Abstract
Defects in human leukocyte antigen class I antigen processing machinery (APM) component expression can have a negative impact on the clinical course of tumors and the response to T cell-based immunotherapy. Since brain metastases of breast cancer are of increasing clinical significance, the APM component expression levels and CD8+ T cell infiltration patterns were analyzed in primary breast and metastatic brain lesions of breast cancer by immunohistochemistry. Comparison of unpaired 50 primary and 33 brain metastases showed lower expression of β2-microglobulin, transporter associated with antigen processing (TAP) 1, TAP2 and calnexin in the brain lesions. Although no significant differences were found in APM component scores between primary breast and brain lesions in 15 paired cases, primary breast lesions of which patients eventually developed brain metastases showed lower levels of β2-microglobulin, TAP1 and calnexin compared with breast lesions without known brain metastases. The extent of CD8+ T cell infiltration was significantly higher in the lesions without metastasis compared with the ones with brain metastases, and was positively associated with the expression of TAP1 and calnexin. Furthermore, mouse tumor cells stably transfected with silencing hairpin (sh)RNA for TAP1 demonstrated a decreased susceptibility to cytotoxic T lymphocytes in vitro and enhanced spontaneous brain metastasis in vivo. These data support the functional significance of TAP1 expression in tumor cells. Taken together, our data suggest that patients with low or defective TAP1 or calnexin in primary breast cancers may be at higher risks for developing brain metastasis due to the defects in T cell-based immunosurveillance.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1137-9) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Brain metastasis, Antigen processing machinery (APM) components, CD8+ T cell, Transporter associated with antigen processing (TAP)1
Introduction
Cerebral metastases are a common complication among patients with systemic cancer. The tumor types most likely to metastasize to the brain are lung, breast and melanoma [1] with 20–30% of brain metastases originating from breast cancer [2]. Among women with breast cancer, 30–40% will develop metastatic disease. Historically, 15–20% of patients with metastatic breast cancer present with clinically symptomatic metastases to the brain. At autopsy, asymptomatic metastatic lesions are found in the brains of more than 30% of breast cancer patients [1, 3–5]. Although risk factors for brain metastasis have been increasingly characterized, such as basal type histology and HER2/neu expression [6], it is necessary to gain better understanding in factors promoting brain metastasis of breast cancer. As therapies for systemic cancer improve and patients survive longer, the risk of cerebral metastasis will increase. Recently, stereotactic radiosurgery has emerged as a possible alternative to whole-brain radiotherapy and surgery [7]. Nevertheless, median overall survival for cerebral metastases from breast cancer remains less than 1 year [8]. Cerebral metastases of cancers, therefore, are major obstacles that must be overcome before cancers can be cured by any means.
Immunotherapy has a great potential for prevention and treatment of brain cancers. Our group is dedicated to the development of vaccine strategies for primary brain tumors, such as malignant gliomas [9, 10]. Yet, these vaccine strategies rely on activated cytotoxic T lymphocytes (CTL) that recognize tumor antigens (TA) presented as a part of the human leukocyte antigen (HLA) class I-TA peptide complex. Antigen processing and presenting machinery components (APMs) play a crucial role in the generation of these complexes. However, defective expression of APMs is a common phenomenon observed in a variety of human tumors [11]. Immunotherapy based on the activation of tumor-specific T cells can be severely limited by the tumor variants lacking APMs. In fact, the frequency of these defects is associated with clinical outcome, such as tumor progression and metastasis, as well as poor patient survival [12–15]. To the best of our knowledge, however, no information is available about the frequency of APM defects in brain metastases of breast cancer.
In this study, we evaluated the expression of HLA class I APM expression between primary breast cancer and brain metastasis, including 15 cases in which paired primary breast and brain metastatic lesions were available. Our data demonstrate that β2-microglobulin, transporter associated with antigen processing (TAP) 1, TAP2 and calnexin are downregulated in brain lesions compared with unpaired breast lesions. Furthermore, primary breast lesions with known history of brain metastases showed lower levels of β2-microglobulin, TAP1 and calnexin compared with breast lesions without known brain metastasis. The extent of CD8+ T cell infiltration in the breast lesions was positively associated with expression of TAP1 and calnexin. Moreover, murine tumor cells in which TAP1 was genetically knocked down demonstrated a decreased sensitivity to CTL-mediated lysis and an increased frequency of spontaneous brain metastasis in vivo, indicating the functional significance of TAP1 expression for immune surveillance. Taken together, these data suggest a potential role of immune surveillance in primary breast lesions for reducing risks for brain metastasis.
Materials and methods
Cell line and animals
The murine B16 melanoma (H-2b) and 4T1 mammary adenocarcinoma (H-2d) cell lines were cultured in RPMI-1640 (Mediatech Inc, Manassas, VA) supplemented with 10% FBS (Mediatech Inc, Manassas, VA) and 1% penicillin/streptomycin (Invitrogen Corp, Carlsbad, CA). Pmel-1 mice (The Jackson Laboratory) are C57BL/6-background (H-2b) mice transgenic for human (h)gp10025–33-specific T cell receptor (TCR), which cross-reacts with mouse (m) gp10025–33 [16]. H-2d Balb/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals were handled in the Animal Facility at the University of Pittsburgh per an Institutional Care and Use Committee-approved protocol.
Tissue samples and protection of human subjects
Archived paraffin-embedded tissue sections of primary breast and brain metastasis lesions were provided with histopathological diagnosis by tissue banks from Magee-Women’s Hospital and the Division of Neuropathology at the University of Pittsburgh School of Medicine, respectively. Among 65 cases of primary breast cancer and 48 cases of brain metastasis used in this study, 15 cases had paired primary and metastatic lesions from the same patient. The other 50 cases of primary breast cancer were followed up for 20 months by median, ranging 10–64 months, after diagnosis and did not have known brain metastases until the day of analysis. These cases are referred to as cases without known metastasis in this manuscript to distinguish them from the other 15 cases with paired brain metastases. De-identified patient information was accessible only through designated honest brokers (Anatomic Pathology Broker System, Approval # IRB 08080284 [PI: Hideho Okada]).
Antibodies and peptides
The mAb HC-10, which recognizes a determinant expressed on β2m-free HLA-A3, HLA-A10, HLA-A28, HLA-A29, HLA-A30, HLA-A31, HLA-A32 and HLA-A33 heavy chains and on all β2m-free HLA-B and HLA-C heavy chains [17, 18]; the mAb HC-A2 which recognizes a determinant expressed on β2m-free HLA-A (excluding HLA-A24), HLA-B7301 and HLA-G heavy chains [17, 19]; the β2m-specific mAb NAMB-1 [20]; the 20S proteasome inducible subunit low molecular weight polypeptide LMP2-specific mAb SY-1 [21]; the TAP1-specific mAb NOB1 [22], the TAP2-specific mAb NOB2 [22], the calnexin-specific mAb TO-5 [23]; and the tapasin-specific mAb TO-3 [23] were developed and characterized as described. All of the above-mentioned mAbs are IgG1 except mAb HC-10 which is an IgG2a. mAb were purified from ascetic fluid by sequential precipitation with ammonium sulfate and caprylic acid. The purity of the mAb preparations was monitored by SDS-PAGE; the activity was monitored by Western blotting. The antihuman CD8-specific mAb clone C8/144B and HRP-conjugated secondary antibody (EnVision + system) were purchased from Dako Cytomation (Glostrup, Denmark). hgp10025–33 peptide (KVPRNQDWL) was synthesized in the University of Pittsburgh Peptide Synthesis Facility with >95% purity as indicated by analytical high-performance liquid chromatography and mass spectrometric analysis.
Immunohistochemistry
Deparaffinized tissue sections were placed in citrate buffer pH 6.0 and heated in a pressure pot for 20 min. Following overnight incubation with an optimal dilution of primary antibody at 4°C, slides were incubated with an optimal amount of HRP-conjugated secondary antibody for 45 min at room temperature. Diaminobenzidine (DAB, DAKO) was used as chromogen, and sections were counterstained with hematoxylin. Staining intensity of APM components was graded as weak: 1, intermediate: 2, or strong: 3. The stained area was classified as follows: no staining, 0; ≤10% of all cells stained as viewed by microscopy, 1; 11–50%, 2; 51–75%, 3; >75%, 4. An average immunoreactive score was calculated by multiplying the staining intensity by the area of staining. A total score of 3 or more was defined as positive expression and a score of less than three as negative. 0–2, (negative, score 0); 3–4, (weakly positive, score 1); 6–8, (moderately positive, score 2); 9–12, (strongly positive, score 3). Intratumoral infiltrated CD8 positive cells were counted in 10 high power fields. Two investigators who blinded to the clinical information analyzed tissue sections independently and the scores of two investigators were averaged.
RNA interference
The mouse B16 melanoma and 4T1 mammary adenocarcinoma cells were transfected at 30–40% confluence with TAP1-targeting shRNA or non-targeting shRNA control (OriGene Inc, Rockville, MD) using Lipofectamine 2000 (Invitrogen Corp) according to the instruction by the manufacturer, followed by 10 μg/ml puromycin selection. The targeting sequence for TAP1 was 5′-TCGTCCAGATGCCTTCGCTATCAGTTATG-3′. The cells from single clones were harvested 14 days later to evaluate the knockdown effect on TAP1 by quantitative real-time PCR. GAPDH was used as the internal control. The primers were obtained from Applied Biosystems: TAP1 (Mm00443188_m1), and GAPDH (Mm99999915_g1).
Generation of hgp100-specific effector cells
Pmel-1 mouse-derived splenocytes (SPC) were stimulated with hgp10025–33 peptide (5 μg/ml) in the presence of 100 U/ml rhIL-2 (PeproTech) [24]. Cells were restimulated under the same conditions at 48 h after the initial stimulation and were harvested on day 7.
CTL assay
B16 cells transfected with TAP1-targeting shRNA (B16-TAP1KO) and ones transfected with non-targeting shRNA control (B16-mock) were first labeled with 51Cr for 1 h. After washes, cells were plated onto 96-well tissue culture plates in triplicates (2 × 103 per well) and incubated with effector cells at 100:1 E/T ratio in 0.2 ml/well complete medium. After incubation for 18 h, the plate was centrifuged at low speed. 25μL supernatant was carefully aspirated and loaded onto a lumaplate. The radioactivity was measured by using a gamma counter.
Spontaneous brain metastasis assay
Balb/c mice received inoculations of 4T1 tumor cells transfected with TAP1-targeting shRNA (4T1-TAP1KO) or ones transfected with non-targeting shRNA control (4T1-mock) (1 × 106 cells/mouse for both groups) in the abdominal mammary gland. On day 30 after the inoculation, mice were killed and perfused with 20 ml PBS. Their brains were removed and finely minced with 18G and 27G needles. After extensive washing with PBS, single cell suspensions from each mouse were plated in a 10-cm tissue culture dish. The cells were cultured in the presence of 2 μg/ml puromycin during the final 7–10 days of the total 28 day culture period to selectively grow tumor cells. The cultured dishes were then fixed with methanol and stained with crystal violet for counting the foci of tumor cells that gave rise from each animal.
Statistical analysis
Statistical analyses were performed using StatMate III and Graphpad Prism 5 softwares. Mann–Whitney U test and t test were used in comparison of two groups. One-way ANOVA was used to analyze data from more than two groups. Kendall’s tau-b test was used to analyze CD8+ T cell infiltration. Pearson’s correlation was used to analyze the association of APM expression. P < 0.05 was considered to be significant.
Results
Expression of HLA class I APM components in primary breast cancer and brain metastatic lesions
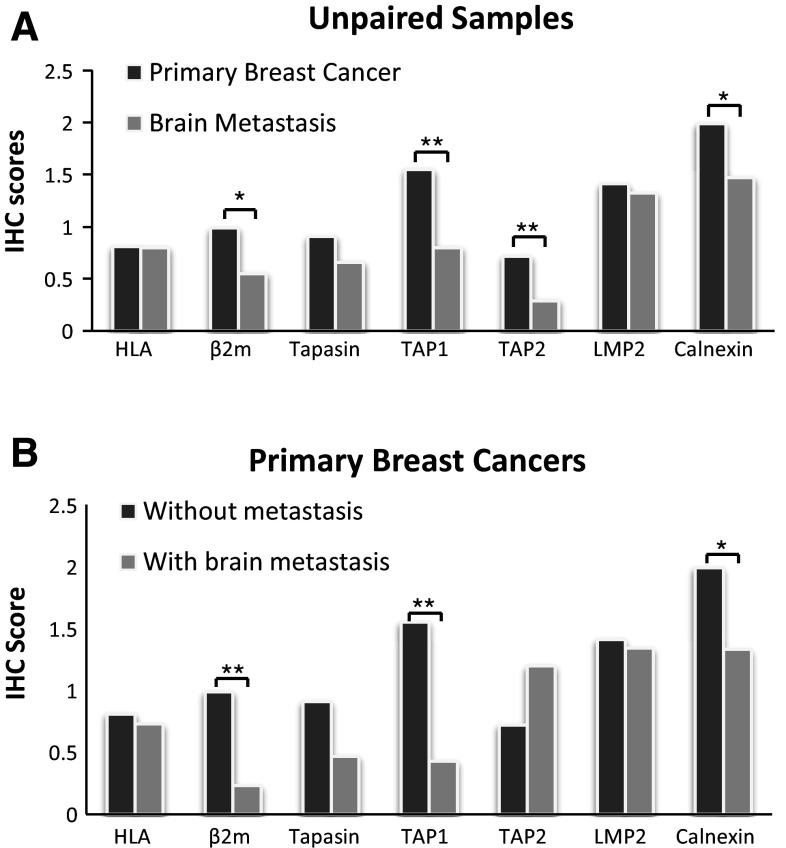
To determine whether HLA class I APM components are differentially expressed in brain metastatic lesions compared with primary breast cancers, 65 cases of primary breast cancer and 48 cases of brain metastasis were evaluated by immunohistochemistry for expression of HLA class I heavy chain, β2-microglobulin, tapasin, TAP1, TAP2, LMP2 and calnexin. Among them, 15 cases had paired primary and metastatic lesions from the same patient. The other 50 cases with primary lesions did not have known brain metastases. As shown as representative cases in Figs. 1 and S1A, tumor cells demonstrated variable expression levels of these molecules. When expression levels were compared between unpaired primary breast and metastatic brain lesions (Table 1 as well as Figs. 1, 2a), the brain lesions demonstrated significantly lower expression levels of β2-microglobulin (P = 0.023), TAP1 (P < 0.001), TAP2 (P = 0.002) and calnexin (P = 0.016). The other markers, HLA class I heavy chain, tapasin and TAP2, did not demonstrate significantly different expression levels between the two groups (Fig. S1).
Fig. 1.
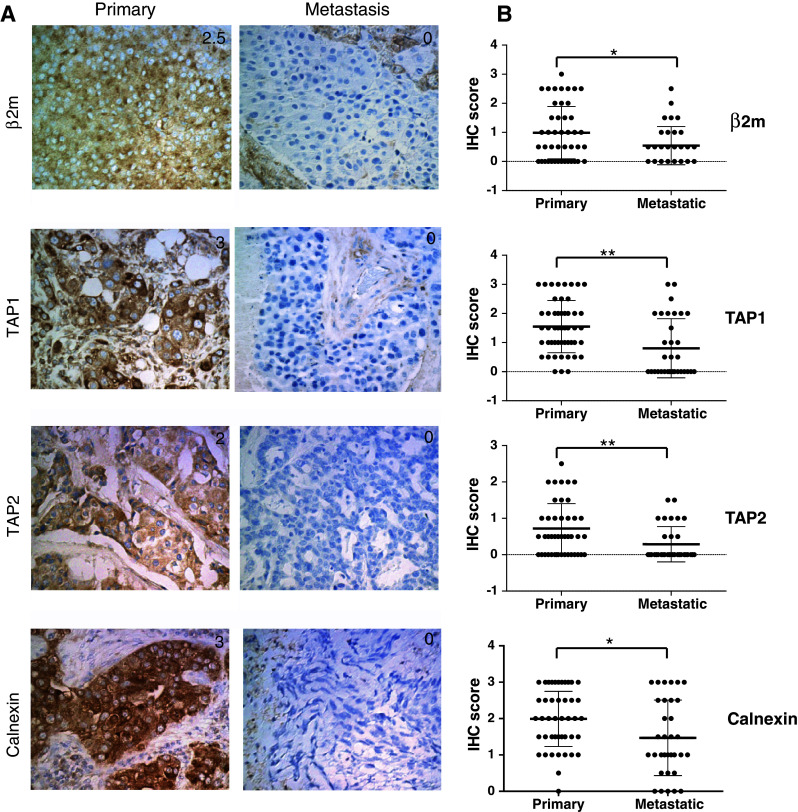
Expression of β2 microglobulin, TAP1, TAP2 and calnexin in primary and brain metastasis of breast cancer. a Representative immunohistochemical (IHC) staining on primary breast cancer tissues (left) and metastatic brain lesions (right) against β2 microglobulin (β2m), transporter associated with antigen processing (TAP)1, TAP2 and calnexin. Numbers in each of the panels indicate the score of staining intensity as defined in the materials and methods section. b Summary of the results, comparing the expression level of each of the APM components in primary breast cancer cases without known brain metastases (Primary, N = 50) and unpaired metastatic brain lesions (Metastatic, N = 33). Thick bars indicate mean scores, and error bars represent ±SD. *P < 0.05, **P < 0.01
Table 1.
Comparison of APM expression between primary breast cancers and brain metastatic lesions
| Unpaired samples* | Paired samples** | |||||
|---|---|---|---|---|---|---|
| Primary (n = 50) | Met (n = 33) | P value | Primary (n = 15) | Met (n = 15) | P value | |
| HLA | 0.81 ± 0.89 | 0.80 ± 0.93 | 0.89 | 0.73 ± 1.02 | 0.5 ± 0.82 | 0.3 |
| β2m | 0.99 ± 0.90 | 0.55 ± 0.65 | 0.023 | 0.23 ± 0.56 | 0.3 ± 0.46 | 0.72 |
| Tapasin | 0.91 ± 0.97 | 0.66 ± 0.81 | 0.36 | 0.47 ± 0.61 | 0.33 ± 0.79 | 0.6 |
| TAP1 | 1.55 ± 0.90 | 0.80 ± 0.90 | <0.001 | 0.43 ± 0.70 | 0.30 ± 0.59 | 0.53 |
| TAP2 | 0.72 ± 0.669 | 0.29 ± 0.48 | 0.002 | 1.20 ± 0.88 | 0.97 ± 0.83 | 0.44 |
| LMP2 | 1.41 ± 0.73 | 1.32 ± 0.80 | 0.49 | 1.34 ± 0.74 | 1.73 ± 0.80 | 0.22 |
| Calnexin | 1.99 ± 0.76 | 1.47 ± 1.04 | 0.016 | 1.33 ± 1.10 | 1.47 ± 1.19 | 0.64 |
Data indicate mean scores ± SD
* Mann–Whitney U test; ** Paired t test. Bold: Statistically significant (P < 0.05)
Fig. 2.
Comparison of antigen-processing machinery (APM) components in primary and brain metastasis of breast cancer. Average scores of APM component expression are shown for: a unpaired samples of primary breast cancers with no known brain metastasis (N = 50) and brain metastases (N = 33); and b primary breast cancers with (N = 15) and without (N = 50) known brain metastasis. *P < 0.05, **P < 0.01
On the other hand, when 15 paired cases were analyzed, none of the evaluated markers demonstrated significant differences between the primary versus metastatic brain lesions (data not shown). However, when the APM expression levels in the primary breast lesions were compared between the cases with or without known brain metastases (Fig. 2b), the cases with known brain metastases demonstrated lower levels of β2-microglobulin (P < 0.001), TAP1 (P < 0.001) and calnexin (P = 0.03) compared with the cases without known brain metastases.
Analyses of all the samples from primary and brain lesions revealed that the expression level of HLA class I heavy chain positively correlated with expression levels of β2-microglobulin (P < 0.0001), tapasin (P < 0.0001), TAP1 (P < 0.0001), TAP2 (P = 0.0005) and LMP2 (P = 0.0189) (Fig. 3).
Fig. 3.
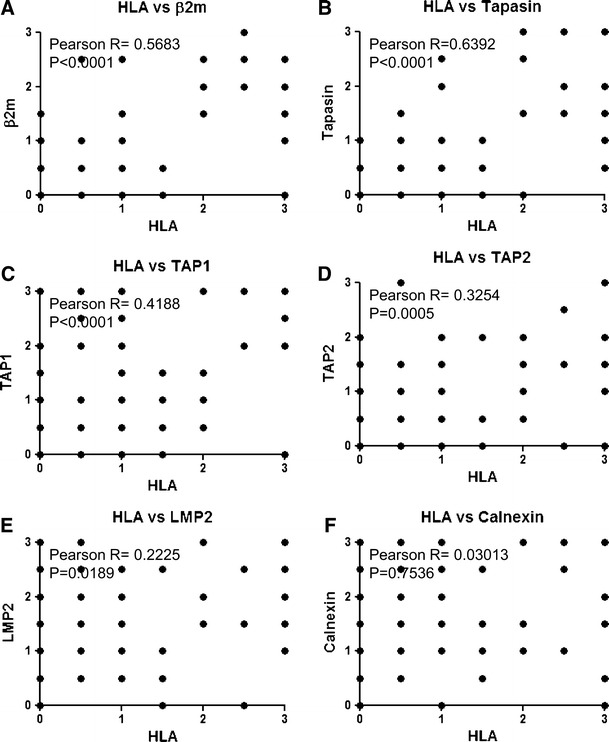
Correlation of HLA class I heavy chain with other APM component expression levels. All the stained samples derived from both primary and metastatic brain lesions were evaluated by Pearson’s correlation analysis
Significance of tumor infiltrating CD8+ T cells in the primary breast cancer
As APM components are critical for intact antigen-presentation to CD8+ T cells [25], it was hypothesized that the degree of APM expression would correlate with that of CD8+ T cell infiltration in these tumors. In most cases (63 out of 65 cases; 96.92%), primary breast lesions were variably infiltrated by CD8+ T cells (Fig. 4a). The extent of CD8+ T cell infiltration in the primary site was positively associated with expression levels of TAP1 (P = 0.004) and calnexin (P = 0.004) (Figs. 4b, S2). Furthermore, the cases that are known to have developed brain metastases demonstrated significantly lower degrees of T cell infiltration compared with the cases without known brain metastases (P < 0.001) (Fig. 4c; Table 2).
Fig. 4.
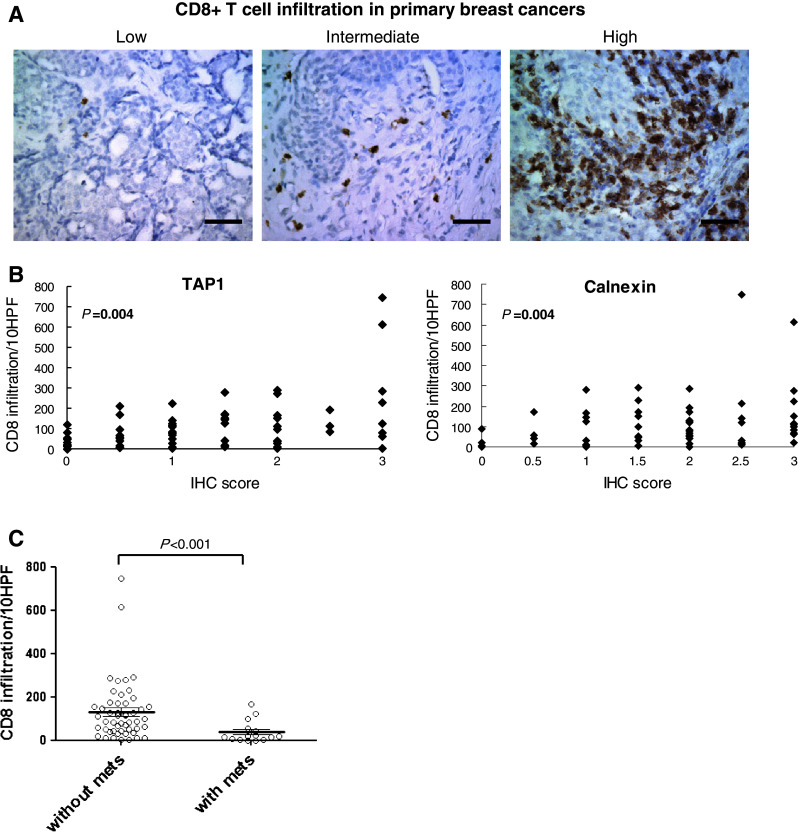
Extent of CD8+ T cell infiltration in primary breast cancer is positively associated with expression levels of TAP1 and Calnexin. a Representative pictures of cases with low (left), intermediate (center), and high (right) CD8+ T cell infiltration levels in primary breast cancer tissues. b Associations between the extent of CD8+ T cell infiltration and expression levels of TAP1 or Calnexin. c Comparison of CD8+ T cell infiltration in primary breast cancer cases without known brain metastases (N = 50) and cases that are known to have experienced brain metastases in the subsequent course (N = 15)
Table 2.
APM expression and CD8+ T cell infiltration in primary breast cancers with or without brain metastases
| Without brain mets (N = 50) | With brain mets (N = 15) | P value* | |
|---|---|---|---|
| HLA | 0.81 ± 0.89 | 0.73 ± 1.02 | 0.61 |
| β2m | 0.99 ± 0.90 | 0.23 ± 0.56 | <0.001 |
| Tapasin | 0.91 ± 0.97 | 0.47 ± 0.61 | 0.13 |
| TAP1 | 1.55 ± 0.90 | 0.43 ± 0.70 | <0.001 |
| TAP2 | 0.72 ± 0.69 | 1.20 ± 0.88 | 0.061 |
| LMP2 | 1.41 ± 0.73 | 1.37 ± 0.74 | 0.87 |
| Calnexin | 1.99 ± 0.76 | 1.33 ± 1.10 | 0.03 |
| CD8+ T cells/10HPF† | 130.04 ± 139.15 | 38.80 ± 50.54 | <0.001 |
Data indicate mean scores ± SD
* Mann–Whitney U test. Bold: Statistically significant (P < 0.05)
† HPF high power field
Significance of TAP1 expression in tumor cells for CTL-mediated lysis and spontaneous brain metastasis in mice
To determine the functional significance of TAP1 expression in tumor cells for CTL-mediated lysis, we used B16 melanoma cells and syngeneic pmel-1-derived gp100-specific CTLs because B16 cells express the murine gp100 [26]. As shown in Fig. 5a, shRNA-mediated knockdown achieved approximately 90% reduction of TAP1 mRNA levels. B16-TAP1KO demonstrated decreased levels of CTL-mediated lysis compared with B16-mock (P = 0.0282) (Fig. 5b).
Fig. 5.
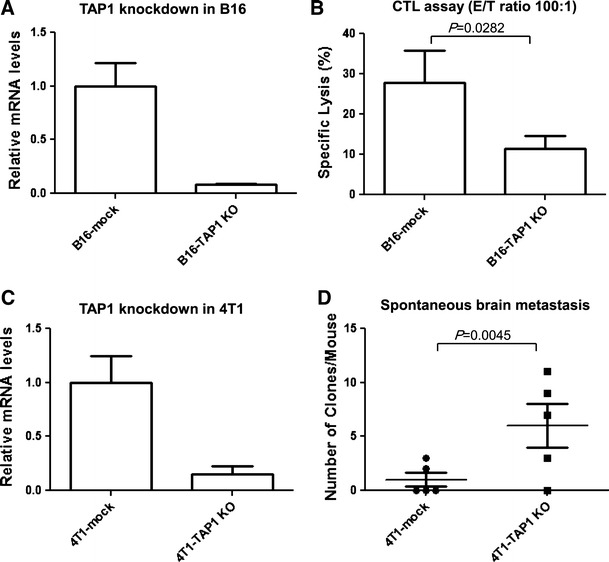
Significance of TAP1 expression in tumor cells for CTL-mediated lysis and brain metastasis. Knockdown of TAP1 in a B16 and c 4T1 cells. B16 and 4T1 cells were stably transfected with TAP1-targeting shRNA (B16-TAP1KO and 4T1-TAP1KO, respectively) or non-targeting shRNA control (B16-mock and 4T1-mock, respectively). The effect of TAP1 knockdown was evaluated by real-time PCR. Boxes, mean value; bars, SD. b CTL assay. 51Cr-labeled B16-TAP1KO or B16-mock cells were cocultured with hgp100-specific CTLs for 18 h. The ratio of the effector cells to the target cells was 100:1. Boxes, mean specific lyses; bars, SD. d Clonogenic assay for spontaneous brain metastasis. On day 30 following the inoculation of 4T1-TAP1KO or 4T1-mock cells in the mammary pad, mice were killed and single cell suspensions from the brain were cultured for 28 days. Numbers of tumor clones were counted after crystal violet staining. Horizontal bars indicate mean values, and vertical bars indicate SD. N = 5/group
To determine the role of TAP1 expression in breast cancer cells for spontaneous brain metastasis, we knocked down TAP1 expression in murine 4T1 breast cancer cells (4T1-TAP1KO) (Fig. 5c). Syngeneic Balb/c mice received an inoculation of 4T1-TAP1KO or control 4T1-mock cells into the mammary pad. On day 30, mice were killed and clonogenic assays were performed as detailed in materials and methods. In the group of 5 mice that received 4T1-TAP1KO cells, 4 mice gave rise to positive growth of tumor cells in the clonogenic assay (11, 9, 7 and 3 tumor cell colonies per mouse) with the mean number of tumor cell colonies as 6.00 ± 2.00 (Mean ± SD) per mouse. On the other hand, only 2 of 5 4T1-mock bearing mice showed tumor cell growth (2 and 3 tumor cell colonies per mouse) with the mean clone number as 1.00 ± 0.63 (Mean ± SD) per mouse (P = 0.0045) (Fig. 5d). There was not a significant difference in in vitro growth rates of 4T1-TAP1KO and 4T1-mock cells, and negative control mice without inoculation of tumor cells did not give rise to any growth of puromycin-resistant cells (data not shown), supporting that the increased number of cell colonies is due to the enhanced metastatic activity of 4T1-TAP1KO cells.
Association of APM components with clinicopathological factors
Clinicopathological factors were evaluated for their association with the expression levels of APM components in both primary breast cancers (Table 3) and brain metastases (Table 4). In both primary and brain lesions, patients younger than 60 years old demonstrated significantly lower TAP1 expression levels compared with older (≥60 years old) patients (P = 0.043; brain mets: P = 0.004). In primary breast cancers, patients younger than 60 years old demonstrated lower expression levels of HLA class I heavy chain than older (≥60 years old) patients (P = 0.036). Patients with more advanced AJCC stage (2B or higher) demonstrated lower expression levels of TAP1 (P = 0.006) and TAP2 (P = 0.004) than patients with lower AJCC stage. When APM expression was evaluated in relation to nuclear grade, which is a prognostic factor of breast cancers [27], primary lesions with nuclear grade III demonstrated lower expression of TAP2 compared with ones with grade I or II (P = 0.029). Furthermore, in primary lesions, cases with positive ER (P = 0.022) or PR (P = 0.016) status demonstrated lower expression levels of TAP2.
Table 3.
Clinocopathological factors and scoring of immunohistochemistry (primary lesions)
| HLA | β2m | Tapasin | TAP1 | TAP2 | LMP2 | Calnexin | CD8+ T cells/10HPF | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| <60 (N = 30) | 0.63 ± 0.97* | 0.67 ± 0.90 | 0.68 ± 0.92 | 1.07 ± 1.10* | 0.90 ± 0.88 | 1.33 ± 0.82 | 1.73 ± 1.00 | 121.9 ± 176.37 |
| ≥60 (N = 35) | 0.93 ± 0.84 | 0.94 ± 0.87 | 0.91 ± 0.90 | 1.49 ± 0.83 | 0.77 ± 0.65 | 1.46 ± 0.63 | 1.93 ± 0.78 | 105.1 ± 76.75 |
| Best AJCC stage | ||||||||
| 1, 2A (N = 46) | 0.86 ± 0.89 | 0.92 ± 0.92 | 0.94 ± 0.97 | 1.51 ± 0.95** | 0.97 ± 0.75** | 1.51 ± 0.65 | 1.79 ± 0.83 | 119.24 ± 147.34 |
| 2B+ (N = 18) | 0.61 ± 0.99 | 0.58 ± 0.79 | 0.47 ± 0.70 | 0.78 ± 0.86 | 0.42 ± 0.60 | 1.14 ± 0.85 | 1.89 ± 1.01 | 97.06 ± 85.01 |
| Nuclear grade† | ||||||||
| I (N = 27) | 0.80 ± 0.69 | 0.96 ± 0.87 | 0.86 ± 0.91 | 1.50 ± 0.96 | 0.98 ± 0.81* | 1.57 ± 0.68 | 2.05 ± 1.77 | 143.16 ± 171.9 |
| II (N = 30) | 0.89 ± 1.05 | 0.79 ± 0.93 | 0.90 ± 0.97 | 1.27 ± 0.97 | 0.90 ± 0.71 | 1.32 ± 0.68 | 1.58 ± 0.87 | 78.19 ± 76.8 |
| III (N = 8) | 0.83 ± 1.28 | 0.83 ± 1.12 | 0.72 ± 1.03 | 0.94 ± 1.07 | 0.50 ± 1.00 | 1.33 ± 1.09 | 2.11 ± 1.08 | 101.17 ± 114.32 |
| ER status | ||||||||
| Negative (N = 16) | 0.91 ± 0.99 | 1.00 ± 1.05 | 1.09 ± 1.10 | 1.47 ± 1.07 | 1.19 ± 0.73* | 1.56 ± 0.81 | 1.72 ± 1.13 | 122.09 ± 148.16 |
| Positive (N = 49) | 0.76 ± 0.89 | 0.76 ± 0.84 | 0.76 ± 0.88 | 1.24 ± 0.95 | 0.71 ± 0.74 | 1.35 ± 0.69 | 1.88 ± 0.80 | 109.87 ± 127.12 |
| PR status | ||||||||
| Negative (N = 20) | 0.98 ± 1.02 | 0.90 ± 1.01 | 1.13 ± 1.04 | 1.53 ± 1.08 | 1.13 ± 0.74* | 1.43 ± 0.71 | 1.75 ± 1.13 | 117.23 ± 141.49 |
| Positive (N = 44) | 0.72 ± 0.87 | 0.78 ± 0.85 | 0.66 ± 0.83 | 1.22 ± 0.91 | 0.65 ± 0.65 | 1.38 ± 0.74 | 1.90 ± 0.76 | 113.44 ± 128.81 |
| Her2 status | ||||||||
| Negative (N = 32) | 0.77 ± 0.98 | 0.92 ± 0.97 | 0.72 ± 0.90 | 1.11 ± 0.98 | 0.86 ± 0.84 | 1.28 ± 0.70 | 1.72 ± 0.88 | 94.83 ± 143.75 |
| Positive (N = 33) | 0.82 ± 0.85 | 0.71 ± 0.81 | 0.89 ± 0.93 | 1.47 ± 0.95 | 0.80 ± 0.68 | 1.52 ± 0.75 | 1.96 ± 0.89 | 130.38 ± 118.00 |
Data indicate mean scores ± SD
Both paired and newly diagnosed primary breast cancer samples were included
AJCC staging system was developed by the American Joint Committee on Cancer (AJCC) that uses TNM to describe the extent of cancer in a patient’s body. T describes the size of the tumor and whether it has invaded nearby tissue. N describes whether cancer has spread to nearby lymph nodes, and M describes whether cancer has metastasized (spread to distant parts of the body). Patients in 2B or higher stages are those bearing tumors that are larger than 5 cm in diameter or already have lymph node or distant metastases
† One-way ANOVA, others: Mann–Whitney U test. * P value < 0.05; ** P value < 0.01
Table 4.
Clinocopathological factors and scoring of immunohistochemistry (metastatic lesions)
| HLA | β2m | Tapasin | TAP1 | TAP2 | LMP2 | Calnexin | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <60 (N = 38) | 0.67 ± 0.95 | 0.49 ± 0.60 | 0.49 ± 0.75 | 0.45 ± 0.76** | 0.51 ± 0.73 | 1.41 ± 0.81 | 1.37 ± 1.03 |
| ≥60 (N = 10) | 0.85 ± 0.75 | 0.40 ± 0.66 | 0.80 ± 1.01 | 1.40 ± 1.15 | 0.45 ± 0.50 | 1.60 ± 0.84 | 1.85 ± 1.23 |
| Nuclear grade† | |||||||
| I (N = 16) | 0.91 ± 0.90 | 0.41 ± 0.64 | 0.78 ± 0.93 | 0.69 ± 1.01 | 0.44 ± 0.48 | 1.56 ± 0.87 | 1.44 ± 1.03 |
| II (N = 16) | 0.56 ± 0.85 | 0.34 ± 0.47 | 0.44 ± 0.85 | 0.50 ± 0.73 | 0.72 ± 0.93 | 1.38 ± 0.76 | 1.34 ± 1.08 |
| III (N = 7) | 0.50 ± 1.12 | 0.64 ± 0.56 | 0.42 ± 0.49 | 0.93 ± 1.31 | 0.36 ± 0.56 | 1.29 ± 0.70 | 2.00 ± 1.26 |
| ER status | |||||||
| Negative (N = 20) | 0.48 ± 0.72 | 0.30 ± 0.59 | 0.50 ± 0.71 | 0.45 ± 0.89 | 0.58 ± 0.65 | 1.38 ± 0.76 | 1.08 ± 1.02 |
| Positive (N = 23) | 0.87 ± 1.07 | 0.59 ± 0.63 | 0.64 ± 0.97 | 0.80 ± 0.99 | 0.48 ± 0.76 | 1.54 ± 0.80 | 1.74 ± 1.08 |
| PR status | |||||||
| Negative (N = 21) | 0.62 ± 0.88 | 0.33 ± 0.60 | 0.67 ± 0.93 | 0.62 ± 0.97 | 0.74 ± 0.82 | 1.36 ± 0.78 | 1.00 ± 0.98* |
| Positive (N = 18) | 0.69 ± 0.93 | 0.58 ± 0.65 | 0.44 ± 0.79 | 0.69 ± 0.96 | 0.33 ± 0.54 | 1.44 ± 0.68 | 1.72 ± 1.06 |
| Her2 status | |||||||
| Negative (N = 19) | 0.47 ± 0.70 | 0.42 ± 0.56 | 0.37 ± 0.74 | 0.53 ± 1.02 | 0.40 ± 0.52 | 1.42 ± 0.80 | 1.11 ± 1.02 |
| Positive (N = 18) | 0.83 ± 1.09 | 0.44 ± 0.73 | 0.68 ± 0.93 | 0.72 ± 0.90 | 0.72 ± 0.90 | 1.53 ± 0.72 | 1.56 ± 1.15 |
Data indicate mean scores ± SD
† One-way ANOVA, others: Mann–Whitney U test. * P value < 0.05; ** P value < 0.01
None of these clinicopathological factors were associated with levels of CD8+ T cell infiltration in primary breast cancers (Table 3).
Discussion
The current study describes, for the first time, expression levels of APM components in primary versus brain metastasis of breast cancer. Our results suggest that low or defective expression of APM components β2-microglobulin, TAP1 and calnexin as well as paucity of CD8+ T cell infiltration in the primary breast cancers may dictate high risks of developing brain metastasis. Our results from in vitro and in vivo functional studies support the role of TAP1 in CTL-mediated lysis and reduction of brain metastasis.
Our results showed that defective or low expression of HLA class I APM components occurred frequently in both primary breast cancer and brain metastasis. There have been only few reports on the APM status and cancer metastasis [28, 29], and to our knowledge, our report is the first specifically evaluating the APM status in brain metastases. Although no significant differences were found in the expression levels of APM components between primary breast and metastatic brain lesions in 15 paired cases, these primary breast lesions with known history of brain metastasis showed lower levels of β2-microglobulin, TAP1 and calnexin compared with breast lesions without known brain metastases. These results suggest that metastases do not originate from a subclone of tumor cells that undergo downregulation of APM expression in the primary site (“acquired” phenotype for tumor immune escape), but rather that an entire primary tumor with lower or defective expression of β2-microglobulin, TAP1 and calnexin might be more likely to spread to the brain (“inherent” phenotype for immune escape). Prospective studies in patients with breast cancer are needed to prove this hypothesis.
The three molecules (β2-microglobulin, TAP1 and calnexin) which are downregulated in the cases with known metastases have critical functions in the complex cascade where peptides are processed and loaded on HLA class I molecules [30, 31]. The processing and presentation of HLA class I antigen-derived peptides is accomplished through a series of intracellular events involving multiple APMs [32]. Following proteosome-mediated degradation and cytosolic cleavage of antigen proteins, peptides are transported via TAP1 and TAP2 into the endoplasmic reticulum (ER). In the ER, the HLA class I heavy chain and β2-microglobulin assembly occurs, which is coordinated by the chaperones calnexin, calreticulin, and the thiol oxidoreductase ERp57. Upon peptide loading, the trimer consisting of the HLA class I heavy chain, β2-microglobulin and antigen-peptide is released and transported via Golgi to the cell surface and there exposed to the CD8+ CTL. Downregulation of TAP1 inhibits the transportation of peptide to the ER, whereas downregulation of calnexin impedes proper folding of HLA class I heavy chain-β2-microglobulin complex. β2-microglobulin and TAP1 were also often found to be downregulated among APM components in a variety of tumors [32, 33]. Based on these known mechanisms underlying the assembly of HLA class I complexes, our results demonstrating a positive correlation of HLA class I heavy chain expression level with β2-microglobulin and TAP1 are reasonable, although we recognize that the immunohistochemical analysis in this study does not completely distinguish intracellular versus surface expression of HLA class I. Although our data with mouse models demonstrate the functional significance of TAP1 in CTL recognition and metastasis, further mechanistic studies are warranted to better understand the molecular mechanisms responsible for APM/HLA downregulation in cancer cells.
CTLs play an active role in the recognition and destruction of tumor cells. Their activation is initiated by the interaction with HLA class I molecules that present cognate antigen peptides. The lack or decreased expression of single or multiple components of the HLA class I antigen processing pathway can allow tumor cells to escape from recognition by CD8+ CTLs. Indeed, downregulation of APM components, such as TAP1, TAP2 and tapasin, has been found to be associated with failure of CTL recognition in squamous cell carcinoma of the head and neck [34]. In accordance with these previous studies, in the current study, the extent of CD8+ T cell infiltration in primary breast cancer was positively associated with expression of TAP1 and calnexin. Significance of our results in the clinical samples was further potentiated by the suppressed CTL-mediated lysis of B16-TAP1KO cells. This finding is not unique to a specific cell line, because similar results have also been described in both mouse and human tumor cells [34–36]. These data imply that future immunotherapy studies ought to consider TAP1 expression levels in tumors as a biomarker and/or develop strategies to enhance TAP1 expression, such as gene transfer.
Presence and extent of T cell infiltration were associated with longer survival of cancer patients [37–39]. Our results show that primary breast lesions with known brain metastases were infiltrated by lower numbers of CD8+ T cells compared with breast lesions without known metastases. These results suggest that low or absent CD8+ T cell infiltration in the primary breast cancer could also be a biomarker for breast cancers that have a higher risk of brain metastasis. We also found a trend that patients with more advanced stages had fewer CD8+ T cell infiltration compared to lower stages in their primary breast cancer site, although the trend did not meet the criteria for statistical significance. The limited amounts of available tissues did not allow us to evaluate the status of CD8+ T cell infiltration in the brain metastases. However, recent reports have demonstrated that the extent of CD8+ T cell infiltration in glioblastoma multiforme tissues correlates with long-term survival of patients [40, 41]. Future studies will address whether the degree of CD8+ T cell infiltration in metastatic cancers in the brain provide us with any additional prognostic information.
Dendritic cells (DCs) and other antigen presenting cells are important initiator and modulator of antitumor immune responses. Unfortunately, we were unable to evaluate the status of DC infiltration in primary and metastatic breast cancer tissues due to the limited amounts of available tumor materials. Nonetheless, the literature suggests that metastatic tumor cells in patients’ lymph nodes may affect the maturation of DCs, thereby affecting the antigen-presentation process. Although the number of DC infiltration in breast cancer did not correlate with lymph node metastasis, pathologic stage, or relapse-free survival [42], sentinel lymph nodes with metastatic tumor cells contained fewer mature DCs in than those without metastasis [43]. In addition to DCs, there is a variety of immune response mediators that can significantly impact the immune surveillance against cancer metastasis, such as chemokines, cytokines and T cell effector molecules [44–46]. Additional studies are warranted to gain more comprehensive understanding on the immunological factors that affect the risk and development of cancer metastasis.
In our analyses of clinicopathologic factors and their relation to APM expression levels, lower expression of TAP1 was significantly associated with early onset of the cancers in both primary and metastatic lesions. It is well known that breast cancers in young patients tend to behave more aggressively and possess poorer prognosis compared with cases in old patients [47, 48]. Furthermore, in our study, lower TAP1 or TAP2 in primary breast cancers were both associated with poorer AJCC stage. Thus, based on our results that TAP1 is downregulated in primary breast cancers with known brain metastases and that its expression level is positively associated with intratumoral CD8+ T cell infiltration, TAP1 may be considered as a biomarker that dictates higher risks for developing brain metastasis in patients with breast cancer. Further studies, especially prospective studies, are warranted to establish the value of TAP1 as such a biomarker. Indeed, our experiment using 4T1-TAP1KO cells indicates that the knockdown of TAP1 in the primary breast cancer promotes brain metastases. Since the extent of CD8+ T cell infiltration in primary breast cancer was positively associated with TAP1 expression, it is reasonable to postulate that downregulation or lack of TAP1 allows breast cancer cells to escape from T cell-mediated immuno-surveillance, thereby promoting the brain metastasis.
In addition to TAP1, lower expression of TAP2 in the primary breast cancer was associated with higher AJCC stage and higher nuclear grade which were poor prognostic indicators. On the other hand, TAP2 expression was negatively associated with ER and PR expressions in the primary lesions. As negative ER and PR status has been determined to be a strong predictor of poor prognosis [49–51], these results suggested that TAP2 and ER/PR status might be two independent indicators of breast cancer prognosis and function in independent mechanisms. Recent studies have shown some degrees of discordance in ER and PR status between primary and metastatic lesions [52, 53]. In fact, in brain metastases, there were no associations between the TAP2 expression levels and ER/PR status, likely supporting our notion that these are two independent factors. Although previous studies by others have shown that over-expression of HER2 in tumor cells leads to markedly reduced levels of APM components [54, 55], we didn’t find either positive or inverse correlations between HER2 and APM expression levels in our cases.
Although there is a growing body of evidence demonstrating downregulation of APM components in a variety of cancer types [11–15], the current study now shed lights on the potential roles of these molecules in the metastatic processes of cancer. Extension of these studies will likely allow us to develop novel biomarkers dictating the risks for metastasis and delineate key underlying mechanisms upon which we can develop strategies to prevent and/or reduce the risk of cancer metastasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Expression of HLA class I heavy chain, tapasin and LMP2 expression in primary and brain metastasis of breast cancer. (A) Representative immunohistochemical (IHC) staining on primary breast cancer tissues (left) and metastatic brain lesions (right). (B) Comparing the expression level HLA class I heavy chain, tapasin and LMP2 in primary breast cancer cases without known brain metastases (Primary, N = 50) and unpaired metastatic brain lesions (Metastatic, N = 33) (PPTX 1596 kb)
Absence of associations between the extent of CD8+ T cell infiltration and expression levels of HLA class I heavy chain, β2 microglobulin, tapasin, TAP2 or LMP2. Data were analyzed by Kendall’s tau-b test (PPTX 61 kb)
Acknowledgments
Lindsay Mock and Louise Mazur for collecting tissue slides and clinical data; Xiaojuan Deng for antibody preparation; Mitsugu Fujita for technical assistance. The Musella Foundation for Brain Tumor Research & Information (HO); The Walter L. Copeland Fund of the Pittsburgh Foundation (YL); NIH/NCI 1P01 CA132714 (HO); NIH/NINDS 2P01 NS40923 (HO); This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13(6):1675–1683. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 3.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167(4):913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 5.Stemmler H-J, Heinemann V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist. 2008;13(7):739–750. doi: 10.1634/theoncologist.2008-0052. [DOI] [PubMed] [Google Scholar]
- 6.Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 7.Martin JJ, Kondziolka D. Indications for resection and radiosurgery for brain metastases. Curr Opin Oncol. 2005;17(6):584–587. doi: 10.1097/01.cco.0000183670.69390.78. [DOI] [PubMed] [Google Scholar]
- 8.Kondziolka D, Kano H, Harrison GL, Yang H-C, Liew DN, Niranjan A, Brufsky AM, Flickinger JC, Lunsford LD. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg. 2010 doi: 10.3171/2010.8.JNS10461. [DOI] [PubMed] [Google Scholar]
- 9.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, Fujita M. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29(1):1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;69:264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 13.Cathro H, Smolkin M, Theodorescu D, Jo V, Ferrone S, Frierson H. Relationship between HLA class I antigen processing machinery component expression and the clinicopathologic characteristics of bladder carcinomas. Cancer Immunol Immunother. 2010;59(3):465–472. doi: 10.1007/s00262-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garre ML, Cama A, Basso G, Ferrone S, Gambini C, Pistoia V. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67(11):5471–5478. doi: 10.1158/0008-5472.CAN-06-4735. [DOI] [PubMed] [Google Scholar]
- 15.Vitale M, Rezzani R, Rodella L, Zauli G, Grigolato P, Cadei M, Hicklin DJ, Ferrone S. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58(4):737–742. [PubMed] [Google Scholar]
- 16.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stam N, Spits H, Ploegh H. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137(7):2299–2306. [PubMed] [Google Scholar]
- 18.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. {beta}2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171(4):1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 19.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA- A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35(3):177–188. doi: 10.1016/S0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 20.Maio M, Altomonte M, Tatake R, Zeff RA, Ferrone S. Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J Clin Investig. 1991;88(1):282–289. doi: 10.1172/JCI115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66(3):185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Campoli M, Cho HS, Ogino T, Bandoh N, Shen J, Hur SY, Kageshita T, Ferrone S. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods. 2005;299(1–2):139–151. doi: 10.1016/j.jim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, Okada H. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180(4):2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 25.Racanelli V, Leone P, Frassanito MA, Brunetti C, Perosa F, Ferrone S, Dammacco F. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2010;115(6):1185–1193. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai Y, Yang JC, Spiess P, Nishimura MI, Overwijk WW, Roberts B, Restifo NP, Rosenberg SA. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother. 1997;20(1):15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.le Doussal V, Tubiana-Hulin M, Hacene K, Friedman S, Brunet M. Nuclear characteristics as indicators of prognosis in node negative breast cancer patients. Breast Cancer Res Treat. 1989;14(2):207–216. doi: 10.1007/BF01810737. [DOI] [PubMed] [Google Scholar]
- 28.Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Störkel S, Seliger B. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109(2):265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 29.Bandoh N, Ogino T, Katayama A, Takahara M, Katada A, Hayashi T, Harabuchi Y. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep. 2010;23(4):933–939. doi: 10.3892/or_00000717. [DOI] [PubMed] [Google Scholar]
- 30.Kloetzel P-M. The proteasome and MHC class I antigen processing. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2004;1695(1–3):225–233. doi: 10.1016/j.bbamcr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9(7):503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 32.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57(11):1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehling M, Simon P, Mittelbronn M, Meyermann R, Ferrone S, Weller M, Wiendl H. WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol. 2007;114(2):111–119. doi: 10.1007/s00401-007-0231-8. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176(6):3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 35.Lou Y, Vitalis TZ, Basha G, Cai B, Chen SS, Choi KB, Jeffries AP, Elliott WM, Atkins D, Seliger B, Jefferies WA. Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific immune responses and survival. Cancer Res. 2005;65(17):7926–7933. doi: 10.1158/0008-5472.CAN-04-3977. [DOI] [PubMed] [Google Scholar]
- 36.Lou Y, Basha G, Seipp RP, Cai B, Chen SS, Moise AR, Jeffries AP, Gopaul RS, Vitalis TZ, Jefferies WA. Combining the antigen processing components TAP and tapasin elicits enhanced tumor-free survival. Clin Cancer Res. 2008;14(5):1494–1501. doi: 10.1158/1078-0432.CCR-07-1066. [DOI] [PubMed] [Google Scholar]
- 37.Sarrabayrouse G, Corvaisier M, Ouisse L-H, Bossard C, Mével BL, Potiron L, Meurette G, Gervois N, Jotereau F. Tumor-reactive CD4+CD8αβ+ CD103+ αβT cells: a prevalent tumor-reactive T-cell subset in metastatic colorectal cancers. Int J Cancer. 2011;128(12):2923–2932. doi: 10.1002/ijc.25640. [DOI] [PubMed] [Google Scholar]
- 38.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S, Sood AK. HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14(11):3372–3379. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 40.Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, Parsa AT. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17(11):1381–1385. doi: 10.1016/j.jocn.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17(13):4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 42.He H, Somlo G, Yun Y, Chu PG. Dendritic cell infiltration in lymph node positive breast carcinomas. Breast J. 2009;15(2):218–220. doi: 10.1111/j.1524-4741.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- 43.Mansfield A, Heikkila P, von Smitten K, Vakkila J, Leidenius M. Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch. 2011;459(4):391–398. doi: 10.1007/s00428-011-1145-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21(1):41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21(1):27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 47.Lott ST, Chen N, Chandler DS, Yang Q, Wang L, Rodriguez M, Xie H, Balasenthil S, Buchholz TA, Sahin AA, Chaung K, Zhang B, Olufemi S-E, Chen J, Adams H, Band V, El-Naggar AK, Frazier ML, Keyomarsi K, Hunt KK, Sen S, Haffty B, Hewitt SM, Krahe R, Killary AM. DEAR1 is a dominant regulator of acinar morphogenesis and an independent predictor of local recurrence-free survival in early-onset breast cancer. PLoS Med. 2009;6(5):e1000068. doi: 10.1371/journal.pmed.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga D, Koenig J, Kuhr K, Strunz K, Geyer V, Kurzeder C, Atassi Z, Blettner M, Kreienberg R, Woeckel A. Comparison of early onset breast cancer patients to older premenopausal breast cancer patients. Arch Gynecol Obstet. 2010;282(4):427–432. doi: 10.1007/s00404-009-1339-y. [DOI] [PubMed] [Google Scholar]
- 49.Bauer K, Parise C, Caggiano V. Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer. 2010;10(1):228. doi: 10.1186/1471-2407-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Belle V, Van Calster B, Brouckaert O, Vanden Bempt I, Pintens S, Harvey V, Murray P, Naume B, Wiedswang G, Paridaens R, Moerman P, Amant F, Leunen K, Smeets A, Drijkoningen M, Wildiers H, Christiaens M-R, Vergote I, Van Huffel S, Neven P. Qualitative assessment of the progesterone receptor and HER2 improves the Nottingham Prognostic Index up to 5 years after breast cancer diagnosis. J Clin Oncol. 2010;28(27):4129–4134. doi: 10.1200/JCO.2009.26.4200. [DOI] [PubMed] [Google Scholar]
- 51.Dunnwald L, Rossing M, Li C. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sari E, Guler G, Hayran M, Gullu I, Altundag K, Ozisik Y. Comparative study of the immunohistochemical detection of hormone receptor status and HER-2 expression in primary and paired recurrent/metastatic lesions of patients with breast cancer. Med Oncol. 2011;28(1):57–63. doi: 10.1007/s12032-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 53.Gong Y, Han EY, Guo M, Pusztai L, Sneige N. Stability of estrogen receptor status in breast carcinoma. Cancer. 2011;117(4):705–713. doi: 10.1002/cncr.25506. [DOI] [PubMed] [Google Scholar]
- 54.Herrmann F, Lehr H-A, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64(1):215–220. doi: 10.1158/0008-5472.CAN-2522-2. [DOI] [PubMed] [Google Scholar]
- 55.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N, Choudhury A, Seliger B, Kiessling R. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer. 2011;128(2):390–401. doi: 10.1002/ijc.25613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of HLA class I heavy chain, tapasin and LMP2 expression in primary and brain metastasis of breast cancer. (A) Representative immunohistochemical (IHC) staining on primary breast cancer tissues (left) and metastatic brain lesions (right). (B) Comparing the expression level HLA class I heavy chain, tapasin and LMP2 in primary breast cancer cases without known brain metastases (Primary, N = 50) and unpaired metastatic brain lesions (Metastatic, N = 33) (PPTX 1596 kb)
Absence of associations between the extent of CD8+ T cell infiltration and expression levels of HLA class I heavy chain, β2 microglobulin, tapasin, TAP2 or LMP2. Data were analyzed by Kendall’s tau-b test (PPTX 61 kb)