Abstract
The gastrointestinal (GI) tract is the largest producer of serotonin (5-hydroxytryptamine (5-HT)) in the body, and as such it is intimately connected with GI function and physiology. 5-HT produced by enterochromaffin (EC) cells is an important enteric mucosal signaling molecule and has been implicated in a number of GI diseases, including inflammatory bowel disease and functional disorders such as irritable bowel syndrome. This review will focus on what is known of basic 5-HT physiology and also on the emerging evidence for its novel role in activation of immune response and inflammation in the gut. Utilizing pubmed.gov, search terms such as “5-HT,” “EC cell,” and “colitis,” as well as pertinent reviews, were used to develop a brief overview of EC cell biology and the association between 5-HT and various GI disorders. It is the aim of this review to provide the readers with an update on EC cell biology and current understanding on the role of 5-HT in GI disorders specifically in inflammatory conditions.
INTRODUCTION
The discovery of 5-hydroxytryptamine (5-HT) was accomplished by two independent research endeavors, one searching for vasoconstrictors causing hypertension described a molecule called serotonin, the other characterizing the granules found in intestinal enterochromaffin (EC) cells described a molecule called enteroamine.1, 2 5-HT is a well-known neurotransmitter of the central nervous system and traditionally it is known to influence a range of behavioral, physiological, and cognitive functions. However, most of the 5-HT in the body is synthesized from EC cells in the gastrointestinal (GI) tract and is an important mediator in normal gut physiology. Abnormal regulation of 5-HT (Table 1) in the human gut has been implicated with a diverse array of GI disorders, such as inflammatory bowel disease (IBD),3, 4 and functional disorders such as irritable bowel syndrome (IBS).3, 5, 6 In addition, alteration in 5-HT signaling is shown to be associated with celiac disease,7 colorectal cancer,8, 9 and diverticular disease.10 Despite this association with a variety of GI disorders it is not clear how the changes in 5-HT occur, what role 5-HT has in intestinal pathophysiology, and whether by modulating 5-HT production and signaling is it possible to elicit a therapeutic effect.
Table 1. Studies of EC cell numbers and 5-HT synthesis in IBD and IBS.
| GI disorders | EC cells | 5-HT | TpH mRNA | Reference |
|---|---|---|---|---|
| CD | Increased | Increased | Kidd et al.77 | |
| CD | Increased | Minderhoud et al.78 | ||
| UC | Decreased | Decreased | Coates et al.3 | |
| CD and UC | Decreased | Magro et al.4 | ||
| CD and UC | Increased | El-Salhy et al.60 | ||
| UC | Decreased | Ahonen et al.62 | ||
| IBS | Increased | Ahonen et al.62 | ||
| IBS | Unchanged | Decreased | Coates et al.3 | |
| IBS-C | Increased | Miwa et al.5 |
Abbreviations: CD, Crohn's disease; EC, enterochromaffin; GI, gastrointestinal; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; TpH, tryptophan hydroxylase; UC, ulcerative colitis; 5-HT, 5-hydroxytryptamine.
EC CELLS AND 5-HT
The association between altered EC cells numbers, 5-HT production, and GI diseases highly emphasizes the significance of 5-HT in intestinal pathophysiology. Understanding EC cell biology, mechanisms of 5-HT production, and the precise roles of EC cells/5-HT in intestinal pathology may ultimately lead to improved therapeutic strategies in GI disorders.
Origin and differentiation of EC cells
The GI tract contains the largest endocrine organ in the body; however, in contrast with other endocrine organs the various types of enteroendocrine cells are scattered throughout the intestinal tract. Enteroendocrine cells are responsible for releasing various biologically active compounds such as gastrin, secretin, stomatostatin, cholecystokinin, chromogranins, and 5-HT.11 The best-characterized subset of enteroendocrine cells are EC cells and are found throughout the gut.12 EC cells are found at the base of the crypts in the GI tract, where they originate from stem cells also located near the base of the crypt.13 The turnover of EC cells is considerably slower, EC cells last 15–150 days in comparison with enterocytes, which last 2–4 days in rats.14
There are potentially two distinguishable pools of stem cells within the intestinal crypt from which EC cells could originate.15 The regulators of intestinal cell lineage and maintenance of the intestinal stem cell niche seems to be the Notch, Wnt, and bone morphogenetic protein signaling pathways.16, 17, 18 Lateral inhibition of Notch signaling down regulates cellular expression of Hes-1, the key transcription factor for the enterocyte cell lineage.19 This allows endocrine cells in particular to begin to express transcription factors such as Math 1 and neurogenin 3, which direct a more secretory cell lineage.20, 21, 22, 23 The necessity of neurogenin 3 in the development of EC cells has been demonstrated in neurogenin 3−/− mice, which develop without any endocrine cells.20
5-HT synthesis and release
Synthesis of 5-HT by intestinal EC cells begins with the conversion of dietary tryptophan to 5-hydroxy-L-tryptophan, which is catalyzed by tryptophan hydroxylase (TpH). Recent studies have identified two isoforms of the TpH enzyme, TpH1, which is present in mainly peripheral organs such as the gut, and TpH2, which is associated with the nervous system and present predominantly in the brain stem.24, 25 The second step catalyzed by L-amino acid decarboxylase, which is also present in EC cells, converts 5-hydroxy-L-tryptophan to 5-HT.26 The 5-HT is then packaged into granules by vesicular monoamine transporter 1 at both the apical and basal ends of the EC cell, just below the surface of the plasma membrane.27, 28 Degradation of 5-HT is facilitated by monoamine oxidase A that drives the conversion of 5-HT into 5-hydrosyindoleacetic acid (5-HIAA).
The release of 5-HT from EC cells follows intraluminal distension, vagal-nerve stimulation, ingestion of a meal, or the presence of acid, amino acids, or hypo- or hyper-osmotic solutions in the duodenum. Microvilli present on the apical end of the EC cell project into the lumen and function as sensors of luminal content, turning the physiochemical signals of the lumen into biochemical endocrine signals.28 The released 5-HT from EC cells either enters the lumen or lamina propria where it can act upon enterocytes or cells of the enteric nervous system and initiate secretion and enteric pulsation patterns.28, 29 Cholera toxin has been shown to release 5-HT into the human jejunum30 this is relevant for both the small and large intestine. Short-chain fatty acids, produced by bacteria in the colon, can also stimulate 5-HT release.29 The diet may also influence 5-HT release, D-glucose, and D-galactose, but not fructose induced the release of 5-HT from human BON cells.31 The complex interaction of the microbiota, diet, and the cells of the intestine all have an influence on 5-HT synthesis, release, and degradation, and therefore all may be responsible for the altered 5-HT function seen in many GI diseases.
Turnover of EC cells and the release of 5-HT can be altered by signaling molecules released by surrounding cells. Cells associated with the immune, neural, and vascular system are in close proximity to EC cells.32, 33 Our work using Trichuris muris infection in severe combined immunodeficiency mice exemplifies the role of CD4+ T cells in modulating the EC cell number and 5-HT content.34 Wild-type mice (BLK6/C57) infected with T. muris produce a predominantly Th2 immune response, and this same study found the interleukin-13 receptor on murine EC cells, which solidifies the role of Th2 cytokines in EC cell biology.34 The close proximity of immune cells with EC cells and the ability of 5-HT and cytokines to regulate the function of both the immune and endocrine system are suggestive that this interaction governs many of the pathophysiological aspects associated with GI disease.
Role of 5-HT in immune activation and inflammation
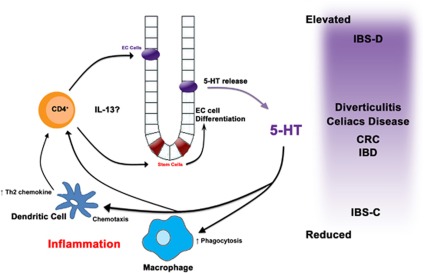
We have previously shown how the immune system can influence 5-HT-expressing EC cell biology, however, in turn 5-HT can also influence the immune system (Figure 1).34, 35, 36, 37 There are many serotonergenic receptors that have been found on various immune cells such as B and T lymphocytes, monocytes, macrophage, and dentritic cells.38 In addition, mast cells, macrophage, and T cells also have the ability to synthesis 5-HT from tryptophan.39, 40, 41 5-HT is also a chemotactic molecule for eosinophils, dendritic cells, and mast cells.42, 43, 44 Previous studies have described 5-HT receptors on human monocyte-derived dendritic cells; immature dendritic cells primarily expressed 5-HT1B, 5-HT1E, and 5-HT2B receptors, whereas mature dendritic cells express 5-HT4 and 5-HT7 receptors.45 This shift in the expression of 5-HT receptors may help to explain the differential functions of 5-HT, for instance 5-HT can function as a chemotactic molecule in immature but not lipopolysaccharides-matured dendritic cells.42 We have found that dendritic cells isolated from mice with decreased ability to synthesize 5-HT (TPH1−/−) in the intestine produced less interleukin-12p70 but cytokine production could be restored by adding 5-HT.46
Figure 1.
Modulation of EC cell biology by immune cells and modulation of immune cells by 5-HT in GI disease. The role of 5-HT in modulating the innate and adaptive immune system can vary by cell type. 5-HT has been shown to enhance phagocytosis in murine macrophages.40 In addition, 5-HT can increase chemotaxis of dendritic cells and promote the release of the Th2-attracting chemokine CCL22 while decreasing the Th1 chemokine CXCL10.42 Finally 5-HT has a proliferative effect on CD4+ T cells, which when coupled with 5-HT effect on dendritic cells create a more permissive environment for a Th2 immune response. CD4+ T cells50 particularly Th2 cytokines, such as interleukin-13,49 in turn may influence on EC cell biology, 5-HT synthesis, and 5-HT release.
In the experimental models of colitis induced by trinitrobenzene sulfonic acid, dinitrobenzenesulfonic acid, and dextran sodium sulfate, an increase in 5-HT content has been observed.47, 48 Studies from our laboratory recently reported reduced severity of colitis in TPH1−/− mice as compared with wild-type mice after dextran sodium sulfate- and dinitrobenzenesulfonic acid-colitis.35 Restoration of 5-HT in TPH1−/− mice by administration of a 5-HT precursor (5-hydroxy-L-tryptophan) enhanced the severity of colitis. These findings are supported by studies from other groups, which have shown that chemical-induced colitis or spontaneous colitis associated with interleukin-10 deficiency is increased in severity when coupled with the 5-HT-enhancing effects of the knockout of 5-HT reuptake transporter (SERT).49, 50 In clinical studies, in patients with diarrhea-predominant IBS duodenal immune activation is associated with reduced levels of SERT mRNA in platelets.51 Taken together, these studies suggest an important role of 5-HT in the pathogenesis of GI disease by influencing pro-inflammatory mediator production and immune modulation.
5-HT IN GI DISORDERS
5-HT and pathophysiology of GI diseases
The role of 5-HT in the pathophysiology of GI diseases can vary, among the most important roles of 5-HT is its influence on the motility of the GI tract and its ability to modulate the immune system. Extensive reviews on receptors for 5-HT (Table 2), such as 5-HT3 and 4, have been developed outlining the pivotal role of 5-HT in both altered motility and sensation of nausea and pain commonly associated with GI disorders.13, 52, 53 Alosetron (5-HT3 receptor antagonist) became the first agent approved by the US Food and Drug Administration for the treatment of diarrhea-predominant IBS. However, the drug unexpectedly was associated with ischemic colitis and, rarely, with severe constipation-induced complications.54 The case of alosetron prompts a rethinking of our approaches to the pharmacological modulation of serotoninergic pathways and warrants more studies on 5-HT in the context of intestinal pathology and pathophysiology.
Table 2. Clinical utility of agonists and antagonists associated with 5-HT metabolism in gastrointestinal disorders.
| Target | Mechanism | Potential and documented clinical utility |
|---|---|---|
| 5-HT1 receptor family | Agonist | FD; IBS-D |
| Antagonist | FD; IBS; GERD | |
| 5-HT2 receptor family | Agonist | None |
| Antagonist | IBS-D (women only) | |
| 5-HT3 receptor | Agonist | GERD; constipation-predominant IBS |
| Antagonist | IBS-D; FD; nocturnal GERD; chemotherapy-induced nausea and vomiting; radiation induced nausea and vomiting; post-operative vomiting | |
| 5-HT4 receptor | Agonist | Chronic constipation; gastroparesis; GERD; IBS-C; IBS-M; FD |
| Antagonist | GERD | |
| 5-HT7 receptor | Agonist | No known applications in GI disorders, however, receptor is thought to mediate colonic relaxation, therefore a potential role in functional GI disorders |
| Antagonist |
Abbreviations: FD, functional dyspepsia; GI, gastrointestinal; IBS-C, constipation-predominant irritable bowel syndrome; IBS-M, mixed IBS; GERD, gastroesophageal reflux disease; IBS-D, diarrhea-predominant IBS; 5-HT, 5-hydroxytryptamine.
The pathophysiology of GI diseases is also associated with aberrant immune responses, such as lymphocyte infiltration and hyperplasia, resulting in inflammation of the enteric mucosa. 5-HT receptors have been found on cells associated with the immune system and has been shown in vitro to affect the proliferation of lymphocytes and recruitment of T-cells.55, 56 The influence of 5-HT on immune cell function may have a critical role in the pathogenesis of various GI diseases as a significant reduction of colonic 5-HT was associated with decreased inflammation in two different animal models of colitis.35 Furthermore, the expression of cytokine receptors on 5-HT-expressing EC cells supports the existence of an immuno–endocrine axis in the context of various GI disorders.34
5-HT in GI disease translational medicine
Understanding 5-HT signaling in GI disorders is very important not only because of the alteration in 5-HT response observed in various GI diseases but also in light of serious and unforeseen complications caused from the use of 5-HT receptor antagonist in clinical practice (Table 2). The location of 5-HT-expressing EC cells within the epithelial mucosa of the GI tract is perfect for the delivery of an oral administered small molecule modulator of 5-HT synthesis. Recent clinical trials using a small molecule inhibitor of TpH1 have shown the ability to alleviate the symptoms associated with IBS, especially diarrhea predominate IBS. Treatment with TpH1 inhibitor decreased blood 5-HT level, relieved pain/discomfort, and increased stool consistency.57, 58 Modulation of tryptophan metabolism, especially 5-HT synthesis may be a novel target for developing therapies for GI disorders.
Modulation of the 5-HT signaling pathway in GI disease has been extensively investigated; however, adverse side effects have restricted the use of many 5-HT receptor targeted drugs.13, 52, 53, 59 Drugs released specifically at the site of inflammation could decrease collateral damage by requiring a lesser amount of drug. For example, addition of covalently linked hydrophilic carriers would enhance the retention of the pro-drug within the GI tract until it reached a specific site. At this point, specific enzymes from the intestinal cells or microflora could cleave the compound allowing its absorption by the intestine.60
5-HT and IBD
IBD includes two chronic GI diseases, ulcerative colitis and Crohn's disease, which are relapsing inflammatory conditions of unknown etiology. Inflammation of the intestinal mucosa has been found to affect 5-HT signaling in both humans and animal models.4, 35, 61, 62 Changes in EC cell numbers and in 5-HT content have been reported in association with both Crohn's disease and ulcerative colitis.61, 63, 64, 65 Approximately 50% of patients with IBD in long-standing remission have IBS-like symptoms, which may be related to these inflammation-induced alterations in EC cells and 5-HT signaling.66 It is also shown that consumption of selective 5-HT reuptake inhibitors is associated with microscopic colitis.67 Increases in 5-HT content have also been observed in experimental models of colitis.47, 48 In our opinion, the intersection between 5-HT-expressing EC cells (5-HT itself) and the immune responses that drive IBD are paramount to the pathogenesis of this disease.
5-HT and IBS
IBS is associated with abdominal pain and cramping, as well as changes in the functionality of the bowel. It is a disorder of dichotomous extremes, with many patients experiencing predominantly constipation, diarrhea, or both. Diarrhea-predominant IBS is associated with elevated 5-HT whereas constipation-predominant IBS is associated with decreased levels of 5-HT in the colon mucosa.13, 52 Alterations in gut motility are paramount to this disorder and contribute to prevalence of both diarrhea and constipation. The etiology of IBS is unclear, however, alterations in 5-HT metabolism have been suggested to contribute to the pathophysiology of this disease.68
There are several studies both in humans and in experimental models that have reported associations of symptoms of IBS and: the number of EC cells, the presence of 5-HT, mRNA levels of TpH, and the expression of SERT in mucosal biopsies.3, 5, 69, 70 In addition, a recent study examining the microbiota of patients who self-reported the severity of IBS, it has been found that the severity was associated with the presence of a Ruminococcus torques-like (94% similarity in 16S rRNA gene sequence) phylotype.71 It is reasonable to assume that the microflora can also influence the function of 5-HT, potentially modulating its content, and signaling in the intestinal mucosa resulting in GI disease.
5-HT and celiac disease
Celiac disease is caused by an immune reaction to gliadin, a prolamin (gluten protein) found in wheat, and is associated with chronic diarrhea and fatigue. Small intestine crypt hyperplasia and an increase in the number of 5-HT-expressing EC cells is also associated with Celiac disease.72, 73 With more EC cells there is also increased 5-HT content in the duodenal mucosa of in both adults and children.74 A recent study by Coleman et al.7 evaluated both fasting and postprandial plasma 5-HT levels in patients after a high-carbohydrate meal. Celiac patients had increased 5-HT-containing EC cell numbers as well as significantly higher peak plasma 5-HT levels.7 These observations point to altered EC cell biology, increased 5-HT release, and impaired 5-HT uptake resulting in altered 5-HT metabolism. This disease promotes a predominantly Th1 cytokine environment, therefore elevated levels of both tumor necrosis factor-α and interferon-γ can decrease the expression of SERT and reduce 5-HT uptake by cells in the intestine.75 Further studies are required to understand the precise role of 5-HT in the pathogenesis of celiac disease.
5-HT and diverticulitis
Although diverticulosis is a common affliction in the western society, there is very little known about its pathogenesis. Altered mobility is an important aspect of the disease, which suggests that alter 5-HT signaling and metabolism may be as well. Diverticulitis develops from diverticulosis, which is the formation of pouches (diverticular) on the outside of the inflamed colon. In a recent study, asymptomatic diverticulosis patients as well as patients with a history of acute diverticulitis were compared with healthy controls.10 The only difference observed in the study was that patients with a history of acute diverticulitis exhibited decreased expression of 5-HT transporter (SERT) expression.10 The requirement of an inflammatory event seems to be associated with a change in 5-HT signaling further emphasizing the intimate relationship between 5-HT metabolism and inflammation. Further studies such as this would be useful to understand the mechanisms involved in the development and progression of this disease.
CONCLUDING REMARKS
The regulation of 5-HT- and 5-HT-expressing EC cells is intimately associated with the inflammatory processes that drive many GI disorders. Recent studies on EC cells and 5-HT have generated a number of concepts that provide insight into how the immune and endocrine systems of the gut interface. It is evident from these studies that mediators from immune cells such as cytokines have an important role in EC cell biology and production of 5-HT in the gut. In addition, 5-HT has a key role in the pathogenesis of experimental colitis and in generation of pro-inflammatory mediators from immune cells. If this is not complex enough, this entire relationship is occurring in the presence of varying diets and mediators secreted by the microbiota, which may directly or indirectly influence the EC cell function.
Recently, Margolis et al.75 has shown that inhibition of 5-HT synthesis using a specific inhibitor of the TpH1 enzyme reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. This finding furthers supports our observation that 5-HT is a critical molecule in pathogenesis of colitis and suggest that targeted inhibition of 5-HT synthesis may ultimately help in the development of improved therapeutic strategies in GI inflammatory disorders.
5-HT exerts a wide range of effects in the gut, largely due to the presence of multiple receptor subtypes that are present on smooth muscle, enteric neurons, enterocytes, and immune cells. Agonist or antagonist for various 5-HT receptor is used in a variety of GI disorders, which include IBS, functional dyspepsia, and chronic constipation (Table 2). The 5-HT7 receptor, coupled to Gs proteins and stimulating cAMP production, is the most recently identified member of the family of 5-HT receptors. Our lab has evaluated the 5-HT7 receptor antagonist (SB-269970) and found that disruption of 5-HT7 receptor signaling significantly reduces the severity of both dextran sodium sulfate and dinitrobenzenesulfonic acid-induced colitis in mice.76 Pharmaco-modulation of 5-HT signaling using specific agonists and antagonists represents one of the best strategies for alleviating many of the symptoms associated with GI disease; however, the effect on the immune system should also be considered.
Guarantor of the article: Waliul I. Khan, MD, PhD.
Specific author contributions: M. Manocha reviewed the published papers which are mentioned in this article, contributed in the experiments on 5-HT, and wrote the article. W. I. Khan wrote the article and obtained the funding.
Financial support: This study is supported by the grants from the Crohn's and Colitis Foundation of Canada (CCFC) and by the Canadian Institutes of Health Research (CIHR) to W. I. Khan.
Potential competing interests: None.
References
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176:1243–1251. [PubMed] [Google Scholar]
- Erspamer V. Historical introduction: the Italian contribution to the discovery of 5-hydroxytryptamine (enteramine, serotonin) J Hypertens Suppl. 1986;4:S3–S5. [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–224. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- Miwa J, Echizen H, Matsueda K, et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- Faure C, Patey N, Gauthier C, et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman NS, Foley S, Dunlop SP, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Ataee R, Ajdary S, Zarrindast M, et al. Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. J Cancer Res Clin Oncol. 2010;136:1461–1469. doi: 10.1007/s00432-010-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, Strom BL, Rosenberg L. Antidepressant use and colorectal cancer risk. Pharmacoepidemiol Drug Saf. 2009;18:1111–1114. doi: 10.1002/pds.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costedio MM, Coates MD, Danielson AB, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GW, Leslie FC, Levison SE, et al. Enteroendocrine cells: neglected players in gastrointestinal disorders. Therap Adv Gastroenterol. 2008;1:51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolund K, Sanden G, Hakanson R, et al. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- de Bruine AP, Dinjens WN, Zijlema JH, et al. Renewal of enterochromaffin cells in the rat caecum. Anat Rec. 1992;233:75–82. doi: 10.1002/ar.1092330110. [DOI] [PubMed] [Google Scholar]
- May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski C, Li VS, Chan AS, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC. Intestinal morphogenesis. Curr Opin Gastroenterol. 2007;23:111–114. doi: 10.1097/MOG.0b013e3280145082. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–454. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, et al. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Hakanson R, Owman C, Sjoberg NO, et al. Amine mechanisms in enterochromaffin and enterochromaffin-like cells of gastric mucosa in various mammals. Histochemie. 1970;21:189–220. doi: 10.1007/BF00304213. [DOI] [PubMed] [Google Scholar]
- Schafermeyer A, Gratzl M, Rad R, et al. Isolation and receptor profiling of ileal enterochromaffin cells. Acta Physiol Scand. 2004;182:53–62. doi: 10.1111/j.1365-201X.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–113. doi: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Bearcroft CP, Perrett D, Farthing MJ. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut. 1996;39:528–531. doi: 10.1136/gut.39.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Cooke HJ, Javed NH, et al. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121:1400–1406. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- Wade PR, Westfall JA. Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 1985;241:557–563. doi: 10.1007/BF00214576. [DOI] [PubMed] [Google Scholar]
- Yang GB, Lackner AA. Proximity between 5-HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol. 2004;146:46–49. doi: 10.1016/j.jneuroim.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Wang H, Steeds J, Motomura Y, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Ghia JE, Wang H, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–481. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together. J Pediatr Gastroenterol Nutr. 2007;45 (Suppl 2:S115–S119. doi: 10.1097/MPG.0b013e31812e66da. [DOI] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol. 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Brown JM, Wu Y, et al. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sato T, Ohashi A, et al. Role of a serotonin precursor in development of gut microvilli. Am J Pathol. 2008;172:333–344. doi: 10.2353/ajpath.2008.070358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O′Connell PJ, Wang X, Leon-Ponte M, et al. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Durk T, Blumenthal B, et al. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4:e6453. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Sikora L, et al. Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol. 2004;173:3599–3603. doi: 10.4049/jimmunol.173.6.3599. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, et al. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- Li N, Ghia JE, Wang H, et al. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257–263. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–G695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- Haub S, Ritze Y, Bergheim I, et al. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter Neurogastroenterol Motil 201022826–834.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation Gastroenterology 20111401434–1443.e1. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2009;16:53–59. doi: 10.1097/med.0b013e32831e9c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Serotonin: its role and receptors in enteric neurotransmission. Adv Exp Med Biol. 1991;294:221–230. doi: 10.1007/978-1-4684-5952-4_20. [DOI] [PubMed] [Google Scholar]
- Ladabaum U. Safety, efficacy and costs of pharmacotherapy for functional gastrointestinal disorders: the case of alosetron and its implications. Aliment Pharmacol Ther. 2003;17:1021–1030. doi: 10.1046/j.1365-2036.2003.01545.x. [DOI] [PubMed] [Google Scholar]
- Stefulj J, Cicin-Sain L, Schauenstein K, et al. Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103–108. doi: 10.1159/000049013. [DOI] [PubMed] [Google Scholar]
- Laberge S, Cruikshank WW, Beer DJ, et al. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310–315. [PubMed] [Google Scholar]
- Brown PM, Drossman DA, Wood AJ, et al. The tryptophan hydroxylase inhibitor LX1031 is effective for patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: where next. Trends Pharmacol Sci. 2008;29:465–471. doi: 10.1016/j.tips.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Rubinstein A. Approaches and opportunities in colon-specific drug delivery. Crit Rev Ther Drug Carrier Syst. 1995;12:101–149. doi: 10.1615/critrevtherdrugcarriersyst.v12.i2-3.10. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, et al. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Ahonen A, Kyosola K, Penttila O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1–7. [PubMed] [Google Scholar]
- Belai A, Boulos PB, Robson T, et al. Neurochemical coding in the small intestine of patients with Crohn′s disease. Gut. 1997;40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AE, Pietroletti R, Taat CW, et al. Increased populations of endocrine cells in Crohn′s ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–396. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Banares F, Esteve M, Espinos JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol. 2007;102:324–330. doi: 10.1111/j.1572-0241.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- Malinen E, Krogius-Kurikka L, Lyra A, et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyana TN, Shukoor S. Gastrointestinal endocrine cell hyperplasia in celiac disease: a selective proliferative process of serotonergic cells. Mod Pathol. 1991;4:419–423. [PubMed] [Google Scholar]
- Wheeler EE, Challacombe DN. Quantification of enterochromaffin cells with serotonin immunoreactivity in the duodenal mucosa in coeliac disease. Arch Dis Child. 1984;59:523–527. doi: 10.1136/adc.59.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe DN, Dawkins PD, Baker P. Increased tissue concentrations of 5-hydroxytryptamine in the duodenal mucosa of patients with coeliac disease. Gut. 1977;18:882–886. doi: 10.1136/gut.18.11.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KF, Pantano C, Ciolino A, et al. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Stevanovic KD, Yang QM, et al. An inhibitor of tryptophan hydroxylase successfully ameliorates TNBS-induced colitis. Gastroenterology. 2011;140:S478–S478. [Google Scholar]
- Kim JJ, Wang HQ, Ghia JE, et al. Inhibition of 5-HT signaling by blocking 5-HT7 receptor function alleviates colitis. Gastroenterology. 2011;140:S26–S26. [Google Scholar]
- Kidd M, Gustafsson BI, Drozdov I, et al. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn′s disease. Neurogastroenterol Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minderhoud IM, Oldenburg B, Schipper ME, et al. Serotonin synthesis and uptake in symptomatic patients with Crohn′s disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–720. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- Chey WD, Pare P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217–1225. doi: 10.1111/j.1572-0241.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- Ford AC, Brandt LJ, Young C, et al. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Van Zanten SV, Saez LR, et al. A dose-ranging, placebo-controlled, randomized trial of alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther. 2001;15:525–537. doi: 10.1046/j.1365-2036.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- Vakil N, Laine L, Talley NJ, et al. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am J Gastroenterol. 2008;103:1906–1919. doi: 10.1111/j.1572-0241.2008.01953.x. [DOI] [PubMed] [Google Scholar]