Abstract
OBJECTIVES:
Obesity is linked to increased mortality from many cancer types, and esophageal adenocarcinoma (EAC) displays one of the strongest epidemiological associations. The aims of this study are to dissect molecular pathways linking obesity with EAC and to determine if obesity is linked to increased aggressiveness of this disease.
METHODS:
Affymetrix microarrays identified altered signaling pathways in an EAC cell line following coculture with visceral adipose tissue or isolated adipocytes from viscerally obese EAC patients (n=6). Differentially expressed genes were subsequently investigated in patient tumor biopsies by quantitative reverse transcriptase PCR and examined with respect to obesity status, tumor biology, and patient survival.
RESULTS:
Visceral adipose tissue induced expression of genes involved in epithelial mesenchymal transition (EMT), plasminogen activator inhibitor (PAI)-1, and transcription factor SNAI2, in an EAC cell line. In EAC patient tumor biopsies from obese patients, we noted elevated expression of these genes, together with reduced expression of epithelial marker E-cadherin. SNAI2 was associated with EAC prognosis.
CONCLUSIONS:
Expression of EMT genes, PAI-1 and SNAI2, was elevated in tumors of obese EAC patients, and SNAI2 was associated with poor survival. Genes deregulated in obesity and associated with prognosis may represent potential targets for treatment stratification of obese EAC patients.
INTRODUCTION
Overweight and obesity are linked to increased incidence and mortality from multiple cancer types,1 with esophageal adenocarcinoma (EAC) displaying one of the strongest epidemiological associations.2 The incidence of this aggressive cancer has risen by 50% in the last 15 years,3 mirroring the alarming rise in obesity, and over 40% of cases of EAC are now estimated to be directly attributable to excess adiposity.4 EAC is a highly lethal disease due in part to early malignant tumor cell dissemination,5 indicating that pathways of tumor invasion and metastasis may be of particular importance. In this study, we sought to investigate if these pathways are modulated by obesity in EAC.
In addition to its role as a proteolysis inhibitor, plasminogen activator inhibitor 1 (PAI-1) has a direct role in cell attachment and detachment to vitronectin through competition with cell surface integrins and uPAR for vitronectin binding sites, thus modulating tumor cell migration.6 Treatment of tumor cells with exogenous PAI-1 has been found to induce a dose-dependent decrease in vitronectin binding and a corresponding increase in migratory and invasive capacity, with these effects abrogated by an antibody against PAI-1.7 PAI-1 is highly induced in SNAIL, SNAI2, and E47-transfected epithelial cells undergoing epithelial mesenchymal transition (EMT)8 and is the most prominently induced transcript in the TGF-β1 and EGF-induced “scatter” phenotype in malignant human keratinocytes,9 indicating an important role for this extracellular matrix remodeling protein in the process of EMT. In addition, PAI-1 is a circulating adipokine, elevated in obesity, and with a direct role in the development of insulin resistance.10 Inhibition of PAI-1 could therefore be particularly relevant in viscerally obese patients with raised circulating levels of this adipokine.
The process of EMT is required for tumor cells to acquire a migratory phenotype via altered expression of cell adhesion molecules and reorganization of the cytoskeleton. SNAI2 is a well-established transcriptional regulator of this process acting often, but not always, via repression of the epithelial cell adhesion molecule E-cadherin.11 In addition, expression of SNAI2 in adipose tissue is required for adipogenesis, and SNAI2-overexpressing mice display adipocyte hypertrophy, while adipose tissue mass is reduced in SNAI2-deficient mice.12 SNAI2 signaling thus represents a common pathway in both adipose tissue and tumor cells, indicating its potential as a novel therapeutic target in obesity-fuelled cancers.
The aim of this study was to investigate molecular mechanisms underlying the association of obesity and EAC, based on the hypothesis that adipose tissue would differentially affect the expression of pro-tumor pathways in EAC cells. Affymetrix microarray technology determined the effect of coculturing EAC cells with adipose tissue and adipocytes, and the expression of genes identified using this coculture system was subsequently investigated in patient tumor biopsies with respect to obesity status, tumor biology, and survival. For the first time, we report increased expression of SNAI2 and PAI-1 in the tumors of obese EAC patients, with SNAI2 significantly associated with poor prognosis. The identification of gene expression pathways responsible for obesity-fuelled cancers could present novel therapeutic targets for stratified treatment of obese cancer patients.
METHODS
Patient cohort
All patients gave informed consent for use of their tissue in this study and the study obtained ethical approval from St James's Hospital Ethical Review Board (Table 1). EAC patients were separated into viscerally obese and non-obese categories by waist circumference (WC) according to the International Diabetes Federation criteria,13 by body mass index (BMI) ≥30 kg/m2, or by visceral fat area (VFA). There currently exists no standard cutoff value for VFA and cancer risk. We therefore used a VFA ≥130 cm2, established as a cutoff for metabolic risk of CVD14, 15 and previously published by our group in EAC patients.16
Table 1. Patient demographics.
| Training cohort | Validation cohort | Patient cohort | |
|---|---|---|---|
| No. of patients | 6 | 12 | 39 |
| Sex (male), n (%) | 6 (100) | 10 (83.3) | 39 (100) |
| Age at surgery, mean (range) | 58.2 (49–63) | 60.2 (55–69) | 64 (48–81) |
| Diagnosis | EAC | EAC | EAC |
| BMI (kg/m2), mean (range) | 30.3 (25.4–39) | 30 (22–39) | 26 (19–39) |
| WC (cm), mean (range) | 107.3 (98–130) | 103.8 (81–130) | 95.5 (77–130) |
| VFA (cm2), mean (range) | 209.9 (120.9–297.6) | 206 (42.5–383.8) | 154 (13–384) |
| Metabolic syndrome, n (%) | 2 (33.3) | 7 (58.3) | 13 (33.3) |
| Neoadjuvant therapy, n (%) | 3 (50) | 6 (50) | 16 (41) |
BMI, body mass index; VFA, visceral fat area; WC, waist circumference.
Tissue preparation
Tumor biopsies from EAC patients were immediately taken from fresh tumor tissue following surgical resection and processed by a designated biobank technician, using standardized operating procedures in order to procure consistently high-quality biospecimens, as previously published.17 Visceral adipose tissue specimens were excised at the commencement of surgery and immediately transported in transport buffer (phosphate-buffered saline, glucose (0.1%), and gentamycin (0.05 mg/ml)) for processing. To generate adipose tissue-conditioned medium, an adapted protocol from Fried and Moustaid-Moussa18 was used. Briefly, visceral adipose tissue was minced, washed in phosphate-buffered saline, and cultured in M199 medium (ISIS, Wicklow, Ireland) (containing 0.05 mg/ml gentamycin) at a ratio of 5 g adipose tissue to 10 ml medium for 72 h, aliquotted, and stored at −80 °C until analyzed. Visceral adipose tissue (10 g) was digested with collagenase buffer (2 mg/ml Type II collagenase (Sigma, Wicklow, Ireland), 4% bovine serum albumin (Sigma) in Krebs–Ringer bicarbonate buffer, ISIS, Wicklow, Ireland, pH 7.4), and extracted adipocytes were ceiling cultured at a dilution of 250,000 cells per well as previously described.19
Cell culture
The OE33 cell line, derived from EAC (ECACC, Health Protection Agency Culture Collections, Salisbury, UK), was maintained in RPMI medium supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), and 10% fetal calf serum at 37 °C and 5% CO2. The OE33 cell line was used at 70% confluency and serum starved in RPMI medium containing 0.5% fetal calf serum for a period of 16 h before each experiment. For NuGO array nugohs1a520180 microarrays and validations, OE33 cell line was seeded at 50,000 cells per well insert and cultured in a non-contact transwell system (Corning, New York, NY, USA) containing visceral adipose tissue, adipocytes, or control M199 medium for a period of 24 h.
Microarray analysis
RNA was extracted from tumor cell lines using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. RNA purity was assessed and complementary DNA was synthesized using random hexamers (Invitrogen, Carlsbad, CA, USA). Gene expression in the OE33 cell line following non-contact coculture with visceral adipose tissue and adipocytes from viscerally obese EAC patients (n=6) was examined using NuGO array nugohs1a520180 microarrays, custom designed by the Nutrigenomics Organisation (NuGO; www.nugo.org) to include a number of nutrition- and obesity-related genes. We used the ‘entrezg' version 14.0.0 annotation from the MBNI custom cdf database, reflecting the latest remapping of Affymetrix probes based on current data in the NCBI database (http://brainarray.mbni.med.umich.edu). All RNA samples processed on Affymetrix microarrays had an RNA integrity number >6.7 and a 260/280 ratio >1.65. Following microarray hybridization, all arrays were assessed for quality using a set of standard metrics (including RNA degradation), as implemented in the AffyQCReport library in R;20 all arrays passed quality control checks. Arrays were then background corrected and normalized using the GCRMA function,21 and then filtered to remove genes in the lowest 10% of variance using a variance filter. More detailed information on the microarray experiments is available online via the GEO public database (Accession ID: GSE36841).
Quantitative reverse transcriptase PCR
RNA from original array samples and RNA from an independent cohort of cocultured samples was used for array validations, carried out using the following primer sets with dual labeled probes (Metabion, Munich, Germany): PAI-1 (fw: 5′-GCCATGGAACAAGGATGAGATC-3′, rv: 5′-GCCCTGGACCAGCTTCAGA-3′, probe: 5′-Fam-CCACAGACGCGATCTTCGTCCAGC-Tamra-3′), and primer/probe sets (Applied Biosystems, Carlsbad, CA, USA): SPP1 (Hs00959010_m1), SNAI2 (Hs00161904_m1), and E-cadherin (Hs01023894_m1). For gene expression analysis of tumor biopsies, RNA was extracted from tumor biopsies using Qiagen RNeasy Mini Kit, according to the manufacturer's instructions and analyzed using quantitative reverse transcriptase polymerase chain reaction (qPCR), as above.
Statistical analysis
Analysis of alterations in gene expression in tumor cells following coculture with adipose tissue and correlation between clinicopathological features and gene expression was carried out using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). A P-value of <0.05 was taken as a statistically significant association between two variables. Affymetrix microarrays were analyzed using predefined scripts and flexible programming with the R software (www.r-project.org) environment for statistical computing. The LIMMA library in R was used to select significantly altered single genes,22 with a significance threshold of α=0.05 (adjusted for multiple testing). Single-gene changes were ranked by log-fold change (logFC). Gene set enrichment analysis (GSEA) was used to examine the changed pathway expression; the gsealmPerm function from the GSEAlm library23 was modified to test for bidirectional enrichment in KEGG pathways.24 The association of gene expression on patient survival was determined by Kaplan–Meier survival analysis with gene expression values divided into low- and high-expression groups using the median. Significance was determined using the log-rank (Mantel–Cox) test. Cox regression multivariate analysis was subsequently conducted using forward likelihood ratio on SPSS (IBM, New York, NY, USA). Variables tested on univariate analysis included gene expression, pathological tumor and node stage, lymphatic and hematological dissemination, and tumor differentiation. Variables with a significance level of <0.1 were included in the multivariate model; these were gene expression, pathological tumor and node stage.
Results
Coculture with adipose tissue alters the expression of pro-tumor pathways in an EAC cell line
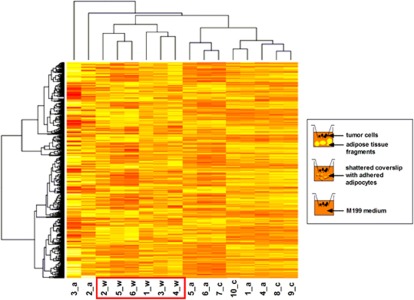
Differential gene regulation in OE33 cells following coculture with visceral adipose tissue, adipocytes, or control medium was investigated using the NuGO array nugohs1a520180 microarray. Principle component analysis and hierarchical clustering both showed that OE33 cocultured with adipose tissue (n=6) segregated together, separately from OE33 cocultured with adipocytes (n=6) and with control medium (n=4), according to gene expression profile (Figure 1). OE33 cocultured with adipocytes and control medium clustered together, demonstrating that adipocyte coculture had little transcriptomic effect on OE33 cells, in comparison with coculture with whole adipose tissue. Indeed, there were no significant gene alterations in OE33 cells following coculture with adipocytes, indicating that global alterations in gene expression are predominantly mediated not by adipocytes but by secreted factors from the adipose tissue stromal vascular fraction. Pathway analysis showed cytokine signaling, focal adhesion/EMT, and glycolysis to be the top three significantly upregulated pathways in OE33 following coculture with adipose tissue, compared with coculture with control medium (adjusted P<0.05) (Table 2). The top 10 differentially expressed genes (with adjusted P<0.1) in each pathway were subsequently ranked by logFC; 9 belonging to the glycolysis pathway were listed, as only this number of genes were altered (adjusted P<0.1). There was a total of five genes with a logFC >2; two belonged to cytokine signaling pathways (CXCL5 and PPBP), and three belonged to focal adhesion/EMT pathways (SPP1, PAI-1, and SNAI2) (Table 2). We subsequently investigated altered expression of these latter three genes, established to have direct and important roles in focal adhesion, EMT, and tumor dissemination, in order to elucidate the relationship between obesity and increased cancer mortality. Integrin-binding signaling molecule, secreted phosphoprotein 1 (SPP1), underwent a 4.9 log-fold change (adjusted P<0.04), EMT transcription factor, SNAI2, underwent a 2.5 log-fold change (adjusted P<0.01) while marker of tumor invasion and metastasis, PAI-1, underwent a 2.9 log-fold change (adjusted P<0.07). Gene expression alterations of these transcripts, in addition to laminin-5 subunits, LAMC2 and LAMB3, and integrin subunit α3 (ITGA3), were measured by qPCR, in both the training cohort (n=6) and in a larger, independent cohort (n=12), which confirmed the findings of the microarray (see Supplementary Figure 1 online).
Figure 1.
Clustering heatmap analysis of genes differentially expressed in esophageal adenocarcinoma (EAC) cells following coculture with whole adipose tissue, adipocytes, or control medium in the highest 5% of variance identified by Affymetrix microarray analysis. Numbers represent patient number, w=OE33 following coculture with whole adipose tissue, a=OE33 following coculture with adipocytes, and c=OE33 following coculture with control M199 medium.
Table 2. Top differentially expressed genes (ranked by logFC) in top three significantly upregulated pathways in esophageal adenocarcinoma OE33 following coculture with adipose tissue from viscerally obese EAC patients.
| Pathway | LogFC | Gene | Description |
|---|---|---|---|
| Cytokine signaling* | 6.941 | CXCL5*** | Chemokine (C–X–C motif) ligand 5 |
| 4.370 | PPBP | Pro-platelet basic protein (chemokine (C–X–C motif) ligand 7) | |
| 1.871 | OSMR** | Oncostatin M receptor | |
| 1.580 | SOCS3* | Suppressor of cytokine signaling 3 | |
| 1.501 | IL1B* | Interleukin 1, beta | |
| 1.082 | CCL7* | Chemokine (C–C motif) ligand 7 | |
| 0.873 | IL4R** | Interleukin 4 receptor | |
| 0.840 | IL1R1 | Interleukin 1 receptor, type I | |
| 0.424 | IL7R* | Interleukin 7 receptor | |
| 0.351 | STAT5A | Signal transducer and activator of transcription 5A | |
| Focal adhesion/EMT* | 4.919 | SPP1* | Secreted phosphoprotein 1 |
| 2.867 | SERPINE1 | Serpin peptidase inhibitor E1 (plasminogen activator inhibitor-1) | |
| 2.496 | SNAI2* | Snail homolog 2 | |
| 1.353 | LAMB3* | Laminin, beta 3 | |
| 1.255 | ITGA3** | Integrin, alpha 3 | |
| 1.149 | LAMC2* | Laminin, gamma 2 | |
| 0.656 | SERPINB6* | Serpin peptidase inhibitor, B6 | |
| 0.504 | ITGA6* | Integrin, alpha 6 | |
| 0.410 | LAMC1 | Laminin, gamma 1 | |
| 0.269 | PXN | Paxillin | |
| Glycolysis* | 1.555 | PDK4* | Pyruvate dehydrogenase kinase, isozyme 4 |
| 1.406 | ALDOC | Aldolase C, fructose-bisphosphate | |
| 1.110 | PFKFB3* | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | |
| 1.071 | HK1** | Hexokinase 1 | |
| 1.036 | PDK1* | Pyruvate dehydrogenase kinase, isozyme 1 | |
| 0.716 | PFKP* | Phosphofructokinase, platelet | |
| 0.697 | PKM2* | Pyruvate kinase, muscle | |
| 0.589 | LDHC | Lactate dehydrogenase C | |
| 0.350 | ALDOA | Aldolase A, fructose-bisphosphate |
EMT, epithelial mesenchymal transition.
Genes differentially expressed between OE33 following coculture with adipose tissue or control M199 medium (*P<0.05, **P<0.01, ***P<0.0001).
Coculture with adipose tissue induces gene expression of PAI-1 and SNAI2 in EAC cell lines
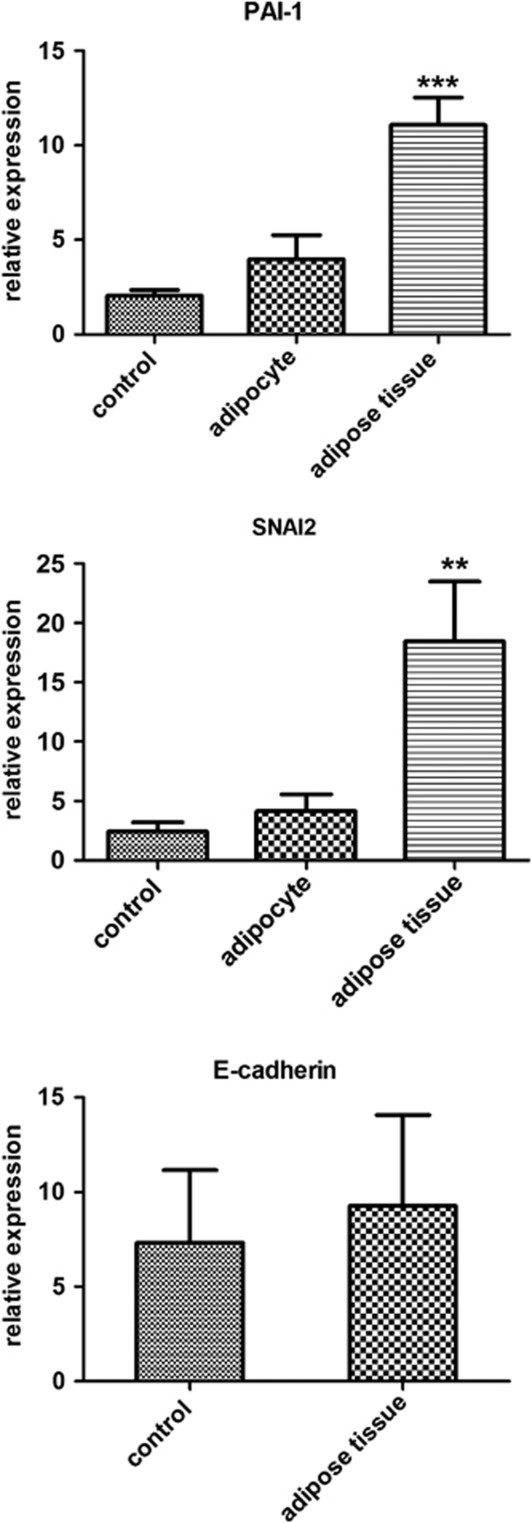
Early tumor dissemination is a characteristic hallmark of EAC, contributing to the dismal five-year survival of this aggressive disease. Pathways in tumor invasion and metastasis could therefore present ideal targets for therapeutic intervention. Investigation in a separate cohort (n=12) using qPCR demonstrated that coculture with adipose tissue induced gene expression of SPP1 by almost 2,000 fold (P<0.02), PAI-1 by 10 fold (P<0.0001), and the expression of SNAI2 by over 7 fold (P<0.004) (Figure 2). In order to establish if increased expression of these genes was associated with transition from the epithelial to mesenchymal phenotype, we measured the expression of epithelial cell marker, E-cadherin. We found that coculture with adipose tissue did not alter the expression of SNAI2 target, E-cadherin, in OE33 cells (Figure 2).
Figure 2.
Coculture of esophageal adenocarcinoma OE33 with visceral adipose tissue induces expression of genes involved in epithelial mesenchymal transition, PAI-1 and SNAI2. Statistical analysis was performed using paired Student's t-test (**P<0.01, ***P<0.001 between control and whole adipose tissue treatment groups).
Tumor gene expression of members of the EMT pathway, PAI-1 and SNAI2, are increased with obesity, while expression of epithelial cell marker, E-cadherin, is decreased
In order to assess whether tumor EMT is affected by the presence of excess adipose tissue in vivo, we compared expression of SPP1, SNAI2, PAI-1, and E-cadherin in tumors of obese and non-obese EAC patients. Gene expression of SPP1 was not associated with VFA (P<0.7), WC (P<0.9), or BMI (P<0.5), and was therefore dropped from further analysis (data not shown). When patients were grouped by VFA, gene expression of PAI-1 was increased fivefold (P<0.05) in tumors from obese (n=26) relative to non-obese (n=13) EAC patients (Table 3). This significant effect was also observed when patients were grouped by WC (P<0.05) and by BMI (P<0.01). Gene expression of EMT transcription factor, SNAI2, was increased twofold in tumor biopsies from obese relative to non-obese EAC patients. This trend approached statistical significance when patients were grouped by BMI (P<0.07), but not when patients were grouped by measures of visceral obesity, WC (P<0.4), and VFA (P<0.3) (Table 3), indicating that elevated SNAI2 expression is associated with overall obesity rather than with visceral adiposity. Gene expression of SNAI2 target, E-cadherin, was decreased almost twofold in tumor biopsies from obese relative to non-obese EAC patients. This association reached statistical significance when patients were grouped by WC (P<0.05) and approached significance when patients were grouped by VFA (P<0.07) (Table 3). However, there was no association when patients were grouped by BMI (P<0.3), indicating that, in contrast to SNAI2, E-cadherin expression is associated with visceral obesity rather than overall adiposity. There was no association between elevated SNAI2 expression and reduced E-cadherin expression on pathological tumor or node stage, tumor differentiation, or measures of tumor dissemination. Neither was there any significant association between increased PAI-1 expression and these markers of aggressive tumor biology; however, a pathologically advanced T stage was accompanied by a twofold increase in PAI-1 expression (P<0.17) (Table 3). As PAI-1 is elevated in obesity, this trend indicates a potential molecular mechanism by which excess adiposity may be associated with advanced tumor stage.
Table 3. PAI-1, SNAI2, and E-cadherin expression in esophageal adenocarcinoma patients with respect to obesity status and tumor differentiation.
| PAI-1 expression, mean (s.e.m.) | P | SNAI2 expression, mean (s.e.m.) | P | E-cadherin expression, mean (s.e.m.) | P | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 0.009 | 0.0693 | 0.2218 | |||
| Non-obese <30 | 1.014 (0.299) | 25.623 (3.549) | 4.546 (0.847) | |||
| Obese ≥30 | 3.691 (1.195) | 61.411 (24.515) | 3.01 (0.656) | |||
| WC (cm) | 0.0397 | 0.3097 | 0.0447 | |||
| Non-obese < 94 cm | 0.74 (0.164) | 26.659 (4.976) | 5.423 (1.232) | |||
| Obese ≥94 cm | 2.833 (0.808) | 46.731 (2.533) | 2.999 (0.468) | |||
| VFA (cm2) | 0.031 | 0.2437 | 0.0602 | |||
| Non-obese <130 cm2 | 0.529 (0.149) | 23.839 (4.134) | 5.49 (1.415) | |||
| Obese ≥130 cm2 | 2.775 (0.74) | 46.655 (14.145) | 3.155 (0.442) | |||
| Tumor diff. | 0.2782 | 0.2809 | 0.5852 | |||
| Well/moderate | 1.517 (0.492) | 73.584 (26.049) | 4.413 (0.639) | |||
| Poor | 2.467 (0.727) | 43.901 (9.297) | 3.817 (0.865) | |||
| PT | 0.1632 | 0.1796 | 0.4062 | |||
| T0–T1 | 1.569 (0.671) | 95.248 (49.316) | 3.158 (0.803) | |||
| T2–T3 | 2.634 (0.748) | 48.598 (12.171) | 4.301 (0.656) | |||
| PN | 0.5241 | 0.7256 | 0.2184 | |||
| N0 | 1.569 (0.671) | 64.717 (28.545) | 3.168 (0.569) | |||
| N1 | 2.166 (0.557) | 54.382 (15.01) | 4.581 (0.792) | |||
| Lymph inv. | 0.3262 | 0.5117 | 0.6864 | |||
| Yes | 2.26 (0.606) | 55.127 (15.53) | 3.90 (0.581) | |||
| No | 1.504 (0.561) | 77.695 (37.67) | 4.45 (1.566) | |||
| Venous inv. | 0.4164 | 0.4169 | 0.8639 | |||
| Yes | 2.299 (0.606) | 48.616 (15.974) | 3.963 (0.569) | |||
| No | 1.504 (0.561) | 72.395 (26.047) | 4.161 (1.103) | |||
| Perineural inv. | 0.6496 | 0.4895 | 0.3857 | |||
| Yes | 2.338 (0.985) | 44.257 (13.09) | 4.195 (0.949) | |||
| No | 1.9 (0.466) | 66.371 (20.284) | 3.403 (0.433) | |||
BMI, body mass index; lymph inv., lymph involvement; perineural inv., perineural involvement, expression relative to a calibrator sample (non-obese patient); pN, pathological node stage; pT, pathological tumor stage; tumor diff., tumor differentiation status; venous inv., venous involvement; VFA, visceral fat area; WC, waist circumference.
Elevated PAI-1 and SNAI2 expression is associated with poor patient prognosis
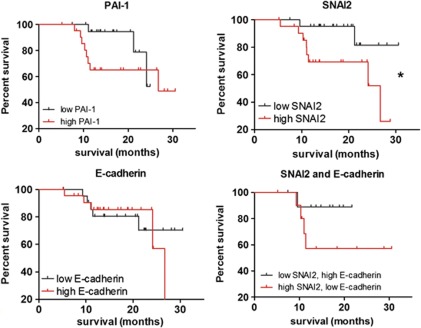
EAC is an aggressive disease associated with high patient mortality due to early malignant tumor dissemination.5 In addition, there is evidence to suggest that obesity is linked to increased cancer mortality.1 It is therefore of great interest that the gene expression of markers of EMT, SNAI2, E-cadherin, and PAI-1, was found to be altered in obese EAC patients. Furthermore, using the log-rank (Mantel–Cox) test, we found that elevated SNAI2 expression was negatively associated with patient survival (P<0.05), identifying this transcriptional regulator of EMT as an obesity-associated prognostic indicator in EAC (Figure 3). Using Cox regression multivariate analysis, we found that SNAI2 approached significance as an independent prognostic indicator, after adjustment for potential confounders (Hazard ratio: 0.221 (95% CI: 0.047–1.045), P=0.057). Notably, SNAI2 expression was more strongly associated with EAC survival than established prognostic indicators pathological tumor and node stage (P=0.38, P=0.128, respectively), indicating that our sample size is insufficient to detect a significant association with survival using the Cox regression multivariate analysis method. Elevated tumor levels of these genes in obesity may represent a molecular pathway by which excess adiposity could negatively impact on EAC prognosis. In contrast, decreased expression of SNAI2 target, E-cadherin, showed no association with patient survival. As SNAI2 is an important transcriptional repressor and negative regulator of E-cadherin, a combined survival analysis investigating high SNAI2 with low E-cadherin was subsequently conducted. Although no association with survival was found (P<0.2), there was some separation between survival curves following 1 year indicating that it may be worthwhile to reanalyze these data after a longer follow-up period (Figure 3). Alternatively, the mechanism of action of SNAI2 in EAC may be independent of E-cadherin repression; we found no correlation between the expression of transcriptional repressor, SNAI2, and expression of its target cell adhesion molecule and epithelial marker, E-cadherin (r2=0.024, P=0.37).
Figure 3.
Elevated tumor gene expression of SNAI2 is associated with poor patient prognosis. Gene expression was separated into above (n=22) and below (n=23) the median expression value, and Kaplan–Meier survival analysis was performed. Statistical analysis was performed using the log-rank (Mantel–Cox) test (*P<0.05).
DISCUSSION
Esophageal adenocarcinoma (EAC) has one of the strongest epidemiological associations with obesity,2 although mechanisms remain largely unknown. In this study, we hypothesized that adipose tissue would differentially affect the expression of pro-tumor pathways in EAC cells. To investigate this hypothesis, we used an ex vivo explant model to identify altered pathways in EAC cells following coculture with adipose tissue or isolated adipocytes from matched obese EAC patients, relative to control medium. We found that coculture with adipose tissue induced the most pronounced gene alterations in EAC cells, implying that the stromal vascular fraction, comprising a range of cell subsets including endothelial and immune cells, is the primary mediator of this pro-tumor effect.
Using Affymetrix microarrays, we identified glycolysis, cytokine signaling, and focal adhesion/EMT to be the top upregulated pathways in EAC cells following coculture with adipose tissue explants, implicating these pathways in obesity-associated cancer. The reliance of tumor cells on glycolysis even in aerobic conditions is termed the “Warburg effect”, a fundamental hallmark of cancer associated with aggressive disease and poor prognosis,25, 26 and adipose tissue-mediated induction of this pathway in tumor cells indicates a mechanism by which obesity promotes cancer mortality. Chronic, low-grade inflammation, a characteristic feature of both obesity27 and cancer,28 may have an important role in their epidemiological association. Circulating levels of CXCL5 and IL-1β are elevated in obesity, and exert pro-inflammatory effects linked to the development of insulin resistance and associated pathologies.29, 30 Upregulation of these cytokines in tumor cells by adipose tissue, combined with upregulation of cytokine receptors, oncostatin M receptor, IL-1R, IL-7R, and IL-4R, highlights a mechanism whereby obesity could support pro-inflammatory signaling pathways in cancer. Finally, with tumor metastasis accounting for the majority of cancer-related deaths, and signaling through the focal adhesion pathway necessary for cells to acquire a motile, mesenchymal-like phenotype via the process of EMT,31 the induction of these pathways in tumor cells by adipose tissue is of profound interest for the elucidation of mechanisms underpinning obesity-associated cancer mortality. We observed a sevenfold increase in expression of EMT transcriptional regulator, SNAI2, together with a 10-fold increase in the expression of PAI-1, leading us to examine the differential expression of these genes in EAC patients.
Gene expression of PAI-1 was significantly elevated fivefold in the tumors of obese EAC patients, suggesting a function for this gene in obesity-fuelled cancer. PAI-1 has a role in tumor cell adhesion, and TGF-β-induced PAI-1 expression in OE33 has been demonstrated to correlate with an invasive cell phenotype in vitro.32 Furthermore, PAI-1 is a circulating adipokine, which is elevated in obesity, and high-fat diet-induced obese mice deficient in PAI-1 have reduced adipose tissue mass and increased insulin sensitivity,33 supporting a potential role for modulation of levels of PAI-1 activity in the treatment of obesity and its comorbidities. We found that PAI-1 tumor gene expression was elevated in patients with more advanced pathological tumor stage, suggesting a mechanism by which PAI-1 is associated with poor prognosis in breast,34, 35 gastric,36 rectal,37, 38 and colorectal carcinoma.39
There is preliminary evidence that SNAI2, implicated in malignant tumor dissemination via initiation of EMT,11 may partially exert its functional effect via upregulation of PAI-1 expression, and this has been demonstrated in canine epithelial cells.8 SNAI2 overexpression is associated with poor prognosis in breast,40, 41 esophageal squamous cell,42 and colorectal carcinoma,43 and we have demonstrated it to be a prognostic indicator in EAC. In addition, this study found that gene expression of SNAI2 was increased twofold, while gene expression of its target, E-cadherin, was decreased twofold, in the tumors of obese EAC patients, implying a role for EMT in obesity-fuelled cancer. Indeed, it has been shown in vitro that overexpression of SNAI2 repressed E-cadherin and induced expression of mesenchymal markers vimentin and fibronectin in the OE33 EAC cell line,44 and that these expression alterations corresponded to a gain in tumor cell proliferation and invasion.45 Interestingly, coculture with adipose tissue-conditioned medium significantly enhanced EAC cell proliferative, migratory, and invasive capacity in vitro,46 indicating the functional effect of these adipose tissue-induced gene alterations. Increased SNAI2 expression, together with nuclear localization of this transcription factor, has been observed in EAC relative to Barrett's esophagus,44 indicating the significance of SNAI2 and the process of EMT in the malignant progression of this disease, as well as in EAC tumor invasiveness. SNAI2 overexpression is not always associated with E-cadherin repression, and this has been demonstrated in breast40, 41 and esophageal squamous cell carcinoma.47 We report that gene expression levels of E-cadherin were not associated with prognosis, indicating that E-cadherin-independent pathways may be important in SNAI2-mediated pathogenesis in EAC.
In summary, we have demonstrated a novel association of PAI-1 and SNAI2 with obesity and of SNAI2 with prognosis in EAC, highlighting a potential mechanism by which excess adiposity may promote malignant tumor dissemination and contribute to the poor prognosis characteristic of this aggressive disease. Although not yet in clinical trials, small molecule PAI-1 inhibitors are being developed as antithrombotic agents and are currently under investigation in animal models of pulmonary fibrosis;48 this study indicates that it would be of interest to examine the effect of these PAI-1 inhibitors in the context of obesity-fuelled cancer. In conclusion, this work has identified PAI-1 and SNAI2 as additional candidates for the development of therapeutic strategies targeting pathways of tumor dissemination in EAC, with the ultimate goal of stratified treatment of obese EAC patients.
Study Highlights

Guarantor of the article: Emma H. Allott, PhD.
Specific author contributions: E.H.A., M.J.M., J.L., G.P.P., and J.V.R. designed the study. E.H.A. and S.Mc.G. carried out the laboratory work. E.H.A., M.J.M., and C.L.D. carried out the statistical analysis of the results. E.H.A., M.J.M., H.M.R., J.L., and G.P.P. interpreted the findings and E.H.A. wrote the manuscript. All authors read the manuscript.
Financial support: This work is supported by a grant from the Health Research Board Ireland and funding from the CROSS charity (charity number: CHY389874).
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- National Cancer Registry, Ireland Incidence, Mortality, Treatment and Survival 1994–2007 , http://www.ncri.ie/data.cgi/choose-methods.php .
- Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- Lagarde SM, ten Kate FJ, Richel DJ, et al. Molecular prognostic factors in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 2007;14:977–991. doi: 10.1245/s10434-006-9262-y. [DOI] [PubMed] [Google Scholar]
- Kjoller L, Kanse SM, Kirkegaard T, et al. Plasminogen activator inhibitor-1 represses integrin- and vitronectin-mediated cell migration independently of its function as an inhibitor of plasminogen activation. Exp Cell Res. 1997;232:420–429. doi: 10.1006/excr.1997.3540. [DOI] [PubMed] [Google Scholar]
- Brooks TD, Slomp J, Quax PH, et al. Antibodies to PAI-1 alter the invasive and migratory properties of human tumour cells in vitro. Clin Exp Metastasis. 2000;18:445–453. doi: 10.1023/a:1011882421528. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Cubillo E, Sarrio D, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- Freytag J, Wilkins-Port CE, Higgins CE, et al. PAI-1 mediates the TGF-beta1+EGF-induced “scatter” response in transformed human keratinocytes. J Invest Dermatol. 2010;130:2179–2190. doi: 10.1038/jid.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves CC, Carneiro F, Hoefler H, et al. Role of the epithelial-mesenchymal transition regulator slug in primary human cancers. Front Biosci. 2009;14:3035–3050. doi: 10.2741/3433. [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Bermejo-Rodriguez C, Gonzalez-Herrero I, et al. Adipose tissue mass is modulated by SLUG (SNAI2) Hum Mol Genet. 2007;16:2972–2986. doi: 10.1093/hmg/ddm278. [DOI] [PubMed] [Google Scholar]
- The International Diabetes Federation The IDF Consensus: Worldwide Definition of the Metabolic Syndrome 2006 . http://www.idf.org/webdata/docs/MetS_def_update2006.pdf .
- Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6:137–159. doi: 10.1079/NRR19930010. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Prud'homme D, Bouchard C, et al. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- Lysaght J, Allott EH, Donohoe CL, et al. T lymphocyte activation in visceral adipose tissue of patients with oesophageal adenocarcinoma. Br J Surg. 2011;98:964–974. doi: 10.1002/bjs.7498. [DOI] [PubMed] [Google Scholar]
- Ennis DP, Pidgeon GP, Millar N, et al. Building a bioresource for esophageal research: lessons from the early experience of an academic medical center. Dis Esophagus. 2010;23:1–7. doi: 10.1111/j.1442-2050.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- Fried SK, Moustaid-Moussa N. Culture of adipose tissue and isolated adipocytes. Methods Mol Biol. 2001;155:197–212. doi: 10.1385/1-59259-231-7:197. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Kumar S, Barnett AH, et al. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000;164:119–128. doi: 10.1677/joe.0.1640119. [DOI] [PubMed] [Google Scholar]
- Parman C, Halling C, Gentleman R.affyQCReport: QC Report Generation for affyBatch objects. R package.
- Wu Z, Irizarry RA, Gentleman R, et al. A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc. 2004;99:909–917. [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:1–25. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Gentleman R. Extensions to gene set enrichment. Bioinformatics. 2007;23:306–313. doi: 10.1093/bioinformatics/btl599. [DOI] [PubMed] [Google Scholar]
- Morine MJ, McMonagle J, Toomey S, et al. Bi-directional gene set enrichment and canonical correlation analysis identify key diet-sensitive pathways and biomarkers of metabolic syndrome. BMC Bioinformatics. 2010;11:499. doi: 10.1186/1471-2105-11-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvortsov S, Schafer G, Stasyk T, et al. Proteomics profiling of microdissected low- and high-grade prostate tumors identifies lamin A as a discriminatory biomarker. J Proteome Res. 2011;10:259–268. doi: 10.1021/pr100921j. [DOI] [PubMed] [Google Scholar]
- Kellenberger LD, Bruin JE, Greenaway J, et al. The role of dysregulated glucose metabolism in epithelial ovarian cancer. J Oncol. 2010;2010:514310. doi: 10.1155/2010/514310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Chavey C, Lazennec G, Lagarrigue S, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–349. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Malizos KN, Oikonomou P, et al. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Gervasi ME, Bakin AV. Role of beta5-integrin in epithelial-mesenchymal transition in response to TGFbeta. Cell Cycle. 2010;9:1647–1659. doi: 10.4161/cc.9.8.11517. [DOI] [PubMed] [Google Scholar]
- Onwuegbusi BA, Rees JR, Lao-Sirieix P, et al. Selective loss of TGFbeta Smad-dependent signalling prevents cell cycle arrest and promotes invasion in oesophageal adenocarcinoma cell lines. PLoS One. 2007;2:e177. doi: 10.1371/journal.pone.0000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Mao SL, Taylor KL, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Dunning AM, Moore DH, et al. Prognostic value of PAI1 in invasive breast cancer: evidence that tumor-specific factors are more important than genetic variation in regulating PAI1 expression. Cancer Epidemiol Biomarkers Prev. 2006;15:2107–2114. doi: 10.1158/1055-9965.EPI-06-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck N, Kates RE, Schmitt M, et al. Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin Breast Cancer. 2004;5:348–352. doi: 10.3816/cbc.2004.n.040. [DOI] [PubMed] [Google Scholar]
- Nekarda H, Schmitt M, Ulm K, et al. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res. 1994;54:2900–2907. [PubMed] [Google Scholar]
- Langenskiold M, Holmdahl L, Angenete E, et al. Differential prognostic impact of uPA and PAI-1 in colon and rectal cancer. Tumour Biol. 2009;30:210–220. doi: 10.1159/000239796. [DOI] [PubMed] [Google Scholar]
- Angenete E, Langenskiold M, Palmgren I, et al. uPA and PAI-1 in rectal cancer—relationship to radiotherapy and clinical outcome. J Surg Res. 2009;153:46–53. doi: 10.1016/j.jss.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Sakakibara T, Hibi K, Koike M, et al. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br J Cancer. 2005;93:799–803. doi: 10.1038/sj.bjc.6602743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Come C, Magnino F, Bibeau F, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Martin TA, Goyal A, Watkins G, et al. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Chatopadhyay T, Raziuddin M, et al. Discovery of deregulation of zinc homeostasis and its associated genes in esophageal squamous cell carcinoma using cDNA microarray. Int J Cancer. 2007;120:230–242. doi: 10.1002/ijc.22246. [DOI] [PubMed] [Google Scholar]
- Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94:1816–1822. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jethwa P, Naqvi M, Hardy RG, et al. Overexpression of Slug is associated with malignant progression of esophageal adenocarcinoma. World J Gastroenterol. 2008;14:1044–1052. doi: 10.3748/wjg.14.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhang S, Jiao X, et al. Slug regulates proliferation and invasiveness of esophageal adenocarcinoma cells in vitro and in vivo. Med Oncol. 2011;28:1089–1100. doi: 10.1007/s12032-010-9652-7. [DOI] [PubMed] [Google Scholar]
- Allott EH, Lysaght J, Cathcart MC, et al. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation Mol Carcinog 2011. doi: 10.1002/mc.21840 [DOI] [PubMed]
- Uchikado Y, Natsugoe S, Okumura H, et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1174–1180. [PubMed] [Google Scholar]
- Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Ther Adv Cardiovasc Dis. 2010;4:315–324. doi: 10.1177/1753944710379126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.