Abstract
Rationale
Among adult liver transplant recipients, sleep disturbances and fatigue are common. Sleep problems following pediatric liver transplantation may contribute to daytime fatigue and lower HRQoL. The aim of this cross-sectional study was to determine the impact of sleep problems on child HRQoL in pediatric liver transplant recipients using validated measures of sleep and quality of life.
Methods
Participants included 47 liver transplant recipients (LTR). The mean age of patients was 10.9 years ± 4.6, and the mean time since transplant was 6.2± 3.9 years. The primary indication for transplantation was biliary atresia (51%).
Results
Based on parent report, pediatric transplant recipients had symptoms of sleep disordered breathing, excessive daytime sleepiness, daytime behavior problems, and restless legs. 40.4% of parents and 43.8% of children reported significantly lower child Total HRQoL. Age, time since transplantation and health status variables were not significantly related to quality of life. Hierarchical regression analyses revealed that the sleep-disordered breathing subscale of the Pediatric Sleep Questionnaire accounted for significant variance in parent-proxy reports on the PedsQL summary scales measuring child Psychosocial Health (R2=0.38, p < 0.001), Physical Health (R2=0.19, p=0.004), and Total HRQoL (R2 =0.35, p < 0.0001). Across the subscales of the PedsQL, sleep-disordered breathing accounted for significant variance on the child self-reported school functioning scale (R2 = 0.18, p=0.033). Clinically significant sleep problems were more common among children with low Total HRQoL. There were no significant group differences based on child self-report.
Conclusions
Sleep problems were common in this cohort of pediatric liver transplant recipients and predicted significant variance in parent-proxy reports of HRQoL. Prospective larger scale studies are needed to assess factors that may lead to sleep difficulties and low HRQoL in this population. Appropriate detection of significant sleep problems may lead to interventions that could benefit the quality of life of pediatric LTR.
INTRODUCTION
Pediatric liver transplant recipients (LTR) have lower health-related quality of life (HRQoL) than healthy children across domains of physical and psychosocial functioning (1–5), particularly in the area of school functioning (2, 3). The risk factors predictive of lower HRQoL in pediatric liver transplant recipients are poorly understood.
Among adult liver transplant recipients, sleep disturbances and fatigue are common. A recent study examined the relationship between fatigue, sleep quality and quality of life in adults before and after liver transplantation (6). Study findings indicated that the majority of post-transplant patients (77%) were classified as having poor sleep quality, which was significantly related to higher body mass index and anxiety. Moreover, high levels of fatigue and poor sleep quality were significantly associated with lower quality of life pre- and post-transplant (6). Similarly, in a separate study, researchers found that poor sleep quality and fatigue were common symptoms after liver transplantation in adult recipients (7). High rates of restless leg symptoms (39%), a frequent cause of decreased sleep quality and quantity, have also been observed in adult liver transplant recipients. (8).
It is well documented that inadequate or disrupted sleep has detrimental effects on HRQoL, physical health, behavior, and learning in children with and without medical comorbidities (9–14). Sleep problems following pediatric liver transplantation may contribute to daytime fatigue, lower HRQoL, cognitive difficulties and behavioral disturbances. Thus, determining the prevalence of sleep problems in this population is important for promoting optimal long term health. To date, no empirical investigations have been conducted to determine the prevalence or impact of sleep problems in pediatric liver transplant recipients. The aim of this cross-sectional study was to determine the impact of sleep problems on child HRQoL in pediatric LTR.
PATIENTS AND METHODS
Study Population
Parents and pediatric LTR were recruited from the Pediatric Liver Transplant Clinic at the University of Michigan to participate in an investigation of child health related quality of life, behavior, sleep quality and health status following liver transplantation. All children between the ages of 2–17 years who were more than 6-months post-liver transplantation were eligible for participation. Potential participants were identified through the University of Michigan electronic medical record and recruited during their Pediatric Liver Transplant Clinic visit.
Procedures
This study was a cross-sectional survey of sleep problems among pediatric LTR. The Institutional Review Board at the University of Michigan Medical School approved all aspects of the study. Parents/guardians completed standardized assessment measures of the patient’s sleep problems and HRQOL during their child’s Pediatric Liver Transplant Clinic appointment. To avoid potential response bias, the order of survey administration was varied across participants. Children >8 years completed a self-report measure of HRQOL. Demographic and medical data were obtained from the University of Michigan electronic medical record.
Measures
Pediatric Sleep Questionnaire (PSQ) (15)
The PSQ is a validated, parent-completed instrument that assesses multidimensional aspects of sleep in children ages 2–18 years old. The PSQ comprises a 22-item Sleep-Related Breathing Disorder (SRBD) Index that assesses symptoms including nighttime breathing difficulties (Snoring), excessive daytime sleepiness (Sleepiness), and inattentive-hyperactive behaviors (Behavior); (15). The PSQ also contains a 6-item subscale measuring periodic limb movements during sleep including restless leg symptoms and growing pains (PLMS/RLS;(16).
Responses on the PSQ are “yes” = 1, “no” =0, or “don’t know” (considered missing). Scores on the PSQ represents the proportion of non-missing items that are positive. A cut-off score of 0.33 (indicating 33% of symptoms are positive), is considered a positive screen (15–17). The PSQ subscales have been validated against polysomnography, and have demonstrated research and clinical utility (15–18). The PSQ is classified as a “well-established” measure of pediatric sleep based on the criteria developed by the American Psychological Association Division 54 Evidence-Based Assessment Task Force (19).
PedsQL 4.0 Generic Core Scales (20)
The PedsQL is a validated, 23-item modular instrument designed to measure HRQoL in children and adolescents. The PedsQL assesses HRQoL across 4 domains: Physical, Emotional, Social and School Functioning. The PedsQL also yields 3 summary scores: Total scale score, Physical Health Summary and Psychosocial Health Summary. It is a 5-level Likert item survey (0–4) with reversed scored answers that are linearly transformed to a 0–100 scale, with higher scores reflecting higher HRQoL. The published validation study identified a value one standard deviation below the population mean for the PedsQL Total Score (69.7 for child self-report and 65.4 for parent-proxy) as a threshold score for an at-risk status for impaired HRQoL relative to the population sample (21).
Health Status Variables
The health status variables obtained for each participant were acute cellular rejection episodes for the year prior to participation, time since transplantation, liver function tests (TBili, AST, ALT), and number of immunosuppressants at the time of participation.
Statistical Methods
Descriptive statistics (i.e., M, SD, percentages) were conducted on demographic, health status variables, PSQ subscales and PedsQL subscales. The prevalence of sleep problems was calculated using the proportion of positive symptom items for the PSQ subscales (15, 22). Paired sample t-tests were used to assess for statistical differences between the parent and child reported HRQoL. Hierarchical multiple regression analyses examining the contribution of sleep problems, as measured by the PSQ, to child HRQoL were conducted in order to test the primary hypothesis.
Finally, differences in scores on the PSQ among children with high HRQoL and low HRQoL as measured by the PedsQL Total Score were examined using independent sample t-tests. All analyses were conducted using SPSS for Windows (Version 18.0, SPSS, Inc., Chicago, IL, USA).
RESULTS
Participant Characteristics
Participants included 47 liver transplant recipients. Clinical and demographic information is presented in Table 1. The mean age of patients at the time of study participation was 10.9 years ± 4.6 (range 2–17.6 years; median 11.2 ± 4.6 years), and the mean time since transplant was 6.2± 3.9 years (range 7-months-15.4 years; median 5.6 ± 3.9 years). The majority of patients were white (55%) and female (55%). The primary indication for transplantation was biliary atresia (51%), and the primary immunosuppressant medication used was tacrolimus (98%).
Table 1.
Pediatric Liver Transplant Recipient Characteristics(n=47)
| Age | 10.9 years ± 4.6 years |
| Time since transplant | 6.2 years ± 3.9 years |
| Gender | n (%) |
| Female | 26 (55) |
| Male | 21 (45) |
| Race | |
| White | 26 (55) |
| African-American | 14 (30) |
| Other | 7 (15) |
| Diagnoses | |
| Biliary Atresia | 24 (51) |
| Acute Liver Failure | 6 (13) |
| Hepatoblastoma | 3 (6) |
| Alagille Syndrome | 2 (4) |
| Choledochal Cyst | 2 (4) |
| Arginosuccinic Aciduria | 2 (4) |
| A1ATP deficiency | 1 (2) |
| Autoimmune Hepatitis | 1 (2) |
| Budd-Chiari Syndrome | 1 (2) |
| Byler Syndrome | 1 (2) |
| Hemangioendothelioma | 1 (2) |
| Tyrosinemia | 1 (2) |
| PFIC | 1 (2) |
| NASH | 1 (2) |
| Immunosuppressant Medications | |
| Tacrolimus | 46 (98) |
| Cellcept | 13 (28) |
| Prednisone | 10 (21) |
| Rejection Episodes | |
| None | 39 (83) |
| 1 | 7 (15) |
| 2 | 1 (2) |
Prevalence of Sleep-Related Symptoms in Pediatric Liver Transplant Recipients
Based on parent report, pediatric LTR in the present study had symptoms of sleep disordered breathing, excessive daytime sleepiness, daytime behavior and restless legs (Table 2).
Table 2.
Percentage of Pediatric Liver Transplant Recipients with Significant Sleep Problems as measured by the PSQ
| Yes (Score≥0.33) | No (Score<0.33) | |
|---|---|---|
| SRBD Index | 23.4% | 76.6% |
| Snoring subscale | 17.0% | 83.0% |
| Sleepiness subscale | 40.4% | 59.6% |
| Behavior subscale | 45.5% | 54.5% |
| PLMS/RLS Index | 29.8% | 70.2% |
The mean SRBD score for the sample of liver transplant recipients was 0.23±0.16, which indicated that 23% of the subscale items were positive. A score suggestive of SRBD was found in 11 (23.4%) of children. The mean PLMS/RLS Index score was 0.23±0.21, with 14 (29.8%) participants exceeding the clinical cut-off score.
With respect to the PSQ subscales that comprise the SRBD Index, the mean Snoring subscale score was 0.17±0.27, with habitual snoring, defined as snoring more than half the time, reported in 8 (17%) of children. The mean EDS subscale score for LTR was 0.28±0.30, with 19 (40.4%) participants exceeding the clinical cut-off score of 0.33. Lastly, the mean Behavioral Index score for the pediatric LT cohort was 0.30±0.33. Significant symptoms of inattention and hyperactivity were reported in 21 (45.5%) of pediatric LTR. Snoring was significantly related to younger age (r = −0.345, p=0.016). There were no other significant associations with age, gender, or time since transplantation. There were no significant differences across domains of Sleepiness, PLMS/RLS or Behavior with respect to age, gender or time since transplantation.
Health Related Quality of Life
No significant differences were found between parent-proxy report and child self-reported quality of life on the PedsQL. Using the published cut-off scores of 65.43 for parent-proxy report and 69.71 for child self-report, 40.4% of parents and 43.8% of children reported low Total HRQoL. Age, time since transplantation and health status variables were not significantly related to quality of life.
Relationship between Sleep problems and HRQoL
Parent-Proxy Report of Child HRQoL
Hierarchical regression analyses were conducted to examine the contribution of SRBD to parent-proxy report of child HRQoL. Age and time since transplant were entered as Block 1, and the SRBD scale was entered into Block 2. The regression analyses revealed that the SRBD subscale of the PSQ accounted for significant variance in parent-proxy reports on the PedsQL summary scales measuring child Psychosocial Health (R2=0.38, p < 0.001), Physical Health (R2=0.19, p=0.004), and Total HRQoL (R2 =0.35, p < 0.0001). In addition, SRBD accounted for significant variance across all subscales of the parent-proxy PedsQL measure (Table 3).
Table 3.
Regression Analyses of Parent-proxy Report of Child HRQoL and Sleep-Related Breathing Disorder (SRBD)
| Model | PedsQL 4.0 | Step | Variable | β | t for within step predictors |
R2 | R2 change for step |
F change | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Total HRQoL | 1 | Age | −0.10 | −0.06 | 0.02 | 0.02 | 0.33 | 0.72 |
| Time Since Transplant |
−0.03 | −0.15 | |||||||
| 2 | SRBD | −0.60** | −4.7 | 0.35 | 0.33 | 21.9** | 0.0001 | ||
| 2 | Psychosocial Health | 1 | Age | −0.06 | −0.35 | 0.02 | 0.02 | 0.034 | .071 |
| Time Since Transplant |
−0.07 | −0.41 | |||||||
| 2 | SRBD | −0.61** | −4.81 | 0.36 | 0.35 | 23.16** | 0.0001 | ||
| 3 | Physical Functioning |
1 | Age | −0.17 | −0.931 | 0.02 | 0.02 | 0.44 | 0.65 |
| Time Since Transplant |
0.09 | 0.47 | |||||||
| 2 | SRBD | −0.44** | −3.04 | 0.19 | 0.17 | 9.24** | 0.004 | ||
| 4 | Emotional Functioning |
1 | Age | −0.01 | −0.03 | 0.02 | 0.02 | 0.48 | 0.623 |
| Time Since Transplant |
−.14 | −.79 | |||||||
| 2 | SRBD | −0.5** | −3.62 | 0.25 | 0.23 | 13.10** | 0.001 | ||
| 5 | Social Functioning | 1 | Age | −.01 | −.05 | 0.003 | 0.003 | 0.07 | 0.93 |
| Time Since Transplant |
−.05 | −.28 | |||||||
| 2 | SRBD | −.42** | −2.86 | 0.16 | 0.16 | 8.17** | 0.007 | ||
| 6 | School Functioning | 1 | Age | −.08 | −.44 | 0.005 | 0.005 | 0.10 | 0.908 |
| Time Since Transplant |
.03 | .17 | |||||||
| 2 | SRBD | −.63** | −4.89 | 0.38 | 0.37 | 23.88** | 0.000 |
p<0.001
To determine which domain of the PSQ had the greatest impact on quality of life, the Sleepiness, Snoring, PLMS/RLS and Behavior subscale scores were entered into hierarchical regression analyses. Age and time since transplant were entered as Block 1 into the overall HRQoL model. Sleepiness, Snoring, PLMS/RLS, and Behavior scores were entered in the 2nd block. There were no significant associations between health status variables and quality of life or sleep at the bivariate level, thus health status variables were not entered into the regression analyses (Table 4).
Table 4.
Hierarchical Regression Analyses of Sleep Problems and Parent-proxy Report of Child HRQoL
| Model | PedsQL Domain | Step | Variables | β | t for within step predictors |
R2 change for step |
F change | Sig. |
|---|---|---|---|---|---|---|---|---|
| 1 | Total HRQoL | 1 | Age | −0.54 | −0.29 | 0.009 | 0.009 | 0.837 |
| Time Since Transplant |
−0.52 | −0.28 | ||||||
| Snoring | −0.12 | −0.847 | 0.366 | 5.410** | 0.002 | |||
| 2 | EDS | −0.20 | −1.22 | |||||
| PLMS/RLS | −0.30 | −1.87† | ||||||
| BEH | −0.27 | −1.78† | ||||||
| 2 | Psychosocial Functioning | 1 | Age | −0.01 | −0.03 | 0.01 | 0.17 | 0.84 |
| Time Since Transplant |
−0.09 | −0.48 | ||||||
| Snoring | −0.14 | −0.95 | 0.36 | 5.20** | 0.00 | |||
| 2 | EDS | −0.18 | −1.09 | |||||
| PLMS/RLS | −0.30 | −1.85† | ||||||
| BEH | −0.27 | −1.80† | ||||||
| 3 | Physical Functioning | 1 | Age | −0.16 | −0.86 | 0.02 | 0.40 | 0.67 |
| Time Since Transplant |
0.04 | 0.24 | ||||||
| Snoring | −0.07 | −0.43 | 0.22 | 2.69* | 0.05 | |||
| 2 | EDS | −0.20 | −1.11 | |||||
| PLMS/RLS | −0.22 | −1.25 | ||||||
| BEH | −0.18 | −1.11 | ||||||
| 4 | Emotional Functioning | 1 | Age | 0.03 | 0.16 | 0.02 | 0.32 | 0.73 |
| Time Since Transplant |
−0.14 | −0.75 | ||||||
| Snoring | −0.23 | −1.53 | 0.33 | 4.61** | 0.00 | |||
| 2 | EDS | −0.09 | −0.53 | |||||
| PLMS/RLS | −0.36 | −2.21* | ||||||
| BEH | −0.14 | −0.93 | ||||||
| 5 | Social Functioning | 1 | Age | −0.03 | −0.14 | 0.01 | 0.17 | 0.84 |
| Time Since Transplant |
−0.08 | −0.41 | ||||||
| Snoring | −0.05 | −0.28 | 0.22 | 2.63* | 0.05 | |||
| 2 | EDS | −0.08 | −0.45 | |||||
| PLMS/RLS | −0.32 | −1.81† | ||||||
| BEH | −0.22 | −1.30 | ||||||
| 6 | School Functioning | 1 | Age | 0.10 | 0.56 | 0.01 | 0.25 | 0.78 |
| Time Since Transplant |
0.02 | 0.13 | ||||||
| Snoring | −0.06 | −0.36 | 0.30 | 3.73** | 0.01 | |||
| 2 | EDS | −0.25 | −1.41 | |||||
| PLMS/RLS | −0.09 | −0.53 | ||||||
| BEH | −0.37 | −2.17* |
β = standardized coefficients.
p<0.05,
p<0.01,
p<0.1
Child Self-Report of HRQoL
Hierarchical regression analyses were conducted to examine the contribution of SRBD to child self-reported HRQoL. Age and time since transplant were entered as Block 1, and the SRBD scale was entered into Block 2. Regression models for Psychosocial Health, Physical Health or Total HRQoL summary scales were not significant. Across the subscales of the PedsQL, the SRBD scale accounted for significant variance on the child self-reported school functioning scale (R2 = 0.18, p=0.033). There were no other significant associations between the child reported HRQoL and the SRBD scale, age, or time since transplantation.
To explore which domain of the PSQ had the greatest impact on the School Functioning domain of the PedsQL, subscale scores were entered into a hierarchical linear regression as described above. This regression model did not account for significant variance in child HRQoL (R2 =0.28, p=0.11).
Between Group Comparisons
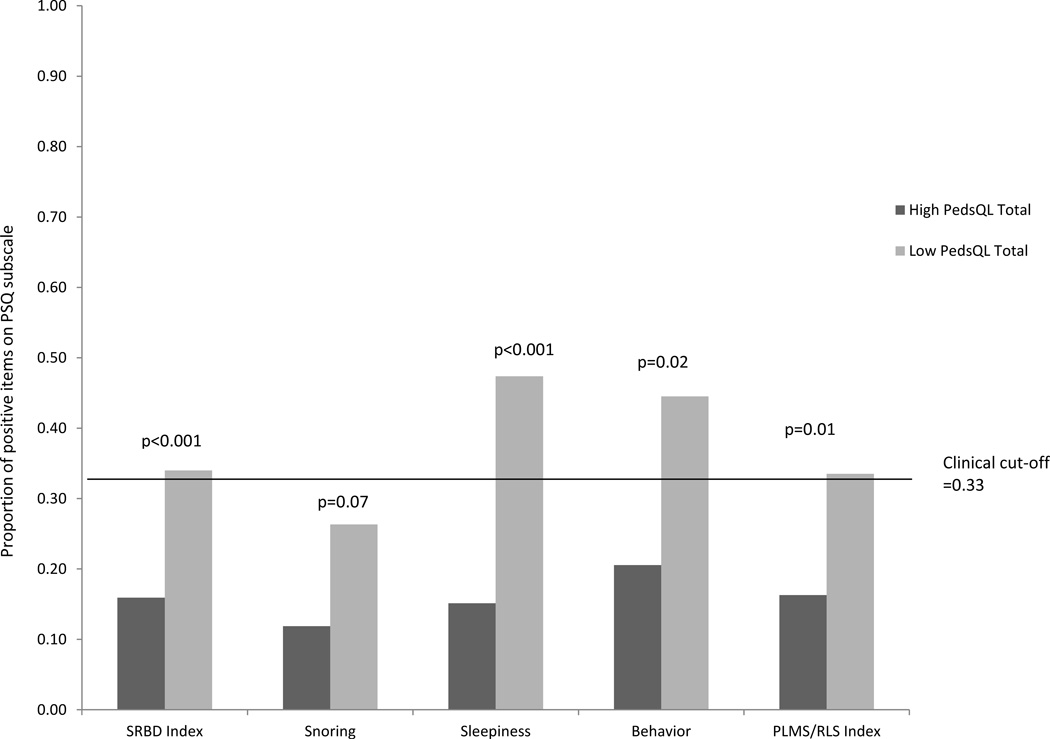
To examine whether sleep problems were differentially present among children with high and low quality of life, we conducted between group analyses using the published cut-off scores of 65.43 for parent-reported Total HRQoL and 69.71 for self- reported Total HRQoL. Clinically significant sleep problems on the PSQ (cut-off score of >0.33) were more common among children with low Total HRQoL (Table 6). There were no significant group differences based on child self-report. Based on parental reports, there were significant differences across all domains of the PSQ except snoring between the high HRQoL group compared to the low HRQoL group (Figure 1).
Figure 1.
Comparison of Sleep Problems between children with high and low HRQoL based on parent report on the PedsQL
Sleep Problems and Health Status
The Snoring subscale of the PSQ was negatively correlated with child age (r = −0.335, p=0.022), and positively correlated with AST (r = 0.366, p=0.013) and total bilirubin (r = 0.433, p=0.003). There were no significant associations between the remaining subscales of the PSQ and child age, time since transplant, weight, height, BMI, liver function tests or immunosuppressant medications.
To determine if elevations on the SRBD scale, which includes questions related to snoring, were related to tonsillar hypertrophy, we conducted a chart review for the 11 patients with SRBD scores in the clinical range (>0.33). Of these, 4 patients had undergone tonsillectomy with (N=3) or without (N=1) adenoidectomy due to tonsillar hypertrophy, elevated EBV and, in one patient, a history of PTLD. The remaining 7 patients did not have evidence of elevated EBV or tonsillar hypertrophy. There was no significant difference in SRBD scores between patients with significant EBV/tonsillar hypertrophy and those who did not (p=0.07).
DISCUSSION
The present study examined the prevalence of sleep problems in pediatric LTR and the impact of sleep problems on health-related quality of life. Based on parental report, sleep-related disturbances and daytime behavior difficulties were prevalent in our cohort of pediatric liver transplant recipients. Results of the current study are consistent with existing literature indicating that pediatric LTR have low HRQoL compared to healthy peers (3). Approximately 40% of participants had substandard quality of life based on parental and/or self-reports. Likewise, daytime behavior problems consistent with symptoms of inattention/hyperactivity were reported with high frequency. It is unclear whether these daytime symptoms are secondary to poor sleep or related to cognitive dysfunction (23, 24).
Sleep disturbances were common in our sample. Nearly 25% of parents endorsed clinically significant symptoms of SRBD in their child. This is significantly higher than the rate of 11% observed in a general pediatric clinic sample (18). When examining the components of the SRBD scale, it appears that excessive daytime sleepiness was a significant contributor to the elevated scores on this index. Indeed, snoring, which is a primary symptom of SRBD, was comparable to rates (17%) previously reported in children (18). In contrast, excessive daytime sleepiness was reported in nearly half of our sample of pediatric LTR, which is more than double the rate observed among children presenting for well-child visits (18). This suggests that daytime sleepiness is a significant concern among pediatric liver transplant recipients. In addition, nearly a third of pediatric LTR in this study exceeded clinical cut-off scores on the PLMS/RLS Index. This rate is significantly higher than the rates of 2–17% observed in population-based (23) and community-based pediatric samples (25). Preliminary research among adult liver transplant recipients suggests that poor sleep quality, fatigue and RLS are common following transplantation (6–8). The associations between RLS, fatigue and HRQoL in a pediatric transplant population remain an area for further study.
In studies conducted with adult liver transplant recipients, symptoms of sleep difficulties were related to higher body mass index and mood disorders (6, 7). In the current study, sleep difficulties were not related to body mass index, time since transplantation, or other measured health status variable. It is surprising that the child’s health status, as measured in this study, was not related to measures of perceived HRQoL or sleep disruption. This could be related to the possibility that perceived sleep problems and poor HRQoL in this population are not associated with physical health problems, but rather to behavioral/emotional health. The causes of daytime fatigue and PLMS/RLS in our pediatric liver transplant population are unknown. There are various factors that may contribute to poor sleep quality in children, including poor sleep hygiene, mood disturbances, obesity, and underlying primary sleep disorders (e.g., obstructive sleep apnea, PLMS/RLS) (15, 26–28). With respect to PLMS/RLS, which can have a substantial impact on sleep quality and quantity, the etiology in pediatric populations is not definitive. Research suggests that serum ferritin levels below 50 ng/mL may contribute to the severity of RLS symptoms (29). Moreover, given the association between RLS/PLMS and attention-deficit/hyperactivity disorder (25), it has been hypothesized that lower iron status and dopaminergic mechanisms are common factors in the pathophysiology of these conditions (29). Given the high rates of RLS and daytime behavior difficulties reported in this study sample, further investigation of potential risk factors, such as suboptimal ferritin levels, is warranted.
Medications can also contribute to sleep disturbances. Indeed, steroids have been shown to be associated with sleep disturbances (30, 31) and tacrolimus, a calcineurin inhibitor, has been associated with neuropsychological, behavioral side effects and insomnia in pediatric renal transplant recipients (32). In the present study, scores on the PSQ were unrelated to type of immunosuppressant medications. However, very few participants were receiving steroid treatment (N=10). Further large scale studies are required to elucidate potential predictors of sleep disturbances, including PLMS/RLS and daytime fatigue, in a pediatric transplant population.
When examining the impact of sleep problems on HRQoL, a disproportionate number of children with significant sleep problems were among those with low total HRQoL on the PedsQL. The SRBD scale, which measures nighttime breathing difficulties, excessive daytime sleepiness, and inattentive-hyperactive behaviors, accounted for significant variance in child quality of life. Further analyses revealed that the subscales measuring excessive daytime sleepiness and symptoms of periodic limb movement/restless legs had the greatest impact on quality of life. Child-reported school functioning was most impacted by the daytime behavioral domain of the SRBD.
The results of our preliminary study suggest that pediatric LTR may be at increased risk for sleep problems, daytime sleepiness, and associated daytime behavioral difficulties, which have the potential to negatively impact HRQoL. Among healthy children, sleep problems are associated with increased daytime behavior problems, emotional difficulties, and academic and cognitive deficits (33–35). There is increasing research to support that pediatric LTR are at risk for poor school functioning (36, 37) and low HRQOL (3). However, modifiable factors contributing to deficits in cognitive functioning and HRQoL remain relatively unknown. It is possible that lower HRQoL could be ameliorated by the identification and treatment of sleep problems and daytime sleepiness.
Untreated sleep disorders may lead to poor health outcomes, including potential deleterious effects on the cardiovascular and immune systems (28, 38, 39). In addition, the correlation of sleep problems and lower HRQoL raises the possibility that interventions aimed at improving sleep (12, 40–42), could potentially have a positive impact on academic and behavioral functioning in pediatric LTR. Thus, the screening and appropriate treatment of sleep disorders in among pediatric LTR may contribute to better long-term health outcomes.
Our study was limited in being a cross-sectional, small single-center study. The ability to detect group differences based on child self-report was limited by insufficient power. Moreover, sleep problems were measured using a subjective questionnaire completed by parents, as opposed to objective measures of sleep disruption such as overnight polysomnography. Moreover, the association between sleep and HRQoL is unlikely to be unidirectional. Individuals with poor HRQoL, particularly in the areas of emotional functioning, may have increased risk for sleep difficulties, including insomnia and daytime fatigue. Likewise, this study did not investigate the level of family distress, which may also impact a child’s HRQoL and sleep patterns. As the measures used in the current study have not been validated in children <2 years of age, we did not assess sleep disruption and perceived quality of life of pediatric liver transplant recipients <2 years of age. It is premature to generalize findings from this study to the very young pediatric LTR population. Nonetheless, these data suggest there is a need for increased awareness of sleep disturbances among clinicians who care for pediatric liver transplant recipients. Further prospective multi-center studies are needed to determine underlying factors contributing to the association between sleep disturbances and poor HRQoL in pediatric liver transplant recipients.
Despite study limitations, the results of the current pilot study have potential clinical implications. Based on early results of this study, our clinic has begun routinely screening for the presence of sleep problems using the BEARS screening method, which briefly assesses for Breathing, Excessive sleepiness, Arousals, Regularity of sleep and Snoring (43). Patients who have positive screens are referred to our pediatric sleep clinic for further evaluation and treatment as needed. We continue to prospectively study sleep across pre- to post-transplant period to identify prevalence rates and potential risk factors. Future directions include the use of objective sleep measures such as polysomnography and actigraphy.
Summary
In summary, sleep problems were common in this cohort of pediatric liver transplant recipients and predicted significant variance in parent-proxy reports of HRQoL. At this point, it remains unclear if this association is mediated by symptoms of underlying sleep disorders, excessive daytime sleepiness, or associated daytime behavior problems. Yet, the significant relationships between sleep difficulties and HRQoL provide guidance for future assessment and intervention, as sleep disorders are readily modifiable. Prospective larger scale studies are needed to assess factors that may lead to sleep difficulties and low HRQoL in this population. Appropriate detection of significant sleep problems may lead to interventions that could benefit the quality of life of pediatric LTR.
Table 5.
Percentages of children with significant sleep problems by HRQoL level as measured by parent-proxy report on PedsQL
| PSQ Domain | High Total HRQoL (>65.43) |
Low Total HRQoL (≤65.43) |
|---|---|---|
| SRBD Index | 10.0% | 42.0% |
| Snoring subscale | 14.0% | 21.0% |
| Sleepiness subscale | 21.0% | 68.0% |
| Behavior subscale | 32.0% | 57.9% |
| RLS/PLMS Index | 17.9% | 47.0% |
Acknowledgments
This publication was made possible by Grant Number UL1RR024986 from NCRR CTSA funding to Dr. Fredericks. We thank the families who volunteered to participate in this project. We also acknowledge the contributions of Rebecca Gleit, Radhika Raghunathan, and Veronica Juan. We thank Dr. Louise O’Brien for her thoughtful review of a previous version of this manuscript.
Abbreviations
- LTR
liver transplant recipients
- HRQoL
Health-related quality of life
- PedsQL
Pediatric Quality of Life
- PSQ
Pediatric Sleep Questionnaire
- PLMS
Periodic Limb Movements during Sleep
- RLS
Restless Leg Symptoms
- SRBD
Sleep-Related Breathing Disorder
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- TBili
total bilirubin
REFERENCES
- 1.Bucuvalas JC, Britto M, Krug S, Ryckman FC, Atherton H, Alonso MP, et al. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transpl. 2003 Jan;9(1):62–71. doi: 10.1053/jlts.2003.50012. [DOI] [PubMed] [Google Scholar]
- 2.Fredericks EM, Magee JC, Opipari-Arrigan L, Shieck V, Well A, Lopez MJ. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatr Transplant. 2008 May;12(3):289–299. doi: 10.1111/j.1399-3046.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 3.Alonso EM, Limbers CA, Neighbors K, Martz K, Bucuvalas JC, Webb T, et al. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. J Pediatr. 2010 Feb;156(2):270, 276, e1. doi: 10.1016/j.jpeds.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso EM, Neighbors K, Mattson C, Sweet E, Ruch-Ross H, Berry C, et al. Functional outcomes of pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2003 Aug;37(2):155–160. doi: 10.1097/00005176-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Weissberg-Benchell J, Zielinski TE, Rodgers S, Greenley RN, Askenazi D, Goldstein SL, et al. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQL transplant module. Am J Transplant. 2010 Jul;10(7):1677–1685. doi: 10.1111/j.1600-6143.2010.03149.x. [DOI] [PubMed] [Google Scholar]
- 6.Walters AS, Pichietti D, Chervin RD, Konofal E. Childhood and adult factors associated with restless legs syndrome (RLS) diagnosis. Sleep Med. 2009 Feb;10(2):267–268. doi: 10.1016/j.sleep.2007.12.013. author reply 9. [DOI] [PubMed] [Google Scholar]
- 7.van Ginneken BT, van den Berg-Emons RJ, van der Windt A, Tilanus HW, Metselaar HJ, Stam HJ, et al. Persistent fatigue in liver transplant recipients: a two-year follow-up study. Clin Transplant. 2010 Jan-Feb;24(1):E10–E16. doi: 10.1111/j.1399-0012.2009.01083.x. [DOI] [PubMed] [Google Scholar]
- 8.Franco RA, Rizvi SM, Franco J, Graf L, Rizvi F. Excess prevalence of RLS in liver transplant recipients - A tertiary care center experience. Sleep. 2010;Volume 33 Abstract Supplement:[Abstract #0890] [Google Scholar]
- 9.Hart CN, Palermo TM, Rosen CL. Health-related quality of life among children presenting to a pediatric sleep disorders clinic. Behav Sleep Med. 2005;3(1):4–17. doi: 10.1207/s15402010bsm0301_3. [DOI] [PubMed] [Google Scholar]
- 10.Shin C, Kim J, Lee S, Ahn Y, Joo S. Sleep habits, excessive daytime sleepiness and school performance in high school students. Psychiatry Clin Neurosci. 2003 Aug;57(4):451–453. doi: 10.1046/j.1440-1819.2003.01146.x. [DOI] [PubMed] [Google Scholar]
- 11.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002 Mar-Apr;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 12.Lima Junior JM, Silva VC, Freitas MR. Long term results in the life quality of children with obstructive sleep disorders submitted to adenoidectomy/adenotonsillectomy. Braz J Otorhinolaryngol. 2008 Sep-Oct;74(5):718–724. doi: 10.1016/S1808-8694(15)31382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, et al. Neurobehavioral correlates of sleep-disordered breathing in children. Journal of Sleep Research. 2004;13(2):165–172. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Bourke R, Anderson V, Yang JSC, Jackman AR, Killedar A, Nixon GM, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Medicine. 2011;12(5):489–496. doi: 10.1016/j.sleep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000 Feb 1;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 16.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001 Nov;2(6):501–510. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 17.Chervin RD, Weatherly RA, Ruzicka DL, Burns JW, Giordani BJ, Dillon JE, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep. 2006 Apr 1;29(4):495–503. [PMC free article] [PubMed] [Google Scholar]
- 18.Archbold KH, Pituch KJ, Panahi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002 Jan;140(1):97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- 19.Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-Based Review of Subjective Pediatric Sleep Measures. Journal of Pediatric Psychology. 2011 Jan 11; doi: 10.1093/jpepsy/jsq119. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL(TM) 4.0 as a Pediatric Population Health Measure: Feasibility, Reliability, and Validity. Ambulatory Pediatrics. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Chervin RD, Archbold KH, Panahi P, Pituch KJ. Sleep problems seldom addressed at two general pediatric clinics. Pediatrics. 2001 Jun;107(6):1375–1380. doi: 10.1542/peds.107.6.1375. [DOI] [PubMed] [Google Scholar]
- 23.Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents--the Peds REST study. Pediatrics. 2007 Aug;120(2):253–266. doi: 10.1542/peds.2006-2767. [DOI] [PubMed] [Google Scholar]
- 24.Picchietti DL, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008 Oct;9(7):770–781. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Chervin RD, Archbold KH, Dillon JE, Pituch KJ, Panahi P, Dahl RE, et al. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. Sleep. 2002 Mar 15;25(2):213–218. [PubMed] [Google Scholar]
- 26.Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, Dahl RE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002 Mar;109(3):449–456. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and Remission of Sleep-Disordered Breathing and Related Symptoms in 6- to 17-Year Old Children-The Tucson Children's Assessment of Sleep Apnea Study. J Pediatr. Mar 19; doi: 10.1016/j.jpeds.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008 Sep;35(3):533–546. vii. doi: 10.1016/j.pop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Picchietti MA, Picchietti DL. Restless Legs Syndrome and Periodic Limb Movement Disorder in Children and Adolescents. Seminars in Pediatric Neurology. 2008;15(2):91–99. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Turner R, Elson E. Sleep disorders. Steroids cause sleep disturbance. BMJ. 1993 May 29;306(6890):1477–1478. doi: 10.1136/bmj.306.6890.1477-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrousos GA, Kattah JC, Beck RW, Cleary PA. Side effects of glucocorticoid treatment. Experience of the Optic Neuritis Treatment Trial. JAMA. 1993 Apr 28;269(16):2110–2112. [PubMed] [Google Scholar]
- 32.Kemper MJ, Sparta G, Laube GF, Miozzari M, Neuhaus TJ. Neuropsychologic side-effects of tacrolimus in pediatric renal transplantation. Clin Transplant. 2003 Apr;17(2):130–134. doi: 10.1034/j.1399-0012.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 33.Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006 Oct;10(5):323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Chorney DB, Detweiler MF, Morris TL, Kuhn BR. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008 May;33(4):339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- 35.Chung KF, Cheung MM. Sleep-wake patterns and sleep disturbance among Hong Kong Chinese adolescents. Sleep. 2008 Feb 1;31(2):185–194. doi: 10.1093/sleep/31.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krull K, Fuchs C, Yurk H, Boone P, Alonso E. Neurocognitive outcome in pediatric liver transplant recipients. Pediatr Transplant. 2003 Apr;7(2):111–118. doi: 10.1034/j.1399-3046.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 37.Gilmour S, Adkins R, Liddell GA, Jhangri G, Robertson CM. Assessment of psychoeducational outcomes after pediatric liver transplant. Am J Transplant. 2009 Feb;9(2):294–300. doi: 10.1111/j.1600-6143.2008.02480.x. [DOI] [PubMed] [Google Scholar]
- 38.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation's children. Pediatrics. 2007 Feb;119(Suppl 1):S29–S37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- 39.Horne RSC, Yang JSC, Walter LM, Richardson HL, O'Driscoll DM, Foster AM, et al. Elevated Blood Pressure During Sleep and Wake in Children With Sleep-Disordered Breathing. Pediatrics. 2011 Jul 1;128(1):e85–e92. doi: 10.1542/peds.2010-3431. 2011. [DOI] [PubMed] [Google Scholar]
- 40.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009 Aug 15;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 41.Owens JA, Moturi S. Pharmacologic treatment of pediatric insomnia. Child Adolesc Psychiatr Clin N Am. 2009 Oct;18(4):1001–1016. doi: 10.1016/j.chc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell RB, Boss EF. Pediatric obstructive sleep apnea in obese and normal-weight children: impact of adenotonsillectomy on quality-of-life and behavior. Dev Neuropsychol. 2009 Sep;34(5):650–661. doi: 10.1080/87565640903133657. [DOI] [PubMed] [Google Scholar]
- 43.Owens JA, Dalzell V. Use of the 'BEARS' sleep screening tool in a pediatric residents' continuity clinic: a pilot study. Sleep Med. 2005 Jan;6(1):63–69. doi: 10.1016/j.sleep.2004.07.015. [DOI] [PubMed] [Google Scholar]