Abstract
Background
Sudden cardiac death (SCD) in children is a rare but devastating event. Experts have debated the merits of community-based screening programs utilizing an electrocardiogram (ECG) and targeting two potential high-risk groups: school-aged children initiating stimulant medications to treat attention deficit hyperactivity disorder (ADHD) and adolescents participating in sports.
Methods and Results
Simulation models incorporating detailed prevalence, sensitivity and specificity, and treatment algorithms were built to determine the cost-effectiveness of targeted SCD screening. Clinical care algorithms were constructed for asymptomatic children initiating stimulants for ADHD (age 8) or participating in sports (age 14) and presenting with a positive ECG finding suggestive of one of the three most common pediatric disorders causing SCD and identifiable by ECG. Information to develop simulation model assumptions was drawn from the existing literature, Medicaid fees, and expert judgment. Sensitivity analyses examined parameter ranges to identify influential sources of uncertainty. Outcomes included costs and lost life years (LYs) caused by condition-related mortality. Our models estimate that screening for all three conditions simultaneously would reduce sudden death risk by 3.6 to 7.5 × 10−5 with projected life expectancy increases of 0.8–1.6 days per screened individual. The incremental cost-effectiveness of screening is $91,000 to $204,000 per LY. Sensitivity analysis showed that assumed disease prevalence, baseline mortality, and the relative risk of mortality due to stimulant medication use and sports participation had the greatest impact on estimated cost-effectiveness.
Conclusions
Results based on assumptions favoring SCD screening indicated its cost is high relative to its health benefits.
Keywords: cardiomyopathy, electrocardiography, long-QT syndrome, pediatrics, Wolff-Parkinson-White syndrome
Background
Sudden cardiac death in the young (SCD) is a rare but devastating event with an annual incidence among apparently healthy children and adolescents (hereafter “children”) of 0.8 to 6.2 per 100,000.1 Following some success in implementing community-based SCD screening programs for school-aged children in Japan2 and for athletes in Italy,3 Israel,4 and the United States,5 popular interest in pediatric SCD screening has increased.6, 7 Two pediatric subpopulations have been deemed to be at potentially higher risk for SCD than the general population and therefore have been targeted for screening: children initiating stimulant medications for attention-deficit/hyperactivity disorder (ADHD) and adolescents participating in organized sports.8
Proposed screening approaches recommend a combination of a child and family cardiac history, physical, and/or electrocardiogram (ECG) to identify common causes of SCD.9 Unfortunately, published estimates suggest a history and physical have limited utility for identifying pediatric disorders associated with SCD; only 7% of children with SCD have a positive family history10 and fewer than 1% with a positive history and physical examination have a confirmed SCD diagnosis.11
Recent efforts have focused on the efficacy of ECG screening in initial case finding.12 Several cost-effectiveness analyses (CEA) of SCD screening including an ECG have suggested at least borderline cost effectiveness among adolescents participating in sports13, 14 and children with ADHD.15 However, a societal analysis of pre-participation athletic screening in Israel suggests that ECG screening may not have measurably influenced the incidence of SCD in that country.4 Available studies have other limitations. First, none has looked specifically at pediatric cardiac disorders that cause SCD, controlling for their prevalence and age of onset (e.g., hypertrophic cardiomyopathy (HCM) often is phenotypically silent until late adolescence). Second, ECG sensitivity and specificity vary by disorder16 and must be incorporated into CEA models. Third, clinical guidelines and outcomes also vary by disorder; the one CEA that incorporated follow-up evaluation and management following a positive ECG finding did not differentiate by disorder.14
For this research, we built simulation models to project the cardiac health benefits and incremental costs of screening, follow-up diagnostic testing, and management associated with the three most common pediatric cardiac disorders identifiable by ECG and associated with SCD: HCM, Wolff-Parkinson-White syndrome (WPW), and long QT syndrome (LQTS) (see Supplemental Material Figure 1). We chose two illustrative scenarios that focused on populations deemed to be at potentially higher SCD risk: early elementary school children with ADHD (estimated at 4–12% of the pediatric population17), for whom stimulant medications are being considered, and high school freshmen participating in organized sports (the sports participation estimate for high school students of 7.7 million18 and the census high school enrollment estimate of 17.2 million19 imply that approximately 45% of high school students participate in sports, a proportion we assume is applicable to freshmen). Each disorder model followed children from an initial screening and, if any positive findings suggestive of these three disorders were found on ECG, included diagnostic testing, management, and outcomes, taking into account disorder-specific characteristics.
Methods
Screening
For the models, we assume that an initial screening occurs in a community setting such as a primary care office or school, and is staffed by either a primary care clinician or trained technician who conducts a history, physical, and ECG as per recent guidelines jointly endorsed by the American Heart Association (AHA) and the American Academy of Pediatrics (AAP).20 We also assume that children with overt, worrisome symptoms and/or a known and easily elicited family history of a potentially fatal genetic cardiac disorder placing them at risk for SCD already would have been identified and referred for evaluation.
Diagnostic Evaluation and Management
The simulation models incorporate algorithms for each disorder developed by researchers and clinicians at Tufts Medical Center and Children’s Hospital Boston, based on clinical practice improvement efforts at Children’s Hospital Boston (see simplified versions used for decision models in Figures 1–3 and Supplemental Material Figure 1). The entry point for each algorithm is a positive finding on an initial, community-based screening and referral for diagnostic evaluation. Evaluation begins with a detailed history, physical, and ECG, regardless of previous screening results. If all findings are negative, the child exits with no further follow-up. Any suggestive findings result in further diagnostic evaluation and treatment, specific to that disorder. For the purposes of modeling, we assume that the three disorders of interest are statistically independent; they neither co-occur, nor are suspected of co-occurring. Components of each algorithm are discussed in detail below.
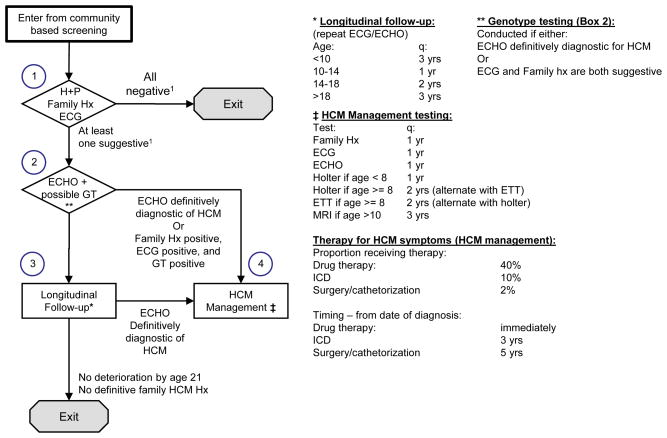
Figure 1.
Hypertrophic Cardiomyopathy Cardiac Subspecialty Evaluation and Management Algorithm
Abbreviations: ECG, electrocardiogram; ECHO, echocardiogram; ETT, exercise tolerance test; Family Hx, detailed family cardiac history; HCM, hypertrophic cardiomyopathy; H+P, targeted patient cardiac history and physical; ICD, implantable cardioverter defibrillator; MRI, magnetic resonance imaging; q, frequency; yr(s), year(s).
1Negative/suggestive relates to HCM criteria.
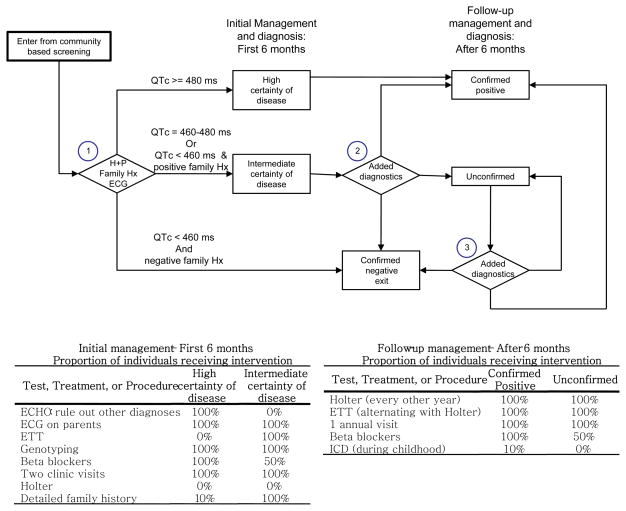
Figure 3.
Long QT Syndrome Cardiac Subspecialty Evaluation and Management Algorithm
Abbreviations: ECG, electrocardiogram; ECHO, echocardiogram; ETT, exercise tolerance test; Family Hx, detailed family cardiac history; H+P, targeted patient cardiac history and physical; ICD, implantable cardioverter defibrillator; LQTS, long QT syndrome; QTc, Bazett’s adjusted QT interval in ms (milliseconds).
(1) HCM (Figure 1 and Supplemental Material)
Children with suggestive findings on examination (Figure 1, Box 1) are referred for echocardiogram (ECHO) and genotype testing (Box 2). If ECHO is definitively diagnostic for HCM, or if the genotype, family history, and ECG are all positive, the child is referred to HCM management (Box 4), which may include medications, implantable cardioverter defibrillator (ICD), or surgery. Otherwise, the child is referred for longitudinal follow-up (Box 3), which involves periodic ECG and ECHO examination and either subsequent referral to HCM management if ECHO indicates deterioration, or exit if no further deterioration is detected by age 21.
(2) WPW (Figure 2 and Supplemental Material)
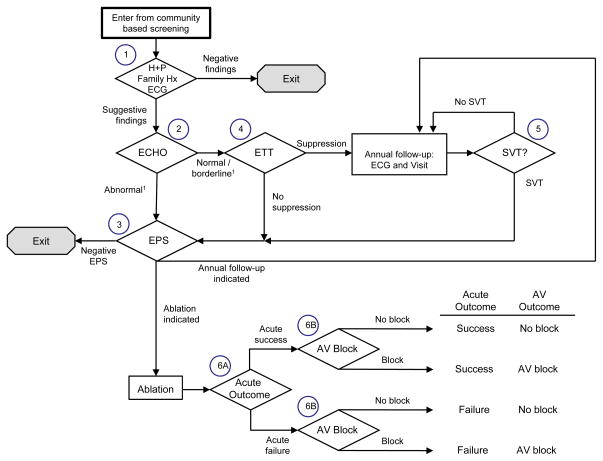
Figure 2.
Wolff-Parkinson-White Syndrome Cardiac Subspecialty Evaluation and Management Algorithm
Abbreviations: AV, atrioventricular; ECG, electrocardiogram; ECHO, echocardiogram; EPS, electrophysiology study; ETT, exercise tolerance test; Family Hx, detailed family cardiac history; H+P, targeted patient cardiac history and physical; SVT, supraventricular tachycardia; WPW, Wolff-Parkinson-White syndrome.
1Normal/borderline/abnormal relates to WPW criteria.
Children with suggestive findings on examination (Figure 2, Box 1) are referred for further evaluation, including ECHO (Box 2). These referrals include true positives, as well as a small number of false positives. If ECHO is normal, the child is referred for exercise tolerance testing (ETT, Box 4). Normalization of the ECG during ETT indicates a lower level of risk and hence results in referral to annual follow-up. Failure to normalize results in referral for electrophysiological study (EPS, Box 3) and possible ablation, based on the results of intraprocedural evaluation. If ECHO shows abnormal cardiac structure or function, the child is referred for an EPS (Box 3). EPS characterizes the WPW pathway and the child undergoes ablation, if appropriate.
(3) LQTS (Figure 3 and Supplemental Material)
On evaluation (Figure 3, Box 1), children are classified based on the probability of an eventual diagnosis of LQTS as “high certainty” (QTc≥ 0.48, or QTc ≥ 0.46 with positive family history), “intermediate certainty” (0.46 ≤ QTc < 0.48 or QTc < 0.46 with positive family history), or “confirmed negative” (QTc < 0.46 with negative family history), in which case they exit the algorithm. Children classified as high or intermediate certainty undergo an initial 6-month management and diagnosis schedule. Children still designated as high certainty after 6 months are considered “confirmed positive”. Children initially classified as intermediate certainty may be reclassified at 6 months as confirmed positive, unconfirmed, or confirmed negative (Box 2, 33.3% probability of each), and those unconfirmed may also be re-designated in subsequent years (Box 3).
Mortality Risk
For all three conditions, we assume that positive findings on community-based screening lead to a cessation of activities potentially contributing to risk (e.g., ADHD medication use or sports participation) and a resulting reduction in SCD risk to baseline levels (see Supplemental Material Tables 1, 2, and 3 for baseline SCD risks, and Supplemental Material Table 4 for associated relative risks). If follow-up diagnostics correctly confirm positive findings, risk is further reduced below its baseline level by treatment of the underlying disorder (see Supplemental Material Tables 1–3). For HCM, we assume ICD implantation confers treatment-related reduction in mortality risk. For WPW, risk is stratified by further testing, and successful ablation therapy eliminates risk, while failed or deferred therapy leaves risk unchanged. For LQTS, treatment-induced risk reduction occurs immediately following screening referral. Finally, for the purposes of these analyses, we assume that mortality rates are the same for the scenarios with and without screening after age 21, by which time individuals with any of the three cardiac disorders would presumably come to the attention of the health care system, even in the absence of targeted SCD screening.
Costs
Supplemental Material Table 4 summarizes cost assumptions used in this analysis. For individuals with HCM, WPW, or LQTS, annual mortality risk reflects a baseline contribution attributable to the disorder (see Supplemental Material Tables 1, 2, and 3) and an incremental contribution associated with stimulant use or sports participation (Supplemental Material Table 4).
Analyses: Simulations
The simulations recorded costs (screening, follow-up diagnosis, management, and treatment) and lost life years (LYs) caused by condition-related mortality. The simulation continued until age 75. Costs were converted to 2010 US dollars, and all outcomes were discounted at an annual rate of 3%.21, 22 The HCM and WPW models were implemented as discrete event simulations (cohorts of 10,000 individuals) in the Microsoft C-Sharp programming language. The LQTS model, which incorporates a management algorithm that is considerably less complex than the corresponding HCM and WPW algorithms, was implemented as a Markov model in Microsoft Excel. Markov models impose simplifying assumptions and track the proportion of the population in various health states rather than modeling each population member separately, as is typical in a discrete event simulation.23
Below, we report the cost-effectiveness of screening (incremental costs divided by incremental LY saved) for all three disorders simultaneously. The analysis sums follow-up costs (follow-up diagnosis, management, and treatment) across the three disorders but assumes that because screening addresses all three disorders, its costs (base case value of $50 per individual) are incurred only once. A standard metric used to determine the economic efficiency of resource use from medical interventions is the incremental cost-effectiveness ratio (ICER) which divides the additional cost of screening versus standard care by its additional benefit in LYs. Typically, medical interventions with ICERs falling below $50,000 to $100,000 per LY gained are considered to be “cost-effective.”
We investigated the robustness of the simulation models by conducting a series of univariate sensitivity analyses that involve changing one assumption at a time, replacing its base case value with alternatives at either end of the plausible range (see Supplemental Material Tables 1–4 for alternative values). By recording the impact of these individual changes on the model-projected cost-effectiveness ratio for screening, we identified those assumptions having the greatest influence on our results. An assumption was deemed influential if an alternative plausible value qualitatively influenced our cost-effectiveness estimates (e.g., the base case cost-effectiveness ratio was “unfavorable” (a large value above a pre-designated threshold) and use of the alternative assumption yielded a cost-effectiveness ratio that was “favorable” (below that threshold)). For this purpose, we used thresholds of $50,000 and $100,000 per LY.
Results
Base Case Results
Our primary results reflect the use of “base case” assumptions, our best estimate for all uncertain model parameters. Table 1 summarizes the impact of targeted SCD screening at ages 8 and 14, reporting incremental costs, and the incremental impact of screening on lifetime risk of SCD, life expectancy, and discounted life expectancy. We estimated these incremental impacts by taking the difference between projections generated by our models with screening implemented and the corresponding projections generated by our models with no screening assumed.
Table 1.
Base Case Results: Incremental Costs and Outcomes per Individual Screened
| Screen at:
|
||
|---|---|---|
| Age 8 | Age 14 | |
| Incremental costs | ||
| HCM | $134 | $136 |
| WPW | $80 | $79 |
| LQTS | $55 | $55 |
| Total* | $170 | $171 |
| Incremental SCD cases prevented due to: | ||
| HCM | 1.1 × 10−5 | 4.7 × 10−5 |
| WPW | 2.0 × 10−5 | 2.3 × 10−5 |
| LQTS | 5.0 × 10−6 | 5.4 × 10−6 |
| Total | 3.6 × 10−5 | 7.5 × 10−5 |
| Incremental LYs saved from preventing SCD due to: | ||
| HCM | 6.8 × 10−4 | 2.7 × 10−3 |
| WPW | 1.1 × 10−3 | 1.2 × 10−3 |
| LQTS | 3.4 × 10−4 | 3.9 × 10−4 |
| Total | 2.1 × 10−3 | 4.3 × 10−3 |
| Incremental discounted LYs saved from preventing SCD due to: | ||
| HCM | 2.6 × 10−4 | 1.2 × 10−3 |
| WPW | 4.5 × 10−4 | 5.5 × 10−4 |
| LQTS | 1.2 × 10−4 | 1.4 × 10−4 |
| Total | 8.3 × 10−4 | 1.9 × 10−3 |
| Incremental cost per discounted LY from screening for all conditions: | $203,979 | $90,828 |
Abbreviations: HCM, hypertrophic cardiomyopathy; LQTS, long QT syndrome; LY, life year; SCD, sudden cardiac death; WPW, Wolff-Parkinson-White syndrome.
The total has twice the per-individual community-based screening cost subtracted out to avoid triple counting this expense.
Incremental SCD cases prevented over a lifetime are consistent with our assumptions. For example, SCD cases prevented by screening at age 8 must not exceed the number of cases that occur between the ages of 8 and 21 (after which time, we make the assumption in the model that the SCD rate is identical for the screened and unscreened populations). Taking the example of WPW in this age period, the number of WPW-related SCD cases is 2.7 SCD per 100,000 population members, which is the product of WPW prevalence (136 WPW cases per 100,000 population members), SCD cases per year among individuals with WPW (1 SCD per 1,000 WPW cases), the relative risk associated with stimulant use (RR = 1.4), and years at risk (14 years). The model-projected benefit (2.0 SCD cases prevented per 100,000 population members) is only modestly less than this upper limit, reflecting our assumptions that WPW is readily detected and can be effectively treated. By comparison, the corresponding figures for HCM screening at age 8 (8.8 SCD cases per 100,000 members of the population, of which our model projects 1.1 will be prevented) reflect our assumptions that HCM may not yet be phenotypically manifest among younger children and that its treatment has limited effectiveness.
The cost-effectiveness of screening at age 14 (approximately $91,000 per LY gained) is notably more favorable than screening at age 8 (approximately $204,000 per LY gained). Incremental costs for screening at these two ages are similar as are the incremental benefits for screening WPW and LQTS. The divergence in the cost-effectiveness results at ages 8 and 14 stems largely from the substantially greater gains from screening for HCM at age 14 (1.2 × 10−3 discounted LYs per individual screened) compared to gains at age 8 (2.6 × 10−4 discounted LYs).
Sensitivity Analysis Results
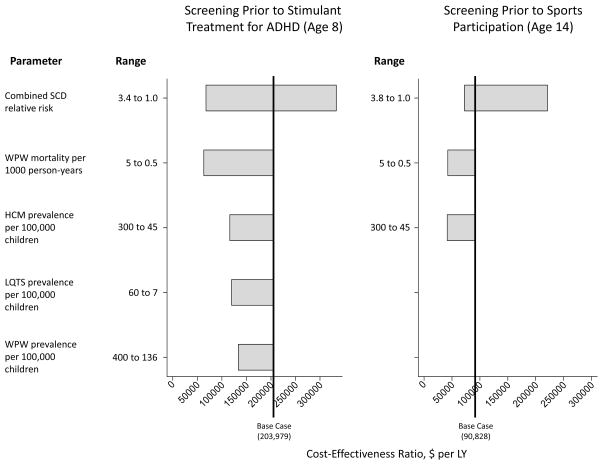
Figure 4 plots the univariate sensitivity analysis results for influential assumptions. At age 8, two alternative assumptions reduce the cost-effectiveness ratio from its base case value of $204,000 per LY to less than $100,000 per LY: the assumed SCD relative risk associated with use of ADHD stimulant medications, and the assumed baseline mortality rate for WPW. Notably, the alternative relative risk assumption for SCD reduces the cost-effectiveness of screening at age 8 to less than $50,000 per LY. As illustrated in Figure 4, three additional alternative assumptions reduce the cost-effectiveness ratio to less than $150,000 per LY.
Figure 4.
Univariate Sensitivity Analysis
Abbreviations: ADHD, attention deficit/hyperactivity disorder; HCM, hypertrophic cardiomyopathy; LQTS, long QT syndrome; LY, life year; SCD, sudden cardiac death; WPW, Wolff-Parkinson-White syndrome.
At age 14, two alternative assumptions reduce the cost-effectiveness ratio from its base case value of $91,000 per LY to less than $50,000 per LY: the assumed HCM prevalence and the assumed mortality rate for WPW. The alternative assumed SCD mortality relative risk associated with sports participation does not reduce the cost-effectiveness ratio below $50,000 per LY but is included in Figure 4 for illustrative purposes because it comes close to doing so. Finally, Table 2 lists sensitivity analysis results for additional assumptions that alter cost-effectiveness by at least 20%.
Table 2.
Univariate Sensitivity Analysis Results – Assumptions Changing Cost-Effectiveness By At Least +/− 20%
| Assumptions | Favorable CE Ratios | Unfavorable CE Ratios | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base Case | For Favorable CE Ratio | For Unfavorable CE Ratio | Inc. Cost | Inc. Discounted LY | Inc. CE | Inc. Cost | Inc. Discounted LY | Inc. CE | |
| Screening Prior to Stimulant Treatment for ADHD (Age 8) | |||||||||
| Base case | $170 | 8.3X10−4 | $203,979 | $170 | 8.3X10−4 | $203,979 | |||
| All SCD - RR with ADHD meds | 1.4 | 6.6 | 1.0 | $170 | 2.5X10−3 | $67,158 | $170 | 5.1X10−4 | $331,496 |
| HCM - Mortality ratea | 10 | 20 | 2 | $168 | 1.1X10−3 | $154,628 | $171 | 6.3X10−4 | $270,773 |
| HCM - Prevalencea | 45 | 300 | 45 | $268 | 2.3X10−3 | $115,781 | $170 | 8.3X10−4 | $203,979 |
| HCM - Community screening specificity | 85% | 100% | 85% | $103 | 8.3X10−4 | $123,444 | $170 | 8.3X10−4 | $203,979 |
| WPW - Mortality ratea | 1 | 5 | 0.5 | $169 | 2.7X10−3 | $62,729 | $170 | 5.5X10−4 | $309,959 |
| WPW - Prevalencea | 136 | 400 | 136 | $226 | 1.7X10−3 | $132,935 | $170 | 8.3X10−4 | $203,979 |
| LQTS - Prevalencea | 7 | 60 | 7 | $201 | 1.8X10−3 | $119,229 | $170 | 8.3X10−4 | $203,979 |
| Cost - Community based screen | $50 | $13 | $100 | $136 | 8.3X10−4 | $163,691 | $237 | 8.3X10−4 | $284,334 |
| Cost - Cardiology visit | $195 | $107 | $299 | $157 | 8.3X10−4 | $188,682 | $208 | 8.3X10−4 | $249,800 |
| Cost - Annual follow-up after ICD implantation | $14,533 | $357 | $206,000 | $161 | 8.3X10−4 | $193,710 | $704 | 8.3X10−4 | $846,429 |
| Screening Prior to Sports Participation (Age 14) | |||||||||
| Base case | $171 | 1.9X10−3 | $90,828 | $171 | 1.9X10−3 | $90,828 | |||
| All SCD - RR with sports participation | 2.8 | 3.8 | 1.0 | $171 | 2.5X10−3 | $67,495 | $170 | 3.0X10−4 | $574,711 |
| HCM - Prevalencea | 45 | 300 | 45 | $354 | 8.6X10−3 | $41,221 | $171 | 1.9X10−3 | $90,828 |
| WPW - Mortality ratea | 1 | 5 | 0.5 | $171 | 4.1X10−3 | $42,176 | $171 | 1.9X10−3 | $90,828 |
| Cost - HCM medication therapy (per year) | $805 | $0 | $13,000 | $172 | 1.9X10−3 | $91,326 | $222 | 1.9X10−3 | $117,787 |
| Cost - Cardiology visit | $195 | $107 | $299 | $153 | 1.9X10−3 | $81,337 | $210 | 1.9X10−3 | $111,914 |
| Cost - Annual follow-up after ICD implantation | $14,533 | $357 | $206,000 | $159 | 1.9X10−3 | $84,563 | $848 | 1.9X10−3 | $451,006 |
Abbreviations: CE, cost effectiveness; HCM, hypertrophic cardiomyopathy; ICD; implantable cardioveter defibrillator; Inc., incremental; LQTS, long QT syndrome; LY, life year; RR, relative risk; SCD, Sudden cardiac death; WPW, Wolff-Parkinson-White Syndrome.
Per 100,000 person year
Discussion
It is undoubtedly true at the individual level that the cost of an ECG is a small price to pay to save the life of a child at risk for SCD. However, the cost borne by society also reflects resources utilized for screening and the costs (and risks) associated with further evaluation. Our analysis demonstrates that the cost-effectiveness of targeted SCD screening for children with ADHD is unfavorable compared to generally-accepted societal thresholds of $50,000 to $100,000 per LY; screening prior to organized sports participation is towards the expensive end of the acceptable range. These generally unfavorable cost-effectiveness ratios reflect the low prevalence of the disorders considered here in combination with the imperfect screening and diagnostic specificity, which results in the referral of a relatively large number of healthy children for follow-up testing. Screening at age 8 is also unfavorable because one of the three conditions considered (HCM) is incompletely expressed phenotypically at that age, making it difficult to identify cases and reducing the mortality benefit.
Recent empirical studies further highlight potential limitations of the utility of ECG screening. Hill and colleagues24 evaluated the accuracy of pediatric cardiologists’ interpretation of pediatric pre-participation athletic cardiac screening and found that accuracy was particularly low for HCM and WPW, two of the disorders considered in our analysis. A pilot test of a screening program in an academic medical center identified a small number of patients with SCD risk factors but also discovered a number of non-specific ECG findings.7 Both studies suggest that the discriminant value of ECG screening may be lower in practice than in published series used to determine ECG positive and negative predictive values and are consistent with our projection of a relatively small effect of SCD screening on population mortality.
This analysis incorporates several “conservative” assumptions that tend to make population SCD screening look more favorable. First, we limited screening to two subpopulations deemed to be at elevated risk. If we had modeled screening of a general population of children, which includes many who would be less likely to benefit from specific interventions (withholding of stimulant medications, avoidance of sport participation), the projected cost-effectiveness would have been even less favorable. Second, we assumed that SCD risks attributed to asymptomatic patients with HCM, WPW, and LQTS are as high as in those patients identified due to symptoms, and that the therapies applied to patients identified by screening will completely eliminate any disease-attributable SCD risks. Third, we assumed that stimulant therapy increases SCD risk even though recently published large-scale retrospective studies investigating this phenomenon could not discern such an association.25,26 Finally, we incorporated the benefits of screening (substantial treatment effect to reduce mortality risk) but omitted some of the negative implications of a positive screening exam (e.g., loss of benefits from discontinued sports participation or ADHD medications). Specifically, although early identification may allow for precautions against SCD events, it may also trigger non-trivial therapies and/or lifestyle or medication restrictions that are based on expert consensus but lack sufficient clinical evidence.27 If ADHD medications reduce the prevalence of life-threatening behaviors (e.g., risky driving behavior), the net mortality benefit assumed here may prove to be overstated since screening could reduce the use of these medications. Nor did we include anxiety associated with medical evaluation and uncertain diagnoses; early work by Bergman and colleagues28 suggests that false positive cardiac findings, even when found to be normal on further evaluation, may result in perceptions of vulnerability on the part of families and patients, leading to unnecessary lifestyle restrictions. Because these children may ultimately be diagnosed by means other than screening (e.g., following emergence of sublethal symptoms, identification of a family member with a genetic cardiac disorder, or by use of diagnostic testing for other unrelated indications), earlier diagnosis may not confer any survival benefit. Thus, our model most likely represents a “best-case scenario” for the cost-effectiveness of SCD screening that is unlikely to be achieved in real world application.
Our study has several limitations. Most notably, because these disorders and SCD events are rare, there are limited empirical findings to inform some of the model assumptions. As a result, some assumptions are uncertain. For example, our central estimates for the prevalence of HCM, WPW, and LQTS are notably lower than the estimates used in an analysis of SCD risk factor screening conducted by Wheeler et al.14 We used estimates based on a comprehensive and recently published meta-analysis conducted by Rodday et al.16 To address this discrepancy, we also included in our sensitivity analysis upper bound values equal to the upper bounds reported by Wheeler et al.14
Second, the algorithms reflect care patterns common in the Boston area, but variation certainly exists across pediatric cardiac centers.
Third, our analysis did not include all disorders that can cause SCD in children (e.g., Brugada syndrome, anomalous coronary artery, dilated cardiomyopathy, and others). However, the pediatric prevalence of each omitted disorders are considerably lower than the prevalence of HCM and WPW (e.g., central estimates reported by Wheeler et al.14 for the prevalence of Brugada syndrome and dilated cardiomyopathy are each more than three-fold less than corresponding estimates for HCM and WPW). In other cases, ECG sensitivity (e.g., to detect anomalous coronary anatomy) for its identification is considerably lower than the corresponding values for HCM, WPW, and LQTS, and therefore their inclusion would not substantially alter our findings. Moreover, seeking to identify additional conditions as part of the screening program could reduce specificity, increasing costs further and making cost-effectiveness less favorable.
Finally, although our cost-effectiveness ratios are expressed in terms of cost per LY, we have assessed these ratios using benchmarks originally developed to assess cost-effectiveness expressed in terms of quality adjusted life years (QALYs), a measure that takes into account both mortality and morbidity. In general, using QALYs in place of LYs does not substantially affect cost-effectiveness favorability.29 Moreover, it is particularly unlikely that use of QALYs would have substantially altered our findings. Using LYs means that we did not capture the screening-related benefit of treating disease earlier and mitigating morbidity, but it also means we omitted the screening-related “cost” of treatment side effects, such as non-trivial therapies and/or lifestyle or medication restrictions and anxiety from living with a diagnosis of heart disease, as mentioned earlier.
The simulations presented here, taken together with the detailed sensitivity analyses, suggest that on a societal basis the costs of ECG screening for pediatric SCD are high compared to the potential benefits. Although economic efficiency alone should not dictate medical practice, the finite nature of health care expenditure implies that spending on one priority restricts spending on other priorities. The fact that the cost-effectiveness ratio for pediatric SCD screening is relatively unfavorable (high) implies that there are opportunities to invest limited health care resources in ways that will produce greater health benefits for the population.
Supplementary Material
Acknowledgments
Steven D. Colan, MD, of Children’s Hospital Boston assisted with data acquisition and HCM model development. Alisa Niksch, MD, of The Floating Hospital for Children at Tufts Medical Center assisted with treatment algorithm design and development. Tully Saunders, BS, of Tufts Medical Center assisted with data acquisition, manuscript editing, and administrative responsibilities. Contributors were funded under NHLBI grant number 1RC1HL100546-01.
Funding Sources: This article is based on research conducted by investigators at Tufts Medical Center and Children’s Hospital Boston funded through the National Heart Lung and Blood Institute grant number 1RC1HL100546-01 and through the John R. Grey IV Cardiology Fellowship Endowment Fund and the Sean Roy Johnson Fund for Electrophysiology Research (Sherwin).
Footnotes
Conflict of Interest Disclosures: John K. Triedman is a consultant for Biosense Webster, Inc..
References
- 1.Berger S, Utech L, Fran HM. Sudden death in children and adolescents. Pediatr Clin North Am. 2004;51:1653–1677. doi: 10.1016/j.pcl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Yoshinaga M, Anan R, Tanaka Y, Nomura Y, Oku S, Nishi S, Kawano Y, Tei C, Arima K, Tanaka Y, Yoshinaga M, Anan R, Tanaka Y, Nomura Y, Oku S, Nishi S, Kawano Y, Tei C, Arima K. Usefulness and cost effectiveness of cardiovascular screening of young adolescents. Med Sci Sports Exerc. 2006;38:2–6. doi: 10.1249/01.mss.0000183187.88000.53. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 4.Steinvil A, Chundadze T, Zeltser D, Rogowski O, Halkin A, Galily Y, Perluk H, Viskin S. Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death: Proven fact or wishful thinking? J Am Coll Cardiol. 2011;57:1291–1296. doi: 10.1016/j.jacc.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Baggish AL, Hutter AM, Jr, Wang F, Yared K, Weiner RB, Kupperman E, Picard MH, Wood MJ. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 2010;152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bove A. Making or Breaking Athletic Careers. J Am Coll Cardiol. 2011;57:1297–1298. doi: 10.1016/j.jacc.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 7.Vetter VL, Dugan N, Guo R, Mercer-Rosa L, Gleason M, Cohen M, Vogel RL, Iyer R. A pilot study of the feasibility of heart screening for sudden cardiac arrest in healthy children. Am Heart J. 2011;161:1000–1006. doi: 10.1016/j.ahj.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Kaltman JR, Thompson P, Lantos J, Berul C, Cohen J, Cook N, Drezner J, Frick K, Kannankeril P, Leslie L, Priori S, Saul J, Shapiro-Mendoza C, Siscovick D, Vetter V, Botkin J, Corrado D, Goldman S, Hlatky M, Boineau R, Burns K, Friedman R. Screening for sudden cardiac death in the young: Report from a National Heart, Lung, and Blood Institute Working Group. Circulation. 2011;123:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.017228. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM, Jr, Krauss MD, Maron MS, Mitten MJ, Roberts WO, Puffer JC. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643–455. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 12.Marek J, Bufalino V, Davis J, Marek K, Gami A, Stephan W, Zimmerman F. Feasibility and findings of large-scale electrocardiographic screening in young adults: Data from 32,561 subjects. Heart Rhythm. 2011;10:1555–1559. doi: 10.1016/j.hrthm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Fuller C. Cost effectiveness analysis of screening of high school athletes for risk of sudden cardiac death. Med Sci Sports Exerc. 2000;32:87–90. doi: 10.1097/00005768-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med. 2010;152:276–286. doi: 10.1059/0003-4819-152-5-201003020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denchev P, Kaltman JR, Schoenbaum M, Vitiello B. Modeled economic evaluation of alternative strategies to reduce sudden cardiac death among children treated for attention deficit/hyperactivity disorder. Circulation. 2010;121:1329–1337. doi: 10.1161/CIRCULATIONAHA.109.901256. [DOI] [PubMed] [Google Scholar]
- 16.Rodday AM, Triedman JK, Alexander ME, Cohen JT, Ip S, Newburger JW, Parsons SK, Trikalinos TA, Wong JB, Leslie LK. ECG screening for disorders that cause sudden cardiac death in asymptomatic children: A meta-analysis. Pediatrics. 2012 doi: 10.1542/peds.2011–0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Disease Control. Diagnosed Attention Deficit Hyperactivity Disorder and Learning Disability: United States, 2004–2006. U.S. Department of Health and Human Services; 2008. [PubMed] [Google Scholar]
- 18.National Federation of State High School Associations. [Accessed September 3, 2011];High School Sports Participation Continues Upward Climb. 2011 http://www.nfhs.org/content.aspx?id=5752. Available at: URL: http://www.nfhs.org/content.aspx?id=5752.
- 19.Davis J, Bauman K. School Enrollment in the United States: 2008. United States Census Bureau; 2011. [Google Scholar]
- 20.American Academy of Pediatrics/American Heart Association. [Accessed February 14, 2011];American Academy of Pediatrics/American Heart Association Clarification of Statement on Cardiovascular Evaluation and Monitoring of Children and Adolescents With Heart Disease Receiving Medications for ADHD. 2011 doi: 10.1097/DBP.0b013e31318185dc14. www.aap.org/pressroom/aap-ahastatement.htm. [DOI] [PubMed]
- 21.Gold M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 22.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 23.Kuntz KM, Weinstein MC. Modelling in Economic Evaluation. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care: Merging Theory with Practice. New York: Oxford University Press; 2001. [Google Scholar]
- 24.Hill AC, Miyake CY, Grady S, Dubin AM. Accuracy of Interpretation of Preparticipation Screening Electrocardiograms. J of Peds. 2011;159:783–788. doi: 10.1016/j.jpeds.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Schelleman H, Bilker WB, Strom BL, Kimmel SE, Newcomb C, Guevara JP, Daniel GW, Cziraky MJ, Hennessy S. Cardiovascular Events and Death in Children Exposed and Unexposed to ADHD Agents. Pediatrics. 2011;127:1102–1110. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA. ADHD Drugs and Serious Cardiovascular Events in Children and Young Adults. N Engl J Med. 2011;365:1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estes NA., III ECG Screening for Athletes: Letting Evidence and Reason Advance the Debate. Heart Rhythm. 2011;8:728–730. doi: 10.1016/j.hrthm.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Bergman AB, Stamm SJ. The Morbidity of Cardiac Nondisease in Schoolchildren. N Engl J Med. 1967;276:1008–1013. doi: 10.1056/NEJM196705042761804. [DOI] [PubMed] [Google Scholar]
- 29.Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. 2004;13:429–436. doi: 10.1002/hec.853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.