Background: Keratinocyte migration involves the coordinated expression of various integrin heterodimers.
Results: Loss of α6β4 integrin expression impairs cell migration and decreases α2 and α3 integrin subunit expression via transcriptional and translational mechanisms.
Conclusion: Migration of human keratinocytes requires α6β4 integrin-dependent regulation of integrin subunit expression.
Significance: α6β4 integrin controls integrin expression profiles and thereby regulates migration.
Keywords: Cell Migration, Integrins, Keratinocytes, Translation Regulation, Wound Healing
Abstract
Three major laminin and collagen-binding integrins in skin (α6β4, α3β1, and α2β1) are involved in keratinocyte adhesion to the dermis and dissemination of skin cells during wound healing and/or tumorigenesis. Knockdown of α6 integrin in keratinocytes not only results in motility defects but also leads to decreased surface expression of the α2, α3, and β4 integrin subunits. Whereas α2 integrin mRNA levels are decreased in α6 integrin knockdown cells, α3 and β4 integrin mRNAs levels are unaffected. Expression of either α6 or α3 integrin in α6 integrin knockdown cells restores α2 integrin mRNA levels. Moreover, re-expression of α6 integrin increases β4 integrin protein at the cell surface, which results in an increase in α3 integrin expression via activation of initiation factor 4E-binding protein 1. Our data indicate that the α6β4 integrin is a master regulator of transcription and translation of other integrin subunits and underscore its pivotal role in wound healing and cancer.
Introduction
In intact skin, α6β4 integrin mediates the interaction of basal keratinocytes to laminin-332 located at the interface of the epidermis and dermis. The importance of α6β4 integrin in maintaining skin integrity has been demonstrated in mouse knock-out models and human studies. In mice, the loss of α6β4 integrin leads to separation of the epidermal from the dermal layers of the skin at the site of the basement membrane zone, skin blistering, and post-natal lethality (1–5). In humans, mutations in either the α6 or β4 integrin genes is the cause of junctional epidermolysis bullosa (JEB),2 characterized by skin blistering and pyloric atresia (6–14).
In addition to its role in stable adhesion, α6β4 integrin is up-regulated during wound healing and tumorigenesis where it determines skin cell motile behavior. Several other integrins enriched in the basal cells of the epidermis, including α3β1 integrin, a receptor for laminin-332, and α2β1 integrin, a receptor for collagen I, have also been implicated in regulating wound healing of the skin and/or cell migration during tumorigenesis (15–17). Specifically, α3β1 integrin enhances matrix proteolysis during tumorigenesis and, possibly, wound healing (18). Moreover, blocking α3β1 integrin function inhibits skin cell migration in vitro (19). In the case of α2β1 integrin, the story is more complex. There is evidence that α2β1 integrin regulates cell migration by promoting matrix proteolysis (20). In contrast, in the complete absence of α2β1 integrin, tumor metastasis is enhanced, most likely as a result of an inhibition of cancer cell adhesion to collagen (21). Indeed, the latter result emphasizes that a precise regulation of expression of integrins in skin cells is a key regulator of migration in wound healing and metastasis, yet we know little about how such regulation is accomplished.
In the current study, we analyzed the consequences of a targeted knockdown in expression of α6 integrin. Keratinocytes deficient in α6 integrin not only exhibit the same pattern of aberrant motility that we previously observed in cultures of β4 integrin-deficient cells (22), but they also show a loss in α2β1 and α3β1 integrin expression. The current data indicate that α6β4 integrin regulates the transcription of α2 integrin and the translation of α3 integrin.
EXPERIMENTAL PROCEDURES
Cell Culture and Antibodies
Human epidermal keratinocytes, immortalized with human papilloma virus genes E6 and E7, and immortalized β4 integrin-deficient cells, derived from a patient with JEB, were described previously (22, 23). The cells were maintained in defined keratinocyte serum-free medium supplemented with a 1% penicillin/streptomycin mixture (Invitrogen) at 37 °C. GoH3, a rat monoclonal antibody against α6 integrin, was obtained from Beckman Coulter (Miami, FL). J1B5, a rat monoclonal antibody against α6 integrin, was a generous gift from Dr. Caroline Damsky (University of California San Francisco). Mouse monoclonal antibodies against β4 integrin (3E1), α3 integrin (P1B5), and α2 integrin (P1E6), and the rabbit polyclonal antibodies against α3 integrin and α6 integrin were purchased from Millipore (Billerica, MA). The mouse monoclonal antibody against β4 integrin, CD104, was obtained from BD Pharmingen (San Diego, CA). Rabbit monoclonal antibodies against β-actin and 4EBP1 were obtained from Epitomics, Inc. (Burlingame, CA). The rabbit polyclonal antibodies against mTOR, lamin A/C, AKT, phosphorylated AKT (Thr 308), and phosphorylated 4EBP1 (Ser65) were obtained from Cell Signaling Technology (Beverly, MA). The mouse monoclonal and polyclonal antibodies against the N-terminal domain of BP180 were described previously (24). The mouse monoclonal antibody against BPAG1e was described elsewhere (25).
Lentiviral and Adenoviral Constructs
To express shRNA targeted against α6 integrin expression, the BLOCK-iTTM lentiviral RNAi expression system was used (Invitrogen). Two complementary single-stranded DNA oligonucleotides (21-mers) derived from the human ITGA6 gene were synthesized, annealed, and cloned into the pENTRTM/U6 entry vector (Invitrogen). A LR recombination was performed between the entry construct and the pLenti6/BLOCK-iTTM-DEST vector to generate an expression construct. To produce lentivirus, the expression construct was transfected into the 293FT packaging cell line. The lentiviral stock was titered, and keratinocytes were infected at a multiplicity of infection of 1:10 in cell medium. To generate stable clones lacking α6 integrin expression, infected keratinocytes were selected in 1.75 μg/ml of blasticidin. To re-express α6 integrin in the knockdown clones, adenovirus encoding α6 integrin mRNA refractory to the shRNA was generated. cDNA encoding α6 integrin with 2 kb of its 3′-untranslated region was subcloned into the pEGFP-N1 vector (Clontech, Palo Alto, CA). The α6 cassette was subsequently subcloned into the polylinker of the pENTR4 vector (Invitrogen). Four point mutations were generated within the α6 integrin target shRNA sequence using the QuikChange® II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). These point mutations conserved the amino acid sequence of α6 integrin and prevented the refractory construct from being targeted by RNAi machinery. The entry vector containing the refractory α6 sequence was used in a LR recombination reaction with the pAD/CMV/V5-DEST vector (Invitrogen) to generate an expression clone. The expression clone was transfected into 293A cells using Lipofectamine 2000 (Invitrogen). After 10 days, the crude viral lysate was harvested and used to amplify the adenovirus. The amplified viral stock was titered, and keratinocytes were infected at a multiplicity of infection of 1:50 in cell medium. The cells were used for various analyses 48–72 h after infection. To express α3 integrin in the cells, we used either adenovirus encoding CFP-tagged α3 integrin or retrovirus encoding GFP-tagged α3 integrin. The retrovirus was a generous gift of Dr. Michael DiPersio (Albany Medical College). To generate the α3 integrin adenovirus, the coding sequence of α3 integrin and 1.2 kb of its 3′-untranslated region were amplified from wild-type keratinocytes by PCR and subcloned into the pECFP-N1 vector (Clontech, Palo Alto, CA). The α3 integrin cassette was subsequently subcloned into the polylinker of the pENTR4 vector (Invitrogen), and adenovirus was prepared as described above. Adenovirus encoding myristoylated AKT was a generous gift of Dr. Navdeep Chandel (Northwestern University).
Polysome Isolation
Polysomal RNA was isolated from wild-type keratinocytes and α6 integrin knockdown cells as previously described (26). Total polysomal RNA was quantitated for each cell type, and 1 μg of RNA was used for cDNA synthesis. Real time PCR for the ITGA3 or ITGB4 genes was performed. The ribosomal protein S26 was used for normalization.
Quantitative RT-PCR, SDS-PAGE, and Immunoprecipitation
Total RNA was extracted from keratinocytes using the Gene EluteTM Mammalian total RNA Miniprep kit (Sigma-Aldrich) or RNeasy® Plus Mini kit (Qiagen). cDNA synthesis was performed using 1 μg of total RNA and the qScript cDNA synthesis kit (Quanta Biosciences, Inc., Gaithersburg, MD). Quantitative analysis of α2 integrin, α3 integrin, α6 integrin, or β4 integrin mRNA expression was performed using an Applied Biosystems 7000 sequence detection system with SYBR green master mix kit (Invitrogen). GAPDH or S26 mRNA levels served as normalization controls. The ΔΔCt method was used to evaluate transcript levels in the various samples.
Cell extracts from 1 × 106 cells were prepared by solubilization in 1% SDS, 8 m urea, 10% glycerol, 5% β-mercaptoethanol, 25 mm Tris-HCl, pH 6.5. The proteins were separated by SDS-PAGE, transferred to nitrocellulose, and processed for immunoblotting as previously described (23).
Flow Cytometry
The cells were trypsinized, resuspended in a 1:1 mix of phosphate-buffered saline containing normal goat serum, and incubated with mouse monoclonal antibodies against α2, α3, α6, or β4 integrins at room temperature for 45 min. The cells were washed with phosphate-buffered saline and incubated with FITC-conjugated or Cy5-conjugated goat anti-mouse IgG for 45 min at room temperature in the dark. Integrin cell surface expression was analyzed using a Beckman Coulter CyAn flow cytometer (Beckman Coulter Inc., Brea, CA). As a negative control, primary antibody was omitted.
Fluorescence Microscopy
The cells were plated onto glass coverslips and processed for microscopical analyses as detailed previously (27). All of the immunofluorescence preparations were viewed with a Zeiss laser scanning 510 confocal microscope (Zeiss Inc., Thornwood, NY), equipped with a Plan-Apochromat 63× 1.4 oil immersion objective lens. The samples were analyzed with Zen software. Images were exported as TIFF files, and figures were generated using Adobe Photoshop software.
Rac Activity Assay
The level of Rac1 activity in keratinocytes was measured using the G-LISA Rac activation assay (Cytoskeleton, Denver, CO) according to the manufacturer's protocol. Cell extracts were prepared from cells, and total protein for each sample was diluted to a concentration of 0.5 mg/ml. Duplicate samples were prepared for each assay. Rac1 activity was measured using an ELx808 ultramicroplate spectrophotometer (Bio-Tek Instruments, Winooski, VT). Data analyses were performed using Microsoft Excel.
Attachment Assay and Strength of Adhesion Assay
Keratinocyte adhesion to matrix was measured by an adhesion assay described previously (28). Briefly, wells of a 96-well non-tissue culture-treated plate (Sarstedt, Newton, NC) were coated with 200 μl of laminin-332-rich conditioned medium from the rat bladder cell line, 804G, or with 10 μg/ml rat tail collagen I (BD Biosciences) for 2 h at 37 °C. Each well was rinsed three times with phosphate-buffered saline and blocked with 1% bovine serum albumin in phosphate-buffered saline for 1 h at 37 °C. Various keratinocyte populations (wild-type or α6 shRNA clones) were added to the coated wells at 1 × 105 cells/well. After 1 h at 37 °C, the wells were washed with phosphate-buffered saline to remove nonadhering cells. The adherent cells were fixed in 3.7% formaldehyde in phosphate-buffered saline for 15 min at room temperature. The fixed cells were incubated with crystal violet in 20% methanol for 15 min at room temperature and then solubilized with 1% SDS. Absorbance at 570 nm was measured with a Vmax plate reader (Molecular Devices, Menlo Park, CA).
Strength of adhesion was measured by a trypsinization assay in which 1 × 105 cells were plated onto 60-mm tissue culture-treated dishes and allowed to adhere for 18–24 h. The cells were exposed to diluted trypsin (Invitrogen) at a concentration of 0.001% trypsin-EDTA for 2 and 5 min or 0.05% trypsin-EDTA for 15 min. The detached cells were collected and counted using a hemocytometer.
Cell Motility
Single cell motility was measured as described previously (23). Briefly, the cells were plated onto uncoated 35-mm glass bottomed culture dishes (MatTek Corp., Ashland, MA) 18–24 h prior to motility assays. The cells were viewed on a Nikon TE2000 inverted microscope (Nikon Inc., Melville, NY). Images were taken at 2-min intervals over 2 h, and cell motility behavior was tracked using the MetaMorph Imaging System (Universal Imaging Corp., Molecular Devices, Downingtown, PA). Keratinocyte matrix was prepared as previously described (29). For migration on preformed matrices, the cells were allowed to adhere to the matrix for 2 h and imaged over an additional 2 h.
Statistical Analysis
Statistical significance was determined by two-tailed Student's t test. A p value equal to or less than 0.05 was considered statistically significant.
RESULTS
Knockdown of α6 Integrin in Human Keratinocytes
In keratinocytes, α6 integrin preferentially pairs with β4 integrin to form α6β4 integrin, a receptor for laminin-332 (30). However, in β4 integrin-deficient keratinocytes, expression of α6β1 integrin is induced. To assess the consequences of loss of α6β4 integrin expression, without subsequent α6β1 integrin expression, we generated keratinocyte cell lines with reduced expression of the α6 integrin subunit by infecting cells with a lentivirus that encodes shRNA targeted against the α6 integrin coding sequence. Because α6 integrin undergoes alternative splicing to yield two α6 integrin subunits (α6A and α6B) that contain distinct cytoplasmic domains (31), we designed the shRNA to target both α6A and α6B subunits to prevent any complicating effects from expression of, or compensation by, α6B integrin.
Knockdown of α6 integrin expression was evaluated in three randomly selected clones expressing α6 integrin shRNA (Fig. 1, A–D). α6 integrin protein level was decreased between 65 and 85% in these three α6 integrin shRNA clones, and all three showed decreased α6 integrin immunostaining with little or no localization of β4 integrin at the basal surface of the cells (Fig. 1C). The latter is in contrast to the localization of α6β4 integrin along the substratum-attached surface of control (wild-type) keratinocytes (Fig. 1C). Surface expression of α6 integrin was decreased between 80 and 90% compared with parental keratinocytes, whereas β4 integrin surface expression was almost completely absent in the three shRNA-expressing clones (Fig. 1D). The latter was not due to an off target effect of the α6 integrin shRNA because mRNA levels of β4 integrin were not decreased in any of our α6 shRNA clones (Fig. 1A). Furthermore, we “rescued” expression of both α6 and β4 integrin, as assessed by immunofluorescence staining, FACS analysis, and immunoblotting, by infecting α6 shRNA-expressing cells with adenovirus expressing α6 integrin mRNA that is refractory to the shRNA (Fig. 1, C–E).
FIGURE 1.
Stable knockdown of α6 integrin expression in human keratinocytes. Human keratinocytes were infected with lentivirus encoding α6 integrin shRNA and stably selected for the loss of α6 integrin expression. α6 and β4 integrin expression was measured in three knockdown clones (1–3, as indicated) by quantitative RT-PCR (A). The bars of the graph represent the relative expression (±S.D.) of α6 and β4 integrin mRNA in the shRNA clones normalized to GAPDH and compared with HEK. Samples were measured in triplicate for two individual experiments. B, α6 integrin, β4 integrin, actin, BP180, mTOR, and lamin were evaluated in the same clones by immunoblot. β-Actin, mTOR, and lamin were used as loading controls. C, α6 and β4 integrin localization was determined by confocal immunofluorescence (α6 shRNA clone 1 only; bar, 10 μm). D, surface expression of α6 and β4 integrins in the three knockdown clones was evaluated by FACS (top two panels). The black curves represent secondary antibody alone. In the lower two panels of D and E, wild-type cells, α6 shRNA clone 1 cells, and α6 shRNA clone 1 cells infected with adenovirus encoding a refractory α6 integrin construct to re-express α6 integrin (+refα6) were analyzed by FACS and immunoblot. In E, the graph represents the relative level of β4 integrin protein expression in cells compared with parental HEK, quantified from immunoblots as shown to the left. The individual bars represent the means ± S.E. (n = 3). The p values were generated by Student's t test. **, p ≤ 0.001. The results using control (wild-type) keratinocytes (HEK) are presented in each assay.
Knockdown of α6 integrin, and the resulting depletion of β4 integrin, did not significantly alter total protein expression of α6β4 integrin-associated proteins, including the transmembrane protein BP180 (type XVII collagen) and BPAG1e (Fig. 1B). Instead, the loss of α6β4 integrin altered their localization (supplemental Fig. S1). Staining of both BP180 and BPAG1e appeared diffuse in the α6 shRNA-expressing cells, whereas in control keratinocytes, these proteins were localized to the basal cell surface (supplemental Fig. S1). This is consistent with previous published data in which α6β4 integrin is required for assembly of α6β4 integrin-BP180-BPAG1e complexes (32, 33).
α2 and α3 integrin subunit surface expression was also measured in all three α6 integrin shRNA-expressing clones (Fig. 2, A and B). Surface expression of α3 and α2 integrin was decreased in the clones, averaging 42 and 26%, respectively, of the levels expressed in wild-type cells (Fig. 2, A and B). Similar to β4 integrin mRNA, α3 integrin mRNA levels were not significantly decreased in the α6 shRNA-expressing clones (Fig. 2C). Interestingly, in contrast to α3 and β4 integrin mRNA, α2 mRNA levels were decreased in the cells (Fig. 2D). This effect was not due to nonspecific targeting of our shRNA because re-expression of α6 integrin in the knockdown cells increased α2 integrin mRNA levels and restored α2 integrin surface expression (Fig. 2, E and G; results for one clone only are shown). In addition, overexpression of α3 integrin in the α6 integrin shRNA-expressing cells restored α2 integrin mRNA levels (Fig. 2E), suggesting a hierarchy of expression whereby α6β4 integrin regulates α3 integrin subunit expression that in turn regulates α2 integrin expression.
FIGURE 2.
Loss of α6β4 integrin leads to decreased α2 and α3 integrin expression. α2 and α3 integrin expression was measured in control cells (HEK), α6 shRNA clones 1–3, and α6 shRNA clone 1 cells induced to re-express GFP-tagged α6 integrin (+refα6) by FACS (A, B, F, and G) and quantitative RT-PCR (C–E). The individual bars on the graphs represent the relative levels of α2 or α3 integrin mRNA normalized to GAPDH and compared with HEK (±S.D.). The samples were measured in triplicate in two experiments. In E, α2 integrin mRNA expression was also measured in α6 shRNA clone 1 keratinocytes infected with adenovirus expressing α3 integrin (+α3). In H, α3 integrin surface expression was measured by FACS analysis in β4 integrin-deficient keratinocytes (JEB) and JEB cells re-expressing β4 integrin. The black curves on the FACS analyses represent secondary antibody alone.
Loss of α6 Integrin Alters Keratinocyte Cell Adhesion and Motility
Each of the α6 integrin shRNA clones adhered less robustly to laminin-332- and collagen-coated substrates and exhibited low strength of attachment to their own matrix, when compared with control keratinocytes (Fig. 3A and supplemental Fig. S2). Results for clone 1 cells are shown in this and subsequent figures because all clones behaved similarly. Supplemental Fig. S2 shows results from the other α6 shRNA-expressing clones.
FIGURE 3.
Human keratinocytes lacking α6β4 integrin expression display adhesion and migration defects. In A, control keratinocytes (HEK) and α6 shRNA clone 1 cells were assayed for adhesion to laminin-332- or collagen I-coated dishes at 1 h after plating. The number of adherent cells was measured by reading the absorbance at 570 nm. The average absorbance for HEK was taken as 100%. The individual bars on the graph represent the means ± S.E. (n = 3) with samples measured in triplicate for each experiment. p values were derived by comparing samples to HEK (Student's t test). *, p ≤ 0.05. B, vector diagrams depict the individual migration patterns of wild-type keratinocytes (HEK) (n = 11), α6 shRNA clone 1 cells (n = 12), or α6 shRNA clone 1 cells induced to re-express GFP-tagged α6 integrin (+refα6) (n = 12). The cells were plated on glass and tracked over a 2-h period. C, Rac1 activity in control HEK and α6 shRNA clone 1 cells was measured by the G-LISA Rac1 activation assay. The individual bars of the graph represent the means ± S.E. (n = 3) (in each experiment, duplicate samples were assayed). p value was derived by comparing to HEK (Student's t test). *, p ≤ 0.05. D, graph representing the average velocity of cells plated on glass over a 2-h period. The individual bars of the graph represent the means ± S.E. (n ≥ 50 cells). **, p ≤ 0.001. E, vector diagrams depicting the migration pattern of HEK (n = 28), α6 shRNA clone 1 cells (n = 37), or α6 shRNA clone 1 cells infected with retrovirus encoding GFP-tagged α3 integrin (+α3) plated on matrix deposited by HEK (n = 19). The cells were allowed to adhere to the matrix for 2 h, and the migration of cells was subsequently tracked for 2 h. F, graph represents the average migration index (net displacement/total distance) of cells over a 2-h period. The individual bars of the graph represent the means ± S.E. (n ≥ 50 cells). G, graph represents the average velocity of cells plated on pre-formed matrix over a 2-h period. The individual bars of the graph represent the means ± S.E. (n ≥ 50 cells). p values were derived by Student's t test. *, p ≤ 0.05.
We have previously demonstrated that β4 integrin-deficient keratinocytes display aberrant motility compared with normal keratinocytes, implicating α6β4 integrin in mediating migration behavior (22). Furthermore, we have also presented evidence that α6β4 integrin, through the plakin molecule BPAG1e, regulates Rac1 activity, which in turn signals to the Slingshot phosphatase proteins to dephosphorylate and activate the actin-severing protein cofilin (23, 34). Therefore, to further characterize the α6 integrin shRNA-expressing clones, we evaluated their motility phenotype and the level of their Rac1 activity. Whereas control keratinocytes moved primarily in linear tracks, the α6 shRNA-expressing cells moved in circles, had decreased Rac1 activity, and migrated significantly more slowly than their wild-type counterparts (Fig. 3, B–D, and supplemental Fig. S3). Interestingly, in prior studies we did not observe a comparable difference in velocity between wild-type keratinocytes and skin cells deficient in BPAG1e or BP180 (34, 35). Again the current result was not due to nonspecific effects of the α6 integrin shRNA because velocity and normal migratory behavior was restored in the clones following expression of α6 integrin mRNA that is refractory to the α6 integrin shRNA (Fig. 3, B and D).
The aberrant motile behavior of β4 integrin-deficient cells and cells exhibiting inhibition in Rac1 signaling to cofilin because of BPAG1e or BP180 loss is reversed by plating the cells onto the laminin-332-rich matrix deposited by wild-type keratinocytes (22, 23). Thus, we tracked migration patterns of individual α6 integrin shRNA-expressing cells after plating on matrix deposited by wild-type keratinocytes. As expected, the α6 integrin shRNA cells now migrated in a more linear pattern on this matrix compared with on their own matrix, yet they failed to increase their speed of migration (Fig. 3, E–G). These results suggested that the α6 shRNA-expressing cells lacked a key regulator of cell velocity. In this regard, we and others have reported that migration of keratinocytes on laminin-332-rich matrix is positively regulated by α3β1 integrin, which, like α6β4 integrin, is a receptor for laminin-332 (16, 22, 36–38). Because our α6 shRNA-expressing cells exhibited a loss in α3β1 integrin surface expression, we induced expression of α3 integrin in the cells and tracked the motile behavior of the α3-expressing cells following plating onto matrix deposited by control keratinocytes. These cells migrated in a similar pattern and with a similar rate of migration as control keratinocytes plated onto the same matrix (Fig. 3, E–G).
α6β4 Integrin Regulates Translation of α3 Integrin
We next measured the relative amount of α3 integrin mRNA associated with the polysomes of control and α6 integrin shRNA-expressing cells. Keratinocytes lacking α6β4 integrin expression had significantly less α3 integrin mRNA associated with the polysome fraction compared with control cells (Fig. 4A). Thus, these data suggest that α3 integrin is translationally regulated by α6β4 integrin. In support of this conclusion, re-expression of the refractory α6 integrin mRNA in α6 knockdown cells rescues α3 integrin expression. Likewise, expression of β4 integrin in β4 integrin-deficient JEB cells also resulted in an increase in α3 integrin surface expression (Fig. 2, F and H).
FIGURE 4.
α6β4 integrin regulates the translation of α3 integrin through 4EBP1. A, polysome fractions from control keratinocytes or α6 shRNA clone 1 keratinocytes were isolated, and the levels of α3 integrin (left), β4 integrin (middle), and GAPDH (right) mRNA were determined by quantitative RT-PCR, as indicated. The graphs represent the relative levels of α3, β4, or GAPDH mRNA normalized to the ribosomal protein S26 and compared with HEK. The samples were measured in triplicate, and the graphs represent the means ± S.E. of three independent experiments. B, whole cell extracts from wild-type (HEK), α6 shRNA clone 1 cells, or α6 shRNA cone 1 cells induced to re-express α6 integrin (+refα6) were probed for levels of phosphorylated 4EBP1 and total 4EBP1. C and D, whole cell extracts from wild-type (HEK), α6 shRNA clone 1 cells, or α6 shRNA clone 1 cells infected with adenovirus encoding myristoylated AKT were probed for levels of phosphorylated 4EBP1 and total 4EBP1 (C) or β4 integrin and α3 integrin (D). Actin and lamin A/C were used as loading controls in B–D. Graphs in B–D represent the means expression (normalized to lamin A/C) + S.E. of three independent experiments, quantified from immunoblots.
Others have demonstrated that α6β4 integrin regulates the translation of several genes important for cell survival and migration by mediating the phosphorylation of initiation factor 4E binding protein 1 (4EBP1) through signaling via the PI3K pathway (39, 40). 4EBP1 binds and represses the activity of the translation initiation factor 4E (eIF-4E) (41). Phosphorylation of 4EBP1 disrupts this interaction and releases eIF-4E, which can then recruit translational machinery and initiate translation (42). Therefore, we compared the level of phosphorylated 4EBP1 in extracts of wild-type keratinocytes with that in the α6 integrin knockdown cells. Significantly less 4EBP1 was phosphorylated in the α6 shRNA-expressing keratinocytes compared with wild-type cells (Fig. 4B). Interestingly, total 4EBP1 expression was also decreased in the α6 shRNA-expressing cells (Fig. 4, B and C). Although re-expression of the refractory α6 integrin construct in the shRNA cells failed to restore total 4EBP1 levels, α6 integrin re-expression led to a significant increase in phosphorylation of 4EBP1, such that the level of phosphorylated 4EBP1 mirrored that of control keratinocytes (Fig. 4B). Consistent with these data, re-expression of α6 integrin in the shRNA-expressing cells led to an increase in the level of phosphorylated AKT, suggesting that PI3K signaling was deficient in α6 integrin knockdown cells (supplemental Fig. S4). Moreover, activation of the AKT-mTOR pathway, by inducing the expression of myristoylated AKT, also increased the levels of phosphorylated 4EBP1 in the α6 shRNA-expressing cells without inducing an increase in total 4EBP1 levels (Fig. 4C). Expression of myristoylated AKT also increased the level of α3 integrin protein in the α6 shRNA-expressing cells, supporting the notion that α6β4 integrin signaling to 4EBP1 regulates α3 integrin expression (Fig. 4D).
Interestingly, significantly less β4 integrin mRNA was associated with the polysome fraction from α6 integrin shRNA-expressing cells (Fig. 4A), implicating a feedback loop in which β4 integrin translation is regulated by phosphorylation of 4EBP1. Consistent with this hypothesis, expression of myristoylated AKT increased the level of β4 integrin expression in α6 shRNA-expressing keratinocytes (Fig. 4D).
DISCUSSION
α6 Integrin and Motility
Although a central role for laminin-332 in regulating wound healing through its effects on keratinocyte migration is generally agreed upon, the functions of its two integrin receptors (α6β4 and α3β1) in migration are much debated (19, 22, 36, 37, 43–52). In this study we demonstrate that a loss of α6 integrin expression in human keratinocytes leads to the same aberrant circular migratory phenotype and decrease in Rac1 activation as is exhibited by keratinocytes deficient in β4 integrin (22). However, knockdown of α6 integrin in keratinocytes also results in lower motility rates when the cells migrate on preformed laminin-332-rich matrices. We demonstrate that this is due to decreased expression of α3β1 integrin, because induced up-regulation of α3 integrin is sufficient to rescue the motility rates of α6 integrin knockdown cells moving on preformed keratinocyte matrix.
In contrast to our results, other workers have presented evidence that α6β4 and α3β1 integrins impede migration of keratinocytes and/or wound closure in vitro (37, 47–49). The latter evidence comes primarily from studies using keratinocyte cultures generated from the skin of various integrin knock-out mice. Therein may lay the explanation for the apparent contradiction in results. Whereas human keratinocytes deposit and move over a matrix rich in laminin-332, mouse keratinocytes deposit a matrix rich in both laminin-332 and fibronectin. Recent evidence suggests that fibronectin in mouse skin cell matrix impedes laminin-332-driven migration (52), most likely by enhancing cell adhesion to substrate, such that mouse keratinocytes move much more slowly than human keratinocytes in vitro. The presence of fibronectin in mouse keratinocyte matrix complicates studies where the goal is to analyze integrin function in laminin-332-driven migration/adhesion and invalidates a direct comparison of migration results derived from work using human keratinocytes versus those using mouse keratinocytes. In this regard, our results emphasize the differential roles of α6β4 and α3β1 integrin in human skin cell motility under conditions where laminin-332 is the predominant matrix secreted by the cells. Thus, we conclude that α6β4 integrin regulates directed migration, whereas α3β1 integrin determines migration velocity.
α6β4 Integrin Regulates Transcription or Translation of Other Keratinocyte Integrins
Our analyses of α6 integrin-deficient keratinocytes indicate that α6β4 integrin is a regulator of the transcription and translation of other major skin cell integrin subunits. We demonstrate here that the loss of α6 integrin leads to a concomitant decrease in surface expression of the α3 and α2 integrin subunits. This was observed in all of our clones and is not the consequence of viral-mediated delivery of shRNA, because we fail to observe a comparable decrease in keratinocytes infected with adenovirus encoding BPAG1e shRNA (34). Our finding is particularly intriguing because α2β1, α3β1, and α6β4 integrins are among the most abundant integrins expressed in the keratinocytes of the epidermis (15). Thus, we propose that α6β4 integrin is a master regulator of the expression levels of the other major integrins in the epidermis. Such a functional role for α6β4 integrin has not been previously described, although it is consistent with the previous results of Chung et al. (39), who established that α6β4 integrin-dependent signaling regulates the translation of mRNA encoding genes required for cell survival. Here, we present evidence that α6β4 integrin-dependent signaling, via phosphorylation of 4EBP1 and activation of PI3K, regulates the translation of α3 integrin. The expression of α3 integrin in turn determines the speed of keratinocytes moving over laminin-332. Moreover, our results also indicate that the expression of α3 integrin regulates either the transcription of the α2 integrin gene or the stability of α2 integrin mRNA (Fig. 5). Future experiments are needed to discern the exact mechanism by which α3 integrin regulates α2 integrin expression. Nevertheless, there appears to be a hierarchy in integrin expression in the epidermis, with α6β4 integrin orchestrating the coordinated expression of other epidermal integrins.
FIGURE 5.
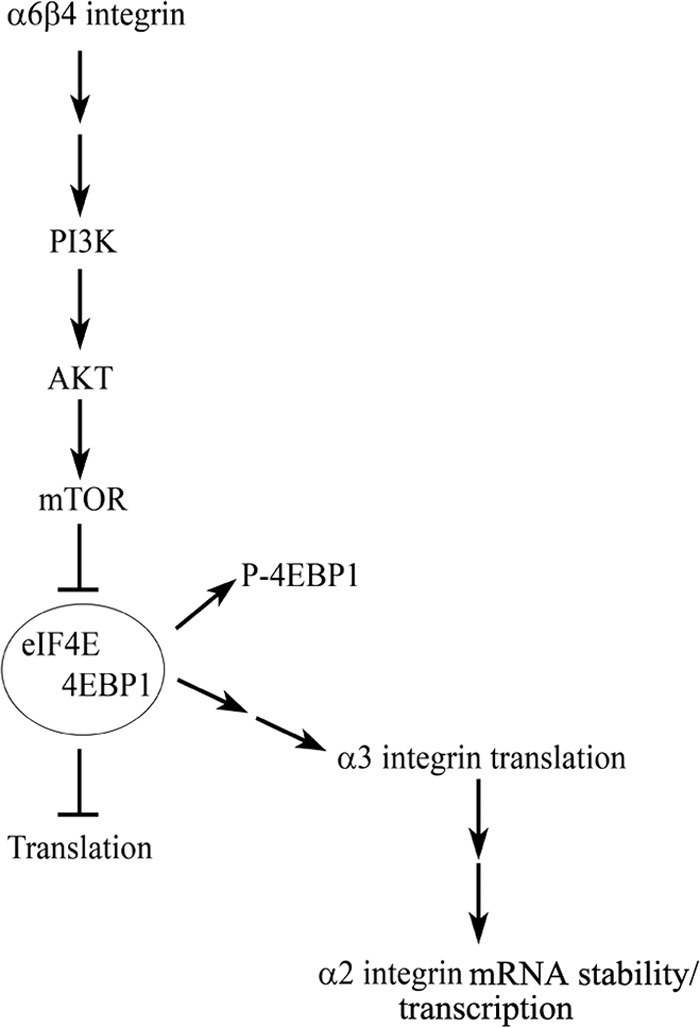
Schematic of α6β4 integrin regulation of α3 and α2 integrin expression. α6β4 integrin signaling to PI3K leads to activation of AKT and its downstream effectors. Activation of this pathway leads to phosphorylation/inactivation of 4EBP1, dissociation of the complex containing initiation factor 4E (eIF4E) and 4EBP1, and ultimately, the translation of α3 integrin. Expression of α3 integrin in turn mediates the expression of α2 integrin by regulating the transcription of the α2 integrin gene and/or α2 integrin mRNA stability.
In summary, our data support the notion that α6β4 integrin is involved in skin cell migration, an area of recent controversy. Moreover, it does so not only via an ability to activate specific signaling pathways, but also by determining the expression of α3β1 integrin, which we show regulates cellular velocity. In addition, our study has uncovered a novel role for α6β4 integrin in regulating the transcription and translation of the subunits of two major laminin- and collagen-binding integrin heterodimers expressed in skin cells in vivo. Indeed, we suggest that this new paradigm for integrin cross-talk plays a central role in determining tissue organization and remodeling in normal, developing, and healing skin, as well as mediating the dissemination of skin cells in cancer by fine tuning matrix interactions and matrix-mediated signal transduction.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 AR054184 (to J. C. R. J.) and RO1 CA77816 (to L. P.). This work was also supported by the Northwestern University Flow Cytometry and Cell Imaging Facilities through Cancer Center Support Grant CA060553.

This article contains supplemental Figs. S1–S4.
- JEB
- junctional epidermolysis bullosa.
REFERENCES
- 1. DiPersio C. M., van der Neut R., Georges-Labouesse E., Kreidberg J. A., Sonnenberg A., Hynes R. O. (2000) α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci. 113, 3051–3062 [DOI] [PubMed] [Google Scholar]
- 2. Dowling J., Yu Q. C., Fuchs E. (1996) β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134, 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Georges-Labouesse E., Messaddeq N., Yehia G., Cadalbert L., Dierich A., Le Meur M. (1996) Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370–373 [DOI] [PubMed] [Google Scholar]
- 4. Brakebusch C., Grose R., Quondamatteo F., Ramirez A., Jorcano J. L., Pirro A., Svensson M., Herken R., Sasaki T., Timpl R., Werner S., Fässler R. (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 19, 3990–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Neut R., Krimpenfort P., Calafat J., Niessen C. M., Sonnenberg A. (1996) Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 13, 366–369 [DOI] [PubMed] [Google Scholar]
- 6. Allegra M., Gagnoux-Palacios L., Gache Y., Roques S., Lestringant G., Ortonne J. P., Meneguzzi G. (2003) Rapid decay of α6 integrin caused by a mis-sense mutation in the propeller domain results in severe junctional epidermolysis bullosa with pyloric atresia. J. Invest. Dermatol. 121, 1336–1343 [DOI] [PubMed] [Google Scholar]
- 7. Pulkkinen L., Bruckner-Tuderman L., August C., Uitto J. (1998) Compound heterozygosity for missense (L156P) and nonsense (R554X) mutations in the β4 integrin gene (ITGB4) underlies mild, nonlethal phenotype of epidermolysis bullosa with pyloric atresia. Am. J. Pathol. 152, 935–941 [PMC free article] [PubMed] [Google Scholar]
- 8. Pulkkinen L., Kim D. U., Uitto J. (1998) Epidermolysis bullosa with pyloric atresia. Novel mutations in the beta4 integrin gene (ITGB4). Am. J. Pathol. 152, 157–166 [PMC free article] [PubMed] [Google Scholar]
- 9. Pulkkinen L., Rouan F., Bruckner-Tuderman L., Wallerstein R., Garzon M., Brown T., Smith L., Carter W., Uitto J. (1998) Novel ITGB4 mutations in lethal and nonlethal variants of epidermolysis bullosa with pyloric atresia. Missense versus nonsense. Am. J. Hum. Genet. 63, 1376–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pulkkinen L., Uitto J. (1998) Hemidesmosomal variants of epidermolysis bullosa. Mutations in the α6β4 integrin and the 180-kD bullous pemphigoid antigen/type XVII collagen genes. Exp. Dermatol. 7, 46–64 [DOI] [PubMed] [Google Scholar]
- 11. Pulkkinen L., Kimonis V. E., Xu Y., Spanou E. N., McLean W. H., Uitto J. (1997) Homozygous α6 integrin mutation in junctional epidermolysis bullosa with congenital duodenal atresia. Hum. Mol. Genet. 6, 669–674 [DOI] [PubMed] [Google Scholar]
- 12. Pulkkinen L., Kurtz K., Xu Y., Bruckner-Tuderman L., Uitto J. (1997) Genomic organization of the integrin β4 gene (ITGB4). A homozygous splice-site mutation in a patient with junctional epidermolysis bullosa associated with pyloric atresia. Lab. Invest. 76, 823–833 [PubMed] [Google Scholar]
- 13. Ruzzi L., Gagnoux-Palacios L., Pinola M., Belli S., Meneguzzi G., D'Alessio M., Zambruno G. (1997) A homozygous mutation in the integrin α6 gene in junctional epidermolysis bullosa with pyloric atresia. J. Clin. Invest. 99, 2826–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vidal F., Aberdam D., Miquel C., Christiano A. M., Pulkkinen L., Uitto J., Ortonne J. P., Meneguzzi G. (1995) Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 10, 229–234 [DOI] [PubMed] [Google Scholar]
- 15. Watt F. M. (2002) Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choma D. P., Milano V., Pumiglia K. M., DiPersio C. M. (2007) Integrin α3β1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J. Invest Dermatol. 127, 31–40 [DOI] [PubMed] [Google Scholar]
- 17. Lamar J. M., Pumiglia K. M., DiPersio C. M. (2008) An immortalization-dependent switch in integrin function up-regulates MMP-9 to enhance tumor cell invasion. Cancer Res. 68, 7371–7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyer V., Pumiglia K., DiPersio C. M. (2005) α3β1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes. A novel mechanism of integrin-mediated MMP gene expression. J. Cell Sci. 118, 1185–1195 [DOI] [PubMed] [Google Scholar]
- 19. Zhang K., Kramer R. H. (1996) Laminin 5 deposition promotes keratinocyte motility. Exp. Cell Res. 227, 309–322 [DOI] [PubMed] [Google Scholar]
- 20. Lochter A., Navre M., Werb Z., Bissell M. J. (1999) α1 and α2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol. Biol. Cell 10, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zutter M. M., Santoro S. A., Staatz W. D., Tsung Y. L. (1995) Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 92, 7411–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sehgal B. U., DeBiase P. J., Matzno S., Chew T. L., Claiborne J. N., Hopkinson S. B., Russell A., Marinkovich M. P., Jones J. C. (2006) Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 281, 35487–35498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kligys K., Claiborne J. N., DeBiase P. J., Hopkinson S. B., Wu Y., Mizuno K., Jones J. C. (2007) The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization, and motility behavior of keratinocytes. J. Biol. Chem. 282, 32520–32528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopkinson S. B., Riddelle K. S., Jones J. C. (1992) Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component. Molecular and cell biologic characterization. J. Invest. Dermatol. 99, 264–270 [DOI] [PubMed] [Google Scholar]
- 25. Klatte D. H., Jones J. C. (1994) Purification of the 230-kD bullous pemphigoid antigen (BP230) from bovine tongue mucosa. Structural analyses and assessment of BP230 tissue distribution using a new monoclonal antibody. J. Invest. Dermatol. 102, 39–44 [DOI] [PubMed] [Google Scholar]
- 26. Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-Kita B., Baker D. P., Hay N., Fish E. N., Platanias L. C. (2008) Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. U.S.A. 105, 4808–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsuruta D., Hopkinson S. B., Lane K. D., Werner M. E., Cryns V. L., Jones J. C. (2003) Crucial role of the specificity-determining loop of the integrin β4 subunit in the binding of cells to laminin-5 and outside-in signal transduction. J. Biol. Chem. 278, 38707–38714 [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez A. M., Gonzales M., Herron G. S., Nagavarapu U., Hopkinson S. B., Tsuruta D., Jones J. C. (2002) Complex interactions between the laminin α4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 99, 16075–16080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langhofer M., Hopkinson S. B., Jones J. C. (1993) The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J. Cell Sci. 105, 753–764 [DOI] [PubMed] [Google Scholar]
- 30. Lee E. C., Lotz M. M., Steele G. D., Jr., Mercurio A. M. (1992) The integrin α6β4 is a laminin receptor. J. Cell Biol. 117, 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hogervorst F., Kuikman I., van Kessel A. G., Sonnenberg A. (1991) Molecular cloning of the human α6 integrin subunit. Alternative splicing of α6 mRNA and chromosomal localization of the α6 and β4 genes. Eur. J. Biochem. 199, 425–433 [DOI] [PubMed] [Google Scholar]
- 32. Litjens S. H., de Pereda J. M., Sonnenberg A. (2006) Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 16, 376–383 [DOI] [PubMed] [Google Scholar]
- 33. Jones J. C., Kurpakus M. A., Cooper H. M., Quaranta V. (1991) A function for the integrin α6β4 in the hemidesmosome. Cell Regul. 2, 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamill K. J., Hopkinson S. B., DeBiase P., Jones J. C. (2009) BPAG1e maintains keratinocyte polarity through β4 integrin-mediated modulation of Rac1 and cofilin activities. Mol. Biol. Cell 20, 2954–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamill K. J., Hopkinson S. B., Jonkman M. F., Jones J. C. (2011) Type XVII collagen regulates lamellipod stability, cell motility, and signaling to Rac1 by targeting bullous pemphigoid antigen 1e to α6β4 integrin. J. Biol. Chem. 286, 26768–26780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank D. E., Carter W. G. (2004) Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 117, 1351–1363 [DOI] [PubMed] [Google Scholar]
- 37. Reynolds L. E., Conti F. J., Silva R., Robinson S. D., Iyer V., Rudling R., Cross B., Nye E., Hart I. R., Dipersio C. M., Hodivala-Dilke K. M. (2008) α3β1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J. Clin. Invest. 118, 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell K., Szekeres C., Milano V., Svenson K. B., Nilsen-Hamilton M., Kreidberg J. A., DiPersio C. M. (2009) α3β1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J. Cell Sci. 122, 1778–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung J., Bachelder R. E., Lipscomb E. A., Shaw L. M., Mercurio A. M. (2002) Integrin (α6β4) regulation of eIF-4E activity and VEGF translation. A survival mechanism for carcinoma cells. J. Cell Biol. 158, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoon S. O., Shin S., Lipscomb E. A. (2006) A novel mechanism for integrin-mediated Ras activation in breast carcinoma cells. The α6β4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res. 66, 2732–2739 [DOI] [PubMed] [Google Scholar]
- 41. McKendrick L., Pain V. M., Morley S. J. (1999) Translation initiation factor 4E. Int. J. Biochem. Cell Biol. 31, 31–35 [DOI] [PubMed] [Google Scholar]
- 42. Gingras A. C., Raught B., Gygi S. P., Niedzwiecka A., Miron M., Burley S. K., Polakiewicz R. D., Wyslouch-Cieszynska A., Aebersold R., Sonenberg N. (2001) Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15, 2852–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldfinger L. E., Hopkinson S. B., deHart G. W., Collawn S., Couchman J. R., Jones J. C. (1999) The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 112, 2615–2629 [DOI] [PubMed] [Google Scholar]
- 44. Nikolopoulos S. N., Blaikie P., Yoshioka T., Guo W., Puri C., Tacchetti C., Giancotti F. G. (2005) Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-κB, causing defects in epidermal growth and migration. Mol. Cell. Biol. 25, 6090–6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santoro M. M., Gaudino G., Marchisio P. C. (2003) The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell 5, 257–271 [DOI] [PubMed] [Google Scholar]
- 46. Russell A. J., Fincher E. F., Millman L., Smith R., Vela V., Waterman E. A., Dey C. N., Guide S., Weaver V. M., Marinkovich M. P. (2003) α6β4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of α3β1 integrin. J. Cell Sci. 116, 3543–3556 [DOI] [PubMed] [Google Scholar]
- 47. Raymond K., Kreft M., Janssen H., Calafat J., Sonnenberg A. (2005) Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J. Cell Sci. 118, 1045–1060 [DOI] [PubMed] [Google Scholar]
- 48. Hintermann E., Yang N., O'Sullivan D., Higgins J. M., Quaranta V. (2005) Integrin α6β4-erbB2 complex inhibits haptotaxis by up-regulating E-cadherin cell-cell junctions in keratinocytes. J. Biol. Chem. 280, 8004–8015 [DOI] [PubMed] [Google Scholar]
- 49. Margadant C., Raymond K., Kreft M., Sachs N., Janssen H., Sonnenberg A. (2009) Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 122, 278–288 [DOI] [PubMed] [Google Scholar]
- 50. Wen T., Zhang Z., Yu Y., Qu H., Koch M., Aumailley M. (2010) Integrin α3 subunit regulates events linked to epithelial repair, including keratinocyte migration and protein expression. Wound Repair Regen. 18, 325–334 [DOI] [PubMed] [Google Scholar]
- 51. Rodius S., Indra G., Thibault C., Pfister V., Georges-Labouesse E. (2007) Loss of α6 integrins in keratinocytes leads to an increase in TGFβ and AP1 signaling and in expression of differentiation genes. J. Cell. Physiol. 212, 439–449 [DOI] [PubMed] [Google Scholar]
- 52. Hamill K. J., Hopkinson S. B., Hoover P., Todorović V., Green K. J., Jones J. C. (2012) Fibronectin expression determines skin cell motile behavior. J. Invest. Derm. 132, 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]






