Steroidogenesis, the processes by which cholesterol is converted to steroid hormones, involves transport proteins, enzymes, redox partners and cofactors. Most steroidogenic enzymes are either forms of cytochrome P450 or are hydroxysteroid dehydrogenases. The P450s may be either Type 1, in mitochondria, or Type 2, in the endoplasmic reticulum; these two types differ in their electron-transfer redox partners as well as in their cellular locations. Hydroxysteroid dehydrogenases may be either shortchain dehydrogenases or aldo-keto reductases, which differ in their structures and catalytic mechanisms. Recent work has identified new enzymes, co-factors and protein modifications, and has described new pathways of steroidogenesis and new sites of steroid synthesis. Thus steroidogenesis is not confined to the adrenals and gonads, and involves more than the production of aldosterone, cortisol and sex steroids. We review the enzymes, factors and pathways of human steroidogenesis and the diseases resulting from their mutations.
Abstract
Steroidogenesis entails processes by which cholesterol is converted to biologically active steroid hormones. Whereas most endocrine texts discuss adrenal, ovarian, testicular, placental, and other steroidogenic processes in a gland-specific fashion, steroidogenesis is better understood as a single process that is repeated in each gland with cell-type-specific variations on a single theme. Thus, understanding steroidogenesis is rooted in an understanding of the biochemistry of the various steroidogenic enzymes and cofactors and the genes that encode them. The first and rate-limiting step in steroidogenesis is the conversion of cholesterol to pregnenolone by a single enzyme, P450scc (CYP11A1), but this enzymatically complex step is subject to multiple regulatory mechanisms, yielding finely tuned quantitative regulation. Qualitative regulation determining the type of steroid to be produced is mediated by many enzymes and cofactors. Steroidogenic enzymes fall into two groups: cytochrome P450 enzymes and hydroxysteroid dehydrogenases. A cytochrome P450 may be either type 1 (in mitochondria) or type 2 (in endoplasmic reticulum), and a hydroxysteroid dehydrogenase may belong to either the aldo-keto reductase or short-chain dehydrogenase/reductase families. The activities of these enzymes are modulated by posttranslational modifications and by cofactors, especially electron-donating redox partners. The elucidation of the precise roles of these various enzymes and cofactors has been greatly facilitated by identifying the genetic bases of rare disorders of steroidogenesis. Some enzymes not principally involved in steroidogenesis may also catalyze extraglandular steroidogenesis, modulating the phenotype expected to result from some mutations. Understanding steroidogenesis is of fundamental importance to understanding disorders of sexual differentiation, reproduction, fertility, hypertension, obesity, and physiological homeostasis.
Introduction
-
Cholesterol Uptake, Storage, and Intracellular Transport
Delivery of cholesterol to mitochondria
Disorders of cholesterol synthesis and trafficking
-
An Overview of Steroidogenic Enzymes
Cytochrome P450
Hydroxysteroid dehydrogenases
-
The Steroidogenic Acute Regulatory Protein
Acute regulation of steroidogenesis
StAR structure and mechanism of action
Disorders of StAR: classic and nonclassic congenital lipoid adrenal hyperplasia
-
Conversion of Cholesterol to Pregnenolone: P450scc and Its Electron Transfer Proteins
P450scc
P450scc deficiency
Chronic maintenance of the steroidogenic machinery
Transport of electrons to P450scc: ferredoxin reductase and ferredoxin
Ferredoxin reductase
Ferredoxin
-
3β-Hydroxysteroid Dehydrogenase/Δ5→Δ4 Isomerase
3β-Hydroxysteroid dehydrogenase deficiency
-
P450c17: 17α-Hydroxylase/17,20-Lyase
Transcriptional regulation of the human CYP17A1 gene encoding P450c17
17α-Hydroxylase/17,20-lyase deficiency
Isolated 17,20-lyase deficiency
-
Electron Transport: P450 Oxidoreductase and Cytochrome b5
P450 oxidoreductase
P450 oxidoreductase deficiency
Cytochrome b5
-
P450c21: Steroid 21-Hydroxylase
CYP21 genes and the genetics of 21-hydroxylase deficiency
21-Hydroxylase deficiency
Molecular genetics of 21-hydroxylase deficiency
-
Isozymes of P450c11: P450c11β and P450c11AS
Isozymes of P450c11
Overview of lesions in isozymes of P450c11
11β-Hydroxylase deficiency
Aldosterone synthase deficiencies
Glucocorticoid-remediable aldosteronism
-
Isozymes of 17β-Hydroxysteroid Dehydrogenase
17βHSD type 1
17βHSD type 2
17βHSD type 3
17βHSD type 4
17βHSD type 5
17βHSD type 6
P450aro: Aromatase
Isozymes of 5α-Reductase
Isozymes of 3α-Hydroxysteroid Dehydrogenase
-
Isozymes of 11β-Hydroxysteroid Dehydrogenase
Lesions in 11βHSD1—apparent cortisone reductase deficiency
Lesions in 11βHSD2—apparent mineralocorticoid excess
Steroid Sulfatase and Sulfotransferases
-
Other Genetic Adrenal Disorders Associated with Steroidogenesis
Adrenal hypoplasia congenita
ACTH resistance syndromes
Familial glucocorticoid resistance
Pseudohypoaldosteronism
-
Tissue-Specific Pathways of Steroidogenesis
Adrenal pathways
Gonadal pathways
The “backdoor pathway” to dihydrotestosterone
Neurosteroids: steroid synthesis in the brain
-
Fetoplacental Steroidogenesis
The fetal adrenal
Placental steroidogenesis
Adrenarche
I. Introduction
Steroid hormones regulate a wide variety of developmental and physiological processes from fetal life to adulthood. Steroid hormones are all synthesized from cholesterol and hence have closely related structures based on the classic cyclopentanophenanthrene 4-ring structure (Fig. 1). These structures were painstakingly determined in the 1930s (1, 2), and precursor/product relationships were identified, leading to the general understanding of the pathways of steroidogenesis. Isolation of some key steroidogenic enzymes from animal sources and the cloning of many of their cDNAs and genes in the 1980s showed that there were fewer steroidogenic enzymes than there were steroidogenic reactions and that, in most cases, a particular steroidogenic reaction was catalyzed by the same enzyme in all tissues, dramatically revising the views derived from steroid chemistry alone; this revolution in the understanding of steroidogenesis was reviewed in 1988 (3). The ensuing 23 yr have witnessed major developments in four areas of steroidogenesis: 1) the cloning of the steroidogenic acute regulatory protein (StAR) and subsequent study of the mechanisms of intracellular cholesterol transport; 2) the expanding array of hydroxysteroid dehydrogenases (HSDs); 3) the expanding roles of electron transfer proteins and other cofactors in disease; and 4) the elucidation of additional pathways of steroidogenesis in classical and extraglandular tissues. All have added substantial complexity and subtlety to the understanding of molecular steroidogenesis. Different physiological categories of steroids (androgens, estrogens, and later mineralocorticoids and glucocorticoids) were recognized more than 70 yr ago (4), but despite efforts to correlate steroid structures with their activities, this area was not understood until the various steroid hormone receptors were identified and cloned (5–10). Thus, the contemporary definition of each class of steroid is based on the receptor(s) to which it binds, rather than on the chemical structure of the steroid.
Fig. 1.
Structure of pregnenolone, illustrating the cycloperhydropentano-phenanthrene structure common to all steroids. The carbon atoms are indicated by numbers, and the rings are designated by letters according to standard convention. Substituents and hydrogens are labeled as α or β if they are positioned behind or in front of the plane of the page, respectively. Pregnenolone is derived from cholesterol, which has a six-carbon side chain attached to carbon no. 20. Pregnenolone is a “Δ5 compound,” having a double bond between carbons no. 5 and 6; the action of 3β-hydroxysteroid dehydrogenase/isomerase moves this double bond from the B ring to carbons 4 and 5 in the A ring, forming Δ4 compounds. Most of the major biologically active steroid hormones are Δ4 compounds. [© R. J. Auchus.]
Substantially more study has been devoted to steroid hormone action than to steroid hormone synthesis, partly because steroids are such widely used drugs and partly because disorders of steroid hormone synthesis were formerly thought to be confined to rare genetic lesions. Work in the past 30 yr has identified the steroidogenic enzymes and their genes (Table 1), reinvigorating studies of steroid biosynthesis by discoveries of roles for altered regulation of steroidogenesis in common disorders such as hypertension and the polycystic ovary syndrome and by discoveries of steroid-modifying enzymes in target tissues that mediate some forms of apparent tissue specificity of hormone action. Thus, the study and understanding of steroidogenesis is germane to broad areas of medicine, physiology, and pharmacology.
Table 1.
Physical characteristics of human genes encoding steroidogenic enzymes
| Enzyme | Gene | Gene size (kb) | Chromosomal location | Exons (n) | mRNA size (kb) |
|---|---|---|---|---|---|
| StAR | STAR | 8 | 8p11.2 | 8 | 1.6 |
| P450scc | CYP11A1 | 30 | 15q23-q24 | 9 | 2.0 |
| P450c11β | CYP11B1 | 9.5 | 8q21-22 | 9 | 4.2 |
| P450c11AS | CYP11B2 | 9.5 | 8q21-22 | 9 | 4.2 |
| P450c17 | CYP17A1 | 6.6 | 10q24.3 | 8 | 1.9 |
| P450c21 | CYP21A2 | 3.4 | 6p 21.1 | 10 | 2.0 |
| P450aro | CYP19A1 | 130 | 15q21.1 | 10 | 1.5–4.5 |
| 3βHSD1 | HSD3B1 | 8 | 1p13.1 | 4 | 1.7 |
| 3βHSD2 | HSD3B2 | 8 | 1p13.1 | 4 | 1.7 |
| 11βHSD1 | HSD11B1 | 7 | 1q32-q41 | 6 | 1.6 |
| 11βHSD2 | HSD11B2 | 6.2 | 16q22 | 5 | 1.6 |
| 17βHSD1 | HSD17B1 | 3.3 | 17q11-q21 | 6 | 1.4, 2.4 |
| 17βHSD2 | HSD17B2 | 63 | 16q24.1-q24.2 | 5 | 1.5 |
| 17βHSD3 | HSD17B3 | 67 | 9q22 | 11 | 1.2 |
| 17βHSD6 (RODH) | HSD17B6 | 24.5 | 12q13 | 5 | 1.6 |
| AKR1C1 | AKR1C1 | 14.3 | 10p14-p15 | 9 | 1.2 |
| AKR1C2 | AKR1C2 | 13.8 | 10p14-p15 | 9 | 1.3 |
| AKR1C3 | AKR1C3 | 13.0 | 10p14-p15 | 9 | 1.2 |
| AKR1C4 | AKR1C4 | 22.1 | 10p14-p15 | 9 | 1.2 |
| 5α-Reductase 1 | SRD5A1 | 36 | 5p15 | 5 | 2.4 |
| 5α-Reductase 2 | SRD5A2 | 56 | 2p23 | 5 | 2.4 |
| SULT2A1 | SULT2A1 | 17 | 19q13.3 | 6 | 2.0 |
| PAPSS2 | PAPSS2 | 85 | 10q24 | 13 | 3.9 |
| P450-oxidoreductase | POR | 69 | 7q11.2 | 16 | 2.5 |
| Ferredoxin | FDX1 | 35 | 11q22 | 5 | 1.0, 1.4, 1.7, 3.2 |
| Ferredoxin reductase | FDXR | 11 | 17q24-q25 | 12 | 2.0 |
| Cytochrome b5 | CYB5A | 32 | 18q23 | 5 | 0.9 |
| H6PDH | H6PD | 36.5 | 1p36 | 5 | 9.1 |
© R. J. Auchus and W. L. Miller.
II. Cholesterol Uptake, Storage, and Intracellular Transport
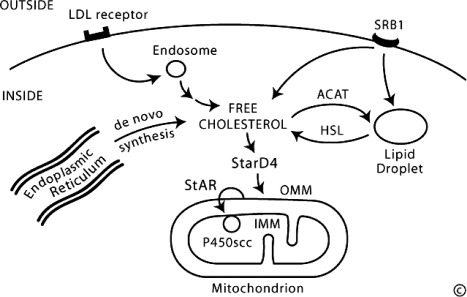
The human adrenal can synthesize cholesterol de novo from acetate (11), but most of its supply of cholesterol comes from plasma low-density lipoproteins (LDLs) derived from dietary cholesterol (12). By contrast, rodent adrenals derive most of their cholesterol from high-density lipoproteins via a receptor termed scavenger receptor B1, but this pathway appears to play a minor role in human steroidogenesis (Fig. 2). The intracellular cholesterol economy is largely regulated by the sterol response element binding protein (SREBPs), a group of transcription factors that regulate genes involved in the biosynthesis of cholesterol and fatty acids (13). Adequate concentrations of LDL will suppress 3-hydroxy-3-methylglutaryl co-enzyme A reductase, the rate-limiting enzyme in cholesterol synthesis. ACTH also stimulates the activity of 3-hydroxy-3-methylglutaryl co-enzyme A reductase, LDL receptors, and uptake of LDL cholesterol. LDL cholesterol esters are taken up by receptor-mediated endocytosis, and are then stored directly or converted to free cholesterol and used for steroid hormone synthesis (14). Cholesterol can be esterified by acyl-coenzyme A (CoA):cholesterol acyltransferase (ACAT), stored in lipid droplets, and accessed by activation of hormone-sensitive lipase (HSL). ACTH stimulates HSL and inhibits ACAT, thus increasing the availability of free cholesterol for steroid hormone synthesis. Cholesterol ester hydrolase and neutral cholesterol ester hydrolase also hydrolyze cytosolic cholesterol esters, but the relative contributions of these three enzymes are not known (15).
Fig. 2.
Principal features of the cellular cholesterol economy. Human cells typically pick up circulating LDLs through receptor-mediated endocytosis, directing the cholesterol to endosomes. Rodent cells pick up high-density lipoproteins via scavenger receptor B1 (SRB1) and direct it to lipid droplets. Cholesterol can also be synthesized de novo from acetate in the endoplasmic reticulum. Irrespective of source, cholesterol can be esterified by ACAT and stored in lipid droplets as cholesterol esters. Free cholesterol, produced by the action of HSL, may be bound by StarD4 for transcytoplasmic transport to membrane destinations, including the OMM. In the adrenals and gonads, StAR is responsible for the rapid movement of cholesterol from the OMM to the IMM, where it can be taken up by the cholesterol side-chain cleavage enzyme, P450scc, and converted to pregnenolone. [© W. L. Miller.]
A. Delivery of cholesterol to mitochondria
The first step in steroidogenesis takes place within mitochondria. The mechanisms by which cholesterol is transported to and loaded into the outer mitochondrial membrane (OMM) remain an active area of research (16, 17). StAR (see Section IV.A), which governs the acute steroidogenic response to tropic stimuli, appears to contribute to this step in a minor fashion; the principal action of StAR is to facilitate the movement of cholesterol from the OMM to the inner mitochondrial membrane (IMM). StAR is the first-described member of a family of proteins that contain so-called START (StAR-related lipid transfer) domains, which are found in most metazoan organisms (18). Fifteen START domain (StarD) proteins appear to serve roles in binding and mediating the intracellular transfer of lipids in mammals (19). StarD4 and StarD5 may play roles in moving cholesterol from intracellular lipid droplets to the OMM, but knockout of the gene for StarD4 in mice does not disrupt steroidogenesis (20).
Free cholesterol is nearly insoluble (critical micellar concentration, ∼25–40 nm) (21). Some cholesterol may be incorporated into vesicular membranes that then fuse with other membranes, thus delivering cholesterol from one intracellular compartment to another, but this appears to be a minor pathway (22). Instead, cholesterol is solubilized by binding to proteins. Early work focused on sterol-carrier protein 2 (SCP-2) and its homolog, SCP-x, but these appear to be nonspecific lipid binding and transfer proteins that play a minor role in the intracellular cholesterol economy (23). Analysis of SREBP-responsive transcripts identified a group of proteins termed StarD4, -5, and -6 that are structurally related to StAR, and appear to play major roles in intracellular cholesterol transport (19). These proteins have closely related cDNA, gene, and protein structures (24). The crystal structure of one of these, StarD4 (25), is essentially the same as the StAR-like domain of a protein called MLN64 (metastatic lymph node clone 64) (26). StarD4, -5, and -6 lack signal sequences that target them to specific subcellular organelles; hence, they appear to be confined to the cytoplasm, where they bind insoluble lipids, permitting the lipid to be transported across aqueous cytosol. Mouse StarD4 (but not StarD5) is regulated by SREBP, and both StarD4 and D5 can exert low levels of StAR-like activity in COS-1 cells cotransfected with the cholesterol side-chain cleavage enzyme system (27). By contrast, StarD6, which appears to be confined to the male germ line, has greater StAR-like activity than StAR itself (28). Thus, the current view is that the family of proteins related to StarD4 are responsible for delivering cholesterol to the OMM from elsewhere in the cell (lipid droplets, endoplasmic reticulum) in most cell types, whereas StAR itself is responsible for delivery from the OMM to the IMM, but only acts in steroidogenic cells.
B. Disorders of cholesterol synthesis and trafficking
There are several genetic disorders in these early steps in steroidogenesis, including adrenoleukodystrophy (ALD) (Schilder disease) and disorders of cholesterol synthesis and metabolism (e.g., Wolman disease, cholesterol ester storage disease, and Smith-Lemli-Opitz syndrome). These diseases typically cause primary adrenal insufficiency. Their impact on fetoplacental development is ameliorated by transplacental cholesterol delivered from the mother (29).
1. Adrenoleukodystrophy
ALD is a relatively common metabolic disorder causing adrenal failure. The prevalence of ALD is probably between 1:20,000 and 1:100,000, although the overall frequency may be as high as 1:17,000 (30). Most cases are caused by mutations in the gene encoding the peroxisomal membrane protein ALDP (ABCD1, Xq28) (31, 32), which belongs to the superfamily of ATP-binding cassette transporters. There is also a rare autosomal recessive form that usually presents in infancy. ALDP imports activated very long chain fatty acid acyl-CoA derivatives into peroxisomes where they are shortened by β-oxidation (33, 34). ALD is thus characterized by high ratios of C26 to C22 very long chain fatty acids in plasma and tissues, permitting accurate diagnosis (35). Carriers can usually be detected by very long chain fatty acid screening, although genetic analysis may be necessary in some cases. X-linked ALD commonly becomes symptomatic in midchildhood, and its variant, adrenomyeloneuropathy, presents in adulthood (36). The same ALDP mutation can cause both ALD and adrenomyeloneuropathy; hence, it is likely that other genetic loci are also involved (37). There is central nervous system (CNS) leukodystrophy causing behavioral changes and diminishing intellectual function progressing to severe dementia. Symptoms of adrenal insufficiency may appear before or after the brain symptoms (30, 38, 39). Adrenomyeloneuropathy typically begins with adrenal insufficiency in childhood and adolescence, and signs of neurological disease follow 10 to 15 yr later. Therapeutic options are limited, centering on hematopoietic stem cell transplantation for early cerebral disease and statin drugs (30, 34).
2. Wolman disease
Wolman disease (primary xanthomatosis) and cholesterol ester storage disease are disorders of lysosomal acid lipase (cholesterol esterase) that hydrolyze cholesterol esters in lysosomes (40). Mutations in the LIPA gene encoding this enzyme cause Wolman disease (41). Because insufficient free cholesterol is available to P450scc, there is adrenal insufficiency. The disease is less severe than congenital lipoid adrenal hyperplasia (lipoid CAH) with respect to steroidogenesis, but because all cells store and utilize cholesterol, it affects all tissues and is fatal. Cholesterol ester storage disease appears to be a rare, milder allelic defect in the same enzyme.
3. Smith-Lemli-Opitz syndrome
Smith-Lemli-Opitz syndrome is a defect in cholesterol biosynthesis, resulting from abnormalities in the sterol Δ7-reductase gene, DHCR7 (42). Associated features of this condition include microcephaly, developmental delay, a typical facial appearance, proximal thumbs, syndactyly of the second and third toes, cardiac abnormalities, and underdeveloped genitalia in males. Adrenal insufficiency is present in some children, especially during times of stress or when LDL-derived cholesterol sources are inadequate (e.g., dietary insufficiency/bile salt depletion) (43).
III. An Overview of Steroidogenic Enzymes
Most enzymes involved in steroid biosynthesis are either cytochrome P450s (CYPs) or HSDs. These steroidogenic enzymes are functionally, if not absolutely, unidirectional, so the accumulation of products does not drive flux back to the precursor. All P450-mediated hydroxylations and carbon-carbon bond cleavage reactions are mechanistically and physiologically irreversible (44). HSD reactions are mechanistically reversible and can run in either direction under certain conditions in vitro, but each HSD drives steroid flux predominantly in either the oxidative or reductive mode in vivo (45). However, two or more HSDs drive the flux of a hydroxysteroid and its cognate ketosteroid in opposite directions, some favoring ketosteroid reduction and others favoring hydroxysteroid oxidation.
A. Cytochrome P450
Cytochrome P450 is a generic term for a group of oxidative enzymes, all of which have about 500 amino acids and contain a single heme group (46). They are termed P450 (pigment 450) because all absorb light at 450 nm in their reduced states complexed with carbon monoxide. The human genome includes genes for 57 cytochrome P450 enzymes (47, 48). Several nomenclature systems have been proposed for these genes and enzymes over the past few decades. The genes are now formally termed CYP genes, and a logical systematic nomenclature for these has been described (http://drnelson.uthsc.edu/cytochromeP450.html); the encoded proteins may be given the same name without the use of italics (thus the CYP11A1 gene encodes CYP11A1), but the classic, more widely understood P450 names for the proteins are preferable (thus the CYP11A1 gene encodes P450scc, where the suffix “scc” denotes “side chain cleavage,” thus identifying the principal activity of the enzyme). The formal gene names are given in Table 1. Seven human cytochrome P450 enzymes are targeted to the mitochondria and are termed “type 1”; the other 50 human P450 enzymes are targeted to the endoplasmic reticulum and are termed “type 2.” All P450 enzymes activate molecular oxygen using their heme center and add electrons from the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). The two types of P450 enzymes are distinguished biochemically by the mechanisms by which they receive electrons from NADPH, as well as by their intracellular locations. Type 1 enzymes receive electrons from NADPH via a flavoprotein termed ferredoxin reductase and a small iron-sulfur protein termed ferredoxin, whereas type 2 P450 enzymes receive electrons from NADPH via a single 2-flavin protein termed P450 oxidoreductase (POR) (49). Each P450 enzyme can metabolize multiple substrates, catalyzing a broad array of oxidations.
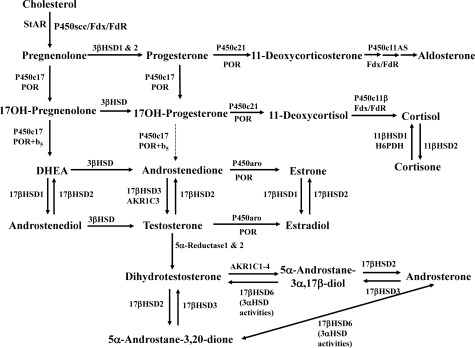
Six P450 enzymes are involved in steroidogenesis (Fig. 3). Mitochondrial P450scc is the cholesterol side-chain cleavage enzyme catalyzing the series of reactions formerly termed “20,22 desmolase.” The two isozymes of mitochondrial P450c11, P450c11β (11β-hydroxylase) and P450c11AS (aldosterone synthase), catalyze 11β-hydroxylase, 18-hydroxylase, and 18-methyl oxidase activities. In the endoplasmic reticulum, P450c17 catalyzes both 17α-hydroxylase and 17,20-lyase activities, P450c21 catalyzes 21-hydroxylation in the synthesis of both glucocorticoids and mineralocorticoids, and P450aro catalyzes aromatization of androgens to estrogens.
Fig. 3.
Major human steroidogenic pathways. Key enzymes and cofactor proteins are shown near arrows indicating chemical reactions. P450scc cleaves cholesterol to pregnenolone, the first committed intermediate in steroid biosynthesis. The steroids in the first column are Δ5-steroids, which constitute the preferred pathway to C19 steroids in human beings. The dashed arrow indicates poor flux from 17α-hydroxyprogesterone to androstenedione via P450c17, and the three small arrows below P450c11AS emphasize the three discrete steps with intermediates corticosterone and 18-hydroxycorticosterone. Not all intermediate steroids, pathways, and enzymes are shown. [© R. J. Auchus]
B. Hydroxysteroid dehydrogenases
The HSDs have molecular masses of about 35 to 45 kDa, do not have heme groups, and require nicotinamide adenine dinucleotide (phosphates) (NADH/NAD+ or NADPH/NADP+) as cofactors to either reduce or oxidize a steroid by two electrons via a hydride transfer mechanism (45). Most examples involve the conversion of a secondary alcohol to a ketone or vice versa, and in the case of the 3βHSD/Δ5→Δ4-isomerases (3βHSD), the dehydrogenation is accompanied by the isomerization of the adjacent carbon-carbon double bond from the Δ5 position (between carbons 5 and 6) to the Δ4 position (between carbons 4 and 5) (Fig. 1). The human steroid 5α-reductases types 1 and 2, which are often grouped with the HSDs for convenience, reduce olefinic carbon-carbon double bonds to the saturated state rather than acting on carbon centers bonded to oxygen. Whereas most steroidogenic reactions catalyzed by P450 enzymes are due to the action of a single form of P450, each of the reactions catalyzed by HSDs can be catalyzed by at least two, often very different, isozymes. The HSDs include the 3α- and 3β-HSDs, the two 11β-HSDs, and a series of 17β-HSDs.
Based on their structures, these enzymes fall into two groups: the short-chain dehydrogenase/reductase (SDR) family, and the aldo-keto reductase (AKR) family (45, 50). The SDR enzymes are β-α-β proteins where up to seven parallel β-strands fan across the center of the molecule, forming the so-called “Rossman fold,” which is characteristic of oxidation/reduction enzymes that use nicotinamide cofactors. The AKR enzymes are soluble proteins that contain a β-barrel or triosephosphate isomerase (TIM)-barrel motif in which eight parallel β-strands lie in a slanted circular distribution like the staves of a barrel. In both cases, the active site contains a critical pair of tyrosine and lysine residues that participate in proton transfer from or to the steroid alcohol during catalysis. The SDR enzymes include 11β-HSDs 1 and 2 as well as 17β-HSDs 1, 2, and 3; the AKR enzymes include 17β-HSD5, which is important in extraglandular activation of androgenic precursors (Table 2).
Table 2.
Properties of HSDs
| Features | AKRs | SDRs |
|---|---|---|
| Quaternary structure | Monomers | Dimers, tetramers |
| Subunit size (kDa) | ∼35 | 25–35 |
| Structural motif | TIM- (β-)barrel | Rossman fold |
| Catalytic motif | Y, K, H, D distant in linear sequence | Y-X-X-X-K motif |
TIM, Triosephosphate isomerase. © R. J. Auchus.
Based on their activities, it is physiologically more useful to classify the HSDs as dehydrogenases or reductases. The dehydrogenases use NAD+ as their cofactor to oxidize hydroxysteroids to ketosteroids, and the reductases mainly use NADPH to reduce ketosteroids to hydroxysteroids. Although these enzymes are typically bidirectional in vitro based on pH and cofactor concentrations, they tend to function mainly in one direction in intact cells, with the direction determined by the cofactor(s) available (45, 50). These directional preferences derive primarily from the relative abundance of the oxidized and reduced form of cofactors and the relative affinity of each enzyme for NAD(H) vs. NADP(H), because cofactor concentrations exceed steroid concentrations by many orders of magnitude (45, 51). Consequently, the directional preference of some “reductive” enzymes can be reduced or reversed by depleting cells of NADPH or by mutations that impair NADPH binding (52, 53).
IV. The Steroidogenic Acute Regulatory Protein
A. Acute regulation of steroidogenesis
Unlike cells that produce polypeptide hormones, which store large amounts of hormone in secretory vesicles ready for rapid release, steroidogenic cells store very little steroid. Thus, a rapid steroidogenic response (e.g., adrenal secretion of aldosterone and cortisol in response to stress or the “pulsing” of sex steroids in response to an LH surge) requires rapid synthesis of new steroid. ACTH promotes steroidogenic cell growth and maintains the steroidogenic machinery at three distinct levels (LH probably acts similarly on gonadal steroidogenic cells, but has not been studied as thoroughly). First, acting over weeks or months, as seen in long-term exposure to ACTH (e.g., in Cushing’s disease), ACTH promotes adrenal growth. This growth occurs primarily by ACTH stimulating the production of cAMP, which in turn promotes the synthesis of IGF-II (54, 55), basic fibroblast growth factor (56), and epidermal growth factor (57). Together, these growth factors stimulate adrenal cellular hypertrophy and hyperplasia, determining the amount of steroidogenic tissue. Second, acting over days, ACTH acts through cAMP, and angiotensin II acts through the calcium/calmodulin pathway to promote the transcription of genes encoding various steroidogenic enzymes and electron-donating cofactor proteins, thus determining the amount of steroidogenic machinery in the cell. Third, ACTH rapidly stimulates StAR gene transcription (58) and phosphorylation of Ser195 in extant StAR (59) to increase the flow of cholesterol from the OMM to the IMM, where it becomes substrate for the first and rate-limiting enzyme, P450scc. This acute response occurs within minutes and is inhibited by inhibitors of protein synthesis (e.g., puromycin or cycloheximide), indicating that a short-lived protein species mediates this process. Orme-Johnson and colleagues (60–62) first showed that this acute steroidogenic response was accompanied by the rapid synthesis of a 37-kDa phosphoprotein. Stocco and Sodeman (63) extended these observations to MA-10 cells and cloned this factor, which they named the “steroidogenic acute regulatory protein,” or StAR (64). The history of the discovery of StAR as this long-sought acute trigger of steroidogenesis has been reviewed elsewhere (65). Although other proteins are involved in the chronic replenishment of mitochondrial cholesterol, abundant biochemical, clinical, and genetic evidence implicates StAR as this labile protein mediator (65).
Some steroidogenesis is independent of StAR; when nonsteroidogenic cells are transfected with the P450scc system, they convert cholesterol to pregnenolone at about 14% of the StAR-induced rate (66, 67). Furthermore, the placenta utilizes mitochondrial P450scc to initiate steroidogenesis (68) but does not express StAR (69). The mechanism of StAR-independent steroidogenesis is unclear; it may occur without a triggering protein, or some other protein may exert StAR-like activity to promote cholesterol flux, but without StAR’s rapid kinetics. A candidate for such a protein is MLN64, a 445-amino acid protein cloned from metastatic breast carcinoma, which has 227 carboxyl-terminal amino acids that are 37% identical and about 50% similar to the sequence of StAR (70). Intact MLN64 lacks StAR-like activity, but deleting the amino-terminal 218 residues produces a protein that closely resembles StAR and has about half of StAR’s ability to promote steroidogenesis, both in transfected cells and when purified and added to steroidogenic mitochondria in vitro (71, 72). MLN64 is expressed in the placenta where its amino-terminal domain is cleaved off, suggesting that an N-terminally deleted form of MLN64 may substitute for StAR in the placenta (72).
StAR is synthesized as a 37-kDa protein that has a typical mitochondrial leader sequence that directs it to the mitochondrion and is cleaved off upon mitochondrial entry to yield a 30-kDa intramitochondrial protein. Overexpression of mouse StAR in mouse Leydig MA-10 cells increased their basal steroidogenic rate (64), and cotransfection of expression vectors for both StAR and the P450scc system in nonsteroidogenic COS-1 cells augmented pregnenolone synthesis above that obtained with the P450scc system alone (66). Mutations in StAR cause the most common form of lipoid CAH (66, 67), in which very little steroid is made; and targeted disruption of the mouse Star gene causes a similar phenotype (73, 74).
B. StAR structure and mechanism of action
The mechanism of StAR’s action has been studied extensively but remains incompletely understood (17, 75, 76). The short half-life of the 37-kDa cytoplasmic precursor and the longer half-life of the “mature” 30-kDa intramitochondrial form of StAR initially suggested that the 30-kDa form was the biologically active moiety. When expressed in cytoplasm or added to mitochondria in vitro, both the 37- and 30-kDa forms of StAR are equally active (77). When StAR is immobilized on the OMM, it is constitutively active, but StAR is inactive when localized to the mitochondrial intramembranous space or to the matrix (78). These data demonstrate that StAR acts exclusively on the OMM (77, 78), and its activity in promoting steroidogenesis is proportional to its residency time on the OMM (78). Thus, it is StAR’s cellular localization, not its cleavage, that determines whether or not it is active. StAR has a sterol-binding pocket that accommodates a single molecule of cholesterol (26). The interaction of StAR with the OMM involves conformational changes (79, 80) that are necessary for StAR to accept and discharge cholesterol molecules. Although StAR can transfer cholesterol between synthetic membranes in vitro (81), suggesting that other protein molecules are not needed for its action, this activity can also be seen with the inactive mutant R182L, which is biologically inactive and causes lipoid CAH (82). Thus StAR’s action to promote steroidogenesis is distinct from its cholesterol-transfer activity.
Substantial data indicate that the action of StAR also requires the translocator protein, TSPO (also known as the peripheral benzodiazepine receptor) on the OMM (83–85). StAR appears to interact with peripheral benzodiazepine receptor (84), voltage-dependent anion channel 1, and phosphate carrier protein (86), all proteins found on the OMM. Each molecule of StAR appears to be recycled, moving hundreds of molecules of cholesterol before the cleavage/inactivation event (87). Although StAR is required for the acute steroidogenic response, steroidogenesis persists in the absence of StAR at about 14% of the StAR-induced rate (67, 75), accounting for the steroidogenic capacity of tissues that lack StAR (e.g., the placenta and the brain). Biophysical and partial proteolysis studies indicate that residues 63–193 of StAR (i.e., the domain that does not contain most of the crucial residues identified by missense mutations) are protease-resistant and constitute a “pause-transfer” sequence, which permits the bioactive loosely folded carboxy-terminal molten globule domain to have increased interaction with the OMM (79).
The sequence of mouse (64) and human (69) StAR initially suggested that it had a novel structure. However, the carboxy-terminal 227 amino acids of MLN64 are 37% identical and about 50% similar to the sequence of StAR (70), and N-218 MLN64 has about half of the ability of StAR to promote steroidogenesis (71, 72). MLN64, a related protein called MENTHO (88, 89), and the NPC proteins disordered in Niemann-Pick type C disease act in the trafficking of cholesterol in peroxisomes and lipid droplets (90). Although the structure of StAR has not been determined directly, a crystal structure at 2.2 Å resolution was determined for N-216 MLN64, which corresponds to N-62 StAR. The structure reveals a globular protein with an α/β helix-grip fold and an elongated hydrophobic pocket measuring about 26 Å deep and 10 Å across at its widest diameter (26). Modeling suggested that N-216 MLN64 could accommodate a single molecule of cholesterol in this pocket, with the 3β-OH group coordinated by the two polar residues at the bottom of the pocket. This structure, the crystal structure of the closely related StarD4 protein (25) and several computational models of StAR (91–93) all feature two long α-helixes at the N and C termini, two short α-helixes, and a set of nine antiparallel β-sheets that form a helix-grip fold (Fig. 4A). The most notable feature is a hollow hydrophobic pocket that has appropriate dimensions and geometry to bind a single molecule of cholesterol. The pocket is defined primarily by the β-sheets and the C-helix, which forms its floor. The interior surface of the pocket contains only two hydrophilic residues, E169 and R188. If a cholesterol molecule is modeled in the pocket, these hydrophyllic residues are perfectly positioned to coordinate with the 3β-hydroxyl group of cholesterol, and direct binding assays show that both N-218 MLN64 and N-62 StAR bind cholesterol with 1:1 stoichiometry (26). Only the exterior surface of the C-terminal α-helix and small segments of the adjacent Ω-loops appear to interact with the OMM to stimulate steroidogenesis (Fig. 4B). The Ω-loops form hydrogen bonds with the C-terminal helix, prohibiting access of cholesterol to StAR’s hydrophobic cholesterol binding pocket. The interaction with the charged phospholipids head groups on the OMM disrupts these hydrogen bonds, permitting the C-helix to swing open and closed, governing access of cholesterol to the sterol-binding pocket. Immobilizing the C-helix by forming disulfide bonds with the adjacent loops ablates activity, and disrupting such artificial disulfide bonds restores activity (80). Thus, the activity of StAR on the OMM requires an acid-induced disruption of hydrogen bonds and a consequent conformational change in StAR to permit it to bind and release cholesterol.
Fig. 4.
Model of N-62 StAR. A, Ribbon diagram shows the N terminus in the upper right-hand corner; the C-terminal helix is in the lower center, extending out of the plane of the diagram. Residues that contribute to the associations between this C-terminal helix and adjacent structures are shown as ball-and-stick representations: carbon atoms are white; nitrogen, blue; oxygen, red; and hydrogen bonds, green. The principal associations involve the C-terminal helix residues Thr263 associating with Asn150, Arg272 associating with Asp106, and Leu275 associating with Gln128. [Reproduced with permission from D. C. Yaworsky et al.: J Biol Chem 280:2045–2054, 2005 (92). © American Society for Biochemistry and Molecular Biology.] B, Model showing StAR interacting with a membrane [Cover picture of Ref. 92.].
C. Disorders of StAR: classic and nonclassic congenital lipoid adrenal hyperplasia
Lipoid CAH is the most severe genetic disorder of steroidogenesis, characterized by the absence of significant concentrations of all steroids, high basal ACTH and plasma renin activity, an absent steroidal response to long-term treatment with high doses of ACTH or human chorionic gonadotropin (hCG), and grossly enlarged adrenals laden with cholesterol and cholesterol esters (94–97). These findings indicate a lesion in the first step in steroidogenesis—the conversion of cholesterol to pregnenolone. It was initially thought that the lesion was in an enzyme involved in this conversion, and before the role of P450scc was understood, lipoid CAH was misnamed “20,22-desmolase deficiency” (97–102). However, the gene for P450scc is normal in these patients (102), as are the mRNAs for adrenodoxin reductase and adrenodoxin (102). Furthermore, placental steroidogenesis persists in lipoid CAH, permitting normal term gestation, which would not be expected to happen if P450scc were involved (103). The normal P450scc system plus the accumulation of cholesterol esters in the affected adrenal suggested that the lesion lay in an upstream factor involved in cholesterol transport into mitochondria (102). The cloning of StAR permitted its study in patients with lipoid CAH, and the identification of disease-causing StAR mutations proved the indispensable role of StAR in adrenal and gonadal (but not placental) steroidogenesis (66, 67, 104).
Lipoid CAH is a StAR gene knockout experiment of nature, revealing the complex physiology of the StAR protein (105). StAR promotes steroidogenesis by increasing the movement of cholesterol into mitochondria, but in the absence of StAR, steroidogenic cells make steroids at about 14% of the StAR-induced level (66, 67, 75, 104). This observation led to the two-hit model of lipoid CAH (67) (Fig. 5). The first hit is the loss of StAR itself, leading to a loss of most, but not all steroidogenesis, leading to a compensatory rise in ACTH and LH. These hormones increase cellular cAMP, which increases biosynthesis of LDL receptors, their consequent uptake of LDL cholesterol, and de novo synthesis of cholesterol. In the absence of StAR, this increased intracellular cholesterol accumulates, causing the second hit, which is the loss of all steroidogenic capacity caused by mitochondrial and cellular damage resulting from the accumulated cholesterol, cholesterol esters, and their autooxidation products (67).
Fig. 5.
Two-hit model of lipoid CAH. A, In normal adrenal cells, cholesterol is derived by endogenous synthesis and from LDLs, as depicted in Fig. 2. The rate-limiting step in steroidogenesis is the flow of cholesterol from the OMM to the IMM, mediated by StAR. B, Early in lipoid CAH, StAR-independent mechanisms still permit some cholesterol to enter the mitochondria; however, steroidogenesis is insufficient, and secretion of ACTH (and LH) increases, stimulating further accumulation of cholesterol esters in lipid droplets. C, The accumulating lipid droplets engorge and damage the cell through physical displacement and by the action of cholesterol autooxidation products. Steroidogenic capacity is destroyed, and secretion of tropic hormones continues. In the ovary, follicular cells remain unstimulated and undamaged until puberty, when they are recruited at the beginning of each cycle, and small amounts of estradiol are produced by StAR-independent means (as in panel B), causing partial feminization, anovulatory cycles, infertility, and hypergonadotropic hypogonadism. [Reprinted with permission from H. S. Bose, et al.: N Engl J Med 335:1870–1878, 1996 (67). © 1996 Massachusetts Medical Society. All rights reserved.]
The two-hit model explains the unusual clinical findings in lipoid CAH. In the fetal testis, which normally makes large amounts of testosterone in fetal life (106), the Leydig cells are destroyed early in gestation, eliminating testosterone biosynthesis; hence, an affected 46,XY fetus does not undergo normal virilization and is born with female external genitalia and a blind vaginal pouch. However, Wolffian duct derivatives are well developed, indicating the presence of some testosterone synthesis early in fetal life (107), as predicted by the two-hit model. The undamaged Sertoli cells produce Müllerian inhibitory hormone, so that the phenotypically female 46,XY fetus has no cervix, uterus, or fallopian tubes. The steroidogenically active fetal zone of the adrenal is similarly affected, eliminating most dehydroepiandrosterone (DHEA) biosynthesis, and hence eliminating the fetoplacental production of estriol, so that midgestation maternal and fetal estriol levels are very low (103). The definitive zone of the fetal adrenal, which differentiates into the zonae glomerulosa and fasciculata, normally produces very little aldosterone, and because fetal salt and water metabolism are primarily maintained by the placenta, stimulation of the glomerulosa by angiotensin II generally does not begin until birth. Consistent with this, many newborns with lipoid CAH who have StAR mutations devoid of measurable function may not have a salt-wasting crisis until after several weeks of life, when chronic stimulation then leads to cellular damage (67, 108). Some mineralocorticoids may also be produced by StAR-independent steroidogenesis, further delaying the onset of salt loss in lipoid CAH.
The two-hit model also explains the spontaneous feminization of affected 46,XX females who are treated in infancy and reach adolescence (109, 110). The fetal ovary makes little or no steroids and contains no detectable mRNAs for the steroidogenic enzymes after the first trimester (106); consequently the ovary remains largely undamaged until it is stimulated by gonadotropins at the time of puberty, when it then produces some estrogen by StAR-independent steroidogenesis. Although the amount of estradiol produced is subnormal, in the absence of opposing action from adrenal androgens, it is sufficient to feminize an adolescent female. Continued stimulation results in cholesterol accumulation and cellular damage, so that biosynthesis of progesterone in the latter part of the cycle is impaired. Because gonadotropin stimulation only recruits individual follicles and does not promote steroidogenesis in the whole ovary, most follicles remain undamaged and available for future cycles. Cyclicity is determined by the hypothalamic-pituitary axis and remains normal. With each new cycle, a new follicle is recruited, and more estradiol is produced by StAR-independent steroidogenesis. Although net ovarian steroidogenesis is impaired, enough estrogen is produced to induce breast development (especially in the absence of androgens), feminization, monthly estrogen withdrawal, and cyclic vaginal bleeding (67, 109, 110). However, progesterone synthesis in the latter half of the cycle is disturbed by the accumulating cholesterol esters so that the cycles are anovulatory. Measurements of estradiol, progesterone, and gonadotropins throughout the cycle in affected adult females with lipoid CAH confirmed this model (110). Similarly, examination of StAR-knockout mice confirms the two-hit model (74).
Numerous mutations in the STAR gene have been found in patients with lipoid CAH (67, 111). Lipoid CAH is relatively common in Japan; about 65–70% of affected Japanese alleles and virtually all affected Korean alleles carry the mutation Q258X (66, 67, 97, 111–113); this observation first identified the crucial role of the C-terminal helix in StAR’s action. The carrier frequency for this mutation in these countries appears to be about one in 300 (67, 112) so that one in every 250,000 to 300,000 newborns is affected, for a total of about 500 patients in Japan and Korea. Other genetic clusters are found among Palestinian Arabs, most of whom carry the mutation R182L (67); in eastern Saudi Arabia, carrying R182H (108); and in parts of Switzerland, carrying the mutation L260P (114). Deletion of only 10 carboxy-terminal residues reduces StAR activity by half (77), and deletion of 28 carboxy-terminal residues by the common Q258X mutation eliminates all activity. By contrast, deletion of the first 62 amino-terminal residues has no effect on StAR activity assayed in vitro, although this deletes the entire mitochondrial leader sequence and forces StAR to remain in the cytoplasm (77).
Most patients with lipoid CAH have had similar clinical findings: an infant with normal-appearing female genitalia experiences failure to thrive and salt loss in the first weeks of life (67, 97, 111). However, other clinical presentations have been described, including apparent sudden infant death syndrome (115) and late initial presentation of salt loss at about 1 yr of age (108). Nonclassic lipoid CAH is an attenuated form of the disease caused by mutations that retain about 20–25% of normal StAR activity. Most of these patients carry StAR mutation R188C, although other mutations can cause this phenotype (116–118). These individuals generally experience mild symptoms of adrenal insufficiency at 2 to 4 yr of age, and 46,XY patients have normal-appearing external genitalia, indicating normal intrauterine Leydig cell function leading to normal male external genital development. Some patients have very mild disorders of mineralocorticoid secretion, characterized by normal electrolytes and elevated plasma renin activity, as well as having rather mild hypergonadotropic hypogonadism. As a result of the predominance of a disorder in glucocorticoid secretion, some of these patients have been mistaken for having a form of familial glucocorticoid deficiency (FGD) (117), which is caused by disorders in the ACTH receptor [melanocortin receptor type 2 (MC2R)] or melanocortin receptor accessory protein (MRAP) (119). Thus, the spectrum of clinical presentation of mutations in the StAR protein is substantially broader than initially appreciated.
Treatment of lipoid CAH consists of physiological replacement with glucocorticoids, mineralocorticoids, and in the newborn period, salt (96, 97). The glucocorticoid requirement is less than in the virilizing adrenal hyperplasias because it is not necessary to suppress excess adrenal androgen production, so that growth in these patients should be normal (97). Genetic males have female external genitalia and should undergo orchiectomy and be raised as females (67, 96, 97). Successful pregnancy has been induced in an adult female with lipoid CAH by clomiphene citrate stimulation, followed by progesterone supplementation to mimic the maternally produced first trimester progesterone that the affected mother could not produce (120).
V. Conversion of Cholesterol to Pregnenolone: P450scc and Its Electron Transfer Proteins
A. P450scc
A cell is said to be steroidogenic if it expresses the cholesterol side-chain cleavage enzyme, P450scc, which catalyzes the first step in steroidogenesis. Conversion of cholesterol to pregnenolone in mitochondria is the first, rate-limiting, and hormonally regulated step in the synthesis of all steroid hormones (121–123). This process involves three distinct chemical reactions, the 22-hydroxylation of cholesterol, 20-hydroxylation of 22(R)-hydroxycholesterol, and oxidative scission of the C20–22 bond of 20(R),22(R)-dihydroxycholesterol (the side-chain cleavage event), yielding pregnenolone and isocaproaldehyde. P450scc can use the hydroxysterol intermediates directly as substrate, providing a useful experimental tool because these hydroxysterols are somewhat water-soluble and do not require StAR for access to P450scc (66). However, these reactions are probably not important in vivo because their kcat/Km ratios are much higher than that of cholesterol (124), and the high KD of approximately 3000 nm drives the dissociation of pregnenolone from P450scc. The reactions catalyzed by P450scc are slow, with a net turnover number of about six (125) to 20 (124) molecules of cholesterol per molecule of P450scc per second. Because 20-hydroxycholesterol, 22-hydroxycholesterol, and 20,22-dihydroxycholesterol could all be isolated from bovine adrenals in significant quantities, and because 3 moles of NADPH were required per mole of cholesterol converted to pregnenolone, it was initially thought that three separate enzymes were involved. However, protein purification and reconstitution of enzymatic activity in vitro showed that a single protein, termed P450scc (where scc refers to the side chain cleavage of cholesterol) converted cholesterol to pregnenolone (126, 127) (for review see Refs. 3 and 128). These three reactions occur on a single active site that is in contact with the IMM. P450scc can also cleave the side chain of other hydroxysterols (e.g., 7-dehydrocholesterol) and can 20- and 22-hydroxylate vitamin D3 (129).
Cloning of the bovine cDNA for P450scc (130) preceded the cloning of the human cDNA (131) and gene (132) (now termed CYP11A1), which lies on chromosome 15q23-q24 and consists of nine exons spanning about 30 kb. The 2-kb mRNA encodes 521 amino acids, including a 39- amino acid mitochondrial leader peptide that is cleaved off during the entry of P450scc into the mitochondria.
Forms of P450scc engineered to lack the mitochondrial leader or that are targeted to the endoplasmic reticulum are inactive, demonstrating that the mitochondrial environment is required for activity (133). A spontaneous deletion of the rabbit cyp11a1 gene for P450scc (134), its knockout in the mouse (135), and rare patients with P450scc mutations (136, 137) result in the loss of all steroidogenesis, indicating that all steroidogenesis is initiated by this one enzyme. Thus the presence of P450scc renders a cell ‘steroidogenic’ and able to make steroids de novo, as opposed to modifying steroids produced elsewhere, which occurs in many types of cells.
Expression of P450scc is induced by cAMP in the adrenal zona fasciculata/reticularis (138), testis (139), and ovary; and by the calcium/protein kinase C system in the zona glomerulosa (140, 141). By contrast, placental P450scc expression is constitutive (68) and requires the action of several members of the CP2 (grainyhead) family of transcription factors (142–145). Side-chain cleavage activity and pregnenolone biosynthesis have been demonstrated in the rat and human brain (146), and abundant P450scc expression is found in the rodent brain, especially in fetal life. Transcription of the CYP11A1 gene encoding P450scc determines the amount of P450scc enzyme and the net steroidogenic capacity of a cell. This transcription is regulated in both tissue-specific and hormonally responsive fashions and can be induced by both the protein kinase A and protein kinase C second messenger systems, which act through different DNA elements in the CYP11A1 promoter (141). The expression of P450scc and other steroidogenic enzymes in the adrenal and gonad require the action of the zinc-finger transcription factor, steroidogenic factor 1 (SF1) (147, 148); by contrast, expression of P450scc in the human placenta is independent of SF1 and requires CP2 proteins (formerly termed LBP proteins) (142–145) and TreP-132 (149, 150). Thus, long-term cellular stimulation over the course of days will increase the content of P450scc and the level of basal steroid produced, as well as the capacity of the cell to mount a steroidogenic response. A comprehensive overview of the factors regulating the transcription of steroidogenic factors is beyond the scope of this review, but some recent, more focused reviews are available (151, 152).
B. P450scc deficiency
Several patients have now been described with mutations in P450scc (136, 137, 153–155). Although these patients may be clinically and hormonally indistinguishable from those with lipoid CAH, their StAR genes are normal. It would seem logical that elimination of P450scc activity would be incompatible with term gestation because the placenta, a fetal tissue, must produce progesterone in the second half of pregnancy to suppress maternal uterine contractions, thus preventing miscarriage. It is most likely that these few fetuses with P450scc mutations reached term gestation because of unusually protracted maintenance of the maternal corpus luteum of pregnancy, which normally involutes in the second trimester. However, maternal progesterone production during these pregnancies has not been investigated directly. Mild or nonclassic P450scc deficiency has been reported recently in patients carrying P450scc mutations that retain about 10–20% of wild-type activity (156, 157). By hormonal and clinical criteria, nonclassic P450scc deficiency is indistinguishable from nonclassic lipoid CAH. However, all patients reported to date with P450scc deficiency have had normal-sized or small adrenals, in contrast to the massive adrenal enlargement that characterized lipoid CAH from severe StAR mutations.
C. Chronic maintenance of the steroidogenic machinery
Whereas the acute regulation of steroidogenesis is determined by the action of StAR, P450scc is the enzymatic rate-limiting step in steroidogenesis. Thus, the chronic regulation of steroidogenesis is quantitatively (how much) determined by P450scc gene expression (158) and qualitatively (which steroids) determined by the expression of downstream enzymes, especially P450c17. The episodic bursts of cAMP resulting from the binding of ACTH and LH to their respective receptors are necessary but not sufficient for the continued expression of the steroidogenic enzymes and the production of steroids. Patients with inactivating mutations in the ACTH receptor (MC2R) (159) or the LH/hCG receptor (160) make negligible steroids from the affected glands.
Conversely, activating mutations of the Gαs protein, which couples receptor binding to cAMP generation, and activating mutations of the LH receptor cause hypersecretion of steroids (161). Indeed, cAMP-responsive elements have been identified in the genes for most of the human steroidogenic P450 enzymes, but this mechanism alone does not explain the diversity of steroid production observed in the various zones of the adrenal cortex, the gonads of both sexes, the placenta, and the brain. Other transcription factors (e.g., AP2, SP1, SP3, NF1C, NR4A1, NR4A2, GATA4, and GATA6) participate in regulating the basal- and cAMP-stimulated transcription of each gene. SF1 coordinates the expression of steroidogenic enzymes in adrenal and gonadal cells (147). By contrast, steroidogenesis in the brain (162) and placenta (142–145) is independent of SF1. Targeted disruption of the SF1 gene in the mouse disrupts steroid biosynthesis and blocks the development of the adrenal glands, gonads, and the ventromedial hypothalamus (163). The action of SF1 is modified by other transcription factors (e.g., WT1 and DAX1) (164) or by its phosphorylation (165) or sumoylation (166). Thus, the development of steroidogenic organs is intimately related to the capacity to produce steroids, and multiple factors acting on the genes for steroidogenic enzymes yield both common features and diversity among the steroidogenic tissues.
D. Transport of electrons to P450scc: ferredoxin reductase and ferredoxin
P450scc functions as the terminal oxidase in a mitochondrial electron transport system (49). Electrons from NADPH are accepted by a flavoprotein, termed ferredoxin reductase (also known as adrenodoxin reductase), which is associated with the IMM. Ferredoxin reductase transfers the electrons to an iron/sulfur protein termed ferredoxin (adrenodoxin), which is found in the mitochondrial matrix or loosely adherent to the IMM. Ferredoxin then transfers the electrons to P450scc (Fig. 6). Ferredoxin reductase and ferredoxin serve as generic electron transfer proteins for all mitochondrial P450s, including the vitamin D 1α- and 24-hydroxylases. Ferredoxin forms a 1:1 complex with ferredoxin reductase, dissociates, then subsequently reforms an analogous 1:1 complex with a mitochondrial P450 such as P450scc, thus functioning as an indiscriminate diffusible electron shuttle mechanism. The human genes for ferredoxin reductase (167, 168) and ferredoxin (169) are expressed in all tissues (170, 171), suggesting that they may have additional roles beyond electron transfer to P450 enzymes.
Fig. 6.
Electron transport to mitochondrial forms of cytochrome P450. The flavin group (FAD) of ferredoxin reductase (FeRed), which is bound to the IMM, accepts two electrons from NADPH, converting it to NADP+. These electrons pass to the iron-sulfur (Fe2S2, diamond with dots) cluster of ferredoxin (Fedx), which is found either in the mitochondrial matrix, as shown, or loosely associated with the IMM. Fedx then donates the electrons to the heme of the P450 (square with Fe). Negatively charged residues in Fedx (−) guide docking and electron transfer with positively charged residues (+) in both FeRed and the P450. For P450scc, three pairs of electrons must be transported to the P450 to convert cholesterol to pregnenolone. [© W. L. Miller.]
E. Ferredoxin reductase
Ferredoxin reductase is widely expressed in human tissues, but its expression is two orders of magnitude higher in steroidogenic tissues (171). The primary RNA transcript from the 11-kb FDXR gene (168) on chromosome 17q24-q25 (172) is alternatively spliced, generating two mRNA species that differ by only 18 bp (167), but only the protein encoded by the shorter mRNA is active in steroidogenesis (173). Unlike most steroidogenic genes, the promoter for ferredoxin reductase contains six copies of GGGCGGG sequences (168), which is the canonical binding site for the transcription factor SP1 typically found in housekeeping genes. Accordingly, cAMP does not regulate transcription of the ferredoxin reductase gene, as is the case for ferredoxin and P450scc (171). Mutations in the human genes for ferredoxin and ferredoxin reductase have not been described, but mutation of the Drosophila ferredoxin reductase homolog dare causes developmental arrest and degeneration of the adult nervous system due to the loss of ecdysteroid production (174).
Crystallography shows that bovine ferredoxin reductase consists of two domains, each comprising a β-sheet core surrounded by α-helices (175). The NADP(H)-binding domain (residues 106 to 331 in bovine numbering) is a compact region, whereas the more open flavin adenine dinucleotide (FAD) domain, formed by the remaining amino- and carboxy-terminal residues, binds the dinucleotide portion of FAD across a Rossman fold with the redox-active flavin isoalloxazine ring abutting the NADP(H) domain. By analogy to related structures, including glutathione and thioredoxin reductases, the nicotinamide ring of NADPH appears to lie adjacent to the flavin ring in position to transfer its two electrons to the FAD. Thus, intramolecular electron transfer occurs in the cleft formed by the angled apposition of these two domains. Within this cleft, basic residues abound, including arginines 240 and 244, which are important for interactions with ferredoxin (176, 177). Hypothetical docking of the two structures suggests that the negative surface of ferredoxin fits elegantly into the positive surface of ferredoxin reductase, even with NADP(H) bound (175). Basic residues are also critical for the interaction of P450scc with the negative surface charges on ferredoxin (178), so that ferredoxin reductase-ferredoxin docking is expected to share some key features with the mitochondrial P450-ferredoxin interaction.
F. Ferredoxin
Ferredoxin is a small (14 kDa), soluble, iron/sulfur (Fe2S2) electron shuttle protein that either resides free in the mitochondrial matrix or is loosely bound to the IMM (179). Encoded by the FDX1 gene on chromosome 11q22 that spans 35 kb, ferredoxin is expressed in many tissues, and its expression in steroidogenic tissues is induced by cAMP in parallel with P450scc (180).
Bovine ferredoxin consists of two domains (181), a core region and an interaction domain. The core region contains residues 1-55 and 91-end (bovine numbering), including the four cysteines whose sulfur atoms tether the Fe2S2 cluster to the protein. Residues 56 to 90 form the interaction domain, which contains a helix at its periphery that includes acidic residues, aspartates 72, 76, and 79, plus glutamate 73, which are critical for the interaction of ferredoxin with P450scc (182). The Fe2S2 cluster lies in a protuberance in the molecule at the junction of its two domains. The charged residues of ferredoxin cluster in the interaction domain, giving the molecule a highly negatively charged surface above the Fe2S2 cluster. This description of ferredoxin is consistent with earlier studies that showed that overlapping sets of negative charges on ferredoxin drive interactions with positive charges on both P450scc and ferredoxin reductase (176). Thus, the preponderance of the evidence indicates that the same surface of ferredoxin interacts with both ferredoxin reductase and the P450 to transport electrons (176, 178). Despite this constraint, it has been possible to construct catalytically active, three-component fusion proteins of the general structure H2N-P450scc-ferredoxin reductase-ferredoxin-COOH, but all have required that the ferredoxin moiety be located at the carboxyl terminus attached to a hydrophilic linker that permits rotational freedom, so that the same surface of the ferredoxin moiety can access both the P450 and the ferredoxin reductase moiety (133, 183, 184).
VI. 3β-Hydroxysteroid Dehydrogenase/Δ5→Δ4 Isomerase (3βHSD)
Once pregnenolone is produced from cholesterol, it may undergo 17α-hydroxylation by P450c17 to yield 17α-hydroxypregnenolone, or it may be converted to progesterone, the first biologically important steroid in the pathway. A 42-kDa microsomal enzyme, 3βHSD, catalyzes both conversion of the hydroxyl group to a keto group on carbon 3 and the isomerization of the double bond from the B ring (Δ5 steroids) to the A ring (Δ4 steroids) (185–187). This 3βHSD converts pregnenolone to progesterone, 17α-hydroxypregnenolone to 17α-hydroxyprogesterone (17OHP), DHEA to androstenedione, and androstenediol to testosterone, all with similar efficiency (Km and Vmax) (188). The Km of the 3βHSD reactions is about 5 μm, which is substantially higher than the 0.8 μm Km for 17-hydroxylation by P450c17 (189), thus favoring the Δ5 pathway. This conversion of Δ5 steroids into their Δ4 congeners consists of two chemical transformations, both performed by 3βHSD. The first reaction is the oxidation of the 3β-hydroxyl group to the ketone; during this process, NAD+ is converted to NADH. The intermediate Δ5, 3-ketosteroid remains tightly bound to the enzyme with nascent NADH, and the presence of NADH in the cofactor-binding site activates the enzyme’s second activity, the Δ5→Δ4-isomerase activity (190).
Although rodents contain multiple 3βHSD isoforms, the human genome has only two active genes and several pseudogenes. These two enzymes are encoded by closely linked genes on chromosome 1p13.1 with identical intron/exon organizations. The type 1 enzyme catalyzes 3βHSD activity in placenta, breast, liver, brain, and some other tissues (186, 191, 192). This isoform is required for placental progesterone production during pregnancy, which may explain why a deficiency of 3βHSD1 has never been described. In contrast, the type 2 enzyme (3βHSD2) is the principal isoform in the adrenals and gonads (191, 193). Deficiency of 3βHSD2 causes the rare form of CAH known as 3βHSD deficiency (194, 195). The presence of the type 1 isozyme in these patients helps to explain the paradox of why 46,XX individuals born with severe 3βHSD2 deficiency can virilize slightly in utero: the 3βHSD block in the adrenal diverts Δ5-steroids away from cortisol and toward DHEA; extraadrenal 3βHSD1 then permits testosterone synthesis despite 3βHSD2 deficiency in the adrenal.
The type 1 and type 2 enzymes share 93.5% amino acid identity and are biochemically and enzymatically very similar. The enzymes are strongly inhibited by Δ4 products (196) and by synthetic Δ4 steroids such as medroxyprogesterone acetate (188). Both enzymes have very similar affinities of about 5 μm for the Δ5, 17-ketosteroids pregnenolone, 17α-hydroxypregnenolone, and DHEA (185, 188) and also convert the 17β-hydroxysteroid androstenediol (androsta-5-ene-3β,17β-diol) to testosterone. These enzymes are primarily membrane-bound and are found in both the microsomal and mitochondrial fractions during subcellular fractionation (185). Soluble forms of the enzyme have been engineered, and studies using these truncated forms demonstrate that H156 of 3βHSD1 is responsible for its higher affinity than 3βHSD2 for the inhibitors epostane and trilostane (197). Ultrastructural studies using immunogold labeling show that, at least in bovine adrenal zona glomerulosa cells, 3βHSD immunoreactivity is found in mitochondria and endoplasmic reticulum as well as in the cytoplasm (198). It is not clear whether this is also true for human 3βHSD or whether this subcellular distribution differs in various types of steroidogenic cells, but this property could be a novel mechanism for regulating the direction of steroidogenesis (199).
3βHSD activity is important in regulating adrenal production of DHEA sulfate (DHEAS). The human fetal adrenal, which produces vast amounts of DHEAS, contains little 3βHSD immunoreactivity (200). Furthermore, the expression of 3βHSD in the innermost regions of the adrenal cortex declines as the zona reticularis develops in childhood to initiate adrenarche (201, 202), and 3βHSD immunoreactivity is low in the adult rhesus (203, 204) and human (201, 205) zona reticularis. Thus, the development of an adrenal cell type (reticularis) that is relatively deficient in 3βHSD activity appears to be a necessary component of adrenarche, in which adrenal production of the Δ5 steroids DHEA and DHEAS rises exponentially (206).
A. 3β-Hydroxysteroid dehydrogenase deficiency
3βHSD deficiency is a rare cause of glucocorticoid and mineralocorticoid deficiency that may be fatal if not diagnosed early in infancy (194). In its classic form, genetic females may have clitoromegaly and mild virilization because the fetal adrenal overproduces large amounts of DHEA, a small portion of which is converted to testosterone via extraadrenal 3βHSD1. Genetic males also synthesize some androgens by peripheral conversion of adrenal and testicular DHEA, but the concentrations are insufficient for complete male genital development so that these males have a small phallus and severe hypospadias. Genetic studies have identified numerous mutations causing 3βHSD deficiency, all found in the type 2 gene (195, 207–210). Mutations have not been found in 3βHSD1, presumably because this would prevent placental biosynthesis of progesterone, resulting in a spontaneous first-trimester abortion.
The presence of peripheral 3βHSD1 activity complicates the hormonal diagnosis of this disorder. One would expect that affected infants should have low concentrations of 17OHP, yet some newborns with 3βHSD deficiency have very high concentrations of serum 17OHP, approaching those seen in patients with classical 21-hydroxylase deficiency (211). The adrenal of a patient with 3βHSD2 deficiency will secrete very large amounts of the Δ5 steroids, pregnenolone, 17α-hydroxypregnenolone, and DHEA. Some of the secreted 17α-hydroxypregnenolone is then converted to 17OHP by 3βHSD1, mainly in the liver. This 17OHP is not effectively taken up by the adrenal for subsequent conversion to cortisol because the circulating concentrations are below the Km of P450c21 (0.8–1.0 μm 17OHP, or about 40,000 ng/dl) (189). The ratio of the Δ5 to the Δ4 compounds remains high, consistent with the adrenal and gonadal deficiency of 3βHSD (211). Thus, the principal diagnostic test in 3βHSD deficiency is iv administration of cosyntropin with measurement of the three Δ5 compounds and the corresponding Δ4 compounds. Similar to 21-hydroxylase deficiency, where heterozygotes cannot be diagnosed by the response of 17OHP to cosyntropin stimulation, steroidal responses to cosyntropin cannot be used to identify carriers of 3βHSD deficiency (212).
Mild or partial defects of adrenal 3βHSD activity have been reported on the basis of ratios of Δ5 steroids to Δ4 steroids after a cosyntropin test that exceed 2 or 3 sd above the mean. These patients are typically young girls with premature adrenarche or young women with a history of premature adrenarche and complaints of hirsutism, virilism, and oligomenorrhea (213–215). However, the 3βHSD2 genes are normal in these patients, and even patients with less severe 3βHSD2 mutations have ratios of Δ5 to Δ4 steroids that exceed 8 sd above the mean (210, 216–219). Thus, ratios of Δ5 to Δ4 steroids are not reliable, and the diagnosis must rest on the cosyntropin-induced rise in Δ5 steroids such as 17α-hydroxypregnenolone to more than 3000 ng/dl (90 nmol/liter) to cosyntropin stimulation, (210, 216–219). The basis of the mildly elevated ratios of Δ5 to Δ4 steroids in these hirsute individuals with normal HSD3B2 genes is unknown.
VII. P450c17: 17α-Hydroxylase/17,20-Lyase
P450c17 is the microsomal P450 enzyme that catalyzes both 17α-hydroxylase and 17,20-lyase activities, principally in the adrenal and gonads. These two activities were once thought to be catalyzed by separate enzymes that differed in the adrenals and gonads. Clinical observations showed that adrenal 17α-hydroxylase activity (reflected by serum cortisol concentrations) was fairly constant throughout life, whereas adrenal 17,20-lyase activity (reflected by serum DHEA and DHEAS concentrations) was low in early childhood but rose abruptly during adrenarche at ages 8 to 10 yr (220, 221). This dissociation between adrenal secretion of 17α-hydroxylase products (cortisol) and 17,20-lyase products (DHEA) suggested that distinct enzymes performed the two transformations. This hypothesis was reinforced by the description of a few patients apparently lacking 17,20-lyase activity but retaining normal 17α-hydroxylase activity (222). Consequently, reports (223) that the 17α-hydroxylase and 17,20-lyase activities of neonatal pig testes copurified and that both 17α-hydroxylase and 17,20-lyase activities reside in a single protein (224, 225) were initially received with great skepticism. This controversy of “one enzyme or two” persisted until the cDNA for bovine P450c17 was cloned and shown to confer both 17α-hydroxylase and 17,20-lyase activities when expressed in nonsteroidogenic COS-1 cells (226). The single 2.1-kb mRNA species yields the 57-kDa P450c17 protein. Cells transfected with vectors expressing P450c17 cDNA acquire both 17α-hydroxylase and 17,20-lyase activities (226, 227), clearly establishing that this one enzyme catalyzes both activities. Thus, the distinction between 17α-hydroxylase and 17,20-lyase is functional and not genetic or structural. P450c17 is encoded by a single gene on chromosome 10q24.3 (228, 229), which is expressed in the adrenals and gonads (230), and not two tissue-specific isozymes as had been thought. This gene, formally called CYP17A1, is structurally related to the gene for P450c21 (21-hydroxylase) (231).
Human P450c17 17α-hydroxylates both pregnenolone and progesterone with approximately equal efficiency (189, 232), but there are prominent differences between Δ4 and Δ5 substrates for all other reactions. The 17,20-lyase activity is about 50 times more efficient for the conversion of 17α-hydroxypregnenolone to DHEA than for the conversion of 17OHP to androstenedione, consistent with the large amounts of DHEA secreted by the adult and fetal human adrenal (189, 232). Thus, under normal circumstances, 17OHP is not an important precursor for human sex steroid synthesis. The rate of the lyase reaction can be increased more than 10-fold by cytochrome b5 (189, 232, 233), but the Δ5 preference persists, and the rate of the lyase reaction never quite reaches the rate of the hydroxylase reactions. Human P450c17 can also 16α-hydroxylate progesterone but not pregnenolone (186), due to the presence of alanine rather than leucine at residue 105 (234). In the presence of b5, P450c17 diverts about 10% of pregnenolone metabolism to a Δ16 andiene product (235), which is of agricultural interest because this reaction forms a pheromone in pigs that results in “boar taint” (236). Experiments to study the chemistry of human P450c17 (and other P450 enzymes) often require manipulations that could be considered nonphysiological; however, the remarkable consistency for substrate preferences and kinetic constants observed for the modified, solubilized P450c17 expressed in Escherichia coli (232, 233), for native P450c17 expressed in yeast microsomes (189) or intact COS-1 cells (237), and for P450c17 obtained from human tissues and cells (189, 238) provide a high degree of confidence in results from work done in vitro.
Mechanistically, P450c17-mediated hydroxylations appear to proceed via the common iron oxene species and “oxygen rebound” mechanism proposed for prototypical P450 hydroxylations (239). The mechanism of the 17,20-lyase reaction involving a carbon-carbon bond cleavage, however, is not known despite considerable study. The failure of hydrogen peroxide alone to support catalysis (as has been shown for some other P450-mediated deacylation reactions) and computer modeling studies consistently suggest that the same heme-oxygen complex might participate in both hydroxylations and the 17,20-lyase reaction (240), but no conclusive evidence exists to exclude any proposed mechanisms. Given the diverse repertoire of reactions catalyzed by P450c17, it is not surprising that synthetic steroids such as dexamethasone (188) and the enantiomer of progesterone (241), as well as planar drugs such as troglitazone (242), also bind to and inhibit P450c17. Guinea pig P450c17 catalyzes the oxidation of spironolactone to 7α-thiospironolactone, which is a covalent inhibitor (“suicide substrate”) of P450c17 (243). The human P450c17 inhibitor abiraterone acetate, a 17-pyridyl pregnenolone analog, is in clinical trials for the treatment of prostate cancer (244, 245).
Because P450c17 has both 17α-hydroxylase activity and 17,20-lyase activity, it is the key branch point in steroid hormone synthesis. Neither activity of P450c17 is present in the adrenal zona glomerulosa; hence, pregnenolone is converted to mineralocorticoids. In the zona fasciculata, the 17α-hydroxylase activity is present, but 17,20-lyase activity is not; hence, pregnenolone is converted to the glucocorticoid cortisol. In the zona reticularis, both activities are present, so that pregnenolone is converted to sex steroids (Fig. 3). The principal factor regulating the 17,20-lyase reaction is electron transport from NADPH via POR.
A. Transcriptional regulation of the human CYP17A1 gene encoding P450c17
Transcription of the human CYP17A1 gene encoding P450c17 in adult tissues is largely limited to the adrenal and gonad, although it may also be expressed in skin (246). Rodent cyp17a1 is expressed in the fetal gastrointestinal tract (247, 248) and brain (249); the transcriptional regulation of rodent cyp17a1 and human CYP17A1 are substantially different (250). Adrenal expression of human CYP17A1 requires the transcription factors SF1, SP1, SP3, CTF2, CTF5, GATA4 and GATA6 (250–255). Recent work indicates that SF1 may activate human CYP17A1 expression in response to sphingosine (256, 257). Members of the SREBP family of factors that coordinate the synthesis of cholesterol and other sterols, especially SREBP1c, may also participate in regulating CYP17A1 transcription, linking the regulation of steroidogenesis with the regulation of cholesterol biosynthesis (258, 259). Regulation of the human gene for cytochrome b5, a protein that interacts with P450c17 to promote 17,20-lyase activity (see Section VIII.C), is regulated similarly to the CYP17A1 gene for P450c17 (260), thus coordinating the two key factors in androgen biosynthesis.
B. 17α-Hydroxylase/17,20-lyase deficiency
The initial description of 17α-hydroxylase deficiency was a case in which both 17α-hydroxylase and 17,20-lyase products were absent (261). Cloning of the gene for human P450c17 (231) permitted identification of CYP17A1 mutations in patients with 17α-hydroxylase deficiency (262). Deficient 17α-hydroxylase activity results in decreased cortisol synthesis, overproduction of ACTH, and stimulation of the steps proximal to P450c17. These patients may have mild symptoms of glucocorticoid deficiency, but this is not life-threatening because the lack of P450c17 results in the overproduction of corticosterone, which also has glucocorticoid activity. This is similar to the situation in rodents, whose adrenals lack P450c17 (158) and consequently produce corticosterone as their glucocorticoid. Affected patients also typically overproduce 11-deoxycorticosterone (DOC) in the zona fasciculata, which causes sodium retention, hypertension, and hypokalemia with suppressed plasma renin activity and suppressed aldosterone secretion from the zona glomerulosa, although the suppression of aldosterone is rather variable. When P450c17 deficiency is treated with glucocorticoids, DOC secretion is suppressed and plasma renin activity and aldosterone concentrations rise to normal (263).
The absence of 17α-hydroxylase and 17,20-lyase activities in complete P450c17 deficiency prevents the synthesis of adrenal and gonadal sex steroids. As a result, affected females are phenotypically normal but fail to undergo adrenarche and puberty (261), and genetic males have absent or incomplete development of the external genitalia (male pseudohermaphroditism, now often termed “46,XY disorder of sex development”) (264). The classical presentation is that of a teenage female with sexual infantilism and hypertension (261, 264). The diagnosis is made by finding low or absent 17-hydroxylated C21 and C19 plasma steroids, which respond poorly to stimulation with cosyntropin. Serum levels of DOC, corticosterone, 18-hydroxycorticosterone, and 18-hydroxy DOC are elevated, hyperresponsive to cosyntropin, and suppressible with glucocorticoids. Heterozygotes can sometimes be identified by cosyntropin stimulation testing (234, 265, 266).
More than 50 distinct mutations causing 17α-hydroxylase deficiency have been identified (267, 268). Four mutations appear recurrently: 1) a duplication of four nucleotides causing a frameshift is found among descendents of Dutch Frieslanders (269); 2) in-frame deletion of residues 487-489 is found throughout Southeast Asia (270, 271); 3) a deletion of phenylalanine at position 53 or 54 (272); and 4) the common W406R and R362C mutations, found among Brazilians of Spanish and Portuguese ancestry, respectively (273). The genetic lesions identified include 12 mutations that cause frameshifts or premature translational termination; as expected, none of these mutants has any detectable 17α-hydroxylase or 17,20-lyase activity. Eleven missense and in-frame mutations have been found, most of which also eliminate all activity, whereas some others, such as P342T, reduce both activities by 80%. Two genome-wide association studies have identified the CYP17A1 locus as a gene associated with hypertension (274, 275). This finding suggests that milder deficiencies in P450c17 might masquerade as primary hypertension in the general population and, if causative, is predicted to be a low-renin and mineralocorticoid antagonist-responsive form.
C. Isolated 17,20-lyase deficiency
Selective deficiency of the 17,20-lyase activity of P450c17 has been reported in about a dozen cases (262), which initially led to the incorrect conclusion that 17α-hydroxylase and 17,20-lyase are separate enzymes. However, the identification of CYP17A1 mutations causing apparent isolated 17,20-lyase deficiency is fraught with difficulty (276), and, to date, none of the initial clinical reports from the 1970s has been confirmed genetically or biochemically. One of the original patients was studied at the genetic level, showing two wholly inactivating mutations (277), which led to a corrected diagnosis of the patient as having complete 17α-hydroxylase deficiency (278). Because both the 17α-hydroxylase and 17,20-lyase activities of P450c17 are catalyzed on the same active site, it was not clear that a syndrome of isolated 17,20-lyase deficiency could exist until two patients with genital ambiguity, normal excretion of 17-hydroxycorticosteroids, and markedly reduced production of C19 steroids were studied at the molecular genetic level (279). One patient was homozygous for the P450c17 mutation R347H, and the other was homozygous for R358Q; both mutations changed the distribution of surface charges in the redox-partner binding site of P450c17 (279). When assayed in vitro, both mutants retained nearly normal 17α-hydroxylase activity but had no detectable 17,20-lyase activity (279, 280), and enzymatic competition experiments showed that the substrate binding site remained normal (280). When an excess of POR and cytochrome b5 was provided, some 17,20-lyase activity was restored, demonstrating that the loss in lyase activity was caused by impaired electron transfer (280). Several additional patients have been described with similar mutations, including girls with failure to manifest adrenarche (281).
By contrast, the P450c17 mutation E305G causes 17,20-lyase deficiency by selectively disrupting binding of 17α-hydroxypregnenolone and preventing DHEA synthesis despite enhanced conversion of 17OHP to androstenedione (282). This unusual variant of isolated 17,20-lyase deficiency provides further genetic evidence that the flux of androgens derived from conversion of 17OHP to androstenedione in the minor Δ4 pathway is not sufficient to form normal male external genitalia. One of the first patients reported to have isolated 17,20-lyase deficiency was recently found to have a homozygous mutation (G539R) in POR, further emphasizing the crucial role of efficient electron transfer in the 17,20-lyase reaction (283).
Computational modeling of P450c17 predicts the effects of all known mutations, including those with partial retention of both activities and those causing selective 17,20-lyase deficiency (240). The model identifies Arg 347 and Arg 358 and several other arginine and lysine residues in the redox partner binding site; mutations of these residues all cause varying degrees of selective loss of 17,20-lyase activity (240, 279, 280, 284). Another example of the critical nature of redox-partner interactions comes from the rare syndrome of cytochrome b5 deficiency. The first patient was a male pseudohermaphrodite with severe methemoglobinemia, but he was not evaluated hormonally (285). A recent, well-studied patient homozygous for the cytochrome b5 mutation W27X had hormonal findings indicative of isolated 17,20-lyase deficiency and clinically inapparent but elevated concentrations of methemoglobin, as expected from the known role of cytochrome b5 in the reduction of methemoglobin (286).
Thus, the central role of electron transfer in 17,20-lyase activity is now well-established. Electron transfer for the lyase reaction is promoted by the action of cytochrome b5 as an allosteric factor rather than as an alternate electron donor (189). The 17,20-lyase activity is also favored by the phosphorylation of serine residues on P450c17 by a cAMP-dependent protein kinase (287–290). The availability of electrons determines whether P450c17 performs only 17α-hydroxylation or also performs 17,20 bond scission; increasing the ratio of POR or cytochrome b5 to P450c17 in vitro or in vivo increases the ratio of 17,20-lyase activity to 17α-hydroxylase activity. Competition between P450c17 and P450c21 for available 17OHP does not appear to be important in determining whether 17OHP undergoes 21-hydroxylation or 17,20 bond scission (291). Thus, the regulation of 17,20-lyase activity, and consequently of DHEA production, depends on factors that facilitate the flow of electrons to P450c17: high concentrations of POR, the presence of cytochrome b5, and serine phosphorylation of P450c17 (292).
VIII. Electron Transport: P450 Oxidoreductase and Cytochrome b5
A. P450 oxidoreductase
All microsomal (type 2) cytochrome P450 enzymes, including steroidogenic P450c17, P450c21, and P450aro, receive electrons from POR, a membrane-bound flavoprotein that is a different protein from the mitochondrial flavoprotein, ferredoxin reductase (49). POR also serves as a reductase for several non-P450 enzymes, including squalene monoxygenase (293), fatty acid elongase (294), heme oxygenase (295), and cytochrome b5 (296). POR is expressed widely in human tissues and serves as the sole electron transfer protein for all microsomal P450s, including xenobiotic-metabolizing hepatic P450s, steroidogenic P450s, and P450s found in other tissues such as the kidney and brain. POR receives two electrons from NADPH and transfers them one at a time to the P450 (297, 298) (Fig. 7).
Fig. 7.
Electron transport to microsomal forms of cytochrome P450. NADPH interacts with POR, bound to the endoplasmic reticulum, and gives up a pair of electrons (e−), which are received by the FAD moiety. Electron receipt elicits a conformational change, permitting the isoalloxazine rings of the FAD and FMN moieties to come close together, so that the electrons pass from the FAD to the FMN. After another conformational change that returns the protein to its original orientation, the FMN domain of POR interacts with the redox-partner binding site of the P450. Electrons from the FMN domain of POR reach the heme group to mediate catalysis. The interaction of POR and the P450 is coordinated by negatively charged acidic residues on the surface of the FMN domain of POR and positively charged basic residues in the concave redox-partner binding site of the P450, similar to the interaction of Fedx with mitochondrial P450s. The active site containing the steroid lies on the side of heme ring (Fe) opposite from the redox-partner binding site. In the case of human P450c17, this interaction is facilitated by the allosteric action of cytochrome b5, and by the serine phosphorylation of P450c17. [© W. L. Miller.]
Much is known about the structure and biochemistry of POR. Crystallographic studies of rat POR reveal two lobes, one binding FAD and the other binding flavin mononucleotide (FMN), and a flexible amino terminus that tethers POR to the endoplasmic reticulum (299). An α-helical connecting domain joins the FAD and the FMN domains, and a disordered “hinge” of about 25 residues lies between the FMN domain and the connecting domain, suggesting that the FMN and FAD domains can move substantially relative to each other. In the crystal structure of rat liver POR (299), the FMN and FAD lie at the base of a cleft formed by the butterfly-shaped apposition of the FAD and FMN domains, reminiscent of the electron transfer surface of ferredoxin reductase (175). Nuclear magnetic resonance and x-ray scattering data have recently confirmed this view that POR undergoes these dramatic conformational changes while receiving and then transferring electrons (299). It is not clear how the surface containing the FMN docks into the redox partner-binding surface of the P450, but the flexible hinge region on which the FMN domain resides suggests that the FMN domain can reorient itself significantly to accommodate docking to the P450 (49, 189). The surface of the electron-donating FMN domain is dominated by acidic residues, whereas the redox-partner binding site of P450 enzymes contains numerous basic residues. In the rat POR, NADPH binds above the FAD in the β-sheet-rich FAD domain, implying direct donation of electrons from the NADPH to the FMN; however, it appears that yeast POR can bind FMN in two different places (300). If this is also true for human POR, it might explain the partial retention of activity by mutants predicted to disrupt FMN binding.
The crystal structure of the complex between the P450 and flavoprotein domains of the bacterial protein P450BM3 serves as a model of this flavoprotein-P450 interaction (301). Negative charges of the FMN domain guide interactions with positive charges on the P450. The FMN approaches no closer than 18 Å from the heme, similar to the 16 Å distance of FAD from the Fe2S2 cluster in the modeled ferredoxin-ferredoxin reductase complex, and presumably similar to the distance of the heme from the Fe2S2 cluster in the P450-ferredoxin complex (175). These distances are too far for electrons to “jump” directly to the heme; rather, electron transfer apparently uses the polypeptide chain as a conduit (301). Basic residues in the redox-partner-binding surface of the recipient P450 are crucial for electron transfer from POR (49, 302), as shown by the mutations in human P450c17 that cause 17,20-lyase deficiency (240, 280). Thus, these structures demonstrate several key principles of the electron transfer proteins involved in human steroidogenesis: NADPH and prosthetic groups lie at the interfaces of protein domains in which electron transfer occurs; the electron transfer surfaces are negatively charged to pair with positive charges on the P450s; the terminal electron transfer moiety (FMN domain or ferredoxin) must be mobile or soluble to pass electrons on to the P450; and electrons flow from the FMN or Fe2S2 cluster along the adjacent polypeptide chain to the heme.
The 69-kb human POR gene, located on chromosome 7q11.2, consists of 16 exons (303). The sequence of this gene in 842 normal persons from four ethnic groups revealed a high degree of polymorphism; most notably, the coding sequence variant A503V was found on approximately 28% of all alleles (304). This sequence variant reduces the 17α-hydroxylase and 17,20-lyase activities of P450c17 to approximately 60% of normal (304, 305) but has no measurable effect on the activities of P450c21 (306) or hepatic CYP1A2 or CYP2C19 (307), but it reduces the activities of CYP2D6 and CYP3A4 to metabolize important drugs in vitro (308, 309).
B. P450 oxidoreductase deficiency
Because POR participates in so many reactions, its mutation might be expected to yield a very severe phenotype. Consistent with this, genetically engineered POR-knockout mice die during fetal development (310, 311). A knockout encompassing the translational start site and N-terminal membrane-insertion domain yielded a soluble, cytoplasmic 66-kDa protein (310). Homozygous knockout mice had either of two phenotypes. Type I embryos appeared normal until embryonic day 10.5 (E10.5) and had grossly normal somite formation, but they had cardiac, neural tube, eye, and limb defects and died by E13.5. Type II embryos had generally retarded development by E8.5 and died shortly thereafter (310). A more complete knockout, encompassing the entire protein-coding region, yielded a similar phenotype, with all mice dying by E9.5 (311). By contrast, liver-specific POR knockout mice created by cre/lox technology were grossly normal—developing, growing, and living as long as normal mice, and having normal reproductive capacity, indicating normal gonadal P450 function (312, 313). However, these mice had hepatomegaly, accumulated hepatic lipids, and had a 99% decrease in bile acids, 65% decrease in serum cholesterol, and 50% decrease in serum triglycerides. Furthermore these animals had dramatically decreased capacities to metabolize acetaminophen, phenobarbital, and testosterone despite having a 5-fold increase in total hepatic P450 content. There is no established role for cytochrome P450 enzymes in the cardiac, neural tube, limb, and eye systems that are disrupted in POR knockout mice or in the disrupted skeletal development characteristic of human POR mutations causing Antley-Bixler syndrome (ABS). It has been proposed that the pleiotropic effects of POR mutation disrupt the metabolism of all-trans retinoic acid because increased maternal ingestion of retinoic acid partially ameliorated the phenotype of the complete POR knockout mouse (311).
Despite its embryonic lethality in mice, POR deficiency is a newly recognized form of CAH (305, 314–319). Beginning in 1985, several patients were described with apparent combined deficiencies of P450c17 and P450c21 (320–324); it was suggested that a mutation in POR was responsible (325), but this was not proven until 2004 (314). The initial report described three patients with ABS, genital ambiguity, and hormonal findings, suggesting partial deficiencies of 17α-hydroxylase and 21-hydroxylase, as well as a fourth patient who was phenotypically normal but had a similar hormonal profile. All had recessive, loss-of-function amino acid replacement mutations in POR (314). One of these patients was born to a woman who had become virilized during the pregnancy, suggesting partial fetoplacental aromatase deficiency. Over 50 POR mutations have now been described, affecting various P450 enzymes to differing degrees, apparently explaining the great variability in the clinical and hormonal findings in POR deficiency (305, 319). In vitro biochemical assays of the recombinant mutant proteins showed that the mutations in the ABS subjects had severely impaired, but not totally absent, activity whereas the mutations found in the phenotypically normal subject with amenorrhea were less severe (314). The serum and urinary steroids indicate defects in both P450c17 and P450c21, and clinical findings vary from severely affected infants with ambiguous genitalia, cortisol deficiency, and ABS to mildly affected women who appear to have a form of polycystic ovary syndrome, or mildly affected men with gonadal insufficiency. ABS is characterized by craniosynostosis, brachycephaly, radio-ulnar or radio-humeral synostosis, bowed femora, arachnodactyly, midface hypoplasia, proptosis, and choanal stenosis. When ABS is seen in association with abnormal steroids and ambiguous genitalia in either sex, the cause is an autosomal recessive mutation in POR (305, 314–317); by contrast, when ABS is seen without a lesion in steroidogenesis or genital development, the cause is an autosomal dominant, gain-of-function mutation in fibroblast growth factor receptor 2 (305) because mutations in the POR and FGFR2 genes segregate completely (305).
Patients with POR deficiency typically have normal electrolytes and mineralocorticoid function, nearly normal levels of cortisol that respond poorly to stimulation with cosyntropin, high concentrations of 17OHP that respond variably to cosyntropin, and low levels of C19 precursors of sex steroids. An important feature of POR deficiency is that there is genital ambiguity in both sexes; females may be virilized and males may be underdeveloped, although there is considerable variation among individuals. Because the 17,20-lyase activity of P450c17 is especially sensitive to perturbations in electron transport (49, 279, 280), defects in fetal testicular steroidogenesis leading to incompletely developed external genitalia in 46,XY males is the predicted outcome. By contrast, the partial virilization seen in 46,XX genetic females appears to be due to two causes. First, because placental aromatase (P450aro) requires POR, some, but not all mothers of infants with POR deficiency experience virilization during pregnancy (305, 314, 318), similar to that experienced by women carrying a fetus with P450aro deficiency (326, 327). This phenotype is common with the R457H mutation prevalent in Japan, but not with the A287P mutation prevalent in Europe. Not surprisingly, the R457H mutant cannot support P450aro activity in vitro, whereas the A287P mutant retains full activity with P450aro (328). The fetus normally disposes of large amounts of adrenal C19 steroids by excreting them through the placenta, which aromatizes them to the maternal estrogens of pregnancy (329). A defect in this placental aromatase activity, either from mutation of POR or P450aro itself, will permit large amounts of fetal C19 steroids to enter and virilize the mother. This is evidenced by the low estriol values seen in women carrying a fetus with POR deficiency (330, 331). Second, an alternative “backdoor” pathway of androgen biosynthesis has been described in fetal marsupials in which 17OHP is eventually converted to dihydrotestosterone (DHT) without utilizing androstenedione and testosterone as intermediates (see Section XVIII) (332, 333). Analysis of urinary steroids from patients with POR deficiency suggests that this pathway also applies to the human fetus (316, 330, 331, 334). The relative importance of these two distinct mechanisms for virilizing the fetus with POR deficiency probably varies with the specific POR mutation involved.
The incidence of POR deficiency is unknown; the rapid description of large numbers of patients (305, 319) and the potentially very subtle clinical manifestations in individuals carrying mutations with partial activity (283, 305, 314, 335) suggest that POR deficiency may be fairly common. Two mutations are especially common: A287P, the predominant mutation in patients of European ancestry, and R457H, the predominant mutation in patients of Japanese ancestry. Because few patients have been studied in the newborn period, it has not been established whether newborn screening of 17OHP designed to detect 21-hydroxylase deficiency will also detect POR deficiency.
The cellular mechanisms by which POR deficiency causes skeletal malformations remain unclear. Four lines of evidence suggest a role for POR-supported cholesterol biosynthesis in bone formation (318). First, cholesterol biosynthesis requires squalene epoxidase, a non-P450 enzyme, and 14α-demethylase (CYP51), which both require POR (293, 336); 14α-demethylase activity was reduced in fibroblasts from a patient with ABS and ambiguous genitalia who was subsequently shown to have POR deficiency (314, 337). Second, ABS has been reported in infants whose mothers ingested the antifungal agent fluconazole (338, 339); fluconazole acts by inhibiting fungal CYP51 activity. Liver-specific knockout of POR in mice decreases the activities of hepatic drug-metabolizing enzymes (312, 313), thereby potentially rendering some drugs teratogenic. Third, skeletal malformations are found in other disorders of cholesterol biosynthesis such as Smith-Lemli-Opitz syndrome (OMIM 270400). Finally, cholesterol derivatization of hedgehog proteins is required for its signaling in bone formation (340, 341). Two recent studies support this hypothesis. First, tissue-specific POR knockout in the limb bud mesenchyme of mice induces the expression of genes throughout the cholesterol biosynthetic pathway, suggesting that cholesterol deficiency could explain the skeletal phenotypes (342). Second, POR knockdown by RNA interference in rat chondrocytes decreased cell proliferation and differentiation, induced apoptosis, and reduced expression of Indian hedgehog, but these effects were reversed by providing cholesterol (343). Thus, cholesterol synthesis is probably involved in the skeletal phenotype of POR deficiency, but other mechanisms may also be operative. Because most clinically used drugs are metabolized by POR-dependent hepatic P450 enzymes, a thorough understanding of the impact of POR mutations and polymorphisms will be needed for a full understanding of the genetic basis of variations in drug metabolism.
C. Cytochrome b5
Cytochrome b5 is a small (12–17 kDa) hemoprotein found as a membrane-bound protein in liver and as a soluble protein lacking the C-terminal membrane anchor in erythrocytes. Cytochrome b5 is expressed in both the adrenals and gonads, where it can interact with P450c17; the adrenal expression is specific to the zona reticularis and may contribute to the genesis of adrenarche (201, 204). Much evidence has shown that cytochrome b5 can augment some activities of certain P450 enzymes, and the mechanism of this effect has been presumed to involve electron transfer from cytochrome b5 to the P450 for the second electron during the P450 cycle (344). Although cytochrome b5 can receive electrons from POR, the redox potentials of cytochrome b5 and one electron-reduced P450 are unfavorable for cytochrome b5-to-P450 electron transfer. Indeed, some of the actions of cytochrome b5 in experimental systems can be observed with apo-cytochrome b5 (345) or Mn+2-b5 (284) (which do not transfer electrons), including the stimulation of 17,20-lyase activity of human P450c17 (189, 232). These experiments suggest that cytochrome b5 does not act alone as an electron donor but rather functions in concert with POR to aid catalysis, possibly by an allosteric mechanism promoting the interaction of P450c17 and POR (Fig. 8).
Fig. 8.
Computational image using the Ribbons program showing the interaction of cytochrome P450c17 (red) with its electron-donating redox partner, (POR) (yellow), facilitated by the allosteric action of cytochrome b5 (green). The heme groups in P450c17 and cytochrome b5, and the NADPH molecule bound to POR, are shown as space-filling models in cyan, whereas the FAD and FMN moieties of POR are shown as ball-and-stick models in white. Note that the heme group in cytochrome b5 faces out of the plane of the page and does not contact either the POR or P450c17. [Reprinted with permission from A. V. Pandey and W. L. Miller: J Biol Chem 280:13265–13271, 2005 (Cover picture of Ref. 289). © American Society for Biochemistry and Molecular Biology.]
Bovine cytochrome b5 was one of the first proteins studied by x-ray crystallography, and a wealth of structural data for b5 have been acquired using molecular dynamics and nuclear magnetic resonance spectroscopy for both the holo- and apo-b5 (346, 347). Analogous to ferredoxin, cytochrome b5 consists of two domains: a heme-liganding core 1 domain (residues 40 to 65, bovine numbering); and a structural core 2 domain, from which the C-terminal membrane-anchoring helix extends. The heme extends more to the periphery of cytochrome b5 than does the Fe2S2 cluster of ferredoxin, and the entire surface is dominated by negatively charged residues rather than just one cluster of negative charges near the heme. In addition, the core 1 domain acquires considerable conformational flexibility in apo-b5, whereas the core 2 domain remains folded as in holo-b5 (347). Finally, the C-terminal membrane-spanning helix (exiting the core 2 domain) is required to stimulate the 17,20-lyase activity of human P450c17, but the signal peptide is not (348). Genetic and biochemical studies have implicated basic residues in P450c17, including R347, R358, and perhaps R449 and K89, as important for its interaction with cytochrome b5 (240, 280, 348), whereas E48 and E49 of cytochrome b5 are required for high 17,20-lyase activity (349). However, the molecular details of how addition of cytochrome b5 to the P450c17.POR complex augments 17,20-lyase activity are not yet known.
IX. P450c21: Steroid 21-Hydroxylase
Microsomal P450c21 catalyzes the 21-hydroxylation of the Δ4 steroids progesterone to DOC and 17OHP to 11-deoxycortisol in the biosynthesis of mineralocorticoids and glucocorticoids, respectively (Fig. 3). The nature of this reaction has been of great clinical interest because 21-hydroxylase deficiency has an incidence of 1 in 15,000 to 1 in 20,000 births, causing more than 90% of all cases of CAH. The resulting clinical symptoms are complex and potentially devastating. Decreased cortisol and aldosterone synthesis often lead to sodium loss, potassium retention, and hypotension, which can culminate in cardiovascular collapse and death, usually within a month after birth if not treated appropriately. Decreased synthesis of cortisol in utero leads to overproduction of ACTH and consequent overstimulation of adrenal steroid synthesis; because the 21-hydroxylase step is impaired, 17OHP accumulates as P450c17 converts very little 17OHP to androstenedione. However, 17-hydroxypregnenolone also accumulates and is readily converted to DHEA, and subsequently to androstenedione and testosterone, resulting in severe prenatal virilization of female fetuses (350–354). Variations in the manifestations of the disease, and especially the identification of patients without apparent defects in mineralocorticoid activity, initially suggested that there were two separate 21 hydroxylating enzymes that were differentially expressed in the zones of the adrenal specifically synthesizing aldosterone or cortisol. However, characterization of the P450c21 protein (355) and gene cloning show there is only one 21-hydroxylase encoded by a single functional gene on chromosome 6p21 (356–359). Because this gene lies in the middle of the major histocompatibility complex locus, disorders of adrenal 21-hydroxylation are closely linked to specific human lymphocyte antigen (HLA) types (360).
The human P450c21 protein, found only in the adrenals, is a microsomal P450 that employs the same POR used by P450c17 to transport electrons from NADPH. Much less is known about the enzymology of P450c21 than of P450c17, but the available evidence suggests that, unlike P450c17, P450c21 is not very sensitive to the abundance of POR or cytochrome b5. It is clear that genotype consistently predicts phenotype in very severe and very mild cases of 21-hydroxylase deficiency (361, 362). In contrast, patients with P450c21 variants [e.g., the common P30L (363) and V281L mutations and less common mutations R339H and P453S], which have 20 to 50% of wild-type activity (352, 364), can have various phenotypes, implying additional factors that can modify the clinical manifestations of 21-hydroxylase deficiency. Such estimations of activity can vary among different reports, especially when different technologies are used to assess the mutant enzymes.
Extraadrenal 21-hydroxylase activity has also been described in a broad range of adult and fetal tissues (365). However, extraadrenal 21-hydroxylation is not mediated by the P450c21 enzyme found in the adrenal (366), but it appears to be catalyzed by CYP2C9, CYP3A4, CYP2C19, and possibly other enzymes as well (367, 368). Human hepatic CYP2C19 and CYP3A4 can 21-hydroxylate progesterone but not 17OHP and thus may account, in part, for the diminished mineralocorticoid requirements in adult patients (368). As a result, patients having absent adrenal 21-hydroxylase activity may still have appreciable concentrations of 21-hydroxylated steroids in their plasma.
A. CYP21 genes and the genetics of 21-hydroxylase deficiency
1. 21-Hydroxylase genes
The locus containing the CYP21 genes is among the most complex in the human genome and explains why 21-hydroxylase deficiency is one of the most common autosomal-recessive diseases. There are two 21-hydroxylase loci, a functional gene formally termed CYP21A2 and a nonfunctional pseudogene formally termed CYP21A1P, which lie on chromosomal locus 6p21.1 in the midst of the HLA locus. These genes, commonly termed CYP21B (functional gene) and CYP21A (pseudogene), are duplicated in tandem with the C4A and C4B genes encoding the fourth component of serum complement (369, 370) (Fig. 9). Although the CYP21A1P locus is transcribed (371, 372), the resultant RNAs do not encode protein (371); only the CYP21A2 gene encodes 21-hydroxylase. The CYP21 genes consist of 10 exons (373), are about 3.4 kb long, and differ in only 87 or 88 of these bases (357–359). This high degree of sequence similarity indicates that these two genes are evolving in tandem through intergenic exchange of DNA. The Cyp21 genes of mice (374) and cattle (375) are also duplicated and linked to leukocyte antigen loci. However, whereas only the CYP21A2 gene functions in human beings, only the cyp21a1 gene functions in mice (376, 377), and both genes function in cattle (378). Sequencing of the gene duplication boundaries shows that the human locus duplicated after mammalian speciation (379), consistent with data that indicate that other mammals have single CYP21 gene copies (380). Because the HLA locus is highly recombinogenic, exchange between the CYP21A1P and CYP21A2 loci is common. Thus, approximately 75–80% of cases of 21-hydroxylase deficiency derive from micro- or macrogene conversion events where some or all of the CYP21A1P pseudogene replaces the corresponding area of the CYP21A2 gene, thus reducing the expression of the encoded P450c21 protein and/or impairing its activity (352, 381).
Fig. 9.
Genetic locus containing the CYP21 genes. The top line shows the p21.1 region of chromosome 6, with the telomere to the left and the centromere to the right. Most HLA genes are found in the class I and class II regions; the class III region containing the CYP21 genes lies between these two. The second line shows the scale (in kilobases) for the diagram immediately below, showing (from left to right) the genes for complement factor C2, properdin factor Bf, and the RD and G11/RP genes; arrows indicate transcriptional orientation. The bottom line shows the 21-hydroxylase locus on an expanded scale, including the C4A and C4B genes for the fourth component of complement, the inactive CYP21A pseudogene (CYP21A1P, 21A) and the active CYP21B gene (CYP21A2, 21B) that encodes P450c21. XA, YA, and YB are adrenal-specific transcripts that lack open reading frames. The XB gene encodes the extracellular matrix protein Tenascin-X; XB-S encodes a truncated adrenal-specific form of the Tenascin-X protein whose function is unknown. ZA and ZB are adrenal-specific transcripts that arise within intron 35 of the C4 genes and have open reading frames, but it is not known whether they are translated into protein; however, the promoter elements of these transcripts are essential components of the CYP21A and CYP21B promoters. The arrows indicate transcriptional orientation. The vertical dotted lines designate the boundaries of the genetic duplication event that led to the presence of A and B regions. [© W. L. Miller.]
The 21-hydroxylase genes lie within the class III region of the major histocompatibility complex locus, permitting HLA typing to be used to track CYP21A2 gene mutations. Salt-losing CAH is associated with HLA-B60 and HLA-40 in some populations (382), and the rare HLA type Bw47 is very strongly associated with salt-losing CAH (383, 384). HLA-Bw51 is associated with simple virilizing CAH in some populations (385), and HLA-B14 is found in approximately 40% of haplotypes for nonclassical CAH carriers (386), often associated with a duplication of the C4B gene (387, 388). HLA-identical individuals in a single family may have different clinical forms of CAH despite HLA identity (389–392), possibly representing extraadrenal 21-hydroxylation, de novo mutations, or multiple genetic crossover events.
2. Other genes in the 21-hydroxylase locus
At least eight additional genes lie in this locus (393) (Fig. 9). The tandemly duplicated C4A and C4B genes encode isoforms of complement component C4; the C4B protein has substantially more hemolytic activity, despite having greater than 99% sequence identity with C4A (394). The C4A gene is 22 kb long, but there are long (22 kb) and short (16 kb) forms of C4B due to a variation in one intron (395). The 3′ ends of the C4 genes are only 2466 bp upstream from the transcriptional start sites of the CYP21 genes (396). Promoter sequences needed for the transcription of the human CYP21A2 gene lie within intron 35 of the C4B gene (397).
A pair of genes, formally termed TNXA (XA) and TNXB (XB), is duplicated with the C4 and CYP21 genes. These genes lie on the strand of DNA opposite from the C4 and CYP21 genes and overlap the 3′ end of CYP21A2. The last exon of XA and XB lies within the 3′ untranslated region of exon 10 in CYP21A1P and CYP21A2, respectively (398). The XA gene was truncated during the duplication of the ancestral C4-CYP21-X genetic unit but is transcribed in the adrenal (379). The XB gene encodes a large extracellular matrix protein called Tenascin X that is expressed in most tissues, especially connective tissue (399, 400). The XB gene spans about 65 kb of DNA and includes 43 exons encoding a 12 kb mRNA (399, 401). The XB gene also encodes a short truncated form of Tenascin-X having unknown function and arising from an intragenic promoter (396). Identification of a CAH patient with a “contiguous gene syndrome” comprising a deletion of both the CYP21A2 and XB genes demonstrated that Tenascin X deficiency results in Ehlers-Danlos syndrome (EDS) (402). Although most forms of EDS are caused by autosomal dominant mutations in collagen genes, recessive forms are caused by mutations in genes for collagen-modifying enzymes, including Tenascin-X, which is associated with and stabilizes collagen fibrils (403, 404). Tenascin-X deficiency causes a clinically distinct, more severe form of EDS, either with or without associated 21-hydroxylase deficiency (405). In addition, RNA transcripts termed YA and YB arise from the CYP21A2 promoter but do not encode protein (371). Transcripts having an open reading frame, termed ZA and ZB, arise from a promoter element within intron 35 of the C4 genes, but it is not clear whether they encode protein (406).
B. 21-Hydroxylase deficiency
21-Hydroxylase deficiency, caused by mutations in P450c21, is the most common form of CAH and one of the most common inborn errors of metabolism. Detailed reviews of the physiology, molecular genetics, and clinical management of 21-hydroxylase deficiency are available (350–354, 407–410); we shall confine this review to material that illustrates issues concerning the genetics and biochemistry of P450c21, rather than try to summarize the extensive clinical literature concerning this disease.
1. Pathophysiology
In the complete absence of P450c21 activity, inability to convert progesterone to DOC results in aldosterone deficiency causing severe hyponatremia, hyperkalemia, and acidosis with concomitant hypotension, shock, cardiovascular collapse, and death in untreated newborns. Because adrenal blood flow is centripetal, high concentrations of cortisol in the adrenocortical capillary effluent bathes the medulla. These high concentrations of cortisol are needed for transcription of medullary phenylethanolamine N-methyltransferase, which catalyzes the conversion of norepinephrine to epinephrine (411); consequently, patients with CAH have low epinephrine concentrations, exacerbating the risk for hypoglycemia associated with cortisol deficiency (412). The inability to convert 17OHP to 11-deoxycortisol results in cortisol deficiency. The fetal adrenal transiently produces cortisol during the first trimester, apparently minimizing adrenal synthesis of androgen precursors (334). In CAH, low fetal cortisol stimulates ACTH secretion, which stimulates adrenal hyperplasia and transcription of the genes for all the steroidogenic enzymes, with consequent accumulation of 21-deoxysteroids, some of which are converted to testosterone, resulting in virilization of affected female fetuses. The degree of virilization generally correlates with the severity of the P450c21 mutation (361). Because normal male fetuses produce abundant testicular testosterone, the additional testosterone produced by adrenals with 21-hydroxylase deficiency does not produce a detectable phenotype.
2. Clinical forms and incidence of 21-hydroxylase deficiency
There is a broad spectrum of clinical manifestations of 21-hydroxylase deficiency, generally termed salt-wasting CAH, simple virilizing CAH, and nonclassical CAH. These different forms of 21-hydroxylase deficiency are not different diseases because there is a continuous spectrum of enzymatic impairments and clinical manifestations. Factors other than the specific P450c21 mutations can influence the clinical phenotype, including the presence of extraadrenal 21-hydroxylation, P450c21 promoter mutations, and variations in androgen metabolism and sensitivity. These discordances between genotype and phenotype are to be expected.
Salt-wasting CAH is caused by gene deletions, large gene conversions, premature stop codons, frame shifts or other mutations ablating more than 98% of enzyme activity, effectively eliminating both glucocorticoid and mineralocorticoid synthesis. Affected females are frequently diagnosed at birth because of masculinization of the external genitalia, but the clinical signs of salt loss are rarely seen before 5 d of age. After appropriate fluid resuscitation, the mineralocorticoids and glucocorticoids can be replaced orally, and the ambiguous genitalia can be corrected with a series of plastic surgical procedures, but the management is complicated and difficult (408, 410). Affected males are not generally recognized until they have a salt-losing crisis at 5 to 15 d or are detected through newborn screening assays of 17OHP, which are now performed in all states in the United States.
Simple virilizing CAH describes virilized females and clinically unrecognized males who have elevated concentrations of 17OHP but do not suffer a salt-losing crisis. These patients have missense mutations, typically I172N, that retain sufficient activity to produce the small quantities of aldosterone required to maintain salt balance. Because the adrenal normally produces 100 to 1000 times as much cortisol as aldosterone, mild defects in P450c21 are less likely to affect mineralocorticoid secretion than cortisol secretion. This mildly impaired aldosterone synthesis is reflected physiologically by the increased plasma renin activity seen in these patients after modest salt restriction. In the absence of newborn screening, comparably affected males typically escape diagnosis until age 3 to 7 yr, when they develop pubic, axillary, and facial hair and phallic growth. These boys grow rapidly and are tall for their age when diagnosed, but their epiphyseal maturation (bone age) advances at a disproportionately rapid rate so that their ultimate adult height is compromised.
Nonclassic CAH, sometimes called cryptic or late-onset CAH, denotes very mild forms of 21-hydroxylase deficiency, most commonly caused by the P450c21 missense mutation V281L (413). The modest overproduction of androgens is asymptomatic in males and evidenced by mild to moderate hirsutism, virilism, menstrual irregularities, and decreased fertility in women (414–417). Some affected persons are identified only by an increased response of plasma 17OHP to ACTH stimulation (415). Nevertheless, these individuals have hormonal evidence of mild mineralocorticoid deficiency, as predicted from the existence of a single adrenal 21-hydroxylase (418), and may have elevated plasma renin activity in response to sodium restriction.
Perinatal screening for elevated concentrations of serum 17OHP has found that the incidence of salt-wasting plus simple virilizing CAH is about 1 in 14,000, yielding a heterozygous carrier rate of 1 in 60 (419). The screening of 1.9 million Texas newborns yielded an overall incidence of 1 in 16,000 (1 in 15,600 Caucasians, 1 in 14,500 Hispanics, and 1 in 42,300 African-Americans) (420). Nonclassical CAH is much more common, but the prevalence varies among populations. One group has reported very high incidences: 1 in 27 for Ashkenazi Jews, 1 in 53 for Hispanics, 1 in 63 for Yugoslavs, 1 in 333 for Italians, and 1 in 1000 for other whites (421–423). Other studies found carrier rates of 1.2% (416) to 6% (424, 425) for Caucasian populations that were not subdivided further. The considerable differences in the reported incidences reflect differences in the small populations examined and the difficulty in distinguishing nonclassical CAH from heterozygous carriers of classical CAH.
C. Molecular genetics of 21-hydroxylase deficiency
21-Hydroxylase deficiency is caused by gene deletions, gene conversions, and apparent point mutations. Most apparent point mutations in CYP21A2 are actually small gene conversion events (350, 351, 426), so that gene conversions account for about 85% of the lesions in 21-hydroxylase deficiency. Most patients with 21-hydroxylase deficiency are compound heterozygotes, having different lesions on their two alleles. Gene deletions and large conversions eliminate all CYP21A2 gene transcription; hence, homozygotes will have salt-losing CAH. Microconversions creating frame shifts or premature translational termination also cause salt-losing CAH. Simple virilizing and nonclassical CAH are associated with amino acid replacements in the P450c21 protein caused by microconversion events. Affected patients are usually compound heterozygotes bearing a severely defective allele and a mildly impaired allele so that the clinical manifestations are based on the nature of the more active allele.
Two unusual and related features of the 21-hydroxylase locus complicate its analysis. First, the gene deletions in this locus are quite unusual in that they extend 30 kb from one of several points in the middle of CYP21A1P to the precisely homologous point in CYP21A2. Second, gene conversions are extremely common in this locus (388, 427). If a segment of gene A replaces the corresponding segment of the related gene B, the structure of recipient gene B is said to be “converted” to that of donor gene A. The hallmark of gene conversion is that the number of closely related genes remains constant, whereas their diversity decreases (428). Two types of gene conversions commonly cause 21-hydroxylase deficiency: large gene conversions that can be mistaken for gene deletions, and small microconversions that resemble point mutations. Depending on the populations examined, different reports find small or large numbers of gene deletions (427, 429, 430). Combining multiple studies shows that about 19% of mutant alleles have gene deletions, 8% have large gene conversions, 67% have microconversions, and 6% have uncharacterized lesions (351) (Fig. 10). A careful study of 155 German patients confirmed these results, finding gene deletions in 20.3%, large gene conversions in 7.1%, microconversions in 71.3% (intron 2 mutation in 30.3%; I172N in 19.7%), and uncharacterized mutations in 1.3% (381). A huge study of 3200 patients in France found the intron 2 mutation in 30%, deletions and large gene conversions in 25%, I172N in 17%, and Q318X in 7% of alleles causing classic CAH, and, in nonclassic CAH, V281L was found in 55%, intron 2 in 9%, large rearrangements in 8%, I172N in 4%, and Q318X in 3% (431). However, there is ascertainment bias in favor of more severely affected patients (388) and most studies address populations primarily of European origin. Thus, the available statistics are weighted in favor of gene deletions and large conversions, which cause the salt-wasting phenotype. About 75% of mutated CYP21 genes are structurally intact by Southern blotting and thus appear to carry point mutations (388, 427). Many mutant CYP21 genes have been sequenced, showing that a relatively small number of mutations that are also found in the CYP21A1P pseudogene cause most cases of CAH, so that most apparent point mutations are actually microconversions (350, 351, 426, 427) (Table 3).
Fig. 10.
Genetic rearrangements causing 21-hydroxylase deficiency. Deletions or duplications of the C4A and C4B genes can occur with or without associated lesions in the CYP21B gene. Note that all point mutations in CYP21B are actually microconversions. Some authors combine the gene deletion and macroconversion groups because these are difficult to distinguish by Southern blotting as both result in a loss of the CYP21B gene, but the genotypes are distinct, as shown. [© W. L. Miller.]
Table 3.
Microconversions of the CYP21A2 gene that cause 21-hydroxylase deficiency
| Mutation | Location | Associated phenotypes | Activity (%) |
|---|---|---|---|
| Pro 30→Leu | Exon 1 | NC/SV | 30–60 |
| A→G | Intron 2 | SV/SW | Minimal |
| 8-bp deletion | Exon 3 | SW | 0 |
| Ile 172→Asn | Exon 4 | SV | 3–7 |
| Ile 236→Asp | |||
| Val 237→Glu | Exon 6 | SW | 0 |
| Met 239→Lys | |||
| Val 281→Leu | Exon 7 | NC | 18 ± 9 |
| Gly 292→Ser | Exon 7 | SW | |
| T insertion @ 306 | Exon 7 | SW | 0 |
| Gly 318→Stop | Exon 8 | SW | 0 |
| Arg 339→His | Exon 8 | NC | 20–50 |
| Arg 356→Trp | Exon 8 | SV/SW | 2 |
| Pro 453→Ser | Exon 10 | NC | 20–50 |
| GG→C @ 484 | Exon 10 | SW | 0 |
NC, Nonclassic; SV, simple virilizing; SW, salt wasting. © W. L. Miller.
Three changes in the pseudogene (8 bp deletion, exon 3; T insertion, exon 7; Gly 318 stop, exon 8) render its product nonfunctional. Each change results in an altered reading frame and/or premature stop codon, eliminating all activity; all of these have been found in CYP21A2 alleles that cause salt-losing CAH. Three closely clustered base changes alter the normal amino acid sequence Ile-Val-Glu-Met at codons 236 to 239 in exon 6 to Asn-Glu-Glu-Lys in both the pseudogene and in a small number of alleles causing severe salt-losing CAH. The most common lesion in classical CAH is an A→G change in intron 2, 13 bases upstream from the 3′ splice acceptor site of this intron (432). This microconversion, which is found in over 25% of severely affected CAH alleles, causes abnormal mRNA splicing, so that a normal protein cannot be produced. However, a small portion of this mRNA may be spliced normally in some patients so that the phenotype is variable; most such patients are salt losers, but some have simple virilizing CAH. This intron 2 microconversion is often associated with the Ser/Thr polymorphism at codon 268; this is a true polymorphism because S268T does not alter enzymatic activity (433). The microconversion R356W, which is found in about 10% of severely affected alleles (434), eliminates virtually all detectable activity (435), apparently because it changes a residue in the binding site for POR. Other rare alleles, which are true mutations rather than gene conversions, have been described in single individuals (436–440).
The microconversion I172N is the most common cause of simple virilizing CAH (436, 441, 442). Ile 172 is an evolutionarily conserved residue that may contribute to the correct conformation of the enzyme. When Ile 172 was changed to Asn, Leu, Gln, or His, the mutants yielded only 3 to 7% of wild-type 21-hydroxylase activity (435, 443). The microconversion P30L is generally associated with nonclassical CAH but is found in some patients with simple virilizing CAH. The P30L/null compound heterozygotes have a very severe and variable form of nonclassical CAH, with minimal virilization at birth but marked androgen excess in postnatal life.
The most common mutation causing nonclassical CAH is V281L (413). This mutation does not alter the affinity of the enzyme for substrate but drastically reduces its Vmax (444). The microconversion P30L is found in about 15 to 20% of nonclassic alleles. In addition, the mutations R339H and P453S have been associated with nonclassical CAH (364, 445). Examination of a large number of CYP21A1P pseudogenes showed that the P453S mutation is polymorphic in about 20% of pseudogenes, and hence also represents a microconversion event.
Each missense mutation appears to occur in a functional domain of P450c21 (440). By analogy with the computationally inferred structure of P450c17 (240), Arg 356 appears to be part of the redox-partner-binding site, Val 281 appears to participate in coordinating the heme moiety, and Cys 428 is the crucial cystine residue in the heme-binding site found in all cytochrome P450 enzymes. The N-terminal region of P450c21, including Pro30, appears to be required for membrane insertion and enzyme stability (446). Finding most mutations in the amino-terminal portion of P450c21 is consistent with finding most gene conversion and gene deletion events occurring in exons 1–8 of the CYP21A2 gene. Changes in exons 9 and 10 are very rare, possibly as a result of evolutionary pressure to retain the overlapping 3′ end of the Tenascin X gene (398, 399).
X. Isozymes of P450c11: P450c11β and P450c11AS
A. Isozymes of P450c11
The final steps in the synthesis of glucocorticoids and mineralocorticoids are catalyzed by two closely related mitochondrial enzymes, P450c11β and P450c11AS (447, 448). These two human isozymes are encoded by tandemly duplicated genes on chromosome 8q21–22 that have 93% amino acid sequence identity (449). There are substantial differences in this enzyme system among various mammals. Cattle have a single enzyme (450) encoded by a single gene (451) that catalyzes the 11β-hydroxylation of 11-deoxycortisol to cortisol, and all three steps required for the synthesis of aldosterone from DOC: 11β-hydroxylase, 18-hydroxylase, and 18 methyl oxidase activities. By contrast, the human genome has two genes named CYP11B1 and CYP11B2 (449) that encode P450c11β (11β-hydroxylase) and P450c11AS (aldosterone synthase), respectively, and rats (but not mice) have three functional CYP11B genes (452). The CYP11B1 and CYP11B2 genes are located about 40 kb apart on chromosome 8q24.3 and share the same intron/exon structure common to all mitochondrial P450 genes. Both forms of P450c11 are found on the IMM and use ferredoxin and ferredoxin reductase to receive electrons from NADPH to mediate catalysis (453). By far the more abundant of the two isozymes is P450c11β, which is the classical 11β-hydroxylase that converts 11-deoxycortisol to cortisol and DOC to corticosterone, and is expressed predominantly in the zona fasciculata, and to a lesser extent in the zona reticularis, but not in the zona glomerulosa (454). P450c11β expression is induced by ACTH via cAMP and is suppressed by glucocorticoids. The less abundant isozyme, P450c11AS, is found only in the zona glomerulosa, where it has 11β-hydroxylase, 18-hydroxylase, and 18-methyl oxidase (aldosterone synthase) activities; thus, P450c11AS is able to catalyze all the reactions needed to convert DOC to aldosterone (455–457). Both enzymes can convert DOC to corticosterone and corticosterone to 18OH-corticosterone, but only P450c11AS can synthesize aldosterone from 18OH-corticosterone. The weak 18-hydroxylase activity of P450c11β (458) explains why an adrenal with suppressed P450c11AS expression continues to synthesize 18OH-corticosterone (459). Transcription of the CYP11B2 gene encoding P450c11AS is induced by potassium and angiotensin II activation of the protein kinase C pathway (460), and requires the transcription factors SF1, NURR1 and NGFIB (461) and possibly other factors (462). Because P450c11AS is normally restricted to the zona glomerulosa, where 17α-hydroxylase activity is absent, the repertoire of steroids that can undergo 18-oxygenation is limited.
B. Overview of lesions in isozymes of P450c11
Patients with disorders in P450c11β have classical 11β-hydroxylase deficiency but can still produce aldosterone (463), whereas patients with disorders in P450c11AS have rare forms of aldosterone deficiency (so-called corticosterone methyl oxidase deficiency) while retaining the ability to produce cortisol (464–466). The clinical descriptions of distinct deficiency syndromes for 11β-hydroxylase, 18-hydroxylase (also called corticosterone methyl oxidase I or CMOI), and 18-oxidase (CMOII) were initially interpreted to mean that three separate enzymes catalyzed these three reactions (467, 468). Mutations in CYP11B1 cause 11β-hydroxylase deficiency isolated to the zonae fasciculata and reticularis (463), whereas defects in CYP11B2 cause both CMOI or CMOII deficiencies (447). Thus, severe defects can impair all P450c11AS activities, leading to the clinical phenotype of CMOI deficiency (456), whereas P450c11β provides 11β-hydroxylase activity in the zona fasciculata.
C. 11β-Hydroxylase deficiency
P450c11β catalyzes the 11β-hydroxylation of 11- deoxycortisol to cortisol and that of DOC to corticosterone in the fasciculata, catalyzes some 18-hydroxylation, but has no 18-methyl oxidase activity. Deficient P450c11β activity accounts for about 5–8% of CAH in persons of European ancestry (469) but accounts for about 15% of cases in both Muslim and Jewish Middle Eastern populations (470). Severe deficiency of P450c11β decreases the secretion of cortisol, causing CAH and virilization of affected females. The defect in the pathway to cortisol results in accumulation of 11-deoxycortisol, and the defect in the 17-deoxy pathway in the synthesis of corticosterone in the fasciculata leads to overproduction of DOC, potentially leading to mineralocorticoid-based hypertension. Although DOC is less potent than aldosterone, patients with 11β-hydroxylase deficiency may secrete it at high levels, so that salt is retained and the serum sodium remains normal. Although overproduction of DOC can cause hypertension, affected newborns may have mild, transient salt loss (470, 471), presumably because normal newborns are relatively resistant to mineralocorticoids (Fig. 11). DOC concentrations, serum potassium, and blood pressure generally correlate poorly with the degree of virilization in affected females or the cardiovascular manifestations (472). Newborns may also have elevated concentrations of 17OHP, which accumulates two steps behind the enzymatic block, presumably because high concentrations of 11-deoxycortisol inhibit P450c21, so that P450c11β deficiency may be detected in newborn screening for P450c21 deficiency (420, 473, 474). The diagnosis is established by elevated basal concentrations of DOC and 11-deoxycortisol, which hyperrespond to cosyntropin (475).
Fig. 11.
Concentrations of aldosterone as a function of age. [© W. L. Miller.]
The CYP11B1 mutations causing 11β-hydroxylase deficiency have been reviewed recently (476). The mutation R448H was found on 11 of 12 affected alleles among Sephardic Jews of Moroccan ancestry (463), Q356X and G379V were the only mutations found among 15 unrelated Tunisian patients (477), and other mutations have also been described in other populations (447). A mild, nonclassic form of 11β-hydroxylase deficiency was found in otherwise asymptomatic women with hirsutism, virilism, and menstrual irregularities (470, 478), but this phenotype is rare: only two of five hyperandrogenemic women with elevated 11-deoxycortisol had CYP11B1 mutations that retained partial activity (479).
D. Aldosterone synthase deficiencies
Disorders of P450c11AS cause aldosterone synthase deficiency, formerly termed corticosterone methyl oxidase (CMO) deficiencies, in which aldosterone biosynthesis is impaired whereas the zona fasciculata and reticularis continue to produce corticosterone and DOC. The absence of aldosterone biosynthesis will generally result in a salt-wasting crisis in infancy, at which time the normal secretory rate of DOC is insufficient to meet the newborn’s mineralocorticoid requirements. These patients may recover spontaneously and grow to adulthood without therapy, probably reflecting the increasing sensitivity to mineralocorticoid action and sodium intake with advancing age (480, 481) (Fig. 11). Consistent with this, plasma renin activity is markedly elevated in affected children but may be normal in affected adults (482).
CMOI deficiency results from a complete loss of P450c11AS activity, eliminating 18-hydroxylase or 18 methyl oxidase activities and consequent biosynthesis of 18OH-corticosterone and aldosterone, while preserving the biosynthesis of corticosterone and cortisol by P450c11β. The diagnosis for CMOI deficiency is based on an increased ratio of corticosterone to 18OH-corticosterone with suppressed aldosterone despite elevated plasma renin activity (465). Few cases of CMOI deficiency have been fully characterized genetically, including a frameshift mutation (483), a premature stop codon (484), and the missense mutation R384P (485).
CMOII deficiency, characterized by increased concentrations of 18OH-corticosterone and very low concentrations of aldosterone, results from amino acid replacements in P450c11AS that selectively delete the 18 methyl oxidase activity while preserving 18-hydroxylase activity. CMOII deficiency is common in Sephardic Jews of Iranian origin, where affected individuals are homozygous for two different mutations, R181W and V386A; individuals homozygous for only one of these mutations were clinically unaffected; both mutations are required to cause disease (464).
There is clinical and genetic overlap between CMOI and CMOII. One patient with findings of CMOII had two mutations on each parental allele: the mother’s allele carried R181W and a deletion/frame-shift mutation that deleted all activity; the father’s allele carried T318M and V386A (466). T318 is a highly conserved Thr residue that participates in cleavage of the dioxygen bond of O2 in all P450 enzymes to create the iron-oxy intermediate required for P450 catalysis (486). When the T318M/V386A double mutant was recreated in vitro, there was no detectable activity, yet the patient had CMOII rather than CMOI as predicted by the in vitro findings (466). Similarly, a patient with apparent CMOI was homozygous for the mutations E198A and V386A, yet when assayed in vitro the double mutant enzyme behaved similarly to the mutant enzyme found in the Iranian Jewish CMOII patients (487). This patient also carried R173K, a normally occurring polymorphism that does not change the enzyme’s Km or Vmax (488). Thus, the distinction between CMOI and CMOII is not precise, and these disorders should be regarded as different degrees of severity on a continuous clinical spectrum.
Although the two CYP11B genes are reminiscent of the genetic anatomy of the CYP21A1P and CYP21A2 genes, gene conversions are rare. Only one gene conversion causing CMO II deficiency has been described (489), probably due to the higher recombinational frequency in the HLA region carrying the CYP21 genes.
E. Glucocorticoid-remediable aldosteronism
Although gene conversion events in the CYP11B locus are rare, an unusual gene duplication, caused by unequal crossing over between the CYP11B1 and CYP11B2 genes, results in glucocorticoid-remediable aldosteronism (GRA) (490–492). The crossover results in one allele having a third, hybrid CYP11B gene in which the ACTH-regulated promoter and the first few exons of CYP11B1 are fused in frame to most of the exons of CYP11B2, resulting in the zona fasciculata expression of a chimeric protein with aldosterone synthase activity (490, 491) (Fig. 12). Crossover has been described in intron 2, intron 3, and exon 4 (491), and the corresponding deleted allele, causing 11-hydroxylase deficiency, has been described (493). As a result, ACTH stimulates the production of an enzyme with 18-hydroxylase and 18-oxidase activities, leading to increased production of aldosterone and 18-oxygenated metabolites of cortisol. The overproduction of aldosterone causes mineralocorticoid hypertension and suppresses plasma renin activity. Because the expression of this hybrid gene is ACTH-responsive, it can be suppressed by glucocorticoids such as dexamethasone, which is used for diagnosis and treatment (494). GRA may account for up to 2% of patients with hypertension (495).
Fig. 12.
Molecular genetics of GRA. Unequal crossing over of the CYP11B2 and CYP11B1 genes yields a chimeric gene with the regulatory sequences of the CYP11B1 promoter (ACTH responsiveness in the zona glomerulosa; filled bar) driving the expression of an enzyme with CYP11B2 sequences, encoding aldosterone synthase activities (unfilled bar). [Figure provided courtesy of Prof. Perrin C. White. (University of Texas Southwestern Medical Center, Dallas, TX)].
Studies of the crossover sites in different patients with GRA, as well as construction of hybrid and site-directed mutants in vitro, have permitted the precise identification of the P450c11AS residues that are specifically required for the 18-oxygenase activity. Residues 288, 296, 301, 302, 325, and, perhaps most importantly, 320 are critical (496, 497). Thus, genetic crossovers 3′ to codon 320 will create a hybrid protein that lacks aldosterone synthase activity, and hence will not cause GRA. These key residues lie in or near the I-helix, which contains the catalytically important T318 residue implicated in oxygen activation for almost all P450s (486); thus, these mutations would be expected to alter active site geometry.
Localized microconversions, similar to those that cause most cases of 21-hydroxylase deficiency, might insert sequences crucial for aldosterone synthase activity into the gene encoding P450c11β. Studies in vitro suggest that changing Ser288 and Val320 would suffice (497), and artificial activating mutations that increase the aldosterone synthase activity of P450c11AS have been created in vitro (498). However, most patients with low-renin hypertension, other than those with glucocorticoid suppressible hypertension, lack mutations in this locus (458, 488, 499).
XI. Isozymes of 17β-Hydroxysteroid Dehydrogenase
Multiple reactions are catalyzed by a group of enzymes collectively known as the 17β-hydroxysteroid dehydrogenases (17βHSDs), sometimes also termed 17-oxidoreductases or 17-ketosteroid reductases (500, 501). These reactions included the interconversions of androstenedione and testosterone, DHEA and androsta-5-ene-3β,17β-diol, estrone and estradiol, androsterone and 5α-androstane-3α,17β-diol, 5α-androstanedione and 5α-DHT, and others. The terminologies for these enzymes vary, depending on the direction of the reaction being considered. These enzymes are often confusing because: 1) there are several different 17βHSDs; 2) some are preferential oxidases, whereas others are preferential reductases; 3) they differ in their substrate preference and sites of expression; 4) there is inconsistent nomenclature, especially with the rodent enzymes; and 5) some proteins termed 17βHSDs actually have very little 17βHSD activity and are principally involved in other reactions (50). There are at least 14 human 17βHSD isoforms; these isoforms vary widely in size, structure, substrate specificity, cofactor utilization, and physiological functions (502). Only those most important in normal steroidogenesis are discussed here (Table 4).
Table 4.
Principal oxidative and reductive steroidogenic reactions catalyzed by 17βHSD types 1–6
| 17βHSD type | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 |
|---|---|---|---|---|---|---|
| Other names | AKR1C3 | RODH | ||||
| Preferred direction | Reduction | Oxidation | Reduction | Oxidation | Reduction | Oxidation |
| Favored cofactor in intact cells | NADPH | NAD+ | NADPH | NAD+ | NADPH | NAD+ |
| Estrone → estradiol | Major | Minor | ||||
| Estradiol → estrone | Major | Trace | ||||
| 16OH-estrone → estriol | Major | |||||
| Estriol → 16OH-estrone | Major | Trace | ||||
| DHEA → androstenediol | Modest | Modest | ||||
| Androstenedione → testosterone | Trace | Major | Minor | |||
| Testosterone → androstenedione | Major | Trace | ||||
| DHT → 5α-androstane-3,20dione | Major | Trace | Modest | |||
| 5α-Androstane-3,20dione → DHT | Major | |||||
| DHT → 5α-androstane-3α,17βdiol | Modest | |||||
| 5α-Androstane-3α,17βdiol → DHT | Modest | |||||
| 5α-Androstane-3α,17βdiol → androsterone | Modest | |||||
| Androsterone → 5α-androstane 3,20dione | Modest |
These enzymes catalyze other reactions as well: 17βHSD6 (RODH) also acts as an isomerase with modest activity to convert 5α-androstane 3α,17βdiol to 5α-androstane 3β,17βdiol and androsterone to 3β-androsterone. © W. L. Miller and R. J. Auchus.
A. 17βHSD type 1
The interconversion of estrone and estradiol by 17βHSD1 has been studied more extensively than any other human steroidogenic enzyme. 17βHSD1, also known as estrogenic 17βHSD, is a cytosolic reductive SDR enzyme that uses NADPH as its cofactor to catalyze reductase activity and is a dimer of 34-kDa subunits. In the late 1980s, three independent groups reported the cloning of its cDNA, the first of any human HSD (503–505). The HSD17B1 gene is located on chromosome 17q11-q21 (506) adjacent to a pseudogene and encodes a 34-kDa protein that is expressed primarily in the placenta and in ovarian granulosa cells of developing follicles (506). 17βHSD1, which is active only as a dimer, primarily accepts steroid substrates with an aromatic A ring, so that it primarily activates estrone to estradiol, although it also has low activity for the conversion of androstenedione to testosterone and DHEA to androsta-5-ene-3β,17β-diol (507). Although 17βHSD1 can oxidize 17β-hydroxysteroids in the presence of NAD+ in vitro at high pH, the enzyme functions in vivo to reduce estrone to estradiol and 16α-hydroxyestrone to estriol (507).
Attempts to identify active site residues using affinity labels and mechanism-based inactivators were reported in 1988 (508), and sequence alignments with other SDR enzymes identified the Tyr-X-X-X-Lys active-site motif at residues 155 to 159 (509), which was then confirmed by crystallography (510, 511). The structure shows that the NADPH cofactor lies across the β-sheet core of the protein in the Rossman fold characteristic of all SDR enzymes. The steroid appears to dangle from the top of the enzyme almost perpendicular to the cofactor, with a hydrophobic pocket holding the body of the steroid in place while the steroid 3-OH group forms hydrogen bonds with His 221 and Glu 282. Where the steroid and cofactor meet, Ser 142, Tyr 155, and Lys 159 help to form a proton-relay system that drives catalysis.
Because estrogen biosynthesis preferentially occurs by the aromatization of androstenedione to estrone, 17βHSD1 appears to be required for the conversion of estrone to biologically active estradiol in the ovary and placenta (506). However, no cases of human 17βHSD1 deficiency have been reported; hence, this role has not been proven unequivocally. One would predict that 17βHSD1 deficiency would be compatible with life because fetuses that cannot produce estrogens because of aromatase deficiency or that have estrogen insensitivity (ERα mutations) are viable (327). Nevertheless, 17βHSD1 is probably critical for ovulation and may be important in the pathogenesis and progression of estrogen-dependent breast cancers (512).
B. 17βHSD type 2
17βHSD2 is a microsomal oxidase that uses NAD+ to inactivate both androgens and estrogens. In contrast to the “activating” role of 17βHSD1 in the placenta and ovary, human endometrium inactivates estradiol by its conversion to estrone. This activity, which is induced by progestins, is not attributable to 17βHSD1, because 17βHSD1 mRNA is not detected in the human uterus (506). Instead, a cDNA encoding microsomal HSD17B2 was cloned (513) and found to be expressed in endometrium, placenta, and other tissues (514). In contrast to 17βHSD1, which is found in placental syncytiotrophoblast cells, 17βHSD2 is expressed in endothelial cells of placental intravillous vessels, consistent with its apparent role in defending the fetal circulation from transplacental passage of maternal estradiol or testosterone (515). 17βHSD2 inactivates sex steroids by oxidizing them to their inactive 17-ketosteroid homolog: estradiol to estrone, testosterone to androstenedione, and DHT to 5α-androstanedione. The widespread tissue distribution and broad substrate specificity of 17βHSD2 suggests that its role in human physiology is to protect tissues from excessive exposure to active steroid hormones by oxidation to inactive 17-ketosteroids (502). This role is somewhat speculative because no human deficiency of 17βHSD2 has been described. 17βHSD2 also oxidizes 20α-dihydroprogesterone to progesterone, but this activity is low relative to its 17βHSD activity (513).
C. 17βHSD type 3
17βHSD3, the androgenic form of 17βHSD, is a 310-amino acid microsomal enzyme that is expressed almost exclusively in the testis (516). Relatively little is known about the enzymology of 17βHSD3, partly because it is a very hydrophobic protein, hampering its expression and purification from bacteria. In transiently transfected HEK-293 cells, human 17βHSD3 reduces DHEA, 5α-androstanedione, and androsterone, the C19 17-ketosteroids that serve as precursors of testosterone and DHT (517). The conversion of DHEA to androsta-5-ene-3β,17β-diol by 17βHSD3 may contribute significantly to testicular testosterone synthesis (238). Estrogens are poor substrates for human 17βHSD3 (507).
When the cDNA derived from the 67-kb gene for 17βHSD3 was cloned, patients with 17-ketosteroid reductase deficiency were found to harbor mutations in this HSD17B3 gene (516–518), proving the central role of this enzyme in male sexual differentiation. 17βHSD3 is the only 17βHSD enzyme whose role in human physiology is genetically established by a deficiency syndrome. Children with 17βHSD3 deficiency, which manifests as a disorder of sexual development only in 46,XY infants, make small amounts of testosterone, indicating that other human 17βHSD enzymes can convert androstenedione to testosterone—but not enough to compensate for 17βHSD3 deficiency during fetal development. Infants with 17βHSD3 deficiency have varying degrees of hypospadias and micropenis with intraabdominal or inguinal testes. The presentation is thus indistinguishable from that of partial androgen insensitivity syndrome or 5α-reductase type 2 deficiency, with most newborns assigned a female sex of rearing (518). Diagnosing the cause of disordered sexual development in an undervirilized 46,XY male is among the most challenging tasks in pediatric endocrinology, and distinguishing among these three diagnoses, despite detailed serum steroid analyses after hCG stimulation, remains problemmatic. Molecular genetic testing helps to establish the diagnosis, which has implications for management of the adolescent (518). As in 5α-reductase type 2 deficiency, children with 17βHSD3 deficiency and functional testes begin to virilize at puberty, and these patients sometimes adopt the male gender as adults. Affected 46,XX girls are normal, and women with 17βHSD3 deficiency produce normal amounts of androgens and estrogens, indicating that the human ovary still produces testosterone in the absence of 17βHSD3 expression (519).
D. 17βHSD type 4
Many additional HSD isoforms have been described, but their activities for steroids are generally poor. An enzyme termed 17βHSD4 was initially identified as an NAD+-dependent oxidase with activities similar to 17βHSD2 (520), but this trifunctional protein is located in peroxisomes (520) and is primarily an enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase (521, 522); its (oxidative) HSD activity toward estradiol is 106 times slower than its 3-hydroxyacyl-CoA dehydrogenase activity (523). Deficiency of 17βHSD4 causes a form of Zellweger syndrome, in which bile acid synthesis is disturbed but steroidogenesis is not affected (522). Thus, this enzyme has some 17βHSD activity, but steroidogenesis is not its principal physiological function.
E. 17βHSD type 5
17βHSD5, originally cloned as a 3α-HSD (524, 525), is an AKR enzyme (AKR1C3), unlike 17βHSD types 1 to 4, which are SDR enzymes. 17βHSD5 catalyzes the reduction of androstenedione to testosterone (524, 526), and is expressed in both steroidogenic and nonsteroidogenic tissues (525, 526). The nature of 17βHSD5 has been confusing because of its multiple activities and inconsistent results from different laboratories. Originally described as hepatic 3α-HSD type 2 for its ability to reduce DHT to 3α-androstanediol (525), this protein was later found to also have 17βHSD activity, including reducing androstenedione to testosterone (527). 17βHSD5 may account for much of the extratesticular, peripheral conversion of androstenedione to testosterone, although its catalytic efficiency as a 17βHSD is poor (528) compared with its 20αHSD activity with progesterone and DOC (520) or its prostaglandin dehydrogenase activity, reducing prostaglandin H2 to prostaglandin F2α (529). Nevertheless, 17βHSD5/AKR1C3 is more highly expressed in the human fetal adrenal during the time of sexual differentiation than 17βHSD3 (334) and may participate in adrenal testosterone production, particularly in virilizing CAHs. The postnatal adrenal also expresses 17βHSD5 in the zona reticularis and accounts for the small amount of testosterone directly produced by the adrenal glands (530).
F. 17βHSD type 6
17βHSD type 6 is also known as RODH for its homology to retinol dehydrogenase enzymes and as 3-hydroxysteroidepimerase for its ability to convert 3α-hydroxysteroids to their 3β-hydroxysteroid epimers via 3-ketosteroid intermediates (531, 532). Encoded by the HSD17B6 gene on chromosome 12q13.3, this enzyme catalyzes oxidative 3αHSD activities and converts androstanediol to DHT in the prostate (533). The role of RODH as the potential oxidative 3αHSD in the testicular alternative pathway to DHT is probable but not proven.
XII. P450aro: Aromatase
Estrogens are produced by the aromatization of androgens, including those derived from adrenal steroidogenesis, by a complex series of reactions catalyzed by a single microsomal aromatase, P450aro (327, 535, 536). This typical cytochrome P450 is encoded by a single gene on chromosome 15q21.1. This gene uses several different promoter sequences, transcriptional start sites, and alternatively chosen first exons to encode aromatase mRNA in different tissues under different hormonal regulation. P450aro is expressed in steroidogenic tissues (ovarian granulosa cells, placenta), in brain, and in nonsteroidogenic tissues, especially fat and bone (535). The CYP19A1 gene for P450aro spans over 75 kb (537) and contains five different transcriptional start sites (538) with individual promoters that permit the tissue-specific regulation of its expression in diverse tissues. P450aro is a glycoprotein, but glycosylation per se does not appear to affect activity (539).
The oxidative demethylation of C19 steroids, mainly androstenedione and testosterone, consumes three equivalents of molecular oxygen and NADPH, yielding formic acid and C18-steroids with an aromatic A-ring, hence the common name for this enzyme, aromatase. As is the case for P450scc, each successive oxygenation proceeds with greater efficiency, aiding in the completion of this transformation that is essential for estrogen biosynthesis in all animals (535). The mechanism of this aromatization appears to involve a hydroxylation at C2 of 19-oxo-androstenedione, followed by an enzyme-assisted rearrangement and tautomerization of the intermediate dienone to the phenolic A-ring (540), accounting for the incorporation of the final oxygen atom from molecular oxygen into the formic acid by-product. The crystallographic structure of human aromatase, the first of any human steroidogenic P450, reveals a tight active site well-suited to accommodate the androgens in the proper geometry (541). The hydrophobic amino terminus is in close apposition to the catalytic cleft, which suggests lipophilic substrates to gain access to the active site from the membrane using this membrane anchor.
Although it has traditionally been thought that aromatase activity is needed for embryonic and fetal development, infants and adults with aromatase deficiency have been described, showing that fetoplacental estrogen is not needed for normal fetal development (326, 542). Studies of patients with aromatase deficiency show that biologically significant estrogen synthesis derives entirely from P450aro (326, 327), although dietary phytoestrogens can provide some estrogen action in mice with targeted deletion of Cyp19a1 (543). Although very few cases of aromatase deficiency have been described, these highly informative “knockouts of nature” illustrate principles of fetoplacental steroidogenesis. In fetuses homozygous for aromatase deficiency, the principal manifestation results from its deficiency in the placenta (326) because ovarian steroidogenesis is quiescent during fetal life (106). The fetal adrenal makes large amounts of C19 steroids, principally DHEAS, much of which is 16α-hydroxylated by CYP3A7 in the fetal liver before undergoing metabolism via steroid sulfatases, 3βHSD1, aromatase, and 17βHSD1 in the placenta to produce estriol, the characteristic estrogen of pregnancy. Although huge amounts of estriol and estradiol are produced by the fetoplacental unit, estrogens are not needed for fetal development, the maintenance of pregnancy, or the onset of parturition; all of these processes proceed normally in fetuses lacking StAR, P450c17, or aromatase, or even in fetuses wholly lacking adrenal glands because of mutations in SF1 or DAX1 (329). However, in the absence of placental aromatase activity, androgenic C19 steroids derived from the fetal adrenal are passed into the maternal circulation, causing marked virilization of the mother (326). Furthermore, in pregnancies in which the mother has poorly treated 21-hydroxylase deficiency, maternal testosterone values can exceed 300 ng/dl (a midpubertal value for males), yet the fetus is not virilized (544) because the maternal testosterone is efficiently metabolized to estradiol by placental aromatase. Thus, placental aromatase is a key enzyme in protecting the fetus and mother from unwanted androgen exposure.
Extraglandular aromatase expression, especially in fat, can convert androgens to estrogens. Aromatase in the epiphyses of growing bone converts testosterone to estradiol; the tall stature, delayed epiphyseal maturation, and osteopenia of males with aromatase deficiency, and their rapid reversal with estrogen replacement provide powerful evidence that estrogen, not androgen, is responsible for epiphyseal maturation in males (327). After birth, individuals with aromatase deficiency grow normally and continue linear growth after completion of puberty, with males producing normal amounts of testosterone. However, when treated with estrogens, aromatase-deficient subjects fuse their epiphyses and cease linear growth (545). These observations have led to the experimental use of aromatase inhibitors in various disorders of accelerated bone maturation.
XIII. Isozymes of 5α-Reductase
Studies in the 1960s showed that testosterone is converted to the more potent androgen, DHT, by an enzyme found in testosterone’s target tissues (546). Studies using fibroblasts suggested that at least two human enzymes with different pH optima and genetics performed these transformations (547). Cloning studies identified two distinct forms of 5α-reductase: the type 1 enzyme, found in the scalp and other peripheral tissues, is encoded by the SRD5A1 gene on chromosome 5p15 (548); whereas the type 2 enzyme, the predominant form found in male reproductive tissues, in encoded by the structurally related SRD5A2 gene on chromosome 2p23 (549, 550). The two isoforms are very hydrophobic 30-kDa microsomal proteins that share 50% identity. The syndrome of 5α-reductase deficiency, a disorder of male sexual differentiation, is due to a wide variety of mutations in the SRD5A2 gene encoding the type 2 enzyme (551, 552). These genes have an unusual pattern of developmental regulation of expression. SRD5A1 is not expressed in the fetus, which explains why fetal deficiency of the type 2 enzyme is not compensated for by the type 1 enzyme (550). SRD5A1 is expressed briefly in the skin of the newborn, then remains unexpressed until its activity and protein are again found in the nongenital skin and liver after puberty. The type 1 enzyme accounts for most hepatic 5α-reduction. SRD5A2 is expressed in fetal genital skin, in the normal prostate, and in prostatic hyperplasia and adenocarcinoma. Thus, the type 1 enzyme may be responsible for the pubertal virilization seen in patients with classic 5α-reductase deficiency, and the type 2 enzyme may be involved in male pattern baldness (550).
The 5α-reductases are important beyond the context of male genital differentiation and androgen action because both isozymes reduce a variety of steroids in degradative pathways. Progesterone, 17OHP, and related C21 steroids are excellent substrates for both 5α-reductases, particularly the type 1; cortisol, cortisone, corticosterone, and related compounds are also good substrates (553). Such 5α- (and 5β-) reduced steroids may be metabolized further and conjugated for excretion in the urine. Inhibitors of the type 2 enzyme have been developed for the treatment of prostatic hyperplasia and the prevention of its recurrence after surgery (554): finasteride selectively inhibits human 5α-reductase type 2, whereas dutasteride inhibits both isoenzymes. These drugs are approved for treatment of prostatic hyperplasia in the United States.
Although the essential function of 5α-reductase type 2 in male sexual differentiation is firmly established, the role of the type 1 isoform is less clear. The abundant expression of the type 1 isoform in liver and its high activity with C21 steroids imply a role of degrading circulating C21 steroids in preparation for excretion in the urine. However, disruption of the corresponding mouse srd5a1 gene results in delayed parturition, which can be rescued with 5α-androstane-3α,17β-diol (555). In immature mice, 5α-reductase type 1 is expressed both in the ovary and in the Leydig cells, where it participates in the synthesis of testicular 5α-androstanediol via two pathways (556). Accumulating evidence suggests that 5α-reductases are at least sometimes expressed in human adrenal (334) and possibly gonads, but their function(s) in normal physiology or in pathological states is not known.
XIV. Isozymes of 3α-Hydroxysteroid Dehydrogenase
The four major human 3αHSDs are AKR enzymes that belong to the AKR1C family and are (usually) catalytically reductive (reviewed in Ref. 50). The 3αHSD types 1, 2, 3, and 4 are trivial names for AKR1C4, 1C3, 1C2, and 1C1, respectively, which are encoded by tandemly duplicated genes on chromosome 10p14-p15. The unfortunate discordance between the numbering of the names for the proteins and genes can cause substantial confusion. Each enzyme has a characteristic tissue distribution (557, 558) and repertoire of catalytic activities (538). AKR1C3, also known as 17βHSD5 (discussed in Section XI.E), catalyzes the 17βHSD reaction with androstenedione, and all of these AKR1C isoforms catalyze additional reactions, such as the 20α-reduction of pregnanes. In the brain, 3αHSDs reduce 5α-dihydroprogesterone to tetrahydroprogesterone (allopregnanolone), which is an allosteric activator of the γ-amino butyric acid (GABAA) receptor-chloride channel complex with a nanomolar affinity (559). AKR1C4 is abundant in liver but has been found in adrenal and gonads; AKR1C2 is found in the prostate (560); and AKR1C1 is abundant in the uterus. The amino acid compositions of isozymes of the type 2 and 3 enzymes differ by a few residues due to allelic variation; these minor differences account for the differing but partially overlapping array of compounds that may be used as substrates (Table 5).
Table 5.
Principal human 3α-hydroxysteroid dehydrogenases
| Enzyme | 3αHSD1 | 3αHSD2 | 3αHSD3 | 3αHSD4 | RODH |
|---|---|---|---|---|---|
| Directional preference | Reductive | Reductive | Reductive | Reductive | Oxidative |
| Gene | AKR1C4 | AKR1C3 | AKR1C2 | AKR1C1 | HSD17B6 |
| Cofactors | NADP(H) | NADP(H) | NADP(H) | NADP(H) | NAD(H) |
| Major reactions | |||||
| Reduction | |||||
| 3αHSDa | High | Moderate | Moderate-high | Moderate | Moderate |
| 3βHSDb | Nil | Nil | Nil | Nil | Moderate |
| 17βHSDc | Nil | Moderate-high | Nil | Low | Low |
| 20αHSDd | Low | Low | Low | Moderate-high | Nil |
| Other substrates | DOC, PGF2α | ||||
| Oxidation | |||||
| 3αHSDa | Low | Nil | Low | Low | High |
| 3βHSDb | Nil | Nil | Nil | Nil | High |
| 17βHSDc | Nil | Low | Nil | Nil | Moderate |
| 20αHSDd | Nil | Nil | Nil | Low | Nil |
| Tissue distribution | Liver (major site), adrenal/gonad (trace) | Prostate, breast, liver, adrenal, testis, lung | Liver, prostate, lung, uterus, brain | Liver, testis, lung, breast, uterus, brain | Prostate |
a Reductive reactions include: DHT → 5α-androstane-3α,17β-diol, 5α-androstane-3,20-dione → androsterone, and 5α-pregnane-3,20-dione → allopregnanolone; oxidative reactions include the reverse reactions.
b Reductive reactions include: DHT → 5α-androstane-3β,17β-diol, 5α-androstane-3,20-dione → 3β-androsterone, and 5α-pregnane-3,20-dione → 5α-pregnan-3β-ol-20-one (3β-allopregnanolone); oxidative reactions include the reverse reactions.
c Reductive reactions include: androsterone → 5α-androstane-3α,17β-diol, androstenedione → testosterone, estrone → estradiol; oxidative reactions include the reverse reactions.
d Reductive reactions include: progesterone → 4-pregnen-20α-ol-3-one (20α-dihydroprogesterone) and DOC → 4-pregnene-20α,21-diol-3-one (20α-dihydroDOC).
© R. J. Auchus and W. L. Miller.
The 3αHSDs appear to be important in the CNS. Selective serotonin reuptake inhibitors, such as the common antidepressants fluoxetine and paroxetine, directly lower the Km of rat brain 3αHSD type 2 for 5α-dihydroprogesterone by almost 10-fold (561), explaining why these drugs augment brain allopregnanolone concentrations and perhaps contributing to their antidepressant activity. X-ray crystallography shows that the β-subunit of the mammalian voltage-gated potassium channel is a tetrameric structure (562) in which each subunit closely resembles a rat liver 3αHSD (AKR1C9) (563), and even contains bound NADP+. These studies suggest a role for HSDs in coupling intracellular redox state to membrane excitation.
The 3αHSDs differ from the 11βHSDs, 3βHSDs, and 17βHSD types 1 to 4 in several respects because all reductive 3αHSDs are AKR enzymes rather than SDR enzymes. As AKR enzymes, they function as monomers with a TIM-barrel structure, binding cofactor with the nicotinamide ring draped across the mouth of the “barrel” rather than lying on a Rossman fold, and their kinetic mechanisms are highly ordered, with dissociation of the cofactor as the final and rate-limiting step (564). NADP(H) is bound tightly because of the interaction of Arg276 with the 2′-phosphate of NADP(H); mutation of Arg276 eliminates a conformational change associated with tight binding (565) and attenuates or reverses the preference for ketosteroid reduction in intact cells (52). The structure of AKR1C9 (564) shows that the active sites of the AKR enzymes also contain tyrosine and lysine residues that facilitate proton transfer during catalysis, but these residues are distantly located in linear sequence rather than confined to the Tyr-X-X-X-Lys motif as in SDR enzymes.
In contrast to the reductive 3αHSDs, the oxidative 3αHSDs belong to the SDR family and are similar to the retinol dehydrogenase or cis-retinol/androgen dehydrogenase (RODH/CRAD) subfamily (566). Although several of these RODH/CRAD enzymes have some 3αHSD activity, the most active enzyme is RODH, the microsomal 3αHSD, 3(α→β)-hydroxysteroid epimerase (formally named 17βHSD6) whose cDNA was first cloned from prostate (533). This enzyme converts the inactive C19 steroid 5α-androstane-3α,17β-diol to DHT, and thus may catalyze the final step in the backdoor pathway from 17OHP to DHT (see Section XVIII.C). Prolonged incubation of 3α-hydroxysteroids with cells expressing 17βHSD6 or with microsomes containing the recombinant enzyme yields both 3α- and 3β-hydroxysteroids and 17β-hydroxysteroids (567); thus, this enzyme has many catalytic activities and may serve a variety of biological functions.
XV. Isozymes of 11β-Hydroxysteroid Dehydrogenase
Although certain steroids are typically categorized as glucocorticoids or mineralocorticoids, cloning and expression of the “mineralocorticoid” (glucocorticoid type 2) receptor showed it had equal affinity for both aldosterone and cortisol (568). However, cortisol does not normally act as a mineralocorticoid in vivo, although cortisol concentrations typically exceed aldosterone concentrations by 100- to 1000-fold. In mineralocorticoid-responsive tissues such as the kidney, cortisol is enzymatically converted to cortisone, a metabolically inactive steroid (569).
The interconversion of cortisol and cortisone is mediated by the two isozymes of 11βHSD, both of which have oxidase and reductase activity, depending on whether NADP+ or NADPH is available as the cofactor (570). Both enzymes are hydrophobic, membrane-bound proteins that bind cortisol/cortisone and corticosterone/11-dehydrocorticosterone, but otherwise their properties and physiological roles differ substantially (570) (Table 6). Interest in these enzymes extends far beyond their deficiency states because they play central roles in metabolism (571, 572).
Table 6.
Principal characteristics of the two isozymes of 11βHSD
| 11βHSD type 1 | 11βHSD type 2 | |||
|---|---|---|---|---|
| Tissues | Liver, testis, lung, fat, proximal nephron | Distal nephron, placenta, colon | ||
| Location | Endoplasmic reticulum, facing lumen | Endoplasmic reticulum, facing cytoplasm | ||
| Reaction | Reduction | Oxidation | Reduction | Oxidation |
| Substrates | Cortisone, dehydrocorticosterone, prednisone | Cortisol, corticosterone, prednisolone | (No significant substrates) | Cortisol, corticosterone, prednisolone |
| Cofactor | NADPH | NADP+ | NAD+ | |
| Coenzyme | H6PDH | |||
| Steroid Km | 0.1–0.3 μm | 1–2 μm | 0.01–0.1 μm | |
© W. L. Miller and R. J. Auchus.
The type 1 enzyme (11βHSD1) (573) is a dimer of 34-kDa subunits expressed mainly in glucocorticoid-responsive tissues such as the liver, testis, lung, fat, and proximal convoluted tubule (571). The type 1 enzyme catalyzes both the oxidation of cortisol to cortisone using NADP+ as cofactor (Km 1–2 μm) and the reduction of cortisone to cortisol using NADPH cofactor (Km 0.1–0.3 μm), with cortisone reduction being the dominant reaction in transfected cells (574, 575); the reaction catalyzed depends on which cofactor is available, but the enzyme can only function with high (micromolar) concentrations of steroid (575, 576). Many synthetic glucocorticoids (e.g., prednisone and cortisone) are 11-ketosteroids that must be reduced to their 11β-hydroxy derivatives to attain biological activity; these transformations are performed mainly in the liver by 11βHSD1. However, when recombinant 11βHSD1 is expressed in vitro, oxidation of cortisol with NADP+ is more efficient, and cortisone reduction is only achieved if NADP+ is removed by an enzymatic NADPH regeneration system (576, 577). Thus, the net flux of steroid driven by 11βHSD1 depends on the relative concentrations of available NADPH and NADP+, which usually favors reduction in cells, especially given the high Km of the enzyme for cortisol (577). The discrepancy between the prominent oxidative preference in vitro and the reductive dominance in vivo derives from the localization of 11βHSD1 in the lumen of the endoplasmic reticulum (578). In the endoplasmic reticulum, the ratio of NADPH to NADP+ is maintained by hexose-6-phosphate dehydrogenase (H6PDH), rather than by cytoplasmic NADP+-coupled dehydrogenases (e.g., glucose-6-phosphate dehydrogenase) (579).
The 41-kDa type 2 enzyme (580) (11βHSD2) has only 21% sequence identity with 11βHSD1, whereas 11βHSD2 and 17βHSD2 share 37% identity and favor steroid oxidation in vivo. Thus, 11βHSD1 and -2 are only distantly related members of the SDR family, yet they perform physiologically related but opposite functions. 11βHSD2 catalyzes only the oxidation of cortisol to cortisone using NAD+ and functions with low (nanomolar) concentrations of steroid (Km 10–100 nm) (581, 582); whether or not 11βHSD2 catalyzes reductive reactions remains undemonstrated. 11βHSD2 is expressed in mineralocorticoid-responsive tissues and thus serves to “defend” the mineralocorticoid receptor by inactivating cortisol to cortisone, so that only “true” mineralocorticoids, such as aldosterone or DOC can exert a mineralocorticoid effect. 11βHSD2 is inactive against aldosterone, DOC, and 9α-fludrocortisol. Thus, 11βHSD2 prevents cortisol from overwhelming renal mineralocorticoid receptors (569), and in the placenta and other fetal tissues 11βHSD2 (583, 584) also inactivates cortisol. The placenta also has abundant NADP+ favoring the oxidative action of 11βHSD1, so that in placenta both enzymes protect the fetus from high maternal concentrations of cortisol (570). 11βHSD1 is located on the luminal side of the endoplasmic reticulum, and hence is not in contact with the cytoplasm. In this unusual cellular location, 11βHSD1 receives NADPH provided by the action of H6PDH (585), linking 11βHSD1 to the pentose monophosphate shunt, thus providing a direct paracrine link between local glucocorticoid production and energy storage as fat (586).
Cortisone and prednisone are inactive prohormones that must be reduced to cortisol or prednisolone by hepatic 11βHSD1 to bind to and activate the glucocorticoid receptor (GR) (45, 587). Cortisol is a potent agonist at the mineralocorticoid receptor in the distal nephron, but its oxidized 11-keto derivative, cortisone, is not a mineralocorticoid. Cortisol does not act as a mineralocorticoid in vivo, although cortisol concentrations can exceed aldosterone concentrations by three orders of magnitude because it is enzymatically converted to cortisone in the cells lining the cortical and medullary collecting ducts. Thus, the type 2 enzyme inactivates the mineralocorticoid activity of cortisol in the kidney tubule (569), and inactivating mutations in the type 2 enzyme cause a syndrome of apparent mineralocorticoid excess (AME) (588). The presence of the type 2 enzyme in the placenta (583) also inactivates endogenous and synthetic corticosteroids such as prednisolone, allowing the use of these agents during pregnancy without affecting the fetus. By contrast, 9-fluorinated steroids such as dexamethasone are minimally inactivated by the type 2 enzyme, primarily because of a shift in the oxidation/reduction preference rather than a reduction in affinity for the enzyme (589). It is this resistance to inactivation by placental 11βHSD2 that is essential for synthetic glucocorticoids to “cross the placenta” and to exert a pharmacological effect on the fetus. Furthermore, the relatively high placental concentrations of NADP+ may also favor the oxidative action of 11βHSD1, so that both placental enzymes protect the fetus from the high maternal concentrations of cortisol that occur during pregnancy (570).
A. Lesions in 11βHSD1—apparent cortisone reductase deficiency
Apparent cortisone reductase deficiency is characterized by high ratios of cortisone to cortisol and of their respective metabolites in blood and urine (589). Such defects in 11βHSD activity impair cortisol feedback at the hypothalamic/pituitary axis, increasing the secretion of ACTH and consequently increasing adrenal C19 steroid secretion, resulting in hyperandrogenism, sexual precocity, and polycystic ovaries (587). Only about 10 such patients have been described (587). This disorder is caused by inactivating mutations in H6PDH (590), which impair NADPH regeneration within the endoplasmic reticulum, rather than by mutations in the coding regions of HSD11B1. It was initially reported that mutations in both 11βHSD1 and H6PDH interacted to cause this disease (590), but variations in 11βHSD1 appear to make a relatively minor contribution to this phenotype or to the polycystic ovary syndrome (591–593). Mutations of H6PDH in patients (594) or knockout of the H6PDH gene in mice (595) appear to be both necessary and sufficient to cause this disorder. Both HSD11B1 and H6PDH deficiencies should be diagnosed from urinary steroids, which show a marked reduction in ratio of metabolites of cortisol to those of cortisone. The genetics and pathophysiology of cortisone reductase deficiency provide an excellent example of the critical role of nicotinamide cofactors in HSD function and biology.
B. Lesions in 11βHSD2—apparent mineralocorticoid excess
Patients with AME have hypervolemic hypertension, salt retention, and hypokalemic alkalosis—the classic picture of hyperaldosteronism—but with suppressed plasma renin activity and without measurable serum mineralocorticoids due to recessive mutations of 11βHSD2 (570). About 30 different mutations in 11βHSD2 have been described in about 60 patients with AME (596, 597). Heterozygous carriers may have an increased risk of hypertension (598). Typical features of children with AME include failure to thrive, delayed puberty, polydipsia, polyuria, muscle weakness, and hypertension. The hypertension is severe, often causing end-organ damage at an early age. Diagnosis is made from the high ratio of urinary metabolites of cortisol to cortisone. Treatment with spironolactone, correction of the hypokalemia, low-salt diets, and diuretics is only partially successful, and 10% of patients die from cerebrovascular accidents (599).
XVI. Steroid Sulfatase and Sulfotransferases
Steroid sulfates may be synthesized directly from cholesterol sulfate or may be formed by sulfation of steroids by cytosolic sulfotransferase (SULT) enzymes (600, 601). At least 44 distinct isoforms of these enzymes have been identified belonging to five families of SULT genes; many of these genes yield alternately spliced products accounting for the large number of enzymes. The SULT enzymes that sulfonate steroids include SULT1E1 (estrogens), SULT2A1 (nonaromatic steroids), and SULT2B1 (sterols). SULT2A1 is the principal SULT expressed in the adrenal, where it sulfates the 3β-hydroxyl group of Δ5 steroids (pregnenolone, 17-hydroxypregnenolone, DHEA, and androsta-5-ene-3β,17β-diol), but not of cholesterol. SULT2B1a will also sulfonate pregnenolone but not cholesterol, whereas cholesterol is the principal substrate for SULT2B1b in the skin, liver, and elsewhere. It is not clear whether most steroid sulfates are simply inactivated forms of steroid or whether they serve specific hormonal roles. Knockout of the mouse SULT1E1 gene is associated with elevated estrogen levels, increased expression of tissue factor in the placenta, and increased platelet activation, leading to placental thrombi and fetal loss that could be ameliorated by anticoagulant therapy (602). Mutations ablating the function of human SULT enzymes have not been described, but single nucleotide polymorphisms that alter the amino acid sequences and catalytic activity affecting drug activity are well-described. African-Americans have a high rate of polymorphisms in SULT2A1 apparently influencing plasma ratios of DHEA:DHEAS, which may correlate with the risk of prostatic and other cancers (603).
Steroid sulfates may also be hydrolyzed to the native steroid by steroid sulfatase. Deletions in the steroid sulfatase gene on chromosome Xp22.3 cause X-linked ichthyosis (604, 605). In the fetal adrenal and placenta, diminished or absent sulfatase deficiency reduces the pool of free DHEA available for placental conversion to estrogen, resulting in low concentrations of estriol in the maternal blood and urine. The accumulation of steroid sulfates in the stratum corneum of the skin causes the ichthyosis. Steroid sulfatase is also expressed in the fetal rodent brain, possibly converting peripheral DHEAS to active DHEA (606, 607).
The obligatory sulfate donor for all the SULT enzymes is 3′-phosphoadenosine-5′-phosphosulfate (PAPS). PAPS is synthesized from ATP by two enzymatic activities: ATP sulfurylase, which catalyzes the conversion of ATP and sulfate (SO4) to adenosine phosphosulfate (APS); and APS kinase, which uses a phosphate from another ATP molecule to convert APS to PAPS (608). In human beings, the two activities are embodied in one enzyme with two isoforms, PAPS synthase types 1 and 2 (PAPSS1, PAPSS2). PAPSS1 is ubiquitously expressed, whereas PAPSS2 is highly expressed in the major sites of DHEA sulfation: the adrenal and liver (608). Deficiency of PAPSS2 prevents DHEA sulfation and causes the adrenal glands to produce much more free DHEA than normal. Because DHEA, unlike DHEAS, is a substrate for the 3βHSD enzymes, the excess DHEA that accumulates when it cannot be converted to DHEAS yields excess androgens. The first patient shown to have compound heterozygous PAPSS2 deficiency was a 6-yr-old girl who presented with premature pubarche and advanced bone age, followed by worsening androgen excess with acne, hirsutism, and secondary amenorrhea at age 13 (609). Her circulating DHEA was markedly elevated, but DHEAS was very low, and androgens including androstenedione, testosterone, and DHT were significantly increased. PAPSS2 is probably also important for cartilage and bone formation because this patient had short stature and abnormal bone development. Furthermore, complete deficiency of PAPSS2 causes spondyloepimetaphyseal dysplasia of the Pakistani type, but steroid metabolism was not assessed in this kindred, and women could not be studied at all (610).
XVII. Other Genetic Adrenal Disorders Associated with Steroidogenesis
A. Adrenal hypoplasia congenita
Adrenal hypoplasia congenita (AHC), also known as congenital adrenal hypoplasia, is a disorder of adrenal development resulting in primary adrenal insufficiency. This condition can occur with several different inheritance patterns and with a variety of associated or syndromic features.
X-linked AHC, caused by mutations of the DAX1 (NR0B1) gene on chromosome Xp21, is the most prevalent form of primary adrenal hypoplasia (611, 612). In AHC, the definitive zone of the fetal adrenal does not develop, and the fetal zone is vacuolated and cytomegalic. About half of boys with AHC present with salt loss and glucocorticoid insufficiency in early infancy; the rest present more insidiously with chronic adrenal insufficiency throughout childhood (613). DAX1 is a nuclear transcription factor involved in adrenal and testicular development, as well as being expressed in the pituitary gonadotropes. About two thirds of boys with AHC have DAX1 point mutations; the other one third have DAX1 gene deletions either in isolation or as part of a contiguous gene deletion syndrome involving a telomeric X-linked mental retardation locus (IL1RAPL1) and/or centromeric loci for glycerol kinase deficiency and sometimes ornithine transcarbamylase and Duchenne muscular dystrophy (612, 613). An adult-onset form of AHC due to point mutations in DAX1 has also been described in several patients (614). Female carriers of DAX1 mutations are unaffected, but half of their sons will be affected. A family history of adrenal failure, unexplained death, or pubertal abnormalities in the male relatives of a boy with adrenal insufficiency should suggest AHC.
Autosomal forms of adrenal hypoplasia exist, but their underlying basis is poorly understood. Heterozygous or homozygous mutations in SF1 (NR5A1) have been reported in 46,XY phenotypic females with either spontaneous or recessively inherited primary adrenal failure, and a heterozygous SF1 mutation has been described in a 46,XX girl with adrenal dysfunction (615–617). However, SF1 mutations have not been found in phenotypic males with adrenal hypoplasia or adrenal steroidogenic defects (613). Primary adrenal failure has been associated with Pena-Shokeir syndrome type I, pseudotrisomy 13, Meckel syndrome, and Pallister-Hall syndrome (GLI3), and with defects in WNT3 (618). Primary adrenal hypoplasia also appears to be part of the IMAGe syndrome (Intrauterine growth retardation, Metaphyseal dysplasia, Adrenal hypoplasia, Genitourinary anomalies), but the underlying etiology of this condition remains unknown (619, 620).
B. ACTH resistance syndromes
Hereditary unresponsiveness to ACTH [familial glucocorticoid deficiency (FGD)] can present as an acute adrenal crisis or with the signs and symptoms of chronic adrenal insufficiency in childhood. Several autosomal recessive causes of FGD have been identified (119). Patients with ACTH unresponsiveness typically continue to produce mineralocorticoids. The typical presenting picture consists of failure to thrive, lethargy, pallor, hyperpigmentation, and hypoglycemia, often associated with seizures. Rare cases may also entail electrolyte abnormalities or increased plasma renin activity, leading to misdiagnosis as a different form of adrenal insufficiency (621).
FGD1 is caused by autosomal recessive mutations in the gene for the G protein-coupled ACTH receptor (MC2R) (119, 622, 623). More than 20 MC2R mutations have been reported, but the mutation S74I is especially prevalent. ACTH levels can be markedly elevated with consequent hyperpigmentation. Tall stature and increased head circumference have been reported in several cases (623, 624). Treatment with replacement doses of glucocorticoids typically prevents adrenal crises but may not suppress elevated ACTH levels completely (119).
FGD2, which is clinically indistinguishable from FGD1, is caused by mutations in the gene for melanocortin 2 receptor accessory protein (MRAP) (119, 625, 626). MRAP is expressed in multiple tissues where it seems to play a role in trafficking the ACTH receptor from the endoplasmic reticulum to the cell membrane. Additional genetic loci for other forms of FGD are under investigation. Some patients with nonclassical lipoid CAH having mild disorders in StAR have been clinically mistaken for having FGD (117).
Triple A (Allgrove) syndrome consists of: 1) ACTH-resistant adrenal (glucocorticoid) deficiency (80% of individuals); 2) achalasia of the cardia (85%); and 3) alacrima (90%) (627). Mineralocorticoid insufficiency is reported in about 15% of cases, and many patients have progressive neurological symptoms such as intellectual impairment, sensorineural deafness, peripheral and cranial neuropathies, optic atrophy, Parkinsonism, and autonomic dysfunction (622, 628, 629). Triple A syndrome is caused by autosomal recessive mutations in AAAS, which encodes a WD-repeat protein termed ALADIN (630, 631). This protein localizes to the cytoplasmic side of the nuclear pore, where it plays a role in nuclear import (632). Defective nuclear import of the heavy chain of ferritin causes oxidative damage, apparently accounting for the pleiotropic effects of ALADIN deficiency (633). Clinical findings can be quite variable, even within the same family, but adrenal insufficiency is rarely the presenting feature.
C. Familial glucocorticoid resistance
Familial glucocorticoid resistance is caused by mutations in the α-isoform of the GR. Decreased glucocorticoid action results in grossly increased ACTH secretion, stimulating the production of cortisol and other adrenal steroids. These very rare patients may have fatigue, hypertension, and hypokalemic alkalosis, suggesting a mineralocorticoid excess syndrome, but they may also have hyperandrogenism (634). Patients may be homozygous for missense mutations (635) or heterozygous for a gene deletion (636), so that in each case some GR activity remains. No patients have been described with homozygous deletion of this receptor. However, a recent report described a newborn infant with profound glucocorticoid resistance who was homozygous for a frameshift mutation at codon 772 in the glucocorticoid-binding domain; the infant had severe hypoglycemia and hypertension but had normal pulmonary development, suggesting that glucocorticoid action is not required for normal human fetal development (637). Heterozygous point mutations with incomplete dominant negative activity or multiple effects on GRα action have also been described (638). These point mutations may interfere with GRα-dependent transcriptional regulation through altered DNA binding, impaired ligand binding, delayed nuclear localization, abnormal nuclear aggregation, and disrupted interaction with coactivators, depending on the position of the mutation (634). GR-knockout mice have disordered hepatic gluconeogenesis and absent adrenomedullary chromaffin cells and die from neonatal respiratory distress syndrome (639). Thus, familial glucocorticoid resistance is typically a syndrome of only partial resistance to the action of glucocorticoids.
D. Pseudohypoaldosteronism
Pseudohypoaldosteronism (PHA) is a rare salt-wasting disorder of infancy characterized by hyponatremia, hyperkalemia, and increased plasma renin activity in the face of elevated aldosterone concentrations. The more common autosomal recessive form of PHA (pseudohypoaldosteronism type II) is caused by inactivating mutations in any of the three subunits (α, β, and γ) of the amiloride-sensitive epithelial sodium channel (ENaC) (640). PHAII is often associated with lower respiratory tract disease because ENaC mutations increase the volume of pulmonary fluid (641). This disease persists into adulthood, requiring vigorous salt-replacement therapy throughout life. Gain-of-function mutations due to carboxy-terminal truncation of β-ENaC cause Liddle’s syndrome, an autosomal dominant form of salt-retaining hypertension (640).
Autosomal dominant type 1 pseudohypoaldosteronism (PHAI) is caused by inactivating mutations in the mineralocorticoid receptor (642, 643). PHAI is milder than PHAII caused by ENaC mutations and remits with age, but it requires sodium replacement therapy in infancy and childhood. Rare point mutations altering the structure of the ligand-binding domain of the mineralocorticoid receptor may result in mild constitutive activation as well as permitting binding and activation of the receptor by progesterone, resulting in severe hypertension that begins in adolescence and worsens with pregnancy (644).
Genetic forms of PHA must be distinguished from the acquired, transient form of PHA often seen in infants with obstructive uropathy, especially shortly after surgical relief of the obstruction (645). That lesion is renal tubular (646), so that mineralocorticoid treatment is generally ineffective; salt replacement generally suffices while the renal lesion resolves.
XVIII. Tissue-Specific Pathways of Steroidogenesis
A. Adrenal pathways
Diagrams of steroidogenic pathways, such as that shown in Fig. 3, typically combine the pathways from multiple cell types to provide an overview of all steroidogenic processes; however, such diagrams are misleading because the pathways differ in each steroidogenic cell type. The three major pathways of steroidogenesis in the human adrenal are shown in Fig. 13. The adrenal zona glomerulosa is characterized by three distinct features: it expresses angiotensin II receptors, it expresses P450c11AS, and it fails to express P450c17. As a result, the zona glomerulosa produces aldosterone under regulation by the renin/angiotensin system. By contrast, the adrenal zona fasciculata does not express angiotensin II receptors or P450c11AS, but instead expresses MC2R (the ACTH receptor) and P450c11β, which cannot convert 18-hydroxycorticosterone to aldosterone and has minimal capacity to convert corticosterone to 18-hydroxycorticosterone (458). Both the zona glomerulosa and zona fasciculata express P450c21, but the zona fasciculata also expresses P450c17, which allows cortisol synthesis. The zona fasciculata, however, expresses little (if any) cytochrome b5 (201); consequently, P450c17 in the zona fasciculata catalyzes 17α-hydroxylation but very little 17,20-lyase activity. Thus, the zona fasciculata produces two glucocorticoids (cortisol and corticosterone) under the influence of ACTH, but very little DHEA. Patients with severe mutations in P450c17 cannot synthesize cortisol but instead increase corticosterone production (647) (as do rodent adrenals, which normally lack P450c17), explaining why they are not glucocorticoid-deficient, despite the lack of cortisol (Fig. 13). The adrenal zona reticularis also expresses MC2R, but very little P450c21 or P450c11β, and as a result, the zona reticularis produces minimal amounts of cortisol. By contrast, the zona reticularis expresses large amounts of P450c17 and cytochrome b5 (201), maximizing 17,20-lyase activity (189), so that DHEA is produced, much of which is sulfated to DHEAS by SULT2A1 (648). The zona reticularis expresses relatively little 3βHSD2, and the Km of 3βHSD2 is approximately 5 μm for pregnenolone and 17-hydroxypregnenolone (188), whereas the Km for both activities of P450c17 is approximately 1 μm (189), so that abundant DHEA is produced. As DHEA accumulates, small amounts are converted to androstenedione, and very small amounts of this androstenedione are converted to testosterone, probably by AKR1C3/17βHSD5. Thus, the pattern of steroid products secreted by each adrenal zone is determined by the enzymes produced in that zone and may be logically deduced from an understanding of their specific enzymatic properties (648).
Fig. 13.
Major steroidogenic pathways in the three zones of the human adrenal cortex. The conversion of cholesterol to pregnenolone by P450scc is common to all three zones. A, In the zona glomerulosa, 3βHSD2 converts pregnenolone to progesterone. P450c17 is absent, but P450c21 produces DOC, which is a substrate for P450c11AS. P450c11AS catalyzes 11-hydroxylation and two 18-oxygenations, which completes aldosterone synthesis. B, The zona fasciculata expresses P450c17, so pregnenolone is hydroxylated to 17α-hydroxypregnenolone (or progesterone to 17-OHP), but the zona fasciculata contains little cytochrome b5, minimizing the 17,20-lyase activity of P450c17, and little DHEA is produced. Instead, 3βHSD2 and P450c17 generate 17-OHP, the preferred substrate for P450c21, yielding 11-deoxycortisol. P450c11β, which is unique to the zona fasciculata, completes the synthesis of cortisol. Corticosterone is normally a minor product (dashed arrows) derived from a parallel pathway without the action of P450c17. C, The zona reticularis has large amounts of P450c17 and cytochrome b5 but little 3βHSD2, so that pregnenolone is sequentially oxidized to 17-hydroxypregnenolone and then DHEA. SULT2A1, using PAPS synthesized by PAPSS2 (see Section XVI), sulfates DHEA, and DHEAS is exported to the circulation. Testosterone synthesis is a very minor pathway (dashed arrows). [© R. J. Auchus.]
B. Gonadal pathways
Testicular synthesis of testosterone follows a pathway that is similar to C19-steroid production in the adrenal zona reticularis, with the notable exceptions that the stimulus for steroidogenesis is transduced by the LH receptor rather than MC2R and that Leydig cells express abundant 3βHSD2 and 17βHSD3, but no SULT2A1. Thus, DHEA produced in the testis is not sulfated but is readily converted to androstenedione and then testosterone (Fig. 14). As in the adrenal, the principal pathway to C19-steroids is via Δ5 steroids to DHEA; the Δ4 pathway from 17OHP to androstenedione makes a minimal contribution (238, 282).
Fig. 14.
Major pathways of gonadal steroidogenesis. A, In testicular Leydig cells, cholesterol is converted to DHEA by the same enzymes using the same cofactors as in the adrenal zona reticularis. Leydig cells contain abundant 17βHSD3, so that Leydig cells efficiently produce testosterone, via androstenedione and/or androstenediol. B, Ovarian granulosa cells contain P450scc and convert cholesterol to pregnenolone. The ovarian theca cells express low levels of P450scc but high amounts of P450c17 and hence acquire C21-steroids from the granulosa cells and produce C19 precursors of sex steroids (the two-cell model of ovarian steroidogenesis). Theca cells do not express aromatase (P450aro); hence, androstenedione must return to the granulosa cells, which contain abundant aromatase and 17βHSD1, completing the synthesis of estradiol. In the luteal phase, 3βHSD2 in the corpus luteum metabolizes nascent pregnenolone to progesterone, the final product. Minor pathways are shown with dashed arrows. [© R. J. Auchus.]
Ovarian steroidogenesis is more complex because the enzymatic steps are partitioned between the granulosa and theca cells, which surround the oocyte and form a follicle. Furthermore, the patterns of steroidogenesis vary during the cycle: estradiol is the principal product in the follicular phase, and progesterone is produced in the luteal phase (Fig. 14). The key point in ovarian steroidogenesis is that granulosa cells do not express P450c17 (158). Thus, in general, steroidogenesis is initiated in granulosa cells under the influence of LH, which, via cAMP, stimulates the expression of P450scc (158). Pregnenolone and progesterone from granulosa cells diffuse into adjacent theca cells, where they can be acted upon by P450c17 and 3βHSD2 to produce androstenedione. Small amounts of this androstenedione are secreted or converted to testosterone (probably by AKR1C3/17βHSD5), but most androstenedione returns to the granulosa cells where it is converted to estrone and then to estradiol by P450aro and 17βHSD1, respectively, under the influence of FSH. Thus, as with the three zones of the adrenal, the patterns of gonadal steroidogenesis are dictated by the cell-specific expression of specific steroidogenic enzymes.
C. The “backdoor pathway” to dihydrotestosterone
Studies of fetal androgen biosynthesis and mechanisms of virilization in the tammar wallaby have revealed the presence of a novel, alternative, so-called “backdoor pathway” that leads from 17OHP to DHT without going through androstenedione or testosterone as intermediate steroids (332, 333). This pathway is initiated when either progesterone or 17OHP is 5α-reduced. The resulting 5α-reduced C21 steroids, dihydroprogesterone (5α-pregnane-3,20-dione) and 5α-pregnan-17α-ol-3,20-dione, are readily acted on by reductive 3αHSDs to yield allopregnanolone [5α-pregnan-3α-ol-20-one (Allo)] and 17α-hydroxylated allopregnanolone (5α-pregnane-3α,17α-diol-20-one; 17OH-Allo). Dihydroprogesterone and Allo are excellent substrates for the 17α-hydroxylase activity of P450c17 (649), and 17OH-Allo is the most efficient substrate known for the 17,20-lyase activity of human P450c17. Furthermore, unlike the conversion of 17α-hydroxypregnenolone to DHEA (189, 232, 233), the cleavage of 17OH-Allo to androsterone is minimally dependent on cytochrome b5 (649). The resulting androsterone may be 3α-oxidized to DHT by the activity of 17βHSD6 [RODH, the microsomal 3αHSD, 3(α→β)-hydroxysteroid epimerase] (533). Thus, this pathway is an alternative, backdoor pathway to DHT, by which DHT is produced without utilizing DHEA, androstenedione, and testosterone as intermediates (333) (Fig. 15). Consequently, the presence of 5α-reductases in steroidogenic cells does not preclude the production of C19 steroids, but rather paradoxically enhances the production of DHT by directing flux to 5α-reduced precursors of DHT. The backdoor pathway enables production of C19 steroids from 17OHP, despite the poor 17,20-lyase activity of human P450c17 for 17OHP, by using 17OH-Allo as the substrate for the 17,20-lyase reaction. The presence of 5α-reductase activity is a key requirement for the backdoor pathway.
Fig. 15.
Reactions catalyzed by human P450c17 and pathways to C19 steroids. A, The four principal A/B-ring configurations of active endogenous steroids and their precursors: Δ5, Δ4, 5α, and 5α,3α (structures shown at bottom). Progesterone and 17α-hydroxyprogesterone can be 5α-reduced, and once the A-ring is saturated, these 5α-reduced steroids are substrates for reductive 3αHSDs of the AKR1C family. Human P450c17 17α-hydroxylates all four classes of C21 steroids, but the 17,20-lyase activity is robust only with 17α-hydroxypregnenolone and 17-hydroxyallopregnanolone (5α-pregnane-3α,17α-diol-20-one), the Δ5 and 5α,3α pathways, respectively. Dihydroprogesterone, 17-hydroxydihydroprogesterone, and allopregnanolone are trivial names for 5α-pregnane-3,20-dione, 5α-pregnan-17α-ol-3,20-dione, and 5α-pregnan-3α-ol-20-one, respectively. B, Two pathways to DHT using the different 17,20-lyase activities of human P450c17. In the conventional or Δ5-pathway (left), the 17,20-lyase activity of P450c17 requires cytochrome b5 to efficiently convert 17α-hydroxypregnenolone to DHEA, and testosterone is reduced in target tissues by 5α-reductase 2 (5αR2) to DHT. In the “backdoor” or 5α,3α-pathway (right), 5α-reduction by 5αR1 and 3α-reduction of C21 steroids occurs in the steroidogenic tissue before the 17,20-lyase reaction. In the best characterized pathway based on the tammar wallaby pouch young, 17-hydroxyallopregnanolone is cleaved to androsterone without requiring cytochrome b5 and reduced to androstanediol. Androstanediol is exported from the testis and metabolized to DHT by the oxidative 3αHSD activity of 17βHSD6. DHT may also be formed from androsterone via a parallel pathway catalyzed by 17βHSD6 and 17βHSD3, with androstanedione as the intermediate. Note that testosterone is not an intermediate in the backdoor pathway to DHT, that different isoforms of 5α-reductase appear to be involved in the two pathways, and that both reductive and oxidative 3αHSD activities are required for the backdoor pathway. Structures of testosterone, DHT, and androstanediol are shown at bottom. [© R. J. Auchus.]
Originally described in marsupials, the backdoor pathway is relevant to human steroidogenesis. The best-studied example of 5α-reduction in a human steroidogenic tissue is the production of 5α-dihydroprogesterone in human corpus luteum by the type 1 enzyme (650). Human enzymes catalyze all of the other reactions required to complete this alternate route to DHT, and good evidence documents production of 5α-reduced androgens by the fetal adrenal, at least in some pathological states. Consequently, it appears that the backdoor pathway is a major route to DHT in pathological states in which 17OHP accumulates, including 21-hydroxylase deficiency and POR deficiency. Mass spectrometric analyses of urinary steroids in patients with POR deficiency confirm that all the steroidal intermediates in the backdoor pathway are produced in infants, children and adults with severe POR deficiency and pregnant women carrying a POR-deficient fetus (316, 331, 651), and adults with mild POR deficiency (283). Androgen production by the backdoor pathway may explain why newborn girls with 21- and 11-hydroxlase deficiencies can be severely virilized, whereas those with 3βHSD2 deficiency, whose adrenals cannot make 17OHP, are minimally virilized (195). The fractional contributions of the conventional and backdoor pathways to DHT production during human sexual differentiation at 8 to 12 wk gestation, and the expression of 5α-reductase in the fetal adrenal and gonad tissues (652), however, are only beginning to be determined.
D. Neurosteroids: steroid synthesis in the brain
Although it has long been known that steroids act on the brain and some early studies found some steroidogenic activities in rat brain homogenates (653), it was not until the early 1980s that clear evidence was presented suggesting that the brain is a site of steroid biosynthesis. DHEA, DHEAS, pregnenolone, and pregnenolone sulfate were found in the brains of rats at higher concentrations than found in peripheral blood, and these concentrations persisted for weeks after adrenalectomy and gonadectomy, suggesting endogenous synthesis (654, 655). Residual skepticism that the brain was the actual site of steroidogenesis was dispelled by finding the mRNAs for P450scc, P450c11β, and ferredoxin in adult rat brain (Ref. 656; for review see Ref. 657). Steroids that are endogenously produced in the brain or elsewhere in the nervous system are termed “neurosteroids.” To date, most studies of neurosteroids have been done with rodents; at present, there is scant evidence for the biosynthesis of steroids in the human brain, although such synthesis seems likely. A wide variety of steroids and steroidogenic enzymes have been found in various regions of the rodent brain; there is no single center that is steroidogenic, so that steroids appear to be widely produced for local action (606, 658). Those actions appear to supplement and reinforce actions regulated by other factors, rather than serving indispensable functions (for review, see Refs. 659 and 660). Thus, ablation of the mouse P450scc gene, similarly to the few cases of human P450scc deficiency, is compatible with anatomically normal development and term gestation, although the animals die shortly after birth from glucocorticoid and mineralocorticoid deficiency (135).
Two steroid pathways seem to predominate in the rodent brain. The first is the conventional pathway from cholesterol to DHEA and DHEAS, involving P450scc, P450c17, and a steroid SULT, whereas the second involves the synthesis of Allo, which is a 3α,5α-reduced derivative of progesterone. Both pathways are initiated by P450scc; whether or not StAR also participates in this first step, or whether brain steroidogenesis, like that in the placenta, is independent of StAR, remains controversial. The mRNAs for StAR and P450scc are colocalized in several regions of the rat brain (661), and StAR mRNA is found in various regions of the human brain (662). StAR mRNA appears in the mouse brain at postnatal d 1 (663), but neither StAR knockout mice nor human patients with lipoid CAH have a phenotype attributable to altered CNS function. Similarly, the mRNA for P450scc is detectable in the fetal mouse brain (656) in specific regions (606), but neither P450scc knockout mice nor the rare patients with P450scc deficiency exhibit obvious neurological phenotypes. P450c17, the key enzyme in the production of DHEA, is found in embryonic mouse neurons as early as E9.5, and is found throughout the developing mouse brain (658), but in the adult it is found only in the hippocampus (664) and spinal cord (665). DHEA and DHEAS appear to exert actions on the genesis, protection, and survival of neurons. DHEA and DHEAS protect the hippocampus against the toxicities of glutamate, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate), and kainite (666). In vitro, concentrations of DHEA and DHEAS equivalent to those found in the fetal mouse brain stimulate neuronal development. When added to primary cultures of embryonic mouse neurons, low nanomolar concentrations of DHEA stimulate the outgrowth of neurites that become axons, whereas DHEAS stimulates the outgrowth of neurites that become dendrites (607). Treatment of rats with sc DHEA pellets increased neurogenesis in the dentate gyrus (667). DHEA and DHEAS exert their actions via nongenomic receptors, such as by antagonizing GABAA receptors (668) or as agonists for σ receptors (669). Evidence for relevance to human biology comes from effects of DHEA increasing neurogenesis and neuronal survival in cultured human neural stem cells (670). However, testing of the roles of DHEA and DHEAS in mice by knocking out the gene for P450c17 has been unsuccessful because this knockout, in contradistinction to human P450c17 deficiency, causes embryonic lethality (671).
The second pathway leads to progesterone and its 3α,5α-reduced derivative, Allo; several bioactive steroids are produced along this pathway. After cholesterol is converted to pregnenolone by P450scc, pregnenolone may be sulfated. Although pregnenolone sulfate was one of the steroids first described in the rat brain (655), advanced mass spectrometric procedures have failed to confirm its presence (672, 673); nevertheless, both SULT2A1 and SULT2B1a mRNA and protein have been found in rat brain (674, 675). SULT2B1 appears to be expressed in the human fetal brain (676), and the presence of pregnenolone sulfate in human brain has been confirmed (677). Pregnenolone sulfate is a negative regulator of GABAA, kainite, and AMPA receptors, and a positive regulator of N-methyl-D-aspartate receptors, thus acting as an excitatory neurosteroid (reviewed in Ref. 660). Pregnenolone sulfate enhances memory in mice (678, 679) and rats (680), but the potential relevance of these observations to human biology remains unknown.
Pregnenolone may be converted to progesterone by 3βHSD, which is found in rat glia and neurons (681, 682). Type 1 3βHSD is found throughout the rat brain and peripheral nervous system (661, 683). Progesterone exerts behavioral effects, probably via its action as an anesthetic, which was one of the first observations of steroids acting on the CNS (684). Progesterone also affects myelinization in the peripheral nervous system by directly stimulating myelin synthesis in Schwann cells and by stimulating neuronal gene expression via its classic nuclear receptor (685, 686). Emerging data indicate that progesterone exerts protective and recuperative effects in spinal cord injury, apparently via both cell surface and classic nuclear receptors (687), although it is possible that some of these effects are mediated by progesterone metabolites. Finally, progesterone appears to stimulate cerebellar development. Rodent Purkinje neurons contain the enzymes needed to produce progesterone and Allo and produce these steroids in the neonatal period during growth of the cerebellar cortex (683), and progesterone promotes dendritic growth and synaptic density in Purkinje cells in newborn rat cerebellar slices in vitro and in vivo (688).
Progesterone may then be sequentially 5α-reduced and 3α-reduced to Allo, a brain pathway that is similar to the backdoor pathway of testicular steroidogenesis. As in the backdoor pathway, the principal 5α-reductase is type 1 (550, 689–691), especially in the fetal rodent brain (692), although type 2 is transiently expressed toward the end of mouse gestation (693, 694). The identity of the 3α-reductase is somewhat unclear. Both a type 2 and type 3 3αHSD were cloned from both fetal and adult human brain, whereas type 3 predominated in the putamen, cerebellum, medulla, and spinal cord (695). The cloned type 2 3αHSD differed from that cloned from prostate by amino acid differences at positions 38 and 89 (524) and differed from that cloned from human liver by differences at amino acids 38, 75, 89, and 175 (557). Whether these three putatively distinct forms of 3αHSD represent allelic variants or sequencing errors of human AKR1C3 cDNA remains undetermined. Allo acts at nanomolar concentrations as an anesthetic, anxiolytic anticonvulsant by binding to GABAA receptors at sites distinct from those bound by GABA, benzodiazepines, and barbiturates, functioning as an allosteric modulator to open the channel and increase chloride flux (696–699). Animal progesterone withdrawal experiments that are designed to model the human menstrual cycle suggest that neurosteroids modulate GABAA receptor subunit composition and function, which may be associated with changes in mood and behavior in some women during the late luteal phase when Allo levels drop (700). The synthesis of multiple neurosteroids is disordered in a mouse model of Niemann-Pick type C disease, a fatal lysosomal storage disease; administration of Allo to affected newborn mice delays onset of symptoms until after untreated littermates are dead, and doubles life expectancy (701).
The brain also 21-hydroxylates steroids (702), leading to the synthesis of DOC and its 3α,5α-reduced product, tetrahydro-DOC, which acts similarly to Allo at GABAA receptors (703); however, the brain does not express significant amounts of P450c21 (366). The enzymes responsible for brain 21-hydroxylase activity include CYP2D4 in the rat and CYP2D6 in the human brain (704); other CYP enzymes may also be involved. In addition, the brain contains P450c11β (656), so that all enzymes needed for the synthesis of corticosterone and cortisol are present, but it is not clear whether the brain is a site of glucocorticoid biosynthesis.
XIX. Fetoplacental Steroidogenesis
A. The fetal adrenal
Adrenocortical steroidogenesis begins around the seventh week of gestation. Steroidogenic enzymes are immunocytochemically detectable in the fetal zone at 50–52 d after conception, and by 8 wk the adrenal contains cortisol and responds to ACTH in primary culture systems (334). Fetal cortisol synthesis is regulated by pituitary ACTH and involves transient expression of adrenal 3βHSD2. Following the ninth week after conception, expression of 3βHSD2 and synthesis of cortisol wane; 3βHSD2 is barely detectable at 10–11 wk and is absent at 14 wk (334). At the same time, the fetal adrenal also produces 17βHSD5 (334), which can convert androstenedione to testosterone. Thus, the fetal adrenal makes cortisol at the same time during gestation that fetal testicular testosterone is virilizing the genitalia of the normal male fetus. This fetal adrenal cortisol apparently suppresses ACTH, which otherwise would drive adrenal testosterone synthesis via 17βHSD5, thus preventing the virilization of female fetuses.
Fetuses with genetic lesions in adrenal steroidogenesis can produce enough adrenal androgen to virilize a female fetus to a nearly male appearance, and this masculinization of the genitalia is complete by the 12th week of gestation. The fetal adrenal is relatively deficient in 3βHSD2 activity after 12 wk (334, 705) but has robust 17,20-lyase activity of P450c17. Low 3βHSD and high 17,20-lyase activity account for the abundant production of DHEA and DHEAS by the fetal adrenal, which are converted to estrogens by the placenta. The fetal adrenal also has considerable SULT activity but little steroid sulfatase activity, also favoring conversion of DHEA to DHEAS. The resulting DHEAS cannot be a substrate for adrenal 3βHSD2; instead, it is secreted, 16α-hydroxylated in the fetal liver by CYP3A7 (706–708), and then acted on by placental 3βHSD1, 17βHSD1, and P450aro to produce estriol. Small amounts of DHEA/DHEAS bypass the liver and are not 16α-hydroxylated, and hence yield estrone and estradiol. Estrogens inhibit adrenal 3βHSD activity, providing a feedback system to promote production of DHEAS (709). Fetal adrenal steroids account for 50% of the estrone and estradiol and 90% of the estriol in the maternal circulation (710).
Although the fetoplacental unit produces huge amounts of DHEA, DHEAS, and estriol, as well as other steroids, they do not appear to serve an essential role. Successful pregnancy is wholly dependent on placental synthesis of progesterone, which suppresses uterine contractility and prevents spontaneous abortion (711); however, fetuses with genetic disorders of adrenal and gonadal steroidogenesis develop normally, reach term gestation, and undergo normal parturition and delivery. Mineralocorticoid production is only required postnatally, estrogens are not required, and androgens are only needed for male sexual differentiation (329). Human fetal glucocorticoids may be needed to suppress the virilization of female fetuses at about 8–12 wk (334), but it appears that glucocorticoids are not needed thereafter (329, 637, 712, 713).
The regulation of steroidogenesis and growth of the fetal adrenal are not fully understood, but both are related to ACTH. ACTH effectively stimulates steroidogenesis by fetal adrenal cells in vitro (54, 714), and excess ACTH is clearly involved in the adrenal growth and overproduction of androgens in fetuses affected with CAH. Prenatal treatment of such fetuses by administering pharmacological doses of dexamethasone to the mother at 6 to 10 wk gestation can significantly reduce fetal adrenal androgen production and thus reduce the virilization of female fetuses; thus, the hypothalamic-pituitary-adrenal axis functions very early in fetal life (407). By contrast, however, anencephalic fetuses lacking pituitary ACTH have adrenals that contain a fairly normal complement of steroidogenic enzymes and retain their capacity for steroidogenesis. Thus, fetal adrenal steroidogenesis may be regulated by both ACTH-dependent and ACTH-independent mechanisms.
B. Placental steroidogenesis
The placenta has two steroidogenic pathways. First, it can initiate steroidogenesis de novo from cholesterol and convert the resulting pregnenolone and progesterone; second, it can take C19 steroids produced by the fetal adrenal and convert them to estrogens. In having these two pathways and lacking P450c17, placental steroidogenesis resembles that in ovarian granulosa cells, but many of the enzymes and their regulation differ.
Human placental synthesis of progesterone begins in midgestation, at the same time that the maternal corpus luteum of pregnancy involutes (the luteoplacental shift), so that progesterone is provided throughout pregnancy. Progesterone suppresses uterine contractility, and hence is essential for the maintenance of pregnancy; drugs that oppose the action of progesterone, such as mifepristone (RU-486), act as abortifacients. Syncytiotrophoblast cells of the placenta use the same P450scc enzyme to convert cholesterol to pregnenolone that is used in the adrenal and gonad (131), but the transcription of its gene is under unique control. Whereas transcription of the genes for P450scc and all other enzymes in the adrenal and gonad requires the action of SF1, little if any SF1 is expressed in the placenta (68, 142). Furthermore, analysis of the expression of P450scc in placental JEG-3 cells shows that promoter elements that bind factors other than SF1 are required for its transcription (68). Several such factors have now been identified, including the CP2 (grainyhead) factors LBP1b (also called TFCP2A or UBP1) and LBP9 (TFCP2L1) (142–145), and the zinc-finger protein TreP-132 (149, 150). In addition, the placenta, unlike the adrenal or gonad, fails to express the gene for StAR (69), and StAR mutations causing lipoid CAH do not disrupt placental steroidogenesis. Thus, the same ill-defined mechanisms of StAR-independent steroidogenesis that are exemplified by the two-hit physiology of lipoid CAH (Fig. 5) appear to be operative in the placenta. A strong candidate for a factor mediating StAR-independent movement of cholesterol into placental mitochondria is MLN64, a 445-amino acid protein first identified in studies of metastatic breast cancer (70). Although full-length MLN64 has minimal StAR-like activity, the carboxy-terminal 234 amino acids of MLN64 are very similar to StAR and have about 50% of StAR’s activity in transfected cells (71). N-218 MLN64 and StAR share very similar biophysical properties, and a truncated form of MLN64 that cross-reacts with StAR antisera and is of about the same size as N-234 or N-218 MLN64 is found in human placenta (72). To conclude the distinctly placental pathway from cholesterol to progesterone, the placenta utilizes 3βHSD1 rather than the 3βHSD2 expressed in the adrenal and gonad. The two isozymes of 3βHSD have similar Km and Vmax values, so that the enzymological efficiency of this reaction is no different in the placenta.
A key feature of human placental steroidogenesis is that (unlike the rat) (715) it fails to express P450c17 (158). As a result, the placenta, like an ovarian granulosa cell, cannot convert pregnenolone to sex steroids. Instead, the placenta receives 16α-hydroxylated DHEA and DHEAS from the fetal circulation and, through the actions of steroid sulfatase, 3βHSD1, 17βHSD1, and aromatase, produces large amounts of estriol. Such placental estrogens provide a useful index of placental welfare, but serve no essential role in pregnancy because disorders of fetal adrenal steroidogenesis that eliminate the supplies of C19 steroid precursors do not interfere with term gestation or with parturition (for review see Ref. 329).
XX. Adrenarche
DHEA, DHEAS, and androstenedione, which are secreted by the adrenal zona reticularis, are generally referred to as adrenal androgens because they can be converted to testosterone, but these steroids have little if any capacity to bind to and activate androgen receptors; hence, they are androgen precursors, but not true androgens. The fetal adrenal secretes large amounts of DHEA and DHEAS, and these steroids are abundant in the newborn, but their concentrations fall rapidly as the fetal zone of the adrenal involutes after birth. After the first year of life, the adrenals of young children secrete very small amounts of DHEA, DHEAS, and androstenedione until the onset of adrenarche, usually around age 7–8, preceding the onset of puberty by about 2 yr. Adrenarche is independent of puberty, the gonads, or gonadotropins, and the mechanism by which the onset of adrenarche is triggered remains unknown (716). Secretion of DHEA and DHEAS increases during and after puberty and reaches maximal values in young adulthood, after which there is a slow, gradual decrease in these steroids in the elderly (“adrenopause”) (Fig. 16) (221). The higher concentration of DHEAS in men is probably attributable to the location of the gene for steroid sulfatase on the X chromosome: men have only one gene, and hence have less steroid sulfatase and consequently have higher DHEAS concentrations (717). Throughout much of adult life, adrenal secretion of DHEAS exceeds that of cortisol; in adult women, adrenal secretion of androstenedione and testosterone is equal to their secretion from the ovary (718). Despite the huge increases in the adrenal secretion of DHEA and DHEAS during adrenarche, circulating concentrations of ACTH and cortisol do not change with age. Thus, ACTH plays a permissive role in adrenarche but does not trigger it. Searches for hypothetical polypeptide hormones that might specifically stimulate the zona reticularis have been unsuccessful (719–721). Adrenarche is a unique phenomenon confined to few higher primates such as chimpanzees or orangutans, but the significance of adrenarche remains unknown (722, 723).
Fig. 16.
Concentrations of DHEAS as a function of age. Note that the x-axis is on a log scale. [Derived from data in N. Orentreich, et al.: J Clin Endocrinol Metab 59:551–555, 1984 (221). © W. L. Miller.]
Recent studies of adrenarche have focused on the roles of 3βHSD and P450c17. The abundance of 3βHSD protein in the zona reticularis appears to decrease with the onset of adrenarche (202, 724, 725), and the adrenal expression of cytochrome b5, which fosters the 17,20-lyase activity of P450c17, is almost exclusively confined to the zona reticularis (203, 205, 726); both of these factors strongly favor the production of DHEA (727). The phosphorylation of P450c17 also increases 17,20-lyase activity (287–289), but the kinase has not been identified (728); hence, its role in adrenarche remains uncertain. Premature and exaggerated adrenarche may be associated with insulin resistance, and girls with premature exaggerated adrenarche appear to be at much higher risk of developing the polycystic ovary syndrome as adults (characterized by hyperandrogenism, fewer ovulatory cycles, insulin resistance, and hypertriglyceridemia) (729–731), and infants born small for gestational age may be at increased risk (732). It has been suggested that replacing the DHEA lost during adrenopause may improve memory and a sense of well-being in the elderly, but meta-analysis of numerous studies has shown little or no effect (534). Thus, studies of physiology, biochemistry, and clinical correlates of adrenarche are pointing to premature adrenarche as an early sign of a metabolic disorder.
Supplementary Material
Acknowledgments
We thank Prof. Synthia H. Mellon (University of California, San Francisco) for help with the section on neurosteroids.
Disclosure Summary: W.L.M. has nothing to disclose. R.J.A. is a consultant for Ortho Biotech and Johnson & Johnson.
- ABS
- Antley-Bixler syndrome
- ACAT
- acyl-CoA:cholesterol acyltransferase
- AHC
- adrenal hypoplasia congenita
- AKR
- aldo-keto reductase
- ALD
- adrenoleukodystrophy
- Allo
- allopregnanolone
- AME
- apparent mineralocorticoid excess
- APS
- adenosine phosphosulfate
- CAH
- congenital adrenal hyperplasia
- CMO
- corticosterone methyl oxidase
- CNS
- central nervous system
- CoA
- coenzyme A
- CYP
- cytochrome P450
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- DHT
- dihydrotestosterone
- DOC
- 11-deoxycorticosterone
- E
- embryonic day
- EDS
- Ehlers-Danlos syndrome
- ENaC
- amiloride-sensitive epithelial sodium channel
- FAD
- flavin adenine dinucleotide
- FGD
- familial glucocorticoid deficiency
- FMN
- flavin mononucleotide
- GABA
- γ-amino butyric acid
- GR
- glucocorticoid receptor
- GRA
- glucocorticoid-remediable aldosteronism
- hCG
- human chorionic gonadotropin
- HLA
- human lymphocyte antigen
- H6PHD
- hexose-6-phosphate dehydrogenase
- HSD
- hydroxysteroid dehydrogenase
- HSL
- hormone-sensitive lipase
- IMM
- inner mitochondrial membrane
- LDL
- low-density lipoprotein
- MC2R
- melanocortin receptor type 2
- MLN64
- metastatic lymph node clone 64
- MRAP
- melanocortin receptor accessory protein
- NAD(P)(H)
- nicotinamide adenine dinucleotide (phosphate) (reduced form)
- 17OHP
- 17-hydroxyprogesterone
- OMM
- outer mitochondrial membrane
- PAPS
- 3′-phosphoadenosine-5′-phosphosulfate
- PAPSS
- PAPS synthase
- PHA
- pseudohypoaldosteronism
- POR
- P450 oxidoreductase
- RODH/CRAD
- retinol dehydrogenase/cis-retinol/androgen dehydrogenase
- SDR
- short-chain dehydrogenase/reductase
- SF1
- steroidogenic factor 1
- SREBP
- sterol response element binding protein
- StAR
- steroidogenic acute regulatory protein
- StarD
- StAR-related lipid transfer domain
- SULT
- sulfotransferase
- TIM
- triosephosphate isomerase.
References
- 1. Steiger M, Reichstein T. 1937. Desoxy-cortico-steron (21-oxy-progesteron) aus Δ 5–3-oxy-atio-cholensaure. Helv Chim Acta 20:1164–1179 [Google Scholar]
- 2. Kendall EC, Mason HL, McKenzie BF, Myers CS, Koelsche GA. 1934. Isolation in crystalline form of the hormone essential to life from the supranetal cortex: its chemical nature and physiologic properties. Trans Assoc Am Physicians 49:147 [Google Scholar]
- 3. Miller WL. 1988. Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318 [DOI] [PubMed] [Google Scholar]
- 4. Seyle H. 1946. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol 6:117–230 [DOI] [PubMed] [Google Scholar]
- 5. O’Malley BW. 2005. A life-long search for the molecular pathways of steroid hormone action. Mol Endocrinol 19:1402–1411 [DOI] [PubMed] [Google Scholar]
- 6. Gustafsson JA. 2005. Steroids and the scientist. Mol Endocrinol 19:1412–1417 [DOI] [PubMed] [Google Scholar]
- 7. Chambon P. 2005. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol 19:1418–1428 [DOI] [PubMed] [Google Scholar]
- 8. Evans RM. 2005. The nuclear receptor superfamily: a Rosetta stone for physiology. Mol Endocrinol 19:1429–1438 [DOI] [PubMed] [Google Scholar]
- 9. Jensen EV. 2005. The contribution of “alternative approaches” to understanding steroid hormone action. Mol Endocrinol 19:1439–1442 [DOI] [PubMed] [Google Scholar]
- 10. Auchus RJ, Miller WL. 2010. The principles, enzymes and pathways of human steroidogenesis. In: DeGroot LJ, Jameson JL. eds. Endocrinology. 6th ed. Philadelphia: WB Saunders; 1784–1804 [Google Scholar]
- 11. Mason JI, Rainey WE. 1987. Steroidogenesis in the human fetal adrenal: a role for cholesterol synthesized de novo. J Clin Endocrinol Metab 64:140–147 [DOI] [PubMed] [Google Scholar]
- 12. Gwynne JT, Strauss JF., 3rd 1982. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev 3:299–329 [DOI] [PubMed] [Google Scholar]
- 13. Horton JD, Goldstein JL, Brown MS. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown MS, Kovanen PT, Goldstein JL. 1979. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Rec Prog Horm Res 35:215–257 [DOI] [PubMed] [Google Scholar]
- 15. Kraemer FB. 2007. Adrenal cholesterol utilization. Mol Cell Endocrinol 265–266:42–45 [DOI] [PubMed] [Google Scholar]
- 16. Chang TY, Chang CC, Ohgami N, Yamauchi Y. 2006. Cholesterol sensing, trafficking and esterification. Annu Rev Cell Dev Biol 22:129–157 [DOI] [PubMed] [Google Scholar]
- 17. Miller WL. 2007. StAR search—what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol 21:589–601 [DOI] [PubMed] [Google Scholar]
- 18. Ponting CP, Aravind L. 1999. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci 24:130–132 [DOI] [PubMed] [Google Scholar]
- 19. Soccio RE, Breslow JL. 2003. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem 278:22183–22186 [DOI] [PubMed] [Google Scholar]
- 20. Riegelhaupt JJ, Waase MP, Garbarino J, Cruz DE, Breslow JL. 2010. Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J Lipid Res 51:1134–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haberland ME, Reynolds JA. 1973. Self-association of cholesterol in aqueous solution. Proc Natl Acad Sci USA 70:2313–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soccio RE, Breslow JL. 2004. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol 24:1150–1160 [DOI] [PubMed] [Google Scholar]
- 23. Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh AL, Martin GG, Chao H, Kier AB, Schroeder F. 2001. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res 40:498–563 [DOI] [PubMed] [Google Scholar]
- 24. Soccio RE, Adams RM, Romanowski MJ, Sehayek E, Burley SK, Breslow JL. 2002. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc Natl Acad Sci USA 99:6943–6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romanowski MJ, Soccio RE, Breslow JL, Burley SK. 2002. Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc Natl Acad Sci USA 99:6949–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsujishita Y, Hurley JH. 2000. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 7:408–414 [DOI] [PubMed] [Google Scholar]
- 27. Soccio RE, Adams RM, Maxwell KN, Breslow JL. 2005. Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. J Biol Chem 280:19410–19418 [DOI] [PubMed] [Google Scholar]
- 28. Bose HS, Whittal RM, Ran Y, Bose M, Baker BY, Miller WL. 2008. StAR-like activity and molten globule behavior of StARD6, a male germ-line protein. Biochemistry 47:2277–2288 [DOI] [PubMed] [Google Scholar]
- 29. Waterham HR. 2006. Defects of cholesterol biosynthesis. FEBS Lett 580:5442–5449 [DOI] [PubMed] [Google Scholar]
- 30. Moser HW, Raymond GV, Dubey P. 2005. Adrenoleukodystrophy: new approaches to a neurodegenerative disease. JAMA 294:3131–3134 [DOI] [PubMed] [Google Scholar]
- 31. Ligtenberg MJ, Kemp S, Sarde CO, van Geel BM, Kleijer WJ, Barth PG, Mandel JL, van Oost BA, Bolhuis PA. 1995. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am J Hum Genet 56:44–50 [PMC free article] [PubMed] [Google Scholar]
- 32. Watkins PA, Gould SJ, Smith MA, Braiterman LT, Wei HM, Kok F, Moser AB, Moser HW, Smith KD. 1995. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am J Hum Genet 57:292–301 [PMC free article] [PubMed] [Google Scholar]
- 33. McGuinness MC, Lu JF, Zhang HP, Dong GX, Heinzer AK, Watkins PA, Powers J, Smith KD. 2003. Role of ALDP (ABCD1) and mitochondria in X-linked adrenoleukodystrophy. Mol Cell Biol 23:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kemp S, Wanders RJA. 2007. X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. Mol Genet Metab 90:268–276 [DOI] [PubMed] [Google Scholar]
- 35. Watkins PA, Naidu S, Moser HW. 1987. Adrenoleukodystrophy: biochemical procedures in diagnosis, prevention and treatment. J Inherit Metab Dis 10:46–53 [DOI] [PubMed] [Google Scholar]
- 36. Moser HW, Moser AE, Singh I, O’Neill BP. 1984. Adrenoleukodystrophy: survey of 303 cases: biochemistry, diagnosis, and therapy. Ann Neurol 16:628–641 [DOI] [PubMed] [Google Scholar]
- 37. Moser HW. 1995. Adrenoleukodystrophy. Curr Opin Neurol 8:221–226 [DOI] [PubMed] [Google Scholar]
- 38. Sadeghi-Nejad A, Senior B. 1990. Adrenomyeloneuropathy presenting as Addison’s disease in childhood. N Engl J Med 322:13–16 [DOI] [PubMed] [Google Scholar]
- 39. Laureti S, Casucci G, Santeusanio F, Angeletti G, Aubourg P, Brunetti P. 1996. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison’s disease in young adult male patients. J Clin Endocrinol Metab 81:470–474 [DOI] [PubMed] [Google Scholar]
- 40. Assmann G, Seedorf U. 1995. Acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. In: Scriver C, Beaudet A, Sly W, Valle D. eds. The metabolic basis of inherited disease. 6th ed. New York: McGraw-Hill; 2563–2587 [Google Scholar]
- 41. Anderson RA, Byrum RS, Coates PM, Sando GN. 1994. Mutations at the lysosomal acid cholesteryl ester hydrolase gene locus in Wolman disease. Proc Natl Acad Sci USA 91:2718–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Correa-Cerro LS, Porter FD. 2005. 3β-Hydroxysterol Δ-7 reductase and the Smith-Lemli-Opitz syndrome. Mol Genet Metab 84:112–126 [DOI] [PubMed] [Google Scholar]
- 43. Andersson HC, Frentz J, Martínez JE, Tuck-Muller CM, Bellizaire J. 1999. Adrenal insufficiency in Smith-Lemli-Optiz syndrome. Am J Med Genet 82:382–384 [PubMed] [Google Scholar]
- 44. Hall PF. 1986. Cytochromes P450 and the regulation of steroid synthesis. Steroids 48:133–196 [DOI] [PubMed] [Google Scholar]
- 45. Agarwal AK, Auchus RJ. 2005. Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology 146:2531–2538 [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez FJ. 1988. The molecular biology of cytochrome P450s. Pharmacol Rev 40:243–288 [PubMed] [Google Scholar]
- 47. Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, et al. 2001. The sequence of the human genome. Science 291:1304–1351 [DOI] [PubMed] [Google Scholar]
- 48. Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860–921 [DOI] [PubMed] [Google Scholar]
- 49. Miller WL. 2005. Regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- 50. Penning TM. 1997. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev 18:281–305 [DOI] [PubMed] [Google Scholar]
- 51. Sherbet DP, Papari-Zareei M, Khan N, Sharma KK, Brandmaier A, Rambally S, Chattopadhyay A, Andersson S, Agarwal AK, Auchus RJ. 2007. Cofactors, redox state, and directional preferences of hydroxysteroid dehydrogenases. Mol Cell Endocrinol 265–266:83–88 [DOI] [PubMed] [Google Scholar]
- 52. Papari-Zareei M, Brandmaier A, Auchus RJ. 2006. Arginine 276 controls the directional preference of AKR1C9 (rat liver 3α-hydroxysteroid dehydrogenase) in human embryonic kidney 293 cells. Endocrinology 147:1591–1597 [DOI] [PubMed] [Google Scholar]
- 53. Sherbet DP, Guryev OL, Papari-Zareei M, Mizrachi D, Rambally S, Akbar S, Auchus RJ. 2009. Biochemical factors governing the steady-state estrone/estradiol ratios catalyzed by human 17β-hydroxysteroid dehydrogenases types 1 and 2 in HEK-293 cells. Endocrinology 150:4154–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Voutilainen R, Miller WL. 1987. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain cleavage enzyme, P450scc, in human steroidogenic tissues. Proc Natl Acad Sci USA 84:1590–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mesiano S, Mellon SH, Jaffe RB. 1993. Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J Clin Endocrinol Metab 76:968–976 [DOI] [PubMed] [Google Scholar]
- 56. Mesiano S, Mellon SH, Gospodarowicz D, Di Blasio AM, Jaffe RB. 1991. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: a model for adrenal growth regulation. Proc Natl Acad Sci USA 88:5428–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coulter CL, Read LC, Carr BR, Tarantal AF, Barry S, Styne DM. 1996. A role for epidermal growth factor in the morphological and functional maturation of the adrenal gland in the fetal rhesus monkey in vivo. J Clin Endocrinol Metab 81:1254–1260 [DOI] [PubMed] [Google Scholar]
- 58. Stocco DM, Wang X, Jo Y, Manna PR. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- 59. Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd 1997. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 [DOI] [PubMed] [Google Scholar]
- 60. Pon LA, Orme-Johnson NR. 1986. Acute stimulation of steroidogenesis in corpus luteum and adrenal cortex by peptide hormones. J Biol Chem 261:6594–6599 [PubMed] [Google Scholar]
- 61. Pon LA, Hartigan JA, Orme-Johnson NR. 1986. Acute ACTH regulation of adrenal corticosteroid biosynthesis: rapid accumulation of a phosphoprotein. J Biol Chem 261:13309–13316 [PubMed] [Google Scholar]
- 62. Epstein LF, Orme-Johnson NR. 1991. Regulation of steroid hormone biosynthesis: identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J Biol Chem 266:19739–19745 [PubMed] [Google Scholar]
- 63. Stocco DM, Sodeman TC. 1991. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem 266:19731–19738 [PubMed] [Google Scholar]
- 64. Clark BJ, Wells J, King SR, Stocco DM. 1994. The purification, cloning and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- 65. Stocco DM, Clark BJ. 1996. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- 66. Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- 67. Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. 1996. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med 335:1870–1878 [DOI] [PubMed] [Google Scholar]
- 68. Moore CC, Hum DW, Miller WL. 1992. Identification of positive and negative placental-specific basal elements, a transcriptional repressor, and a cAMP response element in the human gene for P450scc. Mol Endocrinol 6:2045–2058 [DOI] [PubMed] [Google Scholar]
- 69. Sugawara T, Holt JA, Driscoll D, Strauss JF, 3rd, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ. 1995. Human steroidogenic acute regulatory protein (StAR): functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and an expressed pseudogene to chromosome 13. Proc Natl Acad Sci USA 92:4778–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moog-Lutz C, Tomasetto C, Régnier CH, Wendling C, Lutz Y, Muller D, Chenard MP, Basset P, Rio MC. 1997. MLN64 exhibits homology with the steroidogenic acute regulatory protein (StAR) and is over-expressed in human breast carcinomas. Int J Cancer 71:183–191 [DOI] [PubMed] [Google Scholar]
- 71. Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF., 3rd 1997. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA 94:8462–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bose HS, Whittal RM, Huang MC, Baldwin MA, Miller WL. 2000. N-218 MLN64, a protein with StAR-like steroidogenic activity, is folded and cleaved similarly to StAR. Biochemistry 39:11722–11731 [DOI] [PubMed] [Google Scholar]
- 73. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. 1997. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA 94:11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. 2000. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol 14:1462–1471 [DOI] [PubMed] [Google Scholar]
- 75. Miller WL, Strauss JF., 3rd 1999. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol 69:131–141 [DOI] [PubMed] [Google Scholar]
- 76. Miller WL. 2007. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676 [DOI] [PubMed] [Google Scholar]
- 77. Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., 3rd 1996. Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial targeting sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci USA 93:13731–13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bose HS, Lingappa VR, Miller WL. 2002. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417:87–91 [DOI] [PubMed] [Google Scholar]
- 79. Bose HS, Whittal RM, Baldwin MA, Miller WL. 1999. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci USA 96:7250–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baker BY, Yaworsky DC, Miller WL. 2005. A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem 280:41753–41760 [DOI] [PubMed] [Google Scholar]
- 81. Tuckey RC, Headlam MJ, Bose HS, Miller WL. 2002. Transfer of cholesterol between phospholipid vesicles mediated by the steroidogenic acute regulatory protein (StAR). J Biol Chem 277:47123–47128 [DOI] [PubMed] [Google Scholar]
- 82. Baker BY, Epand RF, Epand RM, Miller WL. 2007. Cholesterol binding does not predict activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem 282:10223–10232 [DOI] [PubMed] [Google Scholar]
- 83. Papadopoulos V. 1993. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: Biological role in steroidogenic cell function. Endocr Rev 14:222–240 [DOI] [PubMed] [Google Scholar]
- 84. Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. 2005. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol 19:540–554 [DOI] [PubMed] [Google Scholar]
- 85. Liu J, Rone MB, Papadopoulos V. 2006. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 281:38879–38893 [DOI] [PubMed] [Google Scholar]
- 86. Bose M, Whittal RM, Miller WL, Bose HS. 2008. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and PCP. J Biol Chem 283:8837–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. 2001. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem 276:46583–46596 [DOI] [PubMed] [Google Scholar]
- 88. Alpy F, Wendling C, Rio MC, Tomasetto C. 2002. MENTHO, a MLN64 homologue devoid of the START domain. J Biol Chem 277:50780–50787 [DOI] [PubMed] [Google Scholar]
- 89. Alpy F, Latchumanan VK, Kedinger V, Janoshazi A, Thiele C, Wendling C, Rio MC, Tomasetto C. 2005. Functional characterization of the MENTAL domain. J Biol Chem 280:17945–17952 [DOI] [PubMed] [Google Scholar]
- 90. Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. 2006. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem 281:31594–31604 [DOI] [PubMed] [Google Scholar]
- 91. Mathieu AP, Fleury A, Ducharme L, Lavigne P, LeHoux JG. 2002. Insights into steroidogenic acute regulatory protein (StAR)-dependent cholesterol transfer in mitochondria: evidence from molecular modeling and structure-based thermodynamics supporting the existence of partially unfolded states of StAR. J Mol Endocrinol 29:327–345 [DOI] [PubMed] [Google Scholar]
- 92. Yaworsky DC, Baker BY, Bose HS, Best KB, Jensen LB, Bell JD, Baldwin MA, Miller WL. 2005. pH-Dependent interactions of the carboxyl-terminal helix of steroidogenic acute regulatory protein with synthetic membranes. J Biol Chem 280:2045–2054 [DOI] [PubMed] [Google Scholar]
- 93. Murcia M, Faráldo-Gómez JD, Maxfield FR, Roux B. 2006. Modeling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J Lipid Res 47:2614–2630 [DOI] [PubMed] [Google Scholar]
- 94. Sandison AT. 1955. A form of lipoidosis of the adrenal cortex in an infant. Arch Dis Childh 30:538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Prader A, Gurtner HP. 1955. Das Syndrom des Pseudohermaphroditismus masculinus bei kongenitaler Nebennierenrindenhyperplasie ohne Androgenuberproduktion (adrenaler Pseudohermaphroditismus masculinus). Helv Paed Acta 10:397–412 [PubMed] [Google Scholar]
- 96. Kirkland RT, Kirkland JL, Johnson CM, Horning MG, Librik L, Clayton GW. 1973. Congenital lipoid adrenal hyperplasia in an eight-year-old phenotypic female. J Clin Endocrinol Metab 36:488–496 [DOI] [PubMed] [Google Scholar]
- 97. Hauffa BP, Miller WL, Grumbach MM, Conte FA, Kaplan SL. 1985. Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20,22 desmolase) in a patient treated for 18 years. Clin Endocrinol (Oxf) 23:481–493 [DOI] [PubMed] [Google Scholar]
- 98. Camacho AM, Kowarski A, Migeon CJ, Brough AJ. 1968. Congenital adrenal hyperplasia due to a deficiency of one of the enzymes involved in the biosynthesis of pregnenolone. J Clin Endocrinol Metab 28:153–161 [DOI] [PubMed] [Google Scholar]
- 99. Degenhart HJ, Visser HK, Boon H, O’Doherty NJ. 1972. Evidence for deficiency of 20α cholesterol hydroxylase activity in adrenal tissue of a patient with lipoid adrenal hyperplasia. Acta Endocrinologica 71:512–518 [DOI] [PubMed] [Google Scholar]
- 100. Koizumi S, Kyoya S, Miyawaki TM, Kidani H, Funabashi T. 1977. Cholesterol side-chain cleavage enzyme activity and cytochrome P450 content in adrenal mitochondria of a patient with congenital lipoid adrenal hyperplasia (Prader disease). Clin Chim Acta 77:301–306 [DOI] [PubMed] [Google Scholar]
- 101. Matteson KJ, Chung BC, Urdea MS, Miller WL. 1986. Study of cholesterol side chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology 118:1296–1305 [DOI] [PubMed] [Google Scholar]
- 102. Lin D, Gitelman SE, Saenger P, Miller WL. 1991. Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J Clin Invest 88:1955–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Saenger P, Klonari Z, Black SM, Compagnone N, Mellon SH, Fleischer A, Abrams CA, Shackelton CH, Miller WL. 1995. Prenatal diagnosis of congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 80:200–205 [DOI] [PubMed] [Google Scholar]
- 104. Tee MK, Lin D, Sugawara T, Holt JA, Guiguen Y, Buckingham B, Strauss JF, 3rd, Miller WL. 1995. T→A transversion 11 bp from a splice acceptor site in the gene for steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. Hum Mol Genet 4:2299–2305 [DOI] [PubMed] [Google Scholar]
- 105. Miller WL. 1997. Congenital lipoid adrenal hyperplasia: the human gene knockout of the steroidogenic acute regulatory protein. J Mol Endocrinol 19:227–240 [DOI] [PubMed] [Google Scholar]
- 106. Voutilainen R, Miller WL. 1986. Developmental expression of genes for the steroidogenic enzymes P450scc (20,22 desmolase), P450c17 (17α-hydroxylase/17,20 lyase) and P450c21 (21-hydroxylase) in the human fetus. J Clin Endocrinol Metab 63:1145–1150 [DOI] [PubMed] [Google Scholar]
- 107. Ogata T, Matsuo N, Saito M, Prader A. 1989. The testicular lesion and sexual differentiation in congenital lipoid adrenal hyperplasia. Helv Paediatr Acta 43:531–538 [PubMed] [Google Scholar]
- 108. Chen X, Baker BY, Abduljabbar MA, Miller WL. 2005. A genetic isolate of congenital lipoid adrenal hyperplasia with atypical clinical findings. J Clin Endocrinol Metab 90:835–840 [DOI] [PubMed] [Google Scholar]
- 109. Bose HS, Pescovitz OH, Miller WL. 1997. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia caused by a homozygous frame-shift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab 82:1511–1515 [DOI] [PubMed] [Google Scholar]
- 110. Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, Sugawara T, Strauss JF., 3rd 1997. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. J Clin Invest 99:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nakae J, Tajima T, Sugawara T, Arakane F, Hanaki K, Hotsubo T, Igarashi N, Igarashi Y, Ishii T, Koda N, Kondo T, Kohno H, Nakagawa Y, Tachibana K, Takeshima Y, Tsubouchi K, Strauss JF, 3rd, Fujieda K. 1997. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum Mol Genet 6:571–576 [DOI] [PubMed] [Google Scholar]
- 112. Yoo HW, Kim GH. 1998. Molecular and clinical characterization of Korean patients with congenital lipoid adrenal hyperplasia. J Pediatr Endocrinol Metab 11:707–711 [DOI] [PubMed] [Google Scholar]
- 113. Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL. 2000. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 85:3636–3639 [DOI] [PubMed] [Google Scholar]
- 114. Flück CE, Maret A, Mallet D, Portrat-Doyen S, Achermann JC, Leheup B, Theintz GE, Mullis PE, Morel Y. 2005. A novel mutation L260P of the steroidogenic acute regulatory protein gene in three unrelated patients of Swiss ancestry with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 90:5304–5308 [DOI] [PubMed] [Google Scholar]
- 115. Gassner HL, Toppari J, Quinteiro González S, Miller WL. 2004. Near-miss apparent SIDS from adrenal crisis. J Pediatr 145:178–183 [DOI] [PubMed] [Google Scholar]
- 116. Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, Achermann JC. 2006. Non-classic congenital lipoid adrenal hyperplasia. A new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab 91:4781–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ. 2009. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab 94:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sahakitrungruang T, Soccio RE, Lang-Muritano M, Walker JM, Achermann JC, Miller WL. 2010. Clinical, genetic, and functional characterization of four patients carrying partial loss-of-function mutations in the steroidogenic acute regulatory protein (StAR). J Clin Endocrinol Metab 95:3352–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Clark AJ, Metherell LA, Cheetham ME, Huebner A. 2005. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol Metab 16:451–457 [DOI] [PubMed] [Google Scholar]
- 120. Khoury K, Barbar E, Ainmelk Y, Ouellet A, Lehoux JG. 2009. Gonadal function, first cases of pregnancy, and child delivery in a woman with lipoid congenital adrenal hyperplasia. J Clin Endocrinol Metab 94:1333–1337 [DOI] [PubMed] [Google Scholar]
- 121. Stone D, Hechter O. 1955. Studies on ACTH action in perfused bovine adrenals: aspects of progesterone as an intermediary in cortico-steroidogenesis. Arch Biochem Biophys 54:121–128 [DOI] [PubMed] [Google Scholar]
- 122. Halkerston ID, Eichhorn J, Hechter O. 1961. A requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J Biol Chem 236:374–380 [PubMed] [Google Scholar]
- 123. Koritz SB, Kumar AM. 1970. On the mechanism of action of adrenocorticotropic hormone—the stimulation of the activity of enzymes involved in pregnenolone synthesis. J Biol Chem 245:152–159 [PubMed] [Google Scholar]
- 124. Tuckey RC, Cameron KJ. 1993. Catalytic properties of cytochrome P-450scc. Biochim Biophys Acta 1163:185–194 [DOI] [PubMed] [Google Scholar]
- 125. Kuwada M, Kitajima R, Suzuki H, Horie S. 1991. Purification and properties of cytochrome P-450 (SCC) from pig testis mitochondria. Biochem Biophys Res Commun 176:1501–1508 [DOI] [PubMed] [Google Scholar]
- 126. Shikita M, Hall PF. 1973. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. I. Purification and properties. J Biol Chem 248:5598–5604 [PubMed] [Google Scholar]
- 127. Shikita M, Hall PF. 1973. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. II. Subunit structure. J Biol Chem 248:5605–5609 [PubMed] [Google Scholar]
- 128. Simpson ER. 1979. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol Cell Endocrinol 13:213–227 [DOI] [PubMed] [Google Scholar]
- 129. Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. 2003. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA 100:14754–14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Morohashi K, Fujii-Kuriyama Y, Okada Y, Sogawa K, Hirose T, Inayama S, Omura T. 1984. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial P450(scc) of bovine adrenal cortex. Proc Natl Acad Sci USA 81:4647–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. 1986. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci USA 83:8962–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Morohashi K, Sogawa K, Omura T, Fujii-Kuriyama Y. 1987. Gene structure of human cytochrome P-450(scc), cholesterol desmolase. J Biochem 101:879–887 [DOI] [PubMed] [Google Scholar]
- 133. Black SM, Harikrishna JA, Szklarz GD, Miller WL. 1994. The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proc Natl Acad Sci USA 91:7247–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yang X, Iwamoto K, Wang M, Artwohl J, Mason JI, Pang S. 1993. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology 132:1977–1982 [DOI] [PubMed] [Google Scholar]
- 135. Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. 2002. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 136. Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. 2001. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab 86:3820–3825 [DOI] [PubMed] [Google Scholar]
- 137. Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL. 2008. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab 93:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. John ME, John MC, Boggaram V, Simpson ER, Waterman MR. 1986. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci USA 83:4715–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mellon SH, Vaisse C. 1989. cAMP regulates P450scc gene expression by a cycloheximide-insensitive mechanism in cultured mouse Leydig MA-10 cells. Proc Natl Acad Sci USA 86:7775–7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Barrett PQ, Bollag WB, Isales CM, McCarthy RT, Rasmussen H. 1989. The role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev 10:496–518 [DOI] [PubMed] [Google Scholar]
- 141. Moore CC, Brentano ST, Miller WL. 1990. Human P450scc gene transcription is induced by cyclic AMP and repressed by 12-O-tetradecanolyphorbol-13-acetate and A23187 by independent cis-elements. Mol Cell Biol 10:6013–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Huang N, Miller WL. 2000. Cloning of factors related to HIV-inducible LBP proteins that regulate steroidogenic factor-1-independent human placental transcription of the cholesterol side-chain cleavage enzyme, P450scc. J Biol Chem 275:2852–2858 [DOI] [PubMed] [Google Scholar]
- 143. Huang N, Miller WL. 2005. LBP proteins mediate SF1-independent expression of P450scc in human placental JEG-3 cells. Mol Endocrinol 19:409–420 [DOI] [PubMed] [Google Scholar]
- 144. Henderson YC, Frederick MJ, Jayakumar A, Choi Y, Wang MT, Kang Y, Evans R, Spring PM, Uesugi M, Clayman GL. 2007. Human LBP-32/MGR is a repressor of the P450scc in human choriocarcinoma cell line JEG-3. Placenta 28:152–160 [DOI] [PubMed] [Google Scholar]
- 145. Henderson YC, Frederick MJ, Wang MT, Hollier LM, Clayman GL. 2008. LBP-1b, LBP-9, and LBP-32/MGR detected in syncytiotrophoblasts from first-trimester human placental tissue and their transcriptional regulation. DNA Cell Biol 27:71–79 [DOI] [PubMed] [Google Scholar]
- 146. Baulieu EE. 1997. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res 52:1–32 [PubMed] [Google Scholar]
- 147. Parker KL, Schimmer BP. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed] [Google Scholar]
- 148. Morohashi Ki. 1999. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol Metab 10:169–173 [DOI] [PubMed] [Google Scholar]
- 149. Gizard F, Lavallée B, DeWitte F, Hum DW. 2001. A novel zinc finger protein TReP-132 interacts with CPB/p300 to regulate human CYP11A1 gene expression. J Biol Chem 276:33881–33892 [DOI] [PubMed] [Google Scholar]
- 150. Gizard F, Lavallee B, DeWitte F, Teissier E, Staels B, Hum DW. 2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J Biol Chem 277:39144–39155 [DOI] [PubMed] [Google Scholar]
- 151. Sewer MB, Dammer EB, Jagarlapudi S. 2007. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 39:371–388 [DOI] [PubMed] [Google Scholar]
- 152. Schimmer BP, White PC. 2010. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol 24:1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Katsumata N, Ohtake M, Hojo T, Ogawa E, Hara T, Sato N, Tanaka T. 2002. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J Clin Endocrinol Metab 87:3808–3813 [DOI] [PubMed] [Google Scholar]
- 154. Hiort O, Holterhus PM, Werner R, Marschke C, Hoppe U, Partsch CJ, Riepe FG, Achermann JC, Struve D. 2005. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J Clin Endocrinol Metab 90:538–541 [DOI] [PubMed] [Google Scholar]
- 155. al Kandari H, Katsumata N, Alexander S, Rasoul MA. 2006. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46,XY patient with adrenal insufficiency, complete sex reversal and agenesis of corpus callosum. J Clin Endocrinol Metab 91:2821–2826 [DOI] [PubMed] [Google Scholar]
- 156. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. 2009. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab 94:936–939 [DOI] [PubMed] [Google Scholar]
- 157. Sahakitrungruang T, Tee MK, Blackett PR, Miller WL. 2010. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling non-classic congenital lipoid adrenal hyperplasia. 10.1210/jc.2010-1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Voutilainen R, Tapanainen J, Chung BC, Matteson KJ, Miller WL. 1986. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 63:202–207 [DOI] [PubMed] [Google Scholar]
- 159. Tsigos C, Arai K, Hung W, Chrousos GP. 1993. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest 92:2458–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Martens JW, Verhoef-Post M, Abelin N, Ezabella M, Toledo SP, Brunner HG, Themmen AP. 1998. A homozygous mutation in the luteinizing hormone receptor causes partial Leydig cell hypoplasia: correlation between receptor activity and phenotype. Mol Endocrinol 12:775–784 [DOI] [PubMed] [Google Scholar]
- 161. Shenker A. 1995. G protein-coupled receptor structure and function: the impact of disease-causing mutations. Baillieres Clin Endocrinol Metab 9:427–451 [DOI] [PubMed] [Google Scholar]
- 162. Zhang P, Rodriguez H, Mellon SH. 1995. Transcriptional regulation of P450scc gene expression in neural and in steroidogenic cells: implications for regulation of neurosteroidogenesis. Mol Endocrinol 9:1571–1582 [DOI] [PubMed] [Google Scholar]
- 163. Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 164. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. 1998. Wilms’ tumor and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445–454 [DOI] [PubMed] [Google Scholar]
- 165. Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA. 1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526 [DOI] [PubMed] [Google Scholar]
- 166. Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- 167. Solish SB, Picado-Leonard J, Morel Y, Kuhn RW, Mohandas TK, Hanukoglu I, Miller WL. 1988. Human adrenodoxin reductase: two mRNAs encoded by a single gene on chromosome 17cen → q25 are expressed in steroidogenic tissues. Proc Natl Acad Sci USA 85:7104–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lin D, Shi YF, Miller WL. 1990. Cloning and sequence of the human adrenodoxin reductase gene. Proc Natl Acad Sci USA 87:8516–8520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Chang CY, Wu DA, Lai CC, Miller WL, Chung BC. 1988. Cloning and structure of the human adrenodoxin gene. DNA 7:609–615 [DOI] [PubMed] [Google Scholar]
- 170. Picado-Leonard J, Voutilainen R, Kao LC, Chung BC, Strauss JF, 3rd, Miller WL. 1988. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem 263:3240–3244 [PubMed] [Google Scholar]
- 171. Brentano ST, Black SM, Lin D, Miller WL. 1992. cAMP post-transcriptionally diminishes the abundance of adrenodoxin reductase mRNA. Proc Natl Acad Sci USA 89:4099–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sparkes RS, Klisak I, Miller WL. 1991. Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23–q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24-q25; and P450c17 to 10q24–q25. DNA Cell Biol 10:359–365 [DOI] [PubMed] [Google Scholar]
- 173. Brandt ME, Vickery LE. 1992. Expression and characterization of human mitochondrial ferredoxin reductase in Escherichia coli. Arch Biochem Biophys 294:735–740 [DOI] [PubMed] [Google Scholar]
- 174. Freeman MR, Dobritsa A, Gaines P, Segraves WA, Carlson JR. 1999. The dare gene: steroid hormone production, olfactory behavior, and neural degeneration in Drosophila. Development 126:4591–4602 [DOI] [PubMed] [Google Scholar]
- 175. Ziegler GA, Vonrhein C, Hanukoglu I, Schulz GE. 1999. The structure of adrenodoxin reductase of mitochondrial P450 systems: electron transfer for steroid biosynthesis. J Mol Biol 289:981–990 [DOI] [PubMed] [Google Scholar]
- 176. Vickery LE. 1997. Molecular recognition and electron transfer in mitochondrial steroid hydroxylase systems. Steroids 62:124–127 [DOI] [PubMed] [Google Scholar]
- 177. Brandt ME, Vickery LE. 1993. Charge pair interactions stabilizing ferredoxin-ferredoxin reductase complexes: identification by complementary site-specific mutations. J Biol Chem 268:17126–17130 [PubMed] [Google Scholar]
- 178. Wada A, Waterman MR. 1992. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J Biol Chem 267:22877–22882 [PubMed] [Google Scholar]
- 179. Hanukoglu I, Suh BS, Himmelhoch S, Amsterdam A. 1990. Induction and mitochondrial localization of cytochrome P450scc system enzymes in normal and transformed ovarian granulosa cells. J Cell Biol 111:1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Voutilainen R, Picado-Leonard J, DiBlasio AM, Miller WL. 1988. Hormonal and developmental regulation of human adrenodoxin mRNA in steroidogenic tissues. J Clin Endocrinol Metab 66:383–388 [DOI] [PubMed] [Google Scholar]
- 181. Müller A, Müller JJ, Muller YA, Uhlmann H, Bernhardt R, Heinemann U. 1998. New aspects of electron transfer revealed by the crystal structure of a truncated bovine adrenodoxin, Adx(4-108). Structure 6:269–280 [DOI] [PubMed] [Google Scholar]
- 182. Coghlan VM, Vickery LE. 1991. Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc. J Biol Chem 266:18606–18612 [PubMed] [Google Scholar]
- 183. Harikrishna JA, Black SM, Szklarz GD, Miller WL. 1993. Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol 12:371–379 [DOI] [PubMed] [Google Scholar]
- 184. Cao PR, Bülow H, Dumas B, Bernhardt R. 2000. Construction and characterization of a catalytic fusion protein system: P-45011β-adrenodoxin reductase-adrenodoxin. Biochim Biophys Acta 1476:253–264 [DOI] [PubMed] [Google Scholar]
- 185. Thomas JL, Myers RP, Strickler RC. 1989. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5 Δ 4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem 33:209–217 [DOI] [PubMed] [Google Scholar]
- 186. Lachance Y, Luu-The V, Labrie C, Simard J, Dumont M, de Launoit Y, Guérin S, Leblanc G, Labrie F. 1990. Characterization of human 3β-hydroxysteroid dehydrogenase/Δ5→Δ4 -isomerase gene and its expression in mammalian cells. J Biol Chem 265:20469–20475 [PubMed] [Google Scholar]
- 187. Lorence MC, Murry BA, Trant JM, Mason JI. 1990. Human 3β-hydroxysteroid dehydrogenase/Δ5→Δ4 isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology 126:2493–2498 [DOI] [PubMed] [Google Scholar]
- 188. Lee TC, Miller WL, Auchus RJ. 1999. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab 84:2104–2110 [DOI] [PubMed] [Google Scholar]
- 189. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 190. Thomas JL, Frieden C, Nash WE, Strickler RC. 1995. An NADH-induced conformational change that mediates the sequential 3β-hydroxysteroid dehydrogenase/isomerase activities is supported by affinity labeling and the time-dependent activation of isomerase. J Biol Chem 270:21003–21008 [DOI] [PubMed] [Google Scholar]
- 191. Lorence MC, Corbin CJ, Kamimura N, Mahendroo MS, Mason JI. 1990. Structural analysis of the gene encoding human 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase. Mol Endocrinol 4:1850–1855 [DOI] [PubMed] [Google Scholar]
- 192. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. 2005. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 –isomerase gene family. Endocr Rev 26:525–582 [DOI] [PubMed] [Google Scholar]
- 193. Rhéaume E, Lachance Y, Zhao HF, Breton N, Dumont M, de Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F. 1991. Structure and expression of a new complementary DNA encoding the almost exclusive 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase in human adrenals and gonads. Mol Endocrinol 5:1147–1157 [DOI] [PubMed] [Google Scholar]
- 194. Bongiovanni AM, Kellenbenz G. 1962. The adrenogenital syndrome with deficiency of 3β-hydroxysteroid dehydrogenase. J Clin Invest 41:2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Moisan AM, Ricketts ML, Tardy V, Desrochers M, Mébarki F, Chaussain JL, Cabrol S, Raux-Demay MC, Forest MG, Sippell WG, Peter M, Morel Y, Simard J. 1999. New insight into the molecular basis of 3β-hydroxysteroid dehydrogenase deficiency: identification of eight mutations in the HSD3B2 gene eleven patients from seven new families and comparison of the functional properties of twenty-five mutant enzymes. J Clin Endocrinol Metab 84:4410–4425 [DOI] [PubMed] [Google Scholar]
- 196. Thomas JL, Berko EA, Faustino A, Myers RP, Strickler RC. 1988. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5/4-ene-isomerase: purification from microsomes, substrate kinetics, and inhibition by product steroids. J Steroid Biochem 31:785–793 [DOI] [PubMed] [Google Scholar]
- 197. Thomas JL, Mason JI, Brandt S, Spencer BR, Jr, Norris W. 2002. Structure/function relationships responsible for the kinetic differences between human type 1 and type 2 3β-hydroxysteroid dehydrogenase and for the catalysis of the type 1 activity. J Biol Chem 277:42795–42801 [DOI] [PubMed] [Google Scholar]
- 198. Cherradi N, Rossier MF, Vallotton MB, Timberg R, Friedberg I, Orly J, Wang XJ, Stocco DM, Capponi AM. 1997. Submitochondrial distribution of three key steroidogenic proteins (steroidogenic acute regulatory protein and cytochrome P450scc and 3β-hydroxysteroid dehydrogenase isomerase enzymes) upon stimulation by intracellular calcium in adrenal glomerulosa cells. J Biol Chem 272:7899–7907 [DOI] [PubMed] [Google Scholar]
- 199. Chapman JC, Polanco JR, Min S, Michael SD. 2005. Mitochondrial 3β-hydroxysteroid dehydrogenase (HSD) is essential for the synthesis of progesterone by corpora lutea: an hypothesis. Reprod Biol Endocrinol 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mesiano S, Coulter CL, Jaffe RB. 1993. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17,20 lyase, and 3β-hydroxysteroid dehydrogenase-isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77:1184–1189 [DOI] [PubMed] [Google Scholar]
- 201. Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. 2000. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 [DOI] [PubMed] [Google Scholar]
- 202. Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. 1996. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81:3558–3565 [DOI] [PubMed] [Google Scholar]
- 203. Mapes S, Corbin CJ, Tarantal A, Conley A. 1999. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD). J Clin Endocrinol Metab 84:3382–3385 [DOI] [PubMed] [Google Scholar]
- 204. Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. 2008. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol 199:367–378 [DOI] [PubMed] [Google Scholar]
- 205. Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. 2009. Development of the human adrenal zona reticularis: morphometric and immunocytochemical studies from birth to adolescence. J Endocrinol 203:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Remer T, Boye KR, Hartmann MF, Wudy SA. 2005. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 [DOI] [PubMed] [Google Scholar]
- 207. Rhéaume E, Simard J, Morel Y, Mebarki F, Zachmann M, Forest MG, New MI, Labrie F. 1992. Congenital adrenal hyperplasia due to point mutations in the type II 3β-hydroxysteroid dehydrogenase gene. Nat Genet 1:239–245 [DOI] [PubMed] [Google Scholar]
- 208. Chang YT, Kappy MS, Iwamoto K, Wang J, Yang X, Pang S. 1993. Mutations in the type II 3β-hydroxysteroid dehydrogenase gene in a patient with classic salt-wasting 3β-HSD deficiency congenital adrenal hyperplasia. Pediatr Res 34:698–700 [DOI] [PubMed] [Google Scholar]
- 209. Simard J, Rhéaume E, Sanchez R, Laflamme N, de Launoit Y, Luu-The V, van Seters AP, Gordon RD, Bettendorf M, Heinrich U, Moshang T, New MI, Labrie F. 1993. Molecular basis of congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase deficiency. Mol Endocrinol 7:716–728 [DOI] [PubMed] [Google Scholar]
- 210. Morel Y, Mébarki F, Rhéaume E, Sanchez R, Forest MG, Simard J. 1997. Structure-function relationships of 3β-hydroxysteroid dehydrogenase: contribution made by the molecular genetics of 3β-hydroxysteroid dehydrogenase deficiency. Steroids 62:176–184 [DOI] [PubMed] [Google Scholar]
- 211. Cara JF, Moshang T, Jr, Bongiovanni AM, Marx BS. 1985. Elevated 17-hydroxy-progesterone and testosterone in a newborn with 3β-hydroxysteroid dehydrogenase deficiency. N Engl J Med 313:618–621 [DOI] [PubMed] [Google Scholar]
- 212. Pang S, Carbunaru G, Haider A, Copeland KC, Chang YT, Lutfallah C, Mason JI. 2003. Carriers for type II 3β-hydroxysteroid dehydrogenase (HSD3B2) deficiency can only be identified by HSD3B2 genotype study and not by hormone test. Clin Endocrinol (Oxf) 58:323–331 [DOI] [PubMed] [Google Scholar]
- 213. Rosenfield RL, Rich BH, Wolfsdorf JI, Cassorla F, Parks JS, Bongiovanni AM, Wu CH, Shackleton CHL. 1980. Pubertal presentation of congenital Δ5-3βhydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 51:345–353 [DOI] [PubMed] [Google Scholar]
- 214. Pang S, Levine LS, Stoner E, Opitz JM, Pollack MS, Dupont B, New MI. 1983. Nonsalt-losing congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase deficiency with normal glomerulosa function. J Clin Endocrinol Metab 56:808–818 [DOI] [PubMed] [Google Scholar]
- 215. Pang SY, Lerner AJ, Stoner E, Levine LS, Oberfield SE, Engel I, New MI. 1985. Late-onset adrenal steroid 3β-hydroxysteroid dehydrogenase deficiency. I. A cause of hirsutism in pubertal and postpubertal women. J Clin Endocrinol Metab 60:428–439 [DOI] [PubMed] [Google Scholar]
- 216. Chang YT, Zhang L, Alkaddour HS, Mason JI, Lin K, Yang X, Garibaldi LR, Bourdony CJ, Dolan LM, Donaldson DL, Pang SY. 1995. Absence of molecular defect in the type II 3β-hydroxysteroid dehydrogenase (3β-HSD) gene in premature pubarche children and hirsute female patients with moderately decreased adrenal 3β-HSD activity. Pediatr Res 37:820–824 [DOI] [PubMed] [Google Scholar]
- 217. Sakkal-Alkaddour H, Zhang L, Yang X, Chang YT, Kappy M, Slover RS, Jorgensen V, Pang S. 1996. Studies of 3β-hydroxysteroid dehydrogenase genes in infants and children manifesting premature pubarche and increased adrenocorticotropin-stimulated Δ5-steroid levels. J Clin Endocrinol Metab 81:3961–3965 [DOI] [PubMed] [Google Scholar]
- 218. Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, Castro-Magana M, Copeland KC, David R, Pang S. 2002. Newly proposed hormonal criteria via genotypic proof for type II 3β-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 87:2611–2622 [DOI] [PubMed] [Google Scholar]
- 219. Mermejo LM, Elias LL, Marui S, Moreira AC, Mendonca BB, de Castro M. 2005. Refining hormonal diagnosis of 3β-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J Clin Endocrinol Metab 90:1287–1293 [DOI] [PubMed] [Google Scholar]
- 220. Apter D, Pakarinen A, Hammond GL, Vihko R. 1979. Adrenocortical function in puberty. Acta Paediatr Scand 68:599–604 [DOI] [PubMed] [Google Scholar]
- 221. Orentreich N, Brind JL, Rizer RL, Vogelman JH. 1984. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- 222. Zachmann M, Völlmin JA, Hamilton W, Prader A. 1972. Steroid 17,20 desmolase deficiency: a new cause of male pseudohermaphroditism. Clin Endocrinol (Oxf) 1:369–385 [DOI] [PubMed] [Google Scholar]
- 223. Nakajin S, Shively JE, Yuan PM, Hall PF. 1981. Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20:4037–4042 [DOI] [PubMed] [Google Scholar]
- 224. Nakajin S, Hall PF. 1981. Microsomal cytochrome P450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J Biol Chem 256:3871–3876 [PubMed] [Google Scholar]
- 225. Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. 1984. C21 steroid side-chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20 lyase cytochrome P450. J Biol Chem 259:3971–3976 [PubMed] [Google Scholar]
- 226. Zuber MX, Simpson ER, Waterman MR. 1986. Expression of bovine 17α-hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS-1) cells. Science 234:1258–1261 [DOI] [PubMed] [Google Scholar]
- 227. Lin D, Harikrishna JA, Moore CC, Jones KL, Miller WL. 1991. Missense mutation Ser106→ Pro causes 17α-hydroxylase deficiency. J Biol Chem 266:15992–15998 [PubMed] [Google Scholar]
- 228. Matteson KJ, Picado-Leonard J, Chung BC, Mohandas TK, Miller WL. 1986. Assignment of the gene for adrenal P450c17 (17α-hydroxylase/17,20 lyase) to human chromosome 10. J Clin Endocrinol Metab 63:789–791 [DOI] [PubMed] [Google Scholar]
- 229. Fan YS, Sasi R, Lee C, Winter JS, Waterman MR, Lin CC. 1992. Localization of the human CYP17 gene (cytochrome P450 17α) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics 14:1110–1111 [DOI] [PubMed] [Google Scholar]
- 230. Chung BC, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shively JE, Miller WL. 1987. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA 84:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Picado-Leonard J, Miller WL. 1987. Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20 lyase): similarity to the gene for P450c21. DNA 6:439–448 [DOI] [PubMed] [Google Scholar]
- 232. Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. 1995. Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J 308:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Katagiri M, Kagawa N, Waterman MR. 1995. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys 317:343–347 [DOI] [PubMed] [Google Scholar]
- 234. Qiao J, Chen X, Zuo CL, Gu YY, Liu BL, Liang J, Lu YL, Tang JF, Wu YX, Chen MD, Chen JL, Wu WL, Song HD. 2010. Identification of steroid biosynthetic defects in genotype-proven heterozygous individuals for 17α-hydroxylase/17,20-lyase deficiency. Clin Endocrinol (Oxf) 72:312–319 [DOI] [PubMed] [Google Scholar]
- 235. Nakajin S, Takahashi M, Shinoda M, Hall PF. 1985. Cytochrome b5 promotes the synthesis of Δ16-C19 steroids by homogeneous cytochrome P-450 C21 side-chain cleavage from pig testis. Biochem Biophys Res Commun 132:708–713 [DOI] [PubMed] [Google Scholar]
- 236. Sinclair PA, Squires EJ. 2005. Testicular sulfoconjugation of the 16-androstene steroids by hydroxysteroid sulfotransferase: its effect on the concentrations of 5α-androstenone in plasma and fat of the mature domestic boar. J Anim Sci 83:358–365 [DOI] [PubMed] [Google Scholar]
- 237. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 238. Flück CE, Miller WL, Auchus RJ. 2003. The 17,20 lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J Clin Endocrinol Metab 88:3762–3766 [DOI] [PubMed] [Google Scholar]
- 239. Ortiz de Montellano PR. 1986. Oxygen activation and reactivity. In: Ortiz de Montellano P. ed. Cytochrome P-450: structure mechanism and biochemistry. New York: Plenum Press; 217–271 [Google Scholar]
- 240. Auchus RJ, Miller WL. 1999. Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): insights into reaction mechanisms and effects of mutations. Mol Endocrinol 13:1169–1182 [DOI] [PubMed] [Google Scholar]
- 241. Auchus RJ, Sampath Kumar A, Andrew Boswell C, Gupta MK, Bruce K, Rath NP, Covey DF. 2003. The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch Biochem Biophys 409:134–144 [DOI] [PubMed] [Google Scholar]
- 242. Arlt W, Auchus RJ, Miller WL. 2001. Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes P450c17 and 3β-hydroxysteroid dehydrogenase. J Biol Chem 276:16767–16771 [DOI] [PubMed] [Google Scholar]
- 243. Kossor DC, Kominami S, Takemori S, Colby HD. 1992. Destruction of testicular cytochrome P-450 by 7α-thiospironolactone is catalyzed by the 17α-hydroxylase. J Steroid Biochem Mol Biol 42:421–424 [DOI] [PubMed] [Google Scholar]
- 244. Lara PN, Jr, Redman MW, Kelly K, Edelman MJ, Williamson SK, Crowley JJ, Gandara DR. 2008. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 26:463–467 [DOI] [PubMed] [Google Scholar]
- 245. O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, Harland S, Robbins A, Halbert G, Nutley B, Jarman M. 2004. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Brit J Cancer 90:2317–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Slominski A, Ermak G, Mihm M. 1996. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab 81:2746–2749 [DOI] [PubMed] [Google Scholar]
- 247. Dalla Valle L, Couët J, Labrie Y, Simard J, Belvedere P, Simontacchi C, Labrie F, Colombo L. 1995. Occurrence of cytochrome P450c17 mRNA and dehydroepiandrosterone biosynthesis in the rat gastrointestinal tract. Mol Cell Endocrinol 111:83–92 [DOI] [PubMed] [Google Scholar]
- 248. Le Goascogne C, Sananès N, Eychenne B, Gouézou M, Baulieu EE, Robel P. 1995. Androgen biosynthesis in the stomach: expression of cytochrome P450 17α-hydroxylase/17,20 lyase messenger ribonucleic acid and protein, and metabolism of pregnenolone and progesterone by parietal cells of the rat gastric mucosa. Endocrinology 136:1744–1752 [DOI] [PubMed] [Google Scholar]
- 249. Compagnone NA, Zhang P, Vigne JL, Mellon SH. 2000. Novel role for the nuclear phosphoprotein SET in transcriptional activation of P450c17 and initiation of neurosteroidogenesis. Mol Endocrinol 14:875–888 [DOI] [PubMed] [Google Scholar]
- 250. Rodriguez H, Hum DW, Staels B, Miller WL. 1997. Transcription of the human genes for cytochrome P450scc and P450c17 is regulated differently in human adrenal NCI-H295 cells than in mouse adrenal Y1 cells. J Clin Endocrinol Metab 82:365–371 [DOI] [PubMed] [Google Scholar]
- 251. Lin CJ, Martens JW, Miller WL. 2001. NF-1c, Sp1 and Sp3 are essential for transcription of the human gene for P450c17 (steroid 17α-hydroxylase/17,20 lyase) in human adrenal NCI-H295A cells. Mol Endocrinol 15:1277–1293 [DOI] [PubMed] [Google Scholar]
- 252. Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR. 2002. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation between p54nrb/NonO, PSF and SF-1, a complex which also participates in repression of transcription. Endocrinology 143:1280–1290 [DOI] [PubMed] [Google Scholar]
- 253. Jimenez P, Saner K, Mayhew B, Rainey WE. 2003. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology 144:4285–4288 [DOI] [PubMed] [Google Scholar]
- 254. Flück CE, Miller WL. 2004. GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol Endocrinol 18:1144–1157 [DOI] [PubMed] [Google Scholar]
- 255. Sewer MB, Jagarlapudi S. 2009. Complex assembly on the human CYP17 promoter. Mol Cell Endocrinol 300:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Urs AN, Dammer E, Sewer MB. 2006. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology 147:5249–5258 [DOI] [PubMed] [Google Scholar]
- 257. Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. 2007. cAMP-stimulated interaction between steroidogenic factor-1 and diacylglycerol kinase facilitates induction of CYP17. Mol Cell Biol 27:6669–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Shea-Eaton WK, Trinidad MJ, Lopez D, Nackley A, McLean MP. 2001. Sterol regulatory element binding protein-1a regulation of the steroidogenic acute regulatory protein gene. Endocrinology 142:1525–1533 [DOI] [PubMed] [Google Scholar]
- 259. Ozbay T, Rowan A, Leon A, Patel P, Sewer MB. 2006. Cyclic adenosine 5′-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1. Endocrinology 147:1427–1437 [DOI] [PubMed] [Google Scholar]
- 260. Huang N, Dardis A, Miller WL. 2005. Regulation of cytochrome b5 gene expression by Sp3, GATA-6, and NF-1 in human adrenal NCI-H295A cells. Mol Endocrinol 19:2020–2034 [DOI] [PubMed] [Google Scholar]
- 261. Biglieri EG, Herron MA, Brust N. 1966. 17-Hydroxylation deficiency in man. J Clin Invest 45:1946–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Yanase T, Simpson ER, Waterman MR. 1991. 17α-Hydroxylase/17,20 lyase deficiency: from clinical investigation to molecular definition. Endocr Rev 12:91–108 [DOI] [PubMed] [Google Scholar]
- 263. Scaroni C, Opocher G, Mantero F. 1986. Renin-angiotensin-aldosterone system: a long-term follow-up study in 17α-hydroxylase deficiency syndrome. Clin Exp Hypertens A 8:773–780 [DOI] [PubMed] [Google Scholar]
- 264. New MI, Suvannakul L. 1970. Male pseudohermaphroditism due to 17α-hydroxylase deficiency. J Clin Invest 49:1930–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. D’Armiento M, Reda G, Kater C, Shackleton CH, Biglieri EG. 1983. 17α-Hydroxylase deficiency: mineralocorticoid hormone profiles in an affected family. J Clin Endocrinol Metab 56:697–701 [DOI] [PubMed] [Google Scholar]
- 266. Wit JM, van Roermund HP, Oostdijk W, Benraad TJ, Thijssen JH, Boer P, Jansen M, Spit M, van den Brande JL. 1988. Heterozygotes for 17α-hydroxylase deficiency can be detected with a short ACTH test. Clin Endocrinol (Oxf) 28:657–664 [DOI] [PubMed] [Google Scholar]
- 267. Auchus RJ. 2001. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am 30:101–119, vii [DOI] [PubMed] [Google Scholar]
- 268. Rosa S, Duff C, Meyer M, Lang-Muritano M, Balercia G, Boscaro M, Topaloglu AK, Mioni R, Fallo F, Zuliani L, Mantero F, Schoenle EJ, Biason-Lauber A. 2007. P450c17 deficiency: clinical and molecular characterization of six patients. J Clin Endocrinol Metab 92:1000–1007 [DOI] [PubMed] [Google Scholar]
- 269. Imai T, Yanase T, Waterman MR, Simpson ER, Pratt JJ. 1992. Canadian Mennonites and individuals residing in the Friesland region of the Netherlands share the same molecular basis of 17α-hydroxylase deficiency. Hum Genet 89:95–96 [DOI] [PubMed] [Google Scholar]
- 270. Fardella CE, Zhang LH, Mahachoklertwattana P, Lin D, Miller WL. 1993. Deletion of amino acids Asp487-Ser488-Phe489 in human cytochrome P450c17 causes severe 17α-hydroxylase deficiency. J Clin Endocrinol Metab 77:489–493 [DOI] [PubMed] [Google Scholar]
- 271. Lam CW, Arlt W, Chan CK, Honour JW, Lin CJ, Tong SF, Choy KW, Miller WL. 2001. Mutation of proline 409 to arginine in the meander region of cytochrome P450c17 causes severe 17α-hydroxylase deficiency. Mol Genet Metab 72:254–259 [DOI] [PubMed] [Google Scholar]
- 272. Miura K, Yasuda K, Yanase T, Yamakita N, Sasano H, Nawata H, Inoue M, Fukaya T, Shizuta Y. 1996. Mutation of cytochrome P-45017 α gene (CYP17) in a Japanese patient previously reported as having glucocorticoid-responsive hyperaldosteronism: with a review of Japanese patients with mutations of CYP17. J Clin Endocrinol Metab 81:3797–3801 [DOI] [PubMed] [Google Scholar]
- 273. Costa-Santos M, Kater CE, Auchus RJ. 2004. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab 89:49–60 [DOI] [PubMed] [Google Scholar]
- 274. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, et al. 2009. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41:666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, et al. 2009. Genome-wide association study of blood pressure and hypertension. Nat Genet 41:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Gupta MK, Geller DH, Auchus RJ. 2001. Pitfalls in characterizing P450c17 mutations associated with isolated 17,20 lyase deficiency. J Clin Endocrinol Metab 86:4416–4423 [DOI] [PubMed] [Google Scholar]
- 277. Yanase T, Waterman MR, Zachmann M, Winter JS, Simpson ER, Kagimoto M. 1992. Molecular basis of apparent isolated 17,20-lyase deficiency: compound heterozygous mutations in the C-terminal region (Arg496→Cys, Gln461→Stop) actually cause combined 17α-hydroxylase/17,20-lyase deficiency. Biochem Biophys Acta 1139:275–279 [DOI] [PubMed] [Google Scholar]
- 278. Zachmann M, Kempken B, Manella B, Navarro E. 1992. Conversion from pure 17,20 desmolase to combined 17,20-desmolase/17α-hydroxylase deficiency with age. Acta Endocrinol (Copenh) 127:97–99 [DOI] [PubMed] [Google Scholar]
- 279. Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20 lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- 280. Geller DH, Auchus RJ, Miller WL. 1999. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- 281. Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. 2002. Differential inhibition of 17α-hydroxylase and 17,20 lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab 87:5714–5721 [DOI] [PubMed] [Google Scholar]
- 282. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. 2003. CYP17 mutation E305G causes isolated 17,20 lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- 283. Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. 2008. Apparent isolated 17,20 lyase deficiency caused by homozygous mutation G539R in P450 oxidoreductase. J Clin Endocrinol Metab 93:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lee-Robichaud P, Akhtar ME, Akhtar M. 1998. Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b5. Biochem J 332:293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Giordano SJ, Kaftory A, Steggles AW. 1994. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Hum Genet 93:568–570 [DOI] [PubMed] [Google Scholar]
- 286. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. 2010. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95:994–999 [DOI] [PubMed] [Google Scholar]
- 287. Zhang LH, Rodriguez H, Ohno S, Miller WL. 1995. Serine phosphorylation of human P450c17 increases 17,20 lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Pandey AV, Mellon SH, Miller WL. 2003. Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- 289. Pandey AV, Miller WL. 2005. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- 290. Biason-Lauber A, Zachmann M, Schoenle EJ. 2000. Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology 141:1446–1454 [DOI] [PubMed] [Google Scholar]
- 291. Yanagibashi K, Hall PF. 1986. Role of electron transport in the regulation of the lyase activity of C-21 side-chain cleavage P450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- 292. Miller WL, Auchus RJ, Geller DH. 1997. The regulation of 17,20 lyase activity. Steroids 62:133–142 [DOI] [PubMed] [Google Scholar]
- 293. Ono T, Bloch K. 1975. Solubilization and partial characterization of rat liver squalene epoxidase. J Biol Chem 250:1571–1579 [PubMed] [Google Scholar]
- 294. Ilan Z, Ilan R, Cinti DL. 1981. Evidence for a new physiological role of hepatic NADPH:ferricytochrome (P-450) oxidoreductase. Direct electron input to the microsomal fatty acid chain elongation system. J Biol Chem 256:10066–10072 [PubMed] [Google Scholar]
- 295. Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. 1995. Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry 34:4421–4427 [DOI] [PubMed] [Google Scholar]
- 296. Enoch HG, Strittmatter P. 1979. Cytochrome b5 reduction by NADPH-cytochrome P-450 reductase. J Biol Chem 254:8976–8981 [PubMed] [Google Scholar]
- 297. Yamano S, Aoyama T, McBride OW, Hardwick JP, Gelboin HV, Gonzalez FJ. 1989. Human NADPH-P450 oxidoreductase: complementary DNA cloning, sequence, vaccinia virus-mediated expression, and localization of the CYPOR gene to chromosome 7. Mol Pharmacol 36:83–88 [PubMed] [Google Scholar]
- 298. Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. 1997. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci USA 94:8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. 2009. Domain motion in cytochrome P450 reductase. Conformational equilibria revealed by NMR and small-angle x-ray scattering. J Biol Chem 284:36628–36637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Lamb DC, Kim Y, Yermalitskaya LV, Yermalitsky VN, Lepesheva GI, Kelly SL, Waterman MR, Podust LM. 2006. A second FMN binding site in yeast NADPH-cytochrome P450 reductase suggests a mechanism of electron transfer by diflavin reductases. Structure 14:51–61 [DOI] [PubMed] [Google Scholar]
- 301. Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL. 1999. Structure of a cytochrome P450-redox partner electron-transfer complex. Proc Natl Acad Sci USA 96:1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. 1995. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure 3:41–62 [DOI] [PubMed] [Google Scholar]
- 303. Scott RR, Gomes LG, Huang N, Van Vliet G, Miller WL. 2007. Apparent manifesting heterozygosity in P450 oxidoreductase deficiency and its effect on coexisting 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:2318–2322 [DOI] [PubMed] [Google Scholar]
- 304. Huang N, Agrawal V, Giacomini KM, Miller WL. 2008. Genetics of P450 oxidoreductase. Sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutants. Proc Natl Acad Sci USA 105:1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL. 2005. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Gomes LG, Huang N, Agrawal V, Mendonça BB, Bachega TA, Miller WL. 2008. The common P450 oxidoreductase variant A503V is not a modifier gene for 21-hydroxylase deficiency. J Clin Endocrinol Metab 93:2913–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Agrawal V, Huang N, Miller WL. 2008. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics 18:569–576 [DOI] [PubMed] [Google Scholar]
- 308. Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL. 2010. Effects of genetic variants of P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet Genomics 20:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Agrawal V, Choi JH, Giacomini KM, Miller WL. 2010. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase (POR). Pharmacogenet Genomics 20:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Shen AL, O’Leary KA, Kasper CB. 2002. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem 277:6536–6541 [DOI] [PubMed] [Google Scholar]
- 311. Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR. 2003. Identification of novel roles of the P450 system in early embryogenesis: effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol 23:6103–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Henderson CJ, Otto DM, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR. 2003. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem 278:13480–13486 [DOI] [PubMed] [Google Scholar]
- 313. Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, Zhang L, Ding X. 2003. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene. Impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenases. J Biol Chem 278:25895–25901 [DOI] [PubMed] [Google Scholar]
- 314. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. 2004. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- 315. Adachi M, Tachibana K, Asakura Y, Yamamoto T, Hanaki K, Oka A. 2004. Compound heterozygous mutation of cytochrome P450 oxidoreductase gene (POR) in two patients with Antley-Bixler syndrome. Am J Med Genet 128A:333–339 [DOI] [PubMed] [Google Scholar]
- 316. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. 2004. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 363:2128–2135 [DOI] [PubMed] [Google Scholar]
- 317. Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, Nakai H, Soneda S, Tachibana K, Matsuo N, Sato S, Homma K, Nishimura G, Hasegawa T, Ogata T. 2005. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab 90:414–426 [DOI] [PubMed] [Google Scholar]
- 318. Flück CE, Miller WL. 2006. P450 oxidoreductase deficiency: a new form of congenital adrenal hyperplasia. Curr Opin Pediatr 18:435–441 [DOI] [PubMed] [Google Scholar]
- 319. Scott RR, Miller WL. 2008. Genetic and clinical features of P450 oxidoreductase deficiency. Horm Res 69:266–275 [DOI] [PubMed] [Google Scholar]
- 320. Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. 1985. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases: a new variant of congenital adrenal hyperplasia. N Engl J Med 313:1182–1191 [DOI] [PubMed] [Google Scholar]
- 321. Małunowicz E, Romer TE, Szarras-Czapnik M, Mielniczuk Z, Gajewska D. 1987. Combined deficiency of 17 α-hydroxylase and 21-hydroxylase in an 8 year-old girl. Endokrynol Pol 38:117–124 [PubMed] [Google Scholar]
- 322. Augarten A, Pariente C, Gazit E, Chayen R, Goldfarb H, Sack J. 1992. Ambiguous genitalia due to partial activity of cytochromes P450c17 and P450c21. J Steroid Biochem Mol Biol 41:37–41 [DOI] [PubMed] [Google Scholar]
- 323. Leiberman E, Hershkovitz E, Lauber-Biason A, Phillip M, Zachmann M. 1997. Subnormal cortisol response to adrenocorticotropin in isolated partial 17,20-lyase activity. J Pediatr Endocrinol Metab 10:387–390 [DOI] [PubMed] [Google Scholar]
- 324. Adachi M, Tachibana K, Asakura Y, Suwa S, Nishimura G. 1999. A male patient presenting with major clinical symptoms of glucocorticoid deficiency and skeletal dysplasia, showing a steroid pattern compatible with 17α-hydroxylase/17/20 lyase deficiency, but without obvious CYP17 gene mutations. Endocr J 46:285–292 [DOI] [PubMed] [Google Scholar]
- 325. Miller WL. 1986. Congenital adrenal hyperplasia. N Engl J Med 314:1321–1322 [PubMed] [Google Scholar]
- 326. Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. 1994. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom). J Clin Endocrinol Metab 78:1287–1292 [DOI] [PubMed] [Google Scholar]
- 327. Grumbach MM, Auchus RJ. 1999. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84:4677–4694 [DOI] [PubMed] [Google Scholar]
- 328. Pandey AV, Kempná P, Hofer G, Mullis PE, Flück CE. 2007. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol Endocrinol 21:2579–2595 [DOI] [PubMed] [Google Scholar]
- 329. Miller WL. 1998. Steroid hormone biosynthesis and actions in the materno-feto-placental unit. Clin Perinatol 25:799–817, v [PubMed] [Google Scholar]
- 330. Shackleton C, Marcos J, Arlt W, Hauffa BP. 2004. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet 129A:105–112 [DOI] [PubMed] [Google Scholar]
- 331. Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T. 2006. Cytochrome P450 oxidoreductase deficiency in three patients initially regarded as having 21-hydroxylase deficiency and/or aromatase deficiency: diagnostic value of urine steroid hormone analysis. Pediatr Res 59:276–280 [DOI] [PubMed] [Google Scholar]
- 332. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 2003. 5α-Androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 144:575–580 [DOI] [PubMed] [Google Scholar]
- 333. Auchus RJ. 2004. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 15:432–438 [DOI] [PubMed] [Google Scholar]
- 334. Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. 2006. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Sahakitrungruang T, Huang N, Tee MK, Agrawal V, Russell WE, Crock P, Murphy N, Migeon CJ, Miller WL. 2009. Clinical, genetic and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J Clin Endocrinol Metab 94:4992–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Debeljak N, Fink M, Rozman D. 2003. Many facets of mammalian lanosterol 14α-demethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch Biochem Biophys 409:159–171 [DOI] [PubMed] [Google Scholar]
- 337. Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Wolf LM, Jabs EW. 2002. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet 110:95–102 [DOI] [PubMed] [Google Scholar]
- 338. Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. 1996. Fluconazole-induced congenital anomalies in three infants. Clin Infect Dis 22:336–340 [DOI] [PubMed] [Google Scholar]
- 339. Aleck KA, Bartley DL. 1997. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet 72:253–256 [PubMed] [Google Scholar]
- 340. Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. 2003. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 33:508–513 [DOI] [PubMed] [Google Scholar]
- 341. Gofflot F, Hars C, Illien F, Chevy F, Wolf C, Picard JJ, Roux C. 2003. Molecular mechanisms underlying limb anomalies associated with cholesterol deficiency during gestation: implications of Hedgehog signaling. Hum Mol Genet 12:1187–1198 [DOI] [PubMed] [Google Scholar]
- 342. Schmidt K, Hughes C, Chudek JA, Goodyear SR, Aspden RM, Talbot R, Gundersen TE, Blomhoff R, Henderson C, Wolf CR, Tickle C. 2009. Cholesterol metabolism: the main pathway acting downstream of cytochrome P450 oxidoreductase in skeletal development of the limb. Mol Cell Biol 29:2716–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Aguilar A, Wu S, De Luca F. 2009. P450 oxidoreductase expressed in rat chondrocytes modulates chondrogenesis via cholesterol- and Indian Hedgehog-dependent mechanisms. Endocrinology 150:2732–2739 [DOI] [PubMed] [Google Scholar]
- 344. Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. 1998. Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J Biol Chem 273:17036–17049 [DOI] [PubMed] [Google Scholar]
- 345. Yamazaki H, Johnson WW, Ueng YF, Shimada T, Guengerich FP. 1996. Lack of electron transfer from cytochrome b5 in stimulation of catalytic activities of cytochrome P450 3A4. Characterization of a reconstituted cytochrome P450 3A4/NADPH-cytochrome P450 reductase system and studies of apo-cytochrome b5. J Biol Chem 271:27438–27444 [DOI] [PubMed] [Google Scholar]
- 346. Muskett FW, Kelly GP, Whitford D. 1996. The solution structure of bovine ferricytochrome b5 determined using heteronuclear NMR methods. J Mol Biol 258:172–189 [DOI] [PubMed] [Google Scholar]
- 347. Falzone CJ, Mayer MR, Whiteman EL, Moore CD, Lecomte JT. 1996. Design challenges for hemoproteins: the solution structure of apocytochrome b5. Biochemistry 35:6519–6526 [DOI] [PubMed] [Google Scholar]
- 348. Lee-Robichaud P, Kaderbhai MA, Kaderbhai N, Wright JN, Akhtar M. 1997. Interaction of human CYP17 (P-45017α, 17α-hydroxylase-17,20 lyase) with cytochrome b5: importance of the orientation of the hydrophobic domain of cytochrome b5. Biochem J 321:857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Naffin-Olivos JL, Auchus RJ. 2006. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762 [DOI] [PubMed] [Google Scholar]
- 350. Miller WL, Morel Y. 1989. Molecular genetics of 21-hydroxylase deficiency. Ann Rev Genet 23:371–393 [DOI] [PubMed] [Google Scholar]
- 351. Morel Y, Miller WL. 1991. Clinical and molecular genetics of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Adv Hum Genet 20:1–68 [DOI] [PubMed] [Google Scholar]
- 352. White PC, Speiser PW. 2000. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 21:245–291 [DOI] [PubMed] [Google Scholar]
- 353. Speiser PW, White PC. 2003. Congenital adrenal hyperplasia. N Engl J Med 349:776–788 [DOI] [PubMed] [Google Scholar]
- 354. Forest MG. 2004. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod Update 10:469–485 [DOI] [PubMed] [Google Scholar]
- 355. Kominami S, Ochi H, Kobayashi Y, Takemori S. 1980. Studies on the steroid hydroxylation system in adrenal cortex microsomes: purification and characterization of cytochrome P450 specific for steroid 21 hydroxylation. J Biol Chem 255:3386–3394 [PubMed] [Google Scholar]
- 356. White PC, New MI, Dupont B. 1984. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA 81:7505–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. 1986. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and genuine gene. Proc Natl Acad Sci USA 83:2841–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. White PC, New MI, Dupont B. 1986. Structure of the human steroid 21-hydroxylase genes. Proc Natl Acad Sci USA 83:5111–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Rodrigues NR, Dunham I, Yu CY, Carroll MC, Porter RR, Campbell RD. 1987. Molecular characterization of the HLA-linked steroid 21-hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J 6:1653–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Dupont B, Oberfield SE, Smithwick EM, Lee TD, Levine LS. 1977. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet 2:1309–1312 [DOI] [PubMed] [Google Scholar]
- 361. Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, Lesser M, New MI, White PC. 1992. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 90:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Wedell A, Thilén A, Ritzén EM, Stengler B, Luthman H. 1994. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 78:1145–1152 [DOI] [PubMed] [Google Scholar]
- 363. Tusie-Luna MT, Speiser PW, Dumic M, New MI, White PC. 1991. A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol 5:685–692 [DOI] [PubMed] [Google Scholar]
- 364. Helmberg A, Tusie-Luna MT, Tabarelli M, Kofler R, White PC. 1992. R339H and P453S: CYP21 mutations associated with nonclassic steroid 21-hydroxylase deficiency that are not apparent gene conversion. Mol Endocrinol 6:1318–1322 [DOI] [PubMed] [Google Scholar]
- 365. Casey ML, MacDonald PC. 1982. Extra-adrenal formation of a mineralocorticoid: deoxycorticosterone and deoxycorticosterone sulfate biosynthesis and metabolism. Endocr Rev 3:396–403 [DOI] [PubMed] [Google Scholar]
- 366. Mellon SH, Miller WL. 1989. Extra-adrenal steroid 21- hydroxylation is not mediated by P450c21. J Clin Invest 84:1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Yamazaki H, Shimada T. 1997. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9 and 3A4. Arch Biochem Biophys 346:161–169 [DOI] [PubMed] [Google Scholar]
- 368. Gomes LG, Huang N, Agrawal V, Mendonça BB, Bachega TA, Miller WL. 2009. Extra-adrenal 21-hydroxylation by CYP2C19 and CYP3A4: effect on 21-hydroxylase deficiency. J Clin Endocrinol Metab 94:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Carroll MC, Campbell RD, Porter RR. 1985. Mapping of steroid 21-hydroxylase genes to complement component C4 genes in HLA, the major histocompatibility locus in man. Proc Natl Acad Sci USA 82:521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, Strominger JL. 1985. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci USA 82:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Bristow J, Gitelman SE, Tee MK, Staels B, Miller WL. 1993. Abundant adrenal-specific transcription of the human P450c21A “pseudogene.” J Biol Chem 268:12919–12924 [PubMed] [Google Scholar]
- 372. Chang SF, Chung BC. 1995. Difference in transcriptional activity of two homologous CYP21A genes. Mol Endocrinol 9:1330–1336 [DOI] [PubMed] [Google Scholar]
- 373. Chung BC, Matteson KJ, Miller WL. 1986. Structure of the bovine gene for P450c21 (steroid 21-hydroxylase) defines a novel cytochrome P450 gene family. Proc Natl Acad Sci USA 83:4243–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Amor M, Tosi M, Duponchel C, Steinmetz M, Meo T. 1985. Liver cDNA probes disclose two cytochrome P450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci USA 82:4453–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Skow LC, Womack JE, Petresh JM, Miller WL. 1988. Synteny mapping of the genes for steroid 21-hydroxylase, α-A-crystallin, and class I bovine leukocyte antigen (BoLA) in cattle. DNA 7:143–149 [DOI] [PubMed] [Google Scholar]
- 376. Parker KL, Chaplin DD, Wong M, Seidman JG, Smith JA, Schimmer BP. 1985. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci USA 82:7860–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Chaplin DD, Galbraith LJ, Seidman JG, White PC, Parker KL. 1986. Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc Natl Acad Sci USA 83:9601–9605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. John ME, Okamura T, Dee A, Adler B, John MC, White PC, Simpson ER, Waterman MR. 1986. Bovine steroid 21-hydroxylase: regulation of biosynthesis. Biochemistry 25:2846–2853 [DOI] [PubMed] [Google Scholar]
- 379. Gitelman SE, Bristow J, Miller WL. 1992. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol 12:2124–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Geffrotin C, Chardon P, de Andres-Cara DF, Feil R, Renard C, Vaiman M. 1990. The swine steroid 21-hydroxylase gene (CYP21): cloning and mapping within the swine leukocyte antigen locus. Anim Genet 21:1–13 [DOI] [PubMed] [Google Scholar]
- 381. Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. 2000. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab 85:1059–1065 [DOI] [PubMed] [Google Scholar]
- 382. Partanen J, Koskimies S, Sipilä I, Lipsanen V. 1989. Major histocompatibility-complex gene markers and restriction fragment analysis of steroid 21-hydroxylase (CYP21) and complement C4 genes in classical adrenal hyperplasia patients in a single population. Am J Hum Genet 44:660–670 [PMC free article] [PubMed] [Google Scholar]
- 383. Dupont B, Pollack MS, Levine LS, O’Neill GJ, Hawkins BR, New MI. 1981. Congenital adrenal hyperplasia: joint report from the Eighth International Histocompatibility Workshop. In: Terasaki P. ed. Histocompatibility testing 1980. Berlin: Springer Verlag; 693–706 [Google Scholar]
- 384. Fleischnick E, Awdeh ZL, Raum D, Granados J, Alosco SM, Crigler JF, Jr, Gerald PS, Giles CM, Yunis EJ, Alper CA. 1983. Extended MHC haplotypes in 21-hydroxylase deficiency congenital adrenal hyperplasia: shared genotypes in unrelated patients. Lancet 1:152–156 [DOI] [PubMed] [Google Scholar]
- 385. Höller W, Scholz S, Knorr D, Bidlingmaier F, Keller E, Albert ED. 1985. Genetic differences in the salt-wasting, simple virilizing, and nonclassical types of congenital adrenal hyperplasia. J Clin Endocrinol Metab 60:757–763 [DOI] [PubMed] [Google Scholar]
- 386. Pollack MS, Levine LS, O’Neill GJ, Pang S, Lorenzen F, Kohn B, Rondanini GF, Chiumello G, New MI, Dupont B. 1981. HLA linkage and B14, DR1, BfS haplotype association with the genes for late onset and cryptic 21-hydroxylase deficiency. Am J Hum Genet 33:540–550 [PMC free article] [PubMed] [Google Scholar]
- 387. Speiser PW, New MI, White PC. 1988. Molecular genetic analysis of nonclassical steroid 21-hydroxylase deficiency associated with HLA-B14DR1. N Engl J Med 319:19–23 [DOI] [PubMed] [Google Scholar]
- 388. Morel Y, André J, Uring-Lambert B, Hauptmann G, Bétuel H, Tossi M, Forest MG, David M, Bertrand J, Miller WL. 1989. Rearrangements and point mutations of P450c21 genes are distinguished by five restriction endonuclease haplotypes identified by a new probing strategy in 57 families with congenital adrenal hyperplasia. J Clin Invest 83:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Rosenbloom AL, Smith DW. 1966. Varying expression for salt-losing in related patients with congenital adrenal hyperplasia. Pediatrics 38:215–219 [PubMed] [Google Scholar]
- 390. Stoner E, Dimartino-Nardi J, Kuhnle U, Levine LS, Oberfield SE, New MI. 1986. Is salt-wasting in congenital adrenal hyperplasia genetic? Clin Endocrinol (Oxf) 24:9–20 [DOI] [PubMed] [Google Scholar]
- 391. Morel Y, David M, Forest MG, Betuel H, Hauptman G, Andre J, Bertrand J, Miller WL. 1989. Gene conversions and rearrangements cause discordance between inheritance of forms of 21-hydroxylase deficiency and HLA types. J Clin Endocrinol Metab 68:592–599 [DOI] [PubMed] [Google Scholar]
- 392. Sinnott PJ, Dyer PA, Price DA, Harris R, Strachan T. 1989. 21-Hydroxylase deficiency families with HLA identical affected and unaffected sibs. J Med Genet 26:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Gomez-Escobar N, Chou CF, Lin WW, Hsieh SL, Campbell RD. 1998. The G11 gene located in the major histocompatibility complex encodes a novel nuclear serine/threonine protein kinase. J Biol Chem 273:30954–30960 [DOI] [PubMed] [Google Scholar]
- 394. Law SK, Dodds AW, Porter RR. 1984. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J 3:1819–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Yu CY, Belt KT, Giles CM, Campbell RD, Porter RR. 1986. Structural basis of the polymorphism of the human complement components C4A and C4B: gene size, reactivity and antigenicity. EMBO J 5:2873–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Tee MK, Thomson AA, Bristow J, Miller WL. 1995. Sequences promoting the transcription of the human XA gene overlapping P450c21A correctly predict the presence of a novel, adrenal-specific, truncated form of Tenascin-X. Genomics 28:171–178 [DOI] [PubMed] [Google Scholar]
- 397. Wijesuriya SD, Zhang G, Dardis A, Miller WL. 1999. Transcriptional regulatory elements of the human gene for cytochrome P450c21 (steroid 21-hydroxylase) lie within intron 35 of the linked C4B gene. J Biol Chem 274:38097–38106 [DOI] [PubMed] [Google Scholar]
- 398. Morel Y, Bristow J, Gitelman SE, Miller WL. 1989. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component/C4 gene locus. Proc Natl Acad Sci USA 86:6582–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. 1993. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol 122:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Burch GH, Bedolli MA, McDonough S, Rosenthal SM, Bristow J. 1995. Embryonic expression of tenascin-X suggests a role in limb, muscle, and heart development. Dev Dyn 203:491–504 [DOI] [PubMed] [Google Scholar]
- 401. Speek M, Barry F, Miller WL. 1996. Alternate promoters and alternate splicing of human Tenascin-X, a gene with 5′ and 3′ ends buried in other genes. Hum Mol Genet 5:1749–1758 [DOI] [PubMed] [Google Scholar]
- 402. Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. 1997. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 17:104–108 [DOI] [PubMed] [Google Scholar]
- 403. Elefteriou F, Exposito JY, Garrone R, Lethias C. 1997. Characterization of the bovine Tenascin-X. J Biol Chem 272:22866–22874 [DOI] [PubMed] [Google Scholar]
- 404. Lethias C, Carisey A, Comte J, Cluzel C, Exposito JY. 2006. A model of tenascin-X integration within the collagenous network. FEBS Lett 580:6281–6285 [DOI] [PubMed] [Google Scholar]
- 405. Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, van Vlijmen IM, van Haren B, Miller WL, Bristow J. 2001. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med 345:1167–1175 [DOI] [PubMed] [Google Scholar]
- 406. Tee MK, Babalola GO, Aza-Blanc P, Speek M, Gitelman SE, Miller WL. 1995. A promoter within intron 35 of the human C4A gene initiates adrenal-specific transcription of a 1 kb RNA: location of a cryptic CYP21 promoter element? Hum Mol Genet 4:2109–2116 [DOI] [PubMed] [Google Scholar]
- 407. New MI, Wilson RC. 1999. Steroid disorders in children: congenital adrenal hyperplasia and apparent mineralocorticoid excess. Proc Natl Acad Sci USA 96:12790–12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Clayton PE, Miller WL, Oberfield SE, Ritzén EM, Sippell WG, Speiser PW. 2002. Consensus statement on 21-hydroxylase deficiency from The Lawson Wilkins Pediatric Endocrine Society and The European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 87:4048–4053 [DOI] [PubMed] [Google Scholar]
- 409. Merke DP, Bornstein SR. 2005. Congenital adrenal hyperplasia. Lancet 365:2125–2136 [DOI] [PubMed] [Google Scholar]
- 410. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HFL, Miller WL, Montori VM, Oberfield SE, Ritzén M, White PC. 2010. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95:4133–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Wurtman RJ, Pohorecky LA. 1971. Adrenocortical control of epinephrine synthesis in health and disease. Adv Metab Disord 5:53–76 [DOI] [PubMed] [Google Scholar]
- 412. Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR. 2000. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med 343:1362–1368 [DOI] [PubMed] [Google Scholar]
- 413. Speiser PW, Knochenhauer ES, Dewailly D, Fruzzetti F, Marcondes JA, Azziz R. 2000. A multicenter study of women with nonclassical congenital adrenal hyperplasia: relationship between genotype and phenotype. Mol Genet Metab 71:527–534 [DOI] [PubMed] [Google Scholar]
- 414. Migeon CJ, Rosenwaks Z, Lee PA, Urban MD, Bias WB. 1980. The attenuated form of congenital adrenal hyperplasia as an allelic form of 21-hydroxylase deficiency. J Clin Endocrinol Metab 51:647–649 [DOI] [PubMed] [Google Scholar]
- 415. Levine LS, Dupont B, Lorenzen F, Pang S, Pollack M, Oberfield SE, Kohn B, Lerner A, Cacciari E, Mantero F, Cassio A, Scaroni C, Chiumello G, Rondanini GF, Gargantini L, Giovannelli G, Virdis R, Bartolotta E, Migliori C, Pintor C, Tato L, Barboni F, New MI. 1981. Genetic and hormonal characterization of the cryptic 21-hydroxylase deficiency. J Clin Endocrinol Metab 53:1193–1198 [DOI] [PubMed] [Google Scholar]
- 416. Chrousos GP, Loriaux DL, Mann DL, Cutler GB., Jr 1982. Late-onset 21-hydroxylase deficiency mimicking idiopathic hirsutism or polycystic ovarian disease: an allelic variant of congenital virilizing adrenal hyperplasia with a milder enzymatic defect. Ann Intern Med 96:143–148 [DOI] [PubMed] [Google Scholar]
- 417. Kohn B, Levine LS, Pollack MS, Pang S, Lorenzen F, Levy D, Lerner AJ, Rondanini GF, Dupont B, New MI. 1982. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 55:817–827 [DOI] [PubMed] [Google Scholar]
- 418. Fiet J, Gueux B, Raux-DeMay MC, Kuttenn F, Vexiau P, Brerault JL, Couillin P, Galons H, Villette JM, Julien R. 1989. Increased plasma 21-deoxycorticosterone (21-DB) levels in late-onset adrenal 21-hydroxylase deficiency suggest a mild defect of the mineralocorticoid pathway. J Clin Endocrinol Metab 68:542–547 [DOI] [PubMed] [Google Scholar]
- 419. Pang SY, Wallace MA, Hofman L, Thuline HC, Dorche C, Lyon IC, Dobbins RH, Kling S, Fujieda K, Suwa S. 1988. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 81:866–874 [PubMed] [Google Scholar]
- 420. Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. 1998. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 101:583–590 [DOI] [PubMed] [Google Scholar]
- 421. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. 1985. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet 37:650–667 [PMC free article] [PubMed] [Google Scholar]
- 422. Sherman SL, Aston CE, Morton NE, Speiser PW, New MI. 1988. A segregation and linkage study of classical and nonclassical 21-hydroxylase deficiency. Am J Hum Genet 42:830–838 [PMC free article] [PubMed] [Google Scholar]
- 423. Dumić M, Brkljacić L, Speiser PW, Wood E, Crawford C, Plavsić V, Baniceviác M, Radmanović S, Radica A, Kastelan A. 1990. An update on the frequency of nonclassic deficiency of adrenal 21-hydroxylase in the Yugoslav population. Acta Endocrinol 122:703–710 [DOI] [PubMed] [Google Scholar]
- 424. Chetkowski RJ, DeFazio J, Shamonki I, Judd HL, Chang RJ. 1984. The incidence of the late-onset congenital adrenal hyperplasia due to 21-hydroxylase deficiency among hirsute women. J Clin Endocrinol Metab 58:595–598 [DOI] [PubMed] [Google Scholar]
- 425. Kuttenn F, Couillin P, Girard F, Billaud L, Vincens M, Boucekkine C, Thalabard JC, Maudelonde T, Spritzer P, Mowszowicz I, Boué A, Mauvais-Jarvis P. 1985. Late-onset adrenal hyperplasia in hirsutism. N Engl J Med 313:224–231 [DOI] [PubMed] [Google Scholar]
- 426. White PC, Tusie-Luna MT, New MI, Speiser PW. 1994. Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat 3:373–378 [DOI] [PubMed] [Google Scholar]
- 427. Matteson KJ, Phillips JA, 3rd, Miller WL, Chung BC, Orlando PJ, Frisch H, Ferrandez A, Burr IM. 1987. P450XXI (steroid 21-hydroxylase) gene deletions are not found in family studies of congenital adrenal hyperplasia. Proc Natl Acad Sci USA 84:5858–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Baltimore D. 1981. Gene conversion: some implications for immunoglobulin genes. Cell 24:592–594 [DOI] [PubMed] [Google Scholar]
- 429. White PC, Vitek A, Dupont B, New MI. 1988. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA 85:4436–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Higashi Y, Tanae A, Inoue H, Hiromasa T, Fujii-Kuriyama Y. 1988. Aberrant splicing and missense mutation cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci USA 85:7486–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Tardy V, Menassa R, Sulmont V, Lienhardt-Roussie A, Lecointre C, Brauner R, David M, Morel Y. 2010. Phenotype-genotype correlations of 13 rare CYP21A2 mutations detected in 46 patients affected with 21-hydroxylase deficiency and in one carrier. J Clin Endocrinol Metab 95:1288–1300 [DOI] [PubMed] [Google Scholar]
- 432. Mornet E, Crété P, Kuttenn F, Raux-Demay MC, Boué J, White PC, Boué A. 1991. Distribution of deletions and seven point mutations on CYP21B genes in three clinical forms of steroid 21-hydroxylase deficiency. Am J Hum Genet 48:79–88 [PMC free article] [PubMed] [Google Scholar]
- 433. Donohoue PA, Sandrini Neto R, Collins MM, Migeon CJ. 1990. Exon 7 Nco I restriction site within CYP21B (steroid 21-hydroxylase) is a normal polymorphism. Mol Endocrinol 4:1354–1362 [DOI] [PubMed] [Google Scholar]
- 434. Higashi Y, Hiromasa T, Tanae A, Miki T, Nakura J, Kondo T, Ohura T, Ogawa E, Nakayama K, Fujii-Kuriyama Y. 1991. Effects of individual mutations in the P-450(C21) pseudogene on P-450(C21) activity and their distribution in patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem 109:638–644 [DOI] [PubMed] [Google Scholar]
- 435. Chiou SH, Hu MC, Chung BC. 1990. A missense mutation of Ile172→Asn or Arg356→Trp causes steroid 21-hydroxylase deficiency. J Biol Chem 265:3549–3552 [PubMed] [Google Scholar]
- 436. Wedell A, Ritzén EM, Haglund-Stengler B, Luthman H. 1992. Steroid 21-hydroxylase deficiency: three additional mutated alleles and establishment of phenotype-genotype relationships of common mutations. Proc Natl Acad Sci USA 89:7232–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Wedell A, Luthman H. 1993. Steroid 21-hydroxylase (P450c21): a new allele and spread of mutations through the pseudogene. Hum Genet 91:236–240 [DOI] [PubMed] [Google Scholar]
- 438. Wedell A, Luthman H. 1993. Steroid 21-hydroxylase deficiency: two additional mutations in salt-wasting disease and rapid screening of disease-causing mutations. Hum Mol Genet 2:499–504 [DOI] [PubMed] [Google Scholar]
- 439. Bleicken C, Loidi L, Dhir V, Parajes S, Quinteiro C, Dominguez F, Grötzinger J, Sippell WG, Riepe FG, Arlt W, Krone N. 2009. Functional characterization of three CYP21A2 sequence variants (p.A265V, p.W302S, p.D322G) employing a yeast co-expression system. Hum Mutat 30:E443–E450 [DOI] [PubMed] [Google Scholar]
- 440. Grischuk Y, Rubtsov P, Riepe FG, Grötzinger J, Beljelarskaia S, Prassolov V, Kalintchenko N, Semitcheva T, Peterkova V, Tiulpakov A, Sippell WG, Krone N. 2006. Four novel missense mutations in the CYP21A2 gene detected in Russian patients suffering from the classical form of congenital adrenal hyperplasia: identification, functional characterization, and structural analysis. J Clin Endocrinol Metab 91:4976–4980 [DOI] [PubMed] [Google Scholar]
- 441. Urabe K, Kimura A, Harada F, Iwanaga T, Sasazuki T. 1990. Gene conversion in steroid 21-hydroxylase genes. Am J Hum Genet 46:1178–1186 [PMC free article] [PubMed] [Google Scholar]
- 442. Amor M, Parker KL, Globerman H, New MI, White PC. 1988. Mutation in the CYP21B gene (Ile-172-Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA 85:1600–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Hu MC, Chung BC. 1990. Expression of human 21- hydroxylase (P450c21) in bacterial and mammalian cell—a system to characterize normal and mutant enzymes. Mol Endocrinol 4:893–898 [DOI] [PubMed] [Google Scholar]
- 444. Wu DA, Chung BC. 1991. Mutations of P450c21 (steroid 21-hydroxylase) at Cys428, Val281, or Ser268 result in complete, partial, or no loss of enzymatic activity. J Clin Invest 88:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Owerbach D, Sherman L, Ballard AL, Azziz R. 1992. Pro453 to Ser mutation in CYP21 is associated with non-classic steroid 21-hydroxylase deficiency. Mol Endocrinol 6:1211–1215 [DOI] [PubMed] [Google Scholar]
- 446. Hsu LC, Hu MC, Cheng HC, Lu JC, Chung BC. 1993. The N-terminal hydrophobic domain of P450c21 is required for membrane insertion and enzyme stability. J Biol Chem 268:14682–14686 [PubMed] [Google Scholar]
- 447. White PC, Curnow KM, Pascoe L. 1994. Disorders of steroid 11β-hydroxylase isozymes. Endocr Rev 15:421–438 [DOI] [PubMed] [Google Scholar]
- 448. Fardella CE, Miller WL. 1996. Molecular biology of mineralocorticoid metabolism. Annu Rev Nutr 16:443–470 [DOI] [PubMed] [Google Scholar]
- 449. Mornet E, Dupont J, Vitek A, White PC. 1989. Characterization of two genes encoding human steroid 11β-hydroxylase (P45011β). J Biol Chem 264:20961–20967 [PubMed] [Google Scholar]
- 450. Yanagibashi K, Haniu M, Shively JE, Shen WH, Hall P. 1986. The synthesis of aldosterone by the adrenal cortex: two zones (fasciculata and glomerulosa) possess one enzyme for 11-, 18-hydroxylation, and aldehyde synthesis. J Biol Chem 261:3556–3562 [PubMed] [Google Scholar]
- 451. Morohashi K, Yoshioka H, Gotoh O, Okada Y, Yamamoto K, Miyata T, Sogawa K, Fujii-Kuriyama Y, Omura T. 1987. Molecular cloning and nucleotide sequence of DNA of mitochondrial P-450(11) of bovine adrenal cortex. J Biochem 102:559–568 [DOI] [PubMed] [Google Scholar]
- 452. Mellon SH, Bair SR, Monis H. 1995. P450c11B3 mRNA, transcribed from a third P450c11 gene, is expressed in a tissue-specific, developmentally and hormonally regulated fashion in the rodent adrenal, and encodes a protein with both 11-hydroxylase and 18-hydroxylase activities. J Biol Chem 270:1643–1649 [DOI] [PubMed] [Google Scholar]
- 453. Chua SC, Szabo P, Vitek A, Grzeschik KH, John M, White PC. 1987. Cloning of cDNA encoding steroid 11β-hydroxylase, P450c11. Proc Natl Acad Sci USA 84:7193–7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. 2010. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 95:2296–2305 [DOI] [PubMed] [Google Scholar]
- 455. Ogishima T, Shibata H, Shimada H, Mitani F, Suzuki H, Saruta T, Ishimura Y. 1991. Aldosterone synthase cytochrome P450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem 266:10731–10734 [PubMed] [Google Scholar]
- 456. Curnow KM, Tusie-Luna MT, Pascoe L, Natarajan R, Gu JL, Nadler JL, White PC. 1991. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 5:1513–1522 [DOI] [PubMed] [Google Scholar]
- 457. Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, Ohnishi T, Ichikawa Y, Nakao K, Imura H, Ulick S, Shizuta Y. 1992. Role of steroid 11β-hydroxylase and 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci USA 89:1458–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Mulatero P, Curnow KM, Aupetit-Faisant B, Foekling M, Gomez-Sanchez C, Veglio F, Jeunemaitre X, Corvol P, Pascoe L. 1998. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab 83:3996–4001 [DOI] [PubMed] [Google Scholar]
- 459. Auchus RJ, Chandler DW, Singeetham S, Chokshi N, Nwariaku FE, Dolmatch BL, Holt SA, Wians FH, Jr, Josephs SC, Trimmer CK, Lopera J, Vongpatanasin W, Nesbitt SD, Leonard D, Victor RG. 2007. Measurement of 18-hydroxycorticosterone during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab 92:2648–2651 [DOI] [PubMed] [Google Scholar]
- 460. Clyne CD, Zhang Y, Slutsker L, Mathis JM, White PC, Rainey WE. 1997. Antiotensin II and potassium regulate human CYP11B2 transcription through common cis-elements. Mol Endocrinol 11:638–649 [DOI] [PubMed] [Google Scholar]
- 461. Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. 2004. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18:279–290 [DOI] [PubMed] [Google Scholar]
- 462. Bassett MH, White PC, Rainey WE. 2004. The regulation of aldosterone synthase expression. Mol Cell Endocrinol 217:67–74 [DOI] [PubMed] [Google Scholar]
- 463. White PC, Dupont J, New MI, Leiberman E, Hochberg Z, Rösler A. 1991. A mutation in CYP11B1 (Arg 448→His) associated with steroid 11β-hydroxylase deficiency in Jews of Moroccan origin. J Clin Invest 87:1664–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Pascoe L, Curnow KM, Slutsker L, Rösler A, White PC. 1992. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methlyoxidase II deficiency. Proc Natl Acad Sci USA 89:4996–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Ulick S, Wang JZ, Morton DH. 1992. The biochemical phenotypes of two inborn errors in the biosynthesis of aldosterone. J Clin Endocrinol Metab 74:1415–1420 [DOI] [PubMed] [Google Scholar]
- 466. Zhang G, Rodriguez H, Fardella CE, Harris DA, Miller WL. 1995. Mutation T318M in P450c11AS causes corticosterone methyl oxidase II deficiency. Am J Hum Genet 57:1037–1043 [PMC free article] [PubMed] [Google Scholar]
- 467. Ulick S. 1976. Diagnosis and nomenclature of the disorders of the terminal portion of aldosterone biosynthetic pathway. J Clin Endocrinol Metab 43:92–96 [DOI] [PubMed] [Google Scholar]
- 468. Veldhuis JD, Kulin HE, Santen RJ, Wilson TE, Melby JC. 1980. Inborn error in the terminal step of aldosterone biosynthesis: corticosterone methyl oxidase type II deficiency in a North American pedigree. N Engl J Med 303:117–121 [DOI] [PubMed] [Google Scholar]
- 469. Chabraoui L, Abid F, Menassa R, Gaouzi A, El Hessni A, Morel Y. 2010. Three novel CYP11B1 mutations in congenital adrenal hyperplasia due to steroid 11β-hydroxylase deficiency in a Moroccan population. Horm Res Paediatr 74:182–189 [DOI] [PubMed] [Google Scholar]
- 470. Zachmann M, Tassinari D, Prader A. 1983. Clinical and biochemical variability in congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. J Clin Endocrinol Metab 56:222–229 [DOI] [PubMed] [Google Scholar]
- 471. Holcombe JH, Keenan BS, Nichols BL, Kirkland RT, Clayton GW. 1980. Neonatal salt loss in the hypertensive form of congenital adrenal hyperplasia. Pediatrics 65:777–781 [PubMed] [Google Scholar]
- 472. New MI, White P, Pang S, Dupont B, Speiser PW. 1989. The adrenal hyperplasias. In: Scriver C, Beaudet A, Sly S, Valle D. eds. The metabolic basis of inherited disease. 6th ed. New York: McGraw-Hill Inc.; 1881–1917 [Google Scholar]
- 473. Peter M, Janzen N, Sander S, Korsch E, Riepe FG, Sander J. 2008. A case of 11β-hydroxylase deficiency detected in a newborn screening program by second-tier LC-MS/MS. Horm Res 69:253–256 [DOI] [PubMed] [Google Scholar]
- 474. Krone N, Grötzinger J, Holterhus PM, Sippell WG, Schwarz HP, Riepe FG. 2009. Congenital adrenal hyperplasia due to11-hydroxylase deficiency—insights from two novel CYP11B1 mutations (p.M92X, p.R453Q). Horm Res 72:281–286 [DOI] [PubMed] [Google Scholar]
- 475. Sonino N, Levine LS, Vecsei P, New MI. 1980. Parallelism of 11-and 18-hydroxylation demonstrated by urinary free hormones in man. J Clin Endocrinol Metab 51:557–560 [DOI] [PubMed] [Google Scholar]
- 476. Parajes S, Loidi L, Reisch N, Dhir V, Rose IT, Hampel R, Quinkler M, Conway GS, Castro-Feijóo L, Araujo-Vilar D, Pombo M, Dominguez F, Williams EL, Cole TR, Kirk JM, Kaminsky E, Rumsby G, Arlt W, Krone N. 2010. Functional consequences of seven novel mutations in the CYP11B1 gene: four mutations associated with nonclassic and three mutations causing classic 11β-hydroxylase deficiency. J Clin Endocrinol Metab 95:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Kharrat M, Trabelsi S, Chaabouni M, Maazoul F, Kraoua L, Ben Jemaa L, Gandoura N, Barsaoui S, Morel Y, M’rad R, Chaabouni H. 2010. Only two mutations detected in 15 Tunisian patients with 11β-hydroxylase deficiency: the p.Q356X and the novel p.G379V. Clin Genet 78:398–401 [DOI] [PubMed] [Google Scholar]
- 478. Azziz R, Boots LR, Parker CR, Jr, Bradley E, Jr, Zacur HA. 1991. 11β-Hydroxylase deficiency in hyperandrogenism. Fertil Steril 55:733–741 [PubMed] [Google Scholar]
- 479. Joehrer K, Geley S, Strasser-Wozak EM, Azziz R, Wollmann HA, Schmitt K, Kofler R, White PC. 1997. CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11β-hydroxylase deficiency. Hum Mol Genet 6:1829–1834 [DOI] [PubMed] [Google Scholar]
- 480. Kayes-Wandover KM, Schindler RE, Taylor HC, White PC. 2001. Type 1 aldosterone synthase deficiency presenting in a middle-aged man. J Clin Endocrinol Metab 86:1008–1012 [DOI] [PubMed] [Google Scholar]
- 481. Martinerie L, Viengchareun S, Delezoide AL, Jaubert F, Sinico M, Prevot S, Boileau P, Meduri G, Lombès M. 2009. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology 150:4414–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Rösler A. 1984. The natural history of salt-wasting disorders of adrenal and renal origin. J Clin Endocrinol Metab 59:689–700 [DOI] [PubMed] [Google Scholar]
- 483. Mitsuuchi Y, Kawamoto T, Miyahara K, Ulick S, Morton DH, Naiki Y, Kuribayashi I, Toda K, Hara T, Orii T, Yasuda K, Miura K, Yamamoto Y, Imura H, Shiruta Y. 1993. Congenitally defective aldosterone biosynthesis in the humans: inactivation of the P-450c18 gene (CYP11B2) due to nucleotide deletion in CMO I deficient patients. Biochem Biophys Res Commun 190:864–869 [DOI] [PubMed] [Google Scholar]
- 484. Peter M, Fawaz L, Drop SL, Visser HK, Sippell WG. 1997. Hereditary defect in biosynthesis of aldosterone: aldosterone synthase deficiency 1964–1997. J Clin Endocrinol Metab 82:3525–3528 [DOI] [PubMed] [Google Scholar]
- 485. Geley S, Jöhrer K, Peter M, Denner K, Bernhardt R, Sippell WG, Kofler R. 1995. Amino acid substitution R384P in aldosterone synthase causes corticosterone methyloxidase type I deficiency. J Clin Endocrinol Metab 80:424–429 [DOI] [PubMed] [Google Scholar]
- 486. Raag R, Martinis SA, Sligar SG, Poulos TL. 1991. Crystal structure of the cytochrome P-450CAM active site mutant Thr252Ala. Biochemistry 30:11420–11429 [DOI] [PubMed] [Google Scholar]
- 487. Portrat-Doyen S, Tourniaire J, Richard O, Mulatero P, Aupetit-Faisant B, Curnow KM, Pascoe L, Morel Y. 1998. Isolated aldosterone synthase deficiency caused by simultaneous E198D and V386A mutations in the CYP11B2 gene. J Clin Endocrinol Metab 83:4156–4161 [DOI] [PubMed] [Google Scholar]
- 488. Fardella CE, Rodriguez H, Montero J, Zhang G, Vignolo P, Rojas A, Villarroel L, Miller WL. 1996. Genetic variation in P450c11AS in Chilean patients with low renin hypertension. J Clin Endocrinol Metab 81:4347–4351 [DOI] [PubMed] [Google Scholar]
- 489. Fardella CE, Hum DW, Rodriguez H, Zhang G, Barry FL, Ilicki A, Bloch CA, Miller WL. 1996. Gene conversion in the CYP11B2 gene encoding aldosterone synthase (P450c11AS) is associated with, but does not cause, the syndrome of corticosterone methyl oxidase II deficiency. J Clin Endocrinol Metab 81:321–326 [DOI] [PubMed] [Google Scholar]
- 490. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. 1992. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355:262–265 [DOI] [PubMed] [Google Scholar]
- 491. Pascoe L, Curnow KM, Slutsker L, Connell JM, Speiser PW, New MI, White PC. 1992. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossover between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA 89:8327–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Dluhy RG, Lifton RP. 1999. Glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 84:4341–4344 [DOI] [PubMed] [Google Scholar]
- 493. Portrat S, Mulatero P, Curnow KM, Chaussain JL, Morel Y, Pascoe L. 2001. Deletion hybrid genes, due to unequal crossing over between CYP11B1 (11β-hydroxylase) and CYP11B2 (aldosterone synthase) cause steroid 11β-hydroxylase deficiency and congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:3197–3201 [DOI] [PubMed] [Google Scholar]
- 494. Litchfield WR, New MI, Coolidge C, Lifton RP, Dluhy RG. 1997. Evaluation of the dexamethasone suppression test for the diagnosis of glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 82:3570–3573 [DOI] [PubMed] [Google Scholar]
- 495. Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. 2000. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile and molecular biology. J Clin Endocrinol Metab 85:1863–1867 [DOI] [PubMed] [Google Scholar]
- 496. Böttner B, Denner K, Bernhardt R. 1998. Conferring aldosterone synthesis to human CYP11B1 by replacing key amino acid residues with CYP11B2-specific ones. Eur J Biochem 252:458–466 [DOI] [PubMed] [Google Scholar]
- 497. Curnow KM, Mulatero P, Emeric-Blanchouin N, Aupetit-Faisant B, Corvol P, Pascoe L. 1997. The amino acid substitutions Ser288Gly and Val320Ala convert the cortisol producing enzyme, CYP11B1, into an aldosterone producing enzyme. Nat Struct Biol 4:32–35 [DOI] [PubMed] [Google Scholar]
- 498. Fardella CE, Rodriguez H, Hum DW, Mellon SH, Miller WL. 1995. Artificial mutations in P450c11AS (aldosterone synthase) can increase enzymatic activity: a model for low-renin hypertension? J Clin Endocrinol Metab 80:1040–1043 [DOI] [PubMed] [Google Scholar]
- 499. Takeda Y, Furukawa K, Inaba S, Miyamori I, Mabuchi H. 1999. Genetic analysis of aldosterone synthase in patients with idiopathic hyperaldosteronism. J Clin Endocrinol Metab 84:1633–1637 [DOI] [PubMed] [Google Scholar]
- 500. Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A. 1997. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148–158 [DOI] [PubMed] [Google Scholar]
- 501. Moghrabi N, Andersson S. 1998. 17β-Hydroxysteroid dehydrogenases: physiological roles in health and disease. Trends Endocrinol Metab 9:265–270 [DOI] [PubMed] [Google Scholar]
- 502. Peltoketo H, Luu-The V, Simard J, Adamski J. 1999. 17β-Hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family: nomenclature and main characteristics of the 17HSD/KSR enzymes. J Mol Endocrinol 23:1–11 [DOI] [PubMed] [Google Scholar]
- 503. Peltoketo H, Isomaa V, Mäentausta O, Vihko R. 1988. Complete amino acid sequence of human placental 17β-hydroxysteroid dehydrogenase deduced from cDNA. FEBS Lett 239:73–77 [DOI] [PubMed] [Google Scholar]
- 504. Gast MJ, Sims HF, Murdock GL, Gast PM, Strauss AW. 1989. Isolation and sequencing of a complementary deoxyribonucleic acid clone encoding human placental 17β-estradiol dehydrogenase: identification of the putative cofactor binding site. Am J Obstet Gynecol 161:1726–1731 [DOI] [PubMed] [Google Scholar]
- 505. Luu-The V, Labrie C, Simard J, Lachance Y, Zhao HF, Couët J, Leblanc G, Labrie F. 1990. Structure of two in tandem human 17β-hydroxysteroid dehydrogenase genes. Mol Endocrinol 4:268–275 [DOI] [PubMed] [Google Scholar]
- 506. Tremblay Y, Ringler GE, Morel Y, Mohandas TK, Labrie F, Strauss JF, 3rd, Miller WL. 1989. Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen→q25. J Biol Chem 264:20458–20462 [PubMed] [Google Scholar]
- 507. Luu-The V, Zhang Y, Poirier D, Labrie F. 1995. Characteristics of human types 1,2 and 3 17β-hydroxysteroid dehydrogenase activities: oxidation/reduction and inhibition. J Steroid Biochem Mol Biol 55:581–587 [DOI] [PubMed] [Google Scholar]
- 508. Auchus RJ, Covey DF, Bork V, Schaefer J. 1988. Solid-state NMR observation of cysteine and lysine Michael adducts of inactivated estradiol dehydrogenase. J Biol Chem 263:11640–11645 [PubMed] [Google Scholar]
- 509. Ghosh D, Wawrzak Z, Weeks CM, Duax WL, Erman M. 1994. The refined three-dimensional structure of 3α, 20β-hydroxysteroid dehydrogenase and possible roles of the residues conserved in short-chain dehydrogenases. Structure 2:629–640 [DOI] [PubMed] [Google Scholar]
- 510. Ghosh D, Pletnev VZ, Zhu DW, Wawrzak Z, Duax WL, Pangborn W, Labrie F, Lin SX. 1995. Structure of human estrogenic 17β-hydroxysteroid dehydrogenase at 2.2 Å resolution. Structure 3:503–513 [DOI] [PubMed] [Google Scholar]
- 511. Sawicki MW, Erman M, Puranen T, Vihko P, Ghosh D. 1999. Structure of the ternary complex of human 17β-hydroxysteroid dehydrogenase type 1 with 3-hydroxyestra-1,3,5,7-tetraen-17-one (equilin) and NADP+. Proc Natl Acad Sci USA 96:840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Sasano H, Frost AR, Saitoh R, Harada N, Poutanen M, Vihko R, Bulun SE, Silverberg SG, Nagura H. 1996. Aromatase and 17β-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 81:4042–4046 [DOI] [PubMed] [Google Scholar]
- 513. Wu L, Einstein M, Geissler WM, Chan HK, Elliston KO, Andersson S. 1993. Expression cloning and characterization of human 17β-hydrosteroid dehydrogenase type 2, a microsomal enzyme possessing 20α-hydroxysteroid dehydrogenase activity. J Biol Chem 268:12964–12969 [PubMed] [Google Scholar]
- 514. Casey ML, MacDonald PC, Andersson S. 1994. 17β-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest 94:2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Takeyama J, Sasano H, Suzuki T, Iinuma K, Nagura H, Andersson S. 1998. 17β-Hydroxysteroid dehydrogenase types 1 and 2 in human placenta: an immunohistochemical study with correlation to placental development. J Clin Endocrinol Metab 83:3710–3715 [DOI] [PubMed] [Google Scholar]
- 516. Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. 1994. Male pseudohermaphroditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nat Genet 7:34–39 [DOI] [PubMed] [Google Scholar]
- 517. Andersson S, Geissler WM, Wu L, Davis DL, Grumbach MM, New MI, Schwarz HP, Blethen SL, Mendonca BB, Bloise W, Witchel SF, Cutler GB, Jr, Griffin JE, Wilson JD, Russel DW. 1996. Molecular genetics and pathophysiology of 17β-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab 81:130–136 [DOI] [PubMed] [Google Scholar]
- 518. Moghrabi N, Hughes IA, Dunaif A, Andersson S. 1998. Deleterious missense mutations and silent polymorphism in the human 17β-hydroxysteroid dehydrogenase 3 gene (HSD17B3). J Clin Endocrinol Metab 83:2855–2860 [DOI] [PubMed] [Google Scholar]
- 519. Mendonca BB, Arnhold IJ, Bloise W, Andersson S, Russell DW, Wilson JD. 1999. 17β-Hydroxysteroid dehydrogenase 3 deficiency in women. J Clin Endocrinol Metab 84:802–804 [DOI] [PubMed] [Google Scholar]
- 520. Adamski J, Normand T, Leenders F, Monté D, Begue A, Stéhelin D, Jungblut PW, de Launoit Y. 1995. Molecular cloning of a novel widely expressed human 80 kDa 17β-hydroxysteroid dehydrogenase IV. Biochem J 311:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Leenders F, Tesdorpf JG, Markus M, Engel T, Seedorf U, Adamski J. 1996. Porcine 80-kDa protein reveals intrinsic 17β-hydroxysteroid dehydrogenase, fatty acyl-CoA-hydratase/dehydrogenase, and sterol transfer activities. J Biol Chem 271:5438–5442 [DOI] [PubMed] [Google Scholar]
- 522. van Grunsven EG, van Berkel E, Ijlst L, Vreken P, de Klerk JB, Adamski J, Lemonde H, Clayton PT, Cuebas DA, Wanders RJ. 1998. Peroxisomal D-hydroxyacyl-CoA dehydrogenase deficiency: resolution of the enzyme defect and its molecular basis in bifunctional protein deficiency. Proc Natl Acad Sci USA 95:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Qin YM, Poutanen MH, Helander HM, Kvist AP, Siivari KM, Schmitz W, Conzelmann E, Hellman U, Hiltunen JK. 1997. Peroxisomal multifunctional enzyme of β-oxidation metabolizing D-3-hydroxyacyl-CoA esters in rat liver: molecular cloning, expression and characterization. Biochem J 321:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. 1997. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol Endocrinol 11:1971–1984 [DOI] [PubMed] [Google Scholar]
- 525. Deyashiki Y, Ogasawara A, Nakayama T, Nakanishi M, Miyabe Y, Sato K, Hara A. 1994. Molecular cloning of two human liver 3α-hydroxysteroid/dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. Biochem J 299:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. 1999. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 140:568–574 [DOI] [PubMed] [Google Scholar]
- 527. El-Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. 1999. Localization of type 5 17β-hydroxysteroid dehydrogenase, 3β-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology 140:1481–1491 [DOI] [PubMed] [Google Scholar]
- 528. Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. 2000. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J 351:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Byrns MC, Steckelbroeck S, Penning TM. 2008. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C2 (type 2 3α-HSD, type 5 17β-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol 75:484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. 2009. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab 94:2192–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Chetyrkin SV, Hu J, Gough WH, Dumaual N, Kedishvili NY. 2001. Further characterization of human microsomal 3α-hydroxysteroid dehydrogenase. Arch Biochem Biophys 386:1–10 [DOI] [PubMed] [Google Scholar]
- 532. Huang XF, Luu-The V. 2000. Molecular characterization of a first human 3(α)-hydroxysteroid epimerase. J Biol Chem 275:29452–29457 [DOI] [PubMed] [Google Scholar]
- 533. Biswas MG, Russell DW. 1997. Expression cloning and characterization of oxidative 17β- and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem 272:15959–15966 [DOI] [PubMed] [Google Scholar]
- 534. Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. 2009. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab 94:3676–3681 [DOI] [PubMed] [Google Scholar]
- 535. Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. 1994. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15:342–355 [DOI] [PubMed] [Google Scholar]
- 536. Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. 2002. Aromatase—a brief overview. Annu Rev Physiol 64:93–127 [DOI] [PubMed] [Google Scholar]
- 537. Mahendroo MS, Means GD, Mendelson CR, Simpson ER. 1991. Tissue specific expression of human P450 arom: the promoter responsible in adipose tissue is different from that utilized in placenta. J Biol Chem 266:11276–11281 [PubMed] [Google Scholar]
- 538. Mahendroo MS, Mendelson CR, Simpson ER. 1993. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J Biol Chem 268:19463–19470 [PubMed] [Google Scholar]
- 539. Shimozawa O, Sakaguchi M, Ogawa H, Harada N, Mihara K, Omura T. 1993. Core glycosylation of cytochrome P-450(arom). Evidence for localization of N terminus of microsomal cytochrome P-450 in the lumen. J Biol Chem 268:21399–21402 [PubMed] [Google Scholar]
- 540. Beusen DD, Carrell HL, Covey DF. 1987. Metabolism of 19-methyl-substituted steroids by human placental aromatase. Biochemistry 26:7833–7841 [DOI] [PubMed] [Google Scholar]
- 541. Ghosh D, Griswold J, Erman M, Pangborn W. 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. 1993. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci USA 90:11673–11677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Fisher CR, Graves KH, Parlow AF, Simpson ER. 1998. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Lo JC, Schwitzgebel VM, Tyrrell JB, Fitzgerald PA, Kaplan SL, Conte FA, Grumbach MM. 1999. Normal female infants born of mothers with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 84:930–936 [DOI] [PubMed] [Google Scholar]
- 545. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. 1995. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- 546. Bruchovsky N, Wilson JD. 1968. The intranuclear binding of testosterone and 5α-androstan-17β-ol-3-one by rat prostate. J Biol Chem 243:5953–5960 [PubMed] [Google Scholar]
- 547. Moore RJ, Wilson JD. 1976. Steroid 5α-reductase in cultured human fibroblasts. Biochemical and genetic evidence for two distinct enzyme activities. J Biol Chem 251:5895–5900 [PubMed] [Google Scholar]
- 548. Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. 1992. Genetic and pharmacologic evidence for more than one human steroid 5α-reductase. J Clin Invest 89:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Andersson S, Berman DM, Jenkins EP, Russell DW. 1991. Deletion of a steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature 354:159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. 1993. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J Clin Invest 92:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Wilson JD, Griffin JE, Russell DW. 1993. Steroid 5α-reductase 2 deficiency. Endocr Rev 14:577–593 [DOI] [PubMed] [Google Scholar]
- 552. Wilson JD. 1999. The role of androgens in male gender role behavior. Endocr Rev 20:726–737 [DOI] [PubMed] [Google Scholar]
- 553. Frederiksen DW, Wilson JD. 1971. Partial characterization of the nuclear reduced nicotinamide adenine dinucleotide phosphate: Δ4–3-ketosteroid 5 α-reductase of rat prostate. J Biol Chem 246:2584–2593 [PubMed] [Google Scholar]
- 554. McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG, Nickel JC, Wang DZ, Taylor AM, Waldstreicher J. 1998. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med 338:557–563 [DOI] [PubMed] [Google Scholar]
- 555. Mahendroo MS, Cala KM, Russell DW. 1996. 5α-reduced androgens play a key role in murine parturition. Mol Endocrinol 10:380–392 [DOI] [PubMed] [Google Scholar]
- 556. Mahendroo M, Wilson JD, Richardson JA, Auchus RJ. 2004. Steroid 5α-reductase 1 promotes 5α-androstane-3α-17β-diol synthesis in immature mouse testis by two pathways. Mol Cell Endocrinol 222:113–120 [DOI] [PubMed] [Google Scholar]
- 557. Khanna M, Qin KN, Wang RW, Cheng KC. 1995. Substrate specificity, gene structure and tissue-specific distribution of multiple human 3α-hydroxysteroid dehydrogenases. J Biol Chem 270:20162–20168 [DOI] [PubMed] [Google Scholar]
- 558. Nishizawa M, Nakajima T, Yasuda K, Kanzaki H, Sasaguri Y, Watanabe K, Ito S. 2000. Close kinship of human 20α-hydroxysteroid dehydrogenase gene with three aldo-keto reductase genes. Genes Cells 5:111–125 [DOI] [PubMed] [Google Scholar]
- 559. Rupprecht R, Hauser CA, Trapp T, Holsboer F. 1996. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol 56:163–168 [DOI] [PubMed] [Google Scholar]
- 560. Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. 2003. Human type 3 3α-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology 144:2922–2932 [DOI] [PubMed] [Google Scholar]
- 561. Griffin LD, Mellon SH. 1999. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 96:13512–13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Gulbis JM, Mann S, MacKinnon R. 1999. Structure of a voltage-dependent K+ channel β subunit. Cell 97:943–952 [DOI] [PubMed] [Google Scholar]
- 563. Hoog SS, Pawlowski JE, Alzari PM, Penning TM, Lewis M. 1994. Three-dimensional structure of rat liver 3α-hydroxysteroid/dihydrodiol dehydrogenase: a member of the aldo-keto reductase superfamily. Proc Natl Acad Sci USA 91:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Cooper WC, Jin Y, Penning TM. 2007. Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehydrogenase (HSD) and identification of all enzyme forms on the reaction coordinate: the example of rat liver 3α-HSD (AKR1C9). J Biol Chem 282:33484–33493 [DOI] [PubMed] [Google Scholar]
- 565. Ratnam K, Ma H, Penning TM. 1999. The arginine 276 anchor for NADP(H) dictates fluorescence kinetic transients in 3α-hydroxysteroid dehydrogenase, a representative aldo-keto reductase. Biochemistry 38:7856–7864 [DOI] [PubMed] [Google Scholar]
- 566. Napoli JL. 2001. 17β-Hydroxysteroid dehydrogenase type 9 and other short-chain dehydrogenases/reductases that catalyze retinoid, 17β- and 3α-hydroxysteroid metabolism. Mol Cell Endocrinol 171:103–109 [DOI] [PubMed] [Google Scholar]
- 567. Chetyrkin SV, Hu J, Gough WH, Dumaual N, Kedishvili NY. 2001. Further characterization of human microsomal 3α-hydroxysteroid dehydrogenase. Arch Biochem Biophys 386:1–10 [DOI] [PubMed] [Google Scholar]
- 568. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. 1987. Cloning of human mineralocorticoid receptor DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- 569. Funder JW, Pearce PT, Smith R, Smith AI. 1988. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242:583–585 [DOI] [PubMed] [Google Scholar]
- 570. White PC, Mune T, Agarwal AK. 1997. 11β-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18:135–156 [DOI] [PubMed] [Google Scholar]
- 571. Seckl JR, Morton NM, Chapman KE, Walker BR. 2004. Glucocorticoids and 11β-hydroxysteroid dehydrogenase in adipose tissue. Rec Prog Horm Res 59:359–393 [DOI] [PubMed] [Google Scholar]
- 572. Walker EA, Stewart PM. 2003. 11β-Hydroxysteroid dehydrogenase: unexpected connections. Trends Endocrinol Metab 14:334–339 [DOI] [PubMed] [Google Scholar]
- 573. Tannin GM, Agarwal AK, Monder C, New MI, White PC. 1991. The human gene for 11β-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J Biol Chem 266:16653–16658 [PubMed] [Google Scholar]
- 574. Agarwal AK, Monder C, Eckstein B, White PC. 1989. Cloning and expression of rat cDNA encoding corticosteroid 11β-dehydrogenase. J Biol Chem 264:18939–18943 [PubMed] [Google Scholar]
- 575. Moore CC, Mellon SH, Murai J, Siiteri PK, Miller WL. 1993. Structure and function of the hepatic form of 11β-hydroxysteroid dehydrogenase in the squirrel monkey, an animal model of glucocorticoid resistance. Endocrinology 133:368–375 [DOI] [PubMed] [Google Scholar]
- 576. Agarwal AK, Tusie-Luna MT, Monder C, White PC. 1990. Expression of 11β-hydroxysteroid dehydrogenase using recombinant vaccinia virus. Mol Endocrinol 4:1827–1832 [DOI] [PubMed] [Google Scholar]
- 577. Walker EA, Clark AM, Hewison M, Ride JP, Stewart PM. 2001. Functional expression, characterization, and purification of the catalytic domain of human 11β-hydroxysteroid dehydrogenase type 1. J Biol Chem 276:21343–21350 [DOI] [PubMed] [Google Scholar]
- 578. Odermatt A, Arnold P, Stauffer A, Frey BM, Frey FJ. 1999. The N-terminal anchor sequences of 11β-hydroxysteroid dehydrogenases determine their orientation in the endoplasmic reticulum membrane. J Biol Chem 274:28762–28770 [DOI] [PubMed] [Google Scholar]
- 579. Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA, Stewart PM. 2005. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11β-hydroxysteroid dehydrogenase type 1. J Mol Endocrinol 34:675–684 [DOI] [PubMed] [Google Scholar]
- 580. Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. 1994. Cloning and tissue distribution of the human 11β-hydroxysteroid dehydrogenase type II enzyme. Mol Cell Endocrinol 105:R11–R17 [DOI] [PubMed] [Google Scholar]
- 581. Brown RW, Chapman KE, Edwards CR, Seckl JR. 1993. Human placental 11 β-hydroxysteroid dehydrogenase: evidence for and partial purification of a distinct NAD+-dependent isoform. Endocrinology 132:2614–2621 [DOI] [PubMed] [Google Scholar]
- 582. Rusvai E, Náray-Fejes-Tóth A. 1993. A new isoform of 11β-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem 268:10717–10720 [PubMed] [Google Scholar]
- 583. Krozowski Z, MaGuire JA, Stein-Oakley AN, Dowling J, Smith RE, Andrews RK. 1995. Immunohistochemical localization of the 11 β-hydroxysteroid dehydrogenase type II enzyme in human kidney and placenta. J Clin Endocrinol Metab 80:2203–2209 [DOI] [PubMed] [Google Scholar]
- 584. Hirasawa G, Sasano H, Suzuki T, Takeyama J, Muramatu Y, Fukushima K, Hiwatashi N, Toyota T, Nagura H, Krozowski ZS. 1999. 11β-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor in human fetal development. J Clin Endocrinol Metab 84:1453–1458 [DOI] [PubMed] [Google Scholar]
- 585. Hewitt KN, Walker EA, Stewart PM. 2005. Hexose-6-phosphate dehydrogenase and redox control of 11β-hydroxysteroid dehydrogenase type 1 activity. Endocrinology 146:2539–2543 [DOI] [PubMed] [Google Scholar]
- 586. McCormick KL, Wang X, Mick GJ. 2006. Evidence that the 11β-hydroxysteroid dehydrogenase (11βHSD-1) is regulated by pentose pathway flux. J Biol Chem 281:341–347 [DOI] [PubMed] [Google Scholar]
- 587. Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 2004. 11β-Hydroxysteroid dehydrogenase type 1: a tissue specific regulator of glucocorticoid response. Endocr Rev 25:831–866 [DOI] [PubMed] [Google Scholar]
- 588. Mune T, Rogerson FM, Nikkilä H, Agarwal AK, White PC. 1995. Human hypertension caused by mutations in the kidney isozyme of 11 β-hydroxysteroid dehydrogenase. Nat Genet 10:394–399 [DOI] [PubMed] [Google Scholar]
- 589. Li KX, Obeyesekere VR, Krozowski ZS, Ferrari P. 1997. Oxoreductase and dehydrogenase activities of the human and rat 11β-hydroxysteroid dehydrogenase type 2 enzyme. Endocrinology 138:2948–2952 [DOI] [PubMed] [Google Scholar]
- 590. Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, Shackleton CH, Stewart PM. 2003. Mutations in the genes encoding 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet 34:434–439 [DOI] [PubMed] [Google Scholar]
- 591. San Millán JL, Botella-Carretero JI, Alvarez-Blasco F, Luque-Ramírez M, Sancho J, Moghetti P, Escobar-Morreale HF. 2005. A study of the hexose-6-phosphate dehydrogenase gene R453Q and 11β-hydroxysteroid dehydrogenase type 1 gene 83557insA polymorphisms in the polycystic ovary syndrome. J Clin Endocrinol Metab 90:4157–4162 [DOI] [PubMed] [Google Scholar]
- 592. White PC. 2005. Genotypes at 11β-hydroxysteroid dehydrogenase type IIB1 and hexose-6-phosphate dehydrogenase loci are not risk factors for apparent cortisone reductase deficiency in a large population-based sample. J Clin Endocrinol Metab 90:5880–5883 [DOI] [PubMed] [Google Scholar]
- 593. Gambineri A, Vicennati V, Genghini S, Tomassoni F, Pagotto U, Pasquali R, Walker BR. 2006. Genetic variation in 11β-hydroxysteroid dehydrogenase type 1 predicts adrenal hyperandrogenism among lean women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:2295–2302 [DOI] [PubMed] [Google Scholar]
- 594. Lavery GG, Walker EA, Tiganescu A, Ride JP, Shackleton CH, Tomlinson JW, Connell JM, Ray DW, Biason-Lauber A, Malunowicz EM, Arlt W, Stewart PM. 2008. Steroid biomarkers and genetic studies reveal inactivating mutations in hexose-6-phosphate dehydrogenase in patients with cortisone reductase deficiency. J Clin Endocrinol Metab 93:3827–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595. Lavery GG, Walker EA, Draper N, Jeyasuria P, Marcos J, Shackleton CH, Parker KL, White PC, Stewart PM. 2006. Hexose-6-phosphate dehydrogenase knock-out mice lack 11β-hydroxysteroid dehydrogenase type 1-mediated glucocorticoid generation. J Biol Chem 281:6546–6551 [DOI] [PubMed] [Google Scholar]
- 596. Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Azar MR, Krozowski ZS, Funder JW, Shackleton CH, Bradlow HL, Wei JQ, Hertecant J, Moran A, Neiberger RE, Balfe JW, Fattah A, Daneman D, Akkurt HI, De Santis C, New MI. 1998. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab 83:2244–2254 [DOI] [PubMed] [Google Scholar]
- 597. Wilson RC, Nimkarn S, New MI. 2001. Apparent mineralocorticoid excess. Trends Endocrinol Metab 12:104–111 [DOI] [PubMed] [Google Scholar]
- 598. Quinkler M, Stewart PM. 2003. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab 88:2384–2392 [DOI] [PubMed] [Google Scholar]
- 599. Palermo M, Quinkler M, Stewart PM. 2004. Apparent mineralocorticoid excess syndrome: an overview. Arq Bras Endocrinol Metabol 48:687–696 [DOI] [PubMed] [Google Scholar]
- 600. Falany CN. 1997. Enzymology of human cytosolic sulfotransferases. FASEB J 11:206–216 [DOI] [PubMed] [Google Scholar]
- 601. Strott CA. 2002. Sulfonation and molecular action. Endocr Rev 23:703–732 [DOI] [PubMed] [Google Scholar]
- 602. Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC. 2005. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med 11:153–159 [DOI] [PubMed] [Google Scholar]
- 603. Nowell S, Falany CN. 2006. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 25:1673–1678 [DOI] [PubMed] [Google Scholar]
- 604. Yen PH, Allen E, Marsh B, Mohandas T, Wang N, Taggart RT, Shapiro LJ. 1987. Cloning and expression of steroid sulfatase cDNA and the frequent occurrence of deletions: implications for X-Y interchange. Cell 49:443–454 [DOI] [PubMed] [Google Scholar]
- 605. Ballabio A, Shapiro LJ. 1995. Steroid sulfatase deficiency and X-linked icthiosis. In: Schriver C, Beaduet A, Sly W, Valle D. eds. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 2999–3022 [Google Scholar]
- 606. Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. 1995. Expression of the steroidogenic enzyme P450scc in the central and peripheral nervous systems during rodent embryogenesis. Endocrinology 136:2689–2696 [DOI] [PubMed] [Google Scholar]
- 607. Compagnone NA, Mellon SH. 1998. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci USA 95:4678–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608. Strott CA. 2002. Sulfonation and molecular action. Endocr Rev 23:703–732 [DOI] [PubMed] [Google Scholar]
- 609. Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W. 2009. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med 360:2310–2318 [DOI] [PubMed] [Google Scholar]
- 610. Thiele H, Sakano M, Kitagawa H, Sugahara K, Rajab A, Höhne W, Ritter H, Leschik G, Nürnberg P, Mundlos S. 2004. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc Natl Acad Sci USA 101:10155–10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ERB, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G. 1994. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed] [Google Scholar]
- 612. Phelan JK, McCabe ER. 2001. Mutations in NR0B1 (DAX1) and NR5A (SF1) responsible for adrenal hypoplasia congenita. Hum Mutat 18:472–487 [DOI] [PubMed] [Google Scholar]
- 613. Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. 2006. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (SF1/Ad4BP, NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab 91:3048–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P. 2000. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest 105:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. 1999. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- 616. Biason-Lauber A, Schoenle EJ. 2000. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL. 2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab 87:1829–1833 [DOI] [PubMed] [Google Scholar]
- 618. Else T, Hammer GD. 2005. Analysis of absence: adrenal agenesis, aplasia and atrophy. Trends Endocrinol Metab 16:458–468 [DOI] [PubMed] [Google Scholar]
- 619. Vilain E, Le Merrer M, Lecointre C, Desangles F, Kay MA, Maroteaux P, McCabe ER. 1999. IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab 84:4335–4340 [DOI] [PubMed] [Google Scholar]
- 620. Bergadá I, Del Rey G, Lapunzina P, Bergadá C, Fellous M, Copelli S. 2005. Familial occurrence of the IMAGe association: additional clinical variants and a proposed mode of inheritance. J Clin Endocrinol Metab 90:3186–3190 [DOI] [PubMed] [Google Scholar]
- 621. Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al-Ali M, Brain CE, Clark AJ, Dattani MT, Achermann JC. 2007. Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients with apparent mineralocorticoid insufficiency. Clin Endocrinol (Oxf) 66:205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622. Clark AJ, Weber A. 1998. Adrenocorticotropin insensitivity syndromes. Endocr Rev 19:828–843 [DOI] [PubMed] [Google Scholar]
- 623. Flück CE, Martens JW, Conte FA, Miller WL. 2002. Clinical, genetic and functional characterization of ACTH receptor mutations using a novel receptor assay. J Clin Endocrinol Metab 87:4318–4323 [DOI] [PubMed] [Google Scholar]
- 624. Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, Cianfarani S, Clayton PE, Savage MO, Clark AJ. 2000. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf) 53:423–430 [DOI] [PubMed] [Google Scholar]
- 625. Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. 2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 626. Webb TR, Clark AJL. 2010. Minireview: the melanocortin 2 receptor accessory proteins. Mol Endocrinol 24:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627. Allgrove J, Clayden GS, Grant DB, Macaulay JC. 1978. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet 1:1284–1286 [DOI] [PubMed] [Google Scholar]
- 628. Grant DB, Barnes ND, Dumic M, Ginalska-Malinowska M, Milla PJ, von Petrykowski W, Rowlatt RJ, Steendijk R, Wales JH, Werder E. 1993. Neurological and adrenal dysfunction in the adrenal insufficiency/alacrima/achalasia (3A) syndrome. Arch Dis Child 68:779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. Houlden H, Smith S, De Carvalho M, Blake J, Mathias C, Wood NW, Reilly MM. 2002. Clinical and genetic characterization of families with triple A (Allgrove) syndrome. Brain 125:2681–2690 [DOI] [PubMed] [Google Scholar]
- 630. Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, de Laet MH, Chaouachi B, Bakiri F, Brottier P, Cattolico L, Penet C, Bégeot M, Naville D, Nicolino M, Chaussain JL, Weissenbach J, Munnich A, Lyonnet S. 2000. Mutant WD-repeat protein in triple-A syndrome. Nat Genet 26:332–335 [DOI] [PubMed] [Google Scholar]
- 631. Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A. 2001. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet 10:283–290 [DOI] [PubMed] [Google Scholar]
- 632. Cronshaw JM, Matunis MJ. 2003. The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci USA 100:5823–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633. Storr HL, Kind B, Parfitt DA, Chapple JP, Lorenz M, Koehler K, Huebner A, Clark AJL. 2009. Deficiency of ferritin heavy-chain nuclear import in Triple A syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol Endocrinol 23:2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634. Charmandari E, Kino T, Chrousos GP. 2004. Familial/sporadic glucocorticoid resistance: clinical phenotype and molecular mechanisms. Ann NY Acad Sci 1024:168–181 [DOI] [PubMed] [Google Scholar]
- 635. Hurley DM, Accili D, Stratakis CA, Karl M, Vamvakopoulos N, Rorer E, Constantine K, Taylor SI, Chrousos GP. 1991. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest 87:680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636. Karl M, Lamberts SW, Detera-Wadleigh SD, Encio IJ, Stratakis CA, Hurley DM, Accili D, Chrousos GP. 1993. Familial glucocorticoid resistance caused by a splice-site deletion in the human glucocorticoid receptor gene. J Clin Endocrinol Metab 76:683–689 [DOI] [PubMed] [Google Scholar]
- 637. McMahon SK, Pretorius CJ, Ungerer JP, Salmon NJ, Conwell LS, Pearen MA, Batch JA. 2010. Neonatal complete generalized glucocorticoid resistance and growth hormone deficiency caused by a novel homozygous mutation in helix 12 of the ligand binding domain of the glucocorticoid receptor gene (NR3C1). J Clin Endocrinol Metab 95:297–302 [DOI] [PubMed] [Google Scholar]
- 638. Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. 2001. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab 86:5600–5608 [DOI] [PubMed] [Google Scholar]
- 639. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621 [DOI] [PubMed] [Google Scholar]
- 640. Chang SS, Grunder S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. 1996. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12:248–253 [DOI] [PubMed] [Google Scholar]
- 641. Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. 1999. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med 341:156–162 [DOI] [PubMed] [Google Scholar]
- 642. Geller DS, Rodriguez-Soriano J, Vallo Boado A, Schifter S, Bayer M, Chang SS, Lifton RP. 1998. Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism. Nat Genet 19:279–281 [DOI] [PubMed] [Google Scholar]
- 643. Sartorato P, Lapeyraque AL, Armanini D, Kuhnle U, Khaldi Y, Salomon R, Abadie V, Di Battista E, Naselli A, Racine A, Bosio M, Caprio M, Poulet-Young V, Chabrolle JP, Niaudet P, De Gennes C, Lecornec MH, Poisson E, Fusco AM, Loli P, Lombès M, Zennaro MC. 2003. Different inactivating mutations of the mineralocorticoid receptor in fourteen families affected by type I pseudohypoaldosteronism. J Clin Endocrinol Metab 88:2508–2517 [DOI] [PubMed] [Google Scholar]
- 644. Geller DS, Farhi A, Pinkerton N, Fradley M, Moritz M, Spitzer A, Meinke G, Tsai FT, Sigler PB, Lifton RP. 2000. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science 289:119–123 [DOI] [PubMed] [Google Scholar]
- 645. Rodríguez-Soriano J, Vallo A, Oliveros R, Castillo G. 1983. Transient pseudohypoaldosteronism secondary to obstructive uropathy in infancy. J Pediatr 103:375–380 [DOI] [PubMed] [Google Scholar]
- 646. Chandar J, Abitbol C, Zilleruelo G, Gosalbez R, Montané B, Strauss J. 1996. Renal tubular abnormalities in infants with hydronephrosis. J Urol 155:660–663 [PubMed] [Google Scholar]
- 647. Kater CE, Biglieri EG. 1994. Disorders of steroid 17α-hydroxylase deficiency. Endocrinol Metab Clin North Am 23:341–357 [PubMed] [Google Scholar]
- 648. Auchus RJ, Rainey WE. 2004. Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60:288–296 [DOI] [PubMed] [Google Scholar]
- 649. Gupta MK, Guryev OL, Auchus RJ. 2003. 5α-Reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys 418:151–160 [DOI] [PubMed] [Google Scholar]
- 650. Milewich L, Mendonca BB, Arnhold I, Wallace AM, Donaldson MD, Wilson JD, Russell DW. 1995. Women with steroid 5α-reductase 2 deficiency have normal concentrations of plasma 5α-dihydroprogesterone during the luteal phase. J Clin Endocrinol Metab 80:3136–3139 [DOI] [PubMed] [Google Scholar]
- 651. Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T. 2006. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 91:2643–2649 [DOI] [PubMed] [Google Scholar]
- 652. Hanley NA, Arlt W. 2006. The human fetal adrenal cortex and the window of sexual differentiation. Trends Endocrinol Metab 17:391–397 [DOI] [PubMed] [Google Scholar]
- 653. Rommerts FFG, van der Molen HJ. 1971. Occurrence and localization of 5α-steroid reductase, 3α- and 17β-hydroxysteroid dehydrogenases in hypothalamus and other brain tissues of the male rat. Biochim Biophys Acta 248:489–502 [Google Scholar]
- 654. Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE. 1981. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA 78:4704–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655. Corpéchot C, Synguelakis M, Talha S, Axelson M, Sjövall J, Vihko R, Baulieu EE, Robel P. 1983. Pregnenolone and its sulfate ester in the rat brain. Brain Res 270:119–125 [DOI] [PubMed] [Google Scholar]
- 656. Mellon SH, Deschepper CF. 1993. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res 629:283–292 [DOI] [PubMed] [Google Scholar]
- 657. Compagnone NA, Mellon SH. 2000. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21:1–56 [DOI] [PubMed] [Google Scholar]
- 658. Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. 1995. Steroidogenic enzyme P450c17 is expressed in the embryonic central nervous system. Endocrinology 136:5212–5223 [DOI] [PubMed] [Google Scholar]
- 659. Mellon SH, Griffin LD. 2002. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab 13:35–43 [DOI] [PubMed] [Google Scholar]
- 660. Mellon SH. 2007. Neurosteroid regulation of central nervous system development. Pharmacol Ther 116:107–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. 1998. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450scc (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 71:2231–2238 [DOI] [PubMed] [Google Scholar]
- 662. Inoue T, Akahira J, Suzuki T, Darnel AD, Kaneko C, Takahashi K, Hatori M, Shirane R, Kumabe T, Kurokawa Y, Satomi S, Sasano H. 2002. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metab 87:5325–5331 [DOI] [PubMed] [Google Scholar]
- 663. King SR, Ginsberg SD, Ishii T, Smith RG, Parker KL, Lamb DJ. 2004. The steroidogenic acute regulatory protein is expressed in steroidogenic cells of the day-old brain. Endocrinology 145:4775–4780 [DOI] [PubMed] [Google Scholar]
- 664. Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665. Kibaly C, Patte-Mensah C, Mensah-Nyagan AG. 2005. Molecular and neurochemical evidence for the biosynthesis of dehydroepiandrosterone in the adult spinal cord. J Neurochem 93:1220–1230 [DOI] [PubMed] [Google Scholar]
- 666. Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. 1998. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA 95:1852–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Karishma KK, Herbert J. 2002. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci 16:445–453 [DOI] [PubMed] [Google Scholar]
- 668. Majewska MD, Demirgören S, Spivak CE, London ED. 1990. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res 526:143–146 [DOI] [PubMed] [Google Scholar]
- 669. Monnet FP, Mahé V, Robel P, Baulieu EE. 1995. Neurosteroids, via σ receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA 92:3774–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670. Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. 2004. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA 101:3202–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Bair SR, Mellon SH. 2004. Deletion of the mouse P450c17 gene causes embryonic lethality. Mol Cell Biol 24:5383–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Liu S, Sjövall J, Griffiths WJ. 2003. Neurosteroids in rat brain: extraction, isolation and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem 75:5835–5846 [DOI] [PubMed] [Google Scholar]
- 673. Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjövall J, Baulieu EE. 2004. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res 45:2287–2302 [DOI] [PubMed] [Google Scholar]
- 674. Shimada M, Yoshinari K, Tanabe E, Shimakawa E, Kobashi M, Nagata K, Yamazoe Y. 2001. Identification of ST2A1 as a rat brain neurosteroid sulfotransferase mRNA. Brain Res 920:222–225 [DOI] [PubMed] [Google Scholar]
- 675. Shimizu C, Fuda H, Yanai H, Strott CA. 2003. Conservation of the hydroxysteroid sulfotransferase SULT2B1 gene structure in the mouse: pre- and postnatal expression, kinetic analysis of isoforms, and comparison with prototypical SULT2A1. Endocrinology 144:1186–1193 [DOI] [PubMed] [Google Scholar]
- 676. Geese WJ, Raftogianis RB. 2001. Biochemical characterization and tissue distribution of human SULT2B1. Biochem Biophys Res Com 288:280–289 [DOI] [PubMed] [Google Scholar]
- 677. Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. 2002. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab 87:5138–5143 [DOI] [PubMed] [Google Scholar]
- 678. Flood JF, Morley JE, Roberts E. 1992. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA 89:1567–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679. Flood JF, Morley JE, Roberts E. 1995. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci USA 92:10806–10810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu EE, Simon H. 1993. Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Res 607:324–328 [DOI] [PubMed] [Google Scholar]
- 681. Guennoun R, Fiddes RJ, Gouézou M, Lombès M, Baulieu EE. 1995. A key enzyme in the biosynthesis of neurosteroids, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) is expressed in rat brain. Brain Res Mol Brain Res 30:287–300 [DOI] [PubMed] [Google Scholar]
- 682. Ibanez C, Guennoun R, Liere P, Eychenne B, Pianos A, El-Etr M, Baulieu EE, Schumacher M. 2003. Developmental expression of genes involved in neurosteroidogenesis: 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in the rat brain. Endocrinology 144:2902–2911 [DOI] [PubMed] [Google Scholar]
- 683. Ukena K, Kohchi C, Tsutsui K. 1999. Expression and activity of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in the rat Purkinje neuron during neonatal life. Endocrinology 140:805–813 [DOI] [PubMed] [Google Scholar]
- 684. Selye H. 1941. The anesthetic effects of steroid hormones. Proc Soc Exp Biol Med 46:116–121 [Google Scholar]
- 685. Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. 1995. Progesterone synthesis and myelin formation by Schwann cells. Science 268:1500–1503 [DOI] [PubMed] [Google Scholar]
- 686. Magnaghi V, Cavarretta I, Galbiati M, Martini L, Melcangi RC. 2001. Neuroactive steroids and peripheral myelin proteins. Brain Res Brain Res Rev 37:360–371 [DOI] [PubMed] [Google Scholar]
- 687. De Nicola AF, Gonzalez SL, Labombarda F, Deniselle MC, Garay L, Guennoun R, Schumacher M. 2006. Progesterone treatment of spinal cord injury: effects on receptors, neurotrophins, and myelination. J Mol Neurosci 28:3–15 [DOI] [PubMed] [Google Scholar]
- 688. Sakamoto H, Ukena K, Tsutsui K. 2001. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci 21:6221–6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Normington K, Russell DW. 1992. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase enzymes. Evidence for distinct physiological functions. J Biol Chem 267:19548–19554 [PubMed] [Google Scholar]
- 690. Russell DW, Wilson JD. 1994. Steroid 5α-reductase: two genes/two enzymes. Ann Rev Biochem 63:25–61 [DOI] [PubMed] [Google Scholar]
- 691. Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. 2001. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA 98:2849–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692. Mahendroo MS, Cala KM, Landrum DP, Russell DW. 1997. Fetal death in mice lacking 5α-reductase type 1 caused by estrogen excess. Mol Endocrinol 11:917–927 [DOI] [PubMed] [Google Scholar]
- 693. Poletti A, Negri-Cesi P, Rabuffetti M, Colciago A, Celotti F, Martini L. 1998. Transient expression of the 5α-reductase type 2 isozyme in the rat brain in late fetal and early postnatal life. Endocrinology 139:2171–2178 [DOI] [PubMed] [Google Scholar]
- 694. Poletti A, Coscarella A, Negri-Cesi P, Colciago A, Celotti F, Martini L. 1998. 5α-Reductase isozymes in the central nervous system. Steroids 63:246–251 [DOI] [PubMed] [Google Scholar]
- 695. Griffin LD, Mellon SH. 1999. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 96:13512–13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696. Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007 [DOI] [PubMed] [Google Scholar]
- 697. Gee KW. 1988. Steroid modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol Neurobiol 2:291–317 [DOI] [PubMed] [Google Scholar]
- 698. Turner DM, Ransom RW, Yang JS, Olsen RW. 1989. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther 248:960–966 [PubMed] [Google Scholar]
- 699. Zhu WJ, Vicini S. 1997. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of deactivated states. J Neurosci 17:4022–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700. Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. 1998. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–930 [DOI] [PubMed] [Google Scholar]
- 701. Griffin LD, Gong W, Verot L, Mellon SH. 2004. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med 10:704–711 [DOI] [PubMed] [Google Scholar]
- 702. Casey ML, Winkel CA, MacDonald PC. 1983. Conversion of progesterone to deoxycorticosterone in the human fetus: steroid 21-hydroxylase activity in fetal tissues. J Steroid Biochem 18:449–452 [DOI] [PubMed] [Google Scholar]
- 703. Lambert JJ, Belelli D, Hill-Venning C, Peters JA. 1995. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci 16:295–303 [DOI] [PubMed] [Google Scholar]
- 704. Kishimoto W, Hiroi T, Shiraishi M, Osada M, Imaoka S, Kominami S, Igarashi T, Funae Y. 2004. Cytochrome P450 2D catalyze steroid 21-hydroxylation in the brain. Endocrinology 145:699–705 [DOI] [PubMed] [Google Scholar]
- 705. Voutilainen R, Ilvesmäki V, Miettinen PJ. 1991. Low expression of 3β-hydroxy-5-ene steroid dehydrogenase gene in human fetal adrenals in vivo; adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. J Clin Endocrinol Metab 72:761–767 [DOI] [PubMed] [Google Scholar]
- 706. Kitada M, Kamataki T, Itahashi K, Rikihisa T, Kanakubo Y. 1987. P-450 HFLa, a form of cytochrome P-450 purified from human fetal livers, is the 16α-hydroxylase of dehydroepiandrosterone 3-sulfate. J Biol Chem 262:13534–13537 [PubMed] [Google Scholar]
- 707. Miller KK, Cai J, Ripp SL, Pierce WM, Jr, Rushmore TH, Prough RA. 2004. Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos 32:305–313 [DOI] [PubMed] [Google Scholar]
- 708. Leeder JS, Gaedigk R, Marcucci KA, Gaedigk A, Vyhlidal CA, Schindel BP, Pearce RE. 2005. Variability of CYP3A7 expression in human fetal liver. J Pharmacol Exp Ther 314:626–635 [DOI] [PubMed] [Google Scholar]
- 709. Fujieda K, Faiman C, Feyes FI, Winter JSD. 1982. The control of steroidogenesis by human fetal adrenal cells in tissue culture: IV. The effects of exposure to placental steroids. J Clin Endocrinol Metab 54:89–94 [DOI] [PubMed] [Google Scholar]
- 710. Siiteri PK, MacDonald PC. 1966. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab 26:751–761 [DOI] [PubMed] [Google Scholar]
- 711. Csapo AI, Pulkkinen MO, Wiest WG. 1973. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol 115:759–765 [DOI] [PubMed] [Google Scholar]
- 712. Pasqualini JR, Nguyen BL, Uhrich F, Wiqvist N, Diczfalusy E. 1970. Cortisol and cortisone metabolism in the human foeto-placental unit at midgestation. J Steroid Biochem 1:209–219 [Google Scholar]
- 713. Kari MA, Raivio KO, Stenman UH, Voutilainen R. 1996. Serum cortisol, dehydroepiandrosterone sulfate, and sterol-binding globulins in preterm neonates: effects of gestational age and dexamethasone therapy. Pediatr Res 40:319–324 [DOI] [PubMed] [Google Scholar]
- 714. Di Blasio AM, Voutilainen R, Jaffe RB, Miller WL. 1987. Hormonal regulation of mRNAs for P450scc (cholesterol side-chain cleavage enzyme) and P450c17 (17α-hydroxylase/17,20 lyase) in cultured human fetal adrenal cells. J Clin Endocrinol Metab 65:170–175 [DOI] [PubMed] [Google Scholar]
- 715. Durkee TJ, McLean MP, Hales DB, Payne AH, Waterman MR, Khan I, Gibori G. 1992. P450–17α and P450scc gene expression and regulation in the rat placenta. Endocrinology 130:1309–1317 [DOI] [PubMed] [Google Scholar]
- 716. Sklar CA, Kaplan SL, Grumbach MM. 1980. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51:548–556 [DOI] [PubMed] [Google Scholar]
- 717. Miller WL. 2009. Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10:3–17 [DOI] [PubMed] [Google Scholar]
- 718. Arlt W, Justl HG, Callies F, Reincke M, Hübler D, Oettel M, Ernst M, Schulte HM, Allolio B. 1998. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab 83:1928–1934 [DOI] [PubMed] [Google Scholar]
- 719. Mellon SH, Shively JE, Miller WL. 1991. Human proopiomelanocortin (79–96), a proposed androgen stimulatory hormone, does not affect steroidogenesis in cultured human fetal adrenal cells. J Clin Endocrinol Metab 72:19–22 [DOI] [PubMed] [Google Scholar]
- 720. Penhoat A, Sanchez P, Jaillard C, Langlois D, Bégeot M, Saez JM. 1991. Human proopiomelanocortin (79–96), a proposed cortical androgen stimulating hormone, does not affect steroidogenesis in cultured human adult adrenal cells. J Clin Endocrinol Metab 72:23–26 [DOI] [PubMed] [Google Scholar]
- 721. Robinson P, Bateman A, Mulay S, Spencer SJ, Jaffe RB, Solomon S, Bennett HPJ. 1991. Isolation and characterization of three forms of joining peptide from adult pituitaries: lack of adrenal androgen stimulating activity. Endocrinology 129:859–867 [DOI] [PubMed] [Google Scholar]
- 722. Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. 1978. Adrenarche: a survey of rodents, domestic animals and primates. Endocrinology 103:2112–2118 [DOI] [PubMed] [Google Scholar]
- 723. Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JSD. 1982. Further studies on adrenarche in nonhuman primates. Endocrinology 111:844–848 [DOI] [PubMed] [Google Scholar]
- 724. Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. 1998. Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab 83:3695–3701 [DOI] [PubMed] [Google Scholar]
- 725. Dardis A, Saraco N, Rivarola MA, Belgorosky A. 1999. Decrease in the expression of the 3β-hydroxysteroid dehydrogenase gene in human adrenal tissue during prepuberty and early puberty: implications for the mechanism of adrenarche. Pediatr Res 45:384–388 [DOI] [PubMed] [Google Scholar]
- 726. Yanase T, Sasano H, Yubisui T, Sakai Y, Takayanagi R, Nawata H. 1998. Immunohistochemical study of cytochrome b5 in human adrenal gland and in adrenocortical adenomas from patients with Cushing’s syndrome. Endocr J 45:89–95 [DOI] [PubMed] [Google Scholar]
- 727. Miller WL. 1999. The molecular basis of premature adrenarche: an hypothesis. Acta Paediatr Suppl 88(Suppl 433):60–66 [DOI] [PubMed] [Google Scholar]
- 728. Tee MK, Dong Q, Miller WL. 2008. Pathways leading to the phosphorylation of P450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology 149:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729. Ibañez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinyé M, Carrascosa A, Vicens-Calvet E. 1993. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab 76:1599–1603 [DOI] [PubMed] [Google Scholar]
- 730. Oppenheimer E, Linder B, DiMartino-Nardi J. 1995. Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab 80:614–618 [DOI] [PubMed] [Google Scholar]
- 731. Ibanez L, Potau N, Zampolli M, Prat N, Virdis R, Vicens-Calvet E, Carrascosa A. 1996. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. J Clin Endocrinol Metab 81:1237–1243 [DOI] [PubMed] [Google Scholar]
- 732. Ibáñez L, Potau N, Marcos MV, de Zegher F. 1999. Exaggerated adrenarche and hyperinsulinism in adolescent girls born small for gestational age. J Clin Endocrinol Metab 84:4739–4741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.