Abstract
Several lines of recent evidence implicate regulatory roles for reactive oxygen species (ROS) in islet function and insulin secretion. The phagocyte-like NADPH oxidase (Nox2) has recently been shown to be one of the sources of ROS in the signaling events leading to glucose stimulated insulin secretion (GSIS). We recently reported inhibition of glucose- or mitochondrial fuel-induced Nox2-derived ROS by a specific inhibitor of protein farnesyl transferse (FTase; FTI-277), suggesting that activation of FTase might represent one of the upstream signaling events to Nox2 activation. Furthermore, FTase inhibitors (FTI-277 and FTI-2628) have also been shown to attenuate GSIS in INS 832/13 cells and normal rodent islets. Herein, we provide further evidence to suggest that inhibition of FTase either by pharmacological (e.g., FTI-277) or gene silencing (siRNA-FTase) approaches markedly attenuates mitochondrial fuel-stimulated insulin secretion (MSIS) in INS 832/13 cells. Together, our findings further establish a link between nutrient-induced Nox2 activation, ROS generation and insulin secretion in the pancreatic β-cell.
Keywords: insulin secretion, mitochondrial fuels, pancreatic β-cells, protein farnesylation
Introduction
Glucose-stimulated insulin secretion (GSIS) from the pancreatic β-cell involves the generation and/or altered distribution of diffusible second messengers such as calcium, cAMP, and lipid hydrolytic products of various phospholipases.1-3 It is noteworthy that a selective increase in intracellular calcium not only initiates insulin secretion but also regulates various enzymes such as protein kinases, phosphodiesterases, adenylyl cyclases, and phospholipases, thereby facilitating insulin secretion. In the context of protein kinases, in addition to calcium-dependent protein kinase(s), several other kinases, including calmodulin-, cyclic nucleotide- and phospholipid-dependent protein kinases, tyrosine kinases and mitogen-activated protein kinases have been described in β-cells.4
In addition to glucose, mitochondrial fuels (e.g., succinate and α-keto-isocaproic acid) also promote insulin secretion in pancreatic clonal β-cells and normal rodent islets.2,5,6 Using Clostridial toxins, which selectively monoglucosylate and inactivate small G-proteins,7 we have provided the first evidence for a requirement of small G-proteins in mitofuel-induced insulin secretion.8 However, potential requirement(s) of post-translational lipidation (e.g., farnesylation) for the G-protein-mediated MSIS remains largely unknown. In the context of G-protein-facilitated and GSIS or MSIS in the islet β-cell, we have recently identified the phagocyte-like NADPH-oxidase (Nox2) as an effector protein for specific G-proteins in isolated β-cell.9 Our data suggested that these secretagogues induce a transient increase in reactive oxygen species (ROS) derived from Nox2 activation, and that inhibition of protein prenylation using GGTI-2147 or FTI-277, selective inhibitors of geranyl geranyl transferases (GGTases) and FTases, respectively, attenuated Nox2-mediated ROS generation in clonal INS 832/13 cells and normal rodent islets.9 Together, these findings further strengthen the proposal of novel “second messenger roles” for ROS in GSIS as proposed originally by Pi and Collins.10 In addition, we have been able to demonstrate that a mixture of mitochondrial fuels [i.e., mono methyl succinate (MMS) and α-keto isocaproate (KIC)] also increased ROS generation in INS 832/13 cells acutely, and that such an increase in ROS was reduced significantly by inhibitors of protein prenylation,9 thus raising an interesting possibility that mitochondrial metabolism leads to Nox2 activation and ROS generation in a protein prenylation-dependent manner. The current study, which is an addendum to the above studies9 further examined if MSIS involves activation of a signaling step involving protein farnesylation.
Results and Discussion
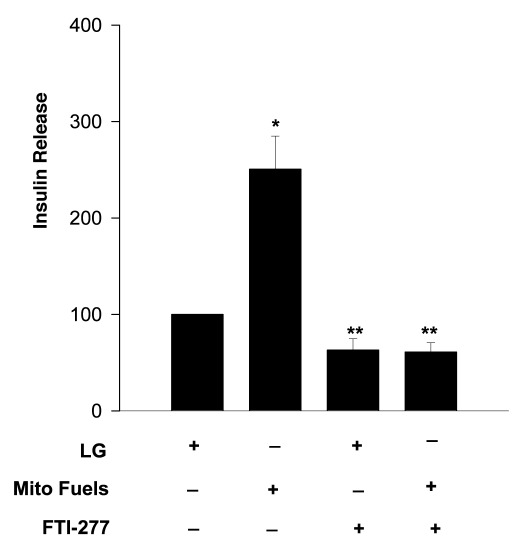
We addressed this question via pharmacological, with the use of a selective inhibitor of FTase (e.g., FTI-277) or siRNA-mediated knockdown of β-subunit of FTase. First, we determined the effects of FTI-277 on MSIS in INS 832/13 cells. Data in Figure 1 indicate nearly 2.5-fold stimulation in insulin secretion in these cells following exposure to a mixture of mitochondrial fuels (bar 1 vs. bar 2). Incubation of these cells with FTI-277 did not inhibit basal insulin secretion significantly (bar 1 vs. bar 3). However, MSIS was markedly attenuated in cells exposed to FTI-277 (bar 2 vs. bar 4) suggesting that an FTase activation step is necessary for MSIS to occur.
Figure 1. FTI-277, a selective FTase inhibitor, attenuates mitochondrial fuel- insulin secretion in INS 832/13 cells. INS 832/13 cells were cultured overnight in low serum- glucose medium containing FTI-277 (5 µM; Calbiochem) or diluent. Cells were incubated in KRB for 1h and then stimulated with either glucose (LG, 2.5 mM) or a mixture of monomethyl succinate (MMS, 15 mM) plus α-keto isocaproic acid (KIC, 5 mM) for 45 min in continuous presence or absence of FTI-277. Insulin released into the medium was quantitated by ELISA. Data are expressed as percent of control, and are mean ± SEM from four to five experiments. *p < 0.05 vs. 2.5 mM glucose and **not significant from 2.5 mM glucose.
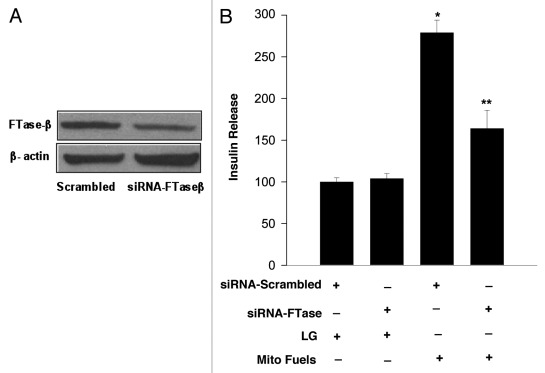
Although FTase and GGTase-I share the same regulatory α-subunit, they are often distinguished by their distinct β-subunits, which dictate their substrate specificity and catalytic function.11–13 To further confirm our pharmacological observations (Fig. 1), we undertook an alternate approach in which we quantitated MSIS in INS 832/13 cells following selective depletion of FTase β-subunit by siRNA methodology.13 Data in Figure 2A demonstrate that the expression of FTase β-subunit was markedly reduced (~65%) under these conditions. Furthermore, as shown in Figure 2B, mitochondrial fuels significantly increased insulin release in cells transfected with scrambled siRNA (bar 1 vs. bar 3). However, MSIS was significantly impaired in FTase β-subunit depleted INS 832/13 cells (bar 3 vs. bar 4). Together, data described above support our overall hypothesis that a farnesylation-dependent signaling step (e.g., Nox2-mediated ROS generation) underlies mitofuel-induced insulin secretion in INS 832/13 cells.
Figure 2. siRNA mediated knockdown of FTase β-subunit attenuates mitochondrial fuel -induced insulin secretion. INS 832/13 cells were cultured in 24-well plates to sub confluence a day before transfection. Cells were transfected using HiPerfect transfection reagent with siRNA duplexes (100 nmol/l) that target FTase β- subunit mRNA (a pool of two target specific 21 nucleotide length (5′→3′ UCACGUFACUUC UACCAUAtt in sense strand and UAUGGUAGAAG-UCACGUGAct in the antisense strand), designed to knock-down gene expression of FTase-β subunit. To assess the specificity of knockdown, a second batch of cells were transfected with non-targeting scrambled siRNA (negative control) that includes at least four nucleotide mismatches with known mouse, rat and human gene duplexes which do not lead to specific degradation of any known cellular mRNA. Srambled and FTase β-siRNAs were from Ambion. HiPerfect transfection reagent was from Qiagen. Additional experimental details are described in.13 Efficiency of FTase β-subunit knockdown was determined by western blot analysis. A representative gel is shown in (A). In (B): Transfected cells were incubated in KRB (pH 7.4) for 1h prior to stimulation either with low glucose (LG, 2.5 mM) or a mixture of mito fuels [MMS (15 mM) plus KIC (5 mM)] for 45 min. Insulin released into the media was quantitated by ELISA. Data are expressed as percent of control and are mean ± SEM from three experiments. *p < 0.05 vs. scrambled low vs. high glucose, **p < 0.05 for siRNA FTase β-subunit low vs. high glucose.
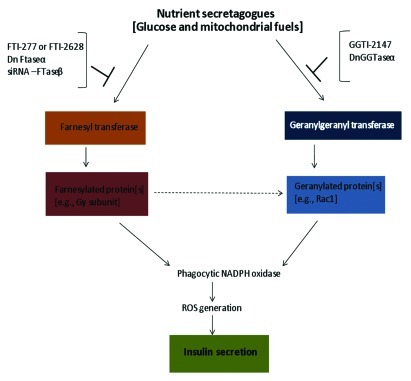
In summary, our current findings described in this brief report, involving site-specific inhibitors of FTase (FTI-277) and siRNA-FTase β-subunit, provide evidence for the requirement of a farnesylation step in MSIS in pancreatic β-cells. Moreover, the current set of observations in addition to our recently published experimental work,9 establish a potential link between activation of a farnesylated protein in the signaling events leading to Nox2 activation, ROS generation and insulin secretion. These studies are a logical extension to our recently published studies to demonstrate that glucose or mitochondrial fuels, but not membrane depolarizing concentrations of KCl, significantly increase ROS generation, and that pharmacological or molecular biological inhibition of Nox2 markedly attenuated ROS generation under these conditions.9 Furthermore, inhibition of protein prenylation reduced ROS generation elicited by glucose or mitochondrial fuels, thus implicating a role for protein prenylation in this signaling step. Together, these observations suggest novel roles for a yet unidentified farnesylated protein in mediating Nox2 activation and ROS generation steps leading to insulin secretion. Potential candidates might include the γ-subunits of trimeric G-proteins as we proposed recently in9 even though this postulation remains to be verified further. Based on our previously published work and the current observations, we propose a model for glucose- or mitochondrial fuel-mediated, protein prenylation-dependent signaling steps leading to Nox2 activation, ROS generation and insulin secretion in the pancreatic β-cell (Fig. 3). Lastly, our findings further corroborate recent findings by Leloup and associates14 to suggest that mitochondrial ROS generation is a requisite for GSIS to occur. Precise understanding of the identity and roles of various forms of Nox (i.e., mitochondrial and extramitochondrial) is necessary to further assess the roles of protein prenylation in coupling extramitochondrial to mitochondrial events leading to insulin secretion.
Figure 3. Schematic representation of potential mechanisms underlying nutrient-mediated, protein prenylation-dependent signaling steps leading to Nox2 activation, ROS generation and insulin secretion in the pancreatic β-cell. Exposure of isolated β-cells to glucose or mitochondrial fuels leads to activation of FTase and GGTase-I,15 which is necessary for the activation and interaction of specific farnesylated and geranylated proteins with their effector proteins (e.g., p21-activated kinase, ERK1/2). GGTI-2147 or a dominant negative mutant of GGTase-1 markedly attenuated glucose-induced Rac1 activation and insulin secretion.12,13 FTI-277 or FTI-2368 or siRNA-FTase-β also attenuated GSIS from isolated β-cells.13 Together, these findings further substantiated our original hypothesis that protein prenylation is necessary for GSIS to occur.12 More recent studies have identified Nox2 as one of the effector proteins in the cascade of events leading to G-protein-mediated GSIS.9 FTI-277 or GGTI-2147 significantly reduced glucose- or mitochondrial fuel-induced ROS generation thereby implicating Nox2 activation as one of the signaling steps involved in nutrient-induced Nox2 activation, ROS generation and insulin secretion.9 Data described in this communication further affirms the postulation that MSIS is also under the control of a farnesylated protein. Potential identity of this farnesylated protein(s), which is involved in glucose- and mitochondrial fuel-induced Nox2 activation, ROS generation and insulin secretion remains to be verified in future investigations.
Acknowledgments
This research is supported by grants from the National Institutes of Health (RO1 DK74921) and Department of Veterans Affairs. A.K. is also the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs. A.M. is supported by a Post-Doctoral Fellowship from Wayne State University. The authors thank Dr Vasudeva Kamath and Ms Sudha Govind for assistance in these studies.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/19121
References
- 1.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67:1185–248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald MJ. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990;39:1461–6. doi: 10.2337/diabetes.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 3.Poitout V. Phospholipid hydrolysis and insulin secretion: A step toward solving the Rubik’s cube. Am J Physiol Endocrinol Metab. 2008;294:E214–6. doi: 10.1152/ajpendo.00638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones PM, Persaud SJ. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic beta-cells. Endocr Rev. 1998;19:429–61. doi: 10.1210/er.19.4.429. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald MJ, Longacre MJ, Stoker SW, Brown LJ, Hasan NM, Kendrick MA. Acetoacetate and beta-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am J Physiol Cell Physiol. 2008;294:C442–50. doi: 10.1152/ajpcell.00368.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru A, Li G, Rabaglia ME, Segu VB, Hofmann F, Aktories K, et al. Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose- and calcium-induced insulin secretion from pancreatic beta cells. Biochem Pharmacol. 1997;54:1097–108. doi: 10.1016/S0006-2952(97)00314-6. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru A, Chen HQ, Tannous M. Novel roles for the rho subfamily of GTP-binding proteins in succinate-induced insulin secretion from betaTC3 cells: further evidence in support of the succinate mechanism of insulin release. Endocr Res. 2003;29:363–76. doi: 10.1081/ERC-120025043. [DOI] [PubMed] [Google Scholar]
- 9.Syed I, Kyathanahalli CN, Kowluru A. Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: role of protein prenylation. Am J Physiol Regul Integr Comp Physiol. 2011;300:R756–62. doi: 10.1152/ajpregu.00786.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010;12(Suppl 2):141–8. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 11.Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL. Protein farnesyl transferase and geranyl geranyltransferase share a common alpha subunit. Cell. 1991;65:429–34. doi: 10.1016/0092-8674(91)90460-G. [DOI] [PubMed] [Google Scholar]
- 12.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowluru A, Veluthakal R, Rhodes CJ, Kamath V, Syed I, Koch BJ. Protein farnesylation-dependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells. Diabetes. 2010;59:967–77. doi: 10.2337/db09-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–81. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goalstone M, Kamath V, Kowluru A. Glucose activates prenyltransferases in pancreatic islet beta-cells. Biochem Biophys Res Commun. 2010;391:895–8. doi: 10.1016/j.bbrc.2009.11.159. [DOI] [PMC free article] [PubMed] [Google Scholar]