Abstract
Using alkaline denaturation-renaturation, exonuclease III digestion and density gradient centrifugations, we have isolated covalently closed circular DNA (cccDNA) molecules from 1-, 8-, 16-, and 24-month C57BL/6 mouse heart tissues. Electron microscopic analyses demonstrated that all these preparations contained small polydisperse circular DNAs (spcDNAs). spcDNAs showed similar size distributions at all ages, but more discrete size classes and slightly larger circles were observed in the 24-month heart spcDNA preparations. Based upon the final yields of spcDNAs, there appeared to be no age-related changes in the quantity of these circular molecules in vivo. Furthermore, [3H]-pBR322 recovery studies revealed no endogenous factors that might have affected the yield of spcDNAs from young and old tissues. To determine if there were any age-related changes in the quantity of repetitive sequences in spcDNAs, we probed heart spcDNAs with B1, B2, IAP, L1 and satellite sequences of the mouse genome. The hybridization results showed that these sequence families were differentially represented at all ages in spcDNAs. B2 sequences were the highest across all the age groups while L1 sequences were the lowest. The quantity of B1-, B2-, IAP-, and L1-spcDNAs appeared to decrease at 24-months. Satellite sequences appeared to decrease from 1-month to 8-months, but no change beyond 8-months.
Full text
PDF







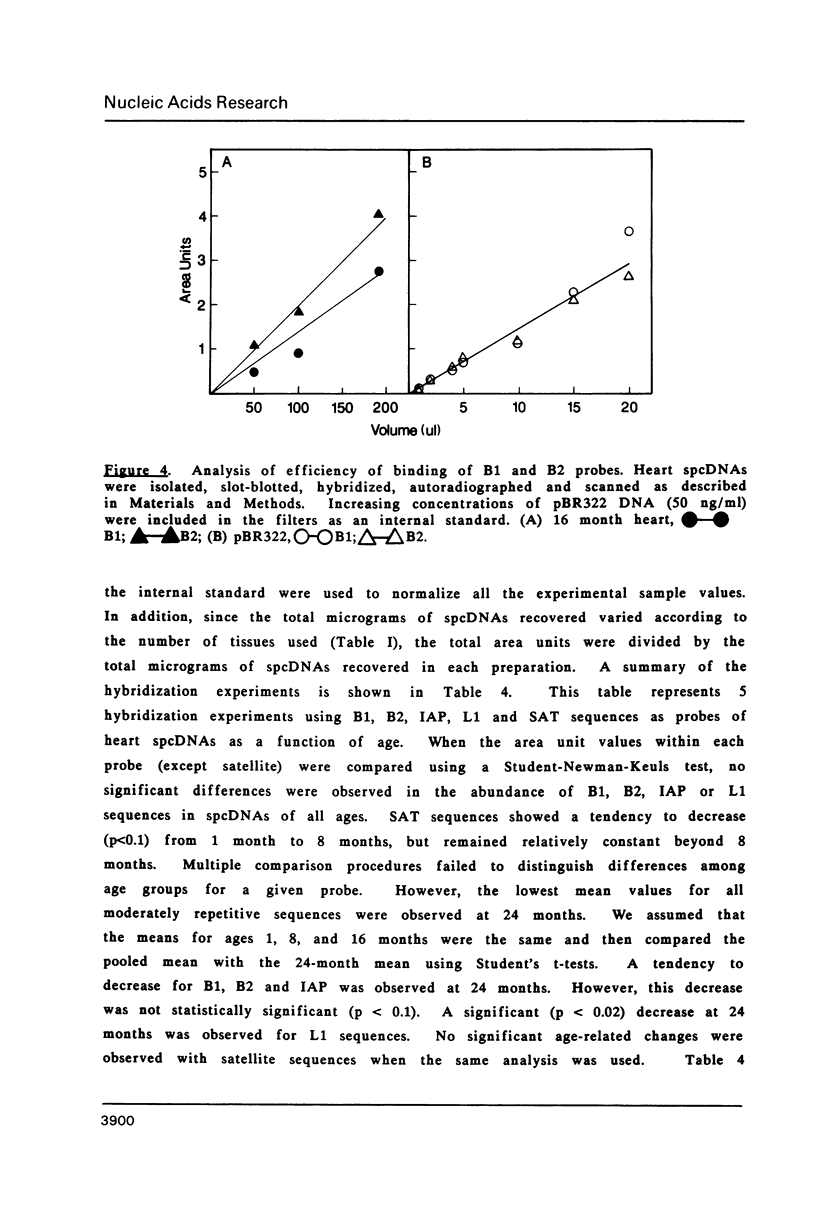
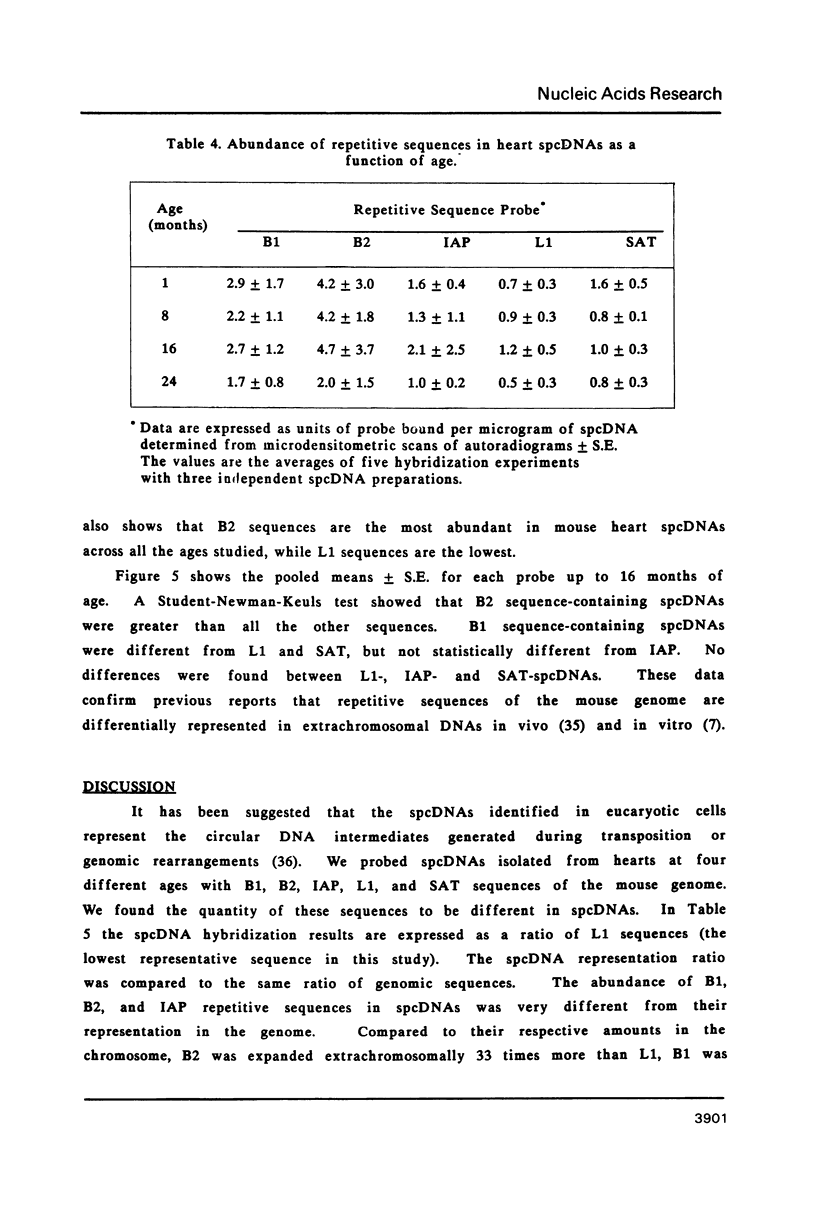
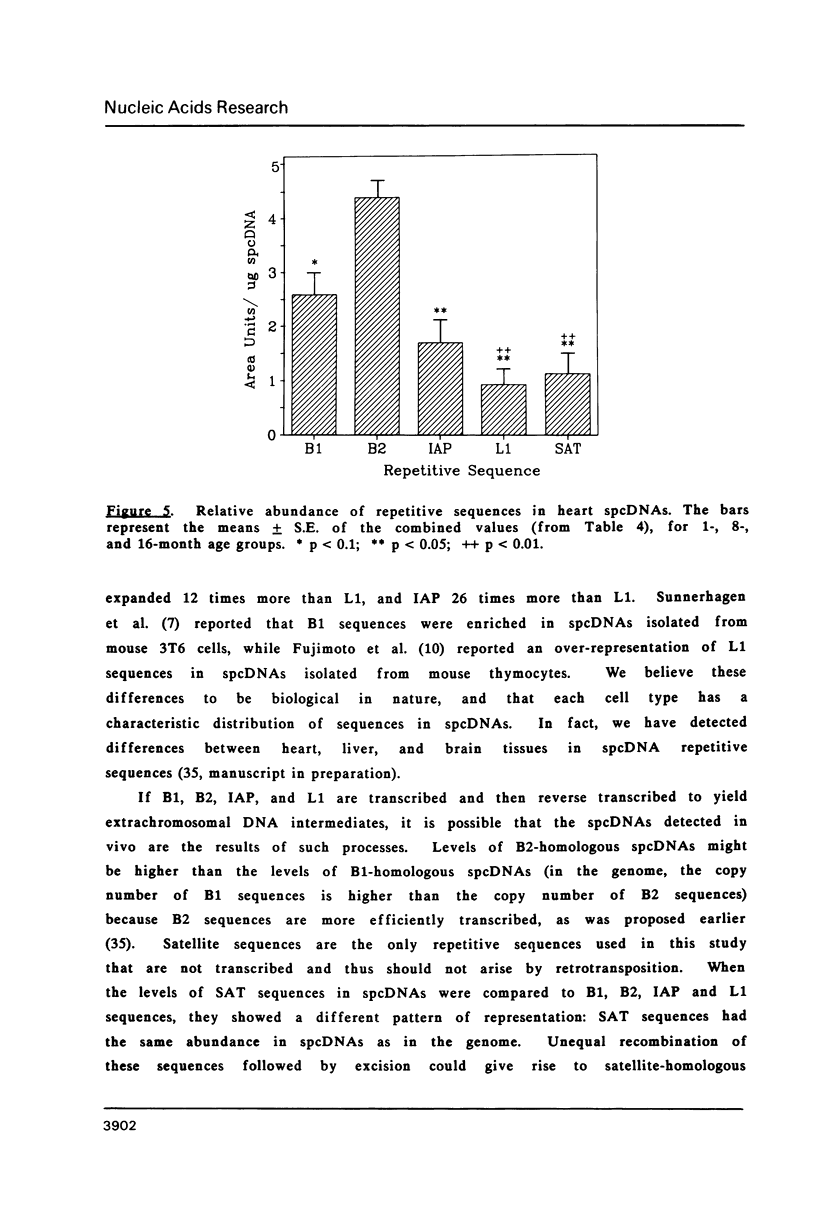
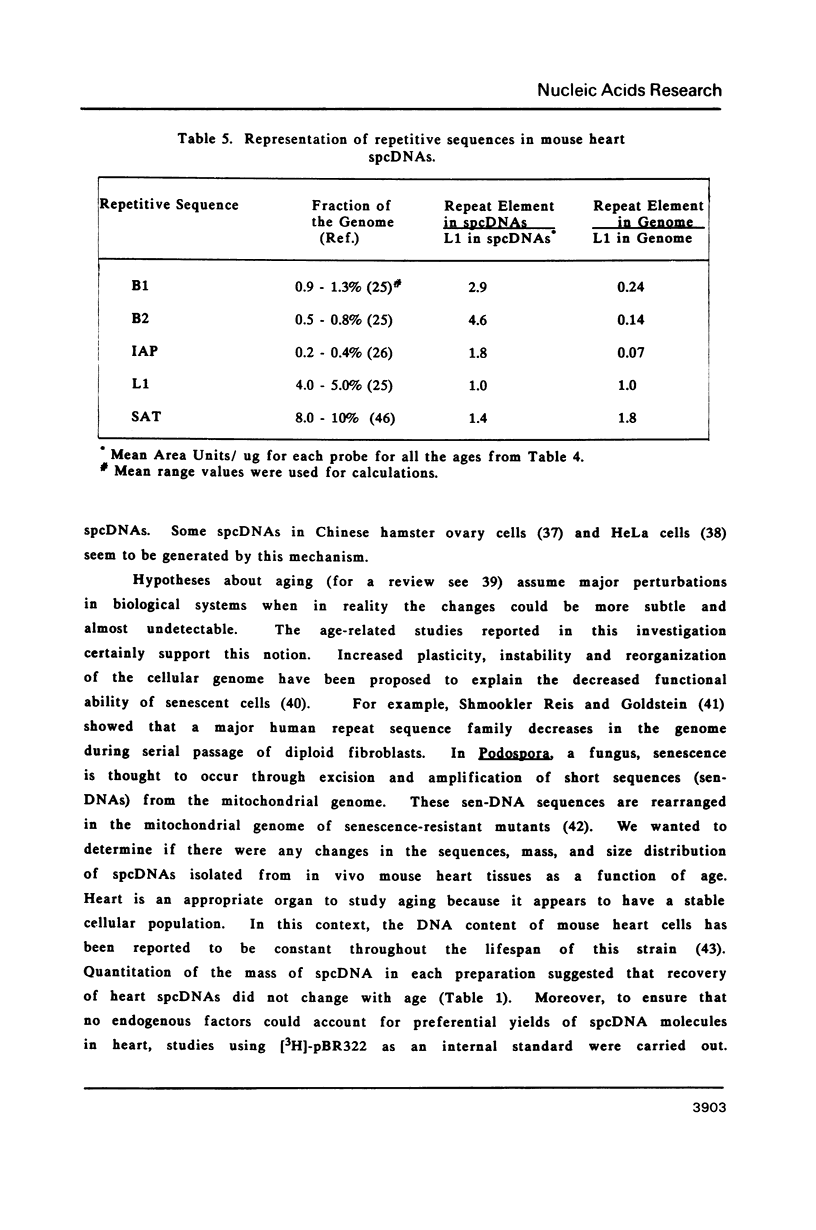



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Hill R. E., Pietras D. F., Woodworth-Gutai M., Kane-Haas C., Houston J. M., Heath J. K., Hastie N. D. Most highly repeated dispersed DNA families in the mouse genome. Mol Cell Biol. 1984 Aug;4(8):1561–1571. doi: 10.1128/mcb.4.8.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen A. H., Humayun M. Z., Karfopoulos S. G., Rush M. G. Molecular characterization of small polydisperse circular deoxyribonucleic acid from an African green monkey cell line. Biochemistry. 1982 Apr 27;21(9):2076–2085. doi: 10.1021/bi00538a015. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky W. Y., Arefyeva A. M., Uryvaeva I. V. Mitotic polyploidization of mouse heart myocytes during the first postnatal week. Cell Tissue Res. 1980;210(1):133–144. doi: 10.1007/BF00232149. [DOI] [PubMed] [Google Scholar]
- Butner K. A., Lo C. W. High frequency DNA rearrangements associated with mouse centromeric satellite DNA. J Mol Biol. 1986 Feb 20;187(4):547–556. doi: 10.1016/0022-2836(86)90333-5. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLap R. J., Rush M. G. Change in quantity and size distribution of small circular DNAs during development of chicken bursa. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5855–5859. doi: 10.1073/pnas.75.12.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores S. C., Moore T. K., Gaubatz J. W. Dispersed repetitive sequences of the mouse genome are differentially represented in extrachromosomal circular DNAs in vivo. Plasmid. 1987 May;17(3):257–260. doi: 10.1016/0147-619x(87)90034-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Tsuda T., Toda M., Yamagishi H. Transposon-like sequences in extrachromosomal circular DNA from mouse thymocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2072–2076. doi: 10.1073/pnas.82.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Theories of biological aging. Exp Gerontol. 1985;20(3-4):145–159. doi: 10.1016/0531-5565(85)90032-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. Characterization of cloned human alphoid satellite with an unusual monomeric construction: evidence for enrichment in HeLa small polydisperse circular DNA. Nucleic Acids Res. 1985 Feb 11;13(3):1027–1042. doi: 10.1093/nar/13.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. L1 sequences in HeLa extrachromosomal circular DNA: evidence for circularization by homologous recombination. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1989–1993. doi: 10.1073/pnas.82.7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Determination of DNA and RNA in homogenized cells and tissues by surface fluorometry. Anal Biochem. 1972 Mar;46(1):135–148. doi: 10.1016/0003-2697(72)90405-8. [DOI] [PubMed] [Google Scholar]
- Koll F., Belcour L., Vierny C. A 1100-bp sequence of mitochondrial DNA is involved in senescence process in Podospora: study of senescent and mutant cultures. Plasmid. 1985 Sep;14(2):106–117. doi: 10.1016/0147-619x(85)90070-8. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Bertelsen A. H., Humayun M. Z., Rush M. G. Members of the Alu family of interspersed, repetitive DNA sequences are in the small circular DNA population of monkey cells grown in culture. J Mol Biol. 1982 Jan 25;154(3):399–415. doi: 10.1016/s0022-2836(82)80003-x. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Rush M. G. Some extrachromosomal circular DNAs containing the Alu family of dispersed repetitive sequences may be reverse transcripts. J Mol Biol. 1984 Mar 25;174(1):31–40. doi: 10.1016/0022-2836(84)90363-2. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T., Yamagishi H., Ogita Z., Kirakawa T., Mitsui Y. Appearance of extrachromosomal circular DNAs during in vivo and in vitro ageing of mammalian cells. Mech Ageing Dev. 1985 Jan;29(1):89–99. doi: 10.1016/0047-6374(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A. Implications of the reorganization of the cell genome for aging or immortalization of dividing cells in vitro. Gerontology. 1980;26(5):276–282. doi: 10.1159/000212428. [DOI] [PubMed] [Google Scholar]
- Peterson C. R., Cryar J. R., Gaubatz J. W. Constancy of ribosomal RNA genes during aging of mouse heart cells and during serial passage of WI-38 cells. Arch Gerontol Geriatr. 1984 Jul;3(2):115–125. doi: 10.1016/0167-4943(84)90004-9. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Shmookler Reis R. J., Goldstein S. Interspersed repetitive and tandemly repetitive sequences are differentially represented in extrachromosomal covalently closed circular DNA of human diploid fibroblasts. Nucleic Acids Res. 1985 Aug 12;13(15):5563–5584. doi: 10.1093/nar/13.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Misra R. Extrachromosomal DNA in eucaryotes. Plasmid. 1985 Nov;14(3):177–191. doi: 10.1016/0147-619x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Loss of reiterated DNA sequences during serial passage of human diploid fibroblasts. Cell. 1980 Oct;21(3):739–749. doi: 10.1016/0092-8674(80)90437-7. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jordan J. M., Vinograd J. In vivo effects of intercalating drugs on the superhelix density of mitochondrial DNA isolated from human and mouse cells in culture. J Mol Biol. 1971 Jul 28;59(2):255–272. doi: 10.1016/0022-2836(71)90050-7. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Cloning and characterization of small circular DNA from Chinese hamster ovary cells. Mol Cell Biol. 1984 Jan;4(1):173–180. doi: 10.1128/mcb.4.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Multiple mechanisms generate extrachromosomal circular DNA in Chinese hamster ovary cells. Nucleic Acids Res. 1986 Apr 25;14(8):3527–3538. doi: 10.1093/nar/14.8.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S. W., Lengyel J. A. Small circular DNA of Drosophila melanogaster: chromosomal homology and kinetic complexity. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6142–6146. doi: 10.1073/pnas.76.12.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S., Helinski D. R. Small circular DNA in Drosophila melanogaster. Cell. 1976 Oct;9(2):333–345. doi: 10.1016/0092-8674(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen P., Sjöberg R. M., Karlsson A. L., Lundh L., Bjursell G. Molecular cloning and characterization of small polydisperse circular DNA from mouse 3T6 cells. Nucleic Acids Res. 1986 Oct 24;14(20):7823–7838. doi: 10.1093/nar/14.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Voliva C. F., Jahn C. L., Comer M. B., Hutchison C. A., 3rd, Edgell M. H. The L1Md long interspersed repeat family in the mouse: almost all examples are truncated at one end. Nucleic Acids Res. 1983 Dec 20;11(24):8847–8859. doi: 10.1093/nar/11.24.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wiberg F. C., Sunnerhagen P., Kaltoft K., Zeuthen J., Bjursell G. Replication and expression in mammalian cells of transfected DNA; description of an improved erythrocyte ghost fusion technique. Nucleic Acids Res. 1983 Nov 11;11(21):7287–7302. doi: 10.1093/nar/11.21.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., Boyer H. W., Helsinki D. R. Size and base composition of RNA in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3744–3748. doi: 10.1073/pnas.70.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Kunisada T., Takeda T. Amplification of extrachromosomal small circular DNAs in a murine model of accelerated senescence. A brief note. Mech Ageing Dev. 1985 Jan;29(1):101–103. doi: 10.1016/0047-6374(85)90051-x. [DOI] [PubMed] [Google Scholar]