Background: Calorie-restricted, fat-depleted Goat−/− mice develop profound hypoglycemia resulting from lack of ghrelin-mediated growth hormone release.
Results: Hypoglycemia is caused by decreased gluconeogenesis and reversed by gluconeogenic precursors (lactate and alanine) or fatty acids.
Conclusion: In absence of fatty acids, growth hormone maintains gluconeogenic precursors, allowing survival.
Significance: Maintenance of blood glucose by ghrelin-growth hormone axis is crucial for evolutionary adaptation to starvation.
Keywords: Carbohydrate Metabolism, Gluconeogenesis, Glucose Metabolism, Growth Hormone, Hormones, Ghrelin, Ghrelin Knock-out Mice, Hypoglycemia, Lipid/Fatty Acid, Starvation
Abstract
When mice are subjected to 7-day calorie restriction (40% of normal food intake), body fat disappears, but blood glucose is maintained as long as the animals produce ghrelin, an octanoylated peptide that stimulates growth hormone secretion. Mice can be rendered ghrelin-deficient by knock-out of the gene encoding either ghrelin O-acyltransferase, which attaches the required octanoate, or ghrelin itself. Calorie-restricted, fat-depleted ghrelin O-acyltransferase or ghrelin knock-out mice fail to show the normal increase in growth hormone and become profoundly hypoglycemic when fasted for 18–23 h. Glucose production in Goat−/− mice was reduced by 60% when compared with similarly treated WT mice. Plasma lactate and pyruvate were also low. Injection of lactate, pyruvate, alanine, or a fatty acid restored blood glucose in Goat−/− mice. Thus, when body fat is reduced by calorie restriction, ghrelin stimulates growth hormone secretion, which allows maintenance of glucose production, even when food intake is eliminated. In humans with anorexia nervosa or kwashiorkor, ghrelin and growth hormone are known to be elevated, just as they are in fat-depleted mice. We suggest that these two hormones prolong survival in starved humans as they do in mice.
Introduction
Chronic starvation is a repeated threat to survival of animals of all species. Indeed, ∼15% of the current human population is estimated to suffer from severe malnutrition, and one-half of all deaths in children less than 5 years of age (∼6 million deaths per year) arise from malnutrition (1, 2). Over the centuries, starvation has exerted profound evolutionary pressure that has selected for a variety of adaptive mechanisms to support life. Paramount among these mechanisms is the necessity to maintain blood sugar concentrations sufficient for brain function (3). The adaptive mechanisms are particularly strained when chronic starvation has depleted the body of its other source of energy, namely fatty acids stored as triglycerides (3, 4).
Recently, our laboratory has begun to study the adaptation to chronic starvation, focusing on the essential roles of two peptide hormones: ghrelin and growth hormone (5–7). Ghrelin is a peptide hormone of 28 amino acids that contains an 8-carbon fatty acid, octanoate, attached in ester linkage to serine-3 (8). Most ghrelin is produced in the stomach. The peptide was discovered based on its ability to stimulate the release of growth hormone from the pituitary, an action that absolutely requires the octanoate adduct (9). Two observations in rodents and humans linked ghrelin to appetite control: 1) administration of excess ghrelin was shown to increase food intake (10–12) and 2) plasma ghrelin concentrations were observed to increase markedly before meals and to decline dramatically after feeding (13, 14). However, an essential role of ghrelin in controlling food intake in rodents was ruled out by gene knock-out experiments that demonstrated little, if any, change in food intake when animals were rendered deficient in ghrelin (15, 16), the ghrelin receptor (17), or ghrelin O-acyltransferase (GOAT), the enzyme that attaches octanoate to ghrelin (5).
Ghrelin was linked to starvation when we showed that Goat knock-out mice were unable to maintain their blood sugar levels in a viable range when the animals were subjected to severe chronic calorie restriction (5). In these experiments, WT mice and Goat−/− homozygotes were fed diets containing only 40% of the calories that they normally would consume. Under these conditions, WT and Goat−/− mice both lost ∼30% of body weight within 72 h. Over this time, body fat stores were drastically depleted, so that body fat accounted for less than 2% of body weight. Despite this depletion, WT mice maintained blood sugar levels in the range of 60 mg/dl and showed normal activity. In contrast, after 3 days of calorie restriction, Goat−/− mice began to exhibit a gradual fall in blood sugar, reaching as low as 20 mg/dl on days 7–9, at which point the animals were moribund.
The adaptation of WT mice to starvation was accompanied by increases in the plasma concentrations of ghrelin and growth hormone, which rose higher each day. These increases did not occur in the Goat−/− mice. Administration of either ghrelin or growth hormone by osmotic minipump restored the ability of the Goat−/− mice to maintain blood sugar levels and survive the severe calorie restriction (5). These previous studies did not address the mechanism for hypoglycemia in the ghrelin-deficient mice.
The current studies were designed to probe the mechanism for the profound hypoglycemia in the calorie-restricted, ghrelin-deficient mice. Our experiments reveal: 1) that hypoglycemia occurs only when an acute starvation of 20 h is superimposed on the adipose-depleted state; 2) that this hypoglycemia is caused by reduced glucose production associated with marked decreases in plasma concentrations of lactate and pyruvate, two major substrates for gluconeogenesis (18, 19); 3) that blood glucose can be restored to WT levels by injection of lactate, pyruvate, and alanine, all of which are readily converted to glucose (3, 19); and 4) that the reductions in plasma lactate levels and glucose production can be reversed by injection of a medium chain fatty acid that cannot be converted to glucose but can provide a necessary source of energy for gluconeogenesis in these fat-depleted mice.
EXPERIMENTAL PROCEDURES
Materials
We obtained an Ascensia Contour (blood glucose meter) from Bayer (Leverkusen, Germany); heparin from Hospira (Lake Forest, IL); [3-3H]glucose (14 Ci/mmol) from American Radiolabeled Chemicals (St. Louis, MO); sodium lactate, sodium pyruvate, sodium octanoate, alanine, p-hydroxymercuribenzoic acid, and aprotinin from Sigma. All other chemicals were from Sigma unless otherwise specified.
Mice
Goat−/− mice were generated as previously described (5). Preproghrelin knock-out mice, hereafter designated Ghrl−/−, were produced by Regeneron Pharmaceuticals, Inc. (20) and generously provided by Tamas L. Horvath (Yale University). Both mouse lines, on a mixed C57BL/6J and DBA background, were housed in colony cages with 12-h light/12-h dark cycles. The dark cycle began at 9:00 or 10:00 p.m. The chow diet consisted of Teklad mouse/rat diet 7002 from Harlan Teklad Global Diets (Houston, TX). This diet contains 3.1 kcal/g of metabolizable energy, of which 18% of calories are from fat, 49% are from carbohydrates, and 33% are from protein. All of the animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center.
Metabolic Parameters
Blood was drawn from tail vein or retro-orbital sinus and collected on ice in EDTA-coated tubes containing p-hydroxymercuribenzoic acid (final concentration, 1 mm) and/or aprotinin (250 kallikrein-inactivating units/ml for glucagon measurement). Plasma was separated immediately and stored without treatment at −80 °C except for samples used for measurement of ghrelin, amino acids, lactate, and pyruvate. For measurement of ghrelin, plasma samples were treated with HCl (final concentration, 0.1 m) before freezing. For measurement of lactate and pyruvate plasma samples were deproteinized using 70% acetone and then lyophilized. Octanoylated ghrelin and des-acyl ghrelin were measured by immunoassay kits that distinguished both forms of the peptide (catalogue number A05117 and A05118; Cayman Chemical, Ann Arbor, MI). Plasma levels of lactate and pyruvate and tissue content of glycogen were measured with commercial assay kits from Biovision (Milpitas, CA). Plasma levels of the following hormones were measured with commercial kits from the indicated vendor: growth hormone (Cayman Chemical), free fatty acids (Wako, Richmond, VA), β-hydroxybutyrate (Pointe, Canton, MI), glucagon and fibroblast growth factor-21 (Millipore, Billerica, MA), and insulin (Crystal Chem, Downers Grove, IL). Plasma amino acid levels were determined with a Hitachi L-8900 amino acid analyzer.
Calorie Restriction
Calorie restriction was carried out as previously described (5). One week before initiation of calorie restriction, 8-week-old male littermates (WT and Goat−/−mice; WT and Ghrl−/−mice) were placed in individual cages and fed the chow diet ad libitum. During this week of acclimation, food intake was monitored to determine the average amount of food consumed daily by each mouse. Thereafter, the mice were subjected to 60% calorie restriction such that each mouse was fed at 6 p.m. every day with an amount of food equal to 40% of the daily amount consumed by the same mouse during the week of acclimation.
High Fat Diet Feeding
Male WT and Goat−/− littermates (4 weeks old) were fed either the standard chow diet (18% calories from fat; see above under “Mice”) or a high fat diet (45% calories from fat; catalogue number D12451, Research Diets, New Brunswick, NJ). After 4 weeks on the diet, the mice from each group were placed in individual cages and subjected to calorie restriction such that each mouse was fed at 6 p.m. with 1.4 g of chow diet. Body composition was measured every 1 or 2 days at 5 p.m. (Bruker Minispec mq7.5NMR analyzer), after which body weight and blood glucose measurements were made at 5:30 p.m. just prior to feeding.
Whole Body Glucose Production in Calorie-restricted Mice
8-week-old male WT and Goat−/− littermates were placed in individual cages and fed the chow diet ad libitum. Basal food intake was measured for each mouse during this week. Four days before being subjected to 60% calorie restriction, each mouse was implanted with a jugular vein catheter that was externalized at the back of the neck. The catheter was filled with a heparin/glycerol mixture (v/v, 1:3) and blocked with a metal plug. Starting at 4 p.m. on day 6 of calorie restriction, each mouse received a continuous infusion of [3-3H]glucose (31 dpm/fmol) based on a modification of previously described isotopic protocols (21, 22). Mice were infused at 0.3 μCi/min from 0 to 20 min, followed by 0.1 μCi/min from 20 to 90 min. The mice were then bled from cut tails at 45, 60, 75, and 90 min after infusion. Blood glucose level was measured at each time point with a Bayer glucose meter. The content of 3H radioactivity in blood was measured as follows: 4 μl of plasma was deproteinized with barium hydroxide and zinc sulfate, after which the supernatant was evaporated, resuspended in 1 ml of water, and subjected to liquid scintillation counting in 10 ml of counting mixture (3a70B; Research Products International). Whole body glucose production was calculated by dividing the steady-state tracer infusion rate (0.1 μCi/min) adjusted for body weight (dpm/kg/min) by the plasma glucose specific activity (dpm/mg) (23).
RESULTS
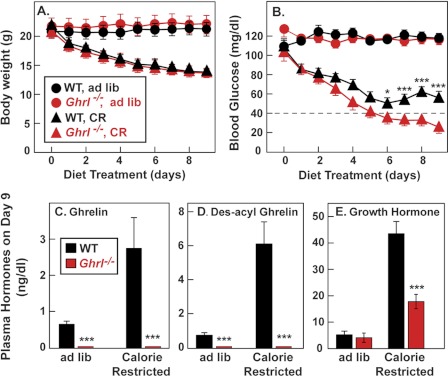
In our previous studies of the role of ghrelin in starvation, we used mice that lacked ghrelin as a result of loss of the Goat gene (5). These animals continued to secrete des-acyl ghrelin. To rule out a contribution of des-acyl ghrelin to the starvation phenotype, we performed a calorie restriction experiment in previously described mice that lack the gene for preproghrelin (designated Ghrl) and hence cannot produce either ghrelin or des-acyl ghrelin. WT and Ghrl−/− mice were placed on a starvation diet containing 40% of their normal caloric intake. The animals were fed each day at 6 p.m. After 2 days of calorie restriction, WT and Ghrl−/− mice became ravenous and ate all of their food within 1 h. Blood was drawn at 5:30 p.m. after the animals had gone nearly 23 h without food. After 4 days of calorie restriction, both WT and Ghrl−/− mice lost ∼25% of their body weight (Fig. 1A), but unlike WT mice, ghrelin knock-out mice developed hypoglycemia, just as previously reported for Goat knock-out mice (Fig. 1B). The mice were killed on day 9 when the blood sugar in the Ghrl−/− mice had reached the range of 16–37 mg/dl. WT mice maintained blood sugars in the 50–60 mg/dl range throughout this period. By day 9, the WT mice exhibited marked increases in plasma ghrelin, des-acyl ghrelin, and growth hormone (Fig. 1, C–E). Ghrelin and des-acyl ghrelin were undetectable in the Ghrl−/− mice, and the rise in growth hormone was much less pronounced.
FIGURE 1.
Comparison of WT and Ghrl−/− mice fed a chow diet or subjected to calorie restriction. Male littermates (8 weeks old) were housed in individual cages and fed ad libitum with a chow diet or subjected to 60% calorie restriction as described under “Experimental Procedures.” Body weight (A) and blood glucose (B) were measured daily at 5:30 p.m. (30 min before feeding). C–E, mice were euthanized at 5:30 p.m. (before feeding) on day 9 of calorie restriction, after which plasma levels of ghrelin (C), des-acyl ghrelin (D), and growth hormone (E) were determined. Each value represents the means ± S.E. of data from six mice. The asterisks denote the levels of statistical significance (Student's t test) between WT and Ghrl−/− mice. *, p < 0.05; ***, p < 0.001.
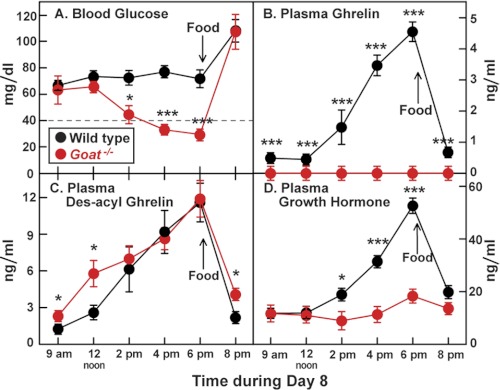
To further investigate the hypoglycemia of ghrelin deficiency, we returned to the Goat knock-out mice. Remarkably, even after 8 days of calorie restriction Goat−/− mice had the same blood sugar level as WT mice when measured at 9 a.m., which was 14 h after the last meal (Fig. 2A). The WT mice maintained this blood sugar level throughout the day. However, in the Goat−/− mice, blood sugar began to decline after noon and reached its nadir at 6 p.m. Blood sugar rose above 100 mg/dl in both strains immediately after feeding. In WT mice, plasma ghrelin, des-acyl ghrelin, and growth hormone levels began to climb after noon and reached a peak at 6 p.m., immediately before the next meal (Fig. 2, B–D). These values declined rapidly after feeding. In the Goat−/− mice, des-acyl ghrelin was the only one of these peptides to rise during the day, and it, too, declined after feeding (Fig. 2C). A nearly identical diurnal result was observed in Ghrl−/− mice except that their des-acyl ghrelin did not rise (supplemental Fig. S1).
FIGURE 2.
Response to starvation in WT and Goat−/− mice after 7 days of calorie restriction. Male WT and Goat−/− littermates (8 weeks old) were subjected to 60% calorie restriction for 7 days as described under “Experimental Procedures,” having received their last food at 6 p.m. on day 7. On day 8, blood was obtained at the indicated time for measurement of glucose (A), ghrelin (B), des-acyl ghrelin (C), and growth hormone (D). Food was given on day 8 at 6 p.m. (arrow). Each value represents the mean ± S.E. of data from four mice. The asterisks denote the levels of statistical significance (Student's t test) between WT and Goat−/− mice. *, p < 0.05; ***, p < 0.001.
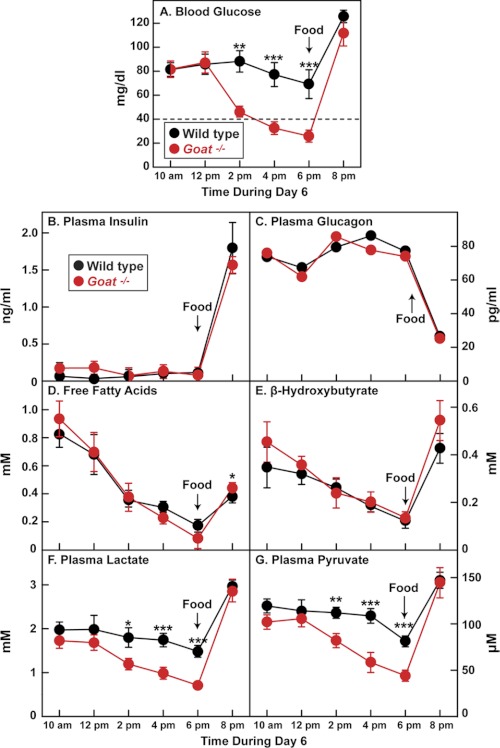
After 6 days of calorie restriction, plasma insulin was below the limit of detection in all mice from 10 a.m. to 6 p.m. and rose markedly after feeding in both mouse strains (Fig. 3B). In direct opposition, plasma glucagon levels were elevated throughout the day in both strains and fell dramatically after feeding (Fig. 3C). Plasma levels of free fatty acids (Fig. 3D) and the ketone body β-hydroxybutyrate (Fig. 3E) were similar in both strains, falling throughout the day and rising after feeding. Plasma levels of the gluconeogenic precursors lactate and pyruvate declined modestly throughout the day in WT mice (Fig. 3, F and G). The decline in the Goat−/− mice was much more profound. By 6 p.m., plasma lactate and pyruvate levels in Goat−/− mice were 48 and 54% of the levels seen in WT mice, respectively (p values, < 0.001).
FIGURE 3.
Changes in plasma parameters in WT and Goat−/− mice after 5 days of calorie restriction. Male WT and Goat−/− littermates (8 weeks old) were subjected to 60% calorie restriction for 5 days as described under “Experimental Procedures,” having received their last food at 6 p.m. on day 5. On day 6, blood was obtained at the indicated time for measurement of glucose (A), insulin (B), glucagon (C), free fatty acids (D), β-hydroxybutyrate (E), lactate (F), and pyruvate (G). Food was given on day 6 at 6 p.m. (arrow). Each value represents the mean ± S.E. of data from six mice except for glucagon, which was measured using pooled samples from six mice. The asterisks denote the levels of statistical significance (Student's t test) between WT and Goat−/− mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Supplemental Table SI compares various metabolic parameters in additional experiments in WT and Goat−/− mice at 9 a.m. and 5:30 p.m. after 7 days of calorie restriction. At 5:30 p.m., when compared with WT mice, Goat−/− mice showed significant decreases in blood sugar, growth hormone, lactate, and pyruvate (experiments A and C). Plasma free fatty acids and β-hydroxybutyrate were equally low in WT and Goat−/− mice at 5:30 p.m. (experiment C). At this time point, glycogen levels in liver and muscle were low in WT mice and even lower in Goat−/− mice (experiment D). In Goat−/− mice, glycogen depletion was extreme; at 5:30 p.m. the levels of liver and muscle glycogen were 4–5% of the levels seen at 9 a.m. Plasma triglycerides at 5:30 p.m. on day 7 in calorie-restricted mice were low in both strains and not significantly different from each other (experiment C).
Fibroblast growth factor-21 (FGF-21)7 is a polypeptide hormone whose production is strongly induced in mouse liver after a 24-h fast (24). However, at 5:30 p.m. after 7 days of calorie restriction and 23.5 h of fasting, FGF-21 levels were actually low in both WT and Goat−/− mice (0.07 ± 0.04 versus 0.09 ± 0.04 ng/ml, respectively; n = 6). In the same experiment, non-calorie-restricted WT and Goat−/− mice fasted for 18 h showed high levels of FGF-21 (2.8 ± 0.48 versus 3.1 ± 0.61 ng/ml, respectively; n = 6). The similarly low levels of FGF-21 in the calorie-restricted WT and Goat−/− mice indicate that the low FGF-21 did not cause the hypoglycemia in the Goat−/− mice.
Table 1 shows the plasma amino acid levels in calorie-restricted mice. At 5:30 p.m., the plasma levels of most amino acids were significantly higher in Goat−/− mice as compared with WT mice. This was particularly true of alanine, the key gluconeogenic amino acid (243 ± 89 μm in Goat−/− mice versus 72 ± 9 μm in WT mice). The levels of the branched chain amino acids (valine, isoleucine, and leucine) were also much higher in Goat−/− mice. Elevation of branched chain amino acids is a hallmark of physiological states associated with increased protein catabolism as occurs in prolonged starvation (19). These findings suggest that hypoglycemia in Goat−/− mice is not caused by the lack of availability of amino acids.
TABLE 1.
Plasma amino acid levels of WT and Goat−/− mice at 9:00 a.m. and 5:30 p.m. on day 7 of calorie restriction
Male WT and Goat−/− littermates (8 weeks old) were subjected to 60% calorie restriction for 7 days. At 9:00 a.m. and 5:30 p.m. on day 7 of calorie restriction, the mice were euthanized, and blood was drawn from each mouse. Plasma amino acid levels were determined by a Hitachi L-8900 amino acid analyzer. Each value represents the mean ± S.E. of data from six mice. The footnotes denote the levels of statistical significance (Student's t test) between WT and Goat−/− mice at 5:30 p.m. There was no statistical difference between WT and Goat−/− mice at 9:00 a.m. Dashes indicate that the amino acid levels were undetectable.
| Amino acid | 9:00 a.m. |
5:30 p.m. |
||
|---|---|---|---|---|
| WT | Goat−/− | WT | Goat−/− | |
| μm | μm | |||
| Gly | 127 ± 13 | 120 ± 17 | 93 ± 14 | 141 ± 33 |
| Ala | 137 ± 15 | 157 ± 51 | 72 ± 9.2 | 243 ± 89a |
| Ser | 58 ± 5.0 | 52 ± 7.5 | 44 ± 5.6 | 107 ± 30a |
| Thr | 70 ± 3.5 | 67 ± 9.1 | 60 ± 7.2 | 182 ± 46a |
| Cys | 11 ± 1.0 | 10 ± 2.2 | 4.2 ± 1.4 | 7.2 ± 1.2 |
| Met | 16 ± 0.8 | 13 ± 1.3 | 11 ± 2.5 | 27 ± 8.0 |
| Val | 98 ± 9.3 | 91 ± 8.7 | 104 ± 27 | 400 ± 100b |
| Iso | 38 ± 5.3 | 32 ± 2.0 | 31 ± 6.7 | 56 ± 10a |
| Leu | 124 ± 15 | 94 ± 14 | 87 ± 42 | 554 ± 147b |
| Asn | — | — | — | — |
| Gln | 202 ± 10 | 183 ± 20 | 173 ± 17 | 279 ± 54 |
| Tyr | 126 ± 4.9 | 130 ± 7.8 | 122 ± 14 | 181 ± 30 |
| Phe | 42 ± 2.8 | 30 ± 3.3 | 37 ± 3.5 | 55 ± 9.9 |
| Trp | 4.6 ± 1.3 | 6.1 ± 1.0 | 8.8 ± 2.0 | 12.1 ± 1.0 |
| Lys | 172 ± 6.2 | 144 ± 17 | 101 ± 25 | 508 ± 152a |
| His | 20 ± 0.9 | 15 ± 3.6 | 18 ± 3.1 | 70 ± 24a |
| Arg | 41 ± 4.6 | 22 ± 3.4 | 20 ± 5.5 | 37 ± 8.1a |
| Asp | 1.7 ± 0.4 | 1.7 ± 0.2 | 3.7 ± 0.7 | 3.5 ± 0.9 |
| Glu | 12 ± 1.9 | 13 ± 1.5 | 9.1 ± 1.8 | 15 ± 3.6 |
| Pro | 179 ± 21 | 142 ± 23 | 214 ± 86 | 275 ± 93 |
a p < 0.05.
b p < 0.01.
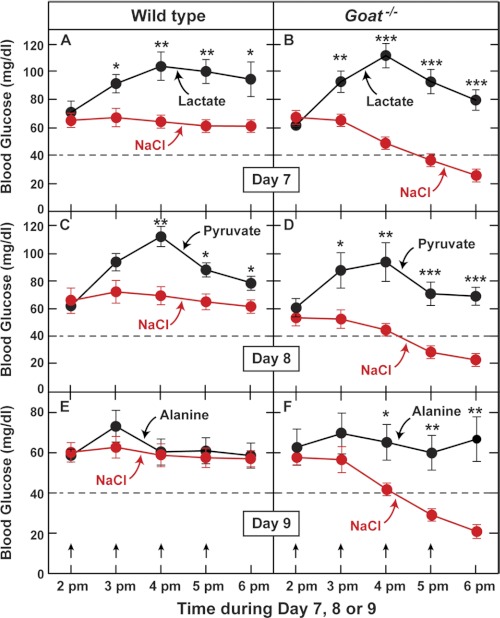
To determine whether hypoglycemia in Goat−/− mice could be reversed by restoring plasma lactate and pyruvate, we subjected the animals to calorie restriction and then injected them intraperitoneally with these substances or with a saline control solution at hourly intervals (Fig. 4). Either lactate or pyruvate raised the blood sugar concentration in WT and Goat−/− mice (Fig. 4, A–D). In the Goat−/− animals, the injections were sufficient to prevent hypoglycemia. Alanine is readily converted to pyruvate and then to glucose (3, 19). Unlike lactate or pyruvate, alanine did not raise the blood sugar in WT mice, but it did prevent hypoglycemia in the Goat−/− animals (Fig. 4, E and F).
FIGURE 4.
Response of calorie-restricted WT and Goat−/− mice to injections of gluconeogenic precursors. Male WT and Goat−/− littermates (8 weeks old) were subjected to 60% calorie restriction for 7–9 days as described under “Experimental Procedures.” On day 7, 8, or 9 as indicated, WT (A, C, and E) and Goat−/− (B, D, and F) mice were injected intraperitoneally with either NaCl or one of the following gluconeogenic precursors: sodium lactate (A and B), sodium pyruvate (C and D), or alanine (E and F). Each mouse received four injections of either NaCl or the indicated gluconeogenic precursor (18 μmol/g of body weight/injection). Each intraperitoneal injection was given at hourly intervals beginning at 2 p.m. in a volume of 150–180 μl (gluconeogenic precursors were dissolved in water and adjusted to pH 7.2). The arrows denote the times of injections. A–F, blood glucose was measured immediately before each injection and at 6 p.m., 1 h after the last injection. Each value represents the mean ± S.E. of data from five mice. The asterisks denote the levels of statistical significance (Student's t test) between WT and Goat−/− mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
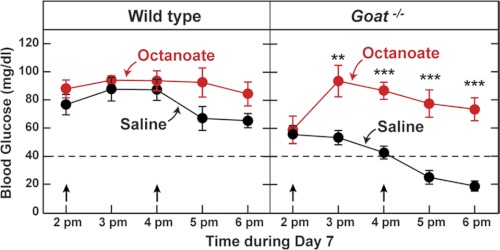
A notable feature of the calorie-restricted mice is the extremely low concentration of free fatty acids that develops in both strains in the afternoon (Fig. 3D). Despite this deficiency, WT mice are able to maintain their blood glucose, whereas Goat−/− mice become hypoglycemic. Fatty acids cannot be converted directly to glucose, but they can supply energy for glucose production (25). To determine whether administration of fatty acids could correct the hypoglycemia in Goat−/− mice, we injected calorie-restricted mice with octanoate. We chose octanoate because it is water-soluble and easier to deliver than are long chain fatty acids. Fig. 5 shows that two intraperitoneal injections of octanoate at 2 and 4 p.m. prevented the drop in blood sugar in the calorie-restricted Goat−/− mice. The plasma ghrelin concentration remained undetectable in the Goat−/− mice injected with octanoate.
FIGURE 5.
Response of calorie-restricted WT and Goat−/− mice to injections of octanoate. Male WT and Goat−/− littermates (8 weeks old) were subjected to 60% calorie restriction for 7 days as described under “Experimental Procedures.” On day 7, WT (left panel) and Goat−/− (right panel) mice were injected intraperitoneally with each mouse receiving two injections (2 and 4 p.m.) of equimolar amounts of either NaCl or sodium octanoate (3 μmol/g of body weight/injection) in a volume of 140–170 μl (octanoate was dissolved in water to a final concentration of 0.3 m and adjusted to pH 7.6). Blood glucose was measured at the indicated time (just prior to injections). Each value represents the mean ± S.E. of data from five mice. The asterisks denote the levels of statistical significance (Student's t test) between WT and Goat−/− mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The arrows denote the times of injections.
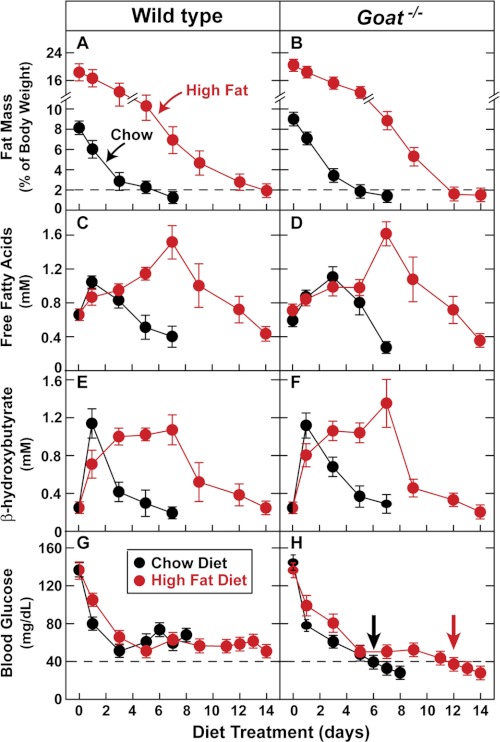
To confirm that the deficiency of fatty acids is responsible for the hypoglycemia in calorie restricted Goat−/− mice, we pretreated a group of mice with a high fat diet for 4 weeks to increase their adipose tissue stores prior to beginning calorie restriction. After the high fat diet, the body weights of the WT and Goat−/− mice increased by an average of 2.8 and 5.1 g, respectively. All of the mice were then switched to a diet composed of 1.4 g of chow, which was the average amount fed to our previous calorie-restricted mice (day 0 in Fig. 6). In the previously chow-fed WT and Goat−/− mice, the percentage of body fat declined below 2% after 5 days of calorie restriction. After the high fat feeding, body fat did not fall below the 2% threshold until 12 days of calorie restriction (Fig. 6, A and B). Likewise, the fall in plasma free fatty acids was also markedly delayed in the fat-fed mice of both strains, reaching the nadir of 0.4 mm after 7 and 14 days in the chow-fed and fat-fed mice, respectively (Fig. 6, C and D). The fall in plasma β-hydroxybutyrate was also delayed in the previously fat-fed mice as compared with the previously chow-fed mice (Fig. 6, E and F). Blood glucose in the previously chow-fed Goat−/− mice fell below 40 mg/dl at day 6, and the animals were moribund at day 8 (Fig. 6H). In contrast, the previously fat-fed Goat−/− mice appeared healthy up until day 12 when their blood glucose fell below 40 mg/dl (arrows in Fig. 6H), and they survived until day 14. Supplemental Fig. S2 shows blood glucose as a function of the percentage of body fat in the calorie-restricted Goat−/− mice. Irrespective of the previous diet, blood glucose did not fall below 40 mg/dl until the fat mass declined below 2% of body weight. This correlation persists even though the previously chow-fed mice required only 6 days to reach this threshold, whereas the previously fat-fed mice required 12 days.
FIGURE 6.
Comparison of WT and Goat−/− mice fed a high fat diet and then subjected to calorie restriction. Four-week-old male littermates were fed ad libitum either a chow diet or a high fat diet for 4 weeks and then subjected to calorie restriction by feeding each mouse 1.4 g of chow diet each day as described under “Experimental Procedures.” Body composition (A and B), plasma free fatty acids (C and D), plasma β-hydroxybutyrate (E and F), and blood glucose (G and H) were measured every 1–2 days at 5:30 p.m. (30 min prior to feeding). Each value represents the mean ± S.E. of data from six mice.
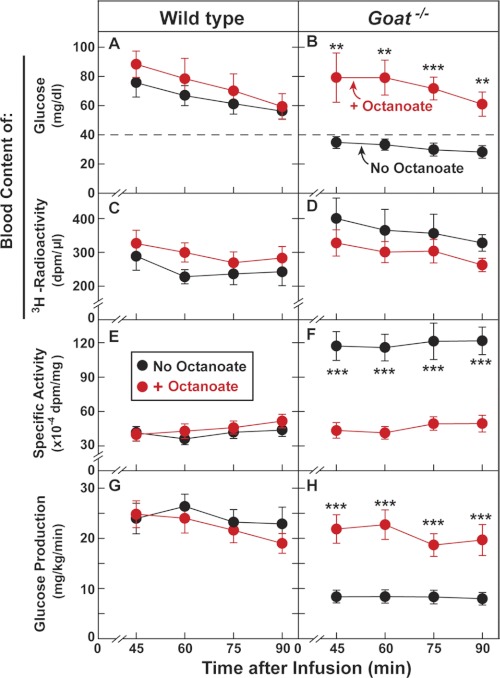
The data of Figs. 5 and 6 suggest that the hypoglycemia in calorie-restricted Goat−/− mice is dependent on a deficiency of plasma free fatty acids that results from the deficiency of body fat. To determine whether the hypoglycemia is caused by reduced glucose production or enhanced glucose clearance, we performed a [3H]glucose infusion experiment (Fig. 7). First, we placed indwelling catheters in the jugular veins of WT and Goat−/− mice. After several days of adaptation to the catheters, the mice were subjected to calorie restriction. On day 6 at 2 and 4 p.m., some of the mice were injected with octanoate intraperitoneally. Beginning at 4 p.m., we infused a tracer loading dose of [3H]glucose into the jugular vein followed by a maintenance infusion of the tracer.
FIGURE 7.
Whole body glucose production in calorie-restricted WT and Goat−/− mice in the absence or presence of octanoate. Eight-week-old male littermates were implanted with jugular vein catheters 4 days before 60% calorie restriction as described under “Experimental Procedures.” On day 6 of calorie restriction, the mice were injected intraperitoneally with either NaCl or sodium octanoate (3 μmol/g of body weight/injection). Each mouse received two injections (2 and 4 p.m.) as described in Fig. 6. After the last injection (4 p.m.), each mouse received a continuous infusion of [3-3H]glucose (31 dpm/fmol). The infusion rate was 0.3 μCi/min from 0–20 min, followed by 0.1 μCi/min from 20–90 min, after which blood was obtained at the indicated time for measurement of its content of glucose (A and B) and 3H radioactivity (C and D). Specific activity of blood glucose (E and F) and whole body glucose production (G and H) were then calculated as described under “Experimental Procedures.” Each value represents the mean ± S.E. of data from six mice. The asterisks denote the levels of statistical significance (Student's t test) between mice injected with octanoate and mice injected with NaCl. **, p < 0.01; ***, p < 0.001.
To present these results, we first focus on the animals that did not receive octanoate (Fig. 7, black circles). During the infusion period, total plasma glucose fell slightly in both strains, but it remained higher in WT mice (Fig. 7A, black circles) than in Goat−/− mice (Fig. 7B, black circles). The level of 3H radioactivity was constant and higher in Goat−/− mice than in WT mice (Fig. 7, C and D). As a result of the higher level of radioactivity and the lower level of total glucose, the specific radioactivity of the [3H]glucose in the Goat−/− plasma was much higher than in WT mice (Fig. 7, E and F). From these data, we were able to calculate glucose production rates using the formula of Wolfe and Chinkes (23) (see “Experimental Procedures”). In WT mice, the calculated production rate was 25 mg/kg/min, whereas in Goat−/− mice the production rate was reduced to 9 mg/kg/min (Fig. 7, G and H). A similar result was obtained in an independent experiment shown in supplemental Fig. S3.
The data for mice that received octanoate is shown in red in Fig. 7. As before, octanoate injections restored blood glucose in Goat−/− mice to the same level seen in WT mice (Fig. 7, A and B). The specific radioactivity of blood glucose was lowered dramatically in Goat−/− mice so that it equaled that in WT mice (Fig. 7, E and F). The octanoate injection did not alter the calculated glucose production rate in WT mice (Fig. 7G), but it markedly increased glucose production in Goat−/− mice so that the rate equaled that of WT mice (Fig. 7H). According to the formula of Wolfe and Chinkes (23), the glucose production rate equals the steady-state [3H]glucose infusion rate divided by the plasma glucose specific radioactivity. Inasmuch as the specific radioactivity declined in the octanoate-injected Goat−/− mice and the [3H]glucose infusion rate remained constant, the glucose production rate must have increased. If the octanoate-induced increase in plasma glucose were caused by a decrease in glucose clearance from plasma without a change in the production rate, the steady-state specific activity of plasma [3H]glucose would not have changed.
At the end of this experiment, we measured the concentration of lactate in the plasma of the mice. In WT mice, the octanoate infusion raised plasma lactate by 45% from 1.63 ± 0.15 to 2.36 ± 0.36 mm. The increase in lactate was larger in the Goat−/− mice (155%), rising from 0.91 ± 0.08 to 2.32 ± 0.28 mm. Considered together, these data indicate that the octanoate injections in the calorie restricted Goat−/− mice raised plasma lactate and restored glucose production to the same level as seen in WT mice.
DISCUSSION
The current results demonstrate that hypoglycemia in ghrelin-deficient mice has two prerequisites: 1) it requires chronic calorie restriction that severely depletes fat stores; and 2) it requires acute starvation for 20 h superimposed on fat depletion. The first requirement was confirmed by experiments in which we first fed Goat−/− mice a high fat diet to increase fat stores (Fig. 6). When these mice were switched to a calorie-restricted diet, it took twice as long to deplete their fat below 2% of body weight as compared with Goat−/− mice that were previously fed normal chow (12 days versus 5 days). Similarly, it took twice as long for the fat-fed mice to develop hypoglycemia defined as blood sugar below 40 mg/dl (12 days versus 6 days). The delay in hypoglycemia also correlated with a delay in two other signs of fat depletion, namely profound declines in plasma free fatty acids and β-hydroxybutyrate.
The second prerequisite, a 20-h fast superimposed on fat depletion, was documented by following blood glucose through a 24-h cycle (Figs. 2 and 3 and supplemental Fig. 1 and supplemental Table SI). WT and Goat−/− mice were fed the calorie-restricted diet at 6 p.m. each day. After a few days, mice of both strains consumed their entire food ration within 1 h. After 7 days, at 9 a.m. mice from both strains had similar levels of blood sugar (60 mg/dl). At this time, in both strains plasma insulin was low, glucagon was high, and the liver had abundant glycogen. Over the next 8 h, in both strains plasma free fatty acids and ketone bodies fell. Liver and muscle glycogen declined drastically. Despite these deficiencies, WT mice maintained blood sugar in the range of 60 mg/dl, but Goat−/− mice exhibited a profound fall to the range of 20–40 mg/dl. The starvation-induced fall in blood sugar in the Goat−/− mice was correlated with a marked fall in plasma lactate and pyruvate, which fell well below the levels seen in WT mice. These levels, along with blood sugar, rose promptly after the mice ingested their next meal.
The pathogenic relevance of the declines in plasma lactate, pyruvate, and free fatty acids was established by experiments in which we corrected the hypoglycemia in Goat−/− mice by injecting lactate, pyruvate, or octanoate (Figs. 4 and 5). The hypoglycemia was also corrected by injecting alanine, which is readily converted to pyruvate and then to glucose (3, 18, 19). Even though plasma alanine was already high in the plasma of Goat−/− mice, injection of extra alanine restored the blood sugar. Interestingly, alanine did not raise the blood sugar in WT mice (Fig. 4, E and F).
Steady-state infusion studies with [3H]glucose demonstrated that hypoglycemia in the Goat−/− mice was attributable to reduced glucose production (Fig. 7 and supplemental Fig. 3). Plasma lactate levels and glucose production were both returned to WT levels after injection of octanoate (Fig. 7). Although fatty acids such as octanoate cannot be converted to glucose, they can supply energy for gluconeogenesis in the liver (25). One possibility is that the increased glucose production in liver leads to increased glucose uptake into muscle with subsequent glycolysis to lactate, thereby restoring the Cori cycle (19). Further experiments will be needed to test this hypothesis.
Many classic studies have characterized the response to acute starvation in animals and humans with normal adipose tissue stores (3). However, much less attention has been paid to starvation superimposed upon adipose tissue depletion. In adipose-replete animals, starvation leads to release of fatty acids from adipose tissue. Although fatty acids cannot be converted to glucose, they help maintain blood glucose by sparing glucose utilization in muscle and by providing energy and reducing equivalents that are required for gluconeogenesis in liver and kidney. In liver, fatty acid oxidation produces ketone bodies, exemplified by β-hydroxybutyrate, which supply energy to muscle and brain. Growth hormone plays a key role in this acute adaptation by stimulating adipose tissue lipolysis, thereby increasing free fatty acid levels in blood. In muscle, the fatty acids are rapidly converted to fatty acyl-CoAs, and a portion is used to form diacylglycerols. Within 1–2 h, these fatty acid-derived products inhibit glucose uptake, thus helping to maintain blood glucose concentrations (26, 27).
Ghrelin does not play an essential role in this early response to starvation because Goat−/− mice are able to maintain normal blood glucose concentrations during acute starvation as long as their adipose tissue can supply fatty acids. The requirement for ghrelin appears when acute starvation occurs in an animal that cannot release sufficient fatty acids, because of adipose tissue depletion that was elicited by previous calorie restriction. Our working model for the role of ghrelin is described below.
As adipose tissue disappears, starvation in WT mice induces progressively higher plasma ghrelin and growth hormone levels (5). These hormones allow the animal to maintain viable levels of blood sugar during fasting even though little or no fatty acids are available. Maintenance of blood sugar is contingent upon continued gluconeogenesis. In the absence of ghrelin, fat-deficient mice cannot maintain normal levels of gluconeogenesis, which occurs largely in liver and kidney. Reduced delivery of glucose to muscle leads to reduced release of lactate, the product of muscle glycolysis (3). As a result of a deficiency of energy and substrate, the liver cannot maintain sufficient rates of gluconeogenesis even though glucagon levels are high, and the liver has responded by increasing the mRNAs encoding gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (5). Ghrelin is required only to release growth hormone because hypoglycemia can be prevented by infusion of growth hormone in the absence of ghrelin (5). A key question for future studies is how elevated growth hormone allows livers of WT mice to maintain sufficient energy to permit sustained gluconeogenesis even in the absence of fatty acids.
Observational evidence indicates that the ghrelin-growth hormone axis is likely to function in protecting humans from hypoglycemia during starvation as it does in mice. Plasma ghrelin and growth hormone levels are both markedly elevated in humans with severe calorie restriction resulting from either famine/protein-energy malnutrition (28–31) or from anorexia nervosa (32–34). Like the calorie-restricted mice in which plasma IGF-1 levels are extremely low despite high growth hormone levels (5), the elevated growth hormone levels in starved humans are also paradoxically associated with low levels of plasma IGF-1, a hormone that is normally elevated in response to growth hormone action in the liver. The combination of high growth hormone and low IGF-1 has been interpreted by some to indicate that starving humans are resistant to the actions of growth hormone (35). Our findings suggest the contrary: they suggest that the elevated growth hormone is actually contributing to the maintenance of blood glucose. Growth hormone must be acting through a signaling mechanism that does not involve IGF-1. Further studies of calorie-restricted WT and Goat−/− mice may reveal the true molecular target of the elevated growth hormone in fat-depleted humans and animals.
Supplementary Material
Acknowledgments
We thank Dr. Ralph DeBerardinis and Christopher Chambers for helpful discussions and amino acid analysis; Srisha Sridharan and Isis Soto for invaluable help with animal studies; and Mi Hwa Kim (University of Texas Southwestern Mouse Metabolic Phenotyping Core Facility) for help with glucose infusions.
This work was supported, in whole or in part, by National Institutes of Health Grant HL20948-35. This work was also supported by the Moss Heart Foundation.

This article contains supplemental Table SI and Figs. S1–S3.
- FGF-21
- fibroblast growth factor-21.
REFERENCES
- 1. Svedberg P. (2011) How many people are malnourished? Annu. Rev. Nutr. 31, 263–283 [DOI] [PubMed] [Google Scholar]
- 2. Bryce J., Boschi-Pinto C., Shibuya K., Black R. E., and the WHO Child Health Epidemiology Reference Group (2005) WHO estimates of the causes of death in children. Lancet 365, 1147–1152 [DOI] [PubMed] [Google Scholar]
- 3. Cahill G. F., Jr. (2006) Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 [DOI] [PubMed] [Google Scholar]
- 4. Nass R. M., Gaylinn B. D., Rogol A. D., Thorner M. O. (2010) Ghrelin and growth hormone: Story in reverse. Proc. Natl. Acad. Sci. U.S.A. 107, 8501–8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao T. J., Liang G., Li R. L., Xie X., Sleeman M. W., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Goldstein J. L., Brown M. S. (2010) Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. U.S.A. 107, 7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao T. J., Sakata I., Li R. L., Liang G., Richardson J. A., Brown M. S., Goldstein J. L., Zigman J. M. (2010) Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. U.S.A. 107, 15868–15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein J. L., Zhao T. J., Li R. L., Sherbet D. P., Liang G., Brown M. S. (2011) Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harbor Symp. Quant. Biol. 76, 10.1101/sqb. 2011.76.010447 [DOI] [PubMed] [Google Scholar]
- 8. Kojima M., Kangawa K. (2005) Ghrelin: Structure and function. Physiol. Rev. 85, 495–522 [DOI] [PubMed] [Google Scholar]
- 9. Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 [DOI] [PubMed] [Google Scholar]
- 10. Tschöp M., Smiley D. L., Heiman M. L. (2000) Ghrelin induces adiposity in rodents. Nature 407, 908–913 [DOI] [PubMed] [Google Scholar]
- 11. Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I. (2001) Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 50, 2438–2443 [DOI] [PubMed] [Google Scholar]
- 12. Wren A. M., Seal L. J., Cohen M. A., Brynes A. E., Frost G. S., Murphy K. G., Dhillo W. S., Ghatei M. A., Bloom S. R. (2001) Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocr. Metab. 86, 5992–5995 [DOI] [PubMed] [Google Scholar]
- 13. Cummings D. E., Purnell J. Q., Frayo R. S., Schmidova K., Wisse B. E., Weigle D. S. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 [DOI] [PubMed] [Google Scholar]
- 14. Nass R., Farhy L. S., Liu J., Prudom C. E., Johnson M. L., Veldhuis P., Pezzoli S. S., Oliveri M. C., Gaylinn B. D., Geysen H. M., Thorner M. O. (2008) Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 93, 1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wortley K. E., Anderson K. D., Garcia K., Murray J. D., Malinova L., Liu R., Moncrieffe M., Thabet K., Cox H. J., Yancopoulos G. D., Wiegand S. J., Sleeman M. W. (2004) Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. U.S.A. 101, 8227–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Y., Ahmed S., Smith R. G. (2003) Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell Biol. 23, 7973–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zigman J. M., Nakano Y., Coppari R., Balthasar N., Marcus J. N., Lee C. E., Jones J. E., Deysher A. E., Waxman A. R., White R. D., Williams T. D., Lachey J. L., Seeley R. J., Lowell B. B., Elmquist J. K. (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 115, 3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Exton J. H. (1972) Gluconeogenesis. Metabolism 21, 945–990 [DOI] [PubMed] [Google Scholar]
- 19. Ruderman N. B. (1975) Muscle amino acid metabolism and gluconeogenesis. Annu. Rev. Med. 26, 245–258 [DOI] [PubMed] [Google Scholar]
- 20. Wortley K. E., del Rincon J. P., Murray J. D., Garcia K., Iida K., Thorner M. O., Sleeman M. W. (2005) Absence of ghrelin protects against early-onset obesity. J. Clin. Invest. 115, 3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. (1975) Glucose turnover values in the dog obtained with various species of labeled glucose. Am. J. Physiol. 229, 1662–1667 [DOI] [PubMed] [Google Scholar]
- 22. Katz H., Homan M., Butler P., Rizza R. (1992) Use of [3-3H]glucose and [6-14C]glucose to measure glucose turnover and glucose metabolism in humans. Am. J. Physiol. 263, E17–E22 [DOI] [PubMed] [Google Scholar]
- 23. Wolfe R. R., Chinkes D. L. (2005) Isotope tracers in metabolic research: Principles and practice of kinetic analysis, pp. 215–257, 2nd Ed., John Wiley and Sons, Inc., New York [Google Scholar]
- 24. Potthoff M. J., Inagaki T., Satapati S., Ding X., He T., Goetz R., Mohammadi M., Finck B. N., Mangelsdorf D. J., Kliewer S. A., Burgess S. C. (2009) FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. U.S.A. 106, 10853–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson J. R., Kreisberg R. A., Felts P. W. (1966) Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc. Natl. Acad. Sci. U.S.A. 56, 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Møller N., Jørgensen J. O. (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 [DOI] [PubMed] [Google Scholar]
- 27. Nørrelund H. (2005) The metabolic role of growth hormone in humans with particular reference in fasting. Growth Horm. IGF Res. 15, 95–122 [DOI] [PubMed] [Google Scholar]
- 28. El-Hodhod M. A., Emam E. K., Zeitoun Y. A., El-Araby A. M. (2009) Serum ghrelin in infants with protein-energy malnutrition. Clin. Nutr. 28, 173–177 [DOI] [PubMed] [Google Scholar]
- 29. Altinkaynak S., Selimoglu M. A., Ertekin V., Kilicarslan B. (2008) Serum ghrelin levels in children with primary protein-energy malnutrition. Pediatr. Int. 50, 429–431 [DOI] [PubMed] [Google Scholar]
- 30. Soliman A. T., Hassan A. E., Aref M. K., Hintz R. L., Rosenfeld R. G., Rogol A. D. (1986) Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr. Res. 20, 1122–1130 [DOI] [PubMed] [Google Scholar]
- 31. Kilic M., Taskin E., Ustundag B., Aygun A. D. (2004) The evaluation of serum leptin level and other hormonal parameters in children with severe malnutrition. Clin. Biochem. 37, 382–387 [DOI] [PubMed] [Google Scholar]
- 32. Nass R., Gaylinn B. D., Thorner M. O. (2011) The role of ghrelin in GH secretion and GH disorders. Mol. Cell Endocrinol. 340, 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka M., Nakahara T., Kojima S., Nakano T., Muranaga T., Nagai N., Ueno H., Nakazato M., Nozoe S., Naruo T. (2004) Effect of nutritional rehabilitation on circulating ghrelin and growth hormone levels in patients with anorexia nervosa. Regul. Pept. 122, 163–168 [DOI] [PubMed] [Google Scholar]
- 34. Misra M., Miller K. K., Kuo K., Griffin K., Stewart V., Hunter E., Herzog D. B., Klibanski A. (2005) Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and health adolescents. Am. J. Physiol. Endocrinol. Metab. 289, E347–E356 [DOI] [PubMed] [Google Scholar]
- 35. Counts D. R., Gwirtsman H., Carlsson L. M., Lesem M., Cutler G. B., Jr. (1992) The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J. Clin. Endocr. Metab. 75, 762–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.