Background: Ibogaine is a noncompetitive inhibitor of SERT that stabilizes the transporter in an inward-open conformation.
Results: Ibogaine binds to a site accessible from the cell exterior that does not overlap with the substrate-binding site.
Conclusion: Ibogaine binds to a novel binding site on SERT and DAT.
Significance: This study provides a mechanistic understanding of an unique inhibitor of SERT and DAT.
Keywords: Addiction, Dopamine Transporters, Drug Action, Electrophysiology, Serotonin Transporters
Abstract
Ibogaine, a hallucinogenic alkaloid proposed as a treatment for opiate withdrawal, has been shown to inhibit serotonin transporter (SERT) noncompetitively, in contrast to all other known inhibitors, which are competitive with substrate. Ibogaine binding to SERT increases accessibility in the permeation pathway connecting the substrate-binding site with the cytoplasm. Because of the structural similarity between ibogaine and serotonin, it had been suggested that ibogaine binds to the substrate site of SERT. The results presented here show that ibogaine binds to a distinct site, accessible from the cell exterior, to inhibit both serotonin transport and serotonin-induced ionic currents. Ibogaine noncompetitively inhibited transport by both SERT and the homologous dopamine transporter (DAT). Ibogaine blocked substrate-induced currents also in DAT and increased accessibility of the DAT cytoplasmic permeation pathway. When present on the cell exterior, ibogaine inhibited SERT substrate-induced currents, but not when it was introduced into the cytoplasm through the patch electrode. Similar to noncompetitive transport inhibition, the current block was not reversed by increasing substrate concentration. The kinetics of inhibitor binding and dissociation, as determined by their effect on SERT currents, indicated that ibogaine does not inhibit by forming a long-lived complex with SERT, but rather binds directly to the transporter in an inward-open conformation. A kinetic model for transport describing the noncompetitive action of ibogaine and the competitive action of cocaine accounts well for the results of the present study.
Introduction
Ibogaine is an alkaloid derived from the African shrub Tabernanthe iboga. The psychoactive properties of ibogaine have been known for centuries. More recently, the drug has achieved some notoriety because of its putative benefits in the treatment of addiction (1–3). The pharmacology of this alkaloid is complex, and many targets have been identified, among them transporters for the neurotransmitters serotonin (5-hydroxytryptamine, 5-HT)3 and dopamine (DA). Ibogaine was shown to inhibit transport by 5-HT and DA transporters (SERT and DAT, respectively) with IC50 values in the micromolar range (4, 5, 56). Signaling by monamines such as DA and 5-HT are thought to occur by volume transmission, which requires diffusion from exocytotic release sites to sites of receptor activation and reuptake (6). SERT and DAT are responsible for reuptake of their cognate substrates (7) through which they curtail the lifetime of extracellular 5-HT and DA after release. SERT and DAT are also important drug targets for therapeutic compounds such as the 5-HT reuptake inhibitors used clinically as antidepressants, and for psychostimulant drugs of abuse such as cocaine and amphetamines (8, 9).
An initial report suggested that ibogaine inhibited SERT and DAT competitively (5). However, more recent studies found that ibogaine differs from other SERT inhibitors because its inhibition is noncompetitive (4). Noncompetitive inhibition of transport was found in two other cases, the adenine nucleotide exchanger of mitochondria and the red cell glucose transporter (10–13) where the inhibitors were found to bind to an “inward-facing” transporter conformation. Accordingly, in SERT, ibogaine appears to stabilize an inward-open state. Cysteine scanning mutagenesis was used to identify a set of residues in the cytoplasmic half of transmembrane helices 1, 5, 6, and 8 that were accessible to cytoplasmic reagents. These positions defined a permeation pathway through which 5-HT and ions diffuse from their binding sites to the cytoplasm (14). The pathway corresponds to positions that are buried in occluded and outward-open crystal structures of LeuT, a prokaryotic SERT homologue (15). However, these positions line a cytoplasmic permeation pathway in a recent inward-open LeuT structure (16). Ibogaine increased the accessibility at all these positions in SERT, consistent with its ability to open the pathway (4, 14). The rate at which these pathway residues reacted (a measure of accessibility) was modulated also by the presence of substrates and other inhibitors. Cocaine and antidepressant drugs decreased reactivity but 5-HT and ibogaine increased it. It was proposed that ibogaine bound to, and stabilized, a conformation of SERT in which the cytoplasmic pathway is open.
Despite the noncompetitive nature of transport inhibition, ibogaine competitively displaced the cocaine analog (−)2-β-carbomethoxy-3β-(4-iodophenyl)tropane (β-CIT) from its binding site (4). β-CIT is a competitive inhibitor of SERT and its binding was also displaced by 5-HT (17). To resolve this apparent contradiction between ibogaine effects on binding and transport it was proposed that ibogaine and cocaine are mutually exclusive because their binding sites exist on different conformations of SERT (4). However, noncompetitive inhibition was also at odds with the fact that ibogaine and 5-HT are structurally similar, implying that they bound at the same site. To account for the inability of 5-HT to overcome ibogaine inhibition, it was proposed that ibogaine binding led to the formation of a long-lived complex in an inward-open conformation to which 5-HT could not bind (4).
Here we refine this view by measuring substrate-induced currents to characterize the inhibitory effects of ibogaine. We recently demonstrated that the uncoupled current carried by SERT in the presence of substrate is dependent on the formation of a specific intermediate in the reaction cycle. The conductive state of SERT is apparently in equilibrium with an inward-open form of SERT with K+ bound (18). This discovery allows the use of the uncoupled current as a measure of the inward-open conformation. The benefits of using electrophysiological methods include a high time resolution, which allows for precise assessment of inhibitor binding and dissociation kinetics.
In this study we address the mechanistic basis of ibogaine action on SERT and extend the analysis to DAT. We present a model compatible with all constraints imposed by published findings and our data. The analysis predicts that ibogaine binds directly to a site accessible from the extracellular milieu in an inward-open conformation of the transporter.
EXPERIMENTAL PROCEDURES
cRNA Preparation
Plasmids encoding hSERT and hDAT were linearized and transcribed into RNA with the T7 RNA polymerase kit mMessage mMachine (Ambion). A total of 5 ng of cRNA was microinjected into each oocyte. Electrophysiological recordings were performed 6–9 days following injection.
Oocyte Preparation
Xenopus laevis frogs (Nasco, Fort Atkinson, WI) were anesthetized with 2 mg/ml of ethyl 3-aminobenzoate methanesulfonate (FLUKA A5040) in H2O. The frog was decapitated and the ovarian lobes were removed and transferred to sterile Ca2+-free OR2 solution (82.5 mm NaCl, 2.5 mm KCl, 2 mm MgCl2, 10 mm HEPES, pH adjusted to 7.4 with NaOH) The lobes were manually reduced to groups of 5–10 oocytes and incubated in OR2, containing 1 mg/ml of collagenase from Clostridium histolyticum (Sigma). Forty-five to 60 min of incubation at 18 °C were sufficient to digest and remove the follicular layer. Oocytes were then selected and transferred to a Ringer solution (100 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, pH adjusted to 7.6 with NaOH). Oocytes were kept at 18 °C for a minimum of 2 h prior to injection. Injected oocytes were kept for 6–9 days at 18 °C in a Ringer solution containing 2.5 mm Na+ pyruvate, 100 μg/ml of penicillin, 100 μg/ml of streptomycin. Solutions were changed daily.
Electrophysiological Recordings in X. laevis Oocytes
A CA-1B high performance oocyte clamp (Dagan Corporation) was employed for the measurements. The recorded signal was digitized with a Digidata 13222A (Axon Instruments). An Intel PC running pCLAMP 9.2 (Axon Instruments) was used for acquisition. Borosilicate glass capillaries were pulled to a final resistance of 0.4–1.2 megaohms and filled with 3 m KCl. Oocytes were impaled and the membrane potential was clamped to a holding potential of −60 mV. For continuous superfusion with ND100 solution (100 mm NaCl, 2 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, pH adjusted to 7.4 with NaOH) a gravity-driven superfusion system (WarnerInstruments, Eight Channel Perfusion Valve Control System (VC-8)) was used. Recordings were started after a stable current baseline was established. The current was sampled with 100 Hz and low pass filtered with 20 Hz.
Transport Assays
Stably transfected HEK-293 cells expressing either hSERT or hDAT were seeded on 48-well plates precoated with poly-d-lysine (0.5 × 105 cells/well) 24 h prior to the experiment. Each well was washed with 500 μl of Krebs-HEPES buffer (KHP) (10 mm HEPES, 130 mm NaCl, 1.3 mm KH2PO4, 1.5 mm CaCl2, 0.5 mm MgSO4, pH 7.4, with NaOH). The cells were incubated in 0.2 ml of KHP buffer containing 0.1 μm [3H]5-HT or 0.01 μm [3H]MPP+, respectively. Unlabeled 5-HT or MPP+ was added to the indicated final concentration (0.3–20 μm 5-HT or 1–15 μm MPP+). The incubation times for [3H]5-HT and [3H]MPP+ were 1 and 3 min, respectively. To obtain an estimate of nonspecific uptake, the transporters were blocked with specific inhibitors 5 min prior and during incubation (mazindol (10 μm) for hDAT or paroxetine (10 μm) for hSERT). After incubation at room temperature, the cells were washed with 0.5 ml of ice-cold KHP buffer. Finally, cells were lysed with 0.5 ml of 1% SDS and transferred into 2 ml of scintillation mixture (Rotiszint eco plus LSC, Art. 0016.3) and counted in a Packard 2300TR TriCarb Liquid Scintillation Analyzer.
Radioligand Binding Assay
HEK293 stably expressing human DAT and hS4TO, a T-REx-293 cell line with human SERT under a Tet-repressor system (19), were harvested and prepared as described (20). SERT containing membranes were prepared in buffer containing 10 mm Tris·HCl (pH 7.5), 1 mm EDTA, 2 mm MgCl2. For DAT, EDTA was omitted from all buffers. For binding to hSERT, the incubation was for 1 h at 20 °C in 0.2 ml of buffer (containing 20 mm Tris·HCl (pH 7.5), 1 mm EDTA, 2 mm MgCl2, 3 mm KCl, 120 mm NaCl) with membranes (10 μg), 2 nm [3H]imipramine (specific activity 76 Ci/mmol), and the indicated concentrations of ibogaine and serotonin. Binding of [3H]CFT ([3H]WIN35,428, 40 Ci/mmol, 10 nm) to DAT containing membranes (12 μg/assay) was measured with the indicated concentrations of dopamine and ibogaine. EDTA was omitted from the reaction because the buffer contained 10 μm ZnCl2. Zn2+ stabilizes the outward-open state of DAT and facilitates inhibitor binding (55). Nonspecific binding was determined with 10 μm paroxetine for SERT and 10 μm mazindol for DAT. After an incubation of 1 h at 20 °C, the bound radioligand was trapped onto GF/B glass microfiber filters (Whatman) that had been soaked in 0.5% polyethyleneimine (Sigma). Radioactivity was counted as outlined above.
Release Experiments
CAD cells were cultured as described previously (21). Cells were transfected with Turbofect (Invitrogen) according to the manufacturer's instructions. Cells were grown overnight on round glass coverslips coated with poly-d-lysine (diameter 5 mm; 4 × 104 cells/well) and incubated with [3H]5-HT (0.4 μm) for 20 min at 37 °C in a final volume of 200 μl of KHB. Coverslips were then transferred to small superfusion chambers (200 μl) and superfused with KHB (25 °C, 0.7 ml/min) as described (22). After a washout period of 40 min to establish a stable baseline efflux of radioactivity, the experiment was started with the collection of fractions (2 min duration), and drugs to stimulate efflux were added at a given time. At the end of the experiment, cells were lysed in 1% SDS and superfusates were measured by liquid scintillation counting. Efflux of 3H was expressed as fractional rate, i.e. the radioactivity released during a fraction was expressed as the percentage of the total radioactivity present in the cells at the beginning of that fraction. Drug-induced efflux was calculated by subtracting the estimated basal efflux from total efflux during the first 6 min of drug exposure and expressed as the percentage of radioactivity in the cell at the beginning of drug exposure (23).
Measurement of DAT S262C Reactivity
The DAT X5C-S262C mutant, in which DAT cysteine residues at positions 90, 135, 306, and 342 were replaced with alanine, Cys-319 with phenylalanine, and Ser-262 with cysteine, was expressed in HeLa cells and membranes from those cells were prepared as described previously (24). Inactivation of [3H]CFT binding to those membranes was measured as described previously for β-CIT binding to SERT (24). Briefly, membranes were incubated with the indicated concentrations of MTSEA for 15 min in the presence of substrate or the indicated inhibitors in pretreated Multiscreen-FB 96-well filtration plates (Millipore, Bedford, MA). After washing away MTSEA and ligands, [3H]CFT (2.6 nm) in binding buffer was added and incubated with the membranes for 1.5 h. The membranes were washed again and bound [3H]CFT was measured by scintillation counting.
Whole Cell Patch Clamp
For patch clamp recordings, HEK-293 cells stably expressing hSERT (19) were seeded at low density for 24 h before measuring currents. To measure substrate-induced hSERT currents, cells were voltage clamped using the whole cell patch clamp technique. Briefly, glass pipettes were filled with a solution consisting of 133 mm potassium gluconate, 5.9 mm NaCl, 1 mm CaCl2, 0.7 mm MgCl2, 10 mm EGTA, and 10 mm HEPES adjusted to pH 7.2 with KOH. The cells were continuously superfused with an external solution of 140 mm NaCl, 3 mm KCl, 2.5 mm CaCl2, 2 mm MgCl2, 20 mm glucose, and 10 mm HEPES adjusted to pH 7.4 with NaOH. Currents were recorded at room temperature (20–24 °C) using an Axopatch 200B amplifier and pClamp 10.2 software (MDS Analytical Technologies). Unless otherwise stated, cells were voltage-clamped to a holding potential of −70 mV and 5-HT was applied for 5 s once every 60 s. Current traces were filtered at 1 kHz and digitized at 2 kHz using a Digidata 1320A (MDS Analytical Technologies). The liquid junction potential was calculated to be +16 mV and measurements were accordingly compensated. Drugs were applied using a DAD-12 (Adams & List, Westbury, NY), which permits complete solution exchange around the cells within 100 ms (25). Current amplitudes in response to 5-HT application were quantified using Clampfit 10.2 software. Passive holding currents were subtracted and the traces were filtered using a 100-Hz digital Gaussian lowpass filter.
Modeling
We incorporated the inhibitor-bound states for cocaine and ibogaine into a previously published kinetic model of SERT function (18). The time-dependent changes in state occupancies were evaluated by numerical integration of the resulting system of differential equations using GNU Octave 3.2.4. The voltage dependence of individual rates were modeled according to Läuger (26) assuming a symmetric barrier as kij = k0ijexp(−zQi,jFV/2RT), with F = 96,485 cmol−1, r = 8.314 JK−1 mol−1, and V the membrane voltage in volts, and T = 293 K. Coupled membrane currents in response to substrate application were calculated as I = (−FΣzQ,ij(pikij − pjkji))NC/NA, with zQ,ij being the net charge transferred during the transition, NC the number of transporters set to 4 × 106, and NA 6.022 × 1023. The uncoupled current was modeled as a current through a Na+ permeable channel with I = PcγNC(V − Vrev), with Pc being the occupancy of the channel state, γ the single channel conductance of 2.4 pS (27), NC the number of channels (4 × 106), V the membrane voltage, and Vrev the reversal potential of Na+ being +80 mV. The extra- and intracellular ion concentrations were set to the values used in patch clamp experiments. To account for the non-instantaneous onset of substrate in patch clamp experiments we modeled the substrate application as exponential rise with a time constant of 50 ms.
In addition a kinetic model for mutually exclusive binding (see supplemental Fig. S2) was simulated. A description of this simulation is included in the supplemental data.
Statistics
All values are given as mean ± S.E. if not stated otherwise. Affinity values obtained by nonlinear fits to the Hill equation are given as EC50 or IC50 values with 95% confidence interval. The statistical significance of differences between two groups were analyzed using the Mann-Whitney U test. p values < 0.05 indicated statistical significance. Differences in slopes were tested with F test.
RESULTS
Mutually Exclusive Binding of Ibogaine and Extracellular Substrate to SERT and DAT
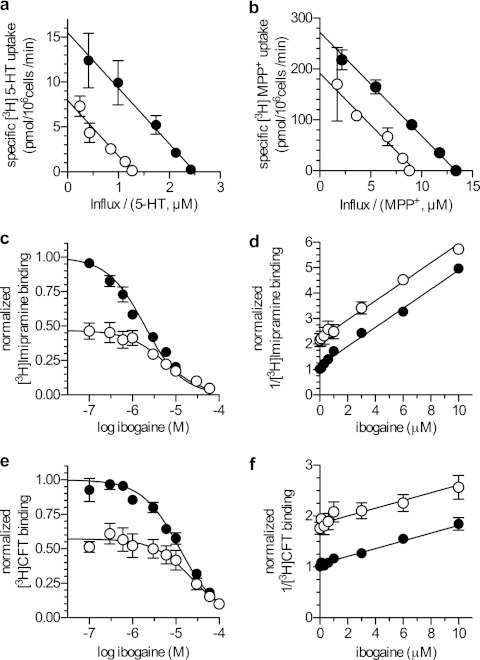
Ibogaine has been reported to inhibit SERT noncompetitively (4). In contrast, all other known SERT inhibitors are competitive with substrate (28). These competitive inhibitors include several compounds that also inhibit the closely related dopamine and norepinephrine transporters (17, 29). Previous reports suggested that both SERT and DAT were sensitive to inhibition by ibogaine (5, 30). Therefore, we compared the kinetics of ibogaine inhibition of SERT and DAT. For DAT, we used MPP+, instead of the physiological substrate DA, because of its superior chemical stability. Fig. 1 confirms that both transporters were sensitive to ibogaine when expressed in HEK-293 cells. It also shows that the inhibition was noncompetitive in both cases. The Eadie-Hofstee plots show that the Vmax for transport (y intercept) was reduced for both transporters, but the slope and hence the Km was essentially unchanged. These measurements confirm and extend the results of Jacobs et al. (4) and do not agree with a previous report of competitive inhibition for both, hSERT and hDAT in synaptosomes (5).
FIGURE 1.
Ibogaine inhibition of transport and binding by SERT and DAT. a, [3H]5-HT influx into cells stably expressing hSERT was measured in the absence (filled circles) or presence (open circles) of 10 μm ibogaine. The incubation time was 1 min and nonspecific uptake was measured in the presence of 10 μm paroxetine. b, [3H]MPP+ influx into cells stably expressing hDAT was measured as above using a 3-min incubation in the absence (filled circles) or presence (open circles) of 10 μm ibogaine. Nonspecific uptake was determined in the presence of 10 μm mazindol and was subtracted to the given values. The data in a and b are shown in form of Eadie-Hofstee plots, where the intercept on the y axis is equivalent to the Vmax and Km is given by the negative of the slope. For SERT the slopes at 0 and 10 μm ibogaine were statistically equivalent (p = 0.90 F test) and therefore globally fit with −6.18 ± 0.33 μm with a y intercepts of 15.51 ± 0.57 pmol/106 cells/min (R2 = 0.99) at 0 μm ibogaine and 7.92 ± 0.38 pmol/106 cells/min (R2 = 0.95) at 10 μm ibogaine. For DAT the slopes were also equivalent (p = 0.24 F test) and fit with −20.37 ± 0.8 μm with a y intercept of 272.30 ± 8.64 pmol/106 cells/min (R2 = 0.99) at 0 μm ibogaine and 191.70 ± 6.75 pmol/106 cells/min (R2 = 0.97) at 10 μm ibogaine. c–f, competition between ibogaine and 2 nm [3H]imipramine bound to SERT (c and d) and between ibogaine and 10 nm [3H]WIN35,428 bound to DAT (e and f) in the presence and absence of 10 μm 5-HT and DA, respectively. c, [3H]imipramine was displaced by ibogaine with an IC50 of 1.94 μm [1.71–2.21] (R2 = 0.95) in the absence of 5-HT and with an IC50 of 5.76 μm [3.72–9.00] (R2 = 0.85) in the presence of 10 μm 5-HT. 10 μm 5-HT reduced initial binding to 47% (95% confidence interval, 43–51). d, Dixon plot obtained by transformation of c. The slopes in d are 0.37 μm−1 (0.35–0.40) and are not statistically different (p = 0.38). e, [3H]WIN35,428 was displaced by ibogaine with an IC50 of 12.33 μm (10.46–14.54) (R2 = 0.99) in the absence of DA and with an IC50 of 23.14 μm (17.49–30.60) (R2 = 0.98) in the presence of 10 μm DA. 10 μm DA reduced initial binding to 57% (54–60). f, Dixon plot obtained by transformation of e. The slopes in f are 0.076 μm−1 (0.059–0.093) and are not statistically different (p = 0.50).
Ibogaine blocks substrate translocation by SERT in a noncompetitive manner (Fig. 1, a and b), but it competes with the binding of the inhibitor [125I]βCIT, a cocaine analog, which labels SERT with high affinity (17). Noncompetitive inhibition is suggestive of a second binding site. SERT contains a minimum of two binding sites: an outward-occluded conformation of LeuT contains a binding site in the outer vestibule (also termed S2 (31)), which is occupied by the planar tricyclic ring of antidepressants (32). S2 has been proposed as the antidepressant site of SERT (33, 34). The primary substrate binding site, S1, is occupied by substrate in the occluded state of LeuT (15) and by several compounds of the SSRI class (selective serotonin reuptake inhibitors) (34–36). We therefore examined whether SERT could bind serotonin and ibogaine simultaneously. This question was addressed in a competition experiment, in which the ability of ibogaine to compete with [3H]imipramine binding to membranes of cells expressing SERT was measured in the absence and presence of serotonin (Fig. 1c). If the data are replotted in a Dixon plot, parallel lines are obtained (Fig. 1d). Similar findings were obtained when ibogaine competed with binding of the cocaine analog [3H]CFT to membranes from cells expressing DAT in the absence and presence of dopamine (Fig. 1, e and f). In the Dixon plot, a parallel shift was seen in the presence of a second inhibitor, as expected if binding is mutually exclusive. Simultaneous binding of two inhibitors, however, should result in intersecting lines (37). It is worth pointing out that both parallel and intersecting Dixon plots have been observed with SERT depending on which pairs of inhibitors are examined (34). Thus based on these observations, we conclude that, despite noncompetitive inhibition of substrate translocation by ibogaine, neither SERT nor DAT bind ibogaine and substrate simultaneously.
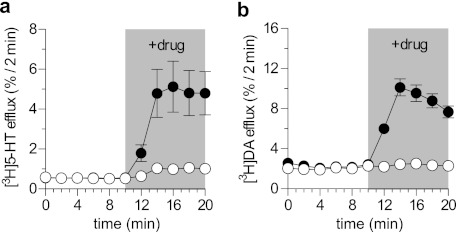
Substrates for membrane transport systems frequently induce efflux of accumulated radiolabeled substrates when added to the extracellular medium (38). Accordingly, we examined whether ibogaine was capable of eliciting efflux, which is also the hallmark action of amphetamines. As is evident from Fig. 2, this was not the case. When cells expressing SERT were preloaded with the substrate [3H]5-HT, superfusion with the SERT substrate p-chloramphetamine (PCA) induced a pronounced efflux. In contrast, ibogaine was ineffective (Fig. 2a). Similar findings were obtained when cells expressing DAT were challenged with d-amphetamine (Fig. 2b). It is worth noting that these experiments were carried out in CAD cells, which also express the vesicular monoamine transporter and are thus highly sensitive to any amphetamine-like releasing action.
FIGURE 2.
Ibogaine does not elicit an amphetamine-like releasing action in SERT and DAT. a and b, effect of ibogaine and PCA (a) or d-amphetamine (b) in a release assay. CAD cells transiently transfected with hSERT (a) or hDAT (b) were preloaded with [3H]5-HT or [3H]DA, and superfused with buffer. 2-min fractions were collected. Ibogaine (50 μm), PCA (3 μm), or d-amphetamine (3 μm) were added after six fractions (at time point 10) and five more 2-min fractions were collected (time points 12–20). Data are fractional release per 2 min in percent. Experiments were performed in quadruplicates; n = 2 (a), n = 3 (b).
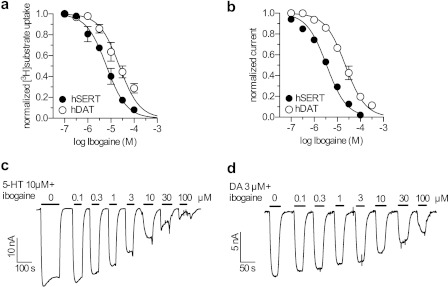
Ibogaine Blocks SERT- and DAT-mediated Currents with Potency Similar as Required for Transport Inhibition
SERT and DAT both carry uncoupled, substrate-induced ionic currents (39, 40). We compared the IC50 values for ibogaine inhibition of substrate transport into HEK-293 cells expressing hSERT or hDAT (Fig. 3a) with those for substrate-induced currents (Fig. 3, b–d). For substrate uptake by SERT, the IC50 was 6 μm (5–8), in good agreement with previous results (4), and for DAT the IC50 was ∼4-fold higher, i.e. 23 μm (18–30). We also found that ibogaine inhibited the steady state currents induced by substrate in both SERT and DAT. In Fig. 3, c and d, currents were induced in X. laevis oocytes expressing hSERT (Fig. 3c) or hDAT (Fig. 3d) by addition of 10 μm 5-HT or 3 μm DA, respectively, together with the indicated concentrations of ibogaine. The oocyte system is better suited for this analysis because signal to noise ratios of currents measured by 2-electrode voltage clamp are superior to those obtained in mammalian cells. In particular, hDAT expressed in HEK-293 cells carried an average DA-induced current amplitude of ∼2 pA (data not shown). This current amplitude is in good agreement with the results of Erreger et al. (41) but is too small to allow reliable analysis of current inhibition.
FIGURE 3.
Inhibition of substrate transport and induced currents by ibogaine. a, for transport, HEK-293 cells expressing hSERT (filled circles) or hDAT (open circles) were incubated with either 0.1 μm [3H]5-HT or 0.01 μm [3H]MPP+ for 1 or 3 min, respectively, in the presence of the indicated concentrations of ibogaine. The IC50 values for ibogaine inhibition of transport were 6.43 μm (5.03–8.21) R2 = 0.91 for SERT and 23.17 μm (17.76–30.23) R2 = 0.78 for DAT. b, measurements of substrate-induced currents were performed using two electrode voltage-clamp with X. laevis oocytes expressing hSERT (filled circles) or hDAT (open circles), clamped to a holding potential of −60 mV. Current was induced by addition of 10 μm 5-HT or 3 μm DA, respectively, and ibogaine was present at the indicated concentrations. The IC50 values for ibogaine inhibition of he substrate-induced current were 3.44 μm (3.10–3.86) R2 = 0.98 for SERT and 22.33 μm (19.32–25.80) R2 = 0.95 for DAT. c, current traces for 5-HT-induced currents and ibogaine inhibition of hSERT. Pulses of 10 μm 5-HT together with the indicated concentrations of ibogaine were applied to X. laevis oocytes expressing hSERT and the substrate-induced currents were measured by two electrode voltage-clamp. Data from this representative experiment and other similar trials were combined by calculating mean currents normalized to maximal substrate-induced inward current at 10 μm 5-HT in the absence of ibogaine, ± S.E., n = 6. The data shown in panel B (filled circles) shows the mean ± S.E. of these experiments. D, current traces for ibogaine inhibition of DAT currents induced by 3 μm DA, measured as in c using oocytes expressing hDAT.
Fig. 3b shows that ibogaine inhibited the currents carried by both transporters and that in each case the inhibition occurred at a similar concentration as required for transport inhibition (compare with Fig. 2a). Inhibition of DAT currents required ∼6-fold higher ibogaine concentrations (22 μm (19–25)) compared with SERT (3.4 μm (3.1–3.9)) (Fig. 3b). The similar ibogaine sensitivity suggests a close functional relationship between substrate-induced current and transport, in agreement with a recent study (18), showing that the 5-HT-induced current in SERT is carried by a state that is occupied primarily when the transporter is transporting 5-HT.
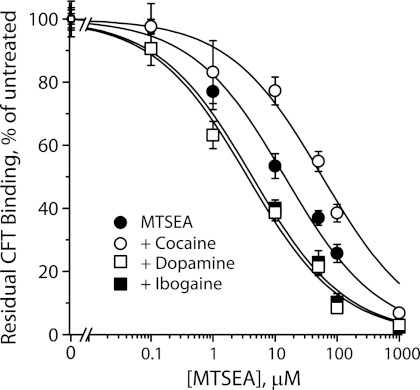
Ibogaine Renders Cytoplasmic Pathway Accessible to Chemical Modification
In SERT, ibogaine was shown to stabilize an inward-open conformation of the transporter (4, 14, 18). In this conformation, accessibility was increased for residues within the permeation pathway through which substrates dissociate to the cytoplasm, and accessibility of residues in the extracellular pathway was decreased (4). To test whether ibogaine had the same effect on DAT, we mutated Ser-262 to cysteine in a background (X5C) previously known to have minimal sensitivity to methanethiosulfonate (MTS) reagents (42). Ser-262 corresponds to a cytoplasmic pathway residue in SERT (Ser-277) known to become more accessible in the presence of ibogaine (4, 14). In the experiment shown in Fig. 4, membranes from cells expressing DAT X5C-S262C were incubated with the indicated concentrations of MTSEA for 15 min, washed, and then assayed for binding of [3H]CFT. Addition of cocaine during the MTSEA treatment shifted the potency of MTSEA for inactivating CFT binding to higher concentrations, as expected if cocaine stabilized an outward-open conformation of DAT as it does for SERT (14, 36, 43). DA addition shifted the MTSEA response to lower concentrations, similar to the effect of 5-HT on SERT S277C and consistent with an increase in accessibility of the cytoplasmic pathway with substrate transport. Ibogaine also increased the sensitivity of the DAT cytoplasmic pathway (Fig. 4) as previously observed for SERT (4, 14, 18) and consistent with ibogaine stabilizing an inward-open of DAT.
FIGURE 4.
Reactivity of the DAT cytoplasmic pathway is increased by ibogaine. Ser-262 in DAT, corresponding to the cytoplasmic pathway residue Ser-277 identified in SERT, was mutated to cysteine in the X5C background (42). Membrane fragments from HeLa cells expressing DAT X5C-S262C were treated with the indicated concentrations of MTSEA for 15 min in the presence or absence of 10 μm cocaine, 10 μm DA, or 20 μm ibogaine as indicated, and then assayed for residual [3H]CFT binding as previously described for SERT (43, 54). From the MTSEA concentrations required for half-maximal inactivation, the rate constants for inactivation were 82 ± 3 s−1 m−1 for MTSEA alone, 13 ± 2 s−1 m−1 in the presence of cocaine, 419 ± 36 s−1 m−1 with DA present, and 348 ± 95 s−1 m−1 in the presence of ibogaine. Results are mean ± S.E. from 3 independent experiments.
Ibogaine Blocks Substrate-induced Currents Only from Extracellular Side
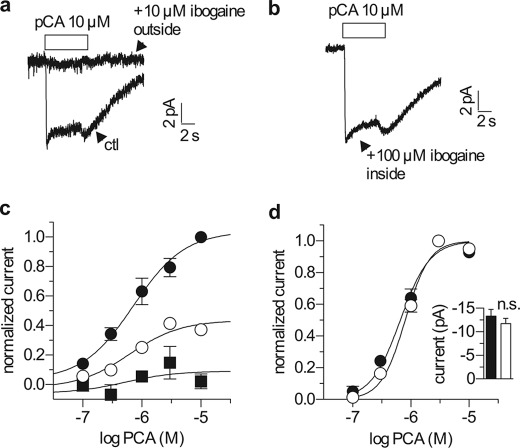
The SERT substrate PCA, like 5-HT, induced uncoupled currents in HEK-293 cells expressing hSERT. Fig. 5 shows an experiment in which HEK-293 cells expressing hSERT were voltage clamped by whole cell patch clamp. Ibogaine decreased the amplitude of the PCA current in a concentration-dependent manner, but did not change the EC50 for PCA (Fig. 5c). The inability of high concentrations of PCA to overcome ibogaine inhibition indicates noncompetitive inhibition, consistent with separate binding sites for ibogaine and substrates on SERT. The EC50 values calculated for PCA were 0.7 μm (0.3–1.5 μm), 0.6 μm (0.2–1.7 μm), and 0.5 μm (0.4–62 μm) in the presence of 0, 1, and 10 μm ibogaine, respectively. This inhibition required extracellular ibogaine: 10 μm ibogaine almost completely prevented PCA-induced current (Fig. 5, a and c). In contrast, when applied from the intracellular side via the pipette solution, ibogaine concentrations up to 100 μm failed to block the current (Fig. 5b). Moreover, the EC50 for PCA and the maximal current amplitude it induced were both unchanged in the presence of 100 μm intracellular ibogaine (Fig. 5d, inset).
FIGURE 5.
Ibogaine blocks substrate-induced currents only from the extracellular side. a, single hSERT expressing cells were voltage clamped to −70 mV using the whole cell patch clamp configuration and continuously superfused with buffer solution as described under “Experimental Procedures.” A 6-s pulse of 10 μm p-chloroamphetamine (a SERT substrate) was applied either in the absence (lower trace) or presence (upper trace) of 10 μm ibogaine in the extracellular medium. b, single hSERT expressing cells were voltage clamped and stimulated with PCA as in a, but with a pipette solution containing 100 μm ibogaine. c, single hSERT expressing cells were voltage clamped as in a and b and stimulated with a range of PCA concentrations. Once per minute they were challenged with a 5-s pulse of 10 μm PCA, either in the absence (filled circles) or after a 10-s pre-application of either 1 or 10 μm ibogaine (open circles and filled squares, respectively). Ibogaine reduced the maximal charge transfer to 0.44 ± 0.04 at 1 μm (n = 5) and 0.09 ± 0.08 at 10 μm (n = 5), whereas the EC50 of PCA was not changed (0.72 μm (0.34–1.55 μm) control (ctl) (n = 10); 0.62 μm (0.23–1.70 μm) at 1 μm ibogaine (n = 5); 0.52 μm (0.44 nm to 62.14 μm) at 10 μm ibogaine (n = 5). d, ibogaine did not influence the concentration response when present in the intracellular pipette solution. Single hSERT expressing cells were voltage clamped and stimulated with PCA as in c using either normal pipette solution (filled circles) or pipette solution containing 100 μm ibogaine (open circles). Ibogaine did not change the EC50 of PCA when applied from the inside (0.66 μm (0.55–0.79 μm) under control (n = 10); 0.80 μm (0.69–0.93 μm) with 100 μm ibogaine inside, n = 10). The inset shows a comparison of the current amplitudes induced by 10 μm PCA both in the absence (−13.3 pA ± 1.4 pA, filled column) and presence of 100 μm internal ibogaine (−11.7 ± 1.1 pA, open column) (p = 0.26, Mann-Whitney U test).
Kinetic Analysis of Inhibition of Substrate-induced Currents
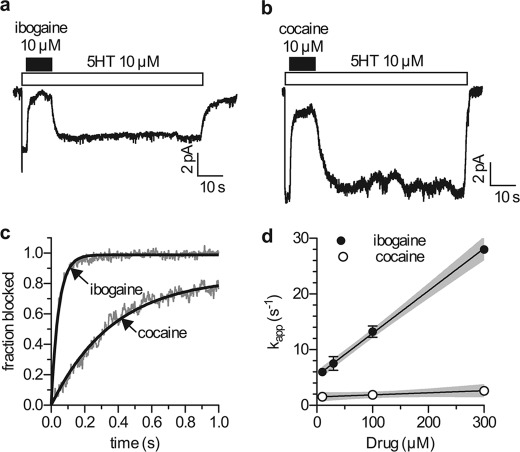
The time resolution of whole cell patch clamp analysis allowed us to measure the kinetics of inhibition by ibogaine and other inhibitors. Fig. 6, a and b, shows the time courses for the action of 10 μm ibogaine and 10 μm cocaine on 5-HT-induced currents in SERT. The uncoupled current was blocked rapidly by both ibogaine and cocaine. Subsequent drug removal led to a rapid increase of current amplitude for ibogaine and cocaine. The onset of the block and the washout followed simple exponential kinetics for both ibogaine and cocaine. Analysis of the rates of current blockage shows that ibogaine acted significantly faster than cocaine (Fig. 6c). Fig. 6d shows that the rate constant for the block (kapp) was only modestly increased as the concentration of cocaine was raised. This is inconsistent with a diffusion-controlled bimolecular reaction. In contrast, the block imposed by ibogaine was greatly accelerated as the ibogaine concentration was increased.
FIGURE 6.
Kinetics of current block by ibogaine and cocaine. Single hSERT expressing cells were voltage clamped to −70 mm using the whole cell patch clamp technique. Cells were continuously superfused with buffer solution. a and b, for the evaluation of blocking kinetics cells were challenged with 5-HT (10 μm). 2 s after 5-HT addition, the blocking agent (ibogaine (a) or cocaine (b)) was applied for 10 s and then washed away for 60 s in the presence of 5-HT. c, comparison of the blocking kinetics of ibogaine (10 μm) and cocaine (10 μm). Gray lines indicate representative traces, and black lines show fits to the traces. d, analysis of the blocking kinetics of ibogaine (filled circles) and cocaine (open circles) over a range of concentrations. Rate constants for the development of the block were calculated over the concentration range and plotted against concentration. The black lines are linear fits through the data points and the gray areas indicate 95% confidence intervals. The slope for ibogaine was significantly different from zero (p < 0.0001 F test), 7.6 × 104 ± 0.4 × 104 s−1 m−1, and the y intercept was 5.3 ± 0.5 s−1. The slope for cocaine was 3 × 103 ± 2 × 103 s−1 m−1, which was not significantly different from zero (p = 0.16 F test), and the y intercept was 1.5 ± 0.4 s−1.
A possible site for ibogaine binding on SERT corresponds to the location proposed as a second substrate binding site in LeuT and DAT (31, 44). This site is in the extracellular pathway outside the main substrate site and separated from it by an ion pair between Arg-30 and Asp-404 in LeuT (15) (Arg-104 and Glu-493 in SERT). Using the positions proposed to constitute the second substrate site as a guide, we tested pathway mutants by measuring their IC50 values for ibogaine inhibition of 5-HT transport. Endogenous amino acids were replaced with cysteine (Trp-103, Tyr-175, Ile-179, Gly-402, Pro-403, Phe-407, Val-489, and Trp-493), phenylalanine (Ile-179, Leu-406, and Val-489), lysine (Asp-400), or aspartate (Lys-490). Although some of these mutations slightly enhanced affinity, none of them decreased inhibitory potency as expected if they were direct contact residues in an ibogaine-binding site (Table 1). The location of these positions relative to the S2 site defined for DAT is shown in supplemental Fig. S1. The mutated positions include some that correspond to the S2 site proposed for DAT (44) and many others in the vicinity of S2. From the undiminished ibogaine sensitivity of these mutants, we conclude that S2 is not the site of ibogaine binding. These results are consistent with a recent LeuT structure in the inward-open conformation (16). In this structure, there is no opening available for extracellular ligands to enter the permeation pathway, which is closed from the binding site to the extracellular surface, leaving no space for a compound the size of ibogaine to bind.
TABLE 1.
Ibogaine inhibition of 5-HT transport by SERT mutants
5-HT influx into cells expressing various mutants of SERT was measured over a range (0.1 to 100 μm) of ibogaine concentrations. C109A is equivalent to wild type SERT but without its sensitivity to inactivation by cysteine reagents. C109A and each of the other mutants were tested in triplicate in two to three experiments to determine the ibogaine concentration that led to half-maximal inactivation of transport activity.
| Mutant | Mean IC50 for Ibogaine | S.E. |
|---|---|---|
| C109A | 7.9 | 0.7 |
| W103C | 4.1 | 0.3 |
| Y175C | 9.0 | 0.4 |
| I179C | 3.3 | 0.2 |
| I179F | 2.3 | 0.2 |
| D400K | 3.0 | 0.6 |
| G402C | 1.9 | 0.2 |
| P403C | 4.3 | 0.2 |
| L406F | 4.8 | 0.2 |
| F407C | 3.6 | 0.1 |
| V489F | 2.9 | 0.3 |
| V489C | 4.2 | 0.1 |
| K490D | 3.8 | 0.1 |
| E493C | 6.9 | 0.7 |
DISCUSSION
The results presented here provide new insight into the mechanism by which ibogaine binds to SERT to induce conformational change. Ibogaine differs from other SERT inhibitors because it inhibits noncompetitively and increases the accessibility of residues in the cytoplasmic substrate permeation pathway. These residues correspond to positions in a permeation pathway recently revealed by an inward-open crystal structure of LeuT (16). In SERT, cysteine residues placed at these positions reacted with aqueous reagents in membrane fragments (when the cytoplasmic face of the transporter was accessible) but not in intact cells, indicating access to these positions only from the cytoplasm. The rate at which these pathway residues reacted was modulated by the presence of substrates and inhibitors. Cocaine and antidepressant drugs decreased reactivity (a measure of accessibility) but 5-HT and ibogaine increased it. Therefore it was proposed that ibogaine bound to, and stabilized, a conformation of SERT in which the cytoplasmic pathway is open, in contrast to earlier x-ray structures of LeuT in which the pathway is closed (15, 46).
Stabilization of the cytoplasmic pathway in a closed state by cocaine is consistent with models of DAT with cocaine and other analogs bound at the substrate-binding site (47). The conformation of DAT in those models corresponds to LeuT structures with a closed cytoplasmic pathway and an open extracellular pathway. However, the ability of ibogaine to close the extracellular pathway and open the cytoplasmic one has not previously been explained in mechanistic or structural terms.
Ibogaine contains within its structure a 5-HT moiety. Accordingly, it was proposed that ibogaine might bind at the substrate site and convert the transporter to a stable, long-lived intermediate in an inward-open conformation (4). In this way, it could act as a noncompetitive inhibitor. Such a long-lived intermediate would need to dissociate ibogaine much more slowly than 5-HT. We calculated that for ibogaine to inhibit in a noncompetitive manner, its dissociation rate would have to be at least 105-fold slower than for substrate (see supplemental Fig. S2). The data in Fig. 6 provide a measure of the ibogaine dissociation rate. Under the conditions used, the koff for ibogaine is ∼0.5 s−1. If we assume that 5-HT binding is diffusion limited with a maximal kon between 108 and 109 s−1 m−1 the maximum 5-HT dissociation rate, consistent with a KD of 3 μm (17, 48), is between 100 and 1000 s−1 (koff = kon × c/KD). Thus, for ibogaine to act as a noncompetitive inhibitor while binding to the same site as 5-HT, it would need to dissociate slower than 0.01 s−1, at least 50 times slower than the measured rate. In contrast, the high affinity ligand citalopram is a competitive inhibitor (48, 49) despite the fact that it dissociates much more slowly than ibogaine. Supplemental Fig. S3 shows the sluggish kinetics that characterizes citalopram binding and dissociation. Although the affinity of citalopram for the lipid bilayer, which provides a reservoir of the inhibitor, probably contributes to the slow dissociation, these data show that a compound that dissociates much slower than ibogaine still appears competitive in kinetic studies.
To account for noncompetitive inhibition, therefore, ibogaine must bind to a distinct site that does not overlap with the 5-HT site. Several results support the idea that ibogaine binds to the extracellular surface of SERT when the transporter is in an inward-open conformation. Although an attractive mechanism might involve ibogaine binding within the cytoplasmic pathway to hold it open, intracellular ibogaine failed to inhibit (Fig. 5b). Even at concentrations 10-fold higher than those necessary to block from outside the cell, intracellular ibogaine had no effect on either the amplitude or concentration dependence of the substrate-induced current (Fig. 5d). Ibogaine stabilizes SERT (and DAT, Fig. 4) in a conformation with increased exposure of the cytoplasmic pathway. This contrasts with the effect of competitive inhibitors such as cocaine and antidepressant drugs, which decreased accessibility in that pathway. The increase in the accessibility of the cytoplasmic pathway is most simply explained by assuming that ibogaine has higher affinity for the inward-open conformation of SERT and thereby stabilizes this conformation. Previous investigations of DAT conformation used decreased reactivity of the I159C mutant, which has a cysteine in the extracellular pathway, to indicate when DAT was in an inward-facing state (50, 51). We propose that accessibility of the DAT cytoplasmic pathway, as monitored in Fig. 4, serves as a more direct indication of whether the transporter is open to the cytoplasm.
The kinetics of the ibogaine-induced current block provide a further argument in support of the conjecture that ibogaine binds to an extracellular site in the inward-open state. We previously showed that the steady state current induced by 5-HT is due to a conductive state of SERT that is in equilibrium with an inward-open intermediate (18). During transport, most of the transporter builds up in this intermediate, which is converted to an outward-open conformation in the rate-limiting step of the transport cycle (TiK → ToK, Fig. 7a). A measure of this return rate is shown in Fig. 6d by the rate constant (∼2 s−1) for current decay after cocaine addition. This rate compares favorably with the overall turnover rate of 1–2 s from previous measurements (40). The rate of cocaine block did not increase significantly at higher cocaine concentrations. This is expected if cocaine can only bind after the rate-limiting conversion to an outward-open form of SERT. However, the ibogaine block occurred much faster (with a rate of ∼6 s−1 at 10 μm). This suggests that ibogaine acted directly on an inward-open conformation of SERT and did not require conversion to an outward-open conformation (Fig. 6d). Importantly, increasing the ibogaine concentration increased the rate of block in a linear manner, consistent with a bimolecular reaction. This concentration-dependent increase in the apparent rate of blockage shows that ibogaine binding did not require the conformational change limiting the rate of cocaine block.
FIGURE 7.
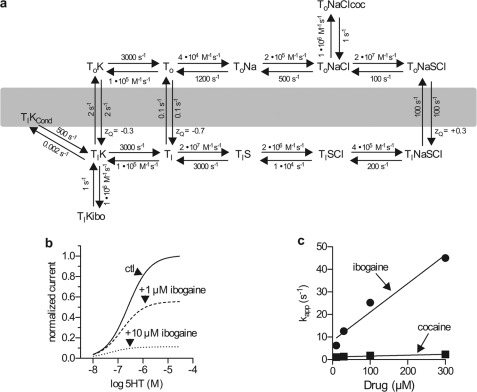
A kinetic model for cocaine and ibogaine inhibition of substrate-induced currents in SERT. a, the model was based on a kinetic model for 5-HT-induced currents (18). KD values of 1 μm were used for both cocaine (coc) and ibogaine (ibo) (4, 36). 5-HT is represented as S. Although cocaine binds in the absence of NaCl, and its affinity is not increased by Cl− (36), binding was arbitrarily assigned to the ToNaCl intermediate to avoid undue complexity in the model. See supplemental data for a more complete analysis of the model. The slow K+-independent conversion of Ti to To is likely to represent H+ export as described previously (45). The conducting state is shown as TiKCond (18). b and c, simulations based on the model in a. b, simulated current amplitudes as a function of substrate concentration in the absence and presence of 1 and 10 μm ibogaine. c, simulated association rate constants (kapp) for ibogaine and cocaine as a function of their concentration.
These results are consistent with the reaction cycle shown in Fig. 7a. This scheme is based on the model proposed in our previous study to explain SERT-dependent currents (18). As previously suggested (18, 52) return of SERT from the TiK form to ToK is shown as the rate-limiting step in transport. Thus, in the presence of substrate, most of the transporter will be in the TiK state. To account for the slow and concentration-independent rate of cocaine blockade in contrast to the faster and concentration-dependent ibogaine block (Fig. 6d), we show cocaine binding to To and ibogaine binding to Ti. The rate of cocaine block is limited by the TiK to ToK transition, but because ibogaine binds directly to Ti, the rate of block increases with ibogaine concentration. Although this scheme shows ibogaine binding to TiK, the rapid equilibrium between Ti forms allows for the possibility that ibogaine binds to a different Ti intermediate. However, the fact that ibogaine blocks the substrate-induced current indicates that the conductive state in equilibrium with TiK is not formed when ibogaine is bound.
We used the scheme shown in Fig. 7a to model the current induced by substrate in the presence of ibogaine (Fig. 7b). Fig. 7b shows the predicted effect of 1 and 10 μm ibogaine. The decrease in maximum current predicted in Fig. 7b mirrors the results shown in Fig. 5c. The rates of cocaine and ibogaine block are also well predicted by this model. Fig. 7c shows the predicted dependence of this rate on inhibitor concentration. Taking into account the 50-ms time constant for solution exchange in our apparatus, the model predicts rates quite similar to those measured in Fig. 6d. The ability of the scheme in Fig. 7a to model the experimental results with a reasonable degree of precision (see supplementary Data) provides some confidence that it accurately represents the interaction of these inhibitors with the reaction cycle of SERT.
Binding of ibogaine to an inward-open state of SERT explains why ibogaine was a noncompetitive transport inhibitor but competitive with equilibrium binding of the cocaine analog β-CIT (4). Cocaine and β-CIT stabilize SERT in an outward-open, To conformation to which ibogaine does not bind. As a consequence of this mutually exclusive binding, ibogaine and β-CIT appear competitive with each other even though the two inhibitors bind to different sites. In contrast, 5-HT binding leads to transport and increases the proportion of SERT in the inward-open, Ti conformation that binds ibogaine. Therefore, external 5-HT should not be able to overcome ibogaine inhibition, even at high concentrations, leading to noncompetitive inhibition. This result is also expected for any process in which substrate converts a protein to an inhibitor binding conformation, as observed with other transporters (10–13). The alternative possibility, that ibogaine and substrate bind simultaneously, was rendered unlikely by the parallel shifts observed in the Dixon plots (Fig. 1, d and f).
If ibogaine does not bind at the same site as substrate, then where does it bind? We initially proposed that it bound at the substrate site because of the resemblance of ibogaine to 5-HT. The present results rule out that possibility and also demonstrate that ibogaine has similar effects on SERT and DAT, despite their dissimilar substrate selectivity. An attractive possibility is that ibogaine sites are created on the extracellular surfaces of SERT and DAT as the extracellular pathway closes. The rocking bundle proposal for transport suggests that a 4-helix bundle tilts to concomitantly open and close the cytoplasmic and extracellular pathways, respectively (53). Movement of the bundle within the protein structure accounts for most of the difference between outward-open and inward-open LeuT structures (16). Contact between this bundle and the rest of the protein in an inward-open conformation could create an ibogaine binding site on the extracellular surface of the transporter that stabilizes the inward-open conformation when occupied. Alternatively, if the pathway does not completely close, the binding site could be formed within a narrowed extracellular pathway, perhaps at a site corresponding to the postulated secondary substrate-binding site in LeuT and DAT (31, 44). However, mutation of 11 residues in the extracellular pathway, including those corresponding to the proposed secondary substrate-binding site in LeuT and DAT, failed to decrease ibogaine potency. Therefore, the precise location of the ibogaine site remains unknown.
Supplementary Material
Acknowledgments
We thank Sacrament of Transition (Maribor, Slovenia) for the kind gift of ibogaine. DAT-X5C was a generous gift from Dr. Jonathan Javitch, Columbia University. Professor Kristian Strømgaard, University of Copenhagen, kindly provided SERT mutants I179F, D400K, L406F, V489F, and K490D.
This work was supported, in whole or in part, by National Institutes of Health Grant DA007259 (to G. R.) and The Austrian Science Fund (FWF) Grants P18706 and SFB35-06 (to H. H. S.) and P17611 (to S. B.).

This article contains supplemental Figs. S1–S3 and additional data.
- 5-HT
- 5-hydroxytryptamine
- DA
- dopamine
- MTS
- methanethiosulfonate
- DAT
- dopamine transporter
- β-CIT
- (−)2-β-carbomethoxy-3β-(4-iodophenyl)tropane
- PCA
- p-chloramphetamine
- MTSEA
- methanethiosulfonate ethylammonium
- CFT
- (−)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane
- MPP+
- 1-methyl-4-phenylpyridinium
- MTSEA
- 2-iminoethyl methanethiosulfonate hydrobromide.
REFERENCES
- 1. Leal M. B., Michelin K., Souza D. O., Elisabetsky E. (2003) Ibogaine attenuation of morphine withdrawal in mice. Role of glutamate N-methyl-d-aspartate receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 781–785 [DOI] [PubMed] [Google Scholar]
- 2. Levi M. S., Borne R. F. (2002) A review of chemical agents in the pharmacotherapy of addiction. Curr. Med. Chem. 9, 1807–1818 [DOI] [PubMed] [Google Scholar]
- 3. Donnelly J. R. (2011) The need for ibogaine in drug and alcohol addiction treatment. J. Legal Med. 32, 93–114 [DOI] [PubMed] [Google Scholar]
- 4. Jacobs M. T., Zhang Y. W., Campbell S. D., Rudnick G. (2007) Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J. Biol. Chem. 282, 29441–29447 [DOI] [PubMed] [Google Scholar]
- 5. Wells G. B., Lopez M. C., Tanaka J. C. (1999) The effects of ibogaine on dopamine and serotonin transport in rat brain synaptosomes. Brain Res. Bull. 48, 641–647 [DOI] [PubMed] [Google Scholar]
- 6. Rice M. E., Cragg S. J. (2008) Dopamine spillover after quantal release. Rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev. 58, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kristensen A. S., Andersen J., Jørgensen T. N., Sørensen L., Eriksen J., Loland C. J., Strømgaard K., Gether U. (2011) SLC6 neurotransmitter transporters. Structure, function, and regulation. Pharmacol. Rev. 63, 585–640 [DOI] [PubMed] [Google Scholar]
- 8. Kahlig K. M., Binda F., Khoshbouei H., Blakely R. D., McMahon D. G., Javitch J. A., Galli A. (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. U.S.A. 102, 3495–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seidel S., Singer E. A., Just H., Farhan H., Scholze P., Kudlacek O., Holy M., Koppatz K., Krivanek P., Freissmuth M., Sitte H. H. (2005) Amphetamines take two to tango. An oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol. Pharmacol. 67, 140–151 [DOI] [PubMed] [Google Scholar]
- 10. Henderson P. J., Lardy H. A., Dorschner E. (1970) Factors affecting the inhibition of adenine nucleotide translocase by bongkrekic acid. Biochemistry 9, 3453–3457 [DOI] [PubMed] [Google Scholar]
- 11. Klingenberg M. (2008) The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 1778, 1978–2021 [DOI] [PubMed] [Google Scholar]
- 12. Czech M. P., Lynn D. G., Lynn W. S. (1973) Cytochalasin B-sensitive 2-deoxy-d-glucose transport in adipose cell ghosts. J. Biol. Chem. 248, 3636–3641 [PubMed] [Google Scholar]
- 13. Gorga F. R., Lienhard G. E. (1981) Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry 20, 5108–5113 [DOI] [PubMed] [Google Scholar]
- 14. Forrest L. R., Zhang Y. W., Jacobs M. T., Gesmonde J., Xie L., Honig B. H., Rudnick G. (2008) Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. U.S.A. 105, 10338–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 16. Krishnamurthy H., Gouaux E. (2012) X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wall S. C., Innis R. B., Rudnick G. (1993) Binding of the cocaine analog 2 β-carbomethoxy-3-β-(4-[125I]iodophenyl)tropane to serotonin and dopamine transporters. Different ionic requirements for substrate and 2 β-carbomethoxy-3-β-(4-[125I]iodophenyl)tropane binding. Mol. Pharmacol. 43, 264–270 [PubMed] [Google Scholar]
- 18. Schicker K., Uzelac Z., Gesmonde J., Bulling S., Stockner T., Freissmuth M., Boehm S., Rudnick G., Sitte H. H., Sandtner W. (2012) Unifying concept of serotonin transporter-associated currents. J. Biol. Chem. 287, 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilber B., Scholze P., Dorostkar M. M., Sandtner W., Holy M., Boehm S., Singer E. A., Sitte H. H. (2005) Serotonin-transporter mediated efflux. A pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology 49, 811–819 [DOI] [PubMed] [Google Scholar]
- 20. Korkhov V. M., Holy M., Freissmuth M., Sitte H. H. (2006) The conserved glutamate (Glu-136) in transmembrane domain 2 of the serotonin transporter is required for the conformational switch in the transport cycle. J. Biol. Chem. 281, 13439–13448 [DOI] [PubMed] [Google Scholar]
- 21. Sucic S., Dallinger S., Zdrazil B., Weissensteiner R., Jørgensen T. N., Holy M., Kudlacek O., Seidel S., Cha J. H., Gether U., Newman A. H., Ecker G. F., Freissmuth M., Sitte H. H. (2010) The N terminus of monoamine transporters is a lever required for the action of amphetamines. J. Biol. Chem. 285, 10924–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pifl C., Drobny H., Reither H., Hornykiewicz O., Singer E. (1995) Mechanism of the dopamine-releasing actions of amphetamine and cocaine. Plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol. Pharmacol. 47, 368–373 [PubMed] [Google Scholar]
- 23. Sitte H. H., Scholze P., Schloss P., Pifl C., Singer E. A. (2000) Characterization of carrier-mediated efflux in human embryonic kidney 293 cells stably expressing the rat serotonin transporter. A superfusion study. J. Neurochem. 74, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y. W., Rudnick G. (2005) Cysteine-scanning mutagenesis of serotonin transporter intracellular loop 2 suggests an α-helical conformation. J. Biol. Chem. 280, 30807–30813 [DOI] [PubMed] [Google Scholar]
- 25. Boehm S. (1999) ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J. Neurosci. 19, 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Läuger P. (1991) Electrogenic Ion Pumps, Sinauer Associates Inc., Sunderland, MA [Google Scholar]
- 27. Lin F., Lester H. A., Mager S. (1996) Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys. J. 71, 3126–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin R. S., Henningsen R. A., Suen A., Apparsundaram S., Leung B., Jia Z., Kondru R. K., Milla M. E. (2008) Kinetic and thermodynamic assessment of binding of serotonin transporter inhibitors. J. Pharmacol. Exp. Ther. 327, 991–1000 [DOI] [PubMed] [Google Scholar]
- 29. Andersen J., Kristensen A. S., Bang-Andersen B., Stromgaard K. (2009) Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporter. Chem. Commun. 3677–3692 [DOI] [PubMed] [Google Scholar]
- 30. Reid M. S., Hsu K., Jr., Souza K. H., Broderick P. A., Berger S. P. (1996) Neuropharmacological characterization of local ibogaine effects on dopamine release. J. Neural Transm. 103, 967–985 [DOI] [PubMed] [Google Scholar]
- 31. Shi L., Quick M., Zhao Y., Weinstein H., Javitch J. A. (2008) The mechanism of a neurotransmitter:sodium symporter-inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell 30, 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh S. K., Yamashita A., Gouaux E. (2007) Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 [DOI] [PubMed] [Google Scholar]
- 33. Zhou Z., Zhen J., Karpowich N. K., Goetz R. M., Law C. J., Reith M. E., Wang D. N. (2007) LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarker S., Weissensteiner R., Steiner I., Sitte H. H., Ecker G. F., Freissmuth M., Sucic S. (2010) The high-affinity binding site for tricyclic antidepressants resides in the outer vestibule of the serotonin transporter. Mol. Pharmacol. 78, 1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersen J., Olsen L., Hansen K. B., Taboureau O., Jørgensen F. S., Jørgensen A. M., Bang-Andersen B., Egebjerg J., Strømgaard K., Kristensen A. S. (2010) Mutational mapping and modeling of the binding site for (S)-citalopram in the human serotonin transporter. J. Biol. Chem. 285, 2051–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tavoulari S., Forrest L. R., Rudnick G. (2009) Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J. Neurosci. 29, 9635–9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segel I. H. (1975) Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems, John Wiley & Sons, New York [Google Scholar]
- 38. Stein W. D. (1967) The Movement of Molecules Across Cell Membranes, Academic Press, New York [Google Scholar]
- 39. Sonders M. S., Zhu S. J., Zahniser N. R., Kavanaugh M. P., Amara S. G. (1997) Multiple ionic conductances of the human dopamine transporter. The actions of dopamine and psychostimulants. J. Neurosci. 17, 960–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mager S., Min C., Henry D. J., Chavkin C., Hoffman B. J., Davidson N., Lester H. A. (1994) Conducting states of a mammalian serotonin transporter. Neuron 12, 845–859 [DOI] [PubMed] [Google Scholar]
- 41. Erreger K., Grewer C., Javitch J. A., Galli A. (2008) Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function. J. Neurosci. 28, 976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrer J. V., Javitch J. A. (1998) Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 95, 9238–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y. W., Rudnick G. (2006) The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem. 281, 36213–36220 [DOI] [PubMed] [Google Scholar]
- 44. Shan J., Javitch J. A., Shi L., Weinstein H. (2011) The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One 6, e16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keyes S. R., Rudnick G. (1982) Coupling of transmembrane proton gradients to platelet serotonin transport. J. Biol. Chem. 257, 1172–1176 [PubMed] [Google Scholar]
- 46. Singh S. K., Piscitelli C. L., Yamashita A., Gouaux E. (2008) A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beuming T., Kniazeff J., Bergmann M. L., Shi L., Gracia L., Raniszewska K., Newman A. H., Javitch J. A., Weinstein H., Gether U., Loland C. J. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat. Neurosci. 11, 780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Humphreys C. J., Wall S. C., Rudnick G. (1994) Ligand binding to the serotonin transporter. Equilibria, kinetics, and ion dependence. Biochemistry 33, 9118–9125 [DOI] [PubMed] [Google Scholar]
- 49. D'Amato R. J., Largent B. L., Snowman A. M., Snyder S. H. (1987) Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. J. Pharm. Exp. Ther. 242, 364–371 [PubMed] [Google Scholar]
- 50. Loland C. J., Grånäs C., Javitch J. A., Gether U. (2004) Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J. Biol. Chem. 279, 3228–3238 [DOI] [PubMed] [Google Scholar]
- 51. Loland C. J., Desai R. I., Zou M. F., Cao J., Grundt P., Gerstbrein K., Sitte H. H., Newman A. H., Katz J. L., Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol. Pharmacol. 73, 813–823 [DOI] [PubMed] [Google Scholar]
- 52. Nelson P. J., Rudnick G. (1979) Coupling between platelet 5-hydroxytryptamine and potassium transport. J. Biol. Chem. 254, 10084–10089 [PubMed] [Google Scholar]
- 53. Forrest L. R., Rudnick G. (2009) The rocking bundle. A mechanism for ion-coupled solute flux by symmetrical transporters. Physiology 24, 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forrest L. R., Tavoulari S., Zhang Y. W., Rudnick G., Honig B. (2007) Identification of a chloride ion binding site in Na+/Cl-dependent transporters. Proc. Natl. Acad. Sci. U.S.A. 104, 12761–12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scholze P., Nørregaard L., Singer E. A., Freissmuth M., Gether U., Sitte H. H. (2002) The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. 277, 21505–21513 [DOI] [PubMed] [Google Scholar]
- 56. Baumann M. H., Rothman R. B., Pablo J. P., Mash D. C. (2001) In vivo Neurobiological effects of Ibogaine and its O-desmethyl metabolite, 12-hydroxyibogamine (Noribogaine), in rats. JPET 297, 2531–2539 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.