Background: Mechanical forces and ErbB receptors are critical for fetal lung development.
Results: Deletion of ErbB1 or down-regulation or ErbB4 prevented stretch-induced type II cell differentiation via ERK.
Conclusion: Interactions between ErbB1 and ErbB4 are critical for stretch-induced type II cell differentiation.
Significance: Learning how mechanical signal regulate fetal lung development is critical to develop strategies to accelerate lung maturation.
Keywords: Cell Differentiation, Cell Surface Receptor, Development, Epidermal Growth Factor Receptor (EGFR), Lung, Mechanotransduction, ErbB Receptors
Abstract
Stretch-induced differentiation of lung fetal type II epithelial cells is mediated through EGFR (ErbB1) via release of HB-EGF and TGF-α ligands. Employing an EGFR knock-out mice model, we further investigated the role of the ErbB family of receptors in mechanotranduction during lung development. Deletion of EGFR prevented endogenous and mechanical stretch-induced type II cell differentiation via the ERK pathway, which was rescued by overexpression of a constitutively active MEK. Interestingly, the expression of ErbB4, the only ErbB receptor that EGFR co-precipitates in wild-type cells, was decreased in EGFR-deficient type II cells. Similar to EGFR, ErbB4 was activated by stretch and participated in ERK phosphorylation and type II cell differentiation. However, neuregulin (NRG) or stretch-induced ErbB4 activation were blunted in EGFR-deficient cells and not rescued after ErbB4 overexpression, suggesting that induction of ErbB4 phosphorylation is EGFR-dependent. Finally, we addressed how shedding of ligands is regulated by EGFR. In knock-out cells, TGF-α, a ligand for EGFR, was not released by stretch, while HB-EGF, a ligand for EGFR and ErbB4, was shed by stretch although to a lower magnitude than in normal cells. Release of these ligands was inhibited by blocking EGFR and ERK pathway. In conclusion, our studies show that EGFR and ErbB4 regulate stretch-induced type II cell differentiation via ERK pathway. Interactions between these two receptors are important for mechanical signals in lung fetal type II cells. These studies provide novel insights into the cell signaling mechanisms regulating ErbB family receptors in lung cell differentiation.
Introduction
Mechanical forces are essential for normal fetal lung development (1–7). Throughout gestation, the lung epithelium actively secretes fluid creating a constant distension pressure of around 2.5 mmHg in the potential airspaces (8). In addition, the fetus makes episodic breathing movements (FBM) (9). It is clear from experimental animals that both tonic hydrostatic distension and cyclic mechanical deformation provide physical signals necessary for normal fetal lung development (10–12).
The ErbB family of tyrosine kinase receptors consists of four receptors: Epidermal growth factor receptor (EGFR2 also called ErbB1), ErbB2, ErbB3, and ErbB4. These receptors have a single transmembrane domain separating an intracellular kinase domain from an extracellular ligand-binding domain. ErbB receptors are activated by a variety of receptor-specific ligands. Ligand binding induces receptor homo- or hetero-dimerization that is essential for activation of the tyrosine kinase and subsequent recruitment of target proteins and initiation of signaling cascade (13). EGF and transforming growth factor (TGF)-α are the ligands for EGFR, heparin binding (HB)-EGF binds to EGFR and ErbB4 and neuregulin (NRG) is the ligand for ErbB3 and ErbB4 (14). Each ligand is synthesized as a transmembrane precursor and is proteolytically cleaved to release the biologically active mature protein (15).
The importance of EGFR in lung development is well established (16). EGFR is critical for branching morphogenesis, alveolarization, and differentiation of type II epithelial cells (17–19). Previous studies from our laboratory demonstrated that mechanical stretch of fetal type II epithelial cells, simulating fetal breathing movements, activates EGFR (20). In addition, we showed that mechanical strain promotes fetal type II cell differentiation via shedding of HB-EGF and TGF-α (21). However, the precise signaling mechanisms through EGFR and interactions with the other ErbB receptors in mechanotransduction during lung development are unknown. ErbB2 and ErbB3 seem to contribute to fetal lung epithelial cell proliferation (22). ErbB4 receptor plays a critical role in central nervous system and cardiac development (23). In addition, ErbB4 regulates late fetal lung development and type II cell differentiation (24–27). Deletion of ErbB4 in vivo leads to delayed fetal lung development (27) and alveolar simplification (26) and down-regulation of ErbB4 receptors in cultured type II cells blocked neuregulin stimulation of surfactant production (24). Therefore, given the role of ErbB4 in epithelial cells maturation, we investigated whether this receptor participates in stretch-induced signaling and type II cell differentiation. In addition, we analyzed whether ErbB4 receptor plays a compensatory role in the absence of EGFR, since it is the prominent dimerization partner in fetal type II cells (25). Lastly, we studied how stretch-induced release of ligands is regulated. Given the severity of lung underdevelopment observed in EGFR knock-out mice we hypothesized that the presence of EGFR is critical for stretch-induced type II cell differentiation.
EXPERIMENTAL PROCEDURES
EGFR Knock-out Mice
EGFR knock-out mice were a generous gift from Dr. Zena Werb (17). Embryos used in this study were derived from intercrosses between EGFR ± mice in a Swiss-Webster genetic background. Animals were housed in the Central Research facilities at Rhode Island Hospital. The following 2 set of primers were used for genotyping: Homozygous: ∼375 bp band (5′-GAT GGA TTG CAC GCA GGT TCT-3′, 5′-AGG TAG CCG GAT CAA GCG TAT-3′). Wild-type: ∼250 bp band (5′-CCT AGC TGT CAC CAA CCC TTT-3′, 5′-GAC GAA GAG CAT CAC AAG GAG-3). The cycling conditions were: 1× @ 94 °C for 2 min; 35× @ 94 °C for 45 s, 59 °C for 1 min, 72 °C for 1 min; 1× @ 72 °C for 5 min. The PCR products were then subjected to 1% agarose gel. EGFR knock-out fetuses were reliably recognized as early as E16 of gestation by their open-eye phenotype.
Cell Isolation and Stretch Protocol
Animal experiments were performed in compliance with the Lifespan Institutional Animal Care and Use Committee, Providence, RI. Fetal mouse lungs were obtained at embryonic day 17 from wild-type and EGFR knock-out timed-pregnant mice after intra-peritoneal administration of pentobarbital sodium. The plug date was considered Day 0.5 of pregnancy. Type II cells were isolated as previously described (28). Briefly, after collagenase digestion, cell suspensions were sequentially filtered through 100-, 30-, and 15-μm nylon meshes using screen cups (Sigma). Clumped non-filtered cells from the 30- and 15 μm nylon meshes were collected after several washes with DMEM, plated on Bioflex multiwall Plates (Flexcell International, Hillsborough, NC) precoated with laminin-1 (2 μg/cm2). Monolayers were maintained for an additional 24 h until reached ∼80% confluency and then mounted in a Flexcell FX-4000 Strain Unit. An equibiaxial cyclical strain regimen of 5% was applied at intervals of 40 cycles/min for different lengths of time. Cells were grown on non-stretched membranes in parallel and were treated in an identical manner to serve as control.
Immunoprecipitation and Western Blotting of ErbB Receptors
Immunoprecipitation experiments were performed as previously described (25). After experiments, monolayers were washed with ice-cold PBS and lysed in RIPA buffer (50 mm Tris (pH 7.4), 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with 1 mm Na3VO4, 1 mm NaF, and protease inhibitor mixture (Thermo Scientific). 300 μg of total protein were incubated with ErbB2 (Abcam cat. ab2428), ErbB3 (Santa Cruz Biotechnology cat. sc-285) or ErbB4 (Santa Cruz Biotechnology cat. sc-283; Cell Signaling cat. 4795) antibodies overnight at 4 °C. The following day, protein-A-Sepharose beads were added and incubated for an additional 3 h. The beads were collected by centrifugation at 3,000 rpm at 4 °C for 1 min, washed three times with PBS (containing 1 mm Na3VO4, 1 mm NaF, and protein inhibitor mixture), denatured in sample buffer for 10 min at 70 °C, and subjected to 4–12% gradient gel and transferred to PVDF membrane. Anti-phosphotyrosine antibody (PY350, Santa Cruz Biotechnology, 1: 500) was used to detect the level of phosphorylation of ErbB2 and ErbB3. ErbB4 receptor activation was analyzed using a phospho-ErbB4 antibody (Cell Signaling cat. 4757). The membranes were then stripped in buffer (Alpha Diagnostic Intl. Inc. San Antonio, TX) for 15 min at room temperature, blocked and reprobed with an antibody against the ErbB receptor to detect the total ErbB protein. Intensity of the bands was evaluated by densitometry and the ratio of phosphorylation to total receptor protein was used to determine the level of activation of each receptor.
Transient Transfection by Electroporation
Type II cells were transiently transfected using Amaxa Nucleofector apparatus as previously described (21). Isolated type II epithelial cells were plated on T75 flasks. The following day, cells were harvested by trypsinization and aliquots of 2 × 106 cells in RPMI with 10% FBS were centrifuged at 100 × g for 10 min; supernatants were discarded, and cell pellets were resuspended in 100 μl of Basic Nucleofector Solution (Primary Mammalian Epithelial Cells Protocol, Amaxa). Each sample was mixed individually with 2 μg of plasmid DNA. For ligand release, plasmids encoding alkaline phosphatase (AP)-tagged expression vectors for HB-EGF or TGF-α, were added. These vectors were a generous gift from Dr. Carl P. Blobel, Weil Medical College of Cornell University, New York and they had been previously described (29). To overexpress ErbB4, a human ErbB4 plasmid containing a GFP tag and an empty vector as a negative control were used (30). ERK pathway was activated using the plasmid pMCL-MKK1-R4F (DeltaN3/S218E/S222D), a generous gift from Dr. Natalie G. Ahn, University of Colorado. To knockdown ErbB4, 2 μm siRNA for ErbB4 (ON-TARGET plus SMART pool mouse ErbB4) and control (siGENOME Control pool non-Targeting 2), both from Dharmacon, Thermo Scientific, were used. Samples were then transferred into the appropriate cuvettes and subjected to electrical pulses using the Amaxa NucleofactorTM II apparatus (Amaxa). Samples containing no DNA were treated in an identical manner and served as negative controls (pulse only). Based on previous studies from our laboratory (31), a T-13 program from Amaxa Biosystems was selected. After electroporation, samples were then transferred into bioflex plates precoated with laminin-1 and left undisturbed for 24 h in a culture incubator. Transfection efficiency using the control vector pmaxGFP was around 50%. 24 h after transfection, monolayers were exposed to mechanical strain for different periods of time.
EGFR Ligand Shedding Assay
This method was adapted from Wang et al. (21). Monolayers transfected with AP-tagged EGFR ligands were exposed to different stretch protocols. After experiments, cell medium (2 ml/well) from static and stretched samples were collected and centrifuged at 16,000 × g for 30 min. Supernatants were saved at −80 °C until further use. Monolayers were lysed by adding 0.5 ml/well of buffer containing 1× PBS, 1% TritonX-100 and protease and phosphatase inhibitors. Lysates were centrifuged and saved at −80 °C. Analysis and quantification of EGFR ligand shedding was performed by running the concentrated supernatants on SDS-polyacrylamide gels and staining the gels for alkaline phosphatase (AP) activity. Because the AP moiety is N-glycosylated, lectin ConA was used to capture and concentrate the shed proteins. 40 μl of ConA Sepharose resin (Amersham Biosciences, cat# 17-0440-03) was added (after equilibrated and resuspended 1:1 in PBS plus protease and phosphatase inhibitors) to every 2 ml of cell supernatant and incubated at 4 °C rotating for overnight. ConA beads were spun down twice at 800 × g for 1 min to remove the supernatant. Glycoproteins were eluted from the beads by adding 20 μl of elution buffer (50 mm Tris-HCl pH 8.0, 0.5 m α-d-methyl mannoside), mixed and then incubated at 37 °C for 2 h. Then, 10 μl of 5× SDS-sample loading buffer containing 25 mm of dithiothreitol (DTT) were added. Samples were not boiled as AP is irreversibly inactivated at temperatures higher than 70 °C. Beads were then spun down and supernatants were loaded on 10% SDS-polyacrylamide gel and a constant current of 100V was applied at 4 °C. When the separation was completed, gels were removed and incubated twice for 30 min in 2.5% Triton X-100, followed by 10 min incubation in alkaline phosphatase buffer (100 mm Tris-HCl pH 9.5, 100 mm NaCl and 20 mm MgCl2). AP activity was visualized by incubating the gels at 37 °C in detection solution (by adding 37.5 mg of NBT and 18.5 mg BCIP to the detection buffer (100 mm Tris-HCl pH 9.5, 100 mm NaCl)). The enzyme reaction was then stopped by removing the detecting buffer. Intensity of the bands was quantified using the Gel-Pro Analyzer 4.0 software (MediaCybernetics, Bethesda, MD). AP activity in the cell lysate was analyzed following the same experimental procedure as described for cell supernatants. Data were expressed as the intensity of each AP supernatant band divided by total AP (supernatant + cell lysate). Preliminary experiments showed that cyclic stretch for 30 min induced maximum release of ligands into the supernatant; therefore, this time point was used for the experiments.
Real-Time PCR
Total RNA was isolated as previously described (5) and purified further using the Turbo DNA-free kit (Ambion, Austin, TX). Total RNA was reverse-transcribed into cDNA using the iScriptTM cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's instructions. Pre-designed TaqMan® SP-C (cat. Mm00488144_m1), SP-B (cat. Mm0045681_m1) and ABCA3 (cat. Mm0550501_m1) primers were purchased from Applied Biosystems. To amplify the cDNA by qRT-PCR, 50 ng of the resulting cDNA were added to a mixture of 10 μl of 2X TaqMan Universal PCR Master Mix (Applied Biosystems) and 1 μl of 20× Gene Expression Assay Mix containing forward and reverse primers and TaqMan-labeled probe (Applied Biosystems). Samples were normalized to the 18 S rRNA. The reactions were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) with an initial denaturation for 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. All assays were performed in triplicate.
Immunohistochemistry
E17 lungs were fixed in 4% paraformaldehyde and processed into serial paraffin sections using routine procedures. To detect the presence of glycogen in epithelial cells, tissue sections from wild-type and knock-out mice were stained with periodic acid Schiff (PAS), examined under the light microscope and photographed. For SP-C immunostaining, tissue sections were blocked with normal goat serum (1:20) for 30 min at room temperature and then incubated with SP-C antibody (Santa Cruz Biotechnology), 1:100 dilution, at 4 °C overnight. Alexa Fluor goat anti-rabbit secondary antibody (Molecular Probes) was used at 1:500 dilution for 1 h at room temperature. To quantify the presence of SP-C-positive cells, 10 random fields from 3 separate animals were evaluated. The number of SP-C positive epithelial cells was divided by the total number of cells present in the acinar tubules/sacs. To assess the intensity of SP-C positive cells, ten 12 bit grayscale images were acquired per specimen with a Nikon E800 microscope (Nikon Inc. Melville, NY) using a 40× PlanApo objective and a Spot RT3 camera. Camera settings were based on the brightest slide and the cameras built-in green filter was used to increase image contrast. (Diagnostic Instruments, Sterling Heights MI). All subsequent images were acquired with the same settings. Image processing and analysis was performed using iVision image analysis software. Positive staining was defined through intensity thresholding for total cell counts and mean intensity measurements.
Statistical Analysis
Results are expressed as means ± S.E. from at least three experiments, using different litters for each experiment. Data were analyzed with ANOVA followed by post hoc tests, and Instat 3.0 (GraphPad Software, San Diego, CA) was used for statistical analysis; p < 0.05 was considered statistically significant.
RESULTS
EGFR Deficiency Prevents Endogenous and Mechanical Stretch-induced Type II Cell Differentiation
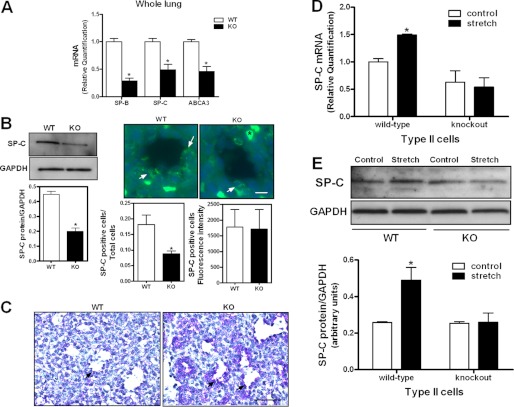
EGFR and mechanical forces are both important for fetal lung development (1–7, 17–19). Therefore, we investigated first differentiation of fetal type II epithelial cells and the effect of mechanical stretch in EGFR knock-out mice. SP-B, SP-C, and ABCA3 mRNA, all genes related to type II epithelial cell differentiation, were reduced by ∼50% in the lung tissue from EGFR knock-out mice when compared with normal lungs (Fig. 1A). Similar results were observed in SP-C protein abundance (Fig. 1B, left panels). We also investigated whether type II cells/total lung cells ratio is different between wild type and mutant lungs. Fig. 1B, right panels shows a 50% reduction in the number of SP-C positive cells in knock-out lung tissue when compared with normal lung; whereas no difference was observed in the intensity of SP-C staining between both tissues. Type II cell differentiation is accompanied by a reduction in cytoplasmic glycogen content. Immunohistochemistry of glycogen expression demonstrated an increased staining in the epithelial cells lining the acinar tubules/sacs in knock-out lungs compared with normal lungs (Fig. 1C), as a marker of delayed differentiation. SP-C is the most specific marker of type II cell differentiation (32). Previous studies from our laboratory have shown that mechanical stretch, simulating fetal breathing movements, increases SP-C expression (3). Therefore, we analyzed the effect of stretch on SP-C mRNA and protein abundance. As shown in Fig. 1D, 5% cyclic stretch for 16 h increased SP-C gene expression by 50% (1 ± 0.06 versus 1.49 ± 0.02) in cells isolated from wild-type mice but not in cells isolated from knock-out mice. Likewise, mechanical stretch increased SP-C protein levels 2-fold (0.26 ± 0.006 versus 0.49 ± 0.07) in type II cells isolated from wild-type mice only (Fig. 1E).
FIGURE 1.
Type II cell differentiation in EGFR knock-out mice. A, E17 lungs from wild-type (WT) and knock-out (KO) mice were processed by qRT-PCR to assess mRNA expression of SP-B, SP-C, and ABCA3 genes. n = 3; *, p < 0.05 versus WT. B, left panels: lungs were isolated on E17 of gestation from WT and KO mice and processed by Western blot to detect SP-C protein levels (n = 3; *, p < 0.01). Right panels, lung tissue from WT and KO mice was processed, as described in “Experimental Procedures,” to evaluate the presence of SP-C-positive cells (green, arrows). Nuclei were counterstained with DAPI (blue) * (red blood cell). Bar, 20 μm. Shown are representative images from three independent experiments. Graphs depict quantification of SP-C-positive cells normalized to the total number of epithelial cells present in the acinar tubules/sacs (n = 3; *, p < 0.05) and fluorescence intensity of SP-C-positive cells between WT and KO tissues. C, PAS staining of E17 lung tissue demonstrating an increase of glycogen-positive epithelial cells (arrows) in the acinar tubules/sacs of knock-out lungs when compared with normal lungs. Bar, 100 μm. D, type II cells from WT and KO mice were isolated on E17 of gestation and exposed to 5% cyclic stretch for 16 h. Samples were analyzed by qRT-PCR to assess SP-C mRNA expression. n = 3; *, p < 0.05 versus control. E, type II cells from WT and KO mice were isolated on E17 of gestation and exposed to 5% cyclic stretch for 16 h. Samples were processed by Western blot to detect SP-C protein abundance. The upper panel shows a representative blot. n = 3; *, p < 0.05 versus control.
ERK Pathway Regulates Stretch-induced Fetal Type II Cell Differentiation
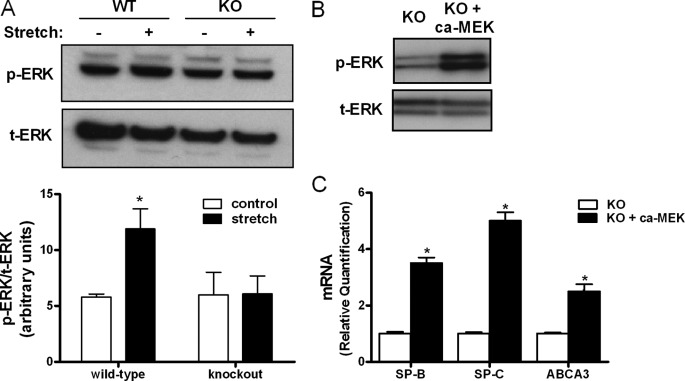
We have previously shown that ERK pathway participates in stretch-induced type II cell differentiation (20). We therefore investigated whether the absence of EGFR affects activation of ERK by stretch. Mechanical stretch of wild-type type II cells increased phosphorylation of ERK by 2-fold (5.8 ± 0.27 versus 11.9 ± 1.8). In contrast, ERK phosphorylation did not change after stretch in cells isolated from knock-out mice (6 ± 2 versus 6.1 ± 1.6) (Fig. 2A). Overexpression of a constitutively active MEK increased ERK phosphorylation by 4-fold when compared with knock-out control cells (Fig. 2B). Activation of ERK pathway in EGFR-deficient cells rescued type II cell differentiation defect by increasing SP-B, SP-C, and ABCA3 gene expression by 3.5-fold, 5-fold, and 2.5-fold, respectively, when compared with knock-out cells transfected with empty vector (Fig. 2C). These data, along with the previous figure, support the role of EGFR and ERK pathway in stretch-induced type II cell differentiation.
FIGURE 2.
ERK pathway regulates stretch-induced fetal type II cell differentiation. A, E17 type II cells isolated from WT and KO mice were exposed to 5% cyclic stretch for 15 min or not (control). Proteins were extracted and the level of ERK activation was evaluated by Western blot using phosphospecific antibody (p-ERK). Blots were reprobed with total ERK antibody (t-ERK) to control for protein loading. The upper panel shows a representative blot. Data in the lower panel are from three independent experiments (*, p < 0.05 versus control). B, E17 type II cells isolated from KO mice were transfected by electroporation with an empty vector (control) or a plasmid encoding a constitutively active MEK. Two days later, proteins were extracted and processed as described above to detected ERK phosphorylation. Blot is representative of two independent experiments. C, samples was transfected with plasmids as described above and processed to assess SP-B, SP-C, and ABCA3 gene expression by qRT-PCR. Results are from three separate experiments. *, p < 0.05.
ErbB4 Receptor Expression Is Decreased in EGFR Knock-out Mice
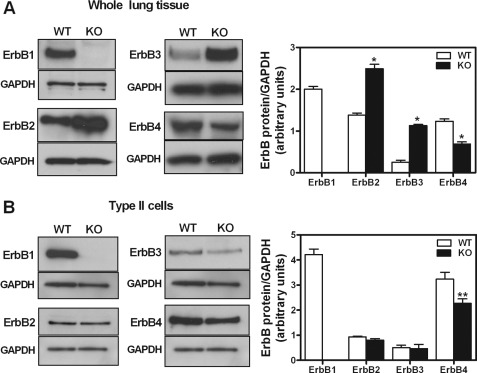
Next, we studied protein abundance of the ErbB family of receptors in EGFR knock-out mice to answer the question whether there is a compensatory response from the other receptors in the absence of EGFR. In whole lung tissue (Fig. 3A) as anticipated, no EGFR protein was present in knock-out mice. ErbB2 receptor increased by 1.8-fold (1.38 ± 0.05 versus 2.49 ± 0.11), and ErbB3 by 4.5-fold (0.25 ± 0.05 versus 1.13 ± 0.03). In contrast, ErbB4 receptor protein abundance decreased by 44% when compared with wild-type mice (1.23 ± 0.06 versus 0.69 ± 0.05). In isolated type II cells (Fig. 3B), no significant differences in ErbB2 or ErbB3 receptor protein between knock-out and wild-type cells were observed. ErbB4 receptor protein expression decreased by 30% in type II cells isolated from knock-out mice (3.24 ± 0.27 versus 2.27 ± 0.18).
FIGURE 3.
Expression of ErbB receptors in EGFR knock-out mice. Whole fetal lung (A) or type II epithelial cells (B) were isolated on E-17 of gestation from wild-type (WT) and knock-out (KO) mice and processed by Western blot to detect protein abundance of ErbB family of receptors. Samples were normalized to GAPDH to control for protein loading. Data in the right panels are from three independent experiments. *, p < 0.02 versus WT; **, p < 0.05 versus WT.
ErbB Receptors Phosphorylation and Dimerization in Response to Stretch in Wild-type Fetal Type II Cells
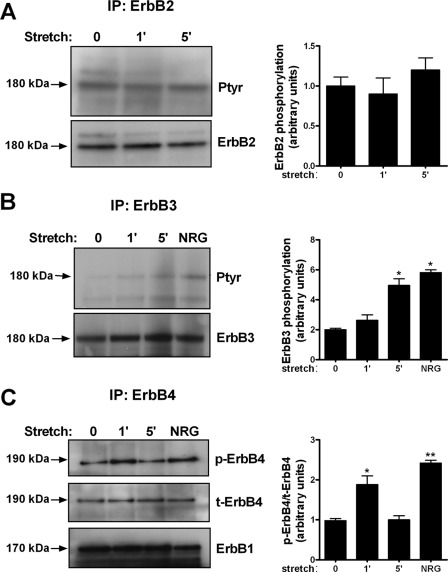
Previous studies from our laboratory have shown that mechanical stretch activates EGFR (20). Therefore, we studied next whether mechanical stretch stimulates other receptors of the ErbB family. For these experiments, monolayers were exposed to 5% cyclic stretch for different periods of time; collected proteins were immunoprecipitated with different ErbB receptor antibodies and immunoblotted with phosphotyrosine (Ptyr) or phospho-ErbB4 antibodies to detect activation. Our results show that mechanical stretch did not activate ErbB2 in type II cells isolated from wild-type rodents (Fig. 4A). ErbB3 phosphorylation increased by 2.5-fold after 5 min of cyclic stretch (2 ± 0.1 versus 4.95 ± 0.45) (Fig. 4B). Similarly, ErbB4 receptor phosphorylation increased by 2-fold after 1 min of stretch (0.98 ± 0.05 versus 1.88 ± 0.21). Compared with stretch, NRG, the ligand for ErbB4, increased ErbB4 phosphorylation by 2.5-fold (Fig. 4C). Interestingly, ErbB4 receptor precipitated EGFR (Fig. 4C, lower panel), whereas no precipitation of EGFR was observed with ErbB2 or ErbB3 receptors under these conditions (data not shown). Specificity of EGFR-ErbB4 interactions was confirmed by precipitating samples with EGFR and immunoblotting with ErbB4 (supplemental Fig. S1). Taken together, the above described results show that mechanical stretch activates ErbB3 and ErbB4. In addition, ErbB4 precipitated EGFR, suggesting tight interactions between these two receptors.
FIGURE 4.
ErbB receptors phosphorylation and dimerization in response to mechanical stretch in normal fetal type II cells. A and B, fetal type II cells were exposed to 5% cyclic stretch for the indicated periods of time. Collected proteins were immunoprecipitated with ErbB2 or ErbB3 receptor antibodies and immunoblotted with anti-phosphotyrosine antibody (Ptyr) to detect activation. Blots were reprobed with total ErbB receptor to control for protein loading. NRG (40 nm) added for 5 min was used as a positive control for receptor stimulation. Blots are representative of three independent experiments. *, p < 0.01 versus control. C, samples were processed as described above except that activation levels of ErbB4 were analyzed using an ErbB4 phosphospecific antibody. Blots were reprobed with total ErbB4 to control for protein loading and with EGFR antibody to investigate their interactions. NRG was used as a positive control. Results are from three independent experiments. *, p < 0.01 versus control; **, p < 0.001 versus control.
ErbB4 Regulates Stretch-induced Type II Cell Differentiation via ERK
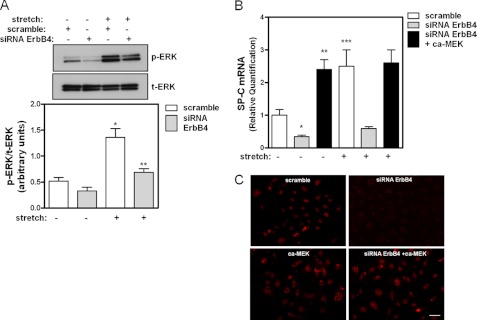
Next, we investigated whether ErbB4 is involved in ERK phosphorylation and thereby regulating stretch-induced fetal lung type II cell differentiation. To answer this question, ErbB4 was down-regulated with siRNA in lung type II cells. Experiments demonstrated undetectable levels of ErbB4 mRNA by qRT-PCR 48 h after transfection with siRNA for ErbB4 (data not shown). Fig. 5A shows that mechanical stretch increased ERK phosphorylation by 2.6-fold (0.52 ± 0.06 versus 1.36 ± 0.17). In samples transfected with siRNA ErbB4, ERK phosphorylation decreased by 40% in non-stretched samples and by 50% after mechanical stretch when compared with scramble stretched samples (1.36 ± 0.17 versus 0.69 ± 0.07). Similar effects were seen for the differentiation marker SP-C analyzed by qRT-PCR (Fig. 5B), SP-B and ABCA3 (supplemental Fig. S2). Furthermore, constitutively active MEK rescued SP-C mRNA and protein expression in ErbB4 down-regulation to the level induced by stretch in normal cells (Fig. 5, B and C). All together, our data clearly demonstrate that ErbB4 receptor regulates stretch-induced fetal type II epithelial cell differentiation via ERK.
FIGURE 5.
ErbB4 participates in stretch-induced ERK phosphorylation and type II cell differentiation. A, E17 type II cells were transfected with scramble siRNA or siRNA for ErbB4; 2 days later, monolayers were exposed to 5% stretch for 15 min. The level of ERK activation was evaluated by Western blot. Upper panel shows a representative blot. n = 3; *, p < 0.01 versus non-stretch scramble, **, p < 0.05 versus stretch scramble. B, fetal type II cells were transfected with scramble siRNA, siRNA ErbB4 or siRNA ErbB4 plus active MEK; 24 h after transfection, cells were exposed to 5% stretch for 16 h. SP-C mRNA abundance was assessed by qRT-PCR. n = 3; *, p < 0.01 versus non-stretch scramble, **, p < 0.01 versus non-stretch siRNA ErbB4, ***, p < 0.05 versus non-stretch scramble. C, fetal type II cells were transfected as indicated; 2 days later, monolayers were fixed and stained by fluorescence immunocytochemistry to detect SP-C protein (red). Pictures are representative of three separate experiments. Bar, 100 μm.
ErbB4 Receptor Is Not Activated in the Absence of EGFR
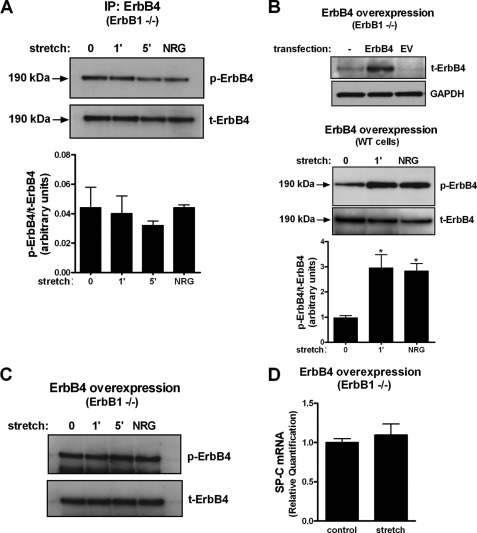
Next, we investigated ErbB4 phosphorylation in EGFR knock-out lungs. Unexpectedly, neither mechanical stretch nor NRG activated ErbB4 in EGFR-deleted cells (Fig. 6A). Because ErbB4 receptor protein is decreased in EGFR knock-out lungs and to rule out this as a possible cause for the lack of activation, we overexpressed ErbB4 in EGFR knock-out type II cells (Fig. 6B, upper panel). In wild-type cells, ErbB4 overexpression potentiated the effect on ErbB4 activation by stretch or NRG (Fig. 6B, lower panel) when compared with wild-type cells containing endogenous EGFR and ErbB4 expression (Fig. 4C). In EGFR-deleted cells, ErbB4 overexpression was not able to rescue stretch-induced ErbB4 phosphorylation (Fig. 6C) or differentiation (Fig. 6D), providing additional support that the lack of ErbB4 phosphorylation is due to the absence of EGFR. All together, these results show that EGFR is necessary for NRG- and stretch-induced ErbB4 phosphorylation.
FIGURE 6.
ErbB4 is not activated in the absence of EGFR. A, fetal type II cells isolated from EGFR knock-out mice were subjected to 5% cyclic stretch for the indicated periods of time. Samples were immunoprecipitated with ErbB4 antibody and immunoblotted with ErbB4 phosphospecific antibody and total ErbB4. Results are from three separate experiments. B, upper panel demonstrates overexpression of ErbB4 protein in cells transfected with ErbB4 as compared with no transfection or transfection with an empty vector (EV). In lower panel fetal type II cells isolated from wild-type mice were transfected with a plasmid encoding ErbB4 gene and then exposed to mechanical stretch or NRG. Samples were immunoprecipitated with ErbB4 antibody and immunoblotted with ErbB4 phosphospecific antibody and total ErbB4. Results are from three separate experiments. *, p < 0.05 versus control. C, EGFR (−/−) type II cells were transfected by electroporation with a plasmid encoding ErbB4 gene and then exposed to similar experimental conditions as described in A. Representative blot shows no phosphorylation of ErbB4 by stretch or neuregulin. D, EGFR (−/−) type II cells transfected with ErbB4 were exposed to 5% cyclic stretch for 16 h. Samples were processed to analyze SP-C mRNA by qRT-PCR. Results are from three independent experiments.
Stretch-mediated Release of Ligands Is Regulated by EGFR and ERK Pathway
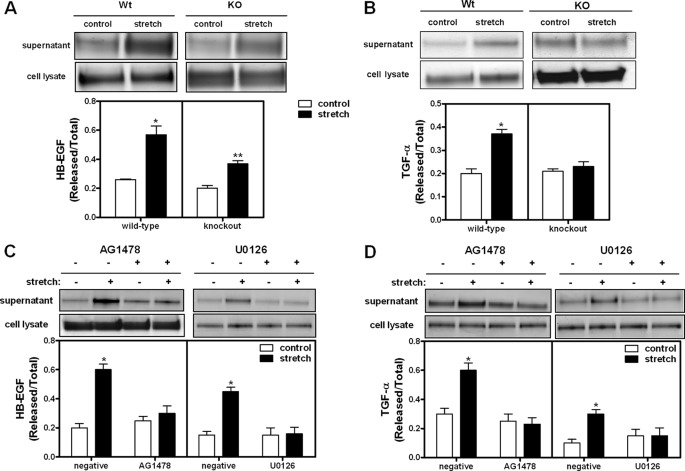
Next, we addressed whether stretch-release of ligands depends on the presence of EGFR. HB-EGF is a ligand for EGFR and ErbB4 whereas TGF-α only binds to EGFR (13). Our results show that, in type II cells isolated from wild-type mice, HB-EGF release was increased by 2.2-fold after 5% cyclic stretch for 30 min (0.26 ± 0.006 versus 0.57 ± 0.06). In EGFR knock-out mice, mechanical stretch still released HB-EGF into the supernatant although to a lower magnitude than in wild-type cells (85% increase, when compared with controls) (0.20 ± 0.02 versus 0.37 ± 0.02) (Fig. 7A). Mechanical stretch shed 85% more TGF-α into the supernatant in wild-type type II cells (0.16 ± 0.02 versus 0.33 ± 0.02). In contrast, no changes were observed in knock-out mice (Fig. 7B). These data suggest that stretch-induced release of ligands is under control of the ErbB receptor they bind to.
FIGURE 7.
Stretch-mediated release of ligands is regulated by EGFR and ERK pathway. A and B, E17 type II cells isolated from wild-type and EGFR knock-out mice were transfected by electroporation with cDNA constructs encoding alkaline phosphatase-tagged HB-EGF or TGF-α ligands. 24 h later, cells were exposed to 5% cyclic stretch for 30 min. Samples were processed as described in “Experimental Procedures” to assess ligand-release into the supernatant using the alkaline phosphatase (AP) shedding assay protocol. Data are expressed as the intensity of each AP supernatant band divided by total AP (supernatant + cell lysate). Upper panels shown representative blots. Data in the lower panels are from three independent experiments. *, p < 0.01 versus unstretched samples; **, p < 0.05 versus unstretched samples. C and D, wild-type fetal type II cells were exposed to similar experimental conditions as described above in the presence or absence of tyrphostin AG1478, an EGFR and ErbB4 phosphorylation inhibitor or U0126, a selective inhibitor of the ERK pathway. Results are from three independent experiments. *, p < 0.01 versus negative controls.
In addition, inhibition of EGFR and ErbB4 phosphorylation (33) using tyrphostin AG1478 (Fig. 7C) or blockade of the ERK pathway with the selective inhibitor U0126 (Fig. 7C) inhibited stretch-induced release of HB-EGF and TGF-α, confirming that shedding of ligands by stretch is mediated via ErbB receptor/ERK signaling pathway.
DISCUSSION
EGFR participates in lung maturation mediated by mechanical forces (20). However, the role of other receptors of the ErbB family in mechanotransduction during fetal lung development is unknown. In this study, we found that EGFR and ErbB4 regulate stretch-induced differentiation of fetal type II epithelial cells via the ERK pathway. In addition, interactions between EGFR and ErbB4 are important for lung development and type II cell differentiation. These conclusions are supported by the fact that even though mechanical strain phosphorylates ErbB4, activation of this receptor by stretch or NRG only occurred in the presence of EGFR. Moreover, EGFR function is not compensated by ErbB4 in EGFR knock-out mice. A final conclusion from our studies is that shedding of ligands by stretch is under control of the ErbB receptor and ERK signaling pathway.
The EGFR knock-out mice provide an excellent model to investigate ErbB receptor signaling in lung development. When compared with waved-2 mice for example (34) in which the EGFR is fully expressed and the ability of the ligand to bind to the receptor and to form dimers remain intact, in EGFR knock-out mice any signaling by homodimers or heterodimers is abolished.
EGFR knock-out mice survive only for a few days after birth and suffer from impaired epithelial development in several organs, including skin, lung, and gastrointestinal tract (17). Specifically, the lungs of these mice are characterized by an impaired branching morphogenesis, deficient alveolarization and septation and immaturity of alveolar type II epithelial cells (18). Consistent with these observations we found a significant decrease in the amounts of SP-B, SP-C, and ABCA3, which are markers of type II cell differentiation, in the lung tissue of EGFR knock-out mice when compared with normal lungs. Furthermore, mechanical stretch increased SP-C mRNA and protein levels only in type II cells isolated from wild-type mice but not in EGFR knock-out cells. Given that differentiation of type II epithelial cells requires EGFR (18) and previous studies from our laboratory, which revealed that stretch-induced type II cell differentiation is mediated via release of HB-EGF and TGF-α ligands (21), we hypothesized and confirmed that the lack of EGFR available for ligand binding prevents type II cell maturation. We also investigated signaling pathways downstream of EGFR, in particular the ERK pathway, previously shown to participate in stretch-induced type II cell differentiation (20). Our results show that mechanical stretch did not activate ERK in EGFR-deficient cells; however, overexpression of a constitutively active MEK rescued type II cell differentiation defect, supporting the role of EGFR-ERK signaling pathway in stretch-induced differentiation of fetal type II epithelial cells.
Genetic redundancy is a problem in gene targeting studies because functionally relevant proteins can compensate for the lack of protein product of a targeted gene (34). Our data show that ErbB2 and ErbB3 protein levels increased in the whole lung tissue of EGFR knock-out mice, suggesting a potential compensatory mechanism for the lack of EGFR. The morphology and cell composition (type II cells/total lung cells ratio) are different between wild type and mutant lungs, with a 50% reduction of type II cells in EGFR knock-out lungs (Fig. 1). Therefore, if protein abundance of ErbB receptors in the whole lung of mutant mice were normalized to SP-C, as a type II cell-specific marker, the compensatory effect for ErbB2 and ErbB3 receptors would be even greater, whereas no changes would be observed for ErbB4. In contrast, we did not observe any overexpression of ErbB receptors in isolated type II cells. These results are not a surprise given that other cell types, such as fibroblasts for example, could compensate the lack of EGFR in type II cells via mesenchymal-epithelial communications (25).
ErbB4 receptor plays an important role in fetal lung development and type II cell differentiation (24–27). We found that down-regulation of ErbB4 in normal type II cells, exposed or not to mechanical stretch, decreased not only ERK phosphorylation but also type II cell differentiation. These results support a key role of this receptor in type II cell differentiation mediated by mechanical forces. Then, we addressed the role of ErbB4 in knock-out mice, as one of the ligands released by stretch, HB-EGF, also binds to ErbB4. We speculated that ErbB4 would play a compensatory role in the absence of EGFR. However, our data show that ErbB4 protein levels were in fact decreased in EGFR knock-out lungs. These results are intriguing given the role of ErbB4 in lung maturation and suggest that EGFR and ErbB4 might depend on the presence of each other in lung development and type II cell differentiation. This observation is consistent with EGFR and ErbB4 being the prominent dimerization partners in the fetal lung (25). The interdependency among ErbB receptors has recently been documented and it has been shown, for example, that the binding affinity of EGF toward EGFR is modulated by cellular co-expression of ErbB2 or ErbB3, even though these receptors do not directly bind to EGF (14). In addition, EGFR is also known to undergo heterodimerization with ErbB2, ErbB3, or ErbB4 in response to EGF stimulation (35). Because of the lack of compensation by ErbB4, our findings also explain the phenotype of severe delay in lung development observed in EGFR knock-out mice.
To further explore the possibility of EGFR-ErbB4 dimerization in fetal lung development, we analyzed first the response of ErbB family of receptors to mechanical stretch and demonstrated that ErbB3 and ErbB4 receptors were activated by stretch and is consistent with previous studies from our laboratory showing activation of EGFR after mechanical stimulation (20). Interestingly, ErbB4 co-precipitated EGFR, further supporting the strong interactions of EGFR and ErbB4 in fetal lung development. Surprisingly, when similar set of experiments were performed in EGFR knock-out cells, neither mechanical stretch nor the ligand for ErbB4 NRG, were able to induce ErbB4 phosphorylation even when ErbB4 was overexpressed. These data strongly suggest that EGFR is required for ErbB4 phosphorylation. Support for these results was also observed in mouse lung epithelial cell lines where EGF activated ErbB4. Because EGF does not bind to ErbB4 directly, the authors speculated that activation of ErbB4 involves transactivation through EGFR (36). Several studies have reported that heterodimerization of ErbB4 with other ErbB receptors forms a high affinity binding complex which enhances the level of autophosphorylation and downstream signaling activation (37). Our data suggest that, at least for fetal lung cells, dimerization of ErbB4 with EGFR is a necessary step to stimulate ErbB4 receptor.
Given that the levels of ErbB4 were decreased in EGFR-deficient cells and to rule out that possibility as a reason for the lack of ErbB4 phosphorylation, ErbB1-deficient type II cells were transfected with a plasmid to overexpress ErbB4. Our results confirm that overexpression of ErbB4 cannot recue the lack of ErbB4 phosphorylation or stretch-mediated type II cell maturation in the absence of EGFR and further supports the finding that EGFR is required for ErbB4 signaling.
Although the precise cellular mechanisms for ErbB receptor activation are not clearly established, receptor-mediated dimerization upon ligand binding provides the best model so far to explain homo- and heterodimerization of the EGFR family of receptors (38). Heterodimers are generally biologically more active than homodimers and selective activation of well characterized signaling transduction pathways depend on receptor dimerization combinations (14). The reason for a preferred heterodimerization of EGFR with ErbB4 in fetal lung epithelial cells is unknown (25). Given the particular conformation of the dimerization loop, most of the previous studies using cell lines have identified ErbB2 as the preferred heterodimerization partner for each of the other ErbB receptors (35, 38). In contrast, our data show a preferred interaction of ErbB4 with EGFR, as previously demonstrated in fetal lung cells (25). We speculate that co-expression of EGFR with ErbB4 increases the affinity for their cognate ligands and increases the duration and intensity of specific signaling pathways, as it has been shown for p85 and Grb2 phosphorylation (39).
Phenotypes of ErbB receptor knock-out mice are the most striking proof of the power of receptor heterodimerization (40). The critical contribution of receptor-receptor interactions to heart development is clearly observed in ErbB2 null mice, where NRG-induced ErbB4 homodimers cannot replace the function of the ErbB2-ErbB4 heterodimer (41). The phenotype of the EGFR knock-out mice and our in vitro studies also show the importance of EGFR-ErbB4 dimerization in fetal lung development. However, these studies have some limitations, and we cannot completely rule out that the opposite hypothesis is also true and ErbB4 is necessary for EGFR activation. Although ErbB4 knock-out mice die early in gestation from cardiac defects (23), the rescued HER4heart transgenic mice (27) would provide a useful model to analyze ErbB family receptors signaling in ErbB4-deficient mice.
Finally, we used this model to investigate how mechanical stretch regulates release of ligands. Our data demonstrate the participation of ErbB receptor and ERK signaling pathway. TGF-α, a ligand for EGFR, is not release in EGFR knock-out cells exposed to mechanical stretch. In contrast, HB-EGF, a ligand for EGFR and ErbB4, was shed in EGFR-deficient cells, albeit to a lower magnitude than in normal cells. Given that EGFR activates ADAM17/TACE, the protease that mediates ectodomain cleavage of various transmembrane proteins (42), it is possible that in the absence of EGFR the baseline activity of TACE is decreased, which, accordingly, would explain the inability to release ligands by stretch. Another interpretation is that each ErbB receptor regulates the release of ligands they bind to. That would explain why HB-EGF is still released in EGFR knock-out cells. Our data are supported by previous studies showing that ectodomain shedding of TGF-α is induced by receptor tyrosine kinase activation and MAP signaling cascades (43, 44).
In summary, our data show that EGFR and ErbB4 are critical for stretch-induced differentiation of fetal type II epithelial cells via the ERK pathway. In addition, phosphorylation of ErbB4 receptor requires the presence of EGFR, suggesting that EGFR-ErbB4 dimerization may be important for fetal lung development. These studies provide novel insights into the cell signaling mechanisms regulating ErbB family of receptors in fetal lung development. These investigations may facilitate development of new strategies to accelerate lung maturation in clinical conditions where lung development is impaired.
Supplementary Material
Acknowledgments
We thank Dr. Zena Werb for providing the EGFR knock-out mice and Brenda Vecchio for manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HD052670 (to J. S.-E.) and P20 RR018728.

This article contains supplemental Figs. S1 and S2.
- EGFR
- epidermal growth factor receptor
- SP-C
- surfactant protein-C
- NRG
- neuregulin
- TGF-α
- transforming growth factor-α
- HB-EGF
- heparin-binding EGF-like growth factor
- ERK
- extracellular-regulated protein kinase.
REFERENCES
- 1. Joe P., Wallen L. D., Chapin C. J., Lee C. H., Allen L., Han V. K., Dobbs L. G., Hawgood S., Kitterman J. A. (1997) Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am. J. Physiol. 272, L95–L105 [DOI] [PubMed] [Google Scholar]
- 2. Liu M., Post M. (2000) Invited review: mechanochemical signal transduction in the fetal lung. J. Appl. Physiol. 89, 2078–2084 [DOI] [PubMed] [Google Scholar]
- 3. Sanchez-Esteban J., Cicchiello L. A., Wang Y., Tsai S. W., Williams L. K., Torday J. S., Rubin L. P. (2001) Mechanical stretch promotes alveolar epithelial type II cell differentiation. J. Appl. Physiol. 91, 589–595 [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Esteban J., Wang Y., Cicchiello L. A., Rubin L. P. (2002) Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L448–L456 [DOI] [PubMed] [Google Scholar]
- 5. Sanchez-Esteban J., Tsai S. W., Sang J., Qin J., Torday J. S., Rubin L. P. (1998) Effects of mechanical forces on lung-specific gene expression. Am. J. Med. Sci. 316, 200–204 [PubMed] [Google Scholar]
- 6. Torday J. S., Sanchez-Esteban J., Rubin L. P. (1998) Paracrine mediators of mechanotransduction in lung development. Am. J. Med. Sci. 316, 205–208 [DOI] [PubMed] [Google Scholar]
- 7. Wirtz H. R., Dobbs L. G. (2000) The effects of mechanical forces on lung functions. Respir. Physiol. 119, 1–17 [DOI] [PubMed] [Google Scholar]
- 8. Scarpelli E. M., Condorelli S., Cosmi E. V. (1975) Lamb fetal pulmonary fluid. I. Validation and significance of method for determination of volume and volume change. Pediatr. Res. 9, 190–195 [DOI] [PubMed] [Google Scholar]
- 9. Harding R. (1997) in The Lung: Scientific Fountations, 2nd Ed. (Crystal R. G., West J. B., Banes P. J., Weiber E. R., eds), pp. 2093–2104, Lippincott-Raven, Philadelphia [Google Scholar]
- 10. Moessinger A. C., Harding R., Adamson T. M., Singh M., Kiu G. T. (1990) Role of lung fluid volume in growth and maturation of the fetal sheep lung. J. Clin. Invest. 86, 1270–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldstein J. D., Reid L. M. (1980) Pulmonary hypoplasia resulting from phrenic nerve agenesis and diaphragmatic amyoplasia. J. Pediatr. 97, 282–287 [DOI] [PubMed] [Google Scholar]
- 12. Wigglesworth J. S., Desai R. (1979) Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum. Dev. 3, 51–65 [DOI] [PubMed] [Google Scholar]
- 13. Alroy I., Yarden Y. (1997) The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 410, 83–86 [DOI] [PubMed] [Google Scholar]
- 14. Singh A. B., Harris R. C. (2005) Autocrine, paracrine, and juxtacrine signaling by EGFR ligands. Cell Signal. 17, 1183–1193 [DOI] [PubMed] [Google Scholar]
- 15. Massagué J., Pandiella A. (1993) Membrane-anchored growth factors. Annu. Rev. Biochem. 62, 515–541 [DOI] [PubMed] [Google Scholar]
- 16. Sibilia M., Kroismayr R., Lichtenberger B. M., Natarajan A., Hecking M., Holcmann M. (2007) The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 75, 770–787 [DOI] [PubMed] [Google Scholar]
- 17. Miettinen P. J., Berger J. E., Meneses J., Phung Y., Pedersen R. A., Werb Z., Derynck R. (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376, 337–341 [DOI] [PubMed] [Google Scholar]
- 18. Miettinen P. J., Warburton D., Bu D., Zhao J. S., Berger J. E., Minoo P., Koivisto T., Allen L., Dobbs L., Werb Z., Derynck R. (1997) Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev. Biol. 186, 224–236 [DOI] [PubMed] [Google Scholar]
- 19. Sibilia M., Wagner E. F. (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269, 234–238 [DOI] [PubMed] [Google Scholar]
- 20. Sanchez-Esteban J., Wang Y., Gruppuso P. A., Rubin L. P. (2004) Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am. J. Respir. Cell Mol. Biol. 30, 76–83 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y., Maciejewski B. S., Soto-Reyes D., Lee H. S., Warburton D., Sanchez-Esteban J. (2009) Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-α. J. Physiol. 587, 1739–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel N. V., Acarregui M. J., Snyder J. M., Klein J. M., Sliwkowski M. X., Kern J. A. (2000) Neuregulin-1 and human epidermal growth factor receptors 2 and 3 play a role in human lung development in vitro. Am. J. Respir. Cell Mol. Biol. 22, 432–440 [DOI] [PubMed] [Google Scholar]
- 23. Gassmann M., Casagranda F., Orioli D., Simon H., Lai C., Klein R., Lemke G. (1995) Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378, 390–394 [DOI] [PubMed] [Google Scholar]
- 24. Zscheppang K., Liu W., Volpe M. V., Nielsen H. C., Dammann C. E. (2007) ErbB4 regulates fetal surfactant phospholipid synthesis in primary fetal rat type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L429–L435 [DOI] [PubMed] [Google Scholar]
- 25. Liu W., Zscheppang K., Murray S., Nielsen H. C., Dammann C. E. (2007) The ErbB4 receptor in fetal rat lung fibroblasts and epithelial type II cells. Biochim. Biophys. Acta 1772, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Purevdorj E., Zscheppang K., Hoymann H. G., Braun A., von Mayersbach D., Brinkhaus M. J., Schmiedl A., Dammann C. E. (2008) ErbB4 deletion leads to changes in lung function and structure similar to bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L516–L522 [DOI] [PubMed] [Google Scholar]
- 27. Liu W., Purevdorj E., Zscheppang K., von Mayersbach D., Behrens J., Brinkhaus M. J., Nielsen H. C., Schmiedl A., Dammann C. E. (2010) ErbB4 regulates the timely progression of late fetal lung development. Biochim. Biophys. Acta 1803, 832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez-Esteban J., Wang Y., Filardo E. J., Rubin L. P., Ingber D. E. (2006) Integrins beta1, alpha6, and alpha3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L343–L350 [DOI] [PubMed] [Google Scholar]
- 29. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zscheppang K., Dork T., Schmiedl A., Jones F. E., Dammann C. E. (2011) Neuregulin receptor ErbB4 functions as a transcriptional cofactor for the expression of surfactant protein B in the fetal lung. Am. J. Respir. Cell Mol. Biol. 45, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y., Maciejewski B. S., Lee N., Silbert O., McKnight N. L., Frangos J. A., Sanchez-Esteban J. (2006) Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L820–L827 [DOI] [PubMed] [Google Scholar]
- 32. Khoor A., Stahlman M. T., Gray M. E., Whitsett J. A. (1994) Temporal-spatial distribution of SP-B and SP-C proteins and mRNAs in developing respiratory epithelium of human lung. J. Histochem. Cytochem. 42, 1187–1199 [DOI] [PubMed] [Google Scholar]
- 33. Egeblad M., Mortensen O. H., van Kempen L. C., Jäättelä M. (2001) BIBX1382BS, but not AG1478 or PD153035, inhibits the ErbB kinases at different concentrations in intact cells. Biochem. Biophys. Res. Commun. 281, 25–31 [DOI] [PubMed] [Google Scholar]
- 34. Fowler K. J., Walker F., Alexander W., Hibbs M. L., Nice E. C., Bohmer R. M., Mann G. B., Thumwood C., Maglitto R., Danks J. A. (1995) A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc. Natl. Acad. Sci. U.S.A. 92, 1465–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graus-Porta D., Beerli R. R., Daly J. M., Hynes N. E. (1997) ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16, 1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zscheppang K., Korenbaum E., Bueter W., Ramadurai S. M., Nielsen H. C., Dammann C. E. (2006) ErbB receptor dimerization, localization, and co-localization in mouse lung type II epithelial cells. Pediatric Pulmonology 41, 1205–1212 [DOI] [PubMed] [Google Scholar]
- 37. Shelly M., Pinkas-Kramarski R., Guarino B. C., Waterman H., Wang L. M., Lyass L., Alimandi M., Kuo A., Bacus S. S., Pierce J. H., Andrews G. C., Yarden Y. (1998) Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J. Biol. Chem. 273, 10496–10505 [DOI] [PubMed] [Google Scholar]
- 38. Schlessinger J. (2002) Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110, 669–672 [DOI] [PubMed] [Google Scholar]
- 39. Moghal N., Sternberg P. W. (1999) Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell Biol. 11, 190–196 [DOI] [PubMed] [Google Scholar]
- 40. Olayioye M. A., Neve R. M., Lane H. A., Hynes N. E. (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19, 3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee K. F., Simon H., Chen H., Bates B., Hung M. C., Hauser C. (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378, 394–398 [DOI] [PubMed] [Google Scholar]
- 42. Blobel C. P. (2005) ADAMs: key components in EGFR signaling and development. Nat. Rev. Mol. Cell Biol. 6, 32–43 [DOI] [PubMed] [Google Scholar]
- 43. Fan H., Derynck R. (1999) Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 18, 6962–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gechtman Z., Alonso J. L., Raab G., Ingber D. E., Klagsbrun M. (1999) The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J. Biol. Chem. 274, 28828–28835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.