Abstract
Purpose
To determine if compliance with referral one year after vision screening failure was associated with care model, demographic, or ocular factors.
Methods
Data were analyzed from 798 children in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study with habitual logMAR visual acuity (VA) ≥ 0.26 (20/40 +2 or worse) in either eye due to uncorrected or under-corrected refractive error and who returned the following year. The parents of 492 children failing in TX and CA were sent letters indicating the need for a complete vision exam (screening model), while 306 children seen primarily in AZ and AL received a free complete exam and eyeglasses if needed (complete care model). Presenting to follow-up with adequate correction (logMAR < 0.26) in each eye was considered compliant. Logistic regression models for compliance were fit to assess if care model, ethnicity, sex, age, uncorrected logMAR in the better eye, or parental income, education or myopia were predictors.
Results
Overall compliance was 28%. Age (p=0.01, odds ratio (OR) = 1.12) and uncorrected logMAR (p<0.001, OR = 1.13) were associated with compliance but care model, ethnicity, and sex were not. Among the 447 children on whom data on parental factors was available, 27% were compliant. In this model, age, ethnicity, sex, parental income, parental education and parental myopia were not associated with compliance, but uncorrected logMAR (p=0.005; OR = 1.13) was predictive. An interaction between unaided VA and care model predicted improved compliance with poorer unaided VA in the complete care model.
Conclusions
Expensive complete care screening programs may not improve compliance over typical notification and referral screening protocols in school-aged children, unless unaided VA is worse than the common 20/40 referral criteria. Unaided VA had less impact on predicted compliance in the screening only protocol.
Keywords: vision screening, refractive error, optical correction, school children, compliance
Decreased visual acuity (VA) associated with uncorrected refractive error is easily remedied by eyeglasses or a contact lens correction. However, uncorrected refractive error remains a significant issue in the United States (US)1 and worldwide2. Based on data from the National Health and Nutrition Examination Survey (NHANES), Vitale and colleagues1 estimated that 11 million individuals 12 years of age or older (estimated prevalence 5.3%; 95% CI 4.9%–5.7%) have poor VA due to uncorrected refractive error. Rates are higher (estimated prevalence 9%; 95% CI 7.8%–10.3%)1 when only those 12 to 19 years of age are considered. Similar prevalence rates (8.2%) have been reported for inner city school-aged children3. However, uncorrected refractive error is less prevalent in younger (age 30 to 72 months) African-American and Hispanic preschool children in the US, with estimates around 4 to 5 % for the poorer seeing eye4.
All but nine states in the US (exceptions: Arizona, Idaho, Iowa, New Hampshire, Montana, North Dakota, South Carolina, South Dakota, Wisconsin)5 currently require periodic school-based vision screenings or a vision screening/eye examination upon school enrollment. For these vision-screening programs to be effective, students who fail the screening need to receive a comprehensive eye examination and adhere to the recommended treatment. Unfortunately, numerous studies have found that many children who fail a vision screening do not receive follow-up care6, 7, and many children with prescribed eyeglasses do not comply with spectacle wear6–8.
Identifying factors associated with compliance could improve outcomes from vision screening programs for school-aged children. In the Collaborative Evaluation of Ethnicity and Refractive Error (CLEERE) study, two different care models were used for the participants who failed the CLEERE vision screening. In the complete care model children who failed the vision screening were provided free eye examinations and free eyeglasses (if indicated) at the time of the vision screening or at a later date agreed upon by their parents. In the screening only care model (more consistent with common vision screening protocols) the parents of the children who failed the vision screening were notified by mail that a complete eye examination was recommended. The purpose of this study was to explore the impact these two different care strategies have on a child’s compliance with the vision screening recommendations. In addition to the care model various demographic and ocular factors were examined for associations with compliance.
METHODS
Participants
Participants were a subset of children enrolled in the CLEERE study, a longitudinal study exploring risk factors in the development of myopia in children in grades 1–8 (ages 6 to 16 years). CLEERE began in 1989 in Orinda, CA as the Orinda Longitudinal Study of Myopia (OLSM). From 1997 to 2009, data were collected at four additional centers (Eutaw, AL [recruitment emphasized African Americans], Irvine, CA [recruitment emphasized Asians], Houston, TX [recruitment emphasized Hispanics] and Tucson, AZ [recruitment emphasized Native Americans]) to improve the generalizability with respect to ethnicity. The study protocols followed the tenets of the Declaration of Helsinki and were reviewed and approved by the local institutional review boards. Parental consent and child assent were obtained prior to enrollment.
CLEERE participants seen between 1989 and 2009 who failed the vision screening portion of the CLEERE protocol due poor VA from uncorrected or under-corrected refractive error and who returned the following year to participate in CLEERE were eligible for analysis. Children who failed and were referred for strabismus or high phorias (as determined by cover test with prism neutralization) or ocular pathology (as determined by history, examination, and assessments made during the vision screening) were excluded from analysis. A vision screening failure due to uncorrected or under-corrected refractive error was defined as VA of ≥ 0.26 logMAR (20/40 +2/5 or worse) in either eye measured with habitual correction (glasses, contact lenses or no correction, however they presented to the test session). Retinoscopy without cycloplegia in each eye and cycloplegic autorefraction of the right eye were reviewed to limit inclusion to only children failing because of uncorrected refractive error. For children who met this criterion more than once during the course of their participation in CLEERE, only the first occurrence was eligible for inclusion (n = 942). Of the 942 children eligible for the study, 144 children were excluded because their unaided logMAR VA was < 0.26 at the return visit. Although these children failed the vision screening based on their habitual VA (logMAR ≥ 0.26 with correction [n = 3] or unaided [n = 141]), they returned one year later without correction and logMAR VA of < 0.26 in each eye. Based on the information available it could not be determined if these children acted on the screening failure notification and received care. The mean spherical equivalent cycloplegic refraction of the right eye at the referral visit and the follow-up visit of those included in the sample (−0.53D ± 1.8; −0.92D ± 1.94, respectively) compared to the 144 that were excluded (+0.58D ± 1.16; +0.47D ± 1.12, respectively) suggest that most, if not all, were misclassified as screening failures at the first visit.
Therefore, this study includes 798 children with data on care model, age, ethnicity, gender, and uncorrected VA. When models were run to determine if compliance was associated with parental income, education or refractive status, complete data were available for 447 of the 798 children and their parents. Parental income was missing from 32%, parental education from 28%, and parental refractive error from 15%. Only 56% of the full data set provided information on all three for the parental variables. Parental income and parental education were collected from a separate survey implemented later in the study. Unlike parental myopia, where multiple attempts were made at data collection, income and education were considered highly sensitive and therefore these data were not actively sought to maintain subject retention.
Procedures
Monocular VA was measured by trained and certified study personnel, using a high contrast projected chart modeled after the chart introduced by Bailey-Lovie9. Each week and every time the screen or projector was moved the chart calibration was confirmed. The letters on the chart ranged from 0.8 logMAR (20/126) to 0.0 (20/20), with 5 letters on each line. The full chart was presented and the examiner was allowed to point to a specific line or letter when testing younger children with limited attention.
The right eye was tested first with the left eye covered with an occluder; the adequacy of occlusion and test distance was monitored by study personnel. Children were asked to read the smallest line of letters they could see; squinting was not allowed. Those who hesitated were directed to begin at the 20/50 or 20/40 line. All participants were encouraged to guess when the letters became difficult and to read all of the letters on each line. Subsequently smaller lines of letters were read until all 5 letters on a line were missed or the child read the 20/20 line (or 20/25 line if in first grade). The VA was recorded in logMAR notation beginning with the last line where all letters were correctly identified and 0.02 subtracted for each letter correctly identified on subsequent lines. The VA for children unable to read the largest line on the chart (20/126) was recorded as 0.99. Children presenting with a refractive correction (i.e., eyeglasses or contact lenses) had their VA assessed both with and without the correction.
Children seen at the Tucson, AZ (n=252) or Eutaw, AL (n=50) sites and four children seen at the Irvine, CA site participated in the complete care model (n=306). These participants were provided free eye examinations at the vision screening (AZ) or at a later date agreed upon by their parents (AL & CA). Free eyeglasses, if needed, were dispensed to the children at school (AZ), by an eye care practitioner (CA, AL) or mailed to their home (AZ). The parents of the children failing the vision screening at Orinda, CA (n=58), Houston, TX (n=338) and the other children in Irvine, CA (n=96), were notified by letter (in the preferred language identified during the consent process) that a complete eye examination was recommended (screening only model, n=492). The decision regarding care model was left up to the discretion of the principal investigator at each site and was based in part on the needs and expectations of the organizations who committed to partner with the CLEERE Study over the course of this longitudinal study.
Regardless of the care model, all these children were seen the following year and compliance with the referral and recommended treatment was defined as returning one year after the vision screening failure (follow-up visit) with VA of < 0.26 logMAR (better than 20/40+2/5) in each eye measured with correction. Non-compliance was defined as presenting to the follow-up visit without correction and logMAR VA of 0.26 or worse in either eye or presenting with a correction that provided and aided logMAR VA or 0.26 or worse in either eye.
Parents of children enrolled in CLEERE completed a medical history form at enrollment and additional survey questions annually. Demographic information and parental refractive status reported on these forms and hypothesized to be associated with compliance were included in the analysis. Ethnic group designation was determined by parental report where parents selected one of the following six ethnic designations (based on categories used by the National Institutes of Health in 1997): American Indian or Alaskan Native; Asian or Pacific Islander; Black, not of Hispanic origin; Hispanic; White, not of Hispanic origin; or other. When parents identified their child with more than one ethnicity, the child was classified by the target ethnic group at the site. If neither ethnic designation was the site’s targeted ethnicity, ethnicity was assigned to the non-white ethnicity. Any missing parent-reported ethnicity was assessed by the investigator by observation, or, in some cases, by questioning the child about their parents and/or grandparents. Agreement between parent-reported ethnicity and investigator-determined ethnicity has been previously reported to be excellent in this investigator group10. Children classified as “other” were not included in this study.
Additional factors explored for associations included the participant’s sex and age. Parental factors hypothesized to be associated with compliance included annual family income (expressed in US dollars), parental educational level and parental history of myopia. Family income was collected by parent survey with six different response categories (< 15K, 15 < 25K, 25K-<35K, 35K < 50K, 50K- <100K and >100K). For the analysis, participants were divided into two categories; those with family incomes of $25K or more and those with family incomes of less $25K. This cut point was selected because the poverty level for a family of 5 (with 3 children, the average number of children in our participating families) is about 25K. The assumption was that those families at or near the poverty level might have better compliance with the complete care model that provided eye care and glasses if needed at no cost.
Parental education level was provided by the parent using 6 response categories (grade school, some high school, completed high school, some college, completed college, and post-graduate or professional). The assumption was that if parental education affected compliance, it would be adequately represented by a dichotomy that represented high and low levels for education. By averaging the highest level completed by the mother and the highest level completed by the father, parental education was categorized as completing at least high school or not completing high school. If information was available for only 1 parent, that parent’s highest education was used in the analysis. Parental myopia was categorized into either no myopic parents or at least one myopic parent.
The demographic, vision and parental factors of interest for this paper are summarized in Table 1 stratified by site for the full data set and the data subset. Table 2 shows the ethnic distribution of the full sample and the subset stratified by site.
Table 1.
Demographic, vision and care model parameters of the samples by site
| Site (n) | Age (yrs) (Mean ± STD) |
SPHEQ (Mean ± STD) |
VA (LogMAR) in best eye (Mean ± STD) |
Female (%) |
Screening & referral Care Model (%) |
Income ≥ 25000 (%) |
Average parent education ≥ high school (%) |
At least one myopic parent (%) |
|---|---|---|---|---|---|---|---|---|
| Full Data Set | ||||||||
| Alabama (50) | 10.9 ± 1.8 | −0.22 ± 1.77 | 0.37 ± 0.24 | 52 | 0 | |||
| Orinda (58) | 10.5 ± 1.9 | −1.57 ± 1.2 | 0.51 ± 0.26 | 47 | 100 | |||
| Houston (338) | 10.6 ± 2 | −1.11 ± 1.72 | 0.48 ± 0.26 | 56 | 100 | |||
| Irvine (100) | 10.6 ± 1.7 | −1.98 ± 2.05 | 0.58 ± 0.29 | 50 | 96 | |||
| Tucson (252) | 11.1 ± 1.7 | −0.23 ± 2.04 | 0.44 ± 0.27 | 57 | 0 | |||
| Subset with Parental Factors | ||||||||
| Alabama (19) | 10.4 ± 1.8 | 0.07 ± 2.18 | 0.38 ± 0.28 | 42 | 0 | 26 | 63 | 32 |
| Orinda (30) | 10.7 ± 1.7 | −1.65 ± 1.34 | 0.53 ± 0.25 | 57 | 100 | 100 | 100 | 90 |
| Houston (220) | 10.2 ± 1.8 | −1.13 ± 1.63 | 0.47 ± 0.25 | 56 | 100 | 43 | 38 | 28 |
| Irvine (37) | 10 ± 1.5 | −1.66 ± 2.05 | 0.56 ± 0.32 | 43 | 92 | 95 | 100 | 68 |
| Tucson (141) | 11.2 ± 1.9 | −0.36 ± 1.99 | 0.46 ± 0.28 | 57 | 0 | 34 | 62 | 67 |
Table 2.
Ethnicity of the participants by site
| Site | Native American |
Asian | African American |
Hispanic | White |
|---|---|---|---|---|---|
| Full Data Set | |||||
| Alabama | 0 (0%) | 0 (0%) | 50 (100%) | 0 (0%) | 0 (0%) |
| Orinda | 0 (0%) | 15 (26%) | 1 (2%) | 0 (0%) | 42 (72%) |
| Houston | 3 (1%) | 0 (0%) | 16 (5%) | 284 (84%) | 35 (10%) |
| Irvine | 1 (1%) | 80 (80%) | 2 (2%) | 3 (3%) | 14 (14%) |
| Tucson | 206 (82%) | 0 (0%) | 2 (1%) | 32 (13%) | 12 (5%) |
| Subset with Parental Factors | |||||
| Alabama | 0 (0%) | 0 (0%) | 19 (100%) | 0 (0%) | 0 (0%) |
| Orinda | 0 (0%) | 7 (23%) | 0 (0%) | 0 (0%) | 23 (77%) |
| Houston | 1 (0%) | 0 (0%) | 9 (4%) | 188 (85%) | 22 (10%) |
| Irvine | 1 (3%) | 24 (65%) | 1 (3%) | 2 (5%) | 9 (24%) |
| Tucson | 111 (79%) | 0 (0%) | 0 (0%) | 19 (13%) | 11 (8%) |
Statistical Analysis
A logistic model of compliance was fit using the full dataset (n = 798), and the subsample with complete parental information (n = 447). Predictors used in the full dataset model included the primary variable of interest, care model, as well as ethnicity, sex, age at follow-up and unaided logMAR VA in the better-seeing eye at the follow-up visit. Additional variables in the subsample model included parental education, family income, and parental myopia. Model fit was determined to be acceptable by the Hosmer-Lemeshow statistic (p = 0.16, for full dataset; p = 0.12, for subset) where a non-significant p-value indicates an acceptable fit of the data. Statistical significance was assessed at p ≤ 0.01 to account for the multiple analyses. For both samples, there were insufficient participants in some of the ethnicity by care model cells (Table 3) to permit an adequate assessment of the interaction of ethnicity and care model. Therefore, modeling of compliance assumed that the effect of care model on compliance is not moderated by ethnicity.
Table 3.
Relationship between compliance at follow-up and care model by ethnicity for the overall sample (n = 798) and the sample with complete parental data (n = 447).
| Full Dataset N = 798 |
Subset with Parental Factors N = 447 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete Care Model |
Screening Only Model |
Overall | Complete Care Model |
Screening Only Model |
Overall | |||||||
| Ethnicity | N | Proportion Compliant |
N | Proportion Compliant |
N | Proportion Compliant |
N | Proportion Compliant |
N | Proportion Compliant |
N | Proportion Compliant |
| Native American | 206 | 0.24 | 4 | 0.50 | 210 | 0.24 | 111 | 0.25 | 2 | 1.0 | 113 | 0.27 |
| Asian | 2 | 0.50 | 93 | 0.34 | 95 | 0.35 | 2 | 0.50 | 29 | 0.21 | 31 | 0.23 |
| African American | 53 | 0.26 | 18 | 0.22 | 71 | 0.25 | 20 | 0.15 | 9 | 0.22 | 29 | 0.17 |
| Hispanic | 33 | 0.39 | 286 | 0.25 | 319 | 0.26 | 19 | 0.42 | 190 | 0.24 | 209 | 0.25 |
| White | 12 | 0.50 | 91 | 0.34 | 103 | 0.36 | 11 | 0.55 | 54 | 0.37 | 65 | 0..40 |
| Overall | 306 | 0.27 | 492 | 0.28 | 798 | 0.28 | 163 | 0.28 | 284 | 0.26 | 447 | 0.27 |
With the minor exception of Irvine, CA, where 96% of subjects experienced the screening only model and 4% the complete care model, type of care was assigned at the site level, not the subject level. Subjects within a site might behave in a more consistent fashion than subjects at different sites. To address the possible within-site correlation, we also fit logistic models with additional parameters that allowed for correlation in responses from subjects at the same site. For model predictors, the statistics from these models were minor variants of those obtained from fitting logistic models assuming no correlation, therefore the results from the logistic models assuming no correlation are presented.
Effect sizes for the predictors are presented as odds ratios. With the categorical predictors (like sex or ethnicity), the odds ratio compares the odds of two levels of the predictor (e.g., female vs. male); with a continuous predictor (like age or logMAR VA), the odds ratio compares the odds of two groups a predictor unit apart. For age the unit is an increase in age by 1 year. To make the effect size associated with VA more meaningful, models were fit using a transformation of VA. The logMAR VA in the better eye was multiplied by 10. As a result, odds ratios related to VA provide effect estimates for a change in logMAR of 0.1 units or one line of letters. An odds ratio of 1 indicates no difference between the groups compared and if the 95% confidence interval (CI) contains 1 there is no statistically significant difference between the groups.
RESULTS
The sample was 54.5% female (435/798). Figure 1 displays the age distribution of the participants in the study. Table 3 shows the proportion of participants who returned for follow-up with an adequate correction (logMAR VA < 0.26, considered compliant) after failing the vision screening one year prior with habitual log MAR VA ≥0.26. The results are presented by ethnicity and care model. In the full sample of 798 only 28% of those failing the vision screening due to uncorrected or under-corrected refractive error were adequately corrected at the follow-up visit one year later. The observed difference between care models was small with 27% of those provided an eye examination and eye glasses at no cost presenting with adequate correction compared to 28% of those notified by letter of the vision screening failure. In the subset of children with complete parental information (n = 447), the results were similar with overall compliance at 27%. Those seen with the complete care model had a compliance rate of 28% and for those seen under the screening only model, compliance was 26%.
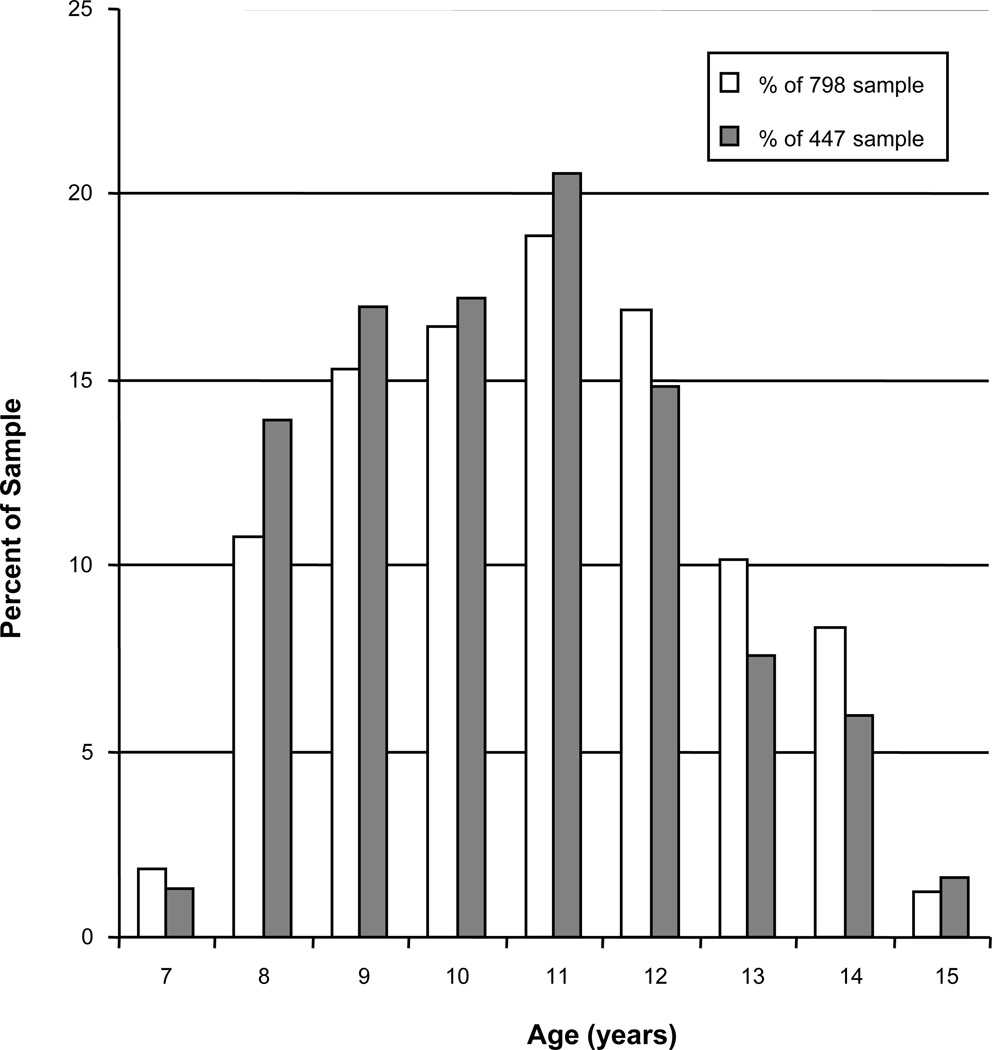
Figure 1.
The age distribution of the sample (□ = sample of 798, ■ = sample of 447) at the follow-up visit.
There were considerably more Hispanic and Native American participants than White, Asian and African American participants. Regardless of the sample used (full or subset), the small numbers of participants in some of the subgroups make comparisons of the two care models within each ethnic group inappropriate.
A logistic model including the full sample was used to explore factors hypothesized to be associated with the outcome variable, adequate VA at the follow-up visit. Care model, ethnicity, and sex were not associated with adequate correction at follow-up, but age and uncorrected logMAR VA in the better eye were associated with adequate correction at follow-up. As shown by the factors in bold in Table 4 for the full sample, the odds of compliance at the follow-up visit increased by 12% (odds ratio 1.12) with a one year increase in age. Also shown in Table 4 is the effect of uncorrected logMAR VA in the better eye at follow-up. In the case of VA, a 0.1 increase in logMAR (one line poorer VA) increases the odds of adequate correction at follow-up by 13%.
Table 4.
Predictors of Adequate Correction at the Follow-up Visit.
| Full Sample N = 798 |
Subset with Parental Factors N = 447 |
|||
|---|---|---|---|---|
| Predictor | Odds Ratio (95% Ci) |
Effect P Value | Odds Ratio (95% Ci) |
Effect P Value |
| Screening* | 0.69 (0.40 – 1.22) |
0.21 | 0.68 (0.32–1.42 |
0.30 |
| Ethnicity | 0.15 | 0.13 | ||
| White | Reference | Reference | ||
| Native American | 0.43 (0.21 – 0.88) |
0.49 (0.20–1.20) |
||
| Asian | 0.88 (0.48 – 1.60) |
0.36 (0.13–0.98) |
||
| African American | 0.53 (0.24 – 1.15) |
0.30 (0.09 – 0.99) |
||
| Hispanic | 0.63 (0.39 – 1.03) |
0.73 (0.37–1.44) |
||
| Female | 0.92 (0.67 – 1.27) |
0.62 | 0.82 (0.53–1.27) |
0.38 |
| Age (years) |
1.12 (1.03 – 1.22) |
0.01 | 1.06 (0.94–1.20) |
0.32 |
| Uncorrected VA Better Eye (0.1 logMAR) |
1.13 (1.06 – 1.20) |
< 0.001 |
1.13 (1.04–1.22) |
0.005 |
| Income <$25,000 | 1.19 (0.72–1.95) |
0.49 | ||
| At least High School Education |
1.66 (0.97–2.83) |
0.06 | ||
| A Myopic Parent | 0.95 (0.57–1.58) |
0.85 | ||
Screening refers to the care model where parents were notified and their child referred after failing the vision screening compared to the complete care model that provided an eye examination and eye glasses, if needed at no cost.
When the model included the parental factors hypothesized to be associated with compliance, the results were similar with the exception of the effect of age. Care model, ethnicity, sex and age were not associated with compliance but uncorrected VA in the better eye remained associated with the same effect size as seen in the model fit using the full sample (13% increase in the odds of compliance for each 1 line poorer logMAR VA). None of the parental factors was associated with compliance. Because of correlation among the parental factors, separate logistic models were run evaluating each parental factor independently. Uncorrected logMAR VA remained significant in each model with similar size effect (12% to 13% increase in odds of compliance for a 0.1 logMAR increase in VA) but no parental factor reached significance at the 0.01 level when tested independently of the other two factors.
Given the demographics of the sites as shown in Table 1, it is possible that there was an interaction between the site and parental income. To examine this possibility, a model of compliance was fit using parental income, site, and the interaction between site and income while controlling for care model, gender, ethnicity, age and visual acuity. Income (p = 0.12), site (p = 0.70), and the interaction between site and income (p= 0.42) were not statistically significant.
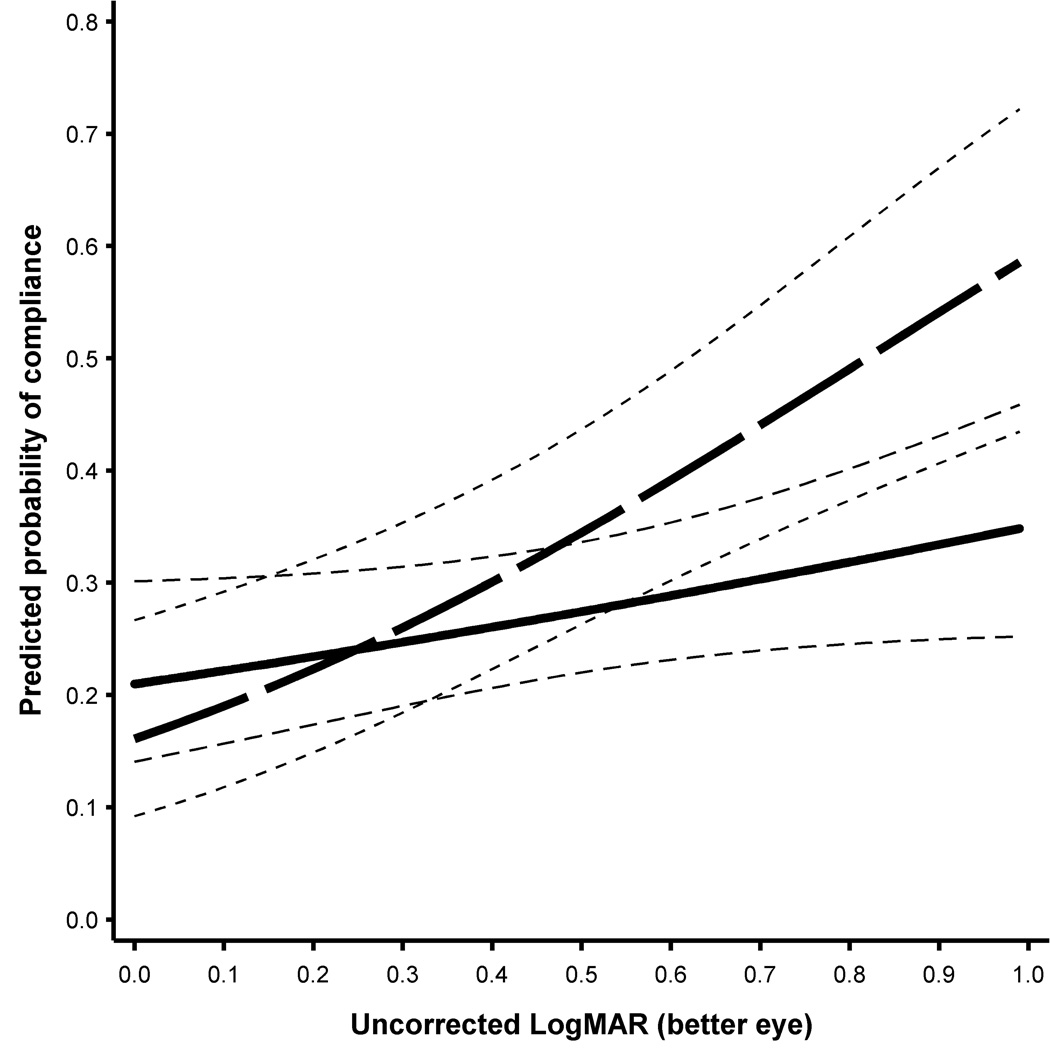
For both the full sample and the subset, the logistic regression model was extended to examine possible interactions between logMAR VA in the better eye at follow-up and the care model. In both models, there was a significant interaction between care model and uncorrected logMAR VA in the better-seeing eye at follow-up (full dataset, p = 0.04; subset, p = 0.01). As seen in Figure 2 for the model fit with the full dataset, those children who received screening and notification of the results (bold black line) showed relatively little change in the probability of compliance as a function of their unaided logMAR VA. For example, the estimated probability of compliance with screening and notification was 0.24 (CI 0.18, 0.31) for an uncorrected logMAR VA of 0.26 (25th percentile), 0.27 (CI 0.21, 0.33) for an uncorrected logMAR VA of 0.44 (50th percentile) and 0.30 (CI 0.24, 0.37) for an uncorrected logMAR VA of 0.66 (75th percentile). However, for those children who received the complete care model (bold dashed line) the poorer the uncorrected VA at follow-up the greater the probability of compliance. The estimated probability of compliance in the complete care model was 0.24 (CI 0.17, 0.34), 0.32 (CI 0.24,0.41), and 0.42 (CI 0.32, 0.52) for the same 25th, 50th and 75th level of uncorrected logMAR VA, respectively.
Figure 2.
Estimated chance of compliance at follow-up as a function of uncorrected logMAR VA in the better eye using the model fit with the full sample (n = 798). Bold dashed line is for the complete care model and the bold solid line is for the screening and notification model. The lighter dashed lines show the estimated 95% confidence intervals.
DISCUSSION
The percentage of children returning one year after vision screening failure with an adequate correction (logMAR < 0.26) was poor regardless of care model; 28% of those seen in the complete care model and 27% of those seen in the screening only model in the full dataset. Rates were similar in the subsample. These rates are slightly higher than those reported by Ethan et al11 (19%) for their control group who were managed similarly to the children in the screening only model reported here, and poorer than their intervention group (47%) who received care similar to those children in the complete care model but included active encouragement and monitoring of compliance by the child’s teacher. The rates reported here are also lower than the 33% compliance rate reported by Messer et al8 seen under the complete care model and drawn from the same large CLEERE dataset. One key difference between these studies is the definition used for compliance. Previous studies classified compliance as presenting to the evaluation with correction8 or observation of eyeglass use in the classroom11 without regard to the VA with correction. Requiring that the correction provide adequate VA at follow-up as a criterion for compliance would be expected to lower the percentage of those classified as compliant.
Although compliance was poor, it was associated with the unaided VA in the better eye. This result is in agreement with that reported by Messer et al8 but not others12–14 for younger children or shorter follow-up intervals in children studied outside the United States. The association between age and compliance reported here is less clear. Age was statistically significant in only one of the two models of compliance. Given that myopia increases with age15–18 and unaided VA becomes worse as myopia increases, it is not surprising to find some evidence that age is associated with compliance.
Factors that were not associated with compliance included sex, parental income, parental education, and number of myopic parents. The lack of association between parental income and compliance is consistent with the results of Messer et al8. The relatively low incomes of the sample could be contributing to this result. Children with at least one myopic parent were no more likely to be compliant with spectacle wear than those with no myopic parents. This result is somewhat counterintuitive in that one might expect parents with poor distance VA to be more proactive about their children’s vision and compliance would improve. No data were available on the compliance of the parent with spectacle wear. Parents who can manage their daily activities without optical correction, may not see the importance or benefit of optical correction for their children.
Eighty-two percent of those in the complete care model were examined at school. Thus most transportation, appointment problems and work issues previously identified as logistical barrier for obtaining eye care19 were eliminated for those in the complete care model. If unaided VA is not considered, eliminating these logistical and financial barriers6, 19 does not improve compliance over screening alone. At first pass, this result suggests expensive programs that provide vision examinations and eyeglasses (if needed) at no cost to those failing a school screening in the United States are not likely to improve compliance of eyeglass wear. However, if unaided VA in the better-seeing eye is considered, care model becomes relevant. Predicted compliance rose only marginally as unaided VA in the better-seeing eye became worse among children in the screening only model. However, predicted compliance was better for those with the poorest unaided VA if free eye examinations at the time of screening and eyeglasses at no cost if needed were provided. This interaction between unaided VA in the better-seeing eye and care model suggests that removing financial and logistical barriers may be most cost effective if selectively applied to those with the poorest unaided VA. The level of unaided VA that would warrant a decision to provide a free examination and eye glasses would need to be based on a minimum acceptable rate of compliance and the availability of resources. This cost-benefit analysis would undoubtedly need to include additional factors.
One limitation of this study was the inability to explore the interaction of ethnicity and care model. This limitation required the modeling of compliance to assume that the effect of care model on compliance was not moderated by ethnicity. The majority of participants in this study were either Native American (primarily participating in the complete care model) or Hispanic (primarily in the screening only model). Both of these groups have a high frequency of astigmatic corrections4, 20–22 and a similar pattern of change in axial length with age22. Although cultural differences between the groups may play a role in the results reported, many of the reasons given for low compliance with spectacle wear (lost or broken, don’t feel glasses are needed, did not like them or concerned about appearance) were similar in a group of Native Americans8 and children of a similar age in Oaxaca, Mexico23 both of whom received eyeglasses at no cost.
The CLEERE study was not specifically designed to examine the question of spectacle wear compliance and care model. Local expectations at the different sites necessitated different protocols for managing vision screening failures and hence, care model could not be randomized within site. However, family income and parental education, two factors that might be expected to be associated with compliance were not found to be associated with compliance. In addition, the propensity for compliance between individuals from the same site was essentially the same as propensity for compliance between individuals from different sites. Nonetheless, the inability to randomize the care model within site may limit the generalizability of the results.
Another limitation was that compliance was defined as presenting to the follow-up with adequate vision correction. It is possible that some of the participants who presented to the follow-up with eyeglasses were in fact compliant, but their prescription had changed since obtaining a correction. Of those classified as non-complaint at follow-up, 82% (472/575) presented to the follow-up with no correction. It should also be noted that the average change in spherical equivalent refractive error between the two visits was less than 0.50D, (−0.53D ± 1.8 at screening failure; −0.92D ± 1.94, at follow-up). These two factors suggest that lack of compliance at follow-up was not likely the result of a prescription change. It should also be noted that the number of children who were not seen the following year after failing the screening was not determined. If these children who were lost to follow-up were also less likely to be compliant, then the compliance rate reported here could be inflated.
The strengths of the study include the collection of all data under a uniform protocol by trained and certified examiners across the sites, a large sample size with complete data for the primary factors, and the ability to limit the dataset to those with reduced VA due to uncorrected or under-corrected refractive error.
CONCLUSIONS
Expensive complete care screening programs may not improve compliance over typical notification and referral screening protocols in school-aged children, unless unaided VA is worse than the common 20/40 referral criteria. Selective application of free examination and eye glasses based on unaided VA in the better eye may be a more cost-effective approach to promote compliance with eyeglass wear. The decision as to what level of unaided VA would warrant a free examination and eye glasses would need to balance the availability of resources with the expected improvement in compliance and would undoubtedly need to include other factors.
ACKNOWLEDGMENTS
Supported by NIH/NEI and the Office of Minority Research/NIH grants U10-EY08893 and R24-EY014792, the Ohio Lions Eye Research Foundation and the E.F. Wildermuth Foundation, Columbus, OH.
The CLEERE Study Group
Clinical Centers
Franklin Primary Health Center, Inc.: Sandral Hullett, MD, MPH (Principal Investigator, 1997–2007); Robert N. Kleinstein, OD, MPH, PhD (Co-Investigator, 1997–2007); Janene Sims, OD (Optometrist, 1997–2001 and 2004–2007); Raphael Weeks, OD (Optometrist, 1999–2007); Sandra Williams (Study Coordinator, 1999–2007); LeeAndra Calvin (Study Coordinator, 1997–1999); Melvin D. Shipp, OD, MPH, DrPH (Co-Investigator, 1997–2004). Drs. Kleinstein and Sims are affiliated with the University of Alabama at Birmingham School of Optometry.
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman, OD, MS (Principal Investigator, 1999–2001); Pamela Qualley, MA (Study Coordinator, 1997–2001); Donald O. Mutti, OD, PhD (Principal Investigator, 1996–1999); Karla Zadnik, OD, PhD (Optometrist, 1996–2001).
University of Houston College of Optometry: Ruth E. Manny, OD, PhD (Principal Investigator, 1997–2007); Suzanne M. Wickum, OD (Optometrist, 1999–2007); Ailene Kim, OD (Optometrist, 2003–2007); Bronwen Mathis, OD (Optometrist, 2002–2007); Janice M. Wensveen, OD, PhD (Optometrist, 1997–2001); Connie J. Crossnoe, OD (Optometrist, 1997–2003); Stephanie L. Tom, OD (Optometrist, 1999–2002); Sally Henry (Study Coordinator, 1997–1998), Jennifer A. McLeod (Study Coordinator, 1998–2004); Mamie Batres (Study Coordinator, 2004–2007); Julio C. Quiralte (Study Coordinator, 1998–2005); Gaby Solis (Study Coordinator, 2005–2007).
Southern California College of Optometry, Fullerton, CA: Susan A. Cotter, OD, MS (Principal Investigator, 2004–2007, Optometrist, 1997–2004); Julie A. Yu, OD (Principal Investigator, 1997–2004; Optometrist 2005–2007); Raymond J. Chu, OD (Optometrist, 2001–2007); John Lee, OD (Optometrist, 2000–2003); Robert J. Lee, OD (Optometrist, 1997–2001); Raymond Maeda, OD (Optometrist, 1999–2003); Carmen N. Barnhardt, OD, MS (Optometrist 2004–2007); Jessica Chang, OD (Optometrist, 2005–2007); Kristine Huang, OD (Optometrist, 2005–2007); Connie Chu, OD (Optometrist, 2004–2005); Soonsi Kwon, OD (Optometrist, 1998–2004); Rachael Emerson (Study Coordinator, 1997–1999); Gen Lee (Study Coordinator, 1999–2003); Tracy Leonhardt (Study Coordinator, 2003–2004); Rebecca Bridgeford (Study Coordinator, 2005–2006).
University of Arizona, Department of Ophthalmology and Vision Science, Tucson, AZ: J. Daniel Twelker, OD, PhD (Principal Investigator, 2000–2010); Dawn Messer, OD, MPH (Optometrist, 2000–2010); Rita Bhakta, OD (Optometrist, 2000–2004); Katie Garvey, OD (Optometrist, 2006–2010); Denise Flores (Study Coordinator, 2000–2007); Mabel Crescioni cDRPH (2009–2010).
Resource Centers and Executive Committee
Chairman’s Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik, OD, PhD (Chairman, 1997–2010); Jodi M. Malone, RN (Study Coordinator, 1997–2010)
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH: Lisa A. Jones-Jordan, PhD (Director, 1997–2010); Linda Barrett (Data Entry Operator, 1997–2008); Austen Tanner (Data Entry 2008–2010) John Hayes, PhD (Biostatistician, 2001–2006); G. Lynn Mitchell, MAS (Biostatistician, 1998–2010); Melvin L. Moeschberger, PhD (Consultant, 1997–2010); Loraine Sinnott, PhD (Biostatistician, 2005–2010); Pamela Wessel (Program Coordinator, 2000–2010); Julie N. Swartzendruber, MA (Program Coordinator, 1998–2000).
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett, MA.
Executive Committee: Karla Zadnik, OD, PhD (Chairman); Lisa A. Jones-Jordan, PhD; Robert N. Kleinstein, OD, MPH, PhD; Ruth E. Manny, OD, PhD; Donald O. Mutti, OD, PhD; J. Daniel Twelker, OD, PhD; Susan A. Cotter, OD, MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- 1.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. JAMA. 2006;295:2158–2163. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 2.Schneider J, Leeder SR, Gopinath B, Wang JJ, Mitchell P. Frequency, course, and impact of correctable visual impairment (uncorrected refractive error) Surv Ophthalmol. 2010;55:539–560. doi: 10.1016/j.survophthal.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103:105–109. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- 4.MEPEDS. Prevalence and causes of visual impairment in African-American and Hispanic preschool children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2009;116:1990–2000. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Star Pupils: Healthy Eyes for Healthy Families. [Accessed: September 29 2011];School Requirments for Children's Vision. Prevent Blindness America. 2010 Available at: http://www.starpupils.org/pba/content/school-requirements-childrens-vision.
- 6.Mark H, Mark T. Parental reasons for non-response following a referral in school vision screening. J Sch Health. 1999;69:35–38. doi: 10.1111/j.1746-1561.1999.tb02341.x. [DOI] [PubMed] [Google Scholar]
- 7.Preslan MW, Novak A. Baltimore Vision Screening Project. Phase 2. Ophthalmology. 1998;105:150–153. doi: 10.1016/s0161-6420(98)91813-9. [DOI] [PubMed] [Google Scholar]
- 8.Messer DH, Mitchell GL, Twelker JD, Crescioni M. Spectacle wear in children given spectacles through a school-based program. Optom Vis Sci. 2012;89:19–26. doi: 10.1097/OPX.0b013e3182357f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey IL, Lovie JE. The design and use of a new near-vision chart. Am J Optom Physiol Opt. 1980;57:378–387. doi: 10.1097/00006324-198006000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Jones L, Mitchell G, Zadnik K The CLEERE Study Group. Agreement between parent-reported and clinician-assessed race in the CLEERE Study. Control Clin Trials. 2001;22:98S. [Google Scholar]
- 11.Ethan D, Basch CE, Platt R, Bogen E, Zybert P. Implementing and evaluating a school-based program to improve childhood vision. J Sch Health. 2010;80:340–345. doi: 10.1111/j.1746-1561.2010.00511.x. quiz 68-70. [DOI] [PubMed] [Google Scholar]
- 12.Horwood AM. Compliance with first time spectacle wear in children under eight years of age. Eye (Lond) 1998;12(Pt. 2):173–178. doi: 10.1038/eye.1998.43. [DOI] [PubMed] [Google Scholar]
- 13.Congdon NG, Patel N, Esteso P, Chikwembani F, Webber F, Msithini RB, Ratcliffe A. The association between refractive cutoffs for spectacle provision and visual improvement among school-aged children in South Africa. Br J Ophthalmol. 2008;92:13–18. doi: 10.1136/bjo.2007.122028. [DOI] [PubMed] [Google Scholar]
- 14.Yabumoto C, Hopker LM, Daguano CR, Basilio FM, Robl R, Rodrigues DB, Jimenez A, Moreira TR, Sakata LM, Sakata K. Factors associated with spectacles-use compliance in a visual screening program for children from Southern Brazil. Invest Opththalmol Vis Sci. 2009;50 E-Abstract 2439. [Google Scholar]
- 15.Sorsby A, Leary GA. A longitudinal study of refraction and its components during growth. Spec Rep Ser Med Res Counc. 1969;309:1–41. [PubMed] [Google Scholar]
- 16.Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44(Suppl 1):S109–S115. doi: 10.1016/s0039-6257(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 17.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, Shipp M, Friedman NE, Kleinstein RN, Walker TW, Jones LA, Moeschberger ML, Mutti DO. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, Everett D. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–987. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- 19.Kimel LS. Lack of follow-up exams after failed school vision screenings: an investigation of contributing factors. J Sch Nurs. 2006;22:156–162. doi: 10.1177/10598405060220030601. [DOI] [PubMed] [Google Scholar]
- 20.Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, Manny RE, Mutti DO, Yu JA, Zadnik K. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–1147. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- 21.Harvey EM, Dobson V, Miller JM, Sherrill DL. Treatment of astigmatism-related amblyopia in 3- to 5-year-old children. Vision Res. 2004;44:1623–1634. doi: 10.1016/j.visres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Twelker JD, Mitchell GL, Messer DH, Bhakta R, Jones LA, Mutti DO, Cotter SA, Klenstein RN, Manny RE, Zadnik K. Children's ocular components and age, gender, and ethnicity. Optom Vis Sci. 2009;86:918–935. doi: 10.1097/opx.0b013e3181b2f903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castanon Holguin AM, Congdon N, Patel N, Ratcliffe A, Esteso P, Toledo Flores S, Gilbert D, Pereyra Rito MA, Munoz B. Factors associated with spectacle-wear compliance in school-aged Mexican children. Invest Ophthalmol Vis Sci. 2006;47:925–928. doi: 10.1167/iovs.05-0895. [DOI] [PubMed] [Google Scholar]