Abstract
OBJECTIVES:
A growing body of evidence indicates that patients with sessile serrated adenoma/polyp (SSA/P) and traditional serrated adenoma (TSA) are at risk for subsequent malignancy. Despite increasing knowledge on histological categorization of serrated polyps (SPs) data are lacking on the actual prevalence and the association of each SP subtype with advanced colorectal neoplasia.
METHODS:
We prospectively determined the prevalence of different SP subtypes and evaluate the association with synchronous advanced neoplasia in asymptomatic average-risk subjects undergoing first-time colonoscopy. All retrieved polyps were examined by two independent pathologists. Serrated lesions were classified into hyperplastic polyps (HP), SSA/P (without and with cytological dysplasia, SSA/P/DIS), and TSA, and were screened for BRAF and K-ras mutations.
RESULTS:
Among 258 polyps detected in 985 subjects, the proportion of SSA/P and TSA was 8.9% and 1.9% with an overall prevalence of 2.3% and 0.6%, respectively. SSA/Ps were small without significant difference in their location between proximal and distal colon; TSA were predominantly left-sided. BRAF mutation was common in SSA/Ps and K-ras mutation was present in all TSA. Independent predictors of advanced neoplasia were male sex (odds ratio (OR)=2.0, 95% confidence interval (CI) 1.0–4.0), increasing age (OR=4.5, 95% CI 1.5–13.4 for 50–69 years and OR=9.9, 95% CI 3.1–31.5 for >70 years), current smoking (OR=2.0, 95% CI 1.3–6.8), >3 tubular adenoma (OR=3.6, 95% CI 1.9–6.4), and SSA/P (OR=6.0, 95% CI 1.9–19.5).
CONCLUSIONS:
The substantial prevalence of BRAF-mutated SSA/P and the independent association with synchronous advanced colorectal neoplasia in asymptomatic average-risk subjects support the overall impact of the serrated pathway on colorectal cancer (CRC) risk in general population. The endoscopic characteristics of SSA/P emphasize the need of high-quality colonoscopy as a key factor for an effective CRC screening program.
INTRODUCTION
Serrated polyps (SPs) of the colon include hyperplastic polyps (HPs), sessile serrated adenomas/polyps (SSA/P), and traditional serrated adenoma (TSA).1 In contrast to adenomatous glands in conventional polyps, the presence of crypts abnormalities such as ectopic crypt formation and compartmentalization seems to be a common denominator in all SPs and different types can be distinguished based on these events.2
SP nomenclature has been evolving during the last 10 years and even among expert pathologists there is significant inter-observer variability in their classification. Most of SSA/Ps were previously identified as HP before the histological differences between SSA/Ps and HPs were fully appreciated. SSA/P account for up 20% of all serrated lesions and are characterized by architectural distortion, with basal crypt branching and dilation, serration, and horizontal crypt orientation. SSA/P differ from TSA because their flat morphology and lack of cytological dysplasia; other histological features of TSA include villiform configuration, eosinophilic cytoplasm, and ectopic crypt formation.2, 3
SSA/Ps are emerging as a new entity thought to be related to colorectal adenocarcinoma by the “serrated pathway” of colorectal cancer (CRC).4, 5 This pathway is characterized by early BRAF mutation, progressive DNA hypermethylation, and the late development of microsatellite instability. Molecular evidence has demonstrated that SSA/Ps are precursors of CRC, especially microsatellite unstable CRC, which account for approximately 15–20% of sporadic CRCs.4, 5 In addition, there is evidence that the presence of large SP is associated with an increased risk of CRC and advanced colorectal neoplasia.6, 7 Microvescicular HPs (MVHPs) and goblet cell HPs represent different subtypes of HP, although this distinction is not currently used in the standard clinical practice. MVHP share with SSA/P frequent BRAF mutation and CpG island methylator phenotype and have been proposed to precede SSA/P in the serrated carcinogenesis;3 in contrast, goblet cell HPs usually display K-ras mutation and whether they could represent precursor lesions in the serrated pathway is unclear.
A review of the literature showed highly variable results in the prevalence of SP, particularly for SSA/Ps. This variability reflects inconsistent diagnostic criteria, inappropriate histological classification of different subtypes,8 variation in polyp detection rate among endoscopists,9 application of enhancing endoscopic modalities, and population selection criteria.10, 11 Indeed, most of the data come from retrospective studies on archive material without the possibility to define the endoscopic techniques applied or clinical indications. Findings from only one small prospective study using chromoendoscopy are available but these are based on a patient population presenting with large bowel symptoms and alarm signs.11 No prospective data on average risk screening population are available. Defining the true prevalence and the clinical significance of different SP subtypes is crucial to estimate the actual cancer risk posed by these lesions and to formulate appropriate screening and surveillance strategies.
Recent studies have shown that large non-dysplastic SPs, mainly HP and SSA/P, are associated with advanced neoplasia and CRC in screening colonoscopies.6, 7 However, in these studies serrated lesions were not well categorized and therefore the association between different type of SPs and advanced colonic neoplasia could not be determined.
The aims of this study were to prospectively determine the prevalence and characteristics of SP subtypes, define their mutation profile, and the association between each subtype with synchronous advanced neoplasia in a large cohort of asymptomatic average risk subjects undergoing first-time colonoscopy.
METHODS
Subjects and study design
This single center prospective study was conducted at the regional hospital of Feltre, Italy. Ethical approval was obtained by the local institutional review board. The study cohort was composed of consecutive average-risk patients who underwent first-time colonoscopy between June 2007 and December 2008. Patients aged ≥50 years were recruited as part of the regional screening program; patients aged <50 years were recruited in collaboration with community general practitioners who provided information about the study. Eligibility was assessed by the study investigators in a preliminary consultation and those who gave their consent were included. Exclusion criteria included alarm symptoms of disease of the lower gastrointestinal tract (recent onset of abdominal pain that normally would require medical evaluation, change in bowel habits), unexplained weight loss, occult anemia, rectal bleeding, and previous colonoscopy performed for any reason; minor abdominal discomfort and/or bloating were not considered as exclusion criteria. Patients with family history of CRC or adenomatous polyps, personal history of inflammatory bowel disease, hereditary non-polyposis CRC, familial and hyperplastic polyposis, previous colonic resection were excluded. Additional exclusion criteria were an unsatisfactory colonic preparation (“poor”, see below) precluding a complete examination. Smoking status was categorized in three groups, never, former, and current smoker.
Colonoscopy and colonic neoplasms
Polyethylene glycol solution (4 l) or sodium phosphate solution (90 ml) was taken orally 24 h before the procedure as standard bowel preparation. The quality of bowel preparation was rated by the endoscopist as “excellent,” “good,” “fair,” or “poor” based on prespecified criteria (excellent: no or minimal stool residue, small amount of clear fluid; good: minimal semisolid stool, large amount of fluid; fair semisolid stool debris cleared after suctioning; poor: solid stool and semisolid debris that cannot be cleared). All procedures were performed by four experienced gastroenterologists. Midazolam, meperidine, and propofol were used for sedation. All the procedures were performed using high-definition colonocopes (Olympus CF-H180AL with 1080i HDTV signal, Olympus Italy, Milan, Italy). Total time, withdrawal time, and examination time (withdrawal time minus time needed for polypectomy, suctioning if applicable) were recorded. Colonic neoplasms were characterized according to the Paris Endoscopic Classification (protruding sessile (Is) and protruding pedunculated (Ip) neoplasms; non-protruding superficial elevated (IIa), flat (IIb), and depressed (IIc) neoplasms);12 endoscopic images were reviewed and criteria agreed among endoscopists before study initiation. In this study, all the non-protruding neoplasms (IIa, IIb, IIc) were categorized as “flat”.
It was also agreed among the investigators that all polyps, even diminutive (size <5 mm), should be removed and retrieved. For purposes of the analysis, the junction of the splenic flexure and the descending colon, as determined by the endoscopist, defined the border between the proximal and the distal colon.
The instrument used for polypectomy was either the cold snare or electrocautery, depending on the size of the polyp and personal preference of the endoscopist. The size of the polyp was estimated with the use of the open-biopsy forcep method. Each polyp resected was submitted in a separate jar for pathological evaluation and molecular characterization.
Pathological findings
All colorectal polyps were reviewed by two experienced gastrointestinal pathologists (RB and GS). CRC included invasive cancer and Tis (carcinoma in situ, intramucosal) category of TNM classification. Conventional adenomas were classified as tubular, tubulovillous, and villous adenoma with low or high grade of dysplasia according to the degree of dysplasia. An advanced neoplasm was defined as a large tubular adenoma (at least 1 cm in maximal diameter), a polyp with villous features, a polyp with high-grade dysplasia, or a cancer. SPs were classified as HP (subdivided in MVHP and goblet cell-rich-type HP according to the mucin content), SSA/P, SSA/P with cytological dysplasia, and TSA using the system described by Snover.3 HPs were characterized by saw-toothed appearance in the upper part of the crypt and normal proliferative zone to the base of the crypt. SSA/Ps were distinguished from HPs by the presence of exaggerated serration, horizontally oriented and dilated crypt bases, and increased proliferation. TSA showed villiform protuberant configuration, serrated epithelial architecture, ectopic crypt foci, eosinophilic cytoplasm in association with features of conventional dysplasia (nuclear crowding and enlargement, pencillate nuclei). SSA/P with cytological dysplasia (SSA/P/DIS) comprised a sessile serrated architecture component and a dysplastic component resembling conventional adenomas. Where there was initial agreement on the diagnosis by both pathologists, the polyp was included without further review. In cases with an initially discordant diagnosis, the polyps were reviewed by the two pathologists together and discussed in an attempt to reach consensus. During the consensus review, special attention was given to the distinctive appearance of extensively dilated and/or horizontally oriented basal crypts, and proliferation pattern. When at least two relevant architectural features were confirmed, the polyp was classified as SSA/P, otherwise as HP.
Molecular characterization
DNA extraction
Genomic DNA was extracted from 5–8 μm thick formalin-fixed, paraffin-embedded sections as previously described.13 Briefly, after deparaffinization and microdissection, lesional tissue was scraped and digested in 50–100 μl buffer containing 50 mM Tris-HCl pH 8.5, 1 mM EDTA pH 8.0, 0.5% (v/v) Tween–20, and proteinase K to a final concentration of 3.3 mg/ml. Digestion was carried out by incubating samples at 55 °C for 16 h with gentle agitation. After proteinase K inactivation at 95 °C for 10 min and centrifugation at room temperature for 10 min, genomic DNA in the supernatant was recovered and then quantified using a conventional spectrophotometer.
BRAF and K-ras mutational analysis
BRAF (V600E) and K-ras codon 12 and 13 mutational analysis were performed using 200–500 ng of the recovered DNA for PCR amplification. For assessing BRAF codon 600 status, a 174-bp fragment was amplified using the following primer pair: 5′-TGTTTTCCTTTACTTACTACACCTCA-3′ (forward) and 5′-TTGAGGCTATTTTTCCACTGA-3′ (reverse) (Sigma, St Louis, MO). A total of 50 cycles were performed at an annealing temperature of 56 °C. The amplification program consisted of five cycles of 1 min each step followed by 45 cycles of 30 s each step. The annealing temperature was 56 °C. Whenever the amplification product was not visible after agarose gel electrophoresis and ethidium bromide staining, a second PCR round of 40 cycles was performed using 1 μl of the amplification mixture. For K-ras codon 12 and 13, a fragment of 190 bp was generated in the first PCR reaction, using the following primer pair: 5′-TTAACCTTATGTGTGACATGTTCT-3′ (forward) and 5′-CAAGATTTACCTCTATTGTTGGAT-3′ (reverse) (Sigma, Poole, UK). PCR cycling was the same as that used for BRAF analysis. If the amplification product was not well visible after the electrophoretic run, 1–2 μl of the PCR product was used as a template to amplify a 171-bp nested fragment using the forward primer from the first reaction together with the 5′-TGGATCATATTCGTCCACAA-3′ reverse primer. The annealing temperature was set to 55 °C for both PCR rounds. PCR products were purified from a low-melting agarose gel by means of the Qiaquick gel Extraction kit according to the manufacturer's protocol (Qiagen, Crawley, West Sussex, UK). Purified DNA samples were then sequenced (BMR Genomics, Padova, Italy).
Statistical analysis
Descriptive statistics showing the percentage distribution of the different characteristics by the explanatory variables were produced. Medians and ranges are presented for continuous variables and proportions for categorical variables. Differences between variables were assessed by χ2 analysis. All P values were two-sided and considered significant when <0.05. Predictors of SP detection and the association between SPs, adenomatous polyps, and advanced neoplasia were assessed by odds ratio (OR) with 95% confidence intervals (CI). ORs were adjusted for gender, age (as a continuous variable), smoking habit, SP subtype through multiple logistic regression equations. Data management and analysis were performed using SPSS version 15 (SPSS, Chicago, IL).
RESULTS
A total of 985 individuals (median age 53 years, interquartile range 43–66 years) who met the inclusion criteria were consecutively enrolled. Among these 38% were men. The mean total procedure time was 23.1±6.4 min, examination time 6.9±1.3 min. Colorectal polyps were detected in 26.1% subjects. A total of 263 polyps were found and resected, of which 258 (98%) had complete histology; 155 (60%) were adenomas and 39 (15%) had advanced histology. When considering the morphology, 92 adenomas (59.3%) were classified as protruding (5.8% peduncolated, 53.5% sessile) and 63 adenomas (40.6%) as flat (39.3% superficial elevated, 1.3% flat). SP was the second most common polyp type, comprising 40% (102 polyps) of all polyps. One polyp was classified as hamartomatous (0.3%). Invasive CRC was diagnosed in three patients (0.3%). The adenoma detection rate (ADR) in the whole population was 14.8%, whereas in subjects aged more than 50 years the ADR was 20.3% (ADR in male 28.3%, ADR in female 16.4%).
Prevalence and characteristics of SPs subtypes
The overall prevalence of SSA/P and TSA was 2.3% and 0.6%, respectively. Characteristics of polyps and categorization according to histology are shown in Table 1. Among SPs, 66.6% (n=68) were diagnosed as HP, 27.4% (n=28) as SSA/P, and 5.8% (n=6) as TSA. Forty-seven SP were detected in the proximal colon (18.2% of all polyps) with a proximal SP detection rate of 10.4%. Inter-observer agreement measured by the Cohen's k score for SP was strong (k=0.83, P<0.001). As expected, the main source of inter-observer variability was in distinguishing SSA/P and HP. SSA/Ps account for 10.8% of all polyps; their endoscopic appearance was protruding sessile in 13 (46.4% Figure 1) and non-protruding (superficial elevated or flat) in 15 (53.5%). SSA/Ps were predominantly small (96.4% of lesions were ≤10 mm) with a mean size of 5.8±3.1 mm, similar to conventional adenoma (mean size 6.3±1.3 mm) but larger than HP (mean size 4.3±2.3 mm). SSA/Ps were larger in the proximal colon compared with distal colon (mean size 5.9±1.3 mm vs. mean size 3.8±2.2 mm, P<0.001). Five SSA/P (18%) showed cytologic dysplasia. TSAs were located predominantly in the left colon (75%) and appear as protruding lesions (Figure 2), without differences in size between proximal and distal locations (P=0.5). Male gender (OR=1.9, 95% CI 1.1–4.8), increasing age (OR=4.7, 95% CI 1.0–21.8 for 50–69 years, OR=7.3, 95% CI 1.5–35.4 for 70 or more years) and current smoking (OR=3.9, 95% CI 1.4–10.6) were associated with the presence of either SSA/P or TSA. Increasing age and current smoking but not gender and former smoking were found to be independent predictors of SSA/P by multivariate analysis (Table 2). When the analysis included only patients with SSA/P and those with normal colonoscopy, we found a higher risk of SSA/P associated with current smoking (OR=5.2, 95% CI 1.5–17.8). Moreover, when compared with patients with tubular adenoma, current smokers were nearly three times more likely to have an SSA/P (OR=2.8, 95% CI 1.3–8.5). Male gender, age, current smoking, patients with three or more tubular adenomas, and the presence of at least one either SSA/P or TSA but not HP were significantly associated with synchronous advanced adenoma. Multivariate analysis confirmed that gender, age, current smoking, number of tubular adenoma are significant risk factors and showed that only the presence of SSA/P, but not TSA, was independently associated with synchronous advanced adenoma (OR=6.0, 95% CI 1.9–19.5; Table 3); this relationship was confirmed even when SSA/P/DIS were removed (OR=5.8, 95% CI 2.3–14.8). Harboring an SSA/P was associated with a higher risk for advanced adenoma in the proximal compared with distal colon (advanced adenoma proximal (OR=8.05, 95% CI 1.8–79) and distal (OR= 2.8, 95% CI 2.05–29.5)). The proximal location of SSA/P showed a stronger association with synchronous advanced adenoma (proximal SSA/P (OR=18, 95% CI 2.91–11.1), distal SSA/P (OR=9.4, 95% CI 2.8–31)).
Table 1. Prevalence, location, and size of polyps according to histology.
| Histology | Subjects (% n=220) | Number (n=258) |
Location (%) |
Size (%) |
|||
|---|---|---|---|---|---|---|---|
| Proximal | Distal | ≤5 mm | 6–9 mm | ≥10 mm | |||
| Hyperplastic | 45 (4.5) | 68 | 29 (42) | 39 (58) | 61 (89.8) | 6 (8.8) | 1 (1.4) |
| MVHP | 20 (2.0) | 28 | 10 (36) | 18 (64) | 22 (78.6) | 5 (17.8) | 1 (3.6) |
| GCHP | 25 (2.5) | 40 | 19 (45) | 21 (55) | 38 (95) | 2 (5) | 0 |
| SSA/P | 23 (2.3) | 28 | 16 (63.6) | 12 (36.4) | 21 (75) | 6 (21.4) | 1 (3.6) |
| TSA | 5 (0.5) | 6 | 2 (25) | 4 (75) | 2 (33.3) | 3 (50) | 1 (16.7) |
| Adenoma | 146 (14.8) | 155 | 66 (42.5) | 89 (57.5) | 103 (66.5) | 34 (21.9) | 18 (11.6) |
| Tubular | 116 (11.8) | 125 | 56 (44.8) | 69 (55.2) | 93 (74.4) | 24 (19.2) | 8 (6.4) |
| Tubulovillous | 28 (2.8) | 28 | 8 (28.6) | 20 (71.4) | 10 (35.8) | 9 (32.1) | 9 (32.1) |
| Villous | 2 (0.2) | 2 | 1 (50) | 1 (50) | 0 | 1 (50) | 1 (50) |
| Hamartomatous | 1 (0.1) | 1 | 0 | 1 (100) | 1 (100) | 0 | 0 |
GCHP, goblet cells hyperplastic polyp; MVHP, microvescicular hyperplastic polyp; SSA/P, sessile serrated adenoma/polyp; TSA, traditional serrated adenoma.
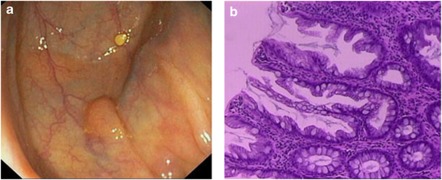
Figure 1.
Endoscopic (a) and histological features (b) of sessile serrated adenoma/polyp (SSA/P). SSA/Ps were predominantly small protruding sessile lesions, with a layer of adherent mucus on the surface. Histology shows serrated features with horizontally oriented and dilated crypt bases (boot-shape).
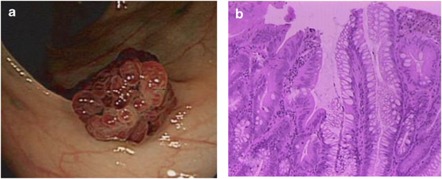
Figure 2.
Endoscopic (a) and histological features (b) of traditional serrated adenoma (TSA). TSAs were usually larger than 5 mm and showed protruberant growth pattern. Histological appearance showing serrated epithelial architecture in association with features of conventional dysplasia (nuclear crowding and pencillate nuclei).
Table 2. Relative risk estimates of SSA/P.
| MLR-OR (95% CI) | |
|---|---|
| Sex | |
| Female | 1 |
| Male | 2.2 (0.9–5.7) |
| Age (years) | |
| <50 | 1 |
| 50–69 | 5.8 (1.3–26.8) |
| ≥70 | 9.3 (1.9–45.4) |
| Smoke | |
| No | 1 |
| Former | 2.6 (0.7–9.3) |
| Current | 4.9 (1.5–16.4) |
CI, confidence interval; MLR, multiple logistic regression; OR, odds ratio; SSA/P, sessile serrated adenoma/polyp.
Table 3. Predictive factors for advanced colorectal neoplasia.
| MLR-OR (95% CI) | |
|---|---|
| Sex | |
| Female | 1 |
| Male | 2.0 (1.0–4.0) |
| Age (years) | |
| <50 | 1 |
| 50–69 | 4.5 (1.5–13.4) |
| ≥70 | 9.9 (3.1–31.5) |
| Smoke | |
| No | 1 |
| Former | 1.4 (0.5–4.0) |
| Current | 2.0 (1.3–6.8) |
| Adenomatous polyps | |
| n>3 | 3.6 (1.9–6.4) |
| SSA | |
| No | 1 |
| Yes | 6.0 (1.9–19.5) |
CI, confidence interval; MLR, multiple logistic regression; OR, odds ratio; SSA, sessile serrated adenoma.
Mutation profile of SP subtypes
The molecular features of SPs are summarized in Table 4. Mutations of BRAF (V600E) were found in 69.5% of SSA/P, in all SSA/P/DIS and 40% of HP but in none of the TSAs. K-ras mutation was present in all TSA. BRAF and K-ras mutations were mutually exclusive in both SSA/Ps and HPs. There was no relationship between BRAF mutation and either SSA/P and SSA/P/DIS location or size. In contrast, BRAF mutation was more common in left-sided MVHPs.
Table 4. BRAF and K-ras mutation in different subtypes of serrated polyps.
|
BRAF mutation (%) |
K-ras mutation (%) |
|||
|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | |
| MVHP | 4/10 (40) | 10/18 (55.5) | 0 | 0 |
| GCHP | 0 | 0 | 2/19 (10.5) | 3/21 (14.2) |
| SSA/P | 10/14 (71.4) | 6/9 (66.6) | 0 | 0 |
| SSA/P/DIS | 3/3 (100) | 2/2 (100) | 0 | 0 |
| TSA | 0 | 0 | 1/2 (50) | 3/4 (75) |
GCHP, goblet cells hyperplastic polyp; MVHP, microvescicular hyperplastic polyp; SSA/P/DIS, sessile serrated adenoma with cytological dysplasia/polyp; TSA, traditional serrated adenoma.
DISCUSSION
A primary goal of CRC screening is the detection and removal of premalignant lesions, which may lead to cancer prevention. Among different subtypes of SPs, SSA/P and TSA have been recently recognized as the precursors of up to 20% of sporadic CRC through the serrated-carcinoma pathway. Despite the increasing knowledge on the categorization of SPs and the progress in defining the molecular pathogenesis, data on the real prevalence of SSA/P and TSA among average-risk individuals are very limited. This study represents the first prospective evaluation of the prevalence of different SP subtypes in a large cohort of average-risk asymptomatic subjects. We found that SSA/P account for 8.9% of all polyps and 22.1% of SP, whereas only 1.5% of all polyps were TSA. The reported proportion of SSA/P among all polyps in other retrospective series ranged from 0.8 to 3.9%.9, 10, 14 This variation can be explained by differences in the selected population,9, 11 in diagnostic threshold,15 and in the only moderate inter-observer concordance.8 SSA/Ps have been underdiagnosed for many years because of their resemblance to HPs and recent data have shown that the proportion of SSA/Ps that were initially unrecognized and misdiagnosed as HPs ranged from 5 to 22%. Moreover, information regarding the prevalence of SP from large retrospective, cross-sectional studies often derived from database recorded before the criteria for SSA/P had been established, without the possibility of performing an analysis for different SP subtypes. The higher concordance rate for SP subtypes reported in our study can be explained by the pre-study agreement on diagnostic criteria and the subspecialty training of our gastrointestinal pathologists.8 In our population, the prevalence of SSA/P was 2.3%. Before this, only one retrospective study has included patients undergoing colonoscopy for the indication of average risk CRC screening and reported a lower proportion of SSA/P and TSA (1.3% and 0.3% of all polyps detected, respectively) with an overall prevalence of SSA/P of 0.6%.9 However, as acknowledged by the authors, pathology misclassification, variation in polyp detection rate among endoscopists and use of standard white-light endoscopy represent limitations, which may have underestimated the prevalence of SPs. These aspects can explain the substantial lower rate of SSA in their work compared with our study.
The percentage of SSA/P found in our population is similar to that by Spring et al.11 who described a small group of consecutive patients. However, in that study, subjects were referred to colonoscopy for lower gastrointestinal-related symptoms, or because a family history of CRC and surveillance after CRC or polyp removal. We enrolled average-risk individuals at their first colonoscopy, excluding patients with symptoms and other factors that might have affected the detection rates of colonic neoplasms. Thus, our findings are more directly applicable to screening cohorts and may have potential implications on the level of awareness endoscopists should have on both the importance of these distinct lesions and the willingness to remove them effectively.
Earlier studies have suggested that SSA/Ps are large in size and occur predominantly in the proximal colon.1, 2, 11, 14, 16, 17 Although we confirmed that proximal lesions appear larger,11 SSAs emerged to be predominantly small with more than half being diminutive (≤5 mm). Interestingly, in our study, SSAs were equally located between left and right colon. These findings are consistent with those of Lu et al.18 The differences with previous literature can be explained in part by the fact that several studies often excluded small specimens from the analysis due to inadequate material, poor orientation, or lack of agreement in the distinction between HP and SSA/Ps. As the diagnosis of SSA/P relies importantly on the morphology of basal crypts, sample quality and orientation have a great impact on the detection of the characteristic morphological features. It has been shown that a correct classification of SP is difficult to apply in tangentially cut, small, and fragmented lesions.16 In our study, small size polyps were removed by cold snare resection and when analyzed, the vast majority of these samples contained well-oriented full-thickness mucosa.
Differences in the detection of SSA/Ps among studies could also be related to different skills of endoscopists and adherence to a “leave no polyp behind” approach, particularly in the presence of diminutive polyps. Recently, Hetzel et al.9 have shown that detection rate of SSA/P and HP varied significantly among endoscopists; this variability together with the inter-pathologist discrepancy in SPs classification could be responsible for the increasing trend of SSA/P detection over the time shown in this study. In our prospective study, the aim was to remove all the polyps detected, including small and diminutive polyps in distal colon. All procedures were performed by experienced examiners; withdrawal time and ADR did not vary among endoscopists and met the targets established as quality indicators for screening colonoscopy.19
One limitation of our study is that chromoendoscopy was not applied. SSA/Ps usually appear flat to sessile with a soft smooth-appearing surface and are known to be best detected by such technique.19 However, we have used high-definition technology, which has been shown to improve the diagnostic yield for superficially elevated, flat lesions.20
A second limitation is that we have an overall low ADR. Data on ADR by high-definition white-light technology in average risk individuals are available from only one recent study by Kahi et al.21 Compared with this study, our lower ADR can be explained by differences in our study population which included only white, predominantly female and younger subjects without family history of CRC or adenoma. Thus, a third limitation could be the applicability of our findings only in population with such geographical and epidemiological characteristics. However, when patients younger than 50 years were removed, ADR was 28.3% in male and 16.4% in female, meeting the recommended threshold.19 Recently, the detection rate of SPs has been suggested as a new measure of quality in colonoscopy.22 Indeed, in our study the overall proportion of colonoscopies with the detection of at least one proximal serrated lesion was similar (10.4 vs. 13%) to what has been recently reported by Kahi et al.23 in a large cohort of average risk patients.
Three recent studies have found an association between the presence of large non-dysplastic SPs with synchronous advanced neoplasia and proximal CRC.6, 7, 24 It is important to note that in all these studies different subtypes of SP were not categorized by histology and the precise association between each type of SP and advanced neoplasia could not be determined. A more recent retrospective study reported that individuals who harbor SSA/P and adenomas have more advanced pathology including cancer compared with patients with adenoma only;25 however, patients with increased risk or with family history were also present in the study cohort, SSA/P cases included large HP and serrated lesions were not histologically reviewed.26 In our study, we were able to demonstrate for the first time that among average-risk subjects harboring one SSA/P but not HP or TSA there was an increased likelihood of synchronous advanced adenoma. Moreover, we found that proximal SSA/Ps are associated with higher rates of synchronous advanced adenomas, compared with patients with distal SSA/Ps, irrespective of their size. An inevitable drawback of our study, including screening average-risk population, is that we could not provide further information about the risk with synchronous CRC because of the small proportion of patients affected.
The molecular characterization of SP in our study showed the presence of BRAF mutation in a significant proportion of distal small SSA/P. Our results agree with two other recent studies where the presence of BRAF mutation was confirmed in diminutive and distal SSA/P.10, 27 The occurrence of BRAF mutation in diminutive left-sided SSA/Ps is consistent with its observation in the earliest steps of the serrated pathway such as serrated hyperplastic aberrant crypt foci.28 MVHPs exhibit morphological similarities with SSA/P, share high frequency of BRAF mutations and are commonly detected in the distal colon.3, 29, 30 We have confirm the presence of BRAF mutation in nearly half of MVHPs regardless their location. These findings suggest that MVHP and SSA/P probably represent a spectrum of related lesions along the serrated neoplasia pathway. In the present study, current smoking was independently associated with the presence of SSA/P of any size or location and we confirmed this relationship when patients with SSA/P were compared with those with normal colonoscopy and with tubular adenomas. These findings are consistent and support what recently found by Anderson et al.31 in a retrospective, case–control study. BRAF mutation has been linked with smoking and observed in early precursors lesions such as serrated aberrant crypt foci.32 Our findings are consistent with earlier reports 33, 34 and provide more data to suggest that smoking may have a role in the development of SPs. However, based on the current data, it is not possible to predict or estimate the incremental effect of smoking on the progression of non-dysplastic SPs to advanced lesions.
In our cohort, the less representative subtypes of SP were TSAs (0.6%), consistent with the present literature,10, 11, 14 and K-ras mutation was found in the majority of TSAs. The morphological characterization of TSAs has been only recently redefined and associated with an alternate pathway of sporadic colorectal carcinogenesis, molecularly characterized by K-ras mutation and with left-side location.35 However, in our study TSA did not significantly influence the risk of synchronous advanced adenoma; this lack of association may be related to the small number of reported cases.
Our results may have potential implications for CRC screening. Given the malignant potential of the various types of SP, with a conversion rate at least as great as that shown for conventional adenomas, the clinical relevance of detection and removal of SSA/P and TSA could be reflected in issues such as high-quality baseline screening colonoscopy and surveillance. Based on our findings, any SSA/P smaller than 10 mm should be completely excised and followed up endoscopically. In order to define appropriate SP surveillance guidelines, we need to improve criteria to stratify the risk. Longitudinal and follow-up studies will clarify how the SP subtype and location influence this risk and also how the importance of polyp number compares to that for conventional adenoma.
As average-risk individuals account for almost 75% of patients with CRC, detection and removal of precursor lesions in this population provide the best opportunities for improving cancer prevention effectiveness. Knowledge of prevalence of SSA/P and TSA in this population will allow us to estimate the true rate of malignant conversion of these lesions.
Our findings are that 14% of all polyps had serrated features with malignant potential, the majority of which were small and spread throughout the colon, provides strong evidence that all such lesions should be completely removed and underline the need for high-quality colonoscopy as a key factor for an effective CRC-screening program.
The appropriate surveillance intervals following the removal of such serrated lesion has yet to be established. Until longitudinal studies have been conducted on progression and recurrence of serrated lesions defined by standardized nomenclature, it seems advisable to provide follow-up surveillance to patients with SSA/P and TSA as the current guidelines for conventional adenoma.
Study Highlights
Guarantor of the article: Andrea Buda, MD, PhD.
Specific author contributions: Conceiving and planning the study, interpreting data, and writing the manuscript: Andrea Buda; conducting the study, performing endoscopy, interpreting data, assisting with the writing of the manuscript: Manuela De Bona; performing the molecular characterization: Isabella Dotti; collecting and database insertion of data: Eva Zabeo; statistical analysis: Pierluca Piselli; performing the pathological examination: Renzo Barbazza; performing the endoscopy: Angelo Bellumat and Flavio Valiante; performing the molecular characterization: Ermanno Nardon; critical review of the manuscript: Chris Probert; interpreting the data: Pignatelli Massimo; pathological examination: Stanta Giorgio; contributing to interpretation of findings: Giacomo Carlo Sturniolo; planning and conducting the study, performing endoscopy: Michele De Boni. All authors approved the final draft submitted.
Financial support: This study was supported by “Arianna, il filo della solidarietà” foundation, Feltre, Italy.
Potential competing interests: None.
References
- Snover DC, Jass JR, Fenoglio-Preiser C, et al. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- Young J, Jenkins M, Parry S, et al. Serrated pathway colorectal cancer in the population: genetic consideration. Gut. 2007;56:1453–1459. doi: 10.1136/gut.2007.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139:1503–1510. doi: 10.1053/j.gastro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695–702. doi: 10.1038/ajg.2008.166. [DOI] [PubMed] [Google Scholar]
- Farris AB, Misdraji J, Srivastava A, et al. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol. 2008;32:30–35. doi: 10.1097/PAS.0b013e318093e40a. [DOI] [PubMed] [Google Scholar]
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- Carr NJ, Mahajan H, Tan KL, et al. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–518. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58 (Suppl 6:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- Bonin S, Hlubek F, Benhattar J, et al. Multicentre validation study of nucleic acids extraction from FFPE tissues. Virchows Arch. 2010;457:309–317. doi: 10.1007/s00428-010-0917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in non-syndromic patients. Histopathology. 2010;56:581–588. doi: 10.1111/j.1365-2559.2010.03520.x. [DOI] [PubMed] [Google Scholar]
- Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice. Virchows Arch. 2007;450:613–618. doi: 10.1007/s00428-007-0413-8. [DOI] [PubMed] [Google Scholar]
- Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- Lu FI, van Niekerk de W, Owen D, et al. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010;34:927–934. doi: 10.1097/PAS.0b013e3181e4f256. [DOI] [PubMed] [Google Scholar]
- Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42–47. doi: 10.1053/j.gastro.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Kahi CJ, Anderson JC, Waxman I, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301–1307. doi: 10.1038/ajg.2010.51. [DOI] [PubMed] [Google Scholar]
- Rex DK, Hewett DG, Snover DC. Detection targets for colonoscopy: from variable detection to validation. Am J Gastroenterol. 2010;105:2665–2669. doi: 10.1038/ajg.2010.330. [DOI] [PubMed] [Google Scholar]
- Kahi CJ, Hewett DG, Norton DL, et al. Prevalence variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–1502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- Vu HT, Lopez R, Bennett A, et al. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54:1216–1223. doi: 10.1097/DCR.0b013e318228f8a9. [DOI] [PubMed] [Google Scholar]
- Snover DC. Sessile serrated adenoma/polyp of the large intestine: a potentially aggressive lesion in need of a new screening strategy. Dis Colon Rectum. 2011;54:1205–1206. doi: 10.1097/DCR.0b013e318228f8bc. [DOI] [PubMed] [Google Scholar]
- Yachida S, Mudali S, Martin SA, et al. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33:1823–1832. doi: 10.1097/PAS.0b013e3181b6da19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007;67:3551–3554. doi: 10.1158/0008-5472.CAN-07-0343. [DOI] [PubMed] [Google Scholar]
- O′Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterol Clin North Am. 2007;36:947–968. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25–46. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol. 2011;45:694–699. doi: 10.1097/MCG.0b013e318207f3cf. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Pleau DC, Rajan TV, et al. Increased frequency of serrated aberrant crypt foci among smokers. Am J Gastroenterol. 2010;105:1648–1654. doi: 10.1038/ajg.2010.109. [DOI] [PubMed] [Google Scholar]
- Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- Young J. Serrated neoplasia of the colorectum and cigarette smoking. Gastroenterology. 2008;135:323–324. doi: 10.1053/j.gastro.2008.03.080. [DOI] [PubMed] [Google Scholar]
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]