Abstract
Heterotrimeric G protein is composed of a Gα-subunit and a Gβγ-dimer. Previous studies have revealed that Gβγ-dimers including the Gγ2 subunit (Gng2/GNG2) are associated with cell proliferation, differentiation, invasion and angiogenesis. At present, however, there is no information on the expression level of Gng2/GNG2 alone in any kind of tumor. In this study, we performed DNA microarray analysis in a benign melanocytic tumor and a malignant melanoma from RET-transgenic mice (RET-mice). Gng2 transcript expression levels in a malignant melanoma were less than 1/10 of the level in a benign tumor. The difference in Gng2 transcript expression levels between benign tumors and malignant melanomas was greatest among all of the G protein γ subunits examined in this study. Moreover, protein expression levels of Gng2 were decreased in malignant melanomas compared with those in benign melanocytic tumors in RET-mice. Analysis of human malignant melanomas also showed reduced GNG2 protein expression levels in five human malignant melanoma cell lines compared with the expression levels in normal human epithelial melanocytes (NHEM). Thus, we demonstrated for the first time that Gng2/GNG2 expression levels are reduced in malignant melanoma, suggesting that GNG2 could be a novel biomarker for malignant melanoma.
Keywords: G-protein, gamma subunit, malignant melanoma
Introduction
The incidence of cutaneous malignant melanoma is increasing at a greater rate than that of any other cancer [1]. Since malignant melanoma is the most serious skin cancer, malignant melanoma is a threat for human life. However, an effective therapy for malignant melanoma has not yet been fully established [2]. Therefore, identification of novel molecules associated with malignant melanoma formation is important for pathological analysis and therapy of malignant melanoma. We previously established oncogenic RET (RFP-RET)-transgenic mice (RET-mice) of line 304/B6 that stepwisely develop cutaneous benign tumors and malignant melanoma [3,4]. Since both benign and malignant tumors often develop simultaneously in an individual RET-mouse, this transgenic mouse line could be a strong tool for DNA microarray analysis without a difference between individuals.
Heterotrimeric G protein, which is composed of a Gα-subunit and a Gβγ-dimer, has been reported to be involved in various biological activities [5]. Gγ2 (Gng2/GNG2) is one of the subunits of the Gβγ-dimer. A previous study showed that Gng2 alone is required for angiogenesis through Vegf signaling in zebrafish [6], suggesting a potential correlation between Gng2 and tumorigenesis. To our knowledge, however, there is no information on the expression level of Gng2/GNG2 alone in any kind of tumor.
In this study, we performed DNA microarray analysis in a benign melanocytic tumor and a malignant melanoma that developed in a RET-mouse of line 304/B6. We then focused on Gng2 and analyzed its expression levels in benign melanocytic tumors and malignant melanomas in mice and humans.
Materials and methods
Mice
Previously established RFP-RET-transgenic mice (RET-mice) of line 304/B6 [7-9] were used. The Animal Care and Use Committee (approval no. 18001 and 2210038) and Recombination DNA Advisory Committee (approval no. 10-08) in Chubu University approved this study.
Cell lines and culture conditions
Normal human epithelial melanocytes (NHEM) were purchased from Cell Applications Inc. and were maintained in melanocyte growth medium containing hydrocortisone and growth supplements. SK-Mel28, MNT-1 [10], G361, HM3KO [11], A375P and A475M human malignant melanoma cells were cultured in RPMI1640 supplemented with 10% fetal bovine serum.
Real-time PCR analysis
Real-time PCR was performed by the method previously described [12]. Primer pairs for realtime PCR were as follows: 5’- GAA GCC AAC ATC GAC AGG AT -3’ and 5’- GTT TTC TGA GGC TGG GAC TG -3’ for Gng2 and 5’- CTT TGC TGA CCT GCT GGA TT -3’ and 5’- TAT GTC CCC CGT TGA CTG AT -3’ for Hprt.
Immunoblot and immunohistochemical analyses
Immunoblot analysis was performed by the method previously described [13]. Rabbit polyclonal antibodies against Gng2 (Proteintech Group) and mouse monoclonal antibodies against α-TUBULIN (Sigma) were used as first antibodies. Immunohistochemistry was performed by the method previously described [14] with anti-Gng2 antibody (Proteintech Group). Densitometric evaluation was performed using the software program WinROOF (MITANI Corporation) as previously reported [15].
Statistical analysis
Statistical analysis in this study was performed according to the method previously described [8]. Results from more than three independent samples (Figure 1A) and experiments (Figure 1B and Figure 2) in each group were statistically analyzed by Student's t-test. We used the SPSS (version 18) software package (SPSS Japan Inc.) for these statistical analyses, and the significance level was set at p < 0.05.
Figure 1.
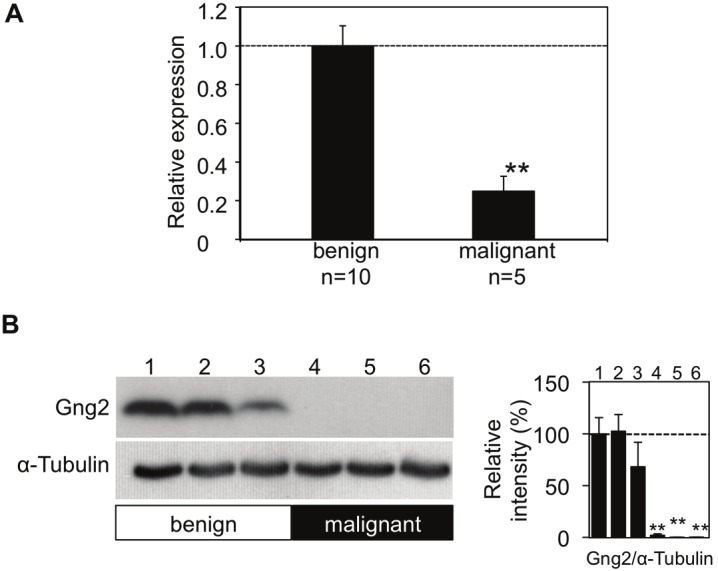
Decreased levels of Gng2 transcription and protein expression in murine malignant melanomas. (A) Transcript expression levels of Gng2 (mean ± SE) in benign melanocytic tumors (n=10) and malignant melanomas (n=5) from RET-mice of line 304/B6 evaluated by real-time PCR are presented. RNA extraction from and RT-PCR for each tumor were independently performed. (B) Protein expression levels of Gng2 in benign tumors (lanes 1-3) and malignant melanomas (lanes 4-6) from RET-mice of line 304/B6 evaluated by immunoblot analyses are presented. Amounts of α-Tubulin as an internal control are also shown. Intensities of bands are presented as percentages (mean ± SD; n=3) relative to the benign tumor (lane 1 in B). **Significantly different (p<0.01) from the benign tumors by Student's t-test.
Figure 2.
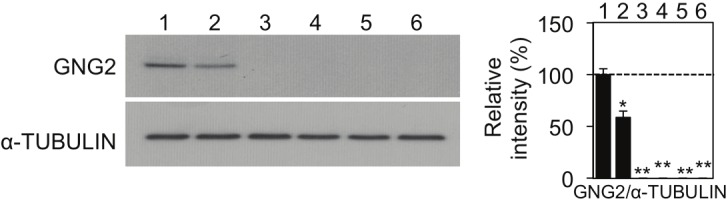
GNG2 expression levels in human melanocytes and malignant melanoma cells. GNG2 protein expressionlevels in normal human epithelial melanocytes (NHEM, lane 1) and A375P (lane 2), SK-Mel28 (lane 3), MNT-1 (lane 4), G361 (lane 5) and HM3KO (lane 6) malignant melanoma cells evaluated by immunoblot analysis are presented.Amounts of α-TUBULIN as an internal control are also shown. Intensities of bands are presented as percentages(mean ± SD; n=3) relative to NHEM (lane 1). * and ** Significantly different (* p< 0.05; ** p<0.01) by Student's t-test.
Results
DNA microarray analysis of tumors from RET-mice of line 304/B6.
We first performed DNA microarray analysis of a benign melanocytic tumor and a malignant melanoma that simultaneously developed in an individual RET-mouse to identify novel candidate molecules associated with malignant melanoma development and progression. Expression levels of CyclinD1, Melanoma cell adhesion molecule (MCAM), Matrix metalloproteinase 2 (MMP-2) and B-cell leukemia/lymphoma 2 (Bcl2) were increased in malignant melanomas from the RET-mice of line 304/B6, whereas expression levels of Transformation related protein 53 (p53), Phosphatase and tensin homolog (PTEN) and MAD homolog 7 (Smad7) were decreased (Table 1). The increases and decreases in these molecules in benign melanocytic tumors versus malignant melanomas from RET-mice of line 304/B6 correspond to previously reported increases and decreases in malignant melanoma-related molecules in humans [16-23]. Therefore, we considered the results of our DNA microarray analysis using tumors from RET-mice of line 304/B6 to be reliable.
Table 1.
A list of gene expression levels in a benign tumor and a malignant melanoma developed in a RET-mouse.
| Gene | Melanocytic tumors in a RET-mouse | ||
|---|---|---|---|
| Expression values in Benign | Expression values in Malignant | Benign/Malignant Ratio | |
| CyclinD1 | 911.5 | 30871.8 | 33.869 |
| Melanoma cell adhesion molecule(MCAM) | 441.6 | 6263.8 | 14.184 |
| Matrix metalloproteinase 2(MMP-2) | 394.3 | 4261.2 | 10.807 |
| B-cell leukemia/lymphoma 2(Bcl2) | 710.4 | 1801.1 | 2.535 |
| Transformation related protein 53(p53) | 523.9 | 209.8 | 0.400 |
| Phosphatase and tensin homolog(PTEN) | 1424.5 | 292.8 | 0.206 |
| MAD homolog 7(Smad7) | 323.9 | 40.1 | 0.124 |
| G protein gamma 2 subunit(Gng2) | 364.7 | 33.3 | 0.091 |
| G protein gamma 3 subunit(Gng3) | 133.5 | 194.9 | 1.460 |
| G protein gamma 4 subunit(Gng4) | 56.4 | 271.5 | 4.814 |
| G protein gamma 5 subunit(Gng5) | 12429.9 | 15896.5 | 1.279 |
| G protein gamma 7 subunit(Gng7) | 710.9 | 666.6 | 0.938 |
| G protein gamma 8 subunit(Gng8) | 98.5 | 216.9 | 2.202 |
| G protein gamma 10 subunit(Gng10) | 1399.2 | 736.3 | 0.526 |
| G protein gamma 11 subunit(Gng11) | 2258.5 | 10041.7 | 4.446 |
Six melanoma-related genes and nine G protein gamma subunit family genes are shown as a result of DNA microarray analysis.
Gng2 transcript expression level in the malignant melanoma was less than 1/10 of that in the benign tumor in a RET-mouse of line 304/B6 (Table 1). The difference in Gng2 expression level between the benign tumor and malignant melanoma was greatest among all of the G protein γ subunits examined in this study (Table 1). Expression levels of other subunits were also different in the malignant melanoma and benign tumor. The expression levels of Gng4 and 11 in the malignant melanoma were more than 4-fold higher than those in the benign tumor (Table 1). Based on the results, we focused on the expression levels of Gng2 in malignant melanomas.
Expression level of Gng2 in tumors from RET-mice of line 304/B6.
In addition to the results of DNA microarray analysis in tumors from an individual RET-mouse of line 304/B6, we performed real-time PCR (Figure 1A), immunoblot analysis (Figure 1B) and immunohistochemical analysis (Figure 3) for multiple tumors from the mice to clarify the expression levels of Gng2 transcripts and proteins. Real-time PCR analysis of tumors from RET-mice of line 304/B6 revealed that Gng2 transcript levels in malignant melanomas were less than 1/3 of those in benign tumors (Figure 1A). Immunoblot and immunohistochemical analyses also showed that Gng2 protein expression levels in malignant melanomas were dramatically decreased compared with those in benign melanocytic tumors from RET-mice of line 304/B6 (Figure 1B and Figure 3).
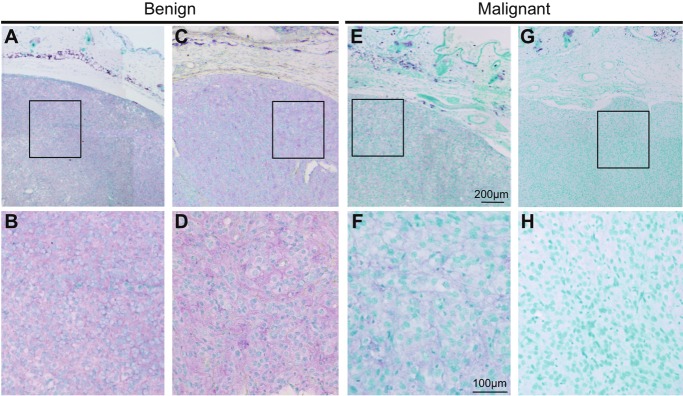
Figure 3.
Immunohistochemical analysis of Gng2 protein expression in murine tumors. Results of immunohistochemistry for Gng2 protein in benign tumors (A-D) and malignant melanomas (E-H) from RET-mice of line 304/B6 are presented. Bottom panels are shown as higher magnification of top panels. Staining of Gng2 protein and the nucleus are presented as purple and green, respectively.
Expression levels of GNG2 protein in human malignant melanoma cell lines.
Based on the results obtained for murine melanocytic tumors, we examined GNG2 protein expression levels in 5 human malignant melanoma cell lines (A375P, SK-Mel28, MNT-1, G361 and HM3KO) and in normal human epithelial melanocytes (NHEM) (Figure 2). GNG2 protein expression levels in 4 of the 5 human malignant melanoma cell lines (SK-Mel28, MNT-1, G361 and HM3KO) were undetectably low (lanes 3-6 in Figure 3). GNG2 protein expression level in A375P cells was significantly lower than that in NHEM (lanes 1 and 2 in Figure 3). These results suggest that GNG2 expression is reduced in human melanoma cell lines compared with that in normal epithelial melanocytes.
Discussion
There has been no report showing levels of GNG2 alone in malignant melanomas in mice and humans. In this study, we first demonstrated that the expression levels of Gng2 were reduced in malignant melanomas compared with those in benign melanocytic tumors from RET-mice of line 304/B6. We then showed that GNG2 expression levels were also reduced in all 5 human melanoma cell lines compared with the level in NHEM.
G proteins are heterotrimeric proteins consisting of α, β and γ subunits. At least 16 α genes, 5 β genes and 12 γ genes have so far been identified in the human genome [24-29]. These genes have been reported to be major transducers from extracellular signaling to intracellular signaling associated with cell proliferation, differentiation, invasion and angiogenesis [5]. Overexpression of the Gβγ-dimer promotes cell proliferation and invasion with activation of c-SRC, FAK and PI3 kinase/AKT molecules [5,6,30,31]. In contrast, reduction of the Gβγ-dimer has been reported to suppress cell invasion [32]. Thus, previous studies showed that Gβγ-dimers have cancer-promoting effects. On the other hand, a previous study showed that level of G protein γ 7 subunit (GNG7) expression was decreased in human esophageal cancer and cell lines [33]. Moreover, suppressed cell growth and tumorigenesis in vitro and in vivo with modulation of p27kip1 expression was observed in GNG7-overexpressed KYSE150 human esophageal carcinoma cells without intrinsic expression of GNG7 [34]. Since both cancerpromoting and -suppressing effects have been reported for Gβγ-dimers and G protein γ subunit alone, the biological significance of the reduced GNG2 expression level in malignant melanoma is unknown. Further study is needed to clarify the biological function of GNG2 for malignant melanoma.
In summary, we found reduced expression levels of Gng2/GNG2 in spontaneously developed murine malignant melanomas and human melanoma cell lines. Our results suggest that GNG2 could be a novel biomarker for malignant melanoma.
Acknowledgments
We thank Kazumi Shigeta, Yumiko Takahashi, Taeko Kawai and Yoko Kato for technical assistance. HM3KO and MNT-1 cells were kindly provided by Dr. Masato Ueda (Ueda Clinic Dermatology, Hyogo, Japan) and Dr. Vincent J. Hearing (National Cancer Institute, Bethesda, USA), respectively. G361 and SK-Mel28 cells were kindly provided by the Cell Resource Center for Biomedical Research, Tohoku University and the Riken Bioresource Center Cell Bank, respectively. This study was supported in part by Grants-in-Aid for Scientific Research (B) (No. 20406003), Grant-in-Aid for Exploratory Research (No. 23650241) and Grant-in-Aid for Young Scientists (B) (No. 22791041 and 22791092) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), COE Project (Health Science Hills) for Private Universities form MEXT and Chubu University (No. S0801055), AA Science Platform Program from the Japan Society for the Promotion of Science (JSPS), Mitsui & Co., Ltd. Environment Fund (No. R08-C097), Research Grant from the Tokyo Biochemical Research Foundation (TBRF), the Naito Foundation Natural Science Scholarship, AEON Environmental foundation, Research Foundation from the Institute of Science and Technology Research in Chubu University and Chubu University grant CG.
References
- 1.Hussein MR. Melanocytic dysplastic naevi occupy the middle ground between benign melanocytic naevi and cutaneous malignant melanomas: emerging clues. J Clin Pathol. 2005;58:453–456. doi: 10.1136/jcp.2004.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Liu W, Akhand AA, Dai Y, Ohbayashi M, Tuzuki T, Suzuki H, Isobe K, Takahashi M, Nakashima I. Linkage between melanocytic tumor development and early burst of Ret protein expression for tolerance induction in metallothionein-I/ret transgenic mouse lines. Oncogene. 1999;18:837–842. doi: 10.1038/sj.onc.1202329. [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Hossain K, Tamakoshi A, Kunisada T, Kambayashi Y, Ogino K, Suzuki H, Takahashi M, Nakashima I. c-Kit-targeting immunotherapy for hereditary melanoma in a mouse model. Cancer Res. 2004;64:801–806. doi: 10.1158/0008-5472.can-03-2532. [DOI] [PubMed] [Google Scholar]
- 5.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- 6.Leung T, Chen H, Stauffer AM, Giger KE, Sinha S, Horstick EJ, Humbert JE, Hansen CA, Robishaw JD. Zebrafish G protein gamma2 is required for VEGF signaling during angiogenesis. Blood. 2006;108:160–166. doi: 10.1182/blood-2005-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto T, Takahashi M, Ito M, Hamatani K, Ohbayashi M, Wajjwalku W, Isobe K, Nakashima I. Aberrant melanogenesis and melanocytic tumour development in transgenic mice that carry a metallothionein/ret fusion gene. EMBO J. 1991;10:3167–3175. doi: 10.1002/j.1460-2075.1991.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato M, Hattori T, Ito H, Kageyama M, Yamashita T, Nitta Y, Nakashima I. Serum-soluble Fas levels as a marker to distinguish allergic and nonallergic rhinitis. J Allergy Clin Immunol. 1999;103:1213–1214. doi: 10.1016/s0091-6749(99)70202-2. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Iwashita T, Akhand AA, Liu W, Takeda K, Takeuchi K, Yoshihara M, Hossain K, Wu J, Du J, Oh C, Kawamoto Y, Suzuki H, Takahashi M, Nakashima I. Molecular mechanism of activation and superactivation of Ret tyrosine kinases by ultraviolet light irradiation. Antioxid Redox Signal. 2000;2:841–849. doi: 10.1089/ars.2000.2.4-841. [DOI] [PubMed] [Google Scholar]
- 10.Kushimoto T, Basrur V, Valencia J, Matsunaga J, Vieira WD, Ferrans VJ, Muller J, Appella E, Hearing VJ. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc Natl Acad Sci USA. 2001;98:10698–10703. doi: 10.1073/pnas.191184798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi A, Funasaka Y, Ueda M, Ichihashi M. c-KIT receptor expression in cutaneous malignant melanoma and benign melanotic naevi. Melanoma Res. 1996;6:25–30. doi: 10.1097/00008390-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ohshima Y, Yajima I, Takeda K, Iida M, Kumasaka M, Matsumoto Y, Kato M. c-RET molecule in malignant melanoma from oncogenic RET-carrying transgenic mice and human cell lines. PLoS One. 2010;5:e10279. doi: 10.1371/journal.pone.0010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato M, Wickner W. Vam10p defines a Sec18p-independent step of priming that allows yeast vacuole tethering. Proc Natl Acad Sci USA. 2003;100:6398–6403. doi: 10.1073/pnas.1132162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumasaka MY, Yajima I, Hossain K, Iida M, Tsuzuki T, Ohno T, Takahashi M, Yanagisawa M, Kato M. A novel mouse model for de novo Melanoma. Cancer Res. 2010;70:24–29. doi: 10.1158/0008-5472.CAN-09-2838. [DOI] [PubMed] [Google Scholar]
- 15.Ohgami N, Ida-Eto M, Shimotake T, Sakashita N, Sone M, Nakashima T, Tabuchi K, Hoshino T, Shimada A, Tsuzuki T, Yamamoto M, Sobue G, Jijiwa M, Asai N, Hara A, Takahashi M, Kato M. c-Ret-mediated hearing loss in mice with Hirschsprung disease. Proc Natl Acad Sci USA. 2010;107:13051–13056. doi: 10.1073/pnas.1004520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 17.Cerroni L, Soyer HP, Kerl H. bcl-2 protein expression in cutaneous malignant melanoma and benign melanocytic nevi. Am J Dermatopathol. 1995;17:7–11. doi: 10.1097/00000372-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- 19.Javelaud D, Delmas V, Moller M, Sextius P, Andre J, Menashi S, Larue L, Mauviel A. Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene. 2005;24:7624–7629. doi: 10.1038/sj.onc.1208900. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JP, Rothbacher U, Sers C. The progression associated antigen MUC18: a unique member of the immunoglobulin supergene family. Melanoma Res. 1993;3:337–340. doi: 10.1097/00008390-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 22.Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, Pistritto G, Nesbit M, Pinkel D, Herlyn M, Bastian BC. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–3206. [PubMed] [Google Scholar]
- 23.Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397–3402. doi: 10.1038/sj.onc.1201881. [DOI] [PubMed] [Google Scholar]
- 24.Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 25.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 26.Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000;7:111–120. doi: 10.1093/dnares/7.2.111. [DOI] [PubMed] [Google Scholar]
- 27.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 28.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 29.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 30.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 31.Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 32.Faivre S, Regnauld K, Bruyneel E, Nguyen QD, Mareel M, Emami S, Gespach C. Suppression of cellular invasion by activated G-protein subunits Galphao, Galphai1, Galphai2, and Galphai3 and sequestration of Gbetagamma. Mol Pharmacol. 2001;60:363–372. doi: 10.1124/mol.60.2.363. [DOI] [PubMed] [Google Scholar]
- 33.Shibata K, Mori M, Tanaka S, Kitano S, Akiyoshi T. Identification and cloning of human G-protein gamma 7, down-regulated in pancreatic cancer. Biochem Biophys Res Commun. 1998;246:205–209. doi: 10.1006/bbrc.1998.8581. [DOI] [PubMed] [Google Scholar]
- 34.Shibata K, Tanaka S, Shiraishi T, Kitano S, Mori M. G-protein gamma 7 is down-regulated in cancers and associated with p 27kip1-induced growth arrest. Cancer Res. 1999;59:1096–1101. [PubMed] [Google Scholar]