Background: TLR4-mediated p38 MAPK and actin cytoskeleton reorganization are important for macrophage phagocytosis.
Results: TLR4-induced Eps8 facilitates TLR4-MyD88 interaction, leading to the activation of p38 MAPK, actin polymerization, and the uptake of bacteria in macrophages.
Conclusion: Eps8 is critical in innate immunity.
Significance: This is the first report to indicate that Eps8 contributes to efficient phagocytosis in macrophages.
Keywords: Innate Immunity, Lipopolysaccharide (LPS), Macrophages, Phagocytosis, Toll-like Receptors (TLRs), Eps8, FAK, Src, Actin, p38 MAPK
Abstract
Toll-like receptors (TLRs) are crucial in macrophage phagocytosis, which is pivotal in host innate immune response. However, the detailed mechanism is not fully defined. Here, we demonstrated that the induction of Src and Eps8 in LPS-treated macrophages was TLR4- and MyD88-dependent, and their attenuation reduced LPS-promoted phagocytosis. Confocal microscopy indicated the colocalization of Eps8 and TLR4 in the cytosol and at the phagosome. Consistently, both Eps8 and TLR4 were present in the same immunocomplex regardless of LPS stimulation. Inhibition of this complex formation by eps8 siRNA or overexpression of pleckstrin homology domain-truncated Eps8 (i.e. 261-p97Eps8) decreased LPS-induced TLR4-MyD88 interaction and the following activation of Src, focal adhesion kinase, and p38 MAPK. Importantly, attenuation of Eps8 impaired the bacterium-killing ability of macrophages. Thus, Eps8 is a key regulator of the LPS-stimulated TLR4-MyD88 interaction and contributes to macrophage phagocytosis.
Introduction
Phagocytosis is a phylogenetically conserved process that is critical for innate immunity. In insects, phagocytosis is accomplished by plasmatocytes and granular cells. In mammals, it is mainly achieved by mononuclear phagocytes (monocytes and macrophages) and polymorphonuclear cells (neutrophils) (1, 2). As neutrophils and monocytes circulate in the blood stream, macrophages reside in tissues, where they serve as sentinels for pathogens. By virtue of a variety of phagocytic receptors, such as Fc γ receptors (FcγRs)3 and complement receptor 3, macrophages can detect the presence of different pathogens in the body (3). Engagement of these receptors activates a series of intracellular signaling pathways that lead to dynamic and rapid cytoskeletal rearrangements as well as membrane trafficking required for macrophage phagocytosis (3–5). In addition to the aforementioned receptors, microbial internalization is usually accompanied by activation of Toll-like receptors (TLRs) that recognize unique pathogen-associated molecular patterns shared by large groups of pathogens (6), indicating that TLR activation and phagocytosis are functionally linked (7).
Lipopolysaccharide (LPS) is present in the extracellular membrane of most Gram-negative bacteria. It activates rest macrophages via binding to the CD14-TLR4-MD2 receptor complex. Mounting evidence reveals that LPS-TLR4 interaction is important in promoting the clearance of bacteria by up-regulating the phagocytic activity of macrophages via at least two distinct pathways (8–11). One is relayed by myeloid differentiation primary response gene 88 (MyD88)-IRAK4-p38 MAPK, resulting in a secondary up-regulation of scavenger receptors for phagocytic clearance of bacteria (9). Another one is conducted by the actin-Cdc42/Rac pathway, in which MyD88 might be dispensable (10). However, how ligand-bound TLR4 transmits signals to the downstream pathways, such as MyD88-p38 MAPK and actin-Cdc42/Rac, remains to be solved.
Eps8 (EGF receptor pathway substrate number 8) is a cytoplasmic protein that contains several protein-protein interaction modules. It includes a split pleckstrin homology (PH) domain, an Src homology 3 domain, and several proline-rich regions (12, 13). Eps8 can form a complex with Sos1, Abi1, and the p85 subunit of PI3K, which allows Sos1 to act as a guanine nucleotide exchange factor to activate Rac, leading to actin remodeling in mouse embryonic fibroblasts (14, 15). In addition, Eps8 associates with IRSp53 to activate Rac, which results in increased cell motility and invasiveness of cancer cells (16). As an actin barbed end (or plus end)-capping protein, Eps8 can also control actin-based motility (17) and regulate the apical morphogenesis of intestinal cells of Caenorhabditis elegans (18). In addition to these functions, Eps8 is important in mitogenesis because its aberrant expression in cells not only increases proliferation in response to epidermal growth factor (EGF) (19) but also causes normal cells to become transformed (12). In addition to EGF receptor, Eps8 is also a substrate of Src tyrosine kinase (20) and is involved in Src-mediated transformation (21, 22). The enzymatic activity of Src positively regulates not only the expression of Eps8 (20, 21) but also the interaction between Eps8 and IRSp53, leading to the activation of Stat3 in Src-transformed cells (23).
To date, studies concerning the function and regulation of Eps8 are mostly limited to fibroblasts and epithelial cells, whereas its role in the macrophage-related innate immunity has not been addressed. Because LPS induces Src expression and activation in macrophages (24) and Eps8 is a regulator of actin cytoskeleton, we wonder whether Eps8 might participate in LPS/TLR4-mediated phagocytosis. In this study, we first demonstrated that the induction of Src and Eps8 in LPS-treated peritoneal macrophages (PEMs) was TLR4- and MyD88-dependent. Second, we proved that Eps8 associated with TLR4 and regulated TLR4-MyD88 interaction in the presence of LPS. Third, our study revealed that Eps8 participated in cell spreading, membrane ruffling, and p38 MAPK activation in LPS-stimulated macrophages, leading to increased phagocytosis. Last, we demonstrated that Eps8 was a critical player in bacterium killing in macrophages. These results for the first time disclosed the importance of Eps8 in LPS-stimulated TLR4-MyD88 signaling and the role of Eps8 in macrophages.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
LPS purified from Escherichia coli serotype 0111:B4 was obtained from Sigma. The primary antibodies used were actin and FAK (Upstate); Pi-Y397 and Pi-Y861 FAK (BIOSOURCE International); Pi-ERK1/2, Pi-JNK, Pi-p38 MAPK, and Pi-Y416 Src (Cell Signaling Technology); Eps8 (BD Transduction Laboratories; Santa Cruz Biotechnology, Inc.); p38 MAPK and TLR4 (Santa Cruz Biotechnology, Inc.); and MyD88 (R&D Systems). The mouse ascites containing monoclonal anti-Src (peptide 2–17) produced by the hybridoma (CRL-2651) was obtained from the American Type Culture Collection (ATCC). Rabbit antiserum against N-terminal p97Eps8 (N-Eps8) was raised against a GST-Eps8 bacterially expressed fusion protein containing murine p97Eps8 residues 121–226.
Bacterial Strain
To generate green fluorescence protein (GFP)-expressing E. coli, DH5α strain was transformed with a tetracycline-resistant derivative of pGFPuv (Clontech), pGFPuv-Tc.
Animals
C3H/HeJ, C3H/HeN, and C57BL/6 were purchased from the Jackson Laboratory (Bar Harbor, ME) and were used at 10–14 weeks of age. MyD88 null mice, on a C57BL/6 background, were generated and provided by Dr. R. Medzhitov (Yale University) (25). Rats (Sprague-Dawley) were utilized to prepare PEMs (26). All experiments using laboratory animals were done in accordance with guidelines of the National Cheng Kung University and the China Medical University.
Cell Culture and Collection of PEMs or Bone Marrow-derived Macrophages (BMDMs)
The murine macrophage cell line, RAW264.7 (ATCC), was cultured and propagated in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT) and 2 mm l-glutamine at 37 °C in a humidified atmosphere of 5% CO2 and air. PEMs were collected by peritoneal lavage from rats (Sprague-Dawley) that had been given an intraperitoneal injection of LPS (1 mg/kg) for 24 h (26) or from mice (C3H/HeJ and C3H/HeN) that had been given an intraperitoneal injection of 1 ml of thioglycollate broth for 4 days. The PEMs were washed with Ca2+- and Mg2+-free phosphate-buffered saline (PBS) and plated in FCS-containing RPMI medium overnight. Then the cells were washed with medium to remove non-adherent cells. BMDMs were collected from wild type and MyD88 null mice and differentiated as described previously (27).
Generation of Macrophages Expressing Nonspecific siRNA, src siRNA, fak siRNA, luciferase siRNA, eps8 siRNA, myd88 siRNA, or 261-p97Eps8
The generation of RAW264.7-based control (Ctrl; expressing nonspecific siRNA), Src-attenuated (siRNA) cells and Src-attenuated cells expressing ectopic Src (siRNA-1/Src6, siRNA-1/Src15) (24) or FAK-attenuated cells (27) was described previously. To generate cells expressing eps8 siRNA (siEps8-346) or myd88 siRNA (siMyD88), RAW264.7 macrophages were transfected with either plasmid pS-meps8-346 (targeted sequence, 5′-AACACAGCTCAAGAACAAGTG-3′) or pLKO.1-mmyd88 (puro) (targeted sequence, 5′-GCCAGCGAGCTAATTGAGAAA-3′) by the Lipofectamine Plus method (Invitrogen) followed by hygromycin or puromycin selection. To generate RAW264.7 cells expressing vector alone and 261-p97Eps8(261), plasmids pBabe and pBabe-eps8(261) (12) were individually transfected into cells by the Lipofectamine Plus method and selected by puromycin.
To generate PEMs transiently expressing luciferase siRNA or eps8 siRNA-503, PEMs (1 × 106) prepared from C57BL/6 mice were infected with lentivirus whose RNA encodes luciferase siRNA (targeted sequence, 5′-CTTCGAAATGTCCGTTCGGTT-3′) or eps8 siRNA (targeted sequence, 5′-CGGAATAAGAAAGCTGAAGTT-3′).
Lysate Preparation and Immunoblot Analysis
Cells were lysed in modified radioimmune precipitation assay buffers as described previously (28), and protein concentration was determined by a BCA protein concentration determination assay (Bio-Rad). Immunoblot analysis was carried out as described previously (21).
Phagocytosis
For each experiment, the GFP-expressing E. coli was grown up from a single colony in ampicillin-containing Luria-Bertani (LB) broth overnight. After centrifugation and washed with PBS, GFP-E. coli was resuspended in RPMI medium at the appropriate concentration without serum and antibiotics. PEMs or RAW264.7 cells (5 × 105) were pretreated with PBS or LPS (100 ng/ml), followed by incubation with GFP-E. coli at a multiplicity of infection (MOI) of 10 at 37 °C for 1 h. Cells were then washed with cold PBS at least three times and analyzed by the FACSCalibur flow cytometry system (BD Biosciences). For uptake of latex beads, RAW264.7 cells were cultured on glass coverslips overnight. Unless indicated, cells were loaded with Amine-modified fluorescent red latex beads (Sigma) and incubated at 37 °C for 1 h. Cells were then thoroughly washed with cold PBS, fixed with methanol, and counterstained with Giemsa stain (modified solution) (Sigma). The uptake of fluorescent latex beads was enumerated under a fluorescence microscope. Three random fields in each coverglass were examined. The phagocytosis index was calculated as the number of ingested beads within 100 cells.
Confocal Microscopy
For confocal microscopic analysis of Eps8 and TLR4 in phagosomes, RAW264.7 cells were cultured on a glass coverslip overnight, incubated without or with LPS-coated latex beads (2-μm diameter; Invitrogen) in serum-free RPMI 1640 medium for 10 min at 37 °C under atmospheric conditions of 5% CO2. Cells were then washed with PBS, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 5 min, and immunostained with primary monoclonal antibodies against Eps8 and TLR4, followed by the incubation of appropriately labeled secondary antibodies. Confocal analysis was carried out with the Leica TCS SP2 confocal imaging system.
Bacterial Killing Assay
Equal numbers (5 × 105) of PEMs or RAW264.7 cells were incubated with GFP-E. coli at an MOI of 100 at 37 °C for 10 min, followed by 10-min gentamicin (100 μg/ml) treatment. After thoroughly washed with PBS, the cells were cultured in normal medium for 0, 0.5, 1, and 1.5 h before they were lysed in 1 ml of ice-cold distilled water. Then the number of viable bacteria inside the solution was enumerated by counting the number of colony-forming units (cfu) in LB agar plates.
Statistical Analysis
Each experiment was performed at least three times in triplicate. Unless indicated, the results were presented as means ± S.E. The significance of difference was assessed by Student's t test. Bonferronic correction was used for controlling type I error in multiple comparisons.
RESULTS
LPS-induced Src Is TLR4-dependent and Plays Important Roles in Regulating Macrophage Phagocytosis
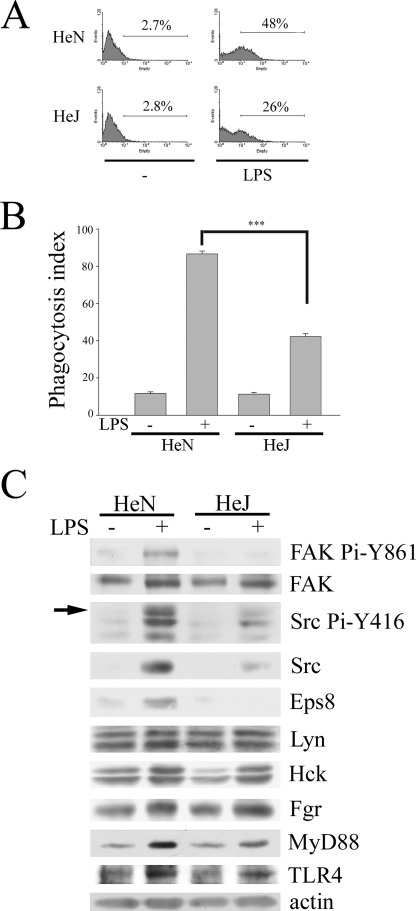
Increasing evidence has revealed the importance of TLRs in promoting the clearance of bacteria by augmenting the phagocytic activity of macrophages. Given that LPS-induced Src expression and activity was iNOS-dependent (24), we wondered whether Src enhancement was TLR4-dependent and was involved in the process of macrophage phagocytosis. To corroborate the role of TLR4 in this process, PEMs from C3H/HeN and C3H/HeJ mice, which express wild type and defective TLR4, respectively (29), were used. To monitor phagocytosis, GFP-expressing E. coli and fluorescent red-conjugated latex beads were utilized in this study. The experiment was carried out in serum-free medium to eliminate interference by the Fc and complement receptors. Although low basal uptake of bacteria (Fig. 1A) or latex beads (Fig. 1B) was observed in C3H/HeN and C3H/HeJ PEMs without LPS stimulation, LPS-mediated phagocytosis was greatly suppressed in C3H/HeJ PEMs as compared with that in C3H/HeN PEMs (Fig. 1, A and B). Concurrently, reduced LPS-elicited Src expression and activation (as reflected by Src Pi-Y416 and FAK Pi-Y861) were detected in C3H/HeJ PEMs relative to C3H/HeN PEMs (Fig. 1C), indicating the involvement of TLR4 in these LPS-mediated effects. Of note, LPS did not significantly alter the expression of myeloid Src family kinases (i.e. Lyn, Hck, and Fgr) in both C3H/HeN and C3H/HeJ PEMs (Fig. 1C).
FIGURE 1.
LPS-induced expression of Src and Eps8 and phagocytosis is TLR4-dependent in macrophages. Peritoneal macrophages were harvested from C3H/HeN and C3H/HeJ mice as described under “Experimental Procedures.” Equal numbers of cells were treated with PBS (−) or LPS (100 ng/ml) for 24 h, followed by 1 h of incubation with either GFP-E. coli (A) or fluorescent red latex beads (B) at 37 °C. Subsequently, macrophages were washed with PBS. The uptake of E. coli or latex beads by macrophages was assayed by FACS analysis (A) or by a fluorescence microscope (B). The phagocytosis index was calculated as the number of ingested beads in 100 cells. The experiment was performed at least three times in triplicate. The results were means ± S.E. ***, p < 0.001. C, equal amounts of lysates (100 μg) from each sample were resolved by SDS-PAGE and probed with antibodies as indicated. The arrow indicates the position of active Src (i.e. Src Pi-Y416).
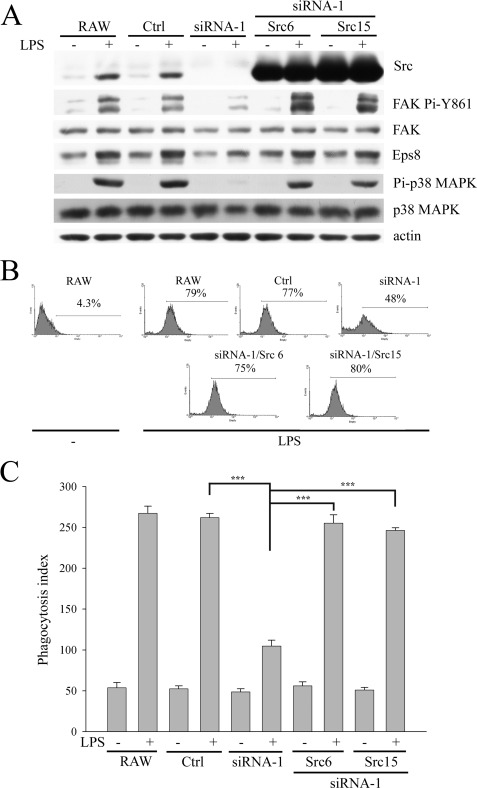
The simultaneously increased Src expression and phagocytosis in LPS-treated C3H/HeN PEMs suggested the involvement of Src in LPS-evoked phagocytosis. Here, the ability of RAW264.7 (RAW) cells and their derived Ctrl, Src-attenuated (siRNA-1), and Src-attenuated cells with ectopic Src (siRNA-1/Src6, siRNA-1/Src15) to phagocytose GFP-E. coli or latex beads after LPS stimulation was determined. Src attenuation impaired LPS-evoked phagocytosis and FAK Pi-Y861, which could be rescued by ectopic Src (Fig. 2). By contrast, knockdown of Lyn (a member of myeloid Src family kinases) did not affect these LPS-triggered events (data not shown). Taken together, this evidence shows that Src plays an important role in LPS-stimulated phagocytosis.
FIGURE 2.
Attenuation of Src decreases LPS-mediated Eps8 expression and macrophage phagocytosis. RAW cells and their derived Ctrl and siRNA-1 cells as well as siRNA-1 cells harboring a plasmid encoding avian Src (siRNA-1/Src6 and siRNA-1/Src15) were stimulated with or without LPS (100 ng/ml) for 48 h. Then either these cells were lysed and their lysates were Western immunoblotted with antibodies as indicated (A), or they were incubated with GFP-E. coli (B) or latex beads (C) at 37 °C for 1 h. The uptake of E. coli by macrophages was assayed by FACS analysis (B), whereas the uptake of latex beads by macrophages was determined by a fluorescence microscope (C). The phagocytosis index was calculated as the number of ingested beads in 100 cells. The experiment was performed at least three times in triplicate. The results were means ± S.E. ***, p < 0.001.
Concomitant Up-regulation of p97Eps8 and Src in LPS-stimulated Macrophages
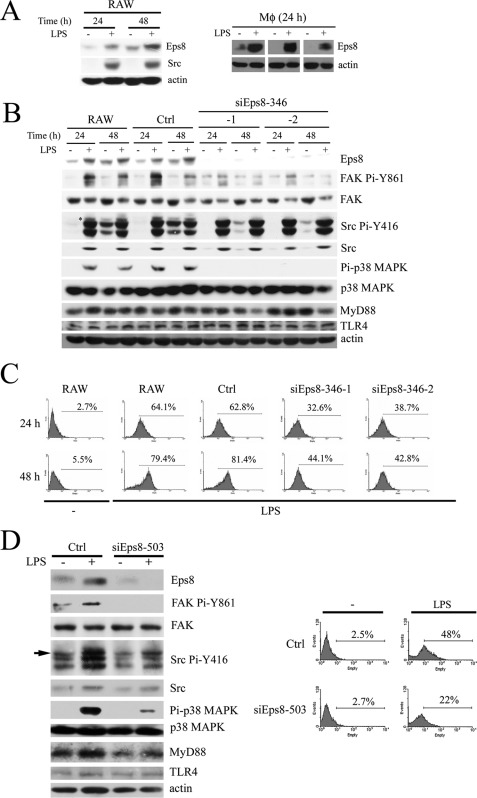
Considered the expression and phosphorylation of p97Eps8 can be mediated by Src activation in murine fibroblasts (21); therefore, its role in the LPS-induced, Src-dependent phagocytosis was determined. As shown in Fig. 3A (left), like Src, LPS augmented the expression of p97Eps8 after 24 and 48 h postexposure. Although expressions of both p97Eps8 and p68Eps8 were observed in fibroblasts (20), the former was the major Eps8 isoform detected in RAW cells regardless of LPS stimulation. Thus, p97Eps8 is referred to here as Eps8. Markedly, Src attenuation inhibited LPS-induced Eps8 expression (Fig. 2A). The presence of Eps8 in PEMs from LPS-challenged rats but not in the control rats (Fig. 3A, right) further excluded the possibility that LPS-induced Eps8 was an in vitro artifact. Like Src, LPS-mediated Eps8 induction was TLR4-dependent in murine PEMs (Fig. 1C). Based on these results, we corroborated that LPS-elevated Eps8 expression was a bona fide physiological event.
FIGURE 3.
LPS-induced Eps8 increases the phagocytic activity of macrophages. A, RAW264.7 cells treated without or with LPS (100 ng/ml) for 24 and 48 h (left). Age-matched rats were intraperitoneally administered with sterile PBS (−) or LPS (1 mg/kg), and their PEMs were collected after 24 h (right). Total lysates (100 μg) from each sample were Western immunoblotted with antibodies as indicated. B, RAW, Ctrl, and Eps8-attenuated (siEps8-346-1 and -2) cells were stimulated without or with LPS (100 ng/ml) for 24 and 48 h. Then either these cells were lysed and their lysates were Western immunoblotted with antibodies as indicated (B), or they were incubated with GFP-E. coli (C) at 37 °C for 1 h to determine their ability to phagocytose E. coli. D, PEMs (1 × 106 cells) from C57BL/6 mice were infected with lentivirus (MOI = 3) encoding luciferase siRNA (Ctrl) or eps8 siRNA (siEps8-503). After 2 days, PEMs were stimulated without (−) or with LPS (100 ng/ml) for 24 h. Then either PEMs were lysed and the lysates (100 μg) were Western immunoblotted with antibodies as indicated (left), or they were incubated with GFP-E. coli at 37 °C for 1 h to determine their ability to uptake E. coli (right). The arrow indicates the position of active Src.
Attenuation of Eps8 Reduces LPS-stimulated Src Activation, FAK Pi-Y861, Actin Polymerization, and Phagocytosis
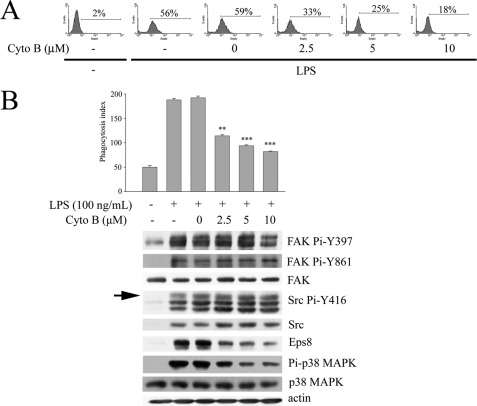
Actin cytoskeleton reorganization is required for phagocytosis. However, the underlying mechanism of TLR-mediated actin polymerization was not fully revealed. As an actin-capping protein and a Rac activator, Eps8 might participate in LPS-stimulated actin polymerization and contribute to macrophage phagocytosis. In order to prove this hypothesis, RAW cells stably expressing either nonspecific siRNA (Ctrl) (20) or eps8-specific siRNA (siEps8-346; supplemental Fig. S1A) were generated. Attenuation of Eps8 hampered LPS-induced Src Pi-Y416 and FAK Pi-Y861 (Fig. 3B), cell spreading and membrane ruffling (supplemental Fig. S2), and also the uptake of either GFP-E. coli (Fig. 3C) or latex beads (supplemental Fig. S1B). Similar results were observed in RAW cells stably expressing different eps8 siRNAs (i.e. siEps8-503 and -1843; supplemental Fig. S1C) and in murine PEMs infected by lentivirus encoding eps8 siRNA-503 (Fig. 3D). These findings revealed that Eps8 was important for the LPS-induced actin cytoskeleton reorganization and phagocytic activity. Interestingly, disruption of actin polymerization by cytochalasin B (an actin polymerization inhibitor) markedly reduced LPS-stimulated phagocytosis of GFP-E. coli (Fig. 4A) and Eps8 expression but not the expression and activity of Src (Fig. 4B), indicating that the actin cytoskeleton itself also contributes to LPS-induced Eps8 expression.
FIGURE 4.
Cytochalasin B inhibits LPS-induced Eps8 expression and macrophage phagocytosis. RAW264.7 cells were pretreated without (−) or with cytochalasin B (Cyto B; 0, 2.5, 5, or 10 μm) for 1 h, followed by incubation without or with LPS (100 ng/ml) for 24 h. Then cells were incubated with GFP-E. coli (A) or fluorescent red latex beads (B, top) at 37 °C for 1 h. Their ability to uptake E. coli and latex beads was assayed individually by FACS analysis and fluorescence microscope. In a separate experiment, cells were lysed, and the lysates were Western immunoblotted with antibodies as indicated (B, bottom). The arrow indicates active Src (i.e. Pi-Y416 Src). The experiment was performed three times in triplicate. The results are means ± S.E. (error bars). **, p < 0.01; ***, p < 0.001, as compared with the LPS-treated control.
FAK Is Required for LPS-induced Eps8 and Macrophage Phagocytosis
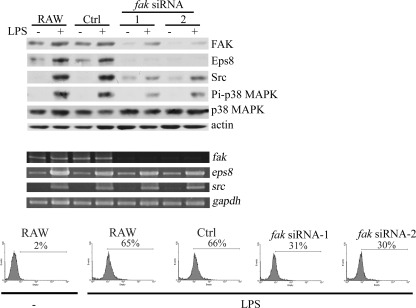
Focal adhesions provide structural tethers linking the actin cytoskeleton to the extracellular matrix, which regulates a variety of cellular processes, including phagocytosis (30). As a critical protein in focal adhesion, FAK has been implicated in integrin-mediated macrophage phagocytosis of Yersinia pseudotuberculosis (31). Given that Eps8 expression was affected by the dynamic of the actin cytoskeleton and that Src knockdown concomitantly reduced FAK Pi-Y861 and phagocytosis mediated by LPS, the role of FAK in LPS-elicited phagocytosis was determined. Attenuation of FAK by stably expressing fak siRNAs targeting different fak sequences (i.e. fak siRNA-1 and -2) diminished not only the expression of Eps8 but also the uptake of GFP-E. coli in LPS-treated RAW cells (Fig. 5). Thus, in addition to Src, FAK acted upstream of Eps8 in LPS-induced phagocytosis.
FIGURE 5.
Attenuation of FAK inhibits LPS-induced Eps8 expression and the uptake of GFP-E. coli in RAW264.7 cells. Generation of RAW stably expressing nonspecific siRNA (Ctrl) and two different fak siRNAs (fak siRNA-1 and -2) cells was described previously (27). These cells were stimulated without (−) or with LPS (100 ng/ml) for 24 h. The cell lysates (100 μg) were Western immunoblotted with antibodies as indicated (top), and the amounts of fak, eps8, and src transcript were analyzed by RT-PCR (middle). gapdh was utilized as an internal control for amplification efficiency. In a parallel experiment, these cells were incubated with GFP-E. coli at 37 °C for 1 h, followed by FACS analysis to determine their ability to uptake E. coli (bottom).
Eps8 Knockdown Impairs LPS-induced TLR4-MyD88 Complex Formation in Macrophages
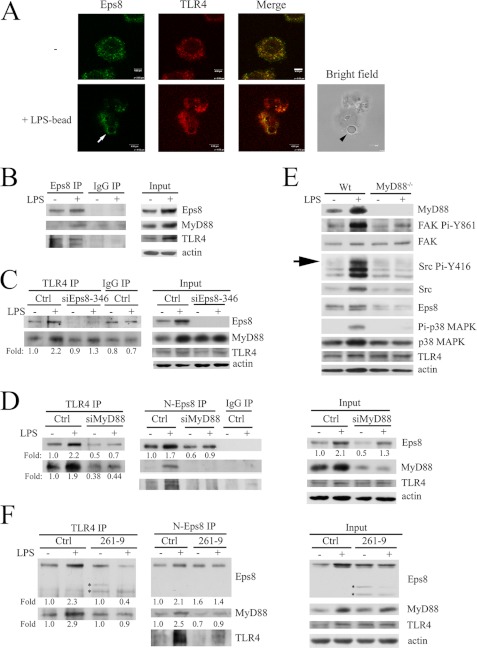
TLR4-MyD88 interaction is required for phagocytosis mediated by LPS. Confocal immunofluorescence microscopy revealed that Eps8 was co-localized with TLR4 in the cytosol and around the engulfed LPS-coated latex beads within RAW cells (Fig. 6A). The co-presence of Eps8 and TLR4 in the same immunocomplex was detected in the lysates of untreated RAW cells (Fig. 6B). Intriguingly, 24-h LPS treatment increased Eps8-TLR4 complex formation, which might be partly attributable to elevated expression of Eps8 and TLR4 (Fig. 6B). Notably, like TLR4, Eps8 also associated with MyD88 in the LPS-treated macrophages (Fig. 6B), suggesting that Eps8, TLR4, and Myd88 might form a complex and that Eps8 might also modulate the interaction between TLR4 and MyD88. Indeed, Eps8 attenuation dramatically reduced LPS-evoked TLR4-MyD88 complex formation, albeit the expression of TLR4 and MyD88 was not affected (Fig. 6C). Remarkably, knockdown of MyD88 decreased LPS-induced Eps8 overexpression and the following Eps8-TLR4 association (Fig. 6D). The failure of Eps8 induction in LPS-treated MyD88 knock-out BMDMs further supported the indispensability of TLR4-MyD88 to elevate Eps8 expression in macrophages exposed to LPS (Fig. 6E).
FIGURE 6.
Eps8 promotes TLR4-MyD88 interaction in macrophages. A, RAW264.7 cells were plated on a glass coverslip overnight, incubated without (top) or with LPS-coated latex beads (bottom) for 10 min, fixed, permeabilized, and stained for Eps8 and TLR4. The confocal images of Eps8, TLR4, and the merged file are shown as indicated. The latex bead, marked by an arrowhead, was observed in bright field. The arrow indicates the phagosome. Scale bar, 4 μm. RAW264.7 (B) and its derived Ctrl and siEps8-346-1 (siEps8-346) cells (C) or MyD88-attenuated (siMyD88) cells (D) were treated with or without LPS (100 ng/ml) for 24 h. The lysates (Input) and the TLR4, Eps8 (Eps8 or N-Eps8), and control (IgG) immunocomplex from each sample were resolved by SDS-PAGE and Western immunoblotting with antibodies as indicated. E, BMDMs from wild type (Wt) and MyD88−/− mice were stimulated without or with LPS for 24 h. Equal amounts of lysates (30 μg) were resolved by SDS-PAGE and probed with antibodies as indicated. The arrow indicates the position of active Src. F, RAW264.7-derived Ctrl and 261-p97Eps8-expressing cells (261-9) were stimulated without or with LPS for 24 h. The lysates (right) and the immunoprecipitates of TLR4 (left) and Eps8 (N-Eps8; middle) from each sample were resolved by SDS-PAGE and Western immunoblotting with antibodies as indicated. Asterisks indicate the position of ectopically expressed 261-p97Eps8. It should be noted that the level of the upper band varied in different experiments. IP, immunoprecipitation.
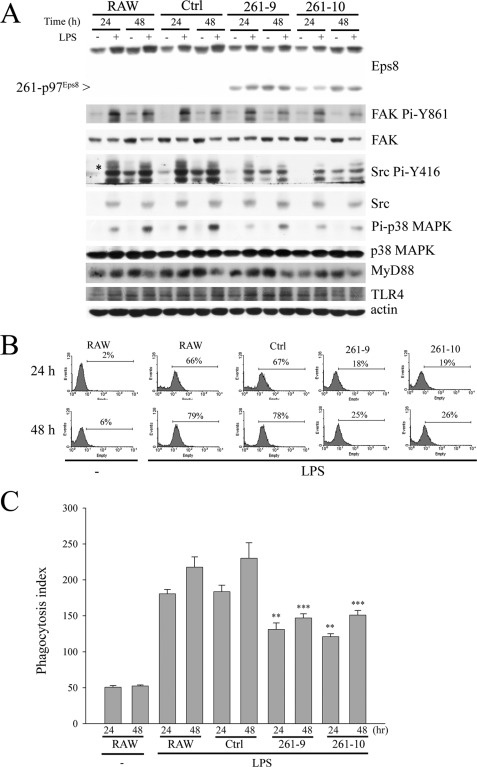
PH-truncated 261-p97Eps8 Inhibits LPS-induced TLR4-MyD88 Complex Formation, Src Pi-Y416, FAK Pi-Y861, and Phagocytosis in Macrophages
Because PH-truncated 261-p97Eps8 contains the domain for actin cytoskeleton organiza tion (17) but fails to associate with the plasma membrane (12), its influence on LPS-induced Eps8-TLR4-MyD88 interaction and the following phagocytosis was determined. Overexpression of 261-p97Eps8 deteriorated the enhancement of Src Pi-Y416 and FAK Pi-Y861 in LPS-treated macrophages (Fig. 7A). Concurrently, 261-p97Eps8 appeared to preferentially associate with TLR4 and hamper LPS-triggered TLR4-Eps8 and TLR4-MyD88 interaction (Fig. 6F) as well as the uptake of GFP-E. coli (Fig. 7B) and latex beads (Fig. 7C) in RAW cells.
FIGURE 7.
PH-truncated 261-p97Eps8 reduces LPS-induced activation of FAK, Src, and p38 MAPK as well as phagocytosis of E. coli and latex beads. A, RAW cells and their derived Ctrl and 261-p97Eps8-expressing (261-9 and 261-10) cells were treated with PBS (−) or LPS (100 ng/ml) for 24 or 48 h. The lysates (100 μg) were resolved by SDS-PAGE and probed with antibodies as indicated. The asterisk indicates the position of active Src. Phagocytosis of GFP-E. coli (B) or latex beads (C) in PBS-pretreated (−) or LPS-pretreated RAW, Ctrl, and 261-9 and -10 cells was done exactly as described above. The experiment was performed at least three times in triplicate. The results are means ± S.E. (error bars). **, p < 0.01; ***, p < 0.001, as compared with the corresponding control.
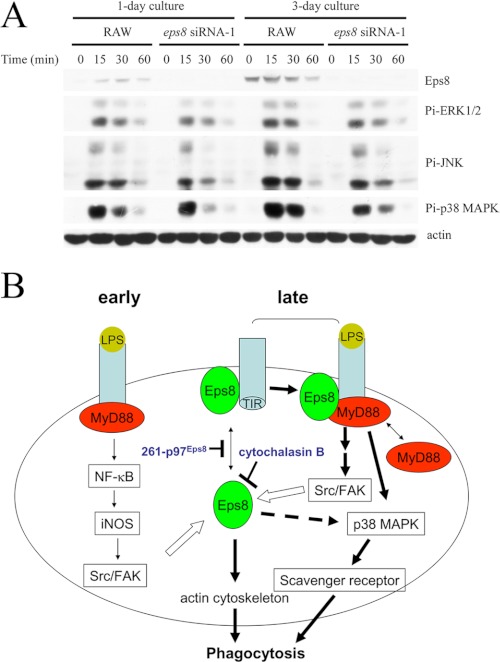
Eps8 Sustains TLR4-mediated Activation of p38 MAPK
Activation of MAPKs is one of the early events observed in LPS-exposed macrophages, and p38 MAPK plays a pivotal role in LPS-stimulated phagocytosis (9, 10). Given that Eps8 facilitated LPS-induced TLR4-MyD88 interaction in macrophages, its participation in TLR4-mediated activation of MAPKs in short term LPS stimulation was investigated. The basal Eps8 expression was low but could be elevated in longer cultured RAW cells, whose Src expression was still not affected (Fig. 8A) (data not shown). Along with Eps8 induction, enhanced activation of MAPKs, especially p38 MAPK, in response to LPS stimulation was observed (Fig. 8A). By contrast, attenuation of Eps8 hindered LPS-elicited activation of p38 MAPK (Fig. 8A). Interestingly, active p38 MAPK could still be detected in RAW and Ctrl cells after 24- and 48-h LPS stimulation but not in the Eps8-attenuated cells (siEps8-346-1 and -2; Fig. 3B). Suppressed p38 MAPK activation also occurred in LPS-stimulated PEMs expressing eps8 siRNA-503 (Fig. 3D) and RAW cells expressing either fak siRNA (Fig. 5E) or 261-p97Eps8 (Fig. 7A), whose Eps8 expression (Fig. 3E) or Eps8-TLR4 complex formation (Fig. 6F) was reduced. These findings indicated that Eps8 participated in TLR4-mediated activation of MAPKs, especially p38 MAPK, in macrophages.
FIGURE 8.
Eps8 promotes LPS-elicited activation of MAPKs in RAW264.7 cells. A, RAW264.7-derived Ctrl and siEps8–346-1 cells were cultured for 1 or 3 days and treated with LPS (100 ng/ml) for various times (i.e. 0, 15, 30, and 60 min). Lysates (100 μg) from these cells were Western immunoblotted with antibodies as indicated. B, a model of the signal transduction pathway of Eps8 in LPS-induced macrophage phagocytosis. The interaction between Eps8 and TLR4 in macrophages without LPS stimulation was rare (early). LPS engagement triggered the association between TLR4 and MyD88 and ignited the activation of Src and FAK in an iNOS-dependent manner. Then Src and FAK enhanced Eps8 expression and increased the portion of Eps8-associated TLR4 (late). Binding of Eps8 subsequently facilitated TLR4 interaction with MyD88. This complex, composed of at least TLR4, Eps8, and MyD88, might specifically promote the activation of p38 MAPK, leading to the expression of scavenger receptors and the enhancement of phagocytosis. In addition, elevated Eps8 might activate p38 MAPK via an uncharacterized but cytochalasin B-sensitive pathway. Finally, Eps8 might modulate actin cytoskeleton organization and facilitate the formation and maturation of phagosomes in LPS-treated macrophages. The closed arrows indicate increased activity of the target proteins, whereas the open arrow indicates protein induction. The double arrow indicates the distribution of Eps8 or MyD88 between cytosol and TLR4-containing complexes.
Complex Formation between Eps8 and FAK in RAW Cells
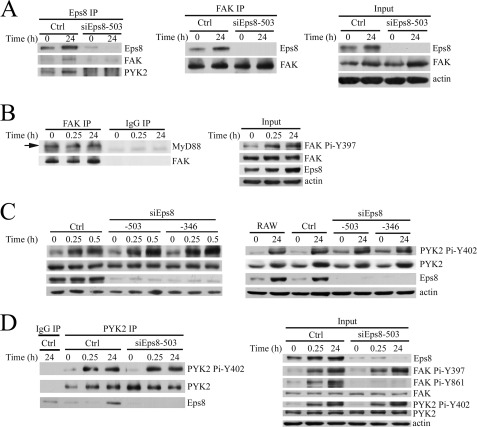
Because C-terminal FAK is capable of directly interacting with MyD88 in vitro (32) and FAK associates with Eps8 in colon cancer cells (22), we were interested to know whether FAK associated with Eps8 and MyD88 in RAW cells. As shown in Fig. 9A, FAK and Eps8 formed a complex in RAW cells. Interestingly, FAK was also associated with MyD88, and this association was not affected by LPS stimulation (Fig. 9B).
FIGURE 9.
Eps8 and FAK form a complex in RAW264.7 cells. A, RAW264.7-derived Ctrl and siEps8–503 cells were treated with LPS (100 ng/ml) for 0 or 24 h. Equal amounts (2.5 mg) of lysates prepared from these cells were immunoprecipitated with antibody against Eps8 (left) or FAK (middle). The lysates (Input) and the Eps8 or FAK immunocomplex from each sample were resolved by SDS-PAGE and Western immunoblotting with antibodies as indicated. B, RAW264.7 cells were treated with LPS (100 ng/ml) for various times. The lysates (Input) and the immunoprecipitates of FAK or control IgG were Western immunoblotted with antibodies as indicated. RAW264.7 and its derived Ctrl, siEps8-503, and siEps8-346 cells were treated with LPS (100 ng/ml) for various times. Their lysates (C; Input in D) and the immunoprecipitates of control IgG and PYK2 (D) were resolved by SDS-PAGE and Western immunoblotting with antibodies as indicated. IP, immunoprecipitation.
PYK2, a FAK-related kinase, is also present in monocytes/macrophages (32, 33) and has been implicated in cell adhesion-mediated interaction with Src in osteoclasts (34) and MyD88-mediated NF-κB activation in LPS/PGN-treated macrophages (32). LPS increases PYK2 Pi-Y402, which is important for regulating PYK2-MyD88 direct interaction and transmits the downstream signaling to activate NF-κB (32). We are interested to know whether Eps8 regulates PYK2 Pi-Y402. Attenuation of Eps8 did not affect the level of PYK2 Pi-Y402 in LPS-exposed RAW cells (Fig. 9C). However, PYK2, like FAK, was detected in the Eps8 immunocomplex (Fig. 9A). And vice versa, Eps8 was present in the PYK2 immunoprecipitates (Fig. 9D).
Attenuation of Eps8 Impairs Intracellular Killing of E. coli in Macrophages
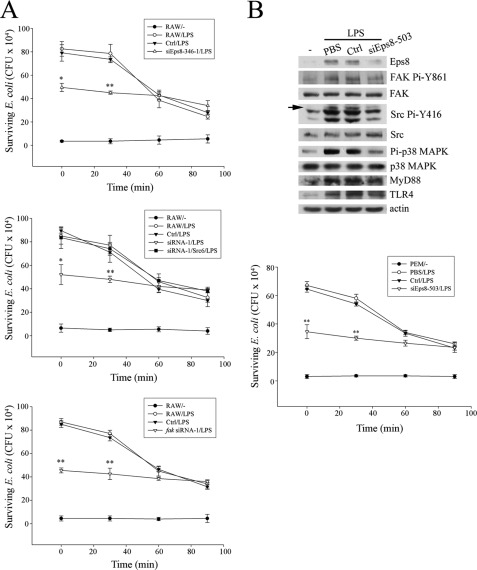
Besides bacterial uptake, the involvement of Eps8, Src, and FAK in the regulation of the bacterium-killing ability of macrophages was also investigated. Here, GFP-E. coli were incubated with either non-stimulated cells or LPS-stimulated RAW cells and their derived cells (i.e. Ctrl, eps8 siRNA-346-1, src siRNA-1, src siRNA-1/Src6, and fak siRNA-1) at an MOI of 100 at 37 °C for 10 min. After washing with PBS, the infected cells were either directly lysed (0 min) to measure the uptake of bacteria or further cultured for various durations (30, 60, and 90 min) and then lysed to assess viable bacteria remaining as cfu. As shown in Fig. 10A, knockdown of Eps8 (top), Src (middle), and FAK (bottom) reduced LPS-induced phagocytosis in RAW cells. More than 60% of bacteria were killed in RAW cells and the Ctrl cells within 90 min of incubation. In contrast, only 31, 25, and 20% of bacteria were killed in Eps8-, Src-, and FAK-attenuated cells, respectively. A similar experiment was also applied to PEMs infected by lentivirus-encoding eps8 siRNA-503 (siEps8–503), and impaired bacterial killing activity evoked by LPS was observed (Fig. 10B). Thus, Eps8, Src, and FAK were in the same axis required for both the uptake and killing of bacteria in macrophages exposed to LPS.
FIGURE 10.
Attenuation of Eps8, Src, or FAK decreases bacterium killing activity of macrophages. A, equal numbers (5 × 105) of RAW cells and their derived Ctrl cells, Eps8-attenuated cells (siEps8-346-1), Src-attenuated cells (siRNA-1), Src-attenuated cells expressing avian c-Src (siRNA-1/Src6), or FAK-attenuated cells (fak siRNA-1) were pretreated with PBS (−) or LPS (100 ng/ml) for 24 h. The assessment of phagocytosis of E. coli and the following bacterium killing in these cells is described under “Experimental Procedures.” B, PEMs (1 × 106) prepared from C57BL/6 mice were infected with lentivirus (MOI = 3) encoding luciferase siRNA (Ctrl) or eps8 siRNA (siEps8-503). After 2 days, PEMs were stimulated without (−) or with LPS (100 ng/ml) for 24 h. The lysates (100 μg) were resolved by SDS-PAGE and probed with antibodies as indicated. The arrow indicates the position of active Src. Bacterium killing activity of Ctrl and siEps8-503 cells was performed exactly as described above. The results in the A and B are means ± S.D. (error bars). *, p < 0.05; **, p < 0.01, as compared with the corresponding control. Experiments were repeated three times in triplicate, and representative results are shown.
DISCUSSION
Macrophages are major phagocytes of the innate immune system. They are equipped with diverse receptors involved in the recognition and binding of pathogens on the cell surface. These receptors include TLRs, integrins, FcγRs, scavenger receptors and lectins that can recognize and engulf large particles. Phagocytosis is tightly regulated by complex rearrangements of actin cytoskeleton in response to microbial-host cell interaction (4, 35). Although a great number of actin-regulatory proteins localized to phagocytic uptake structures have been identified (36, 37), the molecular mechanism by which TLRs elicit signals leading to the activation of these proteins is still unclear. Given that Src is an actin cytoskeleton regulator whose expression and activation can be greatly augmented by long term LPS treatment (24), its involvement in the elevated phagocytic ability of LPS-primed macrophages is investigated. In this study, we first confirmed the requirement of TLR4 in increased Src expression and activation in macrophages following LPS exposure (Fig. 1). The importance of Src in phagocytosis was evidenced by its attenuation reduced the uptake of E. coli and latex beads, whereas its re-expression rescued this defect (Fig. 2). In contrast, the level of the myeloid Src family kinases was not significantly affected by LPS-TLR4 activation (Fig. 1C), and knockdown of Lyn did not affect LPS-stimulated bacterial uptake (data not shown).
As a substrate of Src, FAK localizes prominently within focal adhesion and is well documented to play a role in integrin-mediated cytoskeleton organization, which is required for many cellular processes, including motility and phagocytosis. In insect hemocytes, antibody-mediated inhibition of Src and FAK has been shown to abolish FAK phosphorylation and phagocytosis of E. coli (38). In retina, daily αvβ5 integrin-dependent phagocytosis of spent photoreceptor outer segment fragments by the retinal pigment epithelium was crucial for retinal function, and FAK was pivotal in this process (39). Besides, FAK was involved in low affinity FcγRII-III-mediated phagocytosis (40) as well as in Yersinia uptake by macrophages (31). Herein, we demonstrated that FAK was pivotal in LPS-elicited phagocytosis. This was supported by the simultaneous increment of Src and FAK Pi-Y861 in LPS-treated macrophages (Figs. 1C and 2A), and the depletion of FAK impaired LPS-triggered uptake of GFP-E. coli (Fig. 5). Like Src, attenuation of FAK suppressed LPS-mediated Eps8 expression and the uptake of E. coli without affecting the level of eps8 transcript (Fig. 5), which suggested that Eps8 might be a downstream mediator of FAK in macrophage phagocytosis.
A recent study (41) indicated that the actin-capping activity of Eps8 was essential for dendritic cells to polarize and to form elongated migratory protrusions. However, its role in immunity has not yet been fully defined. Here, we demonstrated that augmented Eps8 expression occurred not only in LPS-treated RAW but also in PEMs recovered from the LPS-challenged rats (Fig. 3A). This Eps8 overexpression required TLR4 (Fig. 1C) and MyD88 (Fig. 6, D and E) in addition to Src and FAK. Furthermore, Eps8 knockdown markedly inhibited LPS-initiated activation of Src and FAK (as reflected by Src Pi-Y416 and FAK Pi-Y861) (Fig. 3B) and phagocytosis (Fig. 3, C and D). Thus, the intimate relationship among Src, FAK, and Eps8 observed in fibroblasts and epithelial cells (22) was also present in LPS-stimulated macrophages and contributed to the promotion of phagocytosis.
Reduced activity of Src and FAK in Eps8-attenuated RAW cells suggested that Eps8 might directly regulate the activity of both kinases as reported previously (22, 42). Given that cytochalasin B exerted no effect on the activity of Src and FAK but significantly inhibited LPS-elicited Eps8 expression as well as phagocytosis of GFP-E. coli (Fig. 4), an alternative pathway must be present in relaying the Eps8-mediated Src/FAK activation. In addition, the expression of Eps8 was not significantly altered in LPS-treated macrophages in response to 2.5 and 10 μm cytochalasin B (Fig. 4B). Therefore, two populations of Eps8 were expected to exist in the cells (i.e. one was cytochalasin B-sensitive, and the other was cytochalasin B-inert). The cytochalasin B-sensitive Eps8 regulated the dynamic of actin filaments that might stabilize Eps8 in return, whereas the cytochalasin B-inert Eps8 seemed to elicit TLR4/MyD88-induced Src, Src Pi-Y416, and FAK Pi-Y861. Consistent with this speculation, co-localization of Eps8 and TLR4 was found in the cytosol and in the phagosome (Fig. 6A), and their presence in the same immunocomplex in either LPS-untreated or -treated macrophages (Fig. 6B) further suggested that Eps8 might regulate the LPS/TLR4-triggered signal transduction leading to the activation of Src and FAK. Concurrently, Eps8 attenuation not only suppressed its interaction with TLR4 but also reduced the interaction of MyD88 with TLR4 in LPS-primed RAW264.7 cells (Fig. 6C), indicating that Eps8-TLR4 either facilitated or stabilized the interaction between TLR4 and MyD88. Like Eps8 attenuation, overexpression of 261-p97Eps8 in RAW cells reduced LPS-evoked complex formation of TLR4-MyD88 (Fig. 6F) and the following increase of Src Pi-Y416 and FAK Pi-Y861 (Fig. 7A) as well as the uptake of either bacteria (Fig. 7B) or latex beads (Fig. 7C). In comparison with Eps8, ectopic 261-p97Eps8 exhibited stronger interaction with TLR4 and hampered the association between TLR4 and Eps8 (Fig. 6F). Apparently, the presence of N-terminal PH-containing domain was important for Eps8 in promoting the interaction between TLR4 and MyD88, Src/FAK enzymatic activity, and the uptake of bacteria in LPS-treated macrophages. Of note, the increased survival of bacteria engulfed by Eps8-attenuated macrophages (Fig. 10) further supported the role of Eps8 in relaying activated TLR4-ignited signals for the maturation of phagosomes and eradication of bacteria.
FAK and PYK2 are two closely related kinases. Both were able to form a complex with Eps8 (Fig. 9). Remarkably, like PYK2 (32), FAK could associate with MyD88 but was not affected by LPS stimulation in RAW cells (Fig. 9B). It should be noted that autophosphorylation of either FAK Tyr-397 or PYK2 Tyr-402 occurred in the very early phase of LPS stimulation and was not influenced by Eps8 knockdown in RAW cells (Fig. 9). Hence, Eps8-mediated macrophage phagocytosis was unlikely through either FAK Pi-Y397 or PYK2 Pi-Y402. Because the C terminus of both tyrosine kinases could directly associate with MyD88 in vitro, we cannot rule out the possibility that FAK and/or PYK2 is required for Eps8-mediated TLR4-MyD88 interaction that results in phagocytosis. Further investigation is required to clarify this issue.
Via MyD88 and IRAK4, TLR-mediated activation of p38 MAPK led to the induction of several phagocytic proteins, including scavenger receptors, which were important for macrophage phagocytosis (9) as well as accelerating TLRs-mediated phagosome maturation (43). By virtue of increasing TLR4-MyD88 interaction, Eps8 specifically relayed a signal to activate p38 MAPK and promote phagocytosis. This was evidenced by the decrement of LPS-induced activation of p38 MAPK caused by Eps8 depletion (Figs. 3B and 8A) and the expression of 261-p97Eps8 (Fig. 7A). Attenuation of Src (Fig. 2A) or FAK (Fig. 5) also suppressed LPS-triggered activation of p38 MAPK, which further supported the importance of Eps8 in positively regulating the TLR4-MyD88-p38MAPK pathway. Due to engagement of various TLRs that activated Src and FAK (27) and increased Eps8 expression (data not shown), Eps8 was speculated to participate in phagocytosis mediated by TLRs other than TLR4.
Taken together with all of the results, a model is proposed and described in Fig. 8B. As depicted in this model, Eps8-TLR4 interaction is rare under normal physiological conditions. In the early phase, LPS engagement triggers the association between TLR4 homodimers (11) and MyD88 and subsequently initiates the activation of Src and FAK in an iNOS-dependent manner (27). Then Src and FAK enhance Eps8 expression and increase the portion of TLR4 associated with Eps8. Binding of Eps8 may alter the conformation of TLR4 and expose its Toll/IL-1 receptor domain for TLR4-TLR4 dimerization and the binding of Toll/IL-1 receptor domain-containing adaptor proteins, such as MyD88 (44). Alternatively, via interaction with FAK (and/or PYK2), elevated Eps8 pulls TLR4 and MyD88 together. Then the complex, composed of TLR4, Eps8, FAK, and MyD88, activates p38 MAPK and promotes phagocytosis. On the other hand, overexpression of PH-truncated 261-p97Eps8 may inhibit this complex formation. In addition, attenuation of either Src or FAK reduced Eps8 expression and p38 MAPK activity in LPS-treated RAW cells. Notably, by virtue of an uncharacterized mechanism, cytochalasin B-sensitive Eps8 could also activate p38 MAPK and phagocytosis in LPS-exposed macrophages. The co-existence of Eps8 and TLR4 in phagosomes suggested that these two proteins might also be directly involved in the formation and maturation of phagosomes that warrant further investigation.
Wiskott-Aldrich syndrome proteins (WASP) and WASP family verprolin-homologous protein (WAVE) family proteins are scaffolds that regulate reorganization of the actin cytoskeleton in response to extracellular stimuli (45). All of the WASP (i.e. WASP and N-WASP) and WAVE family members (i.e. WAVE1, -2, and -3) have a verprolin homology, cofilin homology, acidic (VCA) module at their C terminus through which Arp2/3 complex is activated to nucleate actin polymerization (45). As compared with their family members, WASP and WAVE2 are predominantly expressed in macrophages (46, 47). Whereas WASP is required for β1 integrin-mediated actin reorganization during phagocytosis of pathogens (48), WAVE2 is indispensable for mobilization that needs the participation of Abi1 and IRSp53 in CSF-1-stimulated macrophages (47, 49). Interestingly, to date, no paper concerning the role of WAVE2 in macrophage phagocytosis has been published. Given that Abi1 and IRSp53 are the interacting partners of Eps8 and that Eps8 is involved in LPS-mediated phagocytosis, these findings without doubt open a new area of investigation regarding the contribution of WAVE2 in phagocytosis. Furthermore, how Eps8 cooperates with its modulators to alter cytoskeleton and achieve phagocytosis will also require further studies.
In conclusion, our study showed that Src and its interacting partner Eps8 were induced in LPS-treated macrophages. This induction facilitated TLR4/MyD88-mediated activation of Src, FAK, and p38 MAPK that led to the increased phagocytic activity and bacterial killing effect of macrophages. Our data revealed previously unrecognized roles of Eps8 in macrophage physiology and provided a potential therapeutic target for treatment of a spectrum of inflammatory and infectious diseases.
Supplementary Material
Acknowledgments
We thank Dr. C. H. A. Cheung for critical comments on the manuscript. The lentivirus encoding luciferase siRNA or eps8 siRNA-503 and the DNA constructs of pLKO.1-shLuc, pLKO.1-meps8-503, pLKO.1-meps8-1847, pLKO.1-mfak-1, pLKO.1-mfak-2, pLKO.1-mmyd88-1, and pLKO.1-mmyd88-2 were provided by the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by National Research Program for Genomic Medicine, National Science Council Grants NSC94-3112-B-001-003 and NSC94-3112-B-001-018-Y.
This work was supported in part by National Health Research Institute Grant NHRI-EX101-10131BI (to T.-H. L.); National Science Council Grants NSC100-2325-B-006-011 (to T.-H. L.) and NSC98-2311-B-039-002-MY3 (to M.-C. M.); Comprehensive Cancer Center in Southern Taiwan Grant DOH100-TD-C-111-003 (to T.-H. L.), Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH101-TD-B-111-004 (to M.-C. M.), and China Medical University Grant CMU97-193 (to M.-C. M.).

This article contains supplemental Figs. S1 and S2.
- FcγR
- Fc γ receptor
- BMDM
- bone marrow-derived macrophage
- MOI
- multiplicity of infection
- PEM
- peritoneal macrophage
- PH
- pleckstrin homology
- TLR
- Toll-like receptor
- WASP
- Wiskott-Aldrich syndrome protein(s)
- WAVE
- WASP family verprolin-homologous protein
- FAK
- focal adhesion kinase
- Ctrl
- control
- RAW
- RAW264.7
- iNOS
- inducible nitric-oxide synthase.
REFERENCES
- 1. Brown E. J. (1995) Phagocytosis. BioEssays 17, 109–117 [DOI] [PubMed] [Google Scholar]
- 2. Gillespie J. P., Kanost M. R., Trenczek T. (1997) Biological mediators of insect immunity. Annu. Rev. Entomol. 42, 611–643 [DOI] [PubMed] [Google Scholar]
- 3. Underhill D. M., Ozinsky A. (2002) Phagocytosis of microbes. Complexity in action. Annu. Rev. Immunol. 20, 825–852 [DOI] [PubMed] [Google Scholar]
- 4. Aderem A., Underhill D. M. (1999) Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623 [DOI] [PubMed] [Google Scholar]
- 5. Greenberg S., Grinstein S. (2002) Phagocytosis and innate immunity. Curr. Opin. Immunol. 14, 136–145 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T., Akira S. (2009) The roles of TLRs, RLRs, and NLRs in pathogen recognition. Int. Immunol. 21, 317–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Underhill D. M., Gantner B. (2004) Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 6, 1368–1373 [DOI] [PubMed] [Google Scholar]
- 8. Beutler B. (2002) Toll-like receptors. How they work and what they do. Curr. Opin. Hematol. 9, 2–10 [DOI] [PubMed] [Google Scholar]
- 9. Doyle S. E., O'Connell R. M., Miranda G. A., Vaidya S. A., Chow E. K., Liu P. T., Suzuki S., Suzuki N., Modlin R. L., Yeh W. C., Lane T. F., Cheng G. (2004) Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kong L., Ge B. X. (2008) MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 18, 745–755 [DOI] [PubMed] [Google Scholar]
- 11. Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 12. Maa M. C., Hsieh C. Y., Leu T. H. (2001) Overexpression of p97Eps8 leads to cellular transformation. Implication of pleckstrin homology domain in p97Eps8-mediated ERK activation. Oncogene 20, 106–112 [DOI] [PubMed] [Google Scholar]
- 13. Di Fiore P. P., Scita G. (2002) Eps8 in the midst of GTPases. Int. J. Biochem. Cell Biol. 34, 1178–1183 [DOI] [PubMed] [Google Scholar]
- 14. Scita G., Nordstrom J., Carbone R., Tenca P., Giardina G., Gutkind S., Bjarnegård M., Betsholtz C., Di Fiore P. P. (1999) EPS8 and E3B1 transduce signals from Ras to Rac. Nature 401, 290–293 [DOI] [PubMed] [Google Scholar]
- 15. Scita G., Tenca P., Areces L. B., Tocchetti A., Frittoli E., Giardina G., Ponzanelli I., Sini P., Innocenti M., Di Fiore P. P. (2001) An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J. Cell Biol. 154, 1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Funato Y., Terabayashi T., Suenaga N., Seiki M., Takenawa T., Miki H. (2004) IRSp53-Eps8 complex is important for positive regulation of Rac and cancer cell motility/invasiveness. Cancer Res. 64, 5237–5244 [DOI] [PubMed] [Google Scholar]
- 17. Disanza A., Carlier M. F., Stradal T. E., Didry D., Frittoli E., Confalonieri S., Croce A., Wehland J., Di Fiore P. P., Scita G. (2004) Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat. Cell Biol. 6, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 18. Croce A., Cassata G., Disanza A., Gagliani M. C., Tacchetti C., Malabarba M. G., Carlier M. F., Scita G., Baumeister R., Di Fiore P. P. (2004) A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat. Cell Biol. 6, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 19. Fazioli F., Minichiello L., Matoska V., Castagnino P., Miki T., Wong W. T., Di Fiore P. P. (1993) Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 12, 3799–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maa M. C., Lai J. R., Lin R. W., Leu T. H. (1999) Enhancement of tyrosyl phosphorylation and protein expression of eps8 by v-Src. Biochim. Biophys. Acta 1450, 341–351 [DOI] [PubMed] [Google Scholar]
- 21. Leu T. H., Yeh H. H., Huang C. C., Chuang Y. C., Su S. L., Maa M. C. (2004) Participation of p97Eps8 in Src-mediated transformation. J. Biol. Chem. 279, 9875–9881 [DOI] [PubMed] [Google Scholar]
- 22. Maa M. C., Lee J. C., Chen Y. J., Lee Y. C., Wang S. T., Huang C. C., Chow N. H., Leu T. H. (2007) Eps8 facilitates cellular growth and motility of colon cancer cells by increasing the expression and activity of focal adhesion kinase. J. Biol. Chem. 282, 19399–19409 [DOI] [PubMed] [Google Scholar]
- 23. Liu P. S., Jong T. H., Maa M. C., Leu T. H. (2010) The interplay between Eps8 and IRSp53 contributes to Src-mediated transformation. Oncogene 29, 3977–3989 [DOI] [PubMed] [Google Scholar]
- 24. Maa M. C., Chang M. Y., Chen Y. J., Lin C. H., Yu C. J., Yang Y. L., Li J., Chen P. R., Tang C. H., Lei H. Y., Leu T. H. (2008) Requirement of inducible nitric-oxide synthase in lipopolysaccharide-mediated Src induction and macrophage migration. J. Biol. Chem. 283, 31408–31416 [DOI] [PubMed] [Google Scholar]
- 25. Schnare M., Barton G. M., Holt A. C., Takeda K., Akira S., Medzhitov R. (2001) Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2, 947–950 [DOI] [PubMed] [Google Scholar]
- 26. Leu T. H., Charoenfuprasert S., Yen C. K., Fan C. W., Maa M. C. (2006) Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNFα secretion in macrophages. Mol. Immunol. 43, 308–316 [DOI] [PubMed] [Google Scholar]
- 27. Maa M. C., Chang M. Y., Li J., Li Y. Y., Hsieh M. Y., Yang C. J., Chen Y. J., Li Y., Chen H. C., Cheng W. E., Hsieh C. Y., Cheng C. W., Leu T. H. (2011) The iNOS/Src/FAK axis is critical in Toll-like receptor-mediated cell motility in macrophages. Biochim. Biophys. Acta 1813, 136–147 [DOI] [PubMed] [Google Scholar]
- 28. Maa M. C., Leu T. H. (1998) Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem. Biophys. Res. Commun. 251, 344–349 [DOI] [PubMed] [Google Scholar]
- 29. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice. Mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 30. Defilippi P., Di Stefano P., Cabodi S. (2006) p130Cas. A versatile scaffold in signaling networks. Trends Cell Biol. 16, 257–263 [DOI] [PubMed] [Google Scholar]
- 31. Owen K. A., Thomas K. S., Bouton A. H. (2007) The differential expression of Yersinia pseudotuberculosis adhesins determines the requirement for FAK and/or Pyk2 during bacterial phagocytosis by macrophages. Cell Microbiol. 9, 596–609 [DOI] [PubMed] [Google Scholar]
- 32. Xi C. X., Xiong F., Zhou Z, Mei L., Xiong W. C. (2010) PYK2 interacts with MyD88 and regulates MyD88-mediated NF-κB activation in macrophages. J. Leukoc. Biol. 87, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duong L. T., Nakamura I., Lakkakorpi P. T., Lipfert L., Bett A. J., Rodan G. A. (2001) Inhibition of osteoclast function by adenovirus expressing antisense protein-tyrosine kinase 2. J. Biol. Chem. 276, 7484–7492 [DOI] [PubMed] [Google Scholar]
- 34. Lakkakorpi P. T., Bett A. J., Lipfert L., Rodan G. A., Duong le T. (2003) PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 278, 11502–11512 [DOI] [PubMed] [Google Scholar]
- 35. May R. C., Caron E., Hall A., Machesky L. M. (2000) Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2, 246–248 [DOI] [PubMed] [Google Scholar]
- 36. Allen L. H., Aderem A. (1995) A role for MARCKS, the α isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J. Exp. Med. 182, 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Massol P., Montcourrier P., Guillemot J. C., Chavrier P. (1998) Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 17, 6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Metheniti A., Paraskevopoulou N., Lambropoulou M., Marmaras V. J. (2001) Involvement of FAK-Src complex in the processes of Escherichia coli phagocytosis by insect hemocytes. FEBS Lett. 496, 55–59 [DOI] [PubMed] [Google Scholar]
- 39. Finnemann S. C. (2003) Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 22, 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antonieta Cote-Vélez M. J., Ortega E., Ortega A. (2001) Involvement of pp125FAK and p60SRC in the signaling through Fc γ RII-Fc γ RIII in murine macrophages. Immunol. Lett. 78, 189–194 [DOI] [PubMed] [Google Scholar]
- 41. Frittoli E., Matteoli G., Palamidessi A., Mazzini E., Maddaluno L., Disanza A., Yang C., Svitkina T., Rescigno M., Scita G. (2011) The signaling adaptor Eps8 is an essential actin-capping protein for dendritic cell migration. Immunity 35, 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y. J., Shen M. R., Maa M. C., Leu T. H. (2008) Eps8 decreases chemosensitivity and affects survival of cervical cancer patients. Mol. Cancer Ther. 7, 1376–1385 [DOI] [PubMed] [Google Scholar]
- 43. Blander J. M., Medzhitov R. (2004) Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018 [DOI] [PubMed] [Google Scholar]
- 44. Watters T. M., Kenny E. F., O'Neill L. A. (2007) Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol. Cell Biol. 85, 411–419 [DOI] [PubMed] [Google Scholar]
- 45. Takenawa T., Suetsugu S. (2007) The WASP-WAVE protein network. Connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 [DOI] [PubMed] [Google Scholar]
- 46. Jones G. E., Zicha D., Dunn G. A., Blundell M., Thrasher A. (2002) Restoration of podosomes and chemotaxis in Wiskott-Aldrich syndrome macrophages following induced expression of WASp. Int. J. Biochem. Cell Biol. 34, 806–815 [DOI] [PubMed] [Google Scholar]
- 47. Kheir W. A., Gevrey J. C., Yamaguchi H., Isaac B., Cox D. (2005) A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich membrane protrusions and migration in macrophages. J. Cell Sci. 118, 5369–5379 [DOI] [PubMed] [Google Scholar]
- 48. Wiedemann A., Linder S., Grassl G., Albert M., Autenrieth I., Aepfelbacher M. (2001) Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol. 3, 693–702 [DOI] [PubMed] [Google Scholar]
- 49. Abou-Kheir W., Isaac B., Yamaguchi H., Cox D. (2008) Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization. A role for IRSp53 in mediating the interaction between Rac and WAVE2. J. Cell Sci. 121, 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.