Abstract
Like illness-inducing agents (e.g., lithium chloride), drugs of abuse also suppress intake of a taste solution. To explore the nature of this drug-induced intake reduction, in the current study three aqueous stimuli with different initial values served as the conditioned stimuli (CSs) that were paired with a standard dose of amphetamine in a voluntary intake procedure and lick patterns were analyzed. Consistent with earlier studies, amphetamine significantly reduced intake of all three CSs (quinine, sodium chloride and orange odor). In contrast to studies that analyze orofacial responses, we found that lick cluster size was significantly lowered by amphetamine, indicating that the psychoactive drug induced a conditioned change in CS palatability.
Keywords: licking, microstructure, cluster size, amphetamine, rats
Taste aversions can be acquired following the consumption of toxic food. In the terminology of Pavlovian conditioning, the taste of the food is the conditioned stimulus (CS) and the consequent illness functions as the unconditioned stimulus (US). A wide variety of agents, ranging from radiation to chemicals to vestibular disorientation, have been used to induce illness (see Barker, Best & Domjan, 1977; Braveman and Bronstein, 1985; Milgram, Krames & Alloway, 1977; Reilly & Schachtman, 2009). Conditioned taste aversions (CTAs) are manifest in behavior as a reluctance to consume the CS on subsequent encounters. Indeed, since CTA was introduced into the laboratory in the 1950s (see Freeman & Riley, 2009, for a review), a reduction in the amount of CS consumed is the hallmark of CTA. Furthermore, the strength of the acquired taste aversion is, within limits, directly related to the intensity of the illness inducing agent – the higher the dose the greater the reduction in CS intake (e.g., Elkins, 1973; Garcia, Kimeldorf & Koelling, 1955; Green & Rachlin, 1976; Nachman & Ashe, 1973; Revusky, 1968).
In addition to illness-inducing agents, contingent administration of a drug of abuse (e.g., amphetamine, cocaine, ethanol, morphine, nicotine) can also condition a reduction in CS intake (Cappell & LeBlanc, 1971; Cappell, LeBlanc & Endrenyi, 1973; Carey, 1973; Kumar, Pratt & Stolerman, 1983; Nachman, Lester & Le Magnen, 1970; Riley, Jacobs & LoLordo, 1978). Moreover, as with illness-induced CTAs, the strength of the drug-induced aversion is directly related to the dose of the psychoactive drug (e.g., Berger, 1972; Davison & House, 1975; Nathan & Vogel, 1975). Given that the behavioral effect of the drug of abuse on CS intake appeared identical to the behavioral effect of the illness US, it is not surprising that such drugs were considered to support the development of CTAs. However, this analysis of drug-induced CS suppression ran into the problem that the same drugs were self-administered by human and non-human animals (i.e., they are rewarding). The counterclaim was that drugs of abuse have both aversive and rewarding properties and that the conditioned reduction of CS intake was based on the aversive properties of the drugs. The debate about the nature of the intake suppression was, in part, a debate about the sensitivity of the dependent measure to differentiate between illness- and drug-induced reductions of CS intake. Thus, alternative ways to assess ingestive behavior were needed to resolve this issue and provide a more accurate characterization of performance when a taste is paired with a psychoactive drug.
The first of these alternative methodologies, taste reactivity (TR), was developed for use with rodents by Grill and Norgren (1978) and involved intraoral infusions (via an indwelling oral cannula) of small, controlled volumes of a fluid while orofacial and somatic responses were videotaped for later off-line analysis. The elicted stereotyped behaviors were categorized into two response domains: ingestive (e.g., mouth movements, tongue protrusions, lateral tongue protrusions, lip flaring, paw licking and swallowing) or aversive (e.g., gapes, chin rubs, forelimb flailing, head shakes) responses (Grill & Berridge, 1985; Grill & Norgren, 1978; Grill, Spector, Schwartz, Kaplan & Flynn, 1987). Given the brief duration of the intraoral infusions, TR responses were considered to be particularly sensitive to information carried by the taste of the fluid and to minimize the influence of postingestive factors. Taste stimuli, like sucrose, that are normally preferred elicit ingestive TR responses whereas stimuli, like quinine, that are normally non-preferred elicit aversive TR responses (Berridge & Grill, 1983; Grill & Norgren, 1978; Steiner, Glaser, Hawilo, & Berridge, 2001).
Not only can the TR test be used to assess the unconditioned value of taste stimuli it can also be used to assess the conditioned value of taste stimuli. Of relevance to present purposes, it has been shown that lithium chloride (LiCl, the quintessential illness-inducing agent) results in a reduction in the frequency of ingestive responses and a concomitant increase in the number of aversion TR responses (Berridge, Grill, & Norgren, 1981; Davies & Wellman, 1990; Parker, 1982; Pelchat, Grill, Rozin & Jacobs, 1983; Spector, Breslin & Grill, 1988), a pattern of performance that is interpreted as a conditioned reduction in the palatability of the associated taste CS. Thus, the CTA results obtained using the TR test corresponded well with the results obtained using voluntary intake in the traditional test for CTA learning. If drugs of abuse induce CTAs then they would be expected to elicit the same pattern of TR responses to the CS as found following taste-illness pairings. This prediction, however, has found no support. In an influential series of studies Parker and colleagues reported that drugs of abuse such as amphetamine (Parker, 1982, 1988, 1991; Parker & Carvell, 1986; Zalaquett & Parker, 1989), cocaine (Mayer & Parker, 1993; Parker, 1993), LSD (Parker, 1996), morphine (Parker, 1988, 1991), and nicotine (Parker & Carvel, 1986), in doses that are rewarding in other procedures, were not found to induce a conditioned change in the frequency of aversive TR responses to the associated taste CS. These results provided the foundation for the view that due to the absence of increased aversive responding, the palatability of the CS remains unchanged following taste-drug pairings and that the reduction of CS intake by drugs of abuse is not an instance of CTA; rather it is an example of conditioned taste avoidance (Parker, 1995, 2003; Parker, Limebeer & Rana, 2009).
However, for a number of reasons caution needs to be exercised before accepting this analysis derived from TR methodology. First, there are many obvious differences in the TR procedure relative to the traditional consumption method. Whereas in the latter the fluid deprived subject is allowed to drink voluntarily at its own pace, the former is an involuntary intake method that is typically used with animals that are water replete and in which small amounts (or short durations) of fluid are infused directly into the mouth. Although the TR method of passively presenting fluid affords more precision and experimental control, it is also more unnatural than voluntary drinking and this may have consequences. Second, the CSs used in many of the drug of abuse experiments have been highly preferred sweet-tasting (to humans) stimuli such as 0.5 M sucrose. It is possible that the use of such highly rewarding and palatable stimuli may delay learning (see, for example, Gomez & Grigson, 1999). Finally, the TR response patterns to drug-paired taste CSs are interpreted as null effects, and such outcomes do not always provide a solid basis for strong conclusions. These concerns raise questions about the sensitivity of the dependent measure to detect differences in learning (in this case, drug-induced changes in taste palatability).
A different approach to taste palatability involves detailed analysis of the temporal microstructure of lick patterns in what otherwise is a standard voluntary intake task. When drinking, rats do not lick continuously during the fluid access period. Rather, they make sustained runs (herein termed clusters) of rapid licks followed by pauses of varying durations. For unconditionally preferred taste stimuli like sucrose, the number of licks in a cluster (i.e., cluster size) monotonically increases as concentration increases, even though there is an inverted U-shaped function for intake of sucrose. Conversely, for unconditionally non-preferred taste stimuli like quinine, cluster size monotonically decreases as concentration increases. These results support the conclusion that cluster size is a valid index of taste palatability (e.g., Davis, 1989; 1996; 1998; Davis & Smith, 1992; Hsiao & Fan, 1993; Spector, Klumpp & Kaplan, 1998; Spector & St. John, 1998).
The goal of the present research is to determine whether drug-induced intake suppression is accompanied by a reduction in taste palatability as assayed with lick cluster size. There have been few attempts to examine this issue. Indeed, we are aware of only one such study, that of Dwyer, Boakes and Hayward (2008). These researchers reported that LiCl- and activity-based CTAs each supported a reduction in cluster size but that amphetamine, while conditioning a reduction of CS intake, had no influence on lick cluster size. In all three experiments, Dwyer et al. used 0.1% saccharin as the taste CS. Some evidence shows that the initial (i.e., unconditioned) value of the taste CS may influence drug-induced intake suppression (Gomez & Grigson, 1999; Grigson, 1997). Accordingly, while using the same drug US as Dwyer et al. (1 mg/kg amphetamine sulfate), three different CSs were employed: 0.00003 M quinine, a non-preferred taste (Experiment 1); 0.1 M sodium chloride (NaCl), a stimulus that is preferred relative to water (Experiment 2); and a 0.02% aqueous orange odor, a stimulus that is neutral relative to water1 (Experiment 3). In a companion paper (Arthurs, Lin, Amodeo & Reilly, 2012), we varied the nature of the US (LiCl-induced illness, morphine, amphetamine and sucrose) while using our standard CS (0.15% saccharin). Collectively, these two reports should afford a more complete appreciation of whether a contingently administered drug of abuse can condition a reduction in the palatability of the associated taste CS.
Experiment 1
Because quinine hydrochloride (QHCl) is a non-preferred stimulus, a preliminary study was conducted and established that a 0.00003 M solution was consumed in sufficiently high quantities to allow drug-induced CS suppression to be detected. By comparison, 0.0001 M and 0.00006 M QHCl resulted in intake levels that were too low for present purposes (about 4 ml in 15 min). To afford comparability with the Dwyer et al. (2008) study, we employed the same three dependent measures: intake, total licks, and cluster size. And, as in that report, a cluster was defined as a run of licks with interlick intervals of less than 0.5 s. In addition, a fourth measure, lick efficiency, was included to determine whether lick volume was influenced by CS-drug pairings. Finally, unlike the Dwyer at al. experiment that employed 10 min CS access periods, we used 15 min CS periods, our standard CS duration in drug-induced taste suppression studies (e.g., Lin, Roman & Reilly, 2009; Lin, Arthurs & Reilly, 2011).
Method
Subjects
Twenty-four experimentally naïve male Sprague-Dawley rats, purchased from Charles River Laboratory (Wilmington, MA), served as subjects in the current study. They were individually housed in hanging steel cages located in a vivarium with a 12:12 light cycle (lights on at 7:00 am) and given ad libitum access to food and water until the experiment started, at which time they were placed on a water deprivation schedule as described below. On the day before the first conditioning trial, the body weights of the rats ranged from 366 g to 451 g (mean 404.4 g). The University of Illinois at Chicago Animal Care Committee approved all procedures employed in the present research. At all times, rats were treated according to guidelines recommended by the American Psychological Association (1996) and the National and the Institutes of Health (1996).
Apparatus
All behavioral testing was conducted in 8 identical drinking chambers (Med Associates, St. Albans, VT), measuring 30.5 cm X 24.0 cm X 29.0 cm (length X width X height). In each chamber, the front, back wall, and ceiling were of clear Plexiglas and each side wall was made of three aluminum panels arranged side by side. A house light and a white noise generator (background noise level ~80 dB) were mounted at the top of the middle panel of the left wall in each chamber. Fluid was presented in a retractable sipper tube that could be accessed through an oval hole (1.3 cm wide X 2.6 cm high) in the middle panel of the right wall. In its extended position, the tip of sipper tube was ~3 mm outside the wall. A lickometer circuit allowed monitoring of the time of each lick with a resolution of 10 milliseconds. Each drinking chamber was housed within a sound-attenuating cubicle that was fitted with a ventilation fan. Event control and data collection from the drinking chambers were carried out on-line with a computer running Med-PC software (Med Associates).
Procedure
The rats were acclimated to a water deprivation schedule that allowed 15-min unlimited access to water in the drinking chambers in the morning and 4 hr later 15-min water access in the home cages. Once baseline water intake stabilized in the drinking chambers (about 7 days), the rats were counterbalanced according to baseline water intake into either an unpaired (n = 12) or paired condition (n = 12). Thereafter, the conditioning phase was conducted with 3-day cycles of treatments. On the first day of each cycle, all rats received 15-min access to 0.00003 M QHCl followed 5 min later with an intraperitoneal injection of saline (1 ml/kg body weight; Group Unpaired) or d-amphetamine sulfate (Sigma Aldrich, St. Louis, MO; 1 mg/ml/kg body weight; Group Paired). On the second day, all rats received 15-min access to distilled water in the drinking chambers. To control for experience with the drug US, fluid consumption was followed by injections of saline in Group Paired or amphetamine in Group Unpaired. On the third day of each cycle subjects received water for 15 min but no injections were administered. This 3-day cycle was repeated for a total of two cycles and 24 hr later a taste test was given in which the US injections were omitted as superfluous.
Data Analysis
To capture the phenomenon of drug-induced suppression of fluid intake, four dependent variables were recorded. The first index was intake (a traditional measurement of consummatory behavior), which was obtained by weighing the bottles before and after each session (resolution 0.1 g). The second index was total licks. The third dependent measure calculated for each subject was average lick cluster size. As previously stated, a cluster was defined as a run of licks with interlick intervals of less than 0.5 s. Lick efficiency (licks/g), the final measure was obtained by dividing the total number of licks by amount of fluid consumed on each trial. For baseline water intake, an independent t-test was conducted on each dependent measure to confirm the absence of group differences immediately prior to the first conditioning trial. For the conditioning data, a mixed model analysis of variance (ANOVA) was employed with Group (Unpaired, Paired) as the between-subject variable and Trial as the within-subject variable. A significant main effect or interaction was followed by post hoc analyses using simple main effects with the adjusted error term from the overall ANOVA. The alpha level was set at p < 0.05. All statistical analyses were conducted with Statistica 6.0 software (StatSoft, Inc. Tulsa, OK).
Results and Discussion
Water consumption data from the final baseline water trial are summarized in Table 1. Separate independent t-tests were conducted on intake, total licks, cluster size, and lick efficiency, and found no significant group differences (ps > .40).
Table 1.
Water consumption from Experiments 1, 2 and 3 was characterized using four dependent measures: intake, total licks, cluster size, and lick efficiency. The data, mean (±SE), were taken from the water trial given 24 hr before the first conditioning trial for the control (Unpaired; CS-saline) and experimental (Paired; CS-amphetamine) groups.
| Experiment | Group | Intake | Total licks | Cluster size | Lick efficiency |
|---|---|---|---|---|---|
| 1 | Unpaired | 15.63 (0.70) | 2820.42 (106.32) | 112.03 (14.40) | 182.94 (8.03) |
| Paired | 15.72 (0.91) | 2738.75 (165.82) | 121.22 (15.34) | 175.26 (6.40) | |
| 2 | Unpaired | 14.64 (0.91) | 2876.38 (181.78) | 124.30 (13.43) | 198.36 (11.80) |
| Paired | 14.00 (0.56) | 2792.25 (129.70) | 115.84 (11.81) | 202.34 (10.78) | |
| 3 | Unpaired | 15.59 (0.78) | 2864.33 (151.55) | 133.06 (16.85) | 184.36 (6.61) |
| Paired | 14.20 (0.93) | 2823.58 (183.97) | 136.87 (18.66) | 200.43 (7.82) |
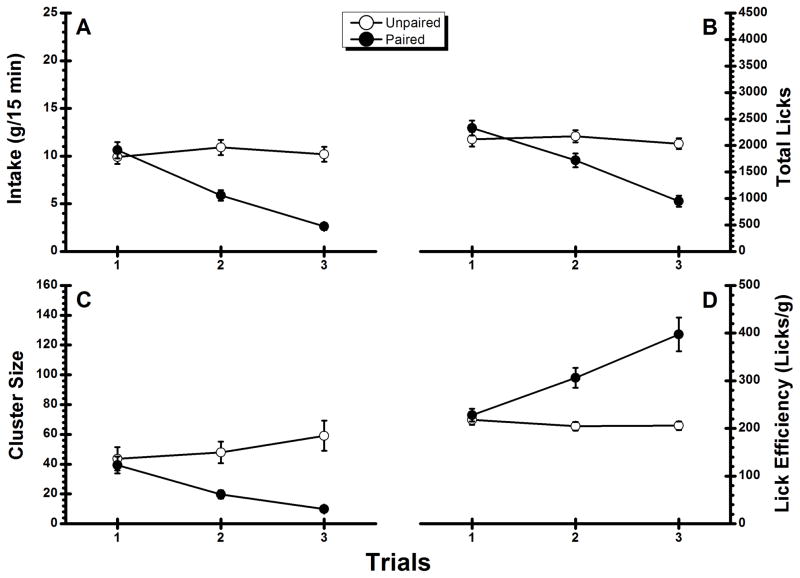
Figure 1A shows the QHCl consumption of Group Unpaired and Group Paired during the three taste trials of the experiment. Inspection of the figure indicates that Group Unpaired consumed similar amounts of QHCl across all three trials whereas intake in Group Paired decreased from each trial to the next. ANOVA conducted on the data summarized in Figure 1A found a significant main effect of Group, F(1, 22) = 24.75, p < .05, a significant main effect of Trial, F(2, 44) = 27.11, p < .05, and a significant Group × Trial interaction, F(2, 44) = 33.32, p < .05. Post-hoc comparisons revealed that while Group Unpaired drank comparable amount of QHCl across all three trials (ps > .05), Group Paired consumed significantly less on each successive trial (ps < .05). The total number of licks per QHCl trial (see Figure 1B) showed an identical pattern of significance as the amount consumed. The ANOVA found a significant main effect of Group, F(1, 22) = 9.94, p < .05, a significant main effect of Trial, F(2, 44) = 34.99, p < .05, and a significant Group X Trial interaction, F(2, 44) = 27.03, p < .05. Post hoc comparisons revealed that although Group Unpaired showed no significant variations across trials (ps >.05), the number of licks significantly decreased over successive QHCl trials in Group Paired (ps < .05). Importantly, the decrease in CS intake by Group Paired was accompanied by a decrease in cluster size (see Figure 1C). The initial statistical analysis revealed a significant main effect of Group, F(1, 22) = 11.88, p < .05 as well as a significant interaction, F(2, 44) = 15.04, p < .05; the main effect of Trial did not achieve significance (p > .10). Post hoc analysis found that cluster size in Group Paired was significantly smaller on Trial 2 and Trial 3 than on Trial 1 (ps < .05); there was no significant difference between cluster size on Trials 2 and 3. Interestingly, although Group Unpaired showed no significant change in fluid consumption across trials, cluster size was significantly larger on Trial 3 than on either Trial 1 or Trial 2 (ps < .05). As shown in Figure 1D, lick efficiency decreased across trials in Group Paired but not in Group Unpaired. Statistical analysis found a significant main effect of Group, F(1, 22) = 17.90, p < .05, a significant main effect of Trial, F(2, 44) = 28.47, p < .05, and a significant Group X Trial interaction, F(2, 44) = 37.65, p < .05. Post-hoc comparisons revealed that Group Paired required significantly more licks to obtain 1 g of CS on each successive trial (ps < .05); the performance of the Unpaired Group was stable across all three trials. Overall, this pattern of results suggests that a reduction in palatability contributes to the amphetamine-induced suppression of QHCl in this voluntary intake procedure. Furthermore, the results demonstrate that contingent administration of amphetamine reduces lick volume as the associated taste CS became less palatable.
Figure 1.
Experiment 1: Mean (±SE) conditioned stimulus (0.00003 M quinine)-directed performance during the two conditioning trials and the taste test trial. A: Intake; B: total number of licks; C: cluster size; D: lick efficiency.
Finally, the present experiment provided internal evidence that 0.00003 M QHCl was non-preferred relative to water. That is, for the unpaired control group we compared the amount of QHCl consumed on the final two taste trials with water intake 24 hr later (see Table 2). Data from the first taste trial were excluded to prevent any distortion that might occur because of a neophobic reaction to the novel taste on Trial 1, a concern most evident in the NaCl data of Experiment 2. A two-way ANOVA found a significant main effect of Trial, F(1, 11) = 4.96, p < .05, and a significant main effect of Stimulus, F(1, 11) = 51.57, p < .001; the interaction was not significant (F < 1). These statistics indicate the fluid intake (collapsed across QHCl and water) declined across the two trials by a numerically small (< 1 g) but nonetheless significant amount. Most importantly, rats drank significantly less QHCl than water. Thus, we conclude that 0.00003 M QHCl was, in the present experiment, non-preferred relative to water.
Table 2.
Mean (±SE) consumption from the Trials 2 and 3 for QHCl and NaCl (or, Trials 5 and 6 for odor) and water intake 24 hr later in the Unpaired control group.
| Experiment | Fluid | Trial 2 (or 5) | Trial 3 (or 6) |
|---|---|---|---|
| Experiment 1 | QHCl | 10.92 (0.80) | 10.18 (0.78) |
| Water | 13.91 (0.80) | 12.93 (0.49) | |
| Experiment 2 | NaCl | 20.69 (1.27) | 19.44 (1.22) |
| Water | 10.36 (0.83) | 11.80 (0.96) | |
| Experiment 3 | Odor | 12.13 (0.54) | 12.65 (0.56) |
| Water | 12.04 (0.64) | 12.50 (0.70) |
Experiment 2
Method
Subjects
The 24 experimentally naïve male rats (Group Unpaired, n = 8; Group Paired, n = 16) were obtained, housed and maintained as described in Experiment 1. Twenty-four hr before the first CS-US trial the mean body weight of the rats was 407.2 g (range: 351–464 g).
Apparatus and procedure
The drinking chambers and behavioral procedure were identical to those employed in Experiment 1, except 0.1 M NaCl served as the CS.
Results and Discussion
Water baseline data are shown in Table 1. Separate t-tests were conducted on intake, total licks, cluster size, and lick efficiency and found no significant effects of Group (ps > .50).
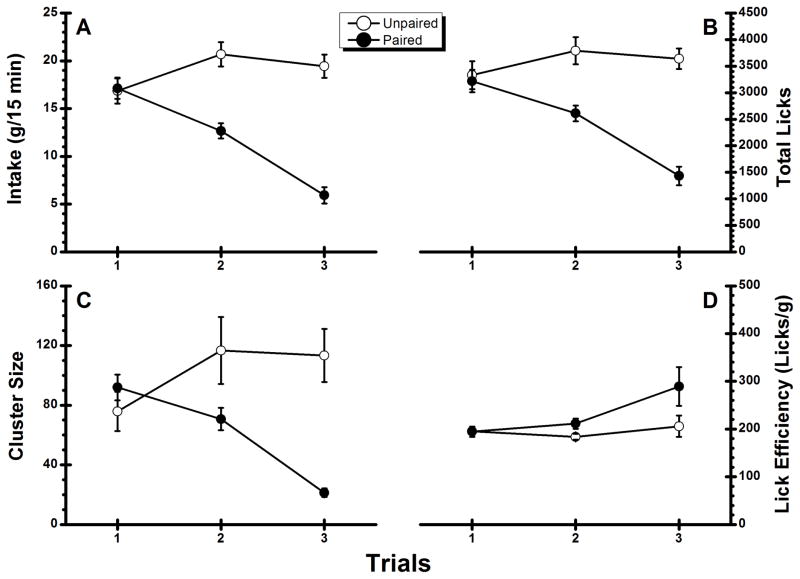
Inspection of Figure 2A suggests that Group Unpaired showed a modest increased in NaCl intake over trials (i.e., habituation of the neophobic response to the novel taste on Trial 1) whereas Group Paired showed a steady decline in consumption across trials. The ANOVA conducted on the data summarized in the figure confirmed these impressions with a significant main effect for Group, F(1, 22) = 28.54, p < .05, a significant main effect of Trial, F(2, 44) = 19.20, p < .05, and a significant Group × Trial interaction F(2, 44) = 40.12, p < .05. Post-hoc comparisons indicated that Group Unpaired significantly increased NaCl intake on Trial 2 relative to Trial 1 (p < .05) and that consumption was stable on Trial 2 and Trial 3 (p > .05). On the other hand, Group Paired significantly decreased the amount of NaCl consumed across each successive trial (ps < .05). A similar pattern was found when total licks (see Figure 2B) were analyzed. That is, there was a significant main effects for Group, F(1, 22) = 25.48, p < .05, a significant main effect of Trial F(2, 44) = 10.86, p < .05, and a significant Group × Trial interaction, F(2, 44) = 17.83, p < .05. Simple main effects confirmed that although total licks were stable in Group Unpaired across all three trials (ps >.05), lick frequency in Group Paired decreased significantly across each successive trial (ps < .05). To examine whether the amphetamine US influenced NaCl palatability, an ANOVA was conducted on the cluster size data summarized in Figure 2C. This analysis revealed a significant main effect of Group, F(1, 22) = 8.63, p < .05, a significant main effect of Trial, F(2, 44) = 7.61, p < .05, and a significant Group × Trial interaction, F(2, 44) = 31.48, p < .05. Post hoc analysis found that Group Unpaired showed an increase in cluster size on Trial 2 relative to Trial 1 (p < .05) and that cluster size was stable on Trials 2 and 3 (p > .05). Group Paired, on the other hand, showed a significant decrease in cluster size from each trial to the next (ps < .05). As is evident from inspection of Figure 2D, lick efficiency was also modulated by contingent injections of amphetamine, an impression that was confirmed with an ANOVA that found a significant Group X Trial interaction, F(2, 44) = 4.58, p < .05. Post hoc analysis revealed that lick efficiency of Group Paired for the CS solution was significantly lower (i.e., more licks required to obtain 1 g of the NaCl tastant) on Trial 3 than Trial 2 but not on Trial 2 relative to Trial 1; the lick efficiency of Group Unpaired was stable across all three NaCl trials. We conclude that a reduction of palatability and lick efficiency occurred when the NaCl CS was paired with an amphetamine US.
Figure 2.
Experiment 2: Mean (±SE) conditioned stimulus (0.1 M sodium chloride)- directed performance during the two conditioning trials and the taste test trial. A: Intake; B: total number of licks; C: cluster size; D: lick efficiency.
As in Experiment 1, additional statistical analyses were conducted to confirm that 0.1 M NaCl was, in the present design, preferred relative to water. The data, summarized in Table 2, were the amount of NaCl consumed by the unpaired control group on Trials 2 and 3 relative to water intake 24 hr after each taste trial. The ANOVA found a significant main effect of Stimulus, F(1, 7) = 110.63, p < .001, but no significant main effect of Trial (F < 1) and no significant interaction (p > .05). These statistics indicate that 0.1 M NaCl was highly preferred relative to water in the present experiment.
Experiment 3
Method
Subjects and apparatus
Twenty-four experimentally naive male rats (Group Unpaired n = 12; Group Paired, n = 12) were obtained from Charles River Laboratory. They were housed, maintained, as well as tested in the same apparatus, as described in Experiment 1. Average body weight of the rats 24-hr before the first conditioning trial was 406.3 g (range: 334–460 g).
Procedure
The conditioning method was identical to that used in Experiment 1, except that the CS was 0.02% aqueous orange (Flavorganics, Newark, NJ) odor and there were 5 odor-amphetamine pairings and 1 odor test trial.
Results and Discussion
Table 1 shows water baseline data on the day immediately prior to the first CS-US trial. Analyses confirmed that no significant group difference was found for intake, total licks, cluster size, or lick efficiency (ps > 0.10).
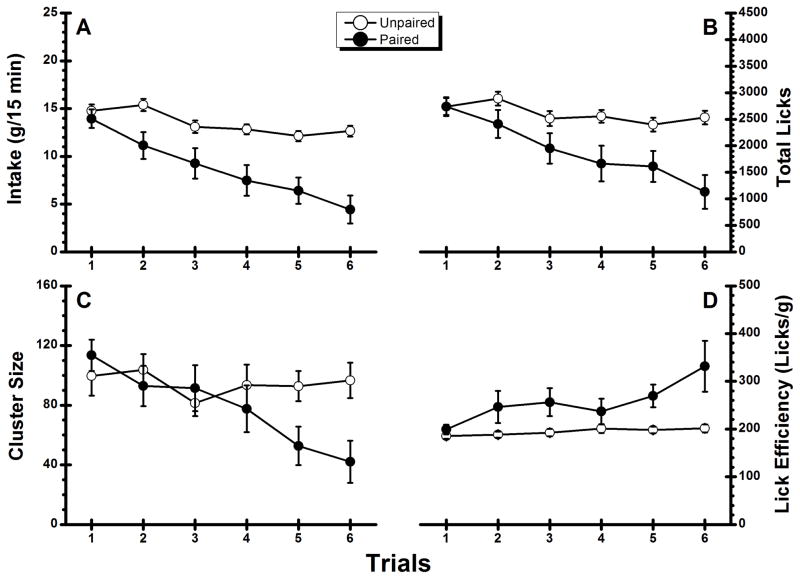
Figure 3A shows the amount of aqueous orange odor consumed across the 6 CS presentations in this experiment. Inspection of the figure suggests that Group Unpaired showed a fairly stable intake at 13–15 g across trials and that the intake of Group Paired was suppressed by amphetamine. These impressions of the data received support from a two-way ANOVA which revealed a significant main effect of Group, F(1, 22) = 14.02, p < .05, a significant main effect of Trial, F(5, 110) = 21.76, p < .05, and a significant Group × Trial interaction, F(5 ,110) = 6.21, p < .05. Post hoc analysis further revealed that while there was no group difference on Trial 1 (p > .20), Group Paired consumed significantly less than Group Unpaired on Trials 2–6 (ps < .05). The same pattern was found when total licks (see Figure 3B) were analyzed. This analysis found significant main effects of Group, F(1, 22) = 6.95, p < .05, a significant main effect of Trial, F(5, 110) = 14.20, p < .05, and a significant interaction, F(5, 110) = 5.92, p < .05. Analysis with simple main effects further revealed that although the two groups made similar number of licks on Trial 1 (p > .05), Group Paired made significantly fewer licks than Group Unpaired on each of the following trials (p < .05). Figure 3C shows the lick cluster size results. The ANOVA conducted on the data summarized in the figure found a significant main effect of Trial, F(5, 110) = 6.40, p < .05, a significant interaction, F(5, 110) = 5.75, p < .05, but the main effect of Group was not significant (p > .20). Following-up on the significant interaction, post hoc comparisons revealed that cluster size in Group Paired was significantly smaller than Group Unpaired on Trial 5 and Trial 6 (ps < .05); no significant groups differences were found on Trials 1–4 (ps > .05). As is indicated by the significant decrease in cluster size in Group Paired, amphetamine reduced the palatability of the aqueous odor after four CS-US pairings. Inspection of the results summarized in Figure 3D suggests that the two groups had similar lick volumes on Trial 1 (i.e., prior to the first US administration) and that whereas lick volume remained constant for Group Unpaired, the number of licks/g of the aqueous odor CS increased across conditioning trials for Group Paired. Surprisingly, the statistical analysis found no significant Group × Trial interaction (p < .10). There was, however, a significant main effect of Group, F(1, 22) = 12.75, p < .05, and a significant main effect of Trial, F(5, 110) = 2.52, p < .05. As shown in Figure 3D, while the number of licks needed to consume one gram of the CS increased over conditioning trials, the variance became larger as well thus reducing statistical power. The high degree of variance is primarily due to the fact that after conditioning, the amount consumed by some rats was so small (i.e., within the resolution of the intake measurement) such that lick efficiency cannot be precisely determined. To detect this treatment effect on lick efficiency, future studies should use a more precise method to measure intake, thereby increasing statistical power.
Figure 3.
Experiment 3: Mean (±SE) conditioned stimulus (0.02% aqueous orange odor)-directed performance across the five conditioning trials and the odor test trial. A: Intake; B: total number of licks; C: cluster size; D: lick efficiency.
Finally, as in previous experiments, we compared the intake of the odor stimulus by Group Unpaired on the final two trials of the experiment relative to water intake 24 hr later. Analysis of the data summarized in Table 2 found no significant main effect of Stimulus (F < 1), no significant main effect of Trial (p > .25) and no significant Stimulus X Trial interaction (F < 1). We take these statistics to demonstrate that the 0.02% aqueous orange odor stimulus was neutral relative to water in the present experiment.
General Discussion
The present results show that CS intake and cluster size each significantly declined in the Paired group following contingent pairings of a drug of abuse (amphetamine) with a non-preferred taste (QHCl; Experiment 1), a preferred taste (NaCl; Experiment 2) and with a neutral, non-taste stimulus (orange odor; Experiment 3). Regarding the performance of the Unpaired group, in the two cases in which the novel stimulus (QHCl and NaCl) supported an initial neophobic reaction, the habituation of that response was manifest not only by an increase in amount consumed but also by an increase in cluster size. The neophobia data were, it should be remembered, obtained from rats that were also given amphetamine, albeit 24 hr after the taste stimulus. Experiments are currently underway to examine in drug naïve rats whether palatability is modulated by taste neophobia.
As previously noted, we are aware of only one published experiment that has employed microstructural analysis of lick patterns, and specifically cluster size, to investigate whether a drug of abuse can condition a reduction in the palatability of the associated taste CS. In the Dwyer et al. (2008) report a saccharin CS was paired with an amphetamine US. In contrast to the present results, Dwyer et al. found that amphetamine did not influence cluster size even though the amount consumed was significantly reduced. While the procedures used in each laboratory share a number of similarities (not least of which was the 1 mg/kg amphetamine US), there were also some notable parametric differences including the length of the conditioning cycle (2 days vs. 3 days), CS duration (10 min vs. 15 min), CS type (saccharin vs. QHCl, NaCl, and orange odor), the number of conditioning trials (1 vs 2 or 5), and CS-US interval (immediate vs. 5 min). These differences, individually or in combination, may have influenced the obtained outcomes. Although one might speculate why the Dwyer et al. experiment obtained a null result, the three experiments in the present research clearly show that contingent administration of amphetamine does indeed support a reduction in cluster size. The present research, to the best of our knowledge, provides the first evidence of a drug-induced conditioned reduction in the palatability of taste and odor stimuli. And, if a reduction in palatability is taken as evidence of an aversion, then amphetamine might be considered to support CTA (and conditioned odor aversion) rather than conditioned taste avoidance. Furthermore, the present results show that as the CS becomes increasingly aversive over successive trial not only does the frequency of licks decrease but so too do the volume of those licks. That is, a drug-induced reduction in palatability is accompanied by a reduction in lick volume until, eventually, the CS becomes so distasteful that the rat quits drinking altogether.
These conclusions about CS palatability are at odds with those derived from studies using TR methodology. As noted in the Introduction, that body of research has consistently been interpreted to support the conclusion that drugs of abuse do not cause a conditioned reduction in taste palatability (but do support conditioned taste avoidance, defined as a reduction in amount of CS consumed). It should be noted that the dose of amphetamine (1 mg/kg) employed in the present experiments cannot account for the discrepancies between methodologies since amphetamine doses of 1–5 mg/kg are considered insufficient to supports a shift in taste palatability as measured by aversive TR response patterns (Parker, 1982, 1984, 1991; Parker & Carvell, 1986; Parker & McLeod, 1992; Zalaquett & Parker, 1989). It is only when amphetamine is used at high doses (e.g., 10 mg/kg) that aversive TR responses are produced (Parker, 1991). As shown in the companion report (Arthurs et al., 2012), a 15 mg/kg dose of morphine supported a significant reduction in cluster size to the associated saccharin CS yet doses as high as 80 mg/kg of morphine are not effective in producing aversive TR responses (Parker, 1991). In drug-based taste learning, aversive TR responses (e.g., gapes, chin rubs and head shakes) are of primary interest. It has been assumed that the occurrence of these responses mimics vomiting and helps to remove from the mouth noxious and extremely unpleasant substances that have been ingested accidentally. On the other hand, the monotonic relationship between cluster size and concentration of non-preferred taste stimuli (e.g., quinine) suggests that lick analysis is sensitive across a wider range of palatability than aversive TR responses. Thus, we are inclined to the view that the null results from TR studies might best be attributed to a lack of sensitivity of aversive TR responses to the detection of drug-induced conditioned reductions in taste palatability.
Finally, the data from Experiment 3, in which aqueous orange odor was paired with amphetamine, merit some discussion. Although significant reductions in amount consumed and total licks occurred after a single CS-US pairing (i.e., just as rapidly as for the taste stimulus used in Experiments 1 and 2), between-group differences in cluster size took four conditioning trials to emerge. How is this pattern of results to be interpreted? We suggest that the aqueous odor, which retronasally stimulates the olfactory epithelia, provides a sensory experience that is novel and perfectly correlated with the US, conditions that typically support rapid learning as indexed by amount consumed and total licks in the present experiment. But, why should more CS-US trials be needed to condition a reduction in palatability? To be clear, the olfactory cue specifically informs when the drinking water is followed by amphetamine intoxication. It is not meaningful, we believe, to claim that the palatability of an odor has been reduced. Rather, it is the palatability of the highly familiar water that is being reduced by the amphetamine US. And, given the well established finding that familiar stimuli are less readily learned about than novel stimuli (Lubow, 1989, 2009), the finding that more CS-US pairings were required to condition a reduction in the palatability of water may not be too surprising. Although this analysis may seem speculative it is a hypothesis that is readily testable since one would expect a similar dissociation to occur if, say, an auditory or visual cue was used in the present consummatory procedure rather than the olfactory cue. Moreover, the above pattern of result should not be dependent upon the use of a drug US. Similar, results would be expected when an aqueous odor is paired with any illness-inducing agent assuming, of course, that the US dose was chosen to avoid complete intake suppression after one conditioning trial.
For the last twenty plus years, a significant number of studies have been conducted with TR methodology the results of which have been interpreted as evidence that drugs of abuse do not reduce the palatability of the associated taste CSs. In turn, this analysis has encouraged the view that such drugs support a form of taste learning that is qualitatively different from LiCl-based CTA learning. This has been the departure point for the development of alternative accounts of drug-based taste learning that claim that the effective US produced by the drug of abuse is either not aversive whatsoever or that it is not aversive in the same way as illness-inducing agents (e.g., Grigson, 1997; Grigson, Twining, Freet, Wheeler & Geddes, 2009; Parker, 1995, 2003; Parker et al., 2009). The present results, as well as those in the companion paper (Arthurs et al., 2012), undermine these accounts and favor interpretations (e.g., Huang & Hsiao, 2008; Riley, 2011) that consider drugs of abuse, which by definition are self-administered by human and non-human animals alike, to have both rewarding and aversive properties and that the nature of the CS might be a factor that determines which aspect of the drug US is associated with a particular type of CS. In the case of taste and odor stimuli, the aversive properties of the drug serve as the US.
Acknowledgments
This work was supported by grants DC06456 from the National Institute of Deafness and Other Communication Disorders. We thank Dr. Dominic Dwyer for kindly supplying the original Med State code from which our programs were derived.
Footnotes
Internal evidence will be presented confirming these statements about the inital value of each stimulus relative to water (see also, Coldwell & Tordoff, 1996a, 1996b; Tordoff, Alarcon & Lawler, 2008).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- American Psychological Association. Guidelines for ethical conduct in the care and use of animals. Washington DC: American Psychological Association; 1996. [Google Scholar]
- Arthurs J, Lin J-Y, Amodeo LR, Reilly S. Reduced palatability in drug-induced taste aversion: II. Aversive and rewarding unconditioned stimuli. Behavioral Neuroscience. 2012;126:xxx–xxx. doi: 10.1037/a0027676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco, TX: Baylor University Press; 1977. [Google Scholar]
- Berger BD. Conditioning of food aversions by injection of psychoactive drugs. Journal of Comparative and Physiological Psychology. 1972;81:21–26. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ. Alternating ingestive and aversive consummatory responses suggest a two-dimensional analysis of palatability in rats. Behavioral Neuroscience. 1983;97:563–573. doi: 10.1037//0735-7044.97.4.563. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. Journal of Comparative and Physiological Psychology. 1981;95:363–382. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- Braveman NS, Bronstein P, editors. Annals of the New York Academy of Sciences. New York: New York Academy of Sciences; 1985. Experimental assessments and clinical applications of conditioned food aversions; p. 443. [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22:352–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: Effects of morphine, alcohol and chlordiazepoxide. Psychopharmacologia. 1973;29:239–246. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Long-term aversions to a saccharin solution induced by repeated amphetamine injections. Pharmacology Biochemistry and Behavior. 1973;1:265–270. doi: 10.1016/0091-3057(73)90115-9. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. American Journal of Physiology. 1996a;271:R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Tordoff MG. Immediate acceptance of minerals and HCl by calcium-deprived rats: brief exposure tests. American Journal of Physiology. 1996b;271:R11–R17. doi: 10.1152/ajpregu.1996.271.1.R11. [DOI] [PubMed] [Google Scholar]
- Davies BT, Wellman PJ. Conditioned taste reactivity in rats after phenylpropanolamine, d-Amphetamine or lithium chloride. Pharmacology Biochemistry and Behavior. 1990;36:973–977. doi: 10.1016/0091-3057(90)90108-t. [DOI] [PubMed] [Google Scholar]
- Davis JD. The microstructure of ingestive behavior. Annals of the New York Academy of Sciences. 1989;575:106–119. doi: 10.1111/j.1749-6632.1989.tb53236.x. [DOI] [PubMed] [Google Scholar]
- Davis JD. Deterministic and probabilistic control of the behavior of rats ingesting liquid diets. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1996;270:R793–R800. doi: 10.1152/ajpregu.1996.270.4.R793. [DOI] [PubMed] [Google Scholar]
- Davis JD. A model for the control of ingestion-20 years later. Progress in Psychobiology and Physiological Psychology. 1998;17:127–173. [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106:217–228. [PubMed] [Google Scholar]
- Davison CS, House WJ. Alcohol as the aversive stimulus in conditioned taste aversion. Bulletin of the Psychonomic Society. 1975;6:49–50. [Google Scholar]
- Dwyer DM, Boakes RA, Hayward AJ. Reduced palatability in lithium- and activity-based, but not in amphetamine-based, taste aversion learning. Behavioral Neuroscience. 2008;122:1051–1060. doi: 10.1037/a0012703. [DOI] [PubMed] [Google Scholar]
- Elkins RL. Individual differences in bait shyness: Effects of drug dose and measurement technique. Psychological Record. 1973;23:349–358. [Google Scholar]
- Freeman KB, Riley AL. The origins of conditioned taste aversion learning: A historical analysis. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 9–33. [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Gomez F, Grigson PS. The suppressive effects of LiCl, sucrose, and drugs of abuse are modulated by sucrose concentration in food-deprived rats. Physiology & Behavior. 1999;67:351–357. doi: 10.1016/s0031-9384(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Green L, Rachlin H. Learned taste aversions in rats as a function of delay, speed, and duration of rotation. Learning and Motivation. 1976;7:283–289. [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behavioral Neuroscience. 1997;111:129–36. [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 74–91. [Google Scholar]
- Grill HJ, Berridge K. Taste reactivity as a measure of neural control of palatability. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology. Vol. 11. New York: Academic Press; 1985. pp. 2–61. [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test: I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Spector AC, Schwartz GJ, Kaplan J, Flynn FW. Evaluating taste effects on ingestive behavior. In: Toates FM, Rowland NE, editors. Techniques in the behavioral and neural sciences: Feeding and drinking. Vol. 1. Amsterdam: Elsevier; 1987. pp. 151–188. [Google Scholar]
- Hsiao S, Fan RJ. Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behavioral Neuroscience. 1993;107:317–326. doi: 10.1037//0735-7044.107.2.317. [DOI] [PubMed] [Google Scholar]
- Huang ACW, Hsiao S. Re-examination of amphetamine-induced conditioned suppression of tastant intake in rats: The task dependent drug effects hypothesis. Behavioral Neuroscience. 2008;122:1207–1216. doi: 10.1037/a0013511. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pratt JA, Stolerman IP. Characteristics of conditioned taste aversion produced by nicotine in rats. British Journal of Pharmacology. 1983;79:245–253. doi: 10.1111/j.1476-5381.1983.tb10518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Role of the insular cortex in morphine-induced conditioned taste avoidance. Brain Research. 2011;1384:80–88. doi: 10.1016/j.brainres.2011.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, Reilly S. Morphine-induced suppression of conditioned stimulus intake: effects of stimulus type and insular cortex lesions. Brain Research. 2009;1292:52–60. doi: 10.1016/j.brainres.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge, England: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 37–57. [Google Scholar]
- Mayer LA, Parker LA. Rewarding and aversive properties of IP versus SC cocaine: Assessment by place and taste conditioning. Psychopharmacology. 1993;112:189–194. doi: 10.1007/BF02244909. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Krames L, Alloway TM, editors. Food aversion learning. New York: Plenum Press; 1977. [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiology & Behavior. 1973;10:73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Nachman M, Lester D, Le Magnen J. Alcohol aversion in the rat: Behavioral assessment of noxious drug effects. Science. 1970;168:1244–1246. doi: 10.1126/science.168.3936.1244. [DOI] [PubMed] [Google Scholar]
- Nathan BA, Vogel JR. Taste aversions induced by d-amphetamine: Dose – response relationship. Bulletin of the Psychonomic Society. 1975;6:287–288. [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Parker LA. Nonconsummatory and consummatory behavioral CRs elicited by lithium- and amphetamine-paired flavors. Learning & Motivation. 1982;13:281–303. [Google Scholar]
- Parker LA. Behavioral conditioned responses across multiple conditioning/ testing trials elicited by lithium-paired and amphetamine-paired flavors. Behavioral and Neural Biology. 1984;41:190–199. doi: 10.1016/s0163-1047(84)90569-7. [DOI] [PubMed] [Google Scholar]
- Parker LA. Positively reinforcing drugs may produce a different kind of CTA than drugs which are not positively reinforcing. Learning & Motivation. 1988;19:207–220. [Google Scholar]
- Parker LA. Taste reactivity responses elicited by reinforcing drugs: a dose- response analysis. Behavioral Neuroscience. 1991;105:955–964. doi: 10.1037//0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behavioral Neuroscience. 1993;107:118–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neuroscience and Biobehavioral Reviews. 1995;19:143–151. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA. LSD produces a place preference and taste avoidance, but does not produce taste aversion. Behavioral Neuroscience. 1996;109:503–508. doi: 10.1037//0735-7044.110.3.503. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: Evidence for two different processes. Learning & Behavior. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine- and amphetamine-paired sucrose solution. Pharmacology, Biochemistry, and Behaviour. 1986;24:883–887. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 92–113. [Google Scholar]
- Parker LA, MacLeod KB. Chin rub CRs may reflect conditioned sickness elicited by a lithium-paired sucrose solution. Pharmacology, Biochemistry and Behavior. 1991;40:983–986. doi: 10.1016/0091-3057(91)90115-i. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. Journal of Comparative Psychology. 1983;97:140–153. [PubMed] [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. [Google Scholar]
- Revusky S. Aversions to sucrose produced by contingent X-irradiation: Temporal and dosage parameters. Journal of Comparative and Physiological Psychology. 1968;95:17–22. doi: 10.1037/h0025416. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: The role of the aversive effects of drugs. Physiology & Behavior. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Riley AL, Jacobs WJ, LoLordo VM. Morphine-induced taste aversions: A consideration of parameters. Physiological Psychology. 1978;6:96–100. [Google Scholar]
- Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversions: A tool for the neural analysis of taste-visceral associations. Behavioral Neuroscience. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ. Role of taste in the microstructure of quinine ingestion by rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1998;274:1687–1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neuroscience and Biobehavioral Reviews. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiology & Behavior. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalaquett C, Parker LA. Further evidence that CTAs produced lithium and amphetamine are qualitatively different. Learning and Motivation. 1989;20:413–427. [Google Scholar]