi: 10.1099/vir.0.80632-0. [DOI] [PubMed] [Google Scholar]
- Powell G., Tosh C.R., Hardie J. Host plant selection by aphids: behavioral, evolutionary and applied perspectives. Annu. Rev. Entomol. 2006;51:309–330. doi: 10.1146/annurev.ento.51.110104.151107. [DOI] [PubMed] [Google Scholar]
- Prado E., Tjallingii W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994;72:157–165. [Google Scholar]
- Prosser W.A., Douglas A.E. The aposymbiotic aphid: an analysis of chlortetracycline-treated pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 1991;37:713–719. [Google Scholar]
- Ramon M., Rolland F., Thevelein J.M., van Dijck P., Lyeman B. ABI4 mediates the effects of exogenous trehalose on Arabidopsis growth and starch breakdown. Plant Mol. Biol. 2007;63:195–206. doi: 10.1007/s11103-006-9082-2. [DOI] [PubMed] [Google Scholar]
- Ramsey J.S., Rider D.S., Walsh T.K., De Vos M., Gordon K.H.J., Ponnala L., Macmil S.L., Roe B.A., Jander G. Comparative analysis of detoxification enzymes in Acrythosiphon pisum and Myzus persicae. Insect Mol. Biol. 2010;19:155–164. doi: 10.1111/j.1365-2583.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- Rashotte A.M. Epicuticular wax in Arabidopsis thaliana: a study of the genetics, chemistry, structure, and interactions with insects. PhD Thesis, University of Arizona; Tucson, USA: 1999. [Google Scholar]
- Rask L., Andréasson E., Ekbom B., Eriksson S., Pontoppidan B., Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Raybould A.F., Moyes C.L. The ecological genetics of aliphatic glucosinolates. Heredity. 2001;87:383–391. doi: 10.1046/j.1365-2540.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- Reese J.C., Tjallingii W.F., van Helden M., Prado E. Waveform comparisons among AC and DC electronic monitoring systems for aphid (Homoptera : Aphididae) feeding behavior. In: Walker G. P., Backus E.A., editors. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior. Thomas Say Publications in Entomology, Entomol. Soc. Am.; Lanham, MD: 2000. pp. 70–101. [Google Scholar]
- Roberts P.A., Thomason I.J. Variability in reproduction of isolates of Meloidogyne incognita and Meloidogyne javanica on resistant tomato genotypes. Plant Dis. 1986;70:547–551. [Google Scholar]
- Roche P., Alston F.H., Maliepaard C., Evans K., Vrielink R., Dunemann F., Markussen T., Tartarini S., Brown L.M., Ryder C., King G.J. RFLP and RAPD markers linked to the rosy leaf curling aphid resistance gene (Sd1) in apple. Theor. Appl. Gen. 1997;94:528–533. [Google Scholar]
- Rogers E.E., Glazebrook J., Ausubel F.M. Mode of action of the Arabidopsis thaliana phytoalexin camalexin and its role in Arabidopsis-pathogen interactions. Mol. Plant-Microbe Interact. 1996;9:748–757. doi: 10.1094/mpmi-9-0748. [DOI] [PubMed] [Google Scholar]
- Rosenthal G.A. L-Canavanine: A higher plant insecticidal allelochemical. Amino Acids. 2001;21:319–330. doi: 10.1007/s007260170017. [DOI] [PubMed] [Google Scholar]
- Rossi M., Goggin F.L., Milligan S.B., Kaloshian I., Ullman D.E., Williamson V.M. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström J., Telang A., Moran N. A. Nutritional enhancement of host plants by aphids — a comparison of three aphid species on grasses. J. Insect Physiol. 2000;46:33–40. doi: 10.1016/s0022-1910(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Sattar S., Song Y., Anstead J. A., Sunkar R., Thompson G.A. Cucumis melo microRNA expression profile during aphid herbivory in a resistant and susceptible interaction. Mol. Plant-Microbe Interact. 2012;25:839–848. doi: 10.1094/MPMI-09-11-0252. [DOI] [PubMed] [Google Scholar]
- Schmelz E.A., Carroll M.J., LeClere S., Phipps S.M., Meredith J., Chourey P.S., Alborn H.T., Teal P.E.A. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA. 2006;103:8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G.F.E. Phospholipase A in plant signal transduction. In: Munnik T., editor. Plant Cell Monographs-Lipid Signaling in Plants, Vol. 16. Berlin-Heidelberg, Germany: Springer-Verlag; 2010. pp. 3–22. [Google Scholar]
- Schuhegger R., Nafisi M., Mansourova M., Petersen B.L., Olsen C.E., Svatos A., Halkier B.A., Glawischnig E. CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol. 2006;141:1248–1254. doi: 10.1104/pp.106.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Zipfel C. News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr. Opin. Plant Biol. 2008;11:389–395. doi: 10.1016/j.pbi.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Shah J., Kachroo P.K., Nandi A., Klessig D.F. A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J. 2001;25:563–574. doi: 10.1046/j.1365-313x.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- Shah J. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 2003;6:365–371. doi: 10.1016/s1369-5266(03)00058-x. [DOI] [PubMed] [Google Scholar]
- J Shah. Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu. Rev. Phytopathol. 2005;43:229–260. doi: 10.1146/annurev.phyto.43.040204.135951. [DOI] [PubMed] [Google Scholar]
- Shukle R.H., Mittapalli O., Morton P.K., Chen M. Characterization and expression analysis of a gene encoding a secreted lipase-like protein expressed in the salivary glands of larval Hessian fly, Mayetiola destructor (Say). J. Insect Physiol. 2009;55:104–111. doi: 10.1016/j.jinsphys.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Singh V., Louis J., Ayre B., Reese J., Shah J. TREHALOSE PHOSPHATE SYNTHASE11-dependent trehalose metabolism regulates Arabidopsis thaliana defense against the phloem-feeding insect, Myzus persicae. Plant J. 2011;67:94–104. doi: 10.1111/j.1365-313X.2011.04583.x. [DOI] [PubMed] [Google Scholar]
- Smith C.M. Plant resistance to arthropods: Molecular and conventional approaches. Springer; The Netherlands: 2005. [Google Scholar]
- So Y.S., Ji H.C., Brewbaker J.L. Resistance to corn leaf aphid (Rhopalosiphum maidis Fitch) in tropical corn (Zea mays L.). Euphytica. 2010;172:373–381. [Google Scholar]
- Somerville C., Browse J., Jaworski J.G., Ohlrogge J.B. Lipids. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: Am. Soc. Plant Biol; 2000. pp. 456–527. [Google Scholar]
- Spiller N.J., Koenders L., Tjallingii W.F. Xylem ingestion by aphids-a strategy for maintaining water balance. Entomol. Exp. Appl. 1990;55:101–104. [Google Scholar]
- Stafford C.A., Walker G.P. Characterization and correlation of DC electrical penetration graph waveforms with feeding behavior of beet leafhopper, Circulifer tenellus. Entomol. Exp. Appl. 2009;130:113–129. [Google Scholar]
- Stewart S.M., Hodge S., Ismail N., Mansfield J.W., Feys B.J., Prospéri J-M., Huguet T., Ben C., Gentzbittel L., Powell G. The RAP1 gene confers effective, race-specific resistance to the pea aphid in Medicago truncatula independent of the hypersensitive reaction. Mol. Plant-Microbe Interact. 2009;22:1645–1655. doi: 10.1094/MPMI-22-12-1645. [DOI] [PubMed] [Google Scholar]
- Stotz H.U., Pittendrigh B.R., Kroymann J., Weniger K., Fritsche J., Bauke A., Mitchell-Olds T. Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol. 2000;124:1007–1017. doi: 10.1104/pp.124.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki N., Sasaki-Sekimoto Y., Obayashi T., Kikuta A., Kobayashi K., Ainai T., Yagi K., Sakurai N., Suzuki H., Masuda T., Takamiya K., Shibata D., Kobayashi Y., Ohta H. 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139:1268–1283. doi: 10.1104/pp.105.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe M.R., Furch A.C.U., Minchin P.E.H., Föller J., Van Bel A.J.E., Hafke J.B. Rapid cooling triggers forisome dispersion just before phloem transport stops. Plant Cell Environ. 2010;33:259–271. doi: 10.1111/j.1365-3040.2009.02079.x. [DOI] [PubMed] [Google Scholar]
- Tjallingii W.F. Continuous recording of stylet penetration activities by aphids. In: Campbell R.K., Eikenbary R.D., editors. Aphid-plant geno-type interactions. Amsterdam: Elsevier; 1990. pp. 89–99. [Google Scholar]
- Tjallingii W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006;57:739–745. doi: 10.1093/jxb/erj088. [DOI] [PubMed] [Google Scholar]
- Tjallingii W.F., Esch T.H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993;18:313–328. [Google Scholar]
- Tsuji J., Jackson E.P., Gage D.A., Hammerschmidt R., Somerville S.C. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae. Plant Physiol. 1992;98:1304–1309. doi: 10.1104/pp.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- van Helden M., Tjallingii W.F. Experimental design and analysis in EPG experiments with emphasis on plant resistance research. In: Walker G.P., Backus E.A., editors. Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Thomas Say Publications in Entomology, Entomol. Soc. Am.; Lanham, MD: 2000. pp. 144–171. [Google Scholar]
- Vasconcelos I.M., Oliveira J.T. Antinutritional properties of plant lectins. Toxicon. 2004;44:385–403. doi: 10.1016/j.toxicon.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Voelckel C., Weisser W.W., Baldwin I.T. An analysis of plant-aphid interactions by different microarray hybridization strategies. Mol. Ecol. 2004;13:3187–3195. doi: 10.1111/j.1365-294X.2004.02297.x. [DOI] [PubMed] [Google Scholar]
- Wagner G.J., Wang E., Shepherd W. New approaches for studying and exploiting an old protuberance, the plant trichome. Annal. Bot. 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G.P. A beginner's guide to electronic monitoring of homopteran probing behavior. In: Walker GP, Backus EA, editors. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior. Thomas Say Publications in Entomology, Entomol. Soc. Am.; Lanham, MD: 2000. pp. 14–40. [Google Scholar]
- Walling L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- Walling L. L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008;146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz C., Giavalisco P., Schad M., Juenger M., Klose J., Kehr J. Proteomics of curcurbit phloem exudate reveals a network of defence proteins. Phytochemistry. 2004;65:1795–1804. doi: 10.1016/j.phytochem.2004.04.006. [DOI] [PubMed] [Google Scholar]
- X Wang. Lipid signaling. Curr. Opin. Plant Biol. 2004;7:329–336. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wang E., Hall J.T., Wagner G.J. Transgenic Nicotiana tabacum L. with enhanced trichome exudate cembratrieneols has reduced aphid infestation in the field. Mol. Breed. 2004;13:49–57. [Google Scholar]
- Whiteman N.K., Jander G. Genome-enabled research on the ecology of plant-insect interactions. Plant Physiol. 2010;154:475–478. doi: 10.1104/pp.110.161117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.C.C., Sternberg L.D.S.L., Hurley K.B. Aphids alter host-plant nitrogen isotope fractionation. Proc. Natl. Acad. Sci. USA. 2011;108:10220–10224. doi: 10.1073/pnas.1007065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A., Fritzius T., Wiemken A., Boller T., R.A Aeschbacher. Trehalose induces the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiol. 2000;124:105–114. doi: 10.1104/pp.124.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T., van Bel A.J.E. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006;57:729–737. doi: 10.1093/jxb/erj089. [DOI] [PubMed] [Google Scholar]
- Will T., Tjallingii W.F., Thonnessen A., van Bel A.J.E. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA. 2007;104:10536–10541. doi: 10.1073/pnas.0703535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T., Kornemann S.R., Furch A.C.U., Tjallingii W.F., van Bel A.J.E. Aphid watery saliva counteracts sieve-tube occlusion: a universal phenomenon? J. Exp. Biol. 2009;212:3305–3312. doi: 10.1242/jeb.028514. [DOI] [PubMed] [Google Scholar]
- Wittstock U., Burow M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. The Arabidopsis Book. 2010:8, e0134. doi: 10.1199/tab.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate S.I., Kempema L.A., Walling L.L. Silverleaf whitefly induces salicylic acid responses and represses effectual jasmonic responses in Arabidopsis. Plant Physiol. 2007;143:866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shi H., Chen L., Wang X., Lü B., Zhang S., Liang Y., Liu R., Qian J., Sun W., You Z., Dong H. Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biol. 2011;11:11. doi: 10.1186/1471-2229-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Goritschnig S., Dong X., Li X. Again-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15:2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Tootle T.L., Tsui F., Klessig D.F., Glazebrook J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell. 1998;10:1021–1030. doi: 10.1105/tpc.10.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Obrycki J.J., Ochieng S.A., Baker T.C., Pickett J.A., Smiley D. Attraction of two lacewing species to volatiles produced by host plants and aphid prey. Naturwissenschaften. 2005;92:277–281. doi: 10.1007/s00114-005-0624-2. [DOI] [PubMed] [Google Scholar]
- Zhu J., Park K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005;31:1733–1746. doi: 10.1007/s10886-005-5923-8. [DOI] [PubMed] [Google Scholar]
- Zhu-Salzman K., Salzman R.A., Ahn J.E., Koiwa H. Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol. 2004;134:420–431. doi: 10.1104/pp.103.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]