Background: Macrophages are key players in the pathogenesis of benign prostatic hyperplasia (BPH). But, molecular mechanisms by which macrophages promote prostate cell proliferation remain unclear.
Results: Macrophages can enhance the growth of prostate stromal cells via an androgen receptor (AR)-CCL3-dependent pathway.
Conclusion: CCL3 is an AR downstream regulator of macrophages in promoting prostate stromal cell growth.
Significance: AR and CCL3 could be targets of opportunity for new therapeutic approaches for the treatment of BPH.
Keywords: Androgen Receptor, Chemokines, Macrophages, Prostate, Stromal Cell, CCL3, Benign Prostatic Hyperplasia
Abstract
Infiltrated macrophages may play important roles in the development and progression of benign prostatic hyperplasia (BPH), but the underlying mechanisms remain largely unknown. We found increased macrophages infiltration in human and mouse BPH tissues. By establishing a co-culture transwell system, we found increased migration of macrophages and proliferation of prostate stromal cells during co-culture. Importantly, stromal androgen receptor (AR) could enhance the migration of macrophages and macrophage-mediated stromal cell proliferation. We identified CCL3 as an AR downstream player, and found CCL3 levels were notably increased in human and mouse BPH prostates. Ablation of prostate stromal AR in a mouse BPH model significantly reduced CCL3 expression levels in prostates. Consistently, targeting AR via an AR degradation enhancer, ASC-J9§, or neutralization of CCL3 with an antibody, resulted in suppression of macrophage migration and prostate stromal cell growth. Our study provides mechanistic insights on the regulation of prostate stromal cells by macrophages via stromal AR/CCL3 signaling pathways, which could potentially allow the development of therapeutic approaches for battling BPH with persistent inflammation.
Introduction
Benign prostatic hyperplasia (BPH)4 is the most common urologic disease of elderly males, and impacts about one-quarter of men in their 50s, one-third in their 60s, and about half of patients with various degrees of bladder outlet obstruction associated with lower urinary tract symptoms (1). Despite the medical significance of BPH in aging males, the pathogenesis of this disorder is unclear, although theories such as altered estrogen/androgen balance (2–5), increased oxidative stress (6), metabolic syndromes (7, 8), chronic inflammation (1, 9, 10), and altered activity of autonomic nerves (11, 12), have been postulated. There are about 9- and 37-fold increases in the proliferative rate of the prostate epithelium and stroma, respectively, in BPH compared with the normal prostate, suggesting that prostate stromal hyper-proliferation is a crucial feature in BPH pathogenesis (13).
The molecular and cellular mechanisms involving stromal and epithelial components of the prostate leading to BPH remain unclear, however, numerous studies show prostatic inflammation is a causative factor in the pathogenesis of BPH (1, 9, 14–16). Moreover, recent clinical trials have suggested a relationship between prostatic inflammations and lower urinary tract symptoms of BPH (17, 18). However, there has been no available model in vivo or in vitro to study roles of macrophages in the microenvironment of BPH via the interaction with prostate stromal cells. In an effort to uncover the processes and molecular mechanisms by which infiltrating macrophages promote prostate stromal cells growth, we have established a co-culture system of macrophages/prostate stromal cells, and demonstrated that macrophage-induced prostate stromal growth involves stromal androgen receptor (AR) → inflammatory chemokine-chemokine (C-C motif) ligand 3 (CCL3) → macrophage infiltration and the stimulation of prostate stromal cell proliferation. Our findings might help us to develop a new potential therapeutic approach to prevent BPH progression.
MATERIALS AND METHODS
Reagents and Antibodies
ASC-J9® (5-hydroxy-1,7-Bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one) from AndroScience Corporation (San Diego, CA) was generated as described previously (19). ASC-J9® was dissolved in DMSO (Sigma) and diluted with corn oil (Sigma). Anti-AR (N20) and anti-CD68 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Mac3 antibody was purchased from BD Biosciences. Anti-CCL3 antibody was purchased from ABGENT (San Diego, CA). Anti-mouse CCL3/MIP-1α (AF-450-NA) neutralizing antibody was purchased from R&D Systems (Minneapolis, MN).
Primary Cultured Mouse Prostate Stromal Cells (mPrSCs) and Immortalization
Mouse prostate tissue specimens were obtained from Probasin-Prolactin transgenic (Pb-PRL-tg) mice and the primary culture protocol was performed as described previously (20). The mPrSCs were cultured with DMEM supplemented with 10% fetal bovine serum (FBS). To obtain the immortalized mPrSC cell line, the lentivirus pWPI-E1A was co-transfected with pMD2.G and pAX2 into 293T cells from American Type Culture Collection (ATCC, Manassas, VA). After a 48-h transfection, the cultured media of 293T cells were harvested and mixed with fresh DMEM culture media (ratio 1:1) and 8 μg/ml of Polybrene (Millipore, Billerica, MA), then incubated with primary cultured cells for 24 h. After 3–5 passages, the surviving cells are the immortalized cells (mPrSC-E1A).
Generation of Pb-PRL-tg and dARKO/Pb-PRL-tg Mice
The floxed Ar mice were generated by insertion of loxP sites flanking to exon-2 region of Ar gene in C57BL/6 background. The stromal double-cre mice were generated by mating of male Fsp1-cre mice (a gift from Dr. N. A. Bhowmick) with female Tgln-cre mice (Jackson Laboratory, Bar Harbor, ME) and backcrossed to C57BL/6 background more than 5–6 generations. Pb-PRL-tg mice were kindly provided by Dr. H. Wennbo and Dr. J. Kindblom and backcrossed into the FVB background (21). The generation of dARKO/Pb-PRL-tg mouse was followed as described previously (22). Mouse prostates were harvested according to the protocols approved by the Division of Laboratory Animal Medicine, University of Rochester Medical Center.
Primary Cultured Mouse Bone Marrow-derived Macrophages (mBMMs)
The mBMMs were obtained as described (47, 48). Briefly, BM cells were expressed from the femur and tibia of 6–8-week-old C57BL/6 mice. After centrifugation at 500 × g, RBCs were lysed by resuspending the cell pellet in ammonium chloride solution (STEMCELL Technologies, Inc., Vancouver, Canada) at 37 °C for 5 min. Cells were then washed three times in RPMI 1640 and plated in 10-cm dishes at 1 × 107 cells per dish in RPMI 1640 medium with 10% FBS and 100 ng/ml of M-CSF (R&D Systems). After 3 days incubation, the media was removed and replaced with fresh media plus 100 ng/ml of M-CSF (macrophage-colony stimulating factor). After an additional 3 days, mBMMs were about 80% confluent and used for experiments.
Cell Line and Co-culture Experiments
The mouse macrophage cell line, RAW264.7, was purchased from ATCC and cultured in RPMI 1640 with 10% FBS. Cultures were grown in a humidified 5% CO2 environment at 37 °C. 24-Well and 6-well transwell plates (3 μm) were used for co-culture experiments (Corning Inc., Corning, NY).
Plasmids and Stable Cell Lines
To generate AR overexpression stable clones of the mPrSC-E1A cell line, the lentivirus vector, pWPI-AR, or pWPI-control, were co-transfected with pMD2.G and pAX2 into 293T cells. After a 48-h transfection, the cultured media of 293T cells was harvested and mixed with fresh DMEM culture media (ratio 1:1) and 8 μg/ml of Polybrene (Millipore), then incubated with mPrSC-E1A cells for 24 h. The viral-infected mPrSC-E1A cells contain green fluorescence protein (GFP), so the mPrSC-AR or mPrSC-vector stable cells were obtained by flow cytometry sorting.
Cell Proliferation Assay
The mPrSC cells were seeded in 24-well plates (103cells/well) and cultured in DMEM for 24 h. Then, cells were fed fresh DMEM and conditioned media from RAW264.7 macrophages (1:1) or control media and incubated for an additional 2 or 4 days. MTT reagent (Promega, Madison, WI) was added at 2 and 4 days per the manufacturer's instructions. After a 4-h reaction, absorbance of the converted dye was measured at a wavelength of 595–620 nm.
Cell Migration Assay
In vitro cell migration assay was performed using 24-well transwell inserts (5 μm) (BD Biosciences) according to the manufacturer's instructions. RAW264.7 cells (1 × 105/well) were seeded in the upper chamber of transwell plates and mPrSC-V/mPrSC-AR cells were seeded in the lower chamber. Cells were incubated for 20 h. The migrated cells of RAW264.7 cells were stained and counted from six random fields.
RNA Extraction and Quantitative Real-time PCR Analysis
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. 1 μg of total RNA was subjected to reverse transcription using Superscript III transcriptase (Invitrogen). RT-PCR has been described previously (23). Primers used were as follows: Ar sense, 5′-GGACAGTACCAGGGACCATG-3′; antisense, 5′-TCCGTAGTGACAGCCAGAAG-3′; Gapdh sense, 5′-GCTCCTGGAAGATGGTGATG-3′; antisense, 5′-GGTGAAGGTCGGTGTGAAC-3′; Ccl2 sense, 5′-TTAAAAACCTGGATCGGAACCAA-3′; antisense, 5′-GCATTAGCTTCAGATTTACGGGT-3′; Ccl3 sense, 5′-TTCTCTGTACCATGACACTCTGC3′; antisense, 5′-CGTGGAATCTTCCGGCTGTAG-3′; Ccl4 sense, 5′-TTCCTGCTGTTTCTCTTACACCT-3′; antisense, 5′-CTGTCTGCCTCTTTTGGTCAG-3′; Cox-2 sense, 5′-TGAGCAACTATTCCAAACCAGC-3′; antisense, 5′-GCACGTAGTCTTCGATCACTATC-3′.
Quantitative real-time PCR was conducted using a Bio-Rad CFX96 system with SYBR Green to determine the level of mRNA expression of a gene of interest. Expression levels were normalized to the expression of Gapdh RNA.
Western Blot Analysis
mPrSC cells were lysed in RIPA buffer (50 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 mm Na3VO4, 1 mm NaF, 1 mm okadaic acid, and 1 mg/ml of aprotinin, leupeptin, and pepstatin). Individual samples (30–35 μg of protein) were prepared for electrophoresis run on 8–10% SDS-PAGE gel and then transferred onto PVDF membranes (Millipore). After blocking the membranes with 5% fat-free milk in TBST (50 mm Tris, pH 7.5, 0.15 m NaCl, and 0.05% Tween 20) for 1 h at room temperature, the membranes were incubated with appropriate dilutions of specific primary antibodies overnight at 4 °C. After washing, the blots were incubated with anti-rabbit or anti-mouse IgG HRPs for 1 h. The blots were developed in ECL mixture (Thermo Fisher Scientific Inc., Rockford, IL).
ELISA
Conditioned media collected from mono-culture or co-cultures were also used for detection of CCL3 by mouse CCL3 ELISA kits (Ray Biotech, Inc.) according to the manufacturer's instructions.
H&E Staining and Immunohistochemistry
Human prostate tissue microarray slides were provided by the Department of Surgical Pathology, University of Rochester Medical Center. Mouse prostate tissues were fixed in 10% (v/v) formaldehyde in PBS and embedded in paraffin, and cut into 5-μm tissue sections. Prostate sections were deparaffinized in xylene solution and rehydrated using gradient ethanol concentrations. For H&E staining, the sections were stained in hematoxylin for 5 min, and washed in running tap water for 5 min. Then the sections were stained in eosin for 30 s, dehydrated, and mounted by routine methods. Immunostaining was performed as described previously (24). For systematic counting of macrophages or CCL3 positive cells, six ocular measuring fields within a tissue were randomly chosen under a microscope at ×400 magnification. The mean number of human CD68/mouse Mac-3/CCL3 positive cells in these six areas was determined as the macrophage count for each case.
Statistics
The data values were presented as the mean ± S.D. Differences in mean values between two groups were analyzed by two-tailed Student's t test. p values ≤ 0.05 were considered statistically significant.
RESULTS
Increased Infiltrated Macrophages in Stroma of Human BPH Prostate Tissues
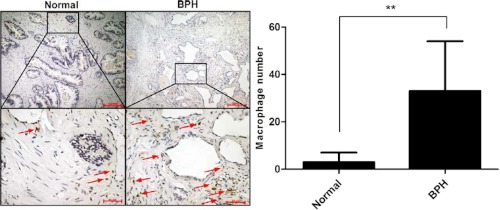
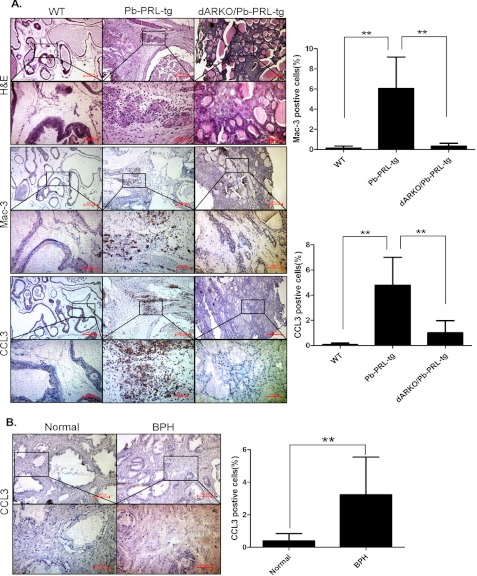
Emerging evidence indicates that increased infiltrated macrophages may contribute to prostate stromal growth in the development and progression of BPH (25). To confirm if the number of infiltrating macrophages is increased in human BPH specimens compared with the normal tissues, we performed immunohistochemical staining analyses of the human BPH and normal prostate control tissues using a macrophage specific antibody, CD68. Our data showed that there were only a few macrophages in normal prostate tissues. But, we found increased macrophages in BPH prostate tissues (Fig. 1, arrows). These clinical results suggest that infiltrating macrophages might be key players in the development and progression of BPH.
FIGURE 1.
Immunohistochemical staining of human normal prostates (n = 7) and BPH (n = 8) tissues using anti-CD68 antibody. Paraffin-embedded tissue sections were used and representation specimens are shown. Red arrows, macrophages.
Stromal AR Enhanced Migration of Macrophages in a Stromal Cells/Macrophages Co-culture System
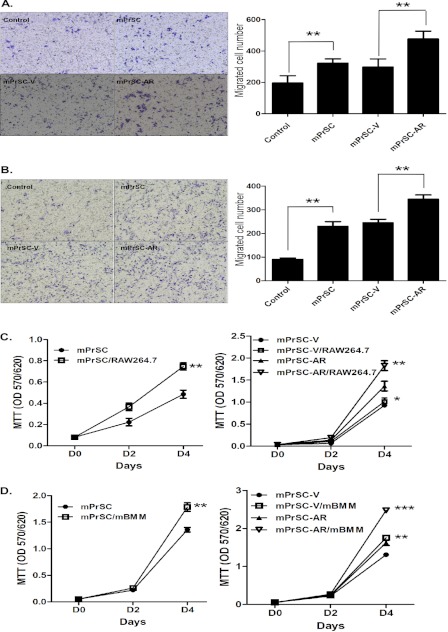
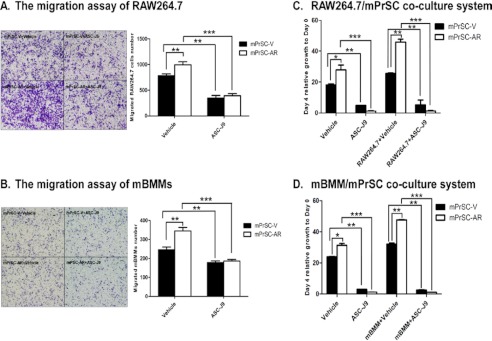
To investigate the potential role of increased infiltrating macrophages in prostate BPH stroma, we established a co-culture model and found more macrophage migration during co-culture of mPrSCs that were isolated from the Pb-PRL-tg mice (21, 26) with RAW264.7 macrophage cells (Fig. 2A). Similar results also were obtained when we replaced RAW264.7 cells with the primary mBMMs (Fig. 2, A and B). These results suggest that the cross-talk of prostate stromal cells and macrophages may prompt induction of chemotactic activity that may drive increased infiltration of macrophages.
FIGURE 2.
Role of stromal AR in macrophage infiltration and macrophage-induced stromal cell proliferation. A and B, RAW264.7 cells (A) or mBMMs (B) (1 × 105/well) were co-cultured with the control medium/mPrSC-V or mPrSC-AR cells for 20 h in transwell plates (pore size 5 μm). Migrated macrophages were stained with toluidine blue and counted from six random fields. Results are expressed at the average number of cells per field and are mean ± S.D. C and D, mPrSC-parental/mPrSC-V/mPrSC-AR cells were co-cultured with RAW264.7 cells (C) or mBMMs (D) and assayed with MTT at days 2 and 4. Different numbers between two groups were analyzed by two-tailed Student's t test. Each experiment was repeated 3 times with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To determine whether stromal AR has any regulatory role in directing the macrophages infiltration during the cross-talk with macrophages, we then established the immortalized mPrSC cells with either AR-infected (mPrSC-AR) or vector-control (mPrSC-V), and confirmed the AR mRNA and protein levels of mPrSC-AR and mPrSC-V cells via quantitative real-time PCR and Western blot analysis (supplemental Fig. S1, A and B). We found the addition of AR in mPrSC cells led to increased migration of RAW264.7 macrophage cells toward prostate stromal cells (Fig. 2A). Similar results were also obtained when we replaced RAW264.7 macrophage cells with the mBMMs (Fig. 2B). Together, results from Fig. 2, A and B, concluded that stromal AR might play an important role in promoting macrophage infiltration during the cross-talk between prostate stromal cells and macrophages.
Stromal AR Enhanced Macrophage-induced Prostate Stromal Cell Proliferation
To investigate the consequence of more infiltrating macrophages migration toward prostate stomal cells during the co-culture system, we examined the cell growth of mPrSC cells during co-culture with RAW264.7 cells using the MTT assay. We found that increased macrophage RAW264.7 cell migration to mPrSC cells led to enhanced cell growth of mPrSC cells (Fig. 2C, left panel). Similar results were obtained when we replaced RAW264.7 cells with mBMMs (Fig. 2D, left panel). Consistently, we found addition of AR in mPrSC cells also led to dramatically enhanced RAW264.7 cell-induced mPrSC cell growth (Fig. 2C, right panel), and similar results were observed when we replaced RAW264.7 cells with mBMMs (Fig. 2D, right panel). Taken together, results from Fig. 2, C and D, suggest a novel role for stromal AR in promoting macrophage-mediated enhancement of prostate stromal cell growth.
Inflammatory Cytokine CCL3 as a Key Mediator for Macrophages Infiltration and Macrophages-induced Stromal Cell Proliferation
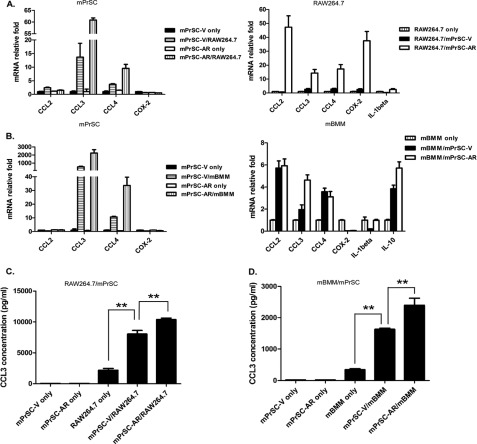
To identify which inflammatory cytokine/chemokine is responsible for macrophage-induced prostate mPrSC stromal cell proliferation, we performed quantitative PCR analysis to screen chemokines/cytokines/other factors that were potentially involved in this cross-talk between macrophages and mPrSC stromal cells (supplemental Fig. S2, A and B). Of these cytokine transcripts, we found a remarkable and consistent increase of CCL3 expression in both prostate stromal cells and macrophages after co-culture (Fig. 3, A and B), suggesting that the cross-talk of both cell types during co-culture triggered induction of CCL3. Previously, it has been shown that chemokines (CC-type and CXC-type) are abundantly expressed in aged prostate stromal cells (27), supporting that induction of chemokines could be an important process for prostate cell proliferation and enlargement. Especially, among CC-type chemokines, CCL3 has been shown to be important for senescence regulation (28), which is also an important mechanism for BPH development (27). But, CCL3 has never been identified as a key player involved in AR-mediated prostate stromal expansion. Therefore, we hypothesized that CCL3 could be a novel regulator of this regulation by prostate stromal AR. More importantly, as shown in Fig. 3, C and D, addition of AR in mPrSC-AR cells increased CCL3 expression as compared with that from mPrSC-V after co-culture with RAW264.7/mBMMs. Increased induction of CCL3 at the protein expression level was confirmed by ELISA. These data identified a previously unrecognized role of prostate stromal AR in promoting CCL3 expression during the cross-talk with macrophages.
FIGURE 3.
CCL3 as potential mediator for macrophage infiltration and macrophage-induced stromal cell proliferation. A and B, quantitative PCR analysis of cytokine/chemokine expression levels in mPrSC-V/mPrSC-AR cells or RAW264.7/mBMMs cells at 48 h after co-culture. The inflammatory chemokine, CCL3, was highly induced after co-culture, and was further increased in the co-cultured mPrSC-AR. C and D, ELISA analysis of CCL3 in the conditioned media isolated from RAW264.7, mPrSC, or the co-culture of both cell lines. Data are mean ± S.D. for each experiment repeated 3 times with similar results. **, p < 0.01.
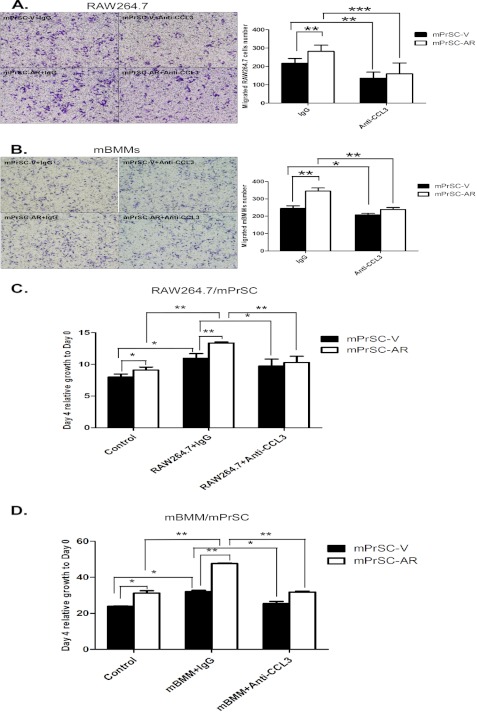
Next, we applied neutralizing antibody against CCL3 to confirm its role in mediating this regulation. As expected, addition of neutralizing CCL3 antibody in the co-culture system resulted in a significant reduction of the migration of RAW264.7 or mBMMs (Fig. 4, A and B), and macrophage-induced mPrSC cell proliferation during co-culture (Fig. 4, C and D). Together, results from Fig. 4, A–D, concluded that CCL3, a downstream gene of prostate stromal AR, plays crucial roles in mediating macrophage recruitment and macrophage-induced prostate stromal cell proliferation.
FIGURE 4.
Neutralization of CCL3 by a specific antibody attenuates macrophage migration and macrophage-induced mPrSC proliferation. A and B, RAW264.7 cells (A) or mBMMs (B) were co-cultured with mPrSC-V or mPrSC-AR cells in a transwell plate in the presence of anti-CCL3 neutralizing antibody or isotype control antibody for cell migration assays. C and D, mPrSC-V or mPrSC-AR cells were co-cultured with RAW264.7 cells (C) or mBMMs (D) in the presence of anti-CCL3 neutralizing antibody or isotype control antibody and assayed with MTT for 4 days. Data are mean ± S.D. for each experiment repeated 3 times with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Stromal AR → Inflammatory Cytokine CCL3 → Prostate Stromal Cell Proliferation in a Prolactin-induced BPH Mouse Model
We then confirmed our above in vitro co-culture results with in vivo data using a prolactin-induced BPH mouse model (21, 26). Using a cre-loxp system, we were able to knockout AR in Pb-PRL-tg mice that could spontaneously develop prostate enlargement after puberty throughout adulthood to mimic human BPH development, and we found that Pb-PRL-tg mice lacking stromal fibromuscular AR developed smaller prostates with a lower proliferation index (22). We performed immunohistochemical analysis with a CCL3-specific antibody and a mouse macrophage-specific antibody Mac-3, in prostate tissues of these mice and their littermate controls, and found increased macrophages and CCL3 in the Pb-PRL-tg BPH mice compared with wild-type littermate controls (Fig. 5A). Consistently, the ablation of the stromal fibromuscular AR in the Pb-PRL-tg BPH mouse (named as dARKO/Pb-PRL-tg) reduced infiltrating macrophages and the CCL3 expression level in prostate tissues (Fig. 5A), confirming our above in vitro co-culture results and supporting a important role of prostate stromal AR in controlling CCL3 expression to influence macrophage infiltration and prostate stroma growth during BPH development.
FIGURE 5.
CCL3 expression in the prolactin-induced BPH mouse model and BPH patients. A, H&E staining and immunohistochemical staining of wild-type (WT) mice (n = 9), Pb-PRL-tg mice (n = 8), and dARKO/Pb-PRL-tg mice (n = 3) using mouse macrophage-specific antibodies anti-Mac-3 and CCL3. B, immunohistochemical staining of human normal prostate tissues (n = 7) and BPH tissues (n = 8) using anti-CCL3 antibody. **, p < 0.01.
Inflammatory Cytokine CCL3 in Human BPH Prostate Tissues
To further confirm the clinical relevance of our data, we then assayed human BPH prostate tissues. We also examined CCL3 expression in these human BPH prostate tissues and found little expression of CCL3 in the normal prostate gland compared with increased CCL3 staining in the BPH prostates (Fig. 5B), suggesting CCL3 might play important roles in the recruitment of macrophages and promoting human BPH development and progression.
Targeting Stromal AR with an AR Degradation Enhancer, ASC-J9®, Led to Suppress Stromal AR-mediated Enhancement of Macrophage Infiltration, Stromal Cell Growth, and CCL3 Induction
To further confirm the functional contribution of AR in this regulation, the AR degradation enhancer, ASC-J9®, which was able to selectively degrade AR proteins in different types of cells, was used to inhibit AR function during co-culture (supplemental Fig. S3) (29–31). We examined ASC-J9® effects (5 μm) on the migration of macrophages and macrophage-induced prostate stromal cell growth. We found that ASC-J9® treatments effectively suppressed the migration of RAW264.7 cells (Fig. 6A) or mBMMs (Fig. 6B), and inhibited RAW264.7-induced (Fig. 6C) or mBMMs-induced mPrSC cell proliferation (Fig. 6D). Consistently, ASC-J9® treatments also significantly decreased the expression levels of CCL3 in mPrSC cells after co-culture with macrophages (supplemental Fig. S4). These data indicate that targeting stromal AR by ASC-J9® can effectively suppress the cross-talk between macrophages and prostate stromal cells, and resulted in suppression of CCL3 induction and prostate stromal cell growth.
FIGURE 6.
Treatment with ASC-J9® blocks stromal AR-mediated enhancement of macrophage infiltration and stromal cell growth. A and B, RAW264.7 cells (A) or mBMMs (B) were co-cultured with mPrSC-V or mPrSC-AR cells in a transwell plate in the presence or absence of ASC-J9® for cell migration assay. C and D, mPrSC-V or mPrSC-AR cells were co-cultured with RAW264.7 cells (C) or mBMMs (D) in the presence or absence of ASC-J9® and assayed with MTT at 4 days. Data are mean ± S.D. for each experiment repeated 3 times with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
During the development and progression of BPH, one of the most common features is prostate enlargement, and previous morphometric analysis studies showed that there is a 5:1 ratio of the stromal to epithelial compartment in BPH patients compared with a 2:1 ratio in nonhyperplastic normal prostate (32, 33). These results strongly suggest that prostate enlargement in BPH is primarily caused by the un-proportional volume increase in the prostate stromal compartment. Histological analysis results also revealed that the increased volume of the stromal compartment is the result of hyperproliferation of prostate stromal cells (34). We thus hypothesized that the aberrant proliferation of prostate stromal cells might be responsible for the BPH pathogenesis.
At present, there is no consensus on the etiology of BPH. However, the majority of etiological postulations point toward prostatic inflammation as one of the potential initiators of BPH (1, 9, 35–38). Although there is still no agreement as to whether inflammation is simply a parallel occurrence or a direct cause, some evidence suggests a significant correlation between inflammation and BPH (1, 36, 39), which is consistent with our study showing that infiltrated macrophages could play a role in promoting stromal cell proliferation (Fig. 2, C and D).
Previously, several studies have shown that altered cytokine/chemokine expression is associated with BPH foci and local stromal responses in human BPH (40–42). Studies by Macoska and colleagues (40) have shown that aging prostate reactive stroma displays an up-regulation of several secreted inflammatory chemokines including CXCL1, CXCL2, CXCL5, CXCL6, and CXCL12, as well as IL-11, IL-33, and cytokine-like 1 (CYTL1). Furthermore, CXCL1, CXCL5, and CXCL6 secretion was elevated in primary BPH stromal fibroblasts, and CXCL1, CXCL5, CXCL6, and CXCL12 all activated a sustained proliferative response in prostate stromal fibroblasts and epithelial cells. The authors postulated that “inflammatory mediators are secreted by prostatic stroma,” which may facilitate “low-level increases in the proliferative rate” potentially explaining the “low-level, but cumulative, proliferation of both epithelial and fibroblastic/myofibroblastic cell types that could promote aging-associated development of BPH“ (40, 43, 44). It is becoming evident that inflammation, elevated chemokine expression, and induction of a tissue repair type of fibrotic/hyperplastic responses in aged prostate are interrelated in the development of BPH. Also, these studies mainly focused on the paracrine action of aged fibroblasts on prostate cell proliferation. However, the role of chemokines in mediating the cross-talk between macrophages and prostate stromal cells remains unclear.
Therefore, our study focused on important inflammatory macrophages of the cell, which may directly promote prostate stroma growth after infiltration into prostate via interaction with prostate stromal cells. Bianchi-Frias et al. quantified transcription levels in microdissected glandular-adjacent stroma from young (age 4 months) and old (age 20–24 months) C57BL/6 mice, and identified macrophages, as well as inflammation-associated genes CCL8, CCL12, CCL5, and CCL7, were significantly increased in the aged prostatic stroma (45), suggesting that macrophages could play an important role in promoting chemokine induction and prostatic stroma expansion.
To determine the potential role of macrophages in promoting stromal cell growth, we established an in vitro co-culture system of macrophages/prostate stromal cells, which allows us to systematically identify key inflammatory mediators that are essential for interaction between macrophages and prostate stromal cells in vitro. We believe our co-culture model can mimic the in vivo interaction of macrophages and stromal cells in an inflammatory microenvironment of BPH. We found that prostate stromal cells could attract macrophage infiltration and the growth of prostate stromal cells could be enhanced by macrophages during co-culture, supporting a potential role for macrophages identified from inflamed stroma by immunohistochemical analysis of BPH prostate tissues (Fig. 1). More importantly, our findings support a new role of prostate stromal AR in this interaction (Fig. 2, A and B), showing that AR further enhances the migration of macrophages, as well as the macrophage-mediated proliferation of prostate stromal cells during co-culture. Our study has established a previously unrecognized role of stromal AR in mediating the cross-talk between macrophages and prostate stromal cells, implicating the possibility that aberrant AR function in prostate stroma may exacerbate inflammatory responses associated with BPH. Also, we have performed a screening of various cytokines, identified CCL3 as a potential mediator, and confirmed that induction of CCL3 is essential for macrophage infiltration and macrophage-mediated proliferation of prostate stromal cells (Fig. 3, A and B). Interestingly, targeting AR via ASC-J9® or using a CCL3-specific neutralizing antibody resulted in suppression of macrophage migration and macrophage-mediated growth of prostate stromal cells. Similarly, the ablation of AR in the stromal-fibromuscular tissue of prostate significantly attenuates the enlargement of prostate tissues, and reduces macrophage infiltration and CCL3 induction in Pb-PRL-tg mice (Fig. 5A). These data also establish a potential role of prostate stromal AR in regulating CCL3 expression during the cross-talk between macrophages and stromal cells. Intriguingly, AR may not promote the induction of CCL3 via a transcriptional regulation because we were not be able to identify a potential AR binding sequence (androgen response element) in the CCL3 promoter region that could be validated by the ChIP binding assay (data not shown). We postulate that during the cross-talk between macrophages and prostate stromal cells, AR may play an assistive role in promoting inflammatory signaling that drives induction of CCL3 instead of directly inducing CCL3 expression via androgens. Our study identifies a potential link between AR and CCL3 in the prostate stroma that contains infiltrated macrophages. This could support a potential BPH model in which inflammatory signaling pathways could act synergistically with AR signals to promote the proliferation of prostate stromal cells. This may be distinct from the regulation of various chemokines identified in senescent fibroblasts as described above, because that study (40) only focused on the paracrine factors of senescent fibroblasts. Therefore, we postulate that inflammatory chemokines, such as CCL3, could be one other driving force factor for prostate stromal expansion during inflammation-associated BPH development and progression, which may also explain why certain patients did not respond well to anti-androgen therapy (46).
Taken, together, our findings delineate a potential mechanism by which infiltrating macrophages promote prostate stromal cell proliferation through stromal AR/CCL3-dependent pathways, which could possibly be one of the key pathways for stromal expansion during BPH development and progression. These results provide significant insights in searching for new combined therapeutic approaches to battle BPH associated with recurrent inflammation using anti-hormone and anti-inflammatory strategies.
In conclusion, there is an urgent need for the identification of new targets or key pathways that are essential for preventing the BPH hyperplastic phenotype. Our findings conclude that CCL3 is a key AR downstream inflammatory mediator, which could enhance the macrophages infiltration and promote proliferation of prostate stromal cells, and the administration of anti-CCL3 neutralizing antibody would attenuate the hyperplastic phenotype. We believe that CCL3 could serve as a potential target for future therapeutic design of BPH treatment instead of the medical castration approach.
Supplementary Material
Acknowledgment
We thank K. Wolf for help in editing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA127300 and CA156700, Department of Defense Grant W81XWH-10-1-0300, Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 from the China Medical University, Taichung, Taiwan, and National Basic Research Program of China Grant 2012CB518305. ASC-J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and C.C. own royalties and equity in AndroScience.

This article contains supplemental Figs. S1–S4.
- BPH
- benign prostatic hyperplasia
- AR
- androgen receptor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- mPrSC
- mouse prostate stromal cell
- tg
- transgenic
- mBMM
- mouse bone marrow-derived macrophages.
REFERENCES
- 1. Kramer G., Mitteregger D., Marberger M. (2007) Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur. Urol. 51, 1202–1216 [DOI] [PubMed] [Google Scholar]
- 2. Nakhla A. M., Khan M. S., Romas N. P., Rosner W. (1994) Estradiol causes the rapid accumulation of cAMP in human prostate. Proc. Natl. Acad. Sci. U.S.A. 91, 5402–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts R. O., Bergstralh E. J., Farmer S. A., Jacobson D. J., Hebbring S. J., Cunningham J. M., Thibodeau S. N., Lieber M. M., Jacobsen S. J. (2006) Polymorphisms in genes involved in sex hormone metabolism may increase risk of benign prostatic hyperplasia. Prostate 66, 392–404 [DOI] [PubMed] [Google Scholar]
- 4. Sciarra F., Toscano V. (2000) Role of estrogens in human benign prostatic hyperplasia. Arch. Androl. 44, 213–220 [DOI] [PubMed] [Google Scholar]
- 5. Wilson J. D., Griffin J. E. (1980) The use and misuse of androgens. Metab. Clin. Exp. 29, 1278–1295 [DOI] [PubMed] [Google Scholar]
- 6. Aydin A., Arsova-Sarafinovska Z., Sayal A., Eken A., Erdem O., Erten K., Ozgök Y., Dimovski A. (2006) Oxidative stress and antioxidant status in nonmetastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 39, 176–179 [DOI] [PubMed] [Google Scholar]
- 7. Kasturi S., Russell S., McVary K. T. (2006) Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr. Urol. Rep. 7, 288–292 [DOI] [PubMed] [Google Scholar]
- 8. Ozden C., Ozdal O. L., Urgancioglu G., Koyuncu H., Gokkaya S., Memis A. (2007) The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur. Urol. 51, 199–203; discussion 204–196 [DOI] [PubMed] [Google Scholar]
- 9. Kramer G., Marberger M. (2006) Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 16, 25–29 [PubMed] [Google Scholar]
- 10. Yi F. X., Wei Q., Li H., Li X., Shi M., Dong Q., Yang Y. R. (2006) Risk factors for prostatic inflammation extent and infection in benign prostatic hyperplasia. Asian J. Androl. 8, 621–627 [DOI] [PubMed] [Google Scholar]
- 11. McVary K. T., Rademaker A., Lloyd G. L., Gann P. (2005) Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 174, 1327–1433 [DOI] [PubMed] [Google Scholar]
- 12. Yun A. J., Doux J. D. (2006) Opening the floodgates. Benign prostatic hyperplasia may represent another disease in the compendium of ailments caused by the global sympathetic bias that emerges with aging. Med. Hypotheses 67, 392–394 [DOI] [PubMed] [Google Scholar]
- 13. Claus S., Wrenger M., Senge T., Schulze H. (1993) Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol. Res. 21, 305–308 [DOI] [PubMed] [Google Scholar]
- 14. Kramer G., Steiner G. E., Handisurya A., Stix U., Haitel A., Knerer B., Gessl A., Lee C., Marberger M. (2002) Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 52, 43–58 [DOI] [PubMed] [Google Scholar]
- 15. Steiner G. E., Newman M. E., Paikl D., Stix U., Memaran-Dagda N., Lee C., Marberger M. J. (2003) Expression and function of proinflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 56, 171–182 [DOI] [PubMed] [Google Scholar]
- 16. Steiner G. E., Stix U., Handisurya A., Willheim M., Haitel A., Reithmayr F., Paikl D., Ecker R. C., Hrachowitz K., Kramer G., Lee C., Marberger M. (2003) Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab. Investig. 83, 1131–1146 [DOI] [PubMed] [Google Scholar]
- 17. Nickel J. C., Roehrborn C. G., O'Leary M. P., Bostwick D. G., Somerville M. C., Rittmaster R. S. (2007) Examination of the relationship between symptoms of prostatitis and histological inflammation. Baseline data from the REDUCE chemoprevention trial. J. Urol. 178, 896–900; discussion 900–891 [DOI] [PubMed] [Google Scholar]
- 18. Nickel J. C., Roehrborn C. G., O'Leary M. P., Bostwick D. G., Somerville M. C., Rittmaster R. S. (2008) The relationship between prostate inflammation and lower urinary tract symptoms. Examination of baseline data from the REDUCE trial. Eur. Urol. 54, 1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu M. H., Ma W. L., Hsu C. L., Chen Y. L., Ou J. H., Ryan C. K., Hung Y. C., Yeh S., Chang C. (2010) Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Science Transl. Med. 2, 32–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X. H., Zhao F. J., Han B. M., Jiang Q., Wang Y. C., Wu J. H., Tang Y. Q., Zhang Y. P., Xia S. J. (2011) Primary stromal cells isolated from human various histological/pathological prostate have different phenotypes and tumor promotion role. Chinese Med. J. 124, 1700–1707 [PubMed] [Google Scholar]
- 21. Kindblom J., Dillner K., Sahlin L., Robertson F., Ormandy C., Törnell J., Wennbo H. (2003) Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology 144, 2269–2278 [DOI] [PubMed] [Google Scholar]
- 22. Lai K. P., Yamashita S., Vitkus S., Shyr C. R., Yeh S., Chang C. (2012) Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor. Mol. Endocrinol. 26, 52–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gu H., Dong Z. X., Wang C. B., Yuan Y. F., Hou J. H. (2006) Role of bFGF and TGF-β1 in primary cultured prostatic stromal cells. Zhonghua nan ke xue = Natl. J. Androl. 12, 917–922 [PubMed] [Google Scholar]
- 24. Wu C. T., Altuwaijri S., Ricke W. A., Huang S. P., Yeh S., Zhang C., Niu Y., Tsai M. Y., Chang C. (2007) Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc. Natl. Acad. Sci. U.S.A. 104, 12679–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sciarra A., Di Silverio F., Salciccia S., Autran Gomez A. M., Gentilucci A., Gentile V. (2007) Inflammation and chronic prostatic diseases. Evidence for a link? Eur. Urol. 52, 964–972 [DOI] [PubMed] [Google Scholar]
- 26. Dillner K., Kindblom J., Flores-Morales A., Shao R., Törnell J., Norstedt G., Wennbo H. (2003) Gene expression analysis of prostate hyperplasia in mice overexpressing the prolactin gene specifically in the prostate. Endocrinology 144, 4955–4966 [DOI] [PubMed] [Google Scholar]
- 27. Macoska J. A. (2011) Chemokines and BPH/LUTS. Differentiation 82, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coppé J. P., Patil C. K., Rodier F., Sun Y., Muñoz D. P., Goldstein J., Nelson P. S., Desprez P. Y., Campisi J. (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai J. J., Lai K. P., Chuang K. H., Chang P., Yu I. C., Lin W. J., Chang C. (2009) Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J. Clin. Invest. 119, 3739–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Z., Chang Y. J., Yu I. C., Yeh S., Wu C. C., Miyamoto H., Merry D. E., Sobue G., Chen L. M., Chang S. S., Chang C. (2007) ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 13, 348–353 [DOI] [PubMed] [Google Scholar]
- 31. Chen S. U., Chou C. H., Lin C. W., Lee H., Wu J. C., Lu H. F., Chen C. D., Yang Y. S. (2010) Signal mechanisms of vascular endothelial growth factor and interleukin-8 in ovarian hyperstimulation syndrome. Dopamine targets their common pathways. Hum. Reprod. 25, 757–767 [DOI] [PubMed] [Google Scholar]
- 32. Bartsch G., Müller H. R., Oberholzer M., Rohr H. P. (1979) Light microscopic stereological analysis of the normal human prostate and benign prostatic hyperplasia. J. Urol. 122, 487–491 [DOI] [PubMed] [Google Scholar]
- 33. Shapiro E., Hartanto V., Lepor H. (1992) Quantifying the smooth muscle content of the prostate using double-immunoenzymatic staining and color assisted image analysis. J. Urol. 147, 1167–1170 [DOI] [PubMed] [Google Scholar]
- 34. Lin V. K., Benaim E. A., McConnell J. D. (2001) α-Blockade down-regulates myosin heavy chain gene expression in human benign prostatic hyperplasia. Urology 57, 170–175 [DOI] [PubMed] [Google Scholar]
- 35. Lee K. L., Peehl D. M. (2004) Molecular and cellular pathogenesis of benign prostatic hyperplasia. J. Urol. 172, 1784–1791 [DOI] [PubMed] [Google Scholar]
- 36. Nickel J. C. (2008) Inflammation and benign prostatic hyperplasia. Urol. Clin. North Am. 35, 109–115; vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sciarra A., Mariotti G., Salciccia S., Autran Gomez A., Monti S., Toscano V., Di Silverio F. (2008) Prostate growth and inflammation. J. Steroid Biochem. Mol. Biol. 108, 254–260 [DOI] [PubMed] [Google Scholar]
- 38. Untergasser G., Madersbacher S., Berger P. (2005) Benign prostatic hyperplasia. Age-related tissue-remodeling. Exp. Gerontol. 40, 121–128 [DOI] [PubMed] [Google Scholar]
- 39. Wang L., Yang J. R., Yang L. Y., Liu Z. T. (2008) Chronic inflammation in benign prostatic hyperplasia. Implications for therapy. Med. Hypotheses 70, 1021–1023 [DOI] [PubMed] [Google Scholar]
- 40. Macoska J. A., Begley L. A., Dunn R. L., Siddiqui J., Wei J. T., Sarma A. V. (2008) Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate 68, 442–452 [DOI] [PubMed] [Google Scholar]
- 41. Penna G., Mondaini N., Amuchastegui S., Degli Innocenti S., Carini M., Giubilei G., Fibbi B., Colli E., Maggi M., Adorini L. (2007) Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51, 524–533; discussion 533 [DOI] [PubMed] [Google Scholar]
- 42. Schauer I. G., Ressler S. J., Tuxhorn J. A., Dang T. D., Rowley D. R. (2008) Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology 72, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Begley L. A., Kasina S., MacDonald J., Macoska J. A. (2008) The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43, 194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Begley L. A., MacDonald J. W., Day M. L., Macoska J. A. (2007) CXCL12 activates a robust transcriptional response in human prostate epithelial cells. J. Biol. Chem. 282, 26767–26774 [DOI] [PubMed] [Google Scholar]
- 45. Bianchi-Frias D., Vakar-Lopez F., Coleman I. M., Plymate S. R., Reed M. J., Nelson P. S. (2010) The effects of aging on the molecular and cellular composition of the prostate microenvironment. PloS One 5, e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nicholson T. M., Ricke W. A. (2011) Androgens and estrogens in benign prostatic hyperplasia. Past, present, and future. Differentiation 82, 184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reddy S. M., Hsiao K. H., Abernethy V. E., Fan H., Longacre A., Lieberthal W., Rauch J., Koh J. S., Levine J. S. (2002) Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J. Immunol. 169, 702–713 [DOI] [PubMed] [Google Scholar]
- 48. Davies J. Q., Gordon S. (2005) Isolation and culture of human macrophages. Methods Mol. Biol. 290, 91–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.