The human fetal adrenal cortex, a pivotal member of the feto-placental unit, is a relatively large organ with steroidogenic activity that is spatially, temporally and functionally regulated. The present review aims to identify what we know and what we need to know about the developmental processes, functional aspects, and physiologic significance of this unique organ.
Abstract
Continuous efforts have been devoted to unraveling the biophysiology and development of the human fetal adrenal cortex, which is structurally and functionally unique from other species. It plays a pivotal role, mainly through steroidogenesis, in the regulation of intrauterine homeostasis and in fetal development and maturation. The steroidogenic activity is characterized by early transient cortisol biosynthesis, followed by its suppressed synthesis until late gestation, and extensive production of dehydroepiandrosterone and its sulfate, precursors of placental estrogen, during most of gestation. The gland rapidly grows through processes including cell proliferation and angiogenesis at the gland periphery, cellular migration, hypertrophy, and apoptosis. Recent studies employing modern technologies such as gene expression profiling and laser capture microdissection have revealed that development and/or function of the fetal adrenal cortex may be regulated by a panoply of molecules, including transcription factors, extracellular matrix components, locally produced growth factors, and placenta-derived CRH, in addition to the primary regulator, fetal pituitary ACTH. The role of the fetal adrenal cortex in human pregnancy and parturition appears highly complex, probably due to redundant and compensatory mechanisms regulating these events. Mounting evidence indicates that actions of hormones operating in the human feto-placental unit are likely mediated by mechanisms including target tissue responsiveness, local metabolism, and bioavailability, rather than changes only in circulating levels. Comprehensive study of such molecular mechanisms and the newly identified factors implicated in adrenal development should help crystallize our understanding of the development and physiology of the human fetal adrenal cortex.
Introduction
-
Structural and Functional Adrenal Development
Prenatal adrenal development
Postnatal involution of the fetal zone of the adrenal cortex
Prenatal adrenal growth and zonation
Functional development: ontogeny of steroidogenesis and functional zonation
Angiogenesis and its regulation
-
Candidate Molecules Implicated in Human Fetal Adrenal Development and Function
Gene expression profiles of human fetal and adult adrenals
Markers unique to zonal cellular subtypes
Extracellular matrix (ECM) environment
Transcriptional regulators
Role of growth factors
Placental factors: role of estrogens
-
Regulation by ACTH, Pituitary Proopiomelanocortin-Derived Peptides, and Angiotensin-II
ACTH and pituitary proopiomelanocortin (POMC)-derived peptides
Angiotensin-II (AT-II)
-
The Fetal Adrenal Cortex in the Human Feto-Placental Unit
Glucocorticoids: roles in fetal maturation and parturition
Progesterone and estrogens: roles in parturition
CRH and the human fetal adrenal cortex
Summary and Future Perspectives
I. Introduction
The human fetal adrenal (HFA) cortex shares its unique structural and functional organization with higher primates such as the rhesus monkey and baboon. The March 1997 issue of Endocrine Reviews reviewed the development and function of the primate fetal adrenal cortex (1). The HFA cortex is an active endocrine organ in which most steroidogenic activity is exerted in a specialized cortical compartment known as the fetal zone (FZ), a unique feature of fetal adrenals in humans and some higher primates but not in other species such as rodents and sheep. Elegant studies during the 1950s and 1960s brought to light the essential role of the HFA in the high estrogenic milieu of pregnancy and led Diczfalusy (2) and coinvestigators to propose the concept of the “feto-placental unit,” in which the FZ of the HFA produces large amounts of adrenal androgens that are used by the placenta for estrogen biosynthesis. However, the roles of the estrogenic milieu in human pregnancy still remain unclear. During the 1960s, Liggins et al. (3, 4) demonstrated that in the sheep, maturation of the fetal hypothalamic-pituitary-adrenal (HPA) axis occurs late in gestation, and cortisol secreted by the fetal adrenal cortex stimulates maturation of fetal organs and initiates the cascade of events leading to parturition. Similarly, the HFA cortex begins to produce cortisol late in gestation. However, it was soon realized that, although cortisol promotes fetal maturation in humans as it does in sheep, the role of cortisol in the regulation of human parturition has not been completely elucidated because fundamental differences exist between sheep and humans in terms of regulation of parturition. Thus, the precise roles of these steroid hormones in the maintenance of pregnancy, fetal maturation, and development, and in the initiation of parturition have been controversial. Nevertheless, recent research developments have allowed significant progress in clarifying many aspects of the biophysiology of the human feto-placental unit.
Consistent with its endocrine capabilities, the human fetus has very large adrenals. Accumulating data indicate that growth of the HFA appears to involve cellular proliferation, hypertrophy, apoptosis, and migration. In addition, angiogenesis, another fundamental process for organ growth, has been recently investigated in the HFA. Genetic studies in mice and humans, as well as studies using materials from human and subhuman primates, and the application of modern technologies, such as gene arrays and laser capture microdissection, have contributed greatly to our understanding of the genes and gene products that play key roles in HFA development and function.
It is well accepted that ACTH is the primary regulator for the development and function of the HFA. However, this tenet does not argue against physiological roles for ACTH-independent mechanisms in the regulation and fine-tuning of the gland. Indeed, such ACTH-independent mechanisms have been identified through recent observations.
Therefore, it seemed appropriate to review the progress in the field since the previous review appeared in 1997. We introduced the topic of the previous review by indicating that “steroid hormones produced by the fetal adrenal cortex regulate intrauterine homeostasis, the maturation of fetal organ systems necessary for extrauterine life, and in some species, the timing of parturition. Appropriate development and function of the fetal adrenal cortex therefore are critical for fetal maturation and perinatal survival. Moreover, the fetal adrenal cortex must itself undergo maturation in preparation for its essential role postnatally, i.e., production of glucocorticoids, androgens, and mineralocorticoids for fetal intrauterine homeostasis and to insure adrenal cortical autonomy once the placenta has separated.” Those comments remain valid. However, as mentioned above, there have been many advances in further understanding the physiological and pathological biology of the initiation of the onset of labor, the interactions of the fetus and placenta, the development of the HFA, the relationship between fetal adrenal architecture and its function, and the role of steroid hormones in intrauterine homeostasis and preparation for extrauterine life. This remains a fascinating area of investigation, which not only will advance our understanding of the biology of the HFA, but should also pave the way for new improvements in treatment of such disorders as premature labor, abnormalities of fetal and newborn adrenal function, and a more comprehensive view of this unique organ.
In this article, we will provide a brief synopsis of earlier studies and present an overview of recent key observations from our laboratories as well as those of other investigators that serve to gain a better understanding of the developmental processes and functional aspects of the organ.
II. Structural and Functional Adrenal Development
The interplay of cell proliferation, differentiation, apoptosis, and cellular senescence affects the development of the HFA. In this section, we summarize the current knowledge of the structural and functional development of the HFA.
A. Prenatal adrenal development
Cells of the human adrenal cortex arise from the intermediate mesoderm. The earliest recognizable manifestation of the adrenal gland is called “the adrenal blastema” (5) or “the adrenal primordium,” which appears distinct from surrounding structures at 33 days post-conception (dpc) (6, 7), lying posteromedial to the urogenital ridge. Hanley et al. (6–8) have demonstrated, using in situ hybridization, that the transcription factors, steroidogenic factor-1 (SF1; also known as Ad4BP or NR5A1) and DAX1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome; NR0B1), are expressed in this adrenal anlage and that hybridization signals from SF1 transcripts are stronger than those from DAX1 at all embryonic stages studied (i.e., 33 through 52 dpc) (7). By the eighth week of gestation, cells in the adrenal blastema organize into anastomosing cords and show ultrastructural characteristics consistent with steroidogenic capability. By 50–52 dpc, the developing HFA acquires two rudimentary, but distinct, zones: the inner FZ that consists of large eosinophilic cells, and the outer definitive zone (DZ), which is comprised of small, densely packed basophilic cells (7, 9). The origin or lineage relationship of cells of both zones remains unknown (5, 10). At about the ninth week of gestation, the developing HFA is completely encapsulated.
During the fetal period, the morphology of the adrenal cortex remains relatively constant. The DZ is composed of a narrow band of small (10–20 mm) cells that exhibit structural characteristics typical of cells in a proliferative state. Inner layers of the DZ form arched cords with finger-like columns of cells reaching the outer rim of the FZ. Cells in the DZ are lipid-poor during midgestation. As gestation advances, the cells accumulate cytoplasmic lipid and begin to resemble steroidogenically active cells. The FZ consists of large (20–50 mm) cells with ultrastructural characteristics typical of steroidogenic cells. In the outer regions of the FZ, the cells are arranged in tightly packed cords. In the central portion, the FZ forms a reticular pattern, with cells spaced more widely and separated by numerous vascular sinusoids. Ultrastructural studies also have revealed a third zone between the DZ and FZ, which we have named the transitional zone (TZ) (11). Cells in this zone show intermediate characteristics (12). TZ cells have the capacity to synthesize cortisol, being analogous to cells of the zona fasciculata of the adult adrenal cortex. By the 30th week of gestation, the HFA cortex manifests a rudimentary form of the adult adrenal cortex; the DZ and TZ begin to resemble the zona glomerulosa and the zona fasciculata, respectively (13).
As early as 6 wk of gestational age, pheochromoblasts derived from the neural crest migrate through the fetal adrenal cortex to form the medulla at a later stage of development (10, 14–16). The medulla is not recognized as a distinct structure in the HFA throughout most of gestation, except for small clusters or nests of chromaffin cells scattered throughout the body of the cortex (17–19). A more structurally discrete medulla does not form until after birth (10, 17).
Chromaffin cells appear to establish part of their phenotype as early as 6 wk gestation, as evidenced by the expression of chromogranin A and tyrosine hydroxylase (16, 20). The widely accepted concept of a key role for adrenal glucocorticoids in chromaffin cell differentiation (21, 22) has been challenged by a recent study in mice lacking the glucocorticoid receptor (23); chromaffin cells in such mice develop quite normally (for review, see Refs. 24 and 25). Studies on interactions between the adrenal cortical cells and chromaffin cells have been reviewed elsewhere (22). However, such interactions of two cell types in the development of the human embryonic and fetal adrenal gland remain to be characterized.
B. Postnatal involution of the fetal zone of the adrenal cortex
Soon after birth, the HFA undergoes rapid involution due to the rapid disappearance of the FZ, with a decrease in androgen secretion (13, 14, 26–29). As a consequence, the total weight of the glands decreases by approximately 50% (12, 30). The atrophy of the FZ appears to occur by apoptosis (31). The number of apoptotic nuclei increases markedly during the postnatal period, whereas it is very low in midgestation HFAs.
There is controversy about whether the timing of the fetal adrenal involution is determined by gestation or by birth. A morphometric study in autopsy cases and an ultrasonographic study during antenatal and neonatal periods indicate that the adrenal gland may shrink more rapidly in infants born at full term (32, 33). Longitudinal observations of infants born preterm showed that urinary excretion of FZ steroids (3β-OH-5ene steroids) persists until term and then decreases as it does in full-term infants, suggesting that the HFA involution is related to gestational age rather than birth (34, 35). In contrast, a more recent study has demonstrated a similar pattern of rapid adrenal involution and/or remodeling in all neonates examined, regardless of their gestational age at birth (26–35 wk) (36). The size of the adrenal gland, as measured by ultrasonography, decreased to its normal infantile size within the first 2 wk after birth (36).
A gender difference does not appear to exist in FZ regression/involution in humans, at least in terms of hormone secretion, given the testicular secretion of androgens in male infants. In a study of Ben-David et al. (36), serum levels of cortisol, dehydroepiandrosterone (DHEA) sulfate (DHEAS), and androstenedione sharply decreased during the first week of life. However, only in males, androstenedione levels increased as of d 21. Garagorri et al. (37) reported that plasma levels of adrenal steroids, with the exception of cortisol, decreased progressively from birth to 6 months of age. Plasma levels of 17-hydroxyprogesterone, 11-desoxycortisol, and cortisol did not reveal gender differences, whereas testosterone and androstenedione levels were higher in males and DHEAS levels were higher in females.
Dramatic remodeling of the postnatal adrenal gland involves a complex combination of FZ regression and development of the zonae glomerulosa and fasciculata (13, 32). Because morphological studies have identified rudimentary zonae glomerulosa and fasciculata during late gestation, the development of these zones may occur from their primordial structures, although there has been a general belief (14) that the adult cortical zones develop from the persistent DZ.
C. Prenatal adrenal growth and zonation
The weight of the developing human adrenal gland increases almost 10-fold from 8 to 10 wk post-conception (9). Although the relative increase in adrenal weight is the greatest during the first trimester (38), the HFA grows rapidly thereafter until term. By 20 wk gestation, the gland becomes as large as the fetal kidney and by 30 wk achieves a relative size 10- to 20-fold that of the adult adrenal gland. A further doubling in fetal adrenal weight occurs thereafter, and by full term the HFA weighs approximately 3–5 g (5, 14).
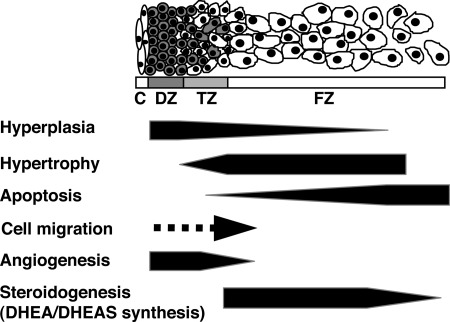
Numerous studies on development of the adrenal cortex have generated at least three general hypothetical models on zonation of the adrenal cortex: 1) the cell migration model; 2) the transformation field model; and 3) the zonal model (for review, see Ref. 39). An increasing number of studies support the cell migration model of adrenal cortical cytogenesis. In this model, each zone is derived from a common pool of progenitor cells, which then migrate and differentiate to populate the cortical zones. It is presumed that the progenitor cells are located in the periphery of the postnatal adrenal gland (i.e., zona glomerulosa/zona fasciculata boundary and/or subcapsular layer) (40–43). In the human adult adrenal cortex, immunoreactivity of Ki-67, an antigen associated with cell proliferation, was observed predominantly in the outer zona fasciculata but also in the zonae glomerulosa and reticularis (44). The adult rat has a layer of cells between the zonae glomerulosa and the fasciculata/reticularis that is nonsteroidogenic and is postulated to play a progenitor role (40, 41, 45). A similar layer of putative progenitor cells also has been visualized in sheep (46) and marmoset (47) adrenals. Studies on adrenals of chimeric and transgenic rodents show variegated expression of chimeric or reporter genes in radial cords of cells from the subcapsular outer rim extending into the cortex, indicating a clonal origin of cells within each radial cord and supporting the migration theory (48–51). Cells in the outer DZ of the HFA exhibit structural characteristics typical of cells in a proliferative state (i.e., small cytoplasmic volume containing free ribosomes; small, dense mitochondria with lamelliform cristae and scant lipid) (52). Most of them are positive for the proliferation markers proliferating cell nuclear antigen and Ki-67 in the midgestation HFA (31, 53). In contrast, cells in the inner FZ are less positive for these markers. Centripetal migration of lipid-containing cells from the DZ to the FZ in the HFA was reported in earlier studies (10, 14). Jirasek (5) described the presence of daughter cells that result from mitoses in the DZ and form cords invading the outer layers of the FZ. Thus, in human fetuses, it is likely that cells proliferate in the periphery of the cortex and subsequently migrate centripetally (by active migration or passive mitotic pressure) and populate the rest of the gland (Fig. 1).
Fig. 1.
Hypothetical model of development of the midgestation HFA gland. The schema shows the HFA cortex structure, which consists of the DZ, TZ, and FZ. The medulla is not recognized as a distinct structure in the HFA throughout most of gestation. Previous studies from our laboratory and others have demonstrated that the HFA cortex is a dynamic organ: hyperplasia occurs primarily in the DZ; hypertrophy occurs primarily in the FZ; and apoptosis occurs primarily in the central portion of the FZ. Thus, cells appear to proliferate in the periphery, migrate centripetally, differentiate to form the specialized cortical compartments, and then undergo senescence when they reach the center of the cortex. Recent studies indicate that angiogenesis (formation of new capillaries from preexisting blood vessels) occurs at the periphery of the gland. C, Capsule.
The origin of progenitor cells during embryonic and fetal adrenal development is unclear. Kim and Hammer (43, 54) and coinvestigators have hypothesized that the precursors in the adrenal primordia (adrenal blastema or the fetal cortex) of the early developing mouse adrenal give rise to Sf1-negative stem cells that reside in the adrenal capsule. In response to mitogenic/morphogenic signals, such Sf1-negative capsular stem cells would exit from the capsular niche into the subcapsular environment, where they commence Sf1 expression and proliferate.
The rapid HFA growth is almost entirely due to enlargement of the FZ; by midgestation (16–20 wk), the FZ clearly dominates in the gland. In contrast to the DZ, mitotic figures in the FZ are scant. The cell number of the FZ is not necessarily higher, but the size of it is much larger than that of the DZ. In the fetal rhesus monkey, Coulter et al. (55) demonstrated that growth of the FZ occurs primarily by hypertrophy, in response to increased endogenous ACTH secretion provoked by metyrapone treatment. Collectively, the FZ appears to grow by hypertrophy under limited cell proliferation.
Apoptosis also appears to occur in the developing HFA cortex. Jirasek (5) provided evidence of cellular apoptosis, determined by morphological criteria, in the HFA primarily in the central portions of the FZ. Spencer et al. (31) detected apoptotic cells by in situ analysis of DNA fragmentation and found that the labeling index of apoptotic nuclei is greater in the central areas of the FZ than in the DZ.
The disparate level of cell proliferation between the DZ and FZ and evidence of centripetal migration favor the migration theory of adrenal cortical development and suggest that the DZ is a pool of progenitor cells from which the inner cortical zones are derived. Thus, we have proposed that the HFA cortex is a dynamic organ in which cells proliferate in the periphery, migrate centripetally, and differentiate to form the specific cortical zones during their inward migration (and possibly continue to proliferate within the zones), only to undergo senescence when they reach the center of the gland (1, 31) (Fig. 1). The size of the fetal adrenal cortex and its constituent, specialized cortical compartments, represents the net effect of forces that modulate these dynamic parameters of growth.
D. Functional development: ontogeny of steroidogenesis and functional zonation
The precise onset of function (i.e., steroidogenesis) and the functional zonation of the HFA cortex have been of great academic and potentially clinical interest. We previously reviewed the literature regarding this issue published before 1997 (1). Evidence is accumulating that the HFA cortex has the capability to produce steroids early in gestation. To avoid repetition, we will focus on recent findings and issues of importance in this section.
1. Cortisol production in early pregnancy
One of the major unsolved questions concerning function of the HFA is when it begins to produce cortisol. Early onset of cortisol production has been suggested by observations of infants affected with deficiency of 21-hydroxylase (CYP21), a steroidogenic enzyme in the cortisol synthesis pathway (Fig. 2) (for review, see Ref. 56). In such patients, the fetal adrenal cortex cannot synthesize adequate amounts of cortisol. The suppressed cortisol inhibits negative feedback at the fetal anterior pituitary, which leads to a compensatory increase in ACTH secretion. The elevated ACTH causes fetal adrenal hyperplasia and increases production of DHEAS because its biosynthesis is not affected by CYP21 deficiency. In the first trimester when sexual differentiation occurs, there is a relative lack of aromatase (CYP19) activity in contrast to high placental aromatase activity seen later in gestation. Thus, the primary clinical manifestations of CYP21 deficiency are those of androgen excess, which are first expressed in utero, resulting in virilization of the external genitalia of female fetuses. In this context, a recent case report by Mendonca et al. (57) provides important insights. They reported the first female case with homozygous disruption of the glucocorticoid receptor as well as haploinsufficiency of the CYP21 gene. Although patients with haploinsufficiency of CYP21 do not usually present with an abnormal genital phenotype (56), the Mendonca et al. (57) case was born with female pseudohermaphroditism. Thus, this case highlights the importance of a defective negative feedback at the corticotroph and of elevated ACTH levels in the pathogenesis of female virilization in CYP21 deficiency. Moreover, maternal administration of the synthetic glucocorticoid dexamethasone, which crosses the placenta, initiated before the seventh to eighth week of gestation, appears to prevent virilization of a female fetus with CYP21 deficiency (58, 59). Because sexual differentiation of external genitalia begins at wk 7 of gestation and is complete by wk 10 (60), the androgen excess observed in CYP21 deficiency most likely occurs during this time window.
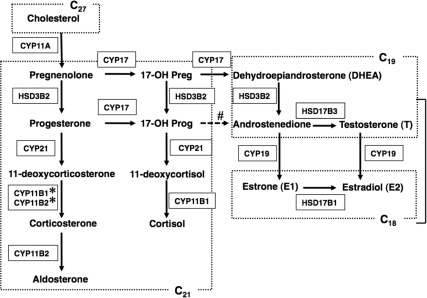
Fig. 2.
Principal pathways of human steroid biosynthesis. Pathways for biosynthesis of progesterone, mineralocorticoids, glucocorticoids, androgens, and estrogens. Names of the enzymes that catalyze each step are indicated in boxes. 17-OH Preg, 17-Hydroxypregnenolone; 17-OH Prog, 17-hydroxyprogesterone. *, 11-Deoxycorticosterone is converted to corticosterone by 11-hydroxylase activity of CYP11B2 (in zona glomerulosa) or by CYP11B1 (in zona fasciculata). #, The conversion of 17-hydroxyprogesterone to androstenedione by CYP17 is limited in humans.
Recently, Goto et al. (9) performed a series of localization and functional studies that strongly support the concept of early fetal adrenal cortisol synthesis. The authors demonstrated by immunohistochemistry that steroid acute regulatory protein (StAR), cytochrome P450 cholesterol side-chain cleavage (CYP11A), 17α-hydroxylase/17,20-lyase (CYP17), CYP21, and 11β-hydroxylase (CYP11B1)/aldosterone synthase (CYP11B2) are not present at 41 dpc. However, at 50–52 dpc they appear within the nascent inner FZ. In the outer DZ, expressions of StAR, CYP11A, CYP21, and CYP11B1/CYP11B2 are lower than in the inner FZ. CYP17 appears largely absent in the DZ. Up to 14 wk post-conception, their expression profiles persist in the FZ, whereas CYP17 is also weakly expressed in the DZ. The ontogenic expression profile of CYP17 is consistent with their previous study, in which scattered expression of CYP17 was noted in the DZ at 18 wk gestation (7). Our previous study (61) using midgestation HFAs (15–24 wk gestation) also demonstrated some isolated DZ cells with CYP17 staining. Adrenal de novo cortisol synthesis requires expression and activity of type 2 3β-hydroxysteroid dehydrogenase/Δ4–5 isomerase (HSD3B2) (Fig. 2). Goto et al. (9) demonstrated that HSD3B2 protein is not positive at 41 dpc. However, at 50–52 dpc, HSD3B2-positive cells appear mostly at the interface between the DZ and FZ. The HSD3B2 expression becomes more widespread throughout the gland and peaks at 8–9 wk post-conception. Thereafter, HSD3B2 immunoreactivity declines, and no protein can be detected at 14 wk post-conception. In parallel with this decreasing pattern of HSD3B2 expression, cortisol content per tissue weight in the first-trimester fetal adrenal, which is 9- to 18-fold higher than in the fetal kidney, decreases by approximately 50% between 8 and 10 wk post-conception (9). Goto et al. (9) further performed ex vivo tissue culture of human first-trimester fetal adrenals under conditions in which culture does not artificially cause HSD3B2 up-regulation, which usually matters in in vitro experiments (1), and showed that ACTH and forskolin increase adrenal cortisol secretion. Additionally, they demonstrated that human fetal corticotrophs at 8 wk post-conception secrete significant amounts of ACTH, which can be suppressed by dexamethasone. Thus, these studies provide not only proof that the HFA can produce cortisol, likely de novo from cholesterol, early in gestation, but also furnish a rationale for prenatal treatment with dexamethasone for female fetuses with CYP21 deficiency. Physiological significance of adrenal cortisol synthesis in the first-trimester fetus remains unclear. Goto et al. (9) hypothesize that the transient, early adrenal cortisol synthesis exerts a negative-feedback effect on ACTH secretion by the anterior pituitary corticotroph, thereby minimizing ACTH-induced androgen secretion to safeguard normal female sexual development.
2. Spatiotemporal expression of steroidogenic pathway components
Attempts have been made to examine spatiotemporal expression of components of steroidogenic pathways in the HFA. Previous (1) and recent data regarding the HFA expression of HSD3B2 are largely consistent with each other but somewhat conflicting in details. A synopsis is as follows: 1) there is a transient HSD3B2 expression in the first trimester (9, 62); 2) during most of the second trimester, the fetal adrenal expression of HSD3B2 is suppressed (11, 62–64); 3) however, after 23–24 wk gestation, HSD3B2 expression is detectable in the DZ and TZ (62, 64, 65); and 4) except in the first trimester, HSD3B2 is not expressed in the FZ (9, 62, 64, 66). A recent cDNA microarray study demonstrated that HSD3B2 mRNA expression in HFAs (15–20 wk) is 21-fold lower than that of adult adrenals (67). Although human adrenal HSD3B2 expression is spatially and temporally regulated during fetal life, the mechanisms regulating HSD3B2 expression remain poorly defined. An intriguing characteristic of FZ cells is that they do not express HSD3B2 in vivo but will readily express it in vitro when stimulated by ACTH (for detailed discussion, see Ref. 1). Thus, there may be a specific repressor that inhibits up-regulation of HSD3B2 in vivo in the HFA. Alternatively, a specific inducer for HSD3B2 expression may exist. The transcription factor nerve growth factor-induced clone B (NGFI-B) is likely one such inducer (68) (see Section III.D.2). Other potential candidates as HSD3B2 regulators include GATA-4 and GATA-6, SF1, and the related molecule liver receptor homolog 1 and signal transducer and activator of transcription family member 5 (for review of HSD3B2 regulation, see Ref. 69).
CYP11A protein is expressed between 14 and 22 wk gestation only in the FZ and TZ, but not in the DZ, and becomes detectable in the DZ after 23–24 wk (11, 64). CYP21 protein localizes in the FZ and TZ between 14 wk and term, and in the DZ starting at 24 wk and continuing to term (64, 70). The distribution of CYP17 is almost limited to the TZ and FZ throughout gestation; CYP17 is largely absent in the DZ (7, 11, 64). Using an antibody that recognizes both CYP11B1 and CYP11B2, Coulter and Jaffe (70) found CYP11B1/B2 protein localization in the TZ and FZ of HFAs (13–24 wk), with higher expression in the FZ than the TZ. However, the DZ lacks CYP11B1/B2 protein. In this study, an antibody that recognizes both CYP11B1 and CYP11B2 was used. The authors further showed that, in the fetal rhesus monkey between 109 d and term, CYP11B1/B2 protein is detectable in all cells of the TZ and FZ but is absent in the DZ until near term. In addition, metyrapone-induced ACTH stimulation in the monkey induces CYP11B1/B2 expression in the DZ and up-regulates its expression in the TZ and FZ (70). Freije et al. (71) detected CYP11B1 mRNA in both the DZ and FZ of the midgestation HFA using RNase protection assays and RT-PCR. By RNase protection assays, CYP11B2 mRNA was weakly positive after prolonged exposure in the DZ but was not detectable in the FZ, whereas the more sensitive RT-PCR detected CYP11B2 mRNA in both the DZ and FZ. Narasaka et al. (64) also investigated protein localization of other steroidogenic components in HFAs (14–40 wk). DHEA sulfotransferase (SULT2A1), which converts DHEA to DHEAS, localizes to the TZ and FZ, but not to the DZ. P450 oxidoreductase and cytochrome b, which regulate the activity of CYP17 (72, 73), are distributed to FZ and TZ cells. In the DZ, both proteins are absent until 22 wk but emerge after 23 wk. SF1 protein is expressed in almost all adrenocortical cells in the DZ, TZ, and FZ (64). Significance of SF1 in the development of the HFA will be discussed in Section III.D.1. StAR protein, which regulates the rate-limiting step in steroid biosynthesis (for review, see Refs. 74 and 75), i.e., the intramitochondrial transport of cholesterol is found in FZ and TZ cells, but not in DZ cells until 22 wk gestation (64).
The current data regarding the ontogeny of expression of steroidogenic components would account for zonal differential steroidogenic activity and its onset. The HFA cortex after midgestation is composed of three functionally distinct zones, each of which expresses different combinations of steroidogenic enzymes and cofactors (Fig. 3): 1) the DZ, which is the likely site of aldosterone synthesis late in gestation because of the persistent lack of CYP17, and the eventual expression of HSD3B2, CYP11A, CYP21, and probably of CYP11B2; 2) the TZ, which appears to be the site of cortisol production late in gestation based on the persistent expression of CYP17, CYP11A, CYP21, and the eventual expression of HSD3B2, and probably of CYP11B1; and 3) the FZ, which expresses CYP11A and CYP17 but not HSD3B2, is the site of Δ5-steroid production, particularly DHEA and DHEAS, throughout most of gestation. The localization and ontogeny of the steroidogenic enzymes and cofactors fit well with the concept that the DZ develops to form the zona glomerulosa, the TZ is the equivalent of the zona fasciculata, and the FZ is analogous to the zona reticularis.
Fig. 3.
Zonal expression of steroidogenic components in the HFA cortex during mid- and late gestation. Schema shows the specific zonal expression of components of the steroidogenic machinery. Black crossbar denotes expression, whereas absence of a bar represents lack of expression. Gray depicts low levels of expression. Figure is derived from previous studies (7, 11, 64, 70).
3. Ontogeny of steroidogenic activity
Data on the ontogeny of HFA steroidogenic activity are available from previous studies that include hormone determinations from cord blood and amniotic fluid, in vitro incubation and superfusion studies of HFA tissues, and perfusion of previable human fetuses with radiolabeled hormones. For detailed description of those studies, the reader is referred to our previous review (1). Based on such data, along with the recent comprehensive analysis of ontogenic expression of the components of the steroidogenic machinery, the ontogeny of steroidogenic activity in the HFA is summarized as follows: 1) DHEAS production appears to begin at around 8–10 wk gestation, continues thereafter, and increases considerably during the second and third trimesters, such that by term the HFA produces around 200 mg of DHEAS per day; 2) de novo cortisol production likely occurs transiently early in gestation (around 7–10 wk gestation); 3) due to the lack of HSD3B2 expression, de novo cortisol biosynthesis appears to be suppressed until late gestation when cortisol production escalates (76–78); and 4) aldosterone synthesis in the HFA may be suppressed during midgestation due to the probable lack of CYP11B2 expression, but likely becomes active by term because 80% of aldosterone in human fetal blood at term appears to originate from the fetal adrenal (79).
In addition, Goto et al. (9) described the capacity of the HFA at 8 wk post-conception to secrete androgens. Androstenedione and testosterone were detected in the media of HFA tissue cultured overnight. In addition, the fetal adrenal secretions of such androgens are responsive to ACTH or forskolin. Because such adrenal androgen production is perfectly timed to influence the development of external genitalia, the investigators suggested that this ACTH-responsive androgen synthesis by the HFA may be partly responsible for the pathogenesis of virilization of the external genitalia seen in female infants with CYP21 deficiency. They also examined expression of the 17β-hydroxysteroid dehydrogenase (HSD17B) isoforms, which can convert androstenedione to testosterone by RT-PCR and found that the type 5 isoform (HSD17B5; officially named AKR1C3) was more readily detected in the early HFA than HSD17B3 that is responsible for testosterone synthesis in the testis.
E. Angiogenesis and its regulation
The HFA is one of the most highly vascularized organs in the human fetus (12, 80). The development of an extensive vasculature in the organ is essential for organ growth and delivery of tropic agents (e.g., ACTH) and steroid hormone precursors to the gland and secretion of hormone products into the peripheral circulation. Here we review studies on HFA vasculature, angiogenesis (the process of formation of new capillaries from preexisting blood vessels), and its regulation.
1. Vasculature of the HFA gland
The extraorganic and intraorganic vascular systems of the human adult adrenal gland have been described (38, 81, 82). In general, the adrenal gland is supplied by arteries that arise from the renal and inferior phrenic arteries and the aorta. Earlier studies of the adult adrenal vasculature demonstrated significant variability in both origin and number of the adrenal arteries, which was consequently confirmed by a selective angiographic study of the adrenal glands in living adults. The adrenal arteries supply blood to a network of arterioles in the adrenal capsule, the subcapsular arteriolar plexus, which gives rise to capsular capillaries that are then in continuity with the thin-walled sinusoids of the zonae glomerulosa, fasciculata, and reticularis. The subcapsular arteriolar plexus also branches into medullary arteries, which traverse the cortex, and conveys blood directly to the adrenal medulla. Studies of the arterial blood supply to the HFA are limited. However, the sources of vessels for the HFA are similar to those for the adult adrenal (83, 84). The arterial blood supply to the HFA also is extremely variable in both the origin and number of the adrenal arteries (84).
The vasculature of the HFA gland is established by the eighth week of gestation when the adrenal is supplied by arteries from the descending aorta, and the capillary sinusoids within the gland form a continuum with the general circulation (5). Goto et al. (9) indicated that vascular channels positive for CD34, a vascular endothelial cell marker, penetrate the human embryonic adrenal gland between the aorta and mesonephros as early as 41 dpc, and vessel density increases at the periphery of the gland at 50 dpc. This timing of adrenal vascular establishment may be critical because the same investigators also provided evidence that the HFA can secrete cortisol after 8 wk post-conception (9) (see Section II.D.1.).
The general vascular pattern within the HFA appears to correspond to that previously described for the adult gland (82, 85–87). Pityński et al. (87), in a study using microcorrosion casts and scanning electron microscopy, described a dense vascular tree that shows a clear centripetal blood flow pattern. Thus, blood from superficial arteries and their branches would run through irregular capillaries of the subcapsular arteriolar plexus and the DZ, and then through the sinusoidal network of the FZ to the central vein. The authors also described the rare presence of medullary arterioles and absence of any portal systems. In addition, they noted the presence of circular impressions on casts of superficial arteries, suggesting existence of muscular sphincters, a circular arrangement of intimal connective tissue fibers, or smooth muscles (87). Thus, the HFA gland has a dense vascular system very similar to that of adults, which enables proximity between adrenocytes and endothelial cells, facilitating delivery of tropic agents, steroid hormone precursors, and hormone products, and is consistent with the fact that the organ is an active endocrine organ during fetal life.
2. Angiogenesis and its regulation
Angiogenesis, the process of formation of new capillaries from preexisting blood vessels, is considered an integral process for organ growth. Because the HFA undergoes a phase of rapid growth in midgestation, angiogenesis likely is essential for the rapid growth of the HFA.
Angiogenesis is partly mediated by proangiogenic factors such as fibroblast growth factor (FGF)-2 and vascular endothelial growth factor (VEGF)-A, both of which are potent mitogens for endothelial cells (88). Angiopoietin (Ang)-1 and Ang2 belong to a more recently identified family of endothelial cell-specific growth factors that also play a crucial role in angiogenesis (88, 89). Ang1 is expressed in a variety of tissues, whereas Ang2 expression is found primarily at sites of vascular remodeling, including the reproductive tract and placenta (90, 91). Both Ang1 and Ang2 bind their Tie2 receptor with high affinity. The currently accepted hypothesis is that Ang1 signals via Tie2 and promotes vessel stabilization and maturation, whereas Ang2 antagonizes Ang1/Tie2 signaling and destabilizes vessels, leading to either angiogenesis or vessel regression, depending on the presence of angiogenic stimuli such as FGF-2 and VEGF-A (88, 89). Studies from our laboratory demonstrated that VEGF-A, FGF-2, Ang1, Ang2, and Tie2 are expressed in the midgestation HFA gland (53, 92–95). Tie2 localizes exclusively to endothelial cells of the organ (95). A recent study by Ishimoto et al. (53) revealed that mRNA expression and protein localization of Ang2 and FGF-2 in the HFA gland show outer-zone predominance. VEGF-A protein localizes throughout the gland, whereas mRNA of VEGF-A follows the outer-zone predominance. In parallel to the outer-zone dominant proangiogenic factor expression, endothelial cell proliferation was restricted to the outer region of the HFA gland. Collectively, these results indicate that the periphery of the HFA is the primary site of angiogenesis (Fig. 1) and that locally produced angiogenic factors such as Ang2, VEGF-A, and FGF-2 likely play a key role in angiogenesis of the HFA. We hypothesize that the outer DZ may benefit from a plastic vascular state to accommodate its proliferative phenotype, and that interactions between adrenal cortical and endothelial cells ensure a coordinate expansion of the vascular network as DZ cells proliferate and the organ grows.
Regulation of proangiogenic factors has been investigated in isolated adrenocortical cells from HFAs (53, 92, 94, 95). ACTH up-regulates VEGF-A in HFA cortical cells (94, 95). ACTH also increases mRNAs encoding Ang1 and Ang2 in HFA cortical cells, with an altered Ang balance in which Ang2 predominates and stimulates Ang2 protein expression (95). Because the growth-stimulatory actions of ACTH in the HFA are likely mediated, at least in part, by locally produced growth factors, acting in an autocrine and/or paracrine fashion (92, 96), and ACTH is not an angiogenic factor per se, these angiogenic factors may mediate the tropic action of ACTH, exerting parallel control over the fetal adrenal vasculature. In addition, Ang2 mRNA can be induced by FGF-2 in HFA cortical cells (53), partly explaining the parallelism observed in the outer-zone predominant expression patterns of Ang2 and FGF-2.
Taken together, these studies highlight the importance of coordinated organ and vasculature growth by interactions between adrenal cortical cells and endothelial cells in which FGF-2 and the vascular endothelial specific growth factors, Ang2 and VEGF-A, likely are involved.
III. Candidate Molecules Implicated in Human Fetal Adrenal Development and Function
Intensive efforts have been made to identify genes and gene products implicated in human adrenal development and function. The recent emergence of new technologies, such as cDNA microarray, laser-capture microdissection, and suppression subtractive hybridization, has provided researchers with new tools to tackle this issue.
A. Gene expression profiles of human fetal and adult adrenals
Marked structural and functional differences are observed between the human fetal and adult adrenal glands. Rainey et al. (67) have contrasted human fetal (15–20 wk) and adult adrenal gene expression profiles using cDNA microarrays. Among 69 transcripts that have a greater than 2.5-fold difference in expression between fetal and adult adrenals, the largest differences are observed for transcripts encoding IGF-II (25-fold higher in the fetal adrenal) and HSD3B2 (21-fold higher in the adult adrenal). Additionally, the transcript expression of the cholesterol biosynthetic enzyme, 24-dehydrocholesterol reductase, exhibits the strongest signal intensity in fetal adrenals. For adult adrenal RNA, the transcript of CYP11B1 has the highest signal intensity ranking (97).
Rehman et al. (98), using real-time RT-PCR, compared fetal and adult gene expression levels of components of the adrenal steroidogenic pathway. The study revealed higher fetal adrenal levels of transcripts encoding the components that favor enhanced synthesis of DHEA and DHEAS, the distinct phenotype of the HFA. Thus, transcripts were higher in the HFA for cytochrome b5 and P450 oxidoreductase that may increase P450c17 activity and SULT2A1 that catalyzes DHEA sulfurylation. In contrast, HSD3B2 mRNA was 127-fold higher in the adult adrenal, consistent with the previous result from the cDNA microarray experiments (67).
More recently, Xing et al. (99) compared mRNA levels of a variety of G protein-coupled receptors between human fetal and adult adrenal glands. They found that transcript levels of six G protein-coupled receptors, including GnRH receptor, angiotensin-II (AT-II) type 2 receptor (AT2), and melanocortin 2 receptor [MC2R; or the ACTH receptor (ACTHR)], were significantly higher in fetal than adult adrenals.
B. Markers unique to zonal cellular subtypes
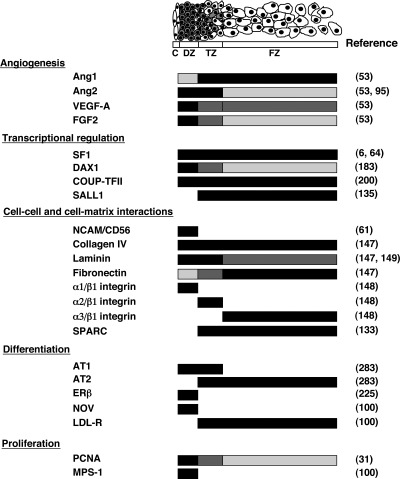
As noted, the HFA is comprised initially of two zones, the DZ and the FZ. A third zone, the TZ, develops between the two zones after midgestation. Identification of cellular markers unique to each zone of the HFA should be useful for purifying cells of the zone of interest and/or providing insight into the mechanisms underlying zonation of the HFA, which facilitates detailed molecular, cellular, and structural analysis toward a better understanding of HFA development and function (Fig. 4).
Fig. 4.
Zonal expression of putative factors implicated in HFA development and/or function. Schema shows specific zonal expression of putative factors implicated in HFA development and/or function. Black crossbar denotes high levels of expression, whereas absence of a bar represents lack of expression. Dark gray depicts moderate levels of expression, and light gray depicts low levels. LDL-R, LDL receptor; PCNA, proliferating cell nuclear antigen. Figure was derived from studies shown in right column.
1. DZ cell markers
Ratcliffe et al. (100) used laser-capture microdissection to obtain cells from the DZ and FZ. They performed subtractive hybridization and found two previously described growth regulatory proteins, nephroblastoma overexpressed (NOV) and metallopanstimulin-1 (MPS-1) that are expressed almost exclusively in the DZ (100).
NOV (CCN3) is a recently described member of the cysteine-rich 61, connective tissue growth factor, NOV (CCN) family of growth regulatory proteins. The family members have described roles in regulating mitosis, cell adhesion, migration, growth arrest, apoptosis, differentiation, and tumorigenesis (101–103). In human adults, NOV is more strongly expressed in the adrenal cortex than in other endocrine tissues (103). Consistent with the notion that NOV is a DZ cell marker, immunohistochemical studies have demonstrated DZ-specific or DZ-predominant localization of NOV in HFAs from first and second trimesters (103, 104). The role of NOV in HFA development remains to be clarified. Several lines of evidence support its role in inhibiting growth while promoting differentiation (105). The amount of NOV increases in benign adrenocortical tumors, whereas it decreases in malignant adrenocortical tumors, suggesting a role for NOV as a growth regulatory protein (103). Alternatively, NOV may be a differentiating factor because, in NCI-H295R adrenocortical cells, expression of NOV is down-regulated by TGF-β1 (106), which negatively regulates adrenal steroidogenesis. NOV also is negatively regulated by WT-1, the Wilm's tumor suppressor gene product (107). Of interest, mice lacking WT-1 fail to develop adrenals (107, 108).
Less is known about MPS-1, a zinc-finger phosphoprotein with DNA-binding properties belonging to the S27E family of ribosomal proteins (109). MPS-1 is highly expressed in a wide variety of actively proliferating cells, cancer cell lines, and malignant tumor cells, whereas it is generally underexpressed in normal adult cells (109–111). Therefore, MPS-1 could play a role in modulating cell proliferation in the DZ. Alternatively, MPS-1 possibly mediates the suppressive effects of TGF-β1 on steroidogenesis in the DZ, because MPS-1 can be up-regulated by TGF-β1 in mammary carcinoma cells (109).
Ratcliffe et al. (100) also performed immunohistochemical screens and identified P-glycoprotein, the product of the multidrug resistance 1 (MDR1; or ABCB1) gene, as a DZ marker. P-glycoprotein is the prototype member of the family of ATP-binding cassette (ABC) transporters, which are ATP-dependent membrane proteins predominantly expressed in excretory organs, such as the liver, intestine, blood-brain barrier, blood-testis barrier, placenta, and kidney (112, 113). The ABC transporters are implicated in the absorption, distribution, and excretion of drugs, xenobiotics, and endogenous compounds. P-glycoprotein is expressed abundantly in the adult and fetal adrenal gland in many species, including human (114–116). Its role in the adrenal gland is unknown. Several studies indicate that P-glycoprotein is involved in active efflux of steroids, particularly aldosterone and cortisol, from steroidogenic cells (117–119). The HFA expression of P-glycoprotein occurs after 22 wk (100). Of particular interest, consistent with this timing of P-glycoprotein expression, the steroidogenic enzymes necessary for aldosterone synthesis begin to be expressed in DZ cells (11). In addition to its pump actions, P-glycoprotein also has been implicated in the protection of cells against apoptosis provoked by a variety of stimuli such as cytotoxic drugs and TNF-α (120, 121). A role for P-glycoprotein during cellular differentiation has also been proposed. P-glycoprotein and another ABC transporter, breast cancer resistance protein (ABCG2), are highly expressed in an enriched population of primitive stem cells: the side population (SP) (113). SP cells were originally discovered in bone marrow by their capacity to exclude rhodamine 123 and Hoechst dye 33342; however, mounting evidence also revealed their presence in other nonhematopoietic tissues. The expression of breast cancer resistance protein and P-glycoprotein are under strict control and may determine the differentiation of SP cells toward other more specialized cell types. Thus, P-glycoprotein expression is down-regulated during differentiation of pluripotent stem cells along the myeloid lineage (122), and overexpression of P-glycoprotein in mouse stem cells induces a myeloproliferative syndrome (123). In this context, P-glycoprotein expression in the DZ is consistent with our hypothesis that cells in the DZ may comprise a progenitor population, some of which migrate centripetally to populate the rest of the gland (1, 61, 100). P-glycoprotein, therefore, may be a useful cell surface marker to distinguish between those cells that have left the progenitor pool and differentiated into steroid-producing cells and the truly undifferentiated progenitor cells.
Recently, Muench et al. (61) found that the neural cell adhesion molecule (NCAM; CD56) can serve as a marker of DZ cells through an extensive series of phenotyping experiments using fluorescence-activated cell sorting. In isolated HFA cells, NCAM was expressed on a cell population of small, dense, low side-scatter cells that lack markers of hematopoietic origin (CD31, CD34, and CD235a), and this cell population was enriched for cells expressing NOV and MPS-1, the DZ cell markers (61). NCAM is widely expressed in the nervous system and plays important roles in neurodevelopment (124, 125). NCAM also has been localized in nonneural tissues, including endocrine organs (126–128). Functions of NCAM in the HFA remain to be clarified. NCAM can modulate cell motility and migration of glioma cells (129). It can also act as a receptor for a diffusible growth factor (130). In the context of the latter possibility, a recent study (131) indicating that NCAM may be a receptor for the heparin-binding growth factor midkine is of particular interest because midkine stimulates selective proliferation of DZ cells (132) where NCAM is localized at the membrane. Muench et al. (61) also described islands of NCAM-positive cells that likely represent chromaffin cells. Chromaffin cells are a major component of the adrenal medulla. However, during primate fetal life, a well-formed medulla is not present before birth (17, 18). It is evident that functions of NCAM in HFA development should be explored in future studies.
2. FZ cell markers
Immunohistochemical studies have revealed several proteins that localize exclusively in the FZ. The low-density lipoprotein (LDL) receptor is one such protein. Despite its FZ-specific immunoreactivity (100, 133), analysis of zonal mRNA expression of the LDL receptor, using laser-capture microdissection and real-time RT-PCR, demonstrates only a 2-fold difference in LDL receptor mRNA levels between the FZ and DZ (133), suggesting that the expression of the LDL receptor may be regulated mainly at the translational level. Given the important role of the LDL receptor in de novo steroidogenesis in the adrenal gland and the DZ's lack of steroidogenic activity until close to term (11), DZ cells would not be expected to express the LDL receptor at midgestation. SALL1 [human homolog of spalt (sal), a region-specific homeotic gene in Drosophila melanogaster] protein is a zinc finger transcriptional repressor and may be part of the NuRD histone deacetylase complex. Defects in the gene SALL1 are a cause of Townes-Brocks syndrome, a disorder with multiorgan dysgenesis including renal and genital malformations (134). Expression of SALL1 appears confined to the pituitary-adrenal-gonadal axis and the placenta (135). Distribution of SALL1 is restricted to the FZ of the midgestation HFA, whereas it is observed in all zones of the adult adrenal cortex, suggestive of its association with steroidogenesis. Interestingly, in a recent study using human adrenocortical H295R cells (136), SALL1 expression was induced by AT-II. Furthermore, SALL1 was shown to decrease expression of CYP11B1 and CYP11B2 in cotransfection reporter assays. SPARC (secreted protein acidic and rich in cysteine), a matricellular protein, also localizes strictly to the FZ of the midgestation HFA (133) (see Section III.C).
3. G protein α-subunits
Heterotrimeric (αβγ) G proteins are central components of the primary mechanism to receive, interpret, and respond to a variety of extracellular stimuli (137, 138). Breault et al. (139) described differential expression of G protein α-subunits in the HFA. Interestingly, the α-subunits had a specific pattern of distribution, other than the αs subunit that was detected in all adrenal cell types except for endothelial cells. The αil-2 subunit was restricted to the DZ, whereas αi3 staining was mainly seen in the FZ. The αq subunit was localized in vascular endothelial cells at the periphery and in FZ cells at the center of the gland. Chromaffin cells expressed αs, αq, and αo1, but not αo2 nor αi. G protein-coupled receptors, including the ACTH receptor and AT-II receptors, play a crucial role in HFA development and function (1, 99). Therefore, the differential expression of G protein α-subunits may partly explain the zonal or cell-type specific responses to stimuli and the uniqueness of the HFA phenotype.
C. Extracellular matrix (ECM) environment
Mounting evidence indicates that the extracellular microenvironment can orchestrate functions such as cell proliferation, migration, differentiation, and apoptosis, essential components of organogenesis (140, 141).
Earlier studies showed growth-promoting effects of ECM components on bovine and human fetal adrenocortical cells in culture (142–146). Crickard et al. (142) showed that isolated fetal adrenocortical cells maintained on an ECM prepared from bovine corneal endothelial cells had a higher growth rate than cells maintained on plastic alone. Recently, Chamoux et al. (147) revisited this issue and demonstrated that isolated HFA cells grown on collagen IV and, to a lesser extent, on laminin favor cell proliferation, whereas cells grown on fibronectin become more apoptotic. In an earlier study, the same group of investigators examined the spatial distribution of these structural components of the ECM in second-trimester HFAs (148). Collagen IV was localized evenly throughout the midgestation HFA. The distribution of fibronectin and laminin exhibited a mirror image of each other; fibronectin was more abundant in the central portion, and laminin was mainly found at the periphery of the gland (148). In addition, several integrin subunits, which can serve as receptors for ECM components, have been localized in the HFA. The α1- and α2-subunits were found mainly in the DZ and in the TZ, respectively. The α3-subunit that binds both fibronectin and laminin was detected only in the FZ. The β2-subunit was observed exclusively in chromaffin cells (148). Similar results for integrin localization were obtained in the other study where distribution of laminin chains, laminin-binding integrins, and non-integrin receptors was examined in the HFA (149). Laminin α2 and α5 chains were weakly localized in the DZ, and laminin α4, β1, and γ1 chains were observed around vessels. A punctate distribution of dystroglycan was seen mainly in the DZ and weakly in the FZ. Chamoux et al. (147) also found that cell morphology can be affected by environmental cues; for example, HFA cells grown on laminin appear rounded, clustered, and smaller, resembling the morphology of DZ cells. They further demonstrated that the ECM components modulate the profile of steroidogenesis by HFA cells. Collagen IV favors ACTH- or AT-II-stimulated cortisol secretion and elevated DHEA secretion stimulated by an agonist for AT2. In contrast, laminin and fibronectin diminished responsiveness to ACTH in relation to cortisol production while increasing ACTH-induced DHEA and DHEAS secretion. Collectively, the differential distribution of the ECM structural proteins may explain some aspects of the zone-specific cellular behavior seen in the HFA.
Matricellular proteins, a term coined by Bornstein (150), are another class of ECM components. They are secreted macromolecules that interact with cell-surface receptors, ECM, growth factors, and/or proteases but do not themselves subserve strictly structural roles, unlike other ECM proteins such as laminin and fibronectin. They disrupt cell-matrix interactions and are involved in tissue remodeling, morphogenesis, and vascular growth. The group includes thrombospondins, tenascin C, and SPARC. Among them, SPARC shows abundant expression in the midgestation HFA and a unique FZ-specific localization (133). SPARC modulates cell proliferation and migration. Its expression appears limited largely to tissues undergoing remodeling, morphogenesis, or tissue repair (151). Thus, SPARC could exert an antiproliferative and antimigratory effect, reflected by the selective expression of SPARC in the FZ, where cell proliferation is less and cell migration should terminate rather than be initiated.
D. Transcriptional regulators
Studies of experimental animal models and humans are beginning to unravel the mechanisms of transcriptional regulation responsible for organogenesis of the human adrenal cortex (for review, see Refs. 152 and 153). An increasing number of transcription factors has been implicated in adrenal development. Among them, SF1 and DAX1, which have been most extensively investigated and play essential roles in normal adrenocortical differentiation and development (154–159). In addition, SF1 is a key transcriptional activator of numerous genes involved in steroidogenesis (154, 158), whereas DAX1 acts as a negative regulator of SF1-induced transactivation (157, 159).
1. SF1 and DAX1
An extensive body of evidence delineates essential roles for SF1 in regulating adrenocortical differentiation and function. SF1 is expressed in the HFA from its earliest stages of development—initially in the adrenogonadal precursors, and subsequently in both the FZ and DZ of the gland (7). SF1 is expressed in all zones of the human adult adrenal cortex. SF1 regulates the transcription of multiple genes involved in steroidogenesis, reproduction, and male sexual differentiation (154, 158). For example, an SF1 response element has been identified within the proximal promoter region of the genes encoding the ACTH receptor, StAR, CYP11A1, CYP17, HSD3B2, CYP21, CYP11B, and SULT2A1 (158, 160). Intriguingly, a recent study of Yazawa et al. (161) demonstrated that stable transfection with an SF1 expression vector, with the aid of cAMP, can induce the differentiation of bone marrow-derived mesenchymal stem cells into steroidogenic cells such as adrenocortical cells and Leydig cells.
SF1 knockout mice lack adrenals and gonads and die shortly after delivery due to severe adrenal insufficiency (162, 163). These mice also exhibit male-to-female sex reversal of their genitalia, impaired gonadotropin expression, and agenesis of the ventromedial hypothalamic nucleus (162, 164). Heterozygous mice with one disrupted allele of the SF1 gene show a decrease in adrenocortical volume, coupled with decreased stress-induced corticosterone response (165). Thus, in mice, SF1 regulates fundamental events in adrenal and gonadal differentiation. Recent evidence suggests a similar role for SF1 in the regulation of adrenal development in humans. SF1 mutations have been identified in an increasing number of patients with impaired adrenal development and/or sexual differentiation disorder (166–175). The first three cases presented with early onset of adrenal insufficiency with or without gonadal dysgenesis (166–168). Biason-Lauber and Schoenle (168) described a prepubertal girl with heterozygous mutation of the SF1 gene that presented with adrenal insufficiency but apparently had normal ovaries, suggesting that ovarian development requires only one functional SF1 allele. More recent individuals presented with various degrees of gonadal dysgenesis with normal adrenal function (171). The underlying mechanisms by which SF1 mutations give rise to gonadal dysgenesis while not affecting adrenal development and function remain to be elucidated.
DAX1 is another important regulator of adrenal development. Mutations in the DAX1 gene give rise to X-linked adrenal hypoplasia congenita, an inherited disorder characterized by manifestations in infancy of adrenal insufficiency and hypoplasia of the HFA with an absence or near absence of the DZ and a structural disorganization of the FZ (176, 177). The tissue distribution of DAX1 (adrenal cortex, gonads, hypothalamus, and pituitary) overlaps that of SF1. Thus, SF1 and DAX1 may be coregulators of steroidogenic tissue development and function. In human embryos, both SF1 and DAX1 are expressed throughout the developing adrenal gland from its inception at 33 dpc (7). In situ hybridization signal intensities of SF1 transcripts were greater than those for DAX1 at all embryonic stages. Lower levels of SF1 and DAX1 expression persisted throughout the HFA cortex at 18 wk gestation (7). These concordant expression patterns very likely reflect the interplay between SF1 and DAX1. DAX1 is one of the target genes for SF1 (178), and DAX1, in turn, inhibits SF1-mediated transcription, likely by recruiting corepressors to the transcriptional complex (179, 180). The adrenal insufficiency in SF1 haploinsufficient mice was partially rescued by knockout of DAX1 (180), suggestive of the importance of a functional antagonism between SF1 and DAX1 in adrenal development. On the other hand, DAX1 expression is unimpaired in SF1 null mice (181), and no change was noted in SF1 expression in the adrenals of DAX1 knockout male mice (180). The mechanism that underlies the differential interplay between SF1 and DAX1 remains unclear.
Recent studies reveal the complexities of DAX1 regulation and function. These include identification of an alternatively spliced variant, DAX1A, and of DAX1 homodimers and heterodimers (177). Niakan et al. (182) have proposed a novel role for DAX1 in the maintenance of a relatively undifferentiated state. They demonstrated that knockdown of murine DAX1 expression by RNA interference, as well as conditional knockout of the DAX1 gene, induced differentiation in murine embryonic stem cells. Battista et al. (183) examined DAX1 localization in the midgestation HFA. DAX1 protein is localized mainly in the nucleus of DZ cells and in the cytoplasm of FZ cells, whereas the number of DAX1-positive cells decreases from the periphery to the center of the gland. Lehmann et al. (184) reported that the localization of DAX1 adrenal hypoplasia congenita mutant proteins is significantly shifted toward the cytoplasm and that cytoplasmic localization of DAX1 adrenal hypoplasia congenita missense mutants directly correlates with the magnitude of the impairment in their transcriptional repression activity. DAX1 functions as a potent negative regulator of steroidogenesis (157). Thus, nuclear DAX1 in the DZ may represent the undifferentiated, nonsteroidogenic phenotype of DZ cells. Because the X-linked form of adrenal hypoplasia congenita is associated with the absence of the DZ, these results are consistent with the hypothesis that the DZ is a pool of undifferentiated, progenitor/stem cells.
2. NGFI-B family members
Members of the NGFI-B family of transcription factors, particularly NURR1 and NGFI-B (also termed Nurr77 or NR4A1), regulate transcription of the steroidogenic enzymes CYP21, HSD3B2, and CYP11B2 (68, 185–188). Immunoreactivity of NURR1 and NGFI-B is detected at high levels in the DZ of the HFA and zona glomerulosa in the postnatal adrenal cortex (188). A microarray study shows that NGFI-B mRNA expression is 7.5-fold higher in adult than fetal adrenals (67). By using transient transfections into NCI-H295R adrenocortical cells, Bassett et al. (188) found that NGFI-B stimulates HSD3B2 reporter activity without an effect on a CYP17 promoter construct. In addition, NGFI-B expression parallels that of HSD3B2 in the human fetal and adult adrenal glands. Likewise, Goto et al. (9) found that HSD3B2 and NGFI-B share a remarkably similar transient pattern of expression in the early HFA. Of particular interest, infection of primary human adrenal FZ cells, which express little or no HSD3B2, with adenovirus containing NGFI-B increased cortisol secretion by 8-fold and induced HSD3B2 mRNA expression by 26-fold over that observed in mock infected cells. These results support a role for NGFI-B as a regulator of the temporal and zone-specific expression of HSD3B2 in the HFA, whereas there still remains the question of the factors controlling the characteristic expression profile of NGFI-B.
3. Other transcription regulators
Expression of several transcription factors is found in the HFA. As described in Section III.B.2, immunoreactive SALL1 is restricted to the FZ of the midgestation HFA, whereas it is observed in all zones of the adult adrenal cortex (135). The GATA family of transcription factors are emerging as novel regulators involved in the development and differentiation of steroidogenic tissues (189, 190). Among the family members, GATA-4 and GATA-6 are expressed in the HFA cortex (191). Adrenal GATA4 expression is down-regulated postnatally, whereas GATA-6 expression persists. GATA-4 and GATA-6 regulate transcription of genes encoding steroidogenic enzymes and cofactors, including CYP17, HSD3B2, SULT2A1, and cytochrome b5 (192–195). Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 (CITED2) is a transcriptional coregulator of cAMP response element binding protein (Cbp) and p300 (196). Immunoreactive CITED2 in an 8-wk human adrenal was restricted to the DZ (197). In NCI-H295R cells, FGF-2, but not ACTH, stimulates CITED2 promoter activity, mRNA, and protein expression (197). Recent in situ hybridization studies showed that transcripts of CITED2 and pre-B cell leukemia transcription factor 1 (PBX1), a homeobox protein (198), were expressed throughout the adrenal gland in a 10-wk human fetus (199). The study also demonstrated, using adrenocortical NCI-H295R cells, that transcription of the CITED2 and PBX1 genes is activated by SF1, and PBX1 is synergistically activated by SF1 and DAX1. Immunoreactivity of chicken ovalbumin upstream promoter transcription factor (COUP-TF)-II is found in the DZ and FZ of the HFA, as well as in all three zones of the adult adrenal cortex, but not in the medulla (200, 201). COUP-TFII immunoreactivity is intense in the zona glomerulosa and weak in the zonae fasciculata and reticularis from 7 months to 8 yr of age, but it subsequently decreases in all of the zones (200). In vitro studies show that COUP-TFs suppress the transcriptional activity of SF1 (202), CYP17 gene transcription in Y-1 murine adrenocortical cells (203), and StAR protein expression in bovine adrenal glomerulosa cells (204). Therefore, COUP-TFs may negatively regulate steroidogenesis.
Studies in mutant mice and human subjects have identified more molecules involved in early stages of adrenal development (i.e., differentiation of the adrenogonadal primordium from the urogenital ridge and formation and differentiation of the adrenal primordium). These molecules, most of which are transcription factors, include GLI3, SALL1, FoxD2, WT1, WNT4, PBX1, Acd, and CITED2 (152, 153). Mice lacking or having defects in each of those genes exhibit an adrenal phenotype. In humans, most of those genes demonstrate no adrenal phenotype when mutated, or human mutations have not been reported. An exception is the Pallister-Hall syndrome, an autosomal dominant disorder caused by GLI3 frameshift mutations that generate a truncated protein. Some patients with Pallister-Hall syndrome manifest absence or hypoplasia of the adrenal glands, as well as kidney malformation (205, 206). The reasons for the phenotypical differences between mice and humans remain unknown. For detailed descriptions of the mutant mice and genes, the reader is referred to recent excellent reviews (152, 153).
E. Role of growth factors
ACTH, the primary regulator of fetal adrenocortical development, stimulates proliferation of adrenocortical cells in vivo, whereas it is not a mitogen for adrenocortical cells in vitro. Therefore, it seems likely that the trophic actions of ACTH may be mediated by locally produced growth factors that act in an autocrine and/or paracrine fashion. Numerous studies have examined in vivo and in vitro expression of such growth factors, their functions, and regulation. These include FGF-2 (also called basic FGF), epidermal growth factor (EGF), IGFs, activins/inhibins, and TGF-β. The literature before 1997 on these issues was reviewed previously (1, 207). In summary, in vitro studies indicate that FGF-2, EGF, and IGF-I/II act as mitogens for human fetal adrenocortical cells, whereas activin-A and TGF-β inhibit proliferation. ACTH up-regulates expression of FGF-2, IGF-II, and the activin-inhibin subunits by isolated human fetal adrenocortical cells. Additionally, IGF-II, activin-A, and TGF-β modulate ACTH-induced steroidogenesis. This section focuses on recent findings.
A comparative tissue microarray analysis revealed genes that are expressed much higher in the HFA than in adult adrenals (67). Among them, the gene for IGF-II is most highly expressed in the HFA compared with the adult adrenal (25-fold higher in the HFA), consistent with previous studies that have demonstrated abundant IGF-II expression in the HFA, suggesting a key role for IGF-II in HFA development. In contrast, IGF-I is down-regulated in vivo in the HFA, and it localizes only in the HFA capsule. IGF-II markedly augments the steroidogenic responsiveness (DHEAS and cortisol production) of isolated FZ cells to ACTH without affecting abundance of mRNA encoding the ACTH receptor (208). In primary cultures of human adrenal FZ cells, IGF-II enhances ACTH-induced up-regulation of CYP11A, CYP17, and HSD3B2, and directly stimulates basal expression of CYP17, but not of CYP11A and HSD3B2, which could partly explain the increased androgen production by the FZ (208). Based on current data, most of the effects of IGF-II are believed to be mediated via the IGF type 1 receptor (208), which can bind to both IGF-I and IGF-II, but not through the type 2 receptor that binds exclusively to IGF-II. Transcript expression of the IGF type 1 receptor is slightly higher in HFAs than adult adrenals (67). Studies from the baboon show that mRNA encoding the IGF type 1 receptor peaks at midgestation (209). However, ontogenic changes in expression of the IGF type 1 receptor in the HFA are not known.
Activins and inhibins are members of the TGF-β superfamily of proteins (210). Activin and inhibin are homodimeric (βA-βA, βB-βB, or βA-βB) and heterodimeric (α-βA or α-βB) glycoproteins, respectively. Immunoreactivity for all three subunits is found in both the DZ and FZ of the HFA (211, 212). In a recent study, mRNA expression of activin type I/II receptor and inhibin receptor (betaglycan and inhibin-binding protein) is observed in HFA tissue, suggesting that the activin/inhibin system is operative in the gland (213). Furthermore, ACTH stimulates secretion of inhibin A and B, but not of activin A, in isolated HFA cortical cells (213). Previously, Spencer et al. (211) indicated that activin-A (βA-βA) inhibits mitogenesis and increased ACTH-stimulated cortisol secretion by cultured FZ cells, but not DZ or adult adrenal cells. Furthermore, a more recent in vitro study shows that activin-A or TGF-β can induce apoptosis of cultured FZ cells (31), compatible with their nature as suppressors of adrenocortical cell proliferation.
Previous studies showed that isolated DZ cells were more responsive to the proliferative effects of FGF-2 and EGF (142, 144). Because DZ cells have a proliferative phenotype in vivo, the findings may reflect that DZ cells per se may be more responsive to proliferative stimuli provided by growth factors. Alternatively, there may be another mechanism that maintains the proliferative DZ cell phenotype. The recent finding of DZ-predominant expression of FGF-2, as revealed by laser-capture microdissection and immunocytochemistry, supports this possibility (53). Furthermore, recent identification of the heparin-binding growth factor, midkine, as a DZ-selective growth factor, provides insight (132). Midkine belongs to a family of heparin-binding growth/differentiation factors that share functional, but not structural, features with the FGF family (214, 215). Although midkine has been most extensively studied in neural tissue, its presence is found in a wide variety of organs and tissues. The expression of midkine is developmentally regulated; it is highly expressed during midgestation. Ishimoto et al. (132) described the expression, function, and regulation of midkine in the HFA. Midkine expression is abundant in the midgestation HFA. Indeed, midkine mRNA levels are 4-fold higher than in adult adrenals, consistent with the recent data in a DNA microarray study (67). Addition of recombinant human midkine stimulates proliferation of isolated DZ cells, but not FZ cells, although FGF-2 induces proliferation of cells from both zones. The study also demonstrates that midkine expression is up-regulated by ACTH. Thus, midkine appears to be another ACTH-inducible growth factor that mediates the trophic actions of ACTH. Pharmacological interventions indicated that phosphatidylinositol 3-kinase, MAPK kinase, and Src family kinases may mediate the midkine-induced DZ cell proliferation (132). In a related manner, a recent study reveals that mRNAs of several Src family kinases (Src, Fyn, and Yes) are expressed in midgestation HFA tissue (216).
F. Placental factors: role of estrogens
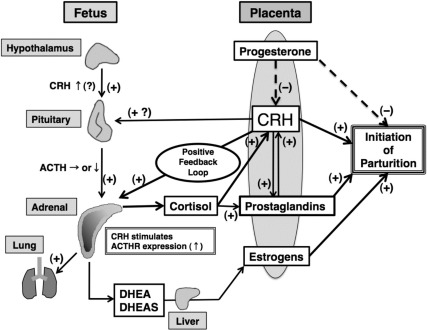
Several observations support the concept that HFA growth and function are influenced by factors derived from the placenta. Of particular note is that the FZ rapidly involutes or is remodeled immediately after birth when placental factors are no longer available. An increasing number of placenta-derived factors have been identified. These include hormones (e.g., human chorionic gonadotropin, CRH, ACTH, and estrogens) and growth factors (e.g., EGF, FGF-2, and IGF-II) as we reviewed previously (1). The major foci of recent studies have been on placental CRH and estrogens. The biology and actions of CRH in the human feto-placental unit will be discussed separately (see Section V.C). Here we discuss whether and how placenta-derived estrogens may regulate the development and function of the HFA.
The human placenta produces large amounts of estrogens by aromatization of C19 precursors, DHEAS and its 16- and 15-hydroxylated metabolites produced by the HFA and fetal liver. Copious amounts of estrogens in the form of estrone, estradiol, estriol, and estetrol can be detected in the circulation of pregnant women (217). The human placental estrogen synthetic pathway is incomplete. The placenta does not produce estrogens de novo from acetate or cholesterol because it lacks the cytochrome P450 enzyme CYP17 (218, 219). Accordingly, human placental estrogen synthesis is dependent on the supply of C19 androgen precursors from other steroidogenic origins. In human pregnancy, the principal C19 precursor for placental estrogen biosynthesis is supplied by fetal and maternal adrenals in the form of DHEAS (217). In the placenta, the sulfatase enzyme acts to remove the sulfate moiety from DHEAS, and then, by the actions of 3β- and 17β-hydroxysteroid dehydrogenase enzymes, DHEA is converted to androstenedione and testosterone, which are subsequently converted to estrone and estradiol, respectively, by the enzyme CYP19. During most of gestation, placental estrone and estradiol are derived from DHEAS that is contributed approximately equally in terms of amounts by the fetal and maternal adrenals.
Estriol, another estrogen produced by the human placenta, increases progressively through pregnancy and exceeds the production rates of estrone and estradiol by late gestation (217). Because the placenta lacks the 16-hydroxylase enzyme, it can only produce estriol from a 16-hydroxylated C19 steroid precursor (2). Some of the DHEAS produced by the HFA is converted to 16α-hydroxy-DHEAS primarily by the fetal liver and, to a limited extent, within the HFA itself (220). In contrast, a supply of 16α-hydroxy-DHEAS by the maternal side is limited. Indeed, more than 90% of estriol is of fetal origin. The placenta converts 16α-hydroxy-DHEAS to 16α-hydroxy-DHEA that is subsequently aromatized to estriol. Thus, placental estriol production (hence, maternal estriol) directly reflects fetal adrenal steroidogenic activity. Estetrol is also a placental estrogen derived from a precursor of fetal origin (221). Estetrol is produced after 15α- and 16α-hydroxylation by the fetal liver. Placental estetrol production is limited, and its physiological roles remain unknown.
Not all of the estrogens in the maternal circulation exist in the biologically active unconjugated form (217, 222, 223). Estrone exists mainly as a sulfate, whereas estradiol is present mainly (70–80%) in the unconjugated form. In contrast, estriol is found primarily (90–95%) in its conjugated form. Therefore, despite the robust production rate of estriol in the late-gestation placenta, target tissues of estrogen action may be exposed to similar bioavailable amounts of estrone, estradiol, and estriol.
The incomplete steroidogenic pathways, existent in the HFA, fetal liver, and placenta, constitute a complete estrogen-synthesizing system, the feto-placental unit (2). It is clear that one of the physiological functions of the HFA is to provide C19 substrate to the placenta for the formation of estrogens. Given the coordinated relay of steroid products between the HFA and placenta, it is tempting to postulate that placental estrogens serve as a regulator of HFA development and function.
The effects of estrogens on target tissues are mediated via nuclear estrogen receptors (ERs), ERα and ERβ. ERβ mRNA, but not ERα mRNA, is expressed in human fetal (224, 225) and postnatal developing adrenals (226). Immunoreactive ERβ is restricted to the DZ of the HFA at mid- and late gestation (225). In contrast, ERβ mRNA and protein are expressed in the postnatal, involuting FZ, and in zona reticularis of post-adrenarchal human adrenals (226). Of interest, in baboon fetal adrenals, both ERα and ERβ proteins are predominantly localized in the DZ at mid- and late gestation (227).
In vitro studies indicate that placental estrogens may influence steroid production by the HFA. Estradiol inhibits production of ACTH-stimulated cortisol, whereas it enhances that of ACTH-induced DHEAS by cultured human fetal adrenocortical cells (228–231). Implications from these studies are summarized in three statements: 1) effects of estradiol on FZ steroidogenesis are exerted at the level of the activity of steroidogenic enzymes rather than their gene transcription; 2) estradiol favors DHEAS production (the FZ phenotype) by inhibiting activity of, but not expression of, HSD3B2; and 3) any estrogen effects on FZ function are not mediated through classic ER interactions.
However, studies in pregnant baboons, performed by Pepe, Albrecht, and colleagues (232), indicate a negative feedback regulation whereby placental estrogens suppress fetal adrenal DHEA production. Recently, they demonstrated that estrogen deprivation, by administration of the aromatase inhibitor CGS 20267, increased fetal adrenal weight and volume and the DHEAS levels in umbilical artery serum, an estimate of the FZ steroidogenic activity (233). Additionally, these changes by estrogen suppression were restored by concomitant administration of CGS 20267 and estradiol. Furthermore, estrogen deprivation caused an increase in FZ volume, whereas it did not affect the volumes of the DZ and TZ or fetal serum cortisol levels. Therefore, these authors suggest that estrogen selectively represses the morphological and functional development of the FZ, thereby maintaining physiological secretion of estrogen precursors by the FZ.
As noted above, there is a significant difference in effects on the fetal adrenal cortex by placental estrogens between humans and baboons. Because the FZ does not appear to express significant amounts of nuclear ERs in both species, future studies may be directed toward nongenomic actions and processes of estrogens including membrane ERs (234, 235).
IV. Regulation by ACTH, Pituitary Proopiomelanocortin-Derived Peptides, and Angiotensin-II
A. ACTH and pituitary proopiomelanocortin (POMC)-derived peptides
Several lines of evidence, such as experiments of nature in humans (e.g., anencephaly), in vitro experiments using human tissue, and in vivo studies in subhuman primates, establish the primary role of ACTH secreted by the fetal pituitary in regulating growth and function of the fetal adrenal cortex in humans and subhuman primates. To avoid repetition, the reader is referred to our previous review (1) for a description of those studies published before 1997. This section will cover key studies and recent data on pituitary ACTH and POMC-derived peptides relevant to HFA development and function.
1. Pituitary ACTH
Recently, Goto et al. (9) addressed the question of how early in gestation the fetal pituitary-adrenal axis becomes functional. The fetal pituitary was negative for ACTH immunoreactivity at 41 dpc. Consistent with a previous study (236), cytoplasmic ACTH became discernible at 50–52 dpc, simultaneously with the onset of adrenal steroidogenic enzyme expression (9). The expression of ACTH overlapped with that of the nuclear glucocorticoid receptor. ACTH expression increased in the anterior pituitary at 8 wk post-conception. Furthermore, overnight in vitro adrenal tissue culture at 8 wk post-conception indicated that the corticotrophs of the early pituitary secrete abundant ACTH, which was suppressed by dexamethasone. These results suggest that the fetal pituitary-adrenal axis is functional at an early fetal age. Despite these results indicating the actions of ACTH on the HFA at early gestation, normal adrenal development is observed before 10–15 wk gestation in anencephalic fetuses (presumably with markedly reduced ACTH) (237, 238), suggesting that the early development of the HFA is ACTH-independent. Thus, ACTH may affect the function of the HFA at early gestation, but not its structural development.
Studies in human and nonhuman primate fetuses indicate that the structural development of the DZ is independent of ACTH. The DZ appears normal in anencephalics despite the absence of ACTH stimulation (237–239). In baboon fetuses, ACTH administration increased, whereas glucocorticoid treatment decreased, the size of the TZ and FZ (240–242). However, neither treatment had an effect on the size of the DZ (241). In contrast, functional maturation of the DZ appears to be regulated by ACTH, at least in primate fetuses (209, 240, 242, 243).
Signals from ACTH are mediated via activation of the ACTHR (also known as the melanocortin 2 receptor or MC2R) (244). ACTHR mRNA was detected in the HFA as early as 8 wk post-conception (9). In our previous study using in situ hybridization, ACTHR mRNA expression levels, as judged by hybridization signal intensities, were highest in the outermost zones (i.e., the DZ) and decreased in the more central areas of the HFA at midgestation (245). Similar results were reported in the baboon fetal adrenal gland (240, 246). Although these results suggest that DZ cells may be more responsive to ACTH, both in vivo and in vitro studies indicate that the DZ cells of human and nonhuman primate fetal adrenals are not responsive to ACTH, as stated above (1, 241). A possible explanation is that signal intensities generated by in situ hybridization can be influenced by cell density and size. Because cells in the DZ are more tightly packed and much smaller than in the FZ, this could affect hybridization signal intensities. In a more recent study, we revisited this issue, using laser capture microdissection coupled with real-time quantitative RT-PCR, and found that ACTHR mRNA is higher in the FZ than in the DZ of the midgestation HFA (133), indicating the importance of using more sensitive, and quantitatively and spatially accurate techniques. Mutations of the ACTHR gene account for approximately 25% of cases with the inherited disease, familial glucocorticoid deficiency (FGD) (247, 248). More recently, MC2R accessory protein (MRAP), a novel interacting partner of ACTHR that is essential in the trafficking of ACTHR to the cell surface (248, 249), was identified, and mutations of the MRAP gene are found to be responsible for 15–20% of cases with FGD (248). FGD is characterized by glucocorticoid deficiency in the absence of mineralocorticoid deficiency. Patients with FGD typically present with hypoglycemic seizures, hyperpigmentation, recurrent infections, failure to thrive, collapse, and coma during the neonatal period or childhood. The adrenal glands in such patients usually are small in size. In most cases, adrenal glands appear to show a failure of development of the zona fasciculata and reticularis, consistent with the glucocorticoid deficiency (247). Intriguingly, there is an analogy between the adrenal histological appearance of FGD and the glucocorticoid-treated late-gestation baboon fetuses as stated above. However, the direct effects of the lack or deficiency of ACTH actions associated with FGD on fetal adrenal development remain unclear.
It has been well-established that ACTH, via binding to the ACTHR, increases intracellular cAMP that activates protein kinase A. The actions of ACTH can be mimicked by cAMP analogs or substances that increase adenylate cyclase activity such as forskolin. The importance of cAMP-protein kinase A pathway also holds true for most of the actions of ACTH in human fetal adrenocortical cells. However, the signaling events downstream from the activation of protein kinase A remain unclear. The contribution of other signaling pathways that mediate the ACTH actions in adrenal cortical cells has been reviewed elsewhere (250, 251).
2. Responsiveness to ACTH: the ACTH paradox
Although ACTH appears to be the principal regulator of HFA cortical development, circulating concentrations of ACTH in the human fetus at midgestation, when the HFA grows rapidly, are relatively low, and ACTH levels in the fetal circulation do not demonstrate a surge during late gestation (77, 252) when cortisol production escalates (76–78). Winters et al. (252) found that circulating ACTH levels in the human fetus decrease at term, whereas a study by Lockwood et al. (77) found a modest increase in plasma ACTH levels in fetal blood collected by percutaneous umbilical blood sampling. More recently, Florio et al. (253) reported that the mean plasma ACTH concentration in umbilical cord blood collected immediately after term birth was 37.25 ± 5.16 pg/ml, which is even lower than the mean of 143 ± 7 pg/ml reported by Winters et al. (252) for term infants. Thus, the rapid growth and profuse steroid production by the HFA cortex are not paralleled by increases in fetal plasma ACTH.
One possible explanation for this “ACTH paradox” is that there may be gestational age-dependent alterations in responsiveness of the fetal adrenals to ACTH. Indeed, studies in fetal sheep showed that adrenal responsiveness to ACTH, presumably through expression of the ACTHR (254, 255), increases during the second and third trimesters (254–259). However, a study in fetal baboons demonstrated a biphasic monomodal developmental ACTHR expression in the adrenal, in which fetal adrenal ACTHR mRNA levels increased approximately 13-fold at midgestation, then decreased by 70% in late gestation (260). In human fetuses, it remains elusive as to whether and how the adrenal expression of ACTHR or adrenal responsiveness to ACTH alters as gestation advances. In this context, a recent in vitro study of Rehman et al. (261) provides insight. They demonstrated that CRH directly up-regulates ACTHR expression in isolated DZ/TZ cells from the HFA. Therefore, the late-gestational increase of placenta-derived CRH would directly stimulate fetal adrenal ACTHR expression and fetal adrenal responsiveness to ACTH, thereby enhancing cortisol and DHEAS synthesis in the HFA (Fig. 5). However, in this paradigm, the reason that increased CRH does not stimulate ACTH secretion by the fetal pituitary remains to be clarified. The same group also demonstrated that simultaneous administration of CRH and ACTH, both in physiological concentrations, results in a synergy in stimulating cortisol secretion (262), ACTHR expression, and I125-labeled ACTH binding (261). Other candidates that may regulate fetal adrenal ACTH responsiveness include IGF-II, FGF-2, and AT-II. In isolated human adrenal FZ cells, neither IGF-I nor IGF-II altered ACTHR expression, although responsiveness to ACTH, cAMP, or forskolin was increased (208), indicating that IGFs modulate the ACTH signal transduction pathway at some point distal to ACTHR binding and activation. The effects of IGFs on ACTHR expression in DZ or TZ cells have not been investigated. Our preliminary data indicate that FGF-2 up-regulates ACTHR mRNA in isolated DZ cells of the HFA (263). Alternatively, there may be a suppressor during midgestation that inhibits ACTHR expression and/or responsiveness to ACTH because the HFA in vivo is constantly exposed to circulating ACTH that up-regulates its own receptor ACTHR in vitro (245, 264). In ovine adrenocortical cells, TGF-β decreases the number of ACTH binding sites (265). In our hands, preliminary results indicate that midkine, a heparin-binding growth factor that is much more abundantly expressed in the HFA than in the adult adrenal (132), down-regulates ACTHR mRNA expression in cultured DZ and FZ cells (263). In addition, a recent study by Roy et al. (266) provided evidence that human α- and β-isoforms of MRAP, a novel interacting partner of ACTHR that is essential in the trafficking of ACTHR to the cell surface (248, 249), modulate ACTH binding and ACTH-induced cAMP production and differentially regulate functional properties of ACTHR (266). Although MRAP is expressed in the human adrenal gland (267), its localization and role in the HFA have not been explored.
Fig. 5.
Schematic representation of hypothetical model describing endocrine cascades in human feto-placental unit. Near term, placenta-derived CRH increases and directly stimulates HFA production of DHEA/DHEAS and cortisol. Increased cortisol, in turn, stimulates production of placental CRH. CRH also up-regulates expression of the ACTHR, thereby increasing HFA responsiveness to ACTH, the level in fetal circulation of which may be constant or decrease near term. HFA and placental CRH constitute positive feedback cascade. DHEA/DHEAS is converted by placenta to estrogen, promoting initiation of parturition partly through up-regulation of contraction-associated proteins. Cortisol also promotes maturation of fetal organs (e.g., the lung) and stimulates production of prostaglandins, uterotonins necessary for parturitional processes. CRH also directly promotes initiation of parturition in part by increasing prostaglandins. When “functional withdrawal” of progesterone occurs, coupled with these changes, parturition is initiated.
Alternatively, the “ACTH paradox” may be explained by the presence of regulators for fetal adrenal cortisol synthesis other than ACTH. Estrogens produced by the placenta may decrease ACTH-induced cortisol synthesis, as was discussed in Section III.F. The expression and regulation of HSD3B2, the key steroidogenic enzyme for cortisol biosynthesis was discussed separately in Section II.D.
3. Pituitary POMC-derived peptides
Although ACTH is not a mitogen for adrenocortical cells in vitro (268, 269), the pituitary ACTH appears to stimulate proliferation of adrenocortical cells in vivo. One likely possibility is that proliferative actions of ACTH may be mediated by other factors, such as growth factors, as was discussed in Section III.E. Alternatively, pituitary POMC-derived peptides (270) other than ACTH could be involved in fetal adrenal growth and development (271). Knockout of the POMC gene in mice resulted in the lack of normal adrenal gland structure (272, 273). Early studies (274, 275) demonstrated adrenal proliferative activity of peptides derived from the N terminus of POMC. Although human POMC (1–76), the major N-terminal POMC peptide secreted by pituitary corticotrophs, had no mitogenic effect on the adrenals (276), shorter N-terminal POMC peptides had tropic activity. In vivo studies in the sheep fetus showed that administration of POMC (1–77) stimulates fetal adrenal growth, whereas it neither affects nor limits adrenal steroidogenesis (277, 278). The tropic actions may be secondary to either direct action of N-terminal POMC (1–77) or a consequence of its proteolytic cleavage. Of particular note in this context is the recent identification of an adrenal serine protease (termed adrenal secretory protease) in the rat adrenal cortex, which cleaves rat POMC to generate a small peptide and is up-regulated at the outer zona fasciculata/glomerulosa boundary (i.e., the site of proliferation) in adrenals undergoing compensatory growth (279). The roles of POMC-derived peptides and adrenal secretory protease in HFA development are yet to be elucidated.
B. Angiotensin-II (AT-II)
AT-II, along with ACTH, has been considered to regulate HFA development. However, currently available data that characterize the functions of AT-II in the HFA are limited compared with those for ACTH.
AT-II, the end-product of the renin-angiotensin system, is a key player in the regulation of blood pressure. By acting as a vasoconstrictor, AT-II increases blood pressure. It also stimulates aldosterone synthesis and secretion by the zona glomerulosa (280, 281). AT-II signals through the two main receptor subtypes, AT1 and AT2. An in situ hybridization study showed that the AT1 receptor mRNA can be detected in the DZ at 8 wk and throughout gestation (282). The expression of AT2 mRNA occurs earlier than that of AT1 mRNA, and is detected at 5–6 wk in a mass of condensed cells that represent the FZ. Autoradiographic receptor binding studies with the use of specific antagonists for each receptor subtype indicate that the main AT-II receptor subtype in the HFA is the AT2 receptor (283). AT2 localizes in the entire FZ in this study in which the earliest adrenal tested was from a 14-wk fetus. AT1 is detected after 16 wk gestation at the periphery of the gland.
Chamoux et al. (284) demonstrated that activation of the AT2 receptor induces apoptosis in isolated FZ cells, suggesting an important role for AT-II in the apoptotic activity observed in vivo in the central part of the HFA. AT2 expression disappears from most tissues after birth, except for the adrenal gland, and is higher in HFAs than in adult adrenals (99), indicating the involvement of AT2 signaling in the development of the HFA. The role of the AT1 receptor in the HFA remains to be clarified. Our preliminary studies show that treatment with AT-II increased ACTHR mRNA in a dose- and time-dependent manner, consistent with a previous study (264). Such increase in ACTHR mRNA was blocked by addition of candesartan, an AT1-selective antagonist, but not by PD123319, an AT2-selective antagonist, suggesting involvement of the AT1 receptor subtype in AT-II-stimulated up-regulation of ACTHR gene expression (285).
AT-II may regulate HFA steroidogenesis. In vitro studies have shown that AT-II enhanced secretion of DHEAS and cortisol increased responsiveness to ACTH, and up-regulated mRNAs encoding CYP11A and CYP17 in isolated human fetal adrenocortical cells (264). Attempts have been made to identify genes that can be regulated by AT-II in adrenocortical cells using microarray analysis, which have recently been reviewed (286). Future studies could include roles of these AT-II-inducible genes in human fetal adrenocortical cells.
V. The Fetal Adrenal Cortex in the Human Feto-Placental Unit
Steroid hormones produced by the HFA, along with progesterone produced by the placenta, play key roles in the maintenance of pregnancy, intrauterine homeostasis, fetal maturation, and the initiation of parturition. The FZ, a unique feature of fetal adrenals in primates, is a site of abundant production of the adrenal androgens DHEA/DHEAS, which serve as a source of C19 steroids for placental estrogen production. Late in gestation, the HFA also secretes cortisol and may produce aldosterone. Studies to date, including experiments of nature, clinical trials, and research in materials from human and nonhuman primates, are beginning to reveal the physiological roles of these steroid hormones in the feto-placental-maternal unit. Additionally, mounting evidence indicates that placental CRH plays a key role in the endocrine cascades, constituted by steroid hormones and other mediators, which leads to the initiation of human parturition.
A. Glucocorticoids: roles in fetal maturation and parturition
The timely birth of an appropriately mature fetus requires a mechanism that synchronizes fetal maturation with initiation of parturient processes. Glucocorticoids are indispensable for cell differentiation and organ maturation in the fetus. In sheep, fetal organ maturation is induced by a prepartum surge of cortisol secretion by the fetal adrenal gland, which also triggers the onset of labor (287, 288). Thus, in sheep, the fetal HPA axis, via cortisol, synchronizes fetal maturation with timing of parturition. In humans, a similar rise of cortisol in the fetal circulation is observed in late gestation (76, 78). However, the roles of the fetal HPA axis and cortisol in human fetal maturation and initiation of parturition remain enigmatic. In this section, possible implication of glucocorticoids in the initiation of human parturition, the beneficial and adverse effects of glucocorticoids, and local glucocorticoid metabolism will be addressed, all of which have received a great deal of attention recently.
1. The length of gestation and glucocorticoids
Pregnancy length may be modulated in conditions in which the fetal HPA axis is disrupted and fetal cortisol synthesis is markedly decreased [e.g., congenital adrenal hyperplasia (CAH) and anencephaly]. Conflicting data exist regarding the length of gestation in patients with CAH. In 1971, Price et al. (289) studied 19 children with CAH due to CYP21 deficiency from 12 families and reported that the length of gestation was not significantly different in their affected cases compared with unaffected siblings. More recently, Gidlöf et al. (290) studied 66 patients with CYP21 defects whose exact number of gestational days were available and found a correlation between gestational age and CYP21 genotype in female, but not male, fetuses with CAH. Females with the most severe form of the disease, a null mutation, had the longest gestation and differed significantly from the normal population (290). Another recent study from the United Kingdom has shown that the frequency of postterm delivery was significantly higher in 31 children with CAH due to CYP21 deficiency than their general or regional frequency (291). Thus, the possible association with CAH and prolonged gestation is still open for debate. In anencephalics, pregnancy does not appear to be prolonged, on average, although labor occurs over a wide time interval (237, 239, 292). In addition, one must be aware that interpretation of data from anencephalic pregnancies is confounded by the influence of polyhydramnios, which causes increased stretch of uterine muscle.
In 1979, Katz et al. (293) reported that intraamniotic glucocorticoid administration resulted in delivery, especially in postterm pregnant women. Administration of synthetic glucocorticoids in women at risk of preterm labor is associated with transient increases in uterine activity (294, 295). In the rhesus monkey, a nonhuman primate model, treatment with dexamethasone does not induce premature delivery as it does in sheep, but instead results in prolonged pregnancy (296). Interpretation of this study is complicated by the possibility that maternal glucocorticoid treatment may have inhibited the fetal HPA axis, and therefore the feto-placental unit.
Thus, due to limited data available from experiments of nature, other clinical data, and nonhuman primate models, it is probably still unsafe to assume that fetal glucocorticoids play a direct causative role in the initiation of human parturition.
2. Fetal maturation
Several lines of research strongly support a key role for glucocorticoids in promoting maturation of human fetal organs before birth (297, 298). The effect of glucocorticoids on the maturation of the fetal lungs is of particular importance. Infant respiratory distress syndrome, an inability to exchange gases due to pulmonary immaturity, is the leading cause of morbidity and mortality of preterm infants. Maternal administration of synthetic glucocorticoids that readily cross the placenta markedly decreases the severity of infant respiratory distress syndrome and significantly improves prognosis of infants delivered preterm. However, experiments of nature suggest that human fetal maturation is independent of fetal adrenal cortisol production. In fetuses with CAH due to CYP21 deficiency, the adrenals produce markedly reduced amounts of cortisol, yet these fetuses are born without any signs of organ immaturity (289). Several possibilities may account for this discrepancy. One explanation is that reduced levels of cortisol produced by the HFA in fetuses with CAH can be compensated by another source of cortisol, possibly from the maternal adrenals. As discussed in Section V.A.3, transplacental passage of maternal cortisol could rise late in gestation in association with decreased placental type 2 11β-hydroxysteroid dehydrogenase (HSD11B2) activity during that period. Alternatively, local glucocorticoid metabolism in human fetal organs may significantly modulate in situ effects of cortisol. In this scenario, altered local glucocorticoid metabolism could compensate for effects of cortisol that is reduced in fetuses with CAH (299). In addition, maturation of the human fetus may not be dependent on glucocorticoids alone; other factors may be involved. Clearly, these possibilities have important implications for future directions of study.
3. HSD11B in human placenta, intrauterine membranes, and fetal tissues
The actions of glucocorticoids are modulated by two distinct isozymes of the enzyme 11β-hydroxysteroid dehydrogenase, HSD11B1 and HSD11B2. HSD11B1 functions predominantly as a reduced nicotinamide adenine dinucleotide phosphate-dependent reductase to generate active cortisol, although it possesses both oxidase (cortisol to cortisone) and reductase activities. In contrast, HSD11B2 is a high affinity oxidized nicotinamide adenine dinucleotide-dependent enzyme that exhibits oxidase activity, inactivating cortisol to cortisone (300).
Cortisol in the human fetus can be derived from the HFA, from the mother by transplacental transfer that is under the control of HSD11B2 activity, or from local conversion from cortisone by HSD11B1 activity within the chorion trophoblasts and amnion epithelium.
For most of gestation, the human placenta expresses HSD11B2 that inactivates cortisol. In human pregnancy, maternal cortisol levels are five to 10 times higher than those of the fetus (301, 302). Thus, the placenta, most likely via HSD11B2, serves as a biochemical barrier, preventing excess maternal cortisol from entering the fetal compartment (303, 304). Concordant with this notion, HSD11B2 has been localized at the feto-maternal interface. Indeed, immunohistochemical studies have localized HSD11B2 to syncytiotrophoblast, invasive extravillous trophoblast, and trophoblasts lining the spiral arteries (305–307). HSD11B2 mRNA is not found in the amnion, chorion, or decidua (308). Conflicting results exist concerning the ontogeny of human placental HSD11B2 expression and activity; placental HSD11B2 expression and activity may decrease or increase as gestation advances (309).
HSD11B2 appears to be regulated by various placental hormones and factors. In vitro studies indicate that inducers of expression and/or activity of HSD11B2 include prostaglandin-E2, prostaglandin-F2α, retinoic acid, protein kinase A activators (e.g., forskolin), and oxygen tension, whereas factors that decrease its expression and/or activity include nitric oxide donors, leukotriene B4, catecholamines, and calcium (300, 309). Intriguingly, placental steroids can modulate HSD11B2 expression and/or activity in the placenta. Progesterone has been found to reduce HSD11B2 activity via a non-receptor-mediated mechanism and concomitantly decrease mRNA encoding HSD11B2 through a progesterone-receptor-mediated mechanism in human syncytiotrophoblast cells (310). Similarly, progesterone decreases HSD11B2 activity in human and baboon placental homogenates (311). In contrast, the role of estrogen in regulating placental HSD11B2 still remains elusive. In vivo studies in baboons (312–314) suggest that estrogen increases HSD11B activity, whereas estradiol inhibits HSD11B2 oxidase activity in cultured human placental trophoblasts (310).
HSD11B1 protein has been localized to chorionic trophoblasts, amniotic epithelial cells, scattered amnion mesenchymal cells, the endothelium of placental and umbilical cord blood vessels, and the decidua (307, 308, 315). Immunoreactive HSD11B1 has also been found in syncytiotrophoblasts of human and baboon placentas (316, 317). Further studies should elucidate the extent to which HSD11B1 in the human placenta is functional because it contains predominantly HSD11B2 enzyme (308, 318). With advancing gestation, both activity and expression of HSD11B1 in the fetal membranes increase gradually in late gestation and further at the time of labor (317, 319). Thus, this possible local production of cortisol by HSD11B1 may play a role in different pathways contributing to the regulation of parturition. Alternatively, HSD11B1 may amplify the local actions of cortisol on the fetal membranes.
Recent in vitro studies have shown that glucocorticoids can stimulate expression of HSD11B1 in human chorionic trophoblasts and amnion fibroblasts (320–322), thereby providing positive feed-forward regulation of HSD11B1 and increasing the local concentration of glucocorticoids.
Furthermore, expression of HSD11B1 and/or HSD11B2 has been found in human fetal tissues including the lung and adrenal gland (323, 324). Studying regulation and function of these glucocorticoid-metabolizing enzymes in such tissues may be a fruitful avenue of future research, leading to better understanding of the roles of glucocorticoids in human fetal development and parturition (299, 323).
4. Glucocorticoids for the fetus: friend or foe?
Glucocorticoids are a “two-edged sword” for the fetus; they promote maturation of organs required for extrauterine survival while they can exert deleterious effects on fetal growth and postnatal development.
A single course of synthetic glucocorticoids is a universally accepted, standard therapy for pregnant women at risk for preterm delivery, which reduces morbidity and mortality in preterm infants by accelerating fetal lung maturation (325, 326). However, multiple courses of antenatal corticosteroid administration may be associated with decreased weight, length, and head circumference at birth (327), suggesting that glucocorticoids have the potential to reduce fetal growth. Synthetic glucocorticoids may affect the HPA axis of the fetus and neonate, as recently reviewed (326).
Because placental HSD11B2 serves as a barrier that prevents maternal glucocorticoids from entering the fetal compartment, decreased HSD11B2 activity in the placenta could lead to an increased amount of maternal glucocorticoids crossing into the fetal compartment, possibly with deleterious effects on fetal development. Apparent mineralocorticoid excess, a congenital disease that results from mutations of the HSD11B2 gene, is associated with moderately reduced fetal growth (328, 329). Reductions in placental HSD11B2 activity or expression have been associated with reduced human fetal growth (309). In addition, a more recent study of Dy et al. (330) suggests that not only placental, but also fetal HSD11B2 activity may be compromised in idiopathic intrauterine growth restriction. Reduced HSD11B2 expression and activity (331, 332), coupled with detectable placental cortisol (332), also are observed in preeclampsia, suggesting that fetal glucocorticoid excess could contribute to the restricted fetal growth that is frequently seen in preeclamptic patients.
Recently, a concept termed “fetal programming” has emerged. A premise of this concept is that adverse influences in utero increase the risk of developing disease in adult life (309, 333). Fetal exposure to excess glucocorticoids may be a link between reduced fetal growth and adult disease. There is much focus on this subject, and an increasing number of studies have been undertaken in recent years. Detailed discussion of fetal programming is beyond the scope of this review. Readers are encouraged to read recent reviews (309, 333, 334).
5. Glucocorticoids and prostaglandins
Prostaglandins are potent endogenous uterotonins that are responsible for parturition. Glucocorticoids stimulate prostaglandin production in human fetal membranes and placenta (335–338), although glucocorticoids inhibit prostaglandin formation in most other tissues. This paradoxical, proinflammatory effect of glucocorticoids involves up-regulation of the key enzyme in prostaglandin biosynthesis, cyclooxygenase 2 (COX2; also known as prostaglandin H synthase 2 or prostaglandin-endoperoxide synthase 2) and suppression of the prostaglandin-inactivating enzyme, 15-hydroxyprostaglandin dehydrogenase (336, 337, 339–344). Additionally, prostaglandins induce HSD11B1 expression and activity within the placenta (345). As discussed above, glucocorticoids increase production of prostaglandins and up-regulate HSD11B1, which activates glucocorticoids, thereby creating a paracrine cause-and-effect spiral that may be involved in human parturition (for reviews, see Refs. 287 and 342). The extent to which this putative feed-forward spiral is operative in vivo remains to be elucidated.
Recently, Sun et al. (346) have proposed a different mechanism by which glucocorticoids may indirectly promote prostaglandin production in the fetal membranes. They demonstrated that surfactant protein A localizes in human amniotic epithelial cells, fibroblasts, and chorionic trophoblasts, and that glucocorticoids induce expression of surfactant protein A, which in turn stimulates prostaglandin E2 synthesis in cultured chorionic trophoblasts (346).
B. Progesterone and estrogens: roles in parturition
Progesterone and estrogens play pivotal roles in maintaining pregnancy and regulating parturition. Generally, progesterone maintains pregnancy by promoting uterine quiescence. In contrast, estrogens oppose the relaxing actions of progesterone and promote myometrial contractility of the uterus and regulate cervical ripening to facilitate parturition.
The essential role of progesterone in maintaining pregnancy is well-established and remarkably universal. Any disruption of progesterone synthesis or action during pregnancy rapidly induces abortion or delivery (347). Progesterone withdrawal is a crucial event in the parturitional processes in most mammals. However, in humans and higher primates, progesterone levels remain elevated throughout pregnancy, and labor is not initiated by a decline in circulating progesterone levels. Thus, progesterone withdrawal in these species is likely to be mediated by mechanisms other than decreased exposure of target tissues to circulating progesterone. In this context, the notion of “functional progesterone withdrawal” was first proposed by Csapo and Pinto-Dantas in 1965 (348) and has been supported by other investigators. Possible underlying mechanisms of functional progesterone withdrawal, including changes in local progesterone metabolism and altered tissue responsiveness for progesterone, have been described in detail in recent reviews (349–353).
Progesterone exerts genomic actions through the classic nuclear progesterone receptors (PRs) that act as ligand-activated transcription factors. The human nuclear PR gene encodes two receptor subtypes, the full-length PR-B and the truncated PR-A (349, 354, 355). In general, PR-B is an activator of transcription, whereas PR-A is a transcriptional repressor (354), and PR-A represses the transcriptional activity of PR-B (356). The induction of labor and delivery by RU486 (347), a nuclear PR-specific antagonist, likely reflects the importance of genomic actions mediated via nuclear PRs for the maintenance of myometrial relaxation during pregnancy. Progesterone maintains pregnancy primarily by directly inhibiting expression of genes encoding contraction-associated proteins (CAPs) including the oxytocin receptor, the prostaglandin-F2α receptor, the gap-junction protein connexin-43, and the prostaglandin-metabolizing enzyme 15-hydroxy-prostaglandin dehydrogenase (357).
Estrogen is considered to oppose the actions of progesterone by stimulating biochemical and physical changes in the uterus and fetal membranes (232, 357). Estrogen stimulates uterine contractions by increasing myometrial excitability and responsiveness to uterotonins such as oxytocin. In the uterine cervix, estrogen induces expression of proteolytic enzymes that degrade the ECM to allow cervical maturation and dilation.
In most species, maternal estrogen levels rise before delivery. Similarly, in humans, the rise of estrogens occurs gradually over the final weeks of pregnancy (358, 359) and is dependent on fetal adrenal steroidogenic activity. Of interest, the pattern of increase in placental estrogens in human pregnancy resembles that of the parturition-initiating fetal cortisol in pregnant sheep.
Conditions with markedly reduced synthesis of placental estrogen (e.g., placental sulfatase deficiency and placental aromatase deficiency) have been considered to illustrate the role of placental estrogens in human pregnancy. Pregnancies affected with placental sulfatase deficiency usually show normal fetal and placental development and normal timing of parturition (360). The majority of pregnancies with placental aromatase deficiency deliver vaginally at term, after spontaneous onset of labor, although such pregnancies are associated with virilization of the mother and female fetus (361–365). These findings suggest a supportive, rather than a critical, role for placental estrogens in regulating human pregnancy and parturition. However, it is known that maternal estrogen levels in those cases of suppressed placental estrogen synthesis, albeit low compared with normal pregnancies, are still in a physiologically significant range and are comparable to levels reached in the midcycle and luteal phase of the menstrual cycle, thereby allowing the target tissues to be exposed to moderately high levels of estrogens. Thus, an implication drawn from these experiments of nature would be that the estrogen levels observed in normal pregnancies far exceed the amounts required to influence parturition. The reason that the feto-placental unit produces such high levels of estrogens through most of pregnancy remains unknown.
Studies in primates support the critical role of placental estrogen in parturition. In the pregnant rhesus monkey, Nathanielsz and colleagues (366, 367) found that increased placental estrogen production, secondary to androstenedione administration, stimulated myometrial contractility and induced preterm delivery. They further showed that infusion of the aromatase inhibitor 4-hydroxyandrostenedione attenuated androstenedione-induced parturition (367). However, peripheral estrogen infusions stimulated myometrial activity but did not cause preterm delivery. Other investigators, in an earlier study, also showed that estradiol treatment alone did not induce preterm delivery in the rhesus monkey, although circulating estradiol levels were markedly elevated (296). Therefore, local production of estrogens from androgen precursors, rather than circulating estrogen levels, may be critical for the initiation of parturition in the rhesus monkey. These data may be applicable to humans, and the actions of estrogen in human pregnancy may be mediated by mechanisms other than changes in circulating estrogen levels. Thus, a likely possibility is that the action of estrogen, as well as that of progesterone, is more regulated by target tissue responsiveness or local metabolism than by circulating estrogen levels.
Extensive research has been undertaken to characterize the molecular mechanism that regulates tissue responsiveness to progesterone and estrogen, and thereby their actions in parturition. Mesiano and coinvestigators (357, 368) have proposed a model that explains how functional progesterone withdrawal and estrogen activation occur in the initiation of human parturition, based on several key findings: 1) ERα mRNA is increased in laboring term myometrium; 2) myometrial ERα mRNA levels positively correlate with the PR-A/PR-B mRNA ratio; and 3) progesterone inhibits myometrial ERα expression. In their scenario, functional progesterone withdrawal (mediated via increased PR-A expression) induces functional estrogen activation (mediated via increased ERα expression), stimulating CAP expression and causing transformation of the myometrium from a quiescent to a contractile state, thereby leading to labor onset.
C. CRH and the human fetal adrenal cortex
CRH was first identified in the hypothalamus (369). CRH stimulates the expression and processing of POMC by pituitary corticotropes and the secretion of ACTH. The human placenta, fetal membranes, and decidua also express CRH that is identical to hypothalamic CRH. CRH is an ancient molecule with remarkable conservation among species (370). However, placental CRH production is restricted to primates.
Placental CRH may be part of the feto-placental stress response machinery. The placenta is commensurate with the hypothalamus in its production of CRH in response to stress. Placental CRH production appears to increase in various situations associated with fetal stress, including intrauterine growth restriction, preeclampsia, and nonreassuring fetal status (371–375). A physiological implication of these findings is that the fetus may be able to mount a stress response through placental CRH.
An increasing number of studies have shown that placental CRH is associated with gestational length and/or the initiation of parturition in humans (376, 377). McLean et al. (376) demonstrated that maternal plasma CRH levels showed an exponential rise from midgestation to term and that women who delivered preterm had a more rapid rate of increase that was detectable from the early second trimester. In contrast, women who delivered postterm had a slower rate of CRH rise that was detectable in advance. These data indicate that maternal plasma CRH levels are predictive of those women who are destined to have normal term, preterm, or postterm delivery. The authors proposed that placental CRH acts as a biological clock that determines the length of pregnancy. Similar results have been reported by other investigators (377, 378).
In feto-maternal tissues, CRH is presumed to exert diverse effects that include modulated secretion of bioactive molecules (e.g., prostaglandins, oxytocin, progesterone, and ACTH) (378), control of feto-placental circulation (378), and pregnant-stage dependent modulation of myometrial contractility. As for the latter, CRH may prevent premature myometrial contractions while it may increase myometrial contractility at term (377, 379). Actions of CRH may be regulated by mechanisms including receptor subtype and its coupling to signaling pathways, and the availability of bioactive CRH (e.g., by CRH-binding protein), as reviewed elsewhere (378).
Unlike hypothalamic CRH, which is under the negative feedback control of glucocorticoids, human placental CRH gene expression and production can be stimulated by glucocorticoids (380–382). Placental CRH production can also be regulated by inhibitory (e.g., progesterone and nitric oxide) and stimulatory (e.g., IL-1, prostaglandins, oxytocin, and AT-II) factors (383–387). Additionally, a variety of neurotransmitters and neuropeptides, which can be activated in response to stress, stimulate placental CRH release in vitro (378, 388, 389).
Placental CRH predominantly enters the maternal circulation, but it is also released into the fetal circulation (390). CRH concentrations increase as gestation advances in the human fetal circulation, although they are lower than those in the maternal circulation (391). The HFA gland, as well as the fetal pituitary, expresses CRH receptors (392–395). Transcripts encoding two isoforms (CRH-R1α and -R2α) have been detected in the HFA (394, 395).
The remarkable increase in placental CRH production at the end of gestation and the capacity of glucocorticoids to enhance this expression have led several investigators to propose a key role for placental CRH in the feto-placental unit and in parturition. Robinson et al. (380) suggested that the marked rise in placental CRH preceding parturition could result from the rise in fetal corticoids that occurs at this time. They also suggested that the increase in placental CRH may stimulate, through fetal ACTH, a further increase in fetal glucocorticoids, “completing a positive feedback loop that would be terminated by delivery.” However, the steroid production by the HFA cortex is not paralleled by increases in fetal plasma ACTH; the surge of cortisol production seen in late gestation occurs despite falling or modestly increasing ACTH levels in the fetal circulation. A late gestational increase in fetal adrenal responsiveness to ACTH could explain this paradox (see Section IV.A.2). Alternatively, the HFA may be under direct control of placental CRH. Indeed, CRH can directly stimulate cortisol and DHEAS synthesis by isolated human fetal adrenocortical cells (262, 393, 395, 396). Furthermore, CRH also can stimulate expression of the ACTHR in isolated definitive/TZ cells from the HFA (261). This finding suggests that placental CRH could directly affect fetal adrenal responsiveness to ACTH, thereby indirectly promoting fetal adrenal steroidogenesis in late gestation despite the limited availability of ACTH in the fetal circulation.
Therefore, a positive endocrine loop may develop between placental CRH and the HFA (Fig. 5). In this paradigm, cortisol derived from the HFA increases placental synthesis of CRH, which in turn stimulates the HFA to produce more cortisol and DHEA/DHEAS. As a consequence, fetal adrenal cortisol increases and placental estrogen synthesis increases. Increased amounts of estrogens stimulate CAP gene expression and transform the myometrium from a quiescent to a contractile state, preparing for successful uterine contractions and parturition. Cortisol promotes maturation of fetal organs such as the lung. The increased CRH potentiates fetal adrenal responsiveness to circulating ACTH, further driving the fetal adrenal production of cortisol and DHEA/DHEAS. Cortisol also stimulates production of prostaglandins to initiate parturition. This positive feed-forward endocrine loop ends when the placenta is expelled at the end of labor.
VI. Summary and Future Perspectives
Development and function of the HFA cortex are unique among mammalian species. Studies in transgenic and knockout mice have made a major contribution to our understanding of the development of various organs and tissues. However, this uniqueness of the HFA somewhat limits extrapolation of data from mouse models to humans. Therefore, the biology and physiology of the HFA have been gradually elucidated, mostly through studies of patients with adrenal developmental disorders, of nonhuman higher primate models, and of HFA tissues. A recent series of investigations, which strongly indicates early cortisol synthesis in the HFA, is such an example and has been one of the major achievements in this field (9). Additionally, use of modern technologies, including gene array methodologies and laser capture microdissection, have revealed factors that were not implicated previously in HFA development and function. The precise roles of these factors and interplay between them remain to be elucidated.
The HFA basically consists of two zones, the outer DZ and inner FZ. DZ cells, which are small and round, have structural characteristics typical of cells in an undifferentiated proliferative state, whereas FZ cells have those typical of differentiated, steroidogenic cells. Recent studies suggest several possible mechanisms by which DZ cells maintain their in vivo phenotype. One possible mechanism is by a growth factor that exerts DZ-selective proliferative effects. Second, there may be gradients of growth or differentiation signals that can regulate cell proliferation and/or differentiation. The undifferentiated proliferative phenotype of DZ cells supports the concept that the DZ contains a pool of progenitor cells. Thus, current data strengthen the tenet that the HFA cortex is a dynamic organ in which cells proliferate in the periphery (i.e., the DZ) and may migrate centripetally, differentiating to form the remainder of the cortex. Recent investigations have identified cellular markers unique to each zone of the HFA (Fig. 4). Identification of these markers would enhance the ability to characterize the proliferative potential of DZ cells and assess their capacity to differentiate into cells of the TZ and FZ.
The development of a vasculature is necessary for support of the rapid organ growth and high endocrine activity of the HFA. Angiogenesis appears to occur at the periphery of the midgestation HFA, as evidenced by predominant endothelial cell proliferation in the DZ and TZ (53). Thus, the DZ likely benefits from a more plastic vascular state to accommodate its proliferative phenotype. Consistent with this, several major proangiogenic factors demonstrate the outer-zone predominant expression. In addition, expression of these factors is under control of ACTH (92, 94, 95), supporting a concept that ACTH coordinates adrenal organ growth and angiogenesis (95, 397).
ACTH secreted by the fetal pituitary is the primary regulator of the development and function of the HFA. However, the rapid growth and profuse steroid production by the HFA cortex, during mid- and late gestation, are not paralleled by elevations in fetal plasma ACTH. Thus, peptide growth factors produced locally within the HFA may partly mediate the actions of ACTH in an autocrine and/or paracrine fashion (1, 132). Alternatively, placenta-derived factors, such as CRH and estrogens, may be involved by an ACTH-independent mechanism.
Genetic studies in mice and humans are beginning to unravel the mechanisms of transcriptional regulation responsible for organogenesis of the HFA. Among the several transcription factors implicated in adrenal development, SF1 and DAX1 have been well characterized and appear to be essential in cortical and functional development of the HFA (152, 153). However, their precise roles remain to be clarified.
The steroid hormonal milieu in mammals undergoes substantial changes in both the maternal and fetal circulations as parturition approaches. Near term, there is a sharp rise in estrogens in the maternal circulation in many mammalian species. However, in humans, estrogen rises gradually throughout pregnancy, without a surge in the last weeks of pregnancy. Intriguingly, circulating estrogen levels during pregnancy are 10–100 times those of nonpregnant women throughout most of gestation. Despite these relatively high levels of estrogen, the myometrium, fetal membranes, and cervix appear refractory to estrogen action. Therefore, factors other than elevated circulating estrogen levels likely are involved. These may include changes in target tissue sensitivity through alteration of ER expression, a change in the relative amounts of ER isoforms, changes in signal transduction, and/or changes in estrogen metabolism, conjugation, or clearance. In most species, parturition is anteceded by a fall in progesterone, the essential hormone that maintains pregnancy. However, in human pregnancy, there are no changes in circulating progesterone levels preceding labor. Consequently, the concept of “functional progesterone withdrawal” emerges to explain this paradox. The precise roles of estrogen and progesterone in the physiology of human parturition remain enigmatic. However, if estrogen and progesterone regulate the human parturitional process, their actions must be mediated by mechanisms other than changes in their circulating levels.
Much of our understanding of human parturition has been built upon animal studies, particularly those in sheep. In that species, Liggins and coinvestigators (3, 4) demonstrated the importance of fetal cortisol, derived from the fetal adrenal cortex after activation of the fetal HPA axis, which both stimulates maturation of fetal organs for extrauterine existence and initiates the process of parturition, ensuring the timely birth of an appropriately mature fetus. In humans, however, the role of cortisol in the parturitional process is less clear, whereas its role in promoting fetal organ maturation appears likely. Cortisol in the human fetal compartment rises near term as in sheep. Mounting evidence indicates that cortisol may act as a “two-edged sword” for the fetus; it can promote maturation of fetal organs necessary for extrauterine life, whereas it can influence adversely fetal growth and postnatal development. Therefore, cortisol biosynthesis and metabolism in the feto-placental unit must be more strictly regulated than previously thought. Indeed, cortisol levels in the fetal compartment appear to be fine-tuned in a spatially and temporally regulated manner, through different mechanisms in the HFA (i.e., regulation of HSD3B2), placenta (i.e., control of HSD11B2 activity), and fetal membranes (i.e., control of HSD11B1 activity).
Endocrine systems that regulate the timing of parturition differ significantly between species. Placental CRH production is a major, unique adaptation that has evolved in anthropoid primates. Placental CRH is likely one of the key determinants of the timing of human parturition. As noted, placenta-derived CRH likely affects the HFA directly by stimulating its responsiveness to ACTH and secretion of cortisol and DHEA/DHEAS, precursors of placental estrogen. Cortisol, in turn, stimulates placental CRH production, forming a positive feedback cascade and generating further cortisol and estrogen near term. Increased estrogen, cortisol, and CRH itself, together with functional progesterone withdrawal, may contribute to the initiation of parturition (Fig. 5). Thus, in addition to the fetal HPA axis, a placental CRH-fetal adrenal axis may exist in regulating human parturition.
Several questions on development and function of the HFA remain to be resolved. Among the key questions are: What are the mechanisms underlying the suppressed HSD3B2 expression in the HFA? What are the master genes that regulate zone-specific gene expression of the HFA? How is the functional progesterone withdrawal related to endocrine interactions evoked by placental CRH, the HFA, and the maternal system? Lastly, but not least, why does the human fetus have huge adrenals with capabilities of robust biosynthesis of DHEA/DHEAS despite the fact that gene knockout experiments of nature indicate that estrogen produced by the feto-placental unit may be irrelevant to the maintenance of pregnancy, fetal development, or appropriately timed labor (398)? Future directions in this field likely would include studies that address these unanswered questions, as well as those on the roles of newly identified factors in the development and function of the HFA.
Acknowledgments
We thank Michiyo Ishimoto and Janice Wong for assistance with manuscript preparation. R.B.J. thanks Egon Diczfalusy in whose laboratory I was first introduced to human fetal endocrinology; Maria Serón Ferré, Sam Mesiano, and Hitoshi Ishimoto, who played such pivotal roles in our research; and the approximately 40 other talented scientists who joined and enriched our laboratory.
This work was supported in part by National Institutes of Health Grant HD08478 (to R.B.J.) and Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science no. 21591423 (to H.I.).
Disclosure Statement: The authors have nothing to disclose.
Footnotes
- ABC
- ATP-binding cassette
- ACTHR
- ACTH receptor
- Ang
- angiopoietin
- AT1
- angiotensin-II type 1 receptor
- AT2
- angiotensin-II type 2 receptor
- AT-II
- angiotensin-II
- CAH
- congenital adrenal hyperplasia
- CAP
- contraction-associated protein
- Cbp
- cAMP response element binding protein
- CITED2
- Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2
- COUP-TF
- chicken ovalbumin upstream promoter transcription factor
- CYP11A
- cytochrome P450 cholesterol side-chain cleavage
- CYP11B1
- 11β-hydroxylase
- CYP11B2
- aldosterone synthase
- CYP17
- 17α-hydroxylase/17,20-lyase
- CYP19
- aromatase
- CYP21
- 21-hydroxylase
- DAX1
- dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- dpc
- days post-conception
- DZ
- definitive zone
- ECM
- extracellular matrix
- EGF
- epidermal growth factor
- ER
- estrogen receptor
- FGD
- familial glucocorticoid deficiency
- FGF
- fibroblast growth factor
- FZ
- fetal zone
- HFA
- human fetal adrenal
- HPA
- hypothalamic-pituitary-adrenal
- HSD11B
- 11β-hydroxysteroid dehydrogenase
- HSD17B
- 17β-hydroxysteroid dehydrogenase
- HSD3B2
- type 2 3β-hydroxysteroid dehydrogenase/Δ4–5 isomerase
- LDL
- low-density lipoprotein
- MC2R
- melanocortin 2 receptor
- MPS-1
- metallopanstimulin-1
- MRAP
- MC2R accessory protein
- NCAM
- neural cell adhesion molecule
- NGFI-B
- nerve growth factor-induced clone B
- NOV
- nephroblastoma overexpressed
- PBX1
- pre-B cell leukemia transcription factor 1
- POMC
- proopiomelanocortin
- PR
- progesterone receptor
- SALL1
- human homolog of spalt (sal), a region-specific homeotic gene in Drosophila melanogaster
- SF1
- steroidogenic factor-1
- SP
- side population
- SPARC
- secreted protein acidic and rich in cysteine
- StAR
- steroid acute regulatory protein
- SULT2A1
- DHEA sulfotransferase
- TZ
- transitional zone
- VEGF
- vascular endothelial growth factor.
References
- 1. Mesiano S, Jaffe RB. 1997. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18:378–403 [DOI] [PubMed] [Google Scholar]
- 2. Diczfalusy E. 1964. Endocrine functions of the human fetoplacental unit. Fed Proc 23:791–798 [PubMed] [Google Scholar]
- 3. Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. 1973. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res 29:111–159 [DOI] [PubMed] [Google Scholar]
- 4. Liggins GC. 1974. Parturition in the sheep and the human. Basic Life Sci 4:423–443 [DOI] [PubMed] [Google Scholar]
- 5. Jirasek JE. 1980. Human fetal endocrines. London: Martinus Nijhoff; 69–82 [Google Scholar]
- 6. Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, Lindsay S, Robson S, Ostrer H, Parker KL, Wilson DI. 1999. Expression of steroidogenic factor 1 and Wilms' tumour 1 during early human gonadal development and sex determination. Mech Dev 87:175–180 [DOI] [PubMed] [Google Scholar]
- 7. Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. 2001. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 15:57–68 [DOI] [PubMed] [Google Scholar]
- 8. Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortés L, McElreavey K, Lindsay S, Robson S, Bullen P, Ostrer H, Wilson DI. 2000. SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev 91:403–407 [DOI] [PubMed] [Google Scholar]
- 9. Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. 2006. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crowder RE. 1957. The development of the adrenal gland in man, with special reference to origin and ultimate location of cell types and evidence in favor of the “cell migration” theory. Contrib Embryol 36:193–210 [Google Scholar]
- 11. Mesiano S, Coulter CL, Jaffe RB. 1993. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17 α-hydroxylase/17, 20-lyase, and 3β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77:1184–1189 [DOI] [PubMed] [Google Scholar]
- 12. McNutt NS, Jones AL. 1970. Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab Invest 22:513–527 [PubMed] [Google Scholar]
- 13. Sucheston ME, Cannon MS. 1968. Development of zonular patterns in the human adrenal gland. J Morphol 126:477–491 [DOI] [PubMed] [Google Scholar]
- 14. Keene MF, Hewer EE. 1927. Observations on the development of the human suprarenal gland. J Anat 61:302–324 [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper MJ, Hutchins GM, Israel MA. 1990. Histogenesis of the human adrenal medulla. An evaluation of the ontogeny of chromaffin and nonchromaffin lineages. Am J Pathol 137:605–615 [PMC free article] [PubMed] [Google Scholar]
- 16. Ehrhart-Bornstein M, Breidert M, Guadanucci P, Wozniak W, Bocian-Sobkowska J, Malendowicz LK, Bornstein SR. 1997. 17α-Hydroxylase and chromogranin A in 6th week human fetal adrenals. Horm Metab Res 29:30–32 [DOI] [PubMed] [Google Scholar]
- 17. Wilburn LA, Goldsmith PC, Chang KJ, Jaffe RB. 1986. Ontogeny of enkephalin and catecholamine-synthesizing enzymes in the primate fetal adrenal medulla. J Clin Endocrinol Metab 63:974–980 [DOI] [PubMed] [Google Scholar]
- 18. Wilburn LA, Jaffe RB. 1988. Quantitative assessment of the ontogeny of met-enkephalin, norepinephrine and epinephrine in the human fetal adrenal medulla. Acta Endocrinol (Copenh) 118:453–459 [DOI] [PubMed] [Google Scholar]
- 19. Yon L, Breault L, Contesse V, Bellancourt G, Delarue C, Fournier A, Lehoux JG, Vaudry H, Gallo-Payet N. 1998. Localization, characterization, and second messenger coupling of pituitary adenylate cyclase-activating polypeptide receptors in the fetal human adrenal gland during the second trimester of gestation. J Clin Endocrinol Metab 83:1299–1305 [DOI] [PubMed] [Google Scholar]
- 20. Molenaar WM, Lee VM, Trojanowski JQ. 1990. Early fetal acquisition of the chromaffin and neuronal immunophenotype by human adrenal medullary cells. An immunohistological study using monoclonal antibodies to chromogranin A, synaptophysin, tyrosine hydroxylase, and neuronal cytoskeletal proteins. Exp Neurol 108:1–9 [DOI] [PubMed] [Google Scholar]
- 21. Seidl K, Unsicker K. 1989. The determination of the adrenal medullary cell fate during embryogenesis. Dev Biol 136:481–490 [DOI] [PubMed] [Google Scholar]
- 22. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. 1998. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19:101–143 [DOI] [PubMed] [Google Scholar]
- 23. Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Brühl B, Beier K, Metz J, Garcia-Arraras JE, Roig-Lopez JL, Monaghan P, Schmid W, Cole TJ, Kellendonk C, Tronche F, Schütz G, Unsicker K. 1999. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development 126:2935–2944 [DOI] [PubMed] [Google Scholar]
- 24. Huber K, Combs S, Ernsberger U, Kalcheim C, Unsicker K. 2002. Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis. Ann NY Acad Sci 971:554–559 [DOI] [PubMed] [Google Scholar]
- 25. Unsicker K, Huber K, Schütz G, Kalcheim C. 2005. The chromaffin cell and its development. Neurochem Res 30:921–925 [DOI] [PubMed] [Google Scholar]
- 26. Grueters A, Korth-Schutz S. 1982. Longitudinal study of plasma dehydroepiandrosterone sulfate in preterm and fullterm infants. J Clin Endocrinol Metab 55:314–320 [DOI] [PubMed] [Google Scholar]
- 27. Honour JH, Wickramaratne K, Valman HB. 1992. Adrenal function in preterm infants. Biol Neonate 61:214–221 [DOI] [PubMed] [Google Scholar]
- 28. Kojima S, Yanaihara T, Nakayama T. 1981. Serum steroid levels in children at birth and in early neonatal period. Am J Obstet Gynecol 140:961–965 [DOI] [PubMed] [Google Scholar]
- 29. Wiener D, Smith J, Dahlem S, Berg G, Moshang T., Jr 1987. Serum adrenal steroid levels in healthy full-term 3-day-old infants. J Pediatr 110:122–124 [DOI] [PubMed] [Google Scholar]
- 30. Lanman JT. 1953. The fetal zone of the adrenal gland: its developmental course, comparative anatomy, and possible physiologic functions. Medicine (Baltimore) 32:389–430 [DOI] [PubMed] [Google Scholar]
- 31. Spencer SJ, Mesiano S, Lee JY, Jaffe RB. 1999. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab 84:1110–1115 [DOI] [PubMed] [Google Scholar]
- 32. Bocian-Sobkowska J. 2000. Morphometric study of the human suprarenal gland in the first postnatal year. Folia Morphol (Warsz) 58:275–284 [PubMed] [Google Scholar]
- 33. Hata K, Nagata H, Nishigaki A, Aoki S, Hata T, Murao F, Kitao M. 1988. Ultrasonographic evaluation of adrenal involution during antenatal and neonatal periods. Gynecol Obstet Invest 26:29–32 [DOI] [PubMed] [Google Scholar]
- 34. Midgley PC, Russell K, Oates N, Holownia P, Shaw JC, Honour JW. 1998. Adrenal function in preterm infants: ACTH may not be the sole regulator of the fetal zone. Pediatr Res 44:887–893 [DOI] [PubMed] [Google Scholar]
- 35. Midgley PC, Russell K, Oates N, Shaw JC, Honour JW. 1996. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr Res 22:729–733 [DOI] [PubMed] [Google Scholar]
- 36. Ben-David S, Zuckerman-Levin N, Epelman M, Shen-Orr Z, Levin M, Sujov P, Hochberg Z. 2007. Parturition itself is the basis for fetal adrenal involution. J Clin Endocrinol Metab 92:93–97 [DOI] [PubMed] [Google Scholar]
- 37. Garagorri JM, Rodríguez G, Lario-Elboj AJ, Olivares JL, Lario-Muñoz A, Orden I. 2008. Reference levels for 17-hydroxyprogesterone, 11-desoxycortisol, cortisol, testosterone, dehydroepiandrosterone sulfate and androstenedione in infants from birth to six months of age. Eur J Pediatr 167:647–653 [DOI] [PubMed] [Google Scholar]
- 38. Neville AM, O'Hare MJ. 1982. The human adrenal cortex. New York: Springer-Verlag; 11–33 [Google Scholar]
- 39. Wolkersdörfer GW, Bornstein SR. 1998. Tissue remodelling in the adrenal gland. Biochem Pharmacol 56:163–171 [DOI] [PubMed] [Google Scholar]
- 40. Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. 1994. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology 135:431–438 [DOI] [PubMed] [Google Scholar]
- 41. Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. 1999. Development of functional zonation in the rat adrenal cortex. Endocrinology 140:3342–3353 [DOI] [PubMed] [Google Scholar]
- 42. Pawlikowski M. 2005. Adrenal cortex—the next biological clock? Neuro Endocrinol Lett 26:193–195 [PubMed] [Google Scholar]
- 43. Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. 2009. In search of adrenocortical stem and progenitor cells. Endocr Rev 30:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sasano H, Imatani A, Shizawa S, Suzuki T, Nagura H. 1995. Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol 8:11–17 [PubMed] [Google Scholar]
- 45. Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. 2003. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta 1619:317–324 [DOI] [PubMed] [Google Scholar]
- 46. Peterson JK, Moran F, Conley AJ, Bird IM. 2001. Zonal expression of endothelial nitric oxide synthase in sheep and rhesus adrenal cortex. Endocrinology 142:5351–5363 [DOI] [PubMed] [Google Scholar]
- 47. Bird IM, Pattison JC. 2004. Expression of AT1-R in marmoset whole adrenal glands and adrenocortical cells in culture. Endocr Res 30:753–757 [DOI] [PubMed] [Google Scholar]
- 48. Iannaccone PM, Weinberg WC. 1987. The histogenesis of the rat adrenal cortex: a study based on histologic analysis of mosaic pattern in chimeras. J Exp Zool 243:217–223 [DOI] [PubMed] [Google Scholar]
- 49. Iannaccone P, Morley S, Skimina T, Mullins J, Landini G. 2003. Cord-like mosaic patches in the adrenal cortex are fractal: implications for growth and development. FASEB J 17:41–43 [DOI] [PubMed] [Google Scholar]
- 50. Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. 1996. Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol 10:585–598 [DOI] [PubMed] [Google Scholar]
- 51. Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC. 1999. Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology 140:5609–5618 [DOI] [PubMed] [Google Scholar]
- 52. Johannisson E. 1968. The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol (Copenh) 58(Suppl 130):1–107 [PubMed] [Google Scholar]
- 53. Ishimoto H, Minegishi K, Higuchi T, Furuya M, Asai S, Kim SH, Tanaka M, Yoshimura Y, Jaffe RB. 2008. The periphery of the human fetal adrenal gland is a site of angiogenesis: zonal differential expression and regulation of angiogenic factors. J Clin Endocrinol Metab 93:2402–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim AC, Hammer GD. 2007. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol 265–266:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Han VK, Jaffe RB. 1996. Functional maturation of the primate fetal adrenal in vivo: I. Role of insulin-like growth factors (IGFs), IGF-I receptor, and IGF binding proteins in growth regulation. Endocrinology 137:4487–4498 [DOI] [PubMed] [Google Scholar]
- 56. White PC, Speiser PW. 2000. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 21:245–291 [DOI] [PubMed] [Google Scholar]
- 57. Mendonca BB, Leite MV, de Castro M, Kino T, Elias LL, Bachega TA, Arnhold IJ, Chrousos GP, Latronico AC. 2002. Female pseudohermaphroditism caused by a novel homozygous missense mutation of the GR gene. J Clin Endocrinol Metab 87:1805–1809 [DOI] [PubMed] [Google Scholar]
- 58. David M, Forest MG. 1984. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 105:799–803 [DOI] [PubMed] [Google Scholar]
- 59. Lajic S, Wedell A, Bui TH, Ritzén EM, Holst M. 1998. Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 83:3872–3880 [DOI] [PubMed] [Google Scholar]
- 60. George FW, Wilson JD. 1988. Sex determination and differentiation. In: Knobil E, Neill JD. eds. The physiology of reproduction. New York: Raven Press; 3–26 [Google Scholar]
- 61. Muench MO, Ratcliffe JV, Nakanishi M, Ishimoto H, Jaffe RB. 2003. Isolation of definitive zone and chromaffin cells based upon expression of CD56 (neural cell adhesion molecule) in the human fetal adrenal gland. J Clin Endocrinol Metab 88:3921–3930 [DOI] [PubMed] [Google Scholar]
- 62. Parker CR, Jr, Faye-Petersen O, Stankovic AK, Mason JI, Grizzle WE. 1995. Immunohistochemical evaluation of the cellular localization and ontogeny of 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase in the human fetal adrenal gland. Endocr Res 21:69–80 [DOI] [PubMed] [Google Scholar]
- 63. Voutilainen R, Ilvesmäki V, Miettinen PJ. 1991. Low expression of 3β-hydroxy-5-ene steroid dehydrogenase gene in human fetal adrenals in vivo; adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. J Clin Endocrinol Metab 72:761–767 [DOI] [PubMed] [Google Scholar]
- 64. Narasaka T, Suzuki T, Moriya T, Sasano H. 2001. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol 174:111–120 [DOI] [PubMed] [Google Scholar]
- 65. Dupont E, Luu-The V, Labrie F, Pelletier G. 1990. Ontogeny of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3 β-HSD) in human adrenal gland performed by immunocytochemistry. Mol Cell Endocrinol 74:R7–R10 [DOI] [PubMed] [Google Scholar]
- 66. Doody KM, Carr BR, Rainey WE, Byrd W, Murry BA, Strickler RC, Thomas JL, Mason JI. 1990. 3β-Hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology 126:2487–2492 [DOI] [PubMed] [Google Scholar]
- 67. Rainey WE, Carr BR, Wang ZN, Parker CR., Jr 2001. Gene profiling of human fetal and adult adrenals. J Endocrinol 171:209–215 [DOI] [PubMed] [Google Scholar]
- 68. Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. 2004. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18:279–290 [DOI] [PubMed] [Google Scholar]
- 69. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. 2005. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr Rev 26:525–582 [DOI] [PubMed] [Google Scholar]
- 70. Coulter CL, Jaffe RB. 1998. Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology 139:5144–5150 [DOI] [PubMed] [Google Scholar]
- 71. Freije WA, Pezzi V, Arici A, Carr BR, Rainey WE. 1997. Expression of 11 β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) in the human fetal adrenal. J Soc Gynecol Investig 4:305–309 [PubMed] [Google Scholar]
- 72. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 73. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 74. Miller WL, Strauss JF., 3rd 1999. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol 69:131–141 [DOI] [PubMed] [Google Scholar]
- 75. Manna PR, Dyson MT, Stocco DM. 2009. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Murphy BE. 1982. Human fetal serum cortisol levels related to gestational age: evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. Am J Obstet Gynecol 144:276–282 [DOI] [PubMed] [Google Scholar]
- 77. Lockwood CJ, Radunovic N, Nastic D, Petkovic S, Aigner S, Berkowitz GS. 1996. Corticotropin-releasing hormone and related pituitary-adrenal axis hormones in fetal and maternal blood during the second half of pregnancy. J Perinat Med 24:243–251 [DOI] [PubMed] [Google Scholar]
- 78. Murphy BE. 1983. Human fetal serum cortisol levels at delivery: a review. Endocr Rev 4:150–154 [DOI] [PubMed] [Google Scholar]
- 79. Bayard F, Ances IG, Tapper AJ, Weldon VV, Kowarski A, Migeon CJ. 1970. Transplacental passage and fetal secretion of aldosterone. J Clin Invest 49:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McClellan M, Brenner RM. 1981. Development of the fetal adrenals in non-human primates: electron microscopy. In: Novy MJ, Resko JA. eds. Fetal endocrinology. New York: Academic Press; 383–403 [Google Scholar]
- 81. Dobbie JW, Symington T. 1966. The human adrenal gland with special reference to the vasculature. J Endocrinol 34:479–489 [DOI] [PubMed] [Google Scholar]
- 82. Ivemark B, Ekström T, Lagergren C. 1967. The vasculature of the developing and mature human adrenal gland. Acta Paediatr Scand 56:601–606 [DOI] [PubMed] [Google Scholar]
- 83. Merklin RJ. 1962. Arterial supply of the suprarenal gland. Anat Rec 144:359–371 [DOI] [PubMed] [Google Scholar]
- 84. Pityński K, Skawina A, Polakiewicz J, Walocha J. 1998. Extraorganic vascular system of adrenal glands in human fetuses. Ann Anat 180:361–368 [DOI] [PubMed] [Google Scholar]
- 85. Hervonen A, Suoranta H. 1972. Vascular supply of human fetal adrenals and the functional significance of the microvascular patterns. Z Anat Entwicklungsgesch 136:311–318 [DOI] [PubMed] [Google Scholar]
- 86. Sasano N, Takezawa Y, Sato H, Horikawa N. 1971. Micro-angiography of normal and pathologic human adrenals in prenatal and aging course. Tohoku J Exp Med 104:129–141 [DOI] [PubMed] [Google Scholar]
- 87. Pityński K, Litwin JA, Nowogrodzka-Zagórska M, Miodoński AJ. 1996. Vascular architecture of the human fetal adrenal gland: a SEM study of corrosion casts. Ann Anat 178:215–222 [DOI] [PubMed] [Google Scholar]
- 88. Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA. 2006. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res 53:89–103 [DOI] [PubMed] [Google Scholar]
- 89. Fiedler U, Augustin HG. 2006. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27:552–558 [DOI] [PubMed] [Google Scholar]
- 90. Geva E, Jaffe RB. 2000. Role of angiopoietins in reproductive tract angiogenesis. Obstet Gynecol Surv 55:511–519 [DOI] [PubMed] [Google Scholar]
- 91. Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. 2002. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab 87:4213–4224 [DOI] [PubMed] [Google Scholar]
- 92. Mesiano S, Mellon SH, Gospodarowicz D, Di Blasio AM, Jaffe RB. 1991. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: a model for adrenal growth regulation. Proc Natl Acad Sci USA 88:5428–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shifren JL, Doldi N, Ferrara N, Mesiano S, Jaffe RB. 1994. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J Clin Endocrinol Metab 79:316–322 [DOI] [PubMed] [Google Scholar]
- 94. Shifren JL, Mesiano S, Taylor RN, Ferrara N, Jaffe RB. 1998. Corticotropin regulates vascular endothelial growth factor expression in human fetal adrenal cortical cells. J Clin Endocrinol Metab 83:1342–1347 [DOI] [PubMed] [Google Scholar]
- 95. Ishimoto H, Ginzinger DG, Jaffe RB. 2006. Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: implications for coordinated adrenal organ growth and angiogenesis. J Clin Endocrinol Metab 91:1909–1915 [DOI] [PubMed] [Google Scholar]
- 96. Mesiano S, Mellon SH, Jaffe RB. 1993. Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J Clin Endocrinol Metab 76:968–976 [DOI] [PubMed] [Google Scholar]
- 97. Rainey WE, Parker CR, Jr, Rehman K, Carr BR. 2002. The adrenal genetic puzzle: how do the fetal and adult pieces differ? Endocr Res 28:611–622 [DOI] [PubMed] [Google Scholar]
- 98. Rehman KS, Carr BR, Rainey WE. 2003. Profiling the steroidogenic pathway in human fetal and adult adrenals. J Soc Gynecol Investig 10:372–380 [DOI] [PubMed] [Google Scholar]
- 99. Xing Y, Nakamura Y, Rainey WE. 2009. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol 300:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ratcliffe J, Nakanishi M, Jaffe RB. 2003. Identification of definitive and fetal zone markers in the human fetal adrenal gland reveals putative developmental genes. J Clin Endocrinol Metab 88:3272–3277 [DOI] [PubMed] [Google Scholar]
- 101. Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. 2009. Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ 51:55–67 [DOI] [PubMed] [Google Scholar]
- 102. Bleau AM, Planque N, Perbal B. 2005. CCN proteins and cancer: two to tango. Front Biosci 10:998–1009 [DOI] [PubMed] [Google Scholar]
- 103. Martinerie C, Gicquel C, Louvel A, Laurent M, Schofield PN, Le Bouc Y. 2001. Altered expression of novH is associated with human adrenocortical tumorigenesis. J Clin Endocrinol Metab 86:3929–3940 [DOI] [PubMed] [Google Scholar]
- 104. Kocialkowski S, Yeger H, Kingdom J, Perbal B, Schofield PN. 2001. Expression of the human NOV gene in first trimester fetal tissues. Anat Embryol (Berl) 203:417–427 [DOI] [PubMed] [Google Scholar]
- 105. Planque N, Perbal B. 2003. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lafont J, Laurent M, Thibout H, Lallemand F, Le Bouc Y, Atfi A, Martinerie C. 2002. The expression of novH in adrenocortical cells is down-regulated by TGFβ 1 through c-Jun in a Smad-independent manner. J Biol Chem 277:41220–41229 [DOI] [PubMed] [Google Scholar]
- 107. Vidal V, Schedl A. 2000. Requirement of WT1 for gonad and adrenal development: insights from transgenic animals. Endocr Res 26:1075–1082 [DOI] [PubMed] [Google Scholar]
- 108. Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. 1999. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126:1845–1857 [DOI] [PubMed] [Google Scholar]
- 109. Fernandez-Pol JA, Klos DJ, Hamilton PD. 1993. A growth factor-inducible gene encodes a novel nuclear protein with zinc finger structure. J Biol Chem 268:21198–21204 [PubMed] [Google Scholar]
- 110. Atsuta Y, Aoki N, Sato K, Oikawa K, Nochi H, Miyokawa N, Hirata S, Kimura S, Sasajima T, Katagiri M. 2002. Identification of metallopanstimulin-1 as a member of a tumor associated antigen in patients with breast cancer. Cancer Lett 182:101–107 [DOI] [PubMed] [Google Scholar]
- 111. Wang YW, Qu Y, Li JF, Chen XH, Liu BY, Gu QL, Zhu ZG. 2006. In vitro and in vivo evidence of metallopanstimulin-1 in gastric cancer progression and tumorigenicity. Clin Cancer Res 12:4965–4973 [DOI] [PubMed] [Google Scholar]
- 112. Leslie EM, Deeley RG, Cole SP. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204:216–237 [DOI] [PubMed] [Google Scholar]
- 113. Huls M, Russel FG, Masereeuw R. 2009. The role of ATP binding cassette transporters in tissue defense and organ regeneration. J Pharmacol Exp Ther 328:3–9 [DOI] [PubMed] [Google Scholar]
- 114. Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. 1987. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Kalken CK, Giaccone G, van der Valk P, Kuiper CM, Hadisaputro MM, Bosma SA, Scheper RJ, Meijer CJ, Pinedo HM. 1992. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol 141:1063–1072 [PMC free article] [PubMed] [Google Scholar]
- 116. Sugawara I, Nakahama M, Hamada H, Tsuruo T, Mori S. 1988. Apparent stronger expression in the human adrenal cortex than in the human adrenal medulla of M(r) 170,000–180,000 P-glycoprotein. Cancer Research 48:4611–4614 [PubMed] [Google Scholar]
- 117. Bello-Reuss E, Ernest S, Holland OB, Hellmich MR. 2000. Role of multidrug resistance P-glycoprotein in the secretion of aldosterone by human adrenal NCI-H295 cells. Am J Physiol Cell Physiol 278:C1256–C1265 [DOI] [PubMed] [Google Scholar]
- 118. van Kalken CK, Broxterman HJ, Pinedo HM, Feller N, Dekker H, Lankelma J, Giaccone G. 1993. Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer 67:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. 1992. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem 267:24248–24252 [PubMed] [Google Scholar]
- 120. Johnstone RW, Cretney E, Smyth MJ. 1999. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 93:1075–1085 [PubMed] [Google Scholar]
- 121. Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. 1998. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci USA 95:7024–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chaudhary PM, Roninson IB. 1991. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 66:85–94 [DOI] [PubMed] [Google Scholar]
- 123. Bunting KD, Galipeau J, Topham D, Benaim E, Sorrentino BP. 1998. Transduction of murine bone marrow cells with an MDR1 vector enables ex vivo stem cell expansion, but these expanded grafts cause a myeloproliferative syndrome in transplanted mice. Blood 92:2269–2279 [PubMed] [Google Scholar]
- 124. Rønn LC, Hartz BP, Bock E. 1998. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol 33:853–864 [DOI] [PubMed] [Google Scholar]
- 125. Shapiro L, Love J, Colman DR. 2007. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci 30:451–474 [DOI] [PubMed] [Google Scholar]
- 126. Ehrhart-Bornstein M, Hilbers U. 1998. Neuroendocrine properties of adrenocortical cells. Horm Metab Res 30:436–439 [DOI] [PubMed] [Google Scholar]
- 127. Jin L, Hemperly JJ, Lloyd RV. 1991. Expression of neural cell adhesion molecule in normal and neoplastic human neuroendocrine tissues. Am J Pathol 138:961–969 [PMC free article] [PubMed] [Google Scholar]
- 128. Zeromski J, Lawniczak M, Galbas K, Jenek R, Golusiński P. 1998. Expression of CD56/N-CAM antigen and some other adhesion molecules in various human endocrine glands. Folia Histochem Cytobiol 36:119–125 [PubMed] [Google Scholar]
- 129. Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Pedersen N. 2002. NCAM regulates cell motility. J Cell Sci 115:283–292 [DOI] [PubMed] [Google Scholar]
- 130. Paratcha G, Ledda F, Ibáñez CF. 2003. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867–879 [DOI] [PubMed] [Google Scholar]
- 131. Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. 2000. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun 270:936–941 [DOI] [PubMed] [Google Scholar]
- 132. Ishimoto H, Muench MO, Higuchi T, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB. 2006. Midkine, a heparin-binding growth factor, selectively stimulates proliferation of definitive zone cells of the human fetal adrenal gland. J Clin Endocrinol Metab 91:4050–4056 [DOI] [PubMed] [Google Scholar]
- 133. Ishimoto H, Ginzinger DG, Matsumoto T, Hattori Y, Furuya M, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB. 2006. Differential zonal expression and adrenocorticotropin regulation of secreted protein acidic and rich in cysteine (SPARC), a matricellular protein, in the midgestation human fetal adrenal gland: implications for adrenal development. J Clin Endocrinol Metab 91:3208–3214 [DOI] [PubMed] [Google Scholar]
- 134. Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. 1998. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet 18:81–83 [DOI] [PubMed] [Google Scholar]
- 135. Ma Y, Chai L, Cortez SC, Stopa EG, Steinhoff MM, Ford D, Morgan J, Maizel AL. 2002. SALL1 expression in the human pituitary-adrenal/gonadal axis. J Endocrinol 173:437–448 [DOI] [PubMed] [Google Scholar]
- 136. Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. 2007. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics 30:26–34 [DOI] [PubMed] [Google Scholar]
- 137. Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. 2003. Insights into G protein structure, function, and regulation. Endocr Rev 24:765–781 [DOI] [PubMed] [Google Scholar]
- 138. Kostenis E, Waelbroeck M, Milligan G. 2005. Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol Sci 26:595–602 [DOI] [PubMed] [Google Scholar]
- 139. Breault L, Chamoux E, Lehoux JG, Gallo-Payet N. 2000. Localization of G protein α-subunits in the human fetal adrenal gland. Endocrinology 141:4334–4341 [DOI] [PubMed] [Google Scholar]
- 140. Adams JC, Watt FM. 1993. Regulation of development and differentiation by the extracellular matrix. Development 117:1183–1198 [DOI] [PubMed] [Google Scholar]
- 141. Streuli C. 1999. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol 11:634–640 [DOI] [PubMed] [Google Scholar]
- 142. Crickard K, Ill CR, Jaffe RB. 1981. Control of proliferation of human fetal adrenal cells in vitro. J Clin Endocrinol Metab 53:790–796 [DOI] [PubMed] [Google Scholar]
- 143. Gospodarowicz D, Delgado D, Vlodavsky I. 1980. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci USA 77:4094–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hornsby PJ, Sturek M, Harris SE, Simonian MH. 1983. Serum and growth factor requirements for proliferation of human adrenocortical cells in culture: comparison with bovine adrenocortical cells. In Vitro 19:863–869 [DOI] [PubMed] [Google Scholar]
- 145. Ill CR, Gospodarowicz D. 1982. Factors involved in supporting the growth and steroidogenic functions of bovine adrenal cortical cells maintained on extracellular matrix and exposed to a serum-free medium. J Cell Physiol 113:373–384 [DOI] [PubMed] [Google Scholar]
- 146. Simonian MH, White ML, Gill GN. 1982. Growth and function of cultured bovine adrenocortical cells in a serum-free defined medium. Endocrinology 111:919–927 [DOI] [PubMed] [Google Scholar]
- 147. Chamoux E, Narcy A, Lehoux JG, Gallo-Payet N. 2002. Fibronectin, laminin, and collagen IV as modulators of cell behavior during adrenal gland development in the human fetus. J Clin Endocrinol Metab 87:1819–1828 [DOI] [PubMed] [Google Scholar]
- 148. Chamoux E, Bolduc L, Lehoux JG, Gallo-Payet N. 2001. Identification of extracellular matrix components and their integrin receptors in the human fetal adrenal gland. J Clin Endocrinol Metab 86:2090–2098 [DOI] [PubMed] [Google Scholar]
- 149. Virtanen I, Korhonen M, Petäjäniemi N, Karhunen T, Thornell LE, Sorokin LM, Konttinen YT. 2003. Laminin isoforms in fetal and adult human adrenal cortex. J Clin Endocrinol Metab 88:4960–4966 [DOI] [PubMed] [Google Scholar]
- 150. Bornstein P. 2000. Matricellular proteins: an overview. Matrix Biol 19:555–556 [DOI] [PubMed] [Google Scholar]
- 151. Brekken RA, Sage EH. 2001. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 19:816–827 [DOI] [PubMed] [Google Scholar]
- 152. Hammer GD, Parker KL, Schimmer BP. 2005. Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- 153. Else T, Hammer GD. 2005. Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16:458–468 [DOI] [PubMed] [Google Scholar]
- 154. Parker KL, Schimmer BP. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed] [Google Scholar]
- 155. Morohashi Ki. 1999. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol Metab 10:169–173 [DOI] [PubMed] [Google Scholar]
- 156. Bakke M, Zhao L, Hanley NA, Parker KL. 2001. SF-1: a critical mediator of steroidogenesis. Mol Cell Endocrinol 171:5–7 [DOI] [PubMed] [Google Scholar]
- 157. Lalli E, Sassone-Corsi P. 2003. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol 17:1445–1453 [DOI] [PubMed] [Google Scholar]
- 158. Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. 2002. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57:19–36 [DOI] [PubMed] [Google Scholar]
- 159. Iyer AK, McCabe ER. 2004. Molecular mechanisms of DAX1 action. Mol Genet Metab 83:60–73 [DOI] [PubMed] [Google Scholar]
- 160. Saner KJ, Suzuki T, Sasano H, Pizzey J, Ho C, Strauss JF, 3rd, Carr BR, Rainey WE. 2005. Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal. Mol Endocrinol 19:184–197 [DOI] [PubMed] [Google Scholar]
- 161. Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. 2006. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology 147:4104–4111 [DOI] [PubMed] [Google Scholar]
- 162. Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 163. Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. 1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi K, Li E. 1995. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- 165. Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. 2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. 1999. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- 167. Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL. 2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab 87:1829–1833 [DOI] [PubMed] [Google Scholar]
- 168. Biason-Lauber A, Schoenle EJ. 2000. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Correa RV, Domenice S, Bingham NC, Billerbeck AE, Rainey WE, Parker KL, Mendonca BB. 2004. A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab 89:1767–1772 [DOI] [PubMed] [Google Scholar]
- 170. Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M, Morel Y. 2004. Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab 89:4829–4832 [DOI] [PubMed] [Google Scholar]
- 171. Köhler B, Lin L, Ferraz-de-Souza B, Wieacker P, Heidemann P, Schröder V, Biebermann H, Schnabel D, Grüters A, Achermann JC. 2008. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat 29:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, Sultan C, Dattani MT, Achermann JC. 2007. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab 92:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Reuter AL, Goji K, Bingham NC, Matsuo M, Parker KL. 2007. A novel mutation in the accessory DNA-binding domain of human steroidogenic factor 1 causes XY gonadal dysgenesis without adrenal insufficiency. Eur J Endocrinol 157:233–238 [DOI] [PubMed] [Google Scholar]
- 174. Hasegawa T, Fukami M, Sato N, Katsumata N, Sasaki G, Fukutani K, Morohashi K, Ogata T. 2004. Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab 89:5930–5935 [DOI] [PubMed] [Google Scholar]
- 175. Coutant R, Mallet D, Lahlou N, Bouhours-Nouet N, Guichet A, Coupris L, Croué A, Morel Y. 2007. Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome. J Clin Endocrinol Metab 92:2868–2873 [DOI] [PubMed] [Google Scholar]
- 176. Phelan JK, McCabe ER. 2001. Mutations in NR0B1 (DAX1) and NR5A1 (SF1) responsible for adrenal hypoplasia congenita. Hum Mutat 18:472–487 [DOI] [PubMed] [Google Scholar]
- 177. McCabe ER. 2007. DAX1: increasing complexity in the roles of this novel nuclear receptor. Mol Cell Endocrinol 265–266:179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K. 1999. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol 13:1267–1284 [DOI] [PubMed] [Google Scholar]
- 179. Crawford PA, Dorn C, Sadovsky Y, Milbrandt J. 1998. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 18:2949–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, Hammer GD. 2002. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143:665–673 [DOI] [PubMed] [Google Scholar]
- 181. Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, Tamai KT, Sassone-Corsi P, Lovell-Badge R, Camerino G, Parker KL. 1996. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol 10:1261–1272 [DOI] [PubMed] [Google Scholar]
- 182. Niakan KK, Davis EC, Clipsham RC, Jiang M, Dehart DB, Sulik KK, McCabe ER. 2006. Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab 88:261–271 [DOI] [PubMed] [Google Scholar]
- 183. Battista MC, Otis M, Côté M, Laforest A, Peter M, Lalli E, Gallo-Payet N. 2005. Extracellular matrix and hormones modulate DAX-1 localization in the human fetal adrenal gland. J Clin Endocrinol Metab 90:5426–5431 [DOI] [PubMed] [Google Scholar]
- 184. Lehmann SG, Lalli E, Sassone-Corsi P. 2002. X-linked adrenal hypoplasia congenita is caused by abnormal nuclear localization of the DAX-1 protein. Proc Natl Acad Sci USA 99:8225–8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. 1993. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol Cell Biol 13:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bassett MH, White PC, Rainey WE. 2004. A role for the NGFI-B family in adrenal zonation and adrenocortical disease. Endocr Res 30:567–574 [DOI] [PubMed] [Google Scholar]
- 187. Martin LJ, Tremblay JJ. 2005. The human 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase type 2 promoter is a novel target for the immediate early orphan nuclear receptor Nur77 in steroidogenic cells. Endocrinology 146:861–869 [DOI] [PubMed] [Google Scholar]
- 188. Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. 2004. The orphan nuclear receptor NGFIB regulates transcription of 3β-hydroxysteroid dehydrogenase. Implications for the control of adrenal functional zonation. J Biol Chem 279:37622–37630 [DOI] [PubMed] [Google Scholar]
- 189. LaVoie HA. 2003. The role of GATA in mammalian reproduction. Exp Biol Med (Maywood) 228:1282–1290 [DOI] [PubMed] [Google Scholar]
- 190. Parviainen H, Kiiveri S, Bielinska M, Rahman N, Huhtaniemi IT, Wilson DB, Heikinheimo M. 2007. GATA transcription factors in adrenal development and tumors. Mol Cell Endocrinol 265–266:17–22 [DOI] [PubMed] [Google Scholar]
- 191. Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, Heikinheimo M. 2002. Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology 143:3136–3143 [DOI] [PubMed] [Google Scholar]
- 192. Flück CE, Miller WL. 2004. GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol Endocrinol 18:1144–1157 [DOI] [PubMed] [Google Scholar]
- 193. Jimenez P, Saner K, Mayhew B, Rainey WE. 2003. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology 144:4285–4288 [DOI] [PubMed] [Google Scholar]
- 194. Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. 2005. GATA factors and the nuclear receptors, steroidogenic factor 1/liver receptor homolog 1, are key mutual partners in the regulation of the human 3β-hydroxysteroid dehydrogenase type 2 promoter. Mol Endocrinol 19:2358–2370 [DOI] [PubMed] [Google Scholar]
- 195. Huang N, Dardis A, Miller WL. 2005. Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol 19:2020–2034 [DOI] [PubMed] [Google Scholar]
- 196. Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. 1999. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev 13:64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Haase M, Schott M, Bornstein SR, Malendowicz LK, Scherbaum WA, Willenberg HS. 2007. CITED2 is expressed in human adrenocortical cells and regulated by basic fibroblast growth factor. J Endocrinol 192:459–465 [DOI] [PubMed] [Google Scholar]
- 198. Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I. 2008. PBX proteins: much more than Hox cofactors. Int J Dev Biol 52:9–20 [DOI] [PubMed] [Google Scholar]
- 199. Ferraz-de-Souza B, Martin F, Mallet D, Hudson-Davies RE, Cogram P, Lin L, Gerrelli D, Beuschlein F, Morel Y, Huebner A, Achermann JC. 2009. CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2, and pre-B-cell leukemia transcription factor 1 in human adrenal development and disease. J Clin Endocrinol Metab 94:678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Suzuki T, Takahashi K, Darnel AD, Moriya T, Murakami O, Narasaka T, Takeyama J, Sasano H. 2000. Chicken ovalbumin upstream promoter transcription factor II in the human adrenal cortex and its disorders. J Clin Endocrinol Metab 85:2752–2757 [DOI] [PubMed] [Google Scholar]
- 201. Shibata H, Ikeda Y, Mukai T, Morohashi K, Kurihara I, Ando T, Suzuki T, Kobayashi S, Murai M, Saito I, Saruta T. 2001. Expression profiles of COUP-TF, DAX-1, and SF-1 in the human adrenal gland and adrenocortical tumors: possible implications in steroidogenesis. Mol Genet Metab 74:206–216 [DOI] [PubMed] [Google Scholar]
- 202. Bakke M, Lund J. 1995. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3′,5′-monophosphate-responsive sequence in the bovine CYP17 gene. Mol Endocrinol 9:327–339 [DOI] [PubMed] [Google Scholar]
- 203. Shibata H, Kurihara I, Kobayashi S, Yokota K, Suda N, Saito I, Saruta T. 2003. Regulation of differential COUP-TF-coregulator interactions in adrenal cortical steroidogenesis. J Steroid Biochem Mol Biol 85:449–456 [DOI] [PubMed] [Google Scholar]
- 204. Buholzer CF, Arrighi JF, Abraham S, Piguet V, Capponi AM, Casal AJ. 2005. Chicken ovalbumin upstream promoter-transcription factor is a negative regulator of steroidogenesis in bovine adrenal glomerulosa cells. Mol Endocrinol 19:65–75 [DOI] [PubMed] [Google Scholar]
- 205. Hall JG, Pallister PD, Clarren SK, Beckwith JB, Wiglesworth FW, Fraser FC, Cho S, Benke PJ, Reed SD. 1980. Congenital hypothalamic hamartoblastoma, hypopituitarism, imperforate anus and postaxial polydactyly—a new syndrome? Part I: clinical, causal, and pathogenetic considerations. Am J Med Genet 7:47–74 [DOI] [PubMed] [Google Scholar]
- 206. Kang S, Graham JM, Jr, Olney AH, Biesecker LG. 1997. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 15:266–268 [DOI] [PubMed] [Google Scholar]
- 207. Mesiano S, Jaffe RB. 1997. Role of growth factors in the developmental regulation of the human fetal adrenal cortex. Steroids 62:62–72 [DOI] [PubMed] [Google Scholar]
- 208. Mesiano S, Katz SL, Lee JY, Jaffe RB. 1997. Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J Clin Endocrinol Metab 82:1390–1396 [DOI] [PubMed] [Google Scholar]
- 209. Aberdeen GW, Pepe GJ, Albrecht ED. 1999. Developmental expression of and effect of betamethasone on the messenger ribonucleic acid levels for peptide growth factors in the baboon fetal adrenal gland. J Endocrinol 163:123–130 [DOI] [PubMed] [Google Scholar]
- 210. Welt C, Sidis Y, Keutmann H, Schneyer A. 2002. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227:724–752 [DOI] [PubMed] [Google Scholar]
- 211. Spencer SJ, Rabinovici J, Mesiano S, Goldsmith PC, Jaffe RB. 1992. Activin and inhibin in the human adrenal gland. Regulation and differential effects in fetal and adult cells. J Clin Invest 90:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Munro LM, Kennedy A, McNicol AM. 1999. The expression of inhibin/activin subunits in the human adrenal cortex and its tumours. J Endocrinol 161:341–347 [DOI] [PubMed] [Google Scholar]
- 213. Vänttinen T, Kuulasmaa T, Liu J, Voutilainen R. 2002. Expression of activin/inhibin receptor and binding protein genes and regulation of activin/inhibin peptide secretion in human adrenocortical cells. J Clin Endocrinol Metab 87:4257–4263 [DOI] [PubMed] [Google Scholar]
- 214. Kadomatsu K, Muramatsu T. 2004. Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204:127–143 [DOI] [PubMed] [Google Scholar]
- 215. Muramatsu T. 2002. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132:359–371 [DOI] [PubMed] [Google Scholar]
- 216. Sirianni R, Carr BR, Andò S, Rainey WE. 2003. Inhibition of Src tyrosine kinase stimulates adrenal androgen production. J Mol Endocrinol 30:287–299 [DOI] [PubMed] [Google Scholar]
- 217. Siiteri PK, MacDonald PC. 1966. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab 26:751–761 [DOI] [PubMed] [Google Scholar]
- 218. Albrecht ED, Pepe GJ. 1990. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 11:124–150 [DOI] [PubMed] [Google Scholar]
- 219. Mesiano S. 2001. Roles of estrogen and progesterone in human parturition. Front Horm Res 27:86–104 [DOI] [PubMed] [Google Scholar]
- 220. Serón-Ferré M, Jaffe RB. 1981. The fetal adrenal gland. Annu Rev Physiol 43:141–162 [DOI] [PubMed] [Google Scholar]
- 221. Holinka CF, Diczfalusy E, Coelingh Bennink HJ. 2008. Estetrol: a unique steroid in human pregnancy. J Steroid Biochem Mol Biol 110:138–143 [DOI] [PubMed] [Google Scholar]
- 222. Ahmed J, Kellie AE. 1973. Oestrogen and oestrogen conjugate concentrations in late pregnancy plasma. J Steroid Biochem 4:1–10 [DOI] [PubMed] [Google Scholar]
- 223. Cassmer O. 1959. Hormone production of the isolated human placenta. Studies on the role of the foetus in the endocrine functions of the placenta. Acta Endocrinol Suppl (Copenh) 32(Suppl 45):1–82 [PubMed] [Google Scholar]
- 224. Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. 1997. Tissue distribution of estrogen receptors α (ER-α) and β (ER-β) mRNA in the midgestational human fetus. J Clin Endocrinol Metab 82:3509–3512 [DOI] [PubMed] [Google Scholar]
- 225. Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. 2001. Expression and cellular localization of estrogen receptors α and β in the human fetus. J Clin Endocrinol Metab 86:2258–2262 [DOI] [PubMed] [Google Scholar]
- 226. Baquedano MS, Saraco N, Berensztein E, Pepe C, Bianchini M, Levy E, Goñi J, Rivarola MA, Belgorosky A. 2007. Identification and developmental changes of aromatase and estrogen receptor expression in prepubertal and pubertal human adrenal tissues. J Clin Endocrinol Metab 92:2215–2222 [DOI] [PubMed] [Google Scholar]
- 227. Albrecht ED, Babischkin JS, Davies WA, Leavitt MG, Pepe GJ. 1999. Identification and developmental expression of the estrogen receptor α and β in the baboon fetal adrenal gland. Endocrinology 140:5953–5961 [DOI] [PubMed] [Google Scholar]
- 228. Mesiano S, Jaffe RB. 1993. Interaction of insulin-like growth factor-II and estradiol directs steroidogenesis in the human fetal adrenal toward dehydroepiandrosterone sulfate production. J Clin Endocrinol Metab 77:754–758 [DOI] [PubMed] [Google Scholar]
- 229. Fujieda K, Faiman C, Feyes FI, Winter JS. 1982. The control of steroidogenesis by human fetal adrenal cells in tissue culture. IV. The effect of exposure to placental steroids. J Clin Endocrinol Metab 54:89–94 [DOI] [PubMed] [Google Scholar]
- 230. Voutilainen R, Kahri AI. 1980. Placental origin of the suppression of 3β-hydroxysteroid dehydrogenase in the fetal zone cells of human fetal adrenals. J Steroid Biochem 13:39–43 [DOI] [PubMed] [Google Scholar]
- 231. Voutilainen R, Kahri AI, Salmenperä M. 1979. The effects of progesterone, pregnenolone, estriol, ACTH and hCG on steroid secretion of cultured human fetal adrenals. J Steroid Biochem 10:695–700 [DOI] [PubMed] [Google Scholar]
- 232. Pepe GJ, Albrecht ED. 1995. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev 16:608–648 [DOI] [PubMed] [Google Scholar]
- 233. Albrecht ED, Aberdeen GW, Pepe GJ. 2005. Estrogen elicits cortical zone-specific effects on development of the primate fetal adrenal gland. Endocrinology 146:1737–1744 [DOI] [PubMed] [Google Scholar]
- 234. Razandi M, Pedram A, Park ST, Levin ER. 2003. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- 235. Boonyaratanakornkit V, Edwards DP. 2007. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med 25:139–153 [DOI] [PubMed] [Google Scholar]
- 236. Baker BL, Jaffe RB. 1975. The genesis of cell types in the adenohypophysis of the human fetus as observed with immunocytochemistry. Am J Anat 143:137–161 [DOI] [PubMed] [Google Scholar]
- 237. Benirschke K. 1956. Adrenals in anencephaly and hydrocephaly. Obstet Gynecol 8:412–425 [PubMed] [Google Scholar]
- 238. Gray ES, Abramovich DR. 1980. Morphologic features of the anencephalic adrenal gland in early pregnancy. Am J Obstet Gynecol 137:491–495 [DOI] [PubMed] [Google Scholar]
- 239. Honnebier WJ, Jöbsis AC, Swaab DF. 1974. The effect of hypophysial hormones and human chorionic gonadotrophin (HCG) on the anencephalic fetal adrenal cortex and on parturition in the human. J Obstet Gynaecol Br Commonw 81:423–438 [DOI] [PubMed] [Google Scholar]
- 240. Leavitt MG, Aberdeen GW, Burch MG, Albrecht ED, Pepe GJ. 1997. Inhibition of fetal adrenal adrenocorticotropin receptor messenger ribonucleic acid expression by betamethasone administration to the baboon fetus in late gestation. Endocrinology 138:2705–2712 [DOI] [PubMed] [Google Scholar]
- 241. Leavitt MG, Albrecht ED, Pepe GJ. 1999. Development of the baboon fetal adrenal gland: regulation of the ontogenesis of the definitive and transitional zones by adrenocorticotropin. J Clin Endocrinol Metab 84:3831–3835 [DOI] [PubMed] [Google Scholar]
- 242. Aberdeen GW, Leavitt MG, Pepe GJ, Albrecht ED. 1998. Effect of maternal betamethasone administration at midgestation on baboon fetal adrenal gland development and adrenocorticotropin receptor messenger ribonucleic acid expression. J Clin Endocrinol Metab 83:976–982 [DOI] [PubMed] [Google Scholar]
- 243. Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Mason JI, Jaffe RB. 1996. Functional maturation of the primate fetal adrenal in vivo. II. Ontogeny of corticosteroid synthesis is dependent upon specific zonal expression of 3β-hydroxysteroid dehydrogenase/isomerase. Endocrinology 137:4953–4959 [DOI] [PubMed] [Google Scholar]
- 244. Buckley DI, Ramachandran J. 1981. Characterization of corticotropin receptors on adrenocortical cells. Proc Natl Acad Sci USA 78:7431–7435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mesiano S, Fujimoto VY, Nelson LR, Lee JY, Voytek CC, Jaffe RB. 1996. Localization and regulation of corticotropin receptor expression in the midgestation human fetal adrenal cortex: implications for in utero homeostasis. J Clin Endocrinol Metab 81:340–345 [DOI] [PubMed] [Google Scholar]
- 246. Aberdeen GW, Babischkin JS, Davies WA, Pepe GJ, Albrecht ED. 1997. Decline in adrenocorticotropin receptor messenger ribonucleic acid expression in the baboon fetal adrenocortical zone in the second half of pregnancy. Endocrinology 138:1634–1641 [DOI] [PubMed] [Google Scholar]
- 247. Clark AJ, Weber A. 1998. Adrenocorticotropin insensitivity syndromes. Endocr Rev 19:828–843 [DOI] [PubMed] [Google Scholar]
- 248. Chan LF, Clark AJ, Metherell LA. 2008. Familial glucocorticoid deficiency: advances in the molecular understanding of ACTH action. Horm Res 69:75–82 [DOI] [PubMed] [Google Scholar]
- 249. Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ. 2009. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 150:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Pepe GJ, Albrecht ED. 1990. Regulation of the primate fetal adrenal cortex. Endocr Rev 11:151–176 [DOI] [PubMed] [Google Scholar]
- 251. Gallo-Payet N, Payet MD. 2003. Mechanism of action of ACTH: beyond cAMP. Microsc Res Tech 61:275–287 [DOI] [PubMed] [Google Scholar]
- 252. Winters AJ, Oliver C, Colston C, MacDonald PC, Porter JC. 1974. Plasma ACTH levels in the human fetus and neonate as related to age and parturition. J Clin Endocrinol Metab 39:269–273 [DOI] [PubMed] [Google Scholar]
- 253. Florio P, Romero R, Chaiworapongsa T, Kusanovic JP, Torricelli M, Lowry PJ, Petraglia F. 2008. Amniotic fluid and umbilical cord plasma corticotropin-releasing factor (CRF), CRF-binding protein, adrenocorticotropin, and cortisol concentrations in intraamniotic infection and inflammation at term. J Clin Endocrinol Metab 93:3604–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Maia M, Vivier J, Hue D, Durand P, Feige JJ, Defaye G. 2002. Expression of the melanocortin receptors MC2-R (ACTH-receptor) and MC5-R during embryonic development of ovine adrenals. Endocr Res 28:631–635 [DOI] [PubMed] [Google Scholar]
- 255. Wang JJ, Valego NK, Su Y, Smith J, Rose JC. 2004. Developmental aspects of ovine adrenal adrenocorticotropic hormone receptor expression. J Soc Gynecol Investig 11:27–35 [DOI] [PubMed] [Google Scholar]
- 256. Durand P, Cathiard AM, Locatelli A, Dazord A, Saez JM. 1981. Spontaneous and adrenocorticotropin (ACTH)-induced maturation of the responsiveness of ovine fetal adrenal cells to in vitro stimulation by ACTH and cholera toxin. Endocrinology 109:2117–2123 [DOI] [PubMed] [Google Scholar]
- 257. Glickman JA, Challis JR. 1980. The changing response pattern of sheep fetal adrenal cells throughout the course of gestation. Endocrinology 106:1371–1376 [DOI] [PubMed] [Google Scholar]
- 258. Jacobs RA, Young IR, Hollingworth SA, Thorburn GD. 1994. Chronic administration of low doses of adrenocorticotropin to hypophysectomized fetal sheep leads to normal term labor. Endocrinology 134:1389–1394 [DOI] [PubMed] [Google Scholar]
- 259. Magyar DM, Devaskar J, Fridshal D, Buster JE, Nathanielsz PW. 1980. Responsiveness and maximum secretory capacity of isolated fetal lamb adrenocortical cells throughout the last third of gestation. Endocrinology 107:1582–1586 [DOI] [PubMed] [Google Scholar]
- 260. Albrecht ED, Aberdeen GW, Babischkin JS, Tilly JL, Pepe GJ. 1996. Biphasic developmental expression of adrenocorticotropin receptor messenger ribonucleic acid levels in the baboon fetal adrenal gland. Endocrinology 137:1292–1298 [DOI] [PubMed] [Google Scholar]
- 261. Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. 2007. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci 14:578–587 [DOI] [PubMed] [Google Scholar]
- 262. Sirianni R, Rehman KS, Carr BR, Parker CR, Jr, Rainey WE. 2005. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab 90:279–285 [DOI] [PubMed] [Google Scholar]
- 263. Ishimoto H, Higuchi T, Asai S, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB. Down-regulation of ACTH receptor gene expression by an ACTH-inducible heparin-binding growth factor, midkine, in human fetal adrenal cells. Program of the 89th Annual Meeting of The Endocrine Society, Toronto, Canada, 2007, P3-64 (Abstract) [Google Scholar]
- 264. Lebrethon MC, Jaillard C, Naville D, Bégeot M, Saez JM. 1994. Regulation of corticotropin and steroidogenic enzyme mRNAs in human fetal adrenal cells by corticotropin, angiotensin-II and transforming growth factor β 1. Mol Cell Endocrinol 106:137–143 [DOI] [PubMed] [Google Scholar]
- 265. Rainey WE, Viard I, Saez JM. 1989. Transforming growth factor β treatment decreases ACTH receptors on ovine adrenocortical cells. J Biol Chem 264:21474–21477 [PubMed] [Google Scholar]
- 266. Roy S, Rached M, Gallo-Payet N. 2007. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms α and β in isogenic human embryonic kidney 293 cells. Mol Endocrinol 21:1656–1669 [DOI] [PubMed] [Google Scholar]
- 267. Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. 2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 268. Di Blasio AM, Fujii DK, Yamamoto M, Martin MC, Jaffe RB. 1990. Maintenance of cell proliferation and steroidogenesis in cultured human fetal adrenal cells chronically exposed to adrenocorticotropic hormone: rationalization of in vitro and in vivo findings. Biol Reprod 42:683–691 [DOI] [PubMed] [Google Scholar]
- 269. Ramachandran J, Suyama AT. 1975. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci USA 72:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Hadley ME, Haskell-Luevano C. 1999. The proopiomelanocortin system. Ann NY Acad Sci 885:1–21 [DOI] [PubMed] [Google Scholar]
- 271. Coulter CL, Ross JT, Owens JA, Bennett HP, McMillen IC. 2002. Role of pituitary POMC-peptides and insulin-like growth factor II in the developmental biology of the adrenal gland. Arch Physiol Biochem 110:99–105 [DOI] [PubMed] [Google Scholar]
- 272. Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. 1999. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5:1066–1070 [DOI] [PubMed] [Google Scholar]
- 273. Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O'Rahilly S. 2004. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 145:4721–4727 [DOI] [PubMed] [Google Scholar]
- 274. Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ. 1982. Stimulation of adrenal mitogenesis by N-terminal proopiocortin peptides. Nature 297:419–422 [DOI] [PubMed] [Google Scholar]
- 275. Lowry PJ, Silas L, McLean C, Linton EA, Estivariz FE. 1983. Pro-γ-melanocyte-stimulating hormone cleavage in adrenal gland undergoing compensatory growth. Nature 306:70–73 [DOI] [PubMed] [Google Scholar]
- 276. Estivariz FE, Hope J, McLean C, Lowry PJ. 1980. Purification and characterization of a γ-melanotropin precursor from frozen human pituitary glands. Biochem J 191:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Ross JT, Bennett HP, James S, McMillen IC. 2000. Infusion of N-proopiomelanocortin-(1–77) increases adrenal weight and messenger ribonucleic acid levels of cytochrome P450 17α-hydroxylase in the sheep fetus during late gestation. Endocrinology 141:2153–2158 [DOI] [PubMed] [Google Scholar]
- 278. Coulter CL, Ross JT, Salkeld MD, Bennett HP, James S, McMillen IC. 2000. N-Proopiomelanocortin (1–77) suppresses expression of steroidogenic acute regulatory protein (StAR) mRNA in the adrenal gland of the fetal sheep. Endocr Res 26:523–529 [DOI] [PubMed] [Google Scholar]
- 279. Bicknell AB, Lomthaisong K, Woods RJ, Hutchinson EG, Bennett HP, Gladwell RT, Lowry PJ. 2001. Characterization of a serine protease that cleaves pro-γ-melanotropin at the adrenal to stimulate growth. Cell 105:903–912 [DOI] [PubMed] [Google Scholar]
- 280. Weir MR, Dzau VJ. 1999. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 12:205S–213S [DOI] [PubMed] [Google Scholar]
- 281. Williams GH. 2005. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev 10:7–13 [DOI] [PubMed] [Google Scholar]
- 282. Schütz S, Le Moullec JM, Corvol P, Gasc JM. 1996. Early expression of all the components of the renin-angiotensin-system in human development. Am J Pathol 149:2067–2079 [PMC free article] [PubMed] [Google Scholar]
- 283. Breault L, Lehoux JG, Gallo-Payet N. 1996. The angiotensin AT2 receptor is present in the human fetal adrenal gland throughout the second trimester of gestation. J Clin Endocrinol Metab 81:3914–3922 [DOI] [PubMed] [Google Scholar]
- 284. Chamoux E, Breault L, Lehoux JG, Gallo-Payet N. 1999. Involvement of the angiotensin II type 2 receptor in apoptosis during human fetal adrenal gland development. J Clin Endocrinol Metab 84:4722–4730 [DOI] [PubMed] [Google Scholar]
- 285. Ishimoto H, Minegishi K, Higuchi T, Asai S, Kim SH, Tanaka M, Yoshimura Y, Jaffe RB. 2008. Regulation of expression of angiotensin II receptor subtypes and ACTH receptor by ACTH and angiotensin II in human fetal adrenal cells. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008, P3-259 (abstract) [Google Scholar]
- 286. Nogueira EF, Bollag WB, Rainey WE. 2009. Angiotensin II regulation of adrenocortical gene transcription. Mol Cell Endocrinol 302:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Challis JRG, Matthews SG, Gibb W, Lye SJ. 2000. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 [DOI] [PubMed] [Google Scholar]
- 288. Challis JR, Sloboda D, Matthews SG, Holloway A, Alfaidy N, Patel FA, Whittle W, Fraser M, Moss TJ, Newnham J. 2001. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and postnatal health. Mol Cell Endocrinol 185:135–144 [DOI] [PubMed] [Google Scholar]
- 289. Price HV, Cone BA, Keogh M. 1971. Length of gestation in congenital adrenal hyperplasia. J Obstet Gynaecol Br Commonw 78:430–434 [DOI] [PubMed] [Google Scholar]
- 290. Gidlöf S, Wedell A, Nordenström A. 2007. Gestational age correlates to genotype in girls with CYP21 deficiency. J Clin Endocrinol Metab 92:246–249 [DOI] [PubMed] [Google Scholar]
- 291. O'Sullivan J, Iyer S, Taylor N, Cheetham T. 2007. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency is associated with a prolonged gestational age. Arch Dis Child 92:690–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Honnebier WJ, Swaab DF. 1973. The influence of anencephaly upon intrauterine growth of fetus and placenta and upon gestation length. J Obstet Gynaecol Br Commonw 80:577–588 [DOI] [PubMed] [Google Scholar]
- 293. Katz Z, Lancet M, Levavi E. 1979. The efficacy of intraamniotic steroids for induction of labor. Obstet Gynecol 54:31–34 [DOI] [PubMed] [Google Scholar]
- 294. Yeshaya A, Orvieto R, Ben-Shem E, Dekel A, Peleg D, Dicker D, Ben-Rafael Z. 1996. Uterine activity after betamethasone administration for the enhancement of fetal lung maturation. Eur J Obstet Gynecol Reprod Biol 67:139–141 [DOI] [PubMed] [Google Scholar]
- 295. Elliott JP, Radin TG. 1995. The effect of corticosteroid administration on uterine activity and preterm labor in high-order multiple gestations. Obstet Gynecol 85:250–254 [DOI] [PubMed] [Google Scholar]
- 296. Novy MJ, Walsh SW. 1983. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol 145:920–931 [DOI] [PubMed] [Google Scholar]
- 297. Gonzales LW, Ertsey R, Ballard PL, Froh D, Goerke J, Gonzales J. 1990. Glucocorticoid stimulation of fatty acid synthesis in explants of human fetal lung. Biochim Biophys Acta 1042:1–12 [DOI] [PubMed] [Google Scholar]
- 298. Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. 2001. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 32:76–91 [DOI] [PubMed] [Google Scholar]
- 299. Garbrecht MR, Klein JM, Schmidt TJ, Snyder JM. 2006. Glucocorticoid metabolism in the human fetal lung: implications for lung development and the pulmonary surfactant system. Biol Neonate 89:109–119 [DOI] [PubMed] [Google Scholar]
- 300. Michael AE, Thurston LM, Rae MT. 2003. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction 126:425–441 [DOI] [PubMed] [Google Scholar]
- 301. Bro-Rasmussen F, Buus O, Trolle D. 1962. Ratio cortisone/cortisol in mother and infant at birth. Acta Endocrinol (Copenh) 40:579–583 [DOI] [PubMed] [Google Scholar]
- 302. Predine J, Merceron L, Barrier G, Sureau C, Milgrom E. 1979. Unbound cortisol in umbilical cord plasma and maternal plasma: a reinvestigation. Am J Obstet Gynecol 135:1104–1108 [DOI] [PubMed] [Google Scholar]
- 303. Benediktsson R, Calder AA, Edwards CR, Seckl JR. 1997. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 46:161–166 [DOI] [PubMed] [Google Scholar]
- 304. Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. 1974. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol 118:538–541 [DOI] [PubMed] [Google Scholar]
- 305. Krozowski Z, MaGuire JA, Stein-Oakley AN, Dowling J, Smith RE, Andrews RK. 1995. Immunohistochemical localization of the 11β-hydroxysteroid dehydrogenase type II enzyme in human kidney and placenta. J Clin Endocrinol Metab 80:2203–2209 [DOI] [PubMed] [Google Scholar]
- 306. Hirasawa G, Takeyama J, Sasano H, Fukushima K, Suzuki T, Muramatu Y, Darnel AD, Kaneko C, Hiwatashi N, Toyota T, Nagura H, Krozowski ZS. 2000. 11β-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human placenta. J Clin Endocrinol Metab 85:1306–1309 [DOI] [PubMed] [Google Scholar]
- 307. Driver PM, Kilby MD, Bujalska I, Walker EA, Hewison M, Stewart PM. 2001. Expression of 11β-hydroxysteroid dehydrogenase isozymes and corticosteroid hormone receptors in primary cultures of human trophoblast and placental bed biopsies. Mol Hum Reprod 7:357–363 [DOI] [PubMed] [Google Scholar]
- 308. Sun K, Yang K, Challis JR. 1997. Differential expression of 11β-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrinol Metab 82:300–305 [DOI] [PubMed] [Google Scholar]
- 309. Murphy VE, Smith R, Giles WB, Clifton VL. 2006. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev 27:141–169 [DOI] [PubMed] [Google Scholar]
- 310. Sun K, Yang K, Challis JR. 1998. Regulation of 11β-hydroxysteroid dehydrogenase type 2 by progesterone, estrogen, and the cyclic adenosine 5′-monophosphate pathway in cultured human placental and chorionic trophoblasts. Biol Reprod 58:1379–1384 [DOI] [PubMed] [Google Scholar]
- 311. Pepe GJ, Albrecht ED. 1984. Comparison of cortisol-cortisone interconversion in vitro by the human and baboon placenta. Steroids 44:229–240 [DOI] [PubMed] [Google Scholar]
- 312. Pepe GJ, Waddell BJ, Stahl SJ, Albrecht ED. 1988. The regulation of transplacental cortisol-cortisone metabolism by estrogen in pregnant baboons. Endocrinology 122:78–83 [DOI] [PubMed] [Google Scholar]
- 313. Pepe GJ, Waddell BJ, Albrecht ED. 1990. Activation of the baboon fetal hypothalamic-pituitary-adrenocortical axis at midgestation by estrogen-induced changes in placental corticosteroid metabolism. Endocrinology 127:3117–3123 [DOI] [PubMed] [Google Scholar]
- 314. Baggia S, Albrecht ED, Pepe GJ. 1990. Regulation of 11β-hydroxysteroid dehydrogenase activity in the baboon placenta by estrogen. Endocrinology 126:2742–2748 [DOI] [PubMed] [Google Scholar]
- 315. Ricketts ML, Verhaeg JM, Bujalska I, Howie AJ, Rainey WE, Stewart PM. 1998. Immunohistochemical localization of type 1 11β-hydroxysteroid dehydrogenase in human tissues. J Clin Endocrinol Metab 83:1325–1335 [DOI] [PubMed] [Google Scholar]
- 316. Pepe GJ, Burch MG, Albrecht ED. 1999. Expression of the 11β-hydroxysteroid dehydrogenase types 1 and 2 proteins in human and baboon placental syncytiotrophoblast. Placenta 20:575–582 [DOI] [PubMed] [Google Scholar]
- 317. Alfaidy N, Li W, MacIntosh T, Yang K, Challis J. 2003. Late gestation increase in 11β-hydroxysteroid dehydrogenase 1 expression in human fetal membranes: a novel intrauterine source of cortisol. J Clin Endocrinol Metab 88:5033–5038 [DOI] [PubMed] [Google Scholar]
- 318. Stewart PM, Rogerson FM, Mason JI. 1995. Type 2 11β-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J Clin Endocrinol Metab 80:885–890 [DOI] [PubMed] [Google Scholar]
- 319. Tanswell AK, Worthington D, Smith BT. 1977. Human amniotic membrane corticosteroid 11-oxidoreductase activity. J Clin Endocrinol Metab 45:721–725 [DOI] [PubMed] [Google Scholar]
- 320. Sun K, He P, Yang K. 2002. Intracrine induction of 11β-hydroxysteroid dehydrogenase type 1 expression by glucocorticoid potentiates prostaglandin production in the human chorionic trophoblast. Biol Reprod 67:1450–1455 [DOI] [PubMed] [Google Scholar]
- 321. Yang Z, Guo C, Zhu P, Li W, Myatt L, Sun K. 2007. Role of glucocorticoid receptor and CCAAT/enhancer-binding protein α in the feed-forward induction of 11β-hydroxysteroid dehydrogenase type 1 expression by cortisol in human amnion fibroblasts. J Endocrinol 195:241–253 [DOI] [PubMed] [Google Scholar]
- 322. Sun K, Myatt L. 2003. Enhancement of glucocorticoid-induced 11β-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology 144:5568–5577 [DOI] [PubMed] [Google Scholar]
- 323. Yang Z, Zhu P, Guo C, Zhu X, Sun K. 2009. Expression of 11β-hydroxysteroid dehydrogenase type 1 in human fetal lung and regulation of its expression by interleukin-1β and cortisol. J Clin Endocrinol Metab 94:306–313 [DOI] [PubMed] [Google Scholar]
- 324. Coulter CL, Smith RE, Stowasser M, Sasano H, Krozowski ZS, Gordon RD. 1999. Expression of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD-2) in the developing human adrenal gland and human adrenal cortical carcinoma and adenoma. Mol Cell Endocrinol 154:71–77 [DOI] [PubMed] [Google Scholar]
- 325. Roberts D, Dalziel S. 2006. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3:CD004454. [DOI] [PubMed] [Google Scholar]
- 326. Tegethoff M, Pryce C, Meinlschmidt G. 2009. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev 30:753–789 [DOI] [PubMed] [Google Scholar]
- 327. Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, Amankwah K, Guselle P, Gafni A, Lee SK, Armson BA. 2008. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 372:2143–2151 [DOI] [PubMed] [Google Scholar]
- 328. Kitanaka S, Tanae A, Hibi I. 1996. Apparent mineralocorticoid excess due to 11β-hydroxysteroid dehydrogenase deficiency: a possible cause of intrauterine growth retardation. Clin Endocrinol (Oxf) 44:353–359 [DOI] [PubMed] [Google Scholar]
- 329. Stewart PM, Krozowski ZS, Gupta A, Milford DV, Howie AJ, Sheppard MC, Whorwood CB. 1996. Hypertension in the syndrome of apparent mineralocorticoid excess due to mutation of the 11β-hydroxysteroid dehydrogenase type 2 gene. Lancet 347:88–91 [DOI] [PubMed] [Google Scholar]
- 330. Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. 2008. Placental 11β-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta 29:193–200 [DOI] [PubMed] [Google Scholar]
- 331. Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. 2002. Oxygen regulation of placental 11β-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metab 87:4797–4805 [DOI] [PubMed] [Google Scholar]
- 332. Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, Surbek D, Hocher B, Mohaupt MG. 2009. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res 65:198–202 [DOI] [PubMed] [Google Scholar]
- 333. Jansson T, Powell TL. 2007. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113:1–13 [DOI] [PubMed] [Google Scholar]
- 334. Seckl JR, Holmes MC. 2007. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab 3:479–488 [DOI] [PubMed] [Google Scholar]
- 335. Casey ML, MacDonald PC, Mitchell MD. 1985. Despite a massive increase in cortisol secretion in women during parturition, there is an equally massive increase in prostaglandin synthesis. A paradox? J Clin Invest 75:1852–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Potestio FA, Zakar T, Olson DM. 1988. Glucocorticoids stimulate prostaglandin synthesis in human amnion cells by a receptor-mediated mechanism. J Clin Endocrinol Metab 67:1205–1210 [DOI] [PubMed] [Google Scholar]
- 337. Sun K, Ma R, Cui X, Campos B, Webster R, Brockman D, Myatt L. 2003. Glucocorticoids induce cytosolic phospholipase A2 and prostaglandin H synthase type 2 but not microsomal prostaglandin E synthase (PGES) and cytosolic PGES expression in cultured primary human amnion cells. J Clin Endocrinol Metab 88:5564–5571 [DOI] [PubMed] [Google Scholar]
- 338. Whittle WL, Gibb W, Challis JR. 2000. The characterization of human amnion epithelial and mesenchymal cells: the cellular expression, activity and glucocorticoid regulation of prostaglandin output. Placenta 21:394–401 [DOI] [PubMed] [Google Scholar]
- 339. Smieja Z, Zakar T, Olson DM. 1993. Stimulation of cultured amnion cell prostaglandin endoperoxide H synthase activity by glucocorticoids and phorbol ester. Am J Obstet Gynecol 169:653–661 [DOI] [PubMed] [Google Scholar]
- 340. Patel FA, Sun K, Challis JR. 1999. Local modulation by 11β-hydroxysteroid dehydrogenase of glucocorticoid effects on the activity of 15-hydroxyprostaglandin dehydrogenase in human chorion and placental trophoblast cells. J Clin Endocrinol Metab 84:395–400 [DOI] [PubMed] [Google Scholar]
- 341. Patel FA, Clifton VL, Chwalisz K, Challis JR. 1999. Steroid regulation of prostaglandin dehydrogenase activity and expression in human term placenta and chorio-decidua in relation to labor. J Clin Endocrinol Metab 84:291–299 [DOI] [PubMed] [Google Scholar]
- 342. Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomorey S, Lye SJ, Gibb W, Challis JR. 2001. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol Reprod 64:1019–1032 [DOI] [PubMed] [Google Scholar]
- 343. Economopoulos P, Sun M, Purgina B, Gibb W. 1996. Glucocorticoids stimulate prostaglandin H synthase type-2 (PGHS-2) in the fibroblast cells in human amnion cultures. Mol Cell Endocrinol 117:141–147 [DOI] [PubMed] [Google Scholar]
- 344. Zakar T, Hirst JJ, Mijovic JE, Olson DM. 1995. Glucocorticoids stimulate the expression of prostaglandin endoperoxide H synthase-2 in amnion cells. Endocrinology 136:1610–1619 [DOI] [PubMed] [Google Scholar]
- 345. Alfaidy N, Xiong ZG, Myatt L, Lye SJ, MacDonald JF, Challis JR. 2001. Prostaglandin F2α potentiates cortisol production by stimulating 11β-hydroxysteroid dehydrogenase 1: a novel feedback loop that may contribute to human labor. J Clin Endocrinol Metab 86:5585–5592 [DOI] [PubMed] [Google Scholar]
- 346. Sun K, Brockman D, Campos B, Pitzer B, Myatt L. 2006. Induction of surfactant protein A expression by cortisol facilitates prostaglandin synthesis in human chorionic trophoblasts. J Clin Endocrinol Metab 91:4988–4994 [DOI] [PubMed] [Google Scholar]
- 347. Mazouni C, Provensal M, Porcu G, Guidicelli B, Heckenroth H, Gamerre M, Bretelle F. 2006. Termination of pregnancy in patients with previous cesarean section. Contraception 73:244–248 [DOI] [PubMed] [Google Scholar]
- 348. Csapo AI, Pinto-Dantas CA. 1965. The effect of progesterone on the human uterus. Proc Natl Acad Sci USA 54:1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Brown AG, Leite RS, Strauss JF., 3rd 2004. Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann NY Acad Sci 1034:36–49 [DOI] [PubMed] [Google Scholar]
- 350. Mesiano S. 2004. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig 11:193–202 [DOI] [PubMed] [Google Scholar]
- 351. Mesiano S. 2007. Myometrial progesterone responsiveness. Semin Reprod Med 25:5–13 [DOI] [PubMed] [Google Scholar]
- 352. Zakar T, Hertelendy F. 2007. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 196:289–296 [DOI] [PubMed] [Google Scholar]
- 353. Mendelson CR. 2009. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Giangrande PH, McDonnell DP. 1999. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 54:291–313; discussion 313–314 [PubMed] [Google Scholar]
- 355. Conneely OM, Lydon JP. 2000. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids 65:571–577 [DOI] [PubMed] [Google Scholar]
- 356. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. 2000. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Mesiano S, Welsh TN. 2007. Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol 18:321–331 [DOI] [PubMed] [Google Scholar]
- 358. Boroditsky RS, Reyes FI, Winter JS, Faiman C. 1978. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol 51:686–691 [PubMed] [Google Scholar]
- 359. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. 1972. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 112:1095–1100 [DOI] [PubMed] [Google Scholar]
- 360. Bedin M, Fournier T, Cabrol D, Bréart G, Kottler ML, Cedard L. 1987. Incidence of placental sulfatase deficiency on the mode of termination of pregnancy. Gynecol Obstet Invest 24:86–91 [DOI] [PubMed] [Google Scholar]
- 361. Shozu M, Akasofu K, Harada T, Kubota Y. 1991. A new cause of female pseudohermaphroditism: placental aromatase deficiency. J Clin Endocrinol Metab 72:560–566 [DOI] [PubMed] [Google Scholar]
- 362. Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood MM, Graham-Lorence S, Sun T, Fisher CR, Qin K, Mendelson CR. 1997. Aromatase expression in health and disease. Recent Prog Horm Res 52:185–213; discussion 213–214 [PubMed] [Google Scholar]
- 363. Belgorosky A, Pepe C, Marino R, Guercio G, Saraco N, Vaiani E, Rivarola MA. 2003. Hypothalamic-pituitary-ovarian axis during infancy, early and late prepuberty in an aromatase-deficient girl who is a compound heterocygote for two new point mutations of the CYP19 gene. J Clin Endocrinol Metab 88:5127–5131 [DOI] [PubMed] [Google Scholar]
- 364. Deladoëy J, Flück C, Bex M, Yoshimura N, Harada N, Mullis PE. 1999. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. J Clin Endocrinol Metab 84:4050–4054 [DOI] [PubMed] [Google Scholar]
- 365. Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. 1997. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab 82:1739–1745 [DOI] [PubMed] [Google Scholar]
- 366. Mecenas CA, Giussani DA, Owiny JR, Jenkins SL, Wu WX, Honnebier BO, Lockwood CJ, Kong L, Guller S, Nathanielsz PW. 1996. Production of premature delivery in pregnant rhesus monkeys by androstenedione infusion. Nat Med 2:443–448 [DOI] [PubMed] [Google Scholar]
- 367. Nathanielsz PW, Jenkins SL, Tame JD, Winter JA, Guller S, Giussani DA. 1998. Local paracrine effects of estradiol are central to parturition in the rhesus monkey. Nat Med 4:456–459 [DOI] [PubMed] [Google Scholar]
- 368. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. 2002. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- 369. Vale W, Spiess J, Rivier C, Rivier J. 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- 370. Power ML, Schulkin J. 2006. Functions of corticotropin-releasing hormone in anthropoid primates: from brain to placenta. Am J Hum Biol 18:431–447 [DOI] [PubMed] [Google Scholar]
- 371. Giles WB, McLean M, Davies JJ, Smith R. 1996. Abnormal umbilical artery Doppler waveforms and cord blood corticotropin-releasing hormone. Obstet Gynecol 87:107–111 [DOI] [PubMed] [Google Scholar]
- 372. Goland RS, Conwell IM, Jozak S. 1995. The effect of pre-eclampsia on human placental corticotrophin-releasing hormone content and processing. Placenta 16:375–382 [DOI] [PubMed] [Google Scholar]
- 373. Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. 1993. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab 77:1174–1179 [DOI] [PubMed] [Google Scholar]
- 374. Goland RS, Tropper PJ, Warren WB, Stark RI, Jozak SM, Conwell IM. 1995. Concentrations of corticotrophin-releasing hormone in the umbilical-cord blood of pregnancies complicated by pre-eclampsia. Reprod Fertil Dev 7:1227–1230 [DOI] [PubMed] [Google Scholar]
- 375. McGrath S, McLean M, Smith D, Bisits A, Giles W, Smith R. 2002. Maternal plasma corticotropin-releasing hormone trajectories vary depending on the cause of preterm delivery. Am J Obstet Gynecol 186:257–260 [DOI] [PubMed] [Google Scholar]
- 376. McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. 1995. A placental clock controlling the length of human pregnancy. Nat Med 1:460–463 [DOI] [PubMed] [Google Scholar]
- 377. Smith R. 2007. Parturition. N Engl J Med 356:271–283 [DOI] [PubMed] [Google Scholar]
- 378. Petraglia F, Imperatore A, Challis JR. 14 July 2010. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev 10.1210/er.2009-0019 [DOI] [PubMed] [Google Scholar]
- 379. Grammatopoulos DK. 2007. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci 12:561–571 [DOI] [PubMed] [Google Scholar]
- 380. Robinson BG, Emanuel RL, Frim DM, Majzoub JA. 1988. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci USA 85:5244–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. 2000. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab 85:1937–1945 [DOI] [PubMed] [Google Scholar]
- 382. Korebrits C, Yu DH, Ramirez MM, Marinoni E, Bocking AD, Challis JR. 1998. Antenatal glucocorticoid administration increases corticotrophin- releasing hormone in maternal plasma. Br J Obstet Gynaecol 105:556–561 [DOI] [PubMed] [Google Scholar]
- 383. Ni X, Chan EC, Fitter JT, Smith R. 1997. Nitric oxide inhibits corticotropin-releasing hormone exocytosis but not synthesis by cultured human trophoblasts. J Clin Endocrinol Metab 82:4171–4175 [DOI] [PubMed] [Google Scholar]
- 384. Ni X, Hou Y, King BR, Tang X, Read MA, Smith R, Nicholson RC. 2004. Estrogen receptor-mediated down-regulation of corticotropin-releasing hormone gene expression is dependent on a cyclic adenosine 3′,5′-monophosphate regulatory element in human placental syncytiotrophoblast cells. J Clin Endocrinol Metab 89:2312–2318 [DOI] [PubMed] [Google Scholar]
- 385. Ni X, Hou Y, Yang R, Tang X, Smith R, Nicholson RC. 2004. Progesterone receptors A and B differentially modulate corticotropin-releasing hormone gene expression through a cAMP regulatory element. Cell Mol Life Sci 61:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Petraglia F, Sutton S, Vale W. 1989. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol 160:247–251 [DOI] [PubMed] [Google Scholar]
- 387. Petraglia F, Garuti GC, De Ramundo B, Angioni S, Genazzani AR, Bilezikjian LM. 1990. Mechanism of action of interleukin-1β in increasing corticotropin-releasing factor and adrenocorticotropin hormone release from cultured human placental cells. Am J Obstet Gynecol 163:1307–1312 [DOI] [PubMed] [Google Scholar]
- 388. Plotsky PM. 1987. Regulation of hypophysiotropic factors mediating ACTH secretion. Ann NY Acad Sci 512:205–217 [DOI] [PubMed] [Google Scholar]
- 389. Reis FM, Petraglia F. 2001. The placenta as a neuroendocrine organ. Front Horm Res 27:216–228 [DOI] [PubMed] [Google Scholar]
- 390. Warren WB, Goland RS. 1995. Effects of parturition on corticotropin releasing hormone and products of the pituitary and adrenal in term fetuses at delivery. J Perinat Med 23:453–458 [DOI] [PubMed] [Google Scholar]
- 391. Nodwell A, Carmichael L, Fraser M, Challis J, Richardson B. 1999. Placental release of corticotrophin-releasing hormone across the umbilical circulation of the human newborn. Placenta 20:197–202 [DOI] [PubMed] [Google Scholar]
- 392. Asa SL, Kovacs K, Singer W. 1991. Human fetal adenohypophysis: morphologic and functional analysis in vitro. Neuroendocrinology 53:562–572 [DOI] [PubMed] [Google Scholar]
- 393. Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. 1998. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab 83:2916–2920 [DOI] [PubMed] [Google Scholar]
- 394. Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW. 2001. Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab 86:4512–4519 [DOI] [PubMed] [Google Scholar]
- 395. Sirianni R, Mayhew BA, Carr BR, Parker CR, Jr, Rainey WE. 2005. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab 90:5393–5400 [DOI] [PubMed] [Google Scholar]
- 396. Parker CR, Jr, Stankovic AM, Goland RS. 1999. Corticotropin-releasing hormone stimulates steroidogenesis in cultured human adrenal cells. Mol Cell Endocrinol 155:19–25 [DOI] [PubMed] [Google Scholar]
- 397. Thomas M, Keramidas M, Monchaux E, Feige JJ. 2004. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology 145:4320–4329 [DOI] [PubMed] [Google Scholar]
- 398. Miller WL. 1998. Steroid hormone biosynthesis and actions in the materno-feto-placental unit. Clin Perinatol 25:799–817, v [PubMed] [Google Scholar]