Background: The importance of de novo ceramide biosynthesis in maintaining cardiac function is unknown.
Results: Deletion of serine palmitoyltransferase subunit Sptlc2 reduced cardiac ceramide and caused cardiac dysfunction associated with activation of ER stress.
Conclusion: Reduced ceramide content by Sptlc2 deficiency does not protect against lipid toxicity associated with increased saturated acyl CoAs.
Significance: Development of disease by lipotoxicity is caused by a number of changes in lipidome.
Keywords: Ceramide, Fatty Acid, Heart Failure, Phospholipid, Sphingolipid
Abstract
The role of serine palmitoyltransferase (SPT) and de novo ceramide biosynthesis in cardiac ceramide and sphingomyelin metabolism is unclear. To determine whether the de novo synthetic pathways, rather than ceramide uptake from circulating lipoproteins, is important for heart ceramide levels, we created cardiomyocyte-specific deficiency of Sptlc2, a subunit of SPT. Heart-specific Sptlc2-deficient (hSptlc2 KO) mice had a >35% reduction in ceramide, which was limited to C18:0 and very long chain ceramides. Sphingomyelinase expression, and levels of sphingomyelin and diacylglycerol were unchanged. But surprisingly phospholipids and acyl CoAs contained increased saturated long chain fatty acids. hSptlc2 KO mice had decreased fractional shortening and thinning of the cardiac wall. While the genes regulating glucose and fatty acid metabolism were not changed, expression of cardiac failure markers and the genes involved in the formation of extracellular matrices were up-regulated in hSptlc2 KO hearts. In addition, ER-stress markers were up-regulated leading to increased apoptosis. These results suggest that Sptlc2-mediated de novo ceramide synthesis is an essential source of C18:0 and very long chain, but not of shorter chain, ceramides in the heart. Changes in heart lipids other than ceramide levels lead to cardiac toxicity.
Introduction
Ceramide, a sphingolipid metabolite, is a component of plasma membranes and a component of all classes of lipoproteins (1). As an important precursor for complex sphingolipids in neurons, ceramide is required for development and function of neurons (2). In addition, ceramide is a bioactive lipid mediator that modulates signal transduction by altering the localization and activation of membrane-associated receptors (3). As a signaling molecule, ceramide is involved in cytokine- or hormone-mediated signaling, insulin sensitivity, senescence, and apoptosis (4, 5).
Ceramide is mainly generated from fatty acids (FA)2 that are complexed to serine. FAs, especially saturated FAs, are converted to ceramide via a series of reactions mediated by serine palmitoyltransferase (SPT), 3-ketosphinganine reductase, ceramide synthase, and dihydroceramide desaturase. This de novo sphingolipid biosynthetic pathway is activated in the setting of excess cellular palmitate (6) and is likely to be increased in the setting of obesity and diabetes. Although ceramide is often viewed as a toxic lipid (3, 5, 7), its de novo production also gives the cell an alternative metabolic pathway to reduce concentrations of long chain saturated FAs. Saturated FAs are poorly incorporated into triglyceride (TG) and cause apoptosis (8).
SPT is a rate-limiting step in the de novo ceramide biosynthesis pathway and is composed of mainly two subunits, Sptlc1 and Sptlc2, encoding 53- and 63-kDa proteins, respectively (9). Complete deficiency of Sptlc1 or Sptlc2 is lethal (10). A third subunit, Sptlc3, has been reported but its function needs further characterization (11). Recently, ssSPTa and ssSPTb have been identified as the small SPT subunits conferring full enzyme activity (12). Regulation of Sptlc2 expression was characterized previously by Linn et al. (13). By forming a dimer or possibly multimer in the endoplasmic reticulum, each subunit is stabilized and active to produce ceramide (14).
Maintenance of cellular sphingolipids including ceramide is critical for normal function of higher organisms. Partial SPT inhibition due to mutation in Sptlc1 or Sptlc2 causes hereditary sensory neuropathy (15) and autonomic neuropathy type I (16), respectively, associated with morphological defects in axons. Since no significant reduction in ceramide levels in neurons is found with these defects, it suggests that there is a compensatory mechanism to maintain cellular ceramide when SPT function is reduced (15). Moreover, it implies that some change other than ceramide deficiency such as sphingolipid composition is responsible for the neuropathy. The alternative ceramide synthesis pathway is via sphingomyelinase (SMase)-mediated hydrolysis of sphingomyelin (SM).
Tissues either synthesize ceramide and sphingolipids or acquire them from circulating lipoproteins. Liver-specific Sptlc2 deficiency reduced ceramide and SM levels in plasma and liver, although liver function was normal (17). A peripheral tissue like the heart should be able to acquire metabolic and structural lipids from plasma lipoproteins and would be less dependent on endogenous production. We previously reported that the SPT inhibitor myriocin prevents cardiac toxicity associated with excess ceramide (18). Although this treatment reduced ceramide in lipotoxic hearts, it did not alter ceramide levels in normal hearts. These data suggested that the de novo ceramide pathway was only active in the lipid overloaded heart. In this study, we created mice with a cardiomyocyte-specific deficiency of Sptlc2 (hSptlc2 KO), to test whether SPT deficiency alters heart ceramide levels and/or affects the metabolism of other heart lipids.
EXPERIMENTAL PROCEDURES
Materials
Ceramides, dihydroceramide, sphinganine, sphingosine, sphingosine 1-phosphate (S1P), and sphingomyelin (SM), fatty acyl CoAs, and diacylglycerols were obtained from Avanti Polar Lipids (Alabaster, AL). Free fatty acids and fatty acid methyl esters were obtained from Sigma-Aldrich.
Generation of Cardiomyocyte-specific Sptlc2-deficient Mouse
Creation of floxed Sptlc2 has been described previously by Li et al. (17). Floxed Sptlc2 mice on C57Bl/6J background were crossed with myosin heavy chain (MHC)-Cre mice to generate cardiomyocyte-specific Sptlc2 KO (hSptlc2 KO) mice. Identification of mice genotype was confirmed by PCR of the tissue extracts of tail (Fig. 1A). All mice used were male and fed a standard chow diet (Research Diet, New Brunswick, NJ). All procedures were approved by the Gachon University of Medicine and Science Institutional Animal Care and Use Committee (IACUC).
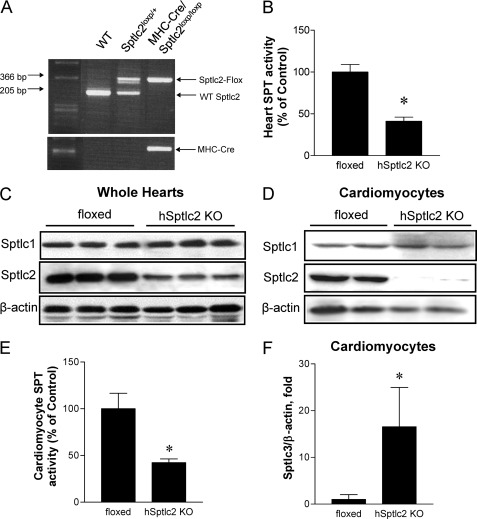
FIGURE 1.
Reduced SPT activity and expression in hSptlc2 KO hearts. Genotyping of floxed Sptlc2 and MHC-Cre transgenic mice confirmed generation of Sptlc2loxP/loxP/MHC-Cre (hSptlc2 KO) mice (A). SPT activity in heart microsome was measured using [14C]serine and palmitoyl CoA as substrates followed by TLC separation of 3-ketosphinganine (B). Sptlc2 proteins in the whole hearts (C) or primary cardiomyocytes (D) of floxed and hSptlc2 KO mice were analyzed by immunoblotting with anti-Sptlc1, anti-Sptlc2 antibody. n = 6, *, p < 0.01 versus 4-month-old floxed mice. SPT activity in primary cardiomyocytes was measured as described in “Experimental Procedures” (E), and Sptlc3 mRNA in cardiomyocytes was quantified by real-time PCR (F). Values are mean ± S.E., n = 4–5, *, p < 0.05 versus cardiomyocytes from floxed mice.
SPT Activity Analysis
Mouse hearts were homogenized in 10 mm HEPES (pH 8.3), 5 mm dithiothreitol, 2.5 mm EDTA (pH 7.4), and 50 μm pyridoxal 5′-phosphate. SPT activity was measured with [14C]serine and palmitoyl CoA as substrates as described previously (19).
Isolation of Primary Cardiomyocytes and Cell Culture
Primary cardiomyocytes and non-cardiomyocytes were prepared as reported previously (20). Briefly, the hearts were isolated from anesthetized mice and quickly connected to a Langendorff perfusion system. Hanks' balanced salt solution buffer was gassed with 95% O2-5% CO2 and heart tissue was digested with 0.5 mg/ml collagenase (Roche Applied Science) in Hanks' balanced salt solution for 20 min. Ventricular tissue was removed and agitated to separate the cells. Cardiomyocytes were isolated by low speed centrifugation at 180 × g for 1 min. After isolation of cardiomyocytes, non-cardiomyocytes were isolated by centrifugation of the supernatant at 1000 × g for 10 min. AC16 cardiomyocytes were grown in DMEM/F12 media containing 12.5% FBS and incubated at 37 °C in an atmosphere containing 5% CO2/95% air (21).
Echocardiography
Cardiac function was measured on conscious male mice by two-dimensional echocardiography using Sonos 5500 system; Philips Medical Systems (Andover, MA). Echocardiographic images were analyzed off-line by a single observer blinded to the genotypes of the mice (22).
Plasma Glucose and Plasma Lipids
Plasma glucose was measured by glucometer (Roche, Indianapolis, IN). Plasma triglycerides (TG) and cholesterol levels were measured enzymatically by TG and cholesterol assay kits (Wako chemicals, Richmond, VA).
Measurement of Cardiac Lipids
For cardiac lipid analysis, the hearts were isolated from the mice, 20 mg of tissues were homogenized in PBS and the internal standard (C17:0 cer, C19:0–19:0 DAG, or C17:0 acyl-CoA, respectively) was added. Ceramide and DAG were extracted by addition of chloroform and methanol (2:1) as described previously by Bligh and Dyer (23). Collected organic phase was evaporated under N2 gas and reconstituted in 100 μl of 0.1% formic acid in methanol, then analyzed by LC/MS/MS. Separately, the lipid extracts were run on silica thin-layer chromatography (TLC), and phospholipids were scraped and the composition of FAs and phospholipids was measured by GC/MS as described previously (24). For analyses of sphingolipid and fatty acyl CoAs, electrospray ionization mode was performed in the positive mode as described previously by Yoo et al. (25) and Magness et al. (26), respectively. Atmospheric pressure chemical ionization (APCI) was performed in the positive mode for DAG analysis as described previously by Ye et al. (27) AC16 cardiomyocyte cells (21) were labeled with 10 μCi [14C]palmitic acid (Perkin Elmer Life Sciences). Lipids were extracted by chloroform: methanol (2:1) and organic phase were loaded onto silica TLC (Partisil LK5D, Whatman) and lipids were separated by mobile phase chloroform:ethanol:water:triethylamine (30:35:7:35, v/v). Autoradiography was performed by exposing the TLC plate to x-ray film.
Gene Expression
Total RNA were isolated from mice heart and homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized by the iScript cDNA synthesis Kit (Bio-RAD). Real-time PCR analysis was performed in ABI7300 equipment (Applied Biosystems Inc, Carlsbad, CA) by using SYBR Green Master Mix (Toyobo, Japan). Expression of mRNA levels was expressed as a ratio normalized to β-actin.
Glucose Uptake Study
Basal glucose uptake was measured by an intravenous injection of 3 μCi of 2-deoxy-d-[14C]glucose (Perkin Elmer Life Sciences). At 60 min following injection, the hearts were perfused with saline, tissues were isolated, and radioactive counts in the tissue extracts were measured by scintillation counting.
Western Blot Analysis
Western blot analyses on heart extracts were performed as described previously (18). Antibodies against phospho-AKT/PKB(ser473), AKT/PKB, phospho-GSK3β(ser9), and GSK3β were purchased from Millipore. Antibodies against phosphor-AMPKα(Thr172), AMPKα, phosphor-Thr980 PERK, GRP78, and β-actin were from Cell Signaling, antibodies against ATF4 was from Santa Cruz Biotechnology, and antibody against CHOP was from Abcam. Antibody against β-actin was used to normalize the sample loading.
Histology
Hearts from hSptlc2 KO and floxed mice were isolated after 6 h fasting and mounted on paraffin. Midventricular sections were stained with Schiff reagent (Polyscientific, Bay Shore, NY) to identify glycogen in the hearts (periodic acid-Schiff (PAS) staining) (28) or by Masson's Trichrome method to identify collagen for fibrosis (20).
TUNEL Staining
6-month-old hSptlc2 KO hearts were isolated and embedded in paraffin. The tissue sections were deparaffinized by xylene and washed with ethanol and rehydrate sequentially by immersing the sections through graded ethanol washes (100%, 95%, 85%, 70%). The sections were immersed in 0.85% NaCl for 5 min followed by PBS. Detection of apoptosis was performed by the manufacturer's instruction (DeadEndTMeFluormetric TUNEL System, Promega, Madison, WI). DAPI (Invitrogen) was utilized to visualize all nuclei and localized green fluorescence of apoptotic tissue was detected by confocal fluorescence microscopy.
Statistical Analyses
Results are shown as mean ± S.E. The differences between groups were tested by two-tailed unpaired Student t test. p < 0.05 were considered significant.
RESULTS
Generation of Cardiac-specific Sptlc2 Knock-out Mice
We crossed floxed Sptlc2 mice with myosin heavy chain (MHC)-Cre mice (Fig. 1A). Young MHC-Cre/Sptlc2loxP/loxP (hSptlc2 KO) mice appeared normal and did not have grossly altered physical activity. Moreover, hSptlc2 KO mice did not show any significant changes in body weight, plasma glucose, TG, and cholesterol compared with control floxed mice (supplemental Table S1). In hSptlc2 KO hearts, SPT activity was reduced by 60% (Fig. 1B) and Sptlc2 protein levels were also reduced (Fig. 1C). However, 20% of normal levels of Sptlc2 protein were still observed in hSptlc2 KO hearts. To confirm whether this was due to cellular heterogeneity of whole heart, we isolated primary cardiomyocytes and immunoblotted SPT subunits, Sptlc1 and Sptlc2. As shown in Fig. 1D, Sptlc2 proteins were not expressed in hSptlc2 KO cardiomyocytes. Sptlc1 was not altered in whole hearts and primary cardiomyocytes of hSptlc2 KO mice (Fig. 1, C and D).
Adult murine heart is mainly composed of cardiomyocytes as well as a number of other cells (29). SPT activity was reduced 58% in hSptlc2 KO cardiomyocytes (Fig. 1E). This less than complete loss of activity was associated with increased expression of Sptlc3, a third subunit of SPT, in hSptlc2 KO hearts compared with the floxed control (Fig. 1F). In contrast, expression of SPT subunits was not altered in non-cardiomyocytes from hSptlc2 hearts (supplemental Fig. S1). These results suggest that Sptlc2 deficiency reduced SPT activity and the residual activity was from elevated Sptlc3 expression in cardiomyocytes.
Cardiac Sptlc2 Deficiency Reduces Ceramide and Alters Other Lipid Composition
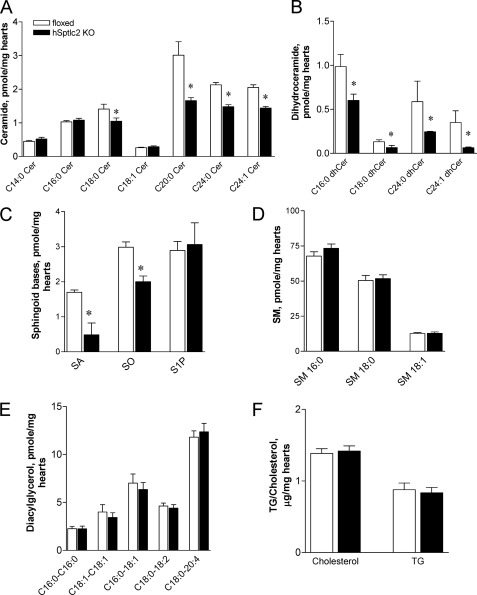
We determined whether hSptlc2 deficiency affected the levels of cardiac ceramide and other sphingolipids. Total ceramide levels in the hearts from hSptlc2 KO mice were reduced by 35% due to a major reduction in C18:0, C20:0, C24:0, C24:1 ceramides (Fig. 2A). Dihydroceramide levels were also reduced by 57% (Fig. 2B). Sphinganine (SA) and sphingosine (SO) were reduced in hSptlc2 KO heart (Fig. 2C). In contrast, shorter chain ceramides, sphingosine 1-phosphate (S1P) (Fig. 2C), and sphingomyelin (SM) (Fig. 2D) were not altered in hSptlc2 KO hearts. However, neither C16 SA nor C16 SO, the products of Sptlc3, was detected in the floxed or hSptlc2 KO hearts (data not shown). Additionally, cardiac diacylglycerol (DAGs), cholesterol, and TG were not altered by hSptlc2 deficiency (Fig. 2, E and F).
FIGURE 2.
Reduced ceramide in hSptlc2 hearts. Lipids in hearts were extracted from 3–4-month-old floxed and hSptlc2 KO mice. Ceramides (cer) (A), dihydroceramide (dhCer) (B), sphinganine (SA), sphingosine (SO), sphingosine 1-phosphate (S1P) (C), sphingomyelin (SM) (D), and diacylglycerol (E) were analyzed by LC/MS/MS. Cardiac cholesterol and TG (F) were measured by enzymatic method as described in “Experimental Procedures.” Values are mean ± S.E., n = 6, *, p < 0.05 versus floxed mice at same ages.
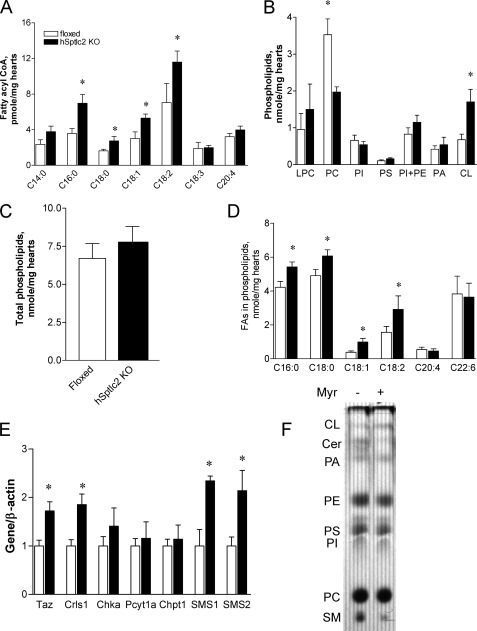
Since fatty acyl CoAs are the substrates for phospholipids, sphingolipids and TG, we examined the fatty acyl CoA profile in the hearts. C16:0, C18:0, C18:1, and C18:2 fatty acyl CoAs were increased in the hearts of hSptlc2 KO mice compared with the floxed mice (Fig. 3A). Although hSptlc2 KO hearts had no change in total phospholipid levels, they had a striking change in phospholipid distribution with decreased phosphatidylcholine (PC) and increased cardiolipin (CL) (Fig. 3, B and C). Additionally hSptlc KO hearts had a greater proportion of long chain saturated FAs in phospholipids (Fig. 3D). While expression of CL synthetic genes: Taffazin (Taz) and cardiolipin synthase (Crls1) and sphingomyelin synthases (SMS1, SMS2) were up-regulated, expression of PC synthetic genes: choline kinase α (Chka), CTP: phosphocholine cytidylyltransferase (Pcyt1a), and choline phosphotransferase 1(Chpt1) were not altered (Fig. 3E).
FIGURE 3.
Cardiac Sptlc2 deficiency increases fatty acyl CoA and alters phospholipid composition. Hearts were isolated from 4-month-old floxed and hSptlc2 KO mice and fatty acyl CoA were extracted and analyzed by LC/MS/MS as described in “Experimental Procedures.” Heart phospholipids were separated by TLC; each phospholipid was scraped and analyzed by GC/MS (B and C). Fatty acids incorporated into phospholipids were analyzed by GC/MS (D). Expression of the gene in cardiolipin synthesis (Taz, Crls1), phosphatidylcholine synthesis (Chka, Pcyt1a, Chpt1) and sphingomyelin synthases (SMS1, SMS2) were measured by RT-PCR (E). AC16 cardiomyocytes were labeled with [14C]palmitate for 4 h and extracted phospholipids were separated by TLC followed by autoradiography (F). Values are mean ± S.E., n = 6, *, p < 0.05 versus floxed mice at same ages. Values are mean ± S.E., n = 6, *, p < 0.05 versus floxed mice at same ages.
To examine whether SPT inhibition alters phospholipid biosynthesis, we labeled AC16 cardiomyocyte cells with [14C]palmitate and examined phospholipid composition of newly synthesized lipids. We found that SPT inhibition by myriocin, an SPT inhibitor, reduced ceramide/SM synthesis, increased CL production, and did not change the amount of labeled PC (Fig. 3F). Thus, while reducing cardiac ceramide, cardiac Sptlc2 deficiency was associated with greater incorporation of labeled palmitate into cardiolipin.
Cardiac Sptlc2 Deficiency Caused Cardiomyopathy
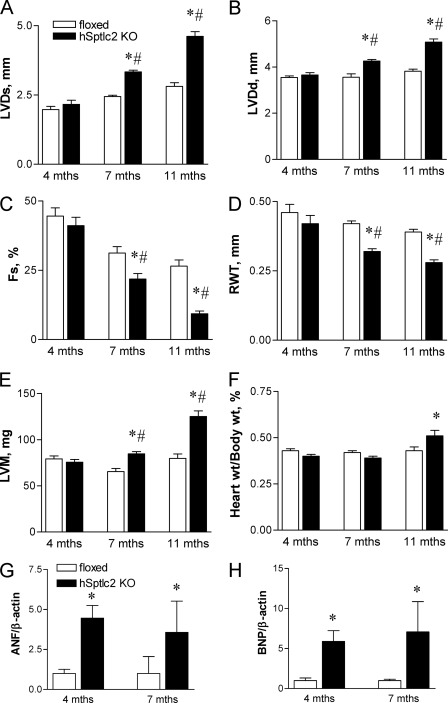
We questioned whether the alterations in cardiac lipids led to changes in function of hSptlc2 KO hearts. Although 4-month-old hSptlc2 KO mice had normal function by echocardiography, older mice developed increased left ventricular systolic dimension (LVDs) and left ventricular diastolic dimension (LVDd) (Fig. 4, A and B). Fractional shortening (Fs) was reduced in 7-month- (31.2 ± 2.3% in floxed versus 21.8 ± 2.0% in hSptlc2 mice) and 11-month-old mice (26.5 ± 2.2% in floxed versus 9.3 ± 1.0% in hSptlc2 mice) (Fig. 4C). In addition, the relative wall thickness (RWT) was reduced (Fig. 4D) and the left ventricular mass (LVM) was increased (Fig. 4E), compared with hearts of floxed controls. hSptlc2 KO hearts were hypertrophied with an increased heart weight/body weight at 11 months (Fig. 4F). This was associated with increased expression of atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP), which were increased in 4- and 7-month old hSptlc2 KO mice, i.e. even before development of cardiac abnormalities found by echocardiography (Fig. 4, G and H). Thus, cardiac-specific deletion of Sptlc2 impaired cardiac function and led to dilated cardiomyopathy.
FIGURE 4.
Development of cardiomyopathy in hSptlc2 KO mice. Cardiac function of floxed and hSptlc2 KO mice at 4- and 7-month-old mice was measured by echocardiography. Left ventricular systolic diameter (LVDs) (A), left ventricular diastolic diameter (LVDd) (B), fractional shortening (Fs) (C), and relative wall thickness (RWT) (D) were measured by echocardiography. Left ventricular mass (LVM) (E) were calculated from the equation of 1.04 × (anterior wall thickness + posterior wall thickness+LVDd)3-LVDd3} × 0.8. Heart weight/body weight was calculated by measuring the weights of hearts from sacrificed mice (F). Expression of cardiac failure markers, ANF (G) and BNP (H) were measured by RT-PCR. Values are mean ± S.E. n = 5–9, *, p < 0.05 versus floxed mice at same age. #, p < 0.05 versus hSptlc2 KO mice at 4 months.
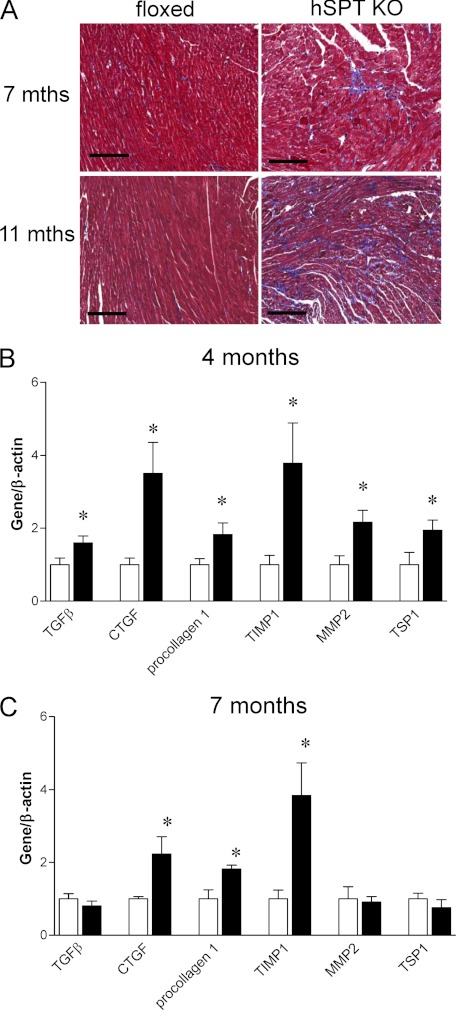
hSptlc2 KO Hearts Have Increased Fibrosis
Hearts from hSptlc2 KO mice had more fibrosis in interstitial areas than did hearts of floxed mice (Fig. 5A). These histological changes were associated with greater expression of the genes involved in formation of extracellular matrix. Hearts from 4-month-old hSptlc2 KO mice had increased mRNA levels of transforming growth factor-β (TGFβ), connective tissue growth factor (CTGF), tissue inhibitor of metalloprotease-1 (TIMP1), matrix metalloprotease-2 (MMP2), and thrombospondin1 (TSP1) (Fig. 5B). In 7-month-old hSptlc2 KO mouse hearts, only CTGF, procollagen1, TIMP1 were up-regulated (Fig. 5C).
FIGURE 5.
Fibrosis in hSptlc2 KO mice. hSptlc2 KO hearts at 7 and 11 months were isolated and stained with Masson's Trichrome for collagen detection (A). Magnification of all panels is at × 20. Expression of fibrotic genes from 4- and 7-month-old floxed and hSptlc2 KO hearts was measured by real time-PCR (B and C). Values are mean ± S.E., n = 5, *, p < 0.05 versus floxed mice. Scale bar, 100 μm.
Effects of Ceramide Reduction on Cardiac Metabolism
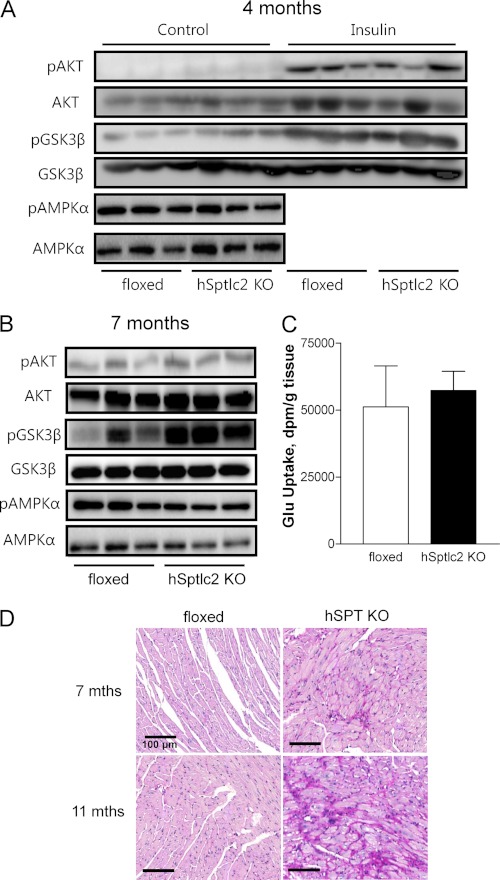
Ceramide has been implicated as a cause of insulin resistance (30, 31). We therefore tested whether ceramide deficiency in the hSptlc2 KO heart led to greater insulin sensitivity and glucose uptake. First we assessed mRNA levels of FA/glucose metabolizing genes - ACO, CPT1, CD36, ACC, LpL, GLUT1, GLUT4, PDK4, PPARα, and PPARγ; none of which were altered (supplemental Table S2). To determine whether reduced ceramide sensitizes cardiac response to insulin, we injected insulin intraperitoneally and checked if there was any change in the insulin signaling pathway. Insulin increased pAKT in both floxed and hSptlc2 hearts to the same levels (Fig. 6A). We then explored whether in vivo glucose uptake was affected by hSptlc2 deficiency. Consistent with the findings in pAKT, in vivo glucose uptake was not changed in hSptlc2 KO hearts (Fig. 6C).
FIGURE 6.
hSptlc2 deficiency increased pGSK3β. Hearts extracts from floxed- and hSptlc2 KO mice at the age of 4- (A) or 7-months (B) were subjected to immunoblotting of pAKT, AKT, pGSK, GSK3β, pAMPKα, and AMPKα in the presence (A) or absence of insulin treatment (A and B). Basal glucose uptake in hearts was measured following intravenous administration of 3 μCi 2-deoxy-d-[14C]glucose. After 40 min, hearts were perfused with saline and excised, and radioactive counts were measured to determine glucose uptake (C). hSptlc2 KO hearts were isolated and stained with periodic acid-Schiff (PAS) staining to identify cardiac glycogen stores in midventricular cross sections (D). Magnification of all panels is at × 20. Values are mean ± S.E., n = 5–6. Scale bar, 100 μm.
Cardiac Sptlc2 Deficiency Increased pGSK3β but Did Not Change Phosphorylation of AMPKα
In failing hearts, pAKT and pGSK3β are increased and this is associated with increased glycogen synthesis (32). To examine whether GSK-3β pathway was associated with Sptlc2 deficiency-mediated cardiac dysfunction, phosphorylation of GSK3β was examined by immunoblot analysis. PGSK3β was elevated in hSptlc2 KO hearts (Fig. 6, A and B). However, pAMPKα, an upstream kinase of GSK3β, was not altered in hSptlc2 KO hearts (Fig. 6, A and B). Inactivation of GSK3β by phosphorylation would be predicted to promote glycogen synthesis in the hearts of hSptlc2 KO mice. We found that hSptlc2 KO hearts had greater glycogen stores than did hearts of floxed control mice (Fig. 6D). AMPKα, an upstream kinase of GSK3α was not altered in hSptlc2 KO hearts (Fig. 6, A and B).
hSptlc2 KO Hearts Have ER Stress
Others have shown in vitro that increases in saturated fatty acyl groups in phospholipids are associated with greater ER stress (33). To determine whether the elevated FA pool altered ER integrity, we examined the expression of unfolded protein response (UPR) genes such as ATF4, CHOP, spliced form of XBP1 (sXBP1), unspliced form of XBP1 (uXBP1) and GRP78 in hearts. Indeed, mRNA levels of ATF4, CHOP, uXBP1, and GRP78 were up-regulated with no change in sXBP1 (Fig. 7A). However, only a subset of UPR protein levels (GRP78, CHOP, and pPERK) were elevated (Fig. 7B). Consistent with these results, we found 2.7-fold more apoptosis in hSptlc2 KO hearts (Fig. 7, C and D).
FIGURE 7.
ER stress was induced in hSptlc2 KO hearts. Total RNAs were isolated from 4-month-old mice and followed by cDNA synthesis. Expression of unfolded protein response (UPR) genes was measured by real time-PCR (A). Western blot analyses of UPR proteins were performed with heart lysates (B). Cardiac ventricular tissues were stained for DNA fragmentation by TUNEL staining (original magnification ×100). Apoptotic cardiomyocytes containing fragmented nuclear chromatin showed bright green nuclear staining (arrows) (C). TUNEL-positive cardiomyocytes were counted and quantified as the number of apoptotic cardiomyocytes per millimeter square of the tissue area (D). A schematic diagram for cardiac dysfunction in hSptlc2 KO mice (E). Values are mean ± S.E., n = 6, *, p < 0.05 versus floxed mice at same ages.
DISCUSSION
Although ceramide is a basic cellular structural and signaling molecule, excess ceramide is widely believed to be a cellular toxin (3–5, 34) associated with toxicity in several tissues including hearts (18). By creating a cardiac-specific deletion of the de novo ceramide synthetic pathway, we gained unique insights into cardiac ceramide production and how the SPT pathway affects cardiac lipids. We found that hSptlc2 KO mice show the following: 1) reduced sphingoid bases and very long chain ceramides, and elevated fatty acyl CoA levels; 2) cardiac dysfunction and fibrosis associated with ER stress and apoptosis, and 3) no change in insulin signaling. Thus, loss of Sptlc2 reduced de novo ceramide synthesis and total heart ceramide content. However, SPT deficiency produced unexpected changes in heart lipid metabolism. In addition, we unexpectedly found cardiac dysfunction that was due either to the altered ceramide composition or increased saturated fatty acyl CoA levels.
hSptlc2 KO mice were viable and did not show any changes in body weight, plasma glucose, TG, and cholesterol. We found that hSptlc2 KO reduced Sptlc2 proteins in whole hearts. Since hearts are composed of various cell types, we isolated primary cardiomyocytes and showed that these cells did not contain Sptlc2 protein. We used these mice to ask two questions: 1) How does Sptlc2 deletion and reduced de novo ceramide synthesis affect heart lipid metabolism? 2) Is this pathway important for normal heart function?
Sphingolipids are important components of plasma membranes and all classes of lipoproteins (35). The liver is the major organ for synthesis and delivery of sphingolipids to the peripheral tissues, but de novo sphingolipid biosynthesis occurs ubiquitously. It is unknown whether loss of de novo synthesis is fully compensated by plasma lipid delivery, akin to cellular cholesterol where uptake and de novo synthesis are finely regulated (36). The alternative route to supply sphingolipids to tissues is via circulating lipoproteins. SM in lipoproteins from intestine or liver is hydrolyzed by cellular SMases to produce ceramide that is a precursor for complex glycosphingolipids and sphingoid bases (37). The importance of each of these pathways and whether they can compensate for each other might be tissue-dependent. Expression of acid and neutral SMase was not altered in hSptlc2 KO hearts (supplemental Fig. S2); this suggested that cardiomyocytes did not compensate by increasing SMases in the setting of Sptlc2 deletion.
Instead Sptlc3, another SPT subunit highly expressed in hearts (11), was dramatically up-regulated in hSptlc2 KO cardiomyocytes suggesting Sptlc3 compensates the deficiency of Sptlc2. Expression of SPT subunits in non-cardiomyocytes was, as expected, not altered. The finding that Sptlc3 up-regulation did not lead to accumulation of C16 sphingoid bases suggested that Sptlc3 is mainly involved in regular ceramide biosynthesis and not production of short chain sphingoid bases. Therefore, the measurement of whole heart lipids reflected the changes in cardiomyocyte sphingolipids (Fig. 2). Cardiac ceramides and sphingoid bases were reduced in hSptlc2 KO hearts. The residual ceramides were likely produced by the basal activities of SMase and up-regulated Sptlc3.
The specific reduction in very long chain and C18:0 ceramides suggests that all ceramides are not equally dependent on the same biosynthetic pathways. Why could SPT deficiency reduce C18:0 and very long chain ceramides without causing a corresponding reduction in other long chain ceramides? The likely option is that the sphingomyelinase pathway preferentially synthesizes ceramides with shorter acyl chains. The ratios of SO/SA and ceramide/dihydroceramide were higher in hSptlc2 hearts. This suggested that SM recycling is increased without transcriptional activation of SMases. Another option is that loss of Spltc2 reduced SA and altered ceramide fluxes perhaps due to different kinetic properties of ceramide synthases. While expression of ceramide synthases (CerS1-CerS6) were not altered (supplemental Fig. S3), each ceramide synthase might have different catalytic affinity for reduced SA. Therefore, it is possible that ceramide synthases preferentially create shorter chain ceramides.
Surprisingly, deletion of Sptlc2 altered other lipids in the heart. Palmitoyl CoA, a direct substrate for the SPT reaction, accumulated in preference to other species. Although it is believed that only a minor fraction of long chain fatty acids are employed for de novo ceramide synthesis (6, 11), these data suggest that loss of SPT actions has more profound changes in lipid metabolism than would be expected.
hSptlc2 KO hearts had increased CL and decreased PC. We found that expression of the genes in CL synthesis were up-regulated. The reasons for this are not obvious, especially since saturated FA decreases CL in cardiomyocytes (38) and CL biosynthetic genes are down-regulated in the failing heart (39). The fact that pharmacological inhibition of SPT also increased CL biosynthesis in AC16 cardiomyocyte cells suggests that CL synthesis is regulated by sphingolipid biosynthesis (Fig. 3F). Both acyl CoAs and CL had an increased concentration of palmitate. Although ceramide biosynthesis would be expected to be a minor pathway for palmitate metabolism, it is likely that more of this saturated fatty acid was shunted into phospholipid biosynthesis. When we inhibited SPT in AC16 cardiomyocytes, we also found greater palmitate incorporation into CL. Thus, inhibition of the de novo ceramide synthesis pathways either directly or indirectly shunted palmitate into phospholipids. Similarly the reasons for reduced PC are an area of future study. Phosphatidic acid (PA) phosphatase converts PA to DAG and would be expected to increase the synthetic ratio of CL/PC. Pah1Δ-deficient yeast had decreased PC due to blockage of PA dephosphorylation and reduced DAG supply for CDP-DAG synthesis (40). However, hSptlc2 deficiency did not alter PA or DAG concentrations. The reduction in PC could be due to its use for production of other lipids; this is consistent with an up-regulation of SM synthases and no change in PC biosynthesis. The fact that decreased PC/PE ratio is a critical modulator of membrane integrity suggests that hSptlc2 deficiency may disrupt membrane integrity and contribute to apoptosis of cardiomyocyte in the same manner (41). Altered phospholipid composition and cardiac function should be verified further.
Increased ceramide is thought to cause cardiac dysfunction (18), so the development of disease in the hSptlc2 KO mice was unexpected. A clue to the causes of this disorder was the changes in ceramide composition. Very long chain C24:0 and C24:1 ceramides are thought to be pro-survival (42–44), whereas elevation of C16:0 long chain ceramide by UV irradiation leads to apoptosis via Bak and Bax (45). Accordingly, reduced very long chain ceramide reduces pro-survival signal and causes induction of apoptosis. Another option for the cause of heart failure is the greater concentration of palmitate in phospholipids and its acyl CoAs. Treatment of cultured cells with palmitate leads to increased palmitate-derived fatty acyl CoA and phospholipids that have been implicated as a cause of ER stress (33). For this reason, when we found greater concentrations of both palmitoyl-CoA and its corresponding phospholipids, we explored whether ER stress-markers were up-regulated and whether there was greater apoptosis in hSptlc2 KO hearts. Our findings are consistent with the in vitro observations of Borradaile et al. demonstrating palmitate-induced ER stress and apoptosis in CHO cells (33).
Several other lipid changes could have been toxic in the hSptlc2 hearts: reduced PC, reduced very long chain ceramide, or increased CL. Recently Cole et al. showed that the absence of phosphatidylethanolamine N-methyltransferase (PEMT) reduced PC biosynthesis. Loss of PEMT improved dilated cardiomyopathy in apoE deficient mice by reducing cardiac TG and lipid droplet accumulation (46). However, no TG change was found in hSptlc2 KO heart, and the reduction in PC was associated with worse heart function. PEMT deficient mice had reduced, not increased CL as was found in hSptlc2 mice. Our findings that increased CL and reduced ceramide by SPT inhibition did not activate ER stress in AC16 cardiomyocytes (Fig. 3F, supplemental Fig. S4) suggest that these lipids are not major culprits responsible for cardiac dysfunction. Instead, it is likely that chronic exposure to elevated fatty acyl CoA is one of major causes for ER-stress mediated cardiac dysfunction found in hSptlc2 KO mice.
hSptlc2 KO mice did not have altered glucose uptake. Accumulation of ceramide has been suggested to cause changes in insulin actions (18, 30), so we expected to find greater insulin sensitivity in these mice, but did not. We also found no change in expression of FA and glucose metabolizing genes. Therefore increased, but not reduced, ceramide affects the insulin signaling pathway.
Heart failure is associated with changes that lead to fibrosis and apoptosis. We suspect that these alterations are secondary to the primary insult, an alteration in heart lipid biology. As was found in other models of chronic heart failure, we found an up-regulation of fibrotic genes and accumulation of collagen in extracellular matrices even before development of cardiac dysfunction. Heart failure is also associated with AKT/GSK3β pathway activation and accumulation of glycogen stores (52, 53). hSptlc2 KO hearts had increased pGSK3β but no changes in its upstream kinases, pAKT or pAMPK. These results suggest that increased pGSK3β in hSptlc2 KO hearts was independent of activation of its upstream kinases, AKT or AMPK.
In conclusion, cardiomyocyte-specific Sptlc2 KO mice had several alterations in cardiac lipids. A reduction in ceramide and altered lipid composition proved that the heart cannot fully compensate for loss of de novo synthesis by uptake of circulating plasma lipoprotein ceramides and Sptlc3 up-regulation. Other changes in the heart lipidome were surprising and suggest that reduced use of palmitate via the SPT pathway shunts the FA into other lipid biosynthetic pathways. We observed an increase in CL and decrease in PC. Increased palmitate in phospholipids and acyl CoA were associated with an expected increase in ER stress. In this model, heart function was diminished and insulin signaling was not altered despite reduced cardiac ceramide. Many changes in the lipidome found in these mice further illustrate the complexity of heart lipid metabolism. These data and those obtained from other models highlight that a single lipid is unlikely to be changed in isolation and that lipotoxicity is likely to be caused by a number of processes.
Supplementary Material
Acknowledgments
We thank the technical assistance of In-Kyung Hong and Hye-Jin Kim for lipid analysis and Ki-Up Lee at Asan Medical center for critical reading of the manuscript and helpful discussion.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2010-0000590, NRF2011-0025903)(to T. S. P.) and HL73029 and HL45095 (to I. J. G.).

This article contains supplemental Figs S1–S4 and Tables S1 and S2.
- FA
- fatty acid
- CL
- cardiolipin
- DAG
- diacylglycerol
- GC/MS
- gas chromatography/mass spectrometry
- LC/MS/MS
- liquid chromatography/tandem mass spectrometry
- MHC
- myosin heavy chain
- PC
- phosphatidylcholine
- S1P
- sphingosine 1-phosphate
- SA
- sphinganine
- SM
- sphingomyelin
- SMase
- sphingomyelinase
- SO
- sphingosine
- SPT
- serine palmitoyltransferase
- TG
- triglyceride.
REFERENCES
- 1. Lightle S., Tosheva R., Lee A., Queen-Baker J., Boyanovsky B., Shedlofsky S., Nikolova-Karakashian M. (2003) Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch. Biochem. Biophys. 419, 120–128 [DOI] [PubMed] [Google Scholar]
- 2. Haughey N. J., Bandaru V. V., Bae M., Mattson M. P. (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim. Biophys. Acta 1801, 878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pettus B. J., Chalfant C. E., Hannun Y. A. (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta 1585, 114–125 [DOI] [PubMed] [Google Scholar]
- 4. Holland W. L., Summers S. A. (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29, 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartke N., Hannun Y. A. (2009) Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50, S91–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanada K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 [DOI] [PubMed] [Google Scholar]
- 7. Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., Unger R. H. (1998) Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273, 32487–32490 [DOI] [PubMed] [Google Scholar]
- 8. Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. U.S.A. 100, 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiss B., Stoffel W. (1997) Human and murine serine-palmitoyl-CoA transferase–cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 249, 239–247 [DOI] [PubMed] [Google Scholar]
- 10. Hojjati M. R., Li Z., Jiang X. C. (2005) Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta 1737, 44–51 [DOI] [PubMed] [Google Scholar]
- 11. Hornemann T., Richard S., Rütti M. F., Wei Y., von Eckardstein A. (2006) Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 281, 37275–37281 [DOI] [PubMed] [Google Scholar]
- 12. Han G., Gupta S. D., Gable K., Niranjanakumari S., Moitra P., Eichler F., Brown R. H., Jr., Harmon J. M., Dunn T. M. (2009) Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. U.S.A. 106, 8186–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linn S. C., Andras L. M., Kim H. S., Wei J., Nagiec M. M., Dickson R. C., Merrill A. H., Jr. (2006) Functional characterization of the promoter for the mouse SPTLC2 gene, which encodes subunit 2 of serine palmitoyltransferase. FEBS Lett. 580, 6217–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hornemann T., Wei Y., von Eckardstein A. (2007) Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? Biochem. J. 405, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCampbell A., Truong D., Broom D. C., Allchorne A., Gable K., Cutler R. G., Mattson M. P., Woolf C. J., Frosch M. P., Harmon J. M., Dunn T. M., Brown R. H., Jr. (2005) Mutant SPTLC1 dominantly inhibits serine palmitoyltransferase activity in vivo and confers an age-dependent neuropathy. Hum. Mol. Genet. 14, 3507–3521 [DOI] [PubMed] [Google Scholar]
- 16. Rotthier A., Auer-Grumbach M., Janssens K., Baets J., Penno A., Almeida-Souza L., Van Hoof K., Jacobs A., De Vriendt E., Schlotter-Weigel B., Löscher W., Vondráček P., Seeman P., De Jonghe P., Van Dijck P., Jordanova A., Hornemann T., Timmerman V. (2010) Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 87, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z., Li Y., Chakraborty M., Fan Y., Bui H. H., Peake D. A., Kuo M. S., Xiao X., Cao G., Jiang X. C. (2009) Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J. Biol. Chem. 284, 27010–27019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., Goldberg I. J. (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park T. S., Panek R. L., Mueller S. B., Hanselman J. C., Rosebury W. S., Robertson A. W., Kindt E. K., Homan R., Karathanasis S. K., Rekhter M. D. (2004) Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110, 3465–3471 [DOI] [PubMed] [Google Scholar]
- 20. Augustus A. S., Buchanan J., Park T. S., Hirata K., Noh H. L., Sun J., Homma S., D'armiento J., Abel E. D., Goldberg I. J. (2006) Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J. Biol. Chem. 281, 8716–8723 [DOI] [PubMed] [Google Scholar]
- 21. Davidson M. M., Nesti C., Palenzuela L., Walker W. F., Hernandez E., Protas L., Hirano M., Isaac N. D. (2005) Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell Cardiol. 39, 133–147 [DOI] [PubMed] [Google Scholar]
- 22. Wang C. Y., Mazer S. P., Minamoto K., Takuma S., Homma S., Yellin M., Chess L., Fard A., Kalled S. L., Oz M. C., Pinsky D. J. (2002) Suppression of murine cardiac allograft arteriopathy by long-term blockade of CD40-CD154 interactions. Circulation 105, 1609–1614 [DOI] [PubMed] [Google Scholar]
- 23. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 24. Wolfe R. R. (1992) Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis, Wiley-Liss, New York [Google Scholar]
- 25. Yoo H. H., Son J., Kim D. H. (2006) Liquid chromatography-tandem mass spectrometric determination of ceramides and related lipid species in cellular extracts. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 843, 327–333 [DOI] [PubMed] [Google Scholar]
- 26. Magnes C., Sinner F. M., Regittnig W., Pieber T. R. (2005) LC/MS/MS method for quantitative determination of long-chain fatty acyl-CoAs. Anal. Chem. 77, 2889–2894 [DOI] [PubMed] [Google Scholar]
- 27. Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., Atcheson B., White M. F., Kraegen E. W., Shulman G. I. (2002) Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 [DOI] [PubMed] [Google Scholar]
- 28. Yoshimura A., Toyoda Y., Murakami T., Yoshizato H., Ando Y., Fujitsuka N. (2005) Glycogen depletion in intrafusal fibres in rats during short-duration high-intensity treadmill running. Acta Physiol. Scand. 185, 41–50 [DOI] [PubMed] [Google Scholar]
- 29. Banerjee I., Fuseler J. W., Price R. L., Borg T. K., Baudino T. A. (2007) Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 293, H1883–1891 [DOI] [PubMed] [Google Scholar]
- 30. Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 31. Chavez J. A., Summers S. A. (2003) Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 419, 101–109 [DOI] [PubMed] [Google Scholar]
- 32. Matsui T., Nagoshi T., Hong E. G., Luptak I., Hartil K., Li L., Gorovits N., Charron M. J., Kim J. K., Tian R., Rosenzweig A. (2006) Effects of chronic Akt activation on glucose uptake in the heart. Am. J. Physiol. Endocrinol. Metab. 290, E789–797 [DOI] [PubMed] [Google Scholar]
- 33. Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., Schaffer J. E. (2006) Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47, 2726–2737 [DOI] [PubMed] [Google Scholar]
- 34. Oskouian B., Saba J. D. (2010) Cancer treatment strategies targeting sphingolipid metabolism. Adv. Exp. Med. Biol. 688, 185–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saito H., Arimoto I., Tanaka M., Sasaki T., Tanimoto T., Okada S., Handa T. (2000) Inhibition of lipoprotein lipase activity by sphingomyelin: role of membrane surface structure. Biochim. Biophys. Acta 1486, 312–320 [DOI] [PubMed] [Google Scholar]
- 36. Maxfield F. R., van Meer G. (2010) Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 22, 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Milhas D., Clarke C. J., Hannun Y. A. (2010) Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 584, 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ostrander D. B., Sparagna G. C., Amoscato A. A., McMillin J. B., Dowhan W. (2001) Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 276, 38061–38067 [DOI] [PubMed] [Google Scholar]
- 39. Saini-Chohan H. K., Holmes M. G., Chicco A. J., Taylor W. A., Moore R. L., McCune S. A., Hickson-Bick D. L., Hatch G. M., Sparagna G. C. (2009) Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid Res. 50, 1600–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han G. S., Wu W. I., Carman G. M. (2006) The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281, 9210–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Z., Agellon L. B., Allen T. M., Umeda M., Jewell L., Mason A., Vance D. E. (2006) The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 3, 321–331 [DOI] [PubMed] [Google Scholar]
- 42. Pewzner-Jung Y., Brenner O., Braun S., Laviad E. L., Ben-Dor S., Feldmesser E., Horn-Saban S., Amann-Zalcenstein D., Raanan C., Berkutzki T., Erez-Roman R., Ben-David O., Levy M., Holzman D., Park H., Nyska A., Merrill A. H., Jr., Futerman A. H. (2010) A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285, 10911–10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., Prieto M., Merrill A. H., Jr., Futerman A. H. (2010) A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 285, 10902–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2010) Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal 22, 1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mullen T. D., Jenkins R. W., Clarke C. J., Bielawski J., Hannun Y. A., Obeid L. M. (2011) Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating postmitochondrial events. J. Biol. Chem. 286, 15929–15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cole L. K., Dolinsky V. W., Dyck J. R., Vance D. E. (2011) Impaired phosphatidylcholine biosynthesis reduces atherosclerosis and prevents lipotoxic cardiac dysfunction in ApoE−/− Mice. Circ. Res. 108, 686–694 [DOI] [PubMed] [Google Scholar]
- 47.Deleted in proof
- 48.Deleted in proof
- 49.Deleted in proof
- 50.Deleted in proof
- 51.Deleted in proof
- 52. Haq S., Choukroun G., Lim H., Tymitz K. M., del Monte F., Gwathmey J., Grazette L., Michael A., Hajjar R., Force T., Molkentin J. D. (2001) Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103, 670–677 [DOI] [PubMed] [Google Scholar]
- 53. Ananthakrishnan R., Moe G. W., Goldenthal M. J., Marín-García J. (2005) Akt signaling pathway in pacing-induced heart failure. Mol. Cell Biochem. 268, 103–110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.