Background: Misfolding of the rBAT subunit of a cystine transporter causes type I cystinuria.
Results: rBAT contains three native intramolecular disulfides (two essential) in vivo only when the carrier subunit b0,+AT is present.
Conclusion: b0,+AT controls both degradation and folding of rBAT.
Significance: Learning how subunit folding and assembly of membrane protein complexes is coordinated is essential to understand protein biogenesis in vivo.
Keywords: Amino Acid Transport, Endoplasmic Reticulum (ER), Membrane Proteins, Membrane Transport, Protein Folding, Cystinuria, Intracellular Trafficking, Membrane Protein Complex, Oxidative Folding, Pegylation
Abstract
We study the amino acid transport system b0,+ as a model for folding, assembly, and early traffic of membrane protein complexes. System b0,+ is made of two disulfide-linked membrane subunits: the carrier, b0,+ amino acid transporter (b0,+AT), a polytopic protein, and the helper, related to b0,+ amino acid transporter (rBAT), a type II glycoprotein. rBAT ectodomain mutants display folding/trafficking defects that lead to type I cystinuria. Here we show that, in the presence of b0,+AT, three disulfides were formed in the rBAT ectodomain. Disulfides Cys-242-Cys-273 and Cys-571-Cys-666 were essential for biogenesis. Cys-673-Cys-685 was dispensable, but the single mutants C673S, and C685S showed compromised stability and trafficking. Cys-242-Cys-273 likely was the first disulfide to form, and unpaired Cys-242 or Cys-273 disrupted oxidative folding. Strikingly, unassembled rBAT was found as an ensemble of different redox species, mainly monomeric. The ensemble did not change upon inhibition of rBAT degradation. Overall, these results indicated a b0,+AT-dependent oxidative folding of the rBAT ectodomain, with the initial and probably cotranslational formation of Cys-242-Cys-273, followed by the oxidation of Cys-571-Cys-666 and Cys-673-Cys-685, that was completed posttranslationally.
Introduction
Around 30% of ORFs in a genome are predicted to encode integral membrane proteins. Recently, the crystal structure of many membrane proteins has prompted detailed mechanistic hypothesis on the structure-function of several membrane protein families (1–3). In contrast, another central question in membrane protein biology, namely biogenesis (e.g. synthesis, membrane insertion, folding, oligomerization, and trafficking), still lags behind structure-function studies (4).
Despite many important contributions to the biogenesis field, there is increasing recognition of a need for more research in this area, both to use new and varied protein models and to develop better experimental methods (4–6). Many key results have been obtained with viral (7), monomeric (5, 8), and oligomeric proteins composed of identical or closely related subunits (9, 10). An underrepresented class is the hetero-oligomeric membrane proteins with subunits showing a wide range of different topologies and structures (11–13). These proteins provide a unique opportunity to analyze how the steps of biogenesis in each subunit are coordinated to attain the native functional complex.
The heteromeric amino acid transporters (HATs)2 are composed of two disulfide-linked polypeptides. The heavy subunits are type II membrane glycoproteins and the light subunits are 12-transmembrane domain unglycosylated proteins. The heterodimer is the functionally relevant unit (14, 15). The physiological role of HATs is highlighted by their involvement in cancer, immune function, and several human inherited diseases such as cystinuria and lysinuric protein intolerance (16–18). Nine mammalian heteromeric amino acid transporter light subunits are known, and each one mediates a different amino acid transport activity (15). The structure of a prokaryotic homologue of the heteromeric amino acid transporter light subunits has been solved, and the transport mechanism is now under close experimental scrutiny (2, 19). The heteromeric amino acid transporter heavy subunit is a helper protein required for trafficking to the plasma membrane (14, 15). The two mammalian heteromeric amino acid transporter heavy subunits are 4F2hc and rBAT. The ectodomain of 4F2hc consists of a (β/α)8 TIM-barrel domain A and an antiparallel eight-stranded β-sheet domain C, similar to α-glucosidases, but 4F2hc shows no glucosidase activity, as predicted (20). A working model of the rBAT ectodomain has been reported (20). rBAT differs from 4F2hc in the presence of the α-glucosidase B-domain between Aβ2 and Aα3 of the TIM barrel and an ∼30 residue C-terminal tail without any noticeable homology.
System b0,+ is formed by rBAT and the heteromeric amino acid transporter light subunit b0,+AT. It exchanges extracellular cystine and dibasic amino acids for intracellular neutral amino acids at the apical membrane of the epithelial cells of the kidney and small intestine (21, 22). Each subunit depends on the other for traffic to the plasma membrane. Without b0,+AT, rBAT is translocated into the endoplasmic reticulum (ER), N-glycosylated, and rapidly degraded, whereas without rBAT, b0,+AT remains stable within the ER, most likely already folded (23–25). Mutations in either rBAT or b0,+AT decrease functional system b0,+ at the plasma membrane, leading to the formation of cystine stones in the kidney, the hallmark of cystinuria (OMIM 220100). Most mutations in b0,+AT cause the partially dominant non-type I cystinuria phenotype, whereas most rBAT mutations cause the recessive type I cystinuria phenotype (26). We have shown that mutations in the rBAT ectodomain lead to folding/trafficking defects underlying type I cystinuria (27).
Here, we report insight into the biogenesis of system b0,+, focusing on the folding of the helper subunit. The intramolecular disulfide connectivity of rBAT and the importance and diverse roles of these disulfides in the biogenesis of the heterodimer are uncovered. Finally, we show that, within the cell, oxidative folding of the helper subunit rBAT does not proceed in the absence of the catalytic subunit b0,+AT.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Reagents were purchased from Sigma unless otherwise indicated. Pro-mix l-[35S] in vitro cell labeling mix (l-[35S]methionine and l-[35S]cysteine) was purchased from PerkinElmer Life Sciences. dMEM media without l-methionine and l-cystine and dialyzed FBS were from Invitrogen. Antibodies against the N termini of human b0,+AT and rBAT are described elsewhere (22, 28).
cDNA Constructs
The vectors for mammalian cell expression of human rBAT and b0,+AT were as described elsewhere (28). The human rBAT mutants were obtained by site-directed mutagenesis (QuikChange, Stratagene) of pCDNA3-rBAT using the following mutagenic oligonucleotides (only sense oligonucleotides are shown): C18S, 5′-GAT GAG TAT GAA GGG ATC CCA GAC AAA CAA CGG G-3′; C114S, 5′-GCC CTC TCT CCA AAG TCC CTA GAC TGG TGG CAG GAG GGG-3′; C242S, 5′-CTG GCA TGA CTC TAC CCA TGA AAA TGG C-3′; C273A, 5′-GGC ACT TTG ACG AAG TGC GAA ACC AAG CTT ATT TTC ATC AG-3′; C571S, 5′-CCT CAA CAG GGG CTG GTT TTC CCA TTT GAG GAA TGA CAG CC-3′; C666S, 5′-GCT TTC AGA GAT AGA TCC TTT GTT TCC AAT CGA GC-3′; C673S, 5′-CCA ATC GAG CAA GCT ATT CCA GTG TAC TGA ACA TAC TGT ATA CC-3′; and C685S, 5′-ATA CCT CGT CTT AGG CAC CTT-3′.
All mutations were confirmed by DNA sequencing using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems).
Cell Culture and Transfection
HeLa and Madin Darby canine kidney IIJ cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (heat-inactivated), 100 units/ml penicillin (Invitrogen), and 0.1 mg/ml streptomycin (Invitrogen) at 37 °C in a humidified atmosphere containing 5% CO2. Stably transfected Madin Darby canine kidney-derived cell lines were described elsewhere (25). Calcium phosphate transient transfection of HeLa cells was performed as described (28). The efficiency of transfection was above 70% in all experiments. For transient transfections, 10-cm diameter plates were incubated with a mixture of DNA containing 2 μg of pEGFP (green fluorescence protein, Clontech), 6 μg of pCDNA3-rBAT (wild-type or the different mutants), and 12 μg of pCDNA3-b0,+AT as described (27, 28). These conditions allow most, if not all, of the expressed rBAT to bind to b0,+AT (27). When rBAT or b0,+AT were transfected alone, 12 or 6 μg of pCDNA3 was added, respectively.
Transport Measurements
Influx experiments of 40 μm l-[35S]cystine (Perkin Elmer) were performed in transfected HeLa cells as described (28).
Endoglycosidase H Assay
The enzyme was obtained from New England Biolabs and used following the protocol of the manufacturer.
Pulse-chase and Immunoprecipitation Protocols
Cells were transfected at 40–50% confluence and seeded the next day in 3.5-cm diameter plates at 60–70% confluence. 36 h after transfection, the cells were incubated for 30 min in prewarmed l-methionine/l-cystine-free media containing 10% dialyzed FBS. Subsequently, cells were labeled with a mixture of [35S]methionine/cysteine (200 μCi/ml) for 30 min (if not otherwise indicated). When indicated, 5 mm DTT was included only during the labeling time. After removal of the labeling media, the cells were incubated with prewarmed media supplemented with 5 mm unlabeled l-methionine/l-cysteine. At this step, two different protocols were used: one for pegylation experiments (see last section) and one for the rest of the experiments. The last one is used in (27) and is detailed here. At the indicated chase times (or just after the pulse), cells were washed twice in cold PBS and once for 5 min in cold PBS containing 20 mm N-ethylmaleimide. Cells were collected and lysed on a rotating wheel in 0.2 ml of NET buffer (150 mm NaCl, 5 mm EDTA, 50 mm Tris (pH 7.4), 0.5% IGEPAL CA-630) with the protease inhibitors aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and pepstatin, and with 20 mm NEM. After 30 min at 4 °C, a postnuclear supernatant was obtained by 10-min centrifugation at 10,000 × g at 4 °C. Where indicated, 1 mm 1-deoxymannojirimycin (dMNJ) (Calbiochem) was included from the beginning of the starving period to the end of the chase.
Immunoprecipitations were performed from equivalent amounts of radioactivity incorporated into proteins by adding an equal volume of immunoabsorbent buffer (200 mm H3BO3, 50 mm Na2B4O7, 150 mm NaCl, 1% IGEPAL CA-630, and 0.1% ovalbumin (pH 8.3)) with the same protease inhibitors as the lysis buffer, and polyclonal antibodies to rBAT or b0,+AT, in combination with protein A-Sepharose. Precipitates were washed four times in borate-NaCl buffer (0.5% IGEPAL CA-630, 0.3 m NaCl, 25 mm Na2B4O7 and 0.1 m H3BO4, pH 8.3) and twice in 40 mm HEPES (pH 8). Samples were run on SDS-PAGE under reducing (100 mm DTT) conditions. Gels were stained with Coomassie Brilliant Blue to control for precipitating antibodies, dried, and placed on an intensifying screen for quantification with a Phosphoimager Typhoon 8600 (Molecular Dynamics).
Data Analysis
The relative intensities of the labeled bands were determined using phosphorimaging as follows: each band was outlined by a rectangle (as tightly fitting to the band as possible), and a rectangle of identical size was drawn in the closest area without any band in the lane. The relative positions of band and background rectangles were maintained within the experiment and in similar experiments. The value for each rectangle was calculated using the local average background correction of the ImageQuant software. The final value of the band was the difference between the value of the rectangle band and the value of the rectangle background. The data were plotted as intensity values of the fraction remaining obtained by dividing by the value at time zero.
mPEG5000-maleimide (mPEG) modification
Several pegylation conditions (e.g. varying time and temperature of incubation and mPEG concentration) were assayed for wild-type rBAT in the absence or presence of b0,+AT. The chosen conditions allowed for maximal pegylation. Additionally, lysis of the cells in trichloroacetic acid immediately after scraping and prior to mPEG modification did not result in significantly different rBAT pegylation.
Pulse and chase were performed as stated above. At the indicated times, cells were washed three times in cold PBS, scraped, and collected for 5 min at 3000 × g at 4 °C. The cell pellet was frozen at −20 °C for at least 1 h. The frozen pellet was lysed in 100 μl of TSD buffer (50 mm Tris HCl (pH 7.5), 1% SDS) (29) with 4 mm mPEG (NOF Corp., Japan) or 20 mm NEM, incubated for 30 min at 30 °C, and centrifuged for 5 min at 17,000 × g at room temperature. For immunoprecipitations, one-third of the supernatant together with 12 volumes of TNN buffer (50 mm Tris HCl 7.5, 250 mm NaCl, 5 mm EDTA, 0.5% IGEPAL CA-630) (29) with the protease inhibitors aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and pepstatin, was incubated overnight at 4 °C with polyclonal antibodies to rBAT or b0,+AT in combination with protein A-Sepharose. Precipitates were washed three times in TNN buffer. Samples were run on SDS-PAGE under reducing (25 mm DTT) or non-reducing conditions. Gels were stained with Coomassie Brilliant Blue to control for precipitating antibodies, dried, and placed on an intensifying screen for quantification with a Phosphoimager Typhoon 8600 (Molecular Dynamics).
RESULTS
Our aim was to study early biogenesis of the rBAT-b0,+AT heterodimer. We used transiently transfected HeLa cells, a valid model for the functional expression of system b0,+ (25, 27). Protein folding in vivo can be monitored by following the oxidation of disulfide bonds (7, 30). Human rBAT has eight cysteines (Fig. 1). Cys-18 is cytosolic. Cys-114 to Cys-685 are extracellular. Cys-114 is disulfide-linked with human b0,+AT (31, 32), Cys-242 and Cys-273 are localized to domain B, Cys-571 is in domain C, and Cys-666 to Cys-685 are in the C-terminal tail (20). Cys-685 is the C-terminal residue. Cys-114 to Cys-685 are conserved in all vertebrate orthologues. Cys-242 and Cys-273 are not conserved in the two annotated urochordate rBAT orthologues.
FIGURE 1.
Cysteine residues in human rBAT. A scheme of human rBAT with the cysteine positions and the corresponding mutations is drawn to scale (top panel). A, B, and C, extracellular TIM barrel domain A, domain B, and domain C, respectively. The N-terminal cytoplasmic segment is pale gray, the transmembrane domain is black, and the C-terminal tail is dark gray. The crystal structure of oligo-1,6-glucosidase from Bacillus cereus (PDB code 1UOK) (47) is shown to observe the opposite position of domain B relative to domain C.
Disulfide Connectivity of rBAT in the Presence of b0,+AT
In the proposed structural model of the rBAT ectodomain, Cys-242 and Cys-273 are at a distance compatible with a disulfide (20), but the intramolecular disulfide connectivity of rBAT is unknown. We used mass tagging under denaturing conditions with the cysteine-specific pegylation reagent mPEG5000-maleimide (mPEG; Mr, 5 kDa) (9, 33) to count the number of reduced cysteines in rBAT. Each mPEG attached should produce an increase in the rBAT molecular mass, easily detectable in SDS-PAGE, although not completely predictable because SDS does not bind mPEG.
First, we made sure that the eight cysteines were accessible to mPEG. rBAT was synthesized in HeLa cells in the presence of [35S]Met/Cys and the reducing agent DTT (supplemental Fig. 1, lanes 1–9). In these conditions, rBAT (which remains core-glycosylated because of its presence in the ER (27)) is reduced because of the more reducing conditions in the ER lumen (7, 30). The cells were lysed in denaturing solution containing mPEG without or with increasing concentrations of the alkylating agent NEM (Mr, 0.125 kDa). rBAT was immunoprecipitated with a specific antibody against the N terminus (27), and the precipitates ran in reducing SDS-PAGE. As expected, an eight-step ladder was detected for wild-type rBAT (supplemental Fig. 1, lanes 2–9), confirming that under these experimental conditions all rBAT cysteines are accessible to mPEG.
To analyze the disulfide connectivity of rBAT, single Cys/Ser rBAT mutants (C18S to C242S, and C571S to C685S) were constructed. Cys-273 was changed to Ala (C273A) because mutation to Ser generated a new and used N-glycosylation acceptor site (data not shown). All cysteine mutants in this study did associate via a disulfide link with b0,+AT (data not shown and Fig. 2), with the expected exception of C114S. The mutants and the wild type, together with b0,+AT, were expressed in HeLa cells, labeled with [35S]Met/Cys, pegylated under denaturing conditions, and immunoprecipitated with a specific antibody against b0,+AT or, for the C114S mutant, against rBAT. As a control, parallel samples were alkylated with NEM. The precipitates were run in reducing SDS-PAGE, and the pegylation pattern was examined. We assumed that cysteines in wild-type rBAT assembled with b0,+AT were oxidized in the form of inter- (with b0,+AT) or intramolecular disulfides. As a starting point, our null hypothesis was that the rBAT ectodomain was fully oxidized in the presence of b0,+AT and that single cysteine mutants of intramolecular disulfides disturbed only the oxidation of its partner cysteine residue. As Cys-18 is cytosolic and Cys-114 is disulfide-linked with b0,+AT, we expected only one mPEG bound to wild-type rBAT. Single mutants should be modified with two mPEGs (to Cys-18 and to the now unpaired cysteine), with the exceptions of C18S (expected to be unmodified) and C114S (expected to be like the wild type). The results were partially consistent with the initial hypothesis (Fig. 2A). Mostly, there was no mPEG attached on C18S, one mPEG on the wild type and C114S, and two mPEGs on C571S to C685S. In contrast, the major C242S and C273A bands had three and four mPEGs attached (Fig. 2A, lanes 4–5), with similar pegylation in both mutants. The simplest interpretation of the data is as follows: 1) if present, Cys-18 is reduced and pegylated; 2) the ectodomain of the wild type, C18S, and C114S rBAT contains three intramolecular disulfide bonds; 3) Cys-571 to Cys-685 form two disulfides among them and, when one of these cysteines is unpaired, this does not appear to affect the other disulfides; and 4) Cys-242 and Cys-273 are disulfide-linked, and unpairing of any of them precludes formation of at least one of the other two disulfides, e.g. the pegylated cysteines in C242S band 3 might be Cys-18, Cys-273, and any Cys from Cys-571 to Cys-685. The proposed structural model of rBAT (20) and the fact that Cys-242 and Cys-273 are the only cysteines not absolutely conserved in the ectodomain also support the Cys-242-Cys-273 disulfide.
FIGURE 2.
Pegylation of cysteine rBAT mutants. HeLa cells were transfected with WT or mutant (CXS/A) rBAT together with b0,+AT or b0,+AT alone. After 36 h the cells were labeled, pelleted immediately, and lysed in denaturing solution with either 4 mm mPEG or 20 mm NEM (for clarity, only WT with NEM is shown. The results for the mutants were the same). After incubation for 30 min at 30 °C, the lysates were immunoprecipitated with the anti-b0,+AT antibody, and the precipitates were run under reducing conditions. C114S was immunoprecipitated with the anti-rBAT antibody. Numbers 0 to 5 highlight the more intense rBAT bands detected in each lane. rBATC, core-glycosylated rBAT (after the pulse, only this rBAT form is detected (27)). A, analysis of single mutants. Dashed lines indicate that the Gray/Color Adjust tool of the View mode of the ImageQuant software (see “Experimental Procedures”) was used to linearly increase the intensity of lanes 3 to 5. Irrelevant lanes were removed between lanes 3 and 4 (dotted line). At least three independent experiments were performed with wild-type rBAT, the rBAT mutants, and b0,+AT alone. B, analysis of double mutants. The first gel (two lanes), from a different experiment, is shown to more clearly see the wild-type pegylation. This is also observed in the second gel. However, here the intensity of wild type lanes 1 and 2 was linearly increased with ImageQuant (dashed line) as in A. Irrelevant lanes were removed between lanes 7 and 8 of the second gel (dotted line). Numbers 4 and 5 highlight bands in C242S-C571S and C242S-C666S with four and five mPEGs attached. Number 1 marks the more intense rBAT band detected in all the other mPEG-treated samples. A representative experiment is shown from at least three for the wild type and each rBAT mutant.
Next, we performed pegylation experiments with double cysteine rBAT mutants (Fig. 2B). C242S-C571S and C242S-C666S (and C242S-C673S, not shown) showed a major band with four attached mPEGs (Fig. 2B, second gel, lanes 6–7). As Cys-18 is pegylated and Cys-114 is linked to b0,+AT, the remaining three pegylated cysteines indicated that there were no intramolecular disulfides in these mutants. Strikingly, the more abundant species in C242S-C273A had only one mPEG attached, as in wild-type rBAT. This indicated that it was not the absence of the Cys-242-Cys-273 disulfide per se but the presence of unpaired Cys-242 or Cys-273 that was responsible for the impaired oxidation of the ectodomain. C571S-C666S, C673S-C685S, C571S-C673S, and C666S-C685S also displayed a pattern with mainly one mPEG attached, like wild type rBAT (Fig. 2B). Assuming the formation of the B-domain Cys-242-Cys-273 disulfide, this implied the presence of non-native disulfides between the C-domain and the terminal tail in these mutants. For instance, the band with one mPEG attached in C571S-C666S should contain the Cys-673-Cys-685 disulfide. Within the one-mPEG band in C571S-C673S, the Cys-666-Cys-685 disulfide should be present. It is highly unlikely that both Cys-673-Cys-685 and Cys-666-Cys-685 are present in the native population of rBAT molecules.
Role of Cysteine Residues in Transporter Biogenesis
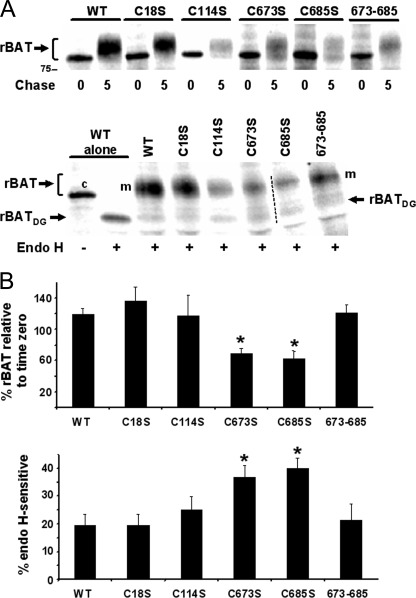
We have reported the maturation kinetics and amino acid transport function of wild-type rBAT-b0,+AT and shown that several rBAT ectodomain cystinuria mutants completely preclude maturation, leading to fast degradation in HeLa cells (27). We did similar pulse-chase experiments in the presence of b0,+AT to dissect the role of cysteines and disulfides of rBAT in the biogenesis of the rBAT-b0,+AT heterodimer. We monitored in-cell stability and maturation of N-glycosylation (as a measure of ER-to-Golgi traffic) of rBAT. The results also revealed Cys-571 to Cys-685 disulfide connectivity (see below). We observed a strong decrease in the rBAT signal coprecipitated with the anti-b0,+AT antibody at 5 h of chase in C242S to C666S single mutants (Fig. 3). The mutants were retained in the ER, as no endoglycosidase H-resistant bands were detected (data not shown). The same happened with double mutants combining C242S to C666S with C242S to C685S, although C242S-C273A disposal was slower (Fig. 3). Therefore, the stability of all these mutants was highly compromised, similar to rBAT type I cystinuria mutants (27). In contrast, C18S, C114S, and wild-type rBAT were not degraded and matured similarly (Fig. 4). This is consistent with the non-conservation of Cys-18, and confirmed that the intermolecular disulfide between rBAT and b0,+AT is not essential (31, 32).
FIGURE 3.
Degradation of cysteine rBAT mutants. A, HeLa cells transiently expressing b0,+AT together with rBAT mutants were labeled and chased for 5 h. Equivalent amounts of radioactivity incorporated into protein were immunoprecipitated with the anti-b0,+AT antibody. The precipitates were run under reducing conditions. The results of representative experiments are presented (top first gel, lanes 1–6; top second gel, lanes 7–12; bottom gel; lanes 1–12). rBATC, core-glycosylated rBAT. The dashed line separates the top two gels. The dotted line indicates that irrelevant lanes were removed at these positions. B, quantification of the rBAT signal coprecipitated with the anti-b0,+AT antibody at time 5 h relative to time zero. The wild-type value, obtained from other experiments (see Fig. 4 and Ref. 27), is shown for comparison. Data are mean ± S.E. of at least three independent experiments for each rBAT species, unless no error bars are depicted. In these cases, the value plotted is the highest from two independent experiments for each mutant. For C242S-C673S and C666S-C673S, the values were 25 and 14%, respectively, in a single experiment.
FIGURE 4.
Stability and maturation of cysteine rBAT mutants. A, the procedures were as in Fig. 3A, except that the anti-rBAT antibody was used for C114S rBAT and for rBAT in the absence of b0,+AT. At 5 h, chase parallel samples were treated with endoglycosidase H (Endo H). The result of a representative experiment is presented. The first two lanes of the bottom gel show the complete endoglycosidase H sensitivity of rBAT when expressed in the absence of b0,+AT. The two gels were processed simultaneously. In the bottom gel, the intensity of the last two lanes was linearly increased with ImageQuant (dashed line). rBAT, core- and mature-glycosylated rBAT; m, mature glycosylated rBAT; c, core glycosylated rBAT; rBATDG, deglycosylated rBAT. B, top panel, the quantification of the rBAT signal coprecipitated with the anti-b0,+AT antibody at time 5 h relative to time zero is shown. The core-glycosylated band and the mature glycosylated rBAT smears from the lanes without endoglycosidase H were quantified together. Bottom, deglycosylated and mature rBAT bands from the endoglycosidase H-treated 5 h chase lanes were quantified. Data were calculated as follows: % endo H-sensitive rBAT = 100 × [rBATDG/(rBATDG + rBATm)]. Both in the top and bottom graphs, data are mean ± S.E. of at least five experiments for each rBAT species, with the exception of C18S (n = 4). Shown are significant differences compared with the wild type (unpaired Student's t test). *, p < 0.005 for the top graph; and *, p < 0.05 for the bottom graph.
The cysteine mutants within the C-terminal tail had a distinct effect on rBAT-b0,+AT biogenesis (Fig. 4). C673S-C685S was the only mutant with wild type-like behavior in stability and maturation. In contrast, C673S and C685S had similar defects. Both were capable of maturation, but to a limited extent, compared with the wild type, and their stability was also compromised, although much less than rBAT mutants containing Cys-242 to Cys-666. These results, combined with the pegylation experiments showing oxidation of all the ectodomain cysteines and the presence of the Cys-242-Cys-273 disulfide, indicated that the three intramolecular disulfides of rBAT in the presence of b0,+AT were Cys-242-Cys-273, Cys-571-Cys-666, and Cys-673-Cys-685. Absence of the first two disulfides dramatically reduced stability, leading to ER retention and degradation. The C-terminal disulfide Cys-673-Cys-685 was not required for biogenesis of the transporter, but the C673S and C685S single mutants displayed reduced stability and maturation. Unpaired Cys-673 or Cys-685 could impair a local folding element, which, however, was maintained when both cysteines were replaced by serine.
Next, we measured l-cystine transport in HeLa cells expressing wild type and cysteine rBAT mutants with b0,+AT (Fig. 5). The data were comparable between groups because of the similar transfection efficiency (27). All mutants that did not mature were unstable (see Fig. 3) and failed to elicit significant cystine transport. C18S and C114S had very similar transport activity compared with the wild type. C673S-C685S, C673S, and C685S were functional. The double mutant induced higher transport activity than the single ones, as expected from the previous results (see Fig. 4). Overall, this functional assay supported our previous conclusions, which help to explain how the cystinuria mutations C666W, C673R, and C673W (34–36) may cause the disease.
FIGURE 5.
Transport activity of cysteine rBAT mutants. HeLa cells were transfected with b0,+AT and the wild type or rBAT mutants, and after 36 h cystine transport was measured for 2 min (see “Experimental Procedures”). Data were calculated as the difference between the uptake in each group minus the uptake in cells transfected with b0,+AT alone (which was not different from the transport in vector-transfected cells). The percentage of transport activity compared with wild-type rBAT of a representative experiment is shown. Data are the mean ± S.E. of four replicas per group. Three independent experiments gave similar results.
Oxidation of Single Intramolecular rBAT Disulfides in the Presence of b0,+AT
Wild-type rBAT and the double mutants containing C571S to C685S had mainly one mPEG attached in the presence of b0,+AT. Minor bands with two and three mPEGs attached were also detected (Fig. 2, and see also below, Figs. 6, 7A, and 8). Three rBAT disulfide mutants (DS) were constructed containing just one of the natively paired cysteines (Cys-242-Cys-273, DS1; Cys-571-Cys-666, DS2; Cys-673-Cys-685, DS3), and also Cys-18 and Cys-114. The DS mutants expressed with b0,+AT were retained in the ER, displayed a greatly compromised stability, and did not induce any transport activity (data not shown). Next, pegylation of these mutants was tested (Fig. 6). More than a third of DS2 and DS3 rBAT molecules did not form the intramolecular disulfide (bands with two and three mPEGs attached). In contrast, DS1 showed mainly the band with one mPEG (less than 5% in bands 2 and 3) (Fig. 6), suggesting that Cys-242-Cys-273, but not Cys-571-Cys-666 and Cys-673-Cys-685, is stably formed without the other two disulfides.
FIGURE 6.
Pegylation of rBAT disulfide mutants (DS) in the presence of b0,+AT. HeLa cells were transfected with WT or mutant rBAT together with b0,+AT. After 36 h the cells were labeled, pelleted immediately, and lysed in denaturing solution with either 4 mm mPEG or 20 mm NEM. For clarity, only WT with NEM is shown. The results for the mutants were the same). After incubation for 30 min at 30 °C, the lysates were immunoprecipitated with the anti-b0,+AT antibody, and the precipitates were run under reducing conditions. Lanes 1–5 and lanes 6–8 belong to two different experiments. For each mutant, at least four experiments were done, and all gave similar results. Numbers 1 to 3 highlight the pegylated bands. rBATC, core-glycosylated rBAT; DS1, DS2, DS3, rBAT mutants with only Cys-242 and Cys-273, Cys-571 and Cys-666, Cys-673 and Cys-685, respectively, together with Cys-18 and Cys-114. In lanes 6–8, the double mutants have the indicated Cys-to-Ser mutations. Bands 1 to 3 were quantified. The relative amount of pegylated bands 2 plus 3 compared with the total amount of pegylated bands (bands 1, 2, and 3) was 4.5 ± 0.9% for DS1; 34.3 ± 3.4% for DS2; 39.1 ± 4% for DS3; 29.2 ± 1.6% for WT; 42.9 ± 3.5% for C242S-C273S; 26.2 ± 3.6% for C571S-C666S; and 27.5 ± 1.3% for C673S-C685S. The data are mean ± S.E. of at least four experiments for each rBAT species. There were significant differences when the value for DS1 was compared with any of the other six rBAT species (unpaired Student's t test, p < 0.005).
FIGURE 7.
Pegylation of rBAT in the absence of b0,+AT. A, HeLa cells were transfected with WT rBAT alone, b0,+AT alone, or WT or the C571S-C666S mutant together with b0,+AT. After 36 h the cells were labeled, chased for 3 h, and immediately pelleted and lysed in denaturing solution containing either 4 mm mPEG or 20 mm NEM. (The result of C571S-C666S with NEM was the same as for WT). After incubation for 30 min at 30 °C, the lysates were immunoprecipitated with the anti-b0,+AT antibody or the anti-rBAT antibody (WT alone group), and the precipitates were run under reducing conditions. One representative experiment of three is shown. Numbers 1 to 4 correspond to the rBAT alone-pegylated bands. Observe that band 3 runs as a smear. The letter m indicates mature glycosylated rBAT band with 1 mPEG attached. ● highlight bands that are not rBAT-specific, as they appear both in rBAT together with b0,+AT and in the b0,+AT alone samples (compare lanes 5 and 12). The lower of these bands is a b0,+AT dimer. The bracket encompasses bands common to lanes 6–11 and 13-14 which, therefore, are not rBAT-specific. Bands marked with ● and brackets were also observed, as expected, in Fig. 2. rBATC, core-glycosylated rBAT. B, HeLa cells were transfected only with wild-type rBAT. After 36 h the cells were labeled, chased for 5 h, and immediately pelleted and lysed in denaturing solution containing either 4 mm mPEG or 20 mm NEM. After incubation for 30 min at 30 °C, equivalent amounts of radioactivity incorporated into protein were immunoprecipitated with the anti-rBAT antibody, and the precipitates were run under reducing conditions. rBATc was observed. dMNJ (1 mm) was present throughout the pulse-chase. Numbers on the right side mark pegylated rBAT bands. One representative experiment of two is shown.
FIGURE 8.
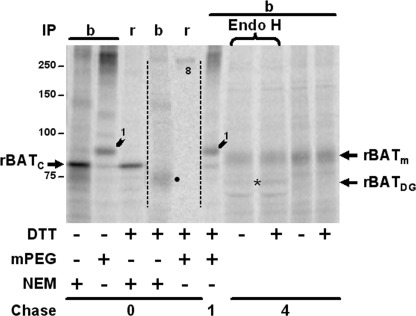
Posttranslational oxidative folding of rBAT in the presence of b0,+AT. HeLa cells were transfected with wild-type rBAT and b0,+AT. After 36 h the cells were labeled in the presence or absence of 5 mm DTT (DTT ±) and chased without DTT. Immediately after labeling or after 1 h of chase, the cells were pelleted and lysed in denaturing solution containing either 4 mm mPEG or 20 mm NEM (only the NEM samples at 0 h chase are shown), and incubated for 30 min at 30 °C. Cells chased for 4 h were processed as in Fig. 4A. They were treated (lanes 7 and 8) or not treated (lanes 9 and 10) with endoglycosidase H (Endo H), immunoprecipitated (IP) with the anti-b0,+AT (b) or the anti-rBAT (r) antibody, and run under reducing conditions. A representative experiment of three is shown. The intensity of lanes 4 and 5 was linearly increased with ImageQuant (dashed line). Numbers 1 and 8 mark the corresponding pegylated bands. Number 8 represents reduced and pegylated wild-type rBAT. ● highlights dimeric b0,+AT as in Fig. 7A. The asterisk marks rBATDG (deglycosylated rBAT). rBATm, mature glycosylated rBAT; rBATC, core-glycosylated rBAT.
Oxidation of rBAT in the Absence of b0,+AT
Misfolded and/or unassembled secretory and membrane proteins are degraded via the endoplasmic reticulum-associated degradation (ERAD) pathway. rBAT is degraded via ERAD in the absence of b0,+AT (27). It is believed that this is due to its unassembled state and that it is b0,+AT assembly per se that prevents rBAT degradation. However, it is not known whether unassembled rBAT is folded. To test this, we performed pegylation experiments in HeLa cells expressing rBAT in the presence or absence of b0,+AT and b0,+AT alone. If rBAT were to be fully oxidized in the absence of b0,+AT, a major band with two mPEGs attached (to Cys-18 and Cys-114) was expected. In fact, pegylated rBAT bands corresponding to one to four mPEGs attached were found. In contrast, the major rBAT band had one mPEG attached when b0,+AT was present (Fig. 7A, compare lanes 2–4 with lanes 6–8 and see also Figs. 2 and 6). Five and six mPEGs attached to unassembled rBAT were observed in some experiments but with much less intensity (data not shown). The major pegylated bands in unassembled rBAT had three and four mPEGs (Fig. 7A, lanes 2–4). This implied that, even if two of the pegylated cysteines were Cys-18 and Cys-114 (a reasonable guess), at least one intramolecular disulfide was not present in unassembled rBAT. Experiments performed in stable MDCK cells expressing much lower amounts of rBAT alone or rBAT together with b0,+AT (25, 27) gave similar results (data not shown). Between 60 and 75% of the unassembled rBAT molecules did not form disulfide-linked homodimers or aggregates, as observed when NEM-treated or pegylated samples from HeLa cells expressing only rBAT were run in non-reducing SDS-PAGE (supplemental Fig. 2). The pegylation of unassembled rBAT did not change during the chase, and the signal decreased evenly in intensity because of degradation (Fig. 7A, lanes 2–4). For comparison, the fate of pegylated wild-type and C571S-C666S rBAT together with b0,+AT is shown. Pegylation of C571S-C666S remained similar during the chase, the signal decreasing evenly because of degradation (Fig. 7A, lanes 9–11). In contrast, the major band carrying one mPEG in wild-type rBAT linked to b0,+AT was maintained and matured because of traffic through the Golgi (Fig. 7A, lanes 7–8). The minor bands with no, two, and three mPEGs attached disappeared during the chase, most likely because of oxidation of the reduced cysteines to render the mature rBAT band with one mPEG (Fig. 7A, lanes 6–8).
We increased the time window for rBAT oxidation by inhibiting its degradation. ER-mannosidases are essential for ERAD (37). The ER-mannosidase inhibitor dMNJ inhibits rBAT degradation. This inhibition does not allow ER exit of unassembled rBAT (27). Inhibition of ERAD by dMNJ did not change the pegylation of unassembled rBAT in the absence of b0,+AT (Fig. 7B), suggesting that a longer time in the ER environment was not sufficient for folding of rBAT alone. We concluded that unassembled rBAT remained within the ER as an ensemble of different, mainly monomeric, redox species, unable to oxidize its ectodomain.
Posttranslational Oxidative Folding of rBAT in the Presence of b0,+AT
We attempted to monitor the oxidative folding pathway of rBAT in the presence of b0,+AT performing short pulses (3 min, within the time range needed to synthesize full-length rBAT) followed by a short chase (supplemental Fig. 3). rBAT was disulfide-linked with b0,+AT immediately after the pulse. Moreover, rBAT was completely oxidated at 1 min of chase, and no intermediates were detected, suggesting that both assembly and ectodomain oxidation occurred either cotranslationally or shortly after translation. Then, we explored the related question of whether rBAT was able to oxidize its ectodomain and to associate with b0,+AT in a posttranslational manner. To this end, we did the pulse in the presence of DTT and the chase without the reducing agent (Fig. 8, lanes 3–6, 8, and 10). This strategy recapitulates (at least qualitatively) the oxidative folding of other proteins (30, 38). After the pulse, rBAT was detected as a totally reduced molecule (Fig. 8, see the 8-pegylated band in lane 5) not disulfide-linked with b0,+AT (Fig. 8, lanes 3–5). After 1 h of chase, rBAT was fully oxidized, as judged by the pegylation pattern, and linked with b0,+AT (Fig. 8; lane 6). A chase as short as 1 min also allowed detection of fully oxidized b0,+AT-linked rBAT without the apparent presence of intermediates (data not shown). b0,+AT-associated rBAT matured similar to rBAT synthesized in the absence of DTT (Fig. 8, compare lanes 7–10). Thus, reduced rBAT was able to posttranslationally associate with b0,+AT, form three intramolecular disulfides, and mature efficiently.
DISCUSSION
We have examined 1) the number and identity of the intramolecular disulfides of rBAT in the presence of b0,+AT; 2) the role of these disulfides and of the individual cysteine residues in stability and traffic of the transporter; and 3) whether the oxidative folding of rBAT differs between its unassembled and assembled states. We used cysteine pegylation to tag reduced cysteines in rBAT and pulse-chase and functional studies to analyze stability and trafficking.
The ectodomain of rBAT has three consecutive disulfide bonds. Cys-242-Cys-273 is in the B-domain, an ∼90-residue-long globular insertion between the third β-strand (Aβ3) and the third α-helix (Aα3) of the TIM barrel A-domain. Modeling of domain B with the Bacillus cereus α-1,6-glucosidase as a template suggested the presence of this disulfide (20). Domain B is present in most α-amylases and forms the substrate-binding cleft together with the central domain A (39, 40). Some α-amylases have a disulfide in domain B, which might be important for enzyme stability (40). In this sense, the double mutant C242S-C273A and the single mutants C242S and C273A are rapidly degraded, indicating a destabilizing effect (Figs. 3 and 5). Pegylation of C242S-C273A and wild-type rBAT is similar, suggesting that C242S-C273A does not influence formation of the other two disulfides. However, the single mutants C242S and C273A (and combinations of C242S with C571S, C666S, and C673S) cause the prevalence of more reduced b0,+AT-linked rBAT forms (Fig. 2, A and B). This is surprising because domains B and C are located at opposite sites of the central TIM barrel (20, 39), and the other two disulfides are Cys-571-Cys-666, joining the C-domain and the C-terminal tail, and Cys-673-Cys-685 in the C-terminal tail. The absence of the Cys-242-Cys-273 disulfide may cause misfolding of domain B but not misfolding of more C-terminal regions unless Cys-242 or Cys-273 are unpaired. Without its native partner, these cysteines may disturb formation of the C-terminal disulfides. The domain B disulfide may form first, preventing Cys-242 and Cys-273 from making contact with more C-terminal, as yet unfolded, regions.
The rBAT tail has no homology to known sequences. Cys-571-Cys-666 connects domain C with this tail. Cys-571 lies within the β1 strand of domain C (Cβ1). Cβ1, Cβ2, and Cβ3 may form the mainly hydrophobic contacts between the C and the A domains (Aα6 to Aα8) (20). Stabilization of the tail closer to domain C and the A-C interface by the Cys-571-Cys-666 disulfide seems to be essential for biogenesis because Cys-571 and Cys-666 single and double mutants are quickly degraded (Fig. 3). Cys-673-Cys-685 is not important per se for biogenesis (Figs. 4 and 5). Although it shows at least a 30% decrease in transport activity, it is difficult to ascertain, using transient transfections, whether this is significant. Strikingly, the single mutants do show reduced stability, maturation, and function compared with wild-type and C673S-C685S rBAT, although they clearly differ from the much more dramatic effects of the other cysteine mutants analyzed (Figs. 3, 4, and 5). In the context of the stabilization role of the Cys-571-Cys-666 disulfide, the 11-residue loop within the Cys-673-Cys-685 disulfide could be important for a late folding event, perhaps related to the ER exit of the transporter (see the compromised maturation of the C673S and C685S transporters in Fig. 4). This loop might still be stable enough in the context of C673S-C685S but not in C673S and C685S. The importance of this loop is highlighted by the fact that deletion of Cys-673 to Cys-685 leads to fast degradation, no maturation and no induction of transport activity3.
The results point to a possible oxidative folding pathway for rBAT-b0,+AT. Given that Cys-114 is the first extracellular cysteine from rBAT, and that it is far away from the next one, Cys-242, it is likely that the intermolecular disulfide between rBAT and b0,+AT forms first and cotranslationally. This is supported by the very fast detection of the disulfide-linked heterodimer (supplemental Fig. 3 and (27)). However, it is not needed for oxidation of the intramolecular disulfides because the C114S mutant and wild-type rBAT look alike in the analysis, and C114S does not decrease transport activity. Therefore, non-covalent interactions between rBAT and b0,+AT suffice for a functional complex. The pegylation of C242S-C273A, C571S-C666S, and C673S-C685S is very similar (Fig. 2B), suggesting that any one of the three disulfides could form independently of the others. However, to avoid non-native contacts between unpaired Cys-242 or Cys-273 with more C-terminal cysteines, formation of Cys-242-Cys-273 may initiate the intramolecular oxidation pathway. This is strongly supported by the fact that Cys-242-Cys-273 is completely oxidized in the absence of the other two disulfides (Fig. 6). In contrast, pegylation of Cys-571-Cys-666 and Cys-673-Cys-685 in the absence of the other two intramolecular disulfides is very similar to wild-type rBAT and double cysteine mutants (Figs. 6, 2B, and 7A). These results suggest that, after the complete oxidation of Cys-242-Cys-273, the oxidation of Cys-571-Cys-666 and/or Cys-673-Cys-685 in wild-type rBAT is posttranslationally stabilized (see also Fig. 7A). Actually, pegylation of C571S-C673S and C666S-C685S is also similar to the native disulfide mutants C571S-C666S and C673S-C685S, indicating the presence of non-native disulfides in C571S-C673S and C666S-C685S, most likely between the corresponding unpaired cysteines (Fig. 2B). The presence of non-native disulfides within the folding on-pathway of some disulfide-rich proteins has been reported both in vitro and in vivo (38, 41, 42). Together, these data raises the possibility that b0,+AT posttranslationally controls the connectivity of C-domain and tail cysteines, irreversibly shifting oxidative folding toward native disulfides Cys-571-Cys-666 and Cys-673-Cys-685. If so, assembly with b0,+AT may stabilize the interactions of the rBAT tail with the A-C interdomain interface and the C domain.
Earlier studies showed that rBAT expressed alone (unassembled rBAT) is degraded via the ERAD pathway, and that heterodimerization blocks degradation (27). b0,+AT could mask an exposed region in unassembled rBAT acting as a degradation determinant, similar to unassembled TCRα (43). An alternative and non-exclusive hypothesis is that unassembled rBAT is recognized as an unfolded polypeptide. Here we show that this is also a possibility because unassembled rBAT does not complete the oxidative folding of its ectodomain (Fig. 7A). The pegylation shifts from the more prominent three and four mPEG bands in unassembled rBAT to the more intense one mPEG band in b0,+AT-associated rBAT. The pegylated bands 1 to 4 (and the minor ones 5 and 6) reflect the presence within the ER of several unassembled rBAT redox species, which could be in equilibrium. In fact, the major bands with three and four mPEGs attached indicate that at least one intramolecular disulfide is not present. It is tempting to speculate that the ensemble of different redox species in rBAT expressed alone represents the in vivo equivalent of the ensemble of redox intermediates detected in folding studies of small disulfide-rich proteins in vitro (42).
There is evidence for in vivo post-assembly folding of selected subunits in other heteromeric protein complexes (11, 12, 44, 45). More challenging is to analyze, for a given heteromeric complex A-B, what the role of one subunit is, e.g. A, in the folding of the other. Subunit B folding may be completely dependent on assembly with A. On the other hand, subunit A may just facilitate folding of subunit B (that could proceed in the absence of A with properly modified conditions in vivo). Actually, this question has recently been convincingly solved only for the Ig heavy chain CH1 domain. CH1 folding strictly depends on the association with the Ig light chain CL domain (44). In an initial approach to the problem, we show that the redox state of rBAT in the absence of b0,+AT does not change during the chase (Fig. 7A, lanes 2–4). More importantly, the same happened when rBAT degradation was delayed (Fig. 7B). This suggests that folding and degradation of unassembled rBAT are not competing events and that b0,+AT assembly, besides providing more time for wild-type rBAT folding, may have a more active role in that process. Analogous to the CL-dependent folding of CH1 (44), assembly with b0,+AT might be mandatory for oxidation of the rBAT ectodomain.
Recently, Sakamoto and coworkers (46) described an ER exit signal at the cytoplasmic C terminus of b0,+AT, active only after association with rBAT. That study, together with this report and our previous one (27), permits the proposal of a working model on how biogenesis events in b0,+AT and rBAT are coordinated to render an ER exit-competent native complex: 1) b0,+AT folds within the ER and remains stable; 2) fast, probably cotranslational, assembly with unfolded rBAT prevents degradation of this subunit and promotes oxidative folding of its ectodomain (folding may also depend on the calnexin system (27)); and 3) the b0,+AT ER exit signal is activated, facilitating traffic of the complex to the plasma membrane. Future studies should address the detailed description of the oxidative and non-oxidative folding pathways of this heteromeric transporter and how end stages of rBAT ectodomain folding may be coupled with activation of the ER exit signal within b0,+AT.
Supplementary Material
Acknowledgments
We thank Robin Rycroft for editorial support. We also thank the laboratory of Prof. Manuel Palacín (Institute for Research in Biomedicine, Barcelona, Spain), and especially Dr. Marta Pineda, for materials and infrastructure support in the beginning of this project.
This study was supported by Spanish Ministerio de Ciencia e Innovación Grants BFU2006-06788/BMC and BFU2009-07215/BMC (to J. C.).

This article contains supplemental Figs. 1–3.
M. Rius and J. Chillarón, unpublished data.
- HAT
- heteromeric amino acid transporter
- rBAT
- related to b0,+ amino acid transporter
- ER
- endoplasmic reticulum
- NEM
- N-ethylmaleimide
- DS
- disulfide mutant
- ERAD
- endoplasmic reticulum-associated degradation
- dMNJ
- 1-deoxymannojirimycin.
REFERENCES
- 1. Abramson J., Smirnova I., Kasho V., Verner G., Kaback H. R., Iwata S. (2003) Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–615 [DOI] [PubMed] [Google Scholar]
- 2. Gao X., Lu F., Zhou L., Dang S., Sun L., Li X., Wang J., Shi Y. (2009) Structure and mechanism of an amino acid antiporter. Science 324, 1565–1568 [DOI] [PubMed] [Google Scholar]
- 3. Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 4. von Heijne G. (2011) Introduction to theme “membrane protein folding and insertion.” Annu. Rev. Biochem. 80, 157–160 [DOI] [PubMed] [Google Scholar]
- 5. Khushoo A., Yang Z., Johnson A. E., Skach W. R. (2011) Ligand-driven vectorial folding of ribosome-bound human CFTR NBD1. Mol. Cell 41, 682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shao S., Hegde R. S. (2011) A flip turn for membrane protein insertion. Cell 146, 13–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatu U., Braakman I., Helenius A. (1993) Membrane glycoprotein folding, oligomerization and intracellular transport. Effects of dithiothreitol in living cells. EMBO J. 12, 2151–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang N., Daniels R., Hebert D. N. (2005) The cotranslational maturation of the type I membrane glycoprotein tyrosinase. The heat shock protein 70 system hands off to the lectin-based chaperone system. Mol. Biol. Cell 16, 3740–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gajewski C., Dagcan A., Roux B., Deutsch C. (2011) Biogenesis of the pore architecture of a voltage-gated potassium channel. Proc. Natl. Acad. Sci. U.S.A. 108, 3240–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wanamaker C. P., Green W. N. (2007) Endoplasmic reticulum chaperones stabilize nicotinic receptor subunits and regulate receptor assembly. J. Biol. Chem. 282, 31113–31123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapman D. C., Williams D. B. (2010) ER quality control in the biogenesis of MHC class I molecules. Semin. Cell Dev. Biol. 21, 512–519 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell W. B., Li J., Murcia M., Valentin N., Newman P. J., Coller B. S. (2007) Mapping early conformational changes in αIIb and β3 during biogenesis reveals a potential mechanism for αIIbβ3 adopting its bent conformation. Blood 109, 3725–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penn A. C., Williams S. R., Greger I. H. (2008) Gating motions underlie AMPA receptor secretion from the endoplasmic reticulum. EMBO J. 27, 3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palacín M., Kanai Y. (2004) The ancillary proteins of HATs. SLC3 family of amino acid transporters. Pflugers Arch. 447, 490–494 [DOI] [PubMed] [Google Scholar]
- 15. Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. (2004) CATs and HATs. The SLC7 family of amino acid transporters. Pflugers Arch. 447, 532–542 [DOI] [PubMed] [Google Scholar]
- 16. Bröer S., Palacín M. (2011) The role of amino acid transporters in inherited and acquired diseases. Biochem. J. 436, 193–211 [DOI] [PubMed] [Google Scholar]
- 17. D'Angelo J. A., Dehlink E., Platzer B., Dwyer P., Circu M. L., Garay J., Aw T. Y., Fiebiger E., Dickinson B. L. (2010) The cystine/glutamate antiporter regulates dendritic cell differentiation and antigen presentation. J. Immunol. 185, 3217–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., Masuko T., Shimizu T., Ishikawa T., Kai K., Takahashi E., Imamura Y., Baba Y., Ohmura M., Suematsu M., Baba H., Saya H. (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19, 387–400 [DOI] [PubMed] [Google Scholar]
- 19. Kowalczyk L., Ratera M., Paladino A., Bartoccioni P., Errasti-Murugarren E., Valencia E., Portella G., Bial S., Zorzano A., Fita I., Orozco M., Carpena X., Vázquez-Ibar J. L., Palacín M. (2011) Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc. Natl. Acad. Sci. U.S.A. 108, 3935–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fort J., de la Ballina L. R., Burghardt H. E., Ferrer-Costa C., Turnay J., Ferrer-Orta C., Usón I., Zorzano A., Fernández-Recio J., Orozco M., Lizarbe M. A., Fita I., Palacín M. (2007) The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J. Biol. Chem. 282, 31444–31452 [DOI] [PubMed] [Google Scholar]
- 21. Chillarón J., Estévez R., Mora C., Wagner C. A., Suessbrich H., Lang F., Gelpí J. L., Testar X., Busch A. E., Zorzano A., Palacín M. (1996) Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J. Biol. Chem. 271, 17761–17770 [DOI] [PubMed] [Google Scholar]
- 22. Fernández E., Carrascal M., Rousaud F., Abián J., Zorzano A., Palacín M., Chillarón J. (2002) rBAT-b(0,+)AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am. J. Physiol. Renal Physiol. 283, F540–F548 [DOI] [PubMed] [Google Scholar]
- 23. Bauch C., Verrey F. (2002) Apical heterodimeric cystine and cationic amino acid transporter expressed in MDCK cells. Am. J. Physiol. Renal Physiol. 283, F181–F189 [DOI] [PubMed] [Google Scholar]
- 24. Pineda M., Wagner C. A., Bröer A., Stehberger P. A., Kaltenbach S., Gelpí J. L., Martín Del Río R., Zorzano A., Palacín M., Lang F., Bröer S. (2004) Cystinuria-specific rBAT(R365W) mutation reveals two translocation pathways in the amino acid transporter rBAT-b0,+AT. Biochem. J. 377, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reig N., Chillarón J., Bartoccioni P., Fernández E., Bendahan A., Zorzano A., Kanner B., Palacín M., Bertran J. (2002) The light subunit of system b(o,+) is fully functional in the absence of the heavy subunit. EMBO J. 21, 4906–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chillarón J., Font-Llitjós M., Fort J., Zorzano A., Goldfarb D. S., Nunes V., Palacín M. (2010) Pathophysiology and treatment of cystinuria. Nat. Rev. Nephrol. 6, 424–434 [DOI] [PubMed] [Google Scholar]
- 27. Bartoccioni P., Rius M., Zorzano A., Palacín M., Chillarón J. (2008) Distinct classes of trafficking rBAT mutants cause the type I cystinuria phenotype. Hum. Mol. Genet. 17, 1845–1854 [DOI] [PubMed] [Google Scholar]
- 28. Font M. A., Feliubadaló L., Estivill X., Nunes V., Golomb E., Kreiss Y., Pras E., Bisceglia L., d'Adamo A. P., Zelante L., Gasparini P., Bassi M. T., George A. L., Jr., Manzoni M., Riboni M., Ballabio A., Borsani G., Reig N., Fernández E., Zorzano A., Bertran J., Palacín M., and International Cystinuria Consortium (2001) Functional analysis of mutations in SLC7A9, and genotype-phenotype correlation in non-Type I cystinuria. Hum. Mol. Genet. 10, 305–316 [DOI] [PubMed] [Google Scholar]
- 29. Tansey W. P. (2007) Denaturing protein immunoprecipitation from mammalian cells. CSH Protoc. doi: 10.1101/pdb.prot4619 [DOI] [PubMed] [Google Scholar]
- 30. Braakman I., Helenius J., Helenius A. (1992) Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 11, 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chillarón J., Roca R., Valencia A., Zorzano A., Palacín M. (2001) Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am. J. Physiol. Renal Physiol. 281, F995–1018 [DOI] [PubMed] [Google Scholar]
- 32. Deora A. B., Ghosh R. N., Tate S. S. (1998) Progressive C-terminal deletions of the renal cystine transporter, NBAT, reveal a novel bimodal pattern of functional expression. J. Biol. Chem. 273, 32980–32987 [DOI] [PubMed] [Google Scholar]
- 33. Guo Z. Y., Chang C. C., Lu X., Chen J., Li B. L., Chang T. Y. (2005) The disulfide linkage and the free sulfhydryl accessibility of acyl-coenzyme A:cholesterol acyltransferase 1 as studied by using mPEG5000-maleimide. Biochemistry 44, 6537–6546 [DOI] [PubMed] [Google Scholar]
- 34. Bisceglia L., Purroy J., Jiménez-Vidal M., d'Adamo A. P., Rousaud F., Beccia E., Penza R., Rizzoni G., Gallucci M., Palacín M., Gasparini P., Nunes V., Zelante L. (2001) Cystinuria type I. Identification of eight new mutations in SLC3A1. Kidney Int. 59, 1250–1256 [DOI] [PubMed] [Google Scholar]
- 35. Egoshi K. I., Akakura K., Kodama T., Ito H. (2000) Identification of five novel SLC3A1 (rBAT) gene mutations in Japanese cystinuria. Kidney Int. 57, 25–32 [DOI] [PubMed] [Google Scholar]
- 36. Font-Llitjós M., Jiménez-Vidal M., Bisceglia L., Di Perna M., de Sanctis L., Rousaud F., Zelante L., Palacín M., Nunes V. (2005) New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J. Med. Genet. 42, 58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanehara K., Kawaguchi S., Ng D. T. (2007) The EDEM and Yos9p families of lectin-like ERAD factors. Semin. Cell Dev. Biol. 18, 743–750 [DOI] [PubMed] [Google Scholar]
- 38. Jansens A., van Duijn E., Braakman I. (2002) Coordinated nonvectorial folding in a newly synthesized multidomain protein. Science 298, 2401–2403 [DOI] [PubMed] [Google Scholar]
- 39. Fitter J. (2005) Structural and dynamical features contributing to thermostability in α-amylases. Cell Mol. Life Sci. 62, 1925–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Janecek S., Svensson B., Henrissat B. (1997) Domain evolution in the α-amylase family. J. Mol. Evol. 45, 322–331 [DOI] [PubMed] [Google Scholar]
- 41. Arias-Moreno X., Arolas J. L., Aviles F. X., Sancho J., Ventura S. (2008) Scrambled isomers as key intermediates in the oxidative folding of ligand binding module 5 of the low density lipoprotein receptor. J. Biol. Chem. 283, 13627–13637 [DOI] [PubMed] [Google Scholar]
- 42. Chang J. Y. (2011) Diverse pathways of oxidative folding of disulfide proteins: underlying causes and folding models. Biochemistry 50, 3414–3431 [DOI] [PubMed] [Google Scholar]
- 43. Bonifacino J. S., Cosson P., Klausner R. D. (1990) Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63, 503–513 [DOI] [PubMed] [Google Scholar]
- 44. Feige M. J., Groscurth S., Marcinowski M., Shimizu Y., Kessler H., Hendershot L. M., Buchner J. (2009) An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol. Cell 34, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Lith M., Benham A. M. (2006) The DMα and DMβ chain cooperate in the oxidation and folding of HLA-DM. J. Immunol. 177, 5430–5439 [DOI] [PubMed] [Google Scholar]
- 46. Sakamoto S., Chairoungdua A., Nagamori S., Wiriyasermkul P., Promchan K., Tanaka H., Kimura T., Ueda T., Fujimura M., Shigeta Y., Naya Y., Akakura K., Ito H., Endou H., Ichikawa T., Kanai Y. (2009) A novel role of the C-terminus of b 0,+ AT in the ER-Golgi trafficking of the rBAT-b 0,+ AT heterodimeric amino acid transporter. Biochem. J. 417, 441–448 [DOI] [PubMed] [Google Scholar]
- 47. Watanabe K., Hata Y., Kizaki H., Katsube Y., Suzuki Y. (1997) The refined crystal structure of Bacillus cereus oligo-1,6-glucosidase at 2.0 A resolution. Structural characterization of proline-substitution sites for protein thermostabilization. J. Mol. Biol. 269, 142–153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.