Abstract
Cancer stem cells (CSCs) have been defined as cells within tumor that possess the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor. They have been identified in blood, breast, brain, colon, melanoma, pancreatic, prostate, ovarian, lung cancers and so on. It is often considered to be associated with chemo-resistance and radio-resistance that lead to the failure of traditional therapies. Most therapies are directed at the fast growing tumor mass but not the slow dividing cancer stem cells. Eradicating cancer stem cells, the root of cancer origin and recurrence, has been thought as a promising approach to improve cancer survival or even to cure cancer patients. Understanding the characteristics of cancer stem cells will help to develop novel therapies to eliminate the initiating cancer stem cell, and the relevant patents on the cancer stem cell and cancer therapy by cancer stem cells will be discussed.
Keywords: Cancer stem cell, biomarker, signal pathway, drug resistance, natural compound, Mesenchymal stem cells, differentiation therapy
Introduction
In the world, cancer remains a major cause of mortality. Despite great progresses have been made in understanding the molecular basis of cancer, the progress in cancer detection and treatment, mortality is still high and there still is not a cure despite great improvements have been made in therapies. The current treatment regimens for cancer have shown limited survival benefits when used for most advanced stage cancers, because these treatments primarily target tumor bulk but not cancer stem cells [1,2]. Indeed, conventional cancer therapies target neoplastic cells that are largely fast-growing, suggesting that cancer stem cells may survive due to their high resistance to drugs and slower proliferation rate [3]. All the traditional cancer therapies including surgery, hormonal therapy, anti-angiogenesis therapy, and immunotherapy show a lack of efficacy in terms of long-term outcome because of their failure to target cancer stem cells and toxicity due to non-specific effects on normal cells. In this review, we will focus on the following aspects: 1, Identification of cancer stem cells and therapies that were developed to target them. In recent years, some molecules (such as CD133, CD44, ABCG2, ALDH) have been defined as the biomarkers of some kind of cancer stem cells, and the aberrant signal pathways (such as Wnt, Notch and Hedgehog signal pathway) have also been suggested as another feature of cancer stem cells. Therapeutics that based on those characters have been developed and some are on clinical trials now. 2, we also discussed the natural compounds that own the ability to target cancer stem cells, the mesenchymal stem cell-mediated gene therapy, to induce cancer stem cell differentiation and some other therapies. Current research is helping us to understand cancer stem cells and in turn this will help to develop novel therapies to eliminate cancer and the initiating cancer stem cell.
Cancer stem cells
Cancer stem cells are cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. It is often considered to be associated with chemoresistance and radio-resistance that lead to the failure of traditional therapy [4]. There appear to be several sources from which cancer stem cells may arise. They may arise from normal ASCs (adipose-derived stromal cells), from more restricted progenitor cells or even from differentiated cells [5]. Normal stem cells are more likely to be the targets of mutants and leading to the formation of CSCs for they already possess active self-renewal pathways. It is also possible for progenitors and other differentiated cells to give rise to CSCs, though they would have to acquire more genetic mutations, especially in self-renewal genes. However, it has been hypotheses that CSCs arising from normal stem cells are more aggressive than those from progenitor cells, though this remains to be proven [6]. In cancer research experiments, tumor cells are sometimes injected into an experimental animal to establish a tumor. The efficient tumor formation requires thousands or tens of thousands of cells to be introduced, however, only a small fraction of the injected cells, the CSCs, have the potential to generate a tumor. In human acute myeloid leukemia the frequency of these cells is less than 1 in 10,000. The first CSC was identified in human acute myeloid leukemia (AML), showed that a rare malignant cell with the ability to repopulate the entire original disease over several transplantations, implying self-renewal and capacity to differentiate, was only found within the immature CD34+CD38-, but not the CD34+CD38+ subpopulation [7]. After that, cancer stem cells were found in some solid tumors subsequently. The first solid CSCs were identified in breast tumors in 2003 [8], and then CSCs were isolated from brain [9], colon [10], melanoma [11], pancreatic [12], prostate [13], ovarian [14], lung [15] and gastric [16] cancers. The emerging picture on CSCs is creating significant excitement and interest in the cancer field. It is believe that the targeting of CSCs offers important and revolutionary advances in the targeting of cancer. Eradicating cancer stem cells, the root of cancer origin and recurrence, has thought as a promising approach to improve cancer survival or even to cure cancer. In the research of killing cancer stem cells, many possible ways were developed to achieve this objective, including molecular targeted therapy, target molecular signaling pathways, natural compounds and their potent to target CSCs, the use of mesenchymal stem cells, and differentiation therapy. Though great progresses have been made in recent year, the accurate mechanism of cancer stem cell is still not clear and the really effective therapy is still not found. Here, we will discuss the new therapeutic approaches to cancer based on the existence of the cancer stem cells.
Biomarkers based therapy
Cancer stem cells have been identified in a growing number of hematopoietic cancer and solid tumors and are typically recognized by virtue of the expression of cell surface markers. These cells have been isolated from the bulk-tumor population by the expression pattern of cell surface proteins (e.g., CD24, CD44, CD133) and cellular activities, such as the efflux of Hoechst dye or aldehyde dehydrogenase activity by flow cytometry and/or fluorescence activated cell sorting (FACS). The identification of markers that allow the prospective isolation of CSCs from whole tumor tissues will lead to the understanding of important biological properties of CSCs and provide the possibility to target them.
CD133 is a glycosylated, 120KD protein with five transmembrane domains and two large extracellular loops. CD133+ phenotype was first used to identify and isolate brain tumor stem cells in malignant tumors and now it has recently been used to define the CSC populations in lung, pancreatic, liver, prostate, gastric, colorectal, and head and neck cancers. The expression of genes known to play important roles in the maintenance of cancer stem cells have been investigated in putative CD133+ CSC populations of multiple tissues. These CD133+ cells undergo multi-lineage differentiation to neurons, astrocytes, and oligodendrocytes in vitro, and can recapitulate the original tumor phenotype in vivo, unlike the CD133−. Some genes associated with cancer stem cell like Nestin, BMI1, Olig2, and Nanog are also found upregulated in CD133+ populations of brain, lung, liver and prostate cancers [17-20]. CSCs is often associated with resistance to traditional chemotherapies, CD133+ cells have had increased survival in vitro and have been enriched in vivo after treatment with cisplatin, etoposide, doxorubicin, and paclitaxel, as the expression of genes known to be markers of stemness, ABC transporters and the DNA repair pathway [21,22].
CD44 is reported as at least one characteristic of CSCs across tissues, including breast, pancreas, gastric, head and neck, ovarian and colon, whereas other markers (e.g., CD24) are not. Early results showed that invasive CD44+ prostate cells also had increased expression of Nanog, BMI1 and SHH, which is similar to CD133+ cells [23]. The standard CD44 (CD44s) molecule is an 85- to 90-kDa transmembrane glycoprotein containing 10 standard exons and four major domains, including the hyaluronan-binding and variably spliced regions, the transmembrane sequence and the intracellular cytoskeletal-signaling domain. Interactions between CD44 and the extracellular matrix glycosaminoglycan hyaluronan (HA) are currently an exciting area of investigation. Several studies show that the binding of CD44 with HA protein is crucial for tumor progression [24] and also some other research suggest that CD44 variants play an important role in metastasis, especially the CD44v6 isoforms.
Aldehyde dehydrogenase is a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids, which leave the liver and are metabolized by the body’s muscle and heart. This cellular function is likely crucial for CSC longevity and probably a key explanation for the reported resistance of CSCs to chemotherapies, especially those that generate toxic aldehyde intermediates [25]. ALDH+ is also investigated as a marker and leukemia CSCs are determined to be highly ALDH positive. ALDH activity is usually measured by using BIODIPY aminoacetaldehyde (BAAA), commonly known as Aldefuor [26]. In the past few years, ALDH has been used to characterize CSCs in breast, lung, head and neck, colon and liver tumors. And several groups have found that shRNA and siRNA knockdown of ALDH1 in colorectal xenografts and lung cells, respectively, sensitized ALDH+ CSCs to CPA and 4-hydroperoxycyclophosphamide treatment. Although CSCs are enriched in ALDH+ populations in several tissues, it is important to acknowledge possible limitations, especially when used as a single marker.
ABCG2 is a member of the ATP binding cassette (ABC) transporters, which can pump a wide variety of endogenous and exogenous compounds out of cells. It was first cloned from doxorubicin-resistant human MCF-7 breast cancer cells and named as breast cancer resistance protein (BCRP) [27]. The side population phenotype, which is characterized by the ability to transport Hoechst 33342 out of cells, has been identified as a characteristic feature of stem cells. ABCG2 plays an important role in promoting stem cell proliferation and the maintenance of the stem cell phenotype. Based on a RNA interference approach, the suppression of ABCG2 could significantly inhibit cancer cell proliferation. Furthermore, the blocking of ABCG2 function by fumitremorgin C, a chemical inhibitor, also inhibited cell proliferation via the prolonged G0/G1 interval [28]. These data suggest that ABCG2 may contribute to cancer cell proliferation. A research learned about diffuse large B-cell lymphoma suggest high levels of ABCG2 is correlated with shorter overall survival and ABCG2 protein levels is correlated with the expression of SHH protein levels which plays a critical role in growth and differentiation during embryonic development [29]. ABCG2 is found to confer the side population (SP) phenotype and is recognized as a universal marker of stem cells [30]. Evidence is growing that the SP is enriched in CSCs because of the upregulation of “stemness” genes when compared with the non-SP cells. Recent studies have shown that ABCG2 is expressed in stem cell populations derived from a wide range of tissues, including pancreas, lung, limbal epithelium, brain and prostate. Since ABCG2 functions as a high capacity transporter with a wide range of substrates, various chemotherapy drugs included, it has been shown to participate in the multidrug resistance of tumors and lead to the cancer relapse. Side population and chemo-resistance suggest a close link between ABCG2 and CSCs.
Besides the CD133, CD44, ALDH, and ABCG2, there have been many other cell surface proteins been identified as the marker of many tumors in the recent years (Table 1). Although CD133, CD44, ALDH, ABCG2 and other proteins have been used to identify cancer stem cells, it is still important to clarify cellular and signaling functions of markers themselves. Biomarkers of the cancer stem cells not only the tools to which were used to identify the cancer stem cells, but also might become the target of many drugs which were developed to cure cancer. In the series research of those proteins, they are proved to be associated with tumor progression, maintenance and metastasis. At present, CSC markers must be clearly defined for each tissue, and investigation is needed to determine whether variations between tumors can be used as indicators of sensitivity to therapy. However, no specified drugs have been developed to target those cell markers, monoclonal antibodies and siRNA and /or shRNA could sensitize cells to traditional therapy or even cure cancer. Finally, clarifying cellular and signaling functions of markers themselves will lead to more therapeutic options to destroy tumor cells that have the ability to repopulate.
Table 1.
Cancer stem marker and their distribution
| Marker | Tumor type | Marker | Tumor type |
|---|---|---|---|
| CD133 | Brain [85] | ALDH | Breast [91] |
| Prostate [86] | Lung [92] | ||
| Pancreas [87] | Head and neck [25] | ||
| Melanoma [88] | Colon [93] | ||
| Colon [88] | Liver [17] | ||
| Liver [89] | Pancreas [94] | ||
| Lung [19] | Gastric [95] | ||
| Ovary [90] | Prostate [96] | ||
| CD44 | Colon [97] | ABCB5 | Melanoma [99] |
| Breast [8] | |||
| Prostate [13] | |||
| Pancreatic [98] | |||
| ABCG2 | Pancreas [100] | CD90 | |
| Lung [101] | |||
| Limbal epithelium [102] | T-acute lymphoblastic leukemia [108] | ||
| Brain [103] | Gliomas [109] | ||
| prostate [104] | Liver [110] | ||
| Liver [105] | |||
| Ovarian [106] | |||
| Retinoblastoma [107] | |||
Target signal pathways
Based on the research of the regulation mechanism of the cancer stem cell, cancer stem cells relied highly on the signal path ways’ stability if they want to maintain the ability to self-renewal and differentiate. Some researchers have suggested that signal path ways’ disorder or excessive activation may lead to the tumorigenicity. Understanding the mechanisms that underlie the self-renewal behavior of CSCs is of greatest importance for discovery and development of anticancer drugs targeting CSCs. During those pathways, Wnt, Notch and Hedgehog signaling pathways may play an important role in the recurrence and maintenance of cancer stem cell. Though experimental evidence for CSC dependence on these pathways is limited, it will be important to develop CSC-selective therapies that avoid potential significant side effects caused by inhibition of normal stem cell function.
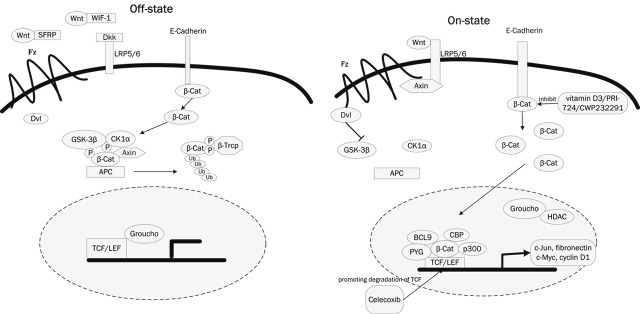
Wnt is a group of secreted signaling proteins that bind receptor molecules on the surface of target cells. The strongest evidence of the importance of the Wnt pathway to CSC biology has been reported in myeloid leukemias and it is also been reported to be implicated in the maintenance of CSCs of melanoma, breast, colon, liver and lung cancers. β-Catenin the essential mediator of Wnt signaling, is involved in two distinct functions in the cell, depending on its cellular localization. The membrane-localized β-Catenin is sequestered by the epithelial cell–cell adhesion protein E-cadherin to maintain cell–cell adhesion and the cytoplasmic accumulation of β-Catenin and its subsequent nuclear translocation eventually leads to activation of Wnt target genes such as c-Jun, c-Myc, fibronectin and cyclin D1. Activated Wnt/β-Catenin signaling is a key feature of epithelial cancers and is perceived as critical for metastasis and epithelial-mesenchymal transitionsm (EMT) [31]. For tumor cells that undergo EMT share characteristics with ESC, there is no surprise that activated Wnt signaling can be linked to stemness [32]. Some research have shown that the necessity of β-Catenin for self-renewal of both normal hematopoietic stem cells and CSCs in chronic myeloid leukemia in a mouse model, and more recently, another research have shown that β-Catenin activation is necessary for myeloid precursor transformation in a HoxA9/Meis1-transduced model of AML [33]. A lot of efforts have been made to identify small molecules capable of disrupting aberrant Wnt/β-Catenin pathway responses induced by loss of APC, which promise such agents would be therapeutically effective against colorectal cancer and other tumors. A broad spectrum of compounds seems useful to specifically modulate Wnt/β-Catenin signals. Those drugs may also help to eliminate drugs-resistant CSC, which is thought to be responsible for tumor relapse and metastasis. For instance, NSAID interferes with Wnt signaling by directly inhibiting the Wnt target COX2 (e.g. aspirin and sulindac) or by promoting degradation of TCF (Celecoxib) [34]. Natural compounds, like vitamins A and D and their derivatives, compete with β-Catenin/TCF interactions, and allow E-cadherin to relocate β-Catenin to the membrane. Furthermore, newly created inhibitors of Wnt/β-Catenin signaling have just entered preclinical trials, such as monoclonal antibodies and small interfering RNAs against Wnt1/2, WIF1 and SFRPs, PRI-724 and CWP232291 (Figure 1). The simultaneous discovery of Tankyrase (Tnks) enzymes as critical regulators of Axin and β-Catenin protein levels that can additionally be drugged has opened new opportunities for achieving this goal, the success of which had depended primarily on efforts to develop inhibitors of TCF/β-Catenin interactions. The compound XAV939 antagonizes Wnt signaling via stimulation of β-catenin degradation and stabilization of axin [35].
Figure 1.
The Wnt/β-Catenin signaling pathway and their inhibitors. A: In the off-state, Wnt ligands usually bind with Soluble frizzled-related proteins (SFRP) and Wnt inhibitory factor-1 (WIF-1) which prevent them from interact with frizzled (Fz) receptors. Dickkopf (Dkk) interacts with low density lipoprotein receptor-related protein 5/6 (LRP5/6) to inhibit binding of Wnt ligands. β-Catenin that is not bound with cadherin is phosphorylated by a complex formed by casein kinase 1α (CK1α), glycogen synthase kinase-3β (GSK3β), adenomatous polyposis coli (APC), and Axin, then it is identified by β-TrCP and lead to the ubiquitin-proteasome pathway. B: In the on state, Wnts bind to and activate FZD (Frizzled) and LRP (LDL-related receptor protein) receptors on target cells. Phosphorylation of β-Catenin is suppressed and β-Catenin escapes from the degradation. Free cytoplasmic β-Catenin translocates to the nucleus, forms a complex with TCF/LEF, and activates the transcription of target genes, such as cyclin D1, c-myc, c-Jun, and fibronectin. The coupounds vitamiD3, PRI-724, CWP232291 inhibit the β-Catenin that lead to the inhibition of Wnt/β-Catenin signaling pathway, and Celecoxi worked through promoting degradation of TCF.
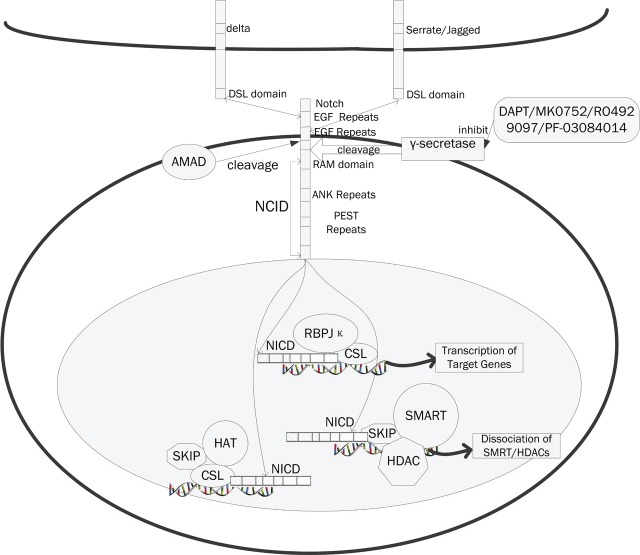
Notch signaling pathway is a highly conserved developmental pathway, which plays a critical role in cell-fate decision, tissue patterning and morphogenesis [36]. There are four human Notch receptors that consist of an extracellular peptide containing epidermal growth factor receptor-like repeats and a transmembrane peptide. Notch 1 and Notch 2 are the most ubiquitously distributed whereas Noth 2 and Notch 4 are more specifically expressed in vascular smooth muscle and endothelial cells. Ligand binding via the Jagged or Delta-like family of membrane proteins leads to cleavage of the receptor by members of the A Disintegrin and Metalloprotease (ADAM) and γ-secretase families of proteases. The Notch pathway plays an important role in maintenance of the stem cell in glioblastoma, breast cancer stem cells and some other tumor stem cells. Since the activation of Notch signaling can upregulate several factors that in turn transmit bidirectional signals among cancer cells expressing both ligands and receptors and it can also transmit signals among cancer, stroma and endothelium cells [37]. In a study learned about Notch signaling pathway in glioblastoma suggested Notch inhibition can lead to a decrease of cancer stem cells in glioblastoma via an endothelial cell intermediate [38]. In the experiment, Notch inhibition depletes CD133+ in glioblastoma and promotes increased responsiveness to radiation. Notch inhibition can be achieved in different level. 1, Inhibition of γ-secretase mediated notch cleavage. Though most of the available γ-secretase inhibitors (GSI), including DAPT, have no preference for substrates, as is commonly observed for small molecule inhibitors, several nonspecific GSI molecules (such as MK0752 and RO4929097) are currently in clinical trials for different cancers. 2, Target Notch ligands such as DLL4 (Delta like ligand 4) or inactivate Notch receptors have also been described. The OMP-21M18 has in tested in Pancreatic Cancer and the combination of anti-human Dll4 and anti-mouse Dll4 results in additive anti-tumor activity in colon tumors. 3, Modulate the Notch signaling by other pathway components. Notch1 is shown to be induced by PI3K/Akt pathway in melanoma development and in human arterial endothelial cells. GSK3α/β acted as the negative regulators of Notch1, and Notch2 was down-regulated by GSK3α/β (Figure 2).
Figure 2.
The Notch signaling pathway and their inhibitors. Notch proteins (and ligands) contain extracellular EGF-like repeats, which interact with the DSL domain of ligands. Activation of Notch upon ligand binding is accompanied by proteolytic processing that releases an intracellular domain of Notch (NICD) from the membrane. The NICD contains the RAM23 domain (RAM), which enhances interaction with CSL protein, NLS (Nuclear Localization Signals), a CDC10/Ankyrin repeat domain ANK. Upon release, the NICD translocates to the nucleus and associates with the CSL family of DNA-binding proteins to form a transcriptional activator, that activate the expression of a set of target genes, including the E (spl) (Enhancer of Split) group and others. The compounds, DAPT, MK0752, RO4929097, PF-03084014, inhibit the Notch signaling pathways through the inhibition of γ-secretase.
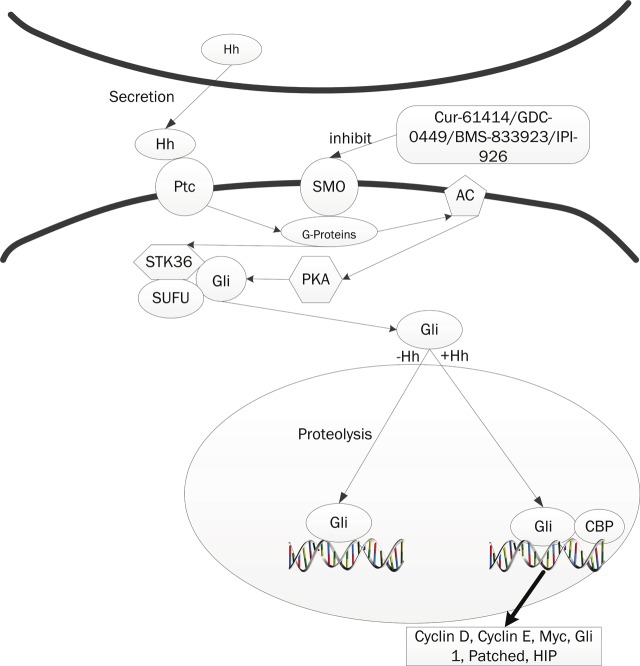
The Hedgehog (Hh) signaling pathway was initially identified in Drosophila as a critical mediator of segmental patterning during embryonic development, and it regulates the proliferation, migration, and differentiation of target cells in a spatial, temporal, and concentration dependent manner [39-42]. Pathway activation is initiated by binding of one of the three secreted and lipid-modified ligands found in mammals, Sonic (SHh), Desert (DHh), and Indian Hedgehog (IHh), to Patched (Ptch1), a 12-pass transmembrane spanning receptor. Emerging data from many tumors including glioblastoma, pancreatic adenocarcinoma, breast cancer, multiple myeloma, and chronic myeloid leukemia (CML) have suggested that Hh signaling regulates cancer stem cells. A study learned about mouse CML suggested the loss of Hh signaling by genetically disrupting Smo resulted in the inhibition of BCR-ABL expressing leukemic stem cells and prolonged survival [43,44]. Another research, learned about multiple myeloma, demonstrated that Hh signaling can act through multiple signaling modes with the same cancer and can medicate interactions between CSCs, differentiated tumor cells and the microenvironment [45]. Sonic hedgehog pathway is also linked to transcription factor NF-κB signaling. It was suggested that overexpression of Hh is activated by NF-κB in pancreatic cancer and pancreatic cancer cell proliferation is accelerated by NF-κB in part through Hh overexpression. Since the discovery of the Drosophila Hedgehog (Hh) mutation [39,46] and gene, Hh signaling has been found to play multiple roles in development, homeostasis and disease. Hhs act by activating the 7-pass transmembrane protein Smoothended (Smo), which then sends signal intracellularly. The discovery of cyclopamine and the subsequent assignment of its activity to the Hh pathway paved the way for rapid development of synthetic Hh pathway inhibitors with drug-like properties and improved bio-activity [47]. Hh inhibitors have transformed approaches which used to study Hh signaling and the treatment of associated tumors such as basal cell carcinoma and medulloblastoma. Encouraged by the identification of a druggable Hh pathway element, two groups screened diverse synthetic chemical libraries for Hh antagonists and identified some of the first Smo antagonists with drug-like properties, the Smo antagonists (SANTs) and Cur-61414 [48,49]. The outcome of these efforts was the entry of several Smo antagonists into clinical trials, including Cur-61414, GDC-0449, and BMS-833923. Also in clinical testing is a derivative of cyclopamine termed IPI-926, which features improved drug-like properties and potency (Figure 3). The existence of a small molecule–binding domain in Smo suggests that its activity is gated by Hh-dependent access to a cellular metabolite with either Smo-inhibitory or Smo-activating properties. Of all the Hh antagonists, the most developed are those targeting Smo [50].
Figure 3.
The Hh signaling pathway and their inhibitors. The Hh-signaling pathway comprises three main components: the Hh ligand; a transmembrane receptor circuit composed of the negative regulator Ptc, and the an activator Smo; and finally a cytoplasmic complex that regulates the Ci or Gli family of transcriptional effectors. Ptc, a twelve-pass membrane protein binds Hh ligand, and in the absence of ligand, Ptc interacts with and inhibits Smo, a sevenpass membrane protein. When Hh binds Ptc, its interactions with Smo are altered, leads to Ci/Gli protein entering the nucleus and acting as a transcriptional activator for the same genes. Cur-61414, GDC-0449, BMS-833923 and IPI-926 could antagonize the smo that lead to the inhibiton of Hh-signaling pathway.
Thus success in identifying chemicals that target signal transduction pathways, such as the Wnt, Notch and Hh pathways, would open up new possibilities for mono-therapeutic or combinatorial therapeutic options that may improve current strategies that target general mechanisms of rapid cell growth. There are many chemicals that have been developed and on clinical trial, and those compounds would be shown on Table 2.
Table 2.
Update on clinical trials for CSC mole targets
| Target | Drug | Cancer | Target | Phase | Date | Gov Identifier |
|---|---|---|---|---|---|---|
| Wnt | vitamin D3 | Basal Cell Carcinoma | β-catenin | III | 201109-201208 | NCT01358045 |
| PRI-724 | advanced solid tumors | CBP/β-catenin | I | 201102-201303 | NCT01302405 | |
| CWP232291 | AML | β-catenin | I | 201107-201212 | NCT01398462 | |
| Notch | MK0752 | Advanced BreastCancer | γ-secretase | I | 200504-201108 | NCT00106145 |
| RO4929097 | Lung Cancer | γ-secretase | II | 201009-201109 | NCT01193868 | |
| PF-03084014 | Leukemia | γ-secretase | I | 200906-201303 | NCT00878189 | |
| OMP-21M18 | Pancreatic Cancer | anti-DLL4 | I | 201008-201401 | NCT01189929 | |
| Hh | GDC-0449 | Solid tumors Colorectal | PTCH and/or | I | 200909-201709 | NCT00968981 |
| SMO | II | 200803-201009 | NCT00636610 | |||
| BMS-833923 | Basal cell | SMO | I | 200807-201312 | NCT00670189 | |
| IPI-926 | Primary Myelofibrosis Fibrosis, Bone Marrow | SMO | II | 201110-201305 | NCT01371617 |
Overcome the mechanisms of resistance
Cancer stem cell is resistant to chemo- and radiation therapy, often lead to the failure of conventional therapy and result relapse. Frequent cancer recurrence may be due to the preferential killing of differentiated cells while leaving CSCs behind. A better understanding of the mechanisms that underlying CSCs resistance to treatment is necessary and may provide a more effective therapy to overcome the resistance.
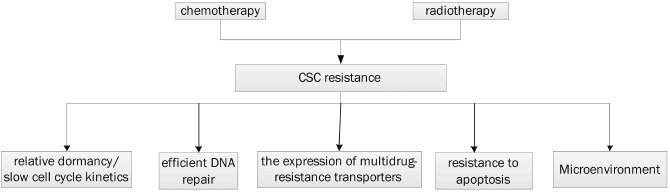
A number of genetic and cellular adaptations have been found confer resistance to classical therapeutic approaches, such as relative dormancy/slow cell cycle kinetics, efficient DNA repair, the expression of multidrug-resistance transporters, and resistance to apoptosis [2,51,52] (Figure 4). One potential modulator of CSC resistance to DNA targeting agents is the family of checkpoint kinases 1/2 (Chk1/2 kinases) and these kinases have higher basal and inducible activities in CSCs than in non-stem cells [53]. CSCs may also derive resistance to chemical mutagens through the expression of drug efflux pumps for they can transport the drugs out of the cells such as the ABC family. The activation of the Akt pathway and the over-amplification of apoptosis inhibitor proteins might also be conferred to CSCs resistance. It is first demonstrated in hepatocellular carcinoma CSCs, which were found to preferentially activate Akt/PKB and bcl-2 cell survival pathways [54].
Figure 4.
Schematic diagram of the mechanisms leading to cancer stem cell resistance to chemo- and radiation therapy.
An important result of the well-documented CSC resistance to radiation and chemotherapy is that these therapies often lead to an increase of resistant CSC subpopulation, perhaps even selecting for more resistant clones within a heterogeneous CSC population. Evidence of radiation-induced enrichment has been shown in both brain and breast CSCs [55-57]. CSC-specific pharmaceutical interventions are being developed that may eliminate both primary and acquired CSC chemo-resistance. This may dramatically improve the treatment of cancer by abrogating the potential for CSC-induced tumor regrowth and systemic disease spread after initial treatment. An experiment showing that pancreatic CSCs could survive and expand after serial exposures to gemcitabine, this chemoresistance was overcome by the use of CD44 or ABC transporter inhibitors [58]. There are some additional strategies to overcome therapeutic resistance during cancer treatment. 1, Concurrent Therapy: It is now well established that combination therapy helps to prevent the development of cancer resistance, but for a select group of cancer types where a single pharmaceutically correctable mutation exists. 2, Surgical Resection following induction: theoretically, CSC-specific induction chemotherapies should offer an immediate reduction in CSC metastatic potential and reduce any hematogenous and lymphatic CSC micrometastases that would otherwise diminish the efficacy of surgical resection [59,60].
The natural compounds and their ability to target cancer stem cell
In the recent years, several compounds were found have the ability to kill cancer stem cells, such as salinomycin, curcumin, sulforaphane, a novel gemini vitamin D analog(BXL0124) and so on.
Salinomycin, a polyether antibiotic acting as a highly selective potassium ionophore and widely used as an anticoccidial drug, was recently shown to act as a specific inhibitor of cancer stem cells [61]. Some studies showed that salinomycin acts as a potent inhibitor of multidrug resistance P-glucoprotein (P-gp170), and the inhibitory effect of salinomycin on P-gp function is mediated by the induction of a conformational change of the ATP transporter. In the experiment, salinomycin elicited a dose-dependent inhibition of cell growth evident both in CEM and A2780 cells, and caused an intracellular accumulation of doxorubicin with a dose-dependent effect in both CEM-VBL10 and CEMV-BL100 MDR cells, indicated that salinomycin may restores vinblastine sensitivity in vinblastine-resistant CEM-VBL10 and CEM-VBL100 cells. Salinomycin has also been demonstrated to significantly rupture the in vitro lung cancer tumorspheres from ALDH positive A549 lung cells, with a significantly down-regulated the expression of stem cell markers OCT-4, NANOG, and SOX2, which may be responsible for blocking self-renewal and proliferation. Although the mechanism of action of salinomycin is not yet clear, it appears that it might induce terminal epithelial differentiation accompanied by cell cycle arrest rather than trigger cytotoxicity.
Curcumin is a well-known dietary polyphenol present in an Indian spice, turmeric, which possesses anti-inflammatory and antioxidant activities, and has been studied as a chemoprevention agent in several cancer models. Curcumin is modulator of ABCG2, although it can also act on ABCB1 and ABCC1 [62]. A study showed curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line. In the experiment, curcumin injected at the tail vein can break through the blood-brain barrier and kills tumor cells inside the brain. This experiment is the first in vivo demonstration of the anticarcinogenic and anti-metastatic activity of curcumin in the brain. Curcumin can induce caspase3- mediated cleavage of β-Catenin, leading to the inactivation of Wnt/ β-Catenin signaling in HCT116 intestinal cancer cells. And another group has strengthened the point that curcumin could decrease β-Catenin/TCF transcription activity in all tested cancer cell lines, including gastric, colon and intestinal cancer cells, which was attributed to the reduction of nuclear β-Catenin and TCF-4 proteins [63]. Recently, it is demonstrated that curcumin was able to target breast stem/progenitor cells, as evidenced by suppressed mammosphere formation along serial passage and by a decrease in the percent of ALDH-positive cells [64].
In a study learned about marine sponges, which harbor novel and undefined compound with antineoplastic activity, were found strongly reduced viability of tumor cells at a dilution of 1:1,000, but was less toxic to normal fibroblasts and endothelials [65]. They identified crambe (Demospongiae: Poecilosclerida) marine sponge extract (CR) as most effective in high dilution, and demonstrated extract of a marine sponge targets CSC characteristics by affecting self-renewal potential, apoptosis resistance, invasive potential, and tumorigenicity in mice.
Sulforaphane was recently described to eliminate pancreatic CSCs by down regulation of NF-ΚB activity without inducing toxic side effects [66]. The phenomenon has been reported in prostate and colon cancer cells [67,68]. In a combination with Sofafenib to kill pancreatic cancer stem cell, SF (sulforaphane) completely eradicated SO-induced NF-ΚB binding, which was associated with abrogated clonogenicity, spheroid formation, ALDH1 activity, migratory capacity, and induction of apoptosis. In vivo, combination therapy reduced the tumor size in a synergistic manner. This was due to induction of apoptosis, inhibition of proliferation and angiogenesis, and down-regulation of SO-induced expression of proteins involved in epithelial-mesenchymal transition. And suggested that SF may be suited to increase targeting of CSCs by SO. It also showed that sulforaphane is effective in targeting breast cancer stem/progenitor cells in vitro and in vivo, at concentrations (0.5-5μM) approximately 10-fold lower of that exhibiting anti-proliferative effect on cancer cell culture. Several studies have reported the activity of sulforaphane of down-regulating Akt pathway in ovarian, prostate and colorectal cancers [69,70]. Recently, PI3K/Akt pathway was demonstrated to play an important role in regulating breast stem/progenitor cells by promoting β-Catenin downstream events through phosphorylation of GSK3β [71].
Vitamin D3 has been shown to reduce the incidence of human breast, prostate and colon cancers, induce apoptosis and cell cycle arrest of various cancer cells. Another group investigated for the inhibitory effect of a novel Gemini vitamin Danalog, BXL0124, on mammary tumor growth and CD44 expression in MCF10DCIS human breast cancer in vitro and in vivo. It is found that BXL0124 markedly decreased the expression of CD44 protein, which is identified as a cancer stem cell marker, through VDR (vitamin D receptor)- and p53-dependent mechanisms [72]. In 2001, it is demonstrated that vitamin D3 promoted the differentiation of colon carcinoma cells by induction of E-cadherin expression and the inhibition of β-Catenin signaling [73]. Ligand-activated vitamin D receptor competed with TCF-4 for β-Catenin binding, thereby reducing levels of c-Myc, peroxisome proliferator-activated receptor, TCF-1 and CD44. These findings would trigger further investigations of vitamin D3 in terms of chemoprevention of CSCs.
The compounds, including Salinomycin, Curcumin, Crambe, Sulforaphane and BXL0124 were discussed above for their direct or indirect effect on target cancer stem cells and further studies are needed to investigate the underling mechanisms (Table 3). For those diet-based compounds are usually multi-targeted, they may mediate other cellular events, such as induction of CSC differentiation, sensing CSCs to chemotherapeutic agents, in addition to their potential impact on self-renewal signaling. Investigating the efficacy of those compounds against CSCs will provide rationale for preclinical and clinical evaluation of these compounds, and will eventually enable us to discover more effective strategies for cancer treatment to reduce cancer resistance and recurrence and to improve patient survival.
Table 3.
Natural compounds and their molecular targets
| Compound | Molecular target |
|---|---|
| Salinomycin | P-gp |
| Curcumin | ABCG2 |
| ABCB1 | |
| ABCC1 | |
| Wnt/ β-Catenin | |
| Sulforaphane | NF-kB |
| Akt pathway | |
| Vitamin D3 | CD44 |
| Wnt/ β-Catenin |
Mesenchymal stem cell-mediated gene therapy for cancer
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types, including: osteoblasts, chondrocytes, and adipocytes [74]. They have generated a significant amount of interest as a result of their apparent ability to home to the tumor site following systemic delivery. The combination of cellular therapy and gene delivery is an attractive option for it will potentially protect the vector from immune surveillance, and will support targeted delivery of a gene or therapeutic proteins to the tumor sites [75].
MSCs potential ability for target gene delivery in the context of cancer is an exciting area of research that has gained considerable momentum in recent years. Some studies reported engineered MSCs specifically targeting multiple tumor types followed by local secretion of pigment epithelium-derived factor, therapeutic proteins (IFNβ, IL-2), TNF-related apoptosis inducing ligand(TRAIL), expression of prodrug activating suicide genes ( herpes simples virus-thymidine kinase, cytosine deaminase) and delivery of replicating oncolytic viruses [76]. Integration of MSCs into the tumor site is thought to be mediated by high local concentrations of inflammatory chemokines and growth factors. The most important chemokine receptor implicated in targeted homing of MSCs is CXCR4, which has potential in cell mobilization and homing [77]. The degree of inflammation in the tumor site plays an important role in the recruitment of MSCs. In a study of MSC-IFNβ-mediated therapy of pancreatic cancer, treatment with anti-inflammatory agents resulted in reduction of MSC engraftment in the tumor.
Irradiation resulted in apoptosis and increased release of inflammatory signals at the site of radiation, including TNFα, PDGF, as well as CLLS and CCR8 [78]. Radiotherapy is a traditional cancer therapy, however, and therefore could work in combination with MSC-based gene delivery to support improved targeting of MSCs to tumors. Along with their tumor tropism, MSCs have been shown to integrate into and persist in the tumor stoma. The integration has supported their use in delivering various biological agents, whose systemic administration is blocked due to their short half-life and toxicity at the doses required for therapy. MSCs can efficiently produce biological products at the tumor sites, and in a number of tumor models, MSCs expressing IFNβ have been shown to result in decreased tumor burden and increased animal survival [79,80]. A significant advantage of MSCs as cellular vehicles is their accessibility for genetic manipulation in vitro.
A study has engineered MSCs to express TNF-related apoptosis-inducing ligand (TRAIL) which causes apoptosis and death of cancer cells, without harming normal cells, by binding to specific TRAIL receptors and leading to activation of the extrinsic apoptosis pathway [81]. Their experiment demonstrate that TRAIL-expressing MSCs are able to kill both SP and non-SP cells in squamous and adenocarcinoma lung cell lines with equal efficacy. This suggested that MSCs can be used as a cellular vehicle to deliver genes to the tumor site. There is also another group demonstrated that MSCs could inhibit the proliferation of SK-MES-1 and A549 cells, and induce the apoptosis of tumor cells in vitro via some soluble factors. In the advanced research, it is suggested that MSCs could really inhibit the lung cancer cell proliferation by the secretion of the soluble factors, which could also interfere in tumor angiogenesis via the down regulation of VEGF expression in tumor cells.
Great progresses have been made in the research of MSC, and suggested promising potential for MSC-mediated delivery of therapeutic agents directly to tumor tissue. MSCs have plenty of advantages as cellular vehicles such as easy to isolate and expand, specifically target tumors and metastases following systemic delivery, easier to be transduced with a range of vectors, have immunosuppressive properties and the ability to express therapeutic proteins in secretory form. The potential for MSC-mediated tumor promotion must be addressed. A better understanding of the MSCs’ biology, and the specific combination of factors controlling their tumor-specific migration and persistence, will support translation to the clinical setting.
Differentiation therapy
Differentiation therapy is an approach to the treatment of advanced or aggressive malignancies so that they can resume the process of maturation and differentiation into mature cells. It aims to force the cancer cell to resume the process of maturation. Differentiation therapy may use either known differentiation inducing agents and/or newly designed differentiation-inducing agents. Vitamin A and its analogue (retinoid) can reverse the malignant progression process through signal modulations mediated by nuclear retinoid receptors and alltrans retinoic acid leads to frequent remission of acute promyelocytic leukemia by inducing promyelocyte differentiation [82] .
The new differentiation-inducing agents are represented by those ligands that can normally induce stem cells to undergo asymmetric mitosis. Those agents can be delivered to the cancer stem cells to force them to switch from a symmetric to an asymmetric mitotic program. Such agents would include gene products of Wnt, Hedgehog, TGF, and EGF. On the other hand, using inhibitors such as antisense or ribozyme agents that block specific factors, which usually either inhibit asymmetric mitosis or activate symmetric mitosis, could cause asymmetric cancer stem line mitosis [94]. For example, the GSK-3β which normally inhibit the native Wnt pathway, could be blocked by antisense or ribozyme agent specific for the GSK-3β (Xenopus), an inhibitor of β-Catenin. Factors which block the allelic pairing/exchange such as SNRPN-inhibitors, zeste-inhibitors, and others could be inhibited by antisense or ribozyme agents. And also, antisense or ribozyme therapy can be used to inhibit expression of gene products that block asymmetric cell division. There are several methods that could be used to induce cancer stem cell differentiation, such as by delivering differentiation-inducing agents, differentiation-inducing ligands to the tumor sites, by activation of positive or inhibition of negative agents in the asymmetric mitotic pathway downstream of the ligand-receptor binding. In addition, there are other methods that induce a cancer stem line to activate differentiationrelated gene products. Therefore, it has been shown that starvation can lead cells to become growth quiescent and at times differentiate or undergo apoptosis if their mitotic program is changed such as c-myc deregulation. Indeed, inhibitors of Wnt signaling, such as ICG-001 showed promising in vitro and in vivo efficacy without toxicity, due to its benefit of differentiation of colon cancer cells [83,84].
Perspective
Though the use of multidisciplinary approaches to cancer therapy, significant strides have been made in the treatment of cancer. There is increasing awareness that cancer stem cells represent a significant challenge to effective treatment of cancer as they are resistant to current clinical drugs. A major challenge for producing agents against CSCs is to distinguish the CSCs from the normal stem cells. This will need the identification of molecular targets unique to cancer stem cells. Better understanding of normal stem cell biology as well as cancer stem cell biology will be essential for the identification of such targets. Though many compounds, methods have been developed to target the cancer stem cell, there still have many blockades to overcome. The ultimate challenge in coming years will be the understanding of the stem cell programmes, particularly the control of self-renewal, in an attempt to develop novel, stem cell-directed therapies. And, reducing the risk of relapses and minimizes long-term side effects for our patients should always remain the ultimate goal of target CSCs.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 3.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 4.Moltzahn FR, Volkmer JP, Rottke D, Ackermann R. "Cancer stem cells"-lessons from Hercules to fight the Hydra. Urol Oncol. 2008;26:581–589. doi: 10.1016/j.urolonc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 12.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 14.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 15.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, Matsuzaki Y, Kitagawa Y. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34:1201–1207. [PubMed] [Google Scholar]
- 17.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 18.Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 19.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133(+) cancer stemlike cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010;289:151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Klarmann GJ, Hurt EM, Mathews LA, Zhang XH, Duhagon MA, Mistree T, Thomas SB, Farrar WL. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clinical & Experimental Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, Shin DM, Donnenberg VS, Whiteside TL, DeLeo AB. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 26.Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, Smith C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S, Zhang L, Han Z. Suppression of ABCG2 inhibits cancer cell proliferation. Int J Cancer. 2010;126:841–851. doi: 10.1002/ijc.24796. [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Singh RR, Cho-Vega JH, Drakos E, Davuluri Y, Khokhar FA, Fayad L, Medeiros LJ, Vega F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YZ, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng ZH, Zon LI, Armstrong SA. The Wnt/beta-Catenin Pathway Is Required for the Development of Leukemia Stem Cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi-Yanaga F, Kahn M. Targeting Wnt Signaling: Can We Safely Eradicate Cancer Stem Cells? Clinical Cancer Research. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 36.Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80:690–701. doi: 10.1016/j.bcp.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J, Tabar V. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- 41.Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–971. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- 42.Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–2645. doi: 10.1101/gad.6.12b.2635. [DOI] [PubMed] [Google Scholar]
- 43.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, Warmuth M. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, Munchhof M, VanArsdale T, Beachy PA, Reya T. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelton CC, Zhu L, Chau D, Yang L, Wang R, Djaballah H, Zheng H, Li YM. Modulation of gamma-secretase specificity using small molecule allosteric inhibitors. Proc Natl Acad Sci U S A. 2009;106:20228–20233. doi: 10.1073/pnas.0910757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peukert S, Miller-Moslin K. Small-molecule inhibitors of the hedgehog signaling pathway as cancer therapeutics. ChemMedChem. 2010;5:500–512. doi: 10.1002/cmdc.201000011. [DOI] [PubMed] [Google Scholar]
- 48.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, Yu LC, Behnke ML, Nair SJ, Hagel M, White K, Conley J, Manna JD, Alvarez-Diez TM, Hoyt J, Woodward CN, Sydor JR, Pink M, MacDougall J, Campbell MJ, Cushing J, Ferguson J, Curtis MS, McGovern K, Read MA, Palombella VJ, Adams J, Castro AC. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52:4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 50.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 51.Blagosklonny MV. Why therapeutic response may not prolong the life of a cancer patient: selection for oncogenic resistance. Cell Cycle. 2005;4:1693–1698. doi: 10.4161/cc.4.12.2259. [DOI] [PubMed] [Google Scholar]
- 52.Frosina G. DNA repair in normal and cancer stem cells, with special reference to the central nervous system. Curr Med Chem. 2009;16:854–866. doi: 10.2174/092986709787549253. [DOI] [PubMed] [Google Scholar]
- 53.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 54.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 55.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 59.Newman EA, Simeone DM, Mulholland MW. Adjuvant treatment strategies for pancreatic cancer. J Gastrointest Surg. 2006;10:916–926. doi: 10.1016/j.gassur.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Liu JM, Mao BY, Hong S, Liu YH, Wang XJ. The postoperative brain tumour stem cell (BTSC) niche and cancer recurrence. Adv Ther. 2008;25:389–398. doi: 10.1007/s12325-008-0050-x. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y. Effects of salinomycin on cancer stem cell in human lung adenocarcinoma A549 cells. Med Chem. 2011;7:106–111. doi: 10.2174/157340611794859307. [DOI] [PubMed] [Google Scholar]
- 62.Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett. 2010;293:65–72. doi: 10.1016/j.canlet.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579:2965–2971. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ottinger S, Kloppel A, Rausch V, Liu L, Kallifatidis G, Gross W, Gebhard MM, Brummer F, Herr I. Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int J Cancer. 2012;130:1671–1681. doi: 10.1002/ijc.26168. [DOI] [PubMed] [Google Scholar]
- 66.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Buchler MW, Zoller M, Salnikov AV, Herr I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 67.Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, Xiao D, Brown CK, Singh SV. D, L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 68.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 69.Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, Reddy BS, Huang MT, Newmark HL, Kong AN. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 70.Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–6866. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 71.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol. 2011;79:360–367. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 75.Petrie Aronin CE, Tuan RS. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:67–74. doi: 10.1002/bdrc.20174. [DOI] [PubMed] [Google Scholar]
- 76.Dwyer RM, Khan S, Barry FP, O'Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1:25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 78.Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M, Marini FC. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 80.Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M, Marini FC. Mesenchymal stromal cells alone or expressing interferon- beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 81.Loebinger MR, Sage EK, Davies D, Janes SM. TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br J Cancer. 2010;103:1692–1697. doi: 10.1038/sj.bjc.6605952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia L, Wurmbach E, Waxman S, Jing Y. Upregulation of Bfl-1/A1 in leukemia cells undergoing differentiation by all-trans retinoic acid treatment attenuates chemotherapeutic agent-induced apoptosis. Leukemia. 2006;20:1009–1016. doi: 10.1038/sj.leu.2404198. [DOI] [PubMed] [Google Scholar]
- 83.Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102:12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 85.Cheng JX, Liu BL, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev. 2009;35:403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the "stem cell marker" CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaksch M, Munera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008;68:7882–7886. doi: 10.1158/0008-5472.CAN-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding W, Mouzaki M, You H, Laird JC, Mato J, Lu SC, Rountree CB. CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis. Hepatology. 2009;49:1277–1286. doi: 10.1002/hep.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, Allan AL. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2009;13:2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomuleasa C, Mosteanu O, Susman S, Cristea V. ALDH as a tumor marker for pancreatic cancer. J Gastrointestin Liver Dis. 2011;20:443–444. author reply 444. [PubMed] [Google Scholar]
- 95.Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY. Salinomycin can effectively kill ALDH(high) stem-like cells on gastric cancer. Biomed Pharmacother. 2011;65:509–515. doi: 10.1016/j.biopha.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Hellsten R, Johansson M, Dahlman A, Sterner O, Bjartell A. Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PLoS One. 2011;6:e22118. doi: 10.1371/journal.pone.0022118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li CW, Heidt DG, Dalerba P, Burant CF, Zhang LJ, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 99.Gazzaniga P, Cigna E, Panasiti V, Devirgiliis V, Bottoni U, Vincenzi B, Nicolazzo C, Petracca A, Gradilone A. CD133 and ABCB5 as stem cell markers on sentinel lymph node from melanoma patients. Ejso. 2010;36:1211–1214. doi: 10.1016/j.ejso.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. doi: 10.1016/S0006-291X(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 101.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 103.Islam MO, Kanemura Y, Tajria J, Mori H, Kobayashi S, Hara M, Yamasaki M, Okano H, Miyake J. Functional expression of ABCG2 transporter in human neural stem/progenitor cells. Neuroscience Research. 2005;52:75–82. doi: 10.1016/j.neures.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 104.Apati A, Orban TI, Varga N, Nemeth A, Schamberger A, Krizsik V, Erdelyi-Belle B, Homolya L, Varady G, Padanyi R, Karaszi E, Kemna EWM, Nemet K, Sarkadi B. High level functional expression of the ABCG2 multidrug transporter in undifferentiated human embryonic stem cells. Biochimica Et Biophysica Acta-Biomembranes. 2008;1778:2700–2709. doi: 10.1016/j.bbamem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Shi GM, Xu Y, Fan J, Zhou J, Yang XR, Qiu SJ, Liao Y, Wu WZ, Ji Y, Ke AW, Ding ZB, He YZ, Wu B, Yang GH, Qin WZ, Zhang W, Zhu J, Min ZH, Wu ZQ. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J Cancer Res Clin Oncol. 2008;134:1155–1163. doi: 10.1007/s00432-008-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dou J, Jiang CL, Wang J, Zhang X, Zhao FS, Hu WH, He XF, Li XL, Zou DD, Gu N. Using ABCG2-molecule-expressing side population cells to identify cancer stem-like cells in a human ovarian cell line. Cell Biol Int. 2011;35:227–234. doi: 10.1042/CBI20100347. [DOI] [PubMed] [Google Scholar]
- 107.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- 108.Yamazaki H, Nishida H, Iwata S, Dang NH, Morimoto C. CD90 and CD110 correlate with cancer stem cell potentials in human T-acute lymphoblastic leukemia cells. Biochem Biophys Res Commun. 2009;383:172–177. doi: 10.1016/j.bbrc.2009.03.127. [DOI] [PubMed] [Google Scholar]
- 109.He J, Liu Y, Zhu T, Zhu J, Dimeco F, Vescovi AL, Heth JA, Muraszko KM, Fan X, Lubman DM. CD90 is identified as a marker for cancer stem cells in primary high-grade gliomas using tissue microarrays. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PWK, Lam CT, Poon RTP, Fan ST. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]