This review provides an overview of the known genetic causes of isolated GnRH deficiency and describes the emerging role played by prokineticin 2 (PROK2) and its receptor, prokineticin receptor 2 (PROKR2) in the neuroendocrine control of reproduction. A rich vein of mutations in PROK2 and PROKR2 has been identified in humans with isolated GnRH deficiency. A majority of these mutations are in the heterozygous state and incomplete penetrance or variable expressivity is typically seen within and across these GnRH deficient pedigrees. The molecular features of the prokineticin family are reviewed and the physiological insights that have been derived by the study of gene mutations in the prokineticin 2 pathway identified in humans and mice are discussed.
Abstract
A widely dispersed network of hypothalamic GnRH neurons controls the reproductive axis in mammals. Genetic investigation of the human disease model of isolated GnRH deficiency has revealed several key genes crucial for GnRH neuronal ontogeny and GnRH secretion. Among these genes, prokineticin 2 (PROK2), and PROK2 receptor (PROKR2) have recently emerged as critical regulators of reproduction in both mice and humans. Both prok2- and prokr2-deficient mice recapitulate the human Kallmann syndrome phenotype. Additionally, PROK2 and PROKR2 mutations are seen in humans with Kallmann syndrome, thus implicating this pathway in GnRH neuronal migration. However, PROK2/PROKR2 mutations are also seen in normosmic GnRH deficiency, suggesting a role for the prokineticin signaling system in GnRH biology that is beyond neuronal migration. This observation is particularly surprising because mature GnRH neurons do not express PROKR2. Moreover, mutations in both PROK2 and PROKR2 are predominantly detected in the heterozygous state with incomplete penetrance or variable expressivity frequently seen within and across pedigrees. In some of these pedigrees, a “second hit” or oligogenicity has been documented. Besides reproduction, a pleiotropic physiological role for PROK2 is now recognized, including regulation of pain perception, circadian rhythms, hematopoiesis, and immune response. Therefore, further detailed clinical studies of patients with PROK2/PROKR2 mutations will help to map the broader biological role of the PROK2/PROKR2 pathway and identify other interacting genes/proteins that mediate its molecular effects in humans.
I. Introduction
In mammalian species studied to date, a sparsely populated yet widely dispersed network of approximately 1500 GnRH neurons residing in the hypothalamus synchronize and coordinate the synthesis of GnRH and thus serve as the body's “pilot light of reproduction” (1). Studies in rodents show that the majority of GnRH neurons reside in the medial preoptic area of the hypothalamus and project their axonal processes into the median eminence of the hypothalamus (2). Coordinated by unknown mechanisms across this neural network, GnRH is secreted in a pulsatile fashion into the hypophyseal portal circulation (3). GnRH neurons are thus strategically positioned in the hypothalamus (e.g., medial preoptic area) to facilitate the receipt of inputs from both external and internal cues. The GnRH neuronal network then integrates this information to maximize the organism's reproductive efficiency by economizing efforts and delaying sexual maturation and fertility until optimal environmental and metabolic conditions exist for bearing and nourishing offspring.
Several human studies have clearly demonstrated that pulsatile GnRH secretion is fully active in utero, continues well into the neonatal period and early infancy, is silenced during childhood, is reactivated at adolescence signaling puberty, and then is maintained during adult reproductive life (4–9). Upon secretion, GnRH binds to its receptor [GnRH receptor (GnRHR)] on the anterior pituitary gonadotropes and stimulates both the synthesis and secretion of the two pituitary dimeric glycoprotein hormones, LH and FSH. These gonadotropic hormones then functionally bifurcate the gonads into their steroidogenic components (Leydig cells in males, and thecal cells in females) that bear LH receptors and the gametogenic supporting cells (Sertoli cells in males, and granulosa cells in females) that bear FSH receptors. Collaboratively, these gonadotropins and their respective gonadal compartments govern the appearance of secondary sexual characteristics and ultimately maturation of the germ cells in both sexes.
Defective development of the GnRH neuronal network or impaired biosynthesis, secretion, and/or action of GnRH results in the clinical syndrome of isolated GnRH deficiency. Clinically, this condition can present either as Kallmann syndrome (KS) when it is associated with anosmia, thus signaling a developmental defect in olfactory bulb neurogenesis and GnRH neuronal migration or as normosmic idiopathic hypogonadotropic hypogonadism (nIHH) when olfaction is normal, indicating a functional defect at the level of the hypothalamo-pituitary axis. In the last decade, considerable progress has been made by leveraging genetic approaches in human GnRH deficiency that have permitted a dissection of the genetic cascade underlying GnRH ontogeny in humans (10). Significant insights into the embryological fate specification, migration, secretion, and action of GnRH neurons have resulted from these discoveries. Recently, a rich vein of mutations in a novel ligand-receptor family, prokineticin 2 (PROK2) and its receptor, prokineticin receptor 2 (PROKR2), have been described in subjects with GnRH deficiency (11–18). This review will provide an overview of the known genetic causes of isolated GnRH deficiency and describe the emerging role played by the prokineticin 2 pathway in the neuroendocrine control of reproduction.
II. Genetic Causes of Isolated GnRH Deficiency in Humans
A. Historical overview of isolated GnRH deficiency
In 1849, Aureliano Maestre de San Juan, a Spanish pathologist, first documented the association of hypogonadism and absence of the olfactory system during the autopsy of a 40-yr-old man (19). Subsequently, postmortem documentation of the association of hypogonadism with defective olfactory system was also reported by Weidenreich in 1914 (20) and Altmann in 1930 (21). Almost 100 yr from its first documentation, Franz J. Kallmann identified the symptoms of hypogonadism and anosmia as a clinical entity and noted its familial nature implying a genetic etiology (22). Since then, GnRH deficiency with the presence of anosmia has been designated as Kallmann syndrome (KS).
In the following decade, De Morsier expanded these observations and confirmed the inherited nature of this disorder that he termed “olfactogenital dysplasia” (23). Multiple lines of investigations in the early 20th century laid the foundations for understanding the role of the hypothalamus and pituitary in regulation of gonadal function in humans. With urinary gonadotropin assays initially (24) and later with the availability of sensitive RIAs to measure LH and FSH (25–27), it was recognized that some patients with hypogonadism had inappropriately low levels of gonadotropins despite their hypogonadal state. Because the level of all other anterior pituitary hormones was normal, the condition was initially referred to as isolated gonadotropin deficiency (28, 29). The seminal discovery of GnRH in 1971 (30, 31) permitted the observation of variable pituitary gonadotropin responses to single boluses of ovine (32) or synthetic GnRH (28, 29) in some patients with isolated gonadotropin deficiency and suggested a hypothalamic defect in these patients. Eventually, the hypothesis of a hypothalamic defect was confirmed when a physiological regimen of exogenous pulsatile GnRH was shown to be both necessary and sufficient to trigger puberty in these patients (29, 32–34).
B. Genetic causes of isolated GnRH deficiency
The familial nature of GnRH deficiency was evident from the earliest studies (22, 35–39). Three possible modes of transmission for isolated GnRH deficiency were soon recognized (OMIM 308700, 147950, and 146110): autosomal recessive, autosomal dominant, and X-linked transmission. The study of an Italian pedigree in which five of six males were affected by ichthyosis and KS offered the first genetic clue for the etiology of GnRH deficiency. All affected subjects showed decreased steroid sulfatase activity, an enzyme coded by a gene located in the distal short arm of chromosome X (40, 41). Subsequently, Bick et al. (42) identified a terminal deletion of the X chromosome with a breakpoint at Xp22.3 in a male infant with KS, ichthyosis, X-linked recessive chondrodysplasia punctata, and choanal atresia, a deletion the infant inherited from his unaffected mother. The child subsequently died, and an autopsy revealed complete absence of the olfactory bulbs and associated tracts, choanal atresia, and a horseshoe kidney. A second male child was then conceived by the same mother. An amniocentesis showed an identical defective X-chromosome, and the pregnancy was terminated at 19 wk gestation. Postmortem examination of the fetus revealed disruption of the olfactory system associated with arrested migration of GnRH neurons into the brain at the level of the cribriform plate (43). Importantly, previous murine studies had already demonstrated that GnRH neurons originate outside the central nervous system in the medial olfactory placode during embryological development and then subsequently migrate to the hypothalamus using the olfactory system to guide their migration (44, 45). Thus, these two brothers with KS initiated the genetic era of unraveling the link between olfaction and reproduction. Two independent groups subsequently identified the first KS gene, KAL1, by positional cloning of the distal portion of the X-chromosome (Xp22.3) (46, 47).
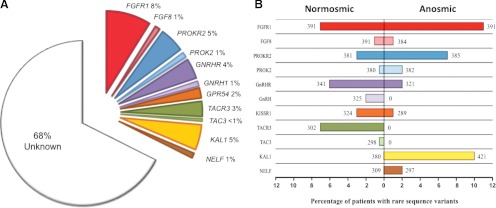
After this landmark discovery of KAL1, several autosomal genes have now been linked to isolated GnRH deficiency. To date, roughly 32% of a large cohort of GnRH-deficient patients (n = 397) at the Massachusetts General Hospital have been linked to at least one gene mutation known to cause human GnRH deficiency (Fig. 1, A and B). This cohort covers a broad clinical spectrum of reproductive phenotypes including: 1) a mild defect of GnRH secretion altering only timing of puberty (delayed puberty); 2) an intermediary or developmental defect presenting as spontaneous puberty with subsequent development of permanent hypogonadism (acquired hypogonadotropic hypogonadism); and 3) a severe defect with complete/partial absence of puberty (48–50). Likewise, GnRH-deficient patients also display a broad spectrum of nonreproductive phenotypes including facial midline defects, renal agenesis, and skeletal abnormalities that can provide key clues as to the underlying causal gene (Table 1). Early developmental genes such as KAL1, FGF8, FGFR1, NELF, CHD7, PROK2, and PROKR2 play a critical role in embryonic neuronal development, and subjects with mutations in these genes present primarily with KS. In addition, these subjects often manifest other associated developmental anomalies (e.g., cleft lip/palate seen with FGFR1 mutations). In contrast, subjects with mutations in genes such as KISS1R (the receptor for the hypothalamic neuropeptide kisspeptin, which plays a critical role as gatekeeper of puberty), GnRH1, GnRHR, TAC3, and TACR3 present primarily with nIHH. Interestingly, some adults with nIHH also harbor mutations in the early developmental genes (FGF8, FGFR1, PROK2, and PROKR2), suggesting an additional role for these genes in regulation of GnRH function in adulthood (Fig. 1, A and B). In addition, mutations in some genes (e.g., LEP, LEPR) also result in isolated GnRH deficiency as part of more complex syndromic presentations. All of these genes are listed in Tables 1 and 2 and are reviewed extensively in other publications (51–56). This review will specifically focus on the role of the newly identified genes, PROK2 and PROKR2, in the neuroendocrine control of reproduction.
Fig. 1.
Genetic causes of isolated GnRH deficiency in a large series of patients at the Massachusetts General Hospital. A, Percentage of patients (n = 397) with isolated GnRH deficiency that harbor rare sequence variants of known genes. B, Histogram of percentage of patients with isolated GnRH deficiency harboring rare sequence variants in each known gene displayed according to their olfactory phenotype. Percentage of patients with normal sense of smell is shown on the left of y-axis, and percentage of patients with anosmia is shown on the right of y-axis. The total number of patients who have been screened for each gene is given on either side of the bar corresponding to their olfactory phenotype. Note that the total cohort of patients shown in panel B is larger than in panel A for some genes (i.e., FGFR1, FGF8, PROK2, and PROKR2). FGFR1, Fibroblast growth factor receptor; FGF8, fibroblast growth factor 8; GPR54, G-protein couple receptor 54; TAC3, tachykinin 3; TACR3, tachykinin receptor 3; KAL1 Kallmann syndrome 1; NELF, nasal embryonic LHRH factor.
Table 1.
Genetics of isolated GnRH deficiency
| Gene (chromosomal locus) | Ref. | OMIM no. | Reproductive phenotypes | Mode of inheritance | Associated phenotypes |
|---|---|---|---|---|---|
| KAL1 (Xp22.3) | 47 | 308700 | KS | X-linked | Unilateral renal agenesis, bimanual synkinesia, high arched palate |
| KISS1R (19p13.3) | 151 | 604161 | nIHH | Autosomal recessive | None |
| FGFR1 (8p11.2-p11.1) | 152 | 136350 | KS | Autosomal dominant | Cleft lip/cleft palate, skeletal anomalies (hand and foot), external ear hypoplasia, dental agenesis |
| FGF8 (10q.24) | 153 | 600483 | nIHH | Autosomal dominant | Cleft lip/cleft palate, skeletal anomalies (hand and foot), external ear hypoplasia, dental agenesis |
| PROK2 (3p21.1) | 11, 12 | 607002 | KS | ?Autosomal recessive | ? Circadian/sleep dysregulation, ? glucose intolerance |
| PROKR2 (20p.13) | 11 | 607123 | nIHH | ?Autosomal recessive | ? Circadian/sleep dysregulation, ? glucose intolerance |
| GnRH-1 (8p21-p11.2) | 154 | 152760 | nIHH | Autosomal recessive | None reported |
| GnRHR (4q21.2) | 155 | 138850 | nIHH | Autosomal recessive | None reported |
| TAC3 (12q13-q21) | 156 | 162332 | nIHH | Autosomal recessive | Microphallus, cryptorchidism, reversal of GnRH deficiency |
| TACR3 (4q25) | 156 | 162330 | nIHH | Autosomal recessive | Microphallus, cryptorchidism, reversal of GnRH deficiency |
| NELF (9q34.3) | 127 | 608137 | KS | Digenic | None reported |
?, Indicates potential association.
Table 2.
Genetics of isolated GnRH deficiency associated with complex syndromes/diseases
| Gene (chromosomal locus) | Ref. | OMIM no. | Reproductive phenotypes | Mode of inheritance | Associated syndrome/phenotype |
|---|---|---|---|---|---|
| LEP (7q31.3) | 157 | 164160 | nIHH | Autosomal recessive | Severe obesity |
| LEPR (1p31) | 158 | 601007 | nIHH | Autosomal recessive | Severe obesity |
| NROB1 (Xp.21.3-p21.2) | 159 | 300473 | nIHH | X-linked | X-linked adrenal hypoplasia congenita |
| CHD7 (8q12.1) | 160 | 608892 | KS, nIHH | Autosomal dominant | Part of CHARGE syndrome |
CHARGE syndrome, Coloboma, heart defect, choanal atresia, growth retardation, genital and ear abnormalities.
III. Discovery of Prokineticin Family
The first report of prokineticin-like peptides dates back to 1980 when venom protein A was isolated from the nontoxic constituents in the venom of black mamba snake (Dendroaspis polylepis) (57). This peptide was subsequently renamed as mamba intestinal toxin, MIT-1 (58, 59). Similarly, an ortholog of human PROK2 was isolated from the skin secretion of the fire-bellied toad (Bombina variegata). This molecule was named Bombina variegata 8 (Bv8) to indicate its species of origin and its molecular mass of 8 kDa (60). In 2001, Zhou and collaborators (61) identified two human cDNAs that encode the human orthologs for the snake toxin MIT-1 and the toad skin-secreted protein, Bv8. The human counterparts were named PROK1 and PROK2 to highlight their potent and specific motility enhancement of the gastrointestinal tract (59, 61). In an attempt to enhance the clarity and maintain consistency with the nomenclature, we will use the designations of PROK1 and PROK2 for the rest of this review. Apart from its gastrointestinal effects, PROK1 was also identified as an angiogenic factor with specific effects on endocrine organs, thus earning its initial name, endocrine gland vascular endothelial growth factor (62). This name reflects the functional resemblance with vascular endothelial growth factor, a widely known angiogenic factor, although these molecules are structurally unrelated (62, 63).
Almost simultaneously with the discovery of the prokineticins, three independent research groups identified and characterized their cognate receptors (64–66). Mature PROK1 and PROK2 are ligands for the highly homologous (85%) G protein-coupled receptors PROKR1 and PROKR2, formerly known as GPR73a and GPR73b, respectively. Intensive research of the prokineticin system over the past decade has revealed a dazzling array of physiological functions of this pathway, including regulation of circadian rhythms, metabolism, angiogenesis, neurogenesis, pain perception, muscle contractility, hematopoiesis, immune response, and reproduction (67–69). In addition, the disruption of prokineticin system has been implicated in several pathological conditions, including cancer (70, 71), immunological response (71, 72), mood disorder (anxiety/depression) (73), and cardiomyopathy (74). In the following sections, we will briefly describe the prokineticin family (PROK1/PROK2 and PROKR1/PROKR2) and then specifically focus on the role of prokineticin 2 pathway on the neuroendocrine control of reproduction.
IV. Prokineticin Ligands: PROK1 and PROK2
In contrast to the high homology exhibited by the prokineticin receptors, the ligands, PROK1 and PROK2, share only 44% amino acid identity. Most of this homology resides in the N-terminal signal peptide and the distinct AVITGA sequence motifs (Fig. 2, A and B) that are highly preserved across several species (fish, frog, snake, and various mammalian species) (75). In view of the distinctively conserved N-terminal AVITGA sequence, Kaser et al. (75) proposed the term “AVIT family” to classify the prokineticins and their nonmammalian orthologs. Despite these structural similarities, PROK1 and PROK2 have diverse physiological effects in view of their differential anatomical distribution. Furthermore, the nonselectivity of the ligands to recognize their receptors (PROKR1 and PROKR2) results in homologous and heterologous ligand/receptor pairings that could potentially trigger divergent downstream signaling pathways (74, 76). These features suggest that the complex intracellular context results in a differential ligand/receptor coupling and, as a consequence, diverse biological actions.
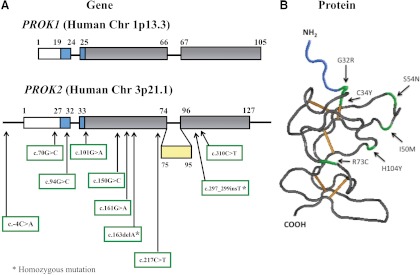
Fig. 2.
Schematic representation of the gene and protein structures of the prokineticin ligands. A, PROK1 and PROK2 are encoded by three and four exons, respectively. Exons 1, 2, and 4 of PROK2 gene encode a mature protein of 81 amino acids. Exon 3 of PROK2 is represented in yellow and undergoes alternative splice processing. Both PROK1 and PROK2 encode a signal peptide that is shown in white. The gene sequence that encodes the AVITGA motif is shown in blue. The amino acid substitutions resulting in various mutations in the PROK2 gene identified to date in GnRH deficiency patients (12, 13, 16, 18) are shown below the schematic of the PROK2 gene. B, The PROK2 protein three-dimensional structure (Protein Data Bank code: 1IMT) was modeled using Cn3D 4.1 software (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). The mature prokineticin ligands exhibit a globular shape as a result of five disulfide bonds (shown in orange). Selected nonsynonymous mutations in PROK2 gene identified in GnRH-deficient patients are shown in green. The AVITGA sequence is shown in blue.
A. Gene sequence
The PROK1 gene maps to regions of human chromosome 1p13.1 and mouse chromosome 3 (77). The gene is organized in three exons encoding a precursor protein of 105 amino acids and a mature form of 86 amino acids (63, 78) (Fig. 2A). The first exon encodes 19 residues corresponding to the signal peptide, and the first five amino acids of the mature protein correspond to the conserved and essential AVITGA sequence domain. The second and third exons encode for a total of ten cysteine residues, suggesting a high degree of tertiary structure to the molecule (six and four cysteine residues, respectively) (Fig. 2, A and B). Despite the highly conserved gene organization, the promoter region in human and mouse diverge, suggesting selective and unique expression patterns and perhaps diverse functions over evolution (77).
The PROK2 gene is located on human chromosome 3p21.1 and mouse chromosome 6 and is arranged in four exons (79, 80). The human and mouse region have five blocks of sequence that are highly conserved (80–100% identity), suggesting a related transcriptional regulation in these species (63, 80). The gene organization of PROK1 and PROK2 is similar except for the third exon in PROK2, which is absent in the PROK1 genomic sequence (60, 61, 80, 81) (Fig. 2A). This third exon can be inserted by alternative splicing, thereby resulting in two mature proteins (76, 80, 81) (Fig. 2A). After the signal peptide (27 amino acids) processing, PROK2 encodes an 81-amino acid protein and a longer isoform (PROK2L) of 102 amino acids with a highly basic insert after residue 74 (80, 81) (Fig. 2A). This insert in PROK2L is rich in arginine and lysine residues and contains several potential cleavage sites for furin and other prohormone convertases (81, 82). To date, the structure and biological action of this long variant remains unknown. The PROK2 promoter region has several E-box sites that are recognized by members of the basic-helix-loop-helix family such as CLOCK and BMAL1 (83) [implicated in the role of PROK2 in circadian regulation in the suprachiasmatic nucleus (SCN)] and NGN1 and MASH1 (implicated in the role of PROK2 in the olfactory bulb) (84).
B. Protein structure
The distinctive N-terminal sequence of both PROK1 and PROK2 (the AVITGA sequence) has been implicated in receptor recognition (76). The high degree of disulfide cross-linking gives rise to a remarkably stable compact protein that is highly resistant to protease degradation (76, 85). Both proteins fold into a polarized ellipsoid structure with one side containing a net positive charge and the opposite with hydrophobic residues (85) (Protein Data Bank, accession number 1IMT) (Fig. 2B). The C- and N-terminal ends are exposed on the surface, whereas the more charged residues are buried inside the molecule (85). The disulfide bond pattern of prokineticins is similar to both colipase, a cofactor for intestinal lipid digestive enzyme lipase, and Dickkopf family members, which are extracellular proteins that organize embryonic head development in Xenopus through the regulation of Wnt/catenin signaling pathways and are important to bone development (86).
C. Anatomical localization
PROK1 and PROK2 are expressed in an impressive array of organs including brain, ovary, testis, placenta, adrenal cortex, peripheral blood cells, intestinal tract, heart, and bone marrow (69, 72) (Tables 3 and 4). Despite their similar pattern of expression, PROK1 and PROK2 have a unique temporal and spatial tissue distribution of expression (87, 88). For example, PROK1 is predominantly expressed in steroidogenic organs: ovary > testis > adrenal cortex > placenta (62); whereas PROK2 is mainly (but not exclusively) expressed in the central nervous system and nonsteroidogenic cells of the testes (87, 88).
Table 3.
Expression pattern of PROK1, PROK2, PROKR1, and PROKR2 in tissues associated with reproductive function
| Tissue | Ref. | PROK1 | PROK2 | PROKR1 | PROKR2 |
|---|---|---|---|---|---|
| Brain | 82, 116 | Olfactory bulb, hypothalamus (medial preoptic area, arcuate nucleus) | Olfactory bulb and ventricles, hypothalamus (arcuate nucleus, mammillary bodies) | Olfactory bulb, piriform and entorhinal cortex, band of Broca, hypothalamus (lateral preoptic area, paraventricular nucleus, arcuate nucleus, median eminence, mammillary nucleus, subfornical organ) | |
| Pituitary | 64, 65 | Present | Present | Present | |
| Ovary | 64, 87 | Granulosa and theca cells | Capillary endothelial cells | Capillary endothelial cells | |
| Uterus | 65, 95, 97 | Glandular epithelium, stromal and smooth muscle cells | Glandular epithelium, stromal and smooth muscle cells | Glandular epithelium, stromal and smooth muscle cells | Glandular epithelium, stromal and smooth muscle cells |
| Placenta | 93, 94 | Present | Present | ||
| Testis | 63, 64, 81, 102 | Leydig cells | Primary spermatocytes | Endothelial cells of interstitium | Endothelial cells of interstitium |
| Prostate | 62, 64 | Prostate cancer | Present | ||
| Human fetal tissue | 61, 66 | Present | Present | Present | Present |
Table 4.
Expression pattern of PROK1, PROK2, PROKR1, and PROKR2 in nonreproductive tissues
| Tissue | Ref. | PROK1 | PROK2 | PROKR1 | PROKR2 |
|---|---|---|---|---|---|
| Brain | 65, 88 | Tractus solitarium, cerebellum | Basal ganglia (accumbens nucleus, islands of Calleja), hypothalamus (suprachiasmatic nucleus), medial amygdala, mesencephalon (Edinger Westphal) | Hypothalamus (zona incerta), mesencephalon | Hippocampus, globus pallidus, amygdala, thalamus, hypothalamus (SCN) |
| Thyroid gland | 64 | Present | Present | ||
| Thymus | 65 | Present | Present | Present | |
| Salivary gland | 64 | Present | |||
| Heart | 141 | Cardiovascular tissue, cardiac cells | Cardiovascular tissue, cardiac cells | Cardiovascular tissue, cardiac cells | |
| Lung | 65 | Present | Present | Present | |
| Liver | 65, 161 | Kupffer cells | Kupffer cells | Kupffer cells | |
| Spleen | 64, 65 | Present | Present | Present | |
| Kidney | 62, 65, 87 | Epithelial tubules | Present | Endothelial cells | |
| Adrenal gland | 64, 65, 162 | Glomerulosa and fasciculate cells | Glomerulosa, fasciculate, and endothelial cells | Glomerulosa, fasciculate, and endothelial cells | Glomerulosa and fasciculate cells |
| Pancreas | 64, 78, 163 | Pancreatic islets and stellate cells | Vascular endothelial cells | Vascular endothelial cells | |
| Stomach | 61, 65, 164 | Present | Present | ||
| Intestinal tract | 72, 165 | Enteric plexus, mucosa of embryonic gut | Enteric plexus | Enteric neural crest cells; plexus cells of Jejunum, ileum, ileocecum, and colon | Enteric plexus of ileocecum |
| Skeletal muscle | 65, 66 | Present | Present | Present | |
| Adipocytes | 65 | Present | Present | Present | |
| Bone marrow and peripheral blood | 71, 166 | B and T cells | Hematopoietic stem cells, monocytes, neutrophils and dendritic cells | Hematopoietic stem cells, mature blood cells | Hematopoietic stem cells, mature blood cells |
From a human reproductive point of view, PROK1 has been reported to have regulatory effects on the gonads (68), whereas PROK2 plays a major role in olfactory bulb development and GnRH neural migration (12, 89) (see Section VII.A). In the human ovary, PROK1 is strongly expressed in the granulosa cells of primordial and primary follicles and then in theca cells during early to mid luteal phase (87, 90). Similar patterns of PROK1 expression have been shown in bovine ovaries (91) and also in primates such as the cynomolgus monkey (Macaca fascicularis) and the chimpanzee (Pan troglodytes) (62). In contrast, PROK2 is undetectable in human ovary (87). In addition to the ovary, PROK1 is also detected in endometrial tissue, where it reaches a maximum level of expression during “the implantation window” of women in reproductive age (72, 92). In contrast, PROK2 expression in the endometrium remains constant across the menstrual cycle (92). During the first trimester of pregnancy, PROK1 levels increase further in the decidualized endometrium compared with midluteal phase of the menstrual cycle (93–95). PROK1 is also highly expressed in term placentas (syncytiotrophoblast and cytotrophoblast), fetal endothelium, and macrophages (96). However, it is rarely detected after menopause or in endometrial carcinoma patients (97). The physiological implications of PROK1 presence in the ovary, uterus, and in various tissues of pregnancy have been comprehensively studied as discussed by others (68, 93, 95). Recently, several reports indicate the involvement of PROK1 signaling in human ectopic endometriosis (98, 99), in ectopic pregnancy (100), and in the immune response of pregnancy (96, 101). PROK1 has also been proposed as a potential biomarker to predict endometrial receptivity in patients undergoing in vitro fertilization (95).
In males, PROK1 is abundantly expressed in the testes from embryonic wk 14 until birth during early testicular development (102). In adult men, in keeping with its steroidogenic theme, PROK1 is expressed in Leydig cells (102), whereas PROK2 expression is restricted to the primary spermatocytes in both humans and mice (81, 87). The PROK2L variant is also expressed in the testes (81). Thus, in the testes, the prokineticin ligands are considered to be angiogenic and mitogenic/survival factors and are potentially involved in the high rate of endothelial cell turnover (87). The expression pattern of PROK1 and PROK2 in reproductive tissues and their expression in nonreproductive tissues are tabulated in Tables 3 and 4, respectively.
V. Prokineticin Receptors: PROKR1 and PROKR2
PROKR1 and PROKR2 are closely related members of the GPCR family (61, 64, 65). The anatomic distribution of prokineticin receptors provides insights to understand the multiple physiological roles that are already attributed to the prokineticins (69, 103).
A. Gene sequence
The PROKR1 gene is located in human chromosome 2p13.3 and mouse chromosome 6. The PROKR2 gene is mapped to human chromosome 20p13 and mouse chromosome 2. Both receptors are encoded by two exons separated by an intron located at the border of transmembrane domain III within the common DRY sequence motif (61). The primary sequence of both prokineticin receptors is remarkably conserved, displaying approximately 85% identity in the amino acid sequence (61, 65, 66).
B. Protein structure
The prokineticin receptors belong to the rhodopsin family of GPCRs. Despite the absence of a crystal structure, the knowledge about the prokineticin signaling pathway has been inferred through pharmacological and biochemical approaches (67, 72, 75). Systems biology approaches are emerging as useful tools to understand the enormous complexities of GPCR signaling (104). Among GPCRs, crystal structures have been solved only for the bovine rhodopsin (105) and the human β-adrenergic receptors (106). The structural knowledge of other GPCR family members has been deduced primarily by homology modeling. Seven trans-membrane- spanning regions in the cell surface characterize the GPCR family. Extracellular agonist molecules (i.e., peptides, neurotransmitters) induce conformational changes, allowing the interaction with heterotrimeric G proteins associated with downstream effectors (107). Most importantly, GPCR-interacting proteins exquisitely regulate the GPCR signal transduction, receptor trafficking, and stability in the cell surface. Because the prokineticin ligands and receptors are differentially expressed in distinct tissues, their intracellular effects are customized in each cell type (107) (Tables 3 and 4).
C. Anatomical localization
The anatomical localization of the prokineticin receptors has been extensively studied, and in comparison to their ligands, both PROKR1 and PROKR2 have a considerably wider tissue distribution (65, 66, 74, 108–110) (Tables 3 and 4). The prokineticin receptors can be detected as early as embryonic d 7 in the mouse (65, 67), suggesting their involvement in early development. PROKR2 is abundantly expressed in the forebrain and testes, whereas PROKR1 is mainly expressed in peripheral tissues such as spleen, prostate, pancreas, heart, and blood cells. As a rule of thumb, tissues with high levels of PROKR1 frequently exhibit low to undetectable levels of PROKR2, and vice versa. In general, prokineticins act as diffusible messengers that reach their receptors in target cells through a paracrine mechanism (e.g., PROK1 is expressed by Leydig cells, whereas PROKR1 and PROKR2 are expressed in endothelial cells of interstitial spaces in the testes). Considering that prokineticin receptors are frequently expressed in the vascular endothelium of endocrine organs, it is tempting to speculate that PROKR1 and/or PROKR2 may play a critical role in hormone secretion and/or hematopoetic regulation (87, 111). An increasing number of reports indicate that prokineticin receptor activation promotes cell migration in multiple cell types including neural progenitors in zones with active neurogenesis (89) and peripheral leukocytes involved in inflammatory response and tumor growth (71, 112).
From a reproductive perspective, targeted deletions of prokr2 in mice show that this system is crucial for olfactory bulb morphogenesis and consequently for GnRH neuronal migration (12, 113). Accordingly, PROKR2 mRNA is present in neural precursors generated in the subventricular zone and across the rostral migratory stream that travels toward the olfactory bulb (89, 114). This neuronal stream continuously populates and replenishes the olfactory bulb, one of the few areas in the central nervous system that is continually regenerating in adult life (115). Within the olfactory bulb, PROK2 is produced and secreted to attract PROKR2-expressing cells during migration (89). Apart from the olfactory bulb, PROKR2 is expressed in the hypothalamic regions that have the highest density of GnRH-expressing neurons, such as the diagonal band of Broca, preoptic area, the paraventricular nucleus, the arcuate nucleus, and the median eminence (108, 116). PROK2 is also distinctly expressed in the SCN in a circadian fashion, and the expression of PROKR2 is complementary with significant expression in prime SCN target areas in the hypothalamus, including the areas with the higher density of GnRH neurons (116). PROK2 and PROKR2 are expressed in the limbic system, although their precise role in the limbic system is unclear (108). PROKR2 is also abundantly expressed in the medial preoptic area, the islands of Calleja, the nucleus accumbens, the amygdala, the globus pallidus, and the thalamic areas (108, 116) (Tables 3 and 4). In contrast to PROKR2, PROKR1 is moderately expressed in the olfactory system, but its physiological role in this system remains to be defined (108). PROKR1 is also detected in the arcuate nucleus, mammillary nucleus (112), astrocytes (109), and in brain blood capillary endothelial cells (110), although their functional role in these cells is yet to be determined.
Angiogenesis is a crucial function across the menstrual cycle and during pregnancy. In the ovary, in keeping with its angiogenic theme, PROKR1 and PROKR2 are mainly expressed in the capillary endothelial cells. Kisliouk et al. (91) demonstrated that PROKR1 (but not PROKR2) is potentially involved in macrophage activation in atretic follicles and in the regressing corpus luteum. This group had previously reported that PROKR2 expression is significantly increased under stress conditions in the endothelial cells of the corpus luteum, whereas PROKR1 mRNA levels remained unchanged (117). In the testes, both receptors are expressed in endothelial cells of the interstitial tissue, responding to PROK1 from Leydig cells and PROK2 from primary spermatocytes (87). However, PROKR1 and PROKR2 mRNA levels are not affected by sex steroid milieu (97). Interestingly, exogenous ligand delivery, either PROK1 or PROK2, produces a dramatic angiogenic effect that correlates with the high endothelial cell turnover in testes despite being a noncyclic tissue (63). In the endometrium, both receptors are abundantly expressed during the proliferative phase. However, under pathological conditions such as endometriosis, PROKR2 mRNA levels increase even more in the proliferative phase of the cycle, whereas PROK1 and PROKR1 remain unchanged (94). During pregnancy, the activation of PROKR1 by PROK1 induces the release of proinflammatory cytokines and acts as a mitogenic factor to differentiate macrophages in the human placenta during the third trimester (68, 96, 101). The anatomical expression of PROKR1 and PROKR2 in tissues associated with reproduction and nonreproductive tissues is tabulated in Tables 3 and 4, respectively.
VI. Prokineticin Signaling
Both PROK1 and PROK2 activate both receptors in the nanomolar range, although PROK2 has moderately higher affinity for both receptors (64–66). Similarly, nonmammalian prokineticin (MIT-1 and Bv8) cannot discriminate between the two receptors. However, they display considerably higher affinity with at least one order of magnitude higher compared with human prokineticins (67). The longer isoform, PROK2L, recognizes both receptors but with a 150-fold decreased affinity compared with PROK2 (76). Further studies will be needed to explore the biological actions as well as the pathophysiological implications of the various PROK2 isoforms. Most importantly, because prokineticin ligands and receptors exhibit a differential anatomical distribution (Tables 3 and 4) and selectivity to recognize their receptors (PROKR1 and PROKR2), it is possible that the intracellular context will customize downstream signaling pathways, resulting in diverse biological actions. Several recent reviews describe this topic in greater detail (67, 69, 72).
Activation of the prokineticin receptors induces intracellular calcium mobilization through several mechanisms. One of them is via Gq coupling that activates phospholipase Cβ and subsequent formation of inositol triphosphate (64) and calcium release from intracellular stores. Phospholipase C inhibition prevents the effects of PROK2 on chemotaxis of mouse macrophages, further supporting the involvement of Gq coupling of the prokineticin receptors (118). In contrast, PROK2 induced-ERK phosphorylation and chemotaxis of human monocytes are inhibited by pertussis toxin, suggesting involvement of the Gi proteins and indicating a species-specific variation of the intracellular effects. Intracellular calcium stimulation by PROK1 also activates the calcineurin pathway, which induces dephosphorylation of the transcription factor, NFAT (nuclear factor of activated T cells), followed by nuclear translocation of NFAT and regulation of gene transcription (101). It remains to be seen whether prokineticin 2 signaling may involve the calcineurin-NFAT pathway. PROK1 and PROK2 expression can be modulated by hypoxia-induced factor-1α and potentially implicated in vascular remodeling (103).
In the peripheral nervous system, PROKR1 is expressed in the dorsal root ganglion (119). In the dorsal root ganglion, prokineticin receptors increase intracellular calcium by inducing the transient receptor potential vanilloid 1 channels in a dose-dependent fashion and are followed by subsequent translocation of protein kinase C (PKC) to the neuronal membrane (119). Furthermore, cross talk between the prokineticin pathway activated PKC and the prostaglandin E2-activated cascade (adenylate cyclase/cAMP/protein kinase A) is thought to contribute to the hyperalgesic effect of PROK2, although the mechanism connecting PKC and cAMP remains elusive (67).
Because the MAPK pathway activation is critical for angiogenesis, Lin et al. (78) examined the prokineticin pathway-induced activation of the p44/p42 MAPK pathway in transfected cell lines. In this report, prokineticin signaling was shown to activate MAPK pathway, suggesting involvement of prokineticin receptor coupling to Gi proteins. Likewise, in cerebellar granule cell cultures, PROK2 stimulates neuronal survival and protects the cultured neurons against excitotoxic death by activating MAPK/phosphatidylinositol-3 kinase pathways (120). In Ishikwawa endometrial epithelial cell lines, PROK1-PROKR1 signaling has been shown to induce inositol phosphate mobilization with sequential phosphorylation of c-Src, epidermal growth factor receptor-MAPK-ERK pathway (95).
Thus, precise intracellular actions of the prokineticins are likely to be determined by the differential interactions with Gq, Gi, and Gs coupling of the receptors in specific cells and at specific developmental time frames (110). However, currently there is sparse evidence of other GPCR modulators regulating prokineticin signaling. Mutations in PROK2 and PROKR2 in humans have been extremely useful in providing critical insights into the signaling cascade (see Section VII). Further study of these human mutations is likely to facilitate identification and expansion of the signal pathway, including effectors and interacting proteins, and this will be imperative to help understand the cell-specific and species-specific biological actions of the prokineticin 2.
VII. Prokineticin 2 Pathway and Neuroendocrine Control of Reproduction
Although our knowledge about the precise molecular mechanisms underlying the biological role of PROK2 in the reproductive neuroendocrine axis remains in its infancy, PROK2 is incriminated by the combination of its clear causation in genetically manipulated mice (11, 12, 113) and its association with the disease state in humans (11, 12). In addition, the role of PROK2 in neuroendocrine hormonal regulation is also supported by the localization of PROK2 in hypothalamic regions critical for GnRH action such as the preoptic area, arcuate nucleus, and median eminence (108). PROK2 is also expressed in the islands of Calleja, nucleus accumbens, amygdala, and premammillary nucleus, which are regions associated with reproductive and feeding behavior (108). In this context, Shapiro and collaborators (121, 122) propose that the above-mentioned structures participate in encoding the appropriate reward component to novel olfactory and emotional information, which is then consolidated into memories by the hippocampus. It is also clear from multiple lines of evidence that circadian signals contribute directly to the neuroendocrine control of reproduction (123, 124). PROK2 is abundantly expressed in the SCN, and PROK2-expressing neurons in the SCN extend their neural processes into the preoptic area where the GnRH neurons reside (83, 108, 116). However, this intense PROK2 expression is undetectable in the SCN during fetal life and increases after the first postnatal week, suggesting that the clock's molecular machinery requires a maturation process (125). Thus, PROK2 is proposed to modulate GnRH function in adults by acting as a key circadian output molecule from the SCN to mature GnRH neurons (116). However, direct evidence for this association in humans is still lacking. This section details the phenotypes of both mice and humans with defects in PROK2/PROKR2 genes.
A. GnRH deficiency in PROK2 and PROKR2 knockout mice
1. Prok2-deficient mice
The prok2 knockout mice develop disrupted olfactory bulb morphogenesis and a dramatic reduction of GnRH-expressing cells in the median preoptic area, with only a few neural projections detected in the median eminence (12). These findings phenocopy the anatomical observation of KAL1 deficiency in humans depicted by the failure of GnRH neurons to enter into the forebrain and herein forming a spherical-shape structure in the nasal septum immediately before the cribriform plate (43). Because the KAL1 gene has never been located in the mouse genome, these mice represent the first murine model of KS. Approximately 50% of prok2 knockout mice show asymmetric development of olfactory bulb. Although some GnRH neurons reach the hypothalamus in these mice, their numbers and/or function are insufficient to initiate reproductive axis competency. This finding suggests that GnRH neurons that reach the hypothalamus in prok2 knockout mice may not be functional and thus implies that PROK2 may impact on GnRH neuronal integrity through additional mechanisms besides olfactory bulb neurogenesis (12, 89). Intriguingly, PROKR2 is not expressed in GnRH neurons themselves, and thus the elucidation of the molecular mechanisms underlying the PROK2/PROKR2 regulation of GnRH neuronal development/function remains a significant investigatory challenge.
The dramatic decrease of hypothalamic GnRH neurons in prok2 knockout mice results in low levels of GnRH secretion, absent gonadotropin secretion, and impaired sexual development and fertility. Twelve-week-old prok2 knockout males have immature testes with small seminiferous tubules that lack lumens and absent haploid spermatocytes (12). Interestingly, under normal conditions, prok2 is heavily expressed in diploid spermatocytes (63, 81) that give rise to the haploid spermatocytes after meiotic division. Thus, it is likely that prok2 could play a role in final stages of spermatogenesis. Female prok2 knockout mice exhibit disrupted estrous cycles as a consequence of incomplete follicular development characterized by the absence of mature follicles and corpora lutea (12). Interestingly, in mice and in humans with PROK2 deficiency, ovarian function can be restored in response to gonadotropin replacement (12). Taken together, these findings indicate that prok2 is likely to contribute to spermatogenesis in males, but its predominant role in females is in producing a hypogonadotropic state.
2. Prokr2-deficient mice
The reproductive phenotype of the prokr2 knockout mice is remarkably similar to the phenotype of the prok2 knockout mice (113). Both prok2- and prokr2-deficient mice exhibit arrest of GnRH neuronal migration in the same physiological window, and the GnRH neurons form a “fibrocellular mass” just beyond the cribiform plate immediately before their entry into the forebrain (12, 113). However, a major difference between the prok2 and prokr2 knockout mice is evident in the olfactory system development: all prokr2 knockout mice have a dramatic decrease in the olfactory bulb size (113), whereas half of the prok2-deficient mice exhibit asymmetric olfactory bulb development (12). In sharp contrast, prokr1 knockout mice display normal olfactory bulb development (113). Prokr2-deficient mice display a striking atrophy of the testes with reduced diameter of seminiferous tubules and lack of sperm in the lumen (113). In addition, histological analysis reveals the presence of spermatogonia and spermatocytes although the spermatids are absent, suggesting a potential role of PROK2 signaling in the last steps of spermatozoa maturation. In wild-type testes, prokr2 is abundantly expressed in endothelial cells of the interstitial space in the testis, which exhibits the highest endothelial cell turnover despite being a noncyclic tissue (63, 87). This observation suggests that prokr2 could be involved in vascular remodeling because prokr2-deficient mice display reduced interstitial space accompanied by small and scattered Leydig cells, compared with their wild-type littermates (113). Similarly, ovarian folliculogenesis is frequently arrested in the preantral phase, and corpora lutea are often absent in female prok2 (12) and prokr2 knockout mice (113). In addition, these mice also show striking atrophy of the endometrial tissue, uterus, and the epithelial layers of the vagina, which is consistent with recent reports implicating a role for PROKR2 in the pathogenesis of ectopic endometrial human tissue (98).
B. PROK2 and PROKR2 mutations in isolated GnRH deficiency in humans
After the description of unexpected GnRH deficiency in murine knockouts of prok2 and prokr2, human PROK2 and PROKR2 genes became potential candidate genes for the etiology of human GnRH deficiency. Subsequently, in a cohort of 192 unrelated KS patients, Dode et al. (11) reported DNA sequence changes in both PROK2 and PROKR2 genes. Remarkably, a significant number of these changes were in the heterozygous state (Tables 5 and 6 and Figs. 2 and 3). In this report, four patients harbored heterozygous changes in PROK2 (one frameshift with a premature stop codon and three missense changes) (Table 5). Ten different PROKR2 mutations (one frameshift causing premature stop codon and nine missense changes) were seen in 14 patients in heterozygous (10 subjects), homozygous (two subjects), and compound heterozygous (two subjects) states (Table 6). No functional studies of the missense mutations were reported in this study.
Table 5.
Genotypes/phenotypes of KS and nIHH subjects with PROK2 mutations
| First author (Ref.) | Patient cohort | No. affected/diagnosis | Mutations | Associated phenotypes |
|---|---|---|---|---|
| Dode (11) | 192 KS | 4/KS | Heterozygous | Severe sleep disorder, obesity |
| Pitteloud (12) | 50 KS, 50 nIHH | 1/KS | Homozygous | Type 2 diabetes, epilepsy |
| Cole (13) | 170 KS | 2/KS | Heterozygous | Synkinesia, type 2 diabetes |
| 154 nIHH | 1/nIHH | Heterozygous | Synkinesia, type 2 diabetes | |
| Abreu (16) | 63 KS | 2/KS | Both homozygous | Pectus excavatum |
| 44 nIHH | 1/nIHH | Heterozygous | Pectus excavatum | |
| Leroy (15) | 2 Patients (case report) | 2/KS | Homozygous | None |
| Sarfati (18) | Comparative study | 3/KS | Heterozygous | Nystagmus, pectus excavatum, high arched palate, bilateral sensorineural deafness |
Table 6.
Genotypes/phenotypes of KS and nIHH subjects with PROKR2 mutations
| First author (Ref.) | Patient cohort | No. affected/diagnosis | Mutations | Associated phenotypes |
|---|---|---|---|---|
| Dode (11) | 192 KS | 14/KS | 2 Homozygous, 2 compound heterozygous, 10 heterozygous | Severe sleep disorder, obesity |
| Cole (13) | 170 KS, 154 nIHH | 6/KS, 5/nIHH | All heterozygous | Pes planus, fibrous dysplasia, hearing loss, epilepsy, synkinesia, pectus excavatum |
| Abreu (16) | 63 KS, 44 nIHH | 5/KS, 1/nIHH | 1 Homozygous, 5 heterozygous | Obesity, metabolic syndrome |
| Sinisi (14) | 1 Patient (case report) | 1/KS | Homozygous | None |
| Canto (17) | 24 KS | 2/KS | Heterozygous | Atopic dermatitis, abnormal eye movements |
| Sarfati (18) | Comparative study | 25/KS | 4 Homozygous, 20 heterozygous, 1 compound heterozygous | High arched palate, type 2 diabetes, nystagmus |
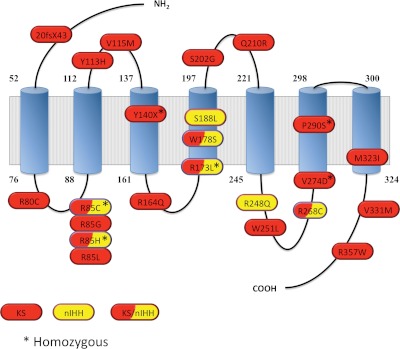
Fig. 3.
PROKR2 gene mutations identified in GnRH-deficient patients. Schematic of the PROKR2 protein generated using the SOSUI secondary structure software (http://bp.nuap.nagoya-u.ac.jp/sosui/sosui_submit.html) showing the seven trans-membrane spans (blue cylinders) and the PROKR2 mutations identified to date in isolated GnRH-deficient patients. Mutations labeled in red have been identified in KS patients; mutations labeled in yellow have been identified in normosmic GnRH-deficient (nIHH) probands; and hatched red and yellow label shows mutations that have been identified in nIHH as well as KS patients (11, 13–18, 135).
Subsequently, in a Portuguese family with three affected siblings with GnRH deficiency (two brothers and one sister), a homozygous deletion in PROK2 was reported (12) (Table 5). This deletion resulted in a 27-amino acid truncated protein, which was functionally demonstrated to be biologically inactive. Interestingly, in this pedigree, both affected brothers with the homozygous mutation had KS, and the affected sister who also harbored the homozygous mutation had nIHH whereas another unaffected brother was heterozygous for the mutation, suggesting that homozygous changes were required to produce the syndrome in this family (12). After this report, a large number of predominantly heterozygous mutations in PROK2 and PROKR2 with considerable clinical and molecular heterogeneity have now been reported in several patients with both KS and nIHH (13–18, 126) (Tables 5 and 6). The reported PROK2 mutations and PROKR2 mutations are depicted in Figs. 2 and 3, respectively.
1. Clinical heterogeneity of PROK2/PROKR2 mutations
a. PROK2 and PROKR2 mutations cause both KS and nIHH.
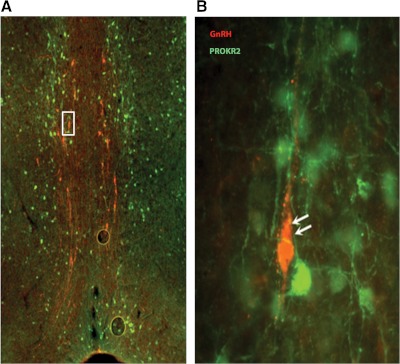
Murine homozygous deletions of prok2 and prokr2 result in a KS phenocopy. Surprisingly, in humans, mutations in PROK2 and PROKR2 cause both KS and nIHH (12, 13, 16) (Tables 5 and 6). The KS phenotype seen in both mice and humans is not surprising, given the role played by prokineticin 2 in olfactory bulb neurogenesis and GnRH neuronal migration during embryonic development. However, the presence of prokineticin 2 pathway mutations in subjects with nIHH suggests an important, yet uncharacterized role for PROK2 in the regulation of GnRH synthesis, secretion, and/or action that is independent of its relationship with olfactory migration. This is also supported by the expression of PROK2 in hypothalamic areas with a “high” density of GnRH neurons. However, given that GnRH neurons in the hypothalamus do not coexpress PROKR2 (Fig. 4) (12), it is presumed that the effect of PROKR2 on GnRH neurons is indirect. Elucidation of the mechanism(s) by which the PROK2 pathway modulates the function of GnRH neurons is currently of significant research interest.
Fig. 4.
PROKR2 is not expressed in the GnRH neurons located in the median preoptic area. A, Double immunostaining showing the GnRH neurons (red) and PROKR2 immunoreactive cells (green) in the preoptic area of PROKR2-GFP transgenic mice. B, A higher magnification view of the boxed area in panel A is shown illustrating the absence of PROKR2 protein expression in GnRH expressing cells.
b. Variable expressivity and incomplete penetrance of reproductive and olfactory phenotypes within families.
Patients with PROK2 and PROKR2 mutations show considerable phenotypic heterogeneity. In general, subjects with homozygous PROK2 and PROKR2 mutations exhibit a reproductive phenotype that is often fairly severe and penetrant (12, 16, 18). It is, however, important to remember that the majority of patients with mutations in this pathway harbor heterozygous mutations. In these pedigrees with heterozygous mutations, variable expressivity or incomplete penetrance of both the reproductive and olfactory phenotypes is evident both within and across families sharing identical mutations. For example, whereas some members with a heterozygous mutation show a fully penetrant KS phenotype, others show a wide spectrum of reproductive and olfactory defects such as delayed puberty, normosmic GnRH deficiency, or isolated anosmia, and still others are seemingly unaffected (11, 13). One possible explanation for these observations is that patients with heterozygous mutations in PROK2/PROKR2 may carry additional genetic mutations in other genes (oligogenicity) that may contribute to the phenotypic presentation (10, 127). Thus, GnRH-deficient subjects with heterozygous mutations in PROK2/PROKR2 represent a unique cohort, and careful genetic investigation is likely to uncover the missing genetic or epigenetic modifiers that interact with this pathway in humans to account for the phenotypic heterogeneity.
c. “Dual” defect and reversal of GnRH deficiency.
Although all GnRH-deficient patients with PROK2/PROKR2 mutations display a hypothalamic defect, in two patients with PROK2 (15) and PROKR2 (14) mutations, persistent oligo/azoospermia has been observed during gonadotropin treatment, suggesting a “dual” defect: hypothalamic GnRH deficiency and a primary gonadal defect. The primary gonadal defect in these patients is in keeping with the unique expression profile of PROK2 and PROKR2 in the testes and, in particular, the expression of PROKR2 in primary spermatocytes (63, 81). More recently, in a pilot genome-wide association study, a tagging single nucleotide polymorphism in close proximity to PROK2 gene has been shown to be associated with oligospermia and azoospermia in men (128). Collectively, these observations also suggest a role for the prokineticin 2 pathway in regulating primary testicular function and spermatogenesis.
Reversal of congenital GnRH deficiency later in adulthood is yet another well recognized phenomenon in patients with both KS and nIHH. Notably, reversal of GnRH deficiency occurs even in the context of deleterious mutations (129). Subjects with mutations in PROKR2 have also been documented to undergo reversal of GnRH deficiency after treatment with sex steroids (13, 14). Although the precise biological basis for this restoration of GnRH secretion remains unclear, it is likely that GnRH and/or PROK2/PROKR2 neuronal plasticity, possibly modulated by sex steroid treatment and/or other epigenetic effects, may be responsible.
d. Nonreproductive phenotypes of GnRH-deficient patients with PROK2/PROKR2 mutations.
GnRH deficiency is often characterized by a number of nonreproductive features (Tables 1 and 2). These clinical and neuroendocrine signatures reflect the expression and function of the underlying causative genes in other organ systems and often provide clues to an unexpected facet of their biology (e.g., renal agenesis and KAL1 mutations, cleft lip/palate, and FGFR1 mutations) (52). Among previously reported nonreproductive features of GnRH deficiency, bimanual synkinesia and hearing loss are seen in a minority of patients with PROK2/PROKR2 mutations, whereas renal agenesis, cleft lip, and cleft palate have not been reported so far (13, 18) (Tables 3 and 4). PROK2 has been proposed as a key candidate linking the reproductive and circadian systems, and both prok2−/− and prokr2−/− mice exhibit disruptions of some of their circadian rhythms, including abnormal thermogenesis, increased nocturnal physical activity, impaired circadian cortisol secretion, and abnormal glucose regulation (130, 131). In keeping with this notion, some GnRH-deficient patients with mutations in PROK2/PROKR2 were reported to have sleep disorders (11, 13, 18). However, detailed circadian assessment using a constant routine protocol in two subjects with homozygous PROK2 mutations did not show any major circadian abnormalities (unpublished data from our group). Similarly, sleep quality and cortisol profile studies in a subset of PROK2/PROKR2 mutation subjects have also failed to confirm this link in humans (18).
In rodents, PROK2 has been linked to ingestive behavior (132) and hypothalamic appetite regulation (133). Although some subjects with PROK2/PROKR2 mutations are obese, no consistent evidence of raised body mass index has been documented in these subjects (18). In addition, both prok2 and prokr2 knockout mice have been associated with increased neonatal death (12, 113). Although increased neonatal mortality has been seen in a family with PROK2 mutations in humans (12), the precise role of the prokineticin pathway in neonatal development in humans remains to be determined.
In summary, no specific nonreproductive signature for the prokineticin 2 pathway is currently evident. Considering the widespread tissue expression of PROK2/PROKR2 (Tables 3 and 4), detailed phenotyping of patients with PROK2 pathway mutations and longitudinal follow-up will be essential to unearth any potential links.
2. Genetic heterogeneity: the puzzle of heterozygous mutations
Whereas homozygous deletions of prok2 and prokr2 in mice result in hypogonadotropic hypogonadism, heterozygous mice do not show any gross reproductive abnormalities (12, 113), thus suggesting a faithful autosomal recessive mode of inheritance in the mouse. In contrast, humans with heterozygous mutations in PROK2 or PROKR2 who present with complete isolated GnRH deficiency are puzzling. This anomaly could represent an autosomal dominant mode of inheritance due to either haploinsufficiency or a dominant-negative effect. However, functional studies of selected PROKR2 mutants to date have failed to show a dominant-negative effect of these mutations (126). Currently, oligogenic inheritance is the most plausible explanation for the phenotypes seen in patients with heterozygous mutations because interaction and synergism between multiple genes causing GnRH deficiency has been demonstrated (10, 15, 134, 135). Accordingly, mutations in genes already linked with GnRH deficiency have been found to be present in some patients with detected heterozygous mutations in PROK2/PROKR2 (11, 13, 17, 18). A comprehensive frequency study of detected protein-altering variants in patients with isolated GnRH deficiency revealed that the likelihood of such oligogenicity was 11% (134). It has been postulated that other nongenetic/environmental modifiers may also contribute to the observed variable phenotypic expression in subjects with heterozygous mutations, although direct evidence for this is still lacking. The potential mechanisms by which heterozygous mutations in PROK2/PROKR2 cause a broad spectrum of reproductive and olfactory phenotypes is shown schematically in Fig. 5. At present, the genetic puzzle of high frequency patients with complete GnRH deficiency who only harbor heterozygous mutations in PROK2/PROKR2 is of significant research interest and is under active evaluation by our group and by others.
Fig. 5.
Variable expressivity and incomplete penetrance in subjects with PROKR2 mutations: representative pedigree and potential mechanisms of how heterozygous PROK2/PROKR2 mutations cause human GnRH deficiency. Schematic representation of potential mechanisms to explain how heterozygous mutations in the PROK2/PROKR2 system cause a broad spectrum of olfactory and reproductive phenotypes. The phenotype severity results from the combination of heterozygous PROK2/PROKR2 mutation along with several factors including haploinsufficiency, oligogenicity, and/or epigenetic modifiers.
3. Molecular heterogeneity
a. Functional heterogeneity of PROK2 and PROKR2 mutations.
The functional effects of mutations in PROK2 was first provided by Pitteloud et al. (12), with the report of a homozygous deletion in exon 2 of the PROK2 gene resulting in a truncated protein of 27 amino acids rather than the mature form (81 amino acids). After stable expression of the WT PROKR2 in a Chinese hamster ovary (CHO) cell line, an aequorin-based luminescent assay was used to measure intracellular calcium activation by the mutant peptide compared with the mature form. Even at high concentrations, the truncated form of PROK2 could not activate the receptor, confirming the deleterious nature of the deletion. Similarly, PROKR2 mutations have also been confirmed to be loss-of-function in transiently transfected cell lines with wild-type and mutant PROKR2, and the receptor biology has been functionally assessed in multiple ways: intracellular calcium influx, MAPK activation, protein expression, cell-surface targeting of the receptor, ligand binding, and bioinformatic prediction of function (13, 126).
There is significant heterogeneity in functional impairment of the different PROK2 and PROKR2 mutations. Among the PROK2 mutations, all but the I50M mutation show significant impairment of intracellular calcium influx. In contrast, there is considerable variation in the functional impact of PROKR2 mutations. Whereas some mutations show significant global impairment of receptor function (L173R, P290S, W178S), others (R85C, R248Q, V331M) preferentially affect the intracellular calcium influx while relatively sparing the MAPK signaling cascade. In contrast, some mutations preferentially affect the MAPK signaling pathway (R357W) (13, 126, 135). The discordant effects of PROKR2 mutations indicate potentially domain-specific effects and will require further evaluation to characterize the structure-activity relationships and identify critical structural elements of the PROKR2.
VIII. Potential Role of Prokineticin 2 Pathway in Nonreproductive Disorders
Although human GnRH deficiency is the only disease entity currently linked with the prokineticin 2 pathway, both PROK1 and PROK2 play important roles in nonreproductive tissues and multiple physiological functions. This section will briefly review the potential role of the prokineticin 2 pathway in nonreproductive disorders.
A. Nociception and inflammatory pain
Both PROKR1 and PROKR2 are expressed in the dorsal root ganglion, in outer layers of the dorsal horns of the spinal cord and in peripheral terminals of nociceptor axons (136). In rodents, local and systemic injections of very low doses of Bv8 (amphibian homolog of PROK2) decreases nociceptive thresholds to thermal, mechanical, and chemical stimuli through activation of both PROKR1 and PROKR2 in the primary sensory neurons (119). This increase in nociceptor excitability results from functional interaction between PROKR1 and transient receptor potential vanilloid 1, two coexpressing receptors in the dorsal root ganglion. Mice lacking prok2 and prokr1 exhibit impaired pain perception to various noxious stimuli (thermal, mechanical, and capsaicin), but preserved tactile sensitivity, suggesting that tactile sensation may signal primarily through PROKR2 (67, 137, 138). Accordingly, mice lacking prokr2 exhibit lower sensitivity to tactile allodynia while retaining sensitivity to noxious stimuli (67).
Recent studies in rodents also highlight a critical role for PROK2 in granulocyte-mediated inflammatory pain (118, 139). Inflammatory granulocytes and macrophages strongly express both PROK2 and PROK2L isoforms, both of which act as potent pronociceptive agents. In rodent models of inflammatory pain induced by complete Freund's adjuvant, marked up-regulation of PROK2 in granulocytes and macrophages correlates with the development and duration of pain (139). In addition, mice lacking prokr1 and prokr2 show significantly less inflammation-induced hyperalgesia, and pretreatment with a nonpeptide PROKR1 antagonist abolishes both hypernociception and inflammatory hyperalgesia induced by PROK2 (139). It remains to be seen whether human subjects with PROK2 and PROKR2 mutations show any significant defects in nociception or inflammatory hyperalgesia.
B. Angiogenesis and vascular function of the heart
Until recently, the mammalian heart was considered to be a fully differentiated organ and therefore thought to be unable to regenerate after cardiovascular insult. However, cardiomyocyte-specific overexpression of prokr1 and prokr2 in transgenic mouse hearts has highlighted an important role for these receptors in cardiac angiogenesis. Recently, Nebigil and collaborators (140) have shown that transient prokr1 gene transfer after coronary artery ligation reduces mortality and preserves left ventricular function by promoting new coronary arteriole formation and augmentation of capillary density in the cardiomyocytes. Furthermore, this cardioprotective effect could be explained by an autocrine/paracrine loop where PROKR1 up-regulates PROK2, which in turn induces the reprogramming of adult epicardium resulting in neovascularization after heart injury (141). In sharp contrast, cardiac-specific prokr2 overexpression in mice leads to dilated cardiomyopathy and induces capillary fenestration and vascular leakage (142). In addition, recent work has also confirmed that PROK2 induces angiogenesis in coronary endothelial cells through PROKR1 activation, whereas PROKR2 activation in coronary endothelial cells results in capillary fenestration (74, 143). Thus, the functional impact of PROK2 on coronary endothelial cells depends on both the expression profile of PROKR1 and PROKR2 and the divergent signaling pathways used by these receptors. The human relevance for these observations is currently unclear.
C. Mood disorders
Disruption of circadian rhythms has previously been linked to mood disorders. In rodents, intracerebroventricular injection of PROK2 results in increased anxiety-like behavior, whereas prok2 knockout mice display reduced anxiety and depression-like behavior. In a recent case-control study of Japanese patients with mood disorders (151 bipolar patients, 319 major depressive disorder patients, and 340 controls), a tagging single nucleotide polymorphism in PROKR2 was significantly associated with major depressive disorder (73). However, considering the small numbers in this report, this observation requires further evaluation in larger samples.
D. Abdominal aortic aneurysm and idiopathic pulmonary arterial hypertension
Prokineticins are potent chemoattractants for monocytes and macrophages both in vitro and in vivo and stimulate the release of proinflammatory cytokines from macrophages and monocytes. In a human study comparing the whole-genome expression profile from abdominal aortic aneurysm rupture sites and anterior sac biopsies (internal control), PROK2 emerged as one of 21 differentially expressed genes (144). Similarly, in a small group of patients with idiopathic pulmonary arterial hypertension, PROK2 expression was highly up-regulated in peripheral B cells compared with controls (145). Both of these associations are weak, probably represent a generic role for PROK2 in inflammation, and hence require further validation.
In summary, the role of PROK2 beyond neuronal migration and the observed reproductive and olfactory phenotypes in humans is yet to be determined. Considering the widespread tissue distribution of both PROK2 and PROKR2, detailed phenotyping of human subjects harboring loss-of-function mutations in PROK2/PROKR2 will help to uncover the broader nonreproductive roles of this pathway in humans.
IX. Conclusion
In the last decade, patients with GnRH deficiency have helped us enormously to define the genetic architecture of GnRH neuronal ontogeny in humans. Several key signaling molecules and novel neuropeptides critical for GnRH neuronal development and functional integrity have been discovered. Some of the newly discovered neuropeptides, e.g., kisspeptin, have assumed significant importance and now can be used as additional physiological probes for assessing GnRH neuronal integrity in vivo (146, 147). Likewise, the recent discovery of mutations in the prokineticin 2 pathway in human GnRH deficiency has helped to expand our understanding of the complex biology of GnRH neuronal development and function.
Nonetheless, several aspects of prokineticin 2 pathway mutations open up further questions and challenges. In the homozygous state, both the murine and human PROK2/PROKR2 mutations provide compelling evidence for the critical role played by this pathway in embryonic migration of GnRH neurons. However, the presence of PROK2/PROKR2 mutations in nIHH subjects and the reproductive abnormalities in prok2 knockout mice with partial olfactory bulb development suggest a potential role for PROK2 beyond GnRH neuronal migration. This fact is particularly interesting given that mature GnRH neurons do not express prokineticin receptors (Fig. 4). At present, the role of other proteins, chaperones, transcription factors, or other second messengers that interact or mediate the molecular effects of PROK2 are unknown and require further detailed investigation.
Careful genetic investigations in humans with extremes of phenotypes have unearthed several key biological insights into physiology and pathogenesis of human disease (148–150). In the same vein, genetic investigations in humans with GnRH deficiency allow us to begin to map the systems biology of GnRH neuronal development and its functional regulation and interactions (167). With expanding genetic and genomic tools and falling sequencing costs, the coming years will be exciting because novel and unexpected candidates involved in GnRH neuronal ontogeny will explode onto the scene. However, defining their biological role and relating them to the known pathways will be extremely challenging and likely to require development of reliable and scalable biological validation systems. Such studies are now imperative so that observations from these unique patient cohorts can be translated to both understand and treat more common reproductive and nonreproductive disorders in humans.
Acknowledgments
We thank Lacey Plummer and Lindsay Cole for their contributions to the work described here. We are also grateful to Chengkang Zhang who kindly provided the GnRH/PROKR2 immunostaining. We apologize to the authors whose work could not be cited in this review due to space limitations but appreciate all of the many contributions to the large body of literature we have tried to summarize here.
This work was supported by National Institutes of Health Grants 5R01HD015788 and 5U54HD028138, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; and C.M. was supported by the Pew Charitable Trust, Latin American Fellows Program in the Biomedical Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bv8
- Bombina variegata 8
- GnRHR
- GnRH receptor
- GPCR
- G protein-coupled receptor
- KS
- Kallmann syndrome
- MIT-1
- mamba intestinal toxin
- NFAT
- nuclear factor of activated T cells
- nIHH
- normosmic idiopathic hypogonadotropic hypogonadism
- PKC
- protein kinase C
- PROK2
- prokineticin 2
- PROKR2
- prokineticin receptor 2
- SCN
- suprachiasmatic nucleus.
References
- 1. Crowley WF, Jr, Jameson JL. 1992. Clinical counterpoint: gonadotropin-releasing hormone deficiency: perspectives from clinical investigation. Endocr Rev 13:635–640 [DOI] [PubMed] [Google Scholar]
- 2. Merchenthaler I, Görcs T, Sétáló G, Petrusz P, Flerkó B. 1984. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res 237:15–29 [DOI] [PubMed] [Google Scholar]
- 3. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. 1978. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- 4. Waldhauser F, Weissenbacher G, Frisch H, Pollak A. 1981. Pulsatile secretion of gonadotropins in early infancy. Eur J Pediatr 137:71–74 [DOI] [PubMed] [Google Scholar]
- 5. Wu FC, Butler GE, Kelnar CJ, Sellar RE. 1990. Patterns of pulsatile luteinizing hormone secretion before and during the onset of puberty in boys: a study using an immunoradiometric assay. J Clin Endocrinol Metab 70:629–637 [DOI] [PubMed] [Google Scholar]
- 6. Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. 1974. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest 54:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filicori M, Santoro N, Merriam GR, Crowley WF., Jr 1986. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62:1136–1144 [DOI] [PubMed] [Google Scholar]
- 8. Spratt DI, O'Dea LS, Schoenfeld D, Butler J, Rao PN, Crowley WF., Jr 1988. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol 254:E658–E666 [DOI] [PubMed] [Google Scholar]
- 9. Grumbach MM. 2005. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab 90:3122–3127 [DOI] [PubMed] [Google Scholar]
- 10. Sykiotis GP, Pitteloud N, Seminara SB, Kaiser UB, Crowley WF., Jr 2010. Deciphering genetic disease in the genomic era: the model of GnRH deficiency. Sci Transl Med 2:32rv2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dodé C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP. 2006. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley WF, Jr, Zhou QY, Pitteloud N. 2008. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinisi AA, Asci R, Bellastella G, Maione L, Esposito D, Elefante A, De Bellis A, Bellastella A, Iolascon A. 2008. Homozygous mutation in the prokineticin-receptor2 gene (Val274Asp) presenting as reversible Kallmann syndrome and persistent oligozoospermia: case report. Hum Reprod 23:2380–2384 [DOI] [PubMed] [Google Scholar]
- 15. Leroy C, Fouveaut C, Leclercq S, Jacquemont S, Boullay HD, Lespinasse J, Delpech M, Dupont JM, Hardelin JP, Dodé C. 2008. Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur J Hum Genet 16:865–868 [DOI] [PubMed] [Google Scholar]
- 16. Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, Garmes HM, Mendonca BB, Latronico AC. 2008. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab 93:4113–4118 [DOI] [PubMed] [Google Scholar]
- 17. Canto P, Munguía P, Söderlund D, Castro JJ, Méndez JP. 2009. Genetic analysis in patients with Kallmann syndrome: coexistence of mutations in prokineticin receptor 2 and KAL1. J Androl 30:41–45 [DOI] [PubMed] [Google Scholar]
- 18. Sarfati J, Guiochon-Mantel A, Rondard P, Arnulf I, Garcia-Piñero A, Wolczynski S, Brailly-Tabard S, Bidet M, Ramos-Arroyo M, Mathieu M, Lienhardt-Roussie A, Morgan G, Turki Z, Bremont C, Lespinasse J, Du Boullay H, Chabbert-Buffet N, Jacquemont S, Reach G, De Talence N, Tonella P, Conrad B, Despert F, Delobel B, Brue T, Bouvattier C, Cabrol S, Pugeat M, Murat A, Bouchard P, Hardelin JP, Dodé C, Young J. 2010. A comparative phenotypic study of Kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab 95:659–669 [DOI] [PubMed] [Google Scholar]
- 19. Maestre de San Juan A. 1856. Teratologia: Falta total de los nervios olfactorios con anosmia en un individuo en quien existia una atrofia congénita de los testículos y miembro viril. Siglo Medico 3:211–221 [Google Scholar]
- 20. Weidenreich F. 1914. Uber partiellen Riechlappendefekt und Eunuchoidismus beim Menschen. Z Morphol Anthropol 18:157–190 [Google Scholar]
- 21. Altmann F. 1930. Uber Eunuchoidismus. Virchows Arch [Pathol Anat] 276:455 [Google Scholar]
- 22. Kallmann F, Schoenfeld W, Barrera S. 1944. The genetic aspects of primary eunuchoidism. Am J Ment Defic 48:203–236 [Google Scholar]
- 23. De Morsier G. 1954. Etudes sur les dysraphies cranio-encephaliques. Schweiz Arch Neurol Psychiatr 74:309–361 [PubMed] [Google Scholar]
- 24. Klinefelter HF, Albright F, Griswold GC. 1943. Experience with a quantitative test for normal or decreased amounts of follicle stimulating hormone in the urine in endocrinological diagnosis. J Clin Endocrinol 3:529–544 [Google Scholar]
- 25. Swerdloff RS, Odell WD. 1968. Gonadotropins: present concepts in the human. Calif Med 109:467–485 [PMC free article] [PubMed] [Google Scholar]
- 26. Odell WD, Parlow AF, Cargille CM, Ross GT. 1968. Radioimmunoassay for human follicle-stimulating hormone: physiological studies. J Clin Invest 47:2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odell WD, Ross GT, Rayford PL. 1967. Radioimmunoassay for luteinizing hormone in human plasma or serum: physiological studies. J Clin Invest 46:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashimoto T, Miyai K, Izumi K, Kumahara Y. 1972. Isolated gonadotropin deficiency with response to luteinizing-hormone-releasing hormone. N Engl J Med 287:1059–1062 [DOI] [PubMed] [Google Scholar]
- 29. Marshall JC, Harsoulis P, Anderson DC, McNeilly AS, Besser GM, Hall R. 1972. Isolated pituitary gonadotrophin deficiency: gonadotrophin secretion after synthetic luteinizing hormone and follicle stimulation hormone-releasing hormone. Br Med J 4:643–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. 1971. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun 44:205–210 [DOI] [PubMed] [Google Scholar]
- 31. Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, Redding TW, Debeljuk L. 1971. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun 43:393–399 [DOI] [PubMed] [Google Scholar]
- 32. Naftolin F, Harris GW, Bobrow M. 1971. Effect of purified luteinizing hormone releasing factor on normal and hypogonadotrophic anosmic men. Nature 232:496–497 [DOI] [PubMed] [Google Scholar]
- 33. Crowley WF, Jr, McArthur JW. 1980. Simulation of the normal menstrual cycle in Kallman's syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH). J Clin Endocrinol Metab 51:173–175 [DOI] [PubMed] [Google Scholar]
- 34. Hoffman AR, Crowley WF., Jr 1982. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- 35. Hockaday TD. 1966. Hypogonadism and life-long anosmia. Postgrad Med J 42:572–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santen RJ, Paulsen CA. 1973. Hypogonadotropic eunuchoidism. II. Gonadal responsiveness to exogenous gonadotropins. J Clin Endocrinol Metab 36:55–63 [DOI] [PubMed] [Google Scholar]
- 37. Santen RJ, Paulsen CA. 1973. Hypogonadotropic eunuchoidism. I. Clinical study of the mode of inheritance. J Clin Endocrinol Metab 36:47–54 [DOI] [PubMed] [Google Scholar]
- 38. White BJ, Rogol AD, Brown KS, Lieblich JM, Rosen SW. 1983. The syndrome of anosmia with hypogonadotropic hypogonadism: a genetic study of 18 new families and a review. Am J Med Genet 15:417–435 [DOI] [PubMed] [Google Scholar]
- 39. Lieblich JM, Rogol AD, White BJ, Rosen SW. 1982. Syndrome of anosmia with hypogonadotropic hypogonadism (Kallmann syndrome): clinical and laboratory studies in 23 cases. Am J Med 73:506–519 [DOI] [PubMed] [Google Scholar]
- 40. Andria G, Ballabio A, Parenti G, DiMaio S, Piccirillo A. 1984. Steroid sulphatase deficiency and hypogonadism. Eur J Pediatr 142:304–305 [DOI] [PubMed] [Google Scholar]
- 41. Andria G, Ballabio A, Parenti G, Di Maio S, Piccirillo A. 1984. Steroid sulphatase deficiency is present in patients with the syndrome ‘ichthyosis and male hypogonadism’ and with ‘Rud syndrome’. J Inherit Metab Dis 7(Suppl 2):159–160 [DOI] [PubMed] [Google Scholar]
- 42. Bick D, Curry CJ, McGill JR, Schorderet DF, Bux RC, Moore CM. 1989. Male infant with ichthyosis, Kallmann syndrome, chondrodysplasia punctata, and an Xp chromosome deletion. Am J Med Genet 33:100–107 [DOI] [PubMed] [Google Scholar]
- 43. Schwanzel-Fukuda M, Bick D, Pfaff DW. 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 [DOI] [PubMed] [Google Scholar]
- 44. Schwanzel-Fukuda M, Pfaff DW. 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- 45. Wray S, Grant P, Gainer H. 1989. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, Brown CJ, Willard HF, Lawrence C, Graziella Persico M, Persico MG, Camerino G, Ballabio A. 1991. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353:529–536 [DOI] [PubMed] [Google Scholar]
- 47. Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, Bougueleret L, Van de Waal HD, Lutfalla G, Weissenbach J, Petit C. 1991. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 67:423–435 [DOI] [PubMed] [Google Scholar]
- 48. Santoro N, Filicori M, Spratt D, Crowley WF., Jr 1986. Gonadotropin-releasing hormone (GnRH) physiology in men and women. Acta Med Hung 43:201–221 [PubMed] [Google Scholar]
- 49. Santoro N, Filicori M, Crowley WF., Jr 1986. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev 7:11–23 [DOI] [PubMed] [Google Scholar]
- 50. Nachtigall LB, Boepple PA, Pralong FP, Crowley WF., Jr 1997. Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med 336:410–415 [DOI] [PubMed] [Google Scholar]
- 51. Hu Y, Guimond SE, Travers P, Cadman S, Hohenester E, Turnbull JE, Kim SH, Bouloux PM. 2009. Novel mechanisms of fibroblast growth factor receptor 1 regulation by extracellular matrix protein anosmin-1. J Biol Chem 284:29905–29920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardelin JP, Dodé C. 2008. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev 2:181–193 [DOI] [PubMed] [Google Scholar]
- 53. Seminara SB. 2007. Kisspeptin in reproduction. Semin Reprod Med 25:337–343 [DOI] [PubMed] [Google Scholar]
- 54. Crowley WF, Jr, Pitteloud N, Seminara S. 2008. New genes controlling human reproduction and how you find them. Trans Am Clin Climatol Assoc 119:29–37; discussion 37–38 [PMC free article] [PubMed] [Google Scholar]
- 55. Bianco SD, Kaiser UB. 2009. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Semple RK, Topaloglu AK. 2010. The recent genetics of hypogonadotrophic hypogonadism—novel insights and new questions. Clin Endocrinol (Oxf) 72:427–435 [DOI] [PubMed] [Google Scholar]
- 57. Joubert FJ, Strydom DJ. 1980. Snake venom. The amino acid sequence of protein A from Dendroaspis polylepis polylepis (black mamba) venom. Hoppe Seylers Z Physiol Chem 361:1787–1794 [DOI] [PubMed] [Google Scholar]
- 58. Schweitz H, Bidard JN, Lazdunski M. 1990. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepis) venom. Toxicon 28:847–856 [DOI] [PubMed] [Google Scholar]
- 59. Schweitz H, Pacaud P, Diochot S, Moinier D, Lazdunski M. 1999. MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett 461:183–188 [DOI] [PubMed] [Google Scholar]
- 60. Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. 1999. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol 374:189–196 [DOI] [PubMed] [Google Scholar]
- 61. Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. 2001. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 59:692–698 [DOI] [PubMed] [Google Scholar]
- 62. LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. 2001. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412:877–884 [DOI] [PubMed] [Google Scholar]
- 63. LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N. 2003. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA 100:2685–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. 2002. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277:19276–19280 [DOI] [PubMed] [Google Scholar]
- 65. Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. 2002. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 293:396–402 [DOI] [PubMed] [Google Scholar]
- 66. Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. 2002. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta 1579:173–179 [DOI] [PubMed] [Google Scholar]
- 67. Negri L, Lattanzi R, Giannini E, Melchiorri P. 2007. Bv8/prokineticin proteins and their receptors. Life Sci 81:1103–1116 [DOI] [PubMed] [Google Scholar]
- 68. Maldonado-Pérez D, Evans J, Denison F, Millar RP, Jabbour HN. 2007. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab 18:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Negri L, Lattanzi R, Giannini E, Canestrelli M, Nicotra A, Melchiorri P. 2009. Bv8/prokineticins and their receptors. A new pronociceptive system. Int Rev Neurobiol 85:145–157 [DOI] [PubMed] [Google Scholar]
- 70. Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, Ho C, Ross J, Tan M, Carano RA, Meng YG, Ferrara N. 2007. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450:825–831 [DOI] [PubMed] [Google Scholar]
- 71. Monnier J, Samson M. 2008. Cytokine properties of prokineticins. FEBS J 275:4014–4021 [DOI] [PubMed] [Google Scholar]
- 72. Ngan ES, Tam PK. 2008. Prokineticin-signaling pathway. Int J Biochem Cell Biol 40:1679–1684 [DOI] [PubMed] [Google Scholar]
- 73. Kishi T, Kitajima T, Tsunoka T, Okumura T, Ikeda M, Okochi T, Kinoshita Y, Kawashima K, Yamanouchi Y, Ozaki N, Iwata N. 2009. Possible association of prokineticin 2 receptor gene (PROKR2) with mood disorders in the Japanese population. Neuromolecular Med 11:114–122 [DOI] [PubMed] [Google Scholar]
- 74. Attramadal H. 2009. Prokineticins and the heart: diverging actions elicited by signalling through prokineticin receptor-1 or -2. Cardiovasc Res 81:3–4 [DOI] [PubMed] [Google Scholar]
- 75. Kaser A, Winklmayr M, Lepperdinger G, Kreil G. 2003. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep 4:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bullock CM, Li JD, Zhou QY. 2004. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol 65:582–588 [DOI] [PubMed] [Google Scholar]
- 77. LeCouter J, Lin R, Frantz G, Zhang Z, Hillan K, Ferrara N. 2003. Mouse endocrine gland-derived vascular endothelial growth factor: a distinct expression pattern from its human ortholog suggests different roles as a regulator of organ-specific angiogenesis. Endocrinology 144:2606–2616 [DOI] [PubMed] [Google Scholar]
- 78. Lin R, LeCouter J, Kowalski J, Ferrara N. 2002. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem 277:8724–8729 [DOI] [PubMed] [Google Scholar]
- 79. Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. 2006. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26:11615–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jilek A, Engel E, Beier D, Lepperdinger G. 2000. Murine Bv8 gene maps near a synteny breakpoint of mouse chromosome 6 and human 3p21. Gene 256:189–195 [DOI] [PubMed] [Google Scholar]
- 81. Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G. 1999. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett 462:177–181 [DOI] [PubMed] [Google Scholar]
- 82. Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C. 2005. Identification and pharmacological characterization of prokineticin 2 β as a selective ligand for prokineticin receptor 1. Mol Pharmacol 67:2070–2076 [DOI] [PubMed] [Google Scholar]
- 83. Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. 2002. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410 [DOI] [PubMed] [Google Scholar]
- 84. Zhang C, Ng KL, Li JD, He F, Anderson DJ, Sun YE, Zhou QY. 2007. Prokineticin 2 is a target gene of proneural basic helix-loop-helix factors for olfactory bulb neurogenesis. J Biol Chem 282:6917–6921 [DOI] [PubMed] [Google Scholar]
- 85. Boisbouvier J, Albrand JP, Blackledge M, Jaquinod M, Schweitz H, Lazdunski M, Marion D. 1998. A structural homologue of colipase in black mamba venom revealed by NMR floating disulphide bridge analysis. J Mol Biol 283:205–219 [DOI] [PubMed] [Google Scholar]
- 86. Niehrs C. 2006. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481 [DOI] [PubMed] [Google Scholar]
- 87. Ferrara N, LeCouter J, Lin R, Peale F. 2004. EG-VEGF and Bv8: a novel family of tissue-restricted angiogenic factors. Biochim Biophys Acta 1654:69–78 [DOI] [PubMed] [Google Scholar]
- 88. Cheng MY, Bittman EL, Hattar S, Zhou QY. 2005. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. 2005. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308:1923–1927 [DOI] [PubMed] [Google Scholar]
- 90. Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. 2005. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab 90:427–434 [DOI] [PubMed] [Google Scholar]
- 91. Kisliouk T, Friedman A, Klipper E, Zhou QY, Schams D, Alfaidy N, Meidan R. 2007. Expression pattern of prokineticin 1 and its receptors in bovine ovaries during the estrous cycle: involvement in corpus luteum regression and follicular atresia. Biol Reprod 76:749–758 [DOI] [PubMed] [Google Scholar]
- 92. Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. 2004. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab 89:2463–2469 [DOI] [PubMed] [Google Scholar]
- 93. Hoffmann P, Feige JJ, Alfaidy N. 2007. Placental expression of EG-VEGF and its receptors PKR1 (prokineticin receptor-1) and PKR2 throughout mouse gestation. Placenta 28:1049–1058 [DOI] [PubMed] [Google Scholar]
- 94. Hoffmann P, Feige JJ, Alfaidy N. 2006. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology 147:1675–1684 [DOI] [PubMed] [Google Scholar]
- 95. Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. 2008. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology 149:2877–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Denison FC, Battersby S, King AE, Szuber M, Jabbour HN. 2008. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology 149:3470–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ngan ES, Lee KY, Yeung WS, Ngan HY, Ng EH, Ho PC. 2006. Endocrine gland-derived vascular endothelial growth factor is expressed in human peri-implantation endometrium, but not in endometrial carcinoma. Endocrinology 147:88–95 [DOI] [PubMed] [Google Scholar]
- 98. Lee KF, Lee YL, Chan RW, Cheong AW, Ng EH, Ho PC, Yeung WS. 2010. Up-regulation of endocrine gland-derived vascular endothelial growth factor but not vascular endothelial growth factor in human ectopic endometriotic tissue. Fertil Steril 93:1052–1060 [DOI] [PubMed] [Google Scholar]
- 99. Tiberi F, Tropea A, Apa R, Romani F, Lanzone A, Marana R. 2010. Prokineticin 1 mRNA expression in the endometrium of healthy women and in the eutopic endometrium of women with endometriosis. Fertil Steril 93:2145–2149 [DOI] [PubMed] [Google Scholar]
- 100. Shaw JL, Denison FC, Evans J, Durno K, Williams AR, Entrican G, Critchley HO, Jabbour HN, Horne AW. 2010. Evidence of prokineticin dysregulation in Fallopian tube from women with ectopic pregnancy. Fertil Steril 94:1601–1608.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cook IH, Evans J, Maldonado-Pérez D, Critchley HO, Sales KJ, Jabbour HN. 2010. Prokineticin-1 (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor 1 (PROKR1) and the calcineurin/NFAT signalling pathway. Mol Hum Reprod 16:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Samson M, Peale FV, Jr, Frantz G, Rioux-Leclercq N, Rajpert-De Meyts E, Ferrara N. 2004. Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. J Clin Endocrinol Metab 89:4078–4088 [DOI] [PubMed] [Google Scholar]
- 103. Zhou QY. 2006. The prokineticins: a novel pair of regulatory peptides. Mol Interv 6:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Heitzler D, Crépieux P, Poupon A, Clément F, Fages F, Reiter E. 2009. Towards a systems biology approach of G protein-coupled receptor signalling: challenges and expectations. C R Biol 332:947–957 [DOI] [PubMed] [Google Scholar]
- 105. Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745 [DOI] [PubMed] [Google Scholar]
- 106. Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. 2007. Crystal structure of the human β2 adrenergic G protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- 107. Ritter SL, Hall RA. 2009. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol 10:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cheng MY, Leslie FM, Zhou QY. 2006. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol 498:796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Koyama Y, Kiyo-oka M, Osakada M, Horiguchi N, Shintani N, Ago Y, Kakuda M, Baba A, Matsuda T. 2006. Expression of prokineticin receptors in mouse cultured astrocytes and involvement in cell proliferation. Brain Res 1112:65–69 [DOI] [PubMed] [Google Scholar]
- 110. Podlovni H, Ovadia O, Kisliouk T, Klipper E, Zhou QY, Friedman A, Alfaidy N, Meidan R. 2006. Differential expression of prokineticin receptors by endothelial cells derived from different vascular beds: a physiological basis for distinct endothelial function. Cell Physiol Biochem 18:315–326 [DOI] [PubMed] [Google Scholar]
- 111. Söderhäll I, Kim YA, Jiravanichpaisal P, Lee SY, Söderhäll K. 2005. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J Immunol 174:6153–6160 [DOI] [PubMed] [Google Scholar]
- 112. Zhong C, Qu X, Tan M, Meng YG, Ferrara N. 2009. Characterization and regulation of bv8 in human blood cells. Clin Cancer Res 15:2675–2684 [DOI] [PubMed] [Google Scholar]
- 113. Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. 2006. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Puverel S, Nakatani H, Parras C, Soussi-Yanicostas N. 2009. Prokineticin receptor 2 expression identifies migrating neuroblasts and their subventricular zone transient-amplifying progenitors in adult mice. J Comp Neurol 512:232–242 [DOI] [PubMed] [Google Scholar]
- 115. Alvarez-Buylla A, Lim DA. 2004. For the long run: maintaining germinal niches in the adult brain. Neuron 41:683–686 [DOI] [PubMed] [Google Scholar]
- 116. Zhang C, Truong KK, Zhou QY. 2009. Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One 4:e7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kisliouk T, Podlovni H, Spanel-Borowski K, Ovadia O, Zhou QY, Meidan R. 2005. Prokineticins (endocrine gland-derived vascular endothelial growth factor and BV8) in the bovine ovary: expression and role as mitogens and survival factors for corpus luteum-derived endothelial cells. Endocrinology 146:3950–3958 [DOI] [PubMed] [Google Scholar]
- 118. Martucci C, Franchi S, Giannini E, Tian H, Melchiorri P, Negri L, Sacerdote P. 2006. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol 147:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. 2002. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol 137:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Melchiorri D, Bruno V, Besong G, Ngomba RT, Cuomo L, De Blasi A, Copani A, Moschella C, Storto M, Nicoletti F, Lepperdinger G, Passarelli F. 2001. The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur J Neurosci 13:1694–1702 [DOI] [PubMed] [Google Scholar]
- 121. Shapiro M. 2009. Memory networks: answering the call of the hippocampus. Curr Biol 19:R329–R330 [DOI] [PubMed] [Google Scholar]
- 122. Shapiro LA, Ng K, Zhou QY, Ribak CE. 2009. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav 14(Suppl 1):74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. de la Iglesia HO, Schwartz WJ. 2006. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153 [DOI] [PubMed] [Google Scholar]
- 124. Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, Ebling FJ. 2009. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One 4:e5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ji Y, Li X. 2009. Cloning and developmental expression analysis of prokineticin 2 and its receptor PKR2 in the Syrian hamster suprachiasmatic nucleus. Brain Res 1271:18–26 [DOI] [PubMed] [Google Scholar]
- 126. Monnier C, Dodé C, Fabre L, Teixeira L, Labesse G, Pin JP, Hardelin JP, Rondard P. 2009. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum Mol Genet 18:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. 2007. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Aston KI, Carrell DT. 2009. Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl 30:711–725 [DOI] [PubMed] [Google Scholar]
- 129. Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF, Jr, Pitteloud N. 2007. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- 130. Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. 2007. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep 30:247–256 [PMC free article] [PubMed] [Google Scholar]
- 131. Jethwa PH, I'Anson H, Warner A, Prosser HM, Hastings MH, Maywood ES, Ebling FJ. 2008. Loss of prokineticin receptor 2 signaling predisposes mice to torpor. Am J Physiol Regul Integr Comp Physiol 294:R1968–R1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. 2004. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol 142:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, Ebling FJ, Vickers SP, Cheetham S, Ghatei MA, Bloom SR, Dhillo WS. 2010. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes 59:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF, Jr, Pitteloud N. 2010. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA 107:15140–15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abreu AP, Kaiser UB, Latronico AC. 2010. The role of prokineticins in the pathogenesis of hypogonadotropic hypogonadism. Neuroendocrinology 91:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Negri L, Lattanzi R, Giannini E, Melchiorri P. 2006. Modulators of pain: Bv8 and prokineticins. Curr Neuropharmacol 4:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F. 2006. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci 26:6716–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hu WP, Zhang C, Li JD, Luo ZD, Amadesi S, Bunnett N, Zhou QY. 2006. Impaired pain sensation in mice lacking prokineticin 2. Mol Pain 2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Giannini E, Lattanzi R, Nicotra A, Campese AF, Grazioli P, Screpanti I, Balboni G, Salvadori S, Sacerdote P, Negri L. 2009. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci USA 106:14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Urayama K, Guilini C, Messaddeq N, Hu K, Steenman M, Kurose H, Ert G, Nebigil CG. 2007. The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis. FASEB J 21:2980–2993 [DOI] [PubMed] [Google Scholar]
- 141. Urayama K, Guilini C, Turkeri G, Takir S, Kurose H, Messaddeq N, Dierich A, Nebigil CG. 2008. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol 28:841–849 [DOI] [PubMed] [Google Scholar]
- 142. Urayama K, Dedeoglu DB, Guilini C, Frantz S, Ertl G, Messaddeq N, Nebigil CG. 2009. Transgenic myocardial overexpression of prokineticin receptor-2 (GPR73b) induces hypertrophy and capillary vessel leakage. Cardiovasc Res 81:28–37 [DOI] [PubMed] [Google Scholar]
- 143. Guilini C, Urayama K, Turkeri G, Dedeoglu DB, Kurose H, Messaddeq N, Nebigil CG. 2010. Divergent roles of prokineticin receptors in the endothelial cells: angiogenesis and fenestration. Am J Physiol Heart Circ Physiol 298:H844–H852 [DOI] [PubMed] [Google Scholar]
- 144. Choke E, Cockerill GW, Laing K, Dawson J, Wilson WR, Loftus IM, Thompson MM. 2009. Whole genome-expression profiling reveals a role for immune and inflammatory response in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 37:305–310 [DOI] [PubMed] [Google Scholar]
- 145. Ulrich S, Taraseviciene-Stewart L, Huber LC, Speich R, Voelkel N. 2008. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross-sectional study. Respir Res 9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. 2009. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 147. Jayasena CN, Dhillo WS. 2009. Kisspeptin offers a novel therapeutic target in reproduction. Curr Opin Investig Drugs 10:311–318 [PubMed] [Google Scholar]
- 148. O'Rahilly S. 2009. Human genetics illuminates the paths to metabolic disease. Nature 462:307–314 [DOI] [PubMed] [Google Scholar]
- 149. Cohen JC, Kimmel M, Polanski A, Hobbs HH. 2003. Molecular mechanisms of autosomal recessive hypercholesterolemia. Curr Opin Lipidol 14:121–127 [DOI] [PubMed] [Google Scholar]
- 150. Lifton RP. 2004. Genetic dissection of human blood pressure variation: common pathways from rare phenotypes. Harvey Lect 100:71–101 [PubMed] [Google Scholar]
- 151. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 152. Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pêcheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP. 2003. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- 153. Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. 2008. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J. 2009. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- 155. de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. 1997. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- 156. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. 1997. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- 158. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. 2007. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Habiby RL, Boepple P, Nachtigall L, Sluss PM, Crowley WF, Jr, Jameson JL. 1996. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalmic and pituitary defects in gonadotropin production. J Clin Invest 98:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RJ, Walker SL, Shi Y, Gusella JF, Layman LC. 2008. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet 83:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Monnier J, Piquet-Pellorce C, Feige JJ, Musso O, Clement B, Turlin B, Theret N, Samson M. 2008. Prokineticin 2/Bv8 is expressed in Kupffer cells in liver and is down regulated in human hepatocellular carcinoma. World J Gastroenterol 14:1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Keramidas M, Faudot C, Cibiel A, Feige JJ, Thomas M. 2008. Mitogenic functions of endocrine gland-derived vascular endothelial growth factor and Bombina variegata 8 on steroidogenic adrenocortical cells. J Endocrinol 196:473–482 [DOI] [PubMed] [Google Scholar]
- 163. Jiang X, Abiatari I, Kong B, Erkan M, De Oliveira T, Giese NA, Michalski CW, Friess H, Kleeff J. 2009. Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology 9:165–172 [DOI] [PubMed] [Google Scholar]
- 164. Bassil AK, Dass NB, Murray CD, Muir A, Sanger GJ. 2005. Prokineticin-2, motilin, ghrelin and metoclopramide: prokinetic utility in mouse stomach and colon. Eur J Pharmacol 524:138–144 [DOI] [PubMed] [Google Scholar]
- 165. Ngan ES, Shum CK, Poon HC, Sham MH, Garcia-Barcelo MM, Lui VC, Tam PK. 2008. Prokineticin-1 (Prok-1) works coordinately with glial cell line-derived neurotrophic factor (GDNF) to mediate proliferation and differentiation of enteric neural crest cells. Biochim Biophys Acta 1783:467–478 [DOI] [PubMed] [Google Scholar]
- 166. LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. 2004. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA 101:16813– 16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Balasubramanian R, Dwyer A, Seminara SB, Pitteloud N, Kaiser UB, Crowley WF., Jr 2010. Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons. Neuroendocrinology 92:81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]