This article provides a comprehensive review on the biological functions of chicken ovalbumin upstream promoter transcription factor-II (COUP-TFII), an orphan member of the nuclear receptor superfamily, in development and diseases. It also summarizes the signaling pathways and the underlying mechanisms that mediate COUP-TFII function. This review also briefly summarizes the current findings on the closely related family member, COUP-TFI.
Abstract
Chicken ovalbumin upstream promoter transcription factors (COUP-TFs) belong to the steroid/thyroid hormone receptor superfamily. Cloning of their cDNAs demonstrated the existence of two distinct but related genes: COUP-TFI (EAR-3, NR2F1) and COUP-TFII (ARP-1, NR2F2). They are referred to as orphan receptors because ligands for COUP-TFs have yet to be identified. Since 1998, extensive studies have demonstrated their physiological importance in cell-fate specification, organogenesis, angiogenesis, and metabolism, as well as a variety of diseases. In this article, we will comprehensively review the biological functions of COUP-TFII and its underlying mechanism in various developmental processes and diseases. In addition, we will briefly summarize some of the current findings of COUP-TFI.
Introduction
Expression Patterns of COUP-TFII in Mouse
Multiple Roles of COUP-TFII in the Developing Mouse
-
Role of COUP-TFII in Cardiovascular Development and Function
COUP-TFII is required for angiogenesis and heart development
COUP-TFII confers vein identity
COUP-TFII regulates coronary vessel identity
COUP-TFII governs lymphangiogenesis
-
COUP-TFII in Reproduction
COUP-TFII is essential for normal female reproductive functions
COUP-TFII is important for the differentiation of Leydig cells for proper male fertility
-
COUP-TFII in Energy Metabolism and Adipogenesis
COUP-TFII plays an important role in energy homeostasis
COUP-TFII plays a central role in adipogenesis
-
COUP-TFII in the Neuronal Development
COUP-TFs determine the cell fate of the progenitor cells in the eye development
COUP-TFs are required in the retinal development
COUP-TFII regulates the cerebellum development
-
COUP-TFII in Organogenesis
COUP-TFII is essential for the radial and anteroposterior (AP) patterning of the stomach
COUP-TFII is necessary for limb and skeletal muscle development
COUP-TFII is essential for the insulin secretion and pancreatic function
-
COUP-TFII in Diseases
COUP-TFII deletion results in the congenital diaphragmatic hernia (CDH)
COUP-TFII is essential for the tumor growth and metastasis
COUP-TFII may play a role in the development of ER-positive breast cancer
COUP-TFI in Early Neural Development
A Comparison between COUP-TFI and COUP-TFII
Summary and Perspective
I. Introduction
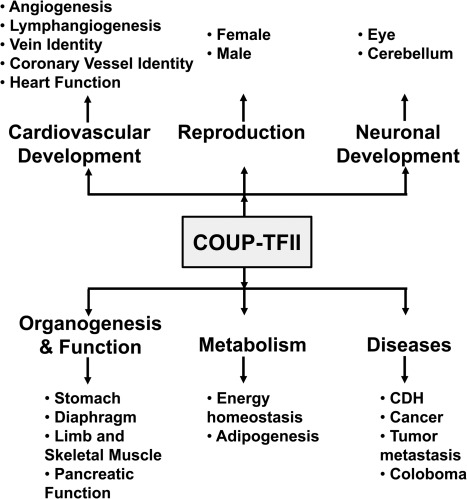
COUP-TFs were first cloned from a HeLa cell cDNA library (1) and shown to be transcriptional factors regulating the expression of the chicken ovalbumin gene in chicken oviducts; hence, they were named chicken ovalbumin upstream promoter transcriptional factors. In mammals, there are two COUP-TFs, COUP-TFI (EAR-3, NR2F1, according to the Nuclear Receptors Nomenclature Committee 1999) (1, 2) and COUP-TFII (ARP-1, NR2F2) (3, 4). Both are encoded by distinct genes located on different chromosomes. They are referred to as orphan nuclear receptors because ligands for COUP-TFs have not been identified. COUP-TFs possess the classic domain structure of nuclear receptors. Specifically, they encompass two highly conserved motifs: 1) a DNA-binding domain (DBD) containing two zinc-fingers; and 2) two highly conserved regions, I and II, in the ligand-binding domain (LBD) (5). Strikingly, there exists a 98% homology within the DBD of COUP-TFI and -II. Additionally, COUP-TFI and -II share 96 and 100% sequence homology in the highly conserved region I and region II, respectively. COUP-TFs form homodimers and bind to a wide range of direct repeat AGGTCA motifs with a variety of spacing and orientation (6, 7). In 20 yr of intensive research since their discovery, COUP-TFs have been implicated in many vital processes, such as organogenesis, angiogenesis, and metabolic homeostasis, as well as in a variety of developmental programs. These developmental processes involve COUP-TFs in different cellular functions, such as the cell fate determination, cell differentiation, cell survival, and cell migration. Because COUP-TFI has recently been reviewed (8), we will discuss COUP-TFII in more detail here. We will summarize the data available so far on COUP-TFII functions in angiogenesis; lymphangiogenesis; adipogenesis; energy metabolism; diaphragm, stomach, limb, skeletal muscle, eye, retinal, and cerebellum development; cancer; and female and male reproduction (Fig. 1), and on COUP-TFI functions in early neural development.
Fig. 1.
Involvement of COUP-TFII in physiological and pathological processes during mouse development. CDH, Congenital diaphragmatic hernia.
II. Expression Patterns of COUP-TFII in Mouse
COUP-TFII, which maps to the central region of chromosome 7 in the mouse (9), has an onset of expression at embryonic day (E) 8.5 in the visceral mesoderm and differentiating myocardium of the sinus venosus and in the mesoderm surrounding the umbilical veins (10) (Tables 1 and 2). At E9.0, COUP-TFII transcripts are detected in the common atrium and in the mesenchyme of the developing umbilical veins, notochord, somite, hindbrain, and the otocyst as well as in the optic vesicle (Table 1). As development proceeds, COUP-TFII can also be detected in the surrounding mesenchyme of the foregut, midgut, hindgut, lung bud, septum transversum, and hepatic primordium by E10.5 (C.-T. Yu, S. Y. Tsai, and M.-J. Tsai, unpublished results).
Table 1.
The expression patterns of COUP-TFII in mice
| Stage | Expression domain | Refs. |
|---|---|---|
| E8.5 | Otic placode, visceral mesoderm, the primitive myocardium of the sinus venosus, the mesoderm surrounding the umbilical veins | 10 |
| E9.5 | The ventral part of the developing neural tube (floorplate), the motor neuron, the common atrium, the mesenchyme of the developing umbilical veins, foregut, gut endoderm, notochord, somite, hindbrain, otocyst, optic vesicle | 51, 98, 99 |
| E10.5 | Similar to that of E9.5, neuroepithelium of midbrain and hindbrain, the dorsal root ganglion, the first branchial arch, the surrounding mesenchyme of foregut, midgut, hindgut, lung bud, septum transversum, hepatic primordium, and the mesonephric tube | C.-T. Yu, S. Y. Tsai, and M.-J. Tsai, unpublished results |
| E14.5 | Notochord, liver, muscle, cochlea, the adrenal gland, retina, the submandibular gland, the mesenchymal compartment of the developing organs such as kidney, lung, pancreas primordium, prostate, stomach, spleen, intestine, bladder, ovary, testis, trachea, esophagus, olfactory epithelium, and salivary gland | Refs. 11 and 98; and C.-T. Yu, S. Y. Tsai, and M.-J. Tsai, unpublished results |
| E18.5 | All tissues derived from endoderm (lung, pancreas, stomach, intestine, bladder, esophagus, and thyroid), kidney, heart, ovary, testis | Refs. 37, 39, 43, 57; and C.-T. Yu, S. Y. Tsai, and M.-J. Tsai, unpublished results |
| Adult | The ventral thalamus, hypothalamus, midbrain, pons, and spinal cord, Purkinje cells in the cerebellum, venous and lymphatic endothelial cells, smooth muscle cells, testis, ovary, the overall expression decreases after the completion of organogenesis | 33, 37, 39, 43, 55, 56 |
Table 2.
A brief comparison of COUP-TFI and COUP-TFII
| COUP-TFI | COUP-TFII | |
|---|---|---|
| Location on chromosome | Chromosome 5 in human, chromosome 13 in mouse (9) | Chromosome 15 in human, chromosome 7 in mouse (9) |
| Amino acid homology in comparison to COUP-TFI | 100% (DBD), 100% (LBD) (5) | 98% (DBD), 96% (LBD) (5) |
| Onset of expression in mouse | E7.5 in the neural ectoderm (100) | E8.5 in the visceral mesoderm and differentiating myocardium (10) |
| Expression pattern in mice | High in the developing neural tissues and low in internal organs (100) | High in the mesenchymal component of the developing organs (11) |
| Loss of function in mice | Perinatal lethality (die by P2) (90) | Embryonic lethality (die by E10) (10) |
| Potential physiological functions | Axon myelination (94) | Angiogenesis (11, 14, 77) |
| Axon guidance and arborization (90, 91, 93) | Adipogenesis and metabolism (46) | |
| Cortex patterning (88, 95) | Cancer progression (84, 86, 87) | |
| Formation of ganglion IX (90) | Eye and cerebellum development (51, 53, 56) | |
| Neuronal fate specification (51, 96) | Venous and arterial specification (15) | |
| Inner ear development (101) | Limb myoblast migration (62) | |
| Neural cell migration (102, 103) | Male and female reproduction (37–39, 41–43) | |
| Eye development (51) | Stomach patterning (72) | |
| Tumor metastasis (14) | ||
| Cellular mechanism | Inhibition of transcription: direct binding of COUP-TFs to DR site (6) | Similar to COUP-TFI (14, 33, 46, 51, 77) |
| Activation of transcription: tethering of COUP-TFs to Sp1-bound at Sp1 site (104) |
Reference numbers are in parentheses.
At later stages of embryogenesis, COUP-TFII expression continues in the mesenchymal compartment of the developing organs such as the kidney, lung, pancreas primordium, prostate, stomach, limb buds, ovary, testis, trachea, esophagus, and salivary gland (11). In general, COUP-TFII is expressed in the mesenchymal cells of the developing organs, not in the terminally differentiated epithelium. As with COUP-TFI, from birth onward, COUP-TFII is expressed at a relatively low level in various developed organs including the uterus, liver, stomach, mammary gland, kidney, prostate, heart, lung, and brain. Taken together, the expression of COUP-TFII is highest at E14–E15 in the mesenchymal compartment of the developing organs and declines sharply after the completion of organogenesis. The spatial and temporal expression pattern of COUP-TFII suggests that COUP-TFII might play a major role in the regulation of mesenchymal-epithelial interaction during organogenesis.
III. Multiple Roles of COUP-TFII in the Developing Mouse
The dynamic expression patterns of COUP-TFII during embryonic development of vertebrates and invertebrates suggest that COUP-TFII might be important for organogenesis. To understand the physiological functions of COUP-TFII in vivo, COUP-TFII null mouse mutants were generated in our laboratory by homologous recombination in embryonic stem cells (10). Targeted disruption of COUP-TFII in mice resulted in early embryonic lethality. As discussed below, recent data using mouse genetics provide novel insights into the different roles of COUP-TFII during organogenesis and development.
IV. Role of COUP-TFII in Cardiovascular Development and Function
A. COUP-TFII is required for angiogenesis and heart development
Heterozygous COUP-TFII (COUP-TFII+/−) mice appeared smaller in comparison to the wild-type littermates at birth, and the number of heterozygous mice was below the expected Mendelian ratio (10). Heterozygous mice that survived past weaning were subfertile (10), and their intercross generated a homozygous COUP-TFII (COUP-TFII−/−) mutant. The homozygous mutants were lethal around E10 (10). Embryos at E9.5 are growth-retarded and have severe hemorrhage in the forebrain throughout the brain vesicles and heart (10). By E10.5, mutants were very bloody and were being resorbed. Histological analyses of COUP-TFII null mutants revealed defects in atrial and vascular development (10). The common atrium appears smaller and fails to expand. In addition, the anterior and posterior cardinal veins are either collapsed or severely malformed. Whole-mount immunostaining for platelet endothelial cell adhesion molecule-1, an endothelial cell marker, shows that the primitive capillary plexus does form but fails to undergo remodeling to generate large and small microcapillaries, suggesting that angiogenesis is defective in the null mutants.
The underlying mechanism contributing to the angiogenic defects has been further investigated. The expression of angiopietin-1 (Ang-1), a secreted growth factor important for angiogenesis, was significantly down-regulated in the mesenchyme of the heart, brain, eye, and somite in COUP-TFII mutants. On the other hand, the expression of Tie2, the receptor for Ang-1, was not altered in these mutants. These data imply that COUP-TFII might regulate the expression of Ang-1 in the mesenchymal compartment and perturb the Ang-1-Tie2 signaling pathway, resulting in the heart and vascular defects observed in COUP-TFII mutants (10). This finding is supported by the fact that mice lacking Ang-1 or Tie2 display similar vascular and cardiac phenotypes as COUP-TFII mutants (12, 13). Furthermore, COUP-TFII is shown to directly regulate Ang-1 expression in cultured cells (14). Taken together, COUP-TFII plays an important role in angiogenesis during development.
B. COUP-TFII confers vein identity
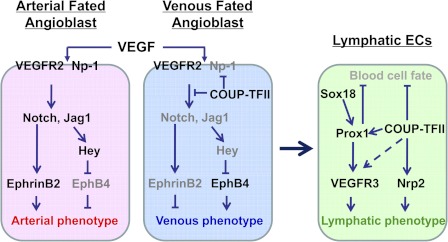
COUP-TFII−/− mutants exhibit the vascular abnormalities that are consistent with COUP-TFII expression pattern in the vessels. COUP-TFII is highly expressed in the endothelium of the veins, but not in the arterial endothelial cells. In addition, COUP-TFII is expressed in the smooth muscle cells of arteries (15). These distinct expression patterns indicate a possible role in the establishment of arterial-venous identity. To address the contribution of COUP-TFII in vein identity, chimera analysis has been carried out and has shown that COUP-TFII null cells were unable to contribute to endothelial formation, suggesting that COUP-TFII has a cell-autonomous function in the formation of the venous endothelium (15). When COUP-TFII was deleted in the endothelium using Tie2-Cre recombinase, the knockout mice died by E12, exhibiting a variety of vascular defects, including hemorrhage, well-dilated vessels, and a defect in arterial-venous specification (15). The general concept of arterial specification is that sonic hedgehog (Shh) lies upstream of vascular endothelial growth factor (VEGF) and Notch signaling to guide the arterial cell fate (16). Shh secreted by the notochord and floor plate of the neural tube is required for the induction of VEGF expression in the somites (16). VEGF binds to its receptor, VEGFR-2, and the coreceptor neuropilin 1 (NP-1), which in turn activates Notch signaling in the endothelial cells, leading to the expression of arterial-specific marker ephrin B2 (efnb2) and the repression of venous marker ephrin receptor B4 (EphB4) expression (17–19). In the endothelial-specific COUP-TFII null mutants, arterial markers such as Notch1, Jagged1 (Jag1), Hey1 and NP-1 were ectopically expressed, whereas venous marker EphB4 was reduced in the veins of the mutants. Functional analysis also showed that mutant veins behaved similarly to an artery, as was evident from the formation of hematopoietic cell clusters at the aorta-gonad-mesonephros (AGM) in the mutant veins and the recruitment of smooth muscle cells surrounding the venous endothelium. The formation of hematopoietic cell clusters and the recruitment of smooth muscle cells are the hallmarks of a functional artery, but not the vein (20). These findings demonstrated that ablation of COUP-TFII in endothelial cells enables veins to acquire arterial characteristics.
In contrast, transgenic embryos with ectopic expression of COUP-TFII in the endothelium exhibited the fusion of veins and arteries (15). The disorganized vessels revealed a significant down-regulation of arterial markers such as NP-1 and Jag1 and up-regulation of a venous marker, EphB4. This suggests that overexpression of COUP-TFII proteins in endothelial cells suppresses the arterial characteristics. These phenotypes resemble the vascular phenotypes exhibited by mouse embryos lacking NP-1, Notch1, or Notch signaling indicating that Notch signaling is likely the downstream target that mediates the defects of COUP-TFII mutants (17, 19, 21, 22). Taken together, the novel feature of this phenotype supported the new working model that, during normal development, COUP-TFII in the venous endothelium suppresses NP-1 and other Notch signaling molecules to confer vein identity (Fig. 2). In contrast, the absence of COUP-TFII in the arterial endothelium allows the expression of Notch signaling molecules to activate the expression of artery-specific genes that confer the artery identity.
Fig. 2.
Model showing arterial, venous, and lymphatic cell fate specification in the developing embryos. In an arterial-fated angioblast, upon VEGF stimulation, the VEGFR-2/Np-1 signaling activates Notch signaling pathway, leading to the induction of arterial marker EphrinB2 and the establishment of arterial phenotype. In contrast, COUP-TFII promotes venous cell fate by suppressing the expression of Np-1 and Notch signaling molecules in a venous-fated angioblast. As development proceeds, a subset of Sox18 and COUP-TFII-expressing venous endothelial cells turn on Prox1 expression, thus inducing the activation of VEGFR-3/Nrp2 signaling. Both COUP-TFII and Prox1 are required for suppressing blood endothelial cell fate and promoting lymphatic endothelial cell fate. [Adapted from a model presented in Kume T: Histol Histopathol 25:637–646, 2010 (105).]
C. COUP-TFII regulates coronary vessel identity
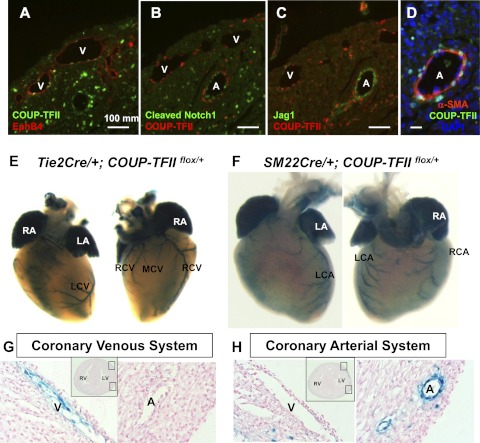
Similar to other vascular systems, coronary vasculature development starts with the formation of the primitive vascular plexus, which is evident by E12.5 (23). Establishment of the vascular network is followed by extensive remodeling giving rise to mature coronary vasculature. Interestingly, based on the locations, two sets of coronary vessels can be identified in the developing mouse hearts: subepicardial vessels, which are positioned closer to the epicardium; and intramyocardial vessels, which are located within the myocardial wall. Corrosion casting studies in developing rat hearts and genetic studies in mice suggested that subepicardial vessels and intramyocardial vessels represent coronary veins and arteries, respectively (24, 25). COUP-TFII is detected in EphB4-expressing coronary venous endothelial cells, but not the Notch1 and Jag1-positive coronary arterial endothelial cells (Fig. 3, A–C). Instead, COUP-TFII is colocalized with α-smooth muscle actin-positive smooth muscle cells surrounding the coronary arteries (Fig. 3D). To trace COUP-TFII expression in specific cell types in vivo, the LacZ gene was placed downstream of the floxed COUP-TFII allele, and a Cre-lox system was used. In the presence of Cre recombinase, the floxed COUP-TFII gene (COUP-TFIIflox/flox) will be removed, allowing for expression of the β-galactosidase. At E18.5, X-gal staining of Tie2Cre; COUP-TFIIflox/+ hearts revealed that the coronary venous system is mainly located on the dorsal side of the hearts, including the coronary sinus and its three major tributaries: the left cardiac vein, the major caudal cardiac vein, and the right cardiac vein (Fig. 3). The cardiac veins drain blood to the coronary sinus, directly to the right atrium (25). As evidenced by X-gal staining of SM22αCre; COUP-TFII flox/+ hearts at postnatal day (P) 0, which marks the smooth muscle cells of the artery, we observed the coronary arterial system consisting of two branches: the left cardiac artery, and the right cardiac artery (Fig. 3). Section analysis further showed that veins are located superficially under the epicardium, whereas arteries are positioned bilaterally within the myocardial wall (Fig. 3, G and H). The expression patterns of COUP-TFII in coronary venous and arterial vessels closely resemble those shown for the venous and arterial system (15).
Fig. 3.
The expression domain of COUP-TFII in mouse hearts. A–D, Immunofluorescent staining of E19.5 hearts with antibodies against COUP-TFII, venous marker EphB4, arterial markers Notch1 and Jag1, and smooth muscle cell marker α-smooth muscle actin (α-SMA) showed that COUP-TFII is expressed in the endothelium of EphB4-positive subepicardial vessels (veins) and the smooth muscle cells of Notch1 and Jag1-expressing intramyocardial vessels (arteries). 4',6-diamidino-2-phenylindole is for nuclei staining. E, The visualization of the coronary venous system by using Tie2Cre transgenic mouse line to label COUP-TFII in the endothelium. F, SM22αCre transgenic mouse line was used to label COUP-TFII in the smooth muscle cells. Whole-mount X-gal staining of SM22Cre; COUP-TFII F/+ hearts at P0 clearly marks the coronary arterial system. G and H, Histological sections of hearts (from panels E and F), showing that COUP-TFII is expressed in the endothelial cells of the coronary veins and the smooth muscle cells of the coronary arteries. A, Artery; LA, Left atrium; LCA, left cardiac artery; LCV, left cardiac vein; LV, left ventricle; MCV, major caudal cardiac vein; RA, right atrium; RCA, right cardiac artery; RCV, right cardiac vein; RV, right ventricle; V, vein.
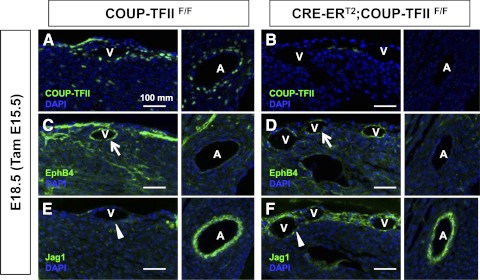
The expression patterns of COUP-TFII in coronary vasculature highly suggest a critical role of COUP-TFII in conferring coronary vessel identity. When the COUP-TFII gene was conditionally inactivated subsequent to the formation of coronary vessels at E15.5, the coronary venous identity was altered at E18.5. The venous marker EphB4 was down-regulated, whereas the arterial marker Jag1 was ectopically expressed in the cardiac veins of COUP-TFII conditional knockout mutants at E18.5 (Fig. 4). In contrast, the expression of Jag1 was unaffected in the artery at E18.5 (Fig. 4). These results indicate that COUP-TFII regulates coronary venous identity and further imply that the coronary vessel identity is reversible during the developmental window of E15.5 to E18.5.
Fig. 4.
Coronary venous identity was altered in the absence of COUP-TFII. Transverse sections of control COUP-TFIIF/F and CRE-ERT2; COUP-TFIIF/F mouse hearts at E18.5 (TAM administrated at E15.5) were analyzed using antibodies against COUP-TFII (A and B), EphB4 (C and D), and Jag1 (E and F). When COUP-TFII is deleted, EphB4 is down-regulated (arrows) and Jag1 is ectopically expressed in the cardiac veins (arrowheads). A, Artery; V, vein.
D. COUP-TFII governs lymphangiogenesis
Although the role of COUP-TFII in angiogenesis is now well recognized, recent evidence also indicates that COUP-TFII plays a key role in lymphangiogenesis, the formation of lymphatic vessels. A century ago, Sabin (26) proposed that lymphatic vasculature has a venous origin. However, the molecular evidence of its origin in mammals has only recently been deciphered (27, 28). Lymphatic endothelial cells (LECs) have been found to develop from venous endothelial cells (27, 28). Srinivasan et al. (27) used lineage tracing experiments and endothelial-specific COUP-TFII mutants to demonstrate that mammalian lymphatic vasculature has a venous origin. During early embryonic development, the SRY-related gene, Sox18, has been shown to be required for Prox1 expression, and the exclusive expression of Prox1 in LECs is shown to be important for the formation and the maintenance of the lymphatic vascular system (29–32). However, Sox18 is expressed in both arterial and venous endothelial cells (30), yet Prox1 is only expressed in the LECs, suggesting that Sox18 is not sufficient for the specification of LEC identity. Instead, other factors with a venous origin must contribute.
COUP-TFII is expressed not only in the venous endothelial cells, but also in the LECs (15, 27). Conditional ablation of COUP-TFII in mice results in the absence of LEC progenitor cells, malformation of primitive lymphatic vessels, lymphatic sacs, and impairment of sprouting lymphangiogenesis (33). Recently, Srinivasan et al. (34) showed that COUP-TFII directly binds to the Prox1 promoter to activate its expression in venous LEC progenitor cells, suggesting that COUP-TFII is necessary for the initiation of Prox1 gene expression in the LEC progenitor cells. Results using an in vitro system further showed that COUP-TFII physically and functionally interact with Prox1 to specify lymphatic cell fate (35, 36) (Fig. 2). Collectively, the above results indicate that, in addition to Prox1, COUP-TFII is indispensable for lymphatic vessel formation and lymphatic cell fate control through direct regulation of Prox1 expression.
Additionally, the Prox1-expressing LECs adopt the blood cell fate in the absence of COUP-TFII during a developmental window between E12.5 and E15.5, suggesting that COUP-TFII also regulates lymphatic identity independent of Prox1 (33). In vitro studies using human primary LECs show that COUP-TFII regulates LEC proliferation and migration. Furthermore, we showed that COUP-TFII controls sprouting lymphangiogenesis through direct transcriptional regulation of neuropilin 2 (Nrp2), the coreceptor for VEGF-C (33). This result clearly indicates that COUP-TFII is involved in the VEGF-C/VEGFR-3/Nrp2 signaling pathway in the lymphatic system. In a spontaneous mouse breast cancer model, inactivation of COUP-TFII substantially suppresses tumor-induced neo-lymphangiogenesis but does not affect the preexisting lymphatic vessels, suggesting that targeted inhibition of COUP-TFII signaling with specific molecules is likely to inhibit only the growth of the newly formed lymphatics (33). Together, these findings suggest a potential therapeutic strategy for COUP-TFII in antilymphangiogenic therapies to prevent lymphatic-induced metastasis.
V. COUP-TFII in Reproduction
A. COUP-TFII is essential for normal female reproductive functions
Recent studies of ovarian, uterine, and placental function in COUP-TFII mutants have shed light on the role of COUP-TFII in female reproduction (37–39).
In the ovary, COUP-TFII is highly expressed in the theca cells surrounding the follicles and weakly expressed in the corpus luteum (37, 39). In the uterus, the COUP-TFII staining was detected in the myometrium and in endometrial stromal cells (37–39). COUP-TFII heterozygous female mice are growth-retarded, and mating pattern analysis shows that heterozygous females have a reduced ability to synthesize progesterone in response to exogenous gonadotropins as a result of a marked reduction in the expression level of steroidogenic enzymes (39). Additionally, analysis of uterine function of uterine-specific COUP-TFII knockout females (PRCre; COUP-TFII flox/flox) showed embryo attachment failure and a reduced uterine decidualization, indicating a defect in endometrial function during the periimplantation period (38). The underlying mechanisms of these defects are due to the impairment of cross talk between the epithelial and stromal compartments of the uterus. In the wild-type uterus, progesterone induces the expression of Indian hedgehog (Ihh) in the epithelium, and the secreted Ihh regulates the expression of stromal COUP-TFII. COUP-TFII in turn controls not only the stromal bone morphogenetic protein 2 (BMP2) to allow decidualization, but also epithelial estrogen receptor α (ERα) activity for proper embryo attachment (38, 40, 41). The mutant mice, having COUP-TFII deleted in the endometrial stromal cells, show a reduced BMP2 level in stromal cells and an enhanced and persistent estrogen receptor activity in the epithelium, leading to defective decidualization and embryo attachment (38, 42). Intriguingly, administration of a pure ERα antagonist, ICI 182780, to the mutant mice partially rescues the implantation defect and restores the expression of BMP2 (42). Collectively, these results suggest that COUP-TFII is one of the major mediators of uterine epithelial-stromal cross talk.
In addition to uterine defects, mice with incomplete deletion of COUP-TFII in the uterine stromal and smooth muscle cells generated by the anti-Müllerian hormone type II receptor (Amhr2)-Cre exhibit a milder phenotype, including a malformed placenta, leading to miscarriage at E10–E12 (37). In mice, the placenta is composed of three main layers by E9.5: the trophoblast giant cells that lie across the maternal deciduas, the spongiotrophoblast, and the labyrinth. COUP-TFII deficiency resulted in an increase in the differentiation of the trophoblast giant cells, a reduction in the cell number of spongiotrophoblasts, and the absence of the labyrinth (37). These results indicate an essential role of endometrial COUP-TFII in placentation. Taken together, these results suggest that COUP-TFII is important for normal female reproductive function.
B. COUP-TFII is important for the differentiation of Leydig cells for proper male fertility
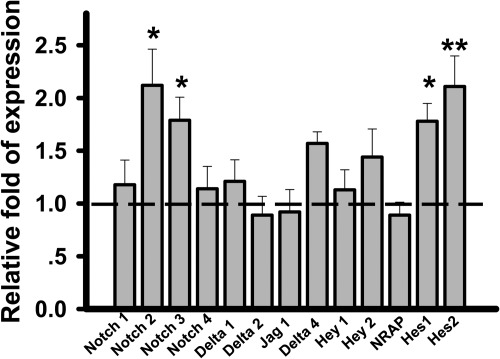
Although extensively studied in models of female reproduction, COUP-TFII is also indispensable for male gonad development and function. Inactivation of COUP-TFII during prepubertal stages of male development by using tamoxifen (TAM)-inducible cytomegalovirus immediate-early enhancer/chicken β-actin/rabbit β-globin hybrid (CAGG)-Cre-ER mouse line results in infertility, hypogonadism, and spermatogenesis arrest (43). Additionally, COUP-TFII null mice display Leydig cell hypoplasia. Leydig cells are the interstitial cells of the testes and were shown to produce testosterone (44). In the testes of COUP-TFII homozygous adult males, Leydig cell differentiation is arrested at the progenitor cell stage, leading to the reduced level of testosterone. Administration of testosterone could largely rescue hypogonadism and spermatogenic defects, but not the Leydig cell hypoplasia in COUP-TFII null mice. It has been shown that Notch signaling is important for the maintenance and differentiation of fetal Leydig progenitor cells (45). Treatment of the γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, or deletion of the Notch downstream target Hes1 facilitates Leydig cell differentiation. Conversely, up-regulation of Notch signaling maintains the status of progenitor cells and restricts their differentiation into mature Leydig cells. Given that COUP-TFII confers vessel identity through inhibiting the activity of Notch signaling, we investigated whether COUP-TFII also functions upstream of Notch signaling to control progenitor Leydig cell differentiation. As shown in Fig. 5, up-regulation of Notch signaling is indeed observed in the testes of the COUP-TFII mutant, as indicated by the increased levels of expression of Notch 2, Notch3, Hes1, and Hes2. As a consequence, these increases likely contribute to the differentiation defects of progenitor Leydig cells in the COUP-TFII mutant mice. However, the detailed mechanism of how COUP-TFII regulates Notch signaling needs to be further defined. Although important for testis organogenesis and progenitor Leydig cell formation, COUP-TFII is not required for Leydig cell maintenance, as is evident by normal function of Leydig cells when COUP-TFII is deleted in the adult stage (43). Thus, COUP-TFII plays roles in progenitor Leydig cell formation and early testis organogenesis.
Fig. 5.
Increased activity of Notch signaling by loss of COUP-TFII. Quantitative RT-PCR analysis of different factors related to Notch signaling in testes from control and COUP-TFII mutant mice. Bar graph shows fold changes of each molecule in COUP-TFII mutants relative to controls (n = 8). The dotted line indicates the expression levels of those genes in control mice. *, P < 0.05; **, P < 0.01.
VI. COUP-TFII in Energy Metabolism and Adipogenesis
A. COUP-TFII plays an important role in energy homeostasis
Increasing evidence suggests that COUP-TFII may have a role in energy metabolism given the fact that COUP-TFII heterozygous mice are lean and resistant to high-fat diet-induced obesity (46). These mice have less white adipose tissue (WAT) and less lipid storage in the liver compared with the wild-type mice. They also have a reduced level of WAT development-related genes, such as PPARγ, C/EBPα, aP2, PEPCK, and LPL, which implies a proadipogenic role for COUP-TFII (46). Additionally, heterozygous mice have similar food intake as wild-type mice but are leaner, in large part due to an increase in basal metabolic rate. COUP-TFII deficiency protects against high-fat diet-induced obesity by an increase in mitochondrial biogenesis in the WAT, muscle mass, and locomotive activity, suggesting higher energy expenditure in the heterozygous mice (46). An increase in insulin sensitivity and an improvement of glucose homeostasis in insulin tolerance and glucose tolerance tests, respectively, correlated with enhanced metabolism in the heterozygous mice (46). Finally, hyperinsulinemic-euglycemic clamp experiments demonstrated an increase in glucose uptake in the skeletal muscle and WAT. The importance of COUP-TFII in glucose homeostasis is further supported by the presence of COUP-TF response elements in the promoters of numerous genes encoding metabolic enzymes (47). Collectively, these data indicate that COUP-TFII is important for energy homeostasis. The studies also highlight a potential therapeutic application of COUP-TFII in regulating energy metabolism and metabolic syndromes.
B. COUP-TFII plays a central role in adipogenesis
The decreased expression of WAT development-related genes in COUP-TFII+/− mice suggests its involvement in the regulation of adipogenesis. Consistent with the in vivo results, knockdown of COUP-TFII in 3T3-L1 cells and mouse embryo fibroblasts results in decreased adipocyte differentiation, indicating a positive role of COUP-TFII in adipogenesis (46). In contrast to the results described above, two other groups recently reported COUP-TFII as a negative regulator in adipogenesis. Xu et al. (48) showed that ectopic expression of COUP-TFII reduced and short hairpin RNA mediated down-regulation of COUP-TFII-induced adipogenesis in 3T3L1 cells. Okamura et al. (49) showed that COUP-TFII recruited the SMRT corepressor complex to the PPARγ gene, leading to inhibition of adipogenesis. The reason for the discrepancy between these studies is unclear at present. It might be due to the timing when COUP-TFII was knocked down in the cell culture system. Because a transient increase in COUP-TFII expression occurs 4–24 h subsequent to the induction of differentiation in 3T3-L1 cells (46, 50), it is likely that COUP-TFII positively regulates adipogenesis at an early expansion phase, whereas the persistent expression of COUP-TFII during differentiation may be inhibitory.
VII. COUP-TFII in the Neuronal Development
A. COUP-TFs determine the cell fate of the progenitor cells in the eye development
During eye morphogenesis, the early progenitor cells in the optic primordia are specified and differentiated into diverse types of neural cells. Both COUP-TFI and COUP-TFII are coexpressed in the progenitor cells of the dorsodistal optic vesicle at E9.5 (51). These two genes compensate for each other functionally because deletion of either gene has little effect on programming the development of progenitor cells (51). The most likely reason for the lack of phenotype in a single gene knockout mutant is the expansion of COUP-TFI expression into the territory where COUP-TFII is expressed when COUP-TFII is deleted and vice versa. At E11.5, COUP-TFI and COUP-TFII display reciprocal expression patterns in the optic cup in which COUP-TFI is expressed in the neural retina cells, whereas COUP-TFII is detected in retinal pigmented epithelial cells (51). Double ablation of COUP-TFI/COUP-TFII in eyes caused the retinal pigmented epithelial cells to adopt a neural retina fate, leading to congenital ocular coloboma and microphthalmia. The abnormal differentiation of the optic vesicle in double knockout mice was attributed to the misexpression of genes essential for early eye development, including Pax6, Otx2, Mif, Pax2, and Vax1/2. In particular, COUP-TFs directly regulate the transcription of Pax6 and Otx2 during eye morphogenesis. Together, the study firmly established a role for COUP-TFs in the specification and differentiation of neural progenitor cells during eye development.
B. COUP-TFs are required in the retinal development
Opsins are cone photopigments that function as the first detection module used in color vision. They mediate the conversion of photons of light into an electrochemical signal. During retinal development, short-wavelength and medium-wavelength opsins display a unique dorsoventral expression profile in the retina (52). COUP-TFs have been implicated in the opsin gene expression based on their expression pattern. COUP-TFI has weak expression levels in the dorsal half of the retina and high expression levels in the ventral half of the retina at E11, whereas COUP-TFII was exclusively expressed in the dorsal area (53). Using both loss- and gain-of-function analyses, Satoh et al. (53) demonstrated that both COUP-TFI and COUP-TFII are essential for suppressing short-wavelength opsin expression in the dorsal retina, but only COUP-TFI is indispensable for suppressing medium-wavelength opsin expression in the ventral retina.
In addition to COUP-TFs, BMP signaling is important for the spatial patterning of cone opsin expression as is evident from the misregulation of opsins in retina-specific BMP receptor knockout (BMPR CKO) mice (53). Furthermore, dorsoventral expression patterns of COUP-TFI and COUP-TFII were severely compromised in the BMPR CKO mouse retina, suggesting that BMP signaling acts upstream of COUP-TFs in the regulation of opsin expression. Collectively, these results indicate that BMP signaling regulates the spatial patterning of mouse cone opsins through downstream effectors COUP-TFs.
C. COUP-TFII regulates the cerebellum development
The cerebellum is part of the central nervous system (CNS) that plays an important role in the coordination of movement and motor learning. Its functions are dependent upon the interaction between different neuronal populations: Purkinje cells, Golgi cells, granule cells, basket cells, and stellate cells. The cerebellum development starts from the dorsal region of the posterior neural tube (54). As development proceeds, precursors from the ventricular neuroepithelium migrate to the cerebellar cortex to become the deep cerebellar nuclei and Purkinje cells (54). Granule cell precursors from a specialized germinal matrix called the rhombic lip migrate tangentially to cover the surface of the cerebellum and form the external granule cell layer (EGL). In addition to the ventricular neuroepithelium, the EGL is a secondary region in which cerebellar neuronal proliferation occurs. By P15, the EGL disappears, and the cerebellum becomes a mature structure with three layers of neurons, including the molecular layer, the Purkinje cell layer, and the granule cell layer (54). Furthermore, the vermis area of the cerebellum is comprised of 10 lobules along the anterior-posterior axis, including lobule I to V/VI and lobule V/VI to X in the anterior and posterior compartments, respectively (54). All these structures are highly conserved between humans and mice.
COUP-TFII is weakly expressed in the cerebellar primordium at E13.5, and its level gradually increases in the anterior part of the Purkinje cell layer as development proceeds (55). At P7, COUP-TFII is detected only in the Purkinje cells of the cerebellum, and its level decreases along the anterior-posterior axis (55, 56). At P21, COUP-TFII is observed in the Purkinje cells of the lobule IX, but not the lobule X. To study the role of COUP-TFII in the cerebellum, neuron-specific enolase-Cre mice were used to ablate COUP-TFII in the brain. As expected, brain-specific COUP-TFII-deficient mice exhibited a smaller cerebellum size, abnormal foliation patterning at P14, and a reduction in branching of Purkinje cell dendrites (56). The improper foliation patterning was possibly due to the incomplete subdivision of lobule VI into VIa and VIb or the loss of lobule VII. Further investigation showed that COUP-TFII is essential in regulating granule cell survival vs. cell death (56). The number of phosphorylated histone H3-positive mitotic cells was reduced, whereas the number of caspase-3-positive apoptotic cells was increased in the EGL of the vermis area, suggesting a reduction in proliferation and an increase in apoptosis of granule cell progenitors. Furthermore, the expression of IGF-I and the activity of its downstream Akt and mTOR signalings were reduced in the cerebellum of the mutant mice. More importantly, supplementation of IGF-I in the slice culture of the cerebellum rescued the defects of the proliferation and apoptosis as well as the dendritic branching of Purkinje cells in the COUP-TFII mutant mice, suggesting that the reduction of granule cell progenitor population and dendritic branching of Purkinje cells were attributed to a decrease in IGF-I expression levels. The same study using chromatin immunoprecipitation analysis further showed that COUP-TFII was directly recruited to the IGF-I promoter in a specificity protein 1 (Sp1)-dependent manner (56). Taken together, COUP-TFII is a crucial regulator of neuronal survival and maturation in the mouse postnatal cerebellum.
VIII. COUP-TFII in Organogenesis
A. COUP-TFII is essential for the radial and anteroposterior (AP) patterning of the stomach
COUP-TFII exhibits a dynamic expression pattern in the developing stomach. It is expressed in both the epithelium and the mesenchyme of the developing stomach with the highest levels in the mesenchyme by E12.5, implying a regulatory role in stomach development (57). COUP-TFII was conditionally ablated in the mesenchyme of the developing stomach by Nkx3.2 Cre mice. COUP-TFII conditional null mutants (Nkx3.2Cre; COUP-TFII flox/flox) exhibit significant dysmorphogenesis of the developing stomach with major defects in the radial and AP patterning. Morphological analysis of the mutant stomach showed that the mutant stomach is posteriorized. However, there are no cell type changes in the epithelium of the fore- and hind-stomach of the mutant animal.
Intriguingly, the radial and AP patterning defects in the stomach elicited by the COUP-TFII conditional null mutants (Nkx3.2Cre; COUP-TFII flox/flox) resemble the phenotypes of Shh null mutants (57, 58). Additionally, COUP-TFII is down-regulated in the caudal region of the stomach of the Shh null mutants as well as in the explants treated with cyclopamine, an inhibitor of hedgehog (Hh) signaling (57, 59). Moreover, COUP-TFII mediates Ihh signaling to regulate embryo attachment and decidualization in the uterus (41). Taken together, these results suggest that COUP-TFII is a downstream target of Hh signaling. The notion that COUP-TFII lies downstream of Hh signaling is supported by an in vitro study in which the expression of COUP-TFII in mouse P19 embryonic carcinoma cells can be induced by a Shh-N (amino-terminal signaling domain) treatment without de novo protein synthesis (60). Furthermore, a Shh-response element was identified in the mCOUP-TFII promoter that binds to a factor distinct from Gli, a gene known to mediate Shh signaling (60, 61). All the results above support the notion that COUP-TFII is a downstream target that mediates the function of Hh in controlling the radial and AP patterning of the stomach.
B. COUP-TFII is necessary for limb and skeletal muscle development
The expression of COUP-TFII is detected in the lateral plate mesoderm of the limb field as early as E9.5 and continues in the limb mesenchyme through E12.5 (62). Interestingly, COUP-TFII is also expressed in the embryonic somites starting at E8.5. At E12.5, COUP-TFII expression can be detected in the muscle bundles of the fore- and hindlimb. Its expression patterns imply an involvement of COUP-TFII in limb and skeletal muscle development. Indeed, tissue-specific ablation of COUP-TFII, specifically in the developing limb bud mesenchyme, results in shorter limbs as well as hypoplastic skeletal muscles (62). Additionally, embryonic chimera analysis indicates that COUP-TFII plays a cell autonomous function during limb bud outgrowth but not limb bud initiation (62). The underlying mechanism contributing to the defects is that proliferating cells were reduced in the limb mesenchyme of the late developmental stage of COUP-TFII conditional knockout mutants, whereas no differences in apoptosis and proliferation were observed between control littermates and COUP-TFII conditional knockout embryos at E9.5 (62). These results support the notion that COUP-TFII is not involved in limb bud initiation but rather is required for the limb bud outgrowth.
Analysis of COUP-TFII null mutants and conditional knockout mutants reveals significant muscle cell migration defects. This abnormal myogenesis is further confirmed by the altered expression of the myogenic differentiation gene myogenin in both somitic and limb muscle lineages (62). Taken together, COUP-TFII is required for the distal outgrowth of embryonic limbs and for the appropriate development of limb musculature.
C. COUP-TFII is essential for the insulin secretion and pancreatic function
Pancreatic β-cell differentiation and function relies on the interaction of a group of transcription factors. The reciprocal cross-regulation between COUP-TFII and hepatocyte nuclear factor 4α (HNF4α), also called maturity-onset diabetes of the young 1 in control of insulin secretion, was well defined in mouse pancreatic islet β-cells (63). HNF4α regulates the expression of COUP-TFII gene through the DR-1 binding site, and HNF4α expression was found to be controlled by COUP-TFII (63). Additionally, COUP-TFII autoregulates its expression (63). The interaction of two factors is further supported by in vivo studies showing that both pancreatic β-cell-specific deletion of heterozygous COUP-TFII mice and HNF4α knockout mice exhibited similar abnormal insulin secretion (64). Moreover, in a high carbohydrate-fed state, high insulin and high glucose suppress COUP-TFII gene expression through Foxo1 signaling and the carbohydrate response element-controlled pathway, respectively (65). In addition, COUP-TFII was shown to regulate the expression of the insulin gene in vitro in a cell culture transfection study (66). These observations suggest that COUP-TFII forms a positive feedback autocrine loop to modulate insulin secretion as well as the transcription of the insulin gene expression.
IX. COUP-TFII in Diseases
A. COUP-TFII deletion results in the congenital diaphragmatic hernia (CDH)
CDH is characterized by the malformation of the diaphragm, with inappropriate protrusion of the viscera contents into the thoracic cavity leading to the abnormal lung growth and development (67, 68). Infants with CDH suffer from pulmonary hypoplasia and pulmonary hypertension (67). The most common forms of CDH, the Bochdalek-type CDH, occurs in the dorsolateral region of the diaphragm, and it accounts for more than 70–90% of the diaphragmatic defects in humans (68, 69). The Bochdalek-type CDH has been mapped to a region in chromosome 15q, which encompass COUP-TFII (15q26) (70, 71), suggesting that COUP-TFII may have a functional association with CDH.
Using Nkx3-2 Cre recombinase to ablate COUP-TFII in the foregut mesentery has shown that a high percentage of COUP-TFII conditional null mutants (Nkx3.2Cre; COUP-TFII flox/flox) exhibit a diaphragmatic hernia in which the developing stomach, intestines, and liver protrude into the thoracic cavity, and the hearts of the COUP-TFII conditional null mutants were pushed laterally to right thorax, displaying dextroposition (72). Additionally, herniation of the liver caused the compression of the left lung (72). The lung hypoplasia and the resulting respiratory insufficiency are the likely underlying causes of the postnatal lethality. This study provided the first demonstration that COUP-TFII might contribute to the formation of CDH in individuals with 15q deletions.
B. COUP-TFII is essential for the tumor growth and metastasis
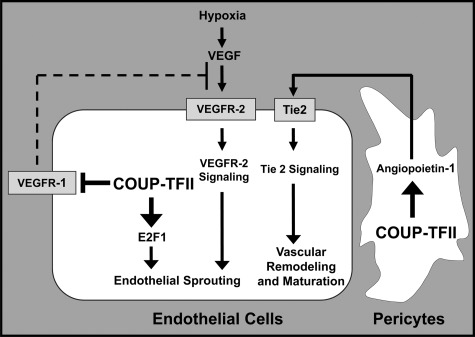
In addition to embryonic angiogenesis, the recent study using Cre-Lox technology to inactivate COUP-TFII in the adult stage showed that COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis (14). It is well accepted that tumor progression, invasion, and metastasis require angiogenesis to provide nutrients and oxygen from the surrounding environment to sustain tumor growth (73–75). Without persistent vessel formation, tumor cells will undergo apoptosis or become necrotic (76). Therefore, further understanding of the orchestration of this angiogenic switch will help in the development of strategies to harness the dynamics of blood vessel formation during cancer progression. Recently, we showed that COUP-TFII serves as one of the major angiogenic regulators within the tumor microenvironment to promote tumor angiogenesis. Ablation of COUP-TFII in adult mice, using TAM-inducible ROSA26Cre-ERT2, severely compromised neoangiogenesis and suppressed tumor growth and metastasis (14, 77). Mechanistic investigations revealed that COUP-TFII directly regulates the transcription of three key genes, E2F1 and VEGFR-1 in endothelial cells and Ang-1 in pericytes, to coordinate endothelial cell proliferation, sprouting, and vascular remodeling (14, 77) (Fig. 6). In the pericyte, COUP-TFII positively regulates Ang-1 expression to modulate a paracrine signal (Ang-1/Tie2 signaling) that impacts vessel remodeling (14). Furthermore, we delineated for the first time that COUP-TFII plays a cell autonomous role in endothelial cells to control vessel sprouting by regulating cell proliferation and migration (77). Loss of COUP-TFII significantly compromised endothelial cell sprouting through transcriptional derepression of VEGFR-1. VEGFR-1 and soluble VEGFR-1 can counteract the positive mitogenic signal of VEGFR-2 by sequestering the VEGF ligand to inhibit angiogenesis (77). As a consequence, up-regulation of VEGFR-1 in COUP-TFII mutants will inhibit VEGF/VEGFR-2 signaling, a major angiogenic driving force for vascular growth. One interesting question that remains to be defined is whether COUP-TFII is involved in tip and stalk cell specification during vessel sprouting. Based on the current understanding, it is hypothesized that a subset of endothelial cells will undergo sprouting to form the new vessel during angiogenic growth. The endothelial cells in the new sprout are called tip cells. Notch and its ligand delta-like 4 have been shown to play a role in sprouting in mice (78, 79). In response to VEGFA, delta-like 4 expression is induced in the tip cells, whereas Notch is activated in the adjacent stalk cells (80, 81). Activation of Notch in stalk cells suppresses VEGFR-2 expression and inhibits the proangiogenic response (79, 81). Because COUP-TFII is a crucial regulator of venous fate determination through inhibition of Notch signaling (15), it is reasonable to speculate that COUP-TFII is preferably expressed in tip cells to ensure the inactivation of Notch signaling. Conversely, to inhibit angiogenic response, Notch signaling and VEGFR-1 expression will be up-regulated in stalk cells due to the absence of COUP-TFII. Thus, these complicated hierarchies of signaling interactions are coordinated, and the interplays among COUP-TFII, Notch signaling, and VEGF/VEGFR-2 signaling remain to be elucidated.
Fig. 6.
Model depicting COUP-TFII functions in tumor angiogenesis. In endothelial cells, the absence of COUP-TFII results in the elevated expression of VEGFR-1, a decoy receptor that sequesters VEGF ligand. This leads to attenuation of VEGFR-2 signaling and endothelial cell sprouting in the COUP-TFII-deficient mice. Additionally, endothelial cell proliferation is impaired due to decreased expression of E2F1. In pericytes, the absence of COUP-TFII results in reduced Ang-1 that serves as a paracrine signal to activate Tie2 receptors on the endothelial cells. Thus, COUP-TFII regulates angiogenesis through growth signaling, VEGF/VEGFR-2, and Ang-1/Tie2 signaling pathway within tumor microenvironment.
Inhibitors of angiogenesis can suppress tumor growth in preclinical models and have entered the clinic as prospective anticancer therapeutics. Despite the enormous effort focused on the development of angiogenic inhibitors for VEGF/VEGFR-2 signaling, the eventual resistance and toxic side effects of VEGF/VEGFR-2 inhibitor treatment remains a significant clinical problem (82). Our studies uncover COUP-TFII as a critical new player, which is essential for controlling the vital and complex circulatory networks, and highlight that COUP-TFII may represent an important target for anticancer interventions. First, COUP-TFII potently controls multiple angiogenic signaling pathways, which renders it as a more efficient approach to inhibit tumor angiogenesis. Second, COUP-TFII has little impact on normal adult physiological functions, suggesting that it will be a well-tolerated approach. Third, COUP-TFII is a nuclear receptor, and its activity could potentially be regulated by ligands (83), rendering it as a useful drug target. Future work will be important to screen and identify the specific antagonist ligand of COUP-TFII and examine whether it could be used for anticancer interventions.
C. COUP-TFII may play a role in the development of ER-positive breast cancer
Except for COUP-TFII function within the tumor microenvironment, increasing evidence has suggested that COUP-TFII might play an important role in breast cancer cell growth. However, the results are somewhat contradictory. Nagasaki et al. (84) showed that COUP-TFII is overexpressed in 59% of breast cancer patients. They found that up-regulation of COUP-TFII in the patients was associated with a worse prognosis, clinical outcome, and lymph node metastasis, and a correlation between COUP-TFII and ERα status was detected (84). In accordance, in vitro observation indicated that COUP-TFII can physically interact with ERα and regulate ERα transcription activity (85), Furthermore, it has been shown that overexpression of COUP-TFI enhances motility and invasiveness of MCF-7 cells and promotes the proliferation of MCF-7 cells through ERα-dependent mechanisms that target cell cycle progression and cell survival (86). Given the biochemical similarities of COUP-TFI and COUP-TFII, these results suggested that COUP-TFII might regulate ERα transcription activity to participate in breast cancer progression.
However, contrary to the oncogenic role of COUP-TFII during breast cancer progression, Riggs et al. (87) demonstrated that COUP-TFII mediated breast cancer cell sensitivity to TAM treatment possibly through inhibition of ERα activity. Reduction of COUP-TFII expression levels will render the breast cancer cells resistant to TAM inhibition. Conversely, overexpression of COUP-TFII in TAM-resistant human breast cancer cell lines increases the antiproliferative effects of TAM and ICI 182780 treatment. These results suggested that COUP-TFII was involved in antiestrogen resistance, and enhancement of COUP-TFII activity in tumor cells in combination with TAM treatment might improve therapy effectiveness of breast cancer patients. The opposing results of COUP-TFII function in breast cancer cells might reflect its complicated roles during the different progression phrases. To precisely elucidate its function in early initiation of breast cancer formation and later relapse phase of TAM resistance, further investigation using appropriate animal model may help in differentiating the distinct role of COUP-TFII during the course of breast cancer progression.
X. COUP-TFI in Early Neural Development
Similar to the important roles of COUP-TFII during development and diseases, growing experimental evidence clearly demonstrates that COUP-TFI is a critical regulator of CNS and peripheral nervous system development. The developmental role of COUP-TFI in the cerebral cortex of the CNS has been intensively studied. The graded expression of COUP-TFI is from low to high along both mediolateral (dorsoventral) and rostrocaudal axes of the cerebral cortex (88–90). The spatially restricted expression pattern of COUP-TFI suggests that COUP-TFI may regulate multiple events during corticogenesis, including neurogenesis, cell differentiation, proliferation, cell death, arborization, axon myelination, and cortex patterning. Homozygous COUP-TFI null mutants die within 48 h after birth due to starvation and dehydration (90). COUP-TFI null mutant mice exhibit improper differentiation of both layer IV neurons, suggesting an essential role of COUP-TFI in neurogenesis (91). This is further supported by the gain-of-function study, in which increases in COUP-TFI dosage suppress Mapk/Erk, phosphatidylinositol-3-kinase/Akt, and β-catenin-mediated Wnt signaling pathways, leading progenitor cells to exit from the cell cycle and their subsequent differentiation (92). COUP-TFI null mutants also display miswired, area-specific connections between the cortex and the thalamus. Normally, axons from subplate neurons project to the thalamus, whereas layer IV neurons receive inputs from the thalamus. The absence of subplate neurons in COUP-TFI null mice results in defective thalamocortical connection, leading to the premature cell death of layer IV neurons (91). Additionally, thalamocortical axons in the mutants fail to reach beyond the internal capsule or extend to the intermediate zone, and fibers of the anterior commissure, hippocampal commissure, and corpus callosum in the mutants fail to cross the midline to connect the two cerebral hemispheres, suggesting defects in axon guidance (90, 93). In addition, axon arborization and innervations are defective in COUP-TFI null mice (91). COUP-TFI null mice also show a delayed differentiation of oligodendrocytes, the myelinating cells of the CNS, suggesting a defect in axon myelination (94). Moreover, the molecular patterning of the cortex, including the regionalized expression of the transcription factor Id2 as well as ROR-β, and adhesion molecule Cadherin 8, is profoundly disturbed in COUP-TFI null mutants, suggesting a role of COUP-TFI in regulating caudoventral identity of the cortex (88). Conditional inactivation of COUP-TFI in the cortex using Emx1-IRES-Cre results in complementary expansion of frontal/motor areas and reduced sensory area size, which is positioned far caudally in the occipital cortex, indicating that COUP-TFI regulates regional patterning of the cortex (95). COUP-TFI suppresses the differentiation of corticospinal motor neuron, thereby controlling its specification (96). Taken together, COUP-TFI plays crucial roles not only in neuronal cell fate specification, but also in axon guidance, arborization, and axon myelination as well as cortex patterning.
Like the defects in the CNS, COUP-TFI null mutants also display defects on axon guidance, nerve projection, and arborization in the peripheral nervous system. Normally, the IX cranial ganglion (the glossopharyngeal ganglion) provides both sensory and motor innervations to the pharynx and the tongue. In COUP-TFI mutants, the glossopharyngeal ganglion is malformed, and the axons from there fail to project to hindbrain (90). This defect is likely due to the excessive cell death in the region dorsal to the developing IX ganglion (90). In addition, oculomotor nerve (ganglion III) of the mutants is shortened and broadened (90). Abnormal branching of the axon trees is also detected at the facial and cervical plexus regions as well as the ophthalmic branch of the trigeminal nerve of COUP-TFI null mutants (90). Together, these observations further support the hypothesis that the abnormal development of glossopharyngeal ganglion in COUP-TFI null mutants impairs sensory and motor functions, which affect feeding behavior and result in perinatal death.
XI. A Comparison between COUP-TFI and COUP-TFII
Although COUP-TFI has been discussed briefly, we have summarized a comparison of COUP-TFI and COUP-TFII functions in Table 2. Given that COUP-TFI and COUP-TFII are highly similar in structure, DNA binding, in vitro functional assays, and mechanism of action (5), one will expect the possible compensatory functions of COUP-TFs. Indeed, during the eye development, inactivation of both COUP-TFI and COUP-TFII is necessary to have significant phenotypes, suggesting that they compensate each other's function (51). In addition, ectopic expression of COUP-TFI in the COUP-TFII-deleted uterus largely rescued implantation and decidualization defects of COUP-TFII mutants, suggesting that COUP-TFI and COUP-TFII are able to functionally replace each other if they are expressed in the same cells (97). However, during embryonic development, they are differentially expressed, but often in a complementary fashion. At the end of gastrulation, COUP-TFI starts to be expressed in the neural ectoderm, whereas COUP-TFII is detected in the visceral mesoderm (11, 90). In contrast to the high expression levels of COUP-TFII in the mesenchymal component of the many developing organs, the expression of COUP-TFI is high throughout the developing neural tissues, but low in the internal organs (5). Both expression levels in the mouse gradually reduce shortly after birth. The expression patterns of COUP-TFs suggest that they will exert different biological functions, in which COUP-TFI will have more roles in neural development and COUP-TFII in organogenesis. Indeed, studies on the genetically manipulated COUP-TFI or COUP-TFII mouse models have identified their different developmental and physiological roles as described in Table 2.
Great insights have been obtained into the physiological function of both COUP-TFI and COUP-TFII in several tissues; however, COUP-TFI and COUP-TFII are expressed in many other tissues in which the physiological function of COUP-TFs still need to be uncovered.
XII. Summary and Perspective
This review has highlighted a unique role of transcription factor COUP-TFII in normal tissue development and homeostasis. Deregulation of its levels during development critically contributes to various congenital anomalies, including CDH (72), congenital ocular coloboma (51), and congenital heart defect (F. -J. Lin, L. -R. You, M. -J. Tsai, and S. Y. Tsai, unpublished results). COUP-TFII is highly expressed in the mesenchymal cell compartment during embryogenesis and plays critical roles during organogenesis, suggesting that COUP-TFII serves as a master regulator to control mesenchymal-epithelial interaction during development. In addition, it also left open the possibility of whether COUP-TFII participates in remodeling the stromal environment to facilitate tissue expansion. Further mechanistic investigation needs to address whether a general downstream target or signaling pathway regulated by COUP-TFII is operated in these processes. Conversely, it also raises an interesting question of whether COUP-TFII is involved in epithelial-mesenchymal transition as well.
Although COUP-TFII plays essential roles during embryogenesis, we showed that deletion of COUP-TFII in the adult lacks a discernible phenotype. In most instances, COUP-TFII is not important for maintenance function in the adult, but it is essential for regeneration or dedifferentiation processes, which often occurred under pathological conditions such as tumor angiogenesis and tumor lymphangiogenesis. Although COUP-TFs are defined as orphan nuclear receptors, a recent study has shown that COUP-TFII is a retinoic acid-activated receptor (83). The ligand-regulated feature has left open the possibility that synthetic agonists or antagonists might be useful in the development of new therapeutic strategies for several human diseases in which COUP-TFII is implicated. In addition, the lack of maintenance function of COUP-TFII in adult organs renders it a potential safe target for treatment of various diseases, including cancer and metabolic syndromes. For instance, agonists can be used for prolymphangiogenic treatment of primary and secondary lymphedemas as well as for enhancing wound healing, whereas antagonists can be used for antilymphangiogenic and/or antiangiogenic therapy targeting tumor growth and metastasis, as well as the prevention of obesity, because COUP-TFII heterozygous mice are resistant to high-fat diet-induced obesity. Specifically, development of selective agents with tissue selectivity would maximize the therapeutic potential. Finally, future studies including genome-wide approaches and detailed analysis of COUP-TFII-dependent genetic programs will be essential to comprehensively understand the relationship between COUP-TFII activity and distinct human diseases.
Acknowledgments
We thank J. Hebert for assistance with the manuscript.
This work was supported by National Institutes of Health Grants R01 HL076448 (to S.Y.T.), R01 HD17379 and DK45641 (to M.-J.T.), P01 DK059820, U19 DK062434 (to M.-J.T. and S.Y.T.), and core facilities of the Diabetes and Endocrinology Research Center (Grant DK079638).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ang-1
- Angiopietin-1
- AP
- anteroposterior
- BMP
- bone morphogenetic protein
- CDH
- congenital diaphragmatic hernia
- CNS
- central nervous system
- COUP-TF
- chicken ovalbumin upstream promoter transcription factor
- DBD
- DNA-binding domain
- E
- embryonic day
- EGL
- external granule cell
- EphB4
- ephrin receptor B4
- ERα
- estrogen receptor α
- Hh
- hedgehog
- HNF4α
- hepatocyte nuclear factor 4α
- Ihh
- Indian hedgehog
- LBD
- ligand-binding domain
- LECs
- lymphatic endothelial cells
- NP-1
- neuropilin 1
- Nrp2
- neuropilin 2
- P
- postnatal day
- Shh
- sonic hedgehog
- Sp1
- specificity protein 1
- TAM
- tamoxifen
- VEGF
- vascular endothelial growth factor
- VEGFR
- VEGF receptor
- WAT
- white adipose tissue.
References
- 1. Wang LH, Tsai SY, Cook RG, Beattie WG, Tsai MJ, O'Malley BW. 1989. COUP transcription factor is a member of the steroid receptor superfamily. Nature 340:163–166 [DOI] [PubMed] [Google Scholar]
- 2. Miyajima N, Kadowaki Y, Fukushige S, Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K, Yamamoto T. 1988. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res 16:11057–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ladias JA, Karathanasis SK. 1991. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science 251:561–565 [DOI] [PubMed] [Google Scholar]
- 4. Wang LH, Ing NH, Tsai SY, O'Malley BW, Tsai MJ. 1991. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr 1:207–216 [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai SY, Tsai MJ. 1997. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18:229–240 [DOI] [PubMed] [Google Scholar]
- 6. Cooney AJ, Tsai SY, O'Malley BW, Tsai MJ. 1992. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol 12:4153–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. 1992. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA 89:1448–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang K, Lin F-J, Tsai SY, Tsai M-J. 2006. Role of chicken ovalbumin upstream promoter-transcription factor I in the development of nervous system. In: Taneja R. ed. Nuclear receptors in development: advances in developmental biology. Vol 16 Philadelphia: Elsevier; 297–312 [Google Scholar]
- 9. Qiu Y, Krishnan V, Zeng Z, Gilbert DJ, Copeland NG, Gibson L, Yang-Feng T, Jenkins NA, Tsai MJ, Tsai SY. 1995. Isolation, characterization, and chromosomal localization of mouse and human COUP-TF I and II genes. Genomics 29:240–246 [DOI] [PubMed] [Google Scholar]
- 10. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. 1999. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13:1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira FA, Qiu Y, Tsai MJ, Tsai SY. 1995. Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J Steroid Biochem Mol Biol 53:503–508 [DOI] [PubMed] [Google Scholar]
- 12. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180 [DOI] [PubMed] [Google Scholar]
- 13. Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376:70–74 [DOI] [PubMed] [Google Scholar]
- 14. Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. 2010. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci USA 107:3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435:98–104 [DOI] [PubMed] [Google Scholar]
- 16. Lawson ND, Vogel AM, Weinstein BM. 2002. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3:127–136 [DOI] [PubMed] [Google Scholar]
- 17. Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128:3675–3683 [DOI] [PubMed] [Google Scholar]
- 18. Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang HU, Chen ZF, Anderson DJ. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741–753 [DOI] [PubMed] [Google Scholar]
- 20. Urness LD, Sorensen LK, Li DY. 2000. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 26:328–331 [DOI] [PubMed] [Google Scholar]
- 21. Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. 2000. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405:966–970 [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895–4902 [DOI] [PubMed] [Google Scholar]
- 23. Lavine KJ, Ornitz DM. 2008. Fibroblast growth factors and Hedgehogs: at the heart of the epicardial signaling center. Trends Genet 24:33–40 [DOI] [PubMed] [Google Scholar]
- 24. Lavine KJ, Long F, Choi K, Smith C, Ornitz DM. 2008. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 135:3161–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciszek B, Skubiszewska D, Ratajska A. 2007. The anatomy of the cardiac veins in mice. J Anat 211:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabin FR. 1902. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic ducts in the pig. Am J Anat 1:367–389 [Google Scholar]
- 27. Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. 2007. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21:2422–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478 [DOI] [PubMed] [Google Scholar]
- 29. Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. 2008. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22:3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. 2008. Sox18 induces development of the lymphatic vasculature in mice. Nature 456:643–647 [DOI] [PubMed] [Google Scholar]
- 31. Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. 2002. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778 [DOI] [PubMed] [Google Scholar]
- 33. Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. 2010. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest 120:1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. 2010. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24:696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. 2009. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14:425–434 [DOI] [PubMed] [Google Scholar]
- 36. Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. 2009. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 113:1856–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY. 2007. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA 104:6293–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. 2007. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. 2005. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol 19:2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simon L, Spiewak KA, Ekman GC, Kim J, Lydon JP, Bagchi MK, Bagchi IC, DeMayo FJ, Cooke PS. 2009. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology 150:3871–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. 2006. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38:1204–1209 [DOI] [PubMed] [Google Scholar]
- 42. Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. 2010. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol 24:930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin J, Tsai MJ, Tsai SY. 2008. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One 3:e3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christensen AK, Mason NR. 1965. The comparative ability of seminiferous tubules and interstitial tissue of rat testis to synthesize androgen from progesterone-4-14C in vitro. Endocrinology 76:646–656 [DOI] [PubMed] [Google Scholar]
- 45. Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. 2008. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135:3745–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Xie X, Qin J, Jeha GS, Saha PK, Yan J, Haueter CM, Chan L, Tsai SY, Tsai MJ. 2009. The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab 9:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Myers SA, Wang SC, Muscat GE. 2006. The chicken ovalbumin upstream promoter-transcription factors modulate genes and pathways involved in skeletal muscle cell metabolism. J Biol Chem 281:24149–24160 [DOI] [PubMed] [Google Scholar]
- 48. Xu Z, Yu S, Hsu CH, Eguchi J, Rosen ED. 2008. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc Natl Acad Sci USA 105:2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, Hamakubo T, Ito S, Aburatani H, Yanagisawa M, Kodama T, Sakai J. 2009. COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proc Natl Acad Sci USA 106:5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. 2005. A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol Endocrinol 19:2437–2450 [DOI] [PubMed] [Google Scholar]
- 51. Tang K, Xie X, Park JI, Jamrich M, Tsai S, Tsai MJ. 2010. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. 2000. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27:513–523 [DOI] [PubMed] [Google Scholar]
- 53. Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S. 2009. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J Neurosci 29:12401–12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang VY, Zoghbi HY. 2001. Genetic regulation of cerebellar development. Nat Rev Neurosci 2:484–491 [DOI] [PubMed] [Google Scholar]
- 55. Qin J, Suh JM, Kim BJ, Yu CT, Tanaka T, Kodama T, Tsai MJ, Tsai SY. 2007. The expression pattern of nuclear receptors during cerebellar development. Dev Dyn 236:810–820 [DOI] [PubMed] [Google Scholar]
- 56. Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ. 2009. Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol 326:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takamoto N, You LR, Moses K, Chiang C, Zimmer WE, Schwartz RJ, DeMayo FJ, Tsai MJ, Tsai SY. 2005. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132:2179–2189 [DOI] [PubMed] [Google Scholar]
- 58. Ramalho-Santos M, Melton DA, McMahon AP. 2000. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127:2763–2772 [DOI] [PubMed] [Google Scholar]
- 59. Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. 2002. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99:14071–14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krishnan V, Pereira FA, Qiu Y, Chen CH, Beachy PA, Tsai SY, Tsai MJ. 1997. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science 278:1947–1950 [DOI] [PubMed] [Google Scholar]
- 61. Krishnan V, Elberg G, Tsai MJ, Tsai SY. 1997. Identification of a novel sonic hedgehog response element in the chicken ovalbumin upstream promoter-transcription factor II promoter. Mol Endocrinol 11:1458–1466 [DOI] [PubMed] [Google Scholar]
- 62. Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY. 2004. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol 24:10835–10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perilhou A, Tourrel-Cuzin C, Zhang P, Kharroubi I, Wang H, Fauveau V, Scott DK, Wollheim CB, Vasseur-Cognet M. 2008. The MODY1 gene for hepatocyte nuclear factor 4α and a feedback loop control COUP-TFII expression in pancreatic β cells. Mol Cell Biol 28:4588–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bardoux P, Zhang P, Flamez D, Perilhou A, Lavin TA, Tanti JF, Hellemans K, Gomas E, Godard C, Andreelli F, Buccheri MA, Kahn A, Le Marchand-Brustel Y, Burcelin R, Schuit F, Vasseur-Cognet M. 2005. Essential role of chicken ovalbumin upstream promoter-transcription factor II in insulin secretion and insulin sensitivity revealed by conditional gene knockout. Diabetes 54:1357–1363 [DOI] [PubMed] [Google Scholar]
- 65. Perilhou A, Tourrel-Cuzin C, Kharroubi I, Henique C, Fauveau V, Kitamura T, Magnan C, Postic C, Prip-Buus C, Vasseur-Cognet M. 2008. The transcription factor COUP-TFII is negatively regulated by insulin and glucose via Foxo1- and ChREBP-controlled pathways. Mol Cell Biol 28:6568–6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwung YP, Crowe DT, Wang LH, Tsai SY, Tsai MJ. 1988. The COUP transcription factor binds to an upstream promoter element of the rat insulin II gene. Mol Cell Biol 8:2070–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harrison MR, Adzick NS, Estes JM, Howell LJ. 1994. A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA 271:382–384 [PubMed] [Google Scholar]
- 68. Stokes KB. 1991. Unusual varieties of diaphragmatic herniae. Prog Pediatr Surg 27:127–147 [DOI] [PubMed] [Google Scholar]
- 69. Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. 2003. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci USA 100:5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schlembach D, Zenker M, Trautmann U, Ulmer R, Beinder E. 2001. Deletion 15q24-26 in prenatally detected diaphragmatic hernia: increasing evidence of a candidate region for diaphragmatic development. Prenat Diagn 21:289–292 [DOI] [PubMed] [Google Scholar]
- 71. Klaassens M, van Dooren M, Eussen HJ, Douben H, den Dekker AT, Lee C, Donahoe PK, Galjaard RJ, Goemaere N, de Krijger RR, Wouters C, Wauters J, Oostra BA, Tibboel D, de Klein A. 2005. Congenital diaphragmatic hernia and chromosome 15q26: determination of a candidate region by use of fluorescent in situ hybridization and array-based comparative genomic hybridization. Am J Hum Genet 76:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ. 2005. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA 102:16351–16356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Folkman J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31 [DOI] [PubMed] [Google Scholar]
- 74. Carmeliet P, Jain RK. 2000. Angiogenesis in cancer and other diseases. Nature 407:249–257 [DOI] [PubMed] [Google Scholar]
- 75. Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- 76. Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. 1999. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284:808–812 [DOI] [PubMed] [Google Scholar]
- 77. Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. 2010. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res 70:8812–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445:776–780 [DOI] [PubMed] [Google Scholar]
- 79. Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. 2006. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–1087 [DOI] [PubMed] [Google Scholar]
- 80. Hainaud P, Contrerès JO, Villemain A, Liu LX, Plouët J, Tobelem G, Dupuy E. 2006. The role of the vascular endothelial growth factor-delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res 66:8501–8510 [DOI] [PubMed] [Google Scholar]
- 81. Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. 2007. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104:3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kerbel RS. 2008. Tumor angiogenesis. N Engl J Med 358:2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, Xu HE. 2008. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol 6:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nagasaki S, Suzuki T, Miki Y, Akahira J, Shibata H, Ishida T, Ohuchi N, Sasano H. 2009. Chicken ovalbumin upstream promoter transcription factor II in human breast carcinoma: possible regulator of lymphangiogenesis via vascular endothelial growth factor-C expression. Cancer Sci 100:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R. 1997. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J Biol Chem 272:31465–31474 [DOI] [PubMed] [Google Scholar]
- 86. Le Dily F, Métivier R, Guéguen MM, Le Péron C, Flouriot G, Tas P, Pakdel F. 2008. COUP-TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res Treat 110:69–83 [DOI] [PubMed] [Google Scholar]
- 87. Riggs KA, Wickramasinghe NS, Cochrum RK, Watts MB, Klinge CM. 2006. Decreased chicken ovalbumin upstream promoter transcription factor II expression in tamoxifen-resistant breast cancer cells. Cancer Res 66:10188–10198 [DOI] [PubMed] [Google Scholar]
- 88. Zhou C, Tsai SY, Tsai MJ. 2001. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev 15:2054–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu Q, Dwyer ND, O'Leary DD. 2000. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci 20:7682–7690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qiu Y, Pereira FA, DeMayo FJ, Lydon JP, Tsai SY, Tsai MJ. 1997. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev 11:1925–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. 1999. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron 24:847–859 [DOI] [PubMed] [Google Scholar]
- 92. Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. 2008. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and β-catenin signaling. Cereb Cortex 18:2117–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Armentano M, Filosa A, Andolfi G, Studer M. 2006. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development 133:4151–4162 [DOI] [PubMed] [Google Scholar]
- 94. Yamaguchi H, Zhou C, Lin SC, Durand B, Tsai SY, Tsai MJ. 2004. The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev Biol 266:238–251 [DOI] [PubMed] [Google Scholar]
- 95. Armentano M, Chou SJ, Tomassy GS, Leingärtner A, O'Leary DD, Studer M. 2007. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci 10:1277–1286 [DOI] [PubMed] [Google Scholar]
- 96. Tomassy GS, De Leonibus E, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis JD, Studer M. 2010. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci USA 107:3576–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu SP, Lee DK, Demayo FJ, Tsai SY, Tsai MJ. 2010. Generation of ES cells for conditional expression of nuclear receptors and coregulators in vivo. Mol Endocrinol 24:1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang P, Bennoun M, Gogard C, Bossard P, Leclerc I, Kahn A, Vasseur-Cognet M. 2002. Expression of COUP-TFII in metabolic tissues during development. Mech Dev 119:109–114 [DOI] [PubMed] [Google Scholar]
- 99. Tang LS, Alger HM, Lin F, Pereira FA. 2005. Dynamic expression of COUP-TFI and COUP-TFII during development and functional maturation of the mouse inner ear. Gene Expr Patterns 5:587–592 [DOI] [PubMed] [Google Scholar]
- 100. Qiu Y, Cooney AJ, Kuratani S, DeMayo FJ, Tsai SY, Tsai MJ. 1994. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci USA 91:4451–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tang LS, Alger HM, Pereira FA. 2006. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development 133:3683–3693 [DOI] [PubMed] [Google Scholar]
- 102. Tripodi M, Filosa A, Armentano M, Studer M. 2004. The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development 131:6119–6129 [DOI] [PubMed] [Google Scholar]
- 103. Adam F, Sourisseau T, Métivier R, Le Page Y, Desbois C, Michel D, Salbert G. 2000. COUP-TFI (chicken ovalbumin upstream promoter-transcription factor I) regulates cell migration and axogenesis in differentiating P19 embryonal carcinoma cells. Mol Endocrinol 14:1918–1933 [DOI] [PubMed] [Google Scholar]
- 104. Pipaón C, Tsai SY, Tsai MJ. 1999. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol Cell Biol 19:2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kume T. 2010. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol 25:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]