In mammals, body growth is rapid in early life but slows with age, thus imposing a limit on adult body size. This growth deceleration is caused by potent suppression of cell proliferation in multiple tissues, but the underlying causes have long remained mysterious. This review summarizes our current understanding of the molecular, cellular, and physiological mechanisms that drive juvenile growth deceleration and allow organs and organisms to target an appropriate adult size.
Abstract
Recent studies have begun to provide insight into a long-standing mystery in biology—why body growth in animals is rapid in early life but then progressively slows, thus imposing a limit on adult body size. This growth deceleration in mammals is caused by potent suppression of cell proliferation in multiple tissues and is driven primarily by local, rather than systemic, mechanisms. Recent evidence suggests that this progressive decline in proliferation results from a genetic program that occurs in multiple organs and involves the down-regulation of a large set of growth-promoting genes. This program does not appear to be driven simply by time, but rather depends on growth itself, suggesting that the limit on adult body size is imposed by a negative feedback loop. Different organs appear to use different types of information to precisely target their adult size. For example, skeletal and cardiac muscle growth are negatively regulated by myostatin, the concentration of which depends on muscle mass itself. Liver growth appears to be modulated by bile acid flux, a parameter that reflects organ function. In pancreas, organ size appears to be limited by the initial number of progenitor cells, suggesting a mechanism based on cell-cycle counting. Further elucidation of the fundamental mechanisms suppressing juvenile growth is likely to yield important insights into the pathophysiology of childhood growth disorders and of the unrestrained growth of cancer. In addition, improved understanding of these growth-suppressing mechanisms may someday allow their therapeutic suspension in adult tissues to facilitate tissue regeneration.
Introduction
-
Cellular Events Responsible for Declining Proliferation
Decreasing growth fraction vs. increasing cell-cycle time
Changes in stem cells, transit-amplifying cells, and terminally differentiated cells
-
Systemic and Local Mechanisms for Body Growth Deceleration
Coordination of body growth: temporal, conditional, and evolutionary
Modulation of growth by systemic mechanisms
Progressive suppression of growth by local mechanisms
A multiorgan postnatal genetic program
-
How Do Organs “Know” When to Stop Growing?
Mechanisms that assess time: biological clocks
Catch-up growth: evidence for mechanisms that assess growth
Mechanisms that assess organ mass: growth-inhibiting chalones
Mechanisms that assess organ function
Mechanisms that count cell divisions
Clinical Implications
Conclusion
I. Introduction
“A person's right and left legs almost always end up the same length, and the hearts of mice and elephants each fit the proper rib cage. How genes set limits on cell size and number continues to mystify.” (1)
One of the remaining fundamental mysteries in biology is how organs and organisms know when to stop growing. In mammals, by the time of adulthood, body growth has generally ceased or slowed dramatically. Importantly, this cessation or near cessation of growth does not occur abruptly, but rather is the end result of a progressive decline in growth rate that begins in early life.
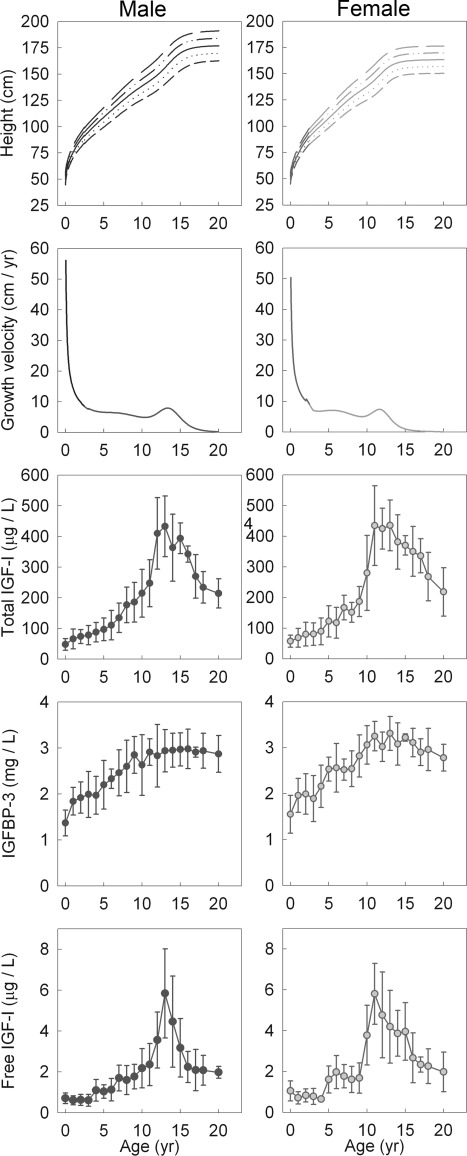
In humans, for example, the linear growth rate (change in body length per unit time) declines dramatically with age (Fig. 1). During fetal life, the linear growth rate is enormous, exceeding 100 cm/yr. By birth, the rate has fallen to approximately 50 cm/yr, and by mid-childhood, 5 cm/yr (2). This decline in growth rate is briefly interrupted by the pubertal growth spurt, raising the growth rate to approximately 10 cm/yr, but then the decline in growth rate resumes, causing linear growth to cease by mid to late adolescence (Fig. 1). Similar declines in linear growth rate occur in other mammals. However, the time course varies markedly. In rodents, this decline occurs over weeks, resulting in a small body size, whereas in humans and other large mammals, the decline occurs gradually, over years, resulting in a larger body size (2, 3). Another difference is that some species, such as the rodent, do not undergo epiphyseal fusion at the time of sexual maturation and have persistent linear growth into adulthood, but nonetheless, this persistent growth continues to slow, reaching very low rates.
Fig. 1.
The decline in the human linear growth rate is not due to declining circulating IGF-I levels. First row, In humans, height increases rapidly in early childhood but eventually plateaus in adolescence (216). Second row, The linear growth velocity (first derivative of the height curve) decreases dramatically during infancy, more gradually during childhood, briefly rises during the pubertal growth spurt, and then resumes its decline, approaching zero (216). Third to fifth rows, As growth is slowing, there is a general increase in total IGF-I and IGFBP-3, both of which are stimulated by GH, as well as an increase in free IGF-I (derived from reference 52).
This growth deceleration occurs not only in linear growth, which reflects growth of chondrocytes in the growth plate, but also in growth of other tissues and organs, and consequently in growth of overall body mass. For example, in rodents, body mass increases exponentially during embryonic life, but then this initial exponential growth is restrained to a linear and then a sublinear trajectory (4). Similarly, in humans, body mass increases exponentially for the first 3 wk after conception, and then the body mass curve deviates below the exponential trajectory (5). Postnatally, human body mass doubles in the first 5 months after birth, doubles again in the next 30 months, and then again in the next 6 yr. Eventually, the body mass growth rate approaches zero as the individual approaches adult size (except for the continuing gain in fat mass that occurs in permissive environments).
Growth deceleration results from a decline in both the rate of cell proliferation (hyperplasia) and the rate of cell enlargement (hypertrophy). In dividing cells, cell growth and cell division are often coordinated to maintain a constant average cell size while cell number increases (6). In rodents, body growth in early juvenile life is primarily due to proliferation, leading to an increase in cell number (7). With increasing age, this hyperplasia slows, at which time hypertrophy occurs in many tissues but also plateaus subsequently (7). Thus, the decline in body growth velocity is first due to a decrease in the rate of cell proliferation, followed by a decrease in the rate of cell enlargement. In the rat, the net result is that between birth and 1 month of age, the whole body DNA content (which reflects the number of nuclei) increases 7-fold, and the amount of protein per nucleus (which reflects cell size) increases 3-fold. The enormous differences in body size among mammals result primarily from differences in cell numbers rather than cell size. For example, the sizes of most cell types in a mouse do not differ much from that of a dog or a cow. The volume of a hepatocyte in all these animals is approximately 2000 fl (8, 9). In contrast, a 70-kg human contains about 1013 cells, compared with a 25-g mouse that contains about 3 × 109 cells (10, 11). Thus, an approximately 3000-fold difference in body mass is accounted for by an approximately 3000-fold difference in cell number (10). In this review, we focus our attention on the mechanisms responsible for the decline in cell proliferation.
II. Cellular Events Responsible for Declining Proliferation
A. Decreasing growth fraction vs. increasing cell-cycle time
The decline in cell proliferation in multiple tissues during juvenile life could be due to an increase in the cell-cycle time, a decrease in the growth fraction, or a combination of both. An increase in the cell-cycle time indicates that a longer time is required to pass through the entire cell cycle. The growth fraction represents the number of cells that remain in the cell cycle divided by the total number of cells in an organ. Therefore, a decrease in growth fraction indicates that fewer cells remain actively dividing, whereas more cells have dropped out of the cell cycle or remain in the G0 phase. In juvenile mice, as proliferation declines in kidney and liver, the cell-cycle time only increases modestly in renal epithelial cells and does not increase appreciably in hepatocytes (12). In contrast, the growth fraction in these organs decreases markedly (12). Similarly, a decrease in growth fraction with age has been reported in the rat liver postnatally (13, 14). These results therefore suggest that declining cell proliferation in juvenile life primarily results not from an increase in cell-cycle time but from a decrease in growth fraction.
B. Changes in stem cells, transit-amplifying cells, and terminally differentiated cells
It has been hypothesized that stem cells exist in adult organs for tissue maintenance purposes (15). The role of these residential stem cells in juvenile growth and growth deceleration is not known. Several possible roles might be imagined. First, growth deceleration might result from a decrease in stem cell proliferation; and second, from a decline in the number of stem cells. Third, the proliferation rate of transit-amplifying progeny derived from these stem cells might decline with age. A fourth possibility is that, in the embryo, proliferation occurs in most cells in each organ, but that with age many cells exit the cell cycle, such that proliferation is eventually restricted to a small set of cells, the residential stem cells.
The mammalian growth plate provides a useful system to analyze these issues because, in this cartilaginous structure, stem-like progenitor cells, transit-amplifying cells, and terminally differentiated cells are spatially segregated into the resting, proliferative, and hypertrophic zones, respectively (16). Declining growth in the juvenile rabbit growth plate is associated with depletion of the stem-like resting zone chondrocytes, both quantitatively (number of cells) and qualitatively (proliferation rate) (17). Interestingly, proliferation also slows with age in the transit-amplifying proliferative chondrocytes (17, 18), which could reflect the declining proliferative capacity of the stem-like cells from which they derive.
The role of stem cells and transit-amplifying cells in growth deceleration has been less well studied in other tissues. In muscle, satellite cells, which function as residential myogenic stem cells, show declining proliferation rates by 2–3 months after birth in rats (19) and turkeys (20). In liver, a hepatic progenitor population, the oval cells (21), contributes to regeneration when injury occurs simultaneously with impaired hepatocyte proliferation (usually due to viruses, toxins, or drugs) (22–24). However, regeneration after partial hepatectomy and normal tissue turnover are driven by proliferation of fully differentiated hepatocytes (25). In organs such as kidney and pancreas, where evidence of adult stem cells is conflicting, it is unclear whether the decrease in cell proliferation during postnatal life is due to the depletion of progenitor cells. The observations that existing fully differentiated cells in kidney (26) and pancreatic β-cells (27) can undergo cell division for tissue repair argue against the existence of adult stem cells and thus suggest that the decrease in proliferation rate with age may not be attributable to a single progenitor cell population.
III. Systemic and Local Mechanisms for Body Growth Deceleration
A. Coordination of body growth: temporal, conditional, and evolutionary
One clue regarding the mechanisms responsible for limiting growth is that growth of different organs and structures appears to be coordinated to maintain proportionality of these body parts. Growth of these various organs appears to be coordinated temporally, conditionally, and evolutionarily (28).
Growth is coordinated temporally in that, as growth slows with age, the deceleration occurs in multiple different organs. The time course of this deceleration is similar in different organs but not identical (29). For example, the central nervous system tends to show more rapid declines in growth than most other tissues (30, 31).
Conditional coordination of growth is observed during conditions such as GH deficiency, hypothyroidism, and malnutrition. Such conditions generally cause widespread inhibition of growth in multiple organs such that body proportions tend to be maintained. Similarly, when growth-inhibiting conditions resolve, catch-up growth is observed in multiple organs, again tending to maintain body proportions (32, 33). Furthermore, targeted ablation of a wide variety of growth-regulating genes in mice either inhibits or stimulates growth of multiple organs. This tendency to maintain body proportions is not absolute in that the growth rates of different organs are typically affected in the same direction but not to an identical extent. For example, in mice lacking the GH receptor, the weights of major organs are reduced proportionately, except for the kidney and spleen, which show a greater reduction in size, and the brain, which shows less reduction in size (34). Similarly, IGF-I deficiency and thyroid hormone deficiency in mice inhibit both brain growth and overall body growth but, in both disorders, brain growth is inhibited less, which results in an increase in brain to body weight (35, 36). In humans, uteroplacental insufficiency similarly causes less inhibition of brain growth compared with overall body growth (37).
Growth also appears to be coordinated during evolution. In large mammals, such as the elephant, the size of multiple organs is proportionally increased compared with small mammals, such as mice. Less dramatic examples can also be found within a species; organs in large dogs are bigger than organs in small dogs (38), and similarly, in comparing organ sizes between chickens of light and heavy breeds (39).
B. Modulation of growth by systemic mechanisms
One possible explanation for coordinated growth of different organs is that body growth is orchestrated by a hormonal or other systemic mechanism. In fact, a hormonal mechanism plays a significant role in growth deceleration of insects. When the larvae reach a “critical size,” juvenile hormone levels fall and ecdysone levels rise, which signals juvenile growth to stop and metamorphosis to commence (40). Might GH play an analogous role in mammalian growth deceleration? Several observations appear to support this hypothesis. Certainly, GH is a potent regulator of postnatal growth and can affect final body size. Mice lacking functional GH receptors reach an adult body mass approximately 60% that of wild-type mice, and mice lacking IGF-I reach an adult body mass approximately 30% of normal (34). Conversely, overexpression of GH and IGF-I in mice leads to an increase in body size (41, 42). Analogous effects on body size are seen in humans with decreased GH, increased GH, or decreased IGF-I. GH deficiency affects primarily postnatal growth, whereas IGF-I deficiency affects both pre- and postnatal somatic growth (43). Interestingly, size differences among different breeds of domestic dogs are associated with different IGF-I alleles (44) and with serum IGF-I levels (45). Furthermore, the normal rapid proliferation rate in early life is temporally associated with a high level of GH. Circulating GH levels in the human, ovine, and rodent fetus are substantially higher than in the adult organism (46). These levels then decline sharply during the neonatal period (46–48). Similarly, the human pubertal growth spurt is driven in large part by an increase in serum GH levels.
However, multiple lines of evidence suggest that growth deceleration is not primarily driven by declining GH levels. First, the elevated fetal levels of GH are not required for the normal rapid pace of fetal growth, which is maintained even in the absence of functional GH receptors in both mice and humans (34, 49). Furthermore, in these mice and humans lacking functional GH receptors, overall postnatal growth is diminished, but the normal pattern of deceleration is maintained (34, 49). Second, in GH-deficient humans, administration of a fixed dose of GH per kilogram of body weight produces a growth curve similar to that of normal individuals, including the normal decline and cessation in growth. Third, overexpression of GH or IGF-I in transgenic mice leads to a substantial increase in body and organ size, but postnatal growth still slows progressively (42, 50, 51). Fourth, the decline in GH levels during early infancy is not accompanied by declines in circulating IGF-I or of IGF binding protein-3 (IGFBP-3) (47, 52), both of which reflect GH action on the liver. Indeed, IGF-I, which mediates most of the growth-promoting effect of GH (34), generally increases in concentration from infancy through early adolescence, as growth is slowing (53) (Fig. 1). This increase in IGF-I is coupled to an increase in IGFBP-3 (47, 52), which stabilizes circulating IGF-I but may also decrease IGF-I bioavailability. However, free IGF-I rises with age (52) (Fig. 1), suggesting that bioavailable IGF-I also likely rises and thus could not account for a declining growth rate. Fifth, both circulating GH and IGF-I concentrations are quite elevated during mid to late adolescence, as growth slows and the individual approaches adult size (47, 52, 54, 55). Serum GH and IGF-I levels do reach a peak during midpuberty and then decline (55, 56), but even when body growth is ceasing, levels are still considerably higher than levels during the rapid growth of earlier childhood.
These findings argue that declining GH and IGF-I, acting in an endocrine manner, do not play major roles in growth deceleration. However, none of these arguments exclude the possibility that IGF-I, acting in a paracrine fashion, plays a role in growth deceleration. Indeed, IGF-I is expressed by many tissues under complex regulation (including, but not limited to, GH) and in mice, IGF-I promotes growth through both endocrine actions (57) and paracrine actions (58). Thus, the role of paracrine IGF-I in growth deceleration remains to be determined.
Nutritional intake is another systemic factor that strongly modulates growth but does not appear to be the predominant mechanism driving normal growth deceleration. Although undernutrition inhibits juvenile growth (29, 59–62), overnutrition does not prevent growth deceleration. In rats (63, 64), pigs (65), and chickens (66, 67), forced overfeeding accelerates weight gain. However, with increasing age, more of the weight gain is attributed to fat deposition rather than protein content (63, 64). Similarly, in transgenic animal models that exhibit hyperphagia and obesity, such as leptin-deficient mice (68) and melanocortin-4 receptor (Mc4r)-deficient mice (69), the majority of weight difference is due to increased fat mass, rather than changes in lean mass or visceral organ mass (69, 70).
A systemic factor, estrogen, does plays a role in growth deceleration, specifically in the growth plate. In humans, the 20-fold decline in linear growth rate that occurs between fetal life and preadolescence appears to be largely independent of sex steroids. However, with puberty, in both males and females, estrogen levels increase, causing linear growth acceleration (due in part to increased GH secretion). Soon afterward, the decline in growth resumes, so that the growth rate approaches zero at approximately 15 yr of age in girls and 18 yr of age in boys. Once linear growth has essentially ceased, the inactive growth plates are converted into bone (71), a process called epiphyseal fusion. In the absence of estrogen effect, this latter phase of linear growth deceleration occurs more gradually. In a man with mutations in estrogen receptor-α, and thus estrogen resistance, persistent growth plate function was observed well into adulthood (72). Importantly, however, in this individual, linear growth did progressively slow and eventually cease. At 30 yr of age, the growth rate had fallen to approximately 0.5 cm/yr (73), and at 35 yr of age, ossification of the growth plates finally occurred (74). Similar slowly progressive declines in linear growth rate have been observed in men with estrogen deficiency due to aromatase mutations (75) and, indeed, in normal rodents whose growth plates appear not to respond to estrogen in a manner similar to humans (76). Studies in rabbits indicate that the normal developmental program of growth plate senescence, which includes a decline in chondrocyte proliferation rate, is accelerated by estrogen, causing earlier exhaustion of the proliferative capacity and consequently earlier fusion (77). Thus, estrogen promotes growth deceleration in the growth plate but is not required for this process.
C. Progressive suppression of growth by local mechanisms
Might some unknown systemic factor other than GH, IGF, or nutritional status drive postnatal growth deceleration? Transplantation experiments between animals of different ages argue against this conjecture. When juvenile organs, including intestine (78, 79), kidney (80), heart (81), thymus (82, 83), spleen (84), and the growth plate (85, 86) are transplanted into adult recipients, these organs continue to grow rapidly as if they were still situated in a juvenile host, and then later show growth deceleration when approaching their adult sizes (78, 80, 82, 84), suggesting that growth deceleration is an intrinsic property of the organ rather than the systemic environment. In contrast, parabiosis between young (2–3 months old) and old (19–26 months old) mice restores the proliferation and regenerative capacity of aged muscle satellite cells and hepatocytes in the older mice (87). However, it is unclear how these studies on regenerative capacity of adult cell proliferation apply to juvenile growth.
In summary, existing evidence does not exclude the possibility that systemic mechanisms contribute to physiological growth deceleration but does indicate that local mechanisms play a central role in this process. Thus, growth deceleration appears to be primarily an intrinsic property of growing organs. It remains possible that such local mechanisms may depend on a systemic signal. For example, although circulating GH and IGF-I concentrations do not decline substantially during human growth deceleration, the local downstream effectors of GH or IGF-I at the organ level, such as receptors and the associated signaling pathways, could be involved. Similarly, declining nutritional intake does not appear to be a principal mechanism driving growth deceleration, but it is possible that local, downstream mediators of this nutritional effect might be involved. For instance, nutritional availability regulates cell growth in part through the mammalian target of rapamycin (mTOR) signaling pathway at the molecular level (88–90). Activation of mTOR kinase under high nutrient/energy conditions leads to the phosphorylation of two major targets, S6K and 4E-BP (91). Phosphorylated S6K in turn acts on a variety of effectors (92) to promote cell growth, cell-cycle progression, and protein synthesis (93–96). Phosphorylated 4E-BP is released from the translation initiation factor eIF4E, allowing its binding with 5′-capped mRNAs to initiate translation (90, 97, 98). Conversely, nutrient deprivation leads to suppression of mTOR kinase activity, which in turn causes inhibition of protein synthesis and cell growth (90, 97, 98). It is possible that the mTOR kinase activity is gradually suppressed in growing organs, thus contributing to growth deceleration. This hypothesis has been tested recently by injecting rapamycin into fetal (embryonic d 19) and postnatal (10-d-old) mice and examining the effects on proliferation (99). Surprising, cellular proliferation in tissues from both ages was resistant to rapamycin treatment and thus occurs independently of mTOR/S6K signaling (99). These findings therefore argue against a role of mTOR signaling in regulating normal organ growth with age.
Although the mechanisms responsible for limiting organ growth appear to be local, rather than systemic, they may not be cell autonomous but rather may involve local interactions among cells, such as paracrine signals. In muscle, experiments involving coculture of myofiber explants with satellite cells suggest that the proliferative capacity of satellite cells depends on the age of the myofiber rather than the satellite cells themselves, suggesting a local, niche-dependent mechanism (100). Similarly, resting zone chondrocytes, which are thought to act as progenitor cells for the growth plate (16), show marked declines in cell proliferation in vivo (17), but when these cells are placed in cell culture, their proliferative capacity is independent of the age of the donor animal (101), suggesting that the decline in proliferative activity is not cell-autonomous but depends on interaction of the cell with neighboring cells, extracellular matrix, or other extracellular factors.
D. A multiorgan postnatal genetic program
Growth retardation and overgrowth phenotypes have been reported in knockout mouse models of many different genes, indicating important roles of these genes and associated signaling pathways in modulating body growth. However, it is yet unclear how these findings can be used to explain juvenile growth deceleration. For example, as discussed above, manipulating GH expression in transgenic animals affects growth rate and adult body size, but it does not imply that GH is responsible for the physiological deceleration and cessation of body growth. Similarly, for most signaling pathways that affect body size, like the oncogenic c-Myc pathway (102–104) and the recently discovered Hippo kinase cascade (105–110), it is not known whether modulation of these regulatory systems contributes to growth deceleration.
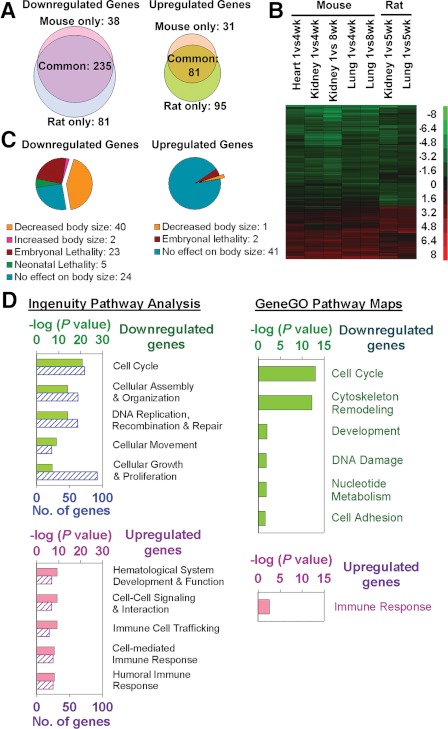
As discussed in Section III.C, body growth deceleration occurs coordinately in many organs throughout the body, and yet this coordination does not appear to be orchestrated by a systemic mechanism. Another possible explanation for this coordination is that multiple organs employ the same or similar local growth-decelerating mechanisms. Consistent with this line of reasoning, recent evidence suggests that there exists a complex growth-limiting genetic program that occurs simultaneously in multiple organs during juvenile life. The presence of this program was first suggested by microarray analysis of global changes in gene expression as juvenile growth slows in mice (111, 112) and rats (113). These studies have uncovered an extensive genetic program that occurs during growth deceleration; is common among kidney, lung, and heart; and is conserved between mice and rats (Fig. 2, A and B). This common program involves the down-regulation of hundreds of genes with age. For many of these genes, knockdown of expression in vitro inhibits cell proliferation, and knockout in vivo inhibits body growth, suggesting that they normally promote growth (Fig. 2, C and D) (113). These down-regulated, growth-promoting genes include growth factors such as Igf2, Mdk, and Ptn and transcription factors such as Ezh2, Mycn, Peg3, and Plagl1. More limited data suggest that this program also occurs in liver (113). Taken together, these findings suggest that juvenile growth deceleration is caused by the down-regulation of a large set of growth-promoting genes, some of which may involve local paracrine mechanisms. Because this genetic program is common to multiple organs, it may explain how growth in different organs slows concordantly, therefore maintaining body proportions. This complex program may also help explain why genetic disorders that produce indefinite, unrestrained body growth have not been observed; the existence of multiple “brakes” ensures that growth deceleration occurs despite the mutation of one or a few genes.
Fig. 2.
A complex growth-related genetic program occurs in multiple organs during juvenile life. A, Venn diagrams showing the number of genes down-regulated and up-regulated with age by microarray analysis in mouse and rats. The analysis included genes that showed age regulation (P < 0.05; ≥2.0-fold) in mouse kidney, lung, and heart or in both rat kidney and lung. The substantial overlap indicates that the program was highly conserved during the 20 million yr since the two species diverged. B, Heat maps based on the same set of genes. Each row corresponds to a single gene. Green, Down-regulation with age; red, up-regulation. Scale values are log2 (fold difference). C, A knockout phenotype was reported for 139 of the genes in this same gene set. For the down-regulated genes, knockout frequently resulted in decreased body size, suggesting that many down-regulated genes in this program are growth promoting. D, Bioinformatic analyses of these age-regulated genes using Ingenuity Pathway Analysis (IPA) 7.1 and GeneGO also suggest that many of the genes that are down-regulated with age serve to regulate proliferation. For IPA, the five most overrepresented molecular, cellular, or physiological functions are shown (solid bars, P value; striped bars, number of significant genes involved). For GeneGO, all significant (P < 0.05) map folders are shown. [Reproduced from J. C. Lui et al.: FASEB J 24:3083–3092, 2010 (113).]
The molecular mechanisms that orchestrate the down-regulation of hundreds of genes in this juvenile program are not known, but recent evidence suggests that epigenetic mechanisms may be involved. In mice between 1 and 4 wk of age, histone H3K4 trimethylation decreases in the promoter region of many down-regulated genes in the multiorgan genetic program (113). Because H3K4 trimethylation is associated with an open chromatin state and increased transcription, its decline with age may contribute to the observed declines in gene expression.
IV. How Do Organs “Know” When to Stop Growing?
The identification of this genetic program could help explain how growth in different organs slows concordantly, but it is insufficient to explain how the organs “know” that they have reached the right sizes to stop growing. The ability of the organs and organism to target a specific adult body size suggests the acquisition of information about elapsed time, organ mass, organ function, or number of cell divisions undergone. In principle, mechanisms to assess any of these types of information would allow the organ to “know” when to stop growing.
A. Mechanisms that assess time: biological clocks
The existence of a biological clock would allow the body to grow for a defined period of time before slowing, thus achieving a certain size. Theoretically, under this mechanism, if growth is transiently inhibited, the final body size would be reduced. Such timing mechanisms may be employed during embryonic development in certain cell types. Embryonic rat cardiac myocytes cultured under different conditions tend to stop dividing at a set time, despite having undergone different numbers of cell division (114). Similar studies in oligodendrocyte precursor cells in the central nervous system (115–117) suggest that time, rather than the number of cell divisions, signals the start of differentiation, indicating the presence of a cell-intrinsic timing mechanism. In particular, cyclin-dependent kinase (Cdk) inhibitor p27/Kip1 has been proposed to act as the timing mechanism because it accumulates progressively with time in cultured oligodendrocyte precursor cells (117). However, when these cells are isolated from p27-deficient mice, they only undergo one or two more cell divisions than wild-type cells before differentiation (118), suggesting that p27/Kip1 is only part of a more complex regulatory mechanism that consists of multiple components with overlapping functions, such that defects in one component do not totally abolish the timing function (119).
B. Catch-up growth: evidence for mechanisms that assess growth
An alternative mechanism for organs and organisms to grow to a reproducible size is by gathering information about growth itself. It is perhaps best demonstrated by the phenomenon of catch-up growth. In humans and other mammals, juvenile growth is inhibited by conditions such as GH deficiency, hypothyroidism, or malnutrition. If such conditions transiently inhibit growth but then resolve, the growth rate generally does not just return to normal but rather exceeds the normal rate for chronological age (120). Even when postnatal growth is delayed for more than 1 yr in rats fed a tryptophan-deficient (Trp−) diet (121) or a corn gluten-based diet (122), switching to a replete diet induces marked catch-up growth, although the animals are well into adulthood. As ingeniously suggested in 1914, this finding suggests that the loss of growth capacity results from the execution of growth itself, rather than increasing age (122).
The mechanisms responsible for catch-up growth have been best studied in the growth plate. Previously, the prevailing explanation for catch-up growth was that it results from a mechanism within the central nervous system that compares the actual body size to an age-appropriate set-point and then adjusts the growth rate accordingly. Thus, if a child is too small for age, the mechanism would sense this deficit and turn up the growth rate, causing catch-up growth (123). However, subsequent studies showed that local growth inhibition, in a single growth plate, is followed by local catch-up growth within that growth plate. This anatomic specificity suggests that the mechanism responsible for catch-up resides not in the central nervous system but rather within the growth plates themselves (124). Studies in rats, rabbits, and humans, after various growth-inhibiting conditions, suggest that this local catch-up growth occurs because growth-inhibiting conditions slow the normal loss of proliferative capacity in the growth plate (125–128). Thus, the loss of growth capacity in the skeletal growth plate is driven, not simply by time, but rather by growth itself.
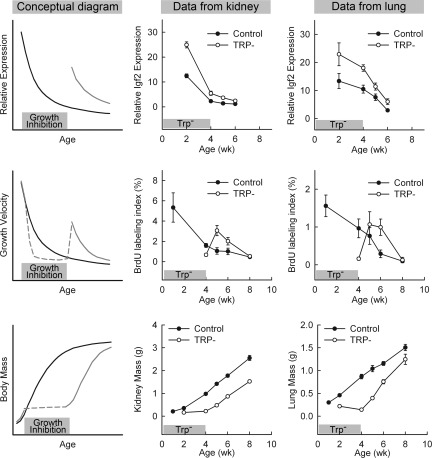
As discussed above, current evidence suggests that the normal process of growth deceleration is driven, at a physiological level, by growth itself and, at a molecular level, by a multiorgan genetic program. Thus, one attractive hypothesis is that growth drives the juvenile multiorgan genetic program, which in turn drives growth deceleration. This hypothesis predicts that experimentally slowing growth by imposing growth-inhibiting conditions would slow the progression of the genetic program and thereby conserve future growth potential. This prediction has recently been tested by slowing growth experimentally in newborn rats for 4 wk using a Trp− diet that suppresses food intake and therefore induces malnutrition (113). At 5 wk of age, 1 wk after release from the Trp− diet, these rats showed increased proliferation in kidney, lung, and liver compared with control rats that had received a replete diet, consistent with the concept that growth-inhibiting conditions slow the loss of growth capacity (Fig. 3). In addition, expression microarray analysis demonstrated that the putative growth-limiting genetic program had been delayed; for a large set of growth-promoting genes that are normally down-regulated with age, the expression levels in rats with prior tryptophan deficiency were higher than in control rats of the same age (113) (Fig. 3). These findings are further supported by a previous study suggesting that hypothyroidism-induced growth retardation also delays down-regulation of growth-promoting genes, although far fewer genes were studied (111). These findings therefore suggest that the putative growth-limiting genetic program is not simply a function of age, but rather depends on the extent of organ growth undergone.
Fig. 3.
Transient inhibition of growth slows the multiorgan juvenile genetic program and also slows the normal loss of growth capacity. Left column represent conceptual diagrams, whereas middle and right columns represent experimental data. During normal mammalian juvenile growth (black curves), multiple growth-promoting genes show declining expression (top left panel). This decline is associated with declining proliferation rate (middle left panel), and therefore plateauing of body mass (bottom left panel). The gray curves in these panels depict the hypothesis that transient growth inhibition (gray box just above x-axis) would delay the normal decline in expression of growth-promoting genes and consequently delay the decline in proliferation rate and thus the increase in body mass. The middle and right columns show data from newborn rats that were placed on a Trp− diet for 4 wk (gray bars) to restrict growth (113). In kidney and lung, transient tryptophan deficiency delayed the decline in expression of many genes, including Igf2 (top row), delayed the decline in proliferation (middle row), and therefore the gain in organ mass (bottom row). [Middle and right panels of top two rows reproduced from J. C. Lui et al.: FASEB J 24:3038–3092, 2010 (113).]
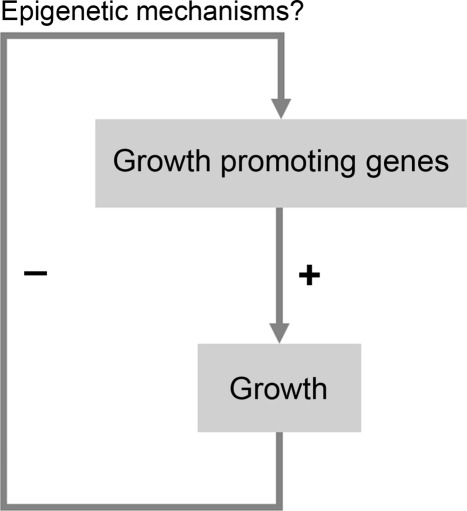
Thus, current findings suggest that mammalian body growth is limited, at least in part, by a negative feedback loop (Fig. 4). Growth causes the down-regulation of a large set of growth-promoting genes, which in turn causes body growth to progressively slow and eventually cease, thus setting a fundamental limit on adult body size. This negative feedback appears to act in multiple organs, providing a possible explanation for how growth slows concordantly throughout the body, thus maintaining body proportions.
Fig. 4.
Model for a mechanism that restricts juvenile body growth. In early life, multiple growth-promoting genes are well expressed, leading to rapid growth. However, growth causes down-regulation of these growth-promoting genes (perhaps through epigenetic mechanisms) which causes growth to slow. Progression of this negative feedback loop would eventually cause the growth rate to approach zero.
As discussed above, organs appear to stop growing based not simply on information about the amount of time elapsed, but based at least in part on the amount of growth undergone. This information about prior growth could involve any of several possible variables. One possibility is that a mechanism exists to assess organ size, either the tissue mass or the number of cells that have accumulated in the organ. A second possibility is that growth continues until the organ achieves a certain level of function. A third possibility is that a mechanism exists to count the number of cell divisions undergone. There is an important distinction between counting the number of cells present and counting the number of cell divisions that have occurred. If growth deceleration is determined by counting the number of cells that have accumulated, it would imply that if we experimentally remove a certain fraction of cells from an organ during the period of growth, the organ will continue to grow until the normal size is achieved. In contrast, if growth deceleration is determined by counting the number of cell divisions undergone, the organ will not sense any experimental removal of cells and would thereafter attain a smaller than normal size. Whether growth is limited by tissue mass, number of cells accumulated, organ function, or the number of cell divisions may depend on the organ involved. Even for a particular organ, more than one type of information might be employed to limit growth.
C. Mechanisms that assess organ mass: growth-inhibiting chalones
Classical studies in salamanders suggest that body growth in these amphibians is dependent on the ultimate tissue mass (129). A tetraploid salamander has cells that are twice as large as those of a diploid salamander, but the tetraploid adult has half as many cells, resulting in a similar body size (129). Similarly, in mice, generation of tetraploids by electrofusion (130) results in embryos with cells of approximately twice the normal size but half the normal cell number, thus achieving an overall size comparable to normal embryos (131). Conversely, in Drosophila, when the cell cycle pace is accelerated or slowed in an imaginal disc by overexpression or ablation of dE2f, the changes in proliferation rate are offset by changes in cell size to achieve a constant overall organ size (132). Recent studies suggest that an additional mechanism called cell competition helps maintain a constant organ mass (133). When growth-promoting dMyc is expressed in some but not all cells in the imaginal wing disc, cell competition occurs such that more slowly proliferating wild-type cells are selectively eliminated by apoptosis, thus, compensating for the increased number of dMyc-expressing cells (103, 134) to maintain a constant cell number.
How might organ size regulate growth? One old theory postulates the existence of “chalones”—soluble factors that are secreted by cells in an organ and negatively regulate growth of that organ (135) (Table 1). According to this theory, because the concentration of a chalone is directly proportional to the cell number in an organ, when the organ reaches a certain size, the concentration of the chalone is sufficient to stop growth (135). Such growth-inhibiting signals could act locally or could circulate and act systemically. Elegant studies demonstrate that myostatin serves as a chalone (136). Myostatin is a TGF-β family protein, which is secreted by muscle cells and acts to inhibit muscle growth (137–139) and myogenic satellite cell renewal (140, 141). Homozygous deletion of the myostatin gene (Mstn) in mice results in dramatic increases in skeletal muscle mass, attributed to both muscle cell hyperplasia and hypertrophy (137, 139, 142). This effect is not limited to the embryonic formation of muscle fibers because postnatal loss or blockade of myostatin has the same effect (143–149). Similar studies in dogs (150), pig (151), sheep (152, 153), cattle (154–156), and human (157) suggest that myostatin is an evolutionarily conserved negative regulator of skeletal muscle growth. Although myostatin was initially reported to be expressed exclusively in skeletal muscle (137), more recent evidence suggests that it is also expressed in cardiomyocytes, with its expression up-regulated postnatally (158), and serves to inhibit cardiomyocyte proliferation (158, 159). These findings therefore suggest that myostatin might also be involved in regulating the size of the heart.
Table 1.
Regulation of growth by chalones and chalone-like signals
| Tissue | Chalone/chalone-like signal | Effect |
|---|---|---|
| Muscle | Myostatin | Inhibits muscle fiber proliferation |
| Inhibits satellite cell renewal | ||
| Activin A | Inhibits muscle fiber proliferation | |
| Heart | Myostatin | Inhibits cardiomyocyte proliferation |
| Adipose | Leptin | Suppresses appetite by activating MSH secretion and inhibiting NPY and AgRP secretion at the hypothalamus |
| Olfactory epithelium | GDF11 | Inhibits olfactory receptor neuron production |
| Hair follicle | BMP2/BMP4 | Inhibits hair follicle stem cell proliferation |
Another member of the TGF-β family, activin A, has recently been shown to function alongside myostatin to negatively regulate muscle growth (160). Interestingly, the activity of both activin A and myostatin could be inhibited by follistatin (161, 162), which makes follistatin a potential therapeutic agent for improving muscle strength (163, 164). It is yet unclear how muscle mass itself might regulate the expression levels of activin A and follistatin as it regulates myostatin.
For many major organs, such as liver, kidney, and lung, it is not known whether chalones are involved in growth regulation. However, recent studies have identified chalone-like growth-inhibiting signals in a limited number of cell types. For example, GDF11 is produced by cells of the neuronal lineage of the olfactory epithelium and inhibit the production of olfactory receptor neurons (165, 166). Similarly, BMP2 and BMP4 serve as local refractory signals to inhibit proliferation of hair follicle stem cells, thus coordinating waves of hair regeneration (167, 168).
Leptin is another hormone that functions similarly to a chalone but acts through a systemic negative-feedback loop. Leptin is primarily produced by adipocytes, and the level of leptin in the circulation is directly proportional to whole-body adipose tissue mass (169, 170). At the hypothalamus, leptin then acts on proopiomelanocortin neurons to activate MSH secretion (171–173) and inhibits neuropeptide Y (NPY) and agouti-related peptide (AgRP) secretion by the AgRP/NPY neurons (174, 175). Collectively, these changes suppress appetite, stimulate energy expenditure, and therefore inhibit accumulation of adipose tissue (169, 170).
In the recently identified multiorgan juvenile genetic program (111), some of the growth-promoting genes that are down-regulated with increasing body growth are growth factors, such as IGF-II, midkine, and pleiotrophin. These findings suggest that local paracrine factors are involved in deceleration of juvenile growth. However, these soluble factors are functionally distinct from chalones. Although chalones are proposed to be growth-inhibitory factors that increase with growth, these are growth-promoting factors that decrease expression with growth, and therefore, in contrast to chalones, their abundance does not reflect the number of cells. However, it is possible that chalones in different organs drive the common genetic program.
D. Mechanisms that assess organ function
Another strategy to target the appropriate adult size of an organ is based on the function of the organ. For example, if the organ is too small to provide adequate function, a mechanism to stimulate growth might be activated. When the organ achieves a size sufficient to provide adequate function, the growth-stimulatory function would cease. Conversely, when the organ achieves adequate size and therefore function, a growth-inhibiting mechanism might be activated.
The existence of a mechanism that senses organ function is suggested by the phenomenon of liver regeneration after partial hepatectomy in mammals (176–178). This regeneration involves a brief burst of cell replication by a large number of differentiated hepatocytes (25). Similarly, the liver also adjusts its size relative to the overall body size after orthotopic transplantation from a small dog to a larger dog (38). Furthermore, in rats connected by parabiosis, partial hepatectomy in one animal stimulates hepatocyte proliferation in the other (179), indicating that liver regeneration involves circulating factors. The concept that liver size may be regulated by function is further supported by its ability to undergo hyperplasia upon increased xenobiotic stress, such as administration of phenobarbital and 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (180), without liver injury (181, 182).
More recent studies suggest that the liver senses organ functionality, at least in part, through the flux of bile acids (183). Bile acids are synthesized in the liver and secreted into the small intestine, where they facilitate digestion. The bile acids are subsequently reabsorbed and returned to the liver, where they are taken up by hepatocytes, thus completing the enterohepatic circulation. When exposed to a mild increase in bile acid flux, hepatocyte proliferation is induced, causing an increase in liver size (183). Similarly, liver regeneration after partial hepatectomy is potentiated in the presence of bile acids (183, 184). These findings were further supported by a complementary study, showing that disruption of the enterohepatic circulation by draining the common bile duct externally during the 7 d before hepatectomy delays subsequent liver regeneration (185). Thus, collectively compelling evidence suggests that liver regeneration and adult liver size can be modulated by organ function. One possibility is that this same mechanism alone could be used to drive rapid juvenile growth of the liver and its subsequent growth deceleration, because the mechanism would cause liver size to keep pace with the growth rate of the rest of the body. Alternatively, organ function might be used only to fine-tune the adult liver size, and a separate mechanism might be used to suppress proliferation in early juvenile life.
For some endocrine organs, such as the thyroid, adrenal, and parathyroid glands, size is also regulated in part by organ function. If the organ produces insufficient hormone, a negative feedback loop serves to stimulate not only increased secretion but also proliferation and growth of the endocrine gland. Although this mechanism may be used to precisely target the optimal adult organ size based on function, other mechanisms may also contribute to juvenile growth deceleration and size determination. For example, the size of the thyroid gland is regulated by a negative feedback loop involving thyroid hormone and TSH, and yet, in mice lacking a functional TSH receptor, the thyroid still grows to approximately 50% of the normal size (186).
Kidney size also seems to be regulated in part by function. Unilateral nephrectomy causes an increase in the size of the remaining kidney (187), perhaps involving a temporary increase in serum creatinine that signals the increased functional need (187). However, this increase in kidney size is primarily due to hypertrophy (cell enlargement) rather than hyperplasia (cell proliferation) and thus may involve mechanisms different from those primarily regulating juvenile growth (188).
E. Mechanisms that count cell divisions
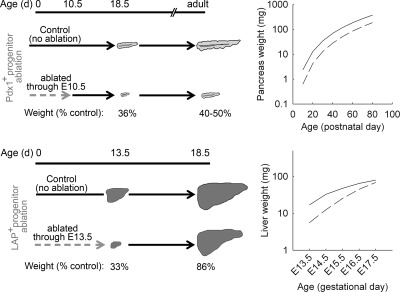
In some developmental systems, growth appears to be limited by a cell-division counter. During the early development of Xenopus laevis, a counting mechanism is in place to ensure that the zygote divides exactly 12 times before cell division slows (189, 190). In mice, reducing the number of pancreatic progenitor cells in early development is not compensated in later development and thus results in a smaller pancreas (Fig. 5) (191). This observation argues against a mechanism that senses organ size or function but is consistent with a mechanism based on cell-cycle counting. As the authors suggested, pancreatic progenitor cell proliferation may be autonomously restricted, and each progenitor cell may be capable of giving rise to only a fixed amount of tissue. In contrast, ablation of progenitor cells during liver development appears not to affect the ultimate organ size, suggesting that hepatic growth is not primarily controlled by cell division counting (Fig. 5). A cell-cycle counting mechanism may also exist in the growth plate. In this structure, the normal decline in chondrocyte proliferation is driven by growth. Because newly formed cartilage in the growth plate is continuously remodeled into bone tissue, the size of the cartilaginous growth plate does not increase with age, arguing against a size-sensing mechanism and suggesting instead a mechanism that senses number of cell divisions undergone (17).
Fig. 5.
Organ size is limited by the number of progenitor cells in pancreas but not in liver. Transgenic mice were generated to allow ablation of Pdx1-expressing (Pdx1+) pancreatic progenitor cells or liver-enriched transcriptional activator protein expressing (LAP+) hepatic progenitor cells (191). Tetracycline could be administered to repress progenitor cell ablation at any time point. In the pancreas (upper panels), progenitor cells were ablated through embryonic day (E) 10.5, reducing pancreas mass to 36% of control values at E18.5. Afterward, the affected pancreases remained proportionately smaller than those of controls. In the liver (lower panels), progenitor cells were ablated through E13.5, causing liver mass to be reduced to 33% of control values. When ablation was repressed by tetracycline administration from E13.5 to E17.5, almost complete recovery of liver size occurred. [Right panels derived from reference 191.]
A cell division counting mechanism might involve some characteristic of the cell that changes with each cell division and then directly or indirectly regulates cellular growth and/or cell division. In principle, this characteristic might involve, for example, DNA, proteins, or other regulatory molecules. One of the most well-studied cell-cycle counting mechanisms is telomere shortening (192). Telomeres are regions of tandem repeats of TTAGGG found at the ends of chromosomes. In all eukaryotes, telomeres shorten with each round of cell division, owing to the incomplete replication of linear chromosomes by conventional DNA polymerases (193). With the exception of a few cell types, including germ cells, stem cells, and certain leukocytes, in which telomerase remains active in adulthood (194), telomeres gradually shorten until a critical length is reached, leading to genome instability and loss of cell viability (192, 195). Telomere shortening plays an important role in cellular senescence, protection against cancer (196), and possibly organismal aging (197, 198) but is unlikely to be centrally involved in regulating juvenile growth. Mice with mutations in Terc, which specifies the RNA component of telomerase, exhibit a variety of phenotypic abnormalities, including decreased life span in late generations, but show normal body growth (199). Similarly, in dyskeratosis congenita, a disorder characterized by abnormally short telomeres due to deficient telomerase activity, short stature does occur but only in a minority of patients (200), particularly those with a severe form of the disease (201).
Another cellular characteristic that might change with cell division is epigenetic changes, such as DNA methylation or histone modification. As mentioned earlier, decreases in H3K4 trimethylation in the promoter region of multiple growth-promoting genes have been observed in multiple organs during juvenile growth deceleration (113). This finding is similar to observations in organismal aging where histone acetylation, which favors gene activation, is found to decline with age (202). It remains unclear whether these epigenetic changes are direct consequences of the cumulative number of cell division and thus might provide a cell-cycle counting mechanism that affects subsequent growth.
V. Clinical Implications
Understanding the fundamental mechanisms that cause growth deceleration and thus govern adult body size is likely to yield insights into the many human genetic disorders that cause childhood growth failure and overgrowth, many of which remain poorly understood. For example, Beckwith-Wiedemann syndrome, an overgrowth disorder, is associated with abnormalities in two imprinted genes that are growth-regulating and down-regulated with age in juvenile life (112)—IGF2, which is growth promoting, and CDKN1C, which inhibits cyclin E-Cdk2 but also activates cyclin D-Cdk4 for cell cycle G1 phase progression (203–205). Conversely, abnormal imprinting and decreased IGF2 expression have been implicated in the growth retardation of Silver-Russell syndrome (206). Simpson-Golabi-Behmel syndrome, an overgrowth disorder, is caused by loss-of-function mutations in GPC3 (207), a growth-inhibiting gene that is down-regulated with age in multiple tissues (113).
Because body growth limitation involves complex, potent mechanisms to suppress cell proliferation, disruption of these mechanisms may contribute to oncogenesis. Multiple growth-promoting genes that are down-regulated with age in multiple tissues and are therefore part of the putative juvenile growth-limiting genetic program have previously been implicated in cancer pathogenesis, for example, N-MYC, a transcription factor (208); EZH2, a histone methyltransferase (209); and midkine and pleiotrophin, two related cytokines (210).
Additionally, elucidating the physiological mechanisms that normally suppress proliferation in juvenile life might suggest new approaches to achieve therapeutic tissue regeneration, either in vivo or ex vivo. It has long been suggested that the regeneration process shares similarities with molecular events of embryonic development. For example, in liver (211, 212) and kidney (213–215), expression of developmental genes reportedly reactivates during injury and regeneration. Interestingly, one gene that is down-regulated postnatally but then reactivated during liver regeneration is Mdk (211). We envision that regeneration may be similarly achieved if quiescent adult cells could be stimulated to proliferate by transiently suspending the mechanisms that suppressed growth during juvenile life.
VI. Conclusion
In summary, mammalian body growth is rapid in early life but slows with age, thus setting a limit on adult body size. This growth deceleration reflects declining proliferation in multiple tissues and is driven in large part by local mechanisms within the growing tissues themselves. Recent findings suggest that body growth deceleration is driven by a growth-limiting genetic program that occurs in multiple organs. This growth-limiting program, which involves the down-regulation of a large set of growth-promoting genes, depends not simply on age but also on organ growth itself. Therefore, the adult body size limit appears to be imposed by a negative feedback loop; organ growth leads to progression of a genetic program, which in turn causes growth of the organ to slow and eventually cease. These findings provide insights into long-standing mysteries regarding body size determination, maintenance of body proportionality, and catch-up growth.
Different organs seem to use different types of information to precisely target the adult body size. For example, skeletal and cardiac muscle growth are negatively regulated by myostatin, the concentration of which depends on muscle mass itself. Liver growth appears to be modulated by bile acid flux, a parameter that reflects organ function. In pancreas, organ size seems to be limited by the initial number of progenitor cells, suggesting a mechanism based on cell-cycle counting.
Further elucidation of the fundamental mechanisms suppressing juvenile growth is likely to yield important insights into the pathophysiology of childhood growth disorders and of the unrestrained growth of cancer. In addition, improved understanding of these growth-suppressing mechanisms may someday allow their therapeutic suspension in adult tissues to facilitate tissue regeneration.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- Agouti-related peptide
- Cdk
- cyclin-dependent kinase
- IGFBP-3
- IGF binding protein-3
- mTOR
- mammalian target of rapamycin
- NPY
- neuropeptide Y
- Trp−
- tryptophan-deficient.
References
- 1. Kennedy D, Norman C. 2005. What don't we know? Science 309:75. [DOI] [PubMed] [Google Scholar]
- 2. Tanner JM, Davies PS. 1985. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr 107:317–329 [DOI] [PubMed] [Google Scholar]
- 3. Hughes PC, Tanner JM. 1970. A longitudinal study of the growth of the black-hooded rat: methods of measurement and rates of growth for skull, limbs, pelvis, nose-rump and tail lengths. J Anat 106:349–370 [PMC free article] [PubMed] [Google Scholar]
- 4. Goedbloed JF. 1972. The embryonic and postnatal growth of rat and mouse. I. The embryonic and early postnatal growth of the whole embryo. A model with exponential growth and sudden changes in growth rate. Acta Anat (Basel) 82:305–306 [PubMed] [Google Scholar]
- 5. Luecke RH, Wosilait WD, Young JF. 1999. Mathematical modeling of human embryonic and fetal growth rates. Growth Dev Aging 63:49–59 [PubMed] [Google Scholar]
- 6. Jorgensen P, Tyers M. 2004. How cells coordinate growth and division. Curr Biol 14:R1014–R1027 [DOI] [PubMed] [Google Scholar]
- 7. Winick M, Noble A. 1965. Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev Biol 12:451–466 [DOI] [PubMed] [Google Scholar]
- 8. Savage VM, Allen AP, Brown JH, Gillooly JF, Herman AB, Woodruff WH, West GB. 2007. Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc Natl Acad Sci USA 104:4718–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgado E, Ocqueteau C, Cury M, Becker L, González U, Muxica L, Günther B. 1990. Three-dimensional morphometry of mammalian cells. II. Areas, volumes, and area-volume ratios. Arch Biol Med Exp Santiago 23:21–27 [PubMed] [Google Scholar]
- 10. Conlon I, Raff M. 1999. Size control in animal development. Cell 96:235–244 [DOI] [PubMed] [Google Scholar]
- 11. Baserga R. 1985. The biology of cell reproduction. Cambridge, MA: Harvard University Press [Google Scholar]
- 12. Chang M, Parker EA, Muller TJ, Haenen C, Mistry M, Finkielstain GP, Murphy-Ryan M, Barnes KM, Sundaram R, Baron J. 2008. Changes in cell-cycle kinetics responsible for limiting somatic growth in mice. Pediatr Res 64:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schultze B, Kellerer AM, Grossmann C, Maurer W. 1978. Growth fraction and cycle duration of hepatocytes in the three-week-old rat. Cell Tissue Kinet 11:241–249 [DOI] [PubMed] [Google Scholar]
- 14. Post J, Hoffman J. 1964. Changes in the replication times and patterns of the liver cell during the life of the rat. Exp Cell Res 36:111–123 [DOI] [PubMed] [Google Scholar]
- 15. Altman J, Das GD. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335 [DOI] [PubMed] [Google Scholar]
- 16. Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. 2002. The role of the resting zone in growth plate chondrogenesis. Endocrinology 143:1851–1857 [DOI] [PubMed] [Google Scholar]
- 17. Schrier L, Ferns SP, Barnes KM, Emons JA, Newman EI, Nilsson O, Baron J. 2006. Depletion of resting zone chondrocytes during growth plate senescence. J Endocrinol 189:27–36 [DOI] [PubMed] [Google Scholar]
- 18. Kember NF, Walker KV. 1971. Control of bone growth in rats. Nature 229:428–429 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki T, Takaishi H, Sakata T, Do MK, Hara M, Sato A, Mizunoya W, Nishimura T, Hattori A, Ikeuchi Y, Tatsumi R. 2010. In vitro measurement of post-natal changes in proliferating satellite cell frequency during rat muscle growth. Anim Sci J 81:245–251 [DOI] [PubMed] [Google Scholar]
- 20. Velleman SG, Zhang X, Coy CS, Song Y, McFarland DC. 2010. Changes in satellite cell proliferation and differentiation during turkey muscle development. Poult Sci 89:709–715 [DOI] [PubMed] [Google Scholar]
- 21. Kinosita R. 1937. Studies on the cancerogenic chemical substances. Trans Soc Pathol Jpn 27:665–727 [Google Scholar]
- 22. Fausto N, Campbell JS. 2003. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120:117–130 [DOI] [PubMed] [Google Scholar]
- 23. Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. 2003. Oval cell-mediated liver regeneration: role of cytokines and growth factors. J Gastroenterol Hepatol 18:4–12 [DOI] [PubMed] [Google Scholar]
- 24. Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. 1999. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 154:537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorgeirsson SS. 1996. Hepatic stem cells in liver regeneration. FASEB J 10:1249–1256 [PubMed] [Google Scholar]
- 26. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. 2008. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291 [DOI] [PubMed] [Google Scholar]
- 27. Dor Y, Brown J, Martinez OI, Melton DA. 2004. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- 28. Widdowson EM. 1970. Harmony of growth. Lancet 1:902–905 [PubMed] [Google Scholar]
- 29. Winick M, Noble A. 1966. Cellular response in rats during malnutrition at various ages. J Nutr 89:300–306 [DOI] [PubMed] [Google Scholar]
- 30. Bogin B. 1999. Evolutionary perspective on human growth. Annu Rev Anthropol 28:109–153 [DOI] [PubMed] [Google Scholar]
- 31. Schultz AH. 1926. Fetal growth of man and other primates. Q Rev Biol 1:465–521 [Google Scholar]
- 32. Kay's SK, Hindmarsh PC. 2006. Catch-up growth: an overview. Pediatr Endocrinol Rev 3:365–378 [PubMed] [Google Scholar]
- 33. Wit JM, Boersma B. 2002. Catch-up growth: definition, mechanisms, and models. J Pediatr Endocrinol Metab 15(Suppl 5):1229–1241 [PubMed] [Google Scholar]
- 34. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- 35. Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. 1995. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14:717–730 [DOI] [PubMed] [Google Scholar]
- 36. Calikoglu AS, Gutierrez-Ospina G, D'Ercole AJ. 1996. Congenital hypothyroidism delays the formation and retards the growth of the mouse primary somatic sensory cortex (S1). Neurosci Lett 213:132–136 [DOI] [PubMed] [Google Scholar]
- 37. Sankaran S, Kyle PM. 2009. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol 23:765–777 [DOI] [PubMed] [Google Scholar]
- 38. Kam I, Lynch S, Svanas G, Todo S, Polimeno L, Francavilla A, Penkrot RJ, Takaya S, Ericzon BG, Starzl TE. 1987. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology 7:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dror Y, Nir I, Nitsan Z. 1977. The relative growth of internal organs in light and heavy breeds. Br Poult Sci 18:493–496 [DOI] [PubMed] [Google Scholar]
- 40. Nijhout HF. 1994. Insect hormones. Princeton, NJ: Princeton University Press [Google Scholar]
- 41. Brem G, Wanke R, Wolf E, Buchmüller T, Müller M, Brenig B, Hermanns W. 1989. Multiple consequences of human growth hormone expression in transgenic mice. Mol Biol Med 6:531–547 [PubMed] [Google Scholar]
- 42. Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, Palmiter RD. 1988. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123:2827–2833 [DOI] [PubMed] [Google Scholar]
- 43. Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. 1996. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367 [DOI] [PubMed] [Google Scholar]
- 44. Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. 2007. A single IGF1 allele is a major determinant of small size in dogs. Science 316:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greer KA, Hughes LM, Masternak MM. 24 September 2010. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr) doi:10.1007/s11357-010-9182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gluckman PD, Grumbach MM, Kaplan SL. 1981. The neuroendocrine regulation and function of growth hormone and prolactin in the mammalian fetus. Endocr Rev 2:363–395 [DOI] [PubMed] [Google Scholar]
- 47. Leger J, Noel M, Limal JM, Czernichow P. 1996. Growth factors and intrauterine growth retardation. II. Serum growth hormone, insulin-like growth factor (IGF) I, and IGF-binding protein 3 levels in children with intrauterine growth retardation compared with normal control subjects: prospective study from birth to two years of age. Study Group of IUGR. Pediatr Res 40:101–107 [DOI] [PubMed] [Google Scholar]
- 48. Miller JD, Esparza A, Wright NM, Garimella V, Lai J, Lester SE, Mosier HD., Jr 1993. Spontaneous growth hormone release in term infants: changes during the first four days of life. J Clin Endocrinol Metab 76:1058–1062 [DOI] [PubMed] [Google Scholar]
- 49. Laron Z, Lilos P, Klinger B. 1993. Growth curves for Laron syndrome. Arch Dis Child 68:768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoeflich A, Nedbal S, Blum WF, Erhard M, Lahm H, Brem G, Kolb HJ, Wanke R, Wolf E. 2001. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinology 142:1889–1898 [DOI] [PubMed] [Google Scholar]
- 51. Blackburn A, Schmitt A, Schmidt P, Wanke R, Hermanns W, Brem G, Wolf E. 1997. Actions and interactions of growth hormone and insulin-like growth factor-II: body and organ growth of transgenic mice. Transgenic Res 6:213–222 [DOI] [PubMed] [Google Scholar]
- 52. Kawai N, Kanzaki S, Takano-Watou S, Tada C, Yamanaka Y, Miyata T, Oka M, Seino Y. 1999. Serum free insulin-like growth factor I (IGF-I), total IGF-I, and IGF-binding protein-3 concentrations in normal children and children with growth hormone deficiency. J Clin Endocrinol Metab 84:82–89 [DOI] [PubMed] [Google Scholar]
- 53. Zapf J, Walter H, Froesch ER. 1981. Radioimmunological determination of insulin like growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest 68:1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rose SR, Municchi G, Barnes KM, Kamp GA, Uriarte MM, Ross JL, Cassorla F, Cutler BG., Jr 1991. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J Clin Endocrinol Metab 73:428–435 [DOI] [PubMed] [Google Scholar]
- 55. Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. 1972. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab 35:665–670 [DOI] [PubMed] [Google Scholar]
- 56. Giustina A, Veldhuis JD. 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- 57. Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. 2002. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. 2007. Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics 30:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sanfilippo S, Imbesi RM, Sanfilippo S., Jr 1995. Effects of a tryptophan deficient diet on the morphology of skeletal muscle fibers of the rat. Preliminary observations at neuroendocrinological and submicroscopical levels. Ital J Anat Embryol 100(Suppl 1):131–141 [PubMed] [Google Scholar]
- 60. Atinmo T, Baldijao C, Pond WG, Barnes RH. 1976. Prenatal and postnatal protein malnutrition in pigs: effects on growth rate, serum protein and albumin. J Anim Sci 43:606–612 [DOI] [PubMed] [Google Scholar]
- 61. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. 2008. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371:340–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260 [DOI] [PubMed] [Google Scholar]
- 63. Drewry MM, Harris RB, Martin RJ. 1988. Developmental changes in response to overfeeding: effect on composition of gain, liver metabolism and adipocyte cellularity in rats. J Nutr 118:194–198 [DOI] [PubMed] [Google Scholar]
- 64. Keenan KP, Laroque P, Soper KA, Morrissey RE, Dixit R. 1996. The effects of overfeeding and moderate dietary restriction on Sprague-Dawley rat survival, pathology, carcinogenicity, and the toxicity of pharmaceutical agents. Exp Toxicol Pathol 48:139–144 [DOI] [PubMed] [Google Scholar]
- 65. Pekas JC. 1985. Animal growth during liberation from appetite suppression. Growth 49:19–27 [PubMed] [Google Scholar]
- 66. Nir I, Nitsan Z, Dror Y, Shapira N. 1978. Influence of overfeeding on growth, obesity and intestinal tract in young chicks of light and heavy breeds. Br J Nutr 39:27–35 [DOI] [PubMed] [Google Scholar]
- 67. Nir I, Shapira N, Nitsan Z, Dror Y. 1974. Force-feeding effects on growth, carcass and blood composition in the young chick. Br J Nutr 32:229–239 [DOI] [PubMed] [Google Scholar]
- 68. Ingalls AM, Dickie MM, Snell GD. 1950. Obese, a new mutation in the house mouse. J Hered 41:317–318 [DOI] [PubMed] [Google Scholar]
- 69. do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Biglane J, da Silva AA, Hall JE. 2009. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 297:R803–R812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- 71. Parfitt AM. 2002. Misconceptions (1): epiphyseal fusion causes cessation of growth. Bone 30:337–339 [DOI] [PubMed] [Google Scholar]
- 72. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 73. Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS. 2008. Impact on bone of an estrogen receptor-α gene loss of function mutation. J Clin Endocrinol Metab 93:3088–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith EP, Specker B, Korach KS. 2010. Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J Steroid Biochem Mol Biol 118:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grumbach MM, Auchus RJ. 1999. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84:4677–4694 [DOI] [PubMed] [Google Scholar]
- 76. Nilsson O, Abad V, Chrysis D, Ritzén EM, Sävendahl L, Baron J. 2002. Estrogen receptor-α and -β are expressed throughout postnatal development in the rat and rabbit growth plate. J Endocrinol 173:407–414 [DOI] [PubMed] [Google Scholar]
- 77. Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. 2001. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA 98:6871–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cooke PS, Yonemura CU, Russell SM, Nicoll CS. 1986. Growth and differentiation of fetal rat intestine transplants: dependence on insulin and growth hormone. Biol Neonate 49:211–218 [DOI] [PubMed] [Google Scholar]
- 79. Montgomery RK, Sybicki MA, Grand RJ. 1981. Autonomous biochemical and morphological differentiation in fetal rat intestine transplanted at 17 and 20 days of gestation. Dev Biol 87:76–84 [DOI] [PubMed] [Google Scholar]
- 80. Pape L, Hoppe J, Becker T, Ehrich JH, Neipp M, Ahlenstiel T, Offner G. 2006. Superior long-term graft function and better growth of grafts in children receiving kidneys from paediatric compared with adult donors. Nephrol Dial Transplant 21:2596–2600 [DOI] [PubMed] [Google Scholar]
- 81. Crickmore MA, Mann RS. 2008. The control of size in animals: insights from selector genes. Bioessays 30:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Metcalf D. 1963. The autonomous behaviour of normal thymus grafts. Aust J Exp Biol Med Sci 41(Suppl):437–447 [DOI] [PubMed] [Google Scholar]
- 83. Metcalf D, Sparrow N, Nakamura K, Ishidate M. 1961. The behaviour of thymus grafts in high and low leukaemia strains of mice. Aust J Exp Biol Med Sci 39:441–453 [DOI] [PubMed] [Google Scholar]
- 84. Metcalf D. 1964. Restricted growth capacity of multiple spleen grafts. Transplantation 2:387–392 [DOI] [PubMed] [Google Scholar]
- 85. Stevens DG, Boyer MI, Bowen CV. 1999. Transplantation of epiphyseal plate allografts between animals of different ages. J Pediatr Orthop 19:398–403 [PubMed] [Google Scholar]
- 86. Glickman AM, Yang JP, Stevens DG, Bowen CV. 2000. Epiphyseal plate transplantation between sites of different growth potential. J Pediatr Orthop 20:289–295 [PubMed] [Google Scholar]
- 87. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- 88. Sarbassov DD, Ali SM, Sabatini DM. 2005. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17:596–603 [DOI] [PubMed] [Google Scholar]
- 89. Ciuffreda L, Di Sanza C, Incani UC, Milella M. 2010. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets 10:484–495 [DOI] [PubMed] [Google Scholar]
- 90. Hay N, Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev 18:1926–1945 [DOI] [PubMed] [Google Scholar]
- 91. Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95:1432–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruvinsky I, Meyuhas O. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31:342–348 [DOI] [PubMed] [Google Scholar]
- 93. Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16:1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. 2004. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24:200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Holz MK, Ballif BA, Gygi SP, Blenis J. 2005. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580 [DOI] [PubMed] [Google Scholar]
- 96. Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. 2007. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors 25:209–226 [DOI] [PubMed] [Google Scholar]
- 97. Shamji AF, Nghiem P, Schreiber SL. 2003. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell 12:271–280 [DOI] [PubMed] [Google Scholar]
- 98. Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. 2003. A nutrient sensor mechanism controls Drosophila growth. Cell 114:739–749 [DOI] [PubMed] [Google Scholar]
- 99. Sanders JA, Lakhani A, Phornphutkul C, Wu KY, Gruppuso PA. 2008. The effect of rapamycin on DNA synthesis in multiple tissues from late gestation fetal and postnatal rats. Am J Physiol Cell Physiol 295:C406–C413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carlson ME, Conboy IM. 2007. Loss of stem cell regenerative capacity within aged niches. Aging Cell 6:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nilsson O, Mitchum RD, Jr, Schrier L, Ferns SP, Barnes KM, Troendle JF, Baron J. 2005. Growth plate senescence is associated with loss of DNA methylation. J Endocrinol 186:241–249 [DOI] [PubMed] [Google Scholar]
- 102. Baena E, Gandarillas A, Vallespinós M, Zanet J, Bachs O, Redondo C, Fabregat I, Martinez-A C, de Alborán IM. 2005. c-Myc regulates cell size and ploidy but is not essential for postnatal proliferation in liver. Proc Natl Acad Sci USA 102:7286–7291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. 2004. Drosophila myc regulates organ size by inducing cell competition. Cell 117:107–116 [DOI] [PubMed] [Google Scholar]
- 104. Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM. 2001. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414:768–773 [DOI] [PubMed] [Google Scholar]
- 105. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9:534–546 [DOI] [PubMed] [Google Scholar]
- 106. Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. 2002. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129:5719–5730 [DOI] [PubMed] [Google Scholar]
- 107. Harvey KF, Pfleger CM, Hariharan IK. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114:457–467 [DOI] [PubMed] [Google Scholar]
- 108. Pan D. 2007. Hippo signaling in organ size control. Genes Dev 21:886–897 [DOI] [PubMed] [Google Scholar]
- 109. Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130:1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Finkielstain GP, Forcinito P, Lui JC, Barnes KM, Marino R, Makaroun S, Nguyen V, Lazarus JE, Nilsson O, Baron J. 2009. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology 150:1791–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lui JC, Finkielstain GP, Barnes KM, Baron J. 2008. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am J Physiol Regul Integr Comp Physiol 295:R189–R196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lui JC, Forcinito P, Chang M, Chen W, Barnes KM, Baron J. 2010. Coordinated postnatal down-regulation of multiple growth-promoting genes: evidence for a genetic program limiting organ growth. FASEB J 24:3083–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Burton PB, Raff MC, Kerr P, Yacoub MH, Barton PJ. 1999. An intrinsic timer that controls cell-cycle withdrawal in cultured cardiac myocytes. Dev Biol 216:659–670 [DOI] [PubMed] [Google Scholar]
- 115. Gao FB, Raff M. 1997. Cell size control and a cell-intrinsic maturation program in proliferating oligodendrocyte precursor cells. J Cell Biol 138:1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gao FB, Durand B, Raff M. 1997. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr Biol 7:152–155 [DOI] [PubMed] [Google Scholar]
- 117. Durand B, Gao FB, Raff M. 1997. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J 16:306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Durand B, Fero ML, Roberts JM, Raff MC. 1998. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol 8:431–440 [DOI] [PubMed] [Google Scholar]
- 119. Durand B, Raff M. 2000. A cell-intrinsic timer that operates during oligodendrocyte development. Bioessays 22:64–71 [DOI] [PubMed] [Google Scholar]
- 120. Boersma B, Wit JM. 1997. Catch-up growth. Endocr Rev. 18:646–661 [DOI] [PubMed] [Google Scholar]
- 121. Segall PE, Timiras PS. 1975. Age-related changes in the thermoregulatory capacity of tryptophan-deficient rats. Fed Proc 34:83–85 [PubMed] [Google Scholar]
- 122. Osborne TB, Mendel LB. 1993. Amino acids in nutrition and growth. 1914. J Am Coll Nutr 12:484–485 [DOI] [PubMed] [Google Scholar]
- 123. Tanner JM. 1963. Regulation of growth in size in mammals. Nature 199:845–850 [DOI] [PubMed] [Google Scholar]
- 124. Baron J, Klein KO, Colli MJ, Yanovski JA, Novosad JA, Bacher JD, Cutler GB., Jr 1994. Catch-up growth after glucocorticoid excess: a mechanism intrinsic to the growth plate. Endocrinology 135:1367–1371 [DOI] [PubMed] [Google Scholar]
- 125. Gafni RI, Weise M, Robrecht DT, Meyers JL, Barnes KM, De-Levi S, Baron J. 2001. Catch-up growth is associated with delayed senescence of the growth plate in rabbits. Pediatr Res 50:618–623 [DOI] [PubMed] [Google Scholar]
- 126. Marino R, Hegde A, Barnes KM, Schrier L, Emons JA, Nilsson O, Baron J. 2008. Catch-up growth after hypothyroidism is caused by delayed growth plate senescence. Endocrinology 149:1820–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Forcinito P, Andrade AC, Finkielstain GP, Baron J, Nilsson O, Lui JC. 2011. Growth-inhibiting conditions slow growth plate senescence. J Endocrinol 208:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Emons JA, Boersma B, Baron J, Wit JM. 2005. Catch-up growth: testing the hypothesis of delayed growth plate senescence in humans. J Pediatr 147:843–846 [DOI] [PubMed] [Google Scholar]
- 129. Fankhauser G. 1945. The effects of changes in chromosome number on amphibian development. Q Rev Biol 20:20–78 [Google Scholar]
- 130. Henery CC, Kaufman MH. 1992. Relationship between cell size and nuclear volume in nucleated red blood cells of developmentally matched diploid and tetraploid mouse embryos. J Exp Zool 261:472–478 [DOI] [PubMed] [Google Scholar]
- 131. Henery CC, Bard JB, Kaufman MH. 1992. Tetraploidy in mice, embryonic cell number, and the grain of the developmental map. Dev Biol 152:233–241 [DOI] [PubMed] [Google Scholar]
- 132. Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. 1998. Coordination of growth and cell division in the Drosophila wing. Cell 93:1183–1193 [DOI] [PubMed] [Google Scholar]
- 133. Stanger BZ. 2008. The biology of organ size determination. Diabetes Obes Metab 10(Suppl 4):16–22 [DOI] [PubMed] [Google Scholar]
- 134. Moreno E, Basler K. 2004. dMyc transforms cells into super-competitors. Cell 117:117–129 [DOI] [PubMed] [Google Scholar]
- 135. Bullough WS, Laurence EB. 1964. Mitotic control by internal secretion: the role of the chalone-adrenalin complex. Exp Cell Res 33:176–194 [DOI] [PubMed] [Google Scholar]
- 136. Rodgers BD, Garikipati DK. 2008. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- 138. Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. 2000. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243 [DOI] [PubMed] [Google Scholar]
- 139. Lee SJ, McPherron AC. 1999. Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev 9:604–607 [DOI] [PubMed] [Google Scholar]
- 140. McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. 2005. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118:3531–3541 [DOI] [PubMed] [Google Scholar]
- 141. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. 2003. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Welle S, Bhatt K, Pinkert CA. 2006. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290:E409–E415 [DOI] [PubMed] [Google Scholar]
- 143. Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. 2005. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102:18117–18122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. 2003. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100:15842–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. 2003. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 35:227–238 [DOI] [PubMed] [Google Scholar]
- 146. Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. 2002. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418–421 [DOI] [PubMed] [Google Scholar]
- 147. Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. 2005. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19:543–549 [DOI] [PubMed] [Google Scholar]
- 148. Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. 2003. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300:965–971 [DOI] [PubMed] [Google Scholar]
- 149. Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. 2007. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292:E985–E991 [DOI] [PubMed] [Google Scholar]
- 150. Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. 2007. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Stinckens A, Luyten T, Van den Maagdenberg K, Janssens S, De Smet S, Georges M, Buys N. 2009. Interactions between genes involved in growth and muscularity in pigs: IGF-2, myostatin, ryanodine receptor 1, and melanocortin-4 receptor. Domest Anim Endocrinol 37:227–235 [DOI] [PubMed] [Google Scholar]
- 152. Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibé B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M. 2006. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38:813–818 [DOI] [PubMed] [Google Scholar]
- 153. Boman IA, Klemetsdal G, Nafstad O, Blichfeldt T, Våge DI. 2010. Impact of two myostatin (MSTN) mutations on weight gain and lamb carcass classification in Norwegian White Sheep (Ovis aries). Genet Sel Evol 42:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. McPherron AC, Lee SJ. 1997. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Grobet L, Poncelet D, Royo LJ, Brouwers B, Pirottin D, Michaux C, Ménissier F, Zanotti M, Dunner S, Georges M. 1998. Molecular definition of an allelic series of mutations disrupting the myostatin function and causing double-muscling in cattle. Mamm Genome 9:210–213 [DOI] [PubMed] [Google Scholar]
- 156. Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, Fries R, Hanset R, Georges M. 1997. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17:71–74 [DOI] [PubMed] [Google Scholar]
- 157. Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ. 2004. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688 [DOI] [PubMed] [Google Scholar]
- 158. McKoy G, Bicknell KA, Patel K, Brooks G. 2007. Developmental expression of myostatin in cardiomyocytes and its effect on foetal and neonatal rat cardiomyocyte proliferation. Cardiovasc Res 74:304–312 [DOI] [PubMed] [Google Scholar]
- 159. Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. 2006. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER. 2010. Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24:1998–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lee SJ. 2007. Quadrupling muscle mass in mice by targeting TGF-β signaling pathways. PLoS One 2:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, Tucker D, Shilling CJ, Therlfall WR, Walker CM, Weisbrode SE, Janssen PM, Clark KR, Sahenk Z, Mendell JR, Kaspar BK. 2009. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med 1:6ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. 2009. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39:283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. 2003. Autoregulation of neurogenesis by GDF11. Neuron 37:197–207 [DOI] [PubMed] [Google Scholar]
- 166. Lander AD, Gokoffski KK, Wan FY, Nie Q, Calof AL. 2009. Cell lineages and the logic of proliferative control. PLoS Biol 7:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. 2008. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Plikus MV, Widelitz RB, Maxson R, Chuong CM. 2009. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol 53:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dhillo WS. 2007. Appetite regulation: an overview. Thyroid 17:433–445 [DOI] [PubMed] [Google Scholar]
- 170. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 171. Thornton JE, Cheung CC, Clifton DK, Steiner RA. 1997. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066 [DOI] [PubMed] [Google Scholar]
- 172. Cheung CC, Clifton DK, Steiner RA. 1997. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138:4489–4492 [DOI] [PubMed] [Google Scholar]
- 173. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- 174. Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. 1997. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525 [DOI] [PubMed] [Google Scholar]
- 175. van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. 2004. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7:493–494 [DOI] [PubMed] [Google Scholar]
- 176. Fausto N, Campbell JS, Riehle KJ. 2006. Liver regeneration. Hepatology 43:S45–S53 [DOI] [PubMed] [Google Scholar]
- 177. Michalopoulos GK, DeFrances MC. 1997. Liver regeneration. Science 276:60–66 [DOI] [PubMed] [Google Scholar]
- 178. Michalopoulos GK. 2010. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Moolten FL, Bucher NL. 1967. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science 158:272–274 [DOI] [PubMed] [Google Scholar]
- 180. Kelley M, Lambert I, Merrill J, Safe S. 1985. 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) and related compounds as inducers of hepatic monooxygenases. Structure-activity effects. Biochem Pharmacol 34:3489–3494 [DOI] [PubMed] [Google Scholar]
- 181. Columbano A, Shinozuka H. 1996. Liver regeneration versus direct hyperplasia. FASEB J 10:1118–1128 [DOI] [PubMed] [Google Scholar]
- 182. Coni P, Simbula G, de Prati AC, Menegazzi M, Suzuki H, Sarma DS, Ledda-Columbano GM, Columbano A. 1993. Differences in the steady-state levels of c-fos, c-jun and c-myc messenger RNA during mitogen-induced liver growth and compensatory regeneration. Hepatology 17:1109–1116 [PubMed] [Google Scholar]
- 183. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. 2006. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 [DOI] [PubMed] [Google Scholar]
- 184. Barone M, Francavilla A, Polimeno L, Ierardi E, Romanelli D, Berloco P, Di Leo A, Panella C. 1996. Modulation of rat hepatocyte proliferation by bile salts: in vitro and in vivo studies. Hepatology 23:1159–1166 [DOI] [PubMed] [Google Scholar]
- 185. Ueda J, Chijiiwa K, Nakano K, Zhao G, Tanaka M. 2002. Lack of intestinal bile results in delayed liver regeneration of normal rat liver after hepatectomy accompanied by impaired cyclin E-associated kinase activity. Surgery 131:564–573 [DOI] [PubMed] [Google Scholar]
- 186. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. 2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Naito A, Yonou H, Kagawa H. 2000. Compensatory renal hypertrophy and changes of renal function following nephrectomy. Hinyokika Kiyo 46:235–240 [PubMed] [Google Scholar]
- 188. Liu B, Preisig PA. 2002. Compensatory renal hypertrophy is mediated by a cell cycle-dependent mechanism. Kidney Int 62:1650–1658 [DOI] [PubMed] [Google Scholar]
- 189. Newport J, Kirschner M. 1982. A major developmental transition in early Xenopus embryos. II. Control of the onset of transcription. Cell 30:687–696 [DOI] [PubMed] [Google Scholar]
- 190. Newport J, Kirschner M. 1982. A major developmental transition in early Xenopus embryos. I. characterization and timing of cellular changes at the midblastula stage. Cell 30:675–686 [DOI] [PubMed] [Google Scholar]
- 191. Stanger BZ, Tanaka AJ, Melton DA. 2007. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445:886–891 [DOI] [PubMed] [Google Scholar]
- 192. Olovnikov AM. 1996. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 31:443–448 [DOI] [PubMed] [Google Scholar]
- 193. Olovnikov AM. 1973. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41:181–190 [DOI] [PubMed] [Google Scholar]
- 194. Collins K, Mitchell JR. 2002. Telomerase in the human organism. Oncogene 21:564–579 [DOI] [PubMed] [Google Scholar]
- 195. Blasco MA. 2005. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622 [DOI] [PubMed] [Google Scholar]
- 196. Garcia CK, Wright WE, Shay JW. 2007. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res 35:7406–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Harley CB, Futcher AB, Greider CW. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460 [DOI] [PubMed] [Google Scholar]
- 198. Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. 1991. In vivo loss of telomeric repeats with age in humans. Mutat Res 256:45–48 [DOI] [PubMed] [Google Scholar]
- 199. Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. 1999. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96:701–712 [DOI] [PubMed] [Google Scholar]
- 200. Dokal I. 2000. Dyskeratosis congenita in all its forms. Br J Haematol 110:768–779 [DOI] [PubMed] [Google Scholar]
- 201. Savage SA, Alter BP. 2009. Dyskeratosis congenita. Hematol Oncol Clin North Am 23:215–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kawakami K, Nakamura A, Ishigami A, Goto S, Takahashi R. 2009. Age-related difference of site-specific histone modifications in rat liver. Biogerontology 10:415–421 [DOI] [PubMed] [Google Scholar]
- 203. Sherr CJ, Roberts JM. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512 [DOI] [PubMed] [Google Scholar]
- 204. LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev 11:847–862 [DOI] [PubMed] [Google Scholar]
- 205. Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18:1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Eggermann T. 2010. Russell-Silver syndrome. Am J Med Genet C Semin Med Genet 154C:355–364 [DOI] [PubMed] [Google Scholar]
- 207. Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. 1996. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12:241–247 [DOI] [PubMed] [Google Scholar]
- 208. Bell E, Chen L, Liu T, Marshall GM, Lunec J, Tweddle DA. 2010. MYCN oncoprotein targets and their therapeutic potential. Cancer Lett 293:144–157 [DOI] [PubMed] [Google Scholar]
- 209. Simon JA, Lange CA. 2008. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 647:21–29 [DOI] [PubMed] [Google Scholar]
- 210. Kadomatsu K, Muramatsu T. 2004. Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204:127–143 [DOI] [PubMed] [Google Scholar]
- 211. Jochheim-Richter A, Rüdrich U, Koczan D, Hillemann T, Tewes S, Petry M, Kispert A, Sharma AD, Attaran F, Manns MP, Ott M. 2006. Gene expression analysis identifies novel genes participating in early murine liver development and adult liver regeneration. Differentiation 74:167–173 [DOI] [PubMed] [Google Scholar]
- 212. Li T, Wan B, Huang J, Zhang X. 2010. Comparison of gene expression in hepatocellular carcinoma, liver development, and liver regeneration. Mol Genet Genomics 283:485–492 [DOI] [PubMed] [Google Scholar]
- 213. Imgrund M, Gröne E, Gröne HJ, Kretzler M, Holzman L, Schlöndorff D, Rothenpieler UW. 1999. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int 56:1423–1431 [DOI] [PubMed] [Google Scholar]
- 214. Zhuang J, Deane JA, Yang RB, Li J, Ricardo SD. 2010. SCUBE1, a novel developmental gene involved in renal regeneration and repair. Nephrol Dial Transplant 25:1421–1428 [DOI] [PubMed] [Google Scholar]
- 215. Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. 2003. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14:1223–1233 [DOI] [PubMed] [Google Scholar]
- 216. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2002. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11:1–190 [PubMed] [Google Scholar]