Abstract
OBJECTIVES:
Cowden syndrome (CS), associated with germline PTEN mutations, is an autosomal-dominant disorder with increased frequencies of thyroid and breast cancers. Recent reports document the occurrence of gastrointestinal (GI) polyps and increased risk of colon cancer in PTEN mutation carriers. Studies to date, however, have not been based on mutation carriers undergoing active, systematic, routine-interval GI surveillance. Our objective is to document the upper and lower GI findings in CS patients undergoing such an active GI surveillance program.
METHODS:
In a 5-year period, 3,000 consecutive patients were referred to our high-risk GI cancer clinic for various reasons. Of these 3,000, 10 met full-blown clinical criteria for the diagnosis of CS. Individuals with identified PTEN mutations underwent annual upper and lower endoscopy surveillance programs using dual white light and narrow-band imaging. All biopsies including archived materials were reviewed by a single dedicated GI pathologist.
RESULTS:
Ten PTEN mutation carriers from different ethnic backgrounds were invited and all participated in the active GI surveillance program. Eight patients had colonic polyps, mostly hyperplastic (eight patients) and hamartomatous (five patients), but also adenomatous (three patients), ganglioneuromatous (three patients), and juvenile polyps (two patients). One patient (10%) had an early-onset rectal cancer (aged 44 years), which was null for PTEN expression on immunohistochemistry. All patients had gastric polyps and nine (90%) had duodenal polyps, mostly hyperplastic and hamartomatous. Additional three patients (30%) had adenomatous duodenal polyps.
CONCLUSIONS:
PTEN mutation–positive CS patients have a higher frequency of upper GI polyps than previously believed. They appear prone to develop adenomatous upper and lower tract dysplastic polyps and cancer. Thus, the polyps encountered during upper or lower endoscopy in these patients should not be automatically considered innocent hamartomas without malignant potential. Active surveillance programs in specialized centers should be considered in these patients.
INTRODUCTION
Cowden syndrome (CS) is an autosomal-dominant disorder characterized by multiple hamartomatous and benign and malignant neoplastic lesions of the skin, mucous membranes, thyroid, breast, endometrium, and brain.1, 2, 3 Germline mutations in the PTEN tumor suppressor gene are found in 80% of classic CS.4, 5 CS is very difficult to ascertain, especially in subtler cases, and recently a clinical scoring system based on a prospective study of 3,042 CS and CS-like individuals has been proposed.6 According to this semiquantitative system, a score of 10, carrying at least a 3% likelihood of finding a PTEN pathogenic mutation, is minimal for considering PTEN testing.6
Classically, although CS patients might harbor ‘polyps' throughout the gastrointestinal tract, they are mostly believed to be hamartomas without malignant potential. Up to now, the National Comprehensive Cancer Network (NCCN) guidelines state that routine gastrointestinal (GI) surveillance in CS patients is not indicated in the absence of symptoms.7 Prior to our recent report,8 there were scattered reports about the occurrence of GI polyposis9, 10, 11, 12, 13 but few reports documenting age-specific prevalence of gastric and colon cancers in CS patients.14, 15, 16, 17 In our prospective series of 127 unrelated individuals with pathogenic PTEN mutations, 69 (54.3%) underwent at least one endoscopy and most had GI polyps comprising multiple histologies.8 Importantly, nine (13%) mutation carriers in this series had prevalent colorectal cancers, yielding an age- and sex-adjusted standardized incidence ratio of >200. All but one with colorectal carcinomas had existing colonic polyposis. What was surprising was that 16 individuals out of 67 who underwent colonoscopy had colonic adenomatous polyps and 2 individuals out of 39 who underwent esophagogastroduodenoscopy (EGD) had upper GI adenomas. It is important to note, however, that in this series, upper or lower GI endoscopies were performed as part of clinical routine, and were not systematically performed on a regular longitudinal basis. Thus, of the 127 mutation carriers, 67 had one or more colonoscopies, and only 39, an EGD. As an important caveat to this study, therefore, an important follow-up study was to document the GI phenotype of PTEN mutation carriers undergoing systematic GI surveillance performed by one or very few endoscopists on a regular longitudinal basis, and having only one or very few pathologists read the histologies.
The High Risk Cancer Clinic (HRCC) at Rabin Medical Center (RMC) is a tertiary referral center of the largest HMO in Israel. All CS patients treated at the HRCC participate in a surveillance program, where they undergo biannual dual upper and lower endoscopy, using narrow-band imaging (NBI) at the advanced endoscopy unit from the age of 25 years old, or earlier, if symptomatic. The technique of NBI allows recognition of flat lesions and helps to recognize adenomatous changes in both the upper and lower digestive tract.18, 19 Here, we sought to document the GI endoscopic and pathologic findings of 10 PTEN mutation–positive CS patients who have been systematically surveillanced annually with dual upper and lower endoscopies at the advanced endoscopy unit by three endoscopists.
METHODS
Study design
Between January 2005 and December 2009, we identified 10 patients with a definitive diagnosis of CS out of 3,000 patients referred to the HRCC. Additional three patients from unrelated families with suspected CS who were not willing to perform genetic testing were not included in the analysis. Patients with a genetic diagnosis (PTEN mutation positive) of CS were offered a surveillance program according to the NCCN guidelines. Additionally, they were offered an intensive GI endoscopy surveillance program. Medical records as well as any endoscopy records and surgical pathology were reviewed. All pathology slides were reviewed by a single expert GI pathologist at RMC. Tumor analysis was performed at the Cleveland Clinic as described below.
Gastrointestinal surveillance program
The program includes annual upper and lower endoscopy examination since the age of 25 years old, or earlier, if symptomatic. The procedures were performed by the advanced endoscopy team at RMC, using both white light (WL) and NBI for each segment with the Olympus TM 180 system (high definition and NBI) (Japan). Resection of large duodenal polyps was performed with a duodenoscope Olympus TM 160.
Genetic testing
PTEN mutation analysis was performed in Eng lab (Genomic Medicine Institute, Cleveland Clinic) or by the local clinical service lab (MYGAL, Israel). The Eng lab's PTEN mutation analysis includes polymerase chain reaction-based mutation analysis of all nine exons, flanking intronic regions and promoter as well as large deletion/rearrangement testing per multiplex ligation-dependent probe amplification as described.6 At MYGAL, Sanger sequencing was applied to all gene exon, including exon–intron boundaries (at least 20 base pairs).
Tumor analysis
Microsatellite instability testing was performed using polymerase chain reaction amplification of seven loci (Promega, Madison, WI), including five mononucleotide repeats (BAT 25, BAT 26, NR-21, NR-24, and MONO-27) and two pentanucleotide repeats (PentaC and PentaD) on genomic DNA extracted from formalin-fixed, paraffin-embedded lesional tissue and a paired normal (germline) sample.
Immunohistochemistry (IHC) for the mismatch repair proteins, MLH1, MSH2, MSH6, and PMS2, was performed on standard 5-micron thick deparaffinized, formalin-fixed tissue sections. Monoclonal mouse primary antibodies for all four proteins (MLH1, MSH2, MSH6 from Biocare Medical, Concord, CA and PMS2 from BD Pharmingen, San Diego, CA) were used at 1:10, 1:100, and 1:500 dilutions, respectively, for 90 min (MLH1, MSH6, and PMS2) and 30 min (MSH2) incubation times at room temperature.
PTEN IHC was performed with the monoclonal antibody 6H2.1 raised against the terminal 100 amino acids of human PTEN as previously described, with minor modifications.17
Human subjects' protection
The study has been approved by the Rabin Medical Center Helsinki committee and by the Cleveland Clinic Committee for Human Subjects' Protection, for the respective research activities.
RESULTS
Utilizing the NCCN-2006/International Cowden Consortium clinical diagnostic criteria, we identified 10 CS patients out of 3,000 patients referred to the HRCC in the last 5 years. All 10 were found to have germline PTEN mutations. These patients belong to six different families. Seven patients (70%) were Jews and the remaining three were Arabs. The current mean age of the patients with CS is 36.4±14.3 years, and seven (70%) are males. The research participants' demographic characteristics and major phenotypic findings and PTEN genotypes are presented in Table 1.
Table 1. Patients' characteristics and features observed in our PTEN mutation–positive patients.
| All | 10 (100%) |
| Age (mean, s.d.) | 40.8±16.0 (years) |
| Gender | |
| Male | 7 (70%) |
| Female | 3 (30%) |
| Origin | |
| Jew | 7 (70%) |
| Arab | 3 (30%) |
| Head | |
| Macrocephaly | 3 (30%) |
| Lhermitte-Duclos | 1 (10%) |
| Skin | |
| Trichilemmomas | 4 (40%) |
| Penile freckling | 2 (28.5%a) |
| Breast | |
| Cancer | 1 (33.3%b) |
| Thyroid | |
| Cancer | 4 (40.0%) |
| Goiter | 3 (33.3%) |
| Genitourinary | |
| Endometrial fibroid | 1 (33.3%b) |
| Mixed germ cell tumor | 1 (33.3%b) |
| Upper GI | |
| Glycogenic acanthosis | 8 (80%) |
| Gastric hyperplastic | 10 (100%) |
| Gastric hamartomatous polyps | 4 (40%) |
| Duodenal hyperplastic | 9 (90%) |
| Duodenal hamartomatous polyps | 1 (10%) |
| Duodenal adenomatous polyps | 3 (30%) |
| Colon and rectum | |
| Hyperplastic | 8 (80%) |
| Hamartomatous polyps | 5 (50%) |
| Juvenile polyps | 2 (20%) |
| Ganglioneuromatous polyps | 3 (30%) |
| Adenomatous polyps | 3 (30%) |
| Cancer (early age) | 1 (10%) |
Percent of males.
Percent of females.
Mutation: 350insA (n=2), 302delT (n=3), R335X (n=1), Q244X (n=1), ivs6/A-G (n=2), in one case specific location of the mutation was not reported.
Colorectal polyps and carcinoma
At least one colonoscopy was performed for all 10 patients; each segment was examined by both WL and NBI (Table 2). The average age at first colonoscopy was 31.7 years (range 9–59 years) and the mean number of colonoscopies per patient was 2.4 (range 1–5). Five patients (50%) underwent colonoscopy because they were symptomatic (rectal bleeding: 3, iron-deficiency anemia: 2). Four patients (40%) had the first procedure because of the diagnosis of CS and one underwent the procedure after upper GI polyps were detected. In Table 2 we specify for each lesion whether it was screen detected or symptom detected.
Table 2. Lower gastrointestinal tract findings in our PTEN mutation–positive patients.
| Patient | Mutation (pedigree) | Origin | Age (years) | Gender | Indication for colonoscopy |
Colorectal findings |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperplastic polyps | Hamartomatous polyps | Ganglioneuroma | Juvenile polyps | Adenomatous polyps | Cancer | ||||||
| 1 | 350insA (1) | Jew | 61 | M | Symptomatic: rectal bleeding | Numerousa, 2–5 mm (SD) | Numerousa, 4–5 mm (SD) | None | None | Several, 3–15 mm,TALGD (SD) | One, rectal (age 44 years) (SY) |
| 2 | 350insA (1) | Jew | 25 | F | Asymptomatic | Numerousa, 1–4 mm (SD) | Fewa, 4 mm (SD) | None | None | None | None |
| 3 | 302delT (2) | Jew | 59 | F | Asymptomatic | Numerousa, 2–5 mm (SD) | None | None | None | Three, 5–8 mm, TALGD (SD) | None |
| 4 | 302delT (2) | Jew | 29 | M | Asymptomatic | None | None | None | None | None | None |
| 5 | 302delT (2) | Jew | 31 | M | Asymptomatic | Fourb, 1–2 mm (SD) | None | None | None | None | None |
| 6 | R335X (3) | Jew | 30 | F | Asymptomatic: upper GI polyposis noted | None | None | None | None | None | None |
| 7 | Q244X (4) | Arab | 29 | M | Symptomatic: iron-deficiency anemia | Numerousa, 1–4 mm (SY) | Numerousa, 4–5 mm (SY) | None | None | None | None |
| 8 | ivs6/A-G (5) | Arab | 22 | M | Symptomatic: iron-deficiency anemia | Numerousa, 2–5 mm(SY) | Fewa, 5–6 mm (SY) | Fivea, 5–6 mm (SY) | Two, 30 mm (SY) | None | None |
| 9 | ivs6/A-G (5) | Arab | 49 | M | Symptomatic: rectal bleeding | Numerousa, 1–8 mm (SY) | None | Sixa, 5–6 mm (SY) | None | None | None |
| 10 | Sp (8) | Jew | 29 | M | Symptomatic: rectal bleeding | Numerousa, 2–8 mm (SY) | Fewa, 5–8 mm (SY) | Two, 6–8 mm (SY) | Two: 8–15 mm (SY) | Two, 6–8 mm, TALGD (SD) | None |
F, female; GI, gastrointestinal; M, male; SD, screen detected; SY, symptom detected; Sp, mutation detected, specific location not reported; TALGD, tubular adenoma low-grade dysplasia.
Pan-colonic.
Rectal only.
Colorectal polyps were identified in 8 of 10 (80%) research participants. The lower GI polyps were found throughout the colon with increased density of polyps in the rectum. Eight subjects (80%) had hyperplastic polyps, five (50%) had hamartomatous polyps, three (30.0%) had ganglioneuromatous polyps, three had adenomatous polyps (one of them at age 29 years), and two (20.0%) had juvenile polyps.
It should be noted, however, that pathology results from cold biopsies are often misleading. In five cases, we used the technique of cold biopsy before snare polypectomy. In three out of those five patients, the superficial biopsy contained “hyperplastic tissue”, while inspection of the whole resected polyp revealed that it contained hamartomatous components.
All the adenomatous lesions were detected during the surveillance program (Table 2). Using both WL and NBI, we were able to identify correctly adenomatous polyps in three patients and to resect these lesions. It is impossible to decide whether it is the careful dual inspection or the added value of NBI that lead to the detection of adenomas.
One out of the ten PTEN mutation–positive patients (10%) had rectal cancer at an early age (44 years). He was found to have synchronous hyperplastic, hamartomatous, and adenomatous polyps. The cancer was found to be microsatellite stable (MSS), and IHC showed normal nuclear expression of MLH1, MSH2, MSH6, and PMS2 in the carcinoma cells and PTEN expression was null by IHC.
Upper GI findings
All patients (100%) underwent at least one EGD (Table 3). The average age at first EGD was 35.6 years (range 21–61 years) and the mean number of endoscopies per patient was 2.7 (range 1–4). Three patients (30%) were symptomatic (iron-deficiency anemia: 2, hematemesis: 1), and in seven patients (70%) the first procedure was performed while asymptomatic as part of the surveillance program.
Table 3. Upper gastrointestinal endoscopic findings in PTEN mutation–positive patients.
| Patient | Mutation (pedigree) | Origin | Age (years) | Gender | Indication for EGD | Esophagus | Stomach |
Duodenum |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogenic acanthosis | Hyperplastic polyps | Hamartomatous polyps | Hyperplastic polyps | Hamartomatous polyps | Brunner gland hyperplasia | Duodenal adenoma | ||||||
| 1 | 350insA (1) | Jew | 61 | M | Asymptomatic | Severe | Multiple 3–5 mm (SD) | Few 5–8 mm (SD) | Multiple 3–5 mm (SD) | One, 8 mm (SD) | One 10 mm (SD) | One 15 mm VALGD (SD) |
| 2 | 350insA (1) | Jew | 25 | F | Asymptomatic | Mild | Multiple 1–3 mm (SD) | None (SD) | Multiple 1–2 mm (SD) | None | None | None |
| 3 | 302delT (2) | Jew | 59 | F | Asymptomatic | Severe | Multiple 3–5 mm (SD) | None | Multiple 3–5 mm (SD) | None | None | None |
| 4 | 302delT (2) | Jew | 29 | M | Asymptomatic | Mild | Few 1–2 mm (SD) | None | Few 1–2 mm (SD) | None | None | One 6 mm TALGD (SD) |
| 5 | 302delT (2) | Jew | 31 | M | Asymptomatic | None | Multiple 3–5 mm (SD) | One 10 mm (SD) | Few 1–2 mm (SD) | None | None | None |
| 6 | R335X (3) | Jew | 30 | F | Symptomatic: Hematemesis | None | Multiple 3–5 mm (SY) | None | Few 1–2 mm (SY) | None | None | None |
| 7 | Q244X (4) | Arab | 29 | M | Symptomatic: Iron-deficiency anemia | Severe | Multiple 3–5 mm (SY) | Few 5–8 mm (SY) | Multiple 1–2 mm (SY) | None | One 10 mm | None |
| 8 | ivs6/A-G (5) | Arab | 22 | M | Symptomatic: Iron-deficiency anemia | Severe | Multiple 3–5 mm (SY) | None | Few, 1–2 mm (SY) | None | None | None |
| 9 | ivs6/A-G (5) | Arab | 49 | M | Asymptomatic | Severe | Multiple 3–5 mm (SD) | Multiple 5–8 mm (SD) | Few 1–2 mm (SD) | None | None | One 5 mm TALGD (SD) |
| 10 | Sp (8) | Jew | 29 | M | Asymptomatic | Mild | Few 1–2 mm (SD) | None | None | None | None | None |
EGD, esophagogastroduodenoscopy; F, female; M, male; SD, screen detected; SY, symptom detected; Sp, mutation detected, specific location not reported; TALGD, tubular adenoma low-grade dysplasia; VALGD, villous adenoma low-grade dysplasia.
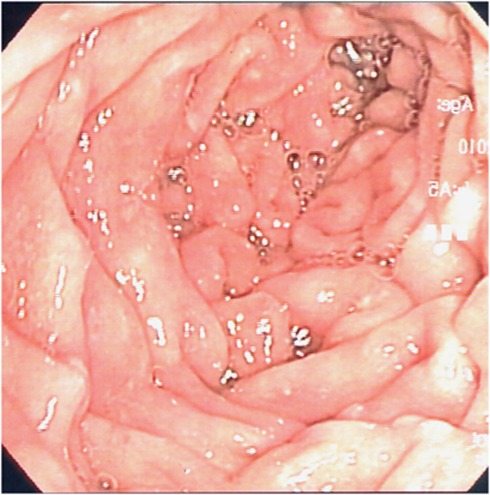
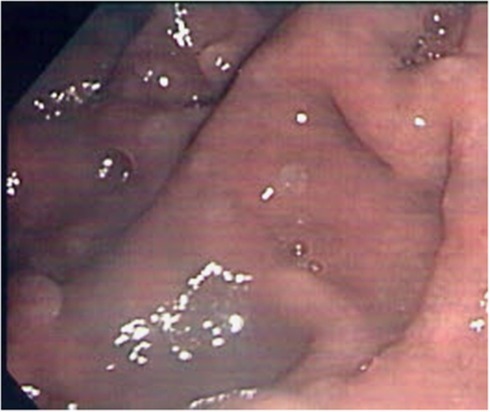
All 10 individuals had abnormal upper GI endoscopic findings, which varied from severe esophageal acanthosis and massive gastroduodenal polyposis to only a few diminutive gastric polyps. Eight patients (80%) had glycogenic acanthosis, of which five had severe acanthosis, and in two cases, the presentation was mild. Gastric polyps were detected in all patients (100%), mostly hyperplastic with varying sizes and number. Duodenal polyps were observed in nine patients (90% Figure 1). Using both WL and NBI, we were able to identify correctly adenomatous polyps in three patients and to resect these lesions (Figure 2). Again, it is impossible to decide whether it is the careful dual inspection or the added value of NBI use that lead to the detection of adenomas.
Figure 1.
Duodenal view in Cowden syndrome patient using white light.
Figure 2.
Duodenal view in Cowden syndrome patient using narrow-band imaging.
In one patient, a carpeting of polyps in the duodenal bulb proved to contain hyperplastic Brunner glands, and in one case, a 15 mm polyp in the second duodenal part contained Brunner gland hyperplasia and gastric metaplasia.
Other clinical features
Clinical features characteristic of or suspicious of CS also were recorded for all subjects (Table 1). Four patients (40%) had thyroid cancer: follicular variant of papillary thyroid carcinoma in a female at aged 13 years and in her father at age 61 years, and two epithelial thyroid carcinomas (of unknown histology) in an adult male aged 40 years and female at age 48. Of the three female patients, one had breast cancer at age 48 years, the second had mixed germcell tumor at age 7 years and the third had endometrial fibroid. Macrocephaly was evident in the three patients (30%) and Lhermitte-Duclos in one.
DISCUSSION
While the increased risk for breast and thyroid cancer is well documented, CS is not considered as a syndrome with an increased colon cancer or upper GI cancer risk, at least in the most studied population, whites of European ancestry. In fact, Online Mendelian Inheritance in Man (OMIM)20 does not report colon or upper GI cancer in CS patients, and currently, the NCCN states that CRC screening in CS patients is not indicated in the absence of symptoms, but rather focuses largely on the increased risks of breast, thyroid, and endometrial cancers.7 A currently updated Gene Reviews21 recommend baseline colonoscopy at age 50 years, unless symptoms arise earlier, and if only hamartomatous polyps are found, the American Cancer Society guidelines for colon cancer screening (i.e., annual fecal occult blood testing and sigmoidoscopy every 5 years or colonoscopy every 10 years) should be followed.21
In late 2010 and this year, we analyzed prospective series of individuals who met classic criteria for the clinical diagnosis of CS (NCCN-2006)7 and relaxed criteria (full criteria minus one) also referred to as CS-like.6, 8 For the first time, upper and lower GI polyps, comprising broad histologies, and even polyposis are an important part of the phenotypic spectrum of individuals with pathogenic germline PTEN mutations (variants of unknown significance were excluded from analysis)8 and a clinical feature signaling the potential of the diagnosis.6 However, the caveat to these analyses is that examination of the upper and/or lower GI tracts was performed as part of clinical care, and not systematically and periodically performed as a routine. The current study directly addresses this caveat because all PTEN mutation–positive individuals here were given active annual upper and lower GI surveillance from the age of 25 years, and starting earlier should there be symptoms. Confirming our previous observation, this current study reveals 8 (80%) with colonic polyps of varying histologies and one male, currently, 61, with rectal carcinoma diagnosed at age 44. As with the Heald et al.8 observations, this cancer was accompanied by prevalent colonic polyposis. Indeed, our patient who was diagnosed with rectal cancer had undergone several endoscopies in the “community” where some of the polyps were biopsied, and were found to be “inflammatory” or hyperplastic, while careful colonoscopy using dual WL and NBI inspection we detected large adenomatous polyps (up to 15 mm) that were removed again with the endoscope. Thus, polyposis may be an indicator of future colorectal cancer risk in individuals with PTEN mutations.
The rectal cancer identified here was MSS and showed normal expression of MLH1, MSH2, MSH6, and PMS2 proteins but was PTEN protein null by IHC. The fact that this MSS rectal tumor was completely null for PTEN protein expression strongly suggests that this rectal cancer was facilitated by the existing germline PTEN mutation.22
Up to our recent GI series by Heald et al.,8 there were several reports of gastric cancers in PTEN mutation carriers,14, 15 but no reports about adenomatous polyps in the upper GI tract. In our current study from active GI surveillance, we report that all patients had upper GI polyps and that three patients (30%) had adenomatous polyps, the latter contrasts with only 2 of 39 (5%) research participants found to have upper GI adenomatous polyps when EGD was performed for clinical reasons8 (P=0.05). It should be noted that the mean age of both series are similar (40 and 39 years). Two plausible explanations for the higher prevalence of GI adenomas include the use of NBI, which was used to advantage to detect all three with upper GI adenomatous polyps in this series, and the active yearly surveillance of both the upper and lower GI tracts. Therefore, as individuals with PTEN mutations may routinely develop numerous upper GI polyps and it is impossible to resect every visualized polyp, it seems reasonable to examine the polyps with dual WL and NBI and endoscopically resect only the large polyps and/or those that seem to be adenomatous.
Finally, although the Heald et al.8 series could not reveal a genotype–GI phenotype correlation, we note here that all 10 individuals in this present have truncating mutations. We caution, however, that the current sample size is relatively small and we are unaware of the general genotypic spectrum in the population served by RMC. Nonetheless, we believe that our two series (Heald et al.;8 this study) begin to support routine GI surveillance on asymptomatic CS patients, confirming the suggestion that surveillance colonoscopies begin before 35 years of age. Because of the prominence of the upper GI phenotype, especially adenomas, upper GI surveillance has an important role as well. However, we do not know at what age to begin and how frequently. Because the PTEN-related GI phenotype includes multiple polyps, it may be conservative to utilize such technological advances as NBI endoscopy to differentiate adenomas. The place of NBI endoscopy and other modern strategies should be further characterized.
From our initial experience with our PTEN mutation–positive CS patients, GI features including adenomas and cancer may be more prevalent than originally thought. However, our study included only 10 patients and only in about half of the cases the findings were detected by the screening program among asymptomatic patients. Therefore, we strongly urge that another independent large series be prospectively accrued for systematic study of prospective routine surveillance of the GI tract in PTEN mutation–positive CS. It is also important to emphasize that various component features arising from identical mutations may result in vastly different phenotypes in individuals of different ancestral backgrounds, and so the international community should come together to perform comparative phenotype studies. Finally, we suspect that lifelong mechanical removal of polyps annually might become unfeasible to patients and to our sagging health-care systems, and call upon a global collaborative trial of novel targeted therapeutic approaches as the use of mammalian target of rapamycin inhibitors (clinical trial NCT00971789).
Study Highlights

Acknowledgments
We thank members of the Eng lab, Genomic Medicine Institute, Cleveland Clinic for technical assistance. Prof Paul Rozen established the high-risk cancer clinic at the Rabin Medical Center and continues to contribute his unique knowledge to the clinic staff. C.E. is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic, and is an ACS Clinical Research Professor, generously funded, in part, by the F.M. Kirby Foundation.
Guarantor of the article: Zohar Levi, MD.
Specific author contributions: Zohar Levi: study concept and design, acquisition and interpretation of data, drafting and critical revision of the manuscript; Yaron Niv: study supervision, drafting and critical revision of the manuscript; Inbal Kedar and Hagit N. Baris: acquisition of data, important intellectual content, and critical revision of the manuscript; Alex Geller, Rachel Gingold, and Eyal Gal: acquisition of data and critical revision of the manuscript; Sara Morgenstern and Susana Horn: technical and material support, and critical revision of the manuscript; Brandie Heald Leach: acquisition of data and critical revision of the manuscript; Mary P. Bronner: acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript; Charis Eng: study concept and design, analysis and interpretation of data, drafting and critical revision of the manuscript.
Financial support: This work was funded, in part, by P01CA124570 from the National Cancer Institute, Bethesda, MD (to C.E.).
Potential competing interests: None.
References
- Schreibman IR, Baker M, Amos C, et al. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100:476–490. doi: 10.1111/j.1572-0241.2005.40237.x. [DOI] [PubMed] [Google Scholar]
- Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Rev Gastroenterol and Hepatol. 2007;4:492–502. doi: 10.1038/ncpgasthep0902. [DOI] [PubMed] [Google Scholar]
- Uppal S, Mistry D, Coatesworth AP. Cowden disease: a review. Int J Clin Pract. 2007;61:645–652. doi: 10.1111/j.1742-1241.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Dahia PL, Caron S, et al. Germline PTEN mutations in Cowden syndrome-like families. J Med Genet. 1998;35:881–885. doi: 10.1136/jmg.35.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XP, Marsh DJ, Morrison CD, et al. Germline inactivation of PTEN and dysregulation of the phosphoinositol-3-kinase/Akt pathway cause human Lhermitte-Duclos disease in adults. Am J Hum Genet. 2003;73:1191–1198. doi: 10.1086/379382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Mester J, Peterson C, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The NCCN Genetic/Familial High-Risk Assessment Breast and Ovarian Guideline (V.1.2007) 2006 National Comprehensive Cancer Network, Inc. http://www.nccn.org/index.asp .
- Heald B, Mester J, Rybicki L, et al. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterol. 2010;139:1927–1933. doi: 10.1053/j.gastro.2010.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen AMN, Fryns JP. Cowden syndrome. J Med Genet. 1995;32:117–119. doi: 10.1136/jmg.32.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra G, Armelao F, Vecchio FM, et al. Cowden's disease withextensive gastrointestinal polyposis. J Clin Gastroenterol. 1994;18:42–47. doi: 10.1097/00004836-199401000-00011. [DOI] [PubMed] [Google Scholar]
- Carlson GJ, Nivatvongs S, Snover DC. Colorectal polyps in Cowden's disease (multiple hamartoma syndrome) Am J Surg Pathol. 1984;8:763–770. doi: 10.1097/00000478-198410000-00005. [DOI] [PubMed] [Google Scholar]
- Umemura K, Takagi S, Ishigaki Y, et al. Gastrointestinal polyposis with esophageal polyposis is useful for early diagnosis of Cowdens disease. World J Gastroenterol. 2008;14:5755–5759. doi: 10.3748/wjg.14.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose K, Araki T, Matsunaka T, et al. Variant manifestation of cowden disease in japan: hamartomatous polyposis of the digestive tract with mutation of the PTEN gene. Am J Hum Genet. 1999;64:308–310. doi: 10.1086/302207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Thihli K, Palma L, Marcus V, et al. A case of Cowden's syndromepresenting with gastric carcinomas and gastrointestinal polyposis. Nat Clin Pract Gastroenterol Hepatol. 2009;6:184–189. doi: 10.1038/ncpgasthep1359. [DOI] [PubMed] [Google Scholar]
- Hamby LS, Lee EY, Schwartz RW. Parathyroid adenoma and gastric carcinoma as manifestations of Cowden's disease. Surgery. 1995;118:115–117. doi: 10.1016/s0039-6060(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Grussendorf-Conen EI, Rübben A, et al. Multiple colon carcinomas in a patient with Cowden syndrome. Int J Mol Med. 2006;18:643–647. [PubMed] [Google Scholar]
- Zhou XP, Loukola A, Salovaara R, et al. PTEN Mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439–447. doi: 10.1016/S0002-9440(10)64200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curvers WL, van den Broek FJ, Reitsma JB, et al. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach Gastrointest Endosc 200969307–317.Review. [DOI] [PubMed] [Google Scholar]
- Lee MM, Enns R.Narrow band imaging for the detection of neoplastic lesions of the colon Can J Gastroenterol 20092315–18.Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM) ; http://www.ncbi.nlm.nih.gov/omim .
- National Center for Biotechnology Information (NCBI) GeneReviews, Eng C., PTEN Hamartoma Tumor Syndrome (PHTS). http://www.ncbi.nlm.nih.gov .
- Nassif N, Lobo GP, Wu X, et al. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617–628. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]