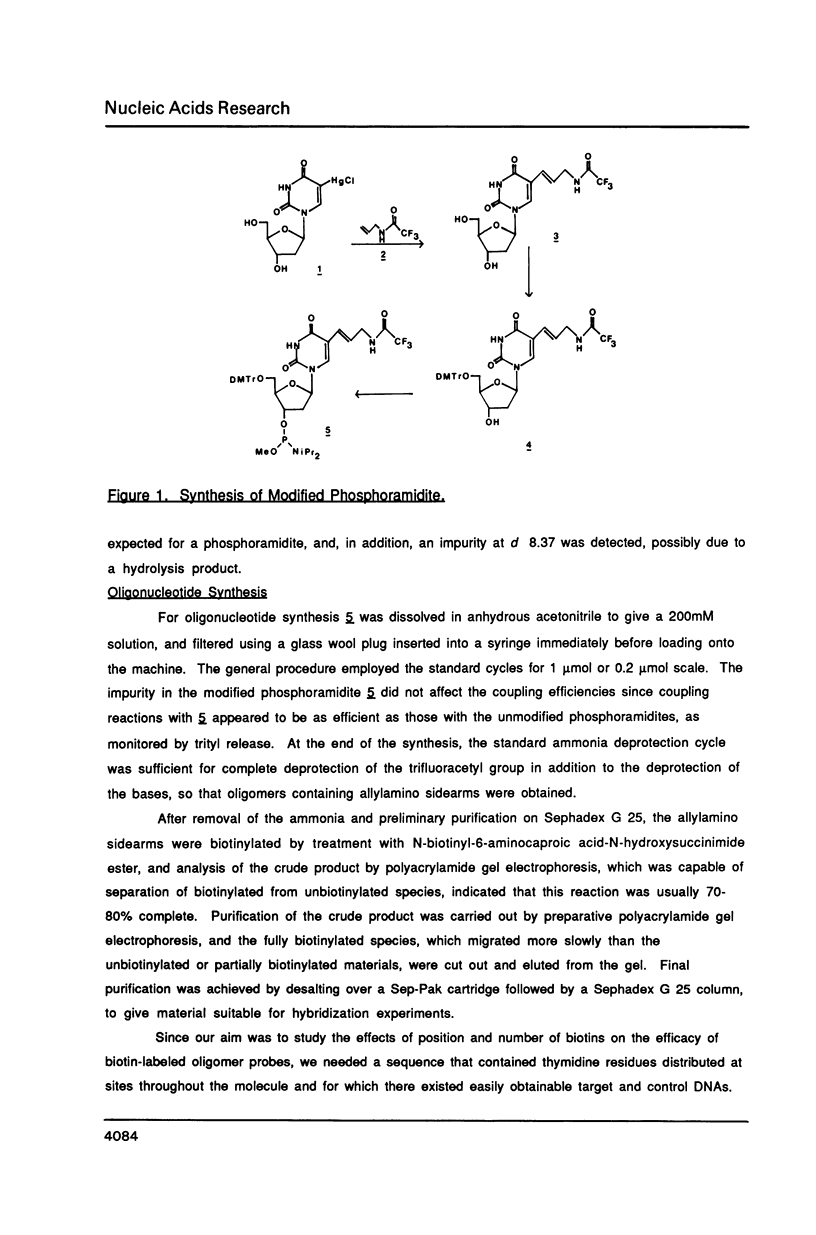
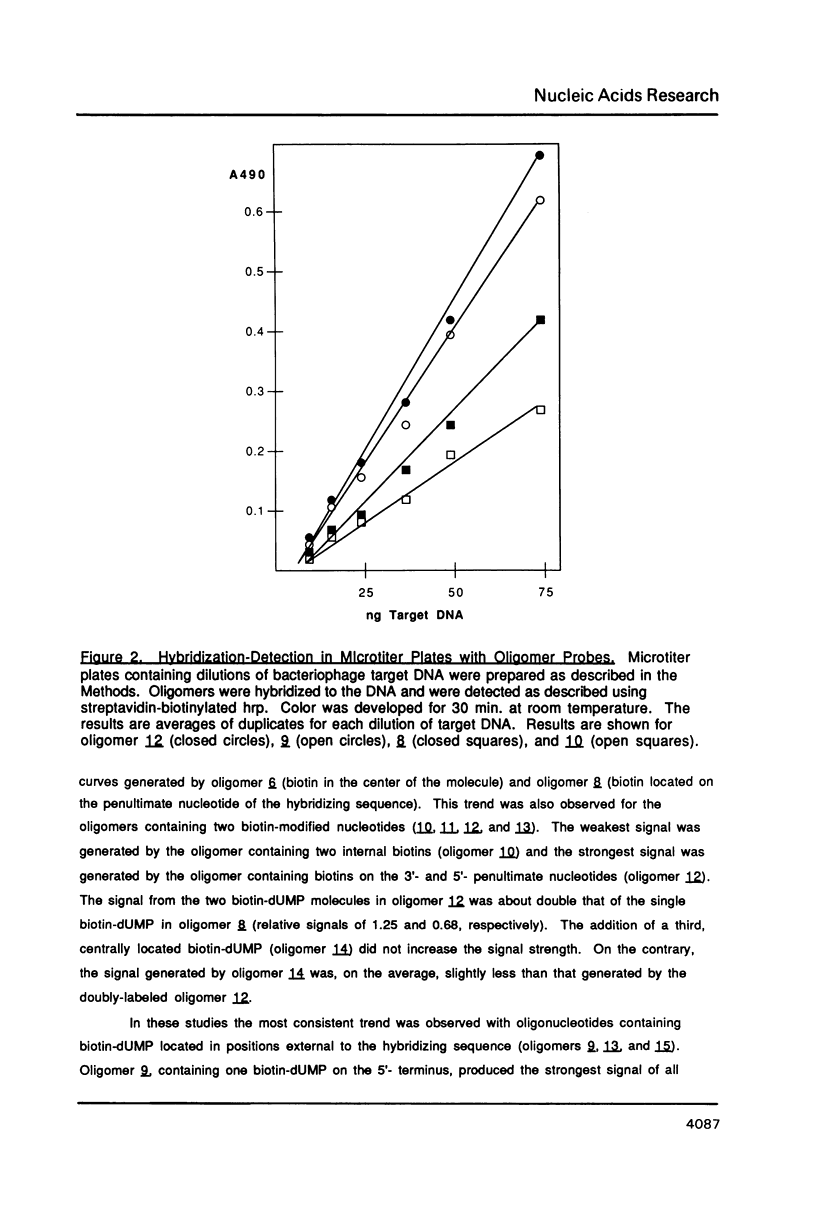
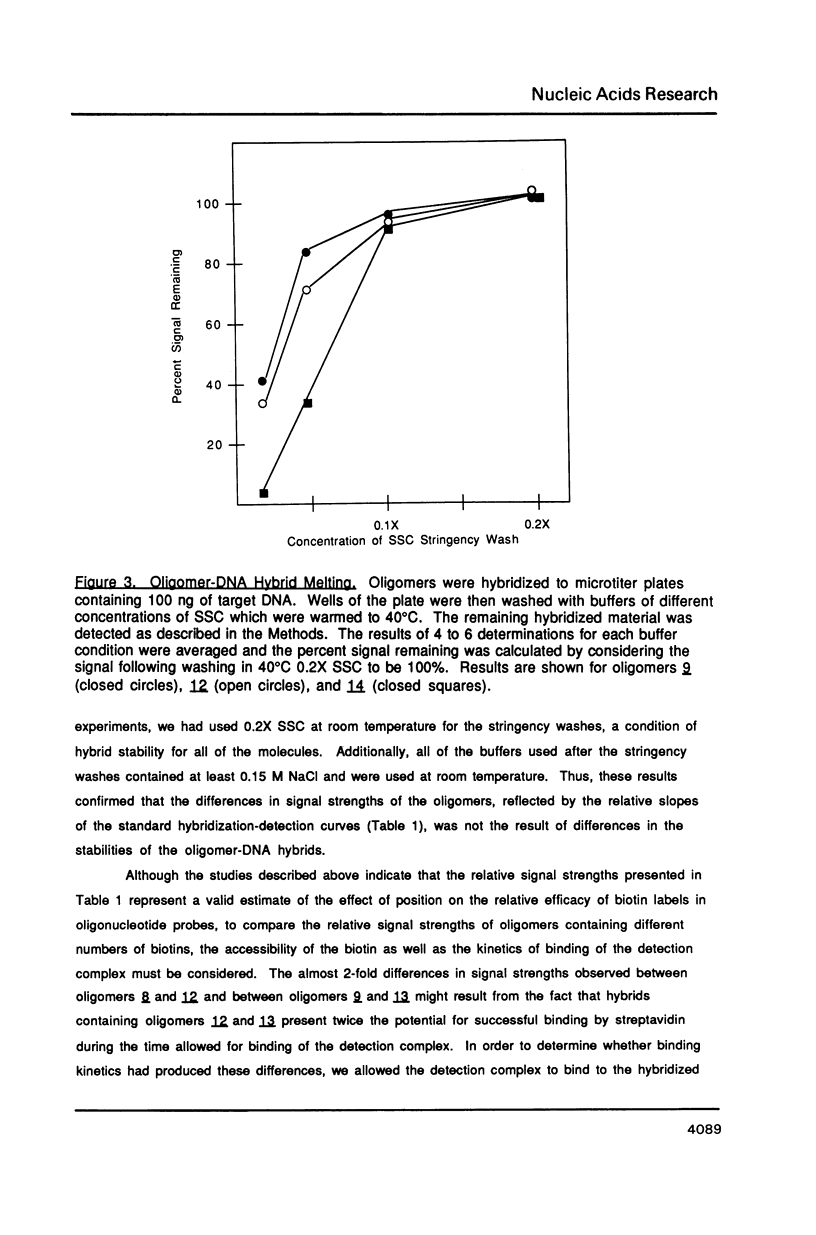
Abstract
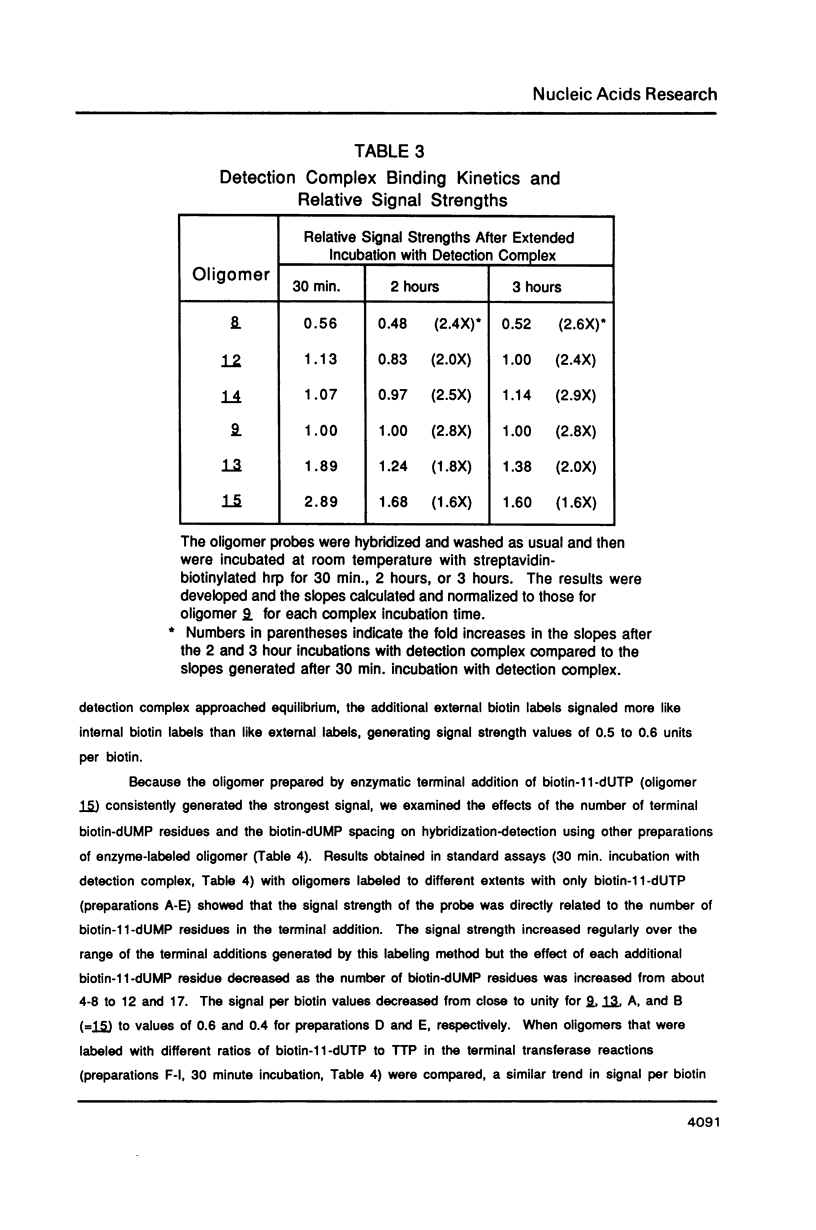
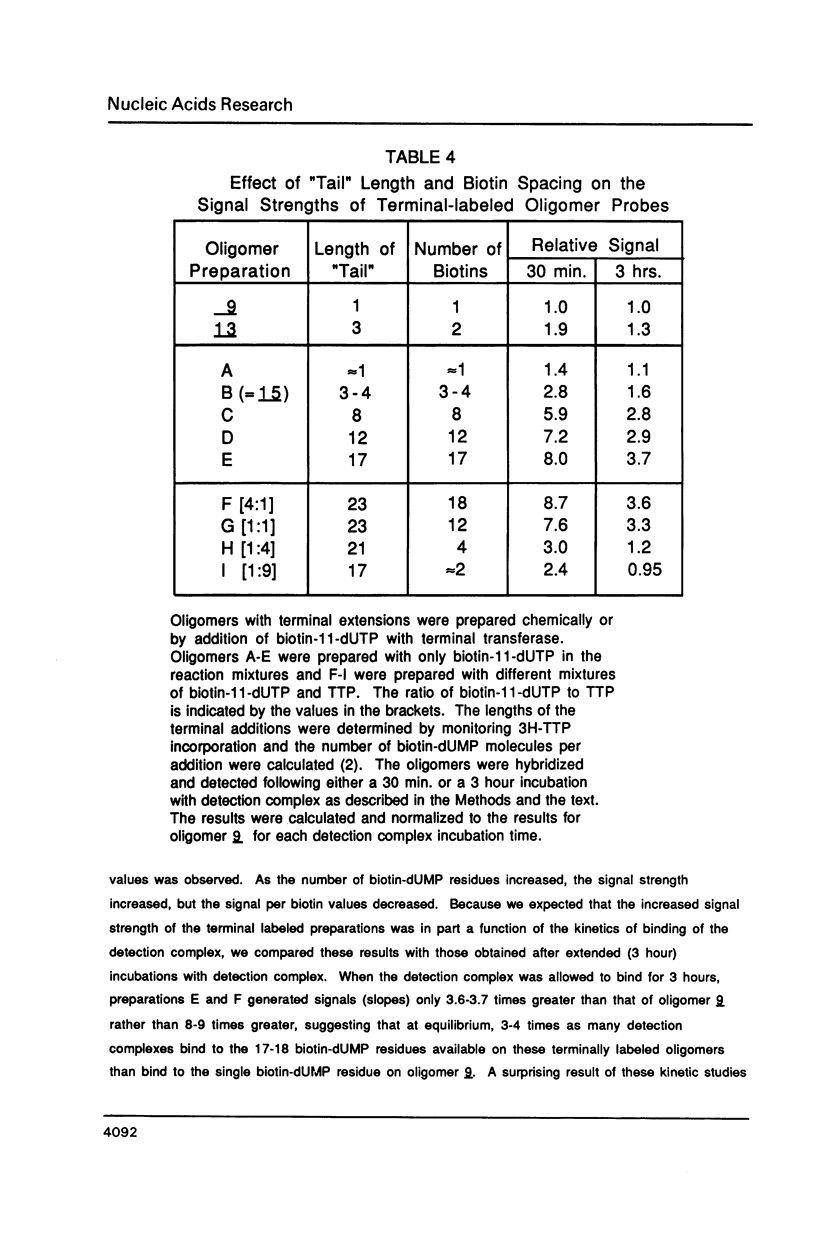
A series of oligonucleotides containing biotin-11-dUMP at various positions were synthesized and compared in quantitative, colorimetric hybridization-detection studies. A deoxyuridine phosphoramidite containing a protected allylamino sidearm was synthesized and used in standard, automated synthesis cycles to prepare oligonucleotides with allylamino residues at various positions within a standard 17-base sequence. Biotin substituents were subsequently attached to the allylamino sidearms by reaction with N-biotinyl-6-aminocaproic acid N-hydroxysuccinimide ester. These oligomers were hybridized to target DNA immobilized on microtiter wells (ELISA plates), and were detected with a streptavidin-biotinylated horseradish peroxidase complex using hydrogen peroxide as substrate and o-phenylenediamine as chromogen. We found that the sensitivity of detection of target DNA by biotin-labeled oligonucleotide probes was strongly dependent upon the position of the biotin label. Oligonucleotides containing biotin labels near or off the ends of the hybridizing sequence were more effective probes than oligonucleotides containing internal biotin labels. An additive effect of increasing numbers of biotin-dUMP residues was found for some labeling configurations.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Detection of specific DNA sequences with short biotin-labeled probes. DNA. 1985 Aug;4(4):327–331. doi: 10.1089/dna.1985.4.327. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Knowles J. R. A simple and efficient method for chemical mutagenesis of DNA. Nucleic Acids Res. 1985 Mar 11;13(5):1733–1745. doi: 10.1093/nar/13.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A., Wallace R. B. Biotin-labeled oligonucleotides: enzymatic synthesis and use as hybridization probes. DNA. 1984 Jun;3(3):269–277. doi: 10.1089/dna.1.1984.3.269. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Yokota H., Kosuda O., Yokoo K., Takemura K., Kikuchi T. Quantification of picogram levels of specific DNA immobilized in microtiter wells. FEBS Lett. 1985 Apr 22;183(2):379–382. doi: 10.1016/0014-5793(85)80814-0. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Marshall M. E., Coleman M. S. A method for biotinylating oligonucleotide probes for use in molecular hybridizations. DNA. 1986 Aug;5(4):333–337. doi: 10.1089/dna.1986.5.333. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Wachter L., Jablonski J. A., Ramachandran K. L. A simple and efficient procedure for the synthesis of 5'-aminoalkyl oligodeoxynucleotides. Nucleic Acids Res. 1986 Oct 24;14(20):7985–7994. doi: 10.1093/nar/14.20.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]



