Abstract
The phenomenon of tumor-to-tumor metastasis has been reported in the literature for over a century. However, it remains fairly uncommon, with fewer than 100 cases being described during that time. Virtually any benign or malignant tumor can be a recipient, but meningiomas have been implicated as the most common intracranial neoplasm to harbor metastasis. The donor neoplasm is most frequently lung or breast carcinoma, while rare cases of metastasis from other primary tumors have been reported. We report here three examples of such rare metastases. This case series reports the first documented instance involving rectal adenocarcinoma. In addition, we report two cases of metastatic prostate adenocarcinoma to a meningioma; to date of which only three cases have been published. The terms “tumor-to-tumor metastasis” and “collision tumor” are addressed, as are details of the pathology. The limitations of standard radiological imaging techniques, such as standard CT and MR, which cannot reliably identify the presence of metastasis within a meningioma are compared with physiology-based neuroimaging methods, such as perfusion MR and MR spectroscopy, which may be more useful in noninvasively differentiating tumor histology.
Keywords: Tumor-to-tumor metastasis, meningioma, adenocarcinoma, neuroimaging, pathology
Introduction
The phenomenon of tumor-to-tumor metastasis has been described in the literature for many years since Fried published the first documented case of bronchogenic carcinoma metastatic to a meningioma in 1930 [1,2]. However, this remains fairly uncommon with fewer than 100 cases being described to date. Virtually any benign or malignant tumor can be a recipient, but meningiomas have been implicated/cited as the most common intracranial neoplasm to harbor metastasis [2-4]. An exhaustive literature search yielded 84 documented cases of this tumor-in-tumor phenomenon, in which, the donor neoplasm is most frequently breast carcinoma, followed by lung [2,3,5]. Less common primary sites yielding such metastasis have been reported, including but not limited to renal and rarely prostate or genitourinary. We present three cases of adenocarcinoma, metastatic to intracranial meningioma, with a review of the literature.
Case reports
Case 1
A 77-year-old male initially presented with irregular bowel movements, rectal bleeding and was later diagnosed via colonoscopy and biopsy with poorly differentiated rectal adenocarcinoma. At the time of diagnosis, work up for distant metastatic disease was negative. The patient underwent a low anterior resection and pathology showed a moderately differentiated adenocarcinoma invading into the serosa. The distal margins of the surgical specimen and 15/25 lymph nodes were all positive for metastatic adenocarcinoma. Postoperatively, the patient was treated with adjuvant chemotherapy and radiation.
Approximately one year later, the patient returned to the gastrointestinal clinic complaining of a mass on his calvarium, short term memory loss, and difficulty speaking. MR imaging of the brain showed multiple intracranial lesions. A large extra-axial enhancing lesion within the left pterional region was observed. This lesion had characteristics of a meningioma and was creating mass effect upon the left anterior temporal lobe and the sylvian fissure. An intra-axial enhancing lesion that was cystic and hemorrhagic was also seen in the left temporal lobe just anteromedial to the previously noted lesion (Figure 1A). A second extra-axial lesion of the vertex was noted to have some characteristics of a meningioma. However, the lesion was destroying the cortex and appeared to be obstructing the superior sagittal sinus suggesting metastasis. At least three other subcentimeter ring-enhancing lesions were seen scattered throughout both hemispheres and were thought to represent metastasis.
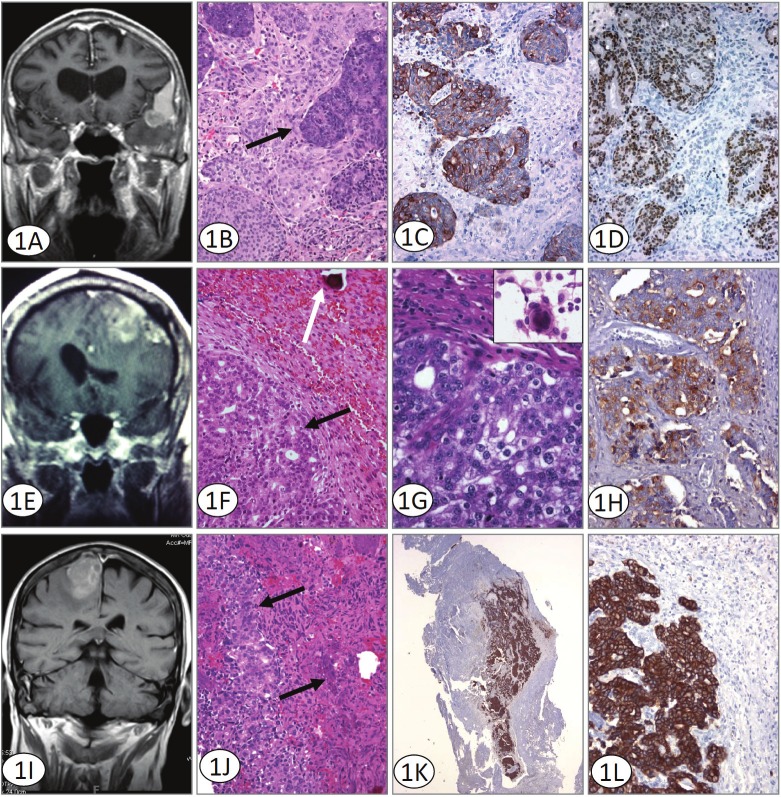
Figure 1.
A. Coronal T1 weighted MRI with contrast shows enhancement of the pterional meningioma, with dural tail sign superiorly and metastatic lesion involving the anteromedial part of the tumor. Although radiographically a collision tumor could be considered, histologically islands of metastatic adenocarcinoma surrounded entirely by meningioma are seen. B. H&E stained sections illustrate metastatic colorectal adenocarcinoma (solid arrow) within otherwise typical meningioma. Original magnification 200x. C. Immunohistochemistry for Cytokeratin 20 supports a colorectal origin for the metastatic component defining solid areas and islands of immunoreactive tumor. Cytokeratin 20 immunoreacted sections. Original magnification 200x. D. Immunohistochemistry for Cdx-2 confirms the origin of the tumor and defines solid areas and islands of immunoreactive tumor. Cdx-2 immunoreacted sections. Original magnification 200x. E. MRI revealed a large, 6cm left frontal mass containing blood adjacent to a prominent area of calvarial hyperostosis. Both intra and extra-axial components were identified. The tumor was creating a mass effect with surrounding vasogenic edema. F and G. H&E stained sections illustrate two morphologically distinct areas; an epithelial, glandular component (black arrow) and a second area with a syncytial pattern and a well defined psammoma body (white arrow) is identified. At higher magnification the larger nuclei and prominent nucleoli of the prostatic adenocarcinoma are evident. The inset shows a well-formed meningothelial whorl seen on smear preparation. (Original magnifications 100x and 400x). H. The metastatic adenocarcinoma component of the tumor is prostate specific antibody immunoreactive. Immunohistochemistry for PSAP Original magnification 200x. I. MRI revealed a dural-based, 2.4 x2.6cm lesion located in the area of the right frontal cortex with some vasogenic edema. The lesion displayed some heterogeneity with apparent discrete areas within the tumor which differed from the larger surrounding tumor. J. H&E stained sections illustrate metastatic prostate adenocarcinoma (solid arrow) within meningioma. Original magnification 200x. K and L. The prostate adenocarcinoma component of the tumor demonstrates a discretely positive cytokeratin Cam 5.2 reaction. Immunohistochemistry for Cytokeratin Cam 5.2. Original magnification 100x and 200x.
A left frontal temporal craniotomy was performed with resection of the two distinct lesions within the left temporal lobe. Postsurgical pathology showed that the extra-axial lesion within the pterional region was in fact a meningioma and the intra-axial lesion within the left temporal lobe was metastatic adenocarcinoma. Within sections from the meningioma however, there was unequivocal metastatic adenocarcinoma. This tumor was directly metastatic to a meningioma without intervening brain tissue and showed solid tumor as well as islands of isolated adenocarcinoma, entirely surrounded by meningioma (Figure 1B). The metastatic adenocarcinoma had characteristic histology and immunohistochemistry for cytokeratin 20 and Cdx-2 confirmed colorectal origin (Figures 1C and 1D). The patient had an uneventful postoperative course and was transferred to the oncology division for further chemotherapy.
Case 2
A 58 year old right-handed male with a history of metastatic prostate cancer (Gleason 8) diagnosed in November, 2006, involving multiple areas, including both the spine and left hip, presented 18 months later complaining of numbness and progressive weakness of the left toes and foot. He was previously treated with both radiation and chemotherapy, and reported that these symptoms were absent prior to treatment. He noted that the weakness progressed caudally to above the knee and that he had eventually begun dragging his foot. Physical exam findings were significant for 4/5 strength with left ankle dorsiflexion and 3-/5 strength with left ankle plantar flexion. He also exhibited a slightly high-steppage gait on the left side.
An MRI of the spine with contrast in August, 2008 demonstrated diffuse skeletal metastases with no evidence of a spinal cord lesion or epidural cord compression. An MRI with contrast of his brain revealed a dural-based, 2.4 x 2.6cm lesion located in the area of the primary motor cortex in the right frontal region with a significant amount of vasogenic edema (Figure 1E). The radiological findings were more consistent with a meningioma rather than metastatic prostate adenocarcinoma. He subsequently underwent a right frontal craniotomy and resection of the tumor.
Histology revealed two morphologically distinct areas; an epithelial, glandular component with larger nuclei and prominent nucleoli (Figure 1F and 1G) and a second area with a syncytial and focally whirling pattern. Immunohistochemistry demonstrated a strong, discretely positive cytokeratin Cam 5.2, PSA and PSAP immunoreactive adenocarcinoma component admixed within a classic meningioma (Figure 1H).
Case 3
A 57 year old with a history of prostate carcinoma, Gleason 8, diagnosed in August 2006 with a positive bone scan in the area of the thoracic spine as well as the calvarium presented three years later for a second opinion regarding his care. At the time of diagnosis, he opted against a recommended biopsy to evaluate for possible metastasis and was treated with hormonal therapy. He complained of new onset dizziness, loss of concentration during driving and conversation, and weakness in the right arm.
An MRI revealed a large, 6cm left frontal mass containing blood adjacent to a prominent area of calvarial hyperostosis. Both intra and extra-axial components were identified. The tumor was creating mass effect with surrounding vasogenic edema (Figure 1I). The lesion was felt to be either a metastatic tumor or a meningioma. He subsequently underwent a left frontal craniotomy with gross total tumor resection with duraplasty and cranioplasty. Histopathology of the tumor demonstrated findings similar to those reported in the previous case with the metastatic prostate carcinoma surrounded by areas of meningioma (Figure 1J). The former staining strongly positive for CAM 5.2 and PSA, intermingled within the meningioma (Figure 1K and 1L).
Discussion
All reported cases are compiled and presented in Table 1. No cases of rectal adenocarcinoma metastatic to a meningioma were identified in our search of the literature, and only three prior cases of prostatic adenocarcinoma have been reported.
Table 1.
Summary of tumor-to-tumor metastasis reported in the literature
| Primary Site | # of Cases | Reference |
|---|---|---|
| Lung | 11 | [21]; [8]; [22]; [23]; [24]; [25]; [1]; [26]; [6]; [14]; [27] |
| Breast | 52 | [28]; [29]; [16]; [30]; [3]; [31]; [32]; [33]; [34]; [35]; [36]; [37]; [38]; [39]; [40]; [41]; [42]; [43]; [44]; [15]; [45]; [46]; [47]; [18] |
| Renal | 7 | [48]; [49]; [50]; [51]; [17]; [3]; [52] |
| Skin | 2 | [9]; [8] |
| Prostate | 5 | [53]; [54]; [55]; and two cases reported in the current series |
| Colon | 1 | [56] |
| Colorectal | 1 | One case - reported in the current series |
| Ovary/Cervix | 2 | [57]; [58] |
| Neuroendocrine | 1 | [59] |
| Salivary Gland | 1 | [60] |
| Stomach | 1 | [61] |
The terms “tumor-to-tumor metastasis” and “collision tumor” have often been confused with one another, and used incorrectly in the literature. Collision tumors are defined as two neighboring neoplasms that invade one another. Tumor-to-tumor metastasis has proved more difficult to define, with several proposed similar criteria to be fulfilled in order to be classified as such. These include a) the metastatic focus must be at least partially enclosed by a rim of benign, histologically distinct host tumor tissue; and b) the existence of a primary carcinoma must be proven and be compatible with the metastasis [6,7]. In addition, Takei and Powell [8] emphasize the importance of the metastatic tumor epicenter when evaluating for true tumor-in-tumor. All three of our cases fulfill these criteria.
Much debate has ensued over the issue of why meningiomas are the most frequent CNS tumors to harbor metastasis. Some attribute the event to a casual occurrence, while others offer more likely theories [3,5]. The increased frequency has been hypothesized to relate to the clinical and biological characteristics of a meningioma, such as higher incidence among intracranial neoplasms, slow growth rate, hypervascularity, and high collagen and lipid content [2,3,9-13]. All of these characteristics create a favorable, noncompetitive environment which promotes tumor growth. By contrast, the speculation that psammoma bodies within meningiomas confer some degree of protection from metastatic implants, has been expounded, however, this is not a widely accepted perspective [14]. More recent studies have focused on the interactions between the meningothelial and metastatic tumor cells. Watanabe, et al [15], suggested that the high expression of the cell adhesion molecule E-cadherin in both meningiomas and breast adenocarcinomas may play a prominent role metastatic seeding. This would explain why breast carcinoma is the most frequently described metastatic neoplasm [3,15]. Molecules involved in the disruption of cell adhesions and immunological influences have also been postulated to make a significant contribution to this phenomenon [3,16].
Characterization of tumor biology is important for prognostic information and appropriate patient management. Routine radiological imaging techniques, such as CT and MR, cannot reliably exclude the presence of metastasis within a meningioma. On CT, metastasis within a meningioma may appear as a hyperdense area or, when associated with a necrotic component, as a hypodense area. Conventional MRI may demonstrate atypical signal characteristics suggesting the presence of another tumor within a meningioma, as is seen in this case [17].
Physiology-based neuroimaging methods, such as perfusion MR and MR spectroscopy, may be useful methods in noninvasively differentiating tumor histology. Perfusion MR imaging relies on hemodynamic differences in microvasculature to discriminate between unique tissue types. Tissues that are highly vascular with densely packed capillaries, such as meningiomas, will show a greater T2 signal intensity drop, and therefore a greater regional cerebral blood volume (rCBV). On the other hand, adenocarcinomas due to their high mucin content, have more diffusely spaced capillaries correlating with a smaller T2 signal intensity drop and rCBV [18]. These differences may facilitate discrimination between tissue types prior to any surgical intervention and can assist in correct patient management.
MR spectroscopy, which provides metabolic composition for tissue samples, together with conventional MR imaging improves the proportion of correctly diagnosed cases of indeterminate intracranial lesions compared to MR imaging alone [19]. Increases in lipid/creatinine and alanine/creatinine ratios have been able to distinguish metastasis and meningiomas from other intracranial tumors, respectively. The degree of malignancy of lesions has been correlated with an increase in the lactate/creatinine ratio [20]. These observations make MR spectroscopy useful in identifying metastatic cells within a meningioma. Even so, histopathological findings remain the only reliable method of diagnosing this unique event. Consequently, when evaluating suspected lesions, pathologists should be cognizant of the numerous meningioma variants, the possibility of an occult primary making its’presence known, in addition to available medical history.
References
- 1.Fried BM. Metastatic Inoculation of a Meningioma by Cancer Cells from a Bronchiogenic Carcinoma. Am J Pathol. 1930;6:47–52. 1. [PMC free article] [PubMed] [Google Scholar]
- 2.Petraki C, Vaslamatzis M, Argyrakos T, Petraki K, Strataki M, Alexopoulos C, Sotsiou F. Tumor to tumor metastasis: report of two cases and review of the literature. Int J Surg Pathol. 2003;11:127–135. doi: 10.1177/106689690301100214. [DOI] [PubMed] [Google Scholar]
- 3.Lanotte M, Benech F, Panciani PP, Cassoni P, Ducati A. Systemic cancer metastasis in a meningioma: report of two cases and review of the literature. Clin Neurol Neurosurg. 2009;111:87–93. doi: 10.1016/j.clineuro.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt HP. Metastases of malignant neoplasms to intracranial tumours: the "tumour-in-a-tumour" phenomenon. Virchows Arch A Pathol Anat Histopathol. 1984;405:155–160. doi: 10.1007/BF00694933. [DOI] [PubMed] [Google Scholar]
- 5.Aghi M, Kiehl TR, Brisman JL. Breast adenocarcinoma metastatic to epidural cervical spine meningioma: case report and review of the literature. J Neurooncol. 2005;75:149–155. doi: 10.1007/s11060-005-1408-4. [DOI] [PubMed] [Google Scholar]
- 6.Pamphlett R. Carcinoma metastasis to meningioma. J Neurol Neurosurg Psychiatry. 1984;47:561–563. doi: 10.1136/jnnp.47.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell LV Jr, Gilbert E, Chamberlain CR Jr, Watne AL. Metastases of cancer to cancer. Cancer. 1968;22:635–643. doi: 10.1002/1097-0142(196809)22:3<635::aid-cncr2820220320>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Takei H, Powell SZ. Tumor-to-tumor metastasis to the central nervous system. Neuropathology. 2009;29:303–308. doi: 10.1111/j.1440-1789.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong A, Koszyca B, Blumbergs PC, Sandhu N, Halcrow S. Malignant melanoma metastatic to a meningioma. Pathology. 1999;31:162–165. doi: 10.1080/003130299105377. [DOI] [PubMed] [Google Scholar]
- 10.Crockard HA, Barnard RO, Isaacson PG. Metastasis of carcinoma to hemangioblastoma cerebelli: case report. Neurosurgery. 1988;23:382–384. doi: 10.1227/00006123-198809000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Best PV. Metastatic Carcinoma in a Meningioma: Report of a Case. J Neurosurg. 1963;20:892–894. doi: 10.3171/jns.1963.20.10.0892. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 13.Shimada S, Ishizawa K, Hirose T. Expression of E-cadherin and catenins in meningioma: ubiquitous expression and its irrelevance to malignancy. Pathol Int. 2005;55:1–7. doi: 10.1111/j.1440-1827.2005.01786.x. [DOI] [PubMed] [Google Scholar]
- 14.Gyori E. Metastatic carcinoma in meningioma. South Med J. 1976;69:514–517. doi: 10.1097/00007611-197604000-00042. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Fujisawa H, Hasegawa M, Arakawa Y, Yamashita J, Ueda F, Suzuki M. Metastasis of breast cancer to intracranial meningioma: case report. Am $lxfS1$ 2002;25:414–417. doi: 10.1097/00000421-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Caroli E, Salvati M, Giangaspero F, Ferrante L, Santoro A. Intrameningioma metastasis as first clinical manifestation of occult primary breast carcinoma. Neurosurg Rev. 2006;29:49–54. doi: 10.1007/s10143-005-0395-4. [DOI] [PubMed] [Google Scholar]
- 17.Han HS, Kim EY, Han JY, Kim YB, Hwang TS, Chu YC. Metastatic renal cell carcinoma in a meningioma: a case report. J Korean Med Sci. 2000;15:593–597. doi: 10.3346/jkms.2000.15.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun P, Garcia J, Tihan T, McDermott MW, Cha S. Perfusion MR imaging of an intracranial collision tumor confirmed by image-guided biopsy. AJNR Am J Neuroradiol. 2006;27:94–97. [PMC free article] [PubMed] [Google Scholar]
- 19.Moller-Hartmann W, Herminghaus S, Krings T, Marquardt G, Lanfermann H, Pilatus U, Zanella FE. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44:371–381. doi: 10.1007/s00234-001-0760-0. [DOI] [PubMed] [Google Scholar]
- 20.Bulakbasi N, Kocaoglu M, Ors F, Tayfun C, Ucoz T. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol. 2003;24:225–233. [PMC free article] [PubMed] [Google Scholar]
- 21.Cserni G, Bori R, Huszka E, Kiss AC. Metastasis of pulmonary adenocarcinoma in right sylvian secretory meningioma. Br J Neurosurg. 2002;16:66–68. doi: 10.1080/026886902753512646. [DOI] [PubMed] [Google Scholar]
- 22.Bori R, Kiss CA, Huszka E, Szucs M, Tusa M, Cserni G. [A rare case of tumor-to-tumor metastasis: secondary deposits of pulmonary adenocarcinoma in a secretory meningioma] . Magy Onkol. 2002;46:261–264. [PubMed] [Google Scholar]
- 23.Bhargava P, McGrail KM, Manz HJ, Baidas S. Lung carcinoma presenting as metastasis to intracranial meningioma: case report and review of the literature. Am $lxfS1$ 1999;22:199–202. doi: 10.1097/00000421-199904000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Jomin M, Dupont A, Wemeau J, Krivosic I, Montagne B, Lesoin F, Adenis L. [Metastasis of visceral tumors in intracranial tumors. Apropos of a metastasis of a lung cancer in an intracranial meningioma] . Neurochirurgie. 1982;28:343–347. [PubMed] [Google Scholar]
- 25.Conzen M, Sollmann H, Schnabel R. Metastasis of lung carcinoma to intracranial meningioma. Case report and review of literature. Neurochirurgia (Stuttg) 1986;29:206–209. doi: 10.1055/s-2008-1054162. [DOI] [PubMed] [Google Scholar]
- 26.Gardiman M, Altavilla G, Marchioro L, Alessio L, Parenti A, Piazza M. Metastasis to intracranial meningioma as first clinical manifestation of occult primary lung carcinoma. Tumori. 1996;82:256–258. [PubMed] [Google Scholar]
- 27.Weems TD, Garcia JH. Intracranial meningioma containing metastatic foci. South Med J. 1977;70:503–505. doi: 10.1097/00007611-197704000-00044. [DOI] [PubMed] [Google Scholar]
- 28.Elmaci L, Ekinci G, Kurtkaya O, Sav A, Pamir MN. Tumor in tumor: metastasis of breast carcinoma to intracranial meningioma. Tumori. 2001;87:423–427. doi: 10.1177/030089160108700613. [DOI] [PubMed] [Google Scholar]
- 29.Zon LI, Johns WD, Stomper PC, Kaplan WD, Connolly JL, Morris JH, Harris JR, Henderson IC, Skarin AT. Breast carcinoma metastatic to a meningioma. Case report and review of the literature. Arch Intern Med. 1989;149:959–962. [PubMed] [Google Scholar]
- 30.Baratelli GM, Ciccaglioni B, Dainese E, Arnaboldi L. Metastasis of breast carcinoma to intracranial meningioma. J Neurosurg Sci. 2004;48:71–73. [PubMed] [Google Scholar]
- 31.Bucciero A, del Basso de Caro M, Vizioli L, Carraturo S, Cerillo A, Tedeschi G. Metastasis of breast carcinoma to intracranial meningioma. Case report and review of the literature. J Neurosurg Sci. 1992;36:169–172. [PubMed] [Google Scholar]
- 32.Chou LW, Ho KH, Fong CM. Intracranial meningioma with metastatic breast carcinoma. Ann Oncol. 1992;3:409–410. [PubMed] [Google Scholar]
- 33.Savoiardo M, Lodrini S. Hypodense area within a meningioma: metastasis from breast cancer. Neuroradiology. 1980;20:107–110. doi: 10.1007/BF00339557. [DOI] [PubMed] [Google Scholar]
- 34.Doron Y, Gruszkiewicz J. Metastasis of invasive carcinoma of the breast to an extradural meningioma of the cranial vault. Cancer. 1987;60:1081–1084. doi: 10.1002/1097-0142(19870901)60:5<1081::aid-cncr2820600526>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Salvati M, Cervoni L. Association of breast carcinoma and meningioma: report of nine new cases and review of the literature. Tumori. 1996;82:491–493. doi: 10.1177/030089169608200517. [DOI] [PubMed] [Google Scholar]
- 36.Lin JW, Su FW, Wang HC, Lee TC, Ho JT, Lin CH, Lin YJ. Breast carcinoma metastasis to intracranial meningioma. J Clin Neurosci. 2009;16:1636–1639. doi: 10.1016/j.jocn.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Miyagi N, Hara S, Terasaki M, Orito K, Yamashita S, Hirohata M, Tokutomi T, Shigemori M. [A rare case of intracranial meningioma with intratumoral metastatic breast cancers] . No Shinkei Geka. 2007;35:901–905. [PubMed] [Google Scholar]
- 38.Nunnery E Jr, Kahn LB, Rudnick SA. Breast carcinoma metastatic to meningioma. Arch Pathol Lab Med. 1980;104:392–393. [PubMed] [Google Scholar]
- 39.Fabaron F, Bainier L, Vende B, Babin P, Morin M. [Metastasis of breast cancer in frontal meningioma] . Ann Radiol (Paris) 1990;33:48–50. [PubMed] [Google Scholar]
- 40.Knuckey NW, Stoll J Jr, Epstein MH. Intracranial and spinal meningiomas in patients with breast carcinoma: case reports. Neurosurgery. 1989;25:112–116. doi: 10.1097/00006123-198907000-00022. discussion 116-117. [DOI] [PubMed] [Google Scholar]
- 41.Bonito D, Giarelli L, Falconieri G, Bonifacio-Gori D, Tomasic G, Vielh P. Association of breast cancer and meningioma. Report of 12 new cases and review of the literature. Pathol Res Pract. 1993;189:399–404. doi: 10.1016/s0344-0338(11)80326-2. [DOI] [PubMed] [Google Scholar]
- 42.Cervoni L, Salvati M, Gagliardi D, Delfini R. Metastasis of breast carcinoma to intracranial meningioma. Case report. Neurosurg Rev. 1994;17:233–236. doi: 10.1007/BF00418442. [DOI] [PubMed] [Google Scholar]
- 43.Volker U, Thierauf P. [Tumor in tumore: breast cancer metastasis in a meningioma] . Pathologe. 1993;14:231–233. [PubMed] [Google Scholar]
- 44.Fornelli A, Bacci A, Collina G, Eusebi V. [Breast carcinoma metastatic to meningioma: review of the literature and description of 2 new cases] . Pathologica. 1995;87:506–512. [PubMed] [Google Scholar]
- 45.Seckin H, Yigitkanli K, Ilhan O, Han U, Bavbek M. Breast carcinoma metastasis and meningioma. A case report. Surg Neurol. 2006;66:324–327. doi: 10.1016/j.surneu.2005.11.056. discussion 327. [DOI] [PubMed] [Google Scholar]
- 46.Lieu AS, Hwang SL, Howng SL. Intracranial meningioma and breast cancer. J Clin Neurosci. 2003;10:553–556. doi: 10.1016/s0967-5868(02)00305-3. [DOI] [PubMed] [Google Scholar]
- 47.Joglekar VM, Davis CH, Blakeney CG. Metastasis of carcinoma to meningioma and glioma. Acta Neurochir (Wien) 1981;58:67–74. doi: 10.1007/BF01401684. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez Morales JC, Gutierrez Morales SE, Astudillo Gonzalez A. 72 year-old man with new seizure while dancing. Brain Pathol. 2009;19:347–348. doi: 10.1111/j.1750-3639.2009.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimiwada T, Motohashi O, Kumabe T, Watanabe M, Tominaga T. Lipomatous meningioma of the brain harboring metastatic renal-cell carcinoma: a case report. Brain Tumor Pathol. 2004;21:47–52. doi: 10.1007/BF02482177. [DOI] [PubMed] [Google Scholar]
- 50.Breadmore R, House R, Gonzales M. Metastasis of renal cell carcinoma to a meningioma. Australas Radiol. 1994;38:144–143. doi: 10.1111/j.1440-1673.1994.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 51.Peison WB, Feigin I. Suprasellar meningioma containing metastatic carcinoma. Report of case. J Neurosurg. 1961;18:688–689. doi: 10.3171/jns.1961.18.5.0688. [DOI] [PubMed] [Google Scholar]
- 52.Chahlavi A, Staugaitis SM, Yahya R, Vogelbaum MA. Intracranial collision tumor mimicking an octreotide-SPECT positive and FDG-PET negative meningioma. J Clin Neurosci. 2005;12:720–723. doi: 10.1016/j.jocn.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein RA, Grumet KA, Wetzel N. Metastasis of prostatic carcinoma to intracranial meningioma. Case report. J Neurosurg. 1983;58:774–777. doi: 10.3171/jns.1983.58.5.0774. [DOI] [PubMed] [Google Scholar]
- 54.Pugsley D, Bailly G, Gupta R, Wilke D, Wood L. A case of metastatic adenocarcinoma of the prostate arising in a meningioma. Can Urol Assoc J. 2009;3:E4–E6. doi: 10.5489/cuaj.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doring L. Metastasis of carcinoma of prostate to meningioma. Virchows Arch A Pathol Anat Histol. 1975;366:87–91. doi: 10.1007/BF00438681. [DOI] [PubMed] [Google Scholar]
- 56.Benedetto N, Perrini P, Scollato A, Buccoliero AM, Di Lorenzo N. Intracranial meningioma containing metastatic colon carcinoma. Acta Neurochir (Wien) 2007;149:799–803. doi: 10.1007/s00701-007-1239-5. discussion 803. [DOI] [PubMed] [Google Scholar]
- 57.Maiuri F, Corriero G, D'Armiento F, Giamundo A. Parasagittal meningioma associated with metastasis by ovarian carcinoma. Acta Neurol (Napoli) 1981;3:512–516. [PubMed] [Google Scholar]
- 58.Wu WQ, Hiszczynskyj R. Metastasis of carcinoma of cervix uteri to convexity meningioma. Surg Neurol. 1977;8:327–329. [PubMed] [Google Scholar]
- 59.Smith TW, Wang SY, Schoene WC. Malignant carcinoid tumor metastatic to a meningioma. Cancer. 1981;47:1872–1877. doi: 10.1002/1097-0142(19810401)47:7<1872::aid-cncr2820470726>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 60.Van Zandijcke M, Casselman J. MR imaging of a metastasis in a meningioma. Acta Neurol Belg. 1996;96:329–331. [PubMed] [Google Scholar]
- 61.Honma K, Hara K, Sawai T. Tumour-to-tumour metastasis. A report of two unusual autopsy cases. Virchows Arch A Pathol Anat Histopathol. 1989;416:153–157. doi: 10.1007/BF01606320. [DOI] [PubMed] [Google Scholar]