Abstract
OBJECTIVES:
RNA silencing utilizing short interfering RNA (siRNA) offers a new and exciting means to overcome the limitations of current treatment options of many diseases. However, delivery of these molecules still poses a great challenge to date.
METHODS:
In the present study, a multicompartmental biodegradable polymer-based nanoparticles-in-microsphere oral system (NiMOS) using gelatin nanoparticles encapsulating a combination of siRNA duplexes specifically targeted against tumor necrosis factor-α (TNF-α) and cyclin D1 (Ccnd1) was employed to study its effects on a dextran sulfate sodium (DSS)-induced acute colitis mouse model mimicking inflammatory bowel disease (IBD). DSS colitis-bearing animals were divided into several control and treatment groups and received either no treatment, blank NiMOS, NiMOS-encapsulating inactive (scrambled), active TNF-α silencing, CyD1 silencing siRNA, or a combination of both active siRNAs by repeated oral administration of three NiMOS doses.
RESULTS:
Successful gene silencing with the aid of dual siRNA treatment led to decreased colonic levels of TNF-α or CyD1, suppressed expression of certain pro-inflammatory cytokines (interleukin-1α and -β, interferon-γ), an increase in body weight, and reduced tissue myeloperoxidase activity, while the silencing effect of CyD1 siRNA or the dual treatment was more potent than that of TNF-α siRNA alone.
CONCLUSION:
Results of this study demonstrate the therapeutic potential of a NiMOS-based oral combined TNF-α and CyD1 gene silencing system for the treatment of IBD as shown in an acute colitis model.
INTRODUCTION
Crohn's disease and ulcerative colitis constitute the two main phenotypes of inflammatory bowel disease (IBD), a group of clinical relapsing conditions of unknown etiology associated with mucosal inflammation of the gastrointestinal tract.1, 2, 3 Current treatment options involving the use of conventional therapy, including anti-inflammatory and immunosuppressive drugs,4 have shown success in management of acute inflammation, but could not effectively maintain remission due to tolerability, toxicity, dependency, and higher relapse rates5 often leaving surgical removal of inflamed tissue as a last resort, which poses physical pain and mental stress for patients. To overcome shortcomings in efficacy and safety, novel treatment options include development of biological therapies involving inhibition of mucosal inflammatory pathways by targeting pro-inflammatory cytokines, their receptors, and adhesion molecules, and thus offer more effective treatment options for the disease.6, 7 Success has been reported in biological therapies, especially in patients refractory to conventional treatment, using specific systemic monoclonal antibodies or soluble receptors aimed at neutralization and reduction of tumor necrosis factor-α (TNF-α),2, 5, 9, 10, 11, 12 a pivotal cytokine in IBD that has a central role in mediation of inflammatory responses.13 However, severe side effects and contraindications, such as opportunistic infections, decreased efficacy, and infusion reactions, have frequently been reported10, 11, 14 so that other therapeutic options have received a great deal of attention. One alternative treatment strategy includes gene therapy, where the gastrointestinal tract additionally poses an ideal starting point and target due to its extremely large surface area and access to luminal sites of inflammation via rectal and oral routes of administration.
This study evaluates the potential of RNA interference (RNAi) as an alternative treatment for systemic diseases such as IBD. Hereby, short interfering RNA (siRNA), which is usually composed of double-stranded 20–25 nucleotides, is associated with a gene silencing mechanism, which interferes with the expression of a specific gene, e.g., one that is overexpressed in a certain disease.15 To date, many studies explore the potential of RNAi in cancer therapy,16, 17, 18 whereas very few focus on the treatment of inflammation.19, 20, 21, 22 RNAi offers great potential for the development of novel drugs. However, one of the biggest hurdles remains in safe and efficacious delivery of siRNA to the organ and cells of interest to avoid degradation of the payload and increase in residence time to allow for sufficient interaction with cells and a resulting cellular uptake to occur. As such, development of proper delivery and encapsulation techniques, including viral vectors,23 nanoparticles,21, 24 and polymer-based22, 25, 26 and liposome-based vehicles,15, 19 is crucial in the success of gene silencing by siRNA. Further, systemic administration has been investigated most commonly,15, 17, 20, 21 while very few studies have been devoted to study mucosal delivery, including rectal, vaginal,19 or oral routes.22
Nanoparticles-in-microsphere oral system (NiMOS) is a multicompartmental delivery system specifically designed for oral administration of nucleic acid contructs.27, 28, 29, 30 In previous studies, we have shown that both reporter (i.e., enhanced green fluorescent protein expressing) and therapeutic (i.e., murine interleukin (IL)-10 expressing) plasmid DNA were transfected in NiMOS administered orally to naïve and acute colitis-bearing Balb/c mice.28, 30, 31 Additionally, we recently showed that TNF-α silencing siRNA, when encapsulated in NiMOS, could be used for the treatment of IBD in dextran sulfate sodium (DSS)-induced colitis-bearing Balb/c mice.32
The specific purpose of the present study was to evaluate the effect of dual siRNA delivery targeting multiple genes of interest as a way to further improve the treatment of IBD. To achieve this goal, we used NiMOS for encapsulation of TNF-α- and cyclin D1 (Ccnd1)-specific siRNA in one formulation, and evaluated dual gene silencing and therapeutic efficacy in DSS-induced colitis model of IBD. CyD1 is a member of the cyclin family and key regulator during the cell cycle by controlling the progression from G1 to S phase.33, 34, 35 It is overexpressed in many human cancers35, 36, 37 and inflammatory diseases;21, 38, 39 however, its exact role in inflammation is not known yet. Because of its importance, it serves as a potential target for treatment options of inflammatory diseases such as IBD.
METHODS
Preparation of siRNA-encapsulated gelatin nanoparticles and NiMOS
Nanoparticles were prepared from gelatin (MW 40,000–50,000, 100–115 mM of free carboxylic acid per 100 g of protein, pI of 4.7–5.2, 225 bloom strength) in the same manner as described before.27, 29, 31 In brief, siRNAs were mixed with aqueous gelatin solutions of pH 7.0 and pre-incubated for 10 min at room temperature, followed by controlled precipitation of siRNA-containing or blank gelatin solutions by using ethanol as the non-solvent. Resulting nanoparticles were centrifuged at 32,000 r.p.m. for 45 min, purified, and lyophilized. Desalted and annealed siRNAs were obtained from Dharmacon (Lafayette, CO), and their sense sequences are shown in the Supplementary Table S1. NiMOS were prepared with a “double emulsion-like” technique, which has been used previously by numerous other researchers in our research laboratory27, 29, 31 and is a standard procedure for production of microparticulate delivery systems in the pharmaceutical industry.40, 41
Characterization of siRNA-encapsulated gelatin nanoparticles and NiMOS
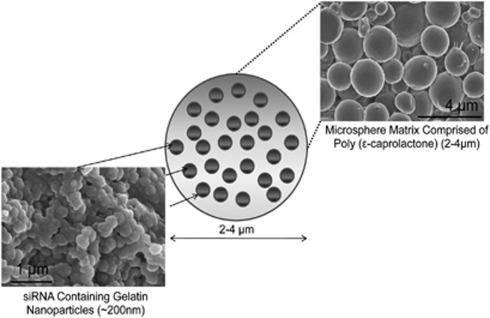
NiMOS consists of biocompatible nucleic acid-encapsulated type B gelatin nanoparticles further entrapped in a poly(ɛ-caprolactone) microsphere (Figure 1).
Figure 1.
Nanoparticles-in-microsphere oral system (NiMOS) for short interfering RNA (siRNA) delivery. Schematic representation of the NiMOS concept and scanning electron micrographs of siRNA-encapsulated type B gelatin nanoparticles further entrapped in poly(ɛ-caprolactone) microsphere.
Particle size analysis
The mean particle size of freshly prepared nanoparticle formulations was determined with the Malvern Zetasizer Nano ZS 90 apparatus (Westborough, MA) at a 90° scattering angle. NiMOS was characterized for particle size and size distribution using the Multisizer 3™ from Beckman Coulter (Fullerton, CA). All sizing measurements were carried out at room temperature.
Scanning electron microscopy
Lyophilized blank or loaded gelatin nanoparticles and NiMOS samples were sputter coated with gold-palladium to minimize surface charging and evaluated according to their surface morphology with a Hitachi S-4800 (Pleasanton, CA) field emission scanning electron microscope.
Determination of siRNA loading in gelatin nanoparticles and NiMOS
siRNA-loaded gelatin nanoparticles and NiMOS were prepared as discussed above. Encapsulation efficiency of siRNA in these formulations was determined by dissolving a known amount of sample (∼50 mg) in phosphate-buffered saline (pH 7.4) containing 0.2 mg/ml protease at 37 °C until a clear solution was obtained. Released double-stranded siRNA was quantified using the PicoGreen® assay (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The intensity of fluorescence, which is proportional to the PicoGreen-bound siRNA was measured at an excitation of 485 nm and emission of 520 nm wavelengths using a Perkin Elmer LS50B spectrophotometer (Waltham, MA). FL WinLab™ (Perkin Elmer) software was used for processing and analysis of resulting data.
For evaluation of siRNA loading in NiMOS, 100 mg of sample was added to 1 ml dichloromethane to dissolve the poly(ɛ-caprolactone) matrix and liberate the gelatin nanoparticles. To this organic poly(ɛ-caprolactone)-dichloromethane mixture, an equal amount of distilled deionized water was added. After careful separation of the aqueous from the organic phase, gelatin nanoparticles underwent the same procedure as described above to measure the final siRNA loading efficiency in the NiMOS using the PicoGreen quantitation assay.
Animal model development and in vivo studies of gene silencing and efficacy
Experimental animals
All animal studies involved were performed in accordance with the experimental protocol approved by the Institutional Animal Care and Use Committee at Northeastern University (Boston, MA). Female Balb/c mice (6–8 weeks, ∼18–20 g) were purchased from Charles River Laboratories (Wilmington, MA). Animals were randomly assigned to groups of 4–5 mice per cage and acclimatized for 10 days before the start of the study. The mice were housed in rooms at a controlled temperature of 22 °C and 26% humidity, with light–dark cycles of 12 h, and fed with water and a standard pellet ad libitum except when fasted overnight to prepare for the oral gavage.
Induction of acute colitis using DSS
Animals were randomly assigned to 14 groups (n=4–5). Acute colitis was induced in mice by addition of 3.5% (w/w) DSS (MW 36,000–50,000; MP Biomedicals United States, Solon, OH) in their drinking water for the duration of the study, whereas one control group (n=8) received regular tap water at the same time. DSS solutions were freshly prepared daily and mice were monitored every day for their health condition.42 The acute disease model was confirmed by evaluation of weight loss, stool consistency, rectal bleeding, and tissue histology. At day 10 and 12 of the study, animals were sacrificed by CO2 inhalation followed by cervical dislocation, and tissue was processed as described below.
Oral siRNA administration in DSS colitis-induced Balb/c mice
On days 2, 4, and 6 of the study, mice of all groups including controls were fasted overnight, followed by administration of blank NiMOS (particles containing no siRNA) or microparticles containing siRNA (inactive sequence, TNF-α, CyD1, or a combination of TNF-α/CyD1) to animals on days 3, 5, and 7 by oral gavage using a blunt-tipped feeding needle inserted into the esophagus equaling three doses of NiMOS formulations per group. An additional control group, serving as DSS control, consisted of animals receiving no oral treatment throughout the course of DSS exposure.
At predetermined time points of 3 and 5 days after the last administration (day 10 and 12, respectively), 4–6 animals per time point and group were killed. After euthanasia, the large intestine was surgically removed and carefully washed with phosphate-buffered saline for histological analysis of the tissue sections and determination of cytokines, murine TNF-α or CyD1 mRNA levels, and expression of CyD1 by Multiplex enzyme-linked immunosorbent assay (ELISA), real-time quantitative PCR (qPCR) and western blotting.
Isolation of RNA from colon tissue and qPCR
Tissue samples were stored in RNAlater (Applied Biosystems, Foster City, CA) at 4 °C for 2 days to allow for tissue penetration, followed by removal of excess liquid and storage of tissue at −70 °C until further processing. Subsequently, RNA was isolated from colon tissue samples using the RNAqueous-4PCR kit (Applied Biosystems) according to the manufacturer's protocol and quantified using the NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE) UV–visible spectrophotometer.
Reverse transcription of isolated RNA samples
A volume corresponding to 1 μg of RNA was used for the complementary DNA (cDNA) synthesis process using the SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) in accordance with the manufacturer's recommendation. Samples containing cDNA were diluted to a final volume of 100 μl and used for subsequent real-time qPCR.
Analysis by real-time qPCR
Real-time qPCR was performed on cDNA samples obtained from large intestinal tissue to determine the levels of mRNA transcript. L32 gene expressing the L32 ribosomal protein served as a control. Pre-diluted cDNA was mixed with 0.2 μM of primer pair detecting the murine TNF-α, murine CyD1, or L32 construct and SYBR Green PCR master mix followed by pipetting into an ABI Prism 96-well optical reaction plate (Applied Biosystems). The sequences of the primers are shown in Supplementary Table S2. The qPCR reaction was performed in the 7300 Real-Time PCR System from Applied Biosystems using the following cycle program: 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Results obtained from the PCR reaction were analyzed by comparative Ct analysis to determine the relative amount of murine TNF-α or CyD1 cDNA in the samples.
Determination of tissue murine cytokine expression levels by ELISA
For subsequent ELISA, samples of the entire large intestine were homogenized in lysis buffer (1 M TRIS, pH 7.4; 0.5 M EDTA, pH 8.0; 5 M NaCl, 10% (w/v) Brij, 10% (v/v) Tween 20, and proteinase inhibitor) on ice for extraction of proteins from the tissue samples. Protein-containing supernatant was separated by centrifugation at 13,000 g for 30 min at 4 °C and stored at −70 °C until analysis. Changes in the concentration of a series of cytokines and chemokines (TNF-α, IL-1α, IL-1β, IL-2, interferon (IFN)-γ, IL-5, IL-6, IL-17, monocyte inflammatory protein (MIP)-1α, monocyte chemotactic protein-1, and granulocyte macrophage colony-stimulating factor) in the large intestine of colitis-induced Balb/c mice were measured with the Q-Plex™ Mouse Cytokine Screen ELISA (Quansys Biosciences, Logan, UT) according to the manufacturer's protocol. Luminescence intensity of each sample was measured and the concentration of each cytokine was analyzed with a five-parameter curve fitting using the Q-View™ software (Quansys Biosciences). Resulting cytokine and chemokine concentrations were normalized against the total protein content of each individual sample as determined by bicinchoninic acid assay (Pierce, Rockford, IL). Values are expressed as pictogram (pg) of murine cytokine or chemokine expressed per mg of total protein content of each sample and represent mean±s.d. of 4–6 mice.
Western blot analysis
Western blot analysis was conducted by separation of total cellular lysates (150 μg) using 4–20% sodium dodecyl sulfate–PAGE and subsequent electrophoretic transfer of proteins to a polyvinylidene fluoride membrane. Membranes were blocked with universal blocking buffer (Fisher Scientific, Pittsburgh, PA) for 15 min at room temperature and probed overnight at 4 °C with CyD1 monoclonal antibody or β-actin antibody serving as a control (Cell Signaling Technology, Danvers, MA). After washing, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature followed by immunodetection using the enhanced chemiluminescence technique (Pierce).
Histological analysis of tissue sections by haematoxylin and eosin staining
Tissue samples were evaluated for mucosal architectural change, cellular infiltration, inflammation, goblet cell depletion, and signs of epithelial regeneration by using light microscopy of haematoxylin and eosin survey staining.42, 43 These values were used to assess the degree of mucosal damage and repair in treatment and control groups. Tissue samples harvested from distal regions of the non-inflamed/inflamed colon were stored in 10% formalin solution at 4 °C for 7 days to ensure complete penetration and fixing of the tissue. After removal from formalin solution, samples were washed with phosphate-buffered saline and transferred to 30% (w/v) sucrose solution for 2 days to protect from freezing damage in preparation of tissue for cryosectioning. Tissue sections with a thickness of 7 μm were stained with haematoxylin and eosin according to the protocols supplied by the manufacturer, followed by imaging using bright-field microscopy (Olympus BX51TRF, Olympus America, Center Valley, PA).
Determination of tissue myeloperoxidase (MPO) activity
Tissue MPO activity was detected with the FluoroMPO assay purchased from Cell Technology (Mountain View, CA). In preparation of this, tissue samples were minced in hexadecyltrimethylammonium bromide buffer (0.5% in 50 mM phosphate buffer) on ice and homogenized. The homogenate was sonicated, subjected to a freeze–thaw cycle, and centrifuged at 10,000 r.p.m. for 3 min. A certain known amount of the tissue lysate supernatant was combined with the detection mix containing H2O2, a non-fluorescent detection reagent, and 1 × assay buffer. The detection reagent was oxidized in the presence of hydrogen peroxide and MPO to produce its fluorescent analog, which was measured at an excitation wavelength of 530 nm and emission of 590 nm. Reported values were normalized to mg of total protein content of the sample.
Statistical data analysis
All data shown are represented as mean±s.d. Statistical differences between DSS control and TNF-α, CyD1, or TNF-α/CyD1 combination NiMOS, and between scramble sequence-containing NiMOS TNF-α, CyD1, or TNF-α/CyD1 combination NiMOS groups were determined on both time points using a T-test with separate variance estimates. P values below 0.05 were considered significant; and only significant differences are shown for the sake of clarity.
RESULTS
Acute colitis was induced in female Balb/c mice (6–8 weeks) by addition of 3.5% (w/w) dextran sodium sulfate (DSS) in their sole source of drinking water for the duration of the study, whereas one control group (n=8) received regular tap water throughout the course of the study. This model has been most frequently used and was first discussed by Okayasu et al.44 in 1990 because of its convenient induction of the disease, lower mortality risk, and defined beginning.42 Simultaneously, three doses of blank or siRNA-encapsulating NiMOS (1.2 mg/kg of body weight) were orally administered to groups of four to five animals every other day starting on day 3 of the study following an overnight fasting period, as food in the stomach and other sections of the gastrointestinal tract is known to interfere with dosing and analysis upon administration of the formulations. RNA silencing of TNF-α and CyD1, and its effects on the severity and symptoms of the acute colitis disease model were evaluated at predetermined time points of 3 and 5 days after the last oral administration of the treatment. Four to five animals per time point and group were killed, their large intestines were surgically removed and washed to prepare for subsequent analysis of the tissue, including assessment of cytokine profile, mRNA expression levels of TNF-α and CyD1, and histological analysis as well as evaluation of therapeutic efficacy of each treatment. As shown previously, TNF-α expression was at its highest level on day 10 of the study before and after which the values were significantly decreased.32 On the basis of these results, day 10 was chosen as the first time point for evaluation of RNA silencing and its effects on the severity and symptoms of the acute colitis disease model as differences between controls and treatment were expected to be at maximum at this time point. A second time point was chosen to be 5 days after the last oral administration (day 12), where DSS control groups were alive, and to see whether the effect of RNA silencing was still persistent. Relevant controls consisted of untreated animals (healthy group and DSS control, n=4) and groups treated the same as test groups receiving active/silencing NiMOS (n=5), but were instead orally administered with blank/unloaded or inactive/scrambled NiMOS (n=5).
TNF-α and CyD1 RNA silencing and protein expression
Real-time qPCR (qPCR) was used to confirm levels of TNF-α or CyD1 upon multiple administration of siRNA-encapsulating NiMOS with sequences active in the cleavage of the selective mRNA. Tissue samples were taken from the same region of the colon across all groups of animals for consistency. Overall, lowest mRNA expression of both TNF-α and CyD1 was observed in the healthy control group on day 10 of the study (Figure 2a) and described as the baseline level. At this time point, TNF-α mRNA levels were reduced when compared with levels observed in animals of the DSS control, blank, scrambled, and CyD1 NiMOS group. Combined siRNA administration had a stronger effect on downregulation of mRNA expression than administration of single siRNA as evidenced by the lower TNF-α level achieved. Elevated levels were observed in groups treated with CyD1 NiMOS, indicating the specificity of the TNF-α siRNA to cause downregulation only in that particular group. However, inactive and CyD1 NiMOS led to upregulation of TNF-α mRNA compared with the remaining test and control groups. On the second time point, all three silencing groups were significantly reduced in comparison with all control groups (untreated or mock treated).
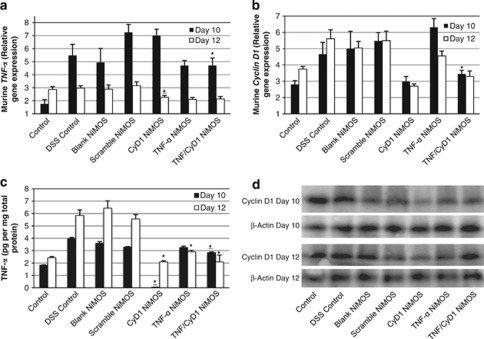
Figure 2.
mRNA and protein expression profiles in control and short interfering RNA (siRNA)-treated mice. Murine tumor necrosis factor-α (TNF-α) and murine cyclin D1 (CyD1) expression upon oral administration of three doses of nanoparticles-in-microsphere oral system (NiMOS; blank or encapsulating inactive (scrambled) siRNA, TNF-α siRNA, CyD1 siRNA, combined TNF-α/CyD1 siRNA) to animals under continuous dextran sulfate sodium (DSS) exposure. Each control or test group consisted of four to five animals. Gene mRNA levels were measured by real-time quantitative PCR analysis, normalized against L32 and depicted as relative gene expression of (a) murine TNF-α and (b) murine CyD1 mRNA performed on samples of the large intestines. Repeated oral administration of the respective siRNA and combination therapy loaded NiMOS led to a reduced mRNA expression of TNF-α and CyD1, respectively, compared with DSS control animals or animals treated with unloaded or scrambled siRNA loaded NiMOS study. (c) Quantitative determination of TNF-α in total cell lysates from colonic tissue by enzyme-linked immunosorbent assay. Levels of the protein were significantly reduced in CyD1 NiMOS groups on day 10 compared with all remaining treatment and control groups including the healthy control group. On day 12, TNF-α levels were reduced in all active siRNA groups compared with the inactive (scrambled) siRNA and blank NiMOS as well as untreated and control and DSS control groups. (d) Western blot of total cell lysates from intestinal tissue for determination of CyD1 with the loading control β-actin. Administration of CyD1 siRNA-loaded NiMOS led to reduction in CyD1 expression compared with the remaining control and test groups on days 10 and 12, whereas protein expression was reduced in TNF-α/CyD1 combined siRNA NiMOS only on day 10 and showed a slight increase on day 12. Values are represented as mean±s.d. (n=4–5). Control=naïve, no colitis; DSS control=colitis, no treatment; blank NiMOS=colitis, blank microspheres; scramble NiMOS=colitis, scrambled siRNA-containing microspheres; CyD1 NiMOS=colitis, CyD1 siRNA-containing microspheres; TNF-α NiMOS=colitis, TNF-α siRNA-containing microspheres; TNF/CyD1 NiMOS=colitis, microspheres containing and combination of both TNF-α and CyD1 siRNA. *P<0.05, vs. Scramble, Statistical comparison was performed on data sets of DSS control vs. TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination NiMOS, and between TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination vs. Scramble NiMOS group. Only significant differences are shown.
As shown in Figure 2b, oral administration of CyD1 NiMOS resulted in substantial downregulation of CyD1 mRNA expression at both time points compared with all other groups. Groups treated with a combination of siRNA NiMOS exhibited a slightly higher expression of CyD1 mRNA potentially due to a dilution effect of CyD1 siRNA compared with CyD1 NiMOS alone.
An ELISA was performed on intestinal tissue lysates for quantification of TNF-α, to identify a potential correlation of mRNA levels and posttranslational protein expression (Figure 2c). It is noteworthy that treatment with CyD1 NiMOS led to a significant decrease in TNF-α levels in these samples on the first test day; a very low value of 49 fg/mg total protein was detected, equaling a 36-fold reduction compared with the healthy control group. On day 12, protein levels were also reduced in this group compared with all other animals tested, followed by the dual siRNA NiMOS group. The control groups consisting of animals administered with blank NiMOS or scrambled siRNA-encapsulated NiMOS in addition to the untreated DSS control group showed no significant difference in the TNF-α expression pattern at both time points, whereas elevated levels of the protein were observed on day 12 in all colitis-bearing control groups. Administration with TNF-α NiMOS resulted in a slight decrease in protein expression on day 10, which was shown before32 and correlates with the mRNA profile. Combined siRNA treatment led to stronger reduction in TNF-α expression than administration with TNF-α siRNA alone, which can be attributed to the effect of CyD1 silencing that was significantly more potent than that of TNF-α silencing. Furthermore, statistically significant differences were observed between dual siRNA NiMOS and scrambled NiMOS groups in both time points.
CyD1 protein expression was detected by western blotting of intestinal cell lysates (Figure 2d). On day 10 and 12, much less CyD1 was detected in the CyD1 NiMOS-treated group in comparison with all controls as well as the TNF-α and combination siRNA NiMOS group, indicating the specificity of the siRNA and success of RNAi. Lower detection of CyD1 was also observed in both remaining test groups compared with the controls, which slightly diminished on day 12. β-Actin loading controls revealed similar intensity of the bands in all groups verifying the proper loading of the samples into the wells.
Transfection efficiency
The cytokine profile of a variety of markers was assessed to better understand the occurring inflammation processes in untreated animals and each treatment group upon oral administration of three doses of blank, scrambled, or silencing NiMOS. For this purpose, cell lysates prepared as described below were assayed using a Q-Plex™ Mouse Cytokine Screen ELISA (Quansys Biosciences) according to the manufacturer's guidelines, and results are expressed as pg per mg of total protein content of each sample (Figure 3). Similarly, as previously mentioned, administration of CyD1 NiMOS led to a significant downregulation of cytokine expression on day 10, ranging from 2.3-fold to almost 52-fold reduction compared with even the healthy control group, which generally exhibited the lowest expression of the cytokines of all animals tested, except in the case of IFN-γ on day 12. On day 12, an overall increase in cytokine concentration was observed in the CyD1 NiMOS group, whereas expression levels were still below that of remaining colitis-bearing control and test animals. Treatment with TNF-α NiMOS caused a significant decrease in expression of IFN-γ, IL-1α, IL-5, and the chemokines monocyte chemotactic protein-1 and MIP-1α compared with colitis-bearing controls on both days, and also lower concentrations of IL-1β, IL-17, and granulocyte macrophage colony-stimulating factor on day 12, which was less pronounced compared with the CyD1 NiMOS group in both cases. Overall, a significant reduction in expression of pro-inflammatory markers was recorded on the second time point in this group compared with all colitis-bearing control animals, indicating success of the treatment in a slightly delayed manner. Combined administration of TNF-α and CyD1 siRNA led to a decline in IL-1α, IL-1β, IL-2, IL-5, IL-17, monocyte chemotactic protein-1, MIP-1α, and granulocyte macrophage colony-stimulating factor expression on day 10 and 12, and in IFN-γ and IL-6 on day 12 compared with the DSS control, mock-treated, and TNF-α NiMOS-treated animals. Results show that the effect of combined TNF-α/CyD1 NiMOS resulted in a stronger, more effective decrease in expression compared with single siRNA treatment of TNF-α alone, but less powerful than the effect of CyD1 administration potentially due to dilution effect with TNF-α NiMOS.
Figure 3.
Colonic cytokine and chemokine profiles. The cytokine expression profile upon oral delivery of cyclin D1 (CyD1), tumor necrosis factor-α (TNF-α), or a combination of both short interfering RNA (siRNA) encapsulated in nanoparticles-in-microsphere oral system (NiMOS) was determined using a chemiluminescent enzyme-linked immunosorbent assay Q-Plex Mouse Cytokine Screen (Quansys Biosciences). Concentrations of the cytokines (a) interferon (IFN)-γ, (b) interleukin (IL)-1α, (c) IL-1β, (d) IL-2, (e) IL-5, (f) IL-6, (g) IL-17 and pro-inflammatory chemokines (h) monocyte chemotactic protein (MCP)-1, (i) monocyte inflammatory protein (MIP)-1α, and (j) granulocyte macrophage colony-stimulating factor (GMCSF) in the large intestine are shown. Administration of CyD1 NiMOS led to a significant reduction in protein expression both time points tested compared with the remaining groups. The effect of combined TNF-α/CyD1 NiMOS and TNF-α was less pronounced, but led to decreased protein levels in comparison with control groups. Values expressed as mean±s.d. (n=5). ΔP<0.05, vs. DSS control; ΔΔP<0.01, vs. DSS control; *P<0.05, vs. Scramble; **P<0.01, vs. Scramble; Statistical comparison was performed on data sets of DSS control vs. TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination NiMOS, and between TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination vs. Scramble NiMOS group. Only significant differences are shown.
Colonic tissue histopathology
An important aspect to investigate feasibility and efficacy of the study involves evaluation of changes in tissue histology of each test group upon induction and treatment of inflammation. Bright-field images of haematoxylin and eosin survey stained tissue sections (original magnification × 10 and × 40) from each control and test group are shown in Figure 4. Tissue from healthy control mice served as a baseline control for comparison purposes as it showed no signs of abnormal tissue architecture or inflammatory infiltration. In comparison with this, tissue sections from colitis-bearing control animals exhibited clear signs of inflammation, including infiltration with leukocytes and gross destruction of normal mucosal structure, such as goblet cell depletion, ulcerations, and membrane thickening, on day 10 and 12. Quite the opposite was observed in tissue from test groups receiving CyD1, TNF-α, or combined TNF/CyD1 silencing NiMOS. Here, the degree of inflammation significantly diminished showing signs of regeneration and tissue morphology similar to the healthy baseline group on both time points tested.
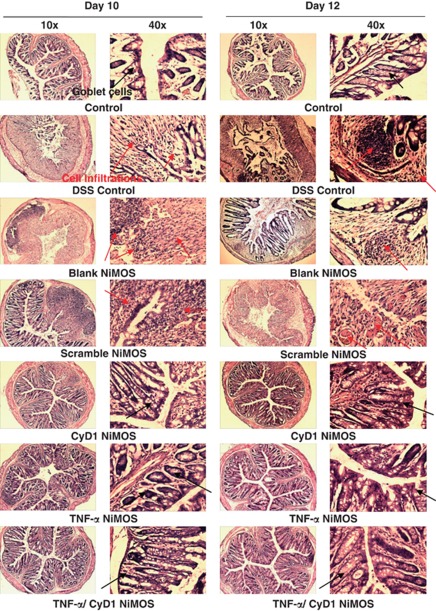
Figure 4.
Microscopic evaluation of colonic tissue histopathology. Bright-field images of haematoxylin and eosin stained sections of the colon harvested from each control and test group. Images are shown at magnifications of × 10 and × 40 from tissue cryosections obtained on day 10 and day 12 of the study. Sections from the first control group show normal and healthy colon tissue. Intestinal tissues from the dextran sulfate sodium (DSS) control group, the group treated with blank and scrambled short interfering RNA (siRNA) nanoparticles-in-microsphere oral system (NiMOS) showed a severe infiltration of white blood cells, abnormal mucosal structure, and a certain degree of goblet cell depletion. Tissue from the group receiving tumor necrosis factor-α (TNF-α), cyclin D1 (CyD1), or combined TNF/CyD1 silencing NiMOS showed signs of regeneration and exhibited a tissue architecture more closely resembling that of healthy tissue in the normal control group. Occurrence of goblet cells is indicated by red arrows; cell infiltration and abnormal tissue histology is indicated by black arrows.
Macroscopic evaluation of anti-inflammatory therapeutic efficacy
The experimental design of the study is illustrated in Figure 5a. Changes in body weight of colitis-induced mice, colon length, and MPO were evaluated after administration of various treatments in comparison with the control (Figure 5b–d). The dips in the weight loss curve (Figure 5c) correspond to the time points of oral administration following the overnight fasting period. As fasting caused a substantial weight loss of ∼10% across all animal groups, NiMOS formulations were administered every other day to allow for a certain degree of weight normalization to occur. In the healthy control group, the weight stabilized after the last fasting period, so that it leveled out at the original body weight, measured at the beginning of the study. On the other hand, the DSS control group as well as animals receiving blank or scrambled siRNA sequence NiMOS exhibited a severe and relative quick weight loss of 28.6, 24.1, and 33.4% of original body weight on day 12. Additionally, a considerable number of animals demonstrated the classical features associated with acute colitis, including gross rectal bleeding, loose and bloody stools in addition to a general lethargic appearance and scrubby fur. In contrast, test groups administered with CyD1, TNF-α, and TNF-α/CyD1 NiMOS showed significantly less change in their original body weight with respective values of 14.5, 8.7, and 8.8% at the end of the study. Furthermore, their overall health seemed much improved over the colitis-bearing control groups closely resembling healthy animals, with no apparent signs of colitis. These observations are in good agreement with the histological evaluation described above.
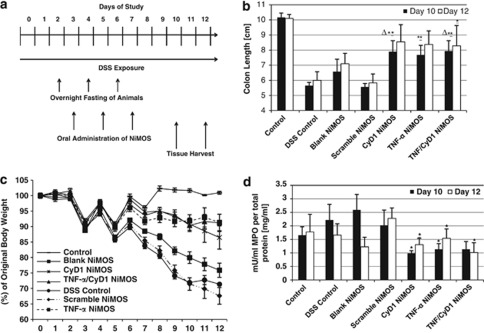
Figure 5.
Macroscopic assessment of anti-inflammatory therapeutic efficacy. (a) Timeline of the study. Animals were continuously exposed to 3.5-wt% dextran sulfate sodium (DSS) throughout the course of the study. Oral administration of short interfering RNA (siRNA)-containing and blank nanoparticles-in-microsphere oral system (NiMOS) was performed on days 3, 5, and 7 followed by tissue harvest on days 10 and 12, as indicated by the medium and long arrows, respectively. (b) Determination of colonic length in control and test groups at both end time points of the study. Silencing NiMOS test groups showed an increase in colon length compared with animals from control groups except the healthy control mice. (c) Percent change of original body weight of Balb/c mice upon continuous exposure to DSS for development of acute colitis model for the duration of the study (12 days). Weight loss was most severe in the DSS control group as well as animals receiving blank or scrambled siRNA sequence NiMOS. The test groups consisting of cyclin D1 (CyD1), tumor necrosis factor-α (TNF-α), and TNF-α/CyD1 siRNA-encapsulating NiMOS exhibited significantly less change in original body weight. (d) Myeloperoxidase (MPO) activity in the large intestine normalized to the total protein content of each sample. Administration of silencing NiMOS led to a reduction in MPO activity in all three test groups on both time points tested whereas elevated levels were measured in the control and DSS control group, as well as groups receiving blank and inactive siRNA sequence-containing NiMOS. Levels represent concentrations obtained from samples on days 10 and 12 of the study (3 and 5 days after administration). Values are expressed as mean±s.d. (n=4–5). ΔP<0.05, vs. DSS control; *P<0.05, vs. Scramble; **P<0.01, vs. Scramble; Statistical comparison was performed on data sets of DSS control vs. TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination NiMOS, and between TNF-α, Cyclin D1, and TNF-α/Cyclin D1 combination vs. Scramble NiMOS group. Only significant differences are shown.
To further evaluate therapeutic efficacy of the treatment, colon lengths were measured and are reported in Figure 5b. Colons were about 45, 35, and 45% shorter in DSS control, blank, and scrambled NiMOS-treated groups, respectively, compared with the healthy control on day 10, which measured a length of ∼10 cm. On the second time point, the length slightly increased in these groups; reduction ranged now between 30 and 41%. In animals treated with silencing NiMOS, an overall increase in colon length compared with colitis control groups was recorded with colon tissue ∼22% shorter than what was measured in healthy animals. On day 12, these values further diminished to about 13% in all test groups and closer to the baseline of healthy mice.
A further marker of inflammatory infiltration was assessed by determination of MPO activity as its occurrence is directly related to presence and activation of neutrophil granulocytes. Overall, administration of active siRNA-containing NiMOS led to a reduction in MPO activity to around 1 mU/ml per total protein content in all three test groups on day 10 with a slight increase up to 1.5 mU/ml on day 12 (Figure 5d). Measured activities in these animals were lower at both test times compared with all colitis-bearing controls and healthy control mice, where an activity of 1.6 and 1.8 mU/ml was detected per total protein content on day 10 and 12, respectively. Highest values of 2.6 mU/ml were observed in blank NiMOS-treated animals on day 10, which was reduced on the second time point. DSS control and inactive siRNA sequence-containing NiMOS-treated groups had comparable activities on day 10, and exhibited reduction and increase, respectively, as the study progressed.
DISCUSSION
Current IBD treatment still faces major limitations associated with its efficacy and safety. Thus, new biological therapeutic strategies are increasingly investigated.4, 10 This present work shows the promise of a novel IBD treatment option by combining the positive aspects of RNAi with the safety of a biodegradable polymeric delivery system to facilitate a localized treatment via oral administration of siRNA rather than using the traditional systemic approach as reported before.15, 21 Mucosal administration including oral delivery has a therapeutic potential for a multitude of diseases and poses several advantages over the traditional method including no relative dilution of the active component (e.g., in the blood stream) due to local delivery enabling the use of lower and fewer doses resulting in overall increased efficacy while minimizing side effects. Other mucosal delivery strategies such as via rectal routes might fail because of non-feasibility in cases of severe diarrhea associated with IBD in addition to the discomfort the disease already causes. Ideally, mucosal therapies should involve a definite mechanism of action with a fast onset and long-lasting effect even after a single administration. As it has many of these characteristics, siRNA has the potential to fulfill this; while duration is dependent on the potency of the siRNA molecule and potential destruction inside the cells.19 However, to fully realize the therapeutic capability of siRNA, suitable delivery systems maximizing cell penetration and prolonged intracellular gene silencing have to be developed. Owing to rapid siRNA degradation, stability and effective delivery still pose great challenges,45 so that development of suitable delivery vehicles are crucial in the success of gene silencing by siRNA.
The study performed by Aouadi et al. was the first to report oral siRNA delivery.22 Hereby, a delivery system based on β-1,3-D-glucan shells originating from baker's yeast was employed after it had undergone a lengthy procedure involving extractions, purification, and loading steps, which enabled the researchers to achieve reduced levels of systemic kinase kinase kinase kinase 4 (Map4k4) in macrophages for attenuation of inflammatory responses.
In this present study, a multicompartmental, biodegradable, and biocompatible polymer-based nanoparticles-in-microsphere delivery system was utilized to encapsulate and safely deliver siRNA within its hydrophilic nanoparticulate core. Type b gelatin was chosen as a matrix for nanoparticles because it promotes physical entrapment of nucleic acids within rather than electrostatic complexation or adsorption on its surface, which could pose difficulties in the release of the nucleic acids, and potentially reduce its efficacy. Release of the payload carrying nanoparticles occurs over time at inflamed sites in the intestine via controlled degradation of the outer layer by action of lipases abundantly present at this location after which they can be endocytosed by enterocytes or other cells at these sites.28, 30 We have successfully incorporated multiple target siRNAs within NiMOS followed by oral administration to DSS-induced colitis mice and evaluation of its potency in silencing of genes involved in inflammation. Specifically, TNF-α was chosen as the primary target cytokine because of its crucial role in the pathogenesis of IBD as the central mediator of inflammation. Furthermore, its pharmacological reduction was demonstrated to diminish severity of the disease in animal models and humans.12 However, it is noteworthy that modulation of DSS-induced colitis by TNF-α reduction is still surrounded by a certain degree of ambiguity. Some studies ascribe a protective role to TNF-α in the initial phase of acute DSS colitis,46 other studies interested in siRNA-mediated gene silencing have reported decreased severity of inflammation after downregulation of this protein.19, 21 The central hypothesis is that downregulation of TNF-α can result in lower levels of other pro-inflammatory markers, which can help to restore the delicate balance between pro- and anti-inflammatory cytokines to allow for alleviation of symptoms and severity of the disease.
Analysis of qPCR on both time points showed decreased levels of murine TNF-α and CyD1. On day 12, mRNA expression of both markers was even further decreased and was smaller than levels recorded in the healthy control group, which can be attributed to the specific RNA silencing effect of NiMOS, and was not observed in remaining colitis-bearing groups. However, on day 12, an overall decrease in TNF-α mRNA expression was seen in all colitis-bearing groups potentially because of a complex interaction of pro-and anti-inflammatory cytokines causing compensatory reactions due to induction of colitis. Results from the ELISA showed a small delay in TNF-α silencing as evidenced by slightly decreased protein expression on day 10 compared with control groups. Much decreased expression of TNF-α and a multitude of other pro-inflammatory cytokines (e.g., IL-1α, IL-1β, IL-5, and IL-17) and chemokines (e.g., monocyte chemotactic protein-1, MIP-1α, and granulocyte macrophage colony-stimulating factor), measured on day 12 and compared with colitis-bearing controls, indicate the success of TNF-α and CyD1 silencing and suggest high potential of both CyD1 and TNF-α siRNA NiMOS for oral administration. The delayed response also reflects the specificity of siRNA encoding for TNF-α, as immune reaction for other markers needs time to occur. The increase in mRNA transcript for both proteins in the scrambled NiMOS group can potentially be ascribed to an unspecific immune response elicited by NiMOS and/or inactive siRNA sequence itself. Owing to the fact that the siRNA sequence was in an unmodified state, it could likely elicit an immune response. To avoid such an off-target effect, modified and as such non-immune modulating siRNA should be included in future studies as a control.
Chemokines are indicators regulating cell infiltration at disease sites, which directly contribute to inflammatory responses. Previous studies demonstrated that systemic administration of MIP-1α can significantly exacerbate colitis in mouse models,47 so that decrease in levels of MIP-1α, as observed in TNF-α NiMOS groups, suggests an improvement of the disease. Overall, it seems possible that lower expression of pro-inflammatory cytokines due to downregulation of TNF-α and CyD1 can result in alleviation of inflammation at the disease site. This is also in very good agreement with the smaller degree of weight loss, increased colon length, lower level of tissue MPO activity, and regenerative tissue histology observed in this group. Simultaneously, elevated levels of pro-inflammatory cytokines in combination with severe weight loss and pathological tissue histology, including loss of goblet cells, infiltration, and destruction of regular mucosal architecture, were detected in non-treated groups and animals treated with blank and scrambled NiMOS.
Because of its upregulation in a number of inflammatory sites, such as in chronic pancreatitis38 and inflamed intestines in IBD,21 CyD1 was chosen as the other target for RNA silencing. The incentive of the study is based on the other hypothesis that encapsulation of multiple siRNA targets, e.g., TNF-α and CyD1, into NiMOS likely result in an even more potent effect on downregulation of pro-inflammatory cytokines, so that combined administration can multiply efficacy of the treatment. Real-time PCR analysis showed significantly reduced levels of CyD1 mRNA expression in CyD1 NiMOS animals on both time points tested compared with all other control or test groups indicating the potency in RNA silencing. Furthermore, the effect of CyD1 silencing was much stronger and even overpowering that of TNF-α silencing, leading to a substantial decrease in expression of pro-inflammatory cytokines and chemokines on both time points tested. Therapeutic efficiency was improved in this group as well as evidenced decrease in weight loss, increased colon length, lower level of MPO, and a tissue histology closely resembling that of healthy control groups.
Furthermore, results from qPCR for TNF-α show that siRNA encoding for CyD1 specifically silences CyD1, as no TNF-α RNA reduction was recorded on day 10. The sequence specificity of RNA silencing with the aid of siRNA particularly encoding for CyD1 or TNF-α is demonstrated by the fact that no silencing of the respective RNA was observed in other control groups. Additionally, the scrambled sequence is irrelevant or inactive as no reduction, aberrant upregulation, or non-specific interferon response was elicited. Although downregulation of pro-inflammatory markers in the TNF-α NiMOS group was slightly delayed, there was an apparent immediate effect in the CyD1 NiMOS group, which demonstrates the potency of CyD1 silencing.
It is noteworthy that combined siRNA treatment caused a stronger downregulation of TNF-α and other pro-inflammatory cytokines than administration with TNF-α siRNA alone, which is a further attribute to the potency of CyD1 silencing. However, this effect was not as pronounced as CyD1 administration alone, which resulted in the strongest reduction of pro-inflammatory markers tested and can be ascribed to a potential dilution effect in the combined treatment containing the same overall amount as the single siRNA. In future studies, more siRNA will be incorporated to maximize and further improve the effect of the dual treatment. Moreover, it can be concluded that the effect of CyD1 silencing was more potent than that of TNF-α indicating the important role of the molecule in inflammation and the potential for further exploration as a target for future therapy strategies. As mentioned before, the nanoparticle in microsphere oral delivery system and the non-modified siRNA sequence used in this study might result in an immune-modulatory effect, which can affect some of the results. Therefore, it is an important control in this study. Although this system needs further improvement and optimization, results presented with this study show the promising nature of NiMOS as a therapeutic treatment option for IBD. Other future projects will involve testing of different time points to study the duration of the RNA silencing effect and addition of targeting moieties to further optimize and improve delivery and efficacy.
Study Highlights

Acknowledgments
This study was supported by a grant (R01-DK080477) from the National Institute of Diabetes, Digestive Diseases, and Kidney Diseases of the National Institutes of Health. We are grateful to Mr Husain Attarwala for assistance in the preparation of the NiMOS, Dr Brian P. Timko in Professor Robert Langer's lab at MIT (Cambridge, MA) for the use of the Coulter particle size analysis instrument, and Dr Dimitry Lukashev from Professor Michail Sitkovsky's lab at Northeastern University for the use of Applied Biosystems 7300 Real-Time PCR System. Furthermore, the assistance of Mr William Fowle from the Nano-Instrumentation Facility at Northeastern University (Boston, MA) with scanning electron microscopy is gratefully acknowledged.
Guarantor of the article: Mansoor M. Amiji, PhD.
Specific author contributions: C.K. and M.M.A. designed research; C.K. performed research; C.K. and M.M.A. analyzed data; and C.K. and M.M.A. wrote the paper.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Ardizzone S, Porro GB. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- Oldenburg B, Hommes D. Biological therapies in inflammatory bowel disease: top-down or bottom-up. Curr Opin Gastroenterol. 2007;23:395–399. doi: 10.1097/MOG.0b013e32815b601b. [DOI] [PubMed] [Google Scholar]
- Stokkers PCF, Hommes DW. Novel biological therapies for inflammatory bowel disease. Curr Treat Options Gastroenterol. 2006;9:201–210. doi: 10.1007/s11938-006-0039-y. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Honda K, Mizutani T, et al. Novel strategies for the treatment of inflammatory bowel disease: selective inhibition of cytokines and adhesion molecules. World J Gastroenterol. 2006;12:4628–4635. doi: 10.3748/wjg.v12.i29.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ. Strategies for targeting tumour necrosis factor in IBD. Best Pract Res Clin Gastroenterol. 2003;17:105–117. doi: 10.1053/bega.2002.0345. [DOI] [PubMed] [Google Scholar]
- van Deventer SJH. New biological therapies in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2003;17:119–130. doi: 10.1053/bega.2003.0360. [DOI] [PubMed] [Google Scholar]
- Papa A, Mocci G, Bonizzi M, et al. Biological therapies for inflammatory bowel disease: controversies and future options. Expert Rev Clin Pharmacol. 2009;2:391–403. doi: 10.1586/ecp.09.12. [DOI] [PubMed] [Google Scholar]
- Hoentjen F, Van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J Gastroenterol. 2009;15:2067–2073. doi: 10.3748/wjg.15.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armuzzi A, De Pascalis B, Lupascu A, et al. Infliximab in the treatment of steroid-dependent ulcerative colitis. Eur Rev Med Pharmacol Sci. 2004;8:231–233. [PubMed] [Google Scholar]
- Mueller C. Tumour necrosis factor in mouse models of chronic intestinal inflammation. Immunology. 2002;105:1–8. doi: 10.1046/j.1365-2567.2002.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, et al. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27:643–651. doi: 10.1038/nbt.1547. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Swiderski P, Herrmann A, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson EB, Blackburn WH, Smith MH, et al. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer. 2010;10:10. doi: 10.1186/1471-2407-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Cristofaro P, Silbermann R, et al. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14:336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Choi B, Hwang Y, Kwon HJ, et al. Tumor necrosis factor alpha small interfering RNA decreases Herpes Simplex virus-induced inflammation in a mouse model. J Dermatol Sci. 2008;52:87–97. doi: 10.1016/j.jdermsci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, et al. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Tesz GJ, Nicoloro SM, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, McCarty D, Fernandes A, et al. Delivery of MDR1 small interfering RNA by self-complementary recombinant adeno-associated virus vector. Mol Ther. 2005;11:523–530. doi: 10.1016/j.ymthe.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–248. doi: 10.1016/j.ijpharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Christian DA, Cai S, Bowen DM, et al. Polymersome carriers: from self-assembly to siRNA and protein therapeutics. Eur J Pharm Biopharm. 2009;71:463–474. doi: 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release. 2006;110:422–430. doi: 10.1016/j.jconrel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS) J Control Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Kaul G, Amiji M. Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies. Pharm Res. 2005;22:951–961. doi: 10.1007/s11095-005-4590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar MD, Amiji MM. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther. 2008;15:1200–1209. doi: 10.1038/gt.2008.67. [DOI] [PubMed] [Google Scholar]
- Bhavsar MD, Amiji MM. Development of novel biodegradable polymeric nanoparticles-in-microsphere formulation for local plasmid DNA delivery in the gastrointestinal tract. Aaps Pharmscitech. 2008;9:288–294. doi: 10.1208/s12249-007-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel C, Amiji M. Oral TNF-α gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Control Release. 2011;150:77–86. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, et al. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, et al. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Alao JP, Gamble SC, Stavropoulou AV, et al. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol Cancer. 2006;5:7. doi: 10.1186/1476-4598-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho JS, Savage K, Lambros MB, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006;19:999–1099. doi: 10.1038/modpathol.3800621. [DOI] [PubMed] [Google Scholar]
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann M, Ishiwata T, Arber N, et al. Increased cyclin D1 expression in chronic pancreatitis. Pancreas. 1998;17:158–162. doi: 10.1097/00006676-199808000-00008. [DOI] [PubMed] [Google Scholar]
- Yang RY, Bie WJ, Haegebarth A, et al. Differential regulation of D-type cyclins in the mouse intestine. Cell Cycle. 2006;5:180–183. doi: 10.4161/cc.5.2.2306. [DOI] [PubMed] [Google Scholar]
- Diez S, de Ilarduya CT. Versatility of biodegradable poly(D,L-lactic-co-glycolic acid) microspheres for plasmid DNA delivery. Eur J Pharm Biopharm. 2006;63:188–197. doi: 10.1016/j.ejpb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Tracy M. Development and scale-up of a microsphere protein delivery system. Biotechnol Prog. 1998;14:108–115. doi: 10.1021/bp9701271. [DOI] [PubMed] [Google Scholar]
- Melgar S, Karlsson A, Michaelsson EM. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- Barbara G, Xing Z, Hogaboam CM, et al. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344–349. doi: 10.1136/gut.46.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative-colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Sioud M. On the delivery of small interfering RNAs into mammalian cells. Expert Opin Drug Deliv. 2005;2:639–651. doi: 10.1517/17425247.2.4.639. [DOI] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeier F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender SL-F, Chance V, Whiting CV, et al. Systemic administration of the chemokine macrophage inflammatory protein 1a exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54:1114–1120. doi: 10.1136/gut.2004.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.