Abstract
OBJECTIVES:
Traditional celiac disease guidelines recommend follow-up endoscopy and duodenal biopsies at 6–12 months after commencing a gluten-free diet (GFD). However, histology may remain abnormal even 1–2 years later. We evaluated the role of capsule endoscopy in patients with celiac disease after treatment with a GFD.
METHODS:
Twelve adult patients with newly diagnosed celiac disease were prospectively enrolled. All patients had baseline symptom assessment, celiac serology (tissue transglutaminase antibody, tTG), and capsule endoscopy. Twelve months after commencing a GFD, patients underwent repeat symptom assessment, celiac serology, upper gastrointestinal endoscopy, and capsule endoscopy.
RESULTS:
At baseline, capsule endoscopy detected endoscopic markers of villous atrophy in the duodenum and extending to a variable distance along the small intestine. On the basis of small bowel transit time, the mean±s.e.m. percentage of small intestine with villous atrophy was 18.2±3.7%. After 12 months on a GFD, repeat capsule endoscopy demonstrated mucosal healing from a distal to proximal direction, and the percentage of small intestine with villous atrophy was significantly reduced to 3.4±1.2% (P=0.0014) and this correlated with improvement in the symptom score (correlation 0.69, P=0.01). There was a significant improvement in symptom score (5.2±1.0 vs. 1.7±0.4, P=0.0012) and reduction in immunoglobulin A–tTG levels (81.5±10.6 vs. 17.5±8.2, P=0.0005). However, 42% of subjects demonstrated persistent villous abnormality as assessed by duodenal histology.
CONCLUSIONS:
After 12 months on a GFD, patients with celiac disease demonstrate an improvement in symptoms, celiac serology, and the extent of disease as measured by capsule endoscopy. Mucosal healing occurs in a distal to proximal direction. The extent of mucosal healing correlates with improvement in symptoms. Duodenal histology does not reflect the healing that has occurred more distally.
INTRODUCTION
Celiac disease is a common disease in the Western world,1, 2 characterized by injury to the small intestine after ingestion of gluten in genetically susceptible individuals. A strict, life-long gluten-free diet (GFD) is the mainstay of treatment. Gluten withdrawal from the diet is associated with improvement in symptoms, disappearance of celiac antibodies and histological recovery.3, 4
After commencing a GFD, symptoms improve within weeks and celiac antibodies may normalize within 6–12 months.4 Guidelines have traditionally recommended that after commencing a GFD, follow-up endoscopy and biopsies should be performed to document the histological improvement and to confirm the clinical remission and dietary compliance.3, 5 Follow-up endoscopy after 6–12 months on a GFD is considered as the gold standard in evaluating dietary compliance.6, 7 However, a significant proportion of adult patients with celiac disease continue to have abnormal duodenal histology even 1–2 years after starting a GFD,4, 8, 9, 10, 11, 12, 13 although much of the data are retrospective and contradictory. Failure of histological improvement is not necessarily due to poor compliance with the GFD.14 Failure to respond to a GFD is associated with long-term complications, such as osteoporosis and lymphoma.15
In the follow-up of patients with celiac disease, commenced on a GFD, there is poor correlation between symptom improvement, normalization of celiac antibodies and histological recovery. Negative celiac serology does not predict histological recovery as assessed by duodenal biopsy.4, 10, 16, 17, 18, 19 Therefore, in documenting mucosal recovery after commencing a GFD, the extent of villous atrophy throughout the small intestine may be more important than the severity of villous atrophy in the duodenum. Simply performing duodenal biopsies at 6–12 months after commencing a GFD may not reflect the extent of mucosal healing that has occurred more distally.
Capsule endoscopy is a safe and painless diagnostic method that provides good quality images of the small intestine, including villi. It has a high sensitivity and specificity in detecting villous atrophy in celiac disease and is useful in assessing the extent of small bowel involvement.20, 21 Follow-up capsule endoscopy at least 6 months after commencing a GFD has demonstrated that the extent of villous atrophy improves, although the correlation with histological recovery as assessed by duodenal biopsies is unknown.21
The aim of this study was to evaluate the role of capsule endoscopy in patients with celiac disease after treatment with a GFD and to determine whether the extent of villous atrophy throughout the small intestine as assessed by capsule endoscopy is more important than the severity of villous atrophy in the duodenum in assessing mucosal recovery.
METHODS
Between July 2006 and April 2009, 22 subjects with positive celiac serology (endomysial antibody or tissue transglutaminase antibody (tTG)) were enrolled in a study examining the role of capsule endoscopy in suspected celiac disease patients with positive celiac serology.20 In total, 8 of these subjects had normal duodenal histology and 14 had duodenal histology demonstrating celiac disease. This study demonstrated that capsule endoscopy is useful in detecting villous atrophy in untreated celiac disease, and patients with positive celiac serology and normal duodenal histopathology are unlikely to have capsule endoscopy markers of villous atrophy. Following this initial study, the 14 subjects with duodenal histology consistent with celiac disease were all commenced on a GFD and 12 months later were invited to participate in this follow-up study and undergo re-evaluation with repeat symptom assessment, celiac serology, upper gastrointestinal endoscopy, and capsule endoscopy. The protocol was approved by the Queen Elizabeth Hospital Ethics and Human Research Committee.
Diagnostic procedures
All subjects gave informed written consent. The five symptoms of fatigue, abdominal bloating, abdominal pain, diarrhea, and weight loss were prospectively recorded and scored as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe), giving a total symptom score out of 15. Blood was taken for celiac serology (immunoglobulin A–tTG).
Subjects underwent upper gastrointestinal endoscopy and duodenal biopsies. Biopsies were interpreted according to the modified Marsh criteria:22, 23 Marsh I consists of raised intraepithelial lymphocytes with >40 lymphocytes per 100 enterocytes, Marsh II consists of raised intraepithelial lymphocytes and crypt hyperplasia, Marsh IIIa partial villous atrophy, Marsh IIIb subtotal villous atrophy, and Marsh IIIc total villous atrophy. Capsule endoscopy was performed within 21 days of the upper gastrointestinal endoscopy. Capsule endoscopy was performed using the Given PillCam SB capsule (Given Imaging, Yoqneam, Israel). The technical aspects have been described elsewhere.24
Analysis of capsule endoscopy
Two investigators, with experience in reading >100 capsule endoscopy studies each, reviewed the capsule endoscopy recordings. The investigators were blinded to the subjects' clinical information and histological findings. If there was any interobserver disagreement, conclusion was reached by consensus. If consensus was unable to be reached, the third investigator reviewed the recording, although this was not required.
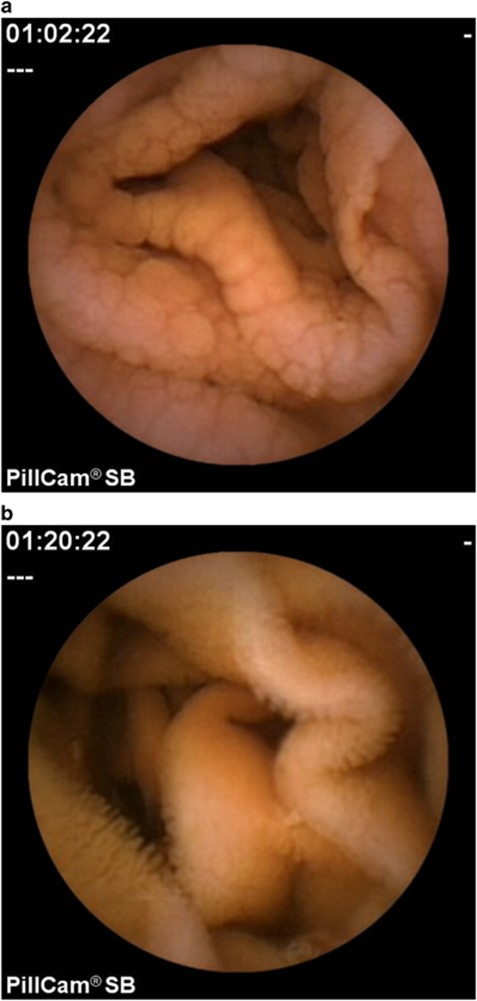
Endoscopic markers of villous atrophy were assessed by analysis of capsule endoscopy findings. These endoscopic markers include loss of mucosal folds, scalloping, mosaic pattern, nodularity, and visible vessels (Figure 1). The extent of small bowel involvement was assessed. The extent of villous atrophy was calculated by measuring the time of villous atrophy as a percentage of total small bowel transit time.
Figure 1.
(a) Capsule endoscopy image in a patient newly diagnosed with celiac disease, demonstrating scalloping and nodularity in the proximal jejunum. (b) After 12 months on a gluten-free diet, the proximal jejunum has returned to normal as demonstrated by the normal villi.
Statistics
Analysis comprised of summary statistics for gender and age, comparative analysis between the findings at capsule endoscopy, and those of histopathology and relationship of capsule endoscopy findings to clinical data. Paired t-tests were used to compare the change in symptom scores, change in histopathology findings and change in the extent of villous atrophy (Marsh grade) during the 12 months follow-up period as well as comparing the histological responders and non-responders. Pearson's correlation was used to calculate the correlation between symptoms and extent of villous atrophy (as measured by capsule endoscopy), and tTG levels. Data values were adjusted for age, gender, and initial values. Quantitative data were expressed as mean±s.e.m.
RESULTS
Of the 14 subjects, who were invited to participate in this follow-up study, 1 was unwilling to participate in the study and a second subject had died from cardiomyopathy while waiting for a cardiac transplant. At the time of the follow-up study, the mean age of the remaining 12 subjects was 45 years, and four were men and eight were women.
Effect of 12 months on a GFD
The 12 patients had a variety of symptoms at baseline, including fatigue, abdominal pain, diarrhea, and anemia. Following 12 months on a GFD, there was a significant improvement in symptom score (5.2±1.0 vs. 1.7±0.4, P=0.0012).
All patients were positive to HLA-DQ2 (10 subjects), HLA-DQ8 (1 subject), or HLA-DQB1*02 (1 subject). At baseline, all were positive to tTG. At the 12-month-follow-up, the tTG antibody level had normalized in 9 of the 12 subjects. Overall, there was a significant reduction in immunoglobulin A–tTG levels (81.5±10.6 vs. 17.5±8.2, P=0.0005) following a GFD.
Capsule endoscopy was performed within 21 days of histological diagnosis of celiac disease and before commencing a GFD. One subject, with Marsh IIIc duodenal histology had a completely normal capsule endoscopy study. The other 11 subjects all had villous abnormalities in the duodenum, extending to a variable distance along the small intestine. On the basis of small bowel transit time, the mean±s.e.m. percentage extent of small intestine involvement with villous atrophy was 18.2±3.7%. Four subjects had erosions in the small intestine (range: 1–4 erosions). These were found in the middle third of the small intestine in one subject, distal third in two subjects, and in both the middle and distal thirds in one subject. After 12 months on a GFD, repeat capsule endoscopy was performed. The cecum was reached in all subjects during the recording time. There was complete interobserver agreement with regards to the extent of villous atrophy. Capsule endoscopy demonstrated significant mucosal healing from a distal to proximal direction, and the percentage of small intestine with villous atrophy was significantly reduced to 3.4±1.2% (P=0.0014). Of the four subjects who had erosions in the small intestine at baseline, one subject had no erosions at follow-up and three subjects still had erosions after 12 months on a GFD (range 1–4 erosions). In addition, a subject with no erosions at baseline developed five erosions in the small intestine at the follow-up capsule endoscopy study. None of the subjects admitted to taking aspirin or anti-inflammatory drugs.
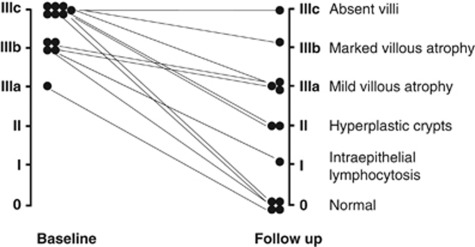
At study enrollment, all subjects had to have villous atrophy (Marsh stage III), to be eligible to participate in the study. Biopsy samples from seven subjects were Marsh IIIc, four were Marsh IIIb, and one was Marsh IIIa. After 12 months on a GFD, subjects underwent repeat endoscopy and 3–6 duodenal biopsy samples were taken for histology. Out of the 12 subjects, 5 were found to still have villous atrophy, at least Marsh IIIa (histological ‘non-responders'), and the other 7 subjects were Marsh II, Marsh I, or Marsh 0 (histological ‘responders') (Figure 2).
Figure 2.
Seven patients showed histological improvement (histological ‘responders') after 12 months on a gluten-free diet. Five did not significantly improve histologically (histological ‘non-responders') and were at least of Marsh grade IIIa after 12 months on a gluten-free diet.
Correlation between improvement in symptoms, serology, histology and extent of villous atrophy
At study enrollment, there was no correlation between the extent of villous atrophy seen at capsule endoscopy and symptom score or celiac serology (tTG) levels. Following 12 months on a GFD, there was a significant reduction in the mean percentage extent of small intestine involvement with villous atrophy as assessed by capsule endoscopy and this correlated with improvement in symptom score (correlation 0.69, P=0.01). However, the reduction in celiac serology (tTG) levels did not correlate with the improvement in symptoms (correlation 0.23, P=0.53) or reduction in the extent of villous atrophy (correlation 0.03, P=0.94).
Comparison of histological ‘responders' vs. ‘non-responders'
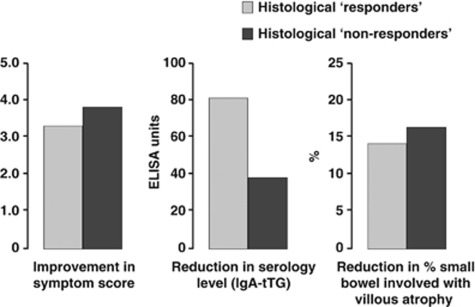
Twelve months after a GFD, 7 of the 12 subjects demonstrated significant histological improvement and the other 5 subjects (42%) demonstrated persistent villous atrophy (at least Marsh IIIa) as assessed by duodenal histology. Both groups demonstrated significant improvement in symptom score, celiac serology (tTG) levels, and reduction in the extent of villous atrophy as assessed by capsule endoscopy. However, both the histological ‘responders' and ‘non-responders' showed similar improvement in symptom score (P=0.58), celiac serology levels (P=0.53) and in extent of villous atrophy (P=0.37), although the sample size is small (Figure 3).
Figure 3.
Both the histological ‘responders' and ‘non-responders' showed similar improvement in symptom score (P=0.58) celiac serology levels (P=0.53), and extent of small bowel villous atrophy (P=0.37) after 12 months on a gluten-free diet. ELISA, enzyme-linked immunosorbent assay; IgA, immunoglobulin A; tTG, tissue transglutaminase.
DISCUSSION
This is the first study to prospectively examine the correlation of baseline and follow-up symptoms, celiac serology, duodenal histology, and capsule endoscopy findings in newly diagnosed celiac disease patients. It demonstrates that after 12 months on a GFD, mucosal healing occurs from a distal to proximal direction and there is a significant correlation between extent of small bowel healing and improvement in symptoms.
A significant proportion of patients (5 out of 12, 42%) demonstrated persistent villous atrophy as assessed by duodenal histology. These five histological ‘non-responders' had similar improvement in symptoms, celiac serology, and reduction in the extent of villous atrophy as assessed by capsule endoscopy, compared with the seven histological ‘responders'. Therefore, follow-up duodenal histology did not reflect the healing that occurred more distally following 12 months on a GFD. This may explain why studies demonstrate that a significant proportion of adult celiac disease patients continue to have abnormal duodenal histology, despite improvements in symptoms and celiac serology, even 1–2 years after starting a GFD.4, 8, 9, 10, 11, 12, 13 We believe that the majority of treated celiac disease patients who demonstrate persistently abnormal duodenal histology, despite improvement in symptoms and celiac serology are incorrectly termed as ‘non-responders' or ‘slow responders'. In our study, these patients all demonstrated significant improvement in the extent of villous atrophy as assessed by capsule endoscopy. The only other study to prospectively follow-up newly diagnosed celiac disease patients, using capsule endoscopy, also demonstrated mucosal healing from a distal to proximal direction, 6–18 months after commencing a GFD, although follow-up duodenal histology or symptoms were not assessed.21
In assessing the response to a GFD, the length of small bowel involved with villous atrophy may be more important than the severity of duodenal villous atrophy. Simply performing follow-up duodenal histology is not an accurate reflection of mucosal healing that has occurred more distally. After 12 months on a GFD, our five histological ‘non-responders' demonstrated significant improvement in symptoms, celiac serology, and the extent of villous atrophy, as assessed by capsule endoscopy, but still had persistent villous atrophy, (at least Marsh IIIa) on duodenal histology. By obtaining biopsy samples from the entire length of small bowel before and after treatment with a GFD, MacDonald et al.25 demonstrated that the distal small bowel rapidly recovered before the proximal small bowel and this may explain the rapid improvement in symptoms, despite persistently abnormal duodenal biopsies. They concluded that symptomatic improvement correlated better with the length of small bowel involved, rather than the severity of duodenal histology.
What is the long-term significance of persistent villous atrophy as assessed by duodenal histology? Holmes et al.26 demonstrated that celiac disease is associated with an increased risk of lymphoma and this risk is reduced with a strict GFD. Kaukinen et al.15 demonstrated that patients with persistent villous atrophy, despite a GFD, may have improvement in symptoms, but are at higher risk of osteoporosis and lymphoma. However, this was a selective sub-population of patients, with histologically refractory celiac disease, comprising 1.9% of their celiac disease database, who had been followed up for a median of 8 years (range 3–30). Rubio-Tapia et al.27 demonstrated in a retrospective study that documented mucosal recovery is associated with a trend towards reduced mortality (P=0.06).
We demonstrated a significant correlation between extent of small bowel healing and improvement in symptoms following a GFD. However, the relevance of this finding is unknown, given that the symptom score is subjective and not validated. We were unable to demonstrate any correlation between the extent of villous atrophy and the symptom score at diagnosis. Using capsule endoscopy, other authors have also been unable to demonstrate any significant association between length of small bowel affected by villous atrophy and severity of symptoms in untreated celiac disease.21, 28 Given that celiac disease patients may present with a wide variety of symptoms, ranging from no symptoms at all to the classical symptoms of diarrhea, steatorrhoea, lassitude, and weight loss, it is plausible that other factors, such as genetic susceptibility, variable immunological tolerance to gluten, or gastrointestinal motility disturbances29 may be important in determining symptoms at diagnosis. Whether patients are diagnosed through family or population screening may also determine the severity of symptoms.
What is the role of capsule endoscopy in celiac disease patients? Capsule endoscopy can visualize the entire length of small intestine. It may have the potential to assess the extent of villous atrophy at diagnosis and to document the extent of small bowel healing after commencing a GFD. It may be useful in assessing dietary compliance and monitoring for complications, such as intestinal lymphoma or ulcerative jejunoileitis.30
In summary, we have demonstrated, using capsule endoscopy, that after 12 months on a GFD, mucosal healing occurs from a distal to proximal direction, and the extent of healing along the length of the small intestine correlated with improvement in the symptom score. Follow-up duodenal histology does not reflect the mucosal healing that occurs more distally. Capsule endoscopy may potentially have a valuable role in the follow-up of people with celiac disease.
Study Highlights

Guarantor of the article: Ilmars Lidums, MBBS, PhD, FRACP.
Specific author contributions: Study concept and design, data collection and analysis, and drafting of the article: Ilmars Lidums; data collection and analysis: Edward Teo; statistical analysis: John Field; technical and intellectual support in the study design and analysis and correction of drafts of the article: Adrian G. Cummins.
Financial support: Given Imaging partially supported the study by supplying Pillcam SB capsules to perform the study, but it does not have any role in the design of the study, analysis, or in writing of the manuscript.
Potential competing interests: None.
References
- Jones R. Coeliac disease in primary care. BMJ. 2007;334:704–705. doi: 10.1136/bmj.39170.410347.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- Ciclitira PJ, King AL, Fraser JS. AGA technical review on Celiac Sprue. American Gastroenterological Association. Gastroenterology. 2001;120:1526–1540. doi: 10.1053/gast.2001.24056. [DOI] [PubMed] [Google Scholar]
- Cummins AG, Alexander BG, Chung A, et al. Morphometric evaluation of duodenal biopsies in celiac disease. Am J Gastroenterol. 2011;106:145–150. doi: 10.1038/ajg.2010.313. [DOI] [PubMed] [Google Scholar]
- Visakorpi JK.Definition of coeliac disease in childrenin Hekkens WTJM, Penna AS (eds).Proceedings of the Second International; Coeliac Symposium, Noordwijkerhout Stenfert Kroese: The Netherlands. Leiden; 197410–16. [Google Scholar]
- Anderson RP. Coeliac disease: current approach and future prospects. Intern Med J. 2008;38:790–799. doi: 10.1111/j.1445-5994.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–S141. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Bardella MT, Velio P, Cesana BM, et al. Coeliac disease: a histological follow-up study. Histopathology. 2007;50:465–471. doi: 10.1111/j.1365-2559.2007.02621.x. [DOI] [PubMed] [Google Scholar]
- Grefte JM, Bouman JG, Grond J, et al. Slow and incomplete histological and functional recovery in adult gluten sensitive enteropathy. J Clin Pathol. 1988;41:886–891. doi: 10.1136/jcp.41.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper AD, Hadjivassiliou M, Hurlstone DP, et al. What is the role of serologic testing in celiac disease? A prospective, biopsy-confirmed study with economic analysis. Clin Gastroenterol Hepatol. 2008;6:314–320. doi: 10.1016/j.cgh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lo W, Memeo L, et al. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet.[see comment] Gastrointest Endosc. 2003;57:187–191. doi: 10.1067/mge.2003.54. [DOI] [PubMed] [Google Scholar]
- Tursi A, Brandimarte G, Giorgetti GM, et al. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: a 2-year prospective study. Endoscopy. 2006;38:702–707. doi: 10.1055/s-2006-925178. [DOI] [PubMed] [Google Scholar]
- Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459–463. doi: 10.1309/EVXT-851X-WHLC-RLX9. [DOI] [PubMed] [Google Scholar]
- Selby WS, Painter D, Collins A, et al. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34:909–914. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]
- Kaukinen K, Peraaho M, Lindfors K, et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237–1245. doi: 10.1111/j.1365-2036.2007.03311.x. [DOI] [PubMed] [Google Scholar]
- Dickey W, Hughes DF, McMillan SA. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am J Gastroenterol. 2000;95:712–714. doi: 10.1111/j.1572-0241.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- Kaukinen K, Sulkanen S, Maki M, et al. IgA-class transglutaminase antibodies in evaluating the efficacy of gluten-free diet in coeliac disease. Eur J Gastroenterol Hepatol. 2002;14:311–315. doi: 10.1097/00042737-200203000-00017. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Mascart F, Mary JY, et al. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–1087. doi: 10.1111/j.1572-0241.2003.07284.x. [DOI] [PubMed] [Google Scholar]
- Valentini RA, Andreani ML, Corazza GR, et al. IgA endomysium antibody: a valuable tool in the screening of coeliac disease but not its follow-up. Ital J Gastroenterol. 1994;26:279–282. [PubMed] [Google Scholar]
- Lidums I, Cummins AG, Teo E. The role of capsule endoscopy in suspected celiac disease patients with positive celiac serology. Dig Dis Sci. 2011;56:499–505. doi: 10.1007/s10620-010-1290-6. [DOI] [PubMed] [Google Scholar]
- Murray JA, Rubio-Tapia A, Van Dyke CT, et al. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol. 2008;6:186–193. doi: 10.1016/j.cgh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue') Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- MacDonald WC, Brandborg LL, Flick AL, et al. Studies of Celiac Sprue. IV. The response of the whole length of the small bowel to a gluten-free diet. Gastroenterology. 1964;47:573–589. [PubMed] [Google Scholar]
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989;30:333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–1420. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondonotti E, Spada C, Cave D, et al. Video capsule enteroscopy in the diagnosis of celiac disease: a multicenter study. Am J Gastroenterol. 2007;102:1624–1631. doi: 10.1111/j.1572-0241.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- Tursi A. Gastrointestinal motility disturbances in celiac disease. J Clin Gastroenterol. 2004;38:642–645. doi: 10.1097/01.mcg.0000118792.58123.c1. [DOI] [PubMed] [Google Scholar]
- Culliford A, Daly J, Diamond B, et al. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55–61. doi: 10.1016/s0016-5107(05)01566-x. [DOI] [PubMed] [Google Scholar]